Abstract
Rationale
Caffeine is widely used as a countermeasure against neurobehavioral impairment during sleep deprivation. However, little is known about the pharmacodynamic profile of caffeine administered repeatedly during total sleep deprivation.
Objectives
To investigate the effects of repeated caffeine dosing on neurobehavioral performance during sleep deprivation, we conducted a laboratory-based, randomized, double-blind, placebo-controlled, crossover, multi-dose study of repeated caffeine administration during 48 h of sleep deprivation. Twelve healthy adults (mean age 27.4 years, six women) completed an 18-consecutive-day in-laboratory study consisting of three 48 h total sleep deprivation periods separated by 3-day recovery periods. During each sleep deprivation period, subjects were awakened at 07:00 and administered caffeine gum (0, 200, or 300 mg) at 6, 18, 30, and 42 h of wakefulness. The Psychomotor Vigilance Test and Karolinska Sleepiness Scale were administered every 2 h.
Results
The 200 and 300 mg doses of caffeine mitigated neurobehavioral impairment across the sleep deprivation period, approaching two-fold performance improvements relative to placebo immediately after the nighttime gum administrations. No substantive differences were noted between the 200 mg and 300 mg caffeine doses, and adverse effects were minimal.
Conclusions
The neurobehavioral effects of repeated caffeine dosing during sleep deprivation were most evident during the circadian alertness trough (i.e., at night). The difference between the 200 mg and 300 mg doses, in terms of the mitigation of performance impairment, was small. Neither caffeine dose fully restored performance to well-rested levels. These findings inform the development of biomathematical models that more accurately account for the time of day and sleep pressure–dependent effects of caffeine on neurobehavioral performance during sleep loss.
Keywords: Caffeine gum, Cognitive performance, Within-subject design, Dose response, Sleep loss
Introduction
Caffeine (1,3,7-trimethylxanthine) is widely used to increase alertness and counteract neurobehavioral performance impairment due to sleep loss (Bonnet and Arand 2012). Yet, important questions remain regarding the effects of repeated caffeine intake during sleep deprivation. Results from studies of repeated caffeine administration during sleep deprivation in the laboratory (Denaro et al. 1990; Doan et al. 2006; Bonnet and Arand 2012) and in the field (McLellan et al. 2005a, b) generally show that 200 mg of caffeine restores performance to near-baseline levels when administered within the first 24 h of sleep deprivation (e.g., Kamimori et al. 2005). After 24 h of sleep deprivation, higher-caffeine doses appear to be required, which increases the probability of side effects (Spaeth et al. 2014). Across the published literature, reported results are inconsistent (Smith et al. 1993; Reyer and Horne 2000; Van Dongen et al. 2001; Wyatt et al. 2004; Kamimori et al. 2005; McLellan et al. 2005a; Doan et al. 2006; McLellan et al. 2007; Spaeth et al. 2014; Dark et al. 2015; Kamimori et al. 2015) and appear to depend on what aspects of neurobehavioral performance are investigated (Tikuisis et al. 2004; McLellan et al. 2005b; Gottselig et al. 2006; Spaeth et al. 2014; Paech et al. 2016).
Incomplete knowledge of the effects of repeated caffeine dosing during sleep deprivation presents a challenge to the development of fatigue prediction models that account for caffeine intake (Benitez et al. 2009; Puckeridge et al. 2011; Ramakrishnan et al. 2013, 2014, 2016; Reifman et al. 2016). Previous work on an individualized caffeine model defined two model parameters that appear to govern the pharmacodynamic effects of caffeine (Ramakrishnan et al. 2013). To be able to quantify the relationship of these model parameters with caffeine dose, performance measurements during sleep deprivation in at least two different dose conditions would be needed. Ideally, such measurements would involve the same subjects, because there are profound, systematic, inter-individual differences in the effects of caffeine (Bodenmann et al. 2012; Quartana and Rupp 2012). Low-caffeine doses up to 300 mg are the most practically relevant in this context (Lieberman et al. 2002; McLellan et al. 2005)—particularly low doses above 100 mg, as the effects of 100 mg have been found to be too short-lived to be operationally useful (Lieberman et al. 2002). Thus, caffeine doses of 200 mg and 300 mg would seem to be reasonable choices for a sleep deprivation experiment designed to quantify the dose relationship of the model parameters in the aforementioned caffeine model (Ramakrishnan et al. 2013). Against this background, we set out to investigate the neurobehavioral effects of 0 mg (placebo), 200 mg, and 300 mg doses of caffeine administered four consecutive times during 48 h of total sleep deprivation (TSD) in the laboratory. Caffeine was administered in randomized, double-blind fashion, at 12-h intervals (early afternoon and late night) capturing two opposite points in the circadian alertness cycle. We implemented a within-subject, counterbalanced crossover design in order to minimize the impact of inter-individual differences and order effects, and measured psychomotor vigilance performance and subjective sleepiness throughout scheduled wakefulness.
Methods
Subjects
N = 12 healthy adults (six women, six men), average age 27.4 years (SD 6.9 years), completed an 18-consecutive-day, in-laboratory study. See the Supporting Information for a power calculation on which the sample size was based. Subjects’ average body weight was 72.4 kg (SD 21.7 kg), and their average body mass index (BMI) was 24.2 (SD 5.2). Subjects’ average self-reported habitual caffeine consumption, calculated using publicly available norms for caffeine dose by type of caffeinated drink or chocolate (Hauri and Linde 1990), was 114 mg (SD 98 mg) per day. One subject reported habitually not consuming any caffeine.
