Abstract
Background:
Unknown origin pneumonia has been furiously spreading since the late of December 2019, subsequently spread to approximately all provinces and areas in China and many countries, which was announced as a Public Health Emergency of International Concern by World Health Organization (WHO). The studies on 2019 Corona Virus Disease-19 (COVID-19) conducted from various fields around the world. Herein, the objective of the present study is to summarize the etiology, epidemiology, clinical manifestations, image findings, traceability analysis, and drug development of COVID-19.
Methods:
The following electronic databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database, VIP Chinese Science and Technology Periodical Database, and Wanfang Data. Other relevant literature will be manually searched as a compliment. We have reviewed etiology, epidemiology, clinical manifestations, image findings, and medication from case reports and retrospective clinical studies relating to COVID-19 published since the outbreak.
Results:
The coronavirus is closely related to bat coronavirus and pangolin coronavirus. Besides, the infection pathway is confirmed to be the respiratory and digestive systems. The virus indicates person-to-person transmission and some patients present asymptomatic. The elderly have a higher mortality rate. Rapid and sensitive nucleic acid testing is usually used as a basis for diagnosis. Currently, there is no specific vaccine and antiviral drug. Intervention actions such as travel bans and quarantine adopted have effectively reduced the spread of the epidemic.
Conclusion:
This systemic review will provide high-quality evidence to summarize etiology, epidemiology, clinical manifestations, image findings, traceability analysis, drug development in patients with COVID-19.
Keywords: 2019 Corona Virus Disease-19, clinical manifestations, drug development, epidemic characteristics, pathogenic mechanism
1. Introduction
Unknown origin pneumonia has been furiously spreading since the late of December 2019, which has been epidemiologically and etiologically investigated by the Chinese Center for Disease Control and Prevention immediately.[1–4] Subsequently, a novel coronavirus, as the pathogen, was identified,[5] which was termed as 2019 novel coronavirus (2019-nCoV) by the WHO. Currently, the virus boasts a new nomenclature: severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), and the disease triggered by it is entitled 2019 Corona Virus Disease-19 (COVID-19); those infected patients mostly involved their lungs, the National Health Commission of the People's Republic of China, therefore, named pneumonia induced by the virus as novel coronavirus pneumonia (NCP), and listed COVID-19 as a Class B infectious disease specified in the Law of the People's Republic of China on the Prevention and Treatment of Infectious Diseases, indicting the prevention and control measures for Class A infectious diseases should be taken. By 18:00 on April 9, 2020, a total of 83,264 confirmed cases and 3344 cumulative deaths have been reported nationwide. Additionally, confirmed patients had been reported in several countries, most involving those people living or visiting Wuhan; and the virus has been well-established that person-to-person transmission is a truth.[2,3,6,7] This article summarizes the relevant etiology, epidemiology, clinical manifestations, imaging findings, and medication on COVID-19 and analyzes and reviews the etiology, epidemiology, clinical manifestations, image findings, and medication of COVID-19 to help comprehensively understand and correctly cope with COVID-19.
2. Ethics
This study was approved by the ethics committee of the West China Hospital, Sichuan University, Chengdu, Sichuan, China. The information of patients involved in the present study has been anonymized.
