Abstract
The brain is organized into networks that reorganize dynamically in response to cognitive demands and exogenous stimuli. In recent years, repetitive transcranial magnetic stimulation (rTMS) has gained increasing use as a noninvasive means to modulate cortical physiology, with effects both proximal to the stimulation site and in distal areas that are intrinsically connected to the proximal target. In light of these network-level neuromodulatory effects, there has been a rapid growth in studies attempting to leverage information about network connectivity to improve neuromodulatory control and intervention outcomes. However, the mechanisms-of-action of rTMS on network-level effects remain poorly understood and is based primarily on heuristics from proximal stimulation findings. To help bridge this gap, the current paper presents a systematic review of 33 rTMS studies with baseline and post-rTMS measures of fMRI resting-state functional connectivity (RSFC). Literature synthesis revealed variability across studies in stimulation parameters, studied populations, and connectivity analysis methodology. Despite this variability, it is observed that active rTMS induces significant changes on RSFC, but the prevalent low-frequency-inhibition/high-frequency-facilitation heuristic endorsed for proximal rTMS effects does not fully describe distal connectivity findings. This review also points towards other important considerations, including that the majority of rTMS-induced changes were found outside the stimulated functional network, suggesting that rTMS effects tend to spread across networks. Future studies may therefore wish to adopt conventions and systematic frameworks, such as the Yeo functional connectivity parcellation atlas adopted here, to better characterize network-level effect that contribute to the efficacy of these rapidly developing noninvasive interventions.
Keywords: Repetitive transcranial magnetic stimulation, Resting-state functional connectivity, Distal effects, Network neuroscience
1. Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a well-established noninvasive technique for neuromodulation that uses a stimulating coil to deliver electromagnetic pulses to induce electric currents in the brain, resulting in modulation of neural tissue. As this field has grown, researchers have come to better understand how variability in the stimulation intensity, frequency, duration, and target location impacts modulatory effects. For example, stimulation frequencies between 5 and 20 Hz have generally produced increases in cortical responses as measured by brain activity (Pascual-Leone et al., 1998; Speer et al., 2000) or motor evoked potentials (Peinemann et al., 2004; Speer et al., 2000), and are often tied to behavioral facilitation (Guse et al., 2010, for a review). Conversely, stimulation at frequencies below 5 Hz tends to produce decreased cerebral blood flow (Pascual-Leone et al., 1998; Speer et al., 2000) and inhibition of neural responses and behavior (Chen et al., 1997; Gerschlager et al., 2001). This, in turn, has created a strong frequency-dependent heuristic that dominates the field (Fitzgerald et al., 2006; Luber and Deng, 2016), even though some studies report findings that do not adhere to this rule-of-thumb (e.g., Eisenegger et al., 2008).
Over the past several years, there has also been a strong movement toward network neuroscience wherein the brain is seen as a connectome of interacting regions that synchronize activity to achieve cognition (Bassett and Sporns, 2017). In particular, extensive neuroimaging research has led to characterization of the brain as a set of large-scale, intrinsically organized networks that interact dynamically to control behavior (Power et al., 2011; Raichle, 2011). For example, when performing a task, the default mode network (DMN), composed of the medial prefrontal cortex, hippocampus, and posterior parietal cortex, tends to become deactivated, while the central executive network (CEN) of lateral frontal and parietal regions becomes activated. In addition, using task-free intrinsic connectivity analyses, Seeley et al. (2007) demonstrated the existence of two dissociable networks, independent from the DMN: the salience network and the executive control networks, which correlated with emotional and cognitive functions, thereby providing further evidence that multiple brain networks can be dissociated that relate to aspects of human behavior. Such findings have led to the dominant view that communication both between and within such brain networks allows for the dynamic control of behavior. This shift in characterization from individual brain regions to integrated networks implies that focal neuromodulation by techniques, such as rTMS, may also affect distal brain areas through intra-network connections, as well as interactions between networks.
Consistent with this network perspective, proximal rTMS-induced changes in neural activity have been associated with changes in anatomically or functionally interconnected distal cerebral regions (D. E. Bohning et al., 1998; Hampson and Hoffman, 2010; Navarro de Lara et al., 2015). For example, by measuring the effects of rTMS applied over the left sensorimotor cortex (M1/S1) on blood-oxygen-level-dependent (BOLD) signals, Bestmann et al. (2003) demonstrated that supra-threshold rTMS increased BOLD signal in the stimulated area and supplementary motor area (SMA), while decreasing signals in the contralateral M1/S1. While these remote effects could be explained by afferent feedback due to the peripheral muscular response evoked by the stimulation, the authors demonstrated that this explanation was insufficient by testing the effect of sub-threshold rTMS. Indeed, while this intensity did not induce any muscular response, changes in BOLD were still observed. Interestingly, these changes were not observed in the stimulated area, but in SMA, bilateral premotor cortex, and the contralateral M1/S1, probably due to a propagation of the electric signal via anatomically connected fibers or through functional connectivity between these sites.
Since such pioneering studies, there has been a steady growth in the number of studies testing “connectivity-based rTMS,” which propose to indirectly target distal brain areas through their connections with accessible, proximal cortical areas. For example, by stimulating parietal regions with strong baseline resting-state functional connectivity (RSFC) to a hippocampal target, Wang et al. (2014) showed that rTMS was able to modify the connectivity between these structures. Similar promising results were found when rTMS was applied over the premotor cortex to modulate the insula (Addicott et al., 2019). Interestingly, this study found that both 1 Hz and 10 Hz rTMS induced increased functional connectivity, despite the fact that these two frequencies of rTMS have previously been associated with opposing effects on the activity of the proximal stimulated region (inhibitory for 1 Hz versus excitatory for 10 Hz). This intriguing result has also been found in other studies investigating the downstream effects of rTMS. For example, by applying 1 Hz and 20 Hz rTMS over the left posterior inferior parietal lobule, a structure belonging to the default mode network, Eldaief et al. (2011) found that 1 Hz rTMS (conventionally inhibitory) increased RSFC between the stimulated node and the hippocampal formation, while 20 Hz rTMS (conventionally excitatory) was associated with decreased connectivity between these nodes. These results suggest that the effect of rTMS on the activity of the stimulated region may not directly correspond to the direction of downstream, distal effects on connectivity. As such, the goals of this pre-registered, systematic review (PROSPERO #CRD42019119982) are to systematically investigate the effect of rTMS frequency on changes in RSFC and to characterize the parameter space used in these studies.
The importance of understanding these relationships is underscored by the emerging view that rTMS efficacy to treat major depressive disorder is strongly predicted by connectivity between the stimulated site —the dorsolateral prefrontal cortex (DLPFC)— and the subgenual anterior cingulate cortex (Fox et al., 2012a,b; Weigand et al., 2018). These studies confirm the importance of studying the effects of rTMS on functional connectivity to optimize targeting approaches, and consequently, the efficacy of rTMS-based interventions. Given the large variability that exists between studies regarding the stimulated site, the type of analysis performed to investigate rTMS-induced functional connectivity changes, and the brain regions showing significant changes, the results in this review are aggregated as “within-” or “out-of-network” changes according to the resting-state functional network of the stimulated site. This approach allows for further characterization of distal effects in relation to the proximal stimulation target based on a widely used parcellation of seven resting-state functional networks (Yeo et al., 2011). The goals of this review are therefore to identify systematic commonalities among studies that are testing whole-brain rTMS effects, and provide prospective recommendations on practices that can best advance understanding and improve the application of this important technique.