Subjects met the following inclusion criteria: age between 18 and 39 years; physically and psychologically healthy as assessed by physical examination, history, and questionnaires; no current medical or drug treatment (except oral contraceptives); no clinically relevant history of psychiatric illness or brain injury; no sleep or circadian disorders; tested negative for commonly abused drugs, alcohol, and tobacco; no history of drug or alcohol abuse in the past year and no history of methamphetamine abuse; no tobacco use for at least 3 years; no travel across time zones within 1 month of study participation; no shift work within 3 months of study participation; not pregnant; no self-reported caffeine use in excess of 400 mg per day on average; no past adverse reactions to caffeine or sleep deprivation; no vision impairment unless corrected to normal; habitual sleep duration between 6 and 10 h; and habitual wake time between 06:00 and 09:00. No female subject reported taking any oral contraceptives.
Subjects were instructed to refrain from caffeine, tobacco, alcohol, drug use, and napping in the week before the study. During this week, they were also required to maintain their habitual sleep and wake times. Compliance was monitored by means of wrist actigraphy (Actiwatch 2, Philips Respironics, Bend, OR), a sleep/wake diary, and a time-stamped voicemail box subjects used to report bed and wake times each day. Actigraphically estimated average daily sleep duration in the week before the study was 7.5 h (SD 0.8 h). Average bedtime was 23:02 (SD 0.9 h), and average wake-up time was 07:30 (SD 0.9 h). One subject reported drinking three cups of tea on the first day of the pre-study week but was allowed to participate because the estimated amount of caffeine ingested was relatively small (total < 100 mg).
The study was approved by the Institutional Review Board (IRB) of Washington State University. Subjects gave written informed consent and were financially compensated for their participation.
Study design
We utilized a double-blind, placebo-controlled, crossover design in which subjects participated in all three caffeine dose conditions (0, 200, and 300 mg). Subjects were randomized to receive the three caffeine doses in different order, counterbalanced across the three 48 h TSD periods. During each TSD period, wakefulness was maintained for 48 h, from 07:00 until 07:00 2 days later, extending 34 h past scheduled wake time.
General procedure
The study was conducted in the sleep laboratory of the Sleep and Performance Research Center at Washington State University Spokane. Light levels were fixed below 100 lx during scheduled wake periods, and lights were off during scheduled sleep periods. Ambient temperature was maintained at 21 °C (SD 1 °C). Subjects were not allowed to leave the laboratory and were isolated from outside influences at all times. They did not have access to live radio or television, phones or computers, the internet, or video games. When not scheduled for performance testing or sleep, subjects were allowed to play board and card games, read, talk with other subjects or staff in the laboratory, or watch DVDs. Research staff ensured that subjects’ social interactions remained emotionally tempered, and an even-keeled environment was maintained at all times. Vigorous physical activity was prohibited. Further, meals or snacks were provided every 4 h during scheduled wakefulness. Except for bathroom breaks, subjects were continuously monitored in-person by research staff.
Subjects remained inside the laboratory for 18 consecutive days, during which time they participated in all three caffeine dose conditions. As depicted in Fig. 1, subjects reported to the laboratory in the early afternoon of day 1 and underwent three baseline days, each with a 10 h nighttime sleep opportunity (21:00–07:00). On day 4, subjects began the first 48 h TSD period and caffeine dose condition, followed by a 5 h morning recovery nap (07:00–12:00) on day 6. Subjects then had three recovery days, each with a 10 h nighttime sleep opportunity (21:00–07:00). This was followed by a second 48 h TSD period and caffeine dose condition and subsequent 5 h morning recovery nap period. This pattern was repeated a third time for the last 48 h TSD period and caffeine dose condition and subsequent 5 h morning recovery nap. Finally, subjects underwent two recovery days, each with a 10 h nighttime sleep opportunity (21:00–07:00). They were discharged in the late morning on day 18.
Fig. 1.
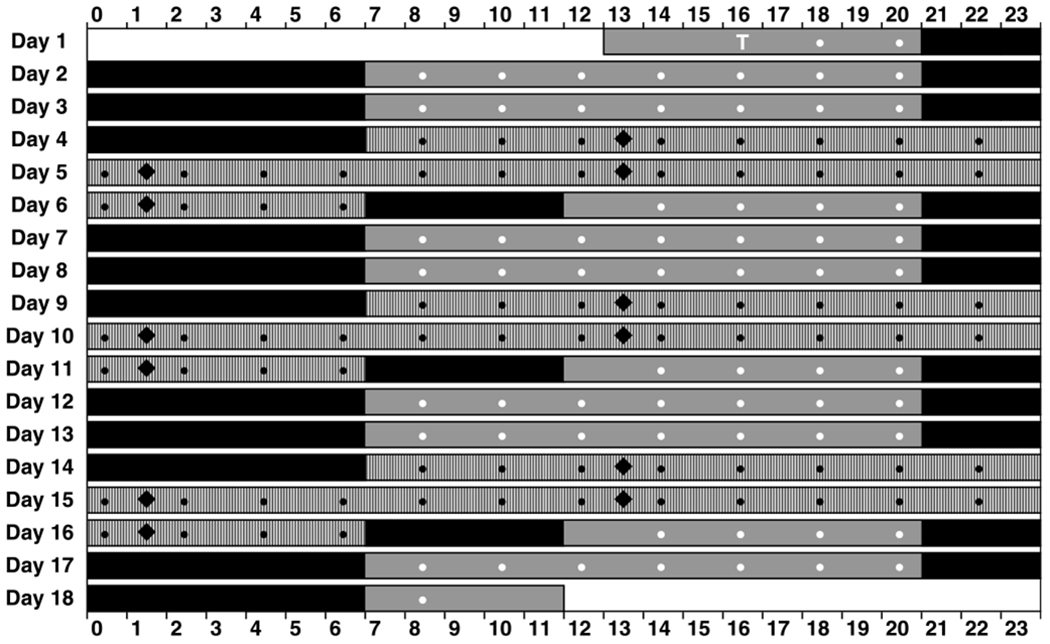
Schematic of the 18 day in-laboratory study protocol. Subjects entered the laboratory at 13:00 on day 1 and remained in the laboratory until 12:00 on day 18. The 48 h TSD periods (hashed bar segments) began at 7:00 on days 4, 9, and 14. Black and white circles indicate neurobehavioral test bouts—only those test bouts indicated in black were included in statistical analyses (the white “T” denotes a practice test bout). Caffeine gum administrations are indicated with black diamonds. Solid black bar segments indicate scheduled sleep periods; solid gray bar segments indicate scheduled wakefulness during baseline or recovery days
Neurobehavioral testing
Throughout the experiment, subjects completed testing while seated at a desk in their individual bedrooms. All tasks were completed on a desktop computer with a 15-in. LCD screen placed approximately 21 in. from the eyes. Subjects were trained on a neurobehavioral performance test battery on the first day of the study (Fig. 1, “T”) and subsequently completed the test battery every 2 h during scheduled wakefulness (Fig. 1, black and white dots). The test battery included a standard, 10-min Psychomotor Vigilance Test (PVT; Lim and Dinges 2008) and a computerized Karolinska Sleepiness Scale (KSS; Åkerstedt et al. 2014). The KSS was administered both before and after the PVT. Other neurobehavioral assays in the test battery included a visual analog scale for mood (VAS-M) and a 4 m Digit Symbol Substitution Task (DSST) (results to be reported elsewhere). The order of test administration in each test battery was VAS-M, KSS, PVT, DSST, VAS-M, and KSS.
