Abstract
Objective:
To assess the impact of Oregon’s policy that allows pharmacist prescription of the pill and patch on contraceptive receipt for Medicaid-insured women.
Study design:
We conducted a difference-in-differences analysis using Oregon Medicaid claims data to compare changes in receipt of all contraceptive services and receipt of the pill or patch for Medicaid-enrolled women (n = 436,258) before and after policy implementation in areas with and without participating pharmacists. We then described filled prescriptions for the contraceptive pill and patch by type of prescribing provider before and after implementation of the policy. We also compared past contraceptive use for women receiving prescriptions from pharmacists and non-pharmacists.
Results:
We found no significant policy effects on receipt of all contraceptive services or on receipt of the pill or patch. More than 98% of prescriptions filled for the pill and patch in the first two years of policy implementation were prescribed by a non-pharmacist provider. Women receiving contraceptive pill and patch prescriptions from pharmacists and nonpharmacists were equally likely to be continuing contraceptive users.
Conclusion:
We identified no increase in receipt of contraceptive services among Medicaid-insured women in the two years following the implementation of a pharmacy access policy. Additional research is needed to investigate other possible benefits of the policy, such as satisfaction, convenience, cost and equity.
Keywords: Contraceptive services, pharmacists, Oregon, Medicaid
1. Introduction
In the United States 45% of pregnancies are unintended [1]. Ensuring access to contraceptive services is a critical aspect of preventing unintended pregnancy and promoting reproductive autonomy. In the U.S. the most frequently used contraceptive method is the pill [2], which has a relatively high typical use failure rate [3]. Problems accessing or using methods are primary reasons that women give for having gaps in contraceptive use [4]. Women who use hormonal methods can become at increased risk of unintended pregnancy if they have gaps in use, such as those that can occur when prescriptions expire.
One strategy for increasing access to contraceptive services is to expand availability at pharmacies. Nine states and the District of Columbia have policies that allow pharmacists to directly prescribe contraception [5]. Oregon’s policy, House Bill (HB) 2879, went into effect in 2016 to allow pharmacists to prescribe the contraceptive pill and patch. Under this policy, pharmacists complete a five-hour online training to become certified to prescribe contraception. Pharmacists can write new prescriptions for adults aged 18 and older. Those younger than 18 can have a prescription renewed by a pharmacist but must have an original prescription from another clinical prescriber. A second bill, HB 2527, went into effect in 2018, expanding pharmacists’ prescribing scope to include the contraceptive injectable and ring, and requiring insurers to reimburse pharmacists for consultation services [6].
Research in other settings has suggested that pharmacy prescribing has the potential to increase access to contraceptive services. Two national surveys indicated widespread interest among pharmacists in providing contraceptive services [7, 8]. In a survey conducted after a similar California law had passed but not yet gone into effect, almost three out of four pharmacists said that they were likely to provide hormonal contraception [9]. Results from a pilot project in Washington state, where several pharmacies provided contraceptive prescriptions through a collaborative drug therapy agreement, demonstrated high feasibility among pharmacists and acceptability among women [10]. Two surveys conducted after California’s policy went into effect indicated that contraceptive prescriptions were available in 5%−11% of pharmacies, suggesting possible gaps in implementation [11, 12].
Little research has been published on implementation in Oregon. Data collected prior to policy enactment suggested that many pharmacists intended to participate in prescribing contraception [13]. Preliminary reports indicated that in the first several months of implementation almost half of all pharmacists in the state had signed up for the required training [14]. By December 2016, pharmacists trained to prescribe were located in 63% of zip codes [13] and by 2019 46% of pharmacies in the state offered contraceptive prescriptions [15]. One recently published study using Oregon Medicaid data reported that a substantial proportion of new pill/patch prescriptions were provided by pharmacists [16]. Additional research is warranted, however, given the early stage of policy implementation and the limited scope of prior research.
The goal of this study was to assess the impact of pharmacist provision on contraceptive access for Medicaid-insured women in Oregon two years following implementation of the policy. Specifically, we (1) evaluated the effect of HB 2879 on overall receipt of contraceptive services and on pill/patch receipt; (2) described pill/patch receipt by type of prescribing provider before and after policy implementation; and (3) assessed whether women receiving prescriptions from pharmacists were more likely to be new users of contraception compared to women with prescriptions from non-pharmacists.
