Abstract
Background
Differentiation of active pulmonary tuberculosis (TB) from non-mycobacterial community-acquired pneumonia (CAP) still remains a diagnostic challenge.
Objective
The study aimed to quantify the IL-18, IFN-γ, IL-18BP, IL-37, and IP-10 levels in serum and Mycobacterium tuberculosis (M.tb) antigens-stimulated blood cultures from TB or CAP patients and explore if the proteins can be a useful basis for discriminating these diseases.
Methods
In total, 124 Polish adults, including mild/moderate (M/MTB) or advanced (ATB) TB patients, and CAP patients, were enrolled in the study. The concentrations of IL-18, IL-18BP, IFN-γ, IL-37, and IP-10 in sera and M.tb-stimulated cultures were measured by ELISA.
Results
The most specific and sensitive serum proteins discriminating TB from CAP were IP-10 and IL-18BP; however, IP-10 had the highest AUC in the ROC curve for the diagnosis. Serum IP-10 and IL-18BP levels increased significantly in M/MTB or ATB groups. The IL-18BP elevation in ATB group was accompanied by an increase in IL-18. No single protein measured in M.tb-stimulated cultures differed TB from CAP patients.
Conclusions
The combined analysis of serum IL-18BP and IP-10 might be considered as an auxiliary tool in the differentiation of TB from CAP.
Keywords: Tuberculosis, Community-acquired pneumonia, IL-18, IL-18BP, IP-10
1. Introduction
According to the estimates of the World Health Organization, tuberculosis (TB) affects about 10 million people in the world and is a cause of 2 million deaths annually (WHO, 2018). Pulmonary TB is the most common form of the disease; however, the disease can develop in other organ systems such as the central nervous system, skeleton, or gastrointestinal tract.
The immunology of TB is complex and multifaceted. The control of M.tb infection mainly depends on the development of Th1 cell immunity involving the participation of activated macrophages, T lymphocytes, and their cytokines that affect interactions and activity of cells engaged in an antimycobacterial immune response. One of the cytokines that have been implicated in both protective and pathological processes associated with M.tb infection is IL-18, which is produced by a wide range of immune cells such as monocytes, macrophages, dendritic cells, epithelial cells, keratinocytes, and synovial fibroblasts as well as T and B lymphocytes (Barksby et al., 2007, Dima et al., 2015, Novick et al., 2013). Because of its property to induce IFN-γ in T cells and natural killer cells, IL-18 is considered a member of the Th1 cytokines family. The IL-18 precursor, pro-IL-18, requires cleavage to an active molecule by the intracellular caspase-1 (Dinarello, 1998, Novick et al., 2013, Wawrocki and Druszczynska, 2017). After cleavage, approximately 20% of the mature cytokine is secreted from macrophages/monocytes, whereas the remaining 80% of the pro-IL-18 remains unprocessed inside the cells (Dinarello et al., 2013, Sugawara et al., 2001). IL-18 binds the ligand-receptor IL-18Rα, inducing the recruitment of IL-18Rβ subunit to form a high-affinity complex, which signals through the Toll/interleukin-1 receptor (TIR) domain (Dinarello, 1999, Fields et al., 2019, Krumm et al., 2014, Plater-Zyberk et al., 2001). This signalling domain recruits the adaptor protein MyD88 that activates an NF-κB pathway and triggers a pro-inflammatory signal. The activity of IL-18 can be suppressed by extracellular interleukin 18 binding protein (IL-18BP) that binds soluble IL-18 with a higher affinity than IL-18Rα and thus prevents IL-18 binding to IL-18 receptor (Nakanishi et al., 2001, Yasuda et al., 2019). IL-37is another endogenous factor that suppresses the action of IL-18. IL-37 has high homology with IL-18 and can bind to IL-18Rα, which then forms a complex with IL-18BP, thereby reducing the activity of IL-18 (Dinarello et al., 2016, Nold et al., 2010).
IL-18 is a potent immunoregulatory cytokine with various immunological properties. It regulates the mechanisms of both innate and adaptive immunity and plays a key role in host defense (Banda et al., 2003, Biet et al., 2002, Kohno et al., 1997, Matsui et al., 1997, Okamura et al., 1995, Wawrocki et al., 2016). The primary role of IL-18 is the induction of IFN-γ production through its synergistic action either with IL-12 or IL-15, which increases the surface expression of IL-18Rβ, a signal-transducing component of the IL-18R complex (Wawrocki and Druszczynska, 2017). Additionally, IL-18 promotes proliferation and activation of NK cells, polarizes T lymphocytes towards the Th1 phenotype, enhances CD8+ T cell cytolytic activity and induces the synthesis of nitric oxide (NO) as well as many cytokines and chemokines (TNF-α, GM-CSF, IL-4, IL-5, IL-9, IL-13, IL-17) (Dima et al., 2015, Dinarello et al., 2013, Gardella et al., 1999, Gutzmer et al., 2003). Although IL-18 functions as an important activator of the protective immune response against intracellular pathogens, it is also implicated in pathological processes leading to tissue damage (Wawrocki and Druszczynska, 2017). Multiple studies have shown that overproduction of IL-18 is involved in the pathogenesis of pulmonary inflammation and lung injury in mice as well as idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD) and granulomatous lung diseases in humans (Dima et al., 2015, Kitasato et al., 2004, Okamoto et al., 2002, Pechkovsky et al., 2006). The IL-18-driven inflammation has been shown to be characterized by emphysema, airway fibrosis, mucus metaplasia, and vascular remodeling of the lungs (Abdel Fattah et al., 2015, Hoshino et al., 2007, Nakajima and Owen, 2012).
Taking into account difficulties in the differentiation of pulmonary TB from non-mycobacterial community-acquired lung infections and biological activity of IL-18, we assessed serum and specific M.tb antigens-stimulated whole blood culture levels of IL-18 and IL-18BP in patients with active pulmonary TB having a mild/moderate (M/MTB) or advanced (ATB) form of tuberculosis and patients suffering from acute non-mycobacterial community-acquired pneumonia (CAP). We also measured serum and M.tb-stimulated concentration of IL-37, IFN-γ, a key cytokine in antimycobacterial immunity, and serum levels of IP-10 (IFN-γ-inducible protein 10), a chemokine mediating leukocyte recruitment and activation. The aim of the study was to assess the usefulness of combined analysis of the proteins as an auxiliary tool in the diagnosis of TB, including the disease caused by a small number of M.tb (paucibacillary TB), that cannot be confirmed by mycobacterial culture. Covering TB patients with mild/moderate (M/MTB) or advanced (ATB) forms of tuberculosis can provide new information on the involvement of IL-18 in the pathology of TB.
2. Materials and methods
2.1. Participants patients
In total, 124 M. bovis BCG-vaccinated adults of both genders, 18–81 years of age, admitted with a clinical diagnosis of lung disease to the Regional Specialized Hospital of Tuberculosis, Lung Diseases, and Rehabilitation in Tuszyn, Poland, from March 2017 to September 2018, were recruited into the study. The baseline demographic information for all participants included in the study is shown in Table 1.
Table 1.
Participant patients characteristics.
|
TB |
CAP | |||
|---|---|---|---|---|
| Total |
Mild/Moderate |
Advanced |
||
| (M/MTB) | (ATB) | |||
| N | 89 | 50 | 39 | 35 |
| Sex M/F | 48/41 | 24/26 | 24/15 | 11/24 |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian |
| Age | ||||
| median | 50 | 55 | 42 | 52 |
| range | 19–81 | 23–79 | 19–81 | 19–85 |
| years (IQR) | 33–63 | 36–66 | 30–58 | 38–68 |
| BCG vaccination | 100% | 100% | 100% | 100% |
| QFT result, N (%) | ||||
| positive | 51 (57%) | 26 (52%) | 25 (64%) | 0 (0%) |
| negative | 38 (43%) | 24 (48%) | 14 (36%) | 35 (100%) |
| M.tb culture, N (%) | ||||
| positive | 49 (55%) | 27 (54%) | 22 (56%) | 0 (0%) |
| negative | 40 (45%) | 23 (43%) | 17 (44%) | 35 (100%) |
| WBC, Counts/mm3 | 7200 | 6000 | 8500 | 7200 |
| RBC, Counts/mm3 | 4,470,000 | 4,570,000 | 4,290,000 | 4,550,000 |
| HGB, g/dl | 13 | 13.2 | 12.8 | 13.5 |
| HCT, % | 39 | 41 | 39 | 41 |
| MCHC, g/dl | 32 | 32 | 32 | 32 |
| PLT, Counts/mm3 | 286,500 | 216,500 | 342,000 | 272,000 |
Abbreviations: ATB – advanced tuberculosis patients; M/MTB – mild/moderate tuberculosis patients; CAP - non-mycobacterial, community-acquired pneumonia patients; QFT – QuantiFERON TB Gold test; WBC - white blood cells; RBC – red blood cells; HGB – hemoglobin; HCT – hematocrit; MCHC - mean corpuscular hemoglobin concentration; PLT – platelets.
