Abstract
Propolis (bee glue) is a bee glue, sticky resinous material released from various plant sources such as bud exudates, flowers, and leaves modified by bee secretions and wax propolis is composed of resins, waxes, polyphenols, polysaccharides, volatile materials, and secondary metabolites that are responsible for various bioactivity such as antibacterial, anti-angiogenic, antiulcer, anti-inflammatory, antioxidant, and anti-viral activities. The physico-chemical characteristics and the natural properties of various kinds of propolis have been studied for the past decade. Novel active anti-microbial compounds have been identified in propolis. Those compounds positively modulated the antimicrobial resistance of multidrug resistant bacteria. Published research has indicated that propolis and its derivatives has many natural antimicrobial compounds with a broad spectrum against different types of bacteria and that it enhanced the efficacy of conventional antibiotics. Besides, the combination of propolis with other compounds such as honey has been studied whereby, such combinations have a synergistic effect against bacterial strains such as Escherichia coli and Staphylococcus aureus. The activity of propolis is very much dependent on seasonal and regional factors, and Middle Eastern propolis have shown best antibacterial efficacy. Propolis and its main flavonoids ingredients should not be overlooked and should be evaluated in clinical trials to better elucidate their potential application in various fields of medicine. Clinical antibacterial potential and its use in new drugs of biotechnological products should be conducted. This review aims at highlighting some of the recent scientific findings associated with the antibacterial properties of propolis and its components.
Keywords: Propolis, antibacterial, antimicrobial resistance, synergistic effects, noval products
1. Introduction
Propolis (bee glue) is a bee glue, sticky resinous material released from various plant sources such as bud exudates, flowers, and leaves modified by bee secretions and wax (Simone-Finstrom et al. 2017). Propolis manufactured by Honeybees, Apes mellifera, from the sap present on needle-leaved trees or evergreens. When bees mix the sap with their own salivary releases and beeswax, propolis is formed as a clingy, darker-green substance utilized by bees to assemble their hives (Martinotti and Ranzato 2015). Since 300 BCE, propolis has been utilized as a nourishment and as a helpful drug for overall wellbeing (Wagh 2013). Propolis has been portrayed in various old scripts as an injury recuperating agent, alone or mixed with other substances (Martinotti and Ranzato 2015). Various natural properties of propolis have been accounted for including free radical scavenging, cytotoxicity, and antimicrobial activity (Pasupuleti et al., 2017, Oryan et al., 2018, Elkhenany et al., 2019). As a result of its wide scope for natural applications, recently, propolis has been broadly utilized as a supplement in drinks to improve human health and avert sicknesses (Ramos and Miranda, 2007). Propolis have been known for a long time for its an anti-viral, anti-inflammatory, anti-bacterial (Sforcin et al. 2017), anesthetic, anti-oxidant, anti-tumoral, anti-cancer, anti-fungal, anti-protozoal, anti-hepatotoxic, anti-mutagenic, anti-septic, in addition to being utilized for its cytotoxic activity, etc. (Toreti et al., 2013, Sforcin, 2016). The in vitro antibacterial activity of propolis was documented against many types of Gram-positive and Gram-negative bacteria and data of synergism exhibited among the various propolis compounds, mainly galangin flavonoids and pinocembrin(Przybyłek and Karpiński 2019).
The antioxidant and antimicrobial characteristics of propolis are extremely important for the food industry because of its potential to delay lipid oxidation and as such increase the shelf life of food products. Such characteristics are attributed to the presence of chemical substances such as: caffeic acid phenylethyl, flavanol, ester flavonoid, pinocembrin, and galangin which exerts its mechanism of action possibly by inhibiting the bacterial RNA polymerase (Funari 2006). Researchers have reported on the antibacterial activity of propolis (Gonsales et al., 2006).
Veiga et al. (2017) reported on the efficacy of poplar propolis against both Gram-positive and Gram-negative microorganisms like multidrug-resistant bacteria (e.g. Methicillin-resistant Staphylococcus aureus (MRSA)). The efficacy of Turkish propolis against tuberculosis was evaluated and the results indicated potential against various types of mycobacteria (Yildirim et al. 2004).
Other studies demonstrated the outstanding activity of propolis against different kinds of microorganisms including, parasites, bacteria, viruses and yeasts (Saeed et al., 2016). Numerous studies have indicated that that propolis has no toxicity and no side effects in animal models or humans (Demir et al. 2016). The Synergetic anti-microbial properties of propolis were investigated by various researchers whereby in most of these in vitro and in vivo experiments the bacterial resistance to conventional antimicrobial agent was significantly reduced (Przybyłek and Karpiński 2019). For instance, Quercetin and some of its derivatives demonstrated antibacterial efficacy against MRSA, Staphylococcus epidermidis and S. aureus (Gajdács 2019). It has been also proven that the clinical antibacterial potential of propolis is related to the vast number of active compounds found in it (more than 200 substances) (Sforcin and Bankova 2011). Thus, propolis have attracted a great deal of interest for the treatment of various human infections. It has been, also, used in development of new drugs of biotechnological products (Przybyłek and Karpiński 2019). Therefore, this review aims at highlighting some of the recent scientific findings associated with the antibacterial properties of propolis and its components.
2. Composition of propolis
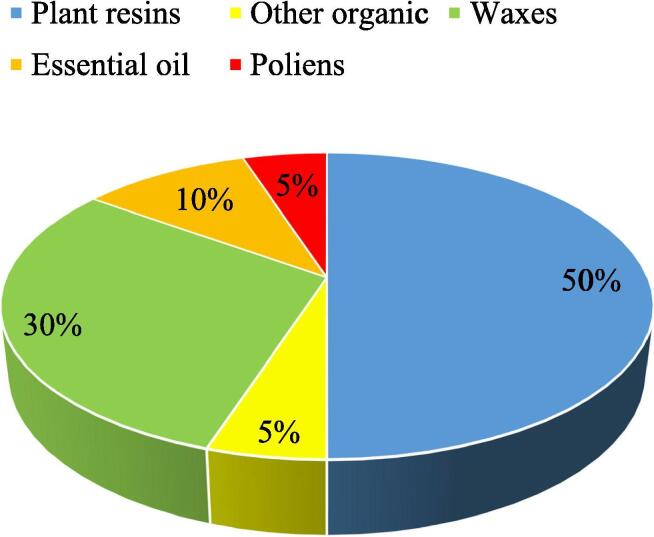
The beneficial use of propolis has prompted an expanded enthusiasm for investigating its chemical composition in relation to its botanical origins. Hence polyphenolic compounds are found in propolis produced by Apis mellifera. Flavonoids, the fundamental polyphenols in propolis, are affected by the source and ecological botanical environment where the bee lives (Toreti et al. 2013, Becerra et al., 2019). Materials accessible to honeybees for making propolis are substances mainly discharged by plants in case of injures or cuts such as lipophilic materials on leaves and leaf buds, pitches, adhesives, gums, and grids (Ristivojević et al. 2015). The synthesis of the plant-based component of propolis dictates its composition, and it is subject to its geographical location. Therefore, propolis’s properties are firmly identified with the vegetation local to the site of the bee dwellings (Bankova and Marcucci, 2000). Propolis is a complex resinous substance which is composed of the following: gum and amber resin (50% to 70%), oil and wax (30% to 50%), pollen (5% to 10%). This is in addition to and other substances like: vitamins B, C and E minerals, sugars, flavonoids, phenol, amino acids, as well as aromatic compounds (Fig. 1) (Ahangari et al. 2018).
