Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease affecting the elderly. AD is associated with a progressive decline in memory and cognitive abilities, drastic changes in behavioural patterns and other psychiatric manifestations. It leads to a significant decline in the quality of life at personal, household as well as national level. Although AD was described about hundred years back and multiple theories have been proposed, its exact pathophysiology is unknown. There is no cure for AD and the life expectancy of AD patients remains low at 3-9 years. An accurate understanding of the molecular mechanism(s) involved in the pathogenesis of AD is imperative to devise a successful treatment strategy. This review explains and summarises the current understanding of different therapeutic strategies based on various molecular pathways known to date. Different strategies based on anti-amyloid pathology, glutamatergic pathway, anti-tau, neuroprotection through neurotrophic factors and cholinergic neurotransmission have been discussed. Further, the use of anti-inflammatory drugs, nutraceuticals, and dietary interventions has also been explained in the management of AD. It further describes different pharmacological and dietary interventions being used in treating and/or managing AD. Additionally, this article provides a thorough review of the literature for improving the therapeutic paradigm of AD.
Keywords: Beta-amyloid, cholinergic neurotransmission, dietary interventions, drug discovery, glutamatergic pathway, neurofibrillary tangles, neuroprotection, nutraceuticals
1. INTRODUCTION
Alzheimer's disease (AD) is the most common cause of dementia and a type of untreatable neurodegenerative condition. It is the third most expensive medical condition [1, 2]. Multiple cognitive and non-cognitive functions get disturbed in AD, thereby impacting the quality of life. A wide range of genetic and environmental factors lead to late-onset AD (LOAD). Beta-amyloid and abnormal tau proteins play a major role in disease development. The basic pathways of the basal forebrain and cerebral cortex are compromised in AD with the neurotransmitter acetylcholine being affected [2]. This degenerative disease is progressive in nature, with the entorhinal cortex being one of the first regions to get affected, which then spreads to other Neighboring parts, such as the hippocampus [3]. The appearance of neurofibrillary tangles (made of tau proteins) and plaques (aggregates of amyloid-beta protein) are the characteristics of the onset of this disease followed by neurodegeneration and synaptic dysfunction [1, 4].
Most clinical studies have focused on reducing the pathogenic amyloid-beta (Aβ) and tau levels. However, such strategies have failed to bring about any long-term relief. The role of Aβ in the normal physiological functioning of the cells, as described by recent research studies have made AD treatment even more complex. Although Aβ pathology has been long thought of as a risk factor for AD, there is still no conclusive evidence of its causal role in AD onset. This has even led some scientists to believe that its aetiology could be due to another pathway that leads to AD, and Aβ might just be one metabolite of its consequence. The mechanism(s) involved in the pathogenesis of AD remain elusive till date. The availability of brain samples from only late-stage AD patients as well as limitations of in vivo models has posed a challenge for pinpointing the molecular changes that take place in prodromal AD. To describe AD, there are many evidence-based theories that have been proposed by researchers. The list includes the theories of free radical damage, inflammatory response, cholinergic damage, and the most popular ones like β-amyloid and anomalous tau protein metabolism, among others.
The lack of understanding of the underlying molecular mechanisms and the unavailability of credible strategies for its early prediction make the prevention and treatment of AD difficult. Understanding the exact pathophysiology of AD would facilitate the finding of appropriate biomarkers and drug‐targets to tackle the growing burden of the disease. The development of efficient and potent therapeutic drugs is the key to tackle the situation. Hence, the search for a diverse range of treatments for this multifactorial disease has shifted the therapy dynamics since the past decade as researchers are inclining towards other non-conventional means of treatment.
2. ANTI-AMYLOID THERAPEUTIC STRATEGIES
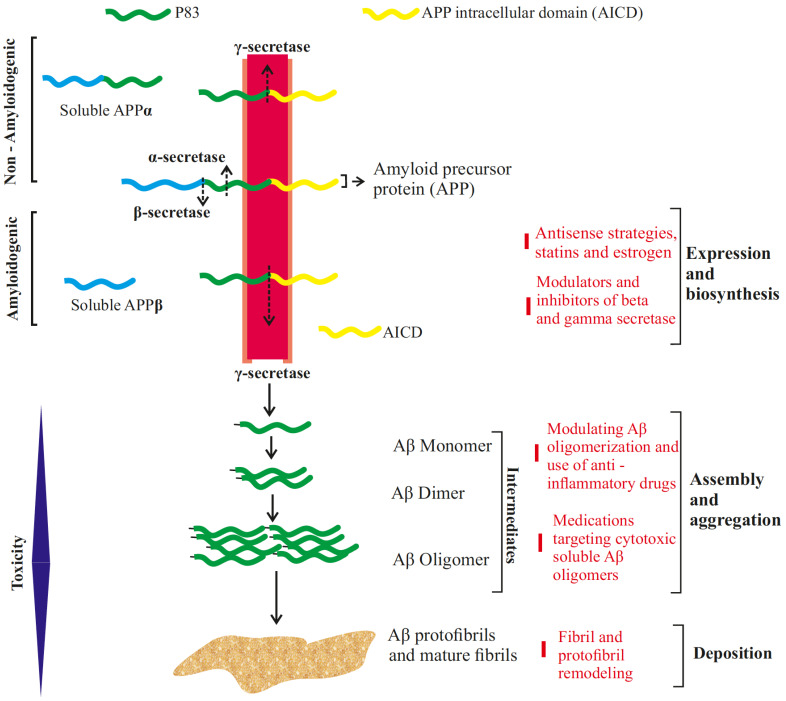
Aβ pathology is one of the major characteristic features of AD, besides tau aggregates. Its role in initiating a cascade of degenerative events is unquestionable. It starts aggregating in the brain of AD patients years before the onset of the symptoms [1]. It is a 4 kD peptide obtained from the integral cellular trans-membrane protein - the amyloid precursor protein (APP). Although not fully understood, APP has its role in a number of cellular processes in the brain ranging from synapse formation, neuroprotection, cell-cell interaction, among others, as observed in various APP knock out experiments [5]. Differential APP processing results in a number of products, each with a distinct role yet to be elucidated. Aβ is generated in the amyloidogenic processing of APP, involving initial cleavage by β-secretase, deviating from the non-amyloidogenic pathway, which employs α-secretase in the initial step (Fig. 1). The end-product of β-secretase is cleaved by γ-secretases, generating Aβ peptide of varying residues such as Aβ40, Aβ42 or Aβ38. Several studies have correlated amyloid toxicity with an elevated ratio of Aβ42/Aβ40 species in AD. The presence of an extra two residues Ile41 and Ala42 in Aβ42 affects the hydrophobicity of monomers and may assist in its aggregation and oligomerization. Thus, mutations in genes such as APP and PSEN1 which affect the differential processing of amyloid precursor protein has garnered interest and has led to the development of novel therapeutic measures to modulate the synthesis of APP [6, 7].
Fig. (1).
Beta-amyloid treatment strategies at various levels of its maturation. The non-amyloidogenic pathway results in the sequential cleavage of transmembrane Amyloid Precursor Protein (APP) by the enzymes α-secretase and γ-secretase resulting in soluble APPα fragment, P83 fragment (proteolytically degraded at later stages) and APP Intracellular Domain fragment (AICD). In amyloidogenic pathway, APP is first processed by β-secretase, releasing soluble APPβ, followed by γ-secretase which cleaves the remaining fragment generating either Aβ1-40 or Aβ1-42, the length of which is determined by the site at which γ-secretase cuts. These Aβ monomers assemble together in series of steps, forming dimers to cytotoxic oligomers, developing into protofibrils and mature fibrils. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Modulating Precursor Synthesis and Aβ Concentration
The elucidation of Aβ synthesis led researchers to target it at various stages of its production. Key enzymes such as γ-secretases and β-secretases were of interest, which led to the development of their respective inhibitors. Early attempts at using γ-secretase inhibitors (GSI) were appealing as it could halt the production of Aβ completely. A number of pre-clinical and clinical trials were conducted using a variety of GSIs. However, these attempts were short-lived as the side effects vastly outnumbered the amelioration offered. Further, the existence of multiple γ-secretase substrates with critical biological functions, in addition to APP, proved a major hindrance. Interaction of γ-secretase with Notch receptors is crucial, as one of its end products, called Notch Intracellular Domain (NICD) is a major transcriptional regulator. Inhibition of NICD is known to cause defects in somite development, neurogenesis, and immune cell maturation, among others in animal studies. GSIs such as Semagacestat and Avagacestat had been of particular interest for their potency and selectivity. However, they faced similar issues for their side effects and efficacy. The rise and fall of GSIs and recognition of the functions of γ-secretase eventually shifted the spotlight towards γ-secretase modulators (GSM). Compared to GSIs, GSMs differ in their activity by regulating the processing of γ-secretases rather than relying on its selectivity towards APP over Notch. Although its interaction with γ -secretases is still not clear, it is believed that it could alter the activity of its proteolytic sub-unit, resulting in a decreased ratio of Aβ42/Aβ40 [6].
Another approach to counter Aβ aggregates is via immunotherapy, which includes active immunisation to produce natural antibodies by stimulating the immune system and passive immunisation through exogenous antibody administration [8]. These antibodies target various intermediary substrates, such as Aβ dimers and oligomers, in the formation of plaques and fibrils (Fig. 1). The inspiration for this approach came from individuals having an innate advantage with their immune system successfully defending against the amyloid based pathology, paving the way for immune-based therapy. Not surprisingly, these donors did not display any cognitive impairment, which is a sure sign of AD. As of now, several anti-amyloid antibodies have reached Phase 2 and Phase 3 clinical trials based on their promising outcomes in early in vivo and in vitro experiments. However, human trials have been disappointing so far with a few notable exception(s), making it clear that immunotherapy for AD is still in infancy and needs a mix of creativity and innovation for this strategy to succeed.
2.1.1. Aducanumab (BIIB037)
It is an IgG1 type human monoclonal antibody (mAb) that primarily binds to Aβ aggregates, soluble oligomers and also insoluble fibrils. However, it does not seem to target Aβ monomers. Aducanumab analogue can cross the blood-brain barrier and engage its target efficiently, thus clearing beta-amyloid toxicity. The effects can be monitored effectively by positron emission tomography (PET). Phagocytic activity of microglia enhances the engulfing of amyloid plaques by binding to the Fc region of the antibody. This drug showed good tolerability and safety outcomes since its inception in early clinical trials. Phase 1 trials on patients with mild to moderate AD reported a decrease in Aβ concentration, as well as improved cognitive scores as assessed by Clinical Dementia Rating-Sum of Boxes and Mini-Mental State Examination. In March 2019, the current license holder of this drug - Biogen had to halt the project in Phase 3 trials, as the data accumulated were apparently inconsistent with the predicted trajectory of the outcome. However, soon after, this drug bounced back to relevancy with the surprising announcement by Biogen in the same year (October 2019) after a re-evaluation of its previous trials highlighting its robustness in reducing amyloid pathology. Common side effects include headache associated with amyloid-related imaging abnormalities (ARIA), especially ARIA-E in which fluid deposition occurs, leading to edema. Even though some additional side effects have been reported, including urinary tract infection, nausea, anxiety, cough and upper respiratory tract infection, it still lacks scientific consensus whether the drug has any involvement with it [4, 9-11].
2.1.2. Bapineuzumab
It is a type of humanized immunoglobulin, IgG1 anti-Aβ mAb that binds to the five N-terminal residues and helps in clearing both fibrillary and soluble form of Aβ. Phase 3 trials, however, did not find any significant reduction of CSF Aβ in mild to moderate AD volunteers compared to placebo controlled subjects. It also failed in efficacy scores, with ARIA-E being the notable adversity. Participants also displayed variable symptoms including headache, gastrointestinal problems and psychological confusion [8, 12, 13].
2.1.3. Solanezumab
It is a humanized IgG1 mAb that binds at the mid-domain of Aβ (around residues 16-26) and increases the clearance rate of monomers. Studies in transgenic PDAPP mice disclosed that the drug reduced Aβ burden without binding to the Aβ deposits, suggesting that the soluble pool of Aβ to be the target [8]. However, human trials have repeatedly failed to replicate the outcomes of pre-clinical tests, as noted in several Phase 3 studies. A series of Phase 3 trials initiated by Eli Lilly (EXPEDITION 1, 2 and 3) demonstrated no significant improvement in cognition as well as reduction of Aβ. Moreover, studies also indicate that increasing the dosage could lead to a rise in Aβ concentrations.
2.1.4. Gantenerumab
It is one of the first fully human IgG1 anti-Aβ mAb that binds to a conformational epitope, which is expressed on Aβ fibrils. This epitope encompasses both N-terminal (3-12) and central (18-27) amino acids of Aβ and thus requires that the peptide be folded with its mid-region near the N-terminus. In PS2APP transgenic mice, gantenerumab significantly reduced Aβ plaques by recruiting microglia and prevented new plaque formation without altering plasma Aβ levels [8]. Consistent with pre-clinical observations, Phase 3 trials have shown robust activity in human subjects. PET imaging revealed that almost 51% of the subjects with an early stage and moderate AD displayed a significant reduction in Aβ plaque when gantenerumab was administered for a period of two years [14].
2.1.5. Crenezumab
The drug is engineered on an IgG4 backbone in order to minimize the Fc gamma receptors activation. When tested on transgenic mice, it resulted in the reduction of Fc gamma receptor-mediated activation of microglia, which enabled a decrease in the level of release of pro-inflammatory cytokine tumor necrosis factor-alpha which is responsible for neurotoxicity. Crenezumab attacks at the mid-domain of the Aβ peptide (residues 13-24) and then binds to the multiple conformations of Aβ, i.e., monomers, oligomers, fibrils [8]. Phase 2 trials assessed the pharmacokinetics as well as evaluated the CSF Aβ levels. Although many were unable to meet their goal points, few studies did find a decrease in CSF Aβ concentration. Phase 3 trials such as CREAD 1 and 2 have also ended pre-maturely and await further evaluation [15-17].