The final diagnosis of lung disease was established eight weeks after the admission of the patients to the hospital. All of the patients were diagnosed with extensive clinical evaluation, including clinical manifestations, TB contact history, sputum smear of acid-fast bacilli (AFB), a culture of M.tb, and chest radiography. This allowed classification of the patients into the following two groups: group 1, consisting of 89 patients (48 men, 41 women) diagnosed with pulmonary TB confirmed by M.tb sputum culture, typical clinical symptoms, characteristic features on radiographs and proper responses to anti-TB treatment (TB patients), and group 2, consisting of 35 patients (11 men, 24 women) suffering from non-mycobacterial, community-acquired pneumonia (CAP) with triple-negative M.tb sputum culture and no previous TB history, treated and cured with wide-range antibiotics, i.e., amoxicillin/clavulanic acid, clarithromycin, clindamycin, ceftriaxone, ciprofloxacin, doxycycline (CAP patients). Based on the extent of the lesions in the lung tissue, the TB patients were classified as having: mild/moderate TB (lesions within the unilateral lung field) (M/MTB) or advanced TB (lesions beyond the unilateral lung field) (ATB). None of the individuals had evidence of HIV infection or being treated with steroids or other immunosuppressive or anti-tubercular drugs at the time of blood sampling. The study was approved by the Ethics Committee of the University in Lodz, Poland (ethical approval number 17/KBBN-UŁ/II/2016; date 2016/11/10). Informed consent to use blood for research purposes was signed by all participants.
2.2. Blood samples
Blood specimens (5 ml), taken from all the patients shortly after the admission to the hospital prior to the start of the treatment, were used to prepare serum and perform a whole-blood interferon-gamma assay (QuantiFERON-TB® Gold Plus (QFT), Qiagen, Germany). The QFT assay was conducted according to the manufacturer’s instructions, as described in detail previously. The results were analyzed by the QuantiFERON-TB Gold Plus Analysis Software and evaluated according to the manufacturer’s criteria. The result was considered positive if the difference between the IFN-γ level in plasma incubated with TB antigen and Nil control was both ≥0.35 IU/ml and ≥25% of Nil control value.
2.3. IL-18, IL-18BP, IL-37, IFN-γ, IP-10 assays
The concentrations of IL-18, IL-18BP, and IL-37 in sera and in the QFT culture supernatants were determined using commercial specific enzyme-linked immunosorbent assays (Human Total IL-18 DuoSet (R&D, Minneapolis, USA), Human IL-18BPa DuoSet (R&D) and Human IL-37/IL-1F7 Duoset (R&D), Human IFN-γDuoset (R&D) and Human IP-10 Duoset ELISA (R&D)), and processed according to the manufacturer’s specifications. After measuring the concentrations of both IL-18 and IL-18BP in each sample, the law of mass action was used to calculate the level of free IL-18. Single IL-18BP molecule binds a single molecule of IL-18, that interaction has a dissociation constant (Kd) of 0.4 nM. Therefore, the level of free IL-18 was calculated from the equation where is [IL-18free], b is = [IL-18BP] – [IL-18] + Kd, and c is -Kd × [IL-18] (Migliorini et al., 2010). The absorbance was measured using the Victor 2 Multi-Label Counter Microplate Reader (Wallac Oy, Turku, Finland).
2.4. Statistical analysis
Non-parametric tests were used to compare protein levels between the diagnostic groups: Mann Whitney U test for two-group comparisons and Kruskal-Wallis tests for multiple groups. Categorical variables were compared using two-tailed chi-square tests. A p-value < 0.05 was considered statistically significant. Receiver operating characteristics (ROC) curve analysis was used to determine the analytic sensitivity and specificity of each studied protein. Optimal sensitivity and specificity were estimated using the Youden’s index. The likelihood ratios were used for assessing the value of performing a diagnostic test. Statistical analyses were done using Statistica 12 PL (Statsoft, Poland), MedCalc (NY, USA) and GraphPad Prism 8 (GraphPad Software, USA) software.
3. Results
3.1. Patient characteristics
The characteristics of the study participants are summarized in Table 1. The group comprised 89 patients diagnosed with active pulmonary tuberculosis (TB) and 35 patients suffering from non-mycobacterial community-acquired pneumonia (CAP). There were no differences between the studied groups regarding the age or BCG vaccination rates. The proportion of men in the TB group was significantly higher than in the CAP group (p < 0.05). Based on the type and extent of pulmonary lesions, TB patients were categorized as having mild/moderate TB (M/MTB; n = 50) or advanced TB (ATB; n = 39). M/MTB patients had a single lobe involved or presented unilateral involvement of two or more lobes with possible cavities reaching a diameter no greater than 4 cm, whereas ATB cases were characterized by bilateral lung changes with massive affectation and multiple cavities. Forty-nine (55%) of TB patients had a positive sputum culture for M.tb. The frequency of culture-confirmed TB was similar among M/MTB (54%) and ATB (56%) group. Forty (45%) of M.tb culture-negative TB patients were diagnosed on the basis of clinical manifestations, typical features on chest radiographs, and proper response to anti-tuberculous treatment. A positive QFT result was observed in 52% and 64% of M/MTB and ATB patients, respectively, and none of the CAP individuals. Four out of 50 (8%) M/MTB patients and 5 out of 39 (13%) ATB patients had a history of healed pulmonary TB. The percentage of underlying diseases did not differ significantly between the studied TB groups. Four out of 50 (8%) M/MTB patients and 2 out of 39 (5%) ATB patients suffered from diabetes. Similar percentages of M/MTB and ATB patients suffered from cardiovascular diseases (18% vs. 15%) and neurological diseases (4% vs. 2.5%). Chronic renal failure was diagnosed in 5 (10%) and 1 (2.5%) M/MTB and ATB patients, respectively. One person from the CAP group had sarcoidosis. There were no differences in the median values of WBC, RBC, PLT counts, and other hematological parameters between M/MTB, ATB, and CAP groups (Table 1).
3.2. Analysis of serum IL-18, IL-18BP, IL-37, IFN-γ, and IP-10 levels
We compared the median concentrations of IL-18 (total and free), IL-18BP, IL-37, IFN- γ, and IP-10 in the sera from the TB (M/MTB and ATB at the same time), and the CAP patients using Kruskal-Wallis tests. The levels of studied proteins measured in the sera are shown in Fig. 1a–e. The concentration of total IL-18 in the sera from the ATB patients (Me 895.63, IQR (416.28, 1146.51) pg/ml) was significantly higher (p = 0.01) than that found in the CAP individuals (Me 530.58, IQR (238.27, 792.12), however, similar levels of total IL-18 were observed in the CAP and M/MTB groups (Me 553.85, IQR (377.58, 830.58) pg/ml) (Fig. 1a). The serum IL-18BP concentration was comparably increased in both TB groups: ATB – Me 44.66, IQR (31.69, 70.00) ng/ml and M/MTB - Me 44.66, IQR (32.45, 66.92) ng/ml, as compared to the level of IL-18BP observed among the CAP patients (Me 35.36, IQR (23.41, 46.26) ng/ml) (p ≤ 0.01) (Fig. 1b). There were no differences in the serum concentrations of free IL-18 between the M/MTB (Me 4.68, IQR (3.01, 10.29) pg/ml) and the ATB (Me 7.63, IQR (4.54, 10.78) as well as among the CAP individuals (Me 5.16, IQR (2.86, 10.29) pg/ml). (Fig. 1c). Similarly, the serum levels of IL-37 among the M/MTB, ATB, and CAP groups were comparable, measuring 66.24, IQR (15.42, 223.00) pg/ml, 102.62, IQR (6.83, 236.57) pg/ml and 63.4, IQR (6.95, 444.63) pg/ml, respectively (Fig. 1d). As shown in Fig. 1e, the serum level of IP-10 was significantly higher in both TB groups (M/MTB – Me 41.53, IQR (23.10, 62.80) pg/ml; ATB - Me 47.37, IQR (14.78, 124.28) pg/ml) than in the group of CAP patients (Me 19.60, IQR (13.51, 35.22) pg/ml. The concentration of IFN- γ observed in the sera from the M/MTB and CAP patients, was similar, measuring 6.07, IQR (3.31, 11.49) pg/ml and 6.38, IQR (4.28, 11.03) pg/ml, respectively, with a slightly elevated level of this cytokine in sera ATB patients (Me 8.59, IQR (4.53, 19.91) pg/ml.
