Abstract
Spread of cancer to the brain remains an unmet clinical need in spite of the increasing number of cases among patients with lung, breast cancer and melanoma most notably. Although research on brain metastasis was considered a minor aspect in the past due to its untreatable nature and invariable lethality, nowadays limited but encouraging examples have questioned this statement making it more attractive for basic and clinical researchers. Evidences of its own biological identity (i.e. specific microenvironment) and particular therapeutic requirements (i.e. presence of blood-brain barrier, blood-tumor barrier, molecular differences with the primary tumor) are thought to be critical aspects that must be functionally exploited using preclinical models.
We present the coordinated effort of 19 laboratories to compile comprehensive information related to brain metastasis experimental models. Each lab has provided details on the cancer cell lines they have generated or characterized as being capable of forming metastatic colonies in the brain, as well as principle methodologies of brain metastasis research. The Brain Metastasis Cell Lines Panel (BrMPanel) represents the first of its class and includes information about the cell line, how tropism to the brain was established and the behavior of each model in vivo.
These and other aspects described are intended to assist investigators in choosing the most suitable cell line for research on brain metastasis. The main goal of this effort is to facilitate research on this unmet clinical need, to improve models through a collaborative environment, and to promote the exchange of information on these valuable resources.
Keywords: Brain metastasis, Cell lines, Organotropism, Resource, Open-source
Introduction
Central nervous system (CNS) metastasis, notably in the brain, are most prevalent in lung cancer (20–56% of patients), breast cancer (5–20% of patients) and melanoma (7–16% of patients), but occur in many other cancer types (1,2). Lesions occur in both the brain parenchyma and the meninges. The incidence of brain metastases is thought to be increasing, owing to improved systemic therapy that however fails to control the disease in the brain, more frequent and improved imaging, and other factors. The development of brain metastases impairs patient survival (3) and is the cause of death in up to 50% of affected patients (1).
Hallmarks of brain metastasis development have been identified (4). The brain metastatic tumor cell must traverse the blood-brain barrier (BBB). The BBB is then modified to a poorly characterized blood-tumor barrier (BTB) which is heterogeneously permeable to most drugs (5,6). Immune cell penetration of the brain is negligible in healthy circumstances, but occurs during brain metastasis development (7). Metastatic tumor cells colonize the brain in an intimate relationship with the BTB, activated astrocytes and microglia, which form a neuro-inflammatory response around damaged areas, neurons, oligodendrocytes, and other specialized brain cells (8–13).
Until recently, brain metastasis therapy was primarily local including surgery, stereotactic radiation therapy, and whole brain radiation therapy. Further, steroids are used to control edema. Systemic brain-permeable kinase inhibitors and immune checkpoint therapies are active and now in use for some cancer types (14–21).
In order to mechanistically understand CNS metastasis formation and to develop preventives and therapeutics, it is essential to use model systems that, as much as possible, faithfully recapitulate the progression in the patient. Thus, the complexities of brain metastases, as listed above, dictate that multiple model systems should be interrogated.
This article shares the experience of 19 laboratories that have established and/or characterized brain-tropic experimental models for parenchymal and leptomeningeal metastasis. This resource is expected to significantly foster brain metastasis research, and is available to the entire scientific community.
Results
Overview of the Brain Metastasis Cell Lines Panel
The BrMPanel (https://apps.cnio.es/app/BrainMetastasis/CellLines) (Supplementary Table 1) is composed of 60 cell lines, derived from patients (32 cell lines, 53.3%), mouse (27 cell lines, 45.0%) or rat (1 cell line, 1.7%), and represent the three main sources of brain metastasis including breast cancer (38 cell lines, 63.3%), lung cancer (8 cell lines, 13.3%) and melanoma (14 cell lines, 23.3%). Each type of cancer has the main molecular drivers or cancer subtypes represented (Figure 1).
Figure 1. Cancer cell lines in the BrMPanel.
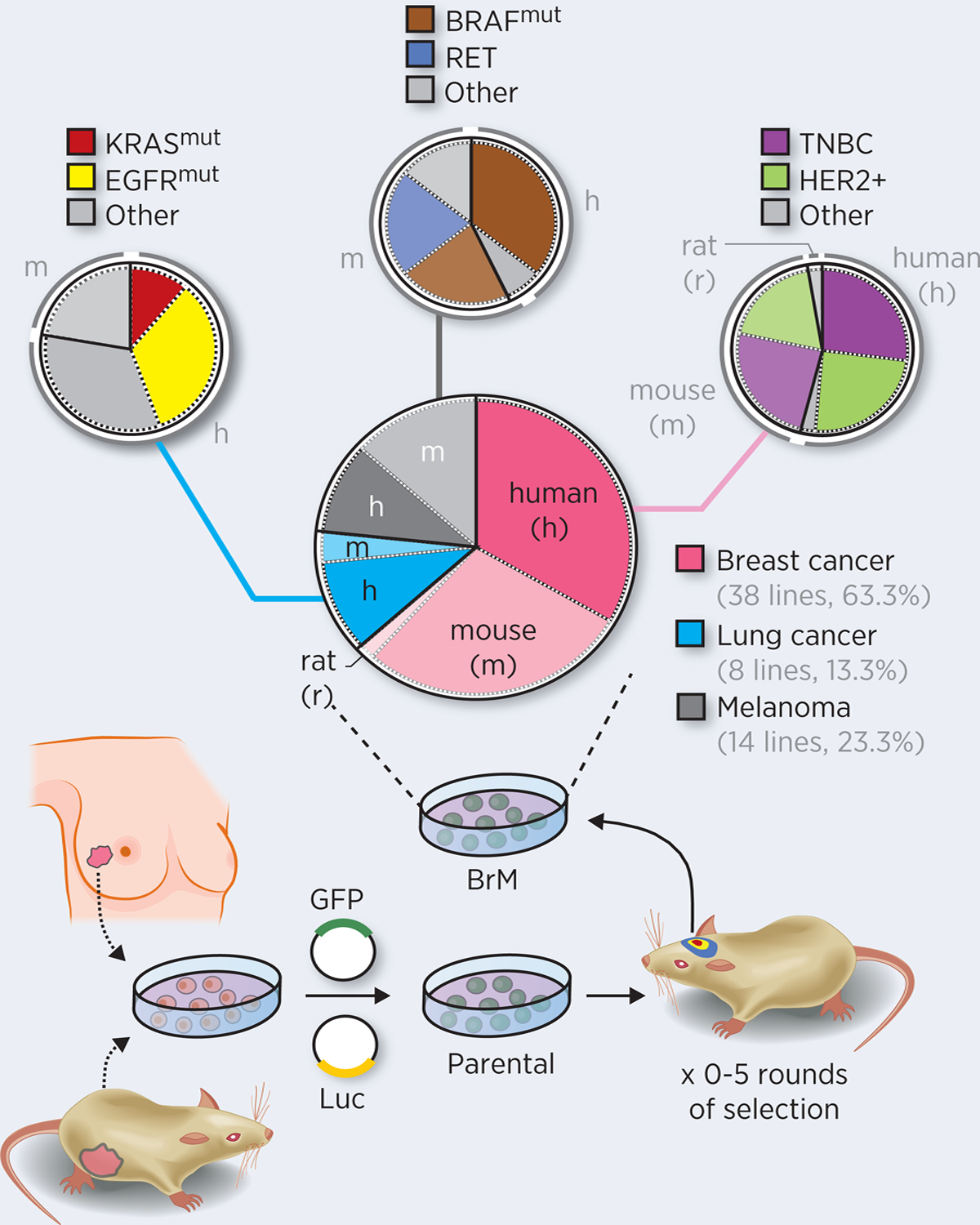
Brain metastatic (BrM) cell lines have been established from human or mice parental cells (derived from primary tumors, pleural fluids or lymph node metastasis). Depending on the model, some cell lines were labelled with fluorescent (i.e. GFP) and/or bioluminescent (Luc) reporters and they underwent subsequent in vivo selection, for 0–5 rounds. Alternatively, spontaneous metastasis obtained from patients or mice (i.e. derived from genetically engineered mouse models (GEMM) or orthotopic injections) could be a direct source of BrM cell lines, without the need of performing in vivo selection. The BrMPanel has 60 cell lines from breast cancer (38 cell lines, 63.3%), lung cancer (8 cell lines, 13.3%) and melanoma (14 cell lines, 23.3%) of human (h), mouse (m), or rat (r) origins. Molecular drivers and cancer subtypes are represented for each cancer type: Breast cancer: 16 entries of HER2+, 42.1%; 19 entries of triple-negative breast cancer patients (TNBC), 50.0%; 3 entries of other, 7.9%; Lung cancer: KRAS mutant and p53 null (1 out of 8 cell lines of 12.5%) or EGFR alterations (3 out of 8 cell lines, 37.5%); Melanoma: 8 BRAF mutant cell lines (57.1%) and 3 Ret alterations (21.4%). Analysis of the molecular profiles correspond to the parental cell lines.