Fig. 1.
Proportion of chemical composition of propolis ().
Source: Przybyłek and Karpiński 2019
For the most part, the composition of propolis is exceptionally inconsistent due to the variety of plant species developing around the hive, from which the honeybees gather the needed exudates (Aminimoghadamfarouj and Nematollahi, 2017, Drescher et al., 2019). The composition of propolis is very much influenced by altitude, illumination, seasonal variations, and the bee nourishment fields (Silva-Carvalho et al. 2015). A great deal of research has been conducted on the chemical and biological composition of propolis. Up to this point, in excess of 300 different compounds have been recognized in propolis collected from various geographical areas (Freires et al. 2016). The primary constituents present in propolis are flavonoids, phenolics, and mixtures of aromatic materials (Devequi-Nunes et al., 2018, Galeotti et al., 2018, Wozniak et al., 2019). Furthermore, propolis also contains some unstable oils, terpenes, and honeybee wax. Altogether, these mixes are thought to contribute in a synergistic manner to the Waxes, 30% Essential oil, 10% Poliens, 5% Other organic substance, Plant resins, 50% 3 compound properties and efficacy of propolis. The quality of propolis is one of its most significant attributes, impacting the physical properties of the nectar such as its thickness, viscosity and ability to crystallize, and also affecting properties crucial to its use including its taste, dissolvability and preservability (Simone-Finstrom and Spivak 2010).
3. Anti-bacterial efficacy of propolis
3.1. 3a. Anti-bacterial properties
The antimicrobial efficacy of propolis and some of its individual components have been documented against bacteria, viruses, fungi and protozoa (Bezerra et al., 2015, Mokhtar et al., 2016, Yildirim et al., 2016, Oryan et al., 2018, Alotaibi et al., 2019, Przybyłek and Karpiński, 2019). In a study, the data related to the anti-bacterial potential of propolis against 600 different bacterial strains have been analyzed. Various research output indicated that propolis was more effective against Gram-positive bacteria in comparison to Gram-negative ones (Table 1). The geographic location from which propolis is collected affected its antibacterial potential. Best propolis activity was noted using Middle Eastern propolis, which was extremely effective against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. (On the contrary, German, Irish and Korean propolis exhibited minimum activity (Przybyłek and Karpiński 2019). Propolis is thought to exert its antibacterial activity by either enhancing the immunity of the organism or by acting directly on the microorganism (Sforcin and Bankova 2011). For the most part, it is seen that the higher antimicrobial action of propolis against Gram-positive bacteria is due to the outer membrane structure of those bacteria (Kędzia 2013). Artepillin C is one of the various phenolic mixes found in propolis and exhibited efficient antibacterial activity against MRSA. Likewise, Wojtyczka et al. showed that Polish propolis slowed down bacterial development and affected biofilm formation (Wojtyczka et al. 2013). Furthermore, various examinations have indicated that Iranian and Brazilian propolis are effective against Gram-positive microbes, spores, bacterial growth, and infections, though they demonstrated restricted action against Gram-negative bacteria (Afrouzan et al. 2018). Interestingly, phytochemicals present in flavonoids may target various components and elements of the bacterial cell (Cushnie and Lamb 2005). In fact, an ethanolic extract of propolis known as kaempferide, is used in the treatment of S. aureus skin infections. Also, kaempferide was highly effective against Enterococcus Faecalis, Listeria monocytogenes and Staphylococcus Saprophyticus (Hernández Tasco et al., 2018, Kharsany et al., 2019). Quercetin, yet another flavonoid, found in propolis, binds to the subunit of E. coli DNA gyrase to hinder bacterial activity (Plaper et al. 2003). Furthermore, it is assumed that propolis can cause fractional bacterial lysis and can affect bacterial proteins. Numerous investigations have confirmed a synergistic action among anti-infection agents and propolis (Dantas Silva et al. 2017). For example, Chloramphenicol in the presence of Bulgarian propolis and Brazilian honey showed synergism against Salmonella typhi (Al-Ani et al. 2018), and the combination of 4 Brazilian red propolis and fluconazole were effective against Candida sp. (Al-Ani et al. 2018). Other flavonoids like pinocembrin and apigenin in propolis were investigated in Chilean propolis and revealed antibacterial activity against Streptococcus mutans (Veloz et al. 2019). Likewise, pinocembrin has shown antibacterial activity against Klebsiella pneumoniae, Listeria monocytogenes, S. aureus, Pseudomonas aeruginosa, Streptococcus sobrinus, E. faecalis, and Streptococcus. mutans (Joray et al., 2015, Kharsany et al., 2019). On the other hand, apigenin showed efficacy against the following Gram-negative bacteria: P. aeruginosa, K. pneumoniae, Salmonella enterica serotype Typhimurium, Proteus mirabilis and Enterobacter aerogenes (Nayaka et al. 2014). Like previously mentioned flavonoids, synergistic antibacterial impact was noted upon the addition of apigenin to β-lactam against MRSA (Akilandeswari and Ruckmani 2016), and when applying apigenin with ceftazidime against ceftazidime-resistant Enterobacter cloacae (Eumkeb and Chukrathok 2013). Interestingly, propolis is also rich with cinnamic acid, which showed potent efficacy against several bacteria. For example, Bacillus spp., Streptococcus pyogenes, Aeromonas spp. Micrococcus flavus, Pseudomonas aeruginosa, Yersinia ruckeri Vibrio spp., E. coli, Mycobacterium tuberculosis, L. monocytogenes, Enterobacter cloacae, Salmonella enterica serotype and Typhimurium (Guzman, 2014, Yilmaz et al., 2018). It is worthy to mention that cinnamic acid exerts its efficacy by disrupting the bacterial cell membrane, thus inhibiting the function of ATPases, bacterial binary fission, and its ability to form biofilms (Vasconcelos et al. 2018).