3. Etiology
Coronaviruses refer to single positive-stranded RNA viruses that roundly or ovally shaped (usually polymorphous) with envelope, which is characterized by the corolla shaped periphery protrusion on the viral envelope and is frequently associated with acute respiratory infections in humans that belong to a more sophisticated class of pathogens.[3,8–11] Zoonotic pathogens,[12] namely severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV) that initiate severe respiratory disease in humans, have spread worldwide and attracted much international attention. The Gao's Group disclosed that SARS-COV-2, different from genetic characteristics of MERS-CoV and SARS-CoV, is a novel coronavirus.[5] The physicochemical characteristics of SARS-COV-2 have not yet been clarified, and it is generally accepted that SARS-COV-2 is sensitive to ultraviolet light and heat and can be effectively killed by lipid solvents such as diethyl ether, while chlorhexidine is with poor effects.[13–16] SARS-COV-2 is an RNA virus that may mutate during the occurrence and development of the disease, thereby making it more difficult for epidemic prevention and control.[2,5,17–20]
4. Epidemiology
Transmission is the central principle in biology and epidemiology of infectious diseases, and for many viruses, the transmission from animals to humans is one of the critical steps in the pathogenesis. SARS-COV-2 owns 96% ribonucleic acid similarity to the coronavirus isolated from Rhinolophus sinicus in Yunnan province, China,[18] with origins traceable to a local seafood wholesale market in Wuhan, China. Bats may be the original reservoir of the virus with animals sold on the market by the intermediate hosts,[9] it is human and animal comorbidity ability remains exploration. The prime source of infection understood presently is patients with SARS-COV-2 infection, and those asymptomatic infected individuals may also become the source of infection. Transrespiratory droplet and contact transmission are the main routes of transmission. SARS-COV-2 has an S protein structure similar to SARS-CoV, which can bind to angiotensin-converting enzyme II (ACE2),[18,21–25] a cellular receptor in humans, to infect cells, it has been shown that ACE2 expression is especially abundant in lung and small intestinal tissues.[20] On February 13, 2020, personnel from the Chinese Center for Disease Control and Prevention isolated 2 novel coronaviruses from the feces of confirmed patients, demonstrating that there was indeed a live virus in the feces, which further suggested that we had the possibility of oral transmission of SARS-COV-2. Whether aerosol or mother-to-child transmission is possible also needs to be further clarified. From the current dissemination rate to its interpersonal transmission ability, the population is generally susceptible. By further studying the affinity of SARS-COV-2 to ACE2, it may be possible to verify its transmission capacity. The first survey by the Chinese Center for Disease Control and Prevention found that the average incubation period of this virus was about 5.2 days, with a maximum of fewer than 14 days, and the basic reproduction number was estimated to be 2.2,[1] which predicted the infectivity of SARS-COV-2, which provided first-hand data for human understanding of the epidemiological characteristics of SARS-COV-2, but epidemiological data may be continuously updated based on the increase of patients and a series of national prevention and control measures. Continue to monitor the epidemiological characteristics of more patients, closely investigate the source of the virus and intermediate hosts, and pay attention to its future evolutionary direction, adaptability, transmission ability, and route, all of which play a considerable role in guiding the prevention and control of the epidemic.
5. Clinical Manifestations
The Gao Group reported 3 patients with pneumonia of unknown cause found in the first time, with clinical manifestations of fever and cough with chest discomfort.[5] Huang et al,[26] reported the clinical features of the first 41 patients diagnosed with COVID-19, with infectious symptoms including fever, cough, and generalized weakness, but their upper respiratory symptoms were not prominent; however, gastrointestinal symptoms such as diarrhea, which are common in SARS-CoV infected patients, were not significant in COVID-19 patients. Chen et al,[27] retrospectively analyzed the clinical manifestations of 99 patients with COVID-19 and found that among 99 patients, fever and cough accounted for >50% of the total patients, shortness of breath accounted for 31%, muscle pain for 11%, and there were also few patients with confusion, headache, sore throat, runny nose, chest pain, diarrhea, nausea, and vomiting (Table 1). As we can see above, common symptoms are fever, cough, dyspnea, and fatigue, which may be accompanied by upper respiratory tract symptoms. COVID-19 Quick Advice for Diagnosis and Treatment Guidelines (standard version) points out fever as a typical symptom. It should be noted that patients with mild disease may have no positive symptoms and signs, and some ill and critically ill patients may have moderate to low fever or even no significant fever. Gastrointestinal symptoms may exist as the first manifestation of the virus due to the expression of ACE2 in the intestine[7]; cough with nausea, vomiting, and abnormal stools was reported in the first diagnosed patient in the United States.[28]
Table 1.
Proportion of clinical symptoms in patients with COVID-19.

With the spread of the epidemic and the increase in the number of cases, COVID-19 patients may have atypical symptoms as the first manifestation.[29,30] For such patients, “early detection, early diagnosis, and early treatment” should be achieved to avoid missed diagnosis. In the early stage of the disease, the total white blood cell count in the peripheral blood of most patients may be normal or reduced, the lymphocyte counts reduced, the high-sensitivity C-reactive protein increased, and normal procalcitonin.[17,31–33]
6. Pathogenesis
6.1. The effect of cytokine storm on COVID-19
SARS-CoV-2 is an RNA virus of β-coronavirus. The S protein on its capsule membrane is a crucial protein for invading target cells. It binds to the target cell receptor ACE2 through the S protein and then begins to replicate and drift in large numbers after entering the target cells. The human body can eliminate SARS-CoV-2 through the occurrence of an immune response. However, the affinity between S protein of SARS-CoV-2 and target cell ACE2 is 10 to 20 times stronger than that of SARS, which may be the main reason for its strong infectivity, infectivity, and pathogenicity.[9] After preliminary clinical observation, critically ill patients with COVID-19 may exhibit a significant increase in inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-γ (IFN-γ),[34] characterized by a cytokine storm syndrome (CSS).