In addition to biologically relevant characteristics, the cell lines included in the BrMPanel express different reporters to track and measure brain metastasis such as luciferase (35 cell lines, 58.3%) and/or fluorescent reporters (green fluorescence reporters (i.e. GFP), 43 cell lines, 71.7%; red fluorescence reporters, 11 cell lines, 18.3%). A limited number of cell lines are not labelled at all (13 cell lines, 21.2%).
Methods for establishing brain tropism
The most popular method to enrich brain tropism is the in vivo selection of cell lines that are preferentially metastatic to the CNS (33/60 cell lines, 55.0%). This approach, described elsewhere (22,23), offers the possibility to select not only aggressive derivatives, but also indolent ones (24). The selection process involves engineering cancer cells with reporters (i.e. luciferase or GFP) so that lesions can be easily visualized, scored and dissected, and finally re-implanted into mice, typically after some period of in vitro growth. Subsequent additional rounds of selection are typically performed. Although less frequent, it is also possible to perform the in vivo selection blind if no reporters are used, based on the macroscopic identification of metastases and MRI (entry#7 and #8 from the BrMPanel) (Supplementary Table 1) (25).
In addition, the BrMPanel contains entries that do not require any selection owing to their intrinsic ability to grow in the brain (27/60 cell lines, 45.0%). However, within this group we have to differentiate between three distinct categories: those that have been directly implanted within the brain (5/27 cell lines, 18.5%), so whether or not they are able to target the brain from a systemic inoculation remains an open question; those that are able to metastasize to the brain from a systemic inoculation into the bloodstream (15/27 cell lines, 55.6%); and those that metastasize from an orthotopic implantation (e.g. a melanoma cell line with the ability to spontaneously metastasize in the brain from intradermal inoculation) (7/27 cell lines, 25.9%).
Syngeneic versus xenograft BrM models
Given the increasing efforts to use immunotherapy in brain malignancies (19–21), syngeneic cell lines, which are compatible with genetically engineered mouse models (GEMM) and with immune competent strains in general, represent a growing interest among cancer researchers studying brain metastasis (26). The use of syngeneic cell lines engineered to express exogenous reporter proteins has been shown to potentially activate an immune response of variable intensity depending on multiple factors, including the specific reporter and the mouse strain (27). Although this has not been specifically reported for any BrM cell line included in the BrMPanel, some researchers are intentionally avoiding the use of any reporter either in syngeneic models (entry#7, #8, #10, #25, #33, #48) (25,28,29) or human models (entry#42, #54) (30–32) and rely on other imaging modalities such as magnetic resonance imaging (MRI) and ultrasound imaging for monitoring local progression (25,33,34) (Table 1 and Supplementary Table 1). In addition, a potential limitation to using syngeneic models is that the endpoint following intracardiac or intracranial injection of the same number of BrM cells is reached faster (2–3 weeks) than in xenograft models (4–6 weeks), which limits the time window to study their growth in the brain, and to assess therapeutic approaches. Notably, although enriched for their brain tropism, extracranial disease is often present in BrM models. In xenografts the incidence of extracranial metastases tends to be less evident than in syngeneic models, which might facilitate the evaluation of brain-specific benefits with a given therapeutic intervention.
Table 1. Techniques to analyze metastasis progression in the brain.
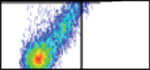
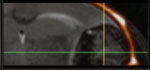
There are multiple available techniques (columns) for assessing the development and treatment of brain metastasis. In selecting the most appropriate approach, it is essential that the researcher considers different parameters (rows). Each cell in the table provides binary information (yes (white background)/ no (red background)) regarding the compatibility of the technique with the specific parameter. However, for several parameters it is important to consider additional quantitative or qualitative aspects. For example, the row testing “sensitivity” can be divided into the different stages of brain metastasis development. The different stages derive from the number of cells in a given metastasis (see (*) at the bottom). The parameters “technical requirement” and “time requirement” do not respond to binary options but are better defined by semiquantitative considerations of feasibility and resource requirements. Finally, the last row reflects potential limitations for a given technique, such as the need to have the BrM cells engineered with particular reporters or the need to use antibodies. We provide a graded code using “+” signs to suggests what is (+) somehow suitable, (++) suitable, and (+++) optimal. A key reference has been added to each technique to provide an example of its implementation.