Psychomotor Vigilance Test
For the PVT, subjects responded to a visual stimulus (incrementing millisecond counter) by pressing a response button. The foreperiod prior to stimulus presentation was randomized between 2 and 10 s in 1 s increments. Subjects were instructed to respond as quickly as possible as soon as the stimulus appeared but not to respond before the stimulus appeared. Following each response, the response time (RT) was displayed (in ms) for 1 s. Performance was quantified as the number of lapses (RTs > 500 ms) as well as the log transformation of the signal-to-noise ratio (LSNR) for each test bout. The LSNR is a measure of the fidelity of cognitive information processing (Chavali et al. 2017), expressed in decibels (dB). It has the useful property that each − 3 dB change reflects a 50% decrease in information processing fidelity and each 3 dB change reflects a twofold improvement in the fidelity of information processing regardless of starting value. This psychometric property allows quantification of impairment irrespective of absolute position on the metric scale (Chavali et al. 2017).
Karolinska Sleepiness Scale
For the KSS, a nine-point Likert scale, subjects rated their current level of sleepiness on a scale from 1 (“extremely alert”) to 9 (“extremely sleepy/fighting sleep”). The KSS was administered immediately before and immediately after each PVT bout.
Caffeine administration
Caffeine or placebo was administered as chewing gum (Stay-Alert, Marketright Inc., Plano, IL), for which the rate of absorption and relative bioavailability has been well characterized (Kamimori et al. 2002). Each gum piece contained caffeine (100 mg) or placebo (0 mg). Gum pieces were administered three at a time in combinations forming overall doses of 0 (placebo), 200, or 300 mg. During each 48 h TSD period, gum was administered double-blind at 13:00 (6 h of wakefulness), 01:00 (18 h of wakefulness), 13:00 (30 h of wakefulness), and 01:00 (42 h of wakefulness). Each of these gum administrations occurred 1 h before the next neurobehavioral test bout; see Fig. 1 (black diamonds). Meals were served every 4 h during scheduled wakefulness and were completed at least 1 h prior to each gum administration. No food was allowed in between meals and/or snacks, and only water was allowed in the 1 h prior to each gum administration. Each gum administration within a given TSD period consisted of the same caffeine dose. Under the supervision of research staff, subjects chewed the three gum pieces simultaneously for precisely 10 min (as verified with a stopwatch) and then discarded. This procedure was expected to result in ingestion of at least 85% of the caffeine dose (Kamimori et al. 2002).
Adverse effects were reported by five different subjects and included seven instances of nausea, two instances of headache, one instance of vomiting (approximately 2 h after gum administration), and one instance of feelings of increased heart rate and dizziness (approximately 1 h after gum administration). Three instances of nausea were associated with the 200 mg dose; all other adverse effects were associated with the 300 mg caffeine dose. The self-reported habitual caffeine use of the subjects that reported adverse effects was in the range of 50–175 mg per day, except for one subject, whose habitual caffeine use was estimated at 380 mg per day (this subject reported two instances of nausea, one instance of headache, and one instance of vomiting). All adverse effects resolved without intervention, except for one instance of nausea which was treated with antacid and one instance of headache which was treated with ibuprofen.
Statistical analyses
The PVT and KSS data from the 24 test bouts within each TSD period were analyzed with mixed-model analysis of variance (ANOVA). Fixed effects for caffeine dose (within-subject factor: 0, 200, or 300 mg), test bout (within-subject factor: 24 bouts), and dose-by-bout interaction were included. A random effect for subjects was placed on the intercept. Statistical tests for the main effect of dose and the dose-by-bout interaction accounted for order effects (see Table 1). Planned contrasts were included for pairwise comparisons between doses overall and in interaction with bout, and for a secondary analysis of just the first three test bouts after each gum administration.
Table 1.