Table 1.
The antibacterial activities of various types of propolis.
| Types of propolis | Bacterial strains | Effects | Ref. |
|---|---|---|---|
| Iranian propolis | P. aeruginosa (PTCC 1707) and S. aureus (PTCC 1431) | Significantly higher inhibitory effect on the Gram-positive bacteria S. aureus compared to P. aeruginosa. | (Aryaei 2018) |
| Tribal propolis | Lactobacillus acidophilus and Streptococcus mutans | Ethanoic extract of propolis (EEP) effective against Lactobacillus acidophilus and Streptococcus mutans | (Airen et al. 2018) |
| Mexican propolis | Salmonella typhimurium (ATCC-13311), Escherichia coli (ATCC-10536), Staphylococcus aureus (ATCC-11632) and Listeria monocytogenes (ATCC-19115) | No effect has been observed on aqueous extract indicating that active ingredients of propolis are not soluble in water. Ethanol extract was observed antimicrobial activity against S. aureus and L. monocytogenes but no effect against for E. coli and S. typhimurium. | (Bucio-Villalobos 2017) |
| Peruvian propolis | Streptococcus Mutans ATCC 250,175 | Autumn collected propolis has higher growth of S. mutans than the growth recorded at extracts collected during summer | (Becerra 2019) |
| Brazilian propolis | Escherichia coli and Staphylococcus aureus | Ethanolic extracts of propolis (EEP) inhibited the growth of Staphylococcus aureus but not Escherichia coli. | (Gonsales 2006) |
| Saudi and Egyptian propolis | Escherichia Coliand Multi-drug Resistant Staphylococcus Aureus, | Ethyl alcohol extraction of propolis collected from Saudi Arabia (EEPS) and from Egypt (EEPE) inhibited antibiotic resistant E.coli and S. aureus. | (Al-Waili et al. 2012) |
| Kenyan propolis | Staphylococcus aureus and Bacillus subtilis | Both strains were highly susceptible for 70% ethanolic extracts of propolis (EEP) | (Muli 2007) |
4. 3b. Synergetic anti-microbial properties
There are numerous other components of propolis, for example, terpenoid lupeol, and flavonoids including: fisetin or decanoic acids, quercetin, kaempferol and chrysin (Pasupuleti et al. 2017). It is worth noting that the most abundant flavanols in food include myricetin, quercetin and kaempferol. Studies investigated the antibacterial and anti-inflammatory effects of kaempferol, chrysin and quercetin (Siriwong et al., 2016, Harasstani et al., 2017, Wang et al., 2018). Some of those have modulated the bacterial resistance to antibiotics as evidenced by quercetin which modulated bacterial resistance to conventional β‐lactam antibiotics against penicillin‐resistant S. aureus (Eumkeb et al., 2010, Harakeh et al., 2017) and treatment of bacterial related gastrointestinal disorders (da Silva et al. 2018). Quercetin and some of its derivatives demonstrated antibacterial efficacy against MRSA, Staphylococcus epidermidis, and S. aureus (Gajdács 2019). Quercetin showed efficacy against Bacillus subtilis (Aderogba et al. 2013). Based on an in vitro study, quercetin showed potency against oral bacteria like Porphyromonas gingivalis (Geoghegan et al. 2010). A synergistic effect was noted upon using quercetin with amoxicillin, whereby the bacterial resistance to this conventional antimicrobial agent was significantly reduced (Siriwong et al. 2016).
5. 3c. Biochemical processing of propolis
Biochemical treatment and processing of propolis can further result in more potent properties when compared to its original source. For instance, a study demonstrated that the removal of lipid from propolis by lipase significantly decreased the fatty acid level in the extract. It, also, lowered the levels of both flavonoids and polyphenols, which are antioxidants and caused an increase in the levels of active flavonoids, such as Artepillin C and kaempferide. Such an activity enhanced the antibacterial potential of propolis against Propionibacterium acne and Staphylococcus epidermidis (Park et al. 2015). In another example, propolis has a high free radical scavenging ability. Nonetheless, ethanolic methods for extracting the active ingredients can reduce its potency, whereas non-ethanolic extracts maintain strong radical scavenging and antimicrobial activity especially against K. pneumoniae, P. aeruginosa, Bacillus cereus and S. aureus. Accordingly, the use of higher temperatures at 70 °C can compensate for using nonethanolic solvent complexes and efficiently extract active compounds from propolis. As a result, the levels of total phenolic compounds are like those present in ethanolic extract (Kubiliene et al. 2015). A study revealed that the combination of honey with propolis from Saudi Arabia and Egypt had a synergistic effect against E. coli and S. aureus. A much better activity was noted for Saudi propolis in comparison to the Egyptian one (Al-Waili et al. 2012). Only few studies have been published on the efficacy of propolis against anaerobic bacteria. Published studies revealed a potent effect against the following anaerobic bacteria: Propionibacterium species, Prevotella, Bacteroides, Fusobacterium, Actinomyces, Porphyromonas and Clostridium (Özen et al., 2010, Shabbir et al., 2016). In fact, the results obtained from three studies using ethanolic extract of polish propolis indicated that Fusobacterium genus was most susceptible anaerobic bacteria tested. However, other anaerobic bacteria studied including: Peptostreptococcus, Clostridium, Actinomyces, Peptococcus, Bacteroides and Propionibacterium, have shown a much higher resistance.