| Histology52 | FACS25 | PCR37 | MRI31 | Bioluminescence22,53 | Two-photon video-microscopy54 | |
|---|---|---|---|---|---|---|
| Techniques to analyze metastasis progression | 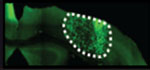 |
 |
 |
 |
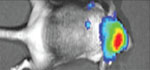 |
 |
| Compatible with in vivo | No | No | No | Yes(+++) (Non-invasive) | Yes(+++) (Non-invasive) | Yes (+++) (Partially-invasive) |
| Sensitivity (*) | Single cell→Big | Medium-Big | Medium-Big | Medium-Big | Medium-Big | Single cell→Small |
| Number of metastases | Yes (+++) | No | No | Yes(++) | Yes (+) | No (In field-of-view only |
| Size | Yes (+++) | No | No | Yes(++) | Yes (+) | Yes (+++) |
| Distribution | Yes (+++) | No | No | Yes(+++) | Yes(++) | No |
| Growth | Yes (+++) (cellular pattern) | No | No | Yes(+++) (over time) | Yes(+++) (over time) | Yes(+++) (cell, pattern/over time) |
| Microenvironment features | Yes (+++) | Yes (++) | Yes (+) | Yes (++) | No | Yes(+++) |
| Technical requirement (Equipment/ expertise) | Low/ Low (+++) | Low/ Low (+++) | Low/ Low (+++) | High/ High | Medium/ Medium (++) | High/ High |
| Time requirement | Medium-high | Low (+++) | Low (+++) | Medium | Low (+++) | High |
| Info about extra-cranial | No | No | No | No | Yes(+++) | No |
| Particular considerations | Antibodies | Antibodies and/ or Engineering BrM cells | Relative quantification | High field strength | Engineering BrM cells | Engineering BrM eel |
Sensitivity: Small (micrometastasis): 5–50 cells
Medium (micrometastasis): <500 cells
Big (macrometastasis): >1000 ceils
Suitability:
Somehow suitable
Suitable
Optimal
Spontaneous models
A spontaneous model of brain metastasis requires that cancer cells must accomplish all steps of the metastatic cascade from a spontaneously arisen or orthotopically implanted tumor. Thus, the main advantage of utilizing such models is that they more faithfully recapitulate the human metastatic process (35,36). In addition, these models also allow one to study the potential influence of the primary or orthotopic tumor on the formation of the pre-metastatic niche, which has been shown to influence BBB permeability, neuroinflammation, dormancy and latency (37). However, spontaneous models often involve a longer latency period to the establishment of brain metastasis, which generally requires the removal of the fast-growing primary or orthotopic tumor, and results in a lower and highly variable incidence of CNS metastasis, which necessitates the use of larger cohorts of mice (37).
Although some breast cancer cell lines included in the BrMPanel (i.e. MDA-MB-231 and 4T1) have been previously reported to form spontaneous brain metastases (38–40), they are not currently used as such. In fact, more convincingly characterized spontaneous models of melanoma brain metastasis have recently been reported. The RMS cell line (entry #51) was originally derived from a spontaneous primary tumor in the Ret transgenic mouse (41,42). Subdermal orthotopic injection of RMS cells results in the formation of aggressive primary tumors, which, after being surgically removed, are the source of brain micrometastases (50% of mice) that evolve to fully developed metastases in 23% of mice after 4–6 months (37,43). Brain-tropic derivatives were also established after intracardiac injection of the RMS cell line (BT-RMS cells, entry #52) or by isolating a spontaneously arisen brain metastasis from an orthotopic tumor (sBT-RMS, entry #53). These additional cell lines increased the incidence of brain metastasis to 80–90% at 2 weeks after systemic inoculation or to 55–60% at 4–6 months after orthotopic inoculation, respectively (37,43).
In addition, the 5610 cell line was originally derived from a spontaneous primary melanoma arising in a Dct::TVA;BrafCA;Cdkn2alox/lox mouse injected with RCAS-Cre (29) and later engineered ex vivo to express Akt1 (entry #49) and Akt1E17K (entry #50) along with luciferase and ZsGreen. Subcutaneous injections of 5610, 5610-Akt1, or 5610-Akt1E17K cells result in the formation of primary tumors in all injected mice but they demonstrate different metastatic capacities. Whereas the parental 5610 cell line does not metastasize, brain (30% of mice) and extracranial (30% of mice) metastases develop in mice injected with either 5610-Akt or 5610-Akt1E17K cells after 2 months (29). Most of these mice have both brain and extracranial metastases, but a reduced number of them have only their brain affected (29). Moreover, the parental 5610 is unable to grow in the brain even if directly implanted by intracranial injection (29). The loss of Pten in the cell line 7788 (entry #48), which also has all previous genetic modifications described in the 5610-AktE17K cells, increases to 100% the mice showing tumor growth upon direct intracranial inoculation, though their tropism upon systemic inoculation is still unknown (29).
PDX models
Patient-derived xenografts (PDX) are models of cancer where the tissue or cells from a patient’s tumor is implanted into humanized or immunodeficient mice. The advantage of this experimental model is the potential to study an avatar of the patient’s tumor that recapitulates the enormous complexity of human cancer. Humanized models, however, remain a challenge due to species-specific differences that preclude proper engraftment and development of human immune cell populations (44).
The BrMPanel has eight PDX models obtained from surgically resected brain metastases derived from breast cancer (F2–7, entry#35 (45); USC-BBM3.1, entry#34), lung cancer (USC-LUBM5, entry#43; SEBTA 001, entry#42 (30,31), and melanoma (H1, entry#54; H1_DL2, entry#55; H1_Tomato, entry#56 (32,46,47); WM4237, entry#59, (48)) that were propagated in vitro for a maximum of 10 passages, during which they were engineered with various reporters (i.e. Luciferase, GFP or tdTomato), and then injected in NSG mice. These PDX models did not require any enrichment in vivo since they were able to generate brain metastases and extracranial metastasis in 3–9 weeks after intracardiac inoculation depending on the model. This also applies to WM4237, obtained from a melanoma brain metastasis in a patient treated with immunotherapy, which is the only PDX model that has been demonstrated to spontaneously metastasize to the brain upon orthotopic injection. As expected, this model requires longer periods of time (17 weeks) and removal of the primary tumor (48).
Ex vivo tumor cell culture in serum-free, “stem-like” culture medium, has been utilized in primary brain tumor research in recent years due to greatly improved persistence of key cellular and molecular features of patients’ tumors (49,50). Limited models of brain metastasis, which are not part of the BrMPanel, have been established under this condition (51). Thus, it remains to be seen whether this strategy will also help to improve models for CNS metastasis research.
Experimental approaches with brain tropic cell lines
Routes of BrM cell line inoculation
There are three types of tumor cell inoculations represented in the BrMPanel: local (intracranial), systemic (intracardiac and intracarotid), and orthotopic (intradermic/ subcutaneous for melanoma and mammary fat pad for breast cancer) (Supplementary Figure 1). The advantages and disadvantages of each type are briefly discussed below. The techniques to evaluate brain colonization (22,25,31,37,52–55) are compared in Table 1.
Local inoculation (5/60, 8.3% of all entries)
Advantage:
This is a simple and fast method to generate brain tumors that allows the study of cancer cells of non-brain origin within their appropriate microenvironment in the brain. It is only suited to study large tumors that can, under certain circumstances, be regarded as models for brain macrometastases. Another advantage is that the exact site of tumor cell growth in the brain can be predetermined.