2. Methods
The present review focuses on studies addressing the effects of rTMS on RSFC. The article search protocol was registered on the international prospective register for systematic review (#42019119982), accessible online at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019119982.
A computer-based search of PubMed and Science Direct was carried out by one author (LB) in December 2018, using the following keywords in the title and abstract fields: “Repetitive transcranial magnetic stimulation” or “rTMS” and “resting-state connectivity” or “resting-state.” A total of 302 articles were collected after the database searches. After removing the duplicates, 113 articles underwent thorough title, abstract and full-text screening for inclusion in the review according to the inclusion and exclusion criteria listed in Table 1. The final review sample consisted of 33 articles.1
Table 1.
Inclusion and exclusion criteria for the article screening.
| Study characteristics | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Healthy volunteers; Patients (all diseases) | None |
| Intervention | rTMS applied over any region of the brain | Single pulse TMS; Paired-pulse TMS |
| Resting-State Acquisition | Acquired both before and after rTMS | Only acquisition before or after rTMS |
| Study design | Between, within, cross-over | Study case |
For each of the 33 included articles, information was extracted on the study design and population characteristics, including the number of subjects and whether they were patients or healthy participants. Regarding the stimulation parameters, the following information was extracted and documented: targeted brain regions, targeting method, type of control comparison (if any), stimulation intensity and frequency, burst and ITI duration, number of TMS pulses per session, number of sessions. Regarding resting-state acquisition, the number of volumes, the subjects’ instructions and the timing of the acquisition related to rTMS were extracted; the type of analysis (seed-based or data driven) was extracted as well as the corresponding outcomes. Two investigators (LB, GA) performed data extraction separately and results were compared. Across the extracted data, 855 cells were in agreement with the remaining 3 cells resolved through discussion. As such, this demonstrates high inter-rater reliability between the two investigators.
To assess whether and how rTMS modulated resting-state functional connectivity, the changes observed before and after the active stimulation condition were extracted by two investigators (LB and JP) who performed this work together. When a control condition was used, RSFC changes associated with sham were also extracted, as well as the comparison between active and sham stimulation, when reported. The direction (increase or decrease) of the connectivity changes was extracted as well as the coordinates of all brain regions where significant changes were observed. Results that were labeled as ‘exploratory’ were not included in this review. To reduce the large between-study variability and to simplify the presentation of the results, results were labeled according to resting-state functional brain network. To do so, the 7-network parcellation defined by Yeo et al. (2011) was used (networks included: dorsal attention (DA), default mode (DM), fronto limbic (FL), limbic (L), somato motor (SM), ventral attention (VA) and visual (V)). Each set of result coordinates was fed into a custom MATLAB script that converted them into the corresponding brain network. When the coordinates were not found to belong to any of these 7 networks, often because they were subcortical structures, they were labeled as “other.” The same process was then performed for all rTMS targets, therefore defining the “stimulated network.” rTMS-induced effects reported in the following sections are presented as: “in-network changes” when the changes were observed in brain regions belonging to the same network as the stimulated region; or “out-of-network changes” when the changes were found in any of the remaining networks.
3. Results
3.1. Descriptive statistics
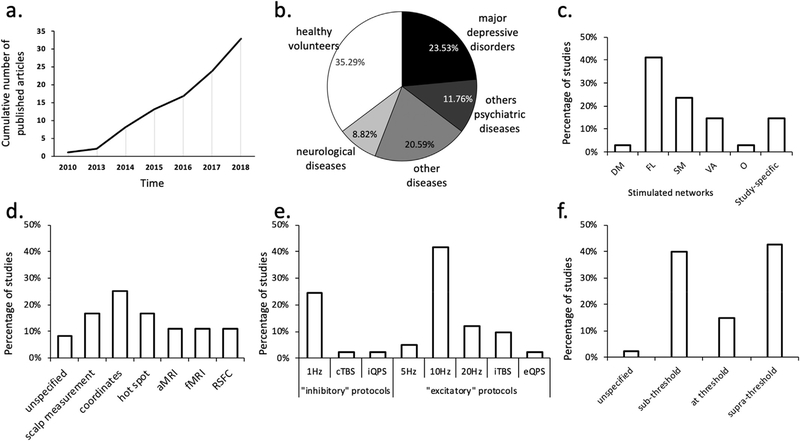
As shown in Fig. 1, across the 33 studies there was considerable variability in the subject populations, TMS targets and stimulation parameters (See Table S1 for detailed description). Within these studies, 35.3% investigated healthy volunteers, while the remaining 65% were distributed over a variety of psychiatric and neurological disorders. The most common of these was major depressive disorder (23.5%), followed by neurological diseases (20.6%) which included writer’s cramp dystonia, Parkinson’s disease, multiple system atrophy, essential tremor and stroke. Other psychiatric disorders (11.8%) including schizophrenia, eating disorders, obsessive compulsive disorders, alcohol abuse disorders comprised the third most common patient group. The remaining category (8.8%) includes other pathologies such as disorder of consciousness, mal de debarquement syndrome, and tinnitus. Across all studies, the average sample size was 25.2 ± 12.7 recruited subjects.
Fig. 1.
Study Characteristics of Reviewed Literature. a) Cumulative number of published articles across years. b) Proportion of patients and healthy volunteers. c) Distribution of stimulated networks with DM: default mode, FL: fronto limbic SM: somato motor, VA: ventral attention, O: others, and study-specific networks. d) Method of determining TMS targeting with aMRI: anatomical MRI, and fMRI functional MRI. e) rTMS frequencies for conventional single frequency and patterned protocols. f) Stimulation intensities relative to motor threshold (resting motor threshold: 90%; or active motor threshold: 7.5% of the included studies).
Across the studies, rTMS was applied most frequently over the frontal cortex (58%), while the motor and premotor areas were also frequently chosen as the stimulation target (28%). The remaining 14% of studies targeted other cortical lobes or the cerebellum. The corresponding stimulated network are presented in Fig. 1c. Moreover, a wide variety of TMS targeting methods were used. These included positioning according to neuroanatomical coordinates (25%), scalp measurement (16.67%), hot spot targeting (as defined by the optimal location on the scalp that evokes a maximum activation of the contralateral targeted muscle, 16.67%), anatomical MRI (11.11%), functional MRI (11.11%), and resting-state connectivity (11.11%). As expected, given the FDA clearance for this frequency of stimulation, 10 Hz rTMS was the most commonly used stimulation frequency (41.4%), followed by 1 Hz (24.4%), 20 Hz (12.2%), and then 5 Hz (4.9%). Relatively few studies investigated the effects of patterned protocols like intermittent and continuous theta burst stimulation (iTBS, cTBS: 9.8% and 2.4%, respectively) and inhibitory and excitatory quadripulse stimulation (iQPS, eQPS: 2.4% for both). Stimulation was applied either above threshold (42.5%), below threshold (40%), or at threshold (15%), with the remaining 2.5% of studies not reporting stimulation intensity. While 42% of the studies investigated the acute effect of a single session of rTMS, the majority of the studies investigated the effect of multiple sessions of rTMS, generally by comparing RSFC before and after the full course of rTMS.
In a similar manner, there was also considerable variability in the resting-state MRI acquisition parameters and the analyses performed to infer connectivity effects. For example, the number of volumes acquired within the resting-state scans varied between 100 and 1248 (mean = 353, standard deviation = 289). During these scans, subjects were asked to keep their eyes open in 36% of the studies and closed in 41% of the studies, while the remaining 23% did not provide this information. To analyze the acquired data, 86% of studies used seed-based analysis techniques, while the remaining 14% used data-driven approaches (See Supplementary Table S2 for detailed descriptions).
