Abstract
Background
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine important for the development of type-2 inflammatory responses at mucosal surfaces.
Objective
In humans, TSLP has been found to be elevated in the lungs of asthmatics, and in mouse models TSLP can promote type-2 airway inflammation, primarily through the activation of dendritic cells. However, the mechanisms underlying its role remain unclear. The objective of this study is to provide a mechanistic analysis of TSLP-mediated type-2 airway inflammation
Methods
To dissect the mechanisms of TSLP-mediated type II responses, mice were treated with TSLP and antigen to evaluate cellular immune responses. Flow cytometric analyses were used to follow responses in the airways, and conditional deletion of TSLPR and adoptive transfer were used to identify the cellular subsets involved in this inflammatory response.
Results
We show that TSLP can directly promote Th2 differentiation in the lung, independent of the draining lymph node. We also identified a population of patrolling monocytes/interstitial macrophages (CD11cIM) that are both necessary and sufficient for TSLP-mediated Th2 differentiation and airway inflammation. Th2-driven airway eosinophilia is attenuated by ablation of CD11cIM, or by selective deficiency of TSLPR signaling in these cells. More importantly, CD11cIM are sufficient for the induction of acute Th2 responses in the lungs that is independent of dendritic cells and T cell priming in the draining LN.
Conclusion
These findings indicate a novel mechanistic role for TSLP and CD11cIM in the development of acute Th2-dependent allergic airway inflammation. This work also demonstrates a new role for TSLP in promoting type-2 responses directly in the lung.
Keywords: TSLP, Th2, Interstitial macrophages, dendritic cells, lung, lymph node
Capsule Summary
Our study showed TSLP promotes rapid primary Th2 driven airway inflammation, which provides a novel mechanism in development of inflammation in tissue; and more importantly, a potential explanation for efficacy of anti-TSLP therapy in moderate-to-severe asthmatic patients.
Introduction
Allergic asthma is a chronic airway inflammatory disease driven by type 2 helper T cells (Th2). Airway eosinophilia, airway hyperresponsiveness, mucus overproduction, and goblet cell metaplasia are the hallmarks for this airway disorder1, 2. Disease progression is thought to be encompassed by a subclinical phase in which chronic antigen drives memory Th2 development, and the subsequent response of established memory Th2 cells results in clinically-relevant airway inflammation. Many cell types and immune mediators have been identified that play important roles in the development of this inflammatory disorder. In addition to immune cells, the airway epithelium is believed to play a critical role in disease development and progression, largely through its ability to produce soluble mediators (e.g., cytokines and chemokines) following encounter with allergens and/or respiratory viruses3. Thymic stromal lymphopoietin (TSLP), IL-25, IL-33, and GM-CSF are the predominant cytokines highly expressed by airway epithelium in response to insult, and importantly contribute to the development of type II airway inflammation4.
TSLP is a predominantly epithelial-derived cytokine whose receptor is comprised of the TSLPR and IL-7Rα subunits. Engagement of this receptor complex leads to the activation of Jak1 and Jak2, as well as Stat3 and Stat5 5–8. Several hematopoietic lineages can respond to TSLP. In addition, airway epithelial cells can also respond, suggesting that TSLP can establish a feedback autocrine or paracrine cascade during inflammatory responses8–10. An important role for TSLP in the development of allergic diseases has been described9, 11, 12. One mechanism underlying the role of TSLP in this process is its ability to drive the maturation of DC to promote Th2 differentiation through induction of OX40L expression13. Consistent with this role of TSLP on APC, specific deletion of TSLPR in dendritic cells inhibited epicutaneous sensitization14.
Several MHCII-expressing cells reside in lungs, including DC and macrophage subsets, such as alveolar macrophages (AM) and interstitial macrophages (IM), that could potentially serve as APC and impact T cell differentiation and activation15–17. While a role for TSLP in DC maturation is somewhat determined, the ability of MHCII+ pulmonary macrophage subsets to respond to TSLP stimulation, and subsequently impact CD4 T cell differentiation and activation, remains obscure.
In this study, we demonstrate that TSLP can trigger acute primary Th2-dependent inflammation when delivered in the airway with antigen. We have found a novel function for a lung myeloid subset, MHCII+ CD11c-expressing patrolling monocytes/interstitial macrophages (CD11cIM). These macrophages have a distinct phenotypic and functional phenotype compared to other known MHCII+ subsets in the lung (including interstitial macrophages, alveolar macrophages, and dendritic cells). This subset is both necessary and sufficient to promote TSLP-mediated acute airway inflammation, and direct TSLP responsiveness by these cells is required. Importantly, CD11cIM can directly promote Th2 differentiation in the lungs independent of DC or lymph nodes. Taken as a whole, our data demonstrate a novel role for TSLP in the functional regulation of pulmonary CD11cIM to act as APC for development of acute allergic airway inflammation through promotion of Th2 differentiation.
Methods
Mice
C57BL/6 and Balb/c mice were purchased from Charles River Laboratory; STAT6−/−, Nur77GFP, CX3CR1GFP, LysmCre, CD11cCre, OTII, Rag1−/−, CD45.1 C57BL/6 were purchased from Jackson Laboratory; CCR2-DTR Tg mice were generously provided by T. Hohl (Memorial Sloan-Kettering Institute), TSLPR deficient and TSLPR floxed mice were as described previously (Carpino, 2004; Han, 2014). Sex-matched male and female mice aged 7–12 weeks were used for experiments. All animals were housed in specific pathogen free facility of Benaroya Research Institute, and all experimental protocols were approved by the BRI IACUC.
Antibodies and Reagents
Antibodies used for flow cytometry and sorting: CD3e (145–2C11), CD4 (RM4–5), CD11b (M1/70), CD11c (N418), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD86 (GL-1), CD103 (2E7), IFN-g (XMG1.2), IL-13 (eBio13A), Ki67 (B56), Ly6C (HK1.4), Ly6G (1A8), MHCII (M5/114.15.2), Siglec-F (E50–2440), and TCRb (H57–597) were purchased from eBioscience, Biolegend, and BD Biosciences; CCR2 Ab (475301) was purchased from R&D System; and F4/80 Ab (CI:A3–1) was purchased from AbD Serotec. CD16/CD32 Ab (2.4G1) for Fc blocking and CD4 Ab (GK1.5) for depletion of CD4+ T cells were purchased from UCSF Monoclonal Antibody Core. CD62L Ab (MEL-14) was purchased from BioXCell.
TSLP was provided by Amgen; whole OVA protein, Diphtheria Toxin, Rat serum IgG, DNase I, PMA, and ionomycin were purchased from Sigma-Aldrich; OVA323–339 was purchased from New England Peptide; recombinant mouse IL-4 was purchased from Peprotech; Alexa647-conjugated OVA was purchased from Invitrogen; Collagenase B was purchased from Roche; fixable viability dye eFluor 780 was purchased from eBioscience; GolgiPlug was purchased from BD Biosciences.