CSS is an over-immune response induced by SARS-CoV-2 in the body, resulting in an imbalance of cytokine levels, such as TNF-α, IL-1, IFN-γ, IFN-α, and IFN-β.[35] Consequently, it is easy to make the immune system out of control and cause systemic inflammatory response syndrome (SIRS), ARDS, septic shock, multiple organ failure (MOF), and even death. de Wit et al[36] reported that the characteristics of CCS caused by SARS-CoV, SARS-CoV-2, or MERS-CoV are different. After SARS-CoV-2 infection, TNF-α, granulocyte colony stimulating factor (G-CSF), and interferon-inducible protein-10 (IP10) cytokines were highly expressed, and the expression levels of TNF-α and G-CSF were firmly related to the degree of illness, which was different from MERS-CoV and SARS-CoV infection. After MERS-CoV infection, lung inflammation was caused by high expression of IFN-γ, TNF-α, IL-17, and other pro-inflammatory cytokines. After SARS-CoV infection, high expression of IL-6, IL-12, IFN-γ other cytokines resulted in the extensive pulmonary inflammatory response.
Moreover, SARS-CoV-2 infection mainly leads to diffuse alveolar injury and transparent membrane formation in the lungs. Although the degree of pulmonary fibrosis is not apparent, the inflammatory response in the lung tissue is severe. Therefore, it is necessary to monitor the patient's inflammatory indicators to assess changes in the condition.
The lungs of patients who died of COVID-19 had the following manifestations[37]: diffuse alveolar injury with fibrous mucinous exudation in both lungs; the epithelial cells of alveoli were exfoliated, the hyaline membrane was formed, and pulmonary edema was observed; the inflammatory infiltration of monocytes in the lung stroma was mainly lymphocytes; multinucleated giant cells and atypical enlarged alveolar cells were found in the alveoli. Besides, after SARS-CoV-2 infection, peripheral blood CD4+ T and CD8+ T cells had low expression numbers, but higher activation degree.
6.2. The role of ACE2 in COVID-19
SARS-CoV-2 binds to the ACE2 receptor through S protein on the capsule, then enters the cell through endocytosis, and infecting the ciliated bronchial epithelial cells and type II alveolar cells in the lung. AP2 related protein kinase 1 (AAK1) is the main regulatory factor of endocytosis. The damage of AAK1 can prevent SARS-CoV-2 from entering the cell and inhibit the replication of the virus. Therefore, AAK1 inhibitor agents may become a potential target drug for anti-COVID-19 therapy in the future. The SARS-CoV-2 infection causes the renin-angiotensin system (RAS) imbalances, and serum Ang II expression level increased. Angiotensin-converting enzyme inhibitor (ACEI) therapy can significantly reduce the high levels of cytokines and lung inflammation caused by SARS-CoV-2 infection.[38] We believe that the regulation of ACEI levels or the treatment of COVID-19 with angiotensin receptor blockers (ARB) may be future research hotspots.
7. Diagnosis
7.1. Radiological findings
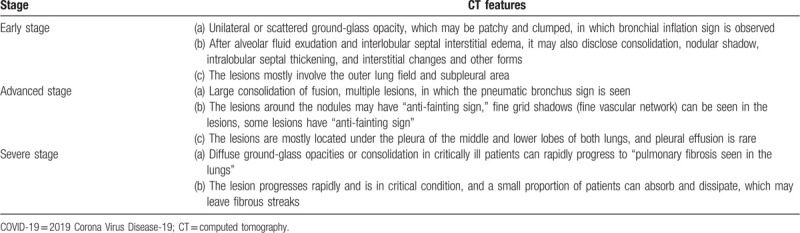
Computed tomography (CT) imaging is a strongly recommended auxiliary diagnostic modality.[28,39–48] In the early stage, due to pulmonary edema and hyaline membrane formation, alveolar septal telangiectasia and congestion, CT mostly shows unilateral or scattered ground-glass opacity, which may be patchy and clumped, in which bronchial inflation sign is observed. After alveolar fluid exudation and interlobular septal interstitial edema, it may also disclose consolidation, nodular shadow, intralobular septal thickening, and interstitial changes and other forms. The lesions mostly involve the outer lung field and subpleural area (Fig. 1).[47] Wang et al[48] analyzed 14 cases in early-stage and found that there was a time difference in clinical symptoms and CT findings in some patients (Fig. 2). In some patients, the density of the lesion is pale, and the extent is small in the early stage, and chest radiography shows that it is easy to miss the diagnosis. Chest CT is recommended to replace chest radiography in those with conditions.