3.2. Quantitative analysis
The primary aim of this review is to assess whether rTMS is capable of modulating RSFC. Active and/or sham stimulation may be reported in these studies, and in some cases the difference between active and sham stimulation is reported. All of these instances are considered here. A central question in this evaluation is to determine whether rTMS-induced changes adhere to the same frequency-dependent inhibitory and facilitatory patterns commonly reported in studies testing behavioral outcomes and neuronal responses in the brain region proximal to the stimulating coil. In a second step, this review will examine whether rTMS changes affect only the stimulated network or spread out to other brain networks.
Across the 22 studies reporting the effects of active rTMS, 16 (72%) were associated with significant changes in connectivity. Among the 20 studies using a control condition, only 12 reported the subsequent effects on connectivity, and only three of those (25%) were associated with significant connectivity changes. For these three studies the control condition used a sham coil, flipped the coil or lowered the stimulation intensity. Among the eight studies that did not report significant connectivity changes, five of them flipped the coil, while the others used a sham coil, a control site, a sham coil over a control site or flipped the coil over the control site. While flipping the coil appears not to induce significant connectivity changes, variability in the approaches prevents drawing conclusions regarding the optimal sham condition.
A chi-square test showed a significant difference between the proportion of reported significant effects for active and sham rTMS (χ2 = 7.17, p = 0.007). These results provide preliminary confirmation that active rTMS modified resting-state connectivity, above and beyond sham rTMS. This result is confirmed by the 12 studies reporting the direct comparison between the effects of active and sham rTMS. Indeed, eight of the twelve (66%) reported stronger changes with active rTMS. To further understand the frequency-dependency of these effects, the following sections organize the findings according to conventional inhibitory protocols (1Hz, cTBS, iQPS) and excitatory protocols (5Hz, 10Hz, 20Hz, iTBS and eQPS).
3.2.1. Effects of stimulation frequency
3.2.1.1. Effects of conventional “inhibitory” protocols (1Hz, cTBS, iQPS)
As shown in Table 2, across the 10 studies that reported results from conventional inhibitory protocols, 7 were associated with increases in connectivity due to active stimulation (see “direction” column), one was associated with decreased connectivity, and one reported change in both directions. The remaining study failed to reveal any changes after active rTMS. This suggests that contrary to proximal effects, these conventionally inhibitory protocols mostly increased neural function as measured by RSFC. In addition, two studies reported the effects of a sham control condition; one of which found no changes in connectivity, while the other found decreased connectivity. Two further studies reported active versus sham, with one finding no difference and the other finding higher RSFC to active stimulation.
Table 2.
Connectivity changes associated with inhibitory protocols (1Hz, cTBS, iQPS) for active rTMS, sham rTMS, and the difference between active and sham rTMS. Empty cells indicate there was no control condition or that the results were not reported. The direction columns indicates whether the study reported increases (“+”), decreases (“−”) or no change (“Ø”) in resting-state connectivity. Location indicates whether the RSFC changes were observed within the stimulated network (In), or out of the stimulated network (Out), as defined by the stimulation target. The following abbreviations were used: DA: dorsal attention, DM: default mode, FL: fronto limbic, L: limbic, O: other, SM: somato motor, V: visual, VA: ventral attention. Where available the affected network is listed.
| Studies: | Stimulated Target | Stimulated Network | Targeting Approach | Active |
Control condition |
Active vs. Sham |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| direction | location | Type | direction | location | direction | location | ||||
| 1Hz rTMS | ||||||||||
| Bharath et al. (2015) | L-M1 | SM | Hot Spot | + | In | None | ||||
| Roland et al. (2016) | L-TPJ | SM | Scalp measurement and MRI | Ø | Sham coil on Control site | Ø | Ø | |||
| Vercammen et al. (2010) | L-TPJ | VA | Scalp measurement | + | In | Sham coil | − | In | ||
| Brabenec et al., 2019 | R-STG | VA | MNI coordinates | + | Out (FL) | Control Site | Higher with active | Out (FL) | ||
| Ji et al. (2017) | L-SMA | VA | MNI coordinates | + | Out (SM) | None | ||||
| Zhang et al. (2018) | L-DLPFC | Central Executive | Connectivity-based | +/− | Out (Anterior DM) | None | ||||
| Popa et al. (2013) | B-cerebellum (lobule VIII) | Cortico-Thalamo Cortical | Unspecified | + | In | None | ||||
| Bharath et al. (2017) | L-premotor | Unknown | Unspecified | + | Out (DA, DM, FL, O, VA) | None | ||||
| cTBS | ||||||||||
| Ji et al. (2017) | L-SMA | VA | MNI coordinates | − | Out (DM, SM) | None | ||||
| iQPS | ||||||||||
| Watanabe et al. (2014) | L-M1 | SM | Hot Spot | + | In and Out (O, L, VA) | None | ||||
3.2.1.2. Effects of conventional “excitatory” protocols
Twenty-five studies using conventionally excitatory protocols were identified (Table 3), 17 of which reported results for active rTMS. Among these 17 studies, nine showed increased connectivity (53%), 3 were associated with decreased functional connectivity (18%) and the five other studies (29%) did not report any changes after active stimulation. Although more variable, this result shows that excitatory protocol mainly increased RSFC. Ten studies reported the effects of sham stimulation, and while eight of them did not observe significant changes, the remaining two reported a decreased connectivity. Two other studies (Yuan et al., 2017; Siddiqi et al., 2018) combined 1 and 10 Hz stimulation within the same protocol but did not report any results for active or sham stimulation, therefore they are not presented in these tables.
Table 3.
Connectivity changes associated with excitatory protocols (5Hz, 10Hz, 20Hz, iTBS, and eQPS) for active rTMS, sham rTMS, and the interaction between active and sham rTMS. Empty cells indicate there was no control condition or that the results were not reported. The direction columns indicates whether the study reported increases (“+”), decreases (“−”) or no change (“Ø”) in resting-state connectivity. Location indicates where the changes in connectivity were observed: in or out of network, as defined by the stimulation target.