2. Material and methods
2.1. Study Population and Data
This study included all Oregon women ages 15–44 enrolled in Medicaid during 2015–2017 (n = 436,258). Linked Medicaid data sources provided information on enrollment, claims, and prescribing provider. We excluded women with residential zip codes that could not be matched to a zip code tabulation area (ZCTA) (n = 602). Certain analyses were restricted to women who filled prescriptions for the pill/patch and were enrolled in Medicaid for at least 80% of the study period. Ethical approval for this study was deferred by the Oregon State University institutional review board (IRB) and approved by the Oregon Health Authority (OHA) IRB. All analyses were conducted using Stata15.
2.2. Measures
We identified receipt of the contraceptive pill/patch based on pharmaceutical claims data, using National Drug Codes (NDCs) for the pill and patch from the Office of Population Affairs (OPA) measure of most and moderately effective methods [17] and the OHA Coordinated Care Organization metric [18]. We also assessed overall receipt of contraceptive services, including the intrauterine device (IUD), implant, injectable, pill, patch, ring and diaphragm, using codes from the OPA measure.
For all women with pill/patch pharmacy claims in 2015–2017, we identified the type of prescribing provider for each claim as pharmacist or non-pharmacist (e.g. physician, nurse practitioner, physician assistant). Provider type was obtained by linking the prescribing provider National Provider Identifier (NPI) in the pharmacy claims data to the Medicaid provider file, which contains Medicaid provider type codes. Prescribing providers with “pharmacist” codes were identified as pharmacists and all others were classified as non-pharmacists. Prescribing NPI was missing for 1.2% (n = 5,414 / 450,240) of pill/patch claims in 2015–2017. We described provider type at the claim level and at the woman level. Women with any pill/patch prescribed by a pharmacist were classified as having pharmacist provision. Women without claims prescribed by a pharmacist but with any claim prescribed by a non-pharmacist provider were classified as having non-pharmacist provision.
We classified women with 2016–2017 pill/patch prescriptions as new or continuing contraceptive users. For women who received a prescription from a pharmacist, we assessed contraceptive use in the twelve months prior to the first pharmacist-prescribed prescription (index prescription) filled in 2016–2017. For women receiving prescriptions only from non-pharmacist providers, we assessed contraceptive use in the twelve months prior to the first filled prescription in 2016–2017 (index prescription). We defined continuing users as those who received any reversible method in the year prior to the index prescription. Ascertainment of methods for this measure is as described above with one modification. We excluded diagnosis or procedure claims for the pill/patch to avoid classifying receipt of a prescription prior to filling the prescription as an indicator of continuing use.
For difference-in-differences analyses we used provider data to classify each Oregon ZCTA as an area with or without pharmacist provision available (also referred to as “intervention” and “non-intervention” areas). ZCTAs match one-to-one with zip codes and provide a geographic approximation of the area represented by each zip code [19]. Each ZCTA was classified based on the presence of at least one pharmacist registered with Medicaid to prescribe hormonal contraception based on provider mailing address. This information was linked to woman-level data using residential zip codes from enrollment data. A pre/post pharmacy access availability variable was defined by ZCTA according to the Medicaid registration date of the first pharmacist in that ZCTA to offer hormonal contraception.
Demographic measures included age, race/ethnicity, and urban/rural residence. Information on race/ethnicity came from all available Medicaid enrollment records from 2008–2018. If multiple categories were reported across records, we categorized race/ethnicity in the following order: Hispanic, Native Hawaiian/Pacific Islander, American Indian/Alaska Native, black, Asian, and white. Non-Hispanic minority women were grouped into a single category for regression models because of low frequencies. We identified women’s residential zip codes as rural or urban according to the Rural Urban Commuting Area (RUCA) classification schematic B [20].
2.3. Data Analysis
We calculated descriptive statistics for women by intervention area and policy time period. Women who contributed person-time to multiple intervention areas or policy time periods were included in multiple groups. For demographic measures that varied over time, we summarized the first available record in descriptive statistics. We calculated monthly probabilities of contraceptive service receipt. We then described monthly trends over 2015–2017 in receipt of any contraceptive service and filled pill/patch prescriptions.