6. Underlying anti-bacterial mechanism of propolis
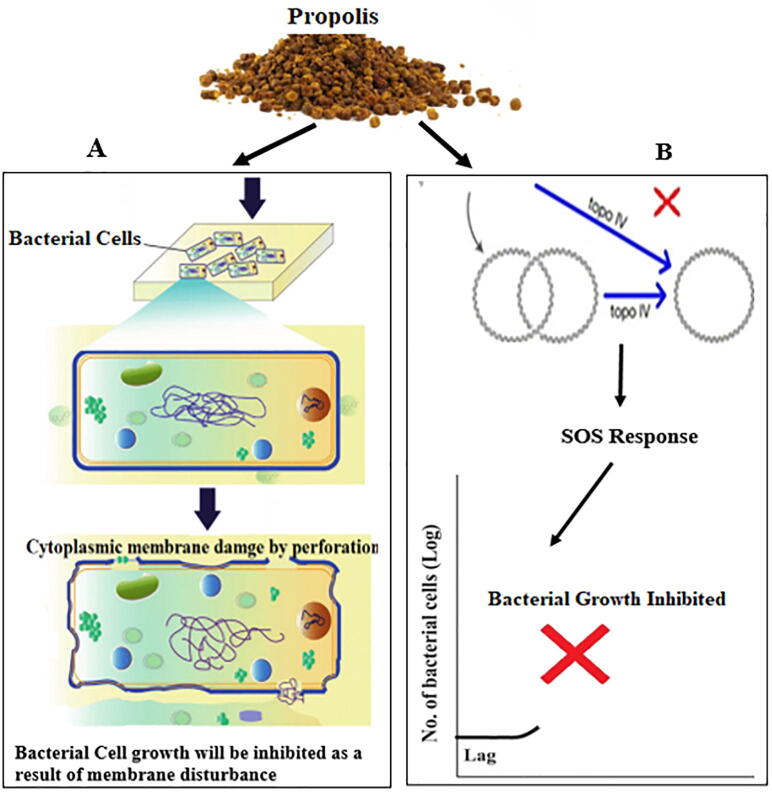
Propolis and some of its derivatives can either act directly on bacteria through several mechanisms or by affecting the immune system of the host (Fig. 2). For instance, cell membrane permeability is likely to be affected by propolis leading to a reduction in membrane-related activity, namely adenosine triphosphate (ATP) production and so compromising bacterial mobility as well as other activities. In fact, the reason behind propolis’s higher efficacy against Gram-positive bacteria as opposed to Gram-negative ones could be the hydrolytic enzymes produced in the outer membrane protein structure of the Gram-negative bacteria. Such enzymes are capable to compromise the function of the active ingredients found in propolis (Sforcin and Bankova, 2011, Kędzia, 2013). Another mechanism is that of cinnamic acid, which inhibits production of ATPases, cell division, and biofilm development by causing bacterial cell membrane damage associated with a disruption of the crucial metabolic pathways with a stress on the pH intracellular inhibition homeostasis (Yilmaz et al. 2018). However, Artepillin antibacterial activity is mediated via the modulation of the NF-kappa B (NF-κB) pathway causing an inhibition of the synthesis of prostaglandin E (2) and nitric oxide (Veiga et al. 2017). However, most of the biological activities of propolis are attributed to its flavonoid compounds, although much of it is still not well understood. It is believed that flavonoids target various bacterial structures to compromise their function, where the B ring of the flavonoids inhibit nucleic acid synthesis powered (Uzel et al. 2005). For example, quercetin inhibits ATPase activity by binding to DNA gyrase B subunit of Escherichia coli. Furthermore, flavonoids can inhibit E. coli growth by their main binding target to topoisomerase II resulting in a cleavage of the DNA. Moreover, various reports have indicated a synergistic effect between antimicrobials and propolis. It is thought that the reason behind flavonoids ability to reduce bacterial resistance to various antibacterial compounds is through propolis binding to cell wall of bacteria, resulting in the lysis of the bacterial cells and their demise (Olegário et al. 2019). In another study, it was reported that the damage E. coli membrane have occurred because of the interaction between the hydrophobic regions of the membrane and its polar head‐ group (He et al., 2014). Yet another mechanism by which propolis might synergistically enhance antimicrobial agents is its ability to inhibit protein synthesis. Synergism has been reported against Salmonella typhi upon the application of both Bulgarian and Brazilian propolis with chloramphenicol, neomycin, and tetracycline by acting on the ribosome (Orsi et al. 2012); it is believed that propolis inhibition of RNA-polymerase might be behind the synergism with agents that inhibit protein synthesis. Flavonoids can modulate the crosstalk through toll-like receptor (TLR) of host microbiota as reported in various published papers related to the influence of flavonoids on TLR gene and protein expression (Bhaskar et al. 2011). Flavonoids can result in activating of various important protein kinases responsible various intracellular signaling pathways regulation (Williams et al. 2004). For example, oxidative stress that lead to the activation of several protein kinases can be suppressed by flavonoids via the modulation of the activity of NF-κB (Comalada et al. 2005), while other flavonoids can regulate interferon-β-dependent pathways (González-Gallego et al., 2018). Another flavonoid, quercetin inhibited the kinase TBK1, present in TRIF-dependent TLR activation (Youn et al. 2006). Cytoplasmic membrane damage was produced upon the combination of ceftazidime with apigenin against ceftazidime‐resistant Enterobacter cloacae leading to a leakage of intracellular components (Eumkeb and Chukrathok 2013). Nucleic acid synthesis inhibition achieved via the inhibition of topoisomerase has been reported using different classes of flavanols, flavan‐3‐ols, and flavones classes. Inhibition of synthesis of cell membrane has also been noted (Jeong et al. 2009). In the study using three 7 flavonoids against both Gram positive and negative bacteria revealed an inhibition of protein synthesis as well as RNA and DNA (Zhang et al. 2008). Published studies elucidated the activity and its underlying mechanism of action of two flavonoids [kaempferol, hesperidin] against E. coli (Harasstani et al. 2017). In an evaluation of the efficacy of twenty‐one synthetic fluoroquinolone‐flavonoid hybrids against three drug‐ resistant (including S. aureus, Bacillus subtilis and E. coli) using efflux pump and DNA gyrase. The data showed that only two compounds out of the 21 one compounds could inhibit DNA gyrase and efflux pump. The combination of quercetin, rutin and morin could lead to the oozing of potassium from the bacterial cytoplasmic membrane (Amin et al., 2015, Al-Ani et al., 2018, Kharsany et al., 2019, Veloz et al., 2019). Three flavonoids quercitin‐3‐glucoside, negletein, and techtochrysin, were investigated against foodborne pathogens, and they caused a 90–95% reduction in biofilms (Majiene et al., 2007, Rajendran et al., 2016, Ming et al., 2017, Przybyłek and Karpiński, 2019).
Fig. 2.
Mechanism action of propolis as anti-bacterial agent (A) The active components of propolis attached on the cytoplasmic membrane of bacterial cell and then the structural integrity has been damaged leading to perforation of the membrane where the cytoplasmic content expelled outside and leading to cell death (B) flavonoids may result in the inhibition of topoisomerase IV-dependent deactivation activity and lead to (associated cellular response and is known as SOS ‘save our ship’ or ‘save our souls’) growth inhibition of bacterial cells.