Fig. 1.
Serum levels of total and free IL-18, IL-18BP, IL-37, IFN-γ and IP-10 in the groups of the study. Boxplots with median (horizontal line within the box), interquartile range (box limits), and extremes (whiskers) of serum levels of total IL-18 (a), IL-18BP (b), free IL-18 (c), IL-37 (d), IFN-γ (e) and IP-10 (f) in the groups of patients with mild/moderate tuberculosis (M/MTB), advanced tuberculosis (ATB) and community-acquired pneumonia (CAP).
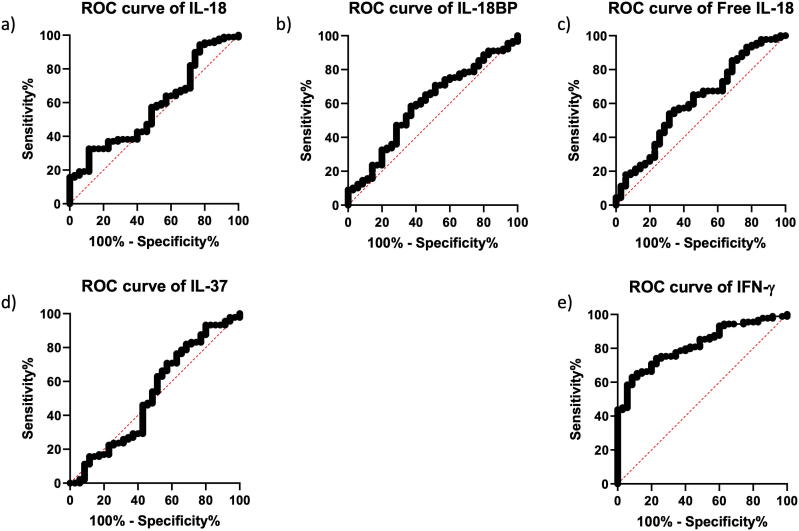
The analysis of ROC was performed to detect the proteins with the expression levels most discriminative of the M/MTB, ATB, and CAP groups. Among the studied serum proteins, IP-10 showed the highest area under the ROC curve (AUC), namely 0.686 (95% CI 0.596–0.766, p = 0.0005) (Table 2, Fig. 2e). The AUCs of the other two proteins – IL-18BP and total IL-18 were also significantly higher than a random assignment, 0.678 (95% CI 0.588–0.759, p = 0.0005), and 0.635 (95% CI 0.544–0.720, p = 0.017), respectively. The best AUC values in M/MTB vs CAP differentiation were observed for IP-10 (AUC = 0.713, 95% CI 0.605–0.806, p = 0.0003) and IL-18BP (AUC = 0.679, 95% CI 0.568–0.776, p = 0.0019), whereas in ATB vs CAP discrimination the best AUC values in the case of IL-18BP and total IL-18 (AUC = 0.685, 95% CI 5.567–0.787, p = 0.0026) (Table 2, Fig. 2).
Table 2.
Predictive values (AUC) of individual protein levels measured in serum.
| Protein | ROC analysis |
ROC analysis |
ROC analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total TB vs CAP |
M/MTB vs CAP |
ATB vs CAP |
|||||||
| AUC | 95% CI | p-value | AUC | 95% CI | p-value | AUC | 95% CI | p-value | |
| total IL-18 | 0.635 | 0.544–0.720 | 0.017 | 0.592 | 0.480–0.697 | 0.160 | 0.685 | 0.567–0.787 | 0.002 |
| free IL-18 | 0.521 | 0.429–0.611 | 0.727 | 0.517 | 0.406–0.626 | 0.796 | 0.579 | 0.459–0.692 | 0.250 |
| IL-18BP | 0.678 | 0.588–0.759 | 0.0005 | 0.679 | 0.568–0.776 | 0.001 | 0.685 | 0.567–0.787 | 0.002 |
| IL-37 | 0.557 | 0.403–0.669 | 0.560 | 0.528 | 0.416–0.637 | 0.675 | 0.535 | 0.416–0.651 | 0.609 |
| IFN-γ | 0.533 | 0.441–0.623 | 0.546 | 0.509 | 0.399–0.620 | 0.881 | 0.597 | 0.478–0.709 | 0.141 |
| IP-10 | 0.686 | 0.596–0.766 | 0.0005 | 0.713 | 0.605–0.806 | 0.0003 | 0.659 | 0.541–0.765 | 0.013 |
Fig. 2.
ROC analysis of serum proteins: total IL-18 (a), IL-18BP (b), free IL-18 (c), IL-37 (d), IP-10 (e), IFN-γ (f).
The most sensitive and specific serum proteins that discriminated total TB from CAP were IP-10 and IL-18BP (Table 3). The sensitivity and specificity of the ELISA assay corresponding to the cut-off of 39.11 pg IP-10/ml were 56.18% and 82.86%, respectively, whereas the cut-off of 41.40 ng IP-18BP /ml corresponded to the sensitivity of 57.30% and specificity of 71.43%. IL-18BP and IP-10 also had the highest sensitivity and specificity in differentiating the M/MTB from CAP and ATB from CAP (Table 3). Serum IFN-γ weakly differentiated the ATB from CAP (the cut-off of 17.68 pg IFN-γ/ml corresponding to the sensitivity of 32.5% and specificity of 91.43%), but it did not discriminate the M/MTB from CAP.
Table 3.
Sensitivity, specificity, and optimal cut-off values of individual proteins measured in serum.
| Protein | Sensitivity |
Specificity |
Cut-off | Likelihood ratio | |
|---|---|---|---|---|---|
| (%) | (%) | ||||
| total TB vs CAP | total IL-18 | 91.01 | 40.00 | 281.40 pg/ml | 1.74 |
| free IL-18 | 48.31 | 62.86 | 6.01 pg/ml | 1.06 | |
| IL-18BP | 57.30 | 71.43 | 41.40 ng/ml | 1.87 | |
| IL-37 | 79.78 | 40.00 | 259.33 pg/ml | 1.07 | |
| IFN-γ | 22.47 | 91.43 | 17.68 pg/ml | 1.30 | |
| IP-10 | 56.18 | 82.86 | 39.11 pg/ml | 1.93 | |
| M/MTB vs CAP | total IL-18 | 92.00 | 40.00 | 281.40 pg/ml | 1.53 |
| free IL-18 | 46.00 | 68.57 | 4.00 pg/ml | 1.46 | |
| IL-18BP | 42.00 | 91.43 | 53.72 ng/ml | 4.9 | |
| IL-37 | 80.00 | 37.14 | 259.33 pg/ml | 1.27 | |
| IFN-γ | 86.00 | 0.00 | 30.82 pg/ml | 0.86 | |
| IP-10 | 66.00 | 77.14 | 35.22 pg/ml | 2.89 | |
| ATB vs CAP | total IL-18 | 90.00 | 40.00 | 281.40 pg/ml | 1.5 |
| free IL-18 | 62.50 | 62.86 | 6.01 pg/ml | 1.68 | |
| IL-18BP | 62.50 | 71.43 | 41.41 ng/ml | 2.19 | |
| IL-37 | 87.50 | 28.57 | 352.64 pg/ml | 1.22 | |
| IFN-γ | 32.50 | 91.43 | 17.68 pg/ml | 3.79 | |
| IP-10 | 55.00 | 82.86 | 39.11 pg/ml | 3.21 |
At the same time, it is worth noting that the analyses of IL-18, IL-18BP, IL-37, IFN-γ and IP-10 levels in the sera from the culture-positive and culture-negative M/MTB or ATB patients showed no significant differences (Table 4).
