Abstract
The animal germline is an immortal cell lineage that gives rise to eggs and/or sperm each generation. Fusion of an egg and sperm, or fertilization, sets off a cascade of developmental events capable of producing an array of different cell types and body plans. How germ cells develop, function, and eventually give rise to entirely new organisms is an important question in biology. A growing body of evidence suggests that phase separation events likely play a significant and multifaceted role in germ cells and development. Here, we discuss the organization, dynamics, and potential functions of phase-separated compartments in germ cells and examine the various ways in which phase separation might contribute to the development of multicellular organisms.
Introduction
Eukaryotic cells produce a multitude of subcellular compartments that concentrate specific molecules in space and time to help organize cellular processes. Some of these compartments, such as mitochondria, are organized and delimited by lipid membranes. However, many other compartments lack a membrane barrier, yet remain distinct from the surrounding nucleoplasm or cytoplasm. These membraneless compartments contribute to a wide range of cellular processes. More prominent examples of membraneless compartments include nucleoli, Cajal bodies, stress granules, processing bodies (P-bodies), and germ granules. However, the list of known membraneless compartments extends far beyond these examples and will likely continue to grow.
The question of how membraneless compartments form is under intense investigation. Mounting evidence suggests that many membraneless compartments such as nucleoli and germ granules form via liquid-liquid phase separation (LLPS), similar to how oil separates from vinegar in a vinaigrette (Banani et al., 2017). LLPS occurs when the contents of a homogenous liquid demix into two distinct liquid phases. In cells, LLPS can occur when a given set of molecules demixes from the surrounding cytoplasm (or nucleoplasm) to form liquid-like droplets, or condensates (Hyman, Weber and Jülicher, 2014). Current models posit that LLPS is driven in part by weak, multivalent interactions between proteins and nucleic acids, and tends to involve proteins containing intrinsically disordered domains, which lack well-defined three-dimensional structures (Shin and Brangwynne, 2017). The resulting liquid-like condensates are structurally dynamic: they exhibit rapid internal molecular rearrangements and rapid exchange of molecules with the surrounding cytoplasm (or nucleoplasm). Due to their dynamic and labile nature, liquid-like condensates are highly sensitive to shifts in temperature, pH, and other environmental factors. LLPS can also be regulated by post-translational modifications and changes in the concentration of constituent molecules. In some cases, phase separation within cells can result in condensates exhibiting gel-like or even solid-like properties (Alberti, 2017). Recently, the field of phase separation has adopted the term “biomolecular condensates” to refer to phase-separated subcellular compartments.
Recent studies show that several proteins involved in key developmental processes such as gene regulation and signal transduction undergo phase separation (Hnisz et al., 2017; Case, Ditlev and Rosen, 2019). It is intriguing, therefore, to speculate that cells use phase separation to coordinate the timing, location, and/or efficacy of developmental processes. Support for this idea is beginning to strengthen, especially as advancements in technology improve our ability to explore the dynamics of biomolecular condensates in living cells and tissues. Here, we review the ways that phase separation might facilitate development. We focus on germ cells, which are known to form a wide array of biomolecular condensates that contribute to germ cell identity and function. Because germ cell condensates are well-studied, diverse, and cell-type-specific, they serve as excellent model systems for studying phase separation in the context of development. We examine how germ cell condensates assemble, disassemble, and change properties in response to developmental cues. We also discuss how these condensates are spatially organized, both internally and in relation to other condensates, and speculate on how this organization could help coordinate different germ cell processes. Finally, we reflect on how different cell types may exploit the unique properties of LLPS and biomolecular condensates to carry out key developmental processes. For in-depth discussions on the role of phase separation in signaling and transcription, we refer the reader to other reviews in this special issue.
Biomolecular Condensates in Germline Development
Metazoans develop via a cascade of highly choreographed events that regulate the timing of cell divisions, cell migrations, cell-to-cell communication, and pattern formation. Remarkably, this cascade of developmental events begins with a single cell - the fertilized egg, or zygote. As the zygote begins to divide, it gives rise to a diverse array of cell types with specialized functions. These cell types can be grouped into two general categories: 1) somatic cells, which make up most of the body and include skin cells, neurons, and blood cells; and 2) germ cells, which produce haploid gametes (eggs and sperm) for reproduction. Whereas somatic cells die out with the organism, gametes can fuse to produce a new zygote and begin the cascade of development anew.
Because germ cells give rise to new life generation after generation, the germline is considered to be “immortal”. Each generation, the germline cycles through the following phases: specification of the primordial germ cells (the precursors of gametes) during embryogenesis; proliferation, meiosis, and differentiation into gametes during later stages of development; and, finally, fusion between an egg and sperm to produce a new, totipotent zygote (Lesch and Page, 2012). In addition to being immortal, germ cells are also unique in that they contain a class of membraneless compartments known as germ granules. Examples of germ granules include P granules in C. elegans, polar granules in Drosophila, and chromatoid bodies in mammals (Table 1) (Mahowald, 1962; Fawcett, Eddy and Phillips, 1970; Strome and Wood, 1982). Germ granules are present in the germ cells of most, if not all, animals and contain many proteins important for the function and development of germ cells (Voronina et al., 2011; Gao and Arkov, 2013).
Table 1.
Prominent membraneless compartments specific to germ cells in animals.
| Compartment | Potential function(s) | Physical properties | Components that condense in vitro and/or in heterologous cells | In vivo FRAP | References for physical properties, condensation, and FRAP | ||
|---|---|---|---|---|---|---|---|
| Component | t1/2 (seconds)a | Mobile fraction (%) | |||||
| P granules (C. elegans) | Maintenance of germ cell totipotency, RNA processing and storage | Spherical, Undergo fusion, Deform under shear stress | LAF-1, MEG-3, PGL-3 | CAR-1 | 2.6 | 16 | Brangwynne et al., 2009; Elbaum-Garfinkle et al., 2015; Saha et al., 2016; Smith et al., 2016 Putnam et al., 2019 |
| GLH-1 | 18.4–30.1 | 100 | |||||
| LAF-1 | 1.5 | ||||||
| MEG-3 | 128.3–384.3 | ||||||
| PGL-1 | 4.7–20.73 | 68 | |||||
| PGL-3 | 21.9 | ||||||
| PIE-1 | 5.4 | 51 | |||||
| Z granules (C. elegans) | Transgenerational epigenetic inheritance, RNA processing | Spherical, Resistant to shear stress | ZNFX-1 | 8 | Wan et al., 2018 | ||
| Mutator foci (C. elegans) | siRNA amplification, Transposon silencing | MUT-16 | MUT-16 | 7.2 | 35 | Uebel et al., 2018 | |
| SIMR foci (C. elegans) | piRNA-based gene silencing | ||||||
| grP-bodies (C. elegans) | RNA storage | Spherical, Highly viscous | CAR-1 | ~ 940 | Hubstenberger et al., 2013 | ||
| Polar granules (Drosophila) | Germ cell specification and development | Spherical, Fusion not observed | Osk | Osk | 6.9 | 43.6 | Jeske et al., 2015; Kistler et al., 2018 |
| Vasa | 23.9 | 46 | |||||
| Nuage (Drosophila) | piRNA processing | Aub | 35 | Snee and Macdonald, 2004; Nosov et al., 2014 | |||
| Vasa | 52.1–59 | 72 | |||||
| Sponge bodies (Drosophila) | RNA processing and/or storage | ||||||
| piNG-body (Drosophila) | piRNA-based gene silencing | Vasa | 5.3 | 71 | Nosov et al., 2014 | ||
| Perinuclear germ granules (zebrafish) | Germ cell development | Spherical, Undergo fusion | Strasser et al., 2008 | ||||
| Balbiani body, or mitochondrial cloud (vertebrates and other animals) | Germ cell specification (Xenopus, zebrafish), Oocyte quality control | Spherical, Resistant to shear stress | Buc (zebrafish) | Buc (zebrafish) | Slow and partial recovery | Boke et al., 2016; Roovers et al., 2018 | |
| Xvelo (Xenopus) | 20 | ||||||
| pi-bodies, or intermitochondrial cement (mammals) | Primary piRNA processing | ||||||
| piP-bodies (mammals) | Secondary piRNA processing | ||||||
| Chromatoid body (mammals) | piRNA-based gene silencing, RNA processing and storage | Ddx4 (human) | Nott et al., 2015 | ||||
FRAP recovery half-times from Brangwynne et al., 2009 were derived from the time constant τ using the following equation: t1/2 = τ*ln2.
Germ granules contain RNA, as well as many proteins involved in RNA regulation and quality control; thus, germ granules are thought to play important roles in various RNA-related processes, including transposon silencing, mRNA surveillance by small regulatory RNAs, mRNA localization, and mRNA storage (Table 1) (Voronina et al., 2011; Gao and Arkov, 2013). Germ granules likely coordinate such processes by concentrating specific proteins and RNAs together in space and time. Germ granules often localize to the outer nuclear periphery, where they associate with nuclear pores (Voronina et al., 2011). One function of perinuclear germ granules may be to surveil and process nascent mRNAs as they exit the nuclear pore (see Significance of Phase Separation in Development). In some animals such as C. elegans, Drosophila, Xenopus, and zebrafish, germ granules are maternally inherited and segregate asymmetrically with germline blastomeres during early embryonic cell divisions. Germ granules are cytoplasmic during this stage of development and contain multiple maternal mRNAs, including some required for germ cell specification. Early embryonic germ granules may help promote germ cell fate by concentrating maternally inherited germ cell factors in germline blastomeres (Voronina et al., 2011).
