Summary
GPCRs are the largest receptor family that are involved in virtually all biological processes. Pharmacologically, they are highly druggable targets, as they cover more than 40% of all drugs in the market. Our knowledge of biased signaling provided insight into pharmacology vastly improving drug design to avoid unwanted effects and achieve higher efficacy and selectivity. However, yet another feature of GPCR biology is left largely unexplored, location bias. Recent developments in this field show promising avenues for evolution of new class of pharmaceuticals with greater potential for higher level of precision medicine. Further consideration and understanding of this phenomenon with deep biochemical and molecular insights would pave the road to success. In this review, we critically analyze this perspective and discuss new avenues of investigation.
Subject Areas: Pharmacology, Biological Science, Molecular Biology, Cell Biology
Graphical Abstract
Pharmacology; Biological Science; Molecular Biology; Cell Biology
Introduction
G-protein-coupled receptors (GPCRs) are the largest family of receptors in eukaryotes to signal for cellular adaptation in response to environmental cues and are involved in virtually all biological processes. They are expressed from approximately 800 genes covering almost 4% of human coding genome and are the target of more than 40% of pharmaceutical agents, validating their significance in pathophysiology (Dupré et al., 2009). They possess a diverse array of ligands spanning from odor molecules, lipid, nucleotide, and carbohydrate metabolites to peptide neurotransmitters and photons. Activation in adhesion GPCRs (adGPCR) is even more complicated; in some cases their basic conformation as active form is inhibited by their own N terminus, whereas in other adGPCRs, a segment of N terminus serves as ligand for their respective receptor (Purcell and Hall, 2018). GPCR regulation and signaling are subject of intensive research. Their subcellular (mostly nuclear) localization and ensuing intra-organelle signaling as well as non-signaling actions, such as co-transcriptional activation, adds a plethora of complexity to GPCR biology.
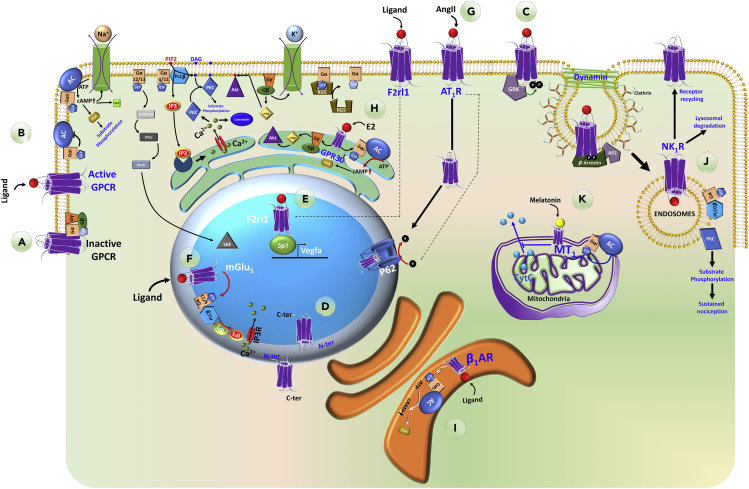
GPCRs are integral membrane proteins in the plasma membrane (PM) consisting of seven transmembrane receptors with an extracellular N terminus and cytoplasmic C terminus. Unstimulated GPCRs are associated with heterotrimeric G-proteins: Gα and Gγ subunits are membrane anchored and a Gβ subunit that tightly interacts and remains bound to Gγ (Figure 1A). Gα is a guanin nucleotide-binding protein with GTPase activity. There are four sub-families of Gα (Gαs, Gαi/o, Gαq/11, Gα12/13) bound to GPCRs in cell and context-dependent manner, and they dictate primary GPCR signaling depending on their sub-family and sub-type. Unstimulated GPCR is in complex with G-proteins, whereas Gα is loaded with GDP. Upon ligand binding to GPCR, inactivated Gα (GDP bound) exchanges GDP with GTP leading to conformational change resulting in release of G-proteins from GPCR. GTP-bound Gα is also separated from Gβγ and diffuses along the PM to signal for downstream effectors (Figure 1B) (Katritch et al., 2013)-(Sato et al., 2006).
Figure 1.
PM and Subcellular Organelle Localization and Signaling of GPCRs
A schematic representation of GPCR cycle and different GPCRs in various cellular organelles.
(A) A GPCR in its inactive confirmation coupled with heterotrimeric G proteins.
(B) Ligand binding to GPCR induces conformational changes releasing heterotrimeric G proteins. Various downstream signaling cascades are activated depending on the specific subfamily of each G protein.
(C) Ligand-bound GPCR attracts GRKs, which phosphorylate the receptor initiating their signal termination process through interaction with β-arrestins and endosomal entry.
(D) The proposed model for conformation of GPCR in the outer and inner nuclear membranes.
(E) F2rl1 is an example of a nuclear GPCR that translocates to the nucleus upon activation and induces Vegfa transcription through interaction with Sp1 transcriptional factor.
(F) mGlu5, another example of a nuclear GPCR initiates downstream signaling inside the nucleus.
(G) Activation of PM AT1R leads to phosphorylation of nuclear pore, which in turn facilitates its nuclear translocation.
(H) GPR30 is an example of ER resident GPCR, which initiates downstream signaling within the ER network.
(I) β1AR is an example of Golgi apparatus GPCR initiates downstream signaling inside the Golgi lumen.
(J) NK1R is an example of endosome-located active GPCR which displays physiological output location bias from endosomal signaling. The receptor could also go through lysosomal degradation or recycle to PM as part of GPCR life cycle.
(K) MT1 is an example of mitochondrial resident GPCR, which initiates downstream signaling inside the mitochondria.
Gαs stimulates activation of Adenylyl Cyclase (AC), a membrane-bound enzyme converting ATP to cAMP. Increased cAMP level leads to activation of protein kinase A (PKA) and subsequent phosphorylation of many targets and cellular response. cAMP can also modulate activity of some of Guanine Exchange Factors (GEFs) and ion channels. On the contrary, Gαi/o sub-family inhibits the activity of AC when it is bound to GTP and lowers cellular cAMP level. Gαq/11 sub-family can activate Phospholipase C-β (PLCβ) in the membrane, which in turn catalyzes the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 acts on endoplasmic reticulum (ER) triggering efflux of Ca2+ to cytoplasm and its subsequent cellular effects such as activation of Ca2+-dependent proteins (e.g., calmodulin, transcription factors). DAG, in parallel with increased Ca2+ level, activates protein kinase C (PKC) in the membrane followed by phosphorylation of its target proteins. Lastly, Gα12/13 interacts with various proteins and exerts its effects mainly by modulation of Rho and Ras-GEFs, cadherins, and ion channels. Released Gβ and Gγ subunits function together in a complex and can signal for various downstream effectors concomitant with Gα activity. Gβγ targets partially depend on the isoforms of their sub-units and span from ion channels to PI3K, AC, and PLC. Gα has inherent GTPase activity, although with different rates depending on its sub-family and isoforms. Regulators of G-protein signaling (RGS) and some downstream effectors of GPCR have GTPase-activating capacity and are able to increase the GTP hydrolysis rate of Gα subunit. Once GTP is hydrolyzed, GDP-bound Gα is able to assemble heterotrimeric Gαβγ and re-associate with GPCR, ready for new stimuli and activation (Ritter and Hall, 2009)-(Hilger et al., 2018).
Conformational changes in GPCR after ligand binding increases its affinity to G protein-coupled receptor kinases (GRKs) and their mediated phosphorylation of GPCRs. Ligand-bound and phosphorylated GPCRs are targeted by arrestins leading to signal attenuation (Figure 1C). GRK-mediated phosphorylation of GPCRs initiates homologous desensitization, whereas heterologous desensitization is the result of longer exposure of ligand to receptor and phosphorylation of wider range of GPCRs via signaling activated PKA and PKC. Arrestin bound to the GPCR recruits clathrin and AP2 to initiate receptor internalization. The forming vesicle is pinched off from PM with aid of accessory proteins such as dynamin. The endosomal GPCR has two fates depending on receptor type and accessory proteins available in cell-type specific manner: (1) recycling of receptor and (2) lysosomal degradation. Once the GPCR is destined for recycling, ligand is released and degraded in endosome and the GPCR is dephosphorylated and the vesicle fuses to the PM leading to receptor resensitization and recycling. When the GPCR is targeted for degradation, endosome is sorted to lysosome and the GPCR is subjected to degradation (Marchese et al., 2008)-(Peterson and Luttrell, 2017).