Order of doses and assignment of weight for statistical analyses
| Period 1 | Period 2 | Period 3 | Order | Weight |
|---|---|---|---|---|
| 0 mg | (200 mg) | (300 mg) | 1 | }2 |
| 0 mg | (300 mg) | (200 mg) | 1 | |
| 200 mg | 0 mg | (300 mg) | 2 | 1 |
| 300 mg | 0 mg | (200 mg) | 3 | 1 |
| 200 mg | 300 mg | 0 mg | 4 | 1 |
| 300 mg | 200 mg | 0 mg | 5 | 1 |
The table shows the six possible permutations of caffeine doses across the three 48 h TSD periods. The bold numbers are the dose of interest; here, it is illustrated for 0 mg (placebo). The italicized numbers show the doses in earlier periods—these determine the different ways any order effects could play out. The numbers in parentheses, in smaller font, are the doses in later periods, which cannot have any impact on order effects. The “Order” column enumerates the different orders. Note that the first two permutations are assigned the same order number, because the difference occurs in the later periods and therefore cannot have any impact on order effects. To determine the temporal profiles of outcome variables for the dose of interest, we computed the marginal means over the five different orders, with the first order weighted twice to account for the two different permutations it represents, as indicated in the “Weight” column. For statistical analyses across the three caffeine doses, all 18 possible permutations leading to 15 different orders were accounted for analogously
There was one missing post-PVT sleepiness rating on the KSS for one subject. The analyses were robust to this missing data point. To verify that the handful of adverse events reported had no substantive effect on the study results, the analyses were repeated with every test bout occurring after an adverse event removed. The impact on the results was negligible, with no changes in statistical significance. Therefore, results of analyses on the full data set are reported here. Graphs display least-squares marginal means and their standard errors, collapsed over TSD periods. Statistical test results are shown as part of the figures.
Results
PVT performance
Results and statistics for the number of lapses and the LSNR on the PVT, across time awake during 48 h of TSD, are shown in Fig. 2. For the placebo condition (0 mg caffeine), the number of lapses increased and the LSNR decreased across time awake, modulated by time of day (circadian rhythm). Within each 24 h period of sleep loss, the number of lapses was highest and the LSNR was lowest at approximately 08:00. Both the time awake and time of day effects were attenuated by the 200 and 300 mg doses of caffeine. The caffeine effect was most evident shortly after nighttime gum administrations.
Fig. 2.
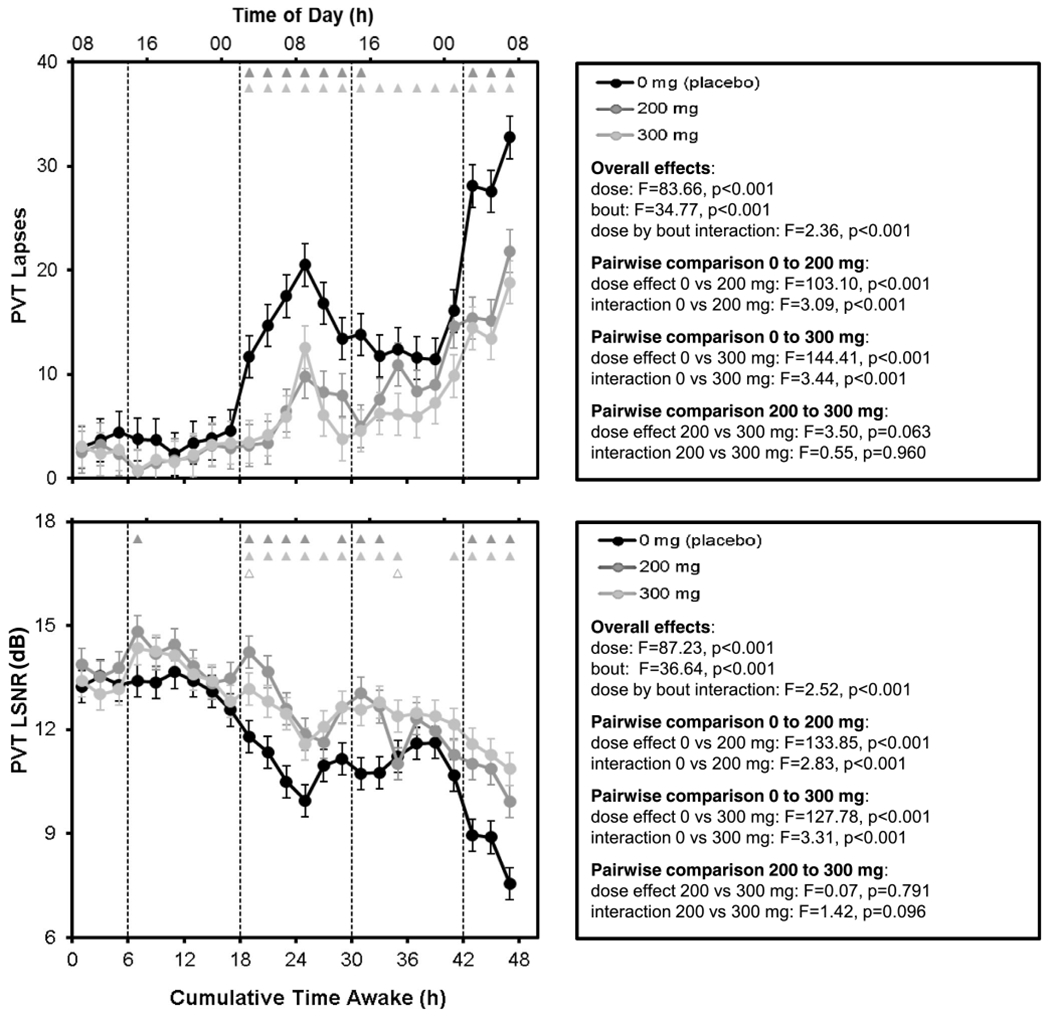
Mean performance (± SE) across time awake for PVT number of lapses (top) and LSNR (bottom) as a function of caffeine dose. Upward corresponds to greater impairment for lapses, while downward corresponds to greater impairment for the LSNR. Dashed vertical lines denote the times of gum administration. Triangles at the top of each panel indicate statistically significant differences by bout between 0 and 200 mg (dark gray), 0 and 300 mg (light gray), and 200 and 300 mg (open light gray)
Pairwise comparisons between doses overall and across time awake showed that the 200 mg and 300 mg caffeine doses were each significantly different than the placebo condition (Fig. 2). The 200 mg and 300 mg doses were not significantly different from each other, although there was a trend for an overall difference between these doses, with the 300 mg dose mitigating performance impairment slightly more.