3. GLUTAMATERGIC PATHWAY BASED THERAPY
Glutamate serves as the major excitatory neurotransmitter in the mammalian brain and is involved in memory as well as several executive functions. Its effect has been implicated in proper working of the synapses, neuroprotective pathways and synaptic plasticity, which includes long-term potentiation (LTP) and long-term depression (LTD). Hence, the progressive degradation of glutamatergic connections in Alzheimer's correlates with the loss of the aforementioned cognitive and executive functions. For example, the loss of hippocampal CA1 neurons in AD, an area implicated in LTP and LTD, results in dementia-like symptoms such as memory deficit in LOAD. Increased excitability of neurons is one of the salient features of glutamate dysfunction, which occurs in AD. Convincingly number of evidences have shown a strong association of Aβ with neuronal hyperactivity. Nevertheless, establishing a causal link, like Aβ toxicity, synaptic failure and the eventual collapse of the glutamatergic system, is still an ongoing pursuit. Although the role of soluble oligomeric Aβ in inducing cytotoxicity is undisputed, studies have also found an abnormality in neuronal firing around regions with localised Aβ plaques, especially the hippocampus. This hyperactive state has been correlated with the severity of mild cognitive impairment, as noted by several researchers [18-20]. These functional observations could be related to changes in cellular morphology, as seen in histopathological studies in AD, highlighting the shrinkage of the dendritic tree, axonal dystrophy, enlargement of axon terminals and decrease in dendritic branching. Interestingly, a decreased glutamatergic activity is observed in later stages of AD, which could be attributed to the loss of synaptic function and neuronal death as the disease progresses, leading to a decline in glutamate facilitated stimulation of neurons [19, 21, 22]. A study by Zott and colleagues gave a plausible insight into the perpetuation and spread of the glutamate dyshomeostasis [23]. They noted that neuronal cells with a relatively elevated baseline activity are more susceptible to Aβ mediated hyperactivity. These neurons are termed active neurons and play a key role in initiating a chain of events, which propagates the pathology to other neurons. Indeed, soluble Aβ dimers were shown to selectively disrupt the reuptake of glutamate in the synaptic cleft. Therefore increasing the membrane potential and baseline activity of the post-synaptic neuron makes it susceptible to Aβ. This model also sheds light on epilepsy like phenotype seen both in humans and animal models in early-stage AD [23]. Altered release of glutamate from pre-synaptic boutons could also be related to Aβ accumulation in its vicinity. Sokolow et al. found that Aβ accumulates within the synaptic boutons co-localised with VGluT1, a vesicular glutamate transporter, hinting at a possible interaction, which could modulate glutamate trafficking. Interestingly, downregulation of both VGluT1 and VGluT2 was observed in several in vivo experiments, but a slight upregulation of VGluT1 was seen when acute effects of Aβ were investigated in early stages. Moreover, only patients with mild cognitive impairment (MCI) displayed VGluT2 downregulation, but not VGluT1. This is suggestive of increased vesicular transport of glutamate in the early stages of AD. This is also in line with the early stage hyperactivity and late-stage hypoactivity of the glutamatergic system [21, 24, 25].
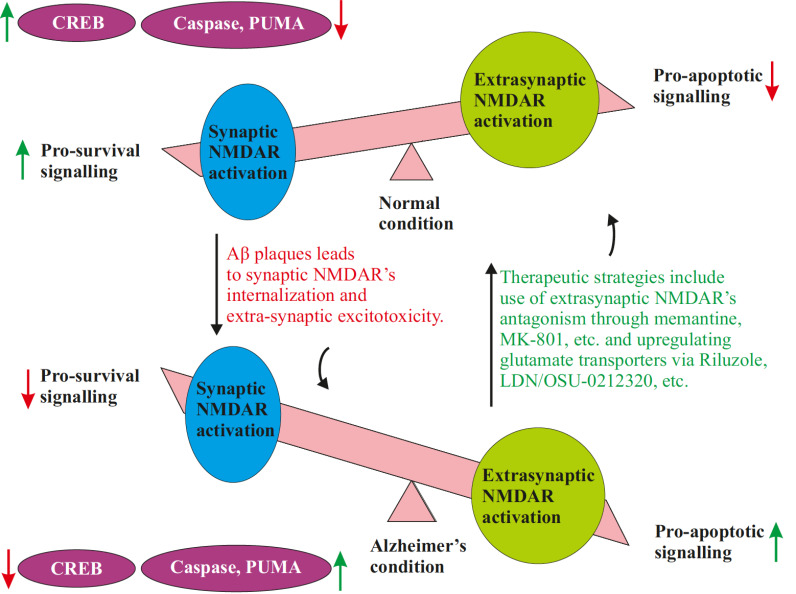
Glutamatergic pathology is also attributed to Aβ interactions with glutamate receptors. Among all the known glutamate receptor subtypes, the NMDAR family has displayed a major association with AD pathology. NMDA receptors play a vital role in learning and memory, and hence, their dysfunction leads to the eventual loss of memory and cognition in late-stage AD. NMDARs are blocked by Mg2+ ions, which get removed via subsequent depolarization, leading to the entry of Ca2+ ions and various Ca2+ mediated transductions. Overstimulation of NMDAR by the efflux of glutamate results in heavy Ca2+ influx, which causes excitotoxicity. This further induces cell-apoptotic signals and an increased reactive oxygen species (ROS) production leading to oxidative damage and neurodegeneration. Such excitotoxicity is mostly linked to extra-synaptic NMDARs, whereas synaptic NMDARs, in general, regulate neuroprotective pathways. Soluble Aβ is also shown to preferentially interact with extra-synaptic NMDARs mediating toxic signaling, mainly by interacting with specific subunit GluN2B, which also serves as a promising drug target (discussed later). Glutamate transporters play an important role in AD pathology as its gene expression is significantly suppressed. Neuropharmacalogists are also trying to find better glycine transporter-1 (GlyT1) inhibitors for additional therapeutic benefits. Aβ has been shown to cause downregulation of synaptic NMDARs while increasing the susceptibility of extra-synaptic NMDARs to glutamate (Fig. 2); thus, the search for selective NMDAR drugs has remained a crucial research area to explore [21, 26].
Fig. (2).
Shifting the balance to pro-survival signaling by selective inhibition of extra synaptic NMDARs. Synaptic NMDARs activation is associated with upregulation of neuroprotective downstream pathways such as CREB while extra-synaptic NMDARs are linked with apoptotic signaling via caspases, PUMA, etc. Drugs which could act as selective antagonist to extra-synaptic NMDARs while showing low affinity to synaptic NMDARs as well as drugs which modulate glutamate levels in extra-synaptic vicinity could restore this disrupted equilibrium in AD. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Targeting NMDA Receptors
Among the glutamate receptor family, NMDARs are the primary drug targets because of their relatively higher permeability to Ca2+ ions as compared to other ligand-gated ionotropic receptors [27]. Although NMDAR inhibition is an effective therapy, reduced activation of NMDARs may result in a depleted amount of neuroprotective signal transduction. Hence, an ideal glutamatergic drug should have an intermediate affinity, i.e., neither it should bind too strongly disrupting the physiology, learning and memory functioning pathways, nor too weakly for any substantial therapeutic benefit. Memantine is an FDA approved non-competitive NMDAR antagonist with low affinity and has been widely used for its neuroprotective effect, providing symptomatic relief and improving cognitive impairment in patients with moderate to severe AD conditions [28]. The NMDA receptors are composed of various subunits such as GluN1, GluN2, GluN3 and GluN4 heterogeneously. These subunits are present in the receptors depending upon their function and location. Out of these, GluN2 sub-types: GluN2A and GluN2B have been the research focus for their involvement in NMDAR associated with AD. The extra-synaptic NMDARs are comprised mainly of the GluN2B subunit, while synaptic NMDARs are dominantly comprised of GluN2A. Thus, drugs that selectively target GluN2B are ideal as they can specifically act on extra-synaptic NMDA receptors alleviating the excitotoxicity without altering the neuroprotection pathways associated with synaptic NMDARs. Drugs such as ifenprodil, which selectively binds to GluN2B has been experimentally shown to reduce Ca2+ mediated neurotoxicity [29, 30]. These demonstrations have provided incentives for developing a more efficacious drug than the already available memantine.
3.2. Targeting Glutamate Transport Channel
The regulation of extra-synaptic glutamate levels is done by excitatory amino acid transport channels (EAATs) which can be found on various glia and neuronal cells, of which the role of astrocytic EAATs is crucial as they comprise 90-95% of the total EAATs in the brain and recycle the majority of glutamate neurotransmitters. In comparison, the amount of neuronal EAATs expressed is relatively low. Excess levels of glutamate are recycled back into glutamine by a process known as a glutamate-glutamine shuttle. Among the five existing subtypes of EAATs, EAAT-2 or glutamate transporter-1 (GLT-1) is predominant and recycles, almost 90% of glutamate [31]. Several studies have reported a marked decrease in GLT-1 expression in AD patients, which has been associated with a decline in cognitive output, as seen with GLT-1 knocked-out mice. In vitro experiments suggest that Aβ might be a causative factor in GLT-1 decline and negatively affect its expression in astrocytes [32-35]. This downregulation of EAATs has been implicated with additional anomalies besides impaired glutamate clearance. Sharma et al. demonstrated alterations in several signaling and metabolic pathways in both neuronal as well as astrocytic EAAT-2 knockout mice [36]. Loss of astrocytic EAAT-2 displayed cognitive as well as spatial memory defect, which is consistent with previous observations. However, neuronal EAAT-2 deletion also displayed late-onset cognitive impairment giving further incentives to look into its role in AD pathogenesis. The transcriptomic analysis also showed that gene expression profile varies between astrocytic and neuronal EAAT-2 knock-outs, with inflammatory and kynurenine pathways (a metabolic pathway associated with tryptophan breakdown and NAD+ production) being disrupted the most among them, respectively. Overall, the deletion of EAAT-2 in the hippocampal region mimics the age-dependent cognitive decline in AD. Such functionality could be regained to an extent when the EAAT-2 expression is restored [36, 37]. Thus, various therapeutic strategies have focused on GLT-1 upregulation, which can be done by targeting its transcription. For instance, beta-lactam antibiotic such as LDN/OSU-0212320, a pyridazine derivative which activates protein kinase C, has been shown to increase GLT-1 gene expression, as well as translation [38, 39]. Such GLT-1 modulating drugs have been used in various other diseases and the current focus has shifted to repurposing their action for the treatment of AD. Riluzole, a drug used to treat ALS, has given positive results when used for AD in pre-clinical trials, having increased glutamate transport, and improved cognition in mice and also downregulated various gene expressions thought to play a role in AD [39].
3.3. Targeting Glycine Transporters
Glycine is a co-agonist of NMDARs. Therefore, activation of hyperactive NMDARs can be modulated by altering the level of glycine. This can be achieved by blocking the Glycine transporters present on astrocytic glia and pre-synaptic neurons. GlyT1 has gained attraction because of its close association with NMDA expressing neurons. Glycine transport inhibitor such as sarcosine has been used in the past to treat schizophrenia symptoms, hence, repurposing these drugs for AD might reveal some therapeutic benefit [40]. ASP2535, a GlyT1 inhibitor, was shown to alleviate cognitive impairment in schizophrenia and AD mouse models [41]. Another GlyT1 inhibitor, BI 425809, has entered in Phase-1 trials for AD and is currently being investigated for more therapeutic relief [42].
4. ANTI-TAU BASED THERAPY
Tau based pathology has been well known and studied since early attempts over the elucidation of AD took place. Tau is a protein that normally binds to microtubule and confers stability to it, which is essential for proper cytoskeletal functioning and cellular trafficking. Tauopathies, which are common in multiple neurodegenerative diseases, result from the formation of intracellular neurofibrillary tangles (NFTs). These are a result of multiple post-translational changes such as hyper-phosphorylation, acetylation, N-glycosylation and C-terminal truncation. Phosphorylated tau has notably been the earliest sign of pathogenesis, which hinders its interaction with tubulin as well as facilitates its relocalization and aggregation. This correlates with the elevation in the levels of tau kinases, such as glycogen synthase kinase - 3β (GSK-3β) or CDK5 in several AD brain samples and serves as a promising site for therapy (discussed later). NFTs are one of the major hallmarks of AD besides Aβ plaques and marks the earliest sign of pathology by its deposition in the entorhinal cortex and hippocampus. The formation of NFT takes place in a series of steps, and results in multiple intermediary fragments, such as pre-tangles, tau oligomers, paired helical filaments, etc., just like Aβ plaques. However, unlike Aβ burden, tau load shows a better correlation to cognitive decline making it an appealing target [43].
Tau, is a major type of microtubule associated proteins (MAPs) which has six isoforms coded by a single gene via alternative splicing. These isoforms are distributed heterogenously throughout the brain and differ in amino acid repeats at the N-terminal coded by different exons. Few studies have suggested that these tau isoforms could have a diverse range of functions in addition to cytoskeletal stabilization such as regulation of synaptic vesicles, nuclear localization, chromatin remodeling, etc. This suggests that tau pathology is not simply a defect in cellular trafficking and there may be other existing pathways, which confer its pathogenesis and symptoms associated with it. It is also known that AD contains a specific mix of tau isoforms (3-repeats and 4-repeats), which separates it from other tau pathologies such as Pick disease. Thus, anti-tau strategies have evolved over time with the knowledge about their underlying mechanisms getting continuously updated [43, 44].