A growing body of evidence suggests that germ granules assemble through phase separation (Table 1). In fact, C. elegans P granules were the first membraneless compartment reported to form via LLPS, and P granules have since emerged as a leading model for the study of biomolecular condensates (Brangwynne et al., 2009). P granules exhibit multiple liquid-like behaviors: they are spherical except when perinuclear, they readily fuse together, they drip off the nucleus in response to shear stress, and fluorescence recovery after photobleaching (FRAP) experiments suggest that they are highly dynamic (Brangwynne et al., 2009; Sheth et al., 2010; Putnam et al., 2019). Germ granules in other animals also show hints of liquid-like behavior, as germ granules in zebrafish can undergo liquid-like fusion events (Strasser et al., 2008), and multiple types of germ granules in Drosophila (polar granules, nuclear germ granules, perinuclear nuage, and piRNA nuage giant bodies, or piNG-bodies) rapidly exchange molecules with the surrounding cytoplasm (Snee and Macdonald, 2004; Nosov, Kibanov and Olenina, 2014; Kistler et al., 2018). Further work characterizing the biophysical properties of different types of germ granules should help determine whether phase separation is a widespread mechanism of germ granule assembly.
In addition to driving germ granule formation, phase separation-related processes likely coordinate other aspects of germline development, as well. For instance, developing vertebrate oocytes compartmentalize protein, RNA, and various membrane-bound organelles into a large, membraneless compartment known as the Balbiani body (Table 1) (Kloc, Bilinski and Etkin, 2004; Jamieson-Lucy and Mullins, 2019). Proposed functions of the Balbiani body include germ cell specification and oocyte quality control (Lei and Spradling, 2016; Jamieson-Lucy and Mullins, 2019). In Xenopus, the Balbiani body is rigid, seemingly non-dynamic, resistant to harsh conditions ex vivo, and stains with an amyloid-specific dye (Chang et al., 2004; Boke et al., 2016). Unlike typical amyloids, however, the Balbiani body is spherical and its formation is reversible, as the Balbiani body disassembles in later stages of oocyte development. To account for all these properties of the Balbiani body, (Woodruff, Hyman and Boke, 2018) propose that Balbiani bodies initially assemble via LLPS, but then rapidly harden into a solid-like state by forming an amyloid-like matrix (Woodruff, Hyman and Boke, 2018). Understanding the mechanisms by which LLPS-generated condensates harden into solid-like states will likely lead to a greater understanding of how such organelles contribute to development. Interestingly, the Balbiani body forms specifically in early oocytes, which have the remarkable ability to remain dormant for months or even years. It is therefore intriguing to speculate that one function of Balbiani body formation and hardening is to preserve the contents of the oocyte during prolonged periods of meiotic arrest (Boke et al., 2016). Other types of condensates may serve similar functions in oocytes of animals that do not form Balbiani bodies. For instance, in C. elegans, oocytes subjected to prolonged arrest form large, P-body-like compartments (often termed grP-bodies) that are thought to store and protect maternal mRNAs in the absence of sperm (Table 1) (Schisa, Pitt and Priess, 2001; Boag et al., 2008; Jud et al., 2008; Noble et al., 2008; Hubstenberger et al., 2013). Thus, phase separation may play a common and important role in preserving the quality of arrested oocytes in animals.
Drivers and Regulators of Phase Separation in Germ Cells
Proteins containing RNA-binding domains, intrinsically disordered regions, and/or low sequence complexity play key roles in the assembly of germ cell condensates. For example, intrinsically disordered proteins rich in serine (MEG-3, MEG-4, and DEPS-1), proteins containing RG-rich RNA-binding domains (PGL-1, PGL-3, and LAF-1), and multiple FG-repeat proteins including the RNA helicase GLH-1 are all known to contribute to P granule formation in C. elegans (Spike et al., 2008; Marnik and Updike, 2019). Some or all of these proteins may help assemble P granules by promoting LLPS. In support of this idea, recombinant MEG-3, PGL-3, and LAF-1 can all phase separate into liquid-like condensates in vitro (Elbaum-Garfinkle et al., 2015; Saha et al., 2016; Smith et al., 2016). Furthermore, when MEG-3, PGL-3, and RNA are all combined in the same buffer, MEG-3 and PGL-3 co-assemble into condensates with substructures similar to those of P granules (see Internal Organization of Germ Granules) (Putnam et al., 2019). GLH-1 and other FG-repeat proteins may undergo phase separation to promote the physical association of P granules with nuclear pores, as tethering of P granules to nuclei requires the presence of both FG-rich proteins in P granules and FG-rich nucleoporins in the nuclear pore complex, and nucleoporin FG domains readily phase separate in vitro (Frey, Richter and Görlich, 2006; Updike and Strome, 2009; Voronina and Seydoux, 2010; Schmidt and Görlich, 2015; Marnik et al., 2019; Chen et al., 2020). GLH-1 is an ortholog of Drosophila Vasa and other Vasa-like helicases, which localize to germ granules in many animals (Voronina et al., 2011). In Drosophila, Vasa interacts with the intrinsically disordered RNA-binding protein Osk to help assemble germ granules (Breitwieser et al., 1996; Jeske et al., 2015; Lehmann, 2016; Kistler et al., 2018). Notably, Ddx4, the human ortholog of Vasa, forms phase-separated condensates in vitro and when expressed ectopically in HeLa cells (Nott et al., 2015). Specific sequence features of Ddx4 and C. elegans LAF-1 govern the phase behavior of these proteins in vitro and in heterologous cells, and it will be interesting to test whether these features impact germ granule formation and/or function (Nott et al., 2015; Schuster et al., 2020).
Post-translational modifications are emerging as important regulators of LLPS (Hofweber and Dormann, 2019; Owen and Shewmaker, 2019). For instance, the C. elegans P granule assembly protein MEG-3 is a substrate for the kinase MBK-2 and the phosphatase PP2A, and phosphorylation and dephosphorylation of MEG-3 is thought to promote P granule dissolution and condensation, respectively (Wang et al., 2014). Additionally, arginine methylation of Ddx4 dramatically impairs Ddx4 condensation in vitro, perhaps by disrupting cation-pi interactions between FG and RG repeats within Ddx4 (Nott et al., 2015). In addition to regulating condensate formation, post-translational modifications may also affect the material properties of condensates. In zebrafish, Balbiani body assembly requires the intrinsically disordered protein Bucky ball (Buc) (Marlow and Mullins, 2008; Bontems et al., 2009). Recently, the multi-Tudor domain-containing protein Tdrd6a was shown to promote the mobility of Buc within Balbiani bodies (Roovers et al., 2018). Tdrd6a interacts directly with methylated arginines in Buc that reside within a tri-RG motif, suggesting that Balbiani body dynamics may be subject to regulation by post-translational modifications (Roovers et al., 2018). Thus, post-translational modifications of intrinsically disordered proteins likely trigger a variety of effects on phase separation in germ cells.
RNA is also likely to play an important role in the phase separation of germ cell condensates. In the case of C. elegans P granules, phase separation is thought to be driven in part by interactions between mRNAs and RNA-binding proteins. Support for this idea originated from studies showing that RNA stimulates the condensation of both MEG-3 and PGL-3 in vitro (Saha et al., 2016; Smith et al., 2016). PGL-3 binds mRNA in vitro with low sequence specificity via the C-terminal RGG domain (Saha et al., 2016). Although MEG-3 lacks a recognizable RNA-binding domain, MEG-3 also binds RNA in vitro, as well as in vivo (Smith et al., 2016; Lee et al., 2020). In vivo, MEG-3 preferentially binds ribosome-poor mRNAs independent of mRNA sequence (Lee et al., 2020). Interestingly, translational repression is sufficient to direct mRNAs to P granules, and increasing cellular pools of ribosome-poor mRNAs (via heat shock or treatment with certain translation inhibitors) causes P granules to enlarge (Lee et al., 2020; Parker et al., 2020). Altogether, these observations suggest that non-specific RNA-protein interactions - particularly between ribosome-poor mRNAs and MEG-3 - help drive P granule condensation in C. elegans germ cells. RNA could potentially regulate the viscosity of P granules, as well: adding short RNAs (15–50 nt) to LAF-1 condensates in vitro increases condensate fluidity, whereas long RNAs (3,000 nt) have the opposite effect (Elbaum-Garfinkle et al., 2015; Wei et al., 2017). A challenge for the future will be to determine the extent to which cells use RNA to tune and regulate phase separation in germ cells and beyond.
Internal Organization of Germ Granules
The composition of germ granules and other germ cell condensates is often non-uniform, with specific proteins and RNAs localizing to distinct subcompartments within a given condensate. Perhaps due to their relatively large size (up to 10 μm), C. elegans grP-bodies exhibit especially striking subdomains, including clusters marked by the poly(A)-binding protein PAB-1, the mRNA decay factor PATR-1, and the RNA-binding protein PGL-1 (Figure 1A) (Jud et al., 2008; Hubstenberger et al., 2013). The mechanistic underpinnings by which condensates like grP-bodies form and maintain internal subcompartments is an area of current investigation. In some cases, condensate subcompartments may be maintained in part by differences in subcompartment surface tensions (Feric et al., 2016; Shin and Brangwynne, 2017). Future studies are likely to reveal the mechanisms by which condensate subcompartments form and may reveal additional mechanisms by which these structures are maintained.
Figure 1. Spatial organization of germ cell condensates.