Mean LSNR differences between the two caffeine conditions and the placebo condition during the first three test bouts after each gum administration, when the caffeine effects were most pronounced, are shown in Fig. 3. Throughout the study, and especially during sleep deprivation, the two caffeine conditions showed improved PVT performance relative to the placebo condition. This effect was particularly pronounced at night and in the morning hours. The 300 mg caffeine condition tended to improve performance more effectively than the 200 mg caffeine after wakefulness was extended beyond approximately 34 h.
Fig. 3.
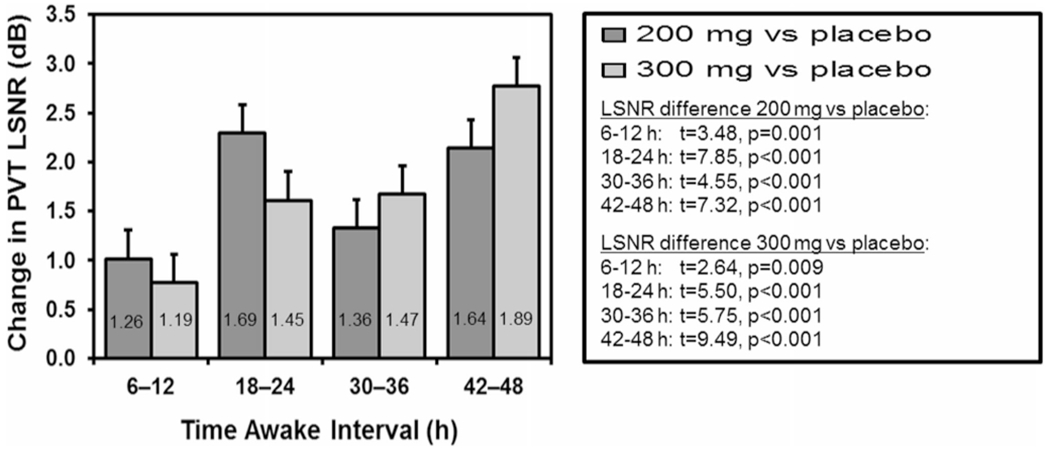
Mean (and SE) of the improvement in PVT performance across the 6-h time interval (three test bouts) immediately after each gum administration, for the 200 mg and 300 mg doses of caffeine relative to the placebo condition. The improvement in PVT performance due to caffeine is expressed in terms of the change in LSNR, where a 3 dB change reflects a twofold improvement regardless of the level of impairment in the placebo condition. Fold improvement values are indicated as numbers inside the mean bars
KSS ratings
Results and statistics for pre- and post-PVT sleepiness ratings on the KSS are shown in Fig. 4. The KSS ratings showed temporal patterns similar to those observed for PVT performance. Overall, pre- and post-PVT sleepiness ratings for the 200 mg and 300 mg caffeine conditions were significantly lower than those for the placebo condition. Pre-PVT sleepiness ratings for the 200 mg dose were also somewhat lower than those for the 300 mg dose, whereas post-PVT sleepiness ratings for the two doses were not significantly different.
Fig. 4.
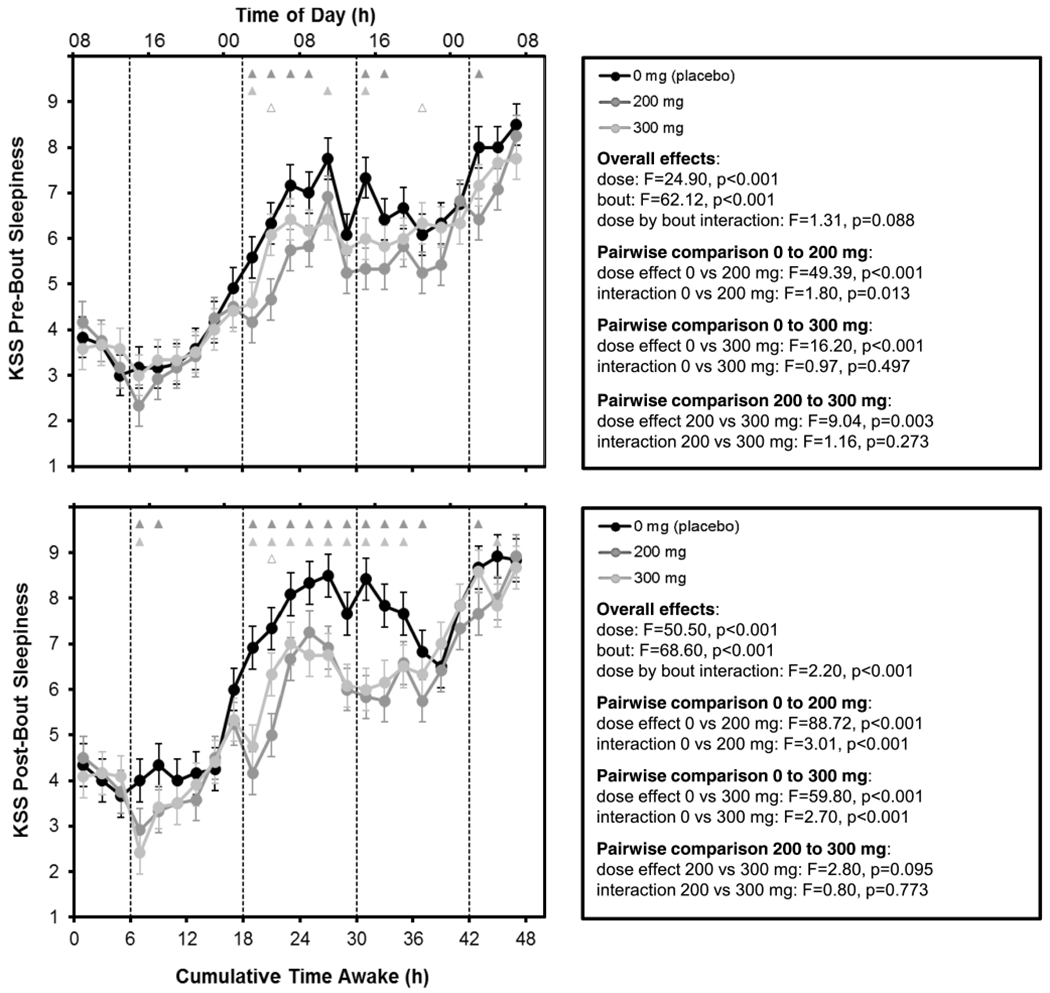
Mean performance (± SE) across time awake for KSS ratings pre-PVT (top) and post-PVT (bottom) as a function of caffeine dose. Upward corresponds to greater sleepiness. Dashed vertical lines denote the times of gum administration. Triangles at the top of each panel indicate statistically significant differences between 0 and 200 mg (dark gray), 0 and 300 mg (light gray), and 200 and 300 mg (open light gray) by bout
Pairwise comparisons of pre-PVT sleepiness ratings between doses overall and across time awake exhibited a drug effect, with both caffeine doses, and especially the 200 mg dose, reducing sleepiness compared to placebo. These effects were relatively small and primarily confined to the period from 19 to 33 h awake. The effects of caffeine on sleepiness were more pronounced in the post-PVT sleepiness ratings, with clear differences between the two caffeine conditions and the placebo condition in the period from 19 to 37 h awake and the emergence of a drug effect in the period from 7 to 11 h awake. However, as with PVT performance, there was essentially no distinction between the 200 mg and 300 mg doses in post-PVT sleepiness.
Discussion
This laboratory-based, randomized, double-blind, placebo-controlled, crossover study of the effect of repeated-dose caffeine on neurobehavioral performance was the first to compare the effects of three different caffeine doses—0 mg (placebo), 200 mg, and 300 mg—head to head in the same subjects during repeated exposures to the same TSD protocol. It was found that 200 and 300 mg doses of caffeine administered at 12 h intervals attenuated neurobehavioral impairment due to time awake (48 h of TSD) and time of day. The effectiveness of caffeine was most evident following the gum administrations at night, when the level of impairment in the placebo condition was highest (Figs. 2 and 4). It would be predicted that reducing the interval between repeated caffeine administrations could more effectively maintain performance—results from studies in which caffeine was administered at 2-h intervals (Kamimori et al. 2005; Paech et al. 2016) showed a more prolonged and consistent attenuation of impairment compared to that observed in the current study.