Table 4.
Total IL-18, IL-18BP, free IL-18, IL-37, IFN-γ and IP-10 levels in the sera from the culture-positive and culture-negative M/MTB or ATB patients.
| Protein | Concentration | |||||
|---|---|---|---|---|---|---|
| Me (IQR) | ||||||
| TB |
M/MTB |
ATB |
||||
| Culture-positive | Culture-negative | Culture-positive | Culture-negative | Culture-positive | Culture-negative | |
| total IL-18 | 719.46 | 490.78 | 588.27 | 483.72 | 967.78 | 549.81 |
| (465.67–1146.10) | (341.27–928.97) | (444.17–963.75) | (311.63–830.58) | (551.64–1297.84) | (376.73–953.65) | |
| free IL-18 | 6.18 | 4.33 | 5.53 | 3.61 | 8.81 | 6.41 |
| (4.23–11.26) | (2.72–8.32) | (3.93–12.8) | (2.24–7.04 | (4.66–11.18) | (3.78–8.41) | |
| IL-18BP | 44.99 | 40.19 | 41.72 | 57.64 | 46.82 | 36.43 |
| (35.27–59.62) | (30.82–77.21) | (29.66–56.92) | (33.22–83.17) | (41.81–80.66) | (26.70–50.42 | |
| IL-37 | 67.32 | 97.34 | 67.32 | 65.20 | 66.23 | 106.42 |
| (6.29–228.52) | (20.43–241.17) | (9.49–236.92) | (15.42–223.00) | (5.94–225.84) | (26.97–292.27) | |
| IFN-γ | 8.80 | 5.71 | 8.45 | 4.70 | 10.36 | 5.86 |
| (4.46–20.69) | (3.27–11.05) | (3.27–16.24) | (3.31–9.38) | (5.46–24.69) | (3.22–13.32) | |
| IP-10 | 51.67 | 37.14 | 41.02 | 42.04 | 64.32 | 16.92 |
| (26.85–80.71) | (14.59–66.07) | (23.10–74.00) | (22.91–57.67) | (31.41–130.46) | (11.10–69.55) | |
The analysis of dependence between the levels of all proteins revealed a certain correlation, that was specific for all study groups (IL-18BP and IP-10), some associations specific for a part of them (i.e., IFN-γ and IP-10 for M/MTB and ATB), and others only for one group (IL-37 and IP-10 for CAP) (Table 3). IL-18 ~ IFN-γ and IL-18BP ~ IFN-γcorrelations were specific for the ATB, but not the M/MTB group. The summary results of the correlation analysis are reported in Table 5.
Table 5.
Correlation and p-values between protein levels measured in serum.
| Proteins | TB |
M/MTB |
ATB |
CAP |
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| IL-18 ~ IL-18BP | 0.237 | 0.025 | 0.036 | 0.801 | 0.445 | 0.004 | 0.121 | 0.488 |
| IL-18 ~ IL-37 | −0.018 | 0.867 | 0.012 | 0.929 | −0.039 | 0.812 | 0.033 | 0.848 |
| IL-18 ~ IFN-γ | 0.360 | 0.001 | 0.206 | 0.150 | 0.462 | 0.003 | −0.147 | 0.398 |
| IL-18BP ~ IL-37 | −0.146 | 0.173 | −0.159 | 0.268 | −0.135 | 0.410 | −0.101 | 0.561 |
| IL-18BP ~ IFN-γ | 0.327 | 0.002 | 0.266 | 0.061 | 0.389 | 0.014 | −0.162 | 0.350 |
| IL-37 ~ IFN-γ | −0.079 | 0.463 | −0.173 | 0.229 | 0.185 | 0.257 | 0.199 | 0.250 |
| IL-18 ~ IP-10 | 0.222 | 0.037 | 0.166 | 0.121 | 0.271 | 0.047 | 0.010 | 0.475 |
| IL-18BP ~ IP-10 | 0.597 | <0.0001 | 0.224 | 0.050 | 0.468 | 0.001 | 0.334 | 0.024 |
| IL-37 ~ IP-10 | 0.068 | 0.526 | 0.118 | 0.203 | −0.012 | 0.470 | 0.276 | 0.050 |
| IFN-γ ~ IP-10 | 0.412 | <0.0001 | 0.235 | 0.047 | 0.537 | 0.001 | 0.199 | 0.125 |
| free IL-18 ~ IL-18BP | −0.420 | <0.0001 | −0.545 | <0.0001 | −0.274 | 0.092 | −0.332 | 0.052 |
| free IL-18 ~ IL-37 | −0.143 | 0.181 | 0.066 | 0.650 | 0.015 | 0.926 | 0.0729 | 0.677 |
| free IL-18 ~ IFN-γ | 0.093 | 0.386 | 0.045 | 0.758 | 0.119 | 0.472 | −0.277 | 0.107 |
| free IL-18 ~ IP-10 | −0.004 | 0.969 | 0.084 | 0.562 | −0.096 | 0.560 | −0.223 | 0.198 |
3.3. M.tb antigens-stimulated IL-18, IL-18BP, and IL-37 responses
The median concentrations of IL-18, IL-18BP, and IL-37 in M.tb antigens-stimulated QFT supernatants are presented in Fig. 3a–e. The antigen-specific responses of the studied proteins were evaluated by subtraction of the unstimulated levels from the antigen-stimulated concentrations. There were no significant differences in the M.tb antigens-stimulated levels of total IL-18 among the patients with TB (M/MTB - Me 723.54, IQR (410.68, 1039.90) pg/ml, ATB - Me 806.98, IQR (452.18, 1121.67) pg/ml) and the CAP individuals (Me 688.11, IQR (332.0, 903.0) pg/ml (Fig. 3a). As shown in Fig. 3b, IL-18BP concentrations in QFT cultures from the M/MTB, ATB and CAP groups were similar, reaching 38.93, IQR (28.71, 51.28) ng/ml, 45.65, IQR (33.13, 81.79) ng/ml and 48.68, IQR (36.05, 68.72) ng/ml, respectively. There were no significant differences in the levels of free IL-18 in M.tb antigens-stimulated QFT whole blood cultures among the studied groups (M/MTB - Me 8.43, IQR (4.96, 10.74) pg/ml, ATB – Me 8.46, IQR (3.28, 11.74) pg/ml, CAP − 5.80, IQR (2.50, 9.76) pg/ml) (Fig. 3c). The concentrations of IL-37 observed in QFT cultures from the M/MTB and ATB groups were comparable, measuring 274.87, IQR (156.63, 548.12) pg/ml, and 260.27, IQR (195.99, 465.44) pg/ml, respectively (Fig. 3d). The level of the cytokine in both TB groups was similar to that observed in the CAP patients (Me 257.36, IQR (128.50, 556.03) pg/ml).
Fig. 3.
Total IL-18, IL-18BP, free IL-18, IL-37 and IFN-γ levels in M.tb antigens-stimulated supernatants. Boxplots with median (horizontal line within the box), interquartile range (box limits), and extremes (whiskers) of serum levels of total IL-18 (a), IL-18BP (b), free IL-18 (c), IL-37 (d),and IFN-γ (e) in the groups of patients with mild/moderate tuberculosis (M/MTB), advanced tuberculosis (ATB) and community-acquired pneumonia (CAP).
The ROC analyses showed that among proteins measured in QFT supernatants, free IL-18 had the best diagnostic value in the differentiation between total TB and CAP with an AUC of 0.614 (95% CI 0.523–0.700, p = 0.045) (Table 6, Fig. 4c). IL-18BP had the highest AUC value (AUC = 0.645, 95% CI 0.533–0.745, p = 0.019) in the M/MTB vs CAP comparison, whereas free IL-18 (AUC = 0.602, 95% CI 0.483–0.714, p = 0.12) in the ATB vs CAP comparison (Table 6, Fig. 4).
Table 6.