(A) PATR-1 and PGL-1 localize to distinct subregions (arrowheads) within germline ribonucleoprotein granules related to P-bodies (grP-bodies) in arrested C. elegans oocytes. Scale bar, 2 μm. Image originally from (Hubstenberger et al., 2013). (B) P granules in the germline blastomeres of early C. elegans embryos. MEG-3 unevenly coats a PGL-3-marked core. Scale bar, 1 μm. Reprinted by permission from Springer Nature: (Putnam et al., 2019). (C) Representative piP-body in mouse prospermatogonia. Dcp1a surrounds a Mael-marked core. Image originally from (Aravin et al., 2009). (D) Homotypic mRNA clustering in Drosophila polar granules. Osk-marked polar granule containing distinct clusters of pgc and nos mRNA. Image originally from (Niepielko, Eagle and Gavis, 2018).
In C. elegans embryos, P granules contain at least two subcompartments: an inner core, and an outer layer marked by the intrinsically disordered protein MEG-3 (Wang et al., 2014; Putnam et al., 2019). MEG-3 and its paralog MEG-4 function specifically during the early stages of embryogenesis to assemble P granules in germ cell precursors (Wang et al., 2014). As a part of this process, MEG-3 condenses into structures that coat the P granule periphery (Figure 1B) (Wang et al., 2014; Putnam et al., 2019). In comparison to the P granule core, which exhibits many liquid-like behaviors, the outer MEG-3 layer is less dynamic and less sensitive to perturbations such as an up-shift in temperature (Putnam et al., 2019). The distinct, gel-like properties of the MEG-3 phase are thought to help nucleate and stabilize the more labile P granule core (Putnam et al., 2019). Embryonic P granules house maternal mRNAs, including some that function in germ cell specification, and the MEG-3 phase helps recruit and retain such mRNAs within P granules (Seydoux and Fire, 1994; Subramaniam and Seydoux, 1999; Schisa, Pitt and Priess, 2001; Smith et al., 2016; Lee et al., 2020; Parker et al., 2020). By preferentially condensing in the germline-destined portion of the cytoplasm during early embryonic divisions, P granules - and the MEG-3 phase, in particular - may help enrich germline-defining mRNAs in the germline founder cell (Lee et al., 2020).
In addition to C. elegans P granules, other germ cell condensates also possess distinct core and surface layers. For instance, polar granules in Drosophila primordial germ cells, piNG-bodies (piRNA nuage giant bodies) in Drosophila male germ cells, and piP-bodies in mouse prospermatogonia all contain proteins that localize to either the condensate core or the condensate periphery (Figure 1C) (Aravin et al., 2009; Kibanov et al., 2011; Vo et al., 2019). Distinctions between the core and surface are also apparent in other types of condensates, including stress granules, nucleoli, and paraspeckles (Shin and Brangwynne, 2017). Therefore, core and surface subdivisions are a common feature of biomolecular condensates. Proteins that localize to the surface of condensates could potentially serve a variety of roles in condensate biology, as they could act as “gatekeepers” that regulate which molecules enter or exit the condensate, as stabilizers of condensate cores, as adaptors that facilitate interactions with other subcellular structures, or as biological surfactants that tune condensate stability.
Additionally, specific RNAs can also localize to distinct subregions within condensates. For instance, RNA-defined subcompartments are a notable feature of Drosophila polar granules. During late oogenesis, several mRNAs important for germ cell development, including nos, gcl, and CycB, accumulate in the oocyte posterior, where they incorporate into polar granules (Lehmann, 2016; Trcek and Lehmann, 2019). Upon entering polar granules, mRNAs organize into discrete homotypic clusters (Figure 1D) (Little et al., 2015; Trcek et al., 2015, 2020; Niepielko, Eagle and Gavis, 2018). Interestingly, homotypic clusters can localize to different positions within polar granules: clusters of cycB mRNA typically reside in the center of Vasa-marked granules, clusters of nos mRNA tend to occupy a middle zone, and clusters of gcl mRNA coat the granule edge (Trcek et al., 2015, 2020). RNA cluster positions appear to depend on the concentration of the mRNA within the granule (Trcek et al., 2020). Homotypic mRNA clusters have also been observed in the Balbiani body/germ plasm of zebrafish and in C. elegans P granules, suggesting that this phenomenon may be common among germ cell condensates (Roovers et al., 2018; Eno, Hansen and Pelegri, 2019; Parker et al., 2020). Future studies exploring the organization of RNA within condensates will likely reveal if and how such organization impacts condensate function.
Germ Granule Remodeling during Development
During development, germ granules undergo regulated changes in composition, morphology, and localization. P granules, for instance, are present during all stages of the C. elegans life cycle, but show distinct characteristics at different stages of germline development. Unlike P granules in early embryos, P granules in later stages of development do not harbor a MEG-3-marked surface phase and do not require MEG-3 and MEG-4 for assembly/maintenance (Wang et al., 2014). Such post-embryonic P granules also lack other proteins found in embryonic P granules, including PIE-1, POS-1, MEG-1, and MEG-2 (Mello et al., 1996; Tabara et al., 1999; Leacock and Reinke, 2008). In addition to losing and gaining various proteins during development, P granules also show changes in subcellular localization. For most of the C. elegans life cycle, P granules associate with clusters of nuclear pores at the outer nuclear periphery of germ cell nuclei (Pitt, Schisa and Priess, 2000; Sheth et al., 2010). In oocytes, however, P granules detach from the nucleus and remain cytoplasmic post-fertilization for the first few cell divisions of embryogenesis before reattaching to the nucleus (Strome and Wood, 1982). Changes in P granule localization and composition likely underlie changes in P granule function: Whereas cytoplasmic P granules in early embryos may assist in germ cell specification, as noted above (Lee et al., 2020), perinuclear P granules in later stages of development are thought to help maintain germ cell identity by surveilling and processing nascent mRNAs as they exit the nucleus (Updike et al., 2014; Knutson et al., 2017).
At least two proteins that localize to P granules in early germline blastomeres (the RNA helicase ZNFX-1 and the Argonaute protein WAGO-4) separate from P granules at a precise time during early embryogenesis (during the birth of primordial germ cells) to form an independent condensate termed the Z granule (Figure 2) (Wan et al., 2018). Z granules are liquid-like in nature and localize adjacent to P granules at the nuclear periphery (Wan et al., 2018). Interestingly, Z granules appear to initially form by demixing from other components in the P granule (Figure 2A) (Wan et al., 2018). How and why Z granules emerge from P granules at a defined point in development is not yet known. P granules contain multiple small RNA pathway proteins including the Argonautes WAGO-1, CSR-1, and PRG-1, and P granules are thought to coordinate small RNA pathways (Batista et al., 2008; Claycomb et al., 2009; Gu et al., 2009; Dodson and Kennedy, 2019; Lev et al., 2019; Ouyang et al., 2019). ZNFX-1 and WAGO-4 also contribute to small RNA-based gene regulation, as they help propagate a subset of small RNA-based gene silencing signals across generations (Ishidate et al., 2018; Wan et al., 2018). Thus, one role of embryonic P granules may be to sort out mRNAs destined for transgenerational silencing, while demixing of ZNFX-1/WAGO-4 to form Z granules may facilitate downstream silencing of these targets.
Figure 2. Z granule formation in C. elegans embryos.

(A) Time-lapse micrographs showing ZNFX-1 demixing from PGL-1 in a primordial germ cell at approximately the 300-cell stage. Reprinted by permission from Springer Nature: (Wan et al., 2018). (B) Z granule formation coincides roughly with nuclear attachment and the onset of germline transcription. In early germline blastomeres (left), ZNFX-1 and WAGO-4 co-localize with P granule proteins. Later in embryogenesis (right), ZNFX-1 and WAGO-4 demix from P granules to form Z granules. Z granule demixing may be driven by newly synthesized mRNAs (purple and blue lines), one or more newly synthesized proteins (blue question mark), post-translational modifications, and/or attachment to the outer nuclear envelope. Note, P granules (and likely Z granules, as well) contain additional proteins not shown here.
Notably, Z granule formation roughly coincides with the onset of germline transcription, as well as the attachment of P granules to nuclei, and these concurrent events could potentially trigger Z granule demixing via a number of mechanisms (Figure 2B) (Strome and Wood, 1982; Seydoux et al., 1996; Wan et al., 2018). It is possible, for instance, that the docking of P granules to nuclei is sufficient to promote Z granule demixing, as membrane surfaces are known to alter phase separation dynamics (Snead and Gladfelter, 2019). Alternatively, Z granule formation may be driven by post-translational modifications and/or by one or more proteins produced as a consequence of renewed germline transcription. Another intriguing possibility is that newly transcribed mRNAs play a direct role in Z granule formation. Time-lapse imaging of mRNAs transcribed from various inducible transgenes suggests that, as nascent mRNAs exit the nuclear pores of germ cell nuclei, they accumulate temporarily in P granules before exiting into the cytoplasm (Sheth et al., 2010). Since RNA can influence the assembly and organization of condensates (Berry et al., 2015; Rhine, Vidaurre and Myong, 2020), (Wan et al., 2018) speculate that, as nascent germline mRNAs enter P granules, they interact with RNA-binding proteins such as ZNFX-1 and WAGO-4 to drive Z granule formation. Other types of perinuclear germ granules (piNG-bodies in Drosophila and chromatoid bodies in mice) also form during waves of transcription, hinting that transcriptional activation may be a common driver of phase separation at or near the outer nuclear membrane (Kibanov et al., 2011; Meikar et al., 2011). In support of this idea, injecting the RNA Polymerase II transcriptional inhibitor α-amanitin into C. elegans gonads causes at least some P granule components to detach from the nucleus, whereas injecting translation inhibitors does not have the same effect (Sheth et al., 2010).