Disadvantage:
This model does not reproduce the metastatic cascade, including the important aspects of organ colonization, such as the initiation of metastasis from isolated clones. Moreover, the inoculation per se induces local neuroinflammation mostly affecting resident cells such as astrocytes and microglia, which might interfere with the role of this process in the initial steps of brain colonization. Therefore, it cannot be regarded as a true “metastasis model” but rather as a technical strategy to avoid the intrinsic difficulties of more advanced approaches (i.e. variable number and location of metastases) when these aspects are not necessarily required to answer the question of interest.
Systemic inoculation (48/60, 80.0% of all entries)
Advantage:
This experimental approach recapitulates all steps required for organ colonization. Although intracardiac inoculation involves phenotypic variability (i.e. variable number of mice with brain metastasis, variable number of metastases per mouse) it is consistent enough to be the most frequently used approach given its technical feasibility (i.e. no requirement for invasive surgery, the injection could be guided by ultrasound imaging (56)), high incidence of brain metastasis among mice injected with brain-homing cells, and sufficiently homogeneous time to reach the experimental endpoint (22,23,57). Intracarotid inoculation minimizes the number of cancer cells that circulate systemically out of the head, thus reducing the incidence of extracranial metastases, which might be useful when the efficacy of different interventions is to be evaluated without the confounding effects of extracranial disseminated disease (58).
Disadvantage:
In particular, intracarotid injection is technically challenging, and requires invasive surgery, which may impact disease pathology.
Systemic inoculation in general obviates the stages of the metastatic cascade preceding organ colonization, and involves the growth of metastatic cells in a cancer naïve host, which does not reproduce the clinical situation. In addition, this strategy to induce metastasis neglects the co-evolution of the cancer and the host from the initial stages of primary tumor growth, and involves the inoculation of a single bolus of a high number of cancer cells rather than the reality of limited numbers of circulating tumor cells disseminated from early stages of the primary tumor development (59,60). In an effort to minimize some of these disadvantages, orthotopic inoculation of the cancer cells has been combined with subsequent systemic inoculations (26).
Orthotopic extracranial inoculation (7/60, 11.7% of all entries)
Advantage:
Although this modality still does not fully recapitulate the spontaneous emergence of cancer, it is nonetheless the second-best approach to study the entire process of metastasis. It reproduces not only the colonization step but also the growth of the original tumor and more realistic systemic dissemination of cancer cells. Since these models have an orthotopic tumor and, frequently, extracranial metastases as well, it more closely recapitulates the clinical scenario and the potential influence that extracranial disease might have on the brain.
Disadvantage:
These models are still not widely used by brain metastasis researchers owing to their limitations, including the highly variable and low rate of incidence of brain metastasis, which is also lower than for any systemic inoculation, increased time to generate brain metastasis, and the frequent need to add surgery to remove the orthotopic tumor, which otherwise involves reaching humane endpoint criteria before brain metastases have developed (37).
Preclinical therapeutic approaches
Preclinical therapeutic approaches in BrM mouse models encompass both local and systemic treatment strategies (Supplementary Table 2), similar to clinical care. Local interventions in BrM models have largely focused on radiation strategies, including modeling whole brain radiation therapy (WBRT) (61–63) and stereotactic radiosurgery (SRS) (64–66). With regards to systemic therapies, a broad spectrum of treatments has been tested in BrM models: traditional chemotherapies, including DNA-damaging agents (67–69); small molecule inhibitors, including tyrosine kinase inhibitors (70–75); nanoparticles and other lipid-based delivery systems (76–80); and biologics, including checkpoint inhibitors (26,81), monoclonal antibodies (34,55,74,75,82) and stem cells (83). Preclinical models described here continue to contribute to our understanding of the BrM tumor microenvironment during therapeutic intervention (53,84–86). Sophisticated studies have also explored optimal partners and their respective timing/sequencing for optimal combinations of systemic and local therapies (87), both mirroring and informing clinical research. Other studies have investigated methods of opening the BBB to improve delivery of drugs that would otherwise be too large to reach intracranial tumors (6,54,80,88,89).
While BrM mouse models allow for a variety of therapeutic strategies, it is imperative that the goal of the tested preclinical treatment modalities is best matched to the appropriate clinical application. For example, investigations with immunotherapies require immunocompetent models (e.g. mouse-derived cells into a syngeneic host). Explorations of inhibitors of the metastatic cascade should utilize intracardiac or intracarotid injections or models that generate spontaneous brain metastasis, depending on the focus of the study, with therapeutics administered at pre-specified time intervals during the metastatic process to determine treatment effects on the respective metastatic stages (51,90,91).
Specific features of BrM cell lines according to their primary origins
Little is known so far about the differences and similarities of various tumor entities with respect to their specific biological mechanisms of brain colonization, and successful growth in the brain. Several reasons might explain this lack of knowledge.
First, in the interest of finding critical aspects of CNS colonization, researchers initially hypothesized and later demonstrated (52) that the high selective pressure imposed by the brain, which is independent of the source of the primary tumor, acts as a potent “filter” that selects those cancer cells that are better fitted to grow in this organ. In fact, this finding does not seem to be determined by particular alterations at the DNA level, which is usually linked to the original source of the metastasis, but rather derived from a transcriptomic rewiring (23,92). Second, research programs devoted to single tumor cell entities have not applied their expertise to additional tumor sources to pinpoint differences. Better understanding of tumor-specific differences might help with the design of clinical trials for brain metastasis, as has been suggested by the early angiogenic dependency of lung cancer brain metastasis (55,93) or the hormonal dependencies of certain subtypes of breast cancer (94).
In an effort to recognize the variety of brain metastasis models in the BrMPanel we briefly describe them according to their primary tumor source.
Breast cancer:
This Panel represents 3 of the 4 molecular subtypes of breast cancer, with Luminal A (ER+, PR+/−, HER2-) being currently absent. Metastatic cells in the BrMPanel harbor all of the major somatic mutations found in patients including those affecting BRCA1, PTEN, HER2, CDK, EGFR and p53, and others which are less prevalent such as BRAF, MYC, KRAS, RB1 and SMAD4. Expression of the epidermal growth factor receptor ErbB2 (HER2) and lack of expression of the estrogen receptor (ER) are independent strong risk factors for the development of brain metastasis in breast cancer patients (95–98). Consistently, the majority of the cell lines represented in this panel are from triple negative breast cancer (ER-negative, progesterone receptor (PR) - and normal HER2) (TNBC) (19/38 entries, 50.0%) and HER2+ subtypes (16/38 entries, 42.1%). Of note, derivatives obtained from the MDA-MB-231 cell line are over-represented in the BrMPanel. The rich representation of breast cancer models in the BrMPanel includes cell lines that progress expeditiously in the brain (entry#1), but also additional HER2+ BrM models with slower progression (entry#2), which will provide the BrM research community with tools to better represent the heterogeneity of brain metastases. Similarly, ER+ BrM cell lines present in the BrMPanel (cell lines#7–10, #18, #30, #31) reflect the characteristic slower progression rates of luminal tumors (1–3 months following IC injection).