Detection of mRNA expression by real-time PCR
Lungs of mice were harvested, and the total RNA was extracted (RNeasy mini kit, Qiagen), reverse transcribed into cDNA (QuantiTect Reverse Trasncription Kit, Qiagen), and performed real time PCR (CYBR Premix Ex Taq, Clontech) for detecting expression of various immune mediators. The primers used for real time PCR: GAPDH forward 5’-TCC ATG ACA ACT TTG GCA TTG-3’, GAPDH reverse 5’-CAG TCT TCT GGG TGG CAG TGA-3’; IL-4 forward 5’-TCA TCG GCA TTT TGA ACG AG-3’, IL-4 reverse 5’-TTT GGC ACA TCC ATC TCC G-3’; IL-13 forward 5’-ATT CCC TGA CCA ACA TCT CCA A-3’, IL-13 reverse 5’-CGG TTA CAG AGG CCA TGC AA-3’; CCL24 forward 5’-CCT GGT AGC CTG CGC GTG TT-3’, CCL24 reverse 5’-CCC CTG CCT TGG GAC AGA TGC-3’; Muc5AC forward 5’-AAA GAC ACC AGT AGT CAC TCA GCA A-3’, Muc5AC reverse 5’-CTG GGA AGT CAG TGT CAA ACC A-3’; IFN-g forward 5’-CCT GCG GCC TAG CTC TGA G-3’, IFN-g reverse 5’-GCC ATG AGG AAG AGC TGC A-3’.
in vivo induction of airway inflammation and cell depletion
Mice were treated intranasally with TSLP (25ng) and OVA (25ug) on day 0, day 3, and day 6. For depletion of CD4+ T cells, wild type mice were treated with 200ug anti-CD4 mAb (GK1.5) or Rat IgG as control one day prior the first two TSLP/OVA administrations. For depletion of CCR2-expressing cell subsets in CCR2-DTR Tg mice, mice were treated with 50ng or 200ng of DT intra-peritoneally one day prior each TSLP/OVA treatment.
Flow cytometry and sorting
Perfused whole lungs of mice were harvested and digested with Collagenase B (2mg/ml) and DNaseI (10 unit/ml) into single cell suspension. The cells were incubated with anti-CD16/CD32 (2.4G2) for Fc blocking, followed by staining with fluorescence-conjugated antibodies for flow cytometry (BD LSRII or Canto) or cell sorting (BD Aria). As described in Fig. 4b, CD11cIM were sorted as CD11cint, Siglec-F−, CD11b+, Ly6G−, and Ly6C−/lo and DC were sorted as CD11chi and Siglec-F− for both in vivo adoptive transfer (approximately 3×105 CD11cIM per transfer/host mouse) and in vitro experiments. The purity of sorted CD11cIM was approximately 85–95%, and sorted DC had approximately 80–90% purity.
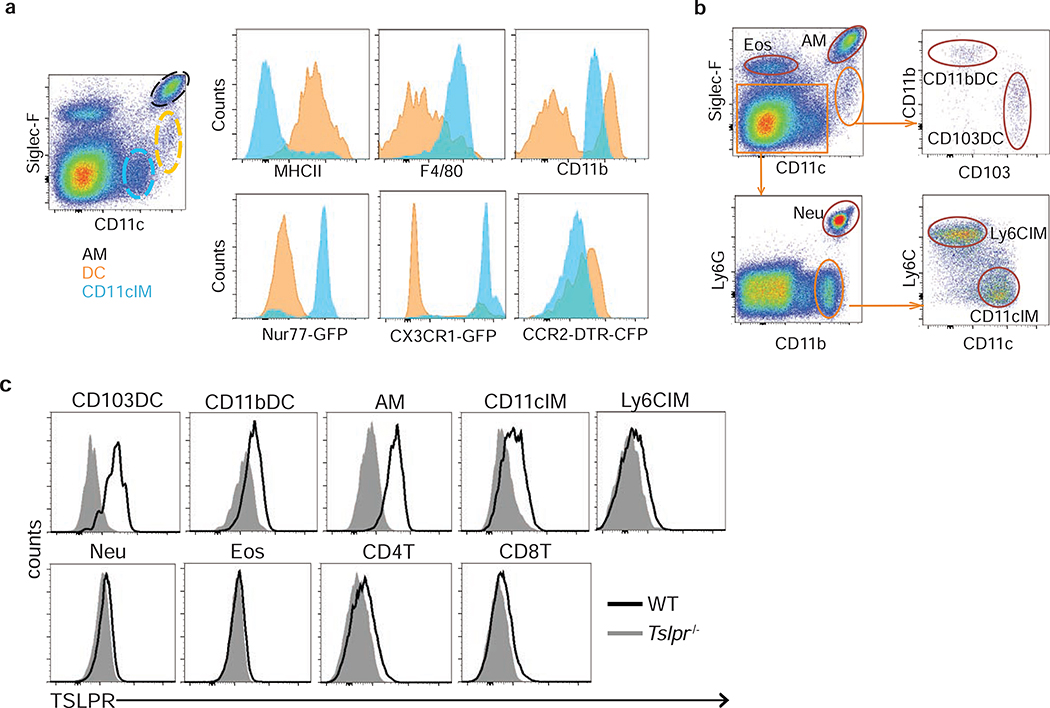
Figure 4. Expression of indicated molecules and TSLPR on pulmonary CD11c-expressing subsets.
(a) pulmonary CD11c-expressing subsets, including AM (black), DC (orange), and CD11cIM (blue), and their expression profiles of indicated molecules. (b) Gating strategy for identifying AM, DC subsets, IM subsets, eosinophils, and neutrophils. (c) Expression of TSLPR on pulmonary DC, AM, IM, granulocytes, and T cells. Representative flow cytometry plots from lungs of naïve mice.
in vitro T cell culture
Spleen and LN were harvested from OTII/Rag1−/−/CD45.1 mice, and OTII cells were purified with EasyPrep (Stem Cell) and followed by cell sorting (BD, Aria) for CD4+ cells. CFSE labeled 5×104 OTII were cultured alone or co-cultured with 2×104 CD11cIM or 5×103 DC. The ratio of OTII: CD11cIM (5:2) and OTII: DC (10:1) were chosen due to the ratio of CD4+ T cell: CD11cIM: DC (10:4:1) in the lungs of naive mice. 3ug/ml OVA323–339 was added for all conditions. 10ng/ml TSLP and/or 0.5ng/ml IL-4 were added for the selective culture conditions. Five days after culture, the OTII cells were re-stimulated with 0.05ug/ml PMA, 1ug/ml ionomycin, and Brefeldin A (GolgiPlug, BD Biosciences) for 2 hours followed by fixable viability dye staining, fixation/permeabilization, and Ab staining for flow cytometry.
Results
TSLP mediates acute eosinophilic airway inflammation
The epithelial cytokine TSLP has been shown to be a critical factor in promoting type-2 inflammatory responses. We and others found that, in a model of airway inflammation, loss of TSLP signaling resulted in a marked decrease in the inflammatory response11, 12. In addition, we have previously shown that intranasal administration of TSLP and antigen resulted in a memory CD4 T cell-dependent airway inflammatory response18. This response is exemplified by elevated levels of Th2 cytokines, mucus overproduction, and eosinophilic infiltration. However, in all of these model systems, the identity of TSLP responsive cells was unclear.