Phase demixing may underlie other instances of germ granule remodeling, as well. For instance, Drosophila polar granules likely undergo internal demixing events during early embryogenesis: Initially, polar granule proteins including Oskar, Aubergine, and Tudor are distributed somewhat evenly within polar granules; however, later in development, these proteins relocate to distinct core and surface subcompartments (Harris and Macdonald, 2001; Jones and Macdonald, 2007; Trcek et al., 2015; Vo et al., 2019). Thus, phase demixing may be a common form of germ granule remodeling. Conversely, germ granule remodeling may also occur via the mixing of two or more different phases. In support of this idea, two different types of germ granules - piP-bodies and early chromatoid bodies - appear to merge together during mouse spermatogenesis to form mature chromatoid bodies (Figure 3A) (Tanaka et al., 2011). The mixing of these two condensates depends on Tdrd7, which encodes a Tudor domain-containing protein that localizes to chromatoid bodies (Hosokawa et al., 2007; Tanaka et al., 2011). In Tdrd7−/− mutants, chromatoid bodies and piP-bodies fail to merge completely, and instead form striking multi-condensate assemblages that, at least in some cases, comprise one piP-body “sandwiched” by two chromatoid bodies (Figure 3B) (Tanaka et al., 2011). Given that Tdrd7 is required for spermatid differentiation, the merging of chromatoid bodies and piP-bodies may be an important step in male germ cell development in mammals (Tanaka et al., 2011). Altogether, these observations suggest that regulated mixing and demixing of phase-separated structures may be a common mechanism by which cells coordinate developmental processes.
Figure 3. Germ granule interactions during mouse spermatogenesis.

(A) Model of germ granule-based organization of the piRNA pathway. In prospermatogonia (ProSg), pi-bodies and piP-bodies facilitate the processing of primary and secondary piRNAs, respectively. piP-bodies are often in close proximity to pi-bodies, and cross-talk between the two compartments (arrows) may promote piRNA amplification. Early chromatoid bodies (CBs) first appear in pachytene spermatocytes (Pa Spc) and are distinct from pi-bodies and piP-bodies. By the round spermatid (RS) stage, chromatoid bodies and piP-bodies have merged to form a mature chromatoid body, which likely functions in piRNA-based gene silencing and other types of RNA processing. PGC, primordial germ cell; Sg, spermatogonium; early Spc, early spermatocyte; ES, elongating spermatid. (B) Electron micrographs of spermatids in Tdrd7+/− and Tdrd7−/− mice. Green arrowhead, mature chromatoid body; yellow arrowheads, early chromatoid bodies; blue arrowhead, piP-body. Image originally from (Tanaka et al., 2011).
Interactions between Germ Cell Condensates
Prior to merging with chromatoid bodies during mouse spermatogenesis, piP-bodies contact another type of condensate termed the pi-body (also referred to as intermitochondrial cement) (Figure 3A) (Aravin et al., 2009; Shoji et al., 2009). In prospermatogonia, piP-bodies often localize adjacent to or partially overlap with pi-bodies (Aravin et al., 2009; Shoji et al., 2009). Interestingly, pi-bodies and piP-bodies are thought to coordinate sequential steps in the processing of pre-pachytene PIWI-interacting RNAs (piRNAs), a class of small RNAs that mediates transposon silencing during early stages of mammalian spermatogenesis (Aravin et al., 2009; Chuma and Pillai, 2009; Shoji et al., 2009; Wang et al., 2020). Thus, pi-bodies and piP-bodies are linked in terms of both location and function. Current models posit that pi-bodies and piP-bodies cooperate to produce and amplify pre-pachytene piRNAs that then silence transposable elements (Figure 3A) (Lehtiniemi and Kotaja, 2018). A second population of piRNAs, pachytene piRNAs, is expressed later in spermatocytes and spermatids and may target mRNAs for silencing within the chromatoid body, as the chromatoid body houses multiple piRNA pathway proteins, including the piRNA-binding PIWI proteins MILI and MIWI and the Tudor domain-containing proteins TDRD1, TDRD6, and TDRD7 (Figure 3A) (Lehtiniemi and Kotaja, 2018). Pachytene piRNAs may silence transposons and also regulate meiotic gene expression (Ernst, Odom and Kutter, 2017). In addition to mice, Drosophila, C. elegans, and likely other animals also appear to compartmentalize the piRNA silencing pathway into distinct, yet interconnected condensates (Dennis et al., 2013; Murota et al., 2014; Dennis, Brasset and Vaury, 2019; Hirakata and Siomi, 2019; Manage et al., 2020). Such organization may help partition different steps of the piRNA pathway in space and time, while still allowing pathway intermediates to flow in an organized and highly choreographed fashion from one step to the next.
Regulated interactions between different condensates are especially evident in the germline of C. elegans. As noted above, ZNFX-1 and WAGO-4 demix from P granules during early embryogenesis to form Z granules, which remain directly adjacent to, and may be in direct contact with, P granules for most of development (Wan et al., 2018). Interestingly, P granules and Z granules reside in multi-condensate assemblages that also include compartments known as Mutator foci and SIMR foci (Figure 4) (Phillips et al., 2012; Wan et al., 2018; Manage et al., 2020). These assemblages are spatially ordered, as each typically contains a single Z granule sandwiched by a P granule on one end and a Mutator focus on the other (Wan et al., 2018). Like P granules and Z granules, Mutator foci are thought to form via phase separation (Uebel et al., 2018). The dynamics of SIMR foci and the exact position of SIMR foci with respect to other compartments remain undetermined. For simplicity, we hereinafter refer to these multi-condensate assemblages as perinuclear nuage. The mechanisms underlying the formation and spatial ordering of C. elegans perinuclear nuage remain unclear. One possibility is that each type of condensate contains a distinct network of intermolecular interactions that partially overlaps with the networks of neighboring condensates (Sanders et al., 2020).
Figure 4. Subcompartments of C. elegans perinuclear nuage.

Model: Nuage subcompartments are spatially ordered and specialize in distinct yet related aspects of RNA processing. pUG RNAs (purple) are enriched in Mutator foci. The exact location of SIMR foci relative to other subcompartments is not yet known (question marks). Inset: Representative micrograph of PGL-1 (marker of P granules), ZNFX-1 (marker of Z granules), and MUT-16 (marker of Mutator foci) in a pachytene germ cell. Reprinted by permission from Springer Nature: (Wan et al., 2018).
Each subcompartment of C. elegans perinuclear nuage contains a distinct set of proteins involved in small RNA-based gene regulation. Mutator foci, for example, contain proteins needed to silence transposable elements (Phillips et al., 2012). Many of these proteins are involved in amplifying small RNA populations by directing the synthesis of secondary small interfering RNAs (siRNAs) via RNA-dependent RNA polymerases (RdRPs) (Billi, Fischer and Kim, 2014). For instance, Mutator foci contain the nucleotidyl transferase RDE-3, which adds tails of alternating uridine and guanosine ribonucleotides (termed pUG tails) to the ends of mRNA fragments (Phillips et al., 2012; Shukla et al., 2020). pUG tails are thought to recruit RdRPs, which then synthesize secondary siRNAs using the pUGylated mRNA fragments as templates (Shukla et al., 2020). RDE-3 and other Mutator foci components, including the RdRP RRF-1 and the RNA helicase MUT-14, are recruited to Mutator foci by the intrinsically disordered protein MUT-16, which nucleates Mutator foci assembly (Phillips et al., 2012; Uebel et al., 2018). Mutator foci are thought to serve as hubs for siRNA amplification because loss of MUT-16 (and therefore Mutator foci) substantially reduces the levels of a major class of secondary siRNAs (Zhang et al., 2011). Further supporting this idea, RNA FISH coupled with immunofluorescence suggests that pUG-tailed RNAs enrich in Mutator foci (Shukla et al., 2020). siRNA amplification in C. elegans germ cells is also mediated by the RdRP EGO-1, which may localize to P granules (Claycomb et al., 2009). EGO-1 produces a specific class of secondary siRNAs that associate with the Argonaute CSR-1 (another P granule component), but also shows some redundancy with RRF-1 (the RdRP in Mutator foci) in the production of other types of secondary siRNAs (Claycomb et al., 2009; Gu et al., 2009). As noted earlier, P granules contain multiple Argonaute proteins, and Z granules contain ZNFX-1 and WAGO-4, which promote the inheritance of siRNA-based gene silencing across generations (Ishidate et al., 2018; Wan et al., 2018). SIMR foci are known to contain the Tudor domain-containing protein SIMR-1 and the small RNA factor RSD-2 (Manage et al., 2020). Together, these observations show that several small RNA-related proteins localize to different subcompartments within C. elegans perinuclear nuage. Given that Z granules and SIMR foci were only recently identified, future studies are likely to provide additional insights into the components and functions of these structures, as well as potentially identify additional types of nuage subcompartments.
Mutator components amplify multiple types of small RNA-based gene-silencing signals, including those triggered by piRNAs (Das et al., 2008; Bagijn et al., 2012; Luteijn et al., 2012; Shirayama et al., 2012). Interestingly, piRNA targeting is thought to occur in P granules, which contain the piRNA-binding protein PRG-1 (Batista et al., 2008; Wang and Reinke, 2008). It is therefore intriguing to speculate that different subcompartments of C. elegans perinuclear nuage orchestrate distinct steps in piRNA-based silencing: target recognition in P granules, and signal amplification in Mutator foci (Figure 4). SIMR foci may also function in piRNA-based silencing, downstream of P granules and upstream of Mutator foci (Manage et al., 2020). Because the different subcompartments of perinuclear nuage may function in related processes, these compartments are likely to communicate with one another, perhaps by relaying messages in the form of RNA. Why compartmentalize piRNA targeting and downstream silencing steps into different condensates? C. elegans piRNAs interact with essentially all germline mRNAs, yet only a subset of these mRNAs are silenced by the piRNA pathway (Shen et al., 2018; Zhang et al., 2018; Reed et al., 2019). Therefore, one function of P granules may be to restrict piRNA activity, perhaps by ensuring that only unwanted mRNAs are routed to other subcompartments for downstream silencing. In support of this idea, the P granule assembly proteins MEG-3 and MEG-4 are required to protect certain germline-expressed mRNAs (e.g. sid-1 and rde-11) from piRNA-based silencing (Dodson and Kennedy, 2019; Ouyang et al., 2019). sid-1 and rde-11 mRNAs normally localize to P granules, suggesting that P granules play a direct role in protecting these mRNAs from silencing (Ouyang et al., 2019). Because piRNAs can trigger robust transgenerational silencing in C. elegans (Ashe et al., 2012; Lee et al., 2012; Luteijn et al., 2012; Shirayama et al., 2012), nuage-based organization of the piRNA pathway may be particularly important for preventing runaway heritable silencing of the wrong germline mRNAs (Dodson and Kennedy, 2019; Lev et al., 2019).