Lung cancer:
All BrM cell lines in the Panel were derived from non-small cell lung cancer (NSCLC) adenocarcinomas. Developing models to study small-cell lung cancer (SCLC), which is a tumor entity with very high CNS tropism in patients (99), is urgently needed. In addition, the limited characterization of molecular profiles in the BrMPanel includes KRAS (G12C), p53 and EGFR (△E746-A750) driver alterations represented. Remarkably, no models involving ALK alterations are present, in spite of the high frequency of brain metastasis in these patients. Finally, the need to increase the lack of mouse-derived BrM models of lung cancer in the field is also evident (2/8, 25%), especially given the growing interest in the use of immune-based approaches in this tumor type.
Melanoma:
The fourteen melanoma BrM models present in the BrMPanel represent key genomic alterations such as BRAF, Cdkn2a, RB1, p53, AKT, PTEN and RET. Interestingly, the melanoma models include the highest representation of syngeneic models within a tumor type (8/14, 57.1%), which could facilitate research on immune-based therapies to dissect the biology of clinically-relevant approaches as demonstrated, at least, in asymptomatic brain metastases (21).
Meningeal brain metastasis
The meninges, or coverings of the CNS consist of two categories: the innermost leptomeninges, and the outer pachymeninges. The pachymeninges consist of the dura mater. Both leptomeninges (from melanoma, breast and lung cancer) and dura (from breast cancer) metastatic cancer cell variants are represented in the BrMPanel.
Leptomeninges metastatic (LeptoM) cell lines:
The delicate leptomeninges consist of the pia mater and arachnoid mater, and contain the circulating cerebrospinal fluid (CSF). The leptomeningeal space represents an anatomical compartment separate from that of the systemic circulation and the brain. Entry into this leptomeningeal space is governed by the blood-CSF-barrier, the choroid plexus. LeptoM cell lines (MDA-MB-231-LeptoM, entry#19; HCC1954-LeptoM, entry#20; PC9-LeptoM, entry#44; LLC-LeptoM, entry#45) were generated through iterative rounds of in vivo selection after injecting them into the cisterna magna. An intermediate derivative obtained after the third round was subsequently injected intracardially into recipient mice for one additional in vivo round. The recovered cell line was termed the LeptoM cell lines. LeptoM cell lines do not generate parenchymal metastases upon IC injection, and, in fact, differ from them transcriptomically (100). As in the clinic, LeptoM cell lines kill mice faster than parenchymal models (2 weeks versus 5 weeks, respectively) (100). Besides the LeptoM cell lines, the B16/F10-BrM (entry#47), RMS (entry#51), BT-RMS (entry#52) and sBT-RMS (entry#53) spontaneously develop limited but consistent meningeal tropism in spite of a purely parenchymal in vivo selection (53).
Dura metastatic (Dura) cell lines:
The tough and fibrous dura mater resides outside of the BBB and is served by the systemic circulation. As such, systemic therapeutics easily reach tumors that seed and grow within this space, in contrast to the leptomeningeal space.
Following internal carotid artery injection of 4T1 cancer cells into syngeneic BALB/c mice, 4T1-Dura3 (entry#13) were established after 3 rounds of in vivo selection from the dura. In parallel, derivatives that grew in the parenchymal space were established (4T1-Par3, entry#12). The cell line 4T1-Dura3 still targets the parenchyma to some extent, but differs from 4T1-Par3 in the immune cell composition as well as inflammation-related pathways (101).
Conclusions and future directions
The BrMPanel is an open resource meant to exchange information and is intended to become a reference for those investigators interested in brain metastasis research. Of note, the BrMPanel is not a cell repository, and those interested in any of these cell lines should directly reach out to the investigator listed in the table for each individual reagent (Supplementary Table 1). The conditions under which cell lines will be shared are entirely dependent on each laboratory/institution.
Importantly, this BrMPanel is a first attempt to summarize such resources and is intended to expand with additional cell lines in the future, from the many laboratories that have reported additional brain metastasis models (102–125). As shown in Figure 1 and mentioned above, some tumor types (lung cancer) and relevant genomic alterations (i.e. ALK translocations) are poorly represented in the Panel, and provide the opportunity to continue with this effort intended to mimic the complete clinical scenario. Consequently, the BrMPanel is a resource open for additional collaborators. Furthermore, we envision similar resources could also emerge for other tropic cell lines to different organs and, with the help of established entities (e.g. Cellosaurus), a comprehensive resource of organotropic cell lines could be made available in the future.
Certainly, these resources could be further enriched through more ambitious projects involving full molecular characterization (i.e. transcriptomic, epigenomics, proteomics, cell surface, karyotyping, etc.), which, if made available to the scientific community, will speed up research and optimize resources. Having the opportunity to identify commonalities between different organotropic cell lines within large repositories might provide new hypotheses to be tested, presumably, increasing the potential for translation to patients. In this regard, although there have been a few clear examples of how the use of cell lines within the BrMPanel can lead to new clinical trials with encouraging results for patients (Supplementary Table 2), there is still room for significant improvement given the dismal prognosis for this disease. For instance, spontaneous models of metastasis would aid in testing preventive strategies and predictive biomarkers before the metastasis becomes clinically relevant. The incorporation of both local and systemic therapies that are part of the standard-of-care might improve the translational potential of basic research. The development of drug screening platforms adapted to the particularities of metastasis and the organ in which they grow (i.e. lab-on-a-chip, organotypic cultures) together with the use of FDA-approved or experimental libraries of compounds preferentially pre-selected for their BBB-permeable properties could drastically increase the limited arsenal against brain metastasis. In addition, a domain that has been poorly explored so far in pre-clinical models of brain metastasis, which is now emerging within the “cancer neuroscience” field (126), is the cognitive deficit associated with the disease itself, a critical concern for patients.
The use of BrM cell lines involves, beyond the general concerns that apply to all models based on cancer cell lines (127), a large number of variables regarding the specific cell line, model, and the particular approach to study metastases. Careful consideration of the underlying question is critical to evaluate whether a simple versus more complex strategy would be more appropriate. For instance, utilizing fully immunocompetent hosts is becoming almost a requirement given the emerging crosstalk between the brain and the immune system as well as the occasional positive responses of CNS metastasis to immunotherapy. However, the use of immunocompetent hosts and mouse cancer cells is not exempt from limitations such as the existing differences between species and the higher complexity of human cancer. In addition, our ability to manipulate the brain immune system is still limited due to relatively recent major discoveries (e.g. brain lymphatic system) and existing open questions regarding basic aspects of the crosstalk between metastasis and different cell types of the adaptive immune system. In addition, the potential impact of exogenous proteins (e.g. reporters) on anti-tumor responses might complicate the ability to track metastasis development. Although some of these aspects could be circumvented (e.g. tolerogenic GEMM (128), novel variants of non-immunogenic reporters (27), the use of humanized mouse models or using imaging methods based on endogenous reporters (i.e. MRI) to minimize the effects of reporters on immunity), a good strategy is to combine and validate experimental findings using several models.