We conducted two difference-in-differences analyses for women ever enrolled in 2015–2017. We assessed monthly receipt of any contraceptive service, constructing a person-month panel dataset with an outcome variable indicating monthly service receipt. For the second analysis we assessed monthly filled pill/patch prescriptions. For each analysis we fitted Poisson regression models with robust standard errors that included a pre/post policy period variable, a variable for residence in an area with pharmacy access to hormonal contraception (intervention area vs. non-intervention area), and an interaction term between these two variables. We accounted for the non-independence of multiple monthly observations per women by estimating models with generalized estimating equations (GEE) using an exchangeable working correlation matrix. To improve on this model, we adjusted for age, race/ethnicity, and rural/urban residence.
We then described trends in pharmacy pill/patch claims by prescribing provider type over the course of 2015–2017 and compared prior contraceptive use by women receiving prescriptions from pharmacists to those receiving prescriptions from non-pharmacists in 2016–2017. We restricted the sample to women with pill/patch prescriptions in 2016–2017 who were enrolled in Medicaid for at least 80% of the days in the twelve calendar months prior to the index prescription in 2016–2017. We fitted bivariable Poisson regression models with robust standard errors with provider type as the outcome and prior contraceptive use as the main independent variable. We also adjusted this model for age, race/ethnicity and urban/rural residence.
3. Results
Before the pharmacy access policy, 32,383 women ages 15–44 were enrolled in non-intervention areas and 317,383 in intervention areas (Table 1). After policy implementation, 41,899 were enrolled in non-intervention areas and 339,390 in intervention areas. Demographic characteristics were similar before and after implementation. In non-intervention and intervention areas, however, the frequency of missing data for race/ethnicity increased over time. Non-intervention areas had a higher proportion of Hispanic women and lower proportion of non-Hispanic white women compared to intervention areas. Women in non-intervention areas were more likely to reside in rural areas (40%) compared to those in intervention areas (15%).
Table 1.
Characteristics of Medicaid enrolled women ages 15–44 before and after policy implementation in areas with and without Medicaid-registered prescribing pharmacists in Oregon, 2015–2017
| Pre-pharmacy access | Post-pharmacy access | |||||
|---|---|---|---|---|---|---|
| Non- intervention areasa |
Intervention areasa |
p-valueb | Non- intervention areas |
Intervention areas |
p-valueb | |
| Measure | ||||||
| (n = 32,382) | (n = 317,383) | (n = 41,899) | (n = 339,390) | |||
| Age, % | <0.001 | <0.001 | ||||
| 15–19 | 19.5 | 17.5 | 20.3 | 18.0 | ||
| 20–24 | 17.1 | 17.5 | 16.6 | 17.3 | ||
| 25–29 | 18.9 | 20.7 | 19.4 | 21.2 | ||
| 30–34 | 17.1 | 17.9 | 17.1 | 17.6 | ||
| 35–39 | 14.5 | 14.5 | 14.5 | 14.6 | ||
| 40–44 | 13.0 | 11.9 | 12.0 | 11.4 | ||
| Race/ethnicity, % | <0.001 | <0.001 | ||||
| Hispanic | 18.5 | 23.2 | 16.2 | 21.4 | ||
| Non-Hispanic white | 69.8 | 58.2 | 67.7 | 56.0 | ||
| All others | 5.0 | 9.2 | 5.1 | 9.5 | ||
| Missing | 6.7 | 9.4 | 11.0 | 13.1 | ||
| Residence, % | <0.001 | <0.001 | ||||
| Urban | 59.9 | 85.4 | 60.0 | 85.4 | ||
| Rural | 40.1 | 14.6 | 40.0 | 14.6 | ||
|
Monthly contraceptive service receipt, % of person-months |
6.7 | 6.4 | 0.006 | 6.8 | 6.4 | <0.001 |
|
Monthly pill/patch receipt, % of person-months |
4.2 | 3.9 | 0.001 | 4.2 | 3.8 | <0.001 |
Intervention and non-intervention areas are those with and without pharmacist prescribing available.
p-values were obtained using bivariable logistic regression with cluster robust standard errors.
The monthly probability of receiving contraceptive services was similar before and after the pharmacy access policy and higher in non-intervention areas (pre-implementation: 6.7% non-intervention, 6.4% intervention; post-implementation: 6.7% non-intervention, 6.4% intervention). Similarly, the monthly probability of filling a pill/patch prescription was similar over time and higher in non-intervention areas (pre-implementation: 4.2% non-intervention, 3.9% intervention; post-implementation: 4.2% non-intervention, 3.8% intervention). The monthly probabilities of receiving any contraceptive service and filling a pill/patch prescription (Figure 1) was relatively constant over 2015–2017 in non-intervention and intervention areas. Difference-in-differences models indicated no significant intervention effects (Table 2). Non-significant interaction terms in all models indicated no effect of the policy on outcomes. Unadjusted model were consistent with results from models adjusted for age, race/ethnicity, and urban/rural residence.