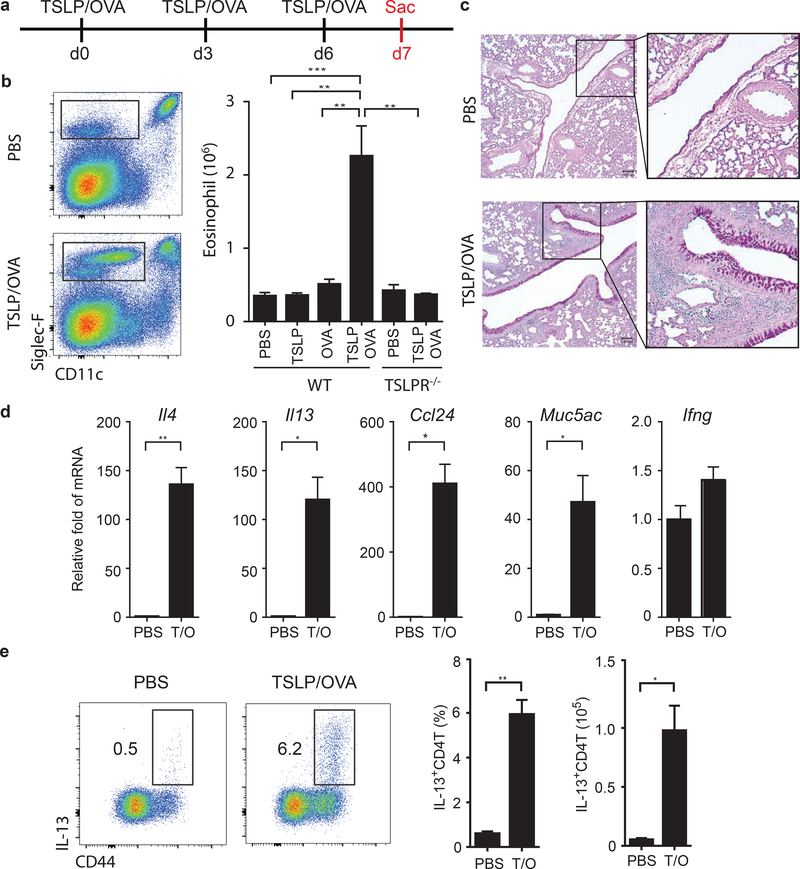
To dissect the mechanisms of TSLP-mediated type II responses in the airways we first analyzed the early stages of the response. Mice were treated with TSLP and antigen (ovalbumin; OVA) intranasally every 3 days (Fig. 1a), and were sacrificed at various time points to examine cellular responses in context of TSLP/OVA. Mice that received TSLP/OVA showed a significant eosinophilia in their lungs by day 7 (Fig. 1b), associated with accumulation of DC and CD4 T cells (Fig. S1a), but no change in neutrophils and AM (Fig. S1a). In addition, goblet cell metaplasia and mucus production was also markedly increased in the airways (Fig. 1c), as well as elevated expression of the type II cytokines IL-4, IL-13, and associated genes CCL24 and Muc5AC, but not the type I cytokine IFN-g (Fig. 1d). A significant increase of IL-13-producing (Fig. 1e), but not IFN-g-producing (Fig. S1b), CD4 T cells were also observed in the lungs. Importantly, this rapid airway inflammation requires both TSLP and OVA antigen (Fig.1b), leading us to hypothesize that TSLP may induce an atypical acute T cell-driven allergic airway inflammation that does not require antigen-specific memory Th2 cells.
Figure 1. TSLP mediates acute eosinophilic airway inflammation.
(a) Experimental design. WT and TSLPR deficient mice were treated intranasally with PBS, TSLP, OVA, or TSLP/OVA on d0, d3, and d6. On d7, the lungs of mice were harvested to evaluate airway inflammation by (b) eosinophilia (n=4–7), (c) lung histology (PAS staining), (d) mRNA expression of inflammatory mediators, Il4, Il13, Ccl24, Muc5ac, and Ifng (n=2–3), and (e) percentage and numbers of IL-13-producing effector CD4 T cells (n=3–4). Data are representative or combined of at least two independent experiments. Error bars represent SEM. * p<0.05, ** p<0.01***, p<0.001, ****p<0.0001. p-value by one-way ANOVA adjusted with Tukey’s multiple comparison test (b) or student’s two-tailed t test (d)(e).
TSLP mediates acute antigen required Th2-driven eosinophilic airway inflammation
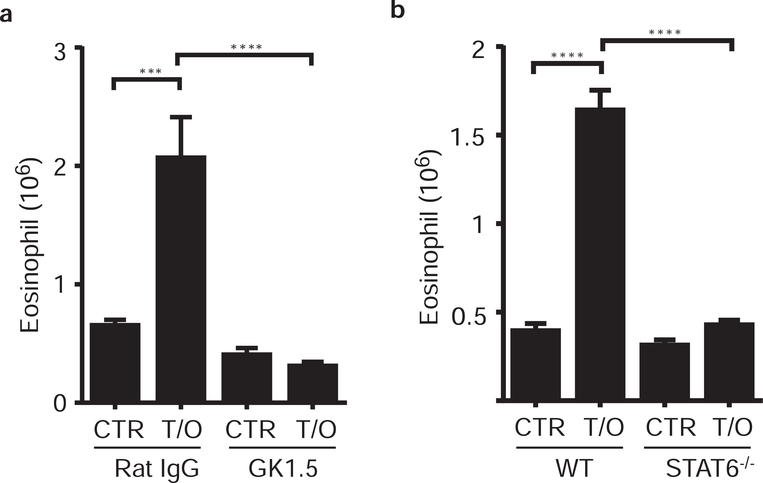
To determine whether this acute airway inflammation is truly a CD4 T cell-dependent allergic response, mice were treated with a depleting anti-CD4 mAb (GK1.5) one-day prior the first and second administration of TSLP/OVA (as described in Fig. 1a). The results showed that CD4 T cell-depleted mice had a diminished TSLP-mediated eosinophilic airway inflammation compared to control mice (Fig. 2a), showing that CD4 T cells are required for the TSLP-mediated acute airway inflammation. In addition, it has been shown that anti-CD4 mAb treatment does not deplete lung tissue resident memory CD4 T cells (Fig. S2a and S2b, and personal communication with DL Farber)19, indicating that tissue resident memory CD4 T cells are not sufficient, and further suggesting a role for acute priming of naïve CD4 T cells in this acute airway inflammation.
Figure 2. Acute TSLP mediated allergic airway inflammation requires T cells and signaling molecule STAT6.
Mice were treated with TSLP/OVA (T/O) or PBS (CTR) every 3 days as described in Fig. 1a, the lungs were harvested at d7 and airway eosinophilia was determined by flow cytometry. Pulmonary eosinophil infiltration in the lungs of (a) wild type C57BL/6 mice pretreated with anti-CD4 (GK1.5) or control (Rat IgG) antibodies (n=4–6), and (b) STAT6 deficient (Balb/c background) and wild type Balb/c mice (n=4–5). Data are representative of at least two independent experiments. ** p<0.01, *** p<0.001, ****p<0.0001. (a) and (b), p-value by two-way ANOVA.
Low doses of endotoxin (LPS) have been shown to favor type II responses20, and commercial OVA preparations are known to have variable amounts of LPS contamination. To investigate the possible role of contaminating LPS in OVA, mice were treated with TSLP/LPS or TSLP/low endotoxin OVA (<1EU/mg). On d7, no eosinophilic airway inflammation was observed in the lungs of mice treated with TSLP/LPS; in contrast, a significant eosinophilic inflammation was shown in the mice treated with TSLP/low endotoxin OVA (Fig. S2c). These data indicate that protein antigen, but not endotoxin, is required for the TSLP-mediated airway inflammation. Furthermore, to rule out the possibility of antigen non-specific type II responses, mice were treated with TSLP plus a self-antigen (mouse serum albumin; MSA). There was no eosinophilic inflammation detected in the lungs of these mice (Fig. S2d), indicating foreign antigen specificity in TSLP mediated type II responses.
The type II cytokines IL-4 and IL-13, signaling through Stat6, are necessary and sufficient for Th2 differentiation21. Therefore, we utilized IL-4, IL-13, and Stat6 deficient mice to further test the requirement of Th2 cells in this inflammatory response. Following TSLP/OVA treatment, deficiency of the signaling molecule Stat6 resulted in blunted eosinophilic airway inflammation (Fig. 2b); however, deficiency of IL-13 or IL-4 alone did not completely block TSLP-induced acute airway inflammation (Fig. S2e and S2f). Along with data showing that signaling through STAT6 is required for Th2 differentiation, these data demonstrate the requirement for Th2 cells in this inflammatory response. These results are consistent with previous studies demonstrating that TSLP can promote STAT6-dependent T cell responses13, 18. Together, these data show that TSLP induces atypical antigen-required Th2 cell-driven airway inflammation that is independent of memory Th2 cells.