Figure 1.
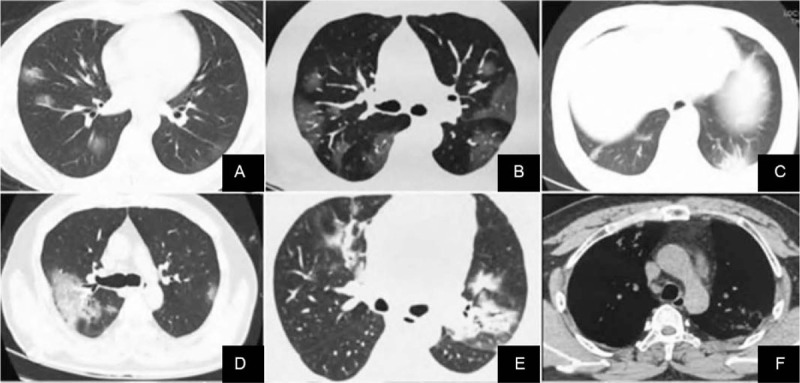
Chest CT images of novel coronavirus 19 pneumonia patients undergoing initial examination. A. Ground glass shadows can be seen in both lungs, and blood vessels are congested and thickened. B. CT showed subpleural ground glass shadows in both lungs with vascular congestion and thickening. C. Hypopleura consolidation with bronchiectasis in the left lower lung. D. The right lung shows large ground glass shadows, thickened interlobular septa, and a small amount of pleural effusion. E. Bilateral lungs show ground glass shadow, consolidation shadow, air bronchogram, and fibrotic lesions. F. The same patient as E, showing enlarged mediastinal lymph nodes. CT = computed tomography.
Figure 2.
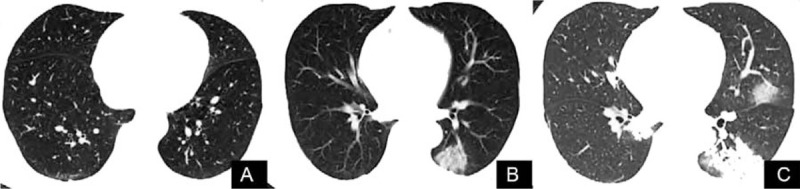
CT images showed that novel coronavirus pneumonia in patients with atypical pneumonia gradually deteriorated. A. At the early stage, no abnormal imaging findings were observed in the CT images. B. CT reexamination 5 days later revealed a localized ground-glass shadow of the left lower lung accompanied by air bronchogram. C. CT examination 10 days later showed scattered ground glass shadows of both lungs and consolidation of the left lower lung. CT = computed tomography.
In the advanced stage, alveolar and interstitial edema are further aggravated due to the accumulation of a large amount of exudate in the alveolar space, and fibrinoid exudation causes alveolar fusion. CT findings develop from an early small shadow to a large consolidation of fusion, and a single lesion develops into multiple lesions (Fig. 3), in which the pneumatic bronchus sign is seen.[39,47,48] However, the lesions around the nodules may have “anti-fainting sign,” fine grid shadows (fine vascular network) can be seen in the lesions, some lesions have “anti-fainting sign,” the lesions are mostly located under the pleura of the middle and lower lobes of both lungs, and pleural effusion is rare.
Figure 3.
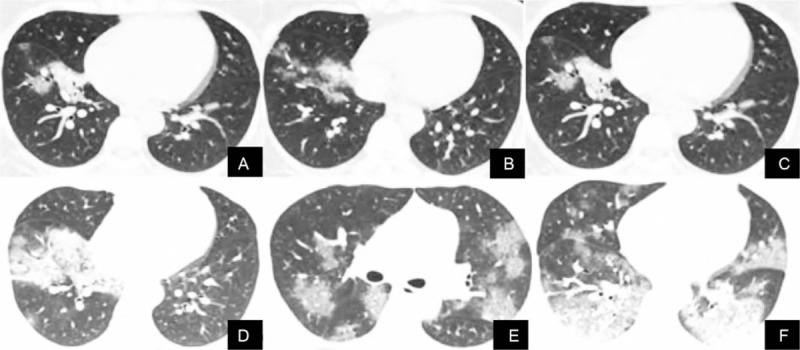
The CT images of a 33-year-old woman with new coronavirus pneumonia shows the progression of the disease. A–B. The first CT scan showed flaky ground glass shadows in the lower lobes of both lungs. C–D. Three days later, the lesions were enlarged, accompanied by consolidation and thickening of interlobular septa. E–F. CT images re-examination 8 days later showed the fusion of large ground glass shadow, consolidation shadow, and interlobar pleural thickening. CT = computed tomography.