Figure 1.
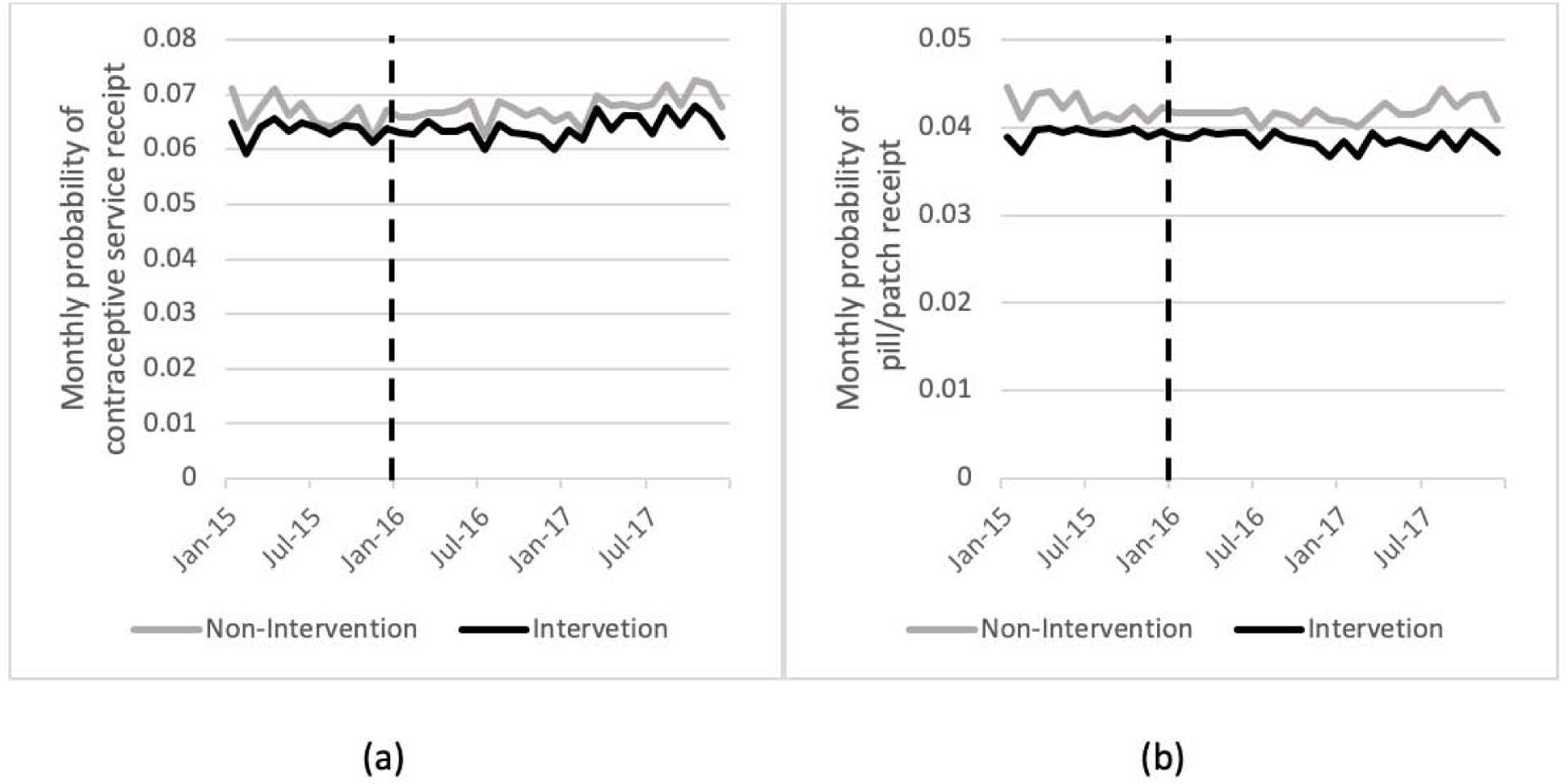
Monthly probabilities (2015–2017) among all Medicaid-enrolled women ages 15–44 of (a) receiving any contraceptive service and (b) filling prescriptions for the pill or patch. Intervention and non-intervention areas are those with and without pharmacist prescribing available. Vertical dashed line indicates pharmacy access policy implementation.