TSLP responsive CD11c+ cells are critical for acute Th2 responses in the lungs, but not in the lymph nodes
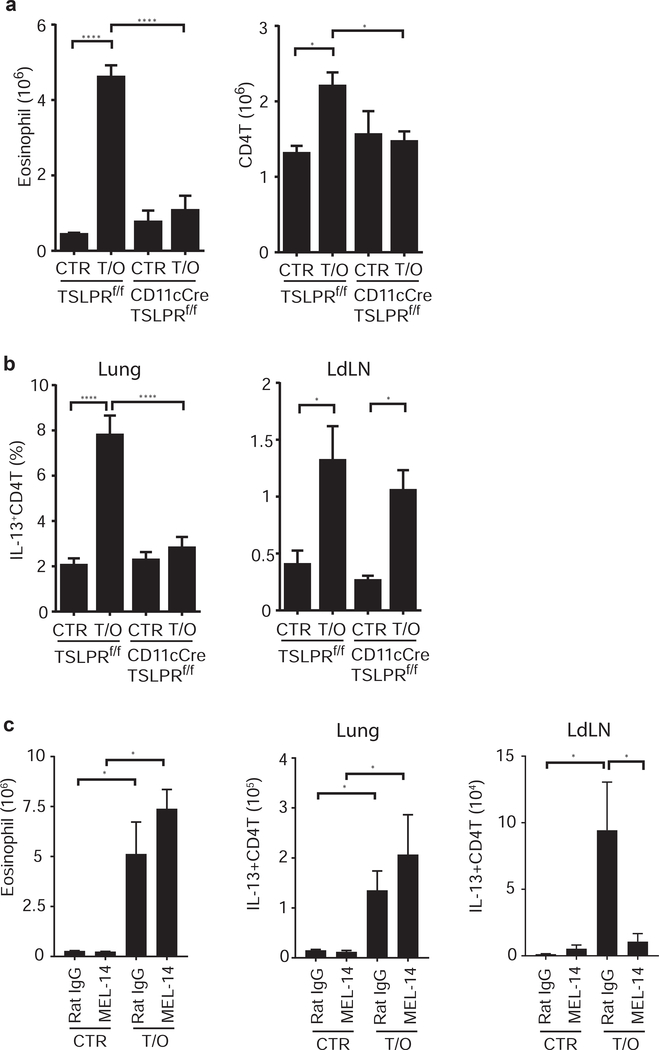
TSLP has been shown to directly act on DC to promote Th2 differentiation both in vitro and in vivo12, 14, 22. The requirement for adaptive immunity indicated potential roles for antigen presenting cells (APC) in the development of acute TSLP-induced inflammation. To address whether TSLP responsive DC are essential for this acute airway inflammatory response, we utilized CD11cCre/TSLPRf/f mice14. With administrations of TSLP/OVA as described in figure 1a, CD11cCre/TSLPRf/f mice displayed diminished eosinophilic allergic airway inflammation compared to their littermate control TSLPRf/f mice (Fig. 3a), showing that TSLP-responding CD11c+ cells are critical for this inflammatory response.
Figure 3. TSLP responsive CD11c+ cells are crucial for the acute airway inflammation.
(a) and (b) CD11cCre/TSLPRf/f mice and littermate TSLPRf/f mice were treated with TSLP/OVA every 3 days (as described in Fig. 1a), the lungs of mice were harvested at d7 to evaluate TSLP-mediated inflammatory responses by (a) infiltration of eosinophils and CD4+ T cells, and (b) IL-13-producing CD4+ T cells in the lungs and the LdLN (n=3–7). (c) WT C57BL/6 mice were treated with anti-CD62L mAb (MEL14) or control (Rat IgG) one day prior each TSLP/OVA administration. The lungs and LdLN of mice were harvested at d8, and pulmonary eosinophilia and IL-13-producing T cells were determined by flow cytometry (n=3–4). Data are representative or combined of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001, ****p<0.0001. p-value by two-way ANOVA adjusted with Tukey’s multiple comparison test.
Interestingly, a blunted Th2 response that is parallel to the diminished airway inflammation in CD11cCre/TSLPRf/f mice was only observed in the lungs (Fig. 3b and S3), while an intact Th2 response was seen in the LdLN (Fig. 3b). These data indicate that the deficiency of TSLPR on CD11c+ cells selectively affects Th2 differentiation in the lungs, but not in the draining LN. It further suggests that T cell priming in lymph nodes is not sufficient for this acute TSLP-mediated airway inflammation.
Furthermore, the uncoupled Th2 responses observed in the lungs and the draining LN led us to hypothesize that T cell priming in LN may not be required for the TSLP-mediated acute airway inflammation. To test this hypothesis, we used antibody blockade to inhibit the lymph node homing receptor CD62L. Mice were treated with anti-CD62L mAb (MEL-14) or control Ab (Rat IgG) one-day prior each TSLP/OVA administration (as described in Fig. 1a). We found no defect in Th2-driven eosinophilic inflammation in the lungs, but diminished Th2 responses in the LdLN, of anti-CD62L treated mice compared to the control mice (Fig.3c), showing that naïve T cell homing into lymph nodes is not required for TSLP-mediated acute airway inflammation. Together, these data indicate that Th2 priming in lymph nodes is neither sufficient nor required for TSLP-mediated acute airway inflammation, suggesting that T cell differentiation in the lung tissue via TSLP-primed CD11c-expressing APC play a crucial role in this atypical acute airway inflammation.
DC subsets are not the only CD11c expressing cell types in the lungs; more specifically, pulmonary CD11c+ cells could be divided into 3 subpopulations by the expression of CD11c and Siglec-F. CD11chiSiglec-Fhi represent Alveolar Macrophages (AM) (Fig. 4a, black). CD11chiSiglec-F− cells that co-express high levels of MHCII are DC (Fig. 4a, orange). The third population of cells has intermediate levels of CD11c and lack Siglec-F expression (CD11cintSiglec-F−; Fig. 4a, blue). CD11cintSiglec-F− cells express CD11b and F4/80 (Fig. 4a), indicating that they are similar to lung Interstitial Macrophages (IM; 15–17. CD11cintSiglec-F− cells also express Nur77, and are CX3CR1hi and CCR2lo (Fig. 4a), a phenotype similar to peripheral Ly6Clo monocytes23–25, and recently described non-classical patrolling monocytes 26–28. Furthermore, a subset of CD11cintSiglec-F− cells expresses high levels of MHCII (Fig. 4a) and was largely protected from i.v. CD45 antibody labeling (Fig. S4a). These data suggest heterogeneity within these cells, which we referred to as CD11c+ Interstitial Monocyte/Macrophages (CD11cIM). Our gating strategy was further adjusted to identify pulmonary macrophage, DC, and granulocyte subsets (Fig. 4b). Staining with anti-Siglec-F, CD11c, CD11b, Ly6G, Ly6C, and CD103 antibodies, we identified 2 subsets of DC (CD11bDC and CD103DC), 2 subsets of IM (CD11cIM and Ly6CIM/originally described as IM), Alveolar Macrophages (AM), and granulocytes Eosinophils (EOS) and Neutrophils (NEU) in the lungs (Fig. 4b and S4b).
We next determined the expression of TSLPR on pulmonary CD11c+ subsets and found expression on various immune cell types, including DC, macrophages, and T cells (Fig. 4c). Interestingly, higher levels of TSLPR expression was detected on the cell subsets that also express CD11c, including CD103DC, CD11bDC, AM, and CD11cIM (Fig. 4c), suggesting that one or more of these cell populations are involved in TSLP-mediated acute airway inflammation, based on the data using CD11c-conditional TSLPR deficient mice (Fig. 3a and S4c).
To further identify the relevant CD11c+ subsets in TSLP-mediated airway inflammation, we generated mice lacking TSLPR expression in AM and neutrophils (LysMCre/TSLPRf/f mice; Fig. S4d) and treated them with TSLP/OVA. Following TSLP/OVA treatment, LysMCre/TSLPRf/f mice developed eosinophilic airway inflammation similar to that seen in their littermate controls (Fig. S4e), indicating that TSLP responsive AM and neutrophils are not required, and further suggesting that CD11cIM or DC in the lungs might be crucial for the TSLP-mediated acute airway inflammation.