In the severe stage, diffuse ground-glass opacities or consolidation in critically ill patients can rapidly progress to “pulmonary fibrosis seen in the lungs” (Fig. 4).[28] Clinical manifestations may be acute respiratory distress syndrome (ARDS), while secondary bacterial, fungal, and other pathogen infections. In this stage, the lesion progresses rapidly and is in critical condition, and a small proportion of patients can absorb and dissipate, which may leave fibrous streaks.[39,47]
Figure 4.
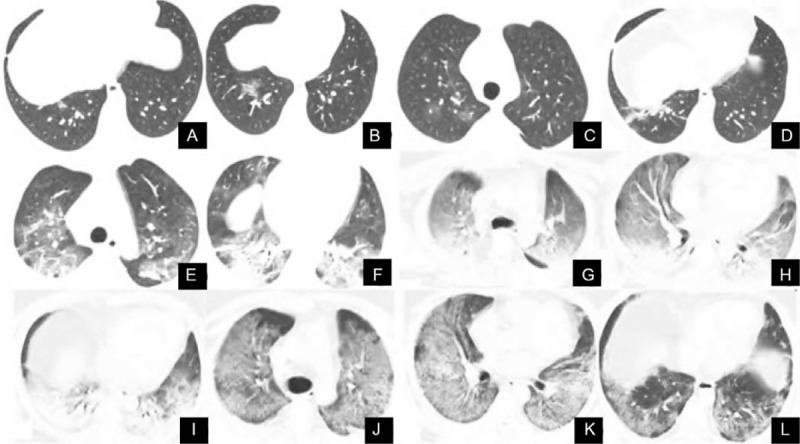
The CT images of a 53-year-old man with new coronavirus pneumonia show the progression of the disease. A. Initial CT examination revealed a small flaky high-density shadow in the lower lobe of the right lung. B. CT examination 3 days later revealed irregular ground glass shadows. C–D. CT scan was re-examined 6 days later, showing multiple thin ground glass shadows, indicating a significant deterioration of the disease. E–F. CT examination 10 days later revealed multiple patchy ground glass shadows in both lungs, accompanied by consolidation and fiber cord shadows. G–I. CT examination 14 days later revealed the appearance of “white lung” with an air bronchogram. J–L. The condition improved after treatment. CT = computed tomography.
In summary, the characteristic changes of chest CT in new-coronary pneumonia are subpleural or peripheral ground-glass opacities or fainting signs, mostly with bronchial ventilation signs (Table 2).
Table 2.
CT findings of COVID-19 patients at different stages.
7.2. Nuclear acid assays
The genome sequence of SARS-CoV-2 was published instantly after the start of the outbreak in Wuhan, China, on January 10, 2020. Reverse transcriptase-polymerase chain reaction (RT-PCR) was utilized to identify SARS-CoV-2 RNA as the main criterion for the diagnosis of COVID-19.[49] Nevertheless, it has the disadvantage of a high false-negative rate which may facilitate the epidemic. Consequently, a combination of disease history, clinical features, laboratory examination, and CT image is vital for making an accuracy diagnosis.[50]
8. Treatment
8.1. Medication
At present, there is no specific vaccine and antiviral drug, and research and development of a vaccine have its cycle and rule, which cannot be applied in clinical practice in the short term. Guidelines recommend that interferon-alpha, Lopinavir/Ritonavir, can be tried.[39] Interferon-alpha enhances immunity, Lopinavir is a human immunodeficiency virus 1 (HIV-1) protease inhibitor with broad-spectrum antiviral activity, and Ritonavir can inhibit Lopinavir metabolism thereby increasing its plasma concentration, so Lopinavir is usually used in combination with Ritonavir. During SARS in 2003, Hong Kong scholars found that Lopinavir combined with Ritonavir reduced the risk of ARDS and death compared with ribavirin monotherapy alone.[19] Early application of Lopinavir/Ritonavir against coronavirus can reduce patient mortality and glucocorticoid dosage, and late application has no significant effect. Therefore, early use of Lopinavir/Ritonavir in patients with confirmed COVID-19 may be beneficial in clinical practice, but its efficacy and safety are still controversial and need to be further evaluated.