Significance of Phase Separation in Development
Biomolecular condensates exhibit a variety of unique properties that cells and organisms could exploit to regulate and drive developmental processes. For instance, condensates are highly sensitive to multiple factors that guide and/or influence development, including post-translational modifications, temperature, pH, and local concentrations of key molecules: small changes in any of these factors can trigger rapid, switch-like effects in terms of phase separation. Switch-like events are common in development and often decide the developmental fate of cells; therefore, one role of phase separation in development may be to drive cell fate decisions by converting subtle developmental cues into robust, all-or-nothing outputs. In support of this idea, phase-separated condensates known as keratohyalin granules (KGs) are thought to promote the terminal differentiation of epidermal keratinocytes in mammals by rapidly dissolving in response to a pH shift (Figure 5A) (Quiroz et al., 2020). Keratinocytes differentiate as they travel from the innermost basal layer of the epidermis to the outermost layer - the skin surface (Fuchs and Raghavan, 2002). During the late stages of differentiation, the intrinsically disordered protein profilaggrin helps assemble KGs, which grow and stiffen over time and eventually occupy a significant portion of the cytoplasm (Quiroz et al., 2020). Once keratinocytes reach the acidic surface of the skin, however, KGs rapidly dissolve, and nuclei and other organelles degrade, leaving flat “ghost” cells that form the skin barrier (Figure 5A) (Quiroz et al., 2020). Current models posit that the change in pH triggers the dissolution of KGs, which in turn promotes enucleation, perhaps by releasing degradation factors residing within KGs into the cytoplasm (Quiroz et al., 2020).
Figure 5. Phase separation and developmental decisions.
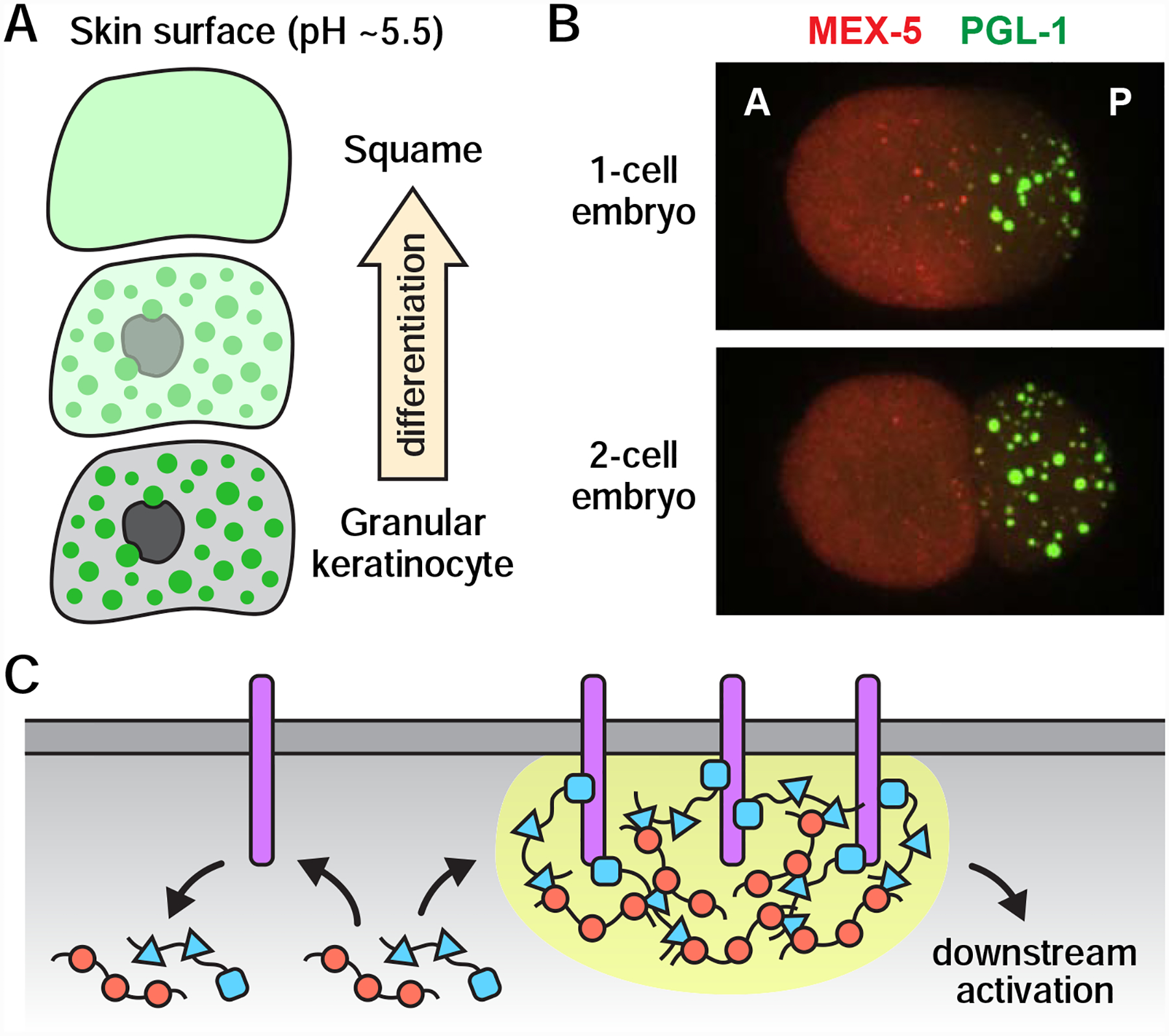
(A) Dissolution of keratohyalin granules (green dots) in response to a pH shift during epidermal differentiation. As keratinocytes approach the acidic skin surface, keratohyalin granules dissolve, nuclei degrade, and the resulting “ghost” cells, or squames, build the skin barrier. (B) Spatial patterning in the C. elegans zygote. An anterior-rich MEX-5 concentration gradient generates a sharp asymmetry in the formation of P granules (marked by PGL-1). As the zygote divides, P granules segregate with the posterior blastomere, which eventually gives rise to the germline. A, anterior; P, posterior. Images originally from (Gallo et al., 2010). Reprinted with permission from AAAS. (C) Model: Phase separation enhances signaling amplification and specificity. Activation of transmembrane proteins (purple) recruits cytoplasmic adaptor proteins (red and blue) to the membrane surface. Multivalent interactions between membrane proteins and cytoplasmic adaptors drive phase separation (yellow), which prolongs the length of time that cytoplasmic signaling molecules reside at the membrane and thereby increases the likelihood of downstream activation. Stochastic interactions between unclustered membrane proteins and cytoplasmic adaptors (left) do not retain cytoplasmic adaptors at the membrane surface and are therefore unlikely to result in downstream activation.
The switch-like nature of phase separation may also be important for spatial patterning, as phase separation is known to occur at precise concentrations and can thereby amplify the effects of weak concentration gradients (Lee et al., 2013). Imagine, for instance, that a condensate assembles only when the concentration of a key component rises above a certain threshold. If that component were distributed in a concentration gradient across a cell, or groups of cells, then phase separation-related processes could turn subtle differences in concentration gradients into a sharp, spatial asymmetry in condensate formation with a boundary dictated by the threshold concentration. A variation on this scenario drives spatial patterning in the C. elegans zygote, which forms multiple protein concentration gradients, including an anterior-rich gradient of the RNA-binding protein MEX-5 (Griffin, 2015). In turn, the MEX-5 gradient gives rise to a pronounced asymmetry in P granule formation: P granules condense only in the posterior half of the zygote, and, therefore, segregate with the posterior blastomere as the zygote divides (Figure 5B) (Schubert et al., 2000; Brangwynne et al., 2009; Gallo et al., 2010). MEX-5 may antagonize P granule formation in the zygote anterior by limiting the availability of RNAs, as MEX-5 binds RNA with high affinity, and RNA is thought to stimulate P granule condensation (Pagano et al., 2007; Lee et al., 2013; Saha et al., 2016; Smith et al., 2016). Notably, P granule asymmetry in zygotes also requires the kinase MBK-2, which phosphorylates the P-granule assembly protein MEG-3 to destabilize P granules (Pellettieri et al., 2003; Quintin et al., 2003; Wang et al., 2014). Thus, C. elegans germ cells exploit the sensitivity of phase-separated P granules to both molecular concentrations and post-translational modifications to drive asymmetric cell division.