4.1. Targeting Tau-phosphorylating Kinases
The involvement of multiple tau phosphorylating kinases has complicated the search for an effective therapeutic target. It is yet to be established if any specific kinase has a major role in tau pathology or whether targeting multiple such kinases would be an appropriate approach. Researchers have identified promising candidates such as GSK-3β, MARK, Fyn and CDK5 in this context [45]. Targeting GSK-3β has yielded positive results encouraging further trials. Tideglusib, a GSK-3β inhibitor, specially reduced tau phosphorylation and showed symptomatic benefits in transgenic mice in vivo [46]. Drugs such as tadalafil, morin, etc. have also been shown to reduce GSK-3β induced tau phosphorylation [47, 48]. Saracatinib, a Fyn kinase inhibitor, has also provided similar alleviations [49].
4.2. Targeting Tau Post-translation Modifications
The formation of NFTs also involves the addition of several compounds to tau besides phosphorylation. Acetylation of lysine group in tau has been shown to facilitate its aggregation. Thus, targeting these acetylation enzymes has been proposed as another potential therapy in tau based pathologies [50]. Salsalate, a drug used for the treatment of rheumatoid arthritis, decreased tau aggregates, improved dementia symptoms and reduced shrinkage in the hippocampal region, which can be attributed to its inhibitory effect on the acetyltransferase p300 enzyme [51]. Additionally, targeting other modifications such as tau glycosylation is also an active area of research. It has been proposed that drug action on enzyme O-GlcNAcase (OGA) that reversibly cleave the β-glycosidic O-linkage with carbohydrate N-acetyl glucosamine (GlcNAc), can positively affect tau aggregates and phosphorylation [52]. In fact, OGA inhibitors such as MK-8719 and ASN120290 have shown relief for non-AD tauopathies such as progressive supranuclear palsy (PSP) and few have even entered the Phase-1 trials for further evaluation [53].
4.3. Tau-focused Immunotherapy
Antibody-mediated immunotherapy has proven advantageous over other therapeutics, mainly for their specificity towards the toxic species while preserving the normal functioning of tau and its associated enzymes. These antibodies preemptively target tau phosphorylation sites, aggregation epitopes as well as toxic assemblies. The efficacy of these antibodies depends on their endocytosis by the cells, which then directs it to tau aggregates leading to the eventual degradation of the tau-antibody complex by lysosomal mediated autophagy. Two different strategies are employed in tau immunotherapy, namely active and passive immunization. The active vaccine approach uses a self-generated antibody via stimulation of the host's immune system. Although convenient and long-lasting, it is prone to auto-immune damage due to its low specificity. In contrast, passive immunization using monoclonal antibodies has fared better in terms of sheer efficacy and specificity. A study by Carranza and his team displayed a significant reduction of toxic oligomeric tau species in Htau mice after the long term administration of tau oligomer-specific mouse monoclonal antibody (TOMA) [54]. These oligomeric tau aggregates correlate better with cognitive decline than NFTs and have been accepted as a suitable biomarker for tauopathy [54, 55].
Tau-based immunotherapeutics are relatively new, and it is worth noting that most of the ameliorating effects described have only been observed in pre-clinical models. Translating these studies in human trials face a long road ahead. Although with the advent of novel techniques, few drugs did manage to enter Phase I and Phase II trials [55].
4.3.1. ACI-35
It is an active vaccine consisting of 16-mer peptide and is administered via liposomal packaging. It contains tau residue at 393-408 and is phosphorylated at two sites on Ser396 and Ser404. In vivo trials with transgenic mice models, C57BL/6 and P301L resulted in IgG with high specificity towards the phosphorylated tau compared to physiologically normal tau; however, the effect of Ser404p tau was not significant, unlike Ser396p. Studies also highlighted the absence of glial scars and pro-inflammatory markers. It entered a Phase 1b trial in 2015 with the objective of testing its efficacy against mild to moderate AD patients [56, 57]. (Clinical Trial Identifier: ISRCTN13033912).
4.3.2. AADvac-1
It is a synthetic peptide derived from tau residues 294–305, which got its epitope selected using the target site of DC8E8 monoclonal antibody. It is an active vaccine, which yielded positive results with a significant reduction of tau aggregates in pre-clinical trials. Phase-1 trials with an alum-adjuvanted vaccine displayed IgG1 in 29 out of 30 patients. An ongoing Phase 2 study is also currently in progress with mild AD patients [43] (Clinical Trial Identifier: NCT02579252).
4.3.3. BMS-986168
This passive immunization therapy uses a stem cell-derived IgG4 monoclonal antibody. It targets the 9-18 amino acid portion of e-tau fragments (end-terminal fragments) obtained from familial AD patients. Preclinical studies reported a decreased Aβ40 as well as interstitial tau species. Four clinical trials with BMS-986168 have been initiated since 2014. A multiple-ascending-dose Phase 1b trial with 48 patients having PSP yielded a significant decrease in CSF e-tau and showed good tolerability [58]. Biogen also initiated a randomized parallel-group Phase 2 trial with 654 patients displaying MCI, which is expected to run till the year 2024 (Clinical Trial Identifier: NCT03352557).
4.3.4. RG7345
It is a humanized monoclonal antibody, which shows specificity for phosphorylated tau at site Ser422, which is implicated in tau relocation towards the somatodendritic region. An experiment conducted in transgenic TauPS2APP mice showed a reduction of phosphorylated tau [59]. In 2015, it entered a Phase 1 trial with 48 healthy participants for the assessment of its pharmacokinetics. However, the study was later discontinued for unknown reasons. (Clinical Trial Identifier: NCT02281786).
4.3.5. C2N-8E12
This humanized IgG4 antibody targets the tau protein at a 25-30 amino-acid site. In vitro studies displayed a reduced misfolding and propagation of tau aggregates when this antibody was administered [60]. In vivo studies by Yanamandra et al. also found positive results, with improved motor functions, reduction of pathogenic tau and lowered brain degeneration [61]. It entered Phase 1 trials in 2015 with a single-ascending dose test conducted on 30 PSP subjects, with results labelling it safe; however, the tolerability limit was not determined [43]. A Phase 2 trial has also been initiated in 2016 with more than 400 early-stage AD participants for assessment based on several cognitive parameters. (Clinical Trial Identifier: NCT02880956).
4.3.6. LY3303560
Also known as Zagotenemab, this monoclonal antibody preferentially binds to tau clusters compared to monomers, possibly via its interaction with a conformational epitope region. Two Phase 1 trials were initiated on MCI patients by Eli Lilly in 2016 and 2017, respectively, with the goal of CSF antibody level assessment and evaluation of the safety and pharmacokinetics of the drug. A Phase 2 trial has also been initiated in 2018, with 285 participants displaying a progressive decline in cognition for a minimum duration of six months [43]. (Clinical Trial Identifier: NCT03518073).
4.3.7. UCB0107
Developed by UCB Biopharma, this monoclonal antibody targets 235–246 amino acid sites, which falls in the mid-segment of tau aggregates. Studies have implied that targeting the N-terminal segment of tau may result in non-specific binding to degenerated tau fragments, which could hinder clearance and tau seeding. In 2018, UCB0107 entered Phase 1 trials with the aim of testing the efficacy and safety of single ascending doses in healthy male volunteers [43, 62]. (Clinical Trials Identifier: NCT03464227).
4.3.8. RO 7105705
It is a monoclonal IgG4 antibody, which possibly interacts with the Ser409 phosphorylation site. Its research is sponsored jointly by AC Immune and Genentech and it is reported to bind N-terminals of all the conformers of tau, including monomers and oligomers. It entered Phase 1 trials in 2016, which compared the dosage effects on mild to moderate AD subjects with placebo controls. (Clinical Trials Identifier: NCT02820896). A Phase 2 trial also started in February 2019, screening its efficacy on moderate AD patients and is expected to be completed by June 2023 [43, 63] (Clinical Trials Identifier: NCT03828747).
4.3.9. JNJ-63733657
This humanized monoclonal antibody shares a similar mechanism with UCB0107, targeting the mid-region of tau aggregates and removes tau spread and seeding. It entered Phase 1, in 2017, testing its tolerability and efficacy in 64 patients with AD phenotype and healthy subjects [43] (Clinical Trials Identifier: NCT03375697).
5. NEUROPROTECTION BASED THERAPY
This type of therapy is aimed at potentiating the intrinsic pro-survival signaling that prevents the cells from all kinds of degenerative cascades, making the cells less vulnerable to accumulating damages. With respect to AD, it includes targeting as well as reversing the destructive effects of Aβ, NFT, inflammation, etc. and restoring synaptic plasticity, inhibiting the pro-apoptotic signals, preventing the excitotoxic effects of Ca2+ ion dyshomeostasis, etc. The main highlights that shall be covered here are neurotrophin (NT) receptors that regulate various pro-survival and pro-apoptotic pathways. It uses a wide array of ligands belonging to NT families such as brain-derived neurotrophic factors (BDNFs), nerve growth factors (NGFs), neurotrophin-3 (NT-3), etc. which bind to one of the two types of NT receptors, namely, tyrosine kinase receptors (Trks) and p75 neurotrophin receptors (p75NTR). The same NT family can co-activate both of these types of receptors; however, different isoforms of Trk get activated by only certain NTs, an example being TrkB having the ligand BDNF.
It is plausible that Aβ binds to these neurotropic receptors and initiate cascading events leading to cell death. Inhibiting such interactions by blocking these receptors without disrupting the normal physiology is challenging. However, recent clinical trials have yielded promising results.
5.1. p75NTR Based Therapy
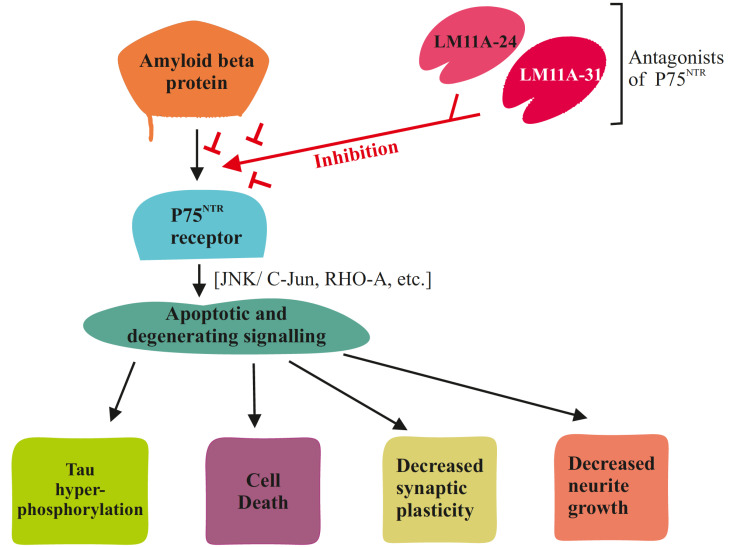
The receptor p75NTR is activated differentially by a wide variety of NT molecules, which results in the activation of multiple downstream pathways such as c-Jun N-terminal kinases (JNKs), caspase, etc., leading to either pro-survival signals such as synaptic stability, or degenerative signals such as synaptic pruning. In AD, the degenerative signaling becomes dominant as the p75NTR levels rise and the activating molecules such as pro-NGF levels also increase, which explain the early degeneration of p75 expressing cells [64-67]. The therapy is thus focused on shifting the balance from degenerative to pro-survival signaling. The use of p75 antagonists in pre-clinical trials has proven efficacious and is supported by the experiments where transgenic mice lacking p75 receptors showed a reduction of Aβ induced degeneration [68-70]. Two key drugs, namely LM11A-24 and LM11A-31, have been shown to increase cognition and decrease NFT levels leading to a reduction in the degeneration of cholinergic neurons of the basal forebrain. This positively affected Aβ induced degeneration as well as Aβ induced tau phosphorylation and minimized the Aβ induced CREB and AKT inhibition, which is vital for proper synaptic function [71] (Fig. 3). Targeting p75 for anti-tau therapy also has an added benefit as it affects the action of multiple tau phosphorylating kinases without directly inhibiting them and disrupting their normal function [72].
Fig. (3).
Inhibition of neurotrophin receptor p75 by LM11A-24 and LM11A-31 which disrupts the various apoptotic signaling pathways. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
5.2. Trk Based Therapy
Trk receptor family includes TrkA, TrkB and TrkC, which are differentially expressed in multiple brain regions such as the entorhinal cortex, hippocampus, basal forebrain, etc. They are activated by different families of NTs. TrkB and TrkC play important roles in various survival pathways, including synapse stabilisation, synapse function, plasticity, LTP, etc. It has been observed that natural agonists of these receptors, such as BDNF (TrkB agonist), progressively decline in the ageing brain and neurodegenerative disorders such as AD. Reduced signaling of these molecules has been associated with major hallmarks of AD, such as Aβ aggregation, NFTs, etc [73-75]. This has invoked attempts to increase the levels of BDNF in patients; however, its low BBB permeability and poor stability have made it a challenging task for researchers [76]. Adopting different diets and physical exercise have shown to positively influence BDNF levels. However, the cognitive benefits imparted by these measures are still under question. Interestingly, a study demonstrated that enhancing BDNF levels in mouse models via exercise is effective in combination with induced neurogenesis in the adult hippocampus [77-81]. Therefore drugs that act as a direct agonists to Trk receptors such as 7, 8-DHF and LM22A-4, which can simulate BDNF effects, have caught the attention of researchers. LM22A-4 has shown similar neuroprotective effects, such as activating synapse functioning pathways (AKT and CREB) [82, 83]. Targeting TrkA receptors, on the other hand, imparts neuroprotection to the cholinergic cell population in the basal forebrain [84]. TrkA ligands such as D3, positively affect the AKT signaling in the hippocampus region, reduced Aβ levels and show improvement in short-term memory [85]. Another TrkA agonist MT2 also is reported to increase cell survival and reduction in amyloid genesis [86].