Deficiency of pulmonary CD11cIM leads to attenuated airway inflammation
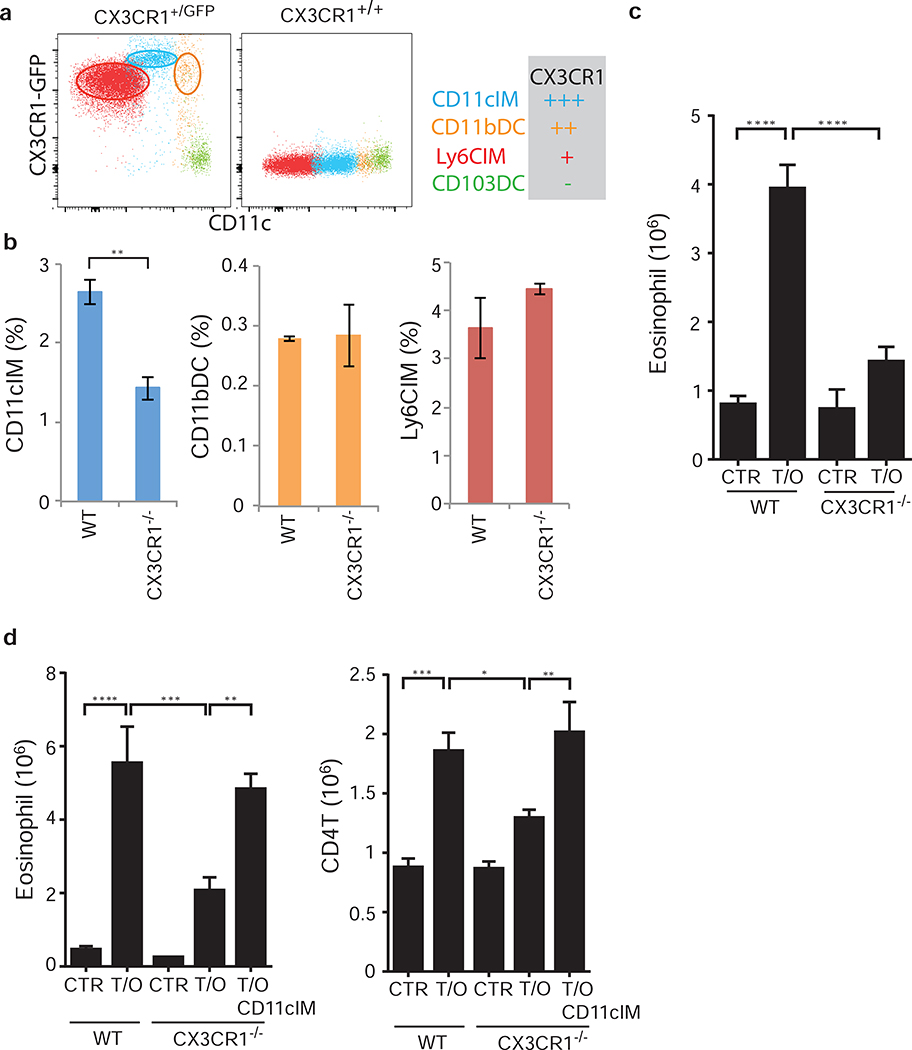
As CD11cIM are very closely related with Ly6Clo monocytes in peripheral blood (Fig. 4a), we examined the expression of the fractalkine receptor CX3CR1, which Ly6Clo monocytes require for migration and survival25. For these studies we used a mouse strain containing an eGFP knockin/knockout in the CX3CR1 locus, allowing analysis of expression in heterozygous mice (CX3CR1+/GFP), and loss of expression in homozygous animals (CX3CR1−/− or CX3CR1GFP/GFP)29. Using eGFP as a surrogate for CX3CR1, we found that CD11cIM expressed high levels of CX3CR1 (Fig. 5a), whereas CD11bDC and Ly6CIM had lower expression and CD103DC had no expression (Fig. 5a). Interestingly, deficiency of CX3CR1 selectively affected the homeostasis of CD11cIM in the lungs of naïve mice, while CD11bDC or Ly6CIM were unaffected (Fig. 5b). Furthermore, CX3CR1-deficient mice displayed an attenuated response to TSLP/OVA treatment, with reduced eosinophilic airway inflammation (Fig. 5c). These data suggested a correlation between reduced numbers of CD11cIM and a diminished type II inflammation in response to TSLP/OVA, thus supporting a role for this subset in TSLP induced allergic airway inflammation.
Figure 5. Deficiency of CD11cIM leads to attenuated airway inflammation.
(a) Expression of CX3CR1 on pulmonary CD11cIM (blue), Ly6CIM (red), CD11bDC (orange), and CD103DC (green) in CX3CR1+/GFP mice. (b) CX3CR1-expressing IM and DC subsets in the lungs of WT and CX3CR1−/− (CX3CR1GFP/GFP) mice. Percentages of total pulmonary cellularity. (c) Eosinophilic inflammation in the lungs of WT and CX3CR1−/− mice in response to TSLP/OVA (n=2–6). (d) Sorted CD11cIM from WT mice were adaptively transferred into the lungs of CX3CR1−/− mice treated with TSLP/OVA (Fig. S4). Airway inflammation was determined by airway eosinophilia in WT, CX3CR1−/−, and CX3CR1−/− mice that received CD11cIM (n=5–7). (a) Representative flow cytometry plots from lungs of mice. (b), (c), and (d) Data are representative or combined of at least two independent experiments. * p<0.05, ** p<0.01, *** p<0.001. p-value by student’s two-tailed t test (b), two-way (c), or one-way (d) ANOVA adjusted with Tukey’s multiple comparison test.
CX3CR1 is expressed by several cell types in the airways, not exclusively on CD11cIM (Fig.5a and data not shown). In fact, direct CX3CR1 signaling on Th2 cells has been described play a critical role for their survival and function in certain experimental disease models30. To address whether the diminished TSLP-induced inflammatory response seen in the CX3CR1−/− mice was due to the deficiency of CD11cIM, CD11cIM were isolated from WT mice by cell sorting (SiglecF−CD11b+Ly6G−Ly6C−CD11cint, sorting strategy as described in Fig. 4b)(Fig. S5a) and intranasally transferred into the lungs of CX3CR1−/− mice one day after each treatment with TSLP/OVA (approximately 300,000 cells per transfer/mouse) (Fig. S5b). CX3CR1-deficient mice that received CD11cIM displayed a restored eosinophilic airway inflammation in response to TSLP/OVA treatment, nearly equivalent to that seen in treated WT mice (Fig. 5d). Although we cannot definitively rule out a potential role for other CX3CR1-expressing cells, these data suggest that the lack of CD11cIM in the lungs of CX3CR1-deficient mice is critical for attenuated TSLP-mediated airway inflammation.
Direct TSLP signaling by CD11cIM is crucial for TSLP dependent airway inflammation
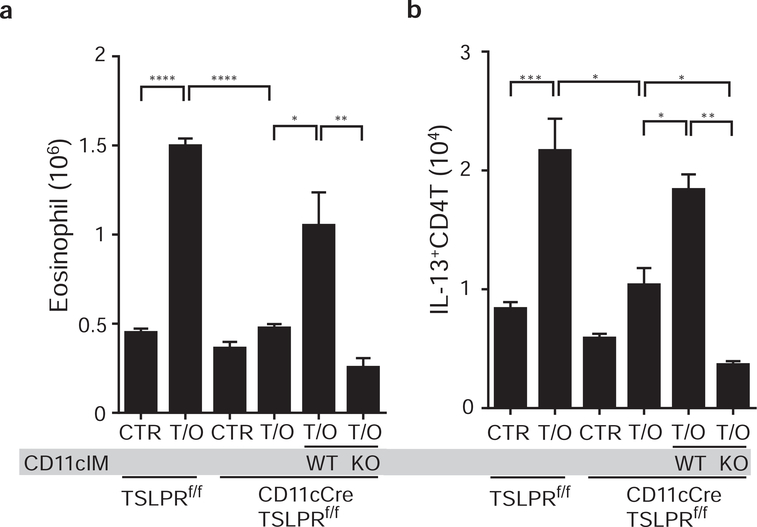
Based on the expression of TSLPR on CD11cIM (Fig. 4c), as well as the requirement for CD11cIM in TSLP-dependent airway response (Fig. 5d), it seemed likely that direct TSLP/TSLPR signaling by CD11cIM was essential. To address this question, we performed a similar adoptive transfer experiment as described in Figure S4. Sorted CD11cIM from WT or TSLPR deficient mice were adoptively transferred into the lungs of CD11cCre/TSLPRf/f mice. The results showed that adoptive transfer of WT CD11cIM was sufficient to restore the reduced eosinophilia and Th2 responses in the lungs of CD11cCre/TSLPRf/f mice (Fig. 6a and 6b, WT), but not transfer of TSLPR deficient CD11cIM (Fig. 6a and 6b, KO). These data indicate that TSLP signaling by CD11cIM is necessary for the inflammation in CD11cCre/TSLPRf/f mice.