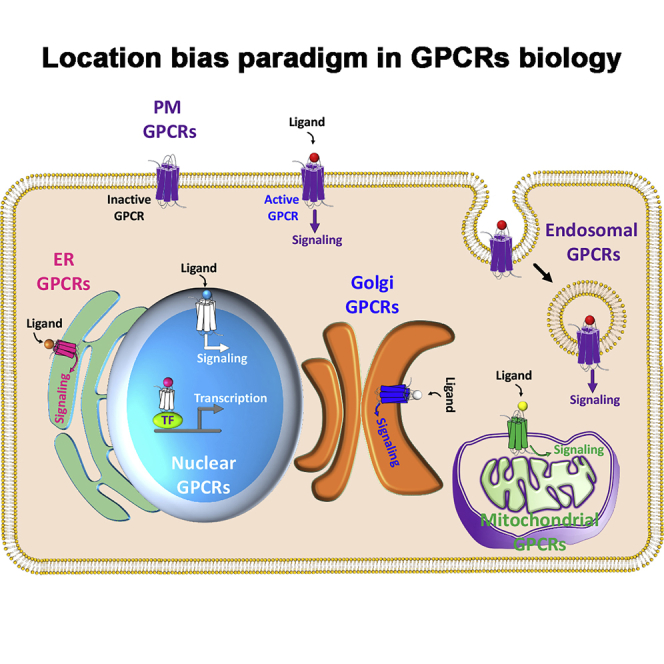
One of the emerging concepts in GPCR biology is their signaling from subcellular organelles, demonstrating another level of specificity and regulation in signal transduction. GPCRs are found in all membranous organelles within cells, acting as a functional receptor modulating signal transduction. Interestingly, some GPCRs are primarily resident to these compartments rather than to the PM. Some signaling cascades are sent to downstream effectors during endosomal entry of activated GPCRs. Mitochondria, Golgi apparatus, and ER have been shown to harbor GPCRs exerting classical signaling cascades, either potentially into the cytoplasm or into the organelle. Unfortunately, conformation of GPCRs in organelles remains largely unresolved resulting in uncertainty as to signaling directionality (intra-organelle versus into cytoplasm). Nuclear GPCRs, on the other hand, are shown to have more dynamic functions. Some are translocated from PM to the nucleus upon ligand binding, whereas others have a ligand-independent nuclear pool. Other than their participation in classical GPCR intra-nuclear signaling, nuclear GPCRs have been shown to interact with transcription factors and regulate gene expression directly (Jong et al., 2018a) (Joyal et al., 2015). In this review, we outline advances in intracellular GPCRs as a means of localizing signaling bias and address some of the challenges that remain unsolved in the field, and their potential therapeutic consequences.
Nuclear GPCRs
Nuclear GPCRs are the most studied permanent intracellular GPCRs in cellular organelles (compared with endosomes). The first report of nuclear GPCR localization was published in 1998 (Lu et al., 1998) showing translocation of AT1 receptor to the nucleus upon ligand stimulation potentially mediated through a classical nuclear localization signal (NLS). Later that year, the second report confirmed using immunogold labeling the presence of EP1 receptor in the cell nucleus independent of ligand binding, with functional consequences resulting in increased intranuclear calcium concentration and gene transcription (Bhattacharya et al., 1998). These studies paved the road for identification of intracellular GPCRs, re-shaping the classical exclusive model of PM receptor for GPCRs.
So far more than 40 GPCRs are shown to be either translocated to or localized in the nucleus (Table 1). These GPCRS have a variety of functional outputs in the nucleus. Most experimental efforts reveal classical GPCR signaling through modulation of downstream effectors such as phosphorylation of different intermittent signaling modules (Cattaneo et al., 2016), or calcium (O'Malley et al., 2003) and cAMP (Valdehita et al., 2010) level fluctuations. Since these signaling activities are known to take place inside the nucleus, it has been assumed that nuclear GPCR conveys signals via inner nuclear membrane (Tadevosyan et al., 2012). This would warrant an inward signaling cascade inside the nucleus upon activation of nuclear GPCRs similar to the cytoplasmic events (Figure 1F). Based on this model, the N terminus of GPCR in inner and outer nuclear membranes would be presumed to locate inside the lumen of nuclear membrane, whereas the C terminus would either be inside the nucleus or in the cytoplasm (Figure 1D); this GPCR orientation remains, however, speculative. Although this model would explain intra-nuclear signaling, it also provides a mode of signal transduction into the cytoplasm from activation of GPCRs on the outer nuclear membrane, but this has not been studied.
Table 1.
List of GPCRs detected on nuclear membrane and/or inside the nucleus
| GPCR | Signaling/Function | Reference |
|---|---|---|
| AT1R | PKC-NOX4-mediated ROS stimulation, NO production | (Lu et al., 1998), (Gwathmey et al., 2009), (Pendergrass et al., 2009), (Morinelli et al., 2007) |
| AT2R | RNA synthesis via IP3R- and NO-dependent pathways | (Tadevosyan et al., 2017) |
| α1A & B-AR | ERK and PKC phosphorylation | (Wright et al., 2008), (Wu et al., 2014) |
| β1&3AR | Gi-mediated RNA synthesis, AC stimulation | (Vaniotis et al., 2011) |
| Apelin R | – | (Lee et al., 2004) |
| Bradykinin B2 R | Interaction with Lamin C | (Lee et al., 2004), (Takano et al., 2014) |
| CCR2 | – | (Favre et al., 2008) |
| CXCR4 | Gi-mediated intra-nuclear Calcium release | (Wang et al., 2005), (Don-Salu-Hewage et al., 2013) |
| ETA&BR | NO production, IP3-dependent increase of calcium | (Boivin et al., 2003), (Vaniotis et al., 2013), (Merlen et al., 2013) |
| FPR2 | ERK2, c-Jun, c-Myc phosphorylation | (Cattaneo et al., 2016) |
| GRLN-R | – | (Leung et al., 2007) |
| GnRH-R | Acetylation and phosphorylation of histone H3 | (Re et al., 2010) |
| CysLT1&2 | ERK1/2 phosphorylation, nuclear calcium signaling | (Nielsen et al., 2005), (Eaton et al., 2012), (Dvash et al., 2015) |
| LPA1R | Gi, PI3K, and AKT-mediated calcium transit and iNOS expression | (Gobeil et al., 2003) |
| S1P1 | ERK and c-Jun dephosphorylation, transcriptional initiation | (Liao et al., 2007), (Estrada et al., 2009) |
| MC2R | – | (Doufexis et al., 2007) |
| MT2 | – | (Lanoix et al., 2006) |
| mAChR | – | (Lind and Cavanagh, 1993) |
| Y1R | – | (Jacques et al., 2003) |
| NTS1 | – | (Toy-Miou-Leong et al., 2004) |
| MOR1 | – | (Khorram-Manesh et al., 2009) |
| Oxtr | Induction of gene transcription | (Kinsey et al., 2007), (Di Benedetto et al., 2014) |
| Ptafr | cAMP reduction, Gi-mediated calcium increase, ERK 1/2 phosphorylation, NF-κB DNA binding, induction of gene transcription | (Marrache et al., 2002), (Bhosle et al., 2016) |
| EP2,3&4 | Gi-mediated calcium increase, ERK1/2 &AKT phosphorylation, induction of gene transcription | (Bhattacharya et al., 1999), (Gobeil et al., 2002) |
| TPR | Coupling to Gs, CREB phosphorylation, induction of gene transcription | (Ramamurthy et al., 2006), (Mir and Le Breton, 2008) |
| F2rl1 | Sp1-mediated gene transcription | (Joyal et al., 2014) |
| NK1&3 | – | (Aline Boer and Gontijo, 2006), (Lessard et al., 2009) |
| UTR | Transcriptional initiation | (Nguyen et al., 2012), (Doan et al., 2012) |
| PTH1R | – | (Watson et al., 2000) |
| VPAC1R | cAMP production | (Valdehita et al., 2010), |
| mGlu5 | Calcium transit, ERK1/2, Arc/Arg3.1, c-fos & CREB phosphorylation | (O'Malley et al., 2003), (Jong et al., 2005), (Kumar et al., 2012), (Vincent et al., 2016) |
| GPR158 | Cyclin D1-mediated cell proliferation | (Patel et al., 2013) |
Our expanded knowledge of nuclear GPCRs compared with other intracellular GPCRs is based on feasible methods of intact nuclear isolation. Isolated nuclei have the ability to behave as functional units, providing reliable examination of GPCR effects in the nucleus. However, we are unable to scrutinize the signal transduction cascade of outer nuclear membrane-localized GPCRs into the cytoplasm. Cells receive external cues from cellular niche and respond to environmental changes in the classical GPCR signaling model. On the contrary, outer nuclear membrane-localized GPCRs would enable cells to respond to the internal nuclear cues and adopt to nuclear changes. Evidence for intra-nuclear mRNA translation (Iborra et al., 2001), as well as presence of various metabolites (Campbell and Wellen, 2018) and lipids (Cascianelli et al., 2008) that can stimulate GPCRs at the nucleus, suggest that GPCR ligands are readily available inside the nucleus to act on their corresponding receptors. Alternatively, GPCR activation and signaling can be delayed until ligands reach the targeted intracellular organelle; in this case, these ligands are either transported or diffuse into the cytoplasm, to activate first PM GPCRs and subsequently nuclear GPCRs.
Alongside GPCRs, growing evidence shows presence of almost every GPCR signaling effectors in the cell nucleus. The classical partners of GPCRs, Gα and Gβγ and their different isoforms, have readily been detected in the nucleus (Zhang et al., 2001) (Boivin et al., 2005) (Dahl et al., 2018) (Sato et al., 2011). This enables formation of a fully functional coupled receptor on the nuclear membrane. In addition to the G-proteins, their immediate effectors such as AC (Yamamoto et al., 1998), PLC isoforms (Schievella et al., 1995) (Freyberg et al., 2001), Ca2+ and K channels (Bootman et al., 2009) (Quesada et al., 2002), as well as their corresponding second messengers including cAMP (Haj Slimane et al., 2014), DAG, and IP3 (Kumar et al., 2008) are observed in the cell nucleus and their activities are reliant on ligand-dependent stimulation of their respective GPCRs. Major components of GPCR signaling downstream cascades are also present and functional at the nuclear level. Various isoforms of ERK, JNK, p38 (Turjanski et al., 2007) (Plotnikov et al., 2011), AKT (Sang et al., 2008), and PKC (Martelli et al., 2003) are resident in the nuclear compartment and/or trafficked as part of their signaling translocation system. On the other hand, GPCR regulating proteins are found in the nucleus as well, including β-Arrestin (Wang et al., 2003), GRK (Johnson et al., 2004) (Jiang et al., 2007), and RGS (Burchett, 2003) (Panicker et al., 2010). The nucleus even harbors proteins such as clathrin (Ybe et al., 2013) required for endosomal entry and recycling of GPCRs. The existence of all major components of GPCR signaling cascades indicate the potential for a fully functional signaling system that could operate independently at the nuclear level.