5.3. Modulating the JNK Pathway
JNKs are a family of protein kinases, which activated during stress signals and involved with plasticity, cell apoptosis, senescence, etc. Studies suggest that Aβ aggregates may activate this pathway, especially the JNK3, which results in early degeneration of neuronal cells via activation of apoptotic signals. Its role in generating NFTs via tau phosphorylation has also been suggested [87-89]. Thus, potential therapies include inhibition of Aβ activated JNK downstream signaling. JNK inhibitors such as bentamapimod, tanzisertib, D-JNKi1, etc., have been previously used for non-AD diseases, but are now underway for getting repurposed to see the effect on AD patients [90]. Moreover, experimental data on JNK inhibitors have shown promising results for AD in pre-clinical models. Novel drugs such as CEP-1347 and SP600125 have improved cognition in mouse models as well as lowered Aβ induced cell degeneration [91-93].
6. CHOLINERGIC NEUROTRANSMISSION BASED THERAPY
Acetylcholinesterase (AChE) is a type of serine hydrolase found at the neuromuscular junctions and also at the cholinergic synapses in the brain. The biological role played by it is in the termination of impulse transmission at cholinergic synapses followed by rapid hydrolysis of the neurotransmitter acetylcholine (ACh) to acetate and choline. In the mammalian brain, the majority of AChE occurs as a tetrameric G4 form, while a marginal quantity exists as the monomeric G1 form [94].
6.1. Targeting Acetylcholinesterase
Anti-cholinesterases are a class of drugs that inhibit cholinesterase enzyme from breaking down ACh and this leads to an increase, both in the level and duration of the neurotransmitter action. According to the mode of its action, AChE inhibitors are divided into two groups: irreversible and reversible. Reversible inhibitors, whether competitive or non-competitive, have therapeutic applications, while toxic effects have been associated with irreversible AChE activity modulators. Irreversible inhibitors of AChE are known to result in convulsions, muscular paralysis, bronchial constriction and even death by asphyxiation. Inhibition of AChE activity helps in maintaining the ACh level by decreasing ACh breakdown rate. Therefore, they help in boosting up the cholinergic neurotransmission in regions of the forebrain, which compensates the loss of functioning neurons [94].
6.1.1. Donepezil
It is a selective and reversible AChE inhibitor that functions after binding to the peripheral anionic site. It shows both symptomatic and causative effects in the treatment of AD, possibly by modulating Aβ levels, as seen in animal studies [95]. It is an approved treatment for Alzheimer’s and is used to treat mild to moderate form of AD. Donepezil also improves cognitive function in patients with severe AD symptoms. The drug is found in both the forms of tablet and oral solution that easily cross the blood-brain barrier with a slow rate of excretion. It has a half-life of about 70 hours, so it is prescribed to take it once a day. Tablets are available in 5 and 10 mg dose strengths and the maximum dose is 23 mg per day. Adverse effects include gastrointestinal problems, as well as an increase in cardiac vagal tone, causing bradycardia [94].
6.1.2. Rivastigmine
It is a powerful and low-reversible carbamate inhibitor that functions by blocking the cholinesterase activity after binding to esteratic part of the active site. It has been introduced in Phase 3 clinical trials under the support of Novartis Pharma [2]. Rivastigmine inhibits both BuChE (Butyrylcholinesterase) and AChE, unlike donepezil. It is used for the treatment of mild-to-moderate forms of AD. The drug can be administered orally in the form of capsules and liquid, providing a good absorption with the bioavailability of 40% in the 3 mg dose. It gets eliminated easily through urine and shows few drug-to-drug interactions. Treatment with rivastigmine has beneficial effects in the treatment of AD but has also been shown to negatively impact cognitive function. Side effects prevail with the cholinergic actions of the drug that include nausea, vomiting, diarrhea, anorexia, headache, syncope, abdominal pain and dizziness [94].
6.1.3. Galantamine
It is an alkaloid that is isolated from the plant Galanthus woronowii. It is a selective, competitive as well as rapidly reversible inhibitor of AChE that interacts with the anionic subsite and the aromatic gorge. Galantamine also induces modulation because it is an allosteric ligand at nicotinic cholinergic receptors. Cognitive impairment in Alzheimer’s correlates with loss of nicotinic receptors and this particular effect appears to be a positive point for the treatment. Absorption of galantamine is rapid, with oral bioavailability lying between 80 and 100% and with seven hours half-life. Treatment is initiated with a 4mg dose twice in a day, which is increased gradually. Side-effects include severe gastrointestinal symptoms [94].
6.1.4. Tacrine
It is an aminocridine that specifically interacts with the subtypes of muscarinic and nicotinic receptors. It shows a reversible activity and gets easily absorbed. It gets cleared by the liver during the metabolic process but with low bioavailability of 2% to 3% when administered orally. Multiple doses of tacrine increase its half-life from 1.4 to 3.6 hours. Patients who were on the effective dosage of the drug showed elevated alanine aminotransferase (ALT) than the upper limit [2].
6.1.5. Metrifonate
It carries an irreversible activity, which leads to an increase in its bioavailability in the CNS. It has reached Phase 3 clinical trials for further evaluation. Its mechanism of action relies on its hydrolysis product 2, 2 - dimethyl dichlorovinyl phosphate (DDVP), which binds to the catalytic sites of the enzyme for AChE inhibition. It evades the cytochrome P50 system in plasma and gets absorbed and distributed in the brain rapidly [2].
7. USE OF ANTI-INFLAMMATORY DRUGS FOR ALZHEIMER’S DISEASE
Altered inflammatory response leading to neurodegeneration has long been studied in AD and their cross-talk with the glial cells has been well implicated in its pathogenesis. Studies have reported elevated levels of cytokines such as TNFα in the AD brain compared to healthy controls, which might downstream a cascade of disruptions in brain metabolism. It is still debatable whether this anomaly is an effect or a cause leading to AD. Epidemiological data suggest that the majority of AD cases are not familial but sporadic in nature and this has prompted researchers to undertake rigorous genetic screening for identifying the risk genes and associated variants. Several studies have demonstrated that certain GWAS genes such as TREM2, CLU, EPHA1, CR1, ABCA7 that are expressed in microglia are highly upregulated in AD condition, which again suggests a strong link to neuro-inflammation. Triggering receptor expressed on myeloid cells 2 (TREM-2) is strongly associated with AD and is possibly a hub gene, regulating a network of other downstream processes. This is supported by the fact that mutations in it, confer a significant risk almost comparable to the widely known APOE ε4 allele. In congruence with this, a study by Sierksma et al. demonstrated the altered microglial expression of a few genes (PC2, TREML2, SYK, GRN, SLC2A5, etc.) in response to Aβ interaction and hinted that their effects may be additive which explains their low genome-wide significance. Thus, it is important to understand the function and downstream regulation of these genes, which could add to the arsenal of effective therapeutics against AD [96, 97].
The role of microglia is quintessential, from clearing the local debris to mediating brain immune response. Generally, microglia stay in a quiescent stage and probe the brain for pathogens and cell debris. Once such an encounter happens, it induces a transition in its morphology, leading to activated microglia state. Although not in consensus, it is believed that in the active state, microglia can adopt a differential characteristic, either taking part in anti-inflammatory (M2) or pro-inflammatory (M1) signaling. M2 state of microglia is neuroprotective in nature, secreting a number of growth factors such as glial-derived neurotrophic factors (GDNF), BDNF and nerve growth factors. In juxtaposition to this, M1 state releases inflammatory mediators as well as free radicals such as ROS, which can cause a number of insults to the surrounding brain region. In AD, Aβ has been shown to interact with microglia and take part in its activation, exacerbating the inflammatory response [98].
Based on the aforementioned role of neuroinflammation in AD, researchers have explored the role of anti-inflammatory drugs as a potential treatment option. Several classes of non-steroidal anti-inflammatory drugs (NSAIDs) have been designed and have garnered attention for their dual role in functioning as GSMs. In vivo experiments have shown that NSAIDs could repress glial activation and cytokine-induced neuroinflammation. Ibuprofen is a well-known NSAID which decreased Aβ plaques and repressed glial activation in animal studies. However, recent meta-analysis questioned its efficacy for its lack of any significant association to cognitive benefit in humans. Other related NSAIDs such as Tarenflurbil and Celecoxib have undergone Phase 3 trials; however, results have mostly been negative. Apart from NSAIDs, glucocorticoids have also been investigated for their anti-inflammatory properties. In vitro and in vivo studies displayed lowered NOS production from microglia as well as decreased Aβ concentration. This, however, failed to show significant benefits in human trials, just like NSAIDs. These studies indicate that there is much room for improvement for anti-inflammatory therapy especially with evidences piling up indicating the role of microglial genes in AD pathophysiology [98-100].
8. NUTRACEUTICALS AND DIETARY INTERVEN-TIONS
Although the therapeutic potential of the discussed drugs in clinical and pre-clinical trials is undisputed, their benefits are not without complications and drawbacks. For instance, most of the tested histone acetylase inhibitor compounds carry toxic side-effects [101]. Hence, the current paradigm shift towards the natural products and their derivatives has driven a multitude of researches to analyse their efficacy on patients (Table 1). These natural compounds are well tolerated with minimal or no side effects. They are easily obtainable and have good bioavailability. Due to their important therapeutic roles, it is quintessential to take nutrition into account during the dietary supplementation in pre-clinical as well as clinical studies as it can lead to inconsistency in results. Individual dietary patterns differ from individual to individual and have given incentives to reconsider previously done epidemiological studies without nutrition adjusted controls.
Table 1.
List of emerging nutraceutical compounds for treatment of Alzheimer's disease.
|
Nutraceutical
Compounds |
Type/Effect | Mechanism of action | Model Used/Mode of Study | Source | Refs. |
|---|---|---|---|---|---|
| Curcumin (curcuminoid) |
Neuroprotective; Reduced Aβ oligomerization and aggregation; Reduction of Tau phosphorylation; Reduced ROS induced damage |
BACE1 inhibition; AchE inhibition | - | Curcuma longa plant | [136] |
| Resveratol (Stilbenoid) |
Anti-oxidant; Neuroprotective; reduced Aβ plaques | SIRT1 activation; AMP-phosphokinase pathway activation | Tg19959 mice | Grapes, peanuts, blueberries, red wine etc. |
[137, 138] |
| Quercetin (Flavonol) |
Reduced Aβ induced toxicity; Anti-taupathic; Improved Long-term and spatial memory | Modulates pro-apoptotic signaling and increases cell survival |
3xTg-AD mice | Ubiquitous among a variety fruits and vegetables such as onions, berries etc. | [139] |
| Dihydromyricetin (DHM)/ Ampelopsin (AMP) (flavanol) | Anti-inflammatory; Improved learning skills | Modulates AMPK/SIRT1 signaling | Sprague Dawley (SD) male rats | Found majorly in Ampelopsis plants | [140] |
| Catechin (flavanol) |
Anti-oxidative; Anti-inflammatory | Free radical scavenging; Metal ion chelation | Clinical trials (Longitudinal) | Green tea | [141] |
| Taurin | Reduced Aβ induced toxicity; Improved cognitive skills | Binds to oligomeric Aβ | C57Bl/6 mice | Animal products such as meat and dairy | [142] |
| Anthocyanin | Suppressed Aβ1–42 cytotoxicity | Inhibition of co-chaperonin GroES fibrills | Neuro2a cells (in vitro) |
Bilberry (Vaccinium myrtillus anthocyanoside (VMA)) | [143] |
| Tannic Acid | Reduced β-amyloid oligomer deposits; Enhanced cognition | Inhibition of β-secretase function | PSAPP mouse | Flowering Plant sources such as Rhus semialata | [144] |
| Arjunolic Acid | Neuroprotection | Anti-cholinesterasic (AChE and BuChE) activity |
- | Combretum leprosum roots | [145, 146] |
| Ferulic Acid (hydroxycinnamic acid) |
Anti-β-Amyloid effect | β-secretase modulator | PSAPP mice (in vivo) and N2a cell line (in vitro) |
Ubiquitous among a variety of plant cell walls, such as flax seeds, barley etc. | [147] |
| Genistein | Anti-β-Amyloid effect | Inhibits ubiquilin 1 which downregulates presenilin 1 expression | Daudi, Jurkat, U937 and K562 cell lines |
Leguminous plants; soybean products | [148] |
| Mangiferin | Reduced NFT aggregates; anti-inflammatory; improved episodic and spatial memory | - | APP/PS1 mice | Mangifera indica | [149] |
8.1. Resveratrol
It is a polyphenolic compound that is commonly extracted from grapes, blueberries, peanuts, etc. It is a phytoalexin in nature, i.e., it is released in response to pathogens that infect them. Known for its antioxidant properties, it has been the focus of many researchers for its ameliorating effect on cancer, cardiovascular diseases and neurodegenerative diseases such as AD. Recent studies have highlighted its effect on the upregulation of SIRT1, a member of sirtuin proteins, which plays an important role in ageing and vitality. SIRT1 signals various neuroprotective pathways that aid in Aβ clearance and aggregation, ROS scavenging, and facilitation of synaptic plasticity involved in learning, memory and autophagy induction [102-104]. This has been supported by various in vivo and in vitro trials, such as the one carried out by Zhao et al., where it was shown that resveratrol fed rats had a marked reduction in Aβ1-42 level in the hippocampus [105]. Another study showed a reduction in microglial inflammation using a resveratrol diet, which plays an important part in ROS induced degenerative cascade [106].