In this sense the emerging, but still very limited number of models that recapitulate the whole metastatic cascade are priorities for further development and should be incorporated into the experimental pipelines more frequently, at least to confirm findings obtained with simpler models.
By combining more sophisticated models with different clinically-relevant therapeutic approaches and appreciating the genomic complexity of human disease, we hope that novel and more effective strategies to target metastases in the brain and elsewhere will soon become a reality.
Supplementary Material
Acknowledgements
Authors want to thank the IT personnel from CNIO Marcos de Miguel and José Luis Fernández for developing the BrMPanel webpage. Authors also want to thank the support from MINECO grants MINECO-Retos SAF2017-89643-R (M.V.), Bristol-Myers Squibb-Melanoma Research Alliance Young Investigator Award 2017 (498103) (M.V.), Fundación Ramón Areces (CIVP19S8163) (M.V.), Worldwide Cancer Research (19-0177) (M.V.), H2020-FETOPEN (828972) (M.V.), Clinic and Laboratory Integration Program CRI Award 2018 (54545) (M.V.), AECC Coordinated Translational Groups 2017 (GCTRA16015SEOA) (M.V.), LAB AECC 2019 (LABAE19002VALI) (M.V.), METAvivor Inc. (P.D.B.), Susan G Komen Career Catalyst Grant (CCR18548205) (P.D.B.), Susan G Komen Career Catalyst Grant (CCR15332673) (J.N.), NIH/NCI (1R01CA223544-01A1) (J.N.), Department of Defense BCRP (BC141728) (J.N.), Cancer Research UK (C5255/A15935) (N.S.), R37 CA227984 (D.C), DoD BCRP BC141980 (D.C), Metavivor Research Foundation (D.C), in collaboration with the Brain Tumor Biorepository at the University of Colorado, PO1 CA114046 (M.H., A.V.), and the Dr. Miriam and Sheldon G. Adelson Medical Research (M.H.), Harvard Ludwig Center, MIT/ HMS Bridge grant (R.K.J.), the National Cancer Institute Outstanding Investigator Award (R35CA197743) (R.K.J.), Susan G. Komen Foundation (PDF15331878) (G.B.F.), Breast Cancer Research Foundation (J.A.J), Cancer Research UK iMAXT Consortium (J.A.J), Ludwig Institute for Cancer Research (J.A.J), Swiss Cancer League and the Swiss Bridge Award (J.A.J), National Cancer Institute (R01CA121118) (S.L.H), Melanoma Research Alliance (347651) (S.L.H). M.V. is a Ramón y Cajal Investigator (RYC-2013-13365) and EMBO YIP (4053).
Footnotes
Conflict of interest statement
The authors declare no potential conflict of interest
References
- 1.Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. 2019;5:5. [DOI] [PubMed] [Google Scholar]
- 2.Valiente M, Ahluwalia MS, Boire A, Brastianos PK, Goldberg SB, Lee EQ, et al. The evolving landscape of brain metastasis. Trends Cancer. 2018;4:176–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rueda OM, Sammut S-J, Seoane JA, Chin S-F, Caswell-Jin JL, Callari M, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. 2020;20:4–11. [DOI] [PubMed] [Google Scholar]
- 5.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5:e1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21:107–12. [DOI] [PubMed] [Google Scholar]
- 11.Winkler F The brain metastatic niche. J Mol Med. 2015;93:1213–20. [DOI] [PubMed] [Google Scholar]
- 12.Wasilewski D, Priego N, Fustero-Torre C, Valiente M. Reactive astrocytes in brain metastasis. Front Oncol. 2017;7:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doron H, Pukrop T, Erez N. A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res. 2019;79:423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119–28. [DOI] [PubMed] [Google Scholar]
- 15.Costa DB, Shaw AT, Ou S-HI, Solomon BJ, Riely GJ, Ahn M-J, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015;33:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler M, Wu Y-L, Hirsh V, O’Byrne K, Yamamoto N, Mok T, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol. 2016;11:380–90. [DOI] [PubMed] [Google Scholar]
- 17.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50:611–21. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. [DOI] [PubMed] [Google Scholar]
- 21.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–81. [DOI] [PubMed] [Google Scholar]
- 22.Bos PD, Zhang XH-F, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen DX, Chiang AC, Zhang XH-F, Kim JY, Kris MG, Ladanyi M, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep. 2016;17:2445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci USA. 2018;115:E1540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limberis MP, Bell CL, Wilson JM. Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 2009;16:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall DG, Stoica G. Characterization of brain and bone-metastasizing clones selected from an ethylnitrosourea-induced rat mammary carcinoma. Clin Exp Metastasis. 1994;12:283–95. [DOI] [PubMed] [Google Scholar]
- 29.Kircher DA, Trombetti KA, Silvis MR, Parkman GL, Fischer GM, Angel SN, et al. AKT1E17K activates focal adhesion kinase and promotes melanoma brain metastasis. Mol Cancer Res. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jassam SA, Maherally Z, Smith JR, Ashkan K, Roncaroli F, Fillmore HL, et al. CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng VWT, Soto MS, Khrapitchev AA, Perez-Balderas F, Zakaria R, Jenkinson MD, et al. VCAM-1-targeted MRI Enables Detection of Brain Micrometastases from Different Primary Tumors. Clin Cancer Res. 2019;25:533–43. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Daphu I, Pedersen PH, Miletic H, Hovland R, Mørk S, et al. A novel brain metastases model developed in immunodeficient rats closely mimics the growth of metastatic brain tumours in patients. Neuropathol Appl Neurobiol. 2011;37:189–205. [DOI] [PubMed] [Google Scholar]
- 33.Chae WH, Niesel K, Schulz M, Klemm F, Joyce JA, Prümmer M, et al. Evaluating Magnetic Resonance Spectroscopy as a Tool for Monitoring Therapeutic Response of Whole Brain Radiotherapy in a Mouse Model for Breast-to-Brain Metastasis. Front Oncol. 2019;9:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Askoxylakis V, Ferraro GB, Kodack DP, Badeaux M, Shankaraiah RC, Seano G, et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz H, Blacher E, Amer M, Livneh N, Abramovitz L, Klein A, et al. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res. 2016;76:4359–71. [DOI] [PubMed] [Google Scholar]
- 38.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–93. [PubMed] [Google Scholar]
- 39.Puchalapalli M, Zeng X, Mu L, Anderson A, Hix Glickman L, Zhang M, et al. NSG Mice Provide a Better Spontaneous Model of Breast Cancer Metastasis than Athymic (Nude) Mice. PLoS One. 2016;11:e0163521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanni P, Nicoletti G, Palladini A, Croci S, Murgo A, Ianzano ML, et al. Multiorgan metastasis of human HER-2+ breast cancer in Rag2−/−;Il2rg−/− mice and treatment with PI3K inhibitor. PLoS One. 2012;7:e39626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–90. [DOI] [PubMed] [Google Scholar]
- 42.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, et al. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–8. [DOI] [PubMed] [Google Scholar]
- 43.Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, et al. Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis. Cell Rep. 2019;28:1785–1798.e6. [DOI] [PubMed] [Google Scholar]