Figure 6. TSLP responsive CD11cIM are required for the airway inflammation.
CD11cIM were sorted from WT or TSLPRko mice and adaptive transferred into the lungs of CD11cCre/TSLPRf/f mice that were treated with TSLP/OVA. Airway inflammation in TSLPRf/f, CD11cCre/TSLPRf/f, and CD11cIM transferred CD11cCre/TSLPRf/f was evaluated by infiltration of (a) eosinophils and (b) IL-13-producing CD4+ T in the lungs (n=2–6). Data are representative or combined of two independent experiments. * p<0.05, ** p<0.01, *** p<0.001, ****p<0.0001. p-value by one-way ANOVA adjusted with Tukey’s multiple comparison test.
We have previously shown that TSLPR deficiency on antigen presenting cells leads to a reduction in antigen uptake in skin DC31. To test whether CD11cIM can take up antigen, and whether this was affected by loss of TSLP signaling, WT and TSLPR−/− mice were treated intranasally with TSLP and fluorescently-conjugated OVA (Alexa647-OVA). One day-post TSLP/OVA treatment, the lungs were harvested and antigen up-take was assessed in various cell subsets. CD11cIM, DC, AM, and neutrophils were capable of taking up antigen, and TSLPR-deficiency did not affect this function (Fig. S6a, S6b and data not shown). Therefore, on the ability of CD11cIM to take up antigen was not TSLP dependent.
CD11cIM are sufficient to drive TSLP-mediated acute allergic inflammation in the absence of DC
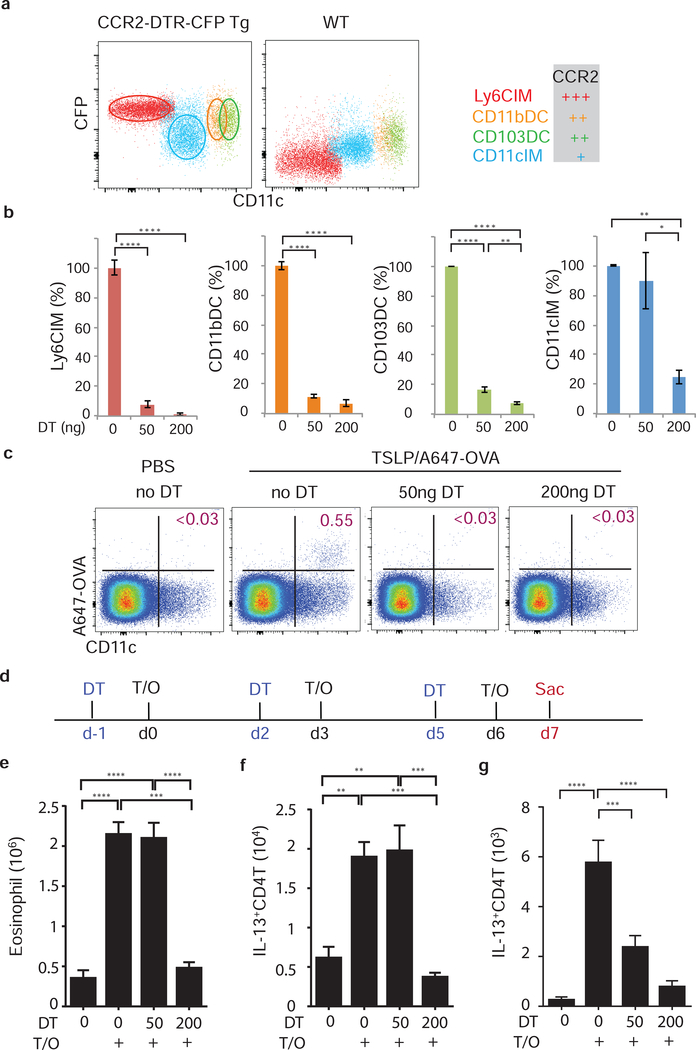
All pulmonary DC and macrophage populations, except AM, express CCR2, although at different levels (Fig. 7a, S7a, and data not shown). We therefore used mice expressing the diphtheria toxin receptor (DTR) from the CCR2 promoter to deplete these cells following DT treatment32. The different levels of CCR2 expression by these subsets allowed us to selectively retain or deplete CD11cIM by changing the dose of DT used. Intra-peritoneal injection of 200ng DT (high dose) depleted all pulmonary CCR2 expressing cells, while injection of 50ng DT (low dose) only depleted the CCR2hi subsets (Ly6CIM, CD11bDC, and CD103DC), sparing the CD11cIM subset (Fig. 7b and S7b).
Figure 7. CD11cIM promote TSLP-mediated airway inflammation independent of DC and lymph node.
(a) Expression of CCR2-DTR-CFP on IM and DC subsets in the lungs of naïve mice. (b) Depletion efficiency of CCR2+ subsets in the lungs of CCR2-DTR-CFP mice that received low (50ng) or high (200ng) dose of DT (n=4). (c)-(g), (c) Antigen delivery into LdLN was determined by tracking OVA-Alexa647+ cells at d1. (d) experimental design. CCR2-DTR-CFP mice were treated with DT (d-1, d2, and d5) one day prior each OVA/TSLP treatment (d0, d3, and d6), and TSLP-mediated inflammation was evaluated by (e) Eosinophilia in the lungs, and IL-13-producing CD4+ T cells in (f) lungs and (g) LdLN at d7 (n=4–6). (a) and (c), representative flow cytometry plots from lungs and LdLN of mice. (b) and (e)-(g) Data are combined of two independent experiments. ** p<0.01, *** p<0.001, ****p<0.0001. p-value by one-way ANOVA adjusted with Tukey’s multiple comparison test.
DT administration at both concentrations led to a lack of antigen delivery into the lung draining LN (LdLN) (Fig. 7c). In control animals, the Alexa647-OVA bearing cells in the LdLN were characterized as CD11c+ and MHCIIhi (Fig. 7c and S7c), likely to be migratory DC33. In addition, the presence or absence of antigen-positive CD11c+MHCIIhi in the LdLN correlated with the presence of DC in the lungs, but not CD11IM (Fig. 7b, 7c, and S7c), indicating that DC deliver antigen from lungs to the lung draining LN. These data demonstrate that both DC and CD11cIM can take up antigen (Fig. S6), only DC migrate to the LdLN, while CD11cIM remain in lungs, suggesting that these subsets function in a spatially different manner during inflammatory responses. These data provide an explanation for the uncoupled Th2 responses seen in the lungs and the lymph nodes shown in Figure 3.