Neither the 200 mg dose nor the 300 mg dose of caffeine mitigated performance impairment due to total sleep deprivation completely in this study. Moreover, the additional benefit of the 300 mg dose compared to the 200 mg dose was small in the dosing regimen investigated here. This was not an artifact due to a floor effect or insensitivity of the neurobehavioral tests; both the PVT and the KSS had ample dynamic range, as seen in the placebo condition. There was also no evidence of a progressive increase in caffeine’s effect over the repeated administrations (Fig. 3).
Our study procedures were designed to minimize prior sleep loss before exposure to TSD. Nonetheless, we found that caffeine produced a small improvement in post-PVT subjective sleepiness during the first approximately 18 h of wakefulness (i.e., prior to actual sleep deprivation). This could mean that caffeine is a performance enhancer (i.e., improves neurobehavioral functioning beyond baseline optimal performance), but evidence from the literature to support this idea is mixed (Snel and Lorist 2011). Sleep homeostatic equilibrium is shaped by long-term sleep/wake history (Rupp et al. 2009; Grant and Van Dongen 2013) and potentially by long-term caffeine intake history (McCauley et al. 2009). Therefore, the improvement from caffeine seen prior to sleep deprivation may also reflect a suboptimal state of sleep homeostatic equilibrium in some or all of the subjects—in other words, subjects may still have carried a small sleep debt at the start of each sleep deprivation period. Whether (and to what extent) additional sleep extension in advance of study participation and between the periods of total sleep deprivation would have been required to fully eliminate any potential sleep debt in the subjects remains to be determined.
Consistent with results from previous studies showing discrepancies between the objective and subjective effects of caffeine (Penetar et al. 1993; Paech et al. 2016), we found that pre-PVT sleepiness ratings on the KSS in the caffeine conditions paralleled those in the placebo condition rather than tracking caffeine effects on PVT performance. Post-PVT sleepiness ratings tracked the impact of caffeine on PVT performance more closely. Differences in pre- versus post-PVT sleepiness ratings have previously been interpreted as evidence of masking in pre-PVT ratings by prior activity, unmasked in post-PVT ratings by standardized test bout conditions designed to minimize behavioral confounds (Van Dongen and Dinges 2000)—but this would not explain the caffeine-specific discrepancies observed here. Rather, such findings suggest that some form of cognitive activity is required to observe caffeine’s beneficial effects, given that neurobehavioral impairment from sleep loss may be neuronal use dependent (Van Dongen et al. 2011). In this view, physiological sleepiness is manifested (or produced) when task performance requires neuronal use (Van Dongen et al. 2016), and only then could its temporary reversal via caffeine and other stimulants be accurately quantified.