To sum up, several possible mechanisms associated with the anti-bacterial efficacy of propolis have been proposed:
1. Nucleic acid synthesis inhibition
2. Cytoplasmic membrane function alteration
3. Energy metabolism inhibition
4. Reducing the affinity to development of biofilms
5. Cell membrane proteins inhibition
6. Compromising membrane permeability
7. Bacterial resistance reduction
7. Clinical antibacterial potential and its use in new drugs of biotechnological products
The complex composition of propolis consisting of more than 200 substances gives it its potential biological efficacy. Those 200 and more ingredients are highly beneficial to human health, and thus gained tremendous popularity throughout the world (Sforcin and Bankova 2011). The in vitro data provides are useful as preliminary findings of the possible application of a natural compound. When the in vitro study gives positive results, then further in vivo study is warranted to produce relevant clinical data. Moreover, in many of both in vitro and in vivo assays, the scientists do not characterize the chemical composition to be able to identify the active ingredient. Hence, one pharmacologic variation in the extracts are highly expected (Heinrich et al. 2008). Many publications have noted the biological potential of propolis, no critical reviews are available related to the application of such data in the context of a product’s clinical use. However, new formulations impregnated with propolis or anyone or more of its active ingredients have been prepared. The Egyptians, recognized propolis’s anti-putrefactive properties, and was used for embalming. The Romans and Greek doctors were the first to recognize its medicinal properties. Propolis was applied as an antiseptic for wound treatment and healing and as a mouthwash. Its use was mainly perpetuated among Arab doctors in the Middle Ages. It was used in the battlefield as an ointment or cream for the treatment of wounds of soldiers in battlefield. Alcoholic and hydroalcoholic extracts of propolis has been impregnated in ointments containing butter, Vaseline, olive oil or lanolin. The concentration of propolis vary depending on the application and should be enough to achieve bacteriostatic or bactericidal effects (Geraldini et al. 2000). One of the main concerns in dentistry is bacterial infections which are associated with dental carries, oral ulcers, gingivitis, periodontitis, and oral ulcers (Geraldini et al. 2000). Also, oral hygiene is extremely important to keep the teeth healthy and reduce associated bacterial infections which may start locally and later will be systemic. Positive data have been published on the antiseptic and healing potential of propolis on patients from various hospitals (Grégio et al. 2005). Thus, propolis has attracted a great deal of interest for the treatment of oral infections. It is worth noting that the first licensed commercial propolis based product was registered in Romania in 1965. Internationally, more than 239 commercial licenses have been reported. Most commercial licenses were obtained in the 1980 s, by the former USSR countries. Currently, a high percentage of commercial licenses (43%) have originated from Japan and 6.2% of those are in the field of dentistry. There was an increase of 660% in the scientific productivity for propolis in Japan between the 1980 and 1990. As a result, of the recognition of propolis’s biological activity, there was a considerable focus on research related to the potential application of propolis and its active ingredients (Parker 2007). Totarol is a diterpenes isolated from propolis with known significant antibacterial efficacy. The underlying mechanism of action of totarol is not fully elucidated. However, it is thought that it inhibits the oxygen consumption by bacteria cells leading to the interruption of the respiratory pathway and electron transport system in the oxidation of bacteria membranes, although this may be very weak assumption against anaerobic bacteria (Shapiro and Guggenheim 1998). Studies conducted on antibacterial activity of diterpene against MRSA revealed that it exerts its effect by interfering with penicillin binding protein 2 expression affecting the synthesis of the adenosine triphosphate in bacteria, and it modulates the integrity of the membrane by reducing the bacterial phospholipid bilayer structural intermolecular forces (Bernabeu et al. 2002). As stated above, propolis has a synergistic effect with antimicrobial drugs, and such an association may lead to the novel commercially available drugs is a field of interest to be pursued by the pharmaceutical industry for the discovery of novel potent products to be used in the treatment of various conditions. It would be very interesting to use propolis with antibiotics to enhance the efficacy of the latter or modulate the antimicrobial resistance of bacteria. For instance, the application of both propolis and ciprofloxacin resulted in synergism in the treatment of keratitis caused by S. aureus (Oksuz et al. 2005). While, a study indicated that propolis, upon the exposure of bacteria to certain antibiotics like: amoxicillin, ampicillin and cefalexin, caused a reduction in the antimicrobial resistance as a consequence to modulating the bacterial wall and, also, indicated synergism with other antibiotics (chloramphenicol, tetracycline and neomycin) which affect the ribosome (Orsi et al., 2006). In addition, the simultaneous application of alcoholic extracts of propolis with antibiotics produced antibacterial synergism against both Escherichia coli and Staphylococcus aureus (Meresta 1985).
8. Conclusions
As discussed above, propolis and its derivatives has shown to be very potent antibacterial agents and a synergetic agent to increase the efficacy of conventional antibiotics. Also, they have helped in modulating the antimicrobial resistance of highly resistant bacteria. As such, they may have a potential in being used in the medical field. However, so far propolis have not been evaluated in clinical trials to better evaluate its potential in various fields of medicine. Few flavonoids have been included in clinical trials and revealed negligible adverse effects and demonstrated biological activity. Some of them like quercetin produced synergistic effect when applied with reference drugs and can be safely investigated further in future clinical trials. It is recommend that the role of flavonoids in the medical field should not be overlooked and more clinical trials should be conducted to further decipher their potential application in the treatment of various medical problems.
Acknowledgement
This work was supported by Yousef Abdul Latif Jameel Scientific Chair of Prophetic Medicine Application, Faculty of Medicine, King Abdulaziz University, Jeddah 21589, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aderogba M.A., Ndhlala A.R., Rengasamy K.R., Van Staden J. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules (Basel, Switzerland) 2013;18(10) doi: 10.3390/molecules181012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrouzan H., Tahghighi A., Zakeri S., Es-haghi A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. Iranian biomedical journal. 2018;22(1) doi: 10.22034/ibj.22.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangari Z., Naseri M., Vatandoost F. Propolis: Chemical Composition and Its Applications in Endodontics. Iranian endodontic journal. 2018;13(3) doi: 10.22037/iej.v13i3.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airen B., Sarkar P.A., Tomar U., Bishen K.A. Antibacterial effect of propolis derived from tribal region on. J Indian Soc Pedod Prev Dent. 2018;36(1):48–52. doi: 10.4103/JISPPD.JISPPD_1128_17. [DOI] [PubMed] [Google Scholar]
- Akilandeswari K., Ruckmani K. “Synergistic antibacterial effect of apigenin with β-lactam antibiotics and modulation of bacterial resistance by a possible membrane effect against methicillin resistant Staphylococcus aureus.” Cellular and molecular biology (Noisy-le-Grand. France) 2016;62(14) [PubMed] [Google Scholar]
- Al-Ani I., Zimmermann S., Reichling J., Wink M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines (Basel, Switzerland) 2018;5(1) doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili N., Al-Ghamdi A., Ansari M.J., Al-Attal Y., Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. International J. Medical Sci. 2012;9(9) doi: 10.7150/ijms.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi A., Ebiloma G.U., Williams R., Alenezi S., Donachie A., Guillaume S., Igoli J.O., Fearnley J., de Koning H.P., Watson D.G. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-47840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, M. U., M. Khurram, B. Khattak and J. Khan (2015). “Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus.” BMC complementary and alternative medicine 15. [DOI] [PMC free article] [PubMed]
- Aminimoghadamfarouj N., Nematollahi A. Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review. Int. J. Mol. Sci. 2017;18(6) doi: 10.3390/ijms18061290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryaei, R. P., P. (2018). “Evaluation of the antibacterial activity of Iranian propolis on the strains of Pseudomonas aeruginosa and Staphylococcus aureus.” Amazonia Investiga 7(14): 5-10.