| Studies: | Stimulated Target | Stimulated Network: | Targeting Approach | Active |
Control condition |
Active vs. Control |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| direction | location | Type | direction | location | direction | location | ||||
| 5Hz | ||||||||||
| Chou et al. (2015) | L-M1 | SM | Hot Spot | + | In and Out (all) | Flipped coil | − | In and Out (all) | Higher with active | Out (DM) |
| (Philip et al. 2018a, b) | L-DLPFC | FL | Scalp measurement | None | ||||||
| 10Hz | ||||||||||
| Dunlop et al. (2015) | B-DMPFC | FL | Talairach Coordinates | None | ||||||
| Dunlop et al. (2016) | B-DMPFC | FL | Talairach Coordinates | None | ||||||
| Jansen et al. (2015) | L-DLPFC | FL | i-fMRI | Spacer | Ø | |||||
| Kang et al. (2016) | L-DLPFC | FL | Scalp measurement | Sham coil | Greater reduction with active | Out (O) | ||||
| Li et al. (2017) | L-DLPFC | FL | Scalp measurement | − | In | Sham coil | Ø | Greater decrease with active | Out (O) | |
| Salomons et al. (2014) | B-DMPFC | FL | Talairach Coordinates | None | ||||||
| Taylor et al. (2018) | L-DLPFC | FL | i-fMRI | None | Ø | |||||
| Tik et al. (2017) | L-DLPFC | FL | MNI coordinates | + | Out (VA, DM, O) | Control Site | Ø | |||
| Lee et al. (2018) | Ipsilesional M1 | SM | Hot Spot | Ø | None | |||||
| Liston et al. (2014) | L-DLPFC | VA | Scalp measurement | + | In and Out (DM) | None | ||||
| Brabenec et al. (2019) | R-pSTG | VA | Scalp measurement | + | Out (O) | Control site | Higher with active | Out (O, FL) | ||
| Schluter et al. (2018) | L-DLPFC | Left FPN | i-fMRI | − | Out (Salience Network) | Flipped coil | Ø | |||
| R-DLPFC | Right FPN | i-fMRI | + | Out (Salience Network) | Flipped coil | Ø | ||||
| Zhang et al. (2018) | L-DLPFC | Central Executive | Connectivity-based | + | In and Out (Anterior and Posterior DMN, Salience Network) | None | ||||
| 20Hz | ||||||||||
| Wang and Voss (2015) | L-lPC | DM | Connectivity-based | + | Out (V) | Low Intensity | − | In | Higher with active | Out |
| Baeken et al. (2014) | L-DLPFC | FL | MRI | Flipped coil | ||||||
| Song et al. (2019) | L-DLPFC | FL | MNI coordinates | Ø | Flipped coil | Ø | ||||
| Xue et al. (2017) | L-DLPFC | FL | MNI coordinates | + | Out (DM, L) | Flipped coil | Ø | Higher with active | Out (O) | |
| Liu et al. (2018) | L-M1 | SM | Unspecified | Ø iTBS | Flipped coil | Ø | ||||
| Baeken et al. (2017) | L-DLPFC | FL | MRI | Sham coil | ||||||
| Vidal-Pineiro et al. (2014) | L-IFG | FL | MNI coordinates | Ø | Sham coil | |||||
| Nettekoven et al. (2014) | L-M1 | SM | Hot Spot | + | In and Out (VA) | Flipped over control site | Higher with active | In & Out (SM,VA) | ||
| Volz et al. (2016) | Ipsilesional M1 | SM | Hot Spot | Ø | Flipped over control site | Ø | Ø | |||
| eQPS | ||||||||||
| Watanabe et al. (2014) | L-M1 | SM | Hot Spot | − | Out (DM, FL, L, V) | None | ||||
3.2.2. Network changes
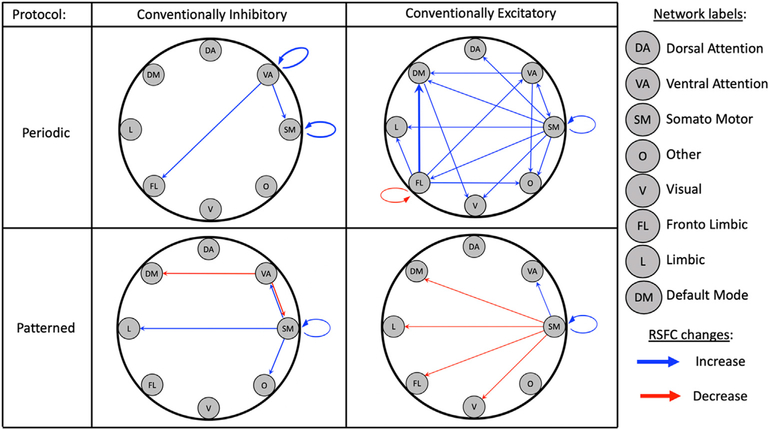
To better understand the nature of rTMS effects across distributed brain networks, it is useful to consider the location of rTMS-induced changes relative to the stimulated network across these studies (see the “location” columns of Tables 2 and 3, and Supplementary Table S3 for the correspondence between the rTMS targets, their coordinates and the corresponding networks). Across the 21 studies that reported significant changes in RSFC associated with active rTMS, effects were most often found outside of the stimulated network (N = 17, 81%), while nine studies (43%) found effects within the stimulated network. Effects were reported both within and outside of the stimulated network in five of these studies (24%). As illustrated visually on Fig. 2, modulatory effects were observed across nearly all pairs of nodes, with a relatively greater proportion of studies targeting the somato-motor network leading to change across all other networks. While, as mentioned earlier the fronto limbic is the most targeted network, the majority of these studies (nine out of 14) did not provide information about the effects of rTMS on RSFC independently of the clinical response, therefore they are not represented in the figure. By categorizing the results according to periodic (1, 5, 10 and 20 Hz) and patterned (cTBS, iQPS, iTBS and eQPS) stimulation protocols, this figure suggests that while conventionally excitatory periodic protocols (5, 10, 20Hz) tend to produce increases in RSFC changes, patterned protocols more frequently lead to decreases in RSFC.
Fig. 2.
rTMS-induced changes in RSFC by resting-state functional networks for each type of stimulation protocol separated into conventional inhibitory/excitatory and periodic versus patterned pulse sequences (Periodic conventionally inhibitory: 1Hz, Periodic conventionally excitatory: 5,10, and 20Hz; Patterned conventionally inhibitory: cTBS and iQPS; Patterned conventionally excitatory: iTBS and eQPS). The starting point of each arrow indicates the stimulated network, and the head of each arrow represents a network where corresponding rTMS-induced changes were observed. The thickness of the arrow indicates the number of studies finding the same result (e.g., FL-DM is thicker as 4 studies were associated with changes between these networks). Blue arrows indicate increased RSFC after rTMS, and red arrows indicate decreased RSFC.
3.3. Sources of variability in the reviewed literature
The aims of this review are to understand the influence of rTMS on RSFC, and as such, a diverse set of articles were included that each included preand post-TMS measures of connectivity. These articles consisted of studies with differing aims, populations, and study parameters. As such, the following section provides an overview of the variability in this literature, with an eye on how this variability may influence RSFC effects.
3.3.1. Study population
As illustrated in Fig. 1, about a third of the reviewed studies applied rTMS in healthy subjects while the remaining studies applied rTMS for clinical purposes. Because the of this variability, it is useful to consider further how connectivity effects may have depended on the population under consideration. Across the 22 studies where rTMS was applied in clinical populations, nine (41%) also reported differences with healthy control subjects (See Supplementary Table S4). All of these studies reported significant differences between the clinical and healthy control populations. Interestingly the four studies investigating psychiatric disorders found increased RSFC in patients, while the four studies investigating neurological disorders found that patients displayed reduced RSFC compared to controls. The remaining study revealed reduced RSFC in patients with consciousness disorders. These differences indicate that rTMS effects on RSFC might depend on the nature of the clinical populations under consideration. Indeed, as highlighted by Huang et al. (2017), the effects of non-invasive brain stimulation are “fragile” and highly variable, they depend on neuroplasticity which can be highly affected by clinical disorders. Furthermore, as demonstrated by Silvanto and Pascual-Leone (2008) the state of the brain at the time of stimulation is also a crucial factor that can dramatically change the effects of rTMS. It is therefore possible that the pattern of findings observed in this review are influenced by the populations under consideration in the accumulated studies. Despite this, the one study reviewed here that did directly contrast RSFC changes after applying rTMS in both patients and controls did not find any difference between the two groups (Jansen et al., 2015).