Holshue et al[28] reported the trial administration of intravenous Remdesivir after the first confirmed patient's deterioration in the United States and found that raloxifene may have a good effect on inhibiting SARS-COV-2. Remdesivir is a novel ribonucleic acid analog under development that is superior to Lopinavir/Ritonavir in combination with interferon-beta in the treatment of MERS-CoV.[11]
At present, clinical trials have been conducted in China to evaluate its effect on the treatment of COVID-19. On February 4, 2020, Li Lanjuan's team released the latest research results in Wuhan: Abidol and Darunavir can effectively inhibit SARS-COV-2, which is a significant finding in treating COVID-19. To date, most of the broad-spectrum antiviral drugs used in clinical practice, and the therapeutic effect mostly needs practical evaluation. Blind use of antibacterial drugs should be avoided, especially in combination with broad-spectrum antibacterial drugs.[39]
The use of glucocorticoids is controversial. It should be used according to the patient's condition. Appropriate use of glucocorticoids can reduce the inflammatory response and promote the absorption of pulmonary lesions. However, it should also be noted that high-dose glucocorticoids will delay the clearance of SARS-COV-2 and have a certain incidence of adverse reactions.[39]
Immunoglobulins are purified blood products from the human body, containing a large number of antibodies, which interact with antigens to neutralize and kill bacteria and viruses. Under the action of large doses, they can also remove immune complexes in the human body and be used in combination with antiviral drugs, which can improve the efficacy for the infection of some serious viral diseases. Therefore, the application of immunoglobulins in treating COVID-19 is worthy of clinical promotion. Patients with COVID-19 can have natural antibodies, and it is necessary to consider convalescent plasma therapy when available. The S protein of SARS-COV-2 has a unique Flyn-like cleavage site (RRAR),[51] which has potential significance in studying its pathogenicity and developing drugs such as vaccines.
We note that SARS-COV-2 can bind to ACE2, providing direction for drug development. For patients with hypertension, ACEI/ARB drugs should be avoided in the selection of antihypertensive drugs. Patients often have anxiety and fear, and the treatment process strengthens the psychological counseling for patients.[39]
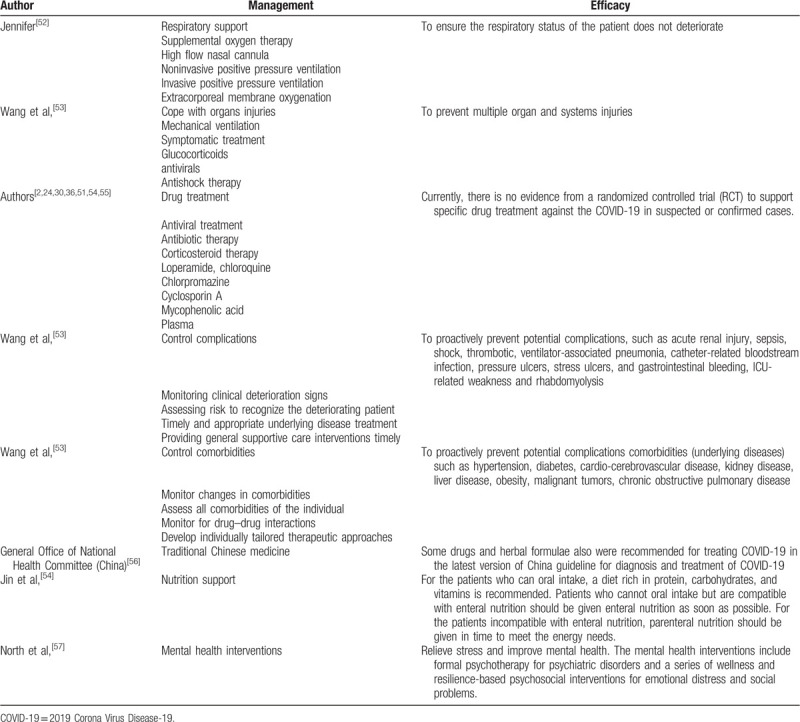
Besides, other treatment options, such as respiratory support, cope with organs injuries, Traditional Chinese Medicine, nutrition support, and mental health interventions, are also very essential for COVID-19 patients and listed in Table 3.
Table 3.
Treatment options for COVID-19 patients.