- 44.Walsh NC, Kenney LL, Jangalwe S, Aryee K-E, Greiner DL, Brehm MA, et al. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Contreras-Zárate MJ, Ormond DR, Gillen AE, Hanna C, Day NL, Serkova NJ, et al. Development of Novel Patient-Derived Xenografts from Breast Cancer Brain Metastases. Front Oncol. 2017;7:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daphu I, Sundstrøm T, Horn S, Huszthy PC, Niclou SP, Sakariassen PØ, et al. In vivo animal models for studying brain metastasis: value and limitations. Clin Exp Metastasis. 2013;30:695–710. [DOI] [PubMed] [Google Scholar]
- 47.Sundstrøm T, Daphu I, Wendelbo I, Hodneland E, Lundervold A, Immervoll H, et al. Automated tracking of nanoparticle-labeled melanoma cells improves the predictive power of a brain metastasis model. Cancer Res. 2013;73:2445–56. [DOI] [PubMed] [Google Scholar]
- 48.Krepler C, Sproesser K, Brafford P, Beqiri M, Garman B, Xiao M, et al. A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell Rep. 2017;21:1953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. [DOI] [PubMed] [Google Scholar]
- 50.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528:93–8. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Venugopal C, Tokar T, McFarlane N, Subapanditha MK, Qazi M, et al. Therapeutic Targeting of the Premetastatic Stage in Human Lung-to-Brain Metastasis. Cancer Res. 2018;78:5124–34. [DOI] [PubMed] [Google Scholar]
- 52.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH-F, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24:1024–35. [DOI] [PubMed] [Google Scholar]
- 54.Connell JJ, Chatain G, Cornelissen B, Vallis KA, Hamilton A, Seymour L, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst. 2013;105:1634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. [DOI] [PubMed] [Google Scholar]
- 56.Balathasan L, Beech JS, Muschel RJ. Ultrasonography-guided intracardiac injection: an improvement for quantitative brain colonization assays. Am J Pathol. 2013;183:26–34. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176:2958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature. 2016;540:588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitz J, Lowes LE, Goodale D, Allan AL. Circulating tumor cell analysis in preclinical mouse models of metastasis. Diagnostics (Basel) 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smart D, Garcia-Glaessner A, Palmieri D, Wong-Goodrich SJ, Kramp T, Gril B, et al. Analysis of radiation therapy in a model of triple-negative breast cancer brain metastasis. Clin Exp Metastasis. 2015;32:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowe W, Wang L, Zhang Z, Varagic J, Bourland JD, Chan MD, et al. MRI evaluation of the effects of whole brain radiotherapy on breast cancer brain metastasis. Int J Radiat Biol. 2019;95:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamilton AM, Wong SM, Wong E, Foster PJ. Cranial irradiation increases tumor growth in experimental breast cancer brain metastasis. NMR Biomed. 2018;31:e3907. [DOI] [PubMed] [Google Scholar]
- 64.Barbone GE, Bravin A, Romanelli P, Mittone A, Bucci D, Gaaβ T, et al. Micro-imaging of Brain Cancer Radiation Therapy Using Phase-contrast Computed Tomography. Int J Radiat Oncol Biol Phys. 2018;101:965–84. [DOI] [PubMed] [Google Scholar]
- 65.Wu C-C, Chaudhary KR, Na YH, Welch D, Black PJ, Sonabend AM, et al. Quality assessment of stereotactic radiosurgery of a melanoma brain metastases model using a mouselike phantom and the small animal radiation research platform. Int J Radiat Oncol Biol Phys. 2017;99:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartmann J, Wölfelschneider J, Stache C, Buslei R, Derer A, Schwarz M, et al. Novel technique for high-precision stereotactic irradiation of mouse brains. Strahlenther Onkol. 2016;192:806–14. [DOI] [PubMed] [Google Scholar]
- 67.Karginova O, Siegel MB, Van Swearingen AED, Deal AM, Adamo B, Sambade MJ, et al. Efficacy of Carboplatin Alone and in Combination with ABT888 in Intracranial Murine Models of BRCA-Mutated and BRCA-Wild-Type Triple-Negative Breast Cancer. Mol Cancer Ther. 2015;14:920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samala R, Thorsheim HR, Goda S, Taskar K, Gril B, Steeg PS, et al. Vinorelbine Delivery and Efficacy in the MDA-MB-231BR Preclinical Model of Brain Metastases of Breast Cancer. Pharm Res. 2016;33:2904–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernatz S, Ilina EI, Devraj K, Harter PN, Mueller K, Kleber S, et al. Impact of Docetaxel on blood-brain barrier function and formation of breast cancer brain metastases. J Exp Clin Cancer Res. 2019;38:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Swearingen AED, Sambade MJ, Siegel MB, Sud S, McNeill RS, Bevill SM, et al. Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro Oncol. 2017;19:1481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saldana SM, Lee H-H, Lowery FJ, Khotskaya YB, Xia W, Zhang C, et al. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS One. 2013;8:e73406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aasen SN, Parajuli H, Hoang T, Feng Z, Stokke K, Wang J, et al. Effective treatment of metastatic melanoma by combining MAPK and PI3K signaling pathway inhibitors. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AMMJ, Song Y, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci USA. 2012;109:E3119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammad AS, Griffith JI, Adkins CE, Shah N, Sechrest E, Dolan EL, et al. Liposomal Irinotecan Accumulates in Metastatic Lesions, Crosses the Blood-Tumor Barrier (BTB), and Prolongs Survival in an Experimental Model of Brain Metastases of Triple Negative Breast Cancer. Pharm Res. 2018;35:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sambade M, Deal A, Schorzman A, Luft JC, Bowerman C, Chu K, et al. Efficacy and pharmacokinetics of a modified acid-labile docetaxel-PRINT(®) nanoparticle formulation against non-small-cell lung cancer brain metastases. Nanomedicine (Lond). 2016;11:1947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anders CK, Adamo B, Karginova O, Deal AM, Rawal S, Darr D, et al. Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer. PLoS One. 2013;8:e61359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittapalli RK, Liu X, Adkins CE, Nounou MI, Bohn KA, Terrell TB, et al. Paclitaxel-hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Mol Cancer Ther. 2013;12:2389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baghirov H, Snipstad S, Sulheim E, Berg S, Hansen R, Thorsen F, et al. Ultrasound-mediated delivery and distribution of polymeric nanoparticles in the normal brain parenchyma of a metastatic brain tumour model. PLoS One. 2018;13:e0191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfannenstiel LW, McNeilly C, Xiang C, Kang K, Diaz-Montero CM, Yu JS, et al. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology. 2019;8:e1507669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Askoxylakis V, Ferraro GB, Badeaux M, Kodack DP, Kirst I, Shankaraiah RC, et al. Dual endothelin receptor inhibition enhances T-DM1 efficacy in brain metastases from HER2-positive breast cancer. NPJ Breast Cancer. 2019;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andreou T, Rippaus N, Wronski K, Williams J, Taggart D, Cherqui S, et al. Hematopoietic stem cell gene therapy for brain metastases using myeloid cell-specific gene promoters. J Natl Cancer Inst. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16:876–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin Cancer Res. 2016;22:5287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smilowitz HM, Sasso D, Lee EW, Goh G, Micca PL, Dilmanian FA. Therapy model for advanced intracerebral B16 mouse melanoma using radiation therapy combined with immunotherapy. Cancer Immunol Immunother. 2013;62:1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aasen SN, Espedal H, Holte CF, Keunen O, Karlsen TV, Tenstad O, et al. Improved Drug Delivery to Brain Metastases by Peptide-Mediated Permeabilization of the Blood-Brain Barrier. Mol Cancer Ther. 2019;18:2171–81. [DOI] [PubMed] [Google Scholar]