8.2. Curcumin
Curcumin belongs to a family of compounds known as polyphenolic curcuminoids. This natural, yellowish chemical is extracted from Curcuma longa tubers and has been widely used in various industrial sectors, ranging from cosmetics to the food industry for its anti-oxidant and anti-inflammatory properties. Its effect in in vitro and in vivo experiments have shown that it is a strong candidate against AD, following its inhibition of AchE and Beta-secretase 1 (BACE1) [107]. It also works against Aβ oligomerization, possibly via the inhibition of PSEN1 activation [108]. This candidate, however, needs further investigation due to it being less stable and with low bioavailability [109].
8.3. Vitamin B
Vitamin B plays an important role in the homocysteine-methionine cycle, which includes the generation of S-adenosylmethionine, a key substrate for the methylation of DNA [110, 111]. DNA methylation epigenetically regulates gene expression, which is relevant because epidemiological studies have linked AD patients with less methylated DNA, leading to over-expression of AD-related genes [112]. Among vitamin B family, vitamin B6, B9 and B12 supplementation have especially been shown with ameliorative properties [113, 114]. However, contrary results have also been shown, in that the intake of only vitamin B is not very efficient in reducing dementia-like symptoms [115]. Even though conflicting data is there, this does not undermine the therapeutic potential of vitamin B supplementation in AD as further validations are still under process.
8.4. Other Vitamins
Although not strongly correlated, vitamin C, D and E also have gained traction following epidemiological studies [116]. Vitamin D has been reported to be low in AD patients and linked with dementia and cognitive deficiency. Its role has been implicated in amyloid plaque clearance, anti-inflammation, reducing oxidative stress and offering neuroprotection mediated by vitamin D receptors (VDR) [117]. Vitamin E, like others, has also been reported to have low CSF concentration in AD patients. Its therapeutic potential has been recognised for its anti-oxidative role, having shown reduced ROS induced damage in animal models [118]. Vitamin C, on the other hand, has been reported for its epigenetic regulation of AD-related genes and ROS scavenging property [119].
8.5. Genistein
Genistein belongs to the isoflavone group. It is a dietary component rich in plants belonging to the Leguminosae family. It has been the research focus for its protective role in cancer, cardiomyopathies as well as neurological diseases [120, 121]. The most interesting aspect of genistein is its similarity to estrogen through which it imbibes its neuroprotective effects. Estrogen rapidly declines in women after the menopause. This has been correlated with higher than average AD cases in post-menopausal women when compared to men. This has also been supported by symptomatic relief brought about by estrogen therapy in AD patients. However, the results are not without its harmful side-effects, and in some cases, have also been oncogenic in nature [122, 123]. Hence, genistein, with its low side-effects, could serve as a potential alternative and even act as a selective agonist to estrogen receptors specifically located in the hippocampal region involved in learning and memory [124]. Genistein has also been shown to reduce Aβ generation and Aβ induced toxicity via modulating the protein kinase C pathway (PKC) and downregulating β-secretase activity [125].
8.6. Calorie Restriction Diet
Changing dietary regimes could have a profound effect in combating various degenerative diseases. Calorie restricted (CR) diet has long been shown to slow down the effects of ageing and Alzheimer's in animal models as well as humans. A reduction of 20-40% in daily calorie intake, which includes decreasing the amount of proteins, carbohydrates and fats (without undernourishment due to lack of essential micronutrients), has resulted in a multitude of benefits. In some cases, it increased the lifespan of rats up to 30% and also improved insulin resistance [126]. In AD mouse models,
CONCLUSION
The past decade has seen a multitude of attempts to push forward the seemingly stagnant status quo in Alzheimer's disease research. While pinpointing the exact aetiology of the disease is important, it is still conceivable to plan interventions based on our limited understanding of AD. Still, we do not know much about the perinatal conditions [130] that can aggravate or program the body of an individual to develop neurodegenerative disorders later in life [131]. AD is multifactorial in nature and raises the question of the feasibility of finding a miraculous all-curing drug. This has led to an array of prophylactic measures, targeting numerous aspects of AD at its various stages of severity (Table 2). Such measures include glutamatergic approaches tackling the excitotoxicity, anti-amyloid and anti-tau based medications acting on extracellular and intracellular aggregates, respectively. Further, cholinergic based approaches that reduce the degradation of cholinergic neurons have also been tested. Techniques that enhance neuroprotective signaling and anti-inflammatory medications also have been shown to be promising (Table 2). While these pharmacological approaches have undeniable benefits, alternative means of treatment such as nutraceuticals and lifestyle changes resulting in epigenomic maintenance [132] have also grasped attention for their relatively lower side-effects (Table 1). How efficacious these measures are and whether they are limited to symptomatic relief is yet an open-ended question that needs rigorous clinical and pre-clinical trials to unveil any meaningful answer.
Table 2.
List of different therapeutic drugs for combating Alzheimer's disease.
| S. No. | Name of Drug | Action of Drug |
Clinical
Status |
Models | Effects | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | CPC-201 (Donepezil + Solifenacin) | Acetylcholine-neurotransmitter based | Phase 2 | Patients with AD type dementia | Cholinergic signaling is improved | [150] | |||||
| 2. | Galantamine | Anti-cholinesterase, Allosteric modulator of nAChRs |
FDA approved | Patients with dementia related to cerebrovascular disease | Prevent and slow down the symptoms | [151] | |||||
| 3. | Bisnorcymserine | Anti-Butyrylcholinesterase, amyloid protein precursor inhibitor | Phase 1 | AD patients above 55 years of age | Neurotransmission of acetylcholine | [152] | |||||
| 4. | TRx0237 (LMTX) |
Anti-tau | Phase 3 | Patients with mild form of AD up to 89 years of age | Inhibits misfolded tau protein aggregation | [153] | |||||
| 5. | NP001 | Anti-inflammatory | Phase 1 | Neuroinflammation in mild to moderate form of AD patients | Activates the immune system | [154] | |||||
| 6. | Solanezumab | Anti-amyloid | Phase 3 | Mild dementia in patients due to AD | Slow down cognitive decline and decrease fibrillary plaques | [155] | |||||
| 7. | BAN2401 | Anti-amyloid immunotherapy | Phase 3 | Transgenic mouse models | Reduction amyloid beta protofibrils, cognitive decline and APOE4 | [156] | |||||
| 8. | Candesartan | Neuroprotective, glial cell functions | FDA approved | Spontaneous hypertensive rats | Targets on vascular functioning, angiotensin II type 1 receptor blocker | [157] | |||||
| 9. | ANAVEX2-73 | Anti-tau, metabolic | High dose – Phase 2, Mid dose -Phase 3 |
In-vivo mouse models of AD | Reduce tau phosphorylation and cell signaling is improved | [158] | |||||
| 10. | Bryostatin-1 | Anti-amyloid, prevent tumor growth | Phase 2 | Mild to moderate form of AD in patients | Improved cellular processes | [159] | |||||
| 11. | Donepezil | Anti-cholinesterase | FDA approved | Mild to moderate form of AD in patients | Slow down the symptoms of AD | [160] | |||||
| 12. | Memantine | Glutamate – NMDA receptor antagonist |
FDA approved | Moderate to severe form of AD in patients | Slow down the symptoms in learning and memory pathways | [161] | |||||
| 13. | Formoterol | Metabolic | Phase 2 | Ts65Dn Mouse model | Improvement in multicellular pathways | [162] | |||||
| 14. | hUCB-MSCs | Regenerative | Phase 1 | Zebrafish, murid rodents, nonhuman primates and invertebrates such as Drosophila and C elegans | Regenerating neurons, proliferation of K562 (an erythromyeloblastoid cell line) and the cytokine secretion pattern | [163] | |||||
| 15. | Semorinemab | Anti-tau monoclonal antibody | Phase 2 | Mouse models and clinical patients of AD | Targets N-terminus of all six isoforms of human type tau | [164] [https://clinicaltrials.gov/ct2/show/NCT03828747] | |||||
| S. No. | Name of Drug | Action of Drug |
Clinical Status |
Models | Effects | Refs. | |||||
| 16. | STA-1 | Neuroprotective | Phase 2 | Patients with vascular dementia, Caenorhabditis elegans | Reduction in oxidative stress | [165] | |||||
| 17. | Probucol | Neuroprotective, anti-inflammatory | Phase 2 | Aged male rats (26 months old) | Synaptic functions improve and APOE gene activity is induced | [166] | |||||
| 18. | DAOIB | Neurotransmitter-NMDA based | Phase 2 | Behavioral and psychological patients of AD | NMDA activity is enhanced | [153] | |||||
| 19. | Ifenprodil | Neuroprotective | Phase 1 | In-vitro and in-vivo models | NMDA receptor antagonist -inhibits receptors containing NR2B subunits | [167] | |||||
| 20. | Riluzole | Neuroprotective, inhibits release of glutamic acid | Phase 2 | 5XFAD Transgenic mouse models of familial AD | Protection of neurons in brain | [168] | |||||
| 21. | Saracatinib | Anti-tau based therapy | Phase 1 | Transgenic mouse models | Fyn kinase inhibitor to inhibit synaptotoxicity | [49] | |||||
| 22. | Morin | Interacts with nucleic acids, proteins and enzymes | - | Rats | Acts on free radicals, reduces oxidative stress and hyperammonemia | [169] | |||||
It is worth pointing out that mitigating AD induced damage via pharmacological interventions relies heavily on its early detection. As yet, no biological marker for AD has been approved for clinical diagnosis. A plethora of molecular and imaging tools have spurted out in recent years, which could gauge the subtle changes that occur years before the onset of this disease. These methods, however, are still in their preliminary stage and their reliability is still under verification. It is known that the two major hallmarks of AD, viz, amyloidosis and tauopathy precede the behavioral identifiers by many decades, such as MCI. The screening methods usually employed include CSF assessment and PET imaging techniques. These diagnostic tools are considered as the gold standard for affirming the AD pathology in both asymptomatic as well as MCI patients. However, their exorbitant cost and invasiveness have incentivized researchers to look for other novel techniques, which could effectively identify diagnostic markers unique to neurodegeneration. Screening plasma-based biomarkers are relatively easy, takes less time and is a lot less invasive. A recent plasma-based amyloid-assay has already shown some efficacy in amyloidosis assessment years before the onset of symptoms. Identifying tau based biomarkers, on the other hand, has been challenging as tauopathy is not unique to AD, as previously discussed. Notwithstanding, a tau fragment, which is phosphorylated at 181 residues (P-tau181), has shown to reliably predict the onset AD [133]. Aside from its robust diagnostic features, recent studies have also validated it as a good prognostic marker, with higher P-tau181 levels correlating effectively with the risk of developing dementia [133, 134]. Thus, plasma-based biomarkers could be a turning point in AD therapeutics even at the prodromal stage [135]. There is one major caveat, however. Neurodegeneration often precedes cognitive deficit and it would appear counter-intuitive for the population to undergo screening for AD detection before the onset of any symptoms. Perhaps, assessment of individual genetic risk profile combined with a routine check-up for approved markers in the future could offer a solution. The experiments in the drug discovery endeavors and further refining the strategies of contemporary and conventional diagnosis would help. Additionally, changes in strategies involved in drug designing, understanding the disease pathways and drug targets would also contribute to finding a possible cure for AD.
ACKNOWLEDGEMENTS
The authors are indebted to the Guest Editor for his invitation to contribute in this special thematic issue entitled “Transcriptional Factor Regulation as a Putative Target in CNS Disorders” of Current Neuropharmacology. We acknowledge the Founder President, Chancellor and Vice Chancellor of Amity University for their encouragement and patronage.
LIST OF ABBREVIATIONS
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- ARIA
Amyloid-related imaging abnormalities
- Aβ
Amyloid beta
- BACE1
Beta-secretase 1
- BDNF
Brain-derived neurotrophic factor
- BuChE
Butyrylcholinesterase
- CR
Calorie restriction
- DDVP
2, 2 - dimethyl dichlorovinyl phosphate
- EAAT
Excitatory amino acid transport channel
- GDNF
Glial derived neurotrophic factor
- GlcNAc
N-acetyl glucosamine
- GLT-1
Glutamate transporter-1
- GlyT1
Glycine transporter-1
- GSI
γ-secretase inhibitor
- GSK-3β
Glycogen synthase kinase - 3β
- GSM
γ-secretase modulator
- JNK
C-Jun N-terminal kinase
- LOAD
Late onset Alzheimer's disease
- LTD
Long-term depression
- LTP
Long-term potentiation
- mAb
Monoclonal antibody
- MAP
Microtubule associated protein
- MCI
Mild Cognitive impairment
- NFT
Neurofibrillary tangle
- NGF
Nerve growth factor
- NICD
Notch Intracellular Domain
- NSAID
Non-steroidal anti-inflammatory drug
- NT
Neurotrophin
- NT-3
Neurotrophin-3
- OGA
O-GlcNAcase
- p75NTR
P75 neurotrophin receptor
- PET
Positron emission tomography
- PKC
Protein kinase C
- PSP
Progressive supranuclear palsy
- P-tau181
Phosphorylated tau181
- ROS
Reactive oxygen species
- TOMA
Tau oligomer-specific monoclonal antibody
- TREM-2
Triggering receptor expressed on myeloid cells 2
- Trk
Tyrosine kinase receptor
- VDR
Vitamin D receptor.