To determine whether CD11cIM are sufficient for TSLP-mediated acute allergic inflammation, CCR2-DTR mice were treated with low or high doses of DT 24hr prior to each TSLP/OVA installation as described in Fig. 7d. As expected, with high dose DT administration, depletion of all CCR2+ cells resulted in markedly blunted airway inflammation (Fig. 7e). Surprisingly, a normal eosinophilic airway inflammation and generation of IL-13 producing CD4 T cells were observed in the lungs of CCR2-DTR mice given low dose DT treatment (Fig. 7e and 7f). These data demonstrate that CD11cIM are sufficient to induce the TSLP-mediated acute airway inflammation in the absence of DC. Furthermore, depletion of DC blocked antigen delivery to the LN (Fig. 7b and 7c), leading to attenuated differentiation of IL-13 producing CD4 T cells in LdLN (Fig. 7g), but no effect in the lungs (Fig. 7f). These data indicate that CD11cIM are crucial for promoting TSLP-mediated acute Th2-driven eosinophilic inflammation in the lungs, which is independent of Th2 cells primed by DCs in the draining LN. This observation substantiates the data shown in Figure 3 that Th2 priming in the LdLN is not required for acute TSLP-driven airway inflammation.
CD11cIM express MHCII and co-stimulatory molecules CD80 and CD86, although majority of CD11cIM express lower levels compared to DC (Fig. S7d and data not shown). Therefore it is possible that these cells could function as APC and directly promote activation and differentiation of naïve CD4 T cells in the lungs. To test the function of CD11IM as APC, CD11cIM and DC (as positive control) were sorted from the lungs of mice, loaded with OVA peptide (OVA323–339), and co-cultured with CFSE-labeled naïve OTII cells. Under neutral conditions, CD11cIM induced proliferation of OTII, as shown by CFSE dilution, but not as well as DC (Fig. S7e; CTR group). Type II cytokines IL-4 and IL-13 were transiently upregulated in the lungs of mice in response to TSLP/OVA (Fig. S7f). Therefore, to further investigate APC function of CD11cIM in the TSLP-conditioned environment, type II cytokine IL-4 and TSLP were added into the culture with OTII cells. With IL-4 in the culture, CD11cIM strongly promoted proliferation and differentiation of OTII cells into IL-13-producing cells (Fig. S7e). Importantly, TSLP increased the potency of IL-4, leading to an increase in both percentage and number of IL-13-producing OTII cells in the presence of CD11cIM (Fig. S7e). These data show that CD11cIM can induce activation and differentiation of effector Th2 cells in vitro, supporting the notion that these cells can function as APC to directly promote Th2 responses in lungs (Fig. 7).
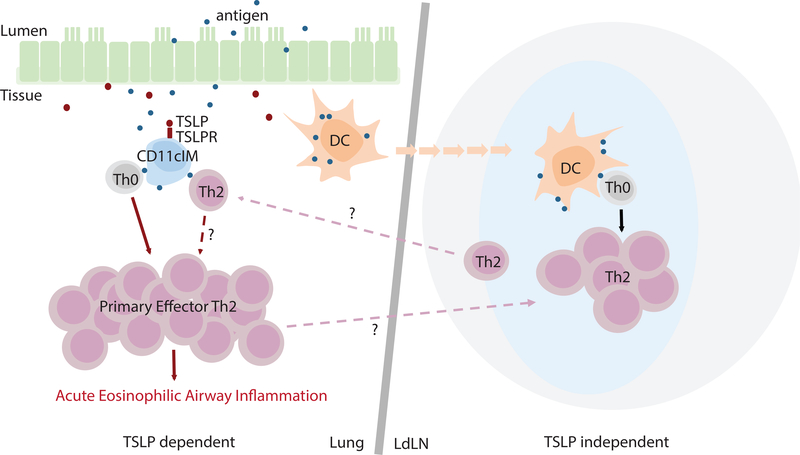
Taken as a whole, our data showed that TSLP has a unique and potent role in the development of acute CD4 T cell dependent airway inflammation independent of DC and LN. More importantly, a previously under characterized cellular subset, pulmonary CD11cIM, was both necessary and sufficient to induce this response directly in the lungs (Fig.8).
Figure 8. Model of TSLP mediated acute CD4 T cell driven airway inflammation via CD11cIM.
Following exposure with TSLP and antigen in the lungs, both CD11cIM and DC uptake antigen. Antigen-bearing DC migrate into the LdLN, whereas CD11cIM remain in the lungs. In the LdLN, Th2 that are generated by DC play a limited role for the acute inflammation in the lungs. In contrast, in the lungs, direct TSLP/TSLPR signal increases the potency of CD11cIM as APC for promoting differentiation of naïve CD4 T cells into primary effector Th2 cells and subsequent acute eosinophilic airway inflammation.
Discussion
TSLP plays an important role in the development of type II immunity; however, the mechanisms underlying its role remain somewhat unclear. In this study we characterized a novel mechanism for TSLP-mediated atypical allergic airway inflammation. We show that a previously under characterized cell subset in lungs that includes patrolling monocytes and non-classical interstitial macrophages (referred to here as CD11cIM) was necessary and sufficient for the development of acute Th2 responses following exposure to TSLP and antigen, independent of DC and T cell priming in draining lymph node. This findings suggest a unique function of TSLP and more importantly, local responses are a critical feature of acute inflammatory responses in the airways.
Allergic asthma is an inflammatory disease of the airways characterized by eosinophilic infiltration, goblet cell metaplasia and mucus overproduction, and the expression of type-2 cytokines (e.g., IL-4 and IL-13). Previous studies have suggested that allergen specific memory Th2 cells, generated in the sensitization phase (first encountered with antigen), are required for subsequent inflammation3. In the current study, we describe a novel role for TSLP in the induction of Th2-dependent allergic airway inflammation during acute allergen challenge, indicating a unique role for TSLP to accelerate the development of type II immune responses. Our data further suggest that TSLP mediates differentiation of primary effector Th2 cells that are sufficient to drive the downstream allergic responses in the airways. This unique role of TSLP as immune modulator for acute Th2 responses in the airways requires CD11c-expressing patrolling monocytes/non-classical interstitial macrophage subsets.
Along with TSLP, other epithelium-derived cytokines (e.g., IL-25 and IL-33) have also been shown to play important roles in initiating acute type II inflammation. Administration of IL-25 or IL-33 directly into the airways can trigger acute type II responses independent of antigen and T cells (Fort, 2001; Hurst 2002; Achmitz 2005), indicating distinct mechanism(s) with respect to TSLP-mediated Th2-driven inflammation. In addition, ILC2 have been shown to respond directly to IL-25, IL-33, and TSLP to promote type II inflammation. Although we did not directly rule out a role of ILC2 in this acute TSLP-mediated inflammation, the diminished inflammation observed following depletion in the CCR2-DTR mice, the lack of response in CD11c-conditional TSLPR deficient mice, as well as depletion of CD4T cells, TSLP signaling by ILC2 is not sufficient for this response.
Our group and others have shown that in concert with TCR stimulation, TSLP can promote Th2 differentiation of naïve CD4 T cells. In the current study, CD4Cre/TSLPRfloxed mice (where TSLPR is deleted from all abTCR+ T cells) showed a modest reduction of inflammation compared to their littermate controls (unpublished data), indicating that direct TSLP signaling by T cells is not a major contributor to the phenotype seen following TSLP/OVA administration.
In LysmCre/TSLPRfloxed mice, TSLPR was only deleted in AM and NEU. DC subsets and CD11cIM displayed only minimal reduction of TSLPR expression compared to controls. A possible explanation is that expression levels of LysM(Cre) are different in these cell types; the cell types have low expression of LysMCre might be able to turn on the reporter activity (such as one allele of R26-stop flox-TdTomato), but not sufficient to affect on two alleles of floxed targeted genes (such as TSLPR flox in our study).