Predictive values (AUC) of individual protein levels measured in QFT supernatants.
| Protein | ROC analysis |
ROC analysis |
ROC analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total TB vs CAP |
M/MTB vs CAP |
ATB vs CAP |
|||||||
| AUC | 95% CI | p-value | AUC | 95% CI | p-value | AUC | 95% CI | p-value | |
| total IL-18 | 0.576 | 0.484–0.664 | 0.182 | 0.555 | 0.443–0.663 | 0.393 | 0.594 | 0.475–0.706 | 0.156 |
| free IL-18 | 0.614 | 0.523–0.700 | 0.045 | 0.632 | 0.520–0.734 | 0.036 | 0.602 | 0.483–0.714 | 0.120 |
| IL-18BP | 0.596 | 0.504–0.683 | 0.092 | 0.645 | 0.533–0.745 | 0.019 | 0.519 | 0.401–0.636 | 0.776 |
| IL-37 | 0.524 | 0.432–0.614 | 0.707 | 0.525 | 0.414–0.635 | 0.703 | 0.526 | 0.407–0.642 | 0.714 |
Fig. 4.
ROC analysis of M.tb antigens-stimulated supernatants proteins: total IL-18 (a), IL-18BP (b), free IL-18 (c), IL-37 (d), and IFN-γ (e).
IL-18BP measured in M.tb antigens-stimulated QFT supernatants was the most sensitive and specific cytokine to discriminate the total TB group from the CAP group (Table 7). The sensitivity and specificity of the ELISA assay corresponding to the cut-off of 45.65 ng IL-18BP/ml were 58.43% and 62.86%, respectively. Similarly, IL-18BP was characterized by the highest sensitivity and specificity in the differentiation between the M/MTB and CAP groups, whereas IL-37 was the most sensitive and specific marker in the ATB vs. CAP differentiation (Table 7).
Table 7.
Sensitivity, specificity, and optimal cut-off values of individual proteins measured in M.tb antigens-stimulated QFT supernatants.
| Protein | Sensitivity |
Specificity |
Cut-off | Likelihood ratio | |
|---|---|---|---|---|---|
| (%) | (%) | ||||
| Total TB vs CAP | total IL-18 | 32.58 | 88.57 | 994.8 pg/ml | 1.42 |
| free IL-18 | 53.93 | 68.57 | 8.12 pg/ml | 1.50 | |
| IL-18BP | 58.43 | 62.86 | 45.65 ng/ml | 1.35 | |
| IL-37 | 70.79 | 42.86 | 193.28 pg/ml | 0.98 | |
| M/MTB vs CAP | total IL-18 | 94.00 | 22.86 | 293.77 pg/ml | 1.22 |
| free IL-18 | 72.00 | 54.29 | 5.91 pg/ml | 1.57 | |
| IL-18BP | 64.00 | 62.86 | 44.02 ng/ml | 1.72 | |
| IL-37 | 30.00 | 57.14 | 417.59 pg/ml | 0.70 | |
| ATB vs CAP | total IL-18 | 37.50 | 88.57 | 994.8 pg/ml | 3.28 |
| free IL-18 | 47.50 | 74.29 | >9.14 pg/ml | 1.85 | |
| IL-18BP | 77.50 | 42.86 | >193.2 ng/ml | 1.36 | |
| IL-37 | 70.00 | 94.29 | >23.23 pg/ml | 12.25 |
The analyses of IL-18, IL-18BP, IL-37, IFN-γ and IP-10 levels in M.tb antigens-stimulated QFT supernatants from culture-positive and culture-negative M/MTB or ATB patients showed no significant differences (Table 8).
Table 8.
Total IL-18, IL-18BP, free IL-18, IL-37 levels in QFT supernatants from culture-positive and culture-negative M/MTB or ATB patients.
| Protein | Concentration | |||||
|---|---|---|---|---|---|---|
| Me (IQR) | ||||||
| TB |
M/MTB |
ATB |
||||
| Culture-positive | Culture-negative | Culture-positive | Culture-negative | Culture-positive | Culture-negative | |
| total IL-18 | 773.24 | 749.81 | 744.29 | 626.18 | 876.88 | 765.47 |
| (449.24–1076.76) | (421.14–1080.21) | (467.68–1039.90) | (372.86–1166.25) | (440.42–1146.72) | (535.28–1038.75) | |
| free IL-18 | 9.00 | 6.05 | 8.48 | 6.69 | 9.74 | 5.64 |
| (6.16–11.85) | (3.33–10.05) | (6.91–10.74) | (4.10–11.78) | (3.70–15.22) | (3.15–10.05) | |
| IL-18BP | 39.76 | 43.69 | 37.08 | 40.77 | 42.50 | 49.26 |
| (27.55–53.02) | (33.88–64.46) | (27.55–53.67) | (33.71–53.67) | (28.07–58.25) | (41.55–81.94) | |
| IL-37 | 271.50 | 274.87 | 271.50 | 276.50 | 265.93 | 256.08 |
| (162.67–421.87) | (178.02–614.73) | (133.84–413.92) | (181.50–778.70) | (204.69–465.44) | (135.87–443.72) | |
The correlation analysis between the levels of all cytokines measured in QFT supernatants showed that free IL-18 ~ IL-18BP correlation was specific for all study groups, whereas the correlation between IL-18 ~ IL-18BP was specific only for the group of M/MTB patients. The summary results of the correlation analysis are reported in Table 9.
Table 9.
Correlation and p-values between the protein levels measured in QFT supernatants.
| Proteins | TB |
M/MTB |
ATB |
CAP |
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| IL-18 ~ IL-18BP | 0.209 | 0.051 | 0.285 | 0.044 | 0.056 | 0.732 | 0.061 | 0.725 |
| IL-18 ~ IL-37 | −0.123 | 0.250 | −0.117 | 0.418 | −0.117 | 0.478 | 0.055 | 0.751 |
| IL-18BP ~ IL-37 | −0.071 | 0.506 | −0.100 | 0.487 | −0.029 | 0.587 | −0.241 | 0.163 |
| Free IL-18 ~ IL-18BP | −0.499 | <0.0001 | −0.358 | 0.011 | −0.623 | <0.0001 | −0.525 | 0.001 |
| Free IL-18 ~ IL-37 | −0.047 | 0.660 | 0.005 | 0.972 | −0.100 | 0.545 | −0.016 | 0.929 |
3.4. Cytokine ratios measured in serum or M.tb antigens-stimulated QFT supernatants
The discriminative power of the ratios in serum or M.tb antigens-stimulated cultures between any two of the studied proteins (total IL-18, free IL-18, IL-18BP, IL-37, and IP-10) was analyzed in the M/MTB, ATB, and CAP groups. The highest discriminative power (of all ratios for proteins measured in serum): (1) between M /MTB and CAP was achieved by free IL-18/IP-10 ratio (AUC = 0.643) as well as IL-18BP/free IL-18 ratio (AUC = 0.615); (2) between ATB and CAP was achieved by IL-18/IL-37 ratio (AUC = 0.603) as well as free IL-18/IP-10 ratio (AUC = 0.568), (3) between M/MTB and ATB was achieved by total IL-18/IL-37 ratio (AUC = 0.606) as well as IL-18BP/IL-37 ratio (AUC = 0.602). On the other hand, the highest discriminative power (of all ratios for proteins measured in the culture supernatants): (1) between M/MTB and CAP and between ATB and CAP was achieved by IL-18BP/free IL-18 ratio (AUC = 0.669 and AUC = 0.584, respectively) as well as free IL-18/IL-37 ratio (AUC = 0.583 and AUC = 0.576, respectively); (2) between M/MTB and ATB was achieved by IL-18BP/free IL-18 ratio (AUC = 0.574) as well as IL-18BP/total IL-18 ratio (AUC = 0.562).
3.5. Penalized multiple logistic regression for comparison between the study groups.
Measuring multiple protein levels in serum and cultured supernatants, we aimed to develop a linear model with the highest discriminative power between the study groups. Due to the small sample size, it was first aimed to increase discriminative power by regressing out potential confounders. Therefore, we first asked the question of whether the abundance of certain circulating cells could affect the concentration of proteins. We performed a bootstrapped (300 replicates) 5-fold cross-validation feature selection analysis based on penalized multivariate linear regression (elastic-net) to find that for the protein levels measured in serum the significant predictors were RBC, HGB, and HCT, whereas for the protein levels measured in culture supernatants the informative features were HGB and HCT. Subsequently, for each protein separately, we regressed out the effect of the selected features with the use of standard linear models. In what follows, we trained four logistic elastic-net models (one for each group-wise comparison in one of the two compartments separately) using the above residuals as predictors. We found IL-18 and IL-18BP to be weak predictors in serum and no predictors in culture supernatants to be informative of the M/MTB versus CAP status. As far as the CAP versus ATB comparison is concerned, we found IL-18BP, free IL-18, and IL-37 in serum and IL-18 as well as IL-18BP to be associated with odds ratio in the culture supernatants. The results are summarized in Supplementary Table S1.