- Bankova, V. d. C., S. L.; Marcucci, M. C. (2000). “Propolis: recent advances in chemistry and plant origin.” Apidologie 31(1): 3-15.
- Becerra T.B., Calla-Poma R.D., Requena-Mendizabal M.F., Millones-Gomez P.A. Antibacterial effect of Peruvian propolis collected during different seasons on the growth of streptococcus mutans. Open Dent. J. 2019;13(1) [Google Scholar]
- Bernabeu A., Shapiro S., Villalaín J. A MAS-NMR study of the location of (+)-totarol, a diterpenoid bioactive molecule, in phospholipid model membranes. Chem. Phys. Lipids. 2002;119(1–2) doi: 10.1016/s0009-3084(02)00050-6. [DOI] [PubMed] [Google Scholar]
- Bezerra, A. M. F. B., K. K. S.; Albuquerque, F. G. F.; Fernandes Filho, G. S; Casimiro, E. M.; Nunes, E.; da Silva, E.; Bezerra, W.; de Almeida, P.; de Araujo, T.; de Abrantes, K.; de Abreu, L.; Maracaja, P.; Araujo, A.; de Silva, R.; (2015). “Red propolis antifungal action on species of Candida of the oral cavity.” Int. Arch. Med. 8.
- Bhaskar S., Shalini V., Helen A. Quercetin regulates oxidized LDL induced inflammatory changes in human PBMCs by modulating the TLR-NF-κB signaling pathway. Immunobiology. 2011;216(3) doi: 10.1016/j.imbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Bucio-Villalobos, C. M. M.-J., O. A. (2017). “ Actividad antibacteriana de un extracto acuoso de propóleo del municipio de Irapuato, Guanajuato, México.” Agronomía Mesoamericana 28(1): 223-227.
- Comalada M., Camuesco D., Sierra S., Ballester I., Xaus J., Gálvez J., Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005;35(2) doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P., Lamb A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005;101(1–3) doi: 10.1016/j.jep.2005.04.014. [DOI] [PubMed] [Google Scholar]
- da Silva, L. M., P. de Souza, S. K. A. Jaouni, S. Harakeh, S. Golbabapour and S. F. de Andrade (2018). “Propolis and Its Potential to Treat Gastrointestinal Disorders.” Evidence-based complementary and alternative medicine : eCAM 2018. [DOI] [PMC free article] [PubMed]
- Dantas Silva R.P., Machado B.A., Barreto G.A., Costa S.S., Andrade L.N., Amaral R.G., Carvalho A.A., Padilha F.F., Barbosa J.D., Umsza-Guez M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0172585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir S., Aliyazicioglu Y., Turan I., Misir S., Mentese A., Yaman S.O., Akbulut K., Kilinc K., Deger O. Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr. Cancer. 2016;68(1):165–172. doi: 10.1080/01635581.2016.1115096. [DOI] [PubMed] [Google Scholar]
- Devequi-Nunes D., Machado B.A.S., Barreto G.A., Rebouças Silva J., da Silva D.F., da Rocha J.L.C., Brandão H.N., Borges V.M., Umsza-Guez M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE. 2018;13(12) doi: 10.1371/journal.pone.0207676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher N., Klein A.M., Schmitt T., Leonhardt S.D. A clue on bee glue: New insight into the sources and factors driving resin intake in honeybees (Apis mellifera) PLoS ONE. 2019;14(2) doi: 10.1371/journal.pone.0210594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhenany, H., N. El-Badri and M. Dhar (2019). “Green propolis extract promotes in vitro proliferation, differentiation, and migration of bone marrow stromal cells.” Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 115. [DOI] [PubMed]
- Eumkeb G., Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2013;20(3–4) doi: 10.1016/j.phymed.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Eumkeb G., Sakdarat S., Siriwong S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2010;18(1) doi: 10.1016/j.phymed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Freires, I. A., S. M. de Alencar and P. L. Rosalen (2016). “A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases.” European journal of medicinal chemistry 110. [DOI] [PubMed]
- Funari, C. S. F., V.O. (2006). “Análise de própolis ” Food Science and Technology 26(1): 171-178.
- Gajdács M. The Continuing Threat of Methicillin-Resistant Staphylococcus aureus. Antibiotics (Basel, Switzerland) 2019;8(2) doi: 10.3390/antibiotics8020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti F., Maccari F., Fachini A., Volpi N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods (Basel, Switzerland) 2018;7(3) doi: 10.3390/foods7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan F., Wong R.W., Rabie A.B. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytotherapy research : PTR. 2010;24(6) doi: 10.1002/ptr.3014. [DOI] [PubMed] [Google Scholar]
- Geraldini C., Salgado E., de Mello Rode S. Ação de diferentes soluções de própolis na superfície dentinária - avaliação ultra-estrutural. Faculdade de Odontologia Sao Jose dos Campos. 2000;3(2):28–32. [Google Scholar]
- Gonsales G.Z.O., Fernandes Junior A., Rodrigues P., Funari S.R.C. Antibacterial activity of propolis collected in different regions of Brazil. J. Venom. Anim. Tox. Incl. Trop. Diseas. 2006;12(2):276–284. [Google Scholar]
- González-Gallego, J. V. G.-M., M.; Sánchez-Campos, S.; Tuñón, M.; (2018). Anti-inflammatory, Immunomodulatory, and Prebiotic Properties of Dietary Flavonoids. Polyphenols: Prevention and Treatment of Human Disease. V. R. P. Ronald Ross Watson, Sherma Zibadi, Academic Press: 327-345.