9. Prognostic factors
The prognosis factors of COVID-19 patients include age,[50,58] sex,[50,58] obesity,[59] smoking,[55,60–62] C-reactive protein (CRP), D-dimer, and lymphocyte count.[50]
About age, it was one of the most critical factors for COVID-19. COVID-19 mainly occurred in humans between 30 and 65, and people older than 65 years have a poor prognosis.[3,5,17,50] As for sex, men are more susceptible to COVID-19 than women.[63] Concerning obesity, it contributes to the increased risk and poorer prognosis of COVID-19.[59] As for smoking, it is very likely to be related to the negative progression and adverse consequences of COVID-19.[55,60–62]
Hematology examination includes CRP, D-dimer, lymphocyte count, and so on. CRP level can reflect the severity of inflammation, which has also been reported to be closely associated with the severity and prognosis of COVID-19.[64] Scholars have reported elevated CRP levels in most COVID-19 cases.[1,65] Wang et al,[65] reported that patients with the severe disease showed an increase in D-dimer concentration and a progressively decrease in lymphocyte count.
10. Conclusions
There has been some understanding of SARS-COV-2, a novel coronavirus, whose epidemiology is not yet fully understood and requires further investigation. Typical symptoms after infection are fever, fatigue, cough, dyspnea, etc., and atypical symptoms may be the first manifestation. Close monitoring of inflammatory markers during treatment avoids excessive inflammatory response. Typical chest CT findings are subpleural or peripheral ground-glass opacities or fainting signs, mostly with bronchial ventilation signs. Its imaging findings change rapidly, and the lesions can increase or decrease in a short time, so the interval between reexaminations should be shorter than that of common pneumonia, and reexamination in 2 to 3 days is recommended in the early stage (when the lesions are unstable). With the gradual increase of confirmed and suspected cases, the lag of nucleic acid detection and the lack of medical resources, whether to use CT instead of nucleic acid detection to diagnose COVID-19, has become a topic of discussion. It should be clear that during the outbreak, when nucleic acid detection is negative, it still needs to be comprehensively evaluated according to the clinical manifestations of patients, relevant examinations and the experience of doctors to avoid missing suspected patients, and causing the spread of the range. Timely and effective therapeutic measures are particularly necessary. At present, there is no specific vaccine and antiviral drug. It is hoped that more effective drugs can be screened on the basis of exerting known drug efficacy, and vaccines and specific drugs can be developed as soon as possible. Timely use of immunoglobulin and antibacterial drugs, rational use of glucocorticoids, and at the same time, psychotherapy should not be forgotten. At present, there are many problems to be discovered and solved urgently for COVID-19. We must recognize that COVID-19 brings great challenges to mankind, and it is urgent to understand and control the epidemic situation.
Author contributions
Funding acquisition: Miao Wang.
Methodology: Xue-Ping Ma, Long Bai.
Supervision: Jia Xu.
Validation: Long Bai.
Writing – original draft: Jia Xu, Xue-Ping Ma.
Writing – review & editing: Jia Xu, Xue-Ping Ma.
Footnotes
Abbreviations: ACE2 = angiotensin-converting enzyme II, AAK1 = AP2 related protein kinase 1, ACEI = angiotensin-converting enzyme inhibitor, ARDS = acute respiratory distress syndrome, COVID-19 = 2019 Corona Virus Disease-19, CSS = cytokine storm syndrome, CT = computed tomography, HIV-1 = human immunodeficiency virus 1, IL-2R = interleukin-2 receptor, IL-6 = interleukin-6, MERS-CoV = middle east respiratory syndrome coronavirus, MOF = multiple organ failure, NCP = novel coronavirus pneumonia, RAS = renin-angiotensin system, SARS-CoV = severe acute respiratory syndrome coronavirus, SARS-COV-2 = severe acute respiratory syndrome coronavirus 2, SIRS = systemic inflammatory response syndrome, WHO = World Health Organization.
How to cite this article: Xu J, Ma XP, Bai L, Wang M, Deng W, Ning N. A systematic review of etiology, epidemiology, clinical manifestations, image findings, and medication of 2019 Corona Virus Disease-19 in Wuhan, China. Medicine. 2020;99:42(e22688).
Disclosure statement: None.