3.3.2. Clinical improvement
Another factor that could influence the aggregated results in this review concerns the response to treatment in those studies that tested rTMS in clinical populations. This is particularly true because both rTMS and clinical response are associated with biochemical and plasticity changes that may share overlapping mechanisms. To further understand these relationships, it is useful to consider whether rTMS-induced RSFC changes were correlated with clinical improvement. As detailed in Supplementary Table S4 it appears that responders and non-responders (as defined by changes in clinical measures) showed different pattern of connectivity changes after rTMS. In particular, some studies observed opposite patterns for responders and non-responders (e.g., Dunlop et al., 2016), or a change in RSFC for only the responders and not for the non-responders (e.g., Taylor et al., 2018). Furthermore, a number of studies also found correlation between RSFC changes and symptoms improvement pointing to important clinical links that will be essential to study further as these therapies continue to develop.
3.3.3. Treatment course
A final domain of these studies that deserves further consideration is the treatment course, and specifically how the sequence of sessions and visits may have affected rTMS effects on RSFC. Indeed, as recently suggested by Schluter et al., 2018), the distribution of rTMS sessions might be a crucial factor for rTMS efficacy. Table 4A summarizes the reviewed papers according to the number of visits and sessions per visit. As can be seen in the Table, there are 13 studies reporting results from acute rTMS in which one rTMS session was given on a single visit (Table 4B). Once-daily rTMS, consisting of one rTMS session conducted on each of multiple visits was reported in 16 studies (Table 4C), while seven studies
Table 4A.
Summary of rTMS treatment sessions and visits across the review sample.
| Acute rTMS | Once-daily rTMS with multiple visits | Multiple sessions per day Over multiple visits | |
|---|---|---|---|
| Number of Visits | 1 | 2–42 | 1–20 |
| Number of Sessions per day | 1 | 1 | More than once a day |
| Number of studies reporting the effect of active rTMS | 13 | 10 | 4 |
| Number of studies with significant changes in RSFC | 12 (92%) | 6 (60%) | 3 (75%) |
Table 4B.
Effect of acute rTMS on RSFC (1 visit 1 session).
| Study Name | rTMS | Decrease | No effect | Increase |
|---|---|---|---|---|
| Bharath et al. (2015) | 1Hz | X | ||
| Bharath et al. (2017) | 1Hz | X | ||
| Brabenec et al. (2019) | 1Hz | X | ||
| Ji et al. (2017) | 1Hz | X | ||
| Zhang et al. (2018) | 1Hz | X | X | |
| Li et al. (2017) | 10Hz | X | ||
| Schluter et al. (2018) | 10Hz | X | ||
| Schluter et al. (2018) | 10Hz | X | ||
| Brabenec et al. (2019) | 10Hz | X | ||
| Zhang et al. (2018) | 10Hz | X | ||
| Song et al. (2019) | 20Hz | X | ||
| Watanabe et al. (2014) | eQPS | X | ||
| Watanabe et al. (2014) | iQPS | X |
→ 12 out of 13 studies (92%) reporting the effect of acute rTMS reported significant changes.
Table 4C.
Effects of once-daily rTMS with multiple visits on RSFC.
| Study Name | # visits | rTMS | Decrease | No effect | Increase |
|---|---|---|---|---|---|
| Roland et al. (2016) | 10 or 20 | 1Hz | X | ||
| Vercammen et al. (2010) | 12 | 1Hz | X | ||
| Chou et al. (2015) | 10 | 5Hz | X | ||
| Tik et al. (2017) | 2 | 10Hz | X | ||
| Lee et al. (2018) | 10 | 10Hz | X | ||
| Liston (2014) | 25 | 10Hz | X | ||
| Xue et al. (2017) | 2 | 20Hz | X | ||
| Liu et al. (2018) | 5 | 20Hz | X | ||
| Wang and Voss (2015) | 5 | 20Hz | X | ||
| Volz et al. (2016) | 5 | iTBS | X |
→ 6 out of 10 (60%) studies reporting the effect of once-daily rTMS reported significant changes.
4. Discussion
This review, performed across a sample of 33 studies, demonstrates that rTMS reliably induces changes in resting-state functional connectivity, but that the direction of these changes is not totally consistent with the common frequency-dependent heuristic observed for proximal effects. In particular, the majority of studies using traditionally inhibitory stimulation protocols, such as 1 Hz, cTBS and iQPS, reported increases in RSFC rather than reductions. Furthermore, results revealed that rTMS-induced changes are not confined to the stimulated functional network, but instead spread to other brain networks, demonstrating the potential of polysynaptic effects that can greatly expand the range and potency of this approach. This review also highlighted heterogeneity across studies regarding stimulation parameters, study population, resting-state fMRI acquisition, functional connectivity analysis, and reporting procedures. Such heterogeneity invites the need for conventions to better characterize the network-level effects observed in these studies. As such, the remainder of this discussion addresses the possible mechanisms underlying distal RSFC effects, how these might differ from proximal CBF and spiking activity effects, and recommendations for frameworks that can continue to move this field forward in the future.
4.1. rTMS modulates resting-state functional connectivity
Across the studies reporting the effects of rTMS, 72% reported significant changes. When compared via Chi-Square, this ratio was significantly greater than for effects of sham or control stimulation across the literature. This result therefore indicates that rTMS applied over superficial brain structures reliably induces distal effects on RSFC, but also invites more internally controlled studies with proper sham, blinding, and other best practices for interventional neuroscience studies.
Various ideas have been proposed to explain the RSFC changes reported in these studies. For example, it has been suggested that rTMS effects propagate through anatomical connections between brain structures (Vink et al., 2018). Here, white matter tracts might facilitate the co-activation of interconnected regions and therefore modulate the functional connectivity between these regions. Alternately, it has been shown that rTMS can entrain endogenous brain oscillations (Thut and Miniussi, 2009), therefore stimulating one brain region might enhance neural synchrony between functionally connected areas and thus alter their connectivity. Yet another theory relates to brain homeostasis, wherein activity of one brain region modulated by rTMS might induce a reorganization of highly connected regions to compensate for this change in an attempt to maintain global brain homeostasis (Watanabe et al., 2014). While this review did not examine changes in BOLD signal associated with rTMS, the distal cortical and subcortical BOLD changes observed in fMRI studies (e.g., Bestmann et al., 2003; Bestmann et al., 2004) could be directly related to the changes in connectivity demonstrated in the current review and warrants further study.
4.2. rTMS effects on RSFC are not totally consistent with the proximal frequency-dependent stimulation heuristic
Stimulation frequency constitutes a central experimental parameter in studies of rTMS. Numerous studies focused on the motor cortex have revealed that frequencies below about 5 Hz, as well as cTBS and iQPS protocols, generally decrease the size of motor evoked potentials (e.g., Chen et al., 1997; Hamada et al., 2008; Huang et al., 2005; Romero et al., 2002). Conversely, higher stimulation frequencies, iTBS and eQPS protocols have typically produced increases in MEP amplitudes. While these findings have led to a common frequency-dependent heuristic that is pervasive in the field (Fitzgerald et al., 2006; Luber and Deng, 2016), relatively little is known about the frequency-dependency of these effects outside of the motor cortex, with a number of studies challenging this rule-of-thumb. For example, when applied over the prefrontal cortex, 1Hz rTMS has been found to increase regional cerebral blood flow (e.g., Eisenegger et al., 2008; Nahas et al., 2001) while 20 Hz rTMS has been shown to decrease brain activity (e.g., George et al., 1999).