- Grégio A.M.T., Lima A.A.S., Ribas M.O., Barbosa A.P.M., Pereira A.C.P., Koike F., Repeke C.E.P. Efeito da Propolis Appis mellifera sobre o processo de reparo de lesões ulceradas na mucosa bucal de ratos. Estudos em Biologia. 2005;27:58. [Google Scholar]
- Guzman J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules (Basel, Switzerland) 2014;19(12) doi: 10.3390/molecules191219292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh S., Khan I., Almasaudi S.B., Azhar E.I., Al-Jaouni S., Niedzweicki A. Role of Nutrients and Phyto-compounds in the Modulation of Antimicrobial Resistance. Curr. Drug Metab. 2017;18(9) doi: 10.2174/1389200218666170719095344. [DOI] [PubMed] [Google Scholar]
- Harasstani O.A., Tham C.L., Israf D.A. Kaempferol and Chrysin Synergies to Improve Septic Mice Survival. Molecules (Basel, Switzerland) 2017;22(1) doi: 10.3390/molecules22010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, M. W., T.; Pan, S.; Xu, X. (2014). “Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study.” Applied Surface Science 305: 515-521.
- Heinrich M., Modarai M., Kortenkamp A. Herbal extracts used for upper respiratory tract infections: are there clinically relevant interactions with the cytochrome P450 enzyme system? Planta Med. 2008;74(6) doi: 10.1055/s-2008-1034292. [DOI] [PubMed] [Google Scholar]
- Hernández Tasco A.J., Ramírez Rueda R.Y., Alvarez C.J., Sartori F.T., Sacilotto A.C.B.C., Ito I.Y., Vichnewski W., Salvador M.J. Antibacterial and antifungal properties of crude extracts and isolated compounds from Lychnophora markgravii. Nat. Prod. Res. 2018;34(6) doi: 10.1080/14786419.2018.1503263. [DOI] [PubMed] [Google Scholar]
- Jeong K.W., Lee J.Y., Kang D.I., Lee J.U., Shin S.Y., Kim Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009;72(4) doi: 10.1021/np800698d. [DOI] [PubMed] [Google Scholar]
- Joray, M. B., L. D. Trucco, M. L. González, G. N. Napal, S. M. Palacios, J. L. Bocco and M. C. Carpinella (2015). “Antibacterial and Cytotoxic Activity of Compounds Isolated from Flourensia oolepis.” Evidence-based complementary and alternative medicine : eCAM 2015. [DOI] [PMC free article] [PubMed]
- Kędzia, B. H.-K., E.; (2013). “The antibiotic activity of native and European propolis.” Postępy Fitoterapii 2: 97-107.
- Kharsany K., Viljoen A., Leonard C., van Vuuren S. The new buzz: Investigating the antimicrobial interactions between bioactive compounds found in South African propolis. J. Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111867. [DOI] [PubMed] [Google Scholar]
- Kubiliene, L., V. Laugaliene, A. Pavilonis, A. Maruska, D. Majiene, K. Barcauskaite, R. Kubilius, G. Kasparaviciene and A. Savickas (2015). “Alternative preparation of propolis extracts: comparison of their composition and biological activities.” BMC complementary and alternative medicine 15. [DOI] [PMC free article] [PubMed]
- Majiene, D. T., S.; Pavilonis, A.; Savickas, A.; Martirosyan, D. M.; (2007). “Antifungal and Antibacterial Activity of Propolis.” Current Nutrition & Food Science 3(4).
- Martinotti, S. and E. Ranzato (2015). “Propolis: a new frontier for wound healing?” Burns & trauma 3. [DOI] [PMC free article] [PubMed]
- Meresta, L. M., T.; (1985). “An attempt to use the extract from propolis in the treatment of mastitis of cows.” Med. Weter 41: 489-492.
- Ming, D., D. Wang, F. Cao, H. Xiang, D. Mu, J. Cao, B. Li, L. Zhong, X. Dong, X. Zhong, L. Wang and T. Wang (2017). “Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus.” Frontiers in microbiology 8. [DOI] [PMC free article] [PubMed]
- Mokhtar A.B., El-Gayar E.K., Habib E.S. In Vitro Anti-protozoal Activity of Propolis Extract and Cysteine Proteases Inhibitor (Phenyl Vinyl Sulfone) On Blastocystis Species. J. Egypt. Soc. Parasitol. 2016;46(2) [PubMed] [Google Scholar]
- Muli, E. M. M., J.M.; (2007). “Antibacterial activity of Apis mellifera L. propolis collected in three regions of Kenya.” Journal of Venomous Animals and Toxins Including Tropical Diseases 13(3): 655-663.
- Nayaka H.B., Londonkar R.L., Umesh M.K., Tukappa A. International journal of bacteriology2014. 2014. Antibacterial Attributes of Apigenin, Isolated from Portulaca oleracea L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuz H., Duran N., Tamer C., Cetin M., Silici S. Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res. 2005;37(6) doi: 10.1159/000087943. [DOI] [PubMed] [Google Scholar]
- Olegário L.S., Andrade J.K.S., Andrade G.R.S., Denadai M., Cavalcanti R.L., da Silva M.A.A.P., Narain N. Chemical characterization of four Brazilian brown propolis: An insight in tracking of its geographical location of production and quality control. Food research international (Ottawa. 2019;Ont.) 123 doi: 10.1016/j.foodres.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Orsi R.O., Fernandes A., Bankova V., Sforcin J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012;26(5) doi: 10.1080/14786419.2010.498776. [DOI] [PubMed] [Google Scholar]
- Orsi, R. O. S., J.M.; Funari, S.R.C.; Fernandes Jr., A.; Bankova, V.; (2006). “Synergistic effect of propolis and antibiotics on the Salmonella Typhi.” Brazilian Journal of Microbiology 37: 108-112.
- Oryan, A., E. Alemzadeh and A. Moshiri (2018). “Potential role of propolis in wound healing: Biological properties and therapeutic activities.” Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 98. [DOI] [PubMed]
- Özen, T. K., A.; Bedir, O.; Koru, Ö.; Sorkun, K.; Tanyuksel, M.; Kılıç, S.; Gencay, O.; Yildiz, O.; Baysallar, M (2010). “In vitro activity of Turkish propolis samples against anaerobic bacteria causing oral cavity infections.” Kafkas Univ Vet Fak Derg 16(2): 293-298.
- Park H., Bae S.H., Park Y., Choi H.S., Suh H.J. Lipase-mediated lipid removal from propolis extract and its antiradical and antimicrobial activity. J. Sci. Food Agric. 2015;95(8) doi: 10.1002/jsfa.6874. [DOI] [PubMed] [Google Scholar]
- Parker, J. F. L., M.M.S.; (2007). “Método para avaliação e pesquisa da atividade antimicrobiana de produtos de origem natural.” Revista Brasileira de Farmacognosia 17(1): 102-107.