4. Discussion
In addressing the need for rapid and accurate diagnostic tests allowing the differentiation of active pulmonary TB from non-mycobacterial community-acquired pneumonia, we estimated if individual protein levels (IL-18, IL-18BP, IL-37, IFN-γ, and IP-10) and/or their ratios, might be considered auxiliary immunological biomarkers.
In a recent study, performed among TB patients and healthy volunteers, we detected an imbalance between the members of the IL-18 signalling complex in active TB, which resulted in a significant increase in the serum levels of total IL-18, IL-18BP, IFN-γ and IP-10 and a simultaneous decrease in the concentration of the anti-inflammatory IL-37 (Wawrocki et al., 2019).
In the current study, we found the potential of IL-18BP and IP-10 in the differentiation of the whole group of TB and CAP patients (Table 2). The highest discriminative power was found for IP-10 and IL-18BP measured in serum. The potential of IL-18BP and IP-10 in the differentiation of pulmonary TB and non-mycobacterial lung infections, to the best of our knowledge, had not been demonstrated before. We detected AUCs at the level of 0.686 for IP-10 and 0.678 for IL-18BP for the discrimination between TB and CAP patients.
As a naturally occurring inhibitor of IL-18, IL-18BP neutralizes the circulating IL-18. The consequences of IL-18 neutralization can include down-regulating Th1 immune response, blocking the expansion of Th17 cells, and inhibiting the infiltration of T lymphocytes into the sites of inflammation (Chiossone et al., 2012, Millward et al., 2010, Plitz et al., 2003). Significant overproduction of serum IL-18BP in the group of TB patients may be interpreted as a feedback mechanism that limits excessive IL-18 activity. However, we hypothesize that the process of modifications in the IL-18 signaling system in active TB is more complex. In the group of M/MTB patients categorized as having mild/moderate TB, the upregulated level of IL-18BP was accompanied by no visible increase in the IL-18 level. At the same time, in the group of patients with advanced TB (ATB), the upregulation of IL-18BP and IL-18 was observed simultaneously (Fig. 1A and B). As a consequence, the correlation between IL and 18 and IFN-γ as well as IL18BP and IFN-γ, was specific for the ATB but not the M/MTB group. Interestingly, the association between free IL-18 and IL-18BP was noted as specific for M/MTB group. Moreover, the highest discriminative power between M/MTB and ATB was achieved by total IL-18/IL-37 ratio (AUC = 0.606) as well as IL-18BP/IL-37 ratio (AUC = 0.602), confirming previously observed imbalance between the members of the IL-18 signaling complex in active pulmonary TB, in the study performed among TB patients and healthy volunteers(Wawrocki et al., 2019). Based on our results, we hypothesize that the elevated levels of IL-18BP were sufficient to counteract the increase in the IL-18 level in the M/MTB group but not sufficient in the ATB patients with a more advanced lung injury. This is probably due to the more efficient production of IL-18 in the ATB than in the M/MTB patients. The role of IL-18 produced by alveolar epithelial cells type II in pathomechanisms of pulmonary TB has been proposed by Pechcovsky et al.. A significant increase in the serum levels of IL-18, IFN-γ and soluble Fas in complicated TB cases as compared to uncomplicated ones was considered as a marker suggesting pulmonary TB, especially in advanced cases. Fas signaling triggers apoptosis and the production and release of mature IL-18. Biologically active IL-18 is able to enhance NK and T cell cytotoxicity and induce the production of matrix metalloproteinases, a process essential for the development of inflammatory reaction and tissue damage. Thus, the overproduction of IL-18 could explain a more advanced lung injury in the ATB patients as compared to the M/MTB group (El-Masry et al., 2007, Pechkovsky et al., 2006).
To explain the simultaneous overproduction of IL-18BP and IL-18 in the ATB patients and the selective intensification of IL-18BP in the M/MTB patients, it is worth mentioning that various isoforms of IL-18BP have different affinity for IL-18. For example, IL-18BPa and IL-18BPc effectively neutralize IL-18, while IL-18BPb and IL-18BPd isoforms lack the ability to bind and neutralize IL-18 (Kim et al., 2000). It is likely that the simultaneous increase in the IL-18BP and IL-18 levels in the ATB group, at least in part, may be a result of the preferential secretion of nonfunctional isoforms of IL-18BP. This observation may point to IL-18BP as a potential target in novel therapeutic approaches. A number of studies demonstrated the use of IL-18BP in the treatment of IL-18-associated diseases characterized by an abnormal ratio of IL-18/IL-18BP in body fluids. The administration of exogenous IL-18BP is a promising therapeutic strategy in psoriasis, experimental autoimmune encephalomyelitis, contact hypersensitivity, rheumatoid arthritis, LPS-induced shock, and atherosclerosis (Banda et al., 2003, Chiossone et al., 2012, Faggioni et al., 2001, Millward et al., 2010, Plitz et al., 2003, Schif-Zuck et al., 2005, Tak et al., 2006). Experimental data indicated that the IL-18BP-driven reduction in cell activity was dose-dependent; however, treatment with low, but not with high, doses of IL-18BP was shown to be the most effective in vivo (Banda et al., 2003).
The results of our study indicate a potential role of IL-18 in the pathomechanisms of pulmonary TB. It is important to note that IL-18 cannot serve as an indicator for differential diagnosis between active TB and non-mycobacterial community-acquired pneumonia, as indicated by the weak discriminative power. We show that the levels of total IL-18 in the sera from the M/MTB and CAP patients were comparable, although significantly higher in ATB patients as compared to the CAP group. Similarly, the discriminative power of the ratios analyzed between any of the two of the studied proteins measured in serum differed between the TB groups. The free IL-18/IP-10 ratio and IL-18BP/free IL-18 measured in serum was the best scoring in the discrimination analysis between M/MTB and CAP groups, whereas the highest importance scores for classification between ATB and CAP were assigned to serum total IL-18/IFN-γ and IL-18BP/IFN-γ. In contrast, IL-18BP/IP10 ratio measured in serum showed the highest discriminative power for distinguishing the entire TB group from the CAP patients. Various studies showed that the cytokines ratios might act as more discriminatory and sensitive TB indicators than assessing a single protein level (Demissie et al., 2004, Goyal et al., 2016, Joshi et al., 2015, Sun et al., 2016, Suter-Riniker et al., 2011, Wassie et al., 2008). Goyal et al. showed a potential role of the IFN-γ/IL-2 ratio in the diagnosis of extrapulmonary TB, Demissie et al. reported a discriminative power of the IL-4/IL-4δ2 in the evaluation of M.tb reactivation risk among latently infected individuals, whereas Wassie et al. demonstrated that IFN-γ/IL-4 and IL-4δ2/IL-4 mRNA ratios could serve as valuable markers for TB susceptibility (Demissie et al., 2004, Goyal et al., 2016, Wassie et al., 2008).