- VGluT1
Vesicular glutamate transporter-1
- VGluT2
Vesicular glutamate transporter-2
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Tomaszewski S., Gauthier S., Wimo A., Rosa-Neto P. Combination therapy of anti-tau and anti-amyloid drugs for disease modification in early-stage alzheimer’s disease: socio-economic considerations modeled on treatments for tuberculosis, hiv/aids and breast cancer. J. Prev. Alzheimers Dis. 2016;3(3):164–172. doi: 10.14283/jpad.2015.85. [DOI] [PubMed] [Google Scholar]
- 2.McGleenon B.M., Dynan K.B., Passmore A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999;48(4):471–480. doi: 10.1046/j.1365-2125.1999.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan U.A., Liu L., Provenzano F.A., Berman D.E., Profaci C.P., Sloan R., Mayeux R., Duff K.E., Small S.A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 2014;17(2):304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., O’Gorman J., Qian F., Arastu M., Li M., Chollate S., Brennan M.S., Quintero-Monzon O., Scannevin R.H., Arnold H.M., Engber T., Rhodes K., Ferrero J., Hang Y., Mikulskis A., Grimm J., Hock C., Nitsch R.M., Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 5.Müller U.C., Deller T., Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017;18(5):281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 6.Xia W. γ-Secretase and its modulators: Twenty years and beyond. Neurosci. Lett. 2019;701:162–169. doi: 10.1016/j.neulet.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Ma Q., Zhang Y.W., Xu H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. 2012;120(Suppl. 1):9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dyck C.H. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol. Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budd Haeberlein S., O’Gorman J., Chiao P., Bussière T., von Rosenstiel P., Tian Y., Zhu Y., von Hehn C., Gheuens S., Skordos L., Chen T., Sandrock A. Clinical development of aducanumab, an anti-aβ human monoclonal antibody being investigated for the treatment of early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2017;4(4):255–263. doi: 10.14283/jpad.2017.39. [DOI] [PubMed] [Google Scholar]
- 10.Ferrero J., Williams L., Stella H., Leitermann K., Mikulskis A., O’Gorman J., Sevigny J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2016;2(3):169–176. doi: 10.1016/j.trci.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 2020;19(2):111–112. doi: 10.1016/S1474-4422(19)30480-6. [DOI] [PubMed] [Google Scholar]
- 12.Vandenberghe R., Rinne J.O., Boada M., Katayama S., Scheltens P., Vellas B., Tuchman M., Gass A., Fiebach J.B., Hill D., Lobello K., Li D., McRae T., Lucas P., Evans I., Booth K., Luscan G., Wyman B.T., Hua L., Yang L., Brashear H.R., Black R.S., Bapineuzumab 3000 and 3001 Clinical Study Investigators Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res. Ther. 2016;8(1):18. doi: 10.1186/s13195-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S., Reichert M., Ketter N., Nejadnik B., Guenzler V., Miloslavsky M., Wang D., Lu Y., Lull J., Tudor I.C., Liu E., Grundman M., Yuen E., Black R., Brashear H.R., Bapineuzumab 301 and 302 Clinical Trial Investigators Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein G., Delmar P., Voyle N., Rehal S., Hofmann C., Abi-Saab D., Andjelkovic M., Ristic S., Wang G., Bateman R., Kerchner G.A., Baudler M., Fontoura P., Doody R. Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: a PET substudy interim analysis. Alzheimers Res. Ther. 2019;11(1):101. doi: 10.1186/s13195-019-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang T., Dang Y., Ostaszewski B., Mengel D., Steffen V., Rabe C., Bittner T., Walsh D.M., Selkoe D.J. Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann. Neurol. 2019;86(2):215–224. doi: 10.1002/ana.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salloway S., Honigberg L.A., Cho W., Ward M., Friesenhahn M., Brunstein F., Quartino A., Clayton D., Mortensen D., Bittner T., Ho C., Rabe C., Schauer S.P., Wildsmith K.R., Fuji R.N., Suliman S., Reiman E.M., Chen K., Paul R. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimers Res. Ther. 2018;10(1):96. doi: 10.1186/s13195-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tariot P.N., Lopera F., Langbaum J.B., Thomas R.G., Hendrix S., Schneider L.S., Rios-Romenets S., Giraldo M., Acosta N., Tobon C., Ramos C., Espinosa A., Cho W., Ward M., Clayton D., Friesenhahn M., Mackey H., Honigberg L., Sanabria Bohorquez S., Chen K., Walsh T., Langlois C., Reiman E.M. Alzheimer’s Prevention Initiative. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. (N. Y.) 2018;4:150–160. doi: 10.1016/j.trci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M., Amariglio R.E., Marshall G.A., Rentz D.M., Johnson K.A., Sperling R.A. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138(Pt 4):1023–1035. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker A., Krauss G.L., Albert M.S., Speck C.L., Jones L.R., Stark C.E., Yassa M.A., Bassett S.S., Shelton A.L., Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien J.L., O’Keefe K.M., LaViolette P.S., DeLuca A.N., Blacker D., Dickerson B.C., Sperling R.A. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Findley C.A., Bartke A., Hascup K.N., Hascup E.R. Amyloid beta-related alterations to glutamate signaling dynamics during Alzheimer’s Disease progression. ASN Neuro. 2019;11:1759091419855541. doi: 10.1177/1759091419855541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ovsepian S.V., O’Leary V.B., Zaborszky L., Ntziachristos V., Dolly J.O. Amyloid Plaques of Alzheimer’s Disease as Hotspots of Glutamatergic Activity. Neuroscientist. 2019;25(4):288–297. doi: 10.1177/1073858418791128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zott B., Simon M.M., Hong W., Unger F., Chen-Engerer H.J., Frosch M.P., Sakmann B., Walsh D.M., Konnerth A. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science. 2019;365(6453):559–565. doi: 10.1126/science.aay0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung J.H.Y., Palpagama T.H., Tate W.P., Peppercorn K., Waldvogel H.J., Faull R.L.M., Kwakowsky A. The Acute Effects of Amyloid-Beta1-42 on Glutamatergic Receptor and Transporter Expression in the Mouse Hippocampus. Front. Neurosci. 2020;13:1427. doi: 10.3389/fnins.2019.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokolow S., Luu S.H., Nandy K., Miller C.A., Vinters H.V., Poon W.W., Gylys K.H. Preferential accumulation of amyloid-beta in presynaptic glutamatergic terminals (VGluT1 and VGluT2) in Alzheimer’s disease cortex. Neurobiol. Dis. 2012;45(1):381–387. doi: 10.1016/j.nbd.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R., Reddy P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017;57(4):1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collingridge G.L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990;11(7):290–296. doi: 10.1016/0165-6147(90)90011-V. [DOI] [PubMed] [Google Scholar]
- 28.Robinson D.M., Keating G.M. Memantine: a review of its use in Alzheimer’s disease. Drugs. 2006;66(11):1515–1534. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Chang L., Song Y., Li H., Wu Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019;13:43. doi: 10.3389/fnins.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44(4):851–859. [PubMed] [Google Scholar]
- 31.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev. 2014;94(4):1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 32.Hefendehl J.K., LeDue J., Ko R.W., Mahler J., Murphy T.H., MacVicar B.A. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat. Commun. 2016;7:13441. doi: 10.1038/ncomms13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scimemi A., Meabon J.S., Woltjer R.L., Sullivan J.M., Diamond J.S., Cook D.G. Amyloid-β1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J. Neurosci. 2013;33(12):5312–5318. doi: 10.1523/JNEUROSCI.5274-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauderback C.M., Hackett J.M., Huang F.F., Keller J.N., Szweda L.I., Markesbery W.R., Butterfield D.A. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1-42. J. Neurochem. 2001;78(2):413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 35.Li S., Mallory M., Alford M., Tanaka S., Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 1997;56(8):901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Sharma A., Kazim S.F., Larson C.S., Ramakrishnan A., Gray J.D., McEwen B.S., Rosenberg P.A., Shen L., Pereira A.C. Divergent roles of astrocytic versus neuronal EAAT2 deficiency on cognition and overlap with aging and Alzheimer’s molecular signatures. Proc. Natl. Acad. Sci. USA. 2019;116(43):21800–21811. doi: 10.1073/pnas.1903566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K., Kong Q., Lin Y., Stouffer N., Schulte D.A., Lai L., Liu Q., Chang L.C., Dominguez S., Xing X., Cuny G.D., Hodgetts K.J., Glicksman M.A., Lin C.L. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J. Exp. Med. 2015;212(3):319–332. doi: 10.1084/jem.20140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothstein J.D., Patel S., Regan M.R., Haenggeli C., Huang Y.H., Bergles D.E., Jin L., Dykes Hoberg M., Vidensky S., Chung D.S., Toan S.V., Bruijn L.I., Su Z.Z., Gupta P., Fisher P.B. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 39.Kong Q., Chang L.C., Takahashi K., Liu Q., Schulte D.A., Lai L., Ibabao B., Lin Y., Stouffer N., Das Mukhopadhyay C., Xing X., Seyb K.I., Cuny G.D., Glicksman M.A., Lin C.L. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J. Clin. Invest. 2014;124(3):1255–1267. doi: 10.1172/JCI66163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strzelecki D., Podgórski M., Kałużyńska O., Stefańczyk L., Kotlicka-Antczak M., Gmitrowicz A., Grzelak P. Adding sarcosine to antipsychotic treatment in patients with stable schizophrenia changes the concentrations of neuronal and glial metabolites in the left dorsolateral prefrontal cortex. Int. J. Mol. Sci. 2015;16(10):24475–24489. doi: 10.3390/ijms161024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada K., Nakato K., Yarimizu J., Yamazaki M., Morita M., Takahashi S., Aota M., Saita K., Doihara H., Sato Y., Yamaji T., Ni K., Matsuoka N. A novel glycine transporter-1 (GlyT1) inhibitor, ASP2535 (4-[3-isopropyl-5-(6-phenyl-3-pyridyl)-4H-1,2,4-triazol-4-yl]-2,1,3-benzoxadiazole), improves cognition in animal models of cognitive impairment in schizophrenia and Alzheimer’s disease. Eur. J. Pharmacol. 2012;685(1-3):59–69. doi: 10.1016/j.ejphar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Moschetti V., Schlecker C., Wind S., Goetz S., Schmitt H., Schultz A., Liesenfeld K.H., Wunderlich G., Desch M. Multiple rising doses of oral bi 425809, a glyt1 inhibitor, in young and elderly healthy volunteers: a randomised, double-blind, phase i study investigating safety and pharmacokinetics. Clin. Drug Investig. 2018;38(8):737–750. doi: 10.1007/s40261-018-0660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14(7):399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jadhav S., Avila J., Schöll M., Kovacs G.G., Kövari E., Skrabana R., Evans L.D., Kontsekova E., Malawska B., de Silva R., Buee L., Zilka N. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019;7(1):22. doi: 10.1186/s40478-019-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider A., Mandelkow E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics. 2008;5(3):443–457. doi: 10.1016/j.nurt.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serenó L., Coma M., Rodríguez M., Sánchez-Ferrer P., Sánchez M.B., Gich I., Agulló J.M., Pérez M., Avila J., Guardia-Laguarta C., Clarimón J., Lleó A., Gómez-Isla T. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 2009;35(3):359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 47.García-Barroso C., Ricobaraza A., Pascual-Lucas M., Unceta N., Rico A.J., Goicolea M.A., Sallés J., Lanciego J.L., Oyarzabal J., Franco R., Cuadrado-Tejedor M., García-Osta A. Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114–123. doi: 10.1016/j.neuropharm.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 48.Gong E.J., Park H.R., Kim M.E., Piao S., Lee E., Jo D.G., Chung H.Y., Ha N.C., Mattson M.P., Lee J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol. Dis. 2011;44(2):223–230. doi: 10.1016/j.nbd.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nygaard H.B., Wagner A.F., Bowen G.S., Good S.P., MacAvoy M.G., Strittmatter K.A., Kaufman A.C., Rosenberg B.J., Sekine-Konno T., Varma P., Chen K., Koleske A.J., Reiman E.M., Strittmatter S.M., van Dyck C.H. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res. Ther. 2015;7(1):35. doi: 10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina M., Hernández F., Avila J. New Features about Tau Function and Dysfunction. Biomolecules. 2016;6(2):E21. doi: 10.3390/biom6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Min S.W., Chen X., Tracy T.E., Li Y., Zhou Y., Wang C., Shirakawa K., Minami S.S., Defensor E., Mok S.A., Sohn P.D., Schilling B., Cong X., Ellerby L., Gibson B.W., Johnson J., Krogan N., Shamloo M., Gestwicki J., Masliah E., Verdin E., Gan L. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015;21(10):1154–1162. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuzwa S.A., Shan X., Macauley M.S., Clark T., Skorobogatko Y., Vosseller K., Vocadlo D.J. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 2012;8(4):393–399. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 53.Sandhu P., Lee J., Ballard J., Walker B., Ellis J., Marcus J., Toolan D., Dreyer D., McAvoy T., Duffy J. Pharmacokinetics and pharmacodynamics to support clinical studies of MK-8719: An O-GlcNAcase inhibitor for Progressive Supranuclear palsy. Alzheimers Dement. 2016;12(7):1028. doi: 10.1016/j.jalz.2016.06.2125. [DOI] [Google Scholar]
- 54.Castillo-Carranza D.L., Gerson J.E., Sengupta U., Guerrero-Muñoz M.J., Lasagna-Reeves C.A., Kayed R. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J. Alzheimers Dis. 2014;40(Suppl. 1):S97–S111. doi: 10.3233/JAD-132477. [DOI] [PubMed] [Google Scholar]