Initiation of transcription upon stimulation of isolated nuclei provides another feature of nuclear GPCR signaling output (Vaniotis et al., 2011). However, it is not clear these transcriptional activities are the result of anything other than signaling cascades initiated inside the nucleus. A study of F2rl1, also referred to as PAR2, provided for the first time another potential mechanism of action for nuclear GPCRs, direct transcriptional regulation (Joyal et al., 2014). F2rl1 is translocated to the nucleus (from PM) upon ligand stimulation and then binds to various transcription factors and genes inside the nucleus; specifically, F2rl1 interacts with Sp1 transcription factor leading to expression of Vegfa (Figure 1E). Besides being present on the nuclear membrane, GPCRs have been detected inside the nucleus; in this case, nuclear F2rl1 is an example for potential distinct mechanisms and functions.
Given that GPCRs contain seven hydrophobic (transmembrane) domains, it is unlikely they are freely “floating” inside the nucleus. There are a number of potential mechanisms to accommodate such hydrophobic proteins inside the nucleus. Lipids are abundantly present inside the nucleus, and more specifically, they can form lipid microdomains inside the interphase nucleus reported to be co-localized with ribonucleoproteins and involved in transcription and transport of transcripts in and out of the nucleus (Maraldi et al., 1992) (Cascianelli et al., 2008). These lipid microdomains could harbor GPCRs, probably through invaginations taking place inward from nuclear envelope (Fricker et al., 1997). Besides lipid microdomains, nuclear vacuoles and some other nuclear bodies have hydrophobic constituents suitable for interactions with lipophilic proteins such as GPCRs (Zimber et al., 2004) (Gobeil et al., 2006).
Other potential explanation for intranuclear GPCR organization is their possible involvement in phase separation. Emergence of colloidal biochemistry enabled identifying many membrane-less organelles capable of having hydrophobic and hydrophilic regions (Rabouille and Alberti, 2017) (Li et al., 2012). Nucleus also has been shown to organize phase separation in order to facilitate function and formation of ribonucleoproteins and RNP bodies for RNA processing (Feric et al., 2016). Interestingly, many of the phase-separating proteins are largely hydrophobic, with multi-domains and linkers (Alberti, 2017). By these criteria, GPCRs are among top candidates for phase separating proteins based on their seven hydrophobic domains, intra and extracellular loop domains, and C- and N-terminal domains as linkers. These multidomain proteins (GPCRs) could be buried with different multivalent proteins covering their lipophilic domains, meanwhile concentrating different protein complex machineries for various cellular functions such as transcription. GPCRs inside the nucleus could operate in different nuclear functions via these mechanisms. The caveat here would be avoiding restriction of functions of GPCRs based on their classical cylindrical 3D structures, as they might adopt different shapes depending on the interacting protein complexes. In this case, their sequential linker-hydrophobic domains would provide them with huge flexibility in modulating a phase-separating function.
Although the number of nuclear GPCRs is increasing rapidly, and we are starting to differentiate their cellular and physiological functions compared with their cell surface counterparts, the field lacks deep non-signaling biochemical and biomolecular analysis of their features and functions specially as it applies to the ones inside the nucleus.
Mitochondrial GPCRs
Mitochondria also harbors GPCRs (Figure 1K). A large and increasing number of these receptors can be found on this organelle (Table 2) and opens new insights into the GPCR localization and signaling bias.
Table 2.
List of GPCRs detected on and/or inside the mitochondria
| GPCR | Signaling/Function | Reference |
|---|---|---|
| P2Y1 | Stimulation of mitochondrial Ca2+ uptake | (Belous et al., 2004) |
| P2Y2 | Inhibition of mitochondrial Ca2+ uptake | (Belous et al., 2004) |
| AT1R | Regulation of superoxide production and increase in mitochondrial respiration | (Valenzuela et al., 2016), (Abadir et al., 2011) |
| AT2R | Nitric oxide formation and decrease in mitochondrial respiration | (Valenzuela et al., 2016), (Abadir et al., 2011) |
| 5-HTR3 | Increase in Ca2+ uptake (in hypoxia) | (Wang et al., 2016) |
| 5-HTR4 | decreases Ca2+ uptake | (Wang et al., 2016) |
| MT1 | Inhibition of adenylyl cyclase through Gαi | (Suofu et al., 2017) |
| CB1 | Inhibition of adenylyl cyclase through Gαi | (Bénard et al., 2012), (Hebert-Chatelain et al., 2016) |
Interestingly, at least two GPCRs have been shown to be present both in the nucleus and on the mitochondria, specifically AT1R and AT2R (Jong et al., 2018b). Their multi-compartmental presence vastly increases the role of a single GPCR in regulation of cell physiologic functions as it adapts to environmental signals at large. Although the role of mitochondrial AT1R remains elusive, in the case of mitochondrial AT2R, its activation leads to increased mitochondrial nitric oxide (NO) production as reported for nuclear AT2R stimulation resulting in formation of nuclear NO (Abadir et al., 2011) (Gwathmey et al., 2009). This is an example of similar downstream output of subcellular GPCR activation regardless of its location. However, increased AT2R-dependent NO in both nucleus and mitochondria is likely to elicit distinct physiological functions. Interestingly, AT1R and AT2R do not have a unique nuclear or mitochondrial localization signal (Abadir et al., 2012). Yet their subcellular localization varies in different tissues and cell types (Jong et al., 2018b). The most plausible mechanism to explain differential localization pattern of GPCRs applies to post-translation modifications (PTMs). Most PTMs information on GPCRs focuses on their conformational and signaling modalities rather than other potential functions, including localization. The fact that there is a difference in localization pattern among various tissues indicates other PTM are tightly regulated to enable tissue-specific function of these GPCRs by their concentrated localization in different subcellular compartments.
The increasing number of mitochondrial GPCRs comes from our technical ability to isolate mitochondria and use them as separate functional units to induce mitochondrial functions, similar to the nucleus. But the same hurdles regarding nuclear GPCRs apply here as well. Specifically, the exact location of GPCRs in outer and inner mitochondrial membranes is not known, as is the case for their topographical orientation. As is the case with nuclear GPCRs, some mitochondrial GPCRs are detected inside the mitochondria as well (Suofu et al., 2017) (Belous et al., 2004), but the relative roles of intra-mitochondrial and mitochondrial membrane-localized GPCRs are not known.
Similar to the nucleus, many receptor-interacting proteins and their downstream effectors are observed to have mitochondrial localization as well. Gα (Suofu et al., 2017) and Gβγ (Fishburn et al., 2000), their effectors such as AC (Yamamoto et al., 1998), PLD1 (Freyberg et al., 2001), ERK (Rasola et al., 2010), AKT (Bijur and Jope, 2003), PKD, DAG (Cowell et al., 2009), C2+ channels (Belous et al., 2004), and even β-arrestins (Suofu et al., 2017) are detected in the mitochondria. Accumulation of major components of GPCR signaling in this organelle ascertains a complete functional system withing the mitochondria.
ER and Golgi GPCRs
ER and Golgi apparatus participate in endogenous route of GPCR trafficking, where they get translated, matured, and post translationally modified and sorted to their destination. It is therefore complicated to ascertain functionally active resident GPCRs in ER and Golgi, since they are readily detectable in both organelles by way of translation and PTM.
One of the best methods to differentiate transient receptor trafficking from these compartments versus functionally active residents is utilizing nanobodies. Nanobodies, also called single-domain antibodies, are able to selectively bind to only active conformation (or ligand occupied) of the receptor and stabilize them in such a conformation providing a versatile tool for structural studies and crystallography of active states of receptor (Steyaert and Kobilka, 2011). Nanobodies have successfully been utilized to detect active receptor pool of β2-adrenoceptor as prototypical GPCR in both endosomes and Golgi apparatus demonstrating that activation of this receptor in these compartments contribute significantly to the overall cellular cAMP response besides the PM localized receptor activation (Irannejad et al., 2017) (Irannejad et al., 2013). Using engineered cells to express nanobodies as active opioid receptor sensors (MOR and DOR), it is as well shown that they localized to both endosomes and Golgi apparatus in neurons (Stoeber et al., 2018). Interestingly, active ORs localize to Golgi outposts throughout the dendrite too, and they contribute to cellular response to membrane permeable agonists such as morphine. This might explain longer and stronger activation of ORs and pain suppression to some agents through cell penetration and receptor activation on both endosomes and Golgi apparatus. Thus, taking advantage of these nanobodies as biosensors they provide an easy and rapid tool to simultaneously interrogate activation of intracellular GPCR in various organelles without requirement for subcellular fractionation.