Table 2.
Difference-in-differences analyses of monthly contraceptive receipt for Medicaid enrolled women ages 15–44 before and after policy implementation in areas with and without Medicaid-registered prescribing pharmacists in Oregon (n = 9.59m person-months; N = 435,656 women), 2015–2017
| Measure | Receipt of any contraceptive method |
Receipt of pill/patch | ||
|---|---|---|---|---|
| RR [95% Cl] | aRR [95% Cl] | RR[95%CI] | aRR[95%CI] | |
| Policy period | ||||
| Pre-pharmacy access | ref | ref | ref | ref |
| Post-pharmacy access | 0.97 [0.94–1.00] | 1.01 [0.98–1.04] | 0.94 [0.90–0.99] | 0.98 [0.93–1.03] |
| Area | ||||
| Non-intervention | ref | ref | Ref | ref |
| Intervention | 0.99 [0.96–1.02] | 1.02 [0.99–1.06] | 0.96 [0.91–1.02] | 0.99 [0.94–1.05] |
| Post* Intervention | ||||
| interaction term | 0.98 [0.95–1.01] | 0.98 [0.95–1.01] | 0.99 [0.94–1.04] | 0.99 [0.94–1.04] |
Notes: RR: relative risk; aRR: adjusted relative risk; adjusted models include variables for age group, race/ethnicity, and urban/rural residence.
Examination of all pill/patch prescriptions filled in 2015–2017 indicated that pharmacist prescribing accounted for a very small share of these prescriptions following policy implementation (Table 3). In each year from 2015–2017, more than 98% of filled pill/patch prescriptions were prescribed by a non-pharmacist provider. In 2016, the first year of implementation, 0.3% (n = 520) of all filled pill/patch claims were prescribed by a pharmacist. An additional 1.1% (n = 1,735) of pill/patch claims in 2016 were missing prescribing provider information. In 2017, the percentage of filled pill/patch claims prescribed by a pharmacist increased to 0.6% (n = 788) and 1.3% of claims were missing prescriber information.
Table 3.
Annual pharmacy claims for the contraceptive pill and patch by prescribing provider type for Medicaid enrolled women ages 15–44, 2015–2017
| Prescriber type | 2015 % (n) |
2016 % (n) |
2017 % (n) |
|---|---|---|---|
| Non-pharmacist | 98.8 (156,974) | 98.5 (149,709) | 98.2 (137,125) |
| Pharmacist | <0.1 (18) | 0.3 (520) | 0.6 (788) |
| Provider type missing | 1.2 (1,915) | 1.1(1,735) | 1.3 (1,764) |
Among women receiving pill/patch prescriptions following policy implementation, 54% were continuing contraceptive users (Table 4). For continuing users, the most frequently used method in the past year was the pill (69%) followed by the injectable (9%), IUD (8%) and implant (7%). Patch users accounted for only 4% of continuing users, 3% were ring users, and < 1% were diaphragm users. Prescribing provider type was similar for new and continuing users. Among both types of users, only 0.6% had a prescription from a pharmacist in 2016–2017. Unadjusted and adjusted regression models (Table 5) provide further evidence of no difference in prescribing provider type between continuing and new users.
Table 4.
Characteristics of Medicaid enrolleda women ages 15–44 with initial pill/patch prescriptions, 2016–2017
| Measure | New users | Continuing users |
p-valueb |
|---|---|---|---|
| (n = 18,175) | (n = 15,563) | ||
| % | % | ||
| Age | <0.001 | ||
| 15–19 | 22.9 | 22.6 | |
| 20–24 | 22.0 | 24.4 | |
| 25–29 | 23.9 | 23.7 | |
| 30–34 | 16.7 | 15.3 | |
| 35–39 | 9.7 | 9.2 | |
| 40–44 | 4.8 | 4.9 | |
| Race/ethnicity | <0.001 | ||
| Hispanic | 20.2 | 17.3 | |
| Non-Hispanic white | 9.3 | 8.3 | |
| All others | 64.5 | 69.1 | |
| Missing | 6.0 | 5.4 | |
| Residence | 0.002 | ||
| Urban | 18.3 | 17.0 | |
| Rural | 81.7 | 83.0 | |
| Most effective method | |||
| received in past year | |||
| IUD | - | 8.1 | |
| Implant | - | 7.0 | |
| Injectable | - | 9.2 | |
| Pill | - | 69.0 | |
| Patch | - | 3.8 | |
| Ring | - | 3.0 | |
| Diaphragm | 100.0 | <0.1 | |
| None | - | ||
| Index prescription | 0.62 | ||
| prescriber type | |||
| Non-pharmacist | 99.4 | 99.4 | |
| Pharmacist | 0.6 | 0.6 | |
| Missing | <0.1 | - |
Enrolled at least 80% of the year prior to the index prescription
p-values were obtained from Chi-square tests
Table 5.