In addition to driving developmental decisions, phase separation may also regulate the fidelity of such decisions. During transmembrane signaling, for example, phase separation is thought to help ensure that only genuine signals (and not stochastic interactions) are able to activate downstream signaling components (Figure 5C) (Huang et al., 2016, 2019; Case et al., 2019). In two different actin regulatory signaling pathways (nephrin signaling in podocyte cells and T cell receptor signaling), receptor activation causes multiple components in the pathway to undergo phase separation at the membrane surface (Banjade and Rosen, 2014; Su et al., 2016). Phase-separating components include the membrane receptor Nephrin and its cytoplasmic targets Nck and N-WASP in podocytes, and the membrane adaptor LAT and its cytoplasmic binding partners Grb2 and Sos1 in activated T cells. In vitro reconstitution assays suggest that multivalent interactions within the condensates help retain cytoplasmic signaling molecules at the membrane and thereby prolong communication between membrane signaling components and their cytoplasmic binding partners (Huang et al., 2016, 2019; Case et al., 2019). Signal transmission from the membrane to the cytoplasm is a kinetically slow, multi-step process; thus, phase separation provides a means for cells to both complete this transmission and to spatially restrict this process to genuine sites of activation.
Proper spatiotemporal regulation of gene expression contributes to many developmental processes, and certain biomolecular condensates are thought to serve as sites of gene regulation. C. elegans P granules, for example, contain several factors involved in post-transcriptional gene regulation (e.g. piRNA- and siRNA-related proteins, the mRNA decapping enzymes DCAP-1 and DCAP-2, and CCF-1, a subunit of the CCR4/NOT deadenylase complex) and are required for maintaining proper germline gene expression programs: Upon the depletion of P granules, germ cells lose their totipotency and begin to accumulate soma-specific transcripts (Updike and Strome, 2010; Updike et al., 2014; Knutson et al., 2017). As noted earlier, P granules associate with nuclear pores for much of development, and nascent mRNAs traffic through perinuclear P granules before entering the cytoplasm (Strome and Wood, 1982; Pitt, Schisa and Priess, 2000; Sheth et al., 2010). Therefore, current models posit that P granules help maintain germ cell totipotency by intercepting nascent soma-specific transcripts as they exit the nuclear pore, thereby preventing their translation (Knutson et al., 2017). Given that many types of germ granules are perinuclear, phase separation may play a widespread role in curating the nascent transcriptome of germ cells.
Phase-separated condensates can adopt a wide range of material properties, which could be exploited by animals to drive and regulate developmental processes. P granules, for instance, exhibit a glycerol-like consistency, whereas the viscosity of grP-bodies is more comparable to peanut butter, and Balbiani bodies are virtually solid (Brangwynne et al., 2009; Hubstenberger et al., 2013; Boke et al., 2016). Notably, different viscosities may be conducive to different functions: liquid-like properties could facilitate dynamic processes such as RNA processing, whereas more solid-like properties could suppress molecular dynamics during periods of dormancy, for instance. In vitro, liquid-like condensates can transition over time into more solid-like states, suggesting that cells may normally regulate condensate viscosity to prevent such transitions from occurring inappropriately (Lin et al., 2015; Molliex et al., 2015; Patel et al., 2015). In support of this idea, loss of the RNA helicase CGH-1 causes the grP-body component CAR-1 to condense into solid, sheet-like structures in the C. elegans germline (Audhya et al., 2005; Boag, Nakamura and Blackwell, 2005; Hubstenberger et al., 2013). It is possible that condensates also undergo phase transitions as a normal part of development, and that such transitions assist in developmental processes. For instance, as keratohyalin granules stiffen during epidermal differentiation, they begin to physically deform the nucleus (Figure 5A) (Quiroz et al., 2020). Stiffening of keratohyalin granules (and subsequent deformation of the nucleus) could be a controlled process that promotes enucleation (Quiroz et al., 2020). Understanding if and how cells might regulate condensate viscosity will likely lead to a better understanding of many biological processes including development.
Open Questions
The topics discussed in this review raise a number of questions worthy of further exploration. How, for example, might cells regulate and exploit the physical properties of condensates to regulate key processes in germ cells and development? Does phase separation underlie the formation and function of other cell-type-specific organelles in addition to germ granules and keratohyalin granules? For development, in particular, it will be interesting to determine how and to what extent biomolecular condensates act as sensors to help organisms process external stimuli. Although in vitro experiments have provided significant insight into phase separation, in vivo experiments will be critical for understanding how biomolecular condensates function and change over the course of development. Genetic manipulations, imaging of living cells and tissues, and the use of phase-separation “sensors” should be particularly informative in terms of tools and approaches (Quiroz et al., 2020). In addition, proximity labeling techniques, which have recently been used to probe the transcriptome and proteome of stress granules, could be useful for extracting a comprehensive list of all the proteins and RNAs that localize to different condensates (Markmiller et al., 2018; Padrón, Iwasaki and Ingolia, 2019).
Future studies of germ cell condensates are likely to provide key insights into the functional significance of phase separation, as germ cells form a diverse array of condensates known to contain many proteins important for germ cell function. This review focuses mainly on the idea that condensates spatially organize cellular processes, serve as storage sites for protein and RNA, and/or facilitate switch-like decisions. However, additional/alternative functions are also possible. For instance, some condensates may function as noise buffers that stabilize protein levels/activity in the cytoplasm (or nucleoplasm) (Klosin et al., 2020). Another possibility is that condensates limit or inactivate certain reactions in the cytoplasm by sequestering a key component. Pinning down the exact function(s) of a given condensate remains difficult, as it would require disrupting the assembly or integrity of that condensate without disrupting any of its individual components. Therefore, additional studies exploring the functional impacts of phase separation are needed and will likely require innovative approaches. Tools enabling the control of phase separation in vivo (e.g. light-activatable “Corelets”) may help advance this endeavor (Bracha et al., 2018; Bracha, Walls and Brangwynne, 2019).
Lastly, we note that germ cell condensates form a variety of subcompartments and multi-condensate assemblages and that these structures are particularly ripe for exploration. Advancements in super-resolution imaging may be especially useful in determining how such structures are organized. In the case of multi-condensate structures, understanding how different condensates might interact and communicate should provide insight into the organization of complex pathways in germ cells and beyond.
Phase separation is a versatile and widespread biophysical phenomenon that contributes to subcellular organization, gene regulation, environmental sensing, and a variety of other cellular processes. Dodson and Kennedy review how animals regulate and exploit the properties of phase separation in the germline and during development.
Acknowledgements
We thank the reviewers for providing valuable and insightful feedback. Research on phase separation in the S.K. lab is supported by the National Institutes of Health, R01GM132286. A.E.D. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2304-17).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberti S (2017) ‘The wisdom of crowds: regulating cell function through condensed states of living matter’, Journal of cell science, 130(17), pp. 2789–2796. [DOI] [PubMed] [Google Scholar]
- Aravin AA et al. (2009) ‘Cytoplasmic compartmentalization of the fetal piRNA pathway in mice’, PLoS genetics, 5(12), p. e1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A et al. (2012) ‘piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans’, Cell, 150(1), pp. 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A et al. (2005) ‘A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans’, The Journal of cell biology, 171(2), pp. 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagijn MP et al. (2012) ‘Function, targets, and evolution of Caenorhabditis elegans piRNAs’, Science, 337(6094), pp. 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF et al. (2017) ‘Biomolecular condensates: organizers of cellular biochemistry’, Nature reviews. Molecular cell biology doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S and Rosen MK (2014) ‘Phase transitions of multivalent proteins can promote clustering of membrane receptors’, eLife, 3. doi: 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ et al. (2008) ‘PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans’, Molecular cell, 31(1), pp. 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J et al. (2015) ‘RNA transcription modulates phase transition-driven nuclear body assembly’, Proceedings of the National Academy of Sciences of the United States of America, 112(38), pp. E5237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Fischer SEJ and Kim JK (2014) ‘Endogenous RNAi pathways in C. elegans’, WormBook: the online review of C. elegans biology, pp. 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR et al. (2008) ‘Protection of specific maternal messenger RNAs by the P body protein CGH-1 (Dhh1/RCK) during Caenorhabditis elegans oogenesis’, The Journal of cell biology, 182(3), pp. 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Nakamura A and Blackwell TK (2005) ‘A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans’, Development, 132(22), pp. 4975–4986. [DOI] [PubMed] [Google Scholar]
- Boke E et al. (2016) ‘Amyloid-like Self-Assembly of a Cellular Compartment’, Cell, 166(3), pp. 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F et al. (2009) ‘Bucky ball organizes germ plasm assembly in zebrafish’, Current biology: CB, 19(5), pp. 414–422. [DOI] [PubMed] [Google Scholar]
- Bracha D et al. (2018) ‘Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds’, Cell, 175(6), pp. 1467–1480.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha D, Walls MT and Brangwynne CP (2019) ‘Probing and engineering liquid-phase organelles’, Nature biotechnology, 37(12), pp. 1435–1445. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP et al. (2009) ‘Germline P granules are liquid droplets that localize by controlled dissolution/condensation’, Science, 324(5935), pp. 1729–1732. [DOI] [PubMed] [Google Scholar]
- Breitwieser W et al. (1996) ‘Oskar protein interaction with Vasa represents an essential step in polar granule assembly’, Genes & development, 10(17), pp. 2179–2188. [DOI] [PubMed] [Google Scholar]