Sichuan provincial science and technology program “Establishment of COVID 19 medical treatment information platform and remote consultation system” (Project No.: 2020YFS0001).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Han H, Luo Q, Mo F, et al. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect Dis 2020;20:655–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsai J, Wilson M. COVID-19: a potential public health problem for homeless populations. Lancet Public Health 2020;5:e186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect 2020;53:365–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruan ZR, Gong P, Han W, et al. A case of 2019 novel coronavirus infected pneumonia with twice negative 2019-nCoV nucleic acid testing within 8 days. Chin Med J (Engl) 2020;133:1487–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020;92:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yao Y, Pan J, Liu Z, et al. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur Respir J 2020;55:2000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ragan I, Hartson L, Pidcoke H, et al. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLoS One 2020;15:e0233947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamzavi IH, Lyons AB, Kohli I, et al. Ultraviolet germicidal irradiation: Possible method for respirator disinfection to facilitate reuse during the COVID-19 pandemic. J Am Acad Dermatol 2020;82:1511–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carrouel F, Conte MP, Fisher J, et al. COVID-19: a recommendation to examine the effect of mouthrinses with beta-cyclodextrin combined with citrox in preventing infection and progression. J Clin Med 2020;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 2020;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271.e8–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol 2020;92:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46:586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luo SH, Liu W, Liu ZJ, et al. A confirmed asymptomatic carrier of 2019 novel coronavirus (SARS-CoV-2). Chin Med J (Engl) 2020;133:1123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020;382:970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shah A, Kashyap R, Tosh P, et al. Guide to understanding the 2019 Novel Coronavirus. Mayo Clin Proc 2020;95:646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med 2020;58:1070–6. [DOI] [PubMed] [Google Scholar]
- [33].Won J, Lee S, Park M, et al. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19). Exp Neurobiol 2020;29:107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: The MuLBSTA Score. Front Microbiol 2019;10:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shinya K, Gao Y, Cilloniz C, et al. Integrated clinical, pathologic, virologic, and transcriptomic analysis of H5N1 influenza virus-induced viral pneumonia in the rhesus macaque. J Virol 2012;86:6055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin Y, Cai L, Cheng Z. Guidelines for rapid recommendations for the diagnosis and treatment of pneumonia with novel coronavirus (2019 nCoV) infection (standard edition). PLA Med J 2020;1:11–20. [Chinese]. [Google Scholar]
- [40].Dai WC, Zhang HW, Yu J, et al. CT imaging and differential diagnosis of COVID-19. Can Assoc Radiol J 2020;71:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): role of Chest CT in diagnosis and management. AJR Am J Roentgenol 2020;6:1–7. [DOI] [PubMed] [Google Scholar]
- [43].Qu J, Yang R, Song L, et al. Atypical lung feature on chest CT in a lung adenocarcinoma cancer patient infected with COVID-19. Ann Oncol 2020;31:825–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang W, Yan F. Patients with RT-PCR confirmed COVID-19 and normal chest CT. Radiology 2020;295:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou S, Wang Y, Zhu T, et al. CT features of Coronavirus Disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2020;214:1287–94. [DOI] [PubMed] [Google Scholar]
- [47].Guan H, Xiong Y, Shen N. Clinical imaging features of wuhan 2019 novel coronavirus (2019 nCoV) pneumonia. Radiol Pract 2020;15:1–6. [Chinese]. [Google Scholar]
- [48].Wang W, Hu H, Song L. Imaging findings and diagnosis of pneumonia with atypical novel coronavirus (2019 nCoV) infection: an analysis of 14 cases. Med knowled 2020;30:7–19. [Chinese]. [Google Scholar]
- [49].Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol 2020;30:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yi Y, Lagniton PNP, Ye S, et al. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci 2020;16:1753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Coutard B, Valle C, de Lamballerie X, et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jennifer AL. Springer, Adult Critical Care Medicine: A Clinical Casebook. Cham, Switzerland: 2019. [Google Scholar]
- [53].Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020;395:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res 2020;22:1653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].General Office of National Health Committee (China) Guideline for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infected Pneumonia (Trial Version 6). 2020. [Google Scholar]
- [57].North CS, Pfefferbaum B. Mental health response to community disasters: a systematic review. JAMA 2013;310:507–18. [DOI] [PubMed] [Google Scholar]
- [58].Mostaza JM, García-Iglesias F, González-Alegre T, et al. Clinical course and prognostic factors of COVID-19 infection in an elderly hospitalized population. Arch Gerontol Geriatr 2020;91:104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr 2020;14:655–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guo FR. Smoking links to the severity of Covid-19: An update of a meta-analysis. J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li Volti G, Caruso M, Polosa R. Smoking and SARS-CoV-2 disease (COVID-19): dangerous liaisons or confusing relationships? J Clin Med 2020;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Silva A, Moreira JC, Martins SR. COVID-19 and smoking: a high-risk association. Cad Saude Publica 2020;36:e00072020. [DOI] [PubMed] [Google Scholar]
- [63].Ciotti M, Angeletti S, Minieri M, et al. COVID-19 outbreak: an overview. Chemotherapy 2019;64:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnson HL, Chiou CC, Cho CT. Applications of acute phase reactants in infectious diseases. J Microbiol Immunol Infect 1999;32:73–82. [PubMed] [Google Scholar]
- [65].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