- 89.Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci USA. 2018;115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmieri D, Duchnowska R, Woditschka S, Hua E, Qian Y, Biernat W, et al. Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin Cancer Res. 2014;20:2727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian Y, Hua E, Bisht K, Woditschka S, Skordos KW, Liewehr DJ, et al. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis. 2011;28:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacob LS, Vanharanta S, Obenauf AC, Pirun M, Viale A, Socci ND, et al. Metastatic Competence Can Emerge with Selection of Preexisting Oncogenic Alleles without a Need of New Mutations. Cancer Res. 2015;75:3713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ilhan-Mutlu A, Osswald M, Liao Y, Gömmel M, Reck M, Miles D, et al. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. 2016;15:702–10. [DOI] [PubMed] [Google Scholar]
- 94.Contreras-Zárate MJ, Day NL, Ormond DR, Borges VF, Tobet S, Gril B, et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene. 2019;38:4685–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 2017;3:666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. [DOI] [PubMed] [Google Scholar]
- 97.Hohensee I, Lamszus K, Riethdorf S, Meyer-Staeckling S, Glatzel M, Matschke J, et al. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183:83–95. [DOI] [PubMed] [Google Scholar]
- 98.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–43. [DOI] [PubMed] [Google Scholar]
- 99.Lukas RV, Gondi V, Kamson DO, Kumthekar P, Salgia R. State-of-the-art considerations in small cell lung cancer brain metastases. Oncotarget. 2017;8:71223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168:1101–1113.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rippaus N, Taggart D, Williams J, Andreou T, Wurdak H, Wronski K, et al. Metastatic site-specific polarization of macrophages in intracranial breast cancer metastases. Oncotarget. 2016;7:41473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22:723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee HW, Lee J-I, Lee SJ, Cho HJ, Song HJ, Jeong DE, et al. Patient-derived xenografts from non-small cell lung cancer brain metastases are valuable translational platforms for the development of personalized targeted therapy. Clin Cancer Res. 2015;21:1172–82. [DOI] [PubMed] [Google Scholar]
- 106.Siam L, Bleckmann A, Chaung H-N, Mohr A, Klemm F, Barrantes-Freer A, et al. The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget. 2015;6:29254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156:986–1001. [DOI] [PubMed] [Google Scholar]
- 108.Morsi A, Gaziel-Sovran A, Cruz-Munoz W, Kerbel RS, Golfinos JG, Hernando E, et al. Development and characterization of a clinically relevant mouse model of melanoma brain metastasis. Pigment Cell Melanoma Res. 2013;26:743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wall BA, Yu LJ, Khan A, Haffty B, Goydos JS, Chen S. Riluzole is a radio-sensitizing agent in an in vivo model of brain metastasis derived from GRM1 expressing human melanoma cells. Pigment Cell Melanoma Res. 2015;28:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988;48:3478–84. [PubMed] [Google Scholar]
- 111.Yagiz K, Rodriguez-Aguirre ME, Lopez Espinoza F, Montellano TT, Mendoza D, Mitchell LA, et al. A Retroviral Replicating Vector Encoding Cytosine Deaminase and 5-FC Induces Immune Memory in Metastatic Colorectal Cancer Models. Mol Ther Oncolytics. 2018;8:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martínez-Aranda A, Hernández V, Guney E, Muixí L, Foj R, Baixeras N, et al. FN14 and GRP94 expression are prognostic/predictive biomarkers of brain metastasis outcome that open up new therapeutic strategies. Oncotarget. 2015;6:44254–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li B, Wang C, Zhang Y, Zhao XY, Huang B, Wu PF, et al. Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene. 2013;32:2952–62. [DOI] [PubMed] [Google Scholar]
- 114.Jilaveanu LB, Parisi F, Barr ML, Zito CR, Cruz-Munoz W, Kerbel RS, et al. PLEKHA5 as a Biomarker and Potential Mediator of Melanoma Brain Metastasis. Clin Cancer Res. 2015;21:2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wrage M, Hagmann W, Kemming D, Uzunoglu FG, Riethdorf S, Effenberger K, et al. Identification of HERC5 and its potential role in NSCLC progression. Int J Cancer. 2015;136:2264–72. [DOI] [PubMed] [Google Scholar]
- 116.Smith DL, Debeb BG, Thames HD, Woodward WA. Computational modeling of micrometastatic breast cancer radiation dose response. Int J Radiat Oncol Biol Phys. 2016;96:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ardini E, Menichincheri M, Banfi P, Bosotti R, De Ponti C, Pulci R, et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol Cancer Ther. 2016;15:628–39. [DOI] [PubMed] [Google Scholar]
- 118.Zubrilov I, Sagi-Assif O, Izraely S, Meshel T, Ben-Menahem S, Ginat R, et al. Vemurafenib resistance selects for highly malignant brain and lung-metastasizing melanoma cells. Cancer Lett. 2015;361:86–96. [DOI] [PubMed] [Google Scholar]
- 119.Park ES, Kim SJ, Kim SW, Yoon S-L, Leem S-H, Kim S-B, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci USA. 2011;108:17456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Debeb BG, Lacerda L, Anfossi S, Diagaradjane P, Chu K, Bambhroliya A, et al. miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer. J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xing F, Sharma S, Liu Y, Mo YY, Wu K, Zhang YY, et al. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene. 2015;34:4890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21:1401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ben-David U, Siranosian B, Ha G, Tang H, Oren Y, Hinohara K, et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature. 2018;560:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aoyama N, Miyoshi H, Miyachi H, Sonoshita M, Okabe M, Taketo MM. Transgenic mice that accept Luciferase- or GFP-expressing syngeneic tumor cells at high efficiencies. Genes Cells. 2018;23:580–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