- Case LB et al. (2019) ‘Stoichiometry controls activity of phase-separated clusters of actin signaling proteins’, Science, 363(6431), pp. 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Ditlev JA and Rosen MK (2019) ‘Regulation of Transmembrane Signaling by Phase Separation’, Annual review of biophysics, 48, pp. 465–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P et al. (2004) ‘Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum’, Molecular biology of the cell, 15(10), pp. 4669–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W et al. (2020) ‘The Dynamics of P Granule Liquid Droplets Are Regulated by the Caenorhabditis elegans Germline RNA Helicase GLH-1 via Its ATP Hydrolysis Cycle’, Genetics, 215(2), pp. 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuma S and Pillai RS (2009) ‘Retrotransposon silencing by piRNAs: ping-pong players mark their sub-cellular boundaries’, PLoS genetics, p. e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM et al. (2009) ‘The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation’, Cell, 139(1), pp. 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP et al. (2008) ‘Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline’, Molecular cell, 31(1), pp. 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C et al. (2013) ‘“Dot COM”, a nuclear transit center for the primary piRNA pathway in Drosophila’, PloS one, 8(9), p. e72752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C, Brasset E and Vaury C (2019) ‘flam piRNA precursors channel from the nucleus to the cytoplasm in a temporally regulated manner along Drosophila oogenesis’, Mobile DNA, 10, p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson AE and Kennedy S (2019) ‘Germ Granules Coordinate RNA-Based Epigenetic Inheritance Pathways’, Developmental cell, 50(6), pp. 704–715.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S et al. (2015) ‘The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics’, Proceedings of the National Academy of Sciences of the United States of America, 112(23), pp. 7189–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eno C, Hansen CL and Pelegri F (2019) ‘Aggregation, segregation, and dispersal of homotypic germ plasm RNPs in the early zebrafish embryo’, Developmental dynamics: an official publication of the American Association of Anatomists, 248(4), pp. 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C, Odom DT and Kutter C (2017) ‘The emergence of piRNAs against transposon invasion to preserve mammalian genome integrity’, Nature communications, 8(1), p. 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Eddy EM and Phillips DM (1970) ‘Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis’, Biology of reproduction, 2(1), pp. 129–153. [DOI] [PubMed] [Google Scholar]
- Feric M et al. (2016) ‘Coexisting Liquid Phases Underlie Nucleolar Subcompartments’, Cell, 165(7), pp. 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Richter RP and Görlich D (2006) ‘FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties’, Science, 314(5800), pp. 815–817. [DOI] [PubMed] [Google Scholar]
- Fuchs E and Raghavan S (2002) ‘Getting under the skin of epidermal morphogenesis’, Nature reviews. Genetics, 3(3), pp. 199–209. [DOI] [PubMed] [Google Scholar]
- Gallo CM et al. (2010) ‘Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans’, Science, 330(6011), pp. 1685–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M and Arkov AL (2013) ‘Next generation organelles: structure and role of germ granules in the germline’, Molecular reproduction and development, 80(8), pp. 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EE (2015) ‘Cytoplasmic localization and asymmetric division in the early embryo of Caenorhabditis elegans’, Wiley interdisciplinary reviews. Developmental biology, 4(3), pp. 267–282. [DOI] [PubMed] [Google Scholar]
- Gu W et al. (2009) ‘Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline’, Molecular cell, 36(2), pp. 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AN and Macdonald PM (2001) ‘Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C’, Development, 128(14), pp. 2823–2832. [DOI] [PubMed] [Google Scholar]
- Hirakata S and Siomi MC (2019) ‘Assembly and Function of Gonad-Specific Non-Membranous Organelles in Drosophila piRNA Biogenesis’, Non-coding RNA, 5(4). doi: 10.3390/ncrna5040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D et al. (2017) ‘A Phase Separation Model for Transcriptional Control’, Cell, 169(1), pp. 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M and Dormann D (2019) ‘Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics’, The Journal of biological chemistry, 294(18), pp. 7137–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M et al. (2007) ‘Tudor-related proteins TDRD1/MTR-1, TDRD6 and TDRD7/TRAP: domain composition, intracellular localization, and function in male germ cells in mice’, Developmental biology, 301(1), pp. 38–52. [DOI] [PubMed] [Google Scholar]
- Huang WYC et al. (2016) ‘Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS’, Proceedings of the National Academy of Sciences of the United States of America, 113(29), pp. 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WYC et al. (2019) ‘A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS’, Science, 363(6431), pp. 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A et al. (2013) ‘Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development’, Developmental cell, 27(2), pp. 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA and Jülicher F (2014) ‘Liquid-liquid phase separation in biology’, Annual review of cell and developmental biology, 30, pp. 39–58. [DOI] [PubMed] [Google Scholar]
- Ishidate T et al. (2018) ‘ZNFX-1 Functions within Perinuclear Nuage to Balance Epigenetic Signals’, Molecular cell, 70(4), pp. 639–649.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson-Lucy A and Mullins MC (2019) ‘Chapter One - The vertebrate Balbiani body, germ plasm, and oocyte polarity’, in Lehmann R (ed.) Current Topics in Developmental Biology. Academic Press, pp. 1–34. [DOI] [PubMed] [Google Scholar]
- Jeske M et al. (2015) ‘The Crystal Structure of the Drosophila Germline Inducer Oskar Identifies Two Domains with Distinct Vasa Helicase- and RNA-Binding Activities’, Cell reports, 12(4), pp. 587–598. [DOI] [PubMed] [Google Scholar]
- Jones JR and Macdonald PM (2007) ‘Oskar controls morphology of polar granules and nuclear bodies in Drosophila’, Development, 134(2), pp. 233–236. [DOI] [PubMed] [Google Scholar]
- Jud MC et al. (2008) ‘Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway’, Developmental biology, 318(1), pp. 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibanov MV et al. (2011) ‘A novel organelle, the piNG-body, in the nuage of Drosophila male germ cells is associated with piRNA-mediated gene silencing’, Molecular biology of the cell, 22(18), pp. 3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler KE et al. (2018) ‘Phase transitioned nuclear Oskar promotes cell division of Drosophila primordial germ cells’, eLife, 7. doi: 10.7554/eLife.37949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, Bilinski S and Etkin LD (2004) ‘The Balbiani Body and Germ Cell Determinants: 150 Years Later’, in Schatten GP (ed.) Current Topics in Developmental Biology. Academic Press, pp. 1–36. [DOI] [PubMed] [Google Scholar]
- Klosin A et al. (2020) ‘Phase separation provides a mechanism to reduce noise in cells’, Science, 367(6476), pp. 464–468. [DOI] [PubMed] [Google Scholar]
- Knutson AK et al. (2017) ‘Germ Granules Prevent Accumulation of Somatic Transcripts in the Adult Caenorhabditis elegans Germline’, Genetics, 206(1), pp. 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leacock SW and Reinke V (2008) ‘MEG-1 and MEG-2 are embryo-specific P-granule components required for germline development in Caenorhabditis elegans’, Genetics, 178(1), pp. 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF et al. (2013) ‘Spatial organization of the cell cytoplasm by position-dependent phase separation’, Physical review letters, 111(8), p. 088101. [DOI] [PubMed] [Google Scholar]
- Lee C-YS et al. (2020) ‘Recruitment of mRNAs to P granules by condensation with intrinsically-disordered proteins’, eLife, 9. doi: 10.7554/eLife.52896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C et al. (2012) ‘C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts’, Cell, 150(1), pp. 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R (2016) ‘Chapter Thirty-Nine - Germ Plasm Biogenesis—An Oskar-Centric Perspective’, in Wassarman PM (ed.) Current Topics in Developmental Biology. Academic Press, pp. 679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtiniemi T and Kotaja N (2018) ‘Germ granule-mediated RNA regulation in male germ cells’, Reproduction, 155(2), pp. R77–R91. [DOI] [PubMed] [Google Scholar]
- Lei L and Spradling AC (2016) ‘Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells’, Science, 352(6281), pp. 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch BJ and Page DC (2012) ‘Genetics of germ cell development’, Nature reviews. Genetics, 13(11), pp. 781–794. [DOI] [PubMed] [Google Scholar]
- Lev I et al. (2019) ‘Germ Granules Govern Small RNA Inheritance’, Current biology: CB, 29(17), pp. 2880–2891.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y et al. (2015) ‘Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins’, Molecular cell, 60(2), pp. 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC et al. (2015) ‘Independent and coordinate trafficking of single Drosophila germ plasm mRNAs’, Nature cell biology, 17(5), pp. 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ et al. (2012) ‘Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans’, The EMBO journal, 31(16), pp. 3422–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP (1962) ‘Fine structure of pole cells and polar granules in Drosophila melanogaster’, The Journal of experimental zoology, 151(3), pp. 201–215. [Google Scholar]
- Manage KI et al. (2020) ‘A tudor domain protein, SIMR-1, promotes siRNA production at piRNA-targeted mRNAs in C. elegans’, eLife, 9. doi: 10.7554/eLife.56731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmiller S et al. (2018) ‘Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules’, Cell, 172(3), pp. 590–604.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow FL and Mullins MC (2008) ‘Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish’, Developmental biology, 321(1), pp. 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnik EA et al. (2019) ‘Germline Maintenance Through the Multifaceted Activities of GLH/Vasa in Caenorhabditis elegans P Granules’, Genetics, 213(3), pp. 923–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnik EA and Updike DL (2019) ‘Membraneless organelles: P granules in C. elegans’, Traffic. doi: 10.1111/tra.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikar O et al. (2011) ‘Chromatoid body and small RNAs in male germ cells’, Reproduction, 142(2), pp. 195–209. [DOI] [PubMed] [Google Scholar]
- Mello CC et al. (1996) ‘The PIE-1 protein and germline specification in C. elegans embryos’, Nature, 382(6593), pp. 710–712. [DOI] [PubMed] [Google Scholar]
- Molliex A et al. (2015) ‘Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization’, Cell, 163(1), pp. 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murota Y et al. (2014) ‘Yb integrates piRNA intermediates and processing factors into perinuclear bodies to enhance piRISC assembly’, Cell reports, 8(1), pp. 103–113. [DOI] [PubMed] [Google Scholar]