- 55.Bittar A., Sengupta U., Kayed R. Prospects for strain-specific immunotherapy in Alzheimer’s disease and tauopathies. NPJ Vaccines. 2018;3:9. doi: 10.1038/s41541-018-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theunis C., Crespo-Biel N., Gafner V., Pihlgren M., López-Deber M.P., Reis P., Hickman D.T., Adolfsson O., Chuard N., Ndao D.M., Borghgraef P., Devijver H., Van Leuven F., Pfeifer A., Muhs A. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickman D.T., López-Deber M.P., Ndao D.M., Silva A.B., Nand D., Pihlgren M., Giriens V., Madani R., St-Pierre A., Karastaneva H., Nagel-Steger L., Willbold D., Riesner D., Nicolau C., Baldus M., Pfeifer A., Muhs A. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J. Biol. Chem. 2011;286(16):13966–13976. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boxer A.L., Qureshi I., Ahlijanian M., Grundman M., Golbe L.I., Litvan I., Honig L.S., Tuite P., McFarland N.R., O’Suilleabhain P., Xie T., Tirucherai G.S., Bechtold C., Bordelon Y., Geldmacher D.S., Grossman M., Isaacson S., Zesiewicz T., Olsson T., Muralidharan K.K., Graham D.L., O’Gorman J., Haeberlein S.B., Dam T. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 2019;18(6):549–558. doi: 10.1016/S1474-4422(19)30139-5. [DOI] [PubMed] [Google Scholar]
- 59.Collin L., Bohrmann B., Göpfert U., Oroszlan-Szovik K., Ozmen L., Grüninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain. 2014;137(Pt 10):2834–2846. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- 60.Kfoury N., Holmes B.B., Jiang H., Holtzman D.M., Diamond M.I. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012;287(23):19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanamandra K., Jiang H., Mahan T.E., Maloney S.E., Wozniak D.F., Diamond M.I., Holtzman D.M. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann. Clin. Transl. Neurol. 2015;2(3):278–288. doi: 10.1002/acn3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shahpasand K., Sepehri S.A., Nabavi S.M. Ping. L.K.; Zhen Zhou, X. “Tau immunotherapy: Hopes and hindrances. Hum. Vaccin. Immunother. 2018;14(2):277–284. doi: 10.1080/21645515.2017.1393594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katsinelos T., Tuck B.J., Mukadam A.S., McEwan W.A. The Role of antibodies and their receptors in protection against ordered protein assembly in neurodegeneration. Front. Immunol. 2019;10:1139. doi: 10.3389/fimmu.2019.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mufson E.J., He B., Nadeem M., Perez S.E., Counts S.E., Leurgans S., Fritz J., Lah J., Ginsberg S.D., Wuu J., Scheff S.W. Hippocampal proNGF signaling pathways and β-amyloid levels in mild cognitive impairment and Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012;71(11):1018–1029. doi: 10.1097/NEN.0b013e318272caab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakravarthy B., Ménard M., Ito S., Gaudet C., Dal Prà I., Armato U., Whitfield J. Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J. Alzheimers Dis. 2012;30(3):675–684. doi: 10.3233/JAD-2012-120115. [DOI] [PubMed] [Google Scholar]
- 66.Fombonne J., Rabizadeh S., Banwait S., Mehlen P., Bredesen D.E. Selective vulnerability in Alzheimer’s disease: amyloid precursor protein and p75(NTR) interaction. Ann. Neurol. 2009;65(3):294–303. doi: 10.1002/ana.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fahnestock M., Michalski B., Xu B., Coughlin M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci. 2001;18(2):210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 68.Tep C., Lim T.H., Ko P.O., Getahun S., Ryu J.C., Goettl V.M., Massa S.M., Basso M., Longo F.M., Yoon S.O. Oral administration of a small molecule targeted to block proNGF binding to p75 promotes myelin sparing and functional recovery after spinal cord injury. J. Neurosci. 2013;33(2):397–410. doi: 10.1523/JNEUROSCI.0399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knowles J.K., Rajadas J., Nguyen T.V., Yang T., LeMieux M.C., Vander Griend L., Ishikawa C., Massa S.M., Wyss-Coray T., Longo F.M. The p75 neurotrophin receptor promotes amyloid-beta(1-42)-induced neuritic dystrophy in vitro and in vivo. J. Neurosci. 2009;29(34):10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Massa S.M., Xie Y., Yang T., Harrington A.W., Kim M.L., Yoon S.O., Kraemer R., Moore L.A., Hempstead B.L., Longo F.M. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J. Neurosci. 2006;26(20):5288–5300. doi: 10.1523/JNEUROSCI.3547-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang T., Knowles J.K., Lu Q., Zhang H., Arancio O., Moore L.A., Chang T., Wang Q., Andreasson K., Rajadas J., Fuller G.G., Xie Y., Massa S.M., Longo F.M. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS One. 2008;3(11):e3604. doi: 10.1371/journal.pone.0003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen T.V., Shen L., Vander Griend L., Quach L.N., Belichenko N.P., Saw N., Yang T., Shamloo M., Wyss-Coray T., Massa S.M., Longo F.M. Small molecule p75NTR ligands reduce pathological phosphorylation and misfolding of tau, inflammatory changes, cholinergic degeneration, and cognitive deficits in AβPP(L/S) transgenic mice. J. Alzheimers Dis. 2014;42(2):459–483. doi: 10.3233/JAD-140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang F., Kang Z., Li W., Xiao Z., Zhou X. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J. Clin. Neurosci. 2012;19(7):946–949. doi: 10.1016/j.jocn.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 74.Forlenza O.V., Diniz B.S., Teixeira A.L., Ojopi E.B., Talib L.L., Mendonça V.A., Izzo G., Gattaz W.F. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J. Biol. Psychiatry. 2010;11(6):774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 75.Arancio O., Chao M.V. Neurotrophins, synaptic plasticity and dementia. Curr. Opin. Neurobiol. 2007;17(3):325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Pardridge W.M. Neurotrophins, neuroprotection and the blood-brain barrier. Curr. Opin. Investig. Drugs. 2002;3(12):1753–1757. [PubMed] [Google Scholar]
- 77.Paillard T., Rolland Y., de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s Disease and Parkinson’s Disease: A narrative review. J. Clin. Neurol. 2015;11(3):212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prakash A., Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer׳s disease. Eur. J. Pharmacol. 2014;741:104–111. doi: 10.1016/j.ejphar.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 79.Cimini A., Gentile R., D’Angelo B., Benedetti E., Cristiano L., Avantaggiati M.L., Giordano A., Ferri C., Desideri G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013;114(10):2209–2220. doi: 10.1002/jcb.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li N., Liu G.T. The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol. Sin. 2010;31(3):265–272. doi: 10.1038/aps.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D., Kim E., Rompala A., Oram M.K., Asselin C., Aronson J., Zhang C., Miller S.J., Lesinski A., Chen J.W., Kim D.Y., van Praag H., Spiegelman B.M., Gage F.H., Tanzi R.E. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361(6406):eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massa S.M., Yang T., Xie Y., Shi J., Bilgen M., Joyce J.N., Nehama D., Rajadas J., Longo F.M. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Invest. 2010;120(5):1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maliartchouk S., Feng Y., Ivanisevic L., Debeir T., Cuello A.C., Burgess K., Saragovi H.U. A designed peptidomimetic agonistic ligand of TrkA nerve growth factor receptors. Mol. Pharmacol. 2000;57(2):385–391. [PubMed] [Google Scholar]
- 85.Aboulkassim T., Tong X.K., Tse Y.C., Wong T.P., Woo S.B., Neet K.E., Brahimi F., Hamel E., Saragovi H.U. Ligand-dependent TrkA activity in brain differentially affects spatial learning and long-term memory. Mol. Pharmacol. 2011;80(3):498–508. doi: 10.1124/mol.111.071332. [DOI] [PubMed] [Google Scholar]
- 86.Scarpi D., Cirelli D., Matrone C., Castronovo G., Rosini P., Occhiato E.G., Romano F., Bartali L., Clemente A.M., Bottegoni G., Cavalli A., De Chiara G., Bonini P., Calissano P., Palamara A.T., Garaci E., Torcia M.G., Guarna A., Cozzolino F. Low molecular weight, non-peptidic agonists of TrkA receptor with NGF-mimetic activity. Cell Death Dis. 2012;3:e339. doi: 10.1038/cddis.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoon S.O., Park D.J., Ryu J.C., Ozer H.G., Tep C., Shin Y.J., Lim T.H., Pastorino L., Kunwar A.J., Walton J.C., Nagahara A.H., Lu K.P., Nelson R.J., Tuszynski M.H., Huang K. JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron. 2012;75(5):824–837. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savage M.J., Lin Y.G., Ciallella J.R., Flood D.G., Scott R.W. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J. Neurosci. 2002;22(9):3376–3385. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R.J., Shirasaki Y., Greenberg M.E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21(19):7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koch P., Gehringer M., Laufer S.A. Inhibitors of c-Jun N-terminal kinases: an update. J. Med. Chem. 2015;58(1):72–95. doi: 10.1021/jm501212r. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Q., Wang M., Du Y., Zhang W., Bai M., Zhang Z., Li Z., Miao J. Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Ann. Neurol. 2015;77(4):637–654. doi: 10.1002/ana.24361. [DOI] [PubMed] [Google Scholar]
- 92.Harris C.A., Deshmukh M., Tsui-Pierchala B., Maroney A.C., Johnson E.M., Jr Inhibition of the c-Jun N-terminal kinase signaling pathway by the mixed lineage kinase inhibitor CEP-1347 (KT7515) preserves metabolism and growth of trophic factor-deprived neurons. J. Neurosci. 2002;22(1):103–113. doi: 10.1523/JNEUROSCI.22-01-00103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bozyczko-Coyne D., O’Kane T.M., Wu Z.L., Dobrzanski P., Murthy S., Vaught J.L., Scott R.W. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. J. Neurochem. 2001;77(3):849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 94.Colović M.B., Krstić D.Z., Lazarević-Pašti T.D., Bondžić A.M., Vasić V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013;11(3):315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong H., Yuede C.M., Coughlan C.A., Murphy K.M., Csernansky J.G. Effects of donepezil on amyloid-beta and synapse density in the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2009;1303:169–178. doi: 10.1016/j.brainres.2009.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takatori S., Wang W., Iguchi A., Tomita T. Genetic Risk Factors for Alzheimer Disease: Emerging Roles of Microglia in Disease Pathomechanisms. Adv. Exp. Med. Biol. 2019;1118:83–116. doi: 10.1007/978-3-030-05542-4_5. [DOI] [PubMed] [Google Scholar]
- 97.Sierksma A., Lu A., Mancuso R., Fattorelli N., Thrupp N., Salta E., Zoco J., Blum D., Buée L., De Strooper B. Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol. Med. 2019;12(3):e10606. doi: 10.15252/emmm.201910606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fakhoury M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018;16(5):508–518. doi: 10.2174/1570159X15666170720095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Z., Trapp B.D. Microglia and neuroprotection. J. Neurochem. 2016;136(Suppl. 1):10–17. doi: 10.1111/jnc.13062. [DOI] [PubMed] [Google Scholar]
- 100.Dong Y., Li X., Cheng J., Hou L. Drug development for Alzheimer’s Disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019;20(3):E558. doi: 10.3390/ijms20030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coppedè F. The potential of epigenetic therapies in neurodegenerative diseases. Front. Genet. 2014;5:220. doi: 10.3389/fgene.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M.Z., Zheng L.J., Shen J., Li X.Y., Zhang Q., Bai X., Wang Q.S., Ji J.G. SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes. Neural Regen. Res. 2018;13(11):2005–2013. doi: 10.4103/1673-5374.239449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sadi G., Konat D. Resveratrol regulates oxidative biomarkers and antioxidant enzymes in the brain of streptozotocin-induced diabetic rats. Pharm. Biol. 2016;54(7):1156–1163. doi: 10.3109/13880209.2015.1056311. [DOI] [PubMed] [Google Scholar]
- 104.Carrizzo A., Forte M., Damato A., Trimarco V., Salzano F., Bartolo M., Maciag A., Puca A.A., Vecchione C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 105.Zhao H.F., Li N., Wang Q., Cheng X.J., Li X.M., Liu T.T. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Capiralla H., Vingtdeux V., Zhao H., Sankowski R., Al-Abed Y., Davies P., Marambaud P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012;120(3):461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen M., Du Z.Y., Zheng X., Li D.L., Zhou R.P., Zhang K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018;13(4):742–752. doi: 10.4103/1673-5374.230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang C., Browne A., Child D., Tanzi R.E. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J. Biol. Chem. 2010;285(37):28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghosh S., Sinha J.K., Muralikrishna B., Putcha U.K., Raghunath M. Chronic transgenerational vitamin B12 deficiency of severe and moderate magnitudes modulates adiposity-probable underlying mechanisms. Biofactors. 2017;43(3):400–414. doi: 10.1002/biof.1350. [DOI] [PubMed] [Google Scholar]
- 111.Ghosh S., Sinha J.K., Khandelwal N., Chakravarty S., Kumar A., Raghunath M. Increased stress and altered expression of histone modifying enzymes in brain are associated with aberrant behaviour in vitamin B12 deficient female mice. Nutr. Neurosci. 2018;•••:1–10. doi: 10.1080/1028415X.2018.1548676. [DOI] [PubMed] [Google Scholar]