The second part of our studies was to check whether the individual and pairwise related IL-18 (total and free), IL-18BP and IL-37 levels, as well as their ratios, measured in M.tb specific antigen-stimulated QuantiFERON culture supernatants, might be considered auxiliary immunological biomarkers in the differentiation between patients with active TB and patients with community-acquired pneumonia. We found no significant differences in the levels of individual proteins of the IL-18 signaling complex in M.tb antigens-stimulated QFT cultures among the TB patients categorized as having mild/moderate TB or advanced TB and patients with CAP. In contrast, the serum IL-18BP, IP-10, and IL-18 levels were able to differentiate between TB and CAP patients. In this way, we confirmed our earlier suggestion that the unstimulated serum biomarker performance is a better approach to diagnose patients with active TB, differentiate them from CAP patients, but also from healthy individuals with a latent M.tb infection and uninfected (Wawrocki et al., 2019). In the correlation analysis between levels of IL-18, IL-18 BP, and IL-37 measured in QFT supernatants, we demonstrated that free IL-18 and IL-18BP correlation was specific for all study groups. On the other hand, the correlation between total IL-18 and IL-18BP was specific for the M/MTB group, but not for the ATB group. In the interpretation of our results on the discriminative power of the levels of the IL-18 signalling complex proteins in QFT whole blood cultures (for TB and CAP patients), it should be noted that the production of cytokines by peripheral blood mononuclear cells is variable and it is influenced by many heritable and non-heritable factors (Schirmer et al., 2018). Genetic variation in individual genes and pathways responsible for cytokine production capacity can be a significant part of cytokine production variability observed among individuals. Non-heritable factors, including age, body weight, and composition of the human microbiome, can also drive variation in baseline cytokine levels. Thus, cytokine variability may have important implications for clinical practice, and the caution in interpreting the results is required to distinguish the immune response to a specific infectious agent from nonspecific inter-individual variations in cytokine responses.
Given the moderate size of the study groups, we aimed to perform a more speculative analysis in which we train an elastic-net model to detect the most robust predictors of the study groups. In this analysis, we detected only a small number of informative, in essence, the IL-18 and IL-18BP were the only significant ones in discrimination of CAP and either M/MTB or ATB cases. Therefore, we strongly believe that in order to find a comprehensive panel of proteins that are both sensitive and specific in discriminating TB and CAP, further and larger studies are required.
Because of the unpredictable and nonspecific clinical manifestation of TB, the paucibacillary TB cases can be a diagnostic challenge for physicians. In 2018, only 55% of pulmonary TB cases were bacteriologically confirmed globally (WHO, 2018). Accordingly, in this study, 55% of TB cases (52% of M/MTB and 56% of ATB) were confirmed by culture. We assessed the levels of IL-18, IL-18BP, and IL-37 in these patient groups, however, we were observing no differences in the levels of the studied proteins either in serum or M.tb-stimulated QFT cultures from culture-positive and culture-negative M/MTB or ATB patients.
The systematic review of publications concerning immunoenzymatic assessment of selected proteins and cytokines indicates that none of the commercial tests performed by itself provide an accurate diagnosis of TB. What is more, most of the large-scale research and meta-analysis for years focused mainly on assessing IFN-γ levels in commercial IGRA assays. They show that the assessment of the IFN-γ level alone is not a sufficient indicator to confirm M.tb infection. A heterogeneous methodology that does not overlap between studies limits comparability and interpretation of results. A rigorous approach to enzyme immunoassays in the diagnosis of tuberculosis in children and adults is needed. Therefore, it seems extremely important to search for new tuberculous biomarkers and evaluate intricate cytokine panels to confirm the disease, while adhering to the highest research standards. Meta-analyzes also emphasize that world trends in the diagnosis of latent TB should be changed to understand this disease better (Mandalakas et al., 2011, Rogerson et al., 2013, Steingart et al., 2011, Sun et al., 2011, Zeng et al., 2017, Zhou et al., 2015).
In conclusion, our study indicates that a combined analysis of serum IL-18BP and IP-10 could offer good classification performance and might be considered as an auxiliary tool in the differentiation of TB from CAP. At the same time, our results may enrich knowledge about the role of IL-18 in the immunopathogenesis of pulmonary TB.
Funding
This work was supported by the National Science Centre grants no 2015/19/N/NZ6/01385 and 2016/21/B/NZ7/01771.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.09.003.
Contributor Information
Sebastian Wawrocki, Email: sebastian.wawrocki@biol.uni.lodz.pl.
Michal Seweryn, Email: michal.seweryn@biol.uni.lodz.pl.
Grzegorz Kielnierowski, Email: kielnier@o2.pl.
Wieslawa Rudnicka, Email: wieslawa.rudnicka@biol.uni.lodz.pl.
Magdalena Druszczynska, Email: magdalena.druszczynska@biol.uni.lodz.pl.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdel Fattah E., Bhattacharya A., Herron A., Safdar Z., Eissa N.T. Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency. J. Immunol. 2015;194:5407–5416. doi: 10.4049/jimmunol.1402277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda N.K., Vondracek A., Kraus D., Dinarello C.A., Kim S.-H., Bendele A., Senaldi G., Arend W.P. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J. Immunol. 2003;170:2100–2105. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- Barksby H.E., Lea S.R., Preshaw P.M., Taylor J.J. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin. Exp. Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biet F., Locht C., Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 2002 doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- Chiossone L., Audonnet S., Chetaille B., Chasson L., Farnarier C., Berda-Haddad Y., Jordan S., Koszinowski U.H., Dalod M., Mazodier K., Novick D., Dinarello C.A., Vivier E., Kaplanski G. Protection from inflammatory organ damage in a murine model of hemophagocytic lymphohistiocytosis using treatment with IL-18 binding protein. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie A., Abebe M., Aseffa A., Rook G., Fletcher H., Zumla A., Weldingh K., Brock I., Andersen P., Doherty T.M., VACSEL Study Group Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J. Immunol. 2004;172:6938–6943. doi: 10.4049/jimmunol.172.11.6938. [DOI] [PubMed] [Google Scholar]
- Dima E., Koltsida O., Katsaounou P., Vakali S., Koutsoukou A., Koulouris N.G., Rovina N. Implication of Interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD) Cytokine. 2015;74:313–317. doi: 10.1016/j.cyto.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. IL-18: AtH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999;103:11–24. doi: 10.1016/S0091-6749(99)70518-X. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Interleukin-1β, interleukin-18, and the interleukin-1β converting enzyme. Ann. N. Y. Acad. Sci. 1998:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A., Nold-Petry C., Nold M., Fujita M., Li S., Kim S., Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 2016;46:1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013;4:1–10. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Masry S., Lotfy M., Nasif W., El-Kady I., Al-Badrawy M. Elevated serum level of interleukin (IL)-18, interferon (IFN)-γ and soluble fas in patients with pulmonary complications in Tuberculosis. Acta Microbiol. Immunol. Hung. 2007;54:65–77. doi: 10.1556/AMicr.54.2007.1.7. [DOI] [PubMed] [Google Scholar]
- Faggioni R., Cattley R.C., Guo J., Flores S., Brown H., Qi M., Yin S., Hill D., Scully S., Chen C., Brankow D., Lewis J., Baikalov C., Yamane H., Meng T., Martin F., Hu S., Boone T., Senaldi G. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J. Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- Fields, J.K., Günther, S., Sundberg, E.J., 2019. Structural basis of IL-1 family cytokine signaling. Front. Immunol. https://doi.org/10.3389/fimmu.2019.01412. [DOI] [PMC free article] [PubMed]
- Gardella S., Andrei C., Costigliolo S., Poggi A., Zocchi M.R., Rubartelli A. Interleukin-18 synthesis and secretion by dendritic cells are modulated by interaction with antigen-specific T cells. J. Leukoc. Biol. 1999;66:237–241. doi: 10.1002/jlb.66.2.237. [DOI] [PubMed] [Google Scholar]
- Goyal N., Kashyap B., Kaur I.R. Significance of IFN-γ/IL-2 ratio as a circulating diagnostic biomarker in extrapulmonary tuberculosis. Scand. J. Immunol. 2016;83:338–344. doi: 10.1111/sji.12424. [DOI] [PubMed] [Google Scholar]