Association between past-year contraceptive use and prescriber type for Medicaid enrolled women ages 15–44 with pill/patch prescriptions filled in 2016–2017 (n = 33,734)
| Measure | Prescriber type (pharmacist vs. non-pharmacist) |
|
|---|---|---|
| RR [95% Cl] | aRR[95%CI] | |
| Continuing contraceptive user | ||
| No | ref | ref |
| Yes | 0.96 [0.73–1.25] | 0.94 [0.72–1.23] |
Notes: RR: relative risk; aRR: adjusted relative risk; adjusted models include variables for age group, race/ethnicity, and urban/rural residence.
4. Discussion
We found no significant effect of Oregon’s pharmacy access policy on receipt of contraceptive services overall or on receipt of the pill/patch for women enrolled in Medicaid. Women with prescriptions from pharmacists were as likely to be continuing users as women with prescriptions from non-pharmacists.
Our study findings are inconsistent with one prior study reporting that 10% of Medicaid-enrolled pill/patch users received new prescriptions from pharmacists [16]. We identified a similarly low volume of pharmacist prescriptions as Anderson et al. [16], but a much higher volume of initial pill/patch prescriptions from non-pharmacists. Our primary analysis examined all contraceptive and pill/patch services rather than focusing only on new prescriptions and is not directly comparable to Anderson et al. Nevertheless, the population for our analysis of continuing and new users is analogous to that examined by Anderson et al., including only women with initial prescriptions, and yielded substantially discrepant results; we found that more than 99% of women with initial prescriptions received them from non-pharmacists. Our results are thus also inconsistent with estimates based on findings from Anderson et al. [16] that the policy prevented 51 unintended pregnancies and saved $1.6 million dollars over the first two years of implementation [21].
All research findings using administrative data should be interpreted cautiously in light of data limitations. The pharmacy claims dataset lacked information required to link claims to provider data for about 1% of all claims; some claims with missing provider information could have been prescribed by pharmacists who were not yet registered with Medicaid. Nevertheless, > 98% of pill/patch claims in all years were prescribed by non-pharmacists with valid linked provider information, indicating that regardless of missing data, pharmacists accounted for a small proportion of pill/patch prescribing. An additional limitation is the use of zip codes, which vary in population and geographic area, to define intervention and control areas. Some women may find healthcare services outside of their residential zip code easily accessible, particularly in urban areas, leading to cross-over between intervention and control areas. Finally, our study is limited to the healthcare utilization experiences of low-income Medicaid-enrolled women in Oregon and may not generalize to other populations. For example, direct pharmacy access may have a greater impact for low-income women without insurance, for whom a visit to a clinical provider poses a more substantial financial barrier. On the other hand, the prescribing fees charged by many pharmacies likely make services more accessible for higher-income women.
Because we found no policy effect on increasing utilization of contraceptive services, an impact of pharmacist provision on reducing unintended pregnancy was highly unlikely during the first two years of policy implementation. Barriers to utilization of pharmacist prescribing may have included fees charged by pharmacies for prescribing services as well as lack of awareness of the availability of contraceptive prescriptions at pharmacies. Nevertheless, the policy may have positive effects on increasing satisfaction with services, convenience of services, and reducing out-of-pocket costs such as transportation and missed work. Impacts on access to contraceptive services and unintended pregnancy may emerge in subsequent years of implementation as availability of and demand for pharmacist-prescribed hormonal contraception increases. Additional research is needed to understand the potential benefits of policies that expand access to contraceptive services at pharmacies, including those with additional follow-up time to study longer-term impacts.