- Niepielko MG, Eagle WVI and Gavis ER (2018) ‘Stochastic Seeding Coupled with mRNA Self-Recruitment Generates Heterogeneous Drosophila Germ Granules’, Current biology: CB, 28(12), pp. 1872–1881.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SL et al. (2008) ‘Maternal mRNAs are regulated by diverse P body-related mRNP granules during early Caenorhabditis elegans development’, The Journal of cell biology, 182(3), pp. 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosov GA, Kibanov MV and Olenina LV (2014) ‘Dynamic properties of germinal granule ping-body in the testes of Drosophila melanogaster’, Molekuliarnaia biologiia, 48(5), pp. 805–813. [PubMed] [Google Scholar]
- Nott TJ et al. (2015) ‘Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles’, Molecular cell, 57(5), pp. 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JPT et al. (2019) ‘P Granules Protect RNA Interference Genes from Silencing by piRNAs’, Developmental cell, 50(6), pp. 716–728.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen I and Shewmaker F (2019) ‘The Role of Post-Translational Modifications in the Phase Transitions of Intrinsically Disordered Proteins’, International journal of molecular sciences, 20(21). doi: 10.3390/ijms20215501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón A, Iwasaki S and Ingolia NT (2019) ‘Proximity RNA Labeling by APEX-Seq Reveals the Organization of Translation Initiation Complexes and Repressive RNA Granules’, Molecular cell, 75(4), pp. 875–887.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano JM et al. (2007) ‘Molecular basis of RNA recognition by the embryonic polarity determinant MEX-5’, The Journal of biological chemistry, 282(12), pp. 8883–8894. [DOI] [PubMed] [Google Scholar]
- Parker DM et al. (2020) ‘mRNA localization is linked to translation regulation in the Caenorhabditis elegans germ lineage’, Development. doi: 10.1242/dev.186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A et al. (2015) ‘A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation’, Cell, 162(5), pp. 1066–1077. [DOI] [PubMed] [Google Scholar]
- Pellettieri J et al. (2003) ‘Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans’, Developmental cell, 5(3), pp. 451–462. [DOI] [PubMed] [Google Scholar]
- Phillips CM et al. (2012) ‘MUT-16 promotes formation of perinuclear mutator foci required for RNA silencing in the C. elegans germline’, Genes & development, 26(13), pp. 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JN, Schisa JA and Priess JR (2000) ‘P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA’, Developmental biology, 219(2), pp. 315–333. [DOI] [PubMed] [Google Scholar]
- Putnam A et al. (2019) ‘A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos’, Nature structural & molecular biology, 26(3), pp. 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S et al. (2003) ‘The mbk-2 kinase is required for inactivation of MEI-1/katanin in the one-cell Caenorhabditis elegans embryo’, EMBO reports, 4(12), pp. 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz FG et al. (2020) ‘Liquid-liquid phase separation drives skin barrier formation’, Science, 367(6483). doi: 10.1126/science.aax9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KJ et al. (2019) ‘Widespread roles for piRNAs and WAGO-class siRNAs in shaping the germline transcriptome of Caenorhabditis elegans’, Nucleic acids research. doi: 10.1093/nar/gkz1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhine K, Vidaurre V and Myong S (2020) ‘RNA Droplets’, Annual review of biophysics, 49, pp. 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers EF et al. (2018) ‘Tdrd6a Regulates the Aggregation of Buc into Functional Subcellular Compartments that Drive Germ Cell Specification’, Developmental cell, 46(3), pp. 285–301.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S et al. (2016) ‘Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism’, Cell, 166(6), pp. 1572–1584.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW et al. (2020) ‘Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization’, Cell, 181(2), pp. 306–324.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa JA, Pitt JN and Priess JR (2001) ‘Analysis of RNA associated with P granules in germ cells of C. elegans adults’, Development, 128(8), pp. 1287–1298. [DOI] [PubMed] [Google Scholar]
- Schmidt HB and Görlich D (2015) ‘Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity’, eLife, 4. doi: 10.7554/eLife.04251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CM et al. (2000) ‘MEX-5 and MEX-6 function to establish soma/germline asymmetry in early C. elegans embryos’, Molecular cell, 5(4), pp. 671–682. [DOI] [PubMed] [Google Scholar]
- Schuster BS et al. (2020) ‘Identifying sequence perturbations to an intrinsically disordered protein that determine its phase-separation behavior’, Proceedings of the National Academy of Sciences of the United States of America, 117(21), pp. 11421–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G et al. (1996) ‘Repression of gene expression in the embryonic germ lineage of C. elegans’, Nature, 382(6593), pp. 713–716. [DOI] [PubMed] [Google Scholar]
- Seydoux G and Fire A (1994) ‘Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans’, Development, 120(10), pp. 2823–2834. [DOI] [PubMed] [Google Scholar]
- Shen E-Z et al. (2018) ‘Identification of piRNA Binding Sites Reveals the Argonaute Regulatory Landscape of the C. elegans Germline’, Cell, 172(5), pp. 937–951.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U et al. (2010) ‘Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells’, Development, 137(8), pp. 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y and Brangwynne CP (2017) ‘Liquid phase condensation in cell physiology and disease’, Science, 357(6357). doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Shirayama M et al. (2012) ‘piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline’, Cell, 150(1), pp. 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M et al. (2009) ‘The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline’, Developmental cell, 17(6), pp. 775–787. [DOI] [PubMed] [Google Scholar]
- Shukla A et al. (2020) ‘poly(UG)-tailed RNAs in genome protection and epigenetic inheritance’, Nature. doi: 10.1038/s41586-020-2323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J et al. (2016) ‘Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3’, eLife, 5. doi: 10.7554/eLife.21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead WT and Gladfelter AS (2019) ‘The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation’, Molecular cell, 76(2), pp. 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snee MJ and Macdonald PM (2004) ‘Live imaging of nuage and polar granules: evidence against a precursor-product relationship and a novel role for Oskar in stabilization of polar granule components’, Journal of cell science, 117(Pt 10), pp. 2109–2120. [DOI] [PubMed] [Google Scholar]
- Spike CA et al. (2008) ‘DEPS-1 promotes P-granule assembly and RNA interference in C. elegans germ cells’, Development, 135(5), pp. 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser MJ et al. (2008) ‘Control over the morphology and segregation of Zebrafish germ cell granules during embryonic development’, BMC developmental biology, 8, p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S and Wood WB (1982) ‘Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans’, Proceedings of the National Academy of Sciences of the United States of America, 79(5), pp. 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K and Seydoux G (1999) ‘nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans’, Development, 126(21), pp. 4861–4871. [DOI] [PubMed] [Google Scholar]
- Su X et al. (2016) ‘Phase separation of signaling molecules promotes T cell receptor signal transduction’, Science, 352(6285), pp. 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H et al. (1999) ‘pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans’, Development, 126(1), pp. 1–11. [DOI] [PubMed] [Google Scholar]
- Tanaka T et al. (2011) ‘Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis’, Proceedings of the National Academy of Sciences of the United States of America, 108(26), pp. 10579–10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T et al. (2015) ‘Drosophila germ granules are structured and contain homotypic mRNA clusters’, Nature communications, 6, p. 7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T et al. (2020) ‘Sequence-Independent Self-Assembly of Germ Granule mRNAs into Homotypic Clusters’, Molecular cell. doi: 10.1016/j.molcel.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T and Lehmann R (2019) ‘Germ granules in Drosophila’, Traffic, 20(9), pp. 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebel CJ et al. (2018) ‘Distinct regions of the intrinsically disordered protein MUT-16 mediate assembly of a small RNA amplification complex and promote phase separation of Mutator foci’, PLoS genetics, 14(7), p. e1007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL et al. (2014) ‘Germ-granule components prevent somatic development in the C. elegans germline’, Current biology: CB, 24(9), pp. 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike DL and Strome S (2009) ‘A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans’, Genetics, 183(4), pp. 1397–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D and Strome S (2010) ‘P granule assembly and function in Caenorhabditis elegans germ cells’, Journal of andrology, 31(1), pp. 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo HDL et al. (2019) ‘Protein components of ribonucleoprotein granules from Drosophila germ cells oligomerize and show distinct spatial organization during germline development’, Scientific reports, 9(1), p. 19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E et al. (2011) ‘RNA granules in germ cells’, Cold Spring Harbor perspectives in biology, 3(12). doi: 10.1101/cshperspect.a002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E and Seydoux G (2010) ‘The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules’, Development, 137(9), pp. 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G et al. (2018) ‘Spatiotemporal regulation of liquid-like condensates in epigenetic inheritance’, Nature, 557(7707), pp. 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G and Reinke V (2008) ‘A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis’, Current biology: CB, 18(12), pp. 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT et al. (2014) ‘Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans’, eLife, 3, p. e04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X et al. (2020) ‘Mitochondria Associated Germinal Structures in Spermatogenesis: piRNA Pathway Regulation and Beyond’, Cells, 9(2). doi: 10.3390/cells9020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M-T et al. (2017) ‘Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles’, Nature chemistry, 9(11), pp. 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JB, Hyman AA and Boke E (2018) ‘Organization and Function of Non-dynamic Biomolecular Condensates’, Trends in biochemical sciences, 43(2), pp. 81–94. [DOI] [PubMed] [Google Scholar]
- Zhang C et al. (2011) ‘mut-16 and other mutator class genes modulate 22G and 26G siRNA pathways in Caenorhabditis elegans’, Proceedings of the National Academy of Sciences of the United States of America, 108(4), pp. 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D et al. (2018) ‘The piRNA targeting rules and the resistance to piRNA silencing in endogenous genes’, Science, 359(6375), pp. 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]