An abundance of a particular GPCR in these compartments compared with other organelles and PM is an indication to distinguish between resident GPCRs and trafficking ones. This was the case of the first functional ER GPCR: GPR30. GPR30 is predominantly resident of ER with very low levels in other compartments. This made it possible to determine its functionality and signaling in the cells (Figure 1H); GPR30 binds to estrogen and initiates intracellular calcium mobilization (Revankar et al., 2005). If GPR30 would have been present on other intracellular compartments, it would be very difficult to measure its functionality and activation in ER solely. This is the case for mGlu5, which has been detected abundantly in the nucleus and ER, in addition to its PM localization. Caged ligand and nanobodies provide a means to differentiate between activation of intracellular mGlu5 versus PM mGlu5. Uncaging the mGlu5 ligand (Glutamate) near the ER with laser results in sustained intracellular increase of calcium (Purgert et al., 2014). However, these results are not fully conclusive in differentiating between the effect of nuclear and ER mGlu5; one can only infer the overall effects of intracellular mGlu5.
ER and Golgi cannot be readily isolated and treated as functional organelles; this obscures the ability to study GPCR signaling in them. Caged ligands provide an efficient method for this hurdle only if the GPCR is largely localized in one intracellular organelle (Audet et al., 2018). Alternatively, one can use FRET-BRET biosensors coupled with various techniques such as caged ligands and cAMP biosensors (Salahpour et al., 2012) conjugated to specific organelle localization signals to feasibly dissect intra-organelle signaling initiated by their respective GPCRs. However, the most reliable method would be to discover the mechanisms of GPCR subcellular localization. For instance, identifying compartmentalization-specific PTMs in GPCRs would allow us to abrogate their ER or Golgi localization (by substitutional mutations), which would be similar to GPCR knockdown in organelle-specific manner. This would enable us to precisely observe the organelle-specific function of GPCRs.
However, receptor localization to these organelles might not necessarily mean a location bias and translocation of a GPCR to these compartments might have different consequences rather than intra-organelle signaling. Unlike classical pathway of β-arrestins-mediated signal termination by endo-lysosomal pathway, it has been shown that cAMP production in response to PTHR activation is further prolonged with β-arrestin interaction. The signal termination of PTHR is initiated with retromer binding and translocation of the endosomal PTHR to the Golgi apparatus (Feinstein et al., 2011). So, in case of PTHR, Golgi translocation serves as a mode of signal termination rather than location biased signaling. Thus, different processes in signal transduction and GPCR biology should be considered for organelle localized/translocated receptors.
Receptor-coupled proteins and their effector modulators are observed in both ER and Golgi. However, detection of these proteins suffers from the same dilemma as GPCRs regarding their residence verse trafficking as part of their translation and PTM routes. Notwithstanding consideration of this obstacle, functional Gα (Godbole et al., 2017), Gβγ(Jamora et al., 1999) (Klayman and Wedegaertner, 2017), and their effector proteins including AC (Yamamoto et al., 1998), PKA (Godbole et al., 2017), PKC (Jamora et al., 1999), PLD1 (Freyberg et al., 2001), PLA2 (Schievella et al., 1995) and ERK (Wainstein and Seger, 2016) are present in the ER and/or Golgi apparatus. This enables a closed system capable of classical GPCR signaling in these two organelles.
In the case of Golgi localized GPCRs, β1AR has been shown to be present in this compartment and does not translocate from PM upon ligand binding. Activation of Golgi β1AR triggers Golgi internal Gs-mediated cAMP response contributing to overall cellular β1AR-mediated cAMP level changes (Figure 1I) (Irannejad et al., 2017). This effect seems to be physiologically relevant since it is recently shown that blocking of Golgi β1AR inhibits norepinephrine-induced cardiac myocyte hypertrophy (Nash et al., 2019).
Endosomal GPCRs
GPCR internalization and endosomal integration is a typical consequence of GPCR activation. After ligand binding and initiation of signal, usually the GPCR starts to internalize through endosomes, where it can either go through re-sensitization process and recycle back to the PM, or combine with lysosomes to degrade. This mechanism provides a mode of signal attenuation and re-sensitization (Bahouth and Nooh, 2017).
Increasing evidence shows most GPCRs continue to signal while they are in endosomes (Table 3) (Irannejad and Von Zastrow, 2014). One of the consequences of GRK-mediated GPCR phosphorylation (leading to internalization) is recruitment of β-arrestins. β-Arrestin-mediated GPCR signaling is a widely known G-protein-independent signaling mode for GPCRs (Shenoy and Lefkowitz, 2005). It has been shown that endosomal signaling of GPCRs can occur via the classical G-protein-mediated pathways in line with their initial signal (Irannejad et al., 2013); this has a location bias in the control of downstream moieties leading to different transcriptional programs induced by the endosomal GPCR (Tsvetanova and von Zastrow, 2014). The physiological consequence of endosomal GPCR signaling seems to be in line with their initial signal. For example, NK1R signaling in endosomes results in sustained nociception and chronic pain contrary to the initial acute pain sensation (Figure 1J) (Jensen et al., 2017).
Table 3.
Examples of GPCRs with endosomal signaling
| GPCR | Signaling/Function | Reference |
|---|---|---|
| β2-AR | Differential transcriptional program from PM receptor via Gαs | (Tsvetanova and von Zastrow, 2014) |
| PTHR | Sustained activation of adenylyl cyclase through Gαs | (Ferrandon et al., 2009) |
| CaSR | Sustained Gαq-mediated signaling | (Gorvin et al., 2018) |
| D1R | Sustained activation of adenylyl cyclase through Gαs | (Kotowski et al., 2011) |
| LHR | Sustained activation of adenylyl cyclase through Gαs | (Godbole et al., 2017) |
| NK1R | Sustained Gαq-mediated signaling | (Jensen et al., 2017) |
| IGF1R | β-Arrestin1-mediated ERK phosphorylation | (Lin et al., 1998) |
| AT1R | β-Arrestin2-mediated JNK3 activation | (McDonald, 2000) |
| CXCR4 | β-Arrestin2-mediated p38 activation for chemotaxis | (Sun et al., 2002) |
| F2rl1 | Gαq-mediated ERK signaling for hyperexcitability of nociceptors in IBS | (Jimenez-Vargas et al., 2018) |
Although the location bias of endosomal signaling yields to a different extent GPCR activation compared with that at the PM, this could still be considered a continuation of the same initial signal. Given the different functional output of these two location biases, after ligand binding and receptor activation, there is no separate receptor stimulation or conformational dependent activity. The process takes places linearly, and signaling is sustained through the GPCR life cycle.
However, endosomal GPCR signaling could elicit distinct consequences from its PM signaling (Tsvetanova et al., 2015), a true example of location bias signaling output. A great and elegantly verified example is the case of β2-AR signaling, in which its activation results in different transcriptional signature when cAMP is generated from PM versus endosomal-generated cAMP (Tsvetanova and von Zastrow, 2014). Authors used optogenetics to induce cAMP generation specifically either from PM or endosomes (and cytoplasm) and proved that location difference in the cAMP generation leads to a biased signaling output and in differential transcriptional signature. Although endosomal-generated cAMP results mainly in CREB phosphorylation and CREB-target gene induction, PM-generated cAMP has little effect on CREB phosphorylation and its related gene expressions (Tsvetanova and von Zastrow, 2014). As such, different agonists of β2-AR (epinephrine and dopamine) produce signaling bias based on their ability to induce higher endocytosis (epinephrine is strong stimulant of β2-AR endocytosis).
Mechanisms of Cellular Localization/Translocation of GPCRs
Some GPCRs are primarily localized in intracellular organelles without substantial PM expression, others are simultaneous residents in numerous organelles, whereas others are translocated from PM to these organelles upon ligand binding. Different mechanisms of GPCR subcellular localization/translocation are studied for nuclear GPCRs.
Endogenous nucleus-localized GPCRs such as α1A & B-AR (Wright et al., 2008), MT2 (Lanoix et al., 2006) and MOR1 (Khorram-Manesh et al., 2009) are directly targeted to this compartment without appearing at the PM. These GPCRs could originate via two main sources. First one is canonical protein synthesis pathway via translation and maturation in ER and further processing in Golgi apparatus. Terminally mature proteins in ER are able to diffuse laterally to the nuclear membrane and inside the nucleus since the ONM is contiguous with the ER membrane. These proteins are diffused to the ONM where they can get translocated to the INM or inside the nucleus via a diffusion-retention mechanism mostly involving nuclear pore complexes (Ungricht et al., 2015). Nucleus-targeted proteins which traffic to the Golgi apparatus can proceed via retrograde transport to the ER or be directly transported to the nucleus via nuclear-targeted vesicles (Liu et al., 2018). Since glycosylation is a common PTM occurring in GPCR, which mainly takes place in the Golgi apparatus (Goth et al., 2020), this route is highly probable. Processed proteins in the Golgi can directly travel to the nucleus via nuclear destined vesicles or they are retrograded to the ER network and, as explained above, diffuse to the ONM and subsequently to the INM and inside the nucleus. Either of these routes are the most likely localization mechanism of endogenous nuclear GPCRs. The second possibility for endogenous nuclear GPCR localization mechanism is their nuclear translation directly in the nucleus, similar to reported transcription-coupled translation inside the nucleus (Iborra et al., 2001).