Several pulmonary resident macrophage subsets, including AM and IM, have been identified previously, and their functions in regulation of CD4 T cell responses have also been characterized15, 34. Here, we showed that pulmonary CD11cIM are phenotypically similar to IM (defined as Ly6CIM in this study); however, these subsets play a distinct role in regulating CD4 T cell activation. Ly6CIM have been shown to modulate T cell responses indirectly via regulating the functions of DC15, while we show that CD11cIM can directly promote differentiation and activation to generate functional effector Th2 cells. The ability of the CD11cIM to directly promote the differentiation of effector Th2 cells suggests they can act as antigen presenting cells, and have properties similar to the monocyte-derived DCs (moDC)35. One of the major differences between these subsets is the migratory nature of the moDC, which differentiate into APC after infiltrating sites of inflammation, followed by migration to the draining LN35. As we have shown, CD11cIM do not migrate to the draining LN but rather remain in the lung to promote T cell responses. Furthermore, in this study, moDC are included in the CD11bDC gate, and due to their high expression of CCR2, would be depleted in low does DT treated CCR2DTR mice. These data indicate that TSLP-mediated acute inflammation is independent of moDC.
Similar to what has been described for blood Ly6Clo monocytes, pulmonary CD11cIM could be further subset based on the expression levels of MHCII. However, to purify CD11cIM based on the expression MHCII is not ideal for in vitro and in vivo functional assay due to functional requirement of MHCII. To investigate potential functional differences between MHCIIhi and MHCIIlo CD11cIM, searching for better target molecules to identify the two subsets of CD11cIM is required. By mRNA expression, MHCIIhi CD11cIM show enhanced levels of various cytokines, cytokine production related genes, and one of the signature macrophage genes Mertk (compared to MHCIIlo CD11cIM)(data not shown). However, by FACS analysis, the expression of Mertk is not homogenous within MHCIIhi CD11cIM subset. Targeting Mertk is therefore not suitable for isolating the two subsets of CD11cIM for functional analysis. The differences between these two subsets of CD11cIM are currently under investigation.
T cell priming in LN is believed to be required for the development of T cell responses36–38. Therefore it was surprising that elimination of antigen delivery into LdLN or blocking entry of naïve T cells into lymph nodes, only affected Th2 responses in LdLN, but did not diminish TSLP-mediated acute Th2 airway inflammation in the lungs (when CD11cIM were present). These data indicate that T cell priming in the lungs is sufficient for generating Th2 cells and subsequent eosinophilic inflammation. On the flip side, deficiency of TSLPR on CD11c+ cells has no impact on differentiation of Th2 in LdLN, but leads to abolished Th2 responses and subsequent inflammation in the lungs. These results indicate that Th2 cells primed in LN are not sufficient to impact on the development of this TSLP mediated acute type II inflammation in the lungs. Together, these data demonstrate that different mechanisms are likely to be involved in the differentiation and activation of Th2 in lung and LN, and may differentially impact on the subsequent inflammation. Furthermore, the ability of lung-resident APC to directly prime CD4 T cells in the lung is consistent with a pervious study showing lung CD11chiCD11bdim cells (including AM and a subset of DC) could promote Th2 differentiation in situ39. Although, in our hands, adoptive transfer of pulmonary DC (including both CD11b and CD103 DC) into the lungs does not reconstitute TSLP driven acute Th2 responses (data not shown).
It’s well know that blockade of SIPR signaling diminishes egress of the activated T cells from LN. However, the S1PR signal is also required for naïve T cells reside in peripheral tissues (such as lungs), activation of T cells, and DC migration. With these broad impact on T cell responses, blockade of S1PR signal by FTY720 treatment attenuates the TSLP-mediated Th2 responses in the lungs (data not shown) does not provide deeper insight for the mechanisms.
Consistent with this compartmentalized models of T cell priming, the only antigen bearing cells in the LdLN following TSLP/OVA treatment were DC, while other cell types (such as CD11cIM, AM, and neutrophils) that took up antigen were absent. The fate of Th2 cells that develop in either the lung or the LdLN is currently under investigation.
CD11cIM and DC localize differently after antigen uptake, and also have different signal requirements and functionalities to trigger T cell activation and survival. That CD11cIM and DC localize differently after antigen uptake also indicates that they have different roles in promoting T cell responses, and provides an explanation for the uncoupled T cell responses in lungs and LdLN. One possible model to explain these differences would be that T cells activated by CD11cIM serve as short term effector cells but fail to enter the memory cell pool. On the other hand, Th2 cells primed by DC in the LdLN would not be involved in the immediate response, but would differentiate into memory cells, which then traffic back to lung as tissue resident memory cell. Upon subsequent challenge with the same antigen, these resident memory T cells would be directly activated by CD11cIM and other APC subsets. This working hypothesis is currently under investigation.
Expression of TSLP has been shown to correlate with severity of allergic airway inflammation. In mouse experimental model, it has been shown that TSLP is upregulated and required for the high dose HDM-induced allergic airway inflammation, but not in response to low dose HDM exposure40. Furthermore, anti-TSLP mAb (Tezepelumab) has been shown to decrease exacerbations in moderate-to-severe un-controlled asthmatic patients41. Although the mechanism(s) remains largely unclear, our findings showing TSLP induces acute primary Th2 differentiation in lung could provide one of the mechanisms for development of severe asthma and a potential therapeutic strategy.
Together, our data demonstrate that TSLP-activated CD11cIM promote rapid and efficient Th2 responses directly in the lungs, which provide fundamental therapeutic targets to modulate or activate inflammation.
Supplementary Material
Key Messages.
TSLP promotes rapid primary Th2 responses in lung tissue, independent of lymph node. Pulmonary CD11c-expressing interstitial macrophages are required and sufficient for this response.
Acknowledgments
We thank J. Hamerman, A. Lacy-Hulbert, M. Pepper, T. Hohl, F. Roan, and S. Tanaka for helpful discussion, and H. Han, A. Sheih, J.B. Tan, and A. Cho for technical support. This study was supported by US National Institutes of Health grants AI068731 and HL098067 (S.F.Z).
Abbreviations
- TSLP
thymic stromal lymphopoietin
- Th2
type II helper T cells
- IM
Interstitial macrophages
- DC
dendritic cells
- LdLN
lung draining lymph node
Footnotes
All authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001; 344:350–62. [DOI] [PubMed] [Google Scholar]
- 2.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol 2008; 26:205–32. [DOI] [PubMed] [Google Scholar]
- 3.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med 2012; 18:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015; 16:45–56. [DOI] [PubMed] [Google Scholar]
- 5.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000; 192:659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001; 15:1286–92. [DOI] [PubMed] [Google Scholar]
- 7.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A 2010; 107:19455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol 2012; 130:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673–80. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 2007; 25:193–219. [DOI] [PubMed] [Google Scholar]
- 11.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005; 6:1047–53. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005; 202:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest 2014; 124:5442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 2009; 119:3723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011; 35:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013; 14:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol 2009; 182:1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 2014; 7:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002; 196:1645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 1996; 4:313–9. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima M, Ziegler SF. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol 2013; 191:4903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953–64. [DOI] [PubMed] [Google Scholar]
- 24.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 2011; 12:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009; 113:963–72. [DOI] [PubMed] [Google Scholar]
- 26.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science 2015; 350:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodero MP, Poupel L, Loyher PL, Hamon P, Licata F, Pessel C, et al. Immune surveillance of the lung by migrating tissue monocytes. Elife 2015; 4:e07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317:666–70. [DOI] [PubMed] [Google Scholar]
- 29.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20:4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med 2010; 16:1305–12. [DOI] [PubMed] [Google Scholar]
- 31.Larson RP, Zimmerli SC, Comeau MR, Itano A, Omori M, Iseki M, et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J Immunol 2010; 184:2974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 2009; 6:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med 2001; 193:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med 2013; 210:775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013; 38:322–35. [DOI] [PubMed] [Google Scholar]
- 36.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med 1997; 185:2133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 2001; 19:23–45. [DOI] [PubMed] [Google Scholar]
- 38.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol 2003; 4:733–9. [DOI] [PubMed] [Google Scholar]
- 39.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 2002; 110:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med 2012; 209:1505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377:936–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.