The main limitation of this study was the small sample size (N = 12)—a trade-off to obtain the increased experimental control associated with crossover study designs. This limitation may be important given the large, systematic individual differences in vulnerability to sleep loss (Van Dongen et al. 2004) and sensitivity to caffeine (Quartana and Rupp 2012). See the Supporting Information for a statistical assessment of such individual differences in the data set. The crossover design of the study, however, allowed for analyses to focus entirely on within-subject comparisons, thereby substantially reducing the impact of individual differences on the study results.
To minimize volunteer dropout associated with the requirement to undergo three separate 48 h TSD periods, we opted to conduct all three TSD periods (corresponding to the three caffeine conditions) within a single, 18-day study. Our relatively short (3 days) washout period between consecutive TSD periods may have potentiated any order effects, which was mitigated by randomizing and counterbalancing the three caffeine conditions over the three TSD periods. All six possible permutations were represented, albeit with a small sample (N = 2) per permutation; we therefore also controlled for any remaining order effects statistically.
In summary, in the present study, we sought to better understand the pharmacodynamics of repeated caffeine administration during sleep deprivation across increasing levels of sleep loss and at different times of day. To this end, we measured objective performance and subjective sleepiness using a laboratory-based, randomized, double-blind, placebo-controlled, crossover study design. We found that caffeine was an effective countermeasure of objective performance impairment, as evidenced by reduced lapses and increased fidelity of information processing (LSNR) on the PVT, with a particularly pronounced effectiveness immediately following caffeine administration at times when impairment in the placebo condition was highest (at night after 18 h and 42 h of TSD). Subjective ratings of sleepiness mirrored the temporal pattern of PVT performance impairment in the placebo condition, but only post-PVT sleepiness ratings captured the beneficial effects of caffeine. These results will inform further efforts to incorporate caffeine’s effects in biomathematical models of fatigue (Ramakrishnan et al. 2013, 2014, 2016).
Supplementary Material
Acknowledgements
The authors thank Walter Reed Army Institute of Research (WRAIR) for supplying the caffeine and placebo gum used in the study. They are grateful to Dr. Thomas Balkin of WRAIR for serving as the contracting officer’s technical representative. The authors also acknowledge the staff of the Human Sleep and Cognition Laboratory in the Sleep and Performance Research Center at Washington State University for their assistance in data collection.
Funding This research was supported by Office of Naval Research grant N00014-15-1-0019. SR and JR were supported by the Military Operational Medicine Program Area Directorate of the US Army Medical Research and Materiel Command, Fort Detrick, MD.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00213-018-5140-0) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
The study was approved by the Institutional Review Board (IRB) of Washington State University. Subjects gave written, informed consent prior to participation.
Conflict of interest The authors declare that they have no conflict of interest.
Publisher's Disclaimer: Disclaimer The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army or of the US Department of Defense. This paper has been approved for public release with unlimited distribution.
References
- Åkerstedt T, Anund A, Axelsson J, Kecklund G (2014) Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. J Sleep Res 23:240–252 [DOI] [PubMed] [Google Scholar]
- Benitez PL, Kamimori GH, Balkin TJ, Greene A, Johnson ML (2009) Modeling fatigue over sleep deprivation, circadian rhythm, and caffeine with a minimal performance inhibitor model. Methods Enzymol 454:405–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmann S, Hohoff C, Freitag C, Deckert J, Rétey JV, Bacmann V, Landolt HP (2012) Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioral performance and sleep EEG after sleep deprivation. Br J Pharmacol 165:1904–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL (2012) Utility of caffeine: evidence from the laboratory In: Wesensten NJ (ed) Sleep Deprivation, Stimulant Medications, and Cognition. Cambridge University Press, New York, pp 82–92 [Google Scholar]
- Chavali VP, Riedy SM, Van Dongen HPA (2017) Signal-to-noise ratio in PVT performance as a cognitive measure of the effect of sleep deprivation on the fidelity of information processing. Sleep 40:zsx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark HE, Kamimori GH, LaValle CR, Eonta SE (2015) Effects of high habitual caffeine use on performance during one night of sleep deprivation: do high users need larger doses to maintain vigilance? J Caff Res 5:155–166 [Google Scholar]
- Denaro CP, Brown CR, Wilson M, Jacob P, Benowitz NL (1990) Dose-dependency of caffeine metabolism with repeated dosing. Clin Pharmacol Ther 48:277–285 [DOI] [PubMed] [Google Scholar]
- Doan BK, Hickey PA, Lieberman HR, Fischer JR (2006) Caffeinated tube food effect on pilot performance during a 9-hour simulated nighttime U-2 mission. Aviat Space Environ Med 77:1034–1040 [PubMed] [Google Scholar]
- Gottselig JM, Adam M, Rétey JV, Khatami R, Achermann P, Landolt HP (2006) Random number generation during sleep deprivation: effects of caffeine on response maintenance and stereotypy. J Sleep Res 15:31–34 [DOI] [PubMed] [Google Scholar]
- Grant DA, Van Dongen HPA (2013) Individual differences in sleep duration and responses to sleep loss In: Shaw PJ, Tafti M, Thorpy MJ (eds) The Genetic Basis of Sleep and Sleep Disorders. Cambridge University Press, Cambridge, pp 189–196 [Google Scholar]
- Hauri P, Linde S (1990) No more sleepless nights. Wiley, New York [Google Scholar]
- Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, Eddington ND (2002) The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm 234:159–167 [DOI] [PubMed] [Google Scholar]
- Kamimori GH, Johnson D, Thorne D, Belenky G (2005) Multiple caffeine doses maintain vigilance during early morning operations. Aviat Space Environ Med 76:1046–1050 [PubMed] [Google Scholar]
- Kamimori GH, McLellan TM, Tate CM, Voss DM, Niro P, Lieberman HR (2015) Caffeine improves reaction time, vigilance, and logical reasoning during extended periods with restricted opportunities for sleep. Psychopharm 232:2031–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R (2002) Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharm 164:250–261 [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF (2008) Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129:305–322 [DOI] [PubMed] [Google Scholar]
- McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA (2009) A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol 256:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan TM, Kamimori GH, Bell DG, Smith IF, Johnson D, Belenky G (2005a) Caffeine maintains vigilance and marksmanship in simulated urban operations with sleep deprivation. Aviat Space Environ Med 76:39–45 [PubMed] [Google Scholar]
- McLellan TM, Kamimori GH, Voss DM, Bell DG, Cole KG, Johnson D (2005b) Caffeine maintains vigilance and improves run times during night operations for Special Forces. Aviat Space Environ Med 76: 647–654 [PubMed] [Google Scholar]
- McLellan TM, Kamimori GH, Voss DM, Tate C, Smith SJ (2007) Caffeine effects on physical and cognitive performance during sustained operations. Aviat Space Environ Med 78:871–877 [PubMed] [Google Scholar]
- Paech GM, Banks S, Pajcin M, Grant C, Johnson K, Kamimori GH, Vedova CBD (2016) Caffeine administration at night during extended wakefulness effectively mitigates performance impairment but not subjective assessments of fatigue and sleepiness. Pharmacol Biochem Behav 145:27–32 [DOI] [PubMed] [Google Scholar]
- Penetar DH, McCann U, Thorne D, Kamimori G, Galinski C, Sing H, Thomas M, Belenky G (1993) Caffeine reversal of sleep deprivation effects on alertness and mood. Psychopharmacology 112:359–365 [DOI] [PubMed] [Google Scholar]
- Puckeridge M, Fulcher BD, Phillips AJK, Robinson PA (2011) Incorporation of caffeine into a quantitative model of fatigue and sleep. J Theor Biol 273:44–54 [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Rupp TL (2012) Genetic basis of individual vulnerability to sleep loss and responsivity to stimulants In: Wesensten NJ (ed) Sleep deprivation, stimulant medications, and cognition. Cambridge University Press, New York, pp 43–57 [Google Scholar]
- Ramakrishnan S, Rajaraman S, Laxminarayan S, Wesensten NJ, Kamimori GH, Balkin TJ, Reifman J (2013) A biomathematical model of the restoring effects of caffeine on cognitive performance during sleep deprivation. J Theor Biol 319:23–33 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Laxminarayan S, Wesensten NJ, Kamimori GH, Balkin TJ, Reifman J (2014) Dose-dependent model of caffeine effects on human vigilance during total sleep deprivation. J Theor Biol 358:11–24 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Wesensten NJ, Kamimori GH, Moon JE, Balkin TJ, Reifman J (2016) A unified model of performance for predicting the effects of sleep and caffeine. Sleep 39:827–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifman J, Kumar K, Wesensten NJ, Tountas NA, Balkin TJ, Ramakrishnan S (2016) 2B-Alert Web: an open-access tool for predicting the effects of sleep/wake schedules and caffeine consumption on neurobehavioral performance. Sleep 39:2157–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer LA, Horne JA (2000) Early morning driver sleepiness: effectiveness of 200 mg caffeine. Psychophysiology 37:251–256 [PubMed] [Google Scholar]
- Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ (2009) Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep 32(3):311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Brockman P, Flynn R, Maben A, Thomas M (1993) Investigation of the effects of coffee on alertness and performance during the day and night. Neuropsychobiology 27:217–223 [DOI] [PubMed] [Google Scholar]
- Snel J, Lorist MM (2011) Effects of caffeine on sleep and cognition. Prog Brain Res 190:105–117 [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Goel N, Dinges DF (2014) Cumulative neurobehavioral and physiological effects of chronic caffeine intake: individual differences and implications for the use of caffeinated energy products. Nutr Rev 72(Suppl. 1):34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikuisis P, Keefe AA, McLellan TM, Kamimori GH (2004) Caffeine restores engagement speed but not shooting precision following 22 h of active wakefulness. Aviat Space Environ Med 75:771–776 [PubMed] [Google Scholar]
- Van Dongen HPA, Dinges DF (2000) Circadian rhythms in fatigue, alertness, and performance In: Kryger MH, Roth T, Dement WC (eds) Principles and Practice of Sleep Medicine, 3rd edn. WB Saunders, Philadelphia, pp 391–399 [Google Scholar]
- Van Dongen HPA, Price NJ, Mullington JM, Szuba P, Kapoor S, Dinges DF (2001) Caffeine eliminates sleep inertia: evidence for the role of adenosine. Sleep 7:813–819 [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Baynard MD, Maislin G, Dinges DF (2004) Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 27:423–433 [PubMed] [Google Scholar]
- Van Dongen HPA, Belenky G, Krueger JM (2011) Investigating the temporal dynamics and underlying mechanisms of cognitive fatigue In: Ackerman PL (ed) Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications. American Psychological Association, Washington, DC, pp 127–147 [Google Scholar]
- Van Dongen HPA, Balkin TJ, Hursh SR (2016) Performance deficits during sleep loss and their operational consequences In: Kryger MH, Roth T, Dement WC (eds) Principles and Practice of Sleep Medicine, 6th edn. Elsevier, Philadelphia, pp 682–688 [Google Scholar]
- Wyatt JK, Cajochen C, Cecco ARD, Czeisler CA, Dijk DJ (2004) Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep 27:374–382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