Many GPCRs that are translocated from the PM have nuclear localization signals. Both classical monopartite NLS consisting of several consecutive basic amino acids and bipartite NLS, which contains two separate clusters of basic amino acids, are shown as functional mechanisms for nuclear translocation of GPCRs (Bhosle et al., 2019). These NLS sequences are located in cytosolic portion of GPCRs, either in the intracellular loop (ICL) domains or C terminus of the receptor. In case of F2rl1, a single monopartite NLS in the ICL3 as well as C terminus of the receptor was coordinately involved in nuclear translocation as deletion of either of them resulted in aberrant nuclear transport from PM upon ligand stimulation (Joyal et al., 2014). However, in other cases such as PTAFR, the C terminus of the receptor is strictly required for the nuclear translocation despite presence of monopartite NLS sequence elsewhere, the deletion of which did not result in any nuclear translocation defects (Bhosle et al., 2016), suggesting more complex means of translocations is necessary for the nuclear GPCRs rather than simple regulation via a single NLS (mono or bipartite) (Figure 1G).
NLS sequences in GPCRs follow the common path to the nucleus through their interaction with nuclear transport machinery, mainly importins. Different members of the importin family, including Impα1, Impα3, Impα5, Impβ1, and Imp5, have been shown to mediate nuclear translocation of different GPCRs, and occasionally more than one is required for this process (Bhosle et al., 2019). Other members of the Karyopherin family are also involved in nuclear translocation of GPCRs such as transportin 1 (Favre et al., 2008) (Don-Salu-Hewage et al., 2013) (Di Benedetto et al., 2014).
Other modulators of nuclear transport machinery such as Rab GTPases are also shown to regulate GPCR nuclear localization/translocation. For instance, Rab11 in conjugation with Imp5 is required for nuclear localization of PTAFR (Bhosle et al., 2016). On the other hand, endosomal translocation machinery such as SNX family (sortin nexins) that is essential for endosomal trafficking are major players of nuclear translocation of GPCRs from PM, thus providing the first step in the nuclear translocation from PM by their endosomal sorting. Snx11 is such an example for translocation of F2rl1 (Joyal et al., 2014). Additionally, proteins regulating cytoskeletal rearrangement and their associated signaling such as integrins and Rho kinases are reported to play some roles in GPCR nuclear trafficking (Waters et al., 2006).
Other modulators of GPCR endosomal targeting and mediators of ligand-induced GPCR PTM such as β-arrestins are also involved in this process. Arrb1 knockdown or substitutional mutations that abrogate Arrb1 binding to oxytocin receptor inhibits its nuclear translocation (Di Benedetto et al., 2014). It is likely that arrestins are involved in the regulation of GPCRs PTM (post ligand-binding) leading to recruitment of factors initiating their nuclear translocation, rather than their direct involvement as a trafficking modulator.
Although modulators and NLS motifs mediate translocation of nuclear GPCRs from PM, they do not explain how they participate in initiating such an action. Most studies focus on deletions in the NLS regions but lack the mechanisms showing how these NLSs are activated; this is probably through ligand-induced PTMs on other regions. These PTMs could either directly or indirectly recruit importins for nuclear translocation or unfold different regions of the receptor exposing the NLS sequences for recognition by importins.
Unfortunately, there is a dearth of data on the mechanisms of localization/translocation to other subcellular organelles; however, bioinformatics can provide clues. ER GPCRs translated in this compartment remain in the ER by lacking necessary domains for PM localization. This could also be the case for Golgi resident GPCRs, which are trafficked there as part of their maturation, however, do not receive further localization signal and reside in the Golgi. On the other hand, mitochondrial GPCRs need a localization signal to target this organelle. Identifying the underlying mechanism of GPCR subcellular localization/translocation would not only reveal different aspects of their regulation and biology but also would provide a tool to manipulate the system for better understanding of differential effects of these location biases by targeted depletion or accumulation of the receptor in specific compartments.
Implications and Conclusion
Although GPCRs are among the best studied receptors, cell compartment localization can extend biased signaling and adds another layer of complexity in their biology and ensuing cellular and physiological effects. Current efforts in the field try to elucidate potential functions of intracellular GPCRs, without addressing the concept of bias that is enforced by these differential localizations. Investigations into differentially induced signaling cascades in various subcellular organelles by a unique GPCR or the endpoint differential physiological output is required to disseminate the location bias concept. Expanding our understanding of this bias and differentiating it from PM receptors would greatly advance the ability to recognize and design new therapeutics with greater precision. From a translational point of view, it is logical to envisage that targeting a drug to an undesired compartmentalized GPCR can lead to unwanted effects; precise targeting of a specifically localized GPCR would prevent such undesired effects.
Directing a drug to a specific cell location can also enhance efficacy. For example, β-blockers that avoid the β1AR localized to intracellular compartments elicits attenuated efficacy (Nash et al., 2019); rational design of cell-penetrant blockers would alter the treatment potential. The same scenario applies to NK1R (Jensen et al., 2017), by which its endosomal targeting can prolong the antinociceptive effect of pain medications. Targeting intracellular Kinin B1 receptor also shows significant anticancer activity, whereas cell impermeant antagonist do not possess the same effect (Dubuc et al., 2019). The case of Kinin B1 receptor proves that only cell surface study of GPCRs might result in escaping valid therapeutic targets whose intracellular functions can be pathophysiologically relevant.
Besides the implications of such location bias, we have almost no information regarding general GPCR biology in subcellular organelles including processes such as desensitization and resensitization, potential signaling biases integrated withing different organelles, and GPCRs post-translation modifications in these compartments. This calls for extensive research in these areas to elucidate the differences in the biology of differentially localized GPCRs.
All in all, the increasing evidence in the importance of intracellular GPCRs and their location bias calls for serious consideration of this concept. A combination of deeper biochemical and biomolecular studies at basic and clinical levels could improve rational design and precision of pharmacologic agents.
Acknowledgments
The authors apologize to colleagues whose contributions could not be individually cited. The authors acknowledge lab members in the S.C. lab for comments and discussions.
Author Contributions
M.A.M.N. conceptualized, outlined, and drafted the manuscript and figure. S.C. reviewed, outlined, edited, and supervised the manuscript. J.C.R. visualized and prepared the figure. All authors approved the final version of the manuscript.
References
- Abadir P.M., Foster D.B., Crow M., Cooke C.A., Rucker J.J., Jain A., Smith B.J., Burks T.N., Cohn R.D., Fedarko N.S. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. U S A. 2011;108:14849. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadir P.M., Walston J.D., Carey R.M. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38:437. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S. Phase separation in biology. Curr. Biol. 2017;27:R1097. doi: 10.1016/j.cub.2017.08.069. [DOI] [PubMed] [Google Scholar]
- Aline Boer P., Gontijo J.A. Nuclear localization of SP, CGRP, and NK1R in a subpopulation of dorsal root ganglia subpopulation cells in rats. Cell. Mol. Neurobiol. 2006;26:191. doi: 10.1007/s10571-006-9020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N., Dabouz R., Allen B.G., Hébert T.E. Nucleoligands-repurposing G protein-coupled receptor ligands to modulate nuclear-localized G protein-coupled receptors in the cardiovascular system. J. Cardiovasc. Pharmacol. 2018;71:193. doi: 10.1097/FJC.0000000000000535. [DOI] [PubMed] [Google Scholar]
- Bahouth S.W., Nooh M.M. Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal. 2017;36:42. doi: 10.1016/j.cellsig.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belous A., Wakata A., Knox C.D., Nicoud I.B., Pierce J., Anderson C.D., Pinson C.W., Chari R.S. Mitochondrial P2Y-like receptors link cytosolic adenosine nucleotides to mitochondrial calcium uptake. J. Cell Biochem. 2004;92:1062. doi: 10.1002/jcb.20144. [DOI] [PubMed] [Google Scholar]
- Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- Di Benedetto A., Sun L., Zambonin C.G., Tamma R., Nico B., Calvano C.D., Colaianni G., Ji Y., Mori G., Grano M. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc. Natl. Acad. Sci. U S A. 2014;111:16502. doi: 10.1073/pnas.1419349111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Peri K.G., Almazan G., Ribeiro-da-Silva A., Shichi H., Durocher Y., Abramovitz M., Hou X., Varma D.R., Chemtob S. Nuclear localization of prostaglandin E2 receptors. Proc. Natl. Acad. Sci. U S A. 1998;95:15792. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Peri K., Ribeiro-da-Silva A., Almazan G., Shichi H., Hou X., Varma D.R., Chemtob S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J. Biol. Chem. 1999;274:15719. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- Bhosle V., Rivera J.C., Zhou T.E., Omri S., Sanchez M., Hamel D., Zhu T., Rouget R., Rabea A.A., Hou X. Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov. 2016;2:16034. doi: 10.1038/celldisc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosle V.K., Rivera J.C., Chemtob S. New insights into mechanisms of nuclear translocation of G-protein coupled receptors. Small GTPases. 2019;10:254–263. doi: 10.1080/21541248.2017.1282402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur G.N., Jope R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87:1427. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B., Chevalier D., Villeneuve L.R., Rousseau E., Allen B.G. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J. Biol. Chem. 2003;278:29153. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- Boivin B., Villeneuve L.R., Farhat N., Chevalier D., Allen B.G. Sub-cellular distribution of endothelin signaling pathway components in ventricular myocytes and heart: lack of preformed caveolar signalosomes. J. Mol. Cell Cardiol. 2005;38:665. doi: 10.1016/j.yjmcc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Fearnley C., Smyrnias I., MacDonald F., Roderick H.L. An update on nuclear calcium signalling. J. Cell Sci. 2009;122:2337. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- Burchett S.A. In through the out door: nuclear localization of the regulators of G protein signaling. J. Neurochem. 2003;87:551. doi: 10.1046/j.1471-4159.2003.02047.x. [DOI] [PubMed] [Google Scholar]
- Campbell S.L., Wellen K.E. Metabolic signaling to the nucleus in cancer. Mol. Cell. 2018;71:398. doi: 10.1016/j.molcel.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Cascianelli G., Villani M., Tosti M., Marini F., Bartoccini E., Magni M.V., Albi E. Lipid microdomains in cell nucleus. Mol. Biol. Cell. 2008;19:5289. doi: 10.1091/mbc.E08-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo F., Parisi M., Fioretti T., Sarnataro D., Esposito G., Ammendola R. Nuclear localization of Formyl-Peptide Receptor 2 in human cancer cells. Arch. Biochem. Biophys. 2016;603:10. doi: 10.1016/j.abb.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Cowell C.F., Döppler H., Yan I.K., Hausser A., Umezawa Y., Storz P. Mitochondrial diacylglycerol initiates protein-kinase D1-mediated ROS signaling. J. Cell Sci. 2009;122:919. doi: 10.1242/jcs.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E.F., Wu S.C., Healy C.L., Harsch B.A., Shearer G.C., O'Connell T.D. Subcellular compartmentalization of proximal Gq-receptor signaling produces unique hypertrophic phenotypes in adult cardiac myocytes. J. Biol. Chem. 2018;293:8734. doi: 10.1074/jbc.RA118.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan N.D., Nguyen T.T., Létourneau M., Turcotte K., Fournier A., Chatenet D. Biochemical and pharmacological characterization of nuclear urotensin-II binding sites in rat heart. Br. J. Pharmacol. 2012;166:243. doi: 10.1111/j.1476-5381.2011.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don-Salu-Hewage A.S., Chan S.Y., McAndrews K.M., Chetram M.A., Dawson M.R., Bethea D.A., Hinton C.V. Cysteine (C)-X-C receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS One. 2013;8:e57194. doi: 10.1371/journal.pone.0057194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doufexis M., Storr H.L., King P.J., Clark A.J. ‘Interaction of the melanocortin 2 receptor with nucleoporin 50: evidence for a novel pathway between a G-protein-coupled receptor and the nucleus’. FASEB J. 2007;21:4095. doi: 10.1096/fj.06-7927com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C., Savard M., Bovenzi V., Lessard A., Côté J., Neugebauer W., Geha S., Chemtob S., Gobeil F. Antitumor activity of cell-penetrant kinin B1 receptor antagonists in human triple-negative breast cancer cells. J. Cell Physiol. 2019;234:2851. doi: 10.1002/jcp.27103. [DOI] [PubMed] [Google Scholar]
- Dupré D.J., Robitaille M., Rebois R.V., Hébert T.E. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 2009;49:31. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash E., Har-Tal M., Barak S., Meir O., Rubinstein M. Leukotriene C4 is the major trigger of stress-induced oxidative DNA damage. Nat. Commun. 2015;6:10112. doi: 10.1038/ncomms10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton A., Nagy E., Pacault M., Fauconnier J., Bäck M. Cysteinyl leukotriene signaling through perinuclear CysLT1 receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J. Mol. Med. 2012;90:1223. doi: 10.1007/s00109-012-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada R., Wang L., Jala V.R., Lee J.F., Lin C.Y., Gray R.D., Haribabu B., Lee M.J. Ligand-induced nuclear translocation of S1P1 receptors mediates Cyr61 and CTGF transcription in endothelial cells. Histochem. Cell Biol. 2009;131:239. doi: 10.1007/s00418-008-0521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre N., Camps M., Arod C., Chabert C., Rommel C., Pasquali C. Chemokine receptor CCR2 undergoes transportin1-dependent nuclear translocation. Proteomics. 2008;8:4560. doi: 10.1002/pmic.200800211. [DOI] [PubMed] [Google Scholar]
- Feinstein T.N., Wehbi V.L., Ardura J.A., Wheeler D.S., Ferrandon S., Gardella T.J., Vilardaga J.P. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 2011;7:278. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M., Vaidya N., Harmon T.S., Mitrea D.M., Zhu L., Richardson T.M., Kriwacki R.W., Pappu R.V., Brangwynne C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S., Feinstein T.N., Castro M., Wang B., Bouley R., Potts J.T., Gardella T.J., Vilardaga J.P. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 2009;5:734. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn C.S., Pollitt S.K., Bourne H.R. Localization of a peripheral membrane protein: gbeta gamma targets Galpha Z. Proc. Natl. Acad. Sci. U S A. 2000;97:1085. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z., Sweeney D., Siddhanta A., Bourgoin S., Frohman M., Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol. Biol. Cell. 2001;12:943. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M., Hollinshead M., White N., Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 1997;136:531. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil F., Dumont I., Marrache A.M., Vazquez-Tello A., Bernier S.G., Abran D., Hou X., Beauchamp M.H., Quiniou C., Bouayad A. Regulation of eNOS expression in brain endothelial cells by perinuclear EP3 receptors. Circ. Res. 2002;90:682. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- Gobeil F., Bernier S.G., Vazquez-Tello A., Brault S., Beauchamp M.H., Quiniou C., Marrache A.M., Checchin D., Sennlaub F., Hou X. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J. Biol. Chem. 2003;278:38875. doi: 10.1074/jbc.M212481200. [DOI] [PubMed] [Google Scholar]
- Gobeil F., Fortier A., Zhu T., Bossolasco M., Leduc M., Grandbois M., Heveker N., Bkaily G., Chemtob S., Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigmThis paper is one of a selection of papers published in this Special Issue, entitled the Nucleus: a Cell within A Cell. Can. J. Physiol. Pharmacol. 2006;84:287. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- Godbole A., Lyga S., Lohse M.J., Calebiro D. Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat. Commun. 2017;8:5459. doi: 10.1038/s41467-017-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvin C.M., Rogers A., Hastoy B., Tarasov A.I., Frost M., Sposini S., Inoue A., Whyte M.P., Rorsman P., Hanyaloglu A.C. AP2σ mutations impair calcium-sensing receptor trafficking and signaling, and show an endosomal pathway to spatially direct G-protein selectivity. Cell Rep. 2018;22:1054–1066. doi: 10.1016/j.celrep.2017.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth C.K., Petäjä-Repo U.E., Rosenkilde M.M. G protein-coupled receptors in the sweet spot: glycosylation and other post-translational modifications. ACS Pharmacol. Transl. Sci. 2020;3:237. doi: 10.1021/acsptsci.0c00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey T.M., Shaltout H.A., Pendergrass K.D., Pirro N.T., Figueroa J.P., Rose J.C., Diz D.I., Chappell M.C. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am. J. Physiol. Ren. Physiol. 2009;296:F1484. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj Slimane Z., Bedioune I., Lechêne P., Varin A., Lefebvre F., Mateo P., Domergue-Dupont V., Dewenter M., Richter W., Conti M. Control of cytoplasmic and nuclear protein kinase A by phosphodiesterases and phosphatases in cardiac myocytes. Cardiovasc. Res. 2014;102:97. doi: 10.1093/cvr/cvu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Chatelain E., Desprez T., Serrat R., Bellocchio L., Soria-Gomez E., Busquets-Garcia A., Pagano Zottola A.C., Delamarre A., Cannich A., Vincent P. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- Hilger D., Masureel M., Kobilka B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018;25:4. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra F.J., Jackson D.A., Cook P.R. Coupled transcription and translation within nuclei of mammalian cells. Science. 2001;293:1139. doi: 10.1126/science.1061216. [DOI] [PubMed] [Google Scholar]
- Irannejad R., Tomshine J.C., Tomshine J.R., Chevalier M., Mahoney J.P., Steyaert J., Rasmussen S.G., Sunahara R.K., El-Samad H., Huang B., von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R., Pessino V., Mika D., Huang B., Wedegaertner P.B., Conti M., von Zastrow M. Functional selectivity of GPCR-directed drug action through location bias. Nat. Chem. Biol. 2017;13:799. doi: 10.1038/nchembio.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R., Von Zastrow M. GPCR signaling along the endocytic pathway. Curr. Opin. Cell Biol. 2014;27:109. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques D., Sader S., Perreault C., Fournier A., Pelletier G., Beck-Sickinger A.G., Descorbeth M. Presence of neuropeptide Y and the Y1 receptor in the plasma membrane and nuclear envelope of human endocardial endothelial cells: modulation of intracellular calcium. J. Physiol. Pharmacol. 2003;81:288. doi: 10.1139/y02-165. [DOI] [PubMed] [Google Scholar]
- Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J.R., Faulkner D.J., Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Jensen D.D., Lieu T., Halls M.L., Veldhuis N.A., Imlach W.L., Mai Q.N., Poole D.P., Quach T., Aurelio L., Conner J. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 2017;9:eaal3447. doi: 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Benovic J.L., Wedegaertner P.B. Plasma membrane and nuclear localization of G protein coupled receptor kinase 6A. Mol. Biol. Cell. 2007;18:2960. doi: 10.1091/mbc.E07-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Vargas N.N., Pattison L.A., Zhao P., Lieu T., Latorre R., Jensen D.D., Castro J., Aurelio L., Le G.T., Flynn B. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci. U S A. 2018;115:E7438. doi: 10.1073/pnas.1721891115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.R., Scott M.G., Pitcher J.A. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol. Cell Biol. 2004;24:10169. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong Y.J., Kumar V., Kingston A.E., Romano C., O'Malley K.L. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J. Biol. Chem. 2005;280:30469. doi: 10.1074/jbc.M501775200. [DOI] [PubMed] [Google Scholar]
- Jong Y.-J.I., Harmon S.K., O’Malley K.L. GPCR signalling from within the cell. Br. J. Pharmacol. 2018;175:4026. doi: 10.1111/bph.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong Y.-J.I., Harmon S.K., O’Malley K.L. Intracellular GPCRs play key roles in synaptic plasticity. ACS Chem. Neurosci. 2018;9:2162. doi: 10.1021/acschemneuro.7b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal J.S., Nim S., Zhu T., Sitaras N., Rivera J.C., Shao Z., Sapieha P., Hamel D., Sanchez M., Zaniolo K. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat. Med. 2014;20:1165. doi: 10.1038/nm.3669. [DOI] [PubMed] [Google Scholar]
- Joyal J.S., Bhosle V.K., Chemtob S. Subcellular G-protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin. Ther. Targets. 2015;19:717. doi: 10.1517/14728222.2015.1042365. [DOI] [PubMed] [Google Scholar]
- Katritch V., Cherezov V., Stevens R.C. ‘Structure-Function of the G protein–coupled receptor superfamily’. Annu. Rev. Pharmacol. Toxicol. 2013;53:531. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram-Manesh A., Nordlander S., Novotny A., Bengtsson C., Nylund G., Levin M., Nordgren S., Delbro D.S. Nuclear expression of mu-opioid receptors in a human mesothelial cell line. Auton. Autacoid Pharmacol. 2009;29:165. doi: 10.1111/j.1474-8665.2009.00444.x. [DOI] [PubMed] [Google Scholar]
- Kinsey C.G., Bussolati G., Bosco M., Kimura T., Pizzorno M.C., Chernin M.I., Cassoni P., Novak J.F. Constitutive and ligand-induced nuclear localization of oxytocin receptor. J. Cell. Mol. Med. 2007;11:96. doi: 10.1111/j.1582-4934.2007.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klayman L.M., Wedegaertner P.B. Inducible inhibition of Gβγ reveals localization-dependent functions at the plasma membrane and Golgi. J. Biol. Chem. 2017;292:1773. doi: 10.1074/jbc.M116.750430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski S.J., Hopf F.W., Seif T., Bonci A., von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Fahey P.G., Jong Y.J., Ramanan N., O'Malley K.L. Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J. Biol. Chem. 2012;287:5412. doi: 10.1074/jbc.M111.301366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Jong Y.J., O’Malley K.L. Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J. Biol. Chem. 2008;283:14072. doi: 10.1074/jbc.M708551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix D., Ouellette R., Vaillancourt C. Expression of melatoninergic receptors in human placental choriocarcinoma cell lines. Hum. Reprod. 2006;21:1981. doi: 10.1093/humrep/del120. [DOI] [PubMed] [Google Scholar]
- Lee D.K., Lança A.J., Cheng R., Nguyen T., Ji X.D., Gobeil F., Chemtob S., George S.R., O'Dowd B.F. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J. Biol. Chem. 2004;279:7901. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Lessard A., Savard M., Gobeil F., Pierce J.P., Pickel V.M. The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental area. Synapse. 2009;63:484. doi: 10.1002/syn.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P.K., Chow K.B., Lau P.N., Chu K.M., Chan C.B., Cheng C.H., Wise H. The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin receptor. Cell Signal. 2007;19:1011. doi: 10.1016/j.cellsig.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Li P., Banjade S., Cheng H.C., Kim S., Chen B., Guo L., Llaguno M., Hollingsworth J.V., King D.S., Banani S.F. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J.J., Huang M.C., Graler M., Huang Y., Qiu H., Goetzl E.J. Distinctive T cell-suppressive signals from nuclearized type 1 sphingosine 1-phosphate G protein-coupled receptors. J. Biol. Chem. 2007;282:1964. doi: 10.1074/jbc.M608597200. [DOI] [PubMed] [Google Scholar]
- Lin F.-T., Daaka Y., Lefkowitz R.J. β-Arrestins regulate mitogenic sigaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 1998;273:31640. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- Lind G.J., Cavanagh H.D. Nuclear muscarinic acetylcholine receptors in corneal cells from rabbit. Invest. Ophthalmol. Vis. Sci. 1993;34:2943. [PubMed] [Google Scholar]
- Liu Y., Li P., Fan L., Wu M. The nuclear transportation routes of membrane-bound transcription factors. Cell Commun. Signal. 2018;16:12. doi: 10.1186/s12964-018-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Yang H., Shaw G., Raizada M.K. Angiotensin ii-induced nuclear targeting of the angiotensin type 1 (at1) receptor in brain neurons. Endocrinology. 1998;139:365. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- Maraldi N.M., Mazzotti G., Capitani S., Rizzoli R., Zini N., Squarzoni S., Manzoli F.A. Morphological evidence of function-related localization of phospholipids in the cell nucleus. Adv. Enzyme Regul. 1992;32:73. doi: 10.1016/0065-2571(92)90009-o. [DOI] [PubMed] [Google Scholar]
- Marchese A., Paing M.M., Temple B.R., Trejo J. ‘G protein–coupled receptor sorting to endosomes and lysosomes’. Annu. Rev. Pharmacol. Toxicol. 2008;48:601. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache A.M., Gobeil F., Bernier S.G., Stankova J., Rola-Pleszczynski M., Choufani S., Bkaily G., Bourdeau A., Sirois M.G., Vazquez-Tello A. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J. Immunol. 2002;169:6474. doi: 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- Martelli A.M., Faenza I., Billi A.M., Falà F., Cocco L., Manzoli L. Nuclear protein kinase C isoforms: key players in multiple cell functions? Histol. Histopathol. 2003;18:1301. doi: 10.14670/HH-18.1301. [DOI] [PubMed] [Google Scholar]
- McDonald P.H., Chow C.W., Miller W.E., Laporte S.A., Field M.E., Lin F.T., Davis R.J., Lefkowitz R.J. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Merlen C., Farhat N., Luo X., Chatenet D., Tadevosyan A., Villeneuve L.R., Gillis M.A., Nattel S., Thorin E., Fournier A., Allen B.G. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca²⁺ in adult cardiac myocytes. J. Mol. Cell Cardiol. 2013;62:189. doi: 10.1016/j.yjmcc.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir F., Le Breton G.C. A novel nuclear signaling pathway for thromboxane A2 receptors in oligodendrocytes: evidence for signaling compartmentalization during differentiation. Mol. Cell Biol. 2008;28:6329. doi: 10.1128/MCB.00482-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinelli T.A., Raymond J.R., Baldys A., Yang Q., Lee M.H., Luttrell L., Ullian M.E. Identification of a putative nuclear localization sequence within ANG II AT1A receptor associated with nuclear activation. Am. J. Physiol. Cell Physiol. 2007;292:C1398. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- Nash C.A., Wei W., Irannejad R., Smrcka A.V. Golgi localized βi-adrenergic receptors stimulate golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy. Elife. 2019;8:e48167. doi: 10.7554/eLife.48167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.T., Létourneau M., Chatenet D., Fournier A. Presence of urotensin-II receptors at the cell nucleus: specific tissue distribution and hypoxia-induced modulation. Int. J. Biochem. Cell Biol. 2012;44:639. doi: 10.1016/j.biocel.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Nielsen C.K., Campbell J.I., Ohd J.F., Mörgelin M., Riesbeck K., Landberg G., Sjölander A. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–742. [PubMed] [Google Scholar]
- O’Malley K.L., Jong Y.J., Gonchar Y., Burkhalter A., Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J. Biol. Chem. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- Panicker L.M., Zhang J.H., Posokhova E., Gastinger M.J., Martemyanov K.A., Simonds W.F. Nuclear localization of the G protein beta 5/R7-regulator of G protein signaling protein complex is dependent on R7 binding protein. J. Neurochem. 2010;113:1101. doi: 10.1111/j.1471-4159.2010.06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Itakura T., Gonzalez J.M., Schwartz S.G., Fini M.E. GPR158, an orphan member of G protein-coupled receptor family C: glucocorticoid-stimulated expression and novel nuclear role. PLoS One. 2013;8:e57843. doi: 10.1371/journal.pone.0057843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass K.D., Gwathmey T.M., Michalek R.D., Grayson J.M., Chappell M.C. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem. Biophys. Res. Commun. 2009;384:149. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson Y.K., Luttrell L.M. ‘The diverse roles of arrestin scaffolds in g protein–coupled receptor signaling’. Pharmacol. Rev. 2017;69:256. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813:1619. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Purcell R.H., Hall R.A. ‘Adhesion G protein–coupled receptors as drug targets’. Annu. Rev. Pharmacol. Toxicol. 2018;58:429. doi: 10.1146/annurev-pharmtox-010617-052933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgert C.A., Izumi Y., Jong Y.J., Kumar V., Zorumski C.F., O'Malley K.L. Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J. Neurosci. 2014;34:4589. doi: 10.1523/JNEUROSCI.3451-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I., Rovira J.M., Martin F., Roche E., Nadal A., Soria B. Nuclear KATP channels trigger nuclear Ca2+ transients that modulate nuclear function. Proc. Natl. Acad. Sci. U S A. 2002;99:9544. doi: 10.1073/pnas.142039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Alberti S. Cell adaptation upon stress: the emerging role of membrane-less compartments. Curr. Opin. Cell Biol. 2017;47:34. doi: 10.1016/j.ceb.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S., Mir F., Gould R.M., Le Breton G.C. Characterization of thromboxane A2 receptor signaling in developing rat oligodendrocytes: nuclear receptor localization and stimulation of myelin basic protein expression. J. Neurosci. Res. 2006;84:1402. doi: 10.1002/jnr.21061. [DOI] [PubMed] [Google Scholar]