Implications:
We identified no effect of allowing pharmacist prescription of the contraceptive pill and patch on increasing utilization of contraceptive services for Medicaid-insured women in Oregon. Impacts on access to contraceptive services and unintended pregnancy may emerge in subsequent years as availability of and demand for pharmacist-prescribed hormonal contraception increases.
Funding sources:
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health [Award Number F32HD095554]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Daniels K, Daugherty J, Jones J, Mosher W. Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15–44: United States, 2011–2013. National health statistics reports. 2015:1–14. [PubMed] [Google Scholar]
- [3].Sundaram A, Vaughan B, Kost K, et al. Contraceptive Failure in the United States: Estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health. 2017;49:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frost JJ, Singh S, Finer LB. U.S. women’s one-year contraceptive use patterns, 2004. Perspect Sex Reprod Health. 2007;39:48–55. [DOI] [PubMed] [Google Scholar]
- [5].National Alliance of State Pharmacy Associations. Pharmacist Prescribing: Hormonal Contraceptives. 2019. [Google Scholar]
- [6].79th Oregon Legislative Assembly 2017 Regular Session. House Bill 2527. 2017.
- [7].Landau S, Besinque K, Chung F, et al. Pharmacist interest in and attitudes toward direct pharmacy access to hormonal contraception in the United States. J Am Pharm Assoc (2003). 2009;49:43–50. [DOI] [PubMed] [Google Scholar]
- [8].Rafie S, Cieri-Hutcherson NE, Frame TR, et al. Pharmacists’ Perspectives on Prescribing and Expanding Access to Hormonal Contraception in Pharmacies in the United States. Journal of pharmacy practice. 2019:897190019867601. [DOI] [PubMed] [Google Scholar]
- [9].Vu K, Rafie S, Grindlay K, Gutierrez H, Grossman D. Pharmacist Intentions to Prescribe Hormonal Contraception Following New Legislative Authority in California. Journal of pharmacy practice. 2019;32:54–61. [DOI] [PubMed] [Google Scholar]
- [10].Gardner JS, Miller L, Downing DF, Le S, Blough D, Shotorbani S. Pharmacist prescribing of hormonal contraceptives: results of the Direct Access study. J Am Pharm Assoc (2003). 2008;48:212–21; 5 p following 21. [DOI] [PubMed] [Google Scholar]
- [11].Gomez AM. Availability of Pharmacist-Prescribed Contraception in California, 2017. Jama. 2017;318:2253–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Batra P, Rafie S, Zhang Z, et al. An Evaluation of the Implementation of Pharmacist-Prescribed Hormonal Contraceptives in California. Obstet Gynecol. 2018;131:850–5. [DOI] [PubMed] [Google Scholar]
- [13].Rodriguez MI, McConnell KJ, Swartz J, Edelman AB. Pharmacist prescription of hormonal contraception in Oregon: Baseline knowledge and interest in provision. J Am Pharm Assoc (2003). 2016;56:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodriguez MI, Anderson L, Edelman AB. Prescription of hormonal contraception by pharmacists in Oregon: Implementation of House Bill 2879. Obstet Gynecol. 2016;128:168–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rodriguez MI, Garg B, Williams SM, Souphanavong J, Schrote K, Darney BG. Availability of pharmacist prescription of contraception in rural areas of Oregon and New Mexico. Contraception. 2020;101:210–2. [DOI] [PubMed] [Google Scholar]
- [16].Anderson L, Hartung DM, Middleton L, Rodriguez MI. Pharmacist Provision of Hormonal Contraception in the Oregon Medicaid Population. Obstet Gynecol. 2019;133:1231–7. [DOI] [PubMed] [Google Scholar]
- [17].Office of Population Affairs. Most or Moderately Effective Contraceptive Methods. U.S: Department of Health & Human Services; 2019. [Google Scholar]
- [18].Office of Health Analytics. CCO Metrics. Oregon Health Authority; 2019.
- [19].United States Census Bureau. ZIP Code Tabulation Areas (ZCTAs). 2020.
- [20].University of Washington. Rural-Urban Community Area Codes (RUCAs). n.d.
- [21].Rodriguez MI, Hersh A, Anderson LB, Hartung DM, Edelman AB. Association of Pharmacist Prescription of Hormonal Contraception With Unintended Pregnancies and Medicaid Costs. Obstet Gynecol. 2019;133:1238–46. [DOI] [PubMed] [Google Scholar]