Contrary to this proximal heuristic, findings from this review suggest that both conventionally inhibitory and conventionally excitatory rTMS protocols tend to increase resting-state functional connectivity to distal brain areas. Indeed, increased RSFC was found in 70% of the studies using inhibitory protocols, and although more variable, 53% of the studies also demonstrated increased connectivity following excitatory stimulation. When contrasting the effect of conventionally excitatory periodic protocols (5–20 Hz) to the effects of patterned protocols (eQPS, iTBS), it was observed that while periodic excitatory protocols induced the expected increase in RSFC, patterned protocols failed to do so and instead led to inhibitory effects on distal regions. This conclusion extends the preliminary results obtained in (Philip et al., 2018a,b) who found that high frequency rTMS was associated with reduced functional connectivity in depressed patients to a larger selection of clinical studies, as well as non-clinical populations. Therefore, this finding suggests that distal RSFC may change independently from proximal blood flow or spiking activity and that the common frequency-dependent heuristic might not predict changes in connectivity. The mechanisms underlying the directionality of rTMS effects both on neural activity on RSFC remain largely unknown and depends on the intrinsic, dynamic relationships between the stimulated and connected regions as well as the direct and indirect connections between those brain regions (Fox et al., 2012a,b). As such, future studies should prioritize designs that rigorously characterize rTMS effects both on brain activation and on connectivity to better understand these results, by using functionally-targeted, within-subject, sham-controlled designs.
4.3. RSFC changes were mainly found out of the stimulated network
The brain is often characterized as a dynamic system of interacting networks, which allows for complex human behavior. With the continued development of analytic techniques, this last decade has seen the proliferation of network-based neurobiological models for psychiatric disorders such as major depressive disorder (e.g., Kaiser et al., 2015) and posttraumatic stress disorder (Akiki et al., 2017; Beynel, Appelbaum, & Kimbrel, (in Press)) Therefore, understanding the effects of rTMS at a network level will likely be important for improving the efficacy of rTMS applications. In this review, rTMS-induced changes were found mainly outside of the stimulated functional network (81% of the studies). This suggests that distal effects of stimulation extend beyond the stimulated network through network interactions. This result is consistent with analyses of BOLD changes during and after rTMS that have failed to reveal significant changes in brain regions located underneath the coil, but instead found changes in remote brain regions (Baudewig et al., 2001; D. Bohning et al., 1999; O’Shea et al., 2007) [though see (Best- mann et al., 2003; Pascual-Leone et al., 1998) who have also reported BOLD changes underneath the coil]. While it is possible that the overall activity of the stimulated network may change without changing intrinsic patterns of connectivity, such interpretations cannot be definitively supported by these results. Indeed, none of the studies included in this review collected resting-state fMRI during rTMS application, and the majority of them assess the rTMS effects after a full treatment that lasted several weeks. Therefore, these RSFC changes could be due to a functional reorganization occurring after rTMS application. As these studies tended not to analyze whole-brain RSFC, but rather specific ROIs, future studies may wish to investigate whole-brain connectivity measured throughout treatment in order to reduce bias and provide a more comprehensive view of the mechanisms-of-action underlying distal connectivity changes. Moreover, in the large majority of the reviewed papers, rTMS effects were observed in specific brain regions. These brain regions were then converted here into brain networks by mapping the brain coordinates provided in the reports into the 7-network parcellation map defined by Yeo et al. (2011). As such, some approximation could have arisen from this process and future studies might want to explicitly state the activated network to validate this aggregated finding.
4.4. Challenges, limitations, and recommendations for future studies
While this review reveals some interesting preliminary results regarding rTMS-induced changes on RSFC, it has surveyed a relatively new field with highly disparate studies, and therefore these findings must be taken in the context of several important limitations that can be improved upon in future studies.
First, as shown in Fig. 1 and Supplementary Table S1, there is substantial heterogeneity across the surveyed studies regarding rTMS parameters (frequencies/patterns, stimulation intensity and targeting approach). While no recommendations can be provided regarding the optimal stimulation parameters, as this is dependent on the study goals, future studies should continue to innovate with stronger experimental designs. For example, as revealed in a recent meta-analysis, the use of individualized-fMRI targeting leads to increased effect sizes compared to more basic targeting approaches (Lysianne Beynel et al., 2019) and can provide value to future studies in this field. Furthermore, roughly two thirds of the studies lacked strong control conditions, preventing assessment of possible placebo effects and inducing a bias due to the rTMS-induced sensory differences. In particular, TMS-induced clicks produce strong activation in the auditory cortex, as measured by PET (Siebner et al., 1999), and can co-vary with activation of the stimulated area (Fox et al., 2012a,b). Applying rTMS over ‘task irrelevant’ brain areas, such as the vertex, is often proposed as a valid control condition and has been shown not to induce changes in functional connectivity (Jung et al., 2016), suggesting that this may be a useful strategy for future studies. Further improvements in placebo blinding, such as somatosensory-matched electrical stimulation (L. Beynel et al., 2019a,b), can add further value, but also must be considered within the cost and feasibility of a given study. As the field continues to develop, consensus recommendations for the clinical application of rTMS have emerged (e.g., Ekhtiari et al., 2019) creating disciplinary norms that will guide the field towards greater rigor and reproducibility. Moreover, while this review focused on repetitive TMS, other studies have found that single pulse TMS has the ability to modify brain and behavior (Rose et al., 2016) creating an opportunity for future reviews to address how single pulse TMS influences network connectivity.
A second challenge that leads to limitations is the variability in resting-state data collection and analysis approaches. For example, some studies required subjects to keep their eyes closed, other to keep them open, and, in some cases, have participants maintain central fixation. These three acquisition conditions are known to induce dramatically different connectivity patterns in the DMN (Yan et al., 2009) and therefore rTMS studies looking to modulate DMN function may wish to adopt a consistent approach, and particularly to maintain central fixation, as it has been shown to produce the highest test-retest reliability (Patriat et al., 2013). RSFC analysis techniques also differ across this literature, with most studies adopting seed-based analysis, while others used data-driven analyses such as ICA. Among studies using seed-based analysis, it is also important to consider that the RSFC seed location was not always the stimulated site. In order to improve data quality, we recommend continued standardization across these parameters, as well as monitoring and removal of physiological artifacts from respiration and cardiac rhythm, which can be particularly problematic given that they may mask other desired low frequency modulatory signals.
A third challenge to consider is the need for a common system of aggregation to define brain networks. In this review, we opted to utilize the 7-network parcellation map (Yeo et al., 2011). This choice was made because this atlas has been built upon functional connectivity obtained during resting state acquisition, which was highly relevant for this review. While this atlas is widely used, it represents only one of many ways to parcellate the brain into networks. Network parcellations based on the given study sample or individual participants may allow for more robust characterizations of network effects but also adds challenges to aggregation across this growing field. In light of these considerations, it will be important for the field to come to a consensus about the most suitable approach for network classification. This review only presented results from group averages that were provided by each of the study papers. Given the crucial role of brain state during the stimulation (Silvanto and Pascual-Leone, 2008), it will be important for future research to present data from individual subjects to provide better insight into which subjects respond to stimulation and why. This knowledge will continue to build towards the personalization of rTMS treatments that holds the greatest potential for therapeutic benefits.