- Rasola A., Sciacovelli M., Chiara F., Pantic B., Brusilow W.S., Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc. Natl. Acad. Sci. U S A. 2010;107:726. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M., Pampillo M., Savard M., Dubuc C., McArdle C.A., Millar R.P., Conn P.M., Gobeil F., Bhattacharya M., Babwah A.V. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5:e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ritter S.L., Hall R.A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 2009;10:819. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A., Espinoza S., Masri B., Lam V., Barak L.S., Gainetdinov R.R. BRET biosensors to study GPCR biology, pharmacology, and signal transduction. Front. Endocrinol. (Lausanne) 2012;3:105. doi: 10.3389/fendo.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang B.L., Nguyan T.L., Choi J.W., Lee K.H., Cho S.W., Liu Z., Ye K., Ahn J.Y. Nuclear Akt interacts with B23/NPM and protects it from proteolytic cleavage, enhancing cell survival. Proc. Natl. Acad. Sci. U S A. 2008;105:16584–16589. doi: 10.1073/pnas.0807668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Blumer J.B., Simon V., Lanier S.M. Accessory proteins for G proteins: partners in signaling. Annu. Rev. Pharmacol. Toxicol. 2006;46:151. doi: 10.1146/annurev.pharmtox.46.120604.141115. [DOI] [PubMed] [Google Scholar]
- Sato M., Hiraoka M., Suzuki H., Bai Y., Kurotani R., Yokoyama U., Okumura S., Cismowski M.J., Lanier S.M., Ishikawa Y. Identification of Transcription Factor E3 (TFE3) as a receptor-independent activator of Gα16: gene regulation by nuclear Gα subunit and its activator. J. Biol. Chem. 2011;286:17766. doi: 10.1074/jbc.M111.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schievella A.R., Regier M.K., Smith W.L., Lin L.L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J. Biol. Chem. 1995;270:30749. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- Shenoy S.K., Lefkowitz R.J. Seven-transmembrane receptor signaling through beta-arrestin. Sci. STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- Steyaert J., Kobilka B.K. Nanobody stabilization of G protein-coupled receptor conformational states. Curr. Opin. Struct. Biol. 2011;21:567. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M., Jullié D., Lobingier B.T., Laeremans T., Steyaert J., Schiller P.W., Manglik A., von Zastrow M. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron. 2018;98:963. doi: 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Cheng Z., Ma L., Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 2002;277:49212. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Suofu Y., Li W., Jean-Alphonse F.G., Jia J., Khattar N.K., Li J., Baranov S.V., Leronni D., Mihalik A.C., He Y. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U S A. 2017;114:E7997. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A., Vaniotis G., Allen B.G., Hébert T.E., Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J. Physiol. (Lond) 2012;590:1313. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A., Xiao J., Surinkaew S., Naud P., Merlen C., Harada M., Qi X., Chatenet D., Fournier A., Allen B.G., Nattel S. Intracellular angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J. Am. Heart Assoc. 2017;6:e004965. doi: 10.1161/JAHA.116.004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M., Kanoh A., Amako K., Otani M., Sano K., Kanazawa-Hamada M., Matsuyama S. Nuclear localization of bradykinin B2 receptors reflects binding to the nuclear envelope protein lamin C. Eur. J. Pharmacol. 2014;723:507. doi: 10.1016/j.ejphar.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Toy-Miou-Leong M., Bachelet C.M., Pélaprat D., Rostène W., Forgez P. NT agonist regulates expression of nuclear high-affinity neurotensin receptors. J. Histochem. Cytochem. 2004;52:335. doi: 10.1177/002215540405200304. [DOI] [PubMed] [Google Scholar]
- Tsvetanova N.G., Irannejad R., Von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J. Biol. Chem. 2015;290:6689. doi: 10.1074/jbc.R114.617951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova N.G., von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol. 2014;10:1061. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski A.G., Vaqué J.P., Gutkind J.S. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Ungricht R., Klann M., Horvath P., Kutay U. Diffusion and retention are major determinants of protein targeting to the inner nuclear membrane. J. Cell Biol. 2015;209:687. doi: 10.1083/jcb.201409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdehita A., Bajo A.M., Fernández-Martínez A.B., Arenas M.I., Vacas E., Valenzuela P., Ruíz-Villaespesa A., Prieto J.C., Carmena M.J. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides. 2010;31:2035. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Valenzuela R., Costa-Besada M.A., Iglesias-Gonzalez J., Perez-Costas E., Villar-Cheda B., Garrido-Gil P., Melendez-Ferro M., Soto-Otero R., Lanciego J.L., Henrion D. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016;7:e2427. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaniotis G., Del Duca D., Trieu P., Rohlicek C.V., Hébert T.E., Allen B.G. Nuclear β-adrenergic receptors modulate gene expression in adult rat heart. Cell Signal. 2011;23:89. doi: 10.1016/j.cellsig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaniotis G., Glazkova I., Merlen C., Smith C., Villeneuve L.R., Chatenet D., Therien M., Fournier A., Tadevosyan A., Trieu P. Regulation of cardiac nitric oxide signaling by nuclear β-adrenergic and endothelin receptors. J. Mol. Cell Cardiol. 2013;62:58. doi: 10.1016/j.yjmcc.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K., Cornea V.M., Jong Y.J., Laferrière A., Kumar N., Mickeviciute A., Fung J.S., Bandegi P., Ribeiro-da-Silva A., O'Malley K.L., Coderre T.J. Intracellular mGluR5 plays a critical role in neuropathic pain. Nat. Commun. 2016;7:10604. doi: 10.1038/ncomms10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainstein E., Seger R. The dynamic subcellular localization of ERK: mechanisms of translocation and role in various organelles. Curr. Opin. Cell Biol. 2016;39:15. doi: 10.1016/j.ceb.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Wang N., Wu Q.L., Fang Y., Mai H.Q., Zeng M.S., Shen G.P., Hou J.H., Zeng Y.X. Expression of chemokine receptor CXCR4 in nasopharyngeal carcinoma: pattern of expression and correlation with clinical outcome. J. Transl. Med. 2005;3:26. doi: 10.1186/1479-5876-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wu Y., Ge X., Ma L., Pei G. Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J. Biol. Chem. 2003;278:11648. doi: 10.1074/jbc.M208109200. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang H., Xu H., Guo D., Shi H., Li Y., Zhang W., Gu Y. 5-HTR3 and 5-HTR4 located on the mitochondrial membrane and functionally regulated mitochondrial functions. Sci. Rep. 2016;6:37336. doi: 10.1038/srep37336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C.M., Saatian B., Moughal N.A., Zhao Y., Tigyi G., Natarajan V., Pyne S., Pyne N.J. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem. J. 2006;398:55. doi: 10.1042/BJ20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P.H., Fraher L.J., Hendy G.N., Chung U.I., Kisiel M., Natale B.V., Hodsman A.B. Nuclear localization of the type 1 PTH/PTHrP receptor in rat tissues. J. Bone Miner. Res. 2000;15:1033. doi: 10.1359/jbmr.2000.15.6.1033. [DOI] [PubMed] [Google Scholar]
- Wright C.D., Chen Q., Baye N.L., Huang Y., Healy C.L., Kasinathan S., O'Connell T.D. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ. Res. 2008;103:992. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C., Dahl E.F., Wright C.D., Cypher A.L., Healy C.L., O'Connell T.D. Nuclear localization of a1A-adrenergic receptors is required for signaling in cardiac myocytes: an “inside-out” a1-AR signaling pathway. J. Am. Heart Assoc. 2014;3:e000145. doi: 10.1161/JAHA.113.000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Kawamura K., James T.N. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc. Res. Tech. 1998;40:479. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ybe J.A., Fontaine S.N., Stone T., Nix J., Lin X., Mishra S. Nuclear localization of clathrin involves a labile helix outside the trimerization domain. FEBS Lett. 2013;587:142. doi: 10.1016/j.febslet.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.H., Barr V.A., Mo Y., Rojkova A.M., Liu S., Simonds W.F. Nuclear localization of G protein beta 5 and regulator of G protein signaling 7 in neurons and brain. J. Biol. Chem. 2001;276:10284. doi: 10.1074/jbc.M009247200. [DOI] [PubMed] [Google Scholar]
- Zimber A., Nguyen Q.D., Gespach C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell Signal. 2004;16:1085. doi: 10.1016/j.cellsig.2004.03.020. [DOI] [PubMed] [Google Scholar]