- 112.Yokoyama A.S., Rutledge J.C., Medici V. DNA methylation alterations in Alzheimer’s disease. Environ. Epigenet. 2017;3(2):dvx008. doi: 10.1093/eep/dvx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y., Lu C.J., Chien K.L., Chen S.T., Chen R.C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 2007;29(10):2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 114.Corrada M.M., Kawas C.H., Hallfrisch J., Muller D., Brookmeyer R. Reduced risk of Alzheimer’s disease with high folate intake: the Baltimore Longitudinal Study of Aging. Alzheimers Dement. 2005;1(1):11–18. doi: 10.1016/j.jalz.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aisen P.S., Schneider L.S., Sano M., Diaz-Arrastia R., van Dyck C.H., Weiner M.F., Bottiglieri T., Jin S., Stokes K.T., Thomas R.G., Thal L.J. Alzheimer Disease Cooperative Study. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhatti A.B., Usman M., Ali F., Satti S.A. Vitamin supplementation as an adjuvant treatment for Alzheimer’s Disease. J. Clin. Diagn. Res. 2016;10(8):OE07–OE11. doi: 10.7860/JCDR/2016/20273.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Banerjee A., Khemka V.K., Ganguly A., Roy D., Ganguly U., Chakrabarti S. Vitamin D and Alzheimer’s Disease: Neurocognition to therapeutics. Int. J. Alzheimers Dis. 2015;2015:192747. doi: 10.1155/2015/192747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gugliandolo A., Bramanti P., Mazzon E. Role of Vitamin E in the treatment of Alzheimer’s Disease: Evidence from animal models. Int. J. Mol. Sci. 2017;18(12):E2504. doi: 10.3390/ijms18122504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monacelli F., Acquarone E., Giannotti C., Borghi R., Nencioni A., Vitamin C., Vitamin C. Aging and Alzheimer’s Disease. Nutrients. 2017;9(7):E670. doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., Nabavi S.M. Genistein and cancer: current status, challenges, and future directions. Adv. Nutr. 2015;6(4):408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta S.K., Dongare S., Mathur R., Mohanty I.R., Srivastava S., Mathur S., Nag T.C. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol. Cell. Biochem. 2015;408(1-2):63–72. doi: 10.1007/s11010-015-2483-2. [DOI] [PubMed] [Google Scholar]
- 122.Henderson V.W., Brinton R.D. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog. Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeng H., Chen Q., Zhao B. Genistein ameliorates beta-amyloid peptide (25-35)-induced hippocampal neuronal apoptosis. Free Radic. Biol. Med. 2004;36(2):180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 124.Bang O.Y., Hong H.S., Kim D.H., Kim H., Boo J.H., Huh K., Mook-Jung I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol. Dis. 2004;16(1):21–28. doi: 10.1016/j.nbd.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 125.Liao W., Jin G., Zhao M., Yang H. The effect of genistein on the content and activity of α- and β-secretase and protein kinase C in Aβ-injured hippocampal neurons. Basic Clin. Pharmacol. Toxicol. 2013;112(3):182–185. doi: 10.1111/bcpt.12009. [DOI] [PubMed] [Google Scholar]
- 126.Li Y., Daniel M., Tollefsbol T.O. Epigenetic regulation of caloric restriction in aging. BMC Med. 2011;9:98. doi: 10.1186/1741-7015-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wood S.H., van Dam S., Craig T., Tacutu R., O’Toole A., Merry B.J., de Magalhães J.P. Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging. Genome Biol. 2015;16:285. doi: 10.1186/s13059-015-0847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masoro E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Patel N.V., Gordon M.N., Connor K.E., Good R.A., Engelman R.W., Mason J., Morgan D.G., Morgan T.E., Finch C.E. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging. 2005;26(7):995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 130.Ghosh S., Raghunath M., Das B.C., Sinha J.K. High sugar content in baby food: an Indian perspective. Lancet Diabetes Endocrinol. 2019;7(10):748–749. doi: 10.1016/S2213-8587(19)30291-8. [DOI] [PubMed] [Google Scholar]
- 131.Alldred M.J., Chao H.M., Lee S.H., Beilin J., Powers B.E., Petkova E., Strupp B.J., Ginsberg S.D. Long-term effects of maternal choline supplementation on CA1 pyramidal neuron gene expression in the Ts65Dn mouse model of Down syndrome and Alzheimer’s disease. FASEB J. 2019;33(9):9871–9884. doi: 10.1096/fj.201802669RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghosh S., Sinha J.K., Raghunath M. Epigenomic maintenance through dietary intervention can facilitate DNA repair process to slow down the progress of premature aging. IUBMB Life. 2016;68(9):717–721. doi: 10.1002/iub.1532. [DOI] [PubMed] [Google Scholar]
- 133.Janelidze S., Mattsson N., Palmqvist S., Smith R., Beach T.G., Serrano G.E., Chai X., Proctor N.K., Eichenlaub U., Zetterberg H., Blennow K., Reiman E.M., Stomrud E., Dage J.L., Hansson O. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020;26(3):379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 134.Thijssen E.H., La Joie R., Wolf A., Strom A., Wang P., Iaccarino L., Bourakova V., Cobigo Y., Heuer H., Spina S., VandeVrede L., Chai X., Proctor N.K., Airey D.C., Shcherbinin S., Duggan Evans C., Sims J.R., Zetterberg H., Blennow K., Karydas A.M., Teunissen C.E., Kramer J.H., Grinberg L.T., Seeley W.W., Rosen H., Boeve B.F., Miller B.L., Rabinovici G.D., Dage J.L., Rojas J.C., Boxer A.L., Advancing R. Advancing research and treatment for frontotemporal lobar degeneration (artfl) investigators. diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020;26(3):387–397. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bateman R.J., Barthélemy N.R., Horie K. Another step forward in blood-based diagnostics for Alzheimer’s disease. Nat. Med. 2020;26(3):314–316. doi: 10.1038/s41591-020-0797-4. [DOI] [PubMed] [Google Scholar]
- 136.Tang M., Taghibiglou C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017;58(4):1003–1016. doi: 10.3233/JAD-170188. [DOI] [PubMed] [Google Scholar]
- 137.Gomes B.A.Q., Silva J.P.B., Romeiro C.F.R., Dos Santos S.M., Rodrigues C.A., Gonçalves P.R., Sakai J.T., Mendes P.F.S., Varela E.L.P., Monteiro M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018;2018:8152373. doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karuppagounder S.S., Pinto J.T., Xu H., Chen H.L., Beal M.F., Gibson G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sabogal-Guáqueta A.M., Muñoz-Manco J.I., Ramírez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gómez G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun P., Yin J.B., Liu L.H., Guo J., Wang S.H., Qu C.H., Wang C.X. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci. Rep. 2019;39(1):BSR20180902. doi: 10.1042/BSR20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ide K., Matsuoka N., Yamada H., Furushima D., Kawakami K. Effects of tea catechins on Alzheimer’s Disease: Recent updates and perspectives. Molecules. 2018;23(9):E2357. doi: 10.3390/molecules23092357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jang H., Lee S., Choi S.L., Kim H.Y., Baek S., Kim Y. Taurine directly binds to oligomeric Amyloid-β and recovers cognitive deficits in alzheimer model mice. Adv. Exp. Med. Biol. 2017;975(Pt 1):233–241. doi: 10.1007/978-94-024-1079-2_21. [DOI] [PubMed] [Google Scholar]
- 143.Yamakawa M.Y., Uchino K., Watanabe Y., Adachi T., Nakanishi M., Ichino H., Hongo K., Mizobata T., Kobayashi S., Nakashima K., Kawata Y. Anthocyanin suppresses the toxicity of Aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s disease. Nutr. Neurosci. 2016;19(1):32–42. doi: 10.1179/1476830515Y.0000000042. [DOI] [PubMed] [Google Scholar]
- 144.Mori T., Rezai-Zadeh K., Koyama N., Arendash G.W., Yamaguchi H., Kakuda N., Horikoshi-Sakuraba Y., Tan J., Town T. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Facundo V.A., Rios K.A., Medeiros C.M., Militão J.S., Miranda A.L.P., Epifanio R.A., Carvalho M.P., Andrade A.T., Pinto A.C., Rezende C.M. Arjunolic acid in the ethanolic extract of Combretum leprosum root and its use as a potential multi-functional phytomedicine and drug for neurodegenerative disorders: anti-inflammatory and anticholinesterasic activities. J. Braz. Chem. Soc. 2005;16(6B):1309–1312. doi: 10.1590/S0103-50532005000800002. [DOI] [Google Scholar]
- 146.Ghosh J., Sil P.C. Arjunolic acid: a new multifunctional therapeutic promise of alternative medicine. Biochimie. 2013;95(6):1098–1109. doi: 10.1016/j.biochi.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 147.Mori T., Koyama N., Guillot-Sestier M.V., Tan J., Town T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One. 2013;8(2):e55774. doi: 10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Okumura N., Yoshida H., Nishimura Y., Murakami M., Kitagishi Y., Matsuda S. Genistein downregulates presenilin 1 and ubiquilin 1 expression. Mol. Med. Rep. 2012;5(2):559–561. doi: 10.3892/mmr.2011.648. [DOI] [PubMed] [Google Scholar]
- 149.Infante-Garcia C., Ramos-Rodriguez J.J., Delgado-Olmos I., Gamero-Carrasco C., Fernandez-Ponce M.T., Casas L., Mantell C., Garcia-Alloza M. Long-Term mangiferin extract treatment improves central pathology and cognitive deficits in APP/PS1 mice. Mol. Neurobiol. 2017;54(6):4696–4704. doi: 10.1007/s12035-016-0015-z. [DOI] [PubMed] [Google Scholar]
- 150.Chase T.N., Farlow M.R., Clarence-Smith K. Donepezil plus solifenacin (cpc-201) treatment for Alzheimer’s Disease. Neurotherapeutics. 2017;14(2):405–416. doi: 10.1007/s13311-016-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lilienfeld S. Galantamine--a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002;8(2):159–176. doi: 10.1111/j.1527-3458.2002.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nordberg A., Ballard C., Bullock R., Darreh-Shori T., Somogyi M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. 2013. [DOI] [PMC free article] [PubMed]
- 153.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline. Alzheimers Dement. (N. Y.) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller R.G., Block G., Katz J.S., Barohn R.J., Gopalakrishnan V., Cudkowicz M., Zhang J.R., McGrath M.S., Ludington E., Appel S.H., Azhir A. Phase 2 Trial NP001 Investigators. Randomized phase 2 trial of NP001-a novel immune regulator: Safety and early efficacy in ALS. Neurol. Neuroimmunol. Neuroinflamm. 2015;2(3):e100. doi: 10.1212/NXI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., Case M., Dean R.A., Hake A., Sundell K., Poole Hoffmann V., Carlson C., Khanna R., Mintun M., DeMattos R., Selzler K.J., Siemers E. Trial of solanezumab for mild dementia due to Alzheimer’s Disease. N. Engl. J. Med. 2018;378(4):321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 156.Tucker S., Möller C., Tegerstedt K., Lord A., Laudon H., Sjödahl J., Söderberg L., Spens E., Sahlin C., Waara E.R., Satlin A., Gellerfors P., Osswald G., Lannfelt L. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J. Alzheimers Dis. 2015;43(2):575–588. doi: 10.3233/JAD-140741. [DOI] [PubMed] [Google Scholar]
- 157.Torika N., Asraf K., Apte R.N., Fleisher-Berkovich S. Candesartan ameliorates brain inflammation associated with Alzheimer’s disease. CNS Neurosci. Ther. 2018;24(3):231–242. doi: 10.1111/cns.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lahmy V., Meunier J., Malmstrom S., Naert G., Givalois L., Kim S.H., Villard V., Vamvakides A., Maurice T. Blockade of Tau hyperphosphorylation and Abeta(1)(-)(4)(2) generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and sigma(1) receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38(9):1706–1723. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nelson T.J., Sun M.K., Lim C., Sen A., Khan T., Chirila F.V., Alkon D.L. Bryostatin Effects on Cognitive Function and PKCɛ in Alzheimer’s Disease Phase IIa and Expanded Access Trials. J. Alzheimers Dis. 2017;58(2):521–535. doi: 10.3233/JAD-170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cacabelos R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007;3(3):303–333. [PMC free article] [PubMed] [Google Scholar]
- 161.Thomas S.J., Grossberg G.T. Memantine: a review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin. Interv. Aging. 2009;4:367–377. doi: 10.2147/cia.s6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dang V., Medina B., Das D., Moghadam S., Martin K.J., Lin B., Naik P., Patel D., Nosheny R., Wesson Ashford J., Salehi A. Formoterol, a long-acting β2 adrenergic agonist, improves cognitive function and promotes dendritic complexity in a mouse model of Down syndrome. Biol. Psychiatry. 2014;75(3):179–188. doi: 10.1016/j.biopsych.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 163.Bali P., Lahiri D.K., Banik A., Nehru B., Anand A. Potential for stem cells therapy in Alzheimer’s Disease: Do neurotrophic factors play critical role? Curr. Alzheimer Res. 2017;14(2):208–220. doi: 10.2174/1567205013666160314145347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cao J., Hou J., Ping J., Cai D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018;13(1):64. doi: 10.1186/s13024-018-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lozano E., de Lucas M.P., Sáez A.G. sta-1 is repressed by mir-58 family in Caenorhabditis elegans. Worm. 2016;5(4):e1238560. doi: 10.1080/21624054.2016.1238560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Champagne D., Pearson D., Dea D., Rochford J., Poirier J. The cholesterol-lowering drug probucol increases apolipoprotein E production in the hippocampus of aged rats: implications for Alzheimer’s disease. Neuroscience. 2003;121(1):99–110. doi: 10.1016/S0306-4522(03)00361-0. [DOI] [PubMed] [Google Scholar]
- 167.Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr. Drug Targets. 2001;2(3):285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- 168.Okamoto M., Gray J.D., Larson C.S., Kazim S.F., Soya H., McEwen B.S., Pereira A.C. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl. Psychiatry. 2018;8(1):153. doi: 10.1038/s41398-018-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Subash S., Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol. Cell. Biochem. 2009;327(1-2):153–161. doi: 10.1007/s11010-009-0053-1. [DOI] [PubMed] [Google Scholar]