Finally, it should be noted that individual and momentary features related to brain state and morphology have been shown to influence the effects of rTMS (Huang et al., 2017; Silvanto and Pascual-Leone, 2008). Such features constitute an additional source of noise in the aggregate results of this review as well as the individual studies included here. Nevertheless, the statistical significance of results in these studies as well as the overall patterns of results observed in this review suggest that group-level effects of rTMS were often able to emerge in spite of these confounding factors. In addition, results from this review could be influenced by publication bias that was not assessed. However, as many of the included studies were interested in clinical outcomes rather than effects on RSFC, it is possible that publication bias may not have strongly influenced these results.
5. Conclusions
This review supports some preliminary conclusions about the effects of rTMS on RSFC. In particular, it can be inferred from these findings that active rTMS does lead to preferential modulation of RSFC over sham stimulation. Importantly, it is observed that the pattern of distal RSFC effects does not adhere to the conventional inhibitory heuristic that is widely reported with proximal stimulation studies. Finally, based on an aggregation of effects across different canonical brain networks, it is found that rTMS-induced changes most frequently occur in brain networks other than the stimulated network. Overall, this literature demonstrates promise for modulating neural features beyond the proximal target region, but further research will be required to more reliably predict and leverage these distal effects for specific applications.
Supplementary Material
Table 4D.
Effects of multiple rTMS sessions per visit, with one or more visits on RSFC.
| Study Name | # visits | #sessions/day | rTMS | Decrease | No effect | Increase |
|---|---|---|---|---|---|---|
| Popa et al. (2013) | 5 | 2 | 1Hz | X | ||
| Ji et al. (2017) | 1 | 3 | cTBS | X | ||
| Nettekoven et al. (2014) | 4 | 3 | iTBS | X | ||
| Vidal-Pineiro et al. (2014) | 1 | 2 | iTBS | X |
→ 3 out of 4 studies (75%) reporting the effect of multiple sessions reported significant changes.
Acknowledgements
The authors want to thank Flavio Frolich and Justin Riddle from UNC for their useful input on this review.
Funding
This research was funded by grant U01AG050618 from the National Institute of Aging (https://www.nia.nih.gov/).
Footnotes
Declaration of competing interest
The authors do not have any conflict of interest to declare.
While all included studies met the pre-registered inclusion criteria, some did not report the effects of rTMS on RSFC, but rather reported effects on clinical response. As such, these articles appear greyed-out in the following tables.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116596.
References
- Addicott M, Luber B, Nguyen D, Palmer H, Lisanby S, Appelbaum L, 2019. Low and High Frequency rTMS Effects on Resting-State Functional Connectivity between the Postcentral Gyrus and the Insula. Brain Connectivity(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Abdallah CG, 2017. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatr. Rep 19 (11), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeken C, Duprat R, Wu GR, De Raedt R, van Heeringen K, 2017. Subgenual anterior cingulate-medial orbitofrontal functional connectivity in medication-resistant major depression: a neurobiological marker for accelerated intermittent theta burst stimulation treatment? Biol Psychiatry Cogn Neurosci Neuroimaging 2 (7), 556–565. 10.1016/j.bpsc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Baeken C, Marinazzo D, Wu G-R, Van Schuerbeek P, De Mey J, Marchetti I, De Raedt R, 2014. Accelerated HF-rTMS in treatment-resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J. Biol. Psychiatr 15 (4), 286–297. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Sporns O, 2017. Network neuroscience. Nat. Neurosci 20 (3), 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Siebner HR, Bestmann S, Tergau F, Tings T, Paulus W, Frahm J, 2001. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS). Neuroreport 12 (16), 3543–3548. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2003. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage 20 (3), 1685–1696. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2004. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci 19 (7), 1950–1962. [DOI] [PubMed] [Google Scholar]
- Beynel L, Appelbaum G, & Kimbrel NA ((in press)). Neurobiology and neuromodulation of emotion in posttraumatic stress disorder In Tull M and Kimbrell NA, Beynel L, Appelbaum LG, Kimbrel NA (Ed.), Emotion in Posttraumatic Stress Disorder: Academic Press, Cambridge, MA. [Google Scholar]
- Beynel L, Appelbaum LG, Luber B, Crowell CA, Hilbig SA, Lim W, Cabeza R, 2019a. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: a meta-analysis and recommendations for future studies. Neurosci. Biobehav. Rev https://www.elsevier.com/books/emotion-in-posttraumatic-stress-disorder/tull/978-0-12-816022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynel L, Davis SW, Crowell CA, Hilbig SA, Lim W, Nguyen D, Luber B, 2019b. Online repetitive transcranial magnetic stimulation during working memory in younger and older adults: a randomized within-subject comparison. PloS One 14 (3), e0213707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath RD, Biswal BB, Bhaskar MV, Gohel S, Jhunjhunwala K, Panda R, Pal PK, 2015. Repetitive transcranial magnetic stimulation induced modulations of resting state motor connectivity in writer’s cramp. Eur. J. Neurol 22 (5), 796–805. 10.1111/ene.12653e753-794. [DOI] [PubMed] [Google Scholar]
- Bharath RD, Panda R, Reddam VR, Bhaskar MV, Gohel S, Bhardwaj S, Pal PK, 2017. A single session of rTMS enhances small-worldness in writer’s cramp: evidence from simultaneous EEG-fMRI multi-modal brain graph. Front. Hum. Neurosci 11, 443 10.3389/fnhum.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning D, Shastri A, McConnell K, Nahas Z, Lorberbaum J, Roberts D, George M, 1999. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol. Psychiatr 45 (4), 385–394. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, George MS, 1998. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol 33 (6), 336–340. [DOI] [PubMed] [Google Scholar]
- Brabenec L, Klobusiakova P, Barton M, Mekyska J, Galaz Z, Zvoncak V, Rektorova I, 2019. Non-invasive stimulation of the auditory feedback area for improved articulation in Parkinson’s disease. Park. Relat. Disord 61, 187–192. 10.1016/j.parkreldis.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG, 1997. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48 (5), 1398–1403. [DOI] [PubMed] [Google Scholar]
- Chou YH, You H, Wang H, Zhao YP, Hou B, Chen NK, Feng F, 2015. Effect of repetitive transcranial magnetic stimulation on fMRI resting-state connectivity in multiple system Atrophy. Brain Connect. 5 (7), 451–459. 10.1089/brain.2014.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Woodside B, Lam E, Olmsted M, Colton P, Giacobbe P, Downar J, 2015. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin 8, 611–618. 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J, 2016. Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology 41 (5), 1395–1403. 10.1038/npp.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenegger C, Treyer V, Fehr E, Knoch D, 2008. Time-course of “off-line” prefrontal rTMS effects–a PET study. Neuroimage 42 (1), 379–384. 10.1016/j.neuroimage.2008.04.172. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Claus E, 2019. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci. Biobehav. Rev 104, 118–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A, 2011. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc. Natl. Acad. Sci. U. S. A 108 (52), 21229–21234. 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ, 2006. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol 117 (12), 2584–2596. 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A, 2012a. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatr 72 (7), 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A, 2012b. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 62 (4), 2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Stallings LE, Speer AM, Nahas Z, Spicer KM, Vincent DJ, Teneback CC, 1999. Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Hum. Psychopharmacol. Clin. Exp 14 (3), 161–170. [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC, 2001. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57 (3), 449–455. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T, 2010. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J. Neural. Transm 117 (1), 105–122. 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, Ugawa Y, 2008. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J. Physiol 586 (16), 3927–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Hoffman RE, 2010. Transcranial magnetic stimulation and connectivity mapping: tools for studying the neural bases of brain disorders. Front. Syst. Neurosci 4 10.3389/fnsys.2010.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005. Theta burst stimulation of the human motor cortex. Neuron 45 (2), 201–206. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, Jaberzadeh S, 2017. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin. Neurophysiol 128 (11), 2318–2329. [DOI] [PubMed] [Google Scholar]