- Gutzmer R., Langer K., Mommert S., Wittmann M., Kapp A., Werfel T. Human dendritic cells express the IL-18R and are chemoattracted to IL-18. J. Immunol. 2003;171:6363–6371. doi: 10.4049/jimmunol.171.12.6363. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kato S., Oka N., Imaoka H., Kinoshita T., Takei S., Kitasato Y., Kawayama T., Imaizumi T., Yamada K., Young H.A., Aizawa H. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am. J. Respir. Crit. Care Med. 2007;176:49–62. doi: 10.1164/rccm.200603-316OC. [DOI] [PubMed] [Google Scholar]
- Joshi L., Ponnana M., Sivangala R., Chelluri L.K., Nallari P., Penmetsa S., Valluri V., Gaddam S. Evaluation of TNF-α, Il-10 and Il-6 cytokine production and their correlation with genotype variants amongst tuberculosis patients and their household contacts. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0137727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Eisenstein M., Reznikov L., Fantuzzi G., Novick D., Rubinstein M., Dinarello C.A. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitasato Y., Hoshino T., Okamoto M., Kato S., Koda Y., Nagata N., Kinoshita M., Koga H., Yoon D.-Y., Asao H., Ohmoto H., Koga T., Rikimaru T., Aizawa H. Enhanced expression of interleukin-18 and its receptor in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2004;31:619–625. doi: 10.1165/rcmb.2003-0306OC. [DOI] [PubMed] [Google Scholar]
- Kohno K., Kataoka J., Ohtsuki T., Suemoto Y., Okamoto I., Usui M., Ikeda M., Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- Krumm B., Xiang Y., Deng J. Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014 doi: 10.1002/pro.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalakas, A.M., Detjen, A.K., Hesseling, A.C., Benedetti, A., Menzies, D., 2011. Interferon-gamma release assays and childhood tuberculosis: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. https://doi.org/10.5588/ijtld.10.0631. [DOI] [PubMed]
- Matsui K., Yoshimoto T., Tsutsui H., Hyodo Y., Hayashi N., Hiroishi K., Kawada N., Okamura H., Nakanishi K., Higashino K. Propionibacterium acnes treatment diminishes CD4+NK1.1+ T Cells but induces type I T Cells in the liver by induction of IL-12 and IL-18 production from kupffer cells. J. Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- Migliorini P., Anzilotti C., Pratesi F., Quattroni P., Bargagna M., Dinarello C.A., Boraschi D. Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur. Cytokine Netw. 2010;21:264–271. doi: 10.1684/ecn.2010.0210. [DOI] [PubMed] [Google Scholar]
- Millward J.M., Løbner M., Wheeler R.D., Owens T. Inflammation in the central nervous system and Th17 responses are inhibited by IFN-gamma-induced IL-18 binding protein. J. Immunol. 2010;185:2458–2466. doi: 10.4049/jimmunol.0902153. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Owen C.A. Interleukin-18: The master regulator driving destructive and remodeling processes in the lungs of patients with chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med. 2012 doi: 10.1164/rccm.201204-0590ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 regulates both Th1 and Th2 response. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Nold M.F., Nold-Petry C.A., Zepp J.A., Palmer B.E., Bufler P., Dinarello C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D., Kim S., Kaplanski G., Dinarello C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013;25:439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Kato S., Oizumi K., Kinoshita M., Inoue Y., Hoshino K., Akira S., McKenzie A.N.J., Young H.A., Hoshino T. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood. 2002;99:1289–1298. doi: 10.1182/blood.v99.4.1289. [DOI] [PubMed] [Google Scholar]
- Okamura H., Tsutsul H., Komatsu T., Yutsudo M., Tanimoto T., Torigoe K., Okura T., Nukada Y., Hattori K., Akita K., Namba M., Tanabe F., Konishi K., Fukuda S., Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Pechkovsky D.V., Goldmann T., Vollmer E., Müller-Quernheim J., Zissel G. Interleukin-18 expression by alveolar epithelial cells type II in tuberculosis and sarcoidosis. FEMS Immunol. Med. Microbiol. 2006;46:30–38. doi: 10.1111/j.1574-695X.2005.00013.x. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C., Joosten L.A.B., Helsen M.M.A., Sattonnet-Roche P., Siegfried C., Alouani S., van de Loo F.A.J., Graber P., Aloni S., Cirillo R., Lubberts E., Dinarello C.A., van den Berg W.B., Chvatchko Y. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J. Clin. Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitz T., Saint-Mézard P., Satho M., Herren S., Waltzinger C., de Carvalho Bittencourt M., Kosco-Vilbois M.H., Chvatchko Y. IL-18 binding protein protects against contact hypersensitivity. J. Immunol. 2003;171:1164–1171. doi: 10.4049/jimmunol.171.3.1164. [DOI] [PubMed] [Google Scholar]
- Rogerson T.E., Chen S., Kok J., Hayen A., Craig J.C., Sud K., Kable K., Webster A.C. Tests for latent tuberculosis in people with ESRD: a systematic review. Am. J. Kidney Dis. 2013;61:33–43. doi: 10.1053/j.ajkd.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Schif-Zuck S., Westermann J., Netzer N., Zohar Y., Meiron M., Wildbaum G., Karin N. Targeted overexpression of IL-18 binding protein at the central nervous system overrides flexibility in functional polarization of antigen-specific Th2 cells. J. Immunol. 2005;174:4307–4315. doi: 10.4049/jimmunol.174.7.4307. [DOI] [PubMed] [Google Scholar]
- Schirmer, M., Kumar, V., Netea, M.G., Xavier, R.J., 2018. The causes and consequences of variation in human cytokine production in health. Curr. Opin. Immunol. https://doi.org/10.1016/j.coi.2018.05.012. [DOI] [PubMed]
- Steingart K.R., Flores L.L., Dendukuri N., Schiller I., Laal S., Ramsay A., Hopewell P.C., Pai M. Commercial Serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and Meta-Analysis. PLoS Med. 2011 doi: 10.1371/journal.pmed.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S., Uehara A., Nochi T., Yamaguchi T., Ueda H., Sugiyama A., Hanzawa K., Kumagai K., Okamura H., Takada H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 2001;167:6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- Sun L., Xiao J., Miao Q., Feng W.X., Wu X.R., Yin Q.Q., Jiao W.W., Shen C., Liu F., Shen D., Shen A.D. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. FEMS Immunol. Med. Microbiol. 2011;63:165–173. doi: 10.1111/j.1574-695X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- Sun Q., Wei W., Sha W. Potential role for Mycobacterium tuberculosis specific IL-2 and IFN-γ responses in discriminating between latent infection and active disease after long-term stimulation. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0166501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter-Riniker F., Berger A., Mayor D., Bittel P., Iseli P., Bodmer T. Clinical significance of interleukin-2/gamma interferon ratios in Mycobacterium tuberculosis-specific T-cell signatures. Clin. Vaccine Immunol. 2011;18:1395–1396. doi: 10.1128/CVI.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P.P., Bacchi M., Bertolino M., Desson A. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. Eur. J. Drug Metab. Pharmacokinet. 2006;31:109–116. doi: 10.1007/BF03191127. [DOI] [PubMed] [Google Scholar]
- Wassie L., Demissie A., Aseffa A., Abebe M., Yamuah L., Tilahun H., Petros B., Rook G., Zumla A., Andersen P., Doherty T.M., Fetcher H., Chintu C., Mulundu G., Mwaba P., McAdam K.P.W.J., Owiafe P., Warndorff D., Lienhardt C., Brookes R., Hill P. Ex vivo cytokine mRNA levels correlate with changing clinical status of Ethiopian TB patients and their contacts over time. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrocki, S., Druszczynska, M., 2017. Inflammasomes in mycobacterium tuberculosis-driven immunity. Can. J. Infect. Dis. Med. Microbiol. https://doi.org/10.1155/2017/2309478. [DOI] [PMC free article] [PubMed]
- Wawrocki S., Druszczynska M., Kowalewicz-Kulbat M., Rudnicka W. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim. Pol. 2016;63 doi: 10.18388/abp.2015_1153. [DOI] [PubMed] [Google Scholar]
- Wawrocki S., Seweryn M., Kielnierowski G., Rudnicka W., Wlodarczyk M., Druszczynska M. IL-18/IL-37/IP-10 signalling complex as a potential biomarker for discriminating active and latent TB. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0225556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation, 2018. Global tuberculosis report 2018.
- Yasuda, K., Nakanishi, K., Tsutsui, H., 2019. Interleukin-18 in health and disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20030649. [DOI] [PMC free article] [PubMed]
- Zeng N., Wan C., Qin J., Wu Y., Yang T., Shen Y., Wen F., Chen L. Diagnostic value of interleukins for tuberculous pleural effusion: a systematic review and meta-analysis. BMC Pulm. Med. 2017;17:180. doi: 10.1186/s12890-017-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.X., Liu Y.L., Zhai K., Shi H.Z., Tong Z.H. Body fluid interferon-γ release assay for diagnosis of extrapulmonary tuberculosis in adults: A systematic review and meta-analysis. Sci. Rep. 2015;5 doi: 10.1038/srep15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.