- Pasupuleti V.R., Sammugam L., Ramesh N., Gan S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative medicine and cellular longevity. 2017;2017 doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaper A., Golob M., Hafner I., Oblak M., Solmajer T., Jerala R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003;306(2) doi: 10.1016/s0006-291x(03)01006-4. [DOI] [PubMed] [Google Scholar]
- Przybyłek I., Karpiński T.M. Antibacterial Properties of Propolis. Molecules (Basel, Switzerland) 2019;24(11) doi: 10.3390/molecules24112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran N., Subramaniam S., Christena L.R., Muthuraman M.S., Subramanian N.S., Pemiah B., Sivasubramanian A. Antimicrobial flavonoids isolated from Indian medicinal plant Scutellaria oblonga inhibit biofilms formed by common food pathogens. Nat. Prod. Res. 2016;30(17) doi: 10.1080/14786419.2015.1104673. [DOI] [PubMed] [Google Scholar]
- Ramos A.F.N., Miranda J.L. Propolis: a review of its anti-inflammatory and healing actions. Journal of Venomous Animals and Toxins including Tropical Diseases. 2007;13(4):697–710. [Google Scholar]
- Ristivojević P., Trifković J., Andrić F., Milojković-Opsenica D. Poplar-type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015;10(11) [PubMed] [Google Scholar]
- Saeed, F. A., R.S.; Arshad, M.U.; Niaz, B.; Batool, R.; Naz, R.; Ansar Rasul Suleria, H. (2016). “Propolis to curb lifestyle related disorders: An overview.” International journal of food properties 19(2): 420-437.
- Sforcin J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016;30(6):894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M., Bankova V. Propolis: is there a potential for the development of new drugs? J. Ethnopharmacol. 2011;133(2) doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M., Bankova V., Kuropatnicki A.K. Medical Benefits of Honeybee Products. Evid Based Complement Alternat Med. 2017;2017:2702106. doi: 10.1155/2017/2702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir A., Rashid M., Tipu H.N. Propolis, A Hope for the Future in Treating Resistant Periodontal Pathogens. Cureus. 2016;8(7) doi: 10.7759/cureus.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Guggenheim B. Inhibition of Oral Bacteria by Phenolic Compounds. Part 1. QSAR Analysis using Molecular Connectivity - Shapiro - 1998 - Quantitative Structure-Activity Relationships - Wiley Online Library. Quant. Struct. Act. Relat. 1998;17:327–337. [Google Scholar]
- Silva-Carvalho, R., F. Baltazar and C. Almeida-Aguiar (2015). “Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development.” Evidence-based complementary and alternative medicine : eCAM 2015. [DOI] [PMC free article] [PubMed]
- Simone-Finstrom M., Borba R.S., Wilson M., Spivak M. Propolis Counteracts Some Threats to Honey Bee Health. Insects. 2017;8(2) doi: 10.3390/insects8020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone-Finstrom M., Spivak M. Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie. 2010;41(3):295–311. [Google Scholar]
- Siriwong S., Teethaisong Y., Thumanu K., Dunkhunthod B., Eumkeb G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC pharmacology & toxicology. 2016;17(1) doi: 10.1186/s40360-016-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toreti, V. C., H. H. Sato, G. M. Pastore and Y. K. Park (2013). “Recent progress of propolis for its biological and chemical compositions and its botanical origin.” Evidence-based complementary and alternative medicine : eCAM 2013. [DOI] [PMC free article] [PubMed]
- Uzel A., Sorkun K., Onçağ O., Cogŭlu D., Gençay O., Salih B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005;160(2) doi: 10.1016/j.micres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vasconcelos N.G., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018;120 doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- Veiga R.S., De Mendonça S., Mendes P.B., Paulino N., Mimica M.J., Lagareiro Netto A.A., Lira I.S., López B.G., Negrão V., Marcucci M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017;122(4) doi: 10.1111/jam.13400. [DOI] [PubMed] [Google Scholar]
- Veloz, J. J., M. Alvear and L. A. Salazar (2019). “Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis.” BioMed research international 2019. [DOI] [PMC free article] [PubMed]
- Wagh V.D. Advances in pharmacological sciences2013. 2013. Propolis: a wonder bees product and its pharmacological potentials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yao J., Zhou B., Yang J., Chaudry M.T., Wang M., Xiao F., Li Y., Yin W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018;81(1) doi: 10.4315/0362-028X.JFP-17-214. [DOI] [PubMed] [Google Scholar]
- Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radical Biol. Med. 2004;36(7) doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wojtyczka R.D., Dziedzic A., Idzik D., Kępa M., Kubina R., Kabała-Dzik A., Smoleń-Dzirba J., Stojko J., Sajewicz M., Wąsik T.J. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules (Basel, Switzerland) 2013;18(8) doi: 10.3390/molecules18089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak, M. M., L.; Waskiexicz; A.; Rogozinski, T.; Ratajczak; I.; (2019). “The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes.” Revista Brasileira de Farmacognosia 29(3).
- Yildirim, A., G. G. Duran, N. Duran, K. Jenedi, B. S. Bolgul, M. Miraloglu and M. Muz (2016). “Antiviral Activity of Hatay Propolis Against Replication of Herpes Simplex Virus Type 1 and Type 2.” Medical science monitor : international medical journal of experimental and clinical research 22. [DOI] [PMC free article] [PubMed]
- Yildirim Z., Hacievliyagil S., Kutlu N.O., Aydin N.E., Kurkcuoglu M., Iraz M., Durmaz R. Effect of water extract of Turkish propolis on tuberculosis infection in guinea-pigs. Pharmacol. Res. 2004;49(3):287–292. doi: 10.1016/j.phrs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Yilmaz S., Sova M., Ergün S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J. Appl. Microbiol. 2018 doi: 10.1111/jam.14097. [DOI] [PubMed] [Google Scholar]
- Youn H.S., Lee J.Y., Saitoh S.I., Miyake K., Kang K.W., Choi Y.J., Hwang D.H. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (-)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem. Pharmacol. 2006;72(7) doi: 10.1016/j.bcp.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Zhang L., Kong Y., Wu D., Zhang H., Wu J., Chen J., Ding J., Hu L., Jiang H., Shen X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: crystal structure characterization with enzymatic inhibition assay. Protein science : a publication of the Protein Society. 2008;17(11) doi: 10.1110/ps.036186.108. [DOI] [PMC free article] [PubMed] [Google Scholar]