- Jansen JM, van Wingen G, van den Brink W, Goudriaan AE, 2015. Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur. Neuropsychopharmacol 25 (12), 2230–2239. 10.1016/j.euroneuro.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Ji GJ, Yu F, Liao W, Wang K, 2017. Dynamic aftereffects in supplementary motor network following inhibitory transcranial magnetic stimulation protocols. Neuroimage 149, 285–294. 10.1016/j.neuroimage.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Jung J, Bungert A, Bowtell R, Jackson SR, 2016. Vertex stimulation as a control site for transcranial magnetic stimulation: a concurrent TMS/fMRI study. Brain stimulation 9 (1), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry 72 (6), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Lee H, Jhung K, Kim KR, An SK, Yoon KJ, Lee E, 2016. Frontostriatal connectivity changes in major depressive disorder after repetitive transcranial magnetic stimulation: a randomized sham-controlled study. J. Clin. Psychiatr 77 (9), e1137–e1143. 10.4088/JCP.15m10110. [DOI] [PubMed] [Google Scholar]
- Lee J, Park E, Lee A, Chang WH, Kim DS, Shin YI, Kim YH, 2018. Modulating brain connectivity by simultaneous dual-mode stimulation over bilateral primary motor cortices in subacute stroke patients. Neural Plast, 1458061 2018/1458061, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Du L, Sahlem GL, Badran BW, Henderson S, George MS, 2017. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend 174, 98–105. 10.1016/j.drugalcdep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Meng F, Gao J, Zhang L, Zhou Z, Pan G, Luo B, 2018. Behavioral and resting state functional connectivity effects of high frequency rTMS on disorders of consciousness: a sham-controlled study. Front. Neurol 9, 982 10.3389/fneur.2018.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B, Deng Z-D, 2016. Application of non-invasive brain stimulation in psychophysiology In: Berntson GG, Cacioppo JT, Tassinary LG (Eds.), Handbook of Psychophysiology, 4 ed. Cambridge University Press, Cambridge, pp. 116–150. [Google Scholar]
- Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, George MS, 2001. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol. Psychiatr 50 (9), 712–720. [DOI] [PubMed] [Google Scholar]
- Navarro de Lara LI, Windischberger C, Kuehne A, Woletz M, Sieg J, Bestmann S, Laistler E, 2015. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn. Reson. Med 74 (5), 1492–1501. 10.1002/mrm.25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, Grefkes C, 2014. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J. Neurosci 34 (20), 6849–6859. 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MFS, 2007. Functionally specific reorganization in human premotor cortex. Neuron 54 (3), 479–490. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD, 1998. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol 15 (4), 333–343. [DOI] [PubMed] [Google Scholar]
- Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Birn RM, 2013. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage 78, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR, 2004. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol 115 (7), 1519–1526. 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Philip NS, Barredo J, Aiken E, Carpenter LL, 2018a. Neuroimaging mechanisms of therapeutic transcranial magnetic stimulation for major depressive disorder. Biol. Psychiatr.: Cognit. Neurosci. Neuroimaging 3 (3), 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL, 2018b. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatr 83 (3), 263–272. 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa T, Russo M, Vidailhet M, Roze E, Lehericy S, Bonnet C, Gallea C, 2013. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimul 6 (2), 175–179. 10.1016/j.brs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Petersen SE, 2011. Functional network organization of the human brain. Neuron 72 (4), 665–678. 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, 2011. The restless brain. Brain Connect. 1 (1), 3–12. 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland LT, Peelle JE, Kallogjeri D, Nicklaus J, Piccirillo JF, 2016. The effect of noninvasive brain stimulation on neural connectivity in Tinnitus: a randomized trial. Laryngoscope 126 (5), 1201–1206. 10.1002/lary.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A, 2002. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin. Neurophysiol 113 (1), 101–107. [DOI] [PubMed] [Google Scholar]
- Rose NS, LaRocque JJ, Riggall AC, Gosseries O, Starrett MJ, Meyering EE, Postle BR, 2016. Reactivation of latent working memories with transcranial magnetic stimulation. Science 354 (6316), 1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter RS, Jansen JM, van Holst RJ, van den Brink W, Goudriaan AE, 2018. Differential effects of left and right prefrontal high-frequency repetitive transcranial magnetic stimulation on resting-state functional magnetic resonance imaging in healthy individuals. Brain Connect 8 (2), 60–67. 10.1089/brain.2017.0542. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27 (9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A, 2008. State-dependency of transcranial magnetic stimulation. Brain Topogr 21 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Chang D, Zhang J, Peng W, Shang Y, Gao X, Wang Z, 2019. Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain Imaging Behav 13 (2), 421–429. 10.1007/s11682-018-9866-4. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, Post RM, 2000. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatr 48 (12), 1133–1141. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, 2009. New insights into rhythmic brain activity from TMS–EEG studies. Trends Cognit. Sci. 13 (4), 182–189. [DOI] [PubMed] [Google Scholar]
- Tik M, Hoffmann A, Sladky R, Tomova L, Hummer A, Navarro de Lara L, Windischberger C, 2017. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 162, 289–296. 10.1016/j.neuroimage.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A, 2010. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J. Psychiatr. Res 44 (11), 725–731. 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Vidal-Pineiro D, Martin-Trias P, Arenaza-Urquijo EM, Sala-Llonch R, Clemente IC, Mena-Sanchez I, Bartres-Faz D, 2014. Task-dependent activity and connectivity predict episodic memory network-based responses to brain stimulation in healthy aging. Brain Stimul 7 (2), 287–296. 10.1016/j.brs.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JJT, Mandija S, Petrov PI, van den Berg CAT, Sommer IEC, Neggers SFW, 2018. A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Hum. Brain Mapp 39 (11), 4580–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz LJ, Rehme AK, Michely J, Nettekoven C, Eickhoff SB, Fink GR, Grefkes C, 2016. Shaping early reorganization of neural networks promotes motor function after stroke. Cerebr. Cortex 26 (6), 2882–2894. 10.1093/cercor/bhw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Voss JL, 2014. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 345 (6200), 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Voss JL, 2015. Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus 25 (8), 877–883. 10.1002/hipo.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, Ohtomo K, 2014. Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum. Brain Mapp 35 (5), 1896–1905. 10.1002/hbm.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, Fox MD, 2018. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatr 84 (1), 28–37. 10.1016/j.biopsych.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue SW, Guo Y, Peng W, Zhang J, Chang D, Zang YF, Wang Z, 2017. Increased low-frequency resting-state brain activity by high-frequency repetitive TMS on the left dorsolateral prefrontal cortex. Front. Psychol 8, 2266 10.3389/fpsyg.2017.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Zang Y, 2009. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PloS One 4 (5), e5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106 (3), 1125–1165. 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sollmann N, Castrillon G, Kurcyus K, Meyer B, Zimmer C, Krieg SM, 2018. Intranetwork and internetwork effects of navigated transcranial magnetic stimulation using lowand high-frequency pulse application to the dorsolateral prefrontal cortex-A combined rTMS-fMRI approach. J. Clin. Neurophysiol 10.1097/WNP.0000000000000528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.