Abstract
Studies with human subjects indicate that ethanol exposure during fetal development causes long-lasting alterations in motor coordination that are, in part, a consequence of cerebellar damage. Studies with rats exposed to ethanol during the neonatal brain growth spurt have consistently recapitulated these deficits. However, studies with mice have yielded mixed results. We hypothesized that the use of highly sensitive motor function tests, such as the Catwalk test, would reliably detect motor function deficits in mice developmentally exposed to ethanol. Venus-vesicular GABA transporter transgenic mice were ethanol exposed during postnatal days 4–9 using vapor inhalation chambers and then subjected to the Catwalk test during adolescence. Catwalk data were rigorously analyzed using an innovative multistep statistical approach. For comparison, motor coordination and strength were assessed with the triple horizontal bar and rotarod tests. Unexpectedly, we found that out of 186 parameters analyzed in the Catwalk test, only one was affected by ethanol exposure (i.e., reduced coupling between left front paw and the right hind paw). In the triple horizontal bar test, ethanol-exposed mice were able to hold to the bars for less time than controls. Surprisingly, ethanol-exposed mice performed better in the rotarod test than controls. These data indicate that neonatal ethanol exposure of mice causes mixed effects on motor function during adolescence. The Catwalk test suggests that gait is generally preserved in these mice, whereas the triple horizontal bar test revealed deficits on motor strength and the rotarod test an increase in motor coordination.
Keywords: neonatal, vapor chamber, motor, coordination, cerebellum
1. Introduction
Exposure to ethanol during fetal development causes fetal alcohol spectrum disorder (FASD), characterized by persistent damage to neuronal circuits in multiple brain regions, including the cerebellum [1–3]. Cerebellar damage likely contributes to deficits in motor coordination, balance, fine motor skills, language, emotional regulation, and cognitive functions [4–6]. Magnetic resonance imaging studies with humans have demonstrated reductions in the volume of the cerebellar hemispheres and vermis, and diffusion tensor imaging has detected fractional anisotropy reductions in cerebellar peduncles, consistent with damage of the axons that interconnect the brainstem with the cerebellum [7]. These alterations have been linked to deficits in oculomotor control and eyeblink conditioning in children with FASD [4, 8]. Magnetic resonance spectroscopy of children who were exposed to high doses of ethanol during fetal development found a reduction in the levels of N-acetylaspartate, glycerophosphocholine, and phosphocholine, as well as an increase in glutamate and glutamine levels in the deep cerebellar nuclei, consistent with functional alterations in this region [9]. These human studies indicate that the cerebellum plays a central role in the pathophysiology of FASD.
Deficits in cerebellar-dependent behaviors have been well documented in animal models of FASD, particularly in rats exposed to ethanol during the third trimester-equivalent of human pregnancy (first 1–2 weeks of life in rodents; [10]). It is important to characterize the effects of ethanol during the third trimester because some pregnant women consume ethanol during this period of gestation [11–14], which can interfere with key developmental processes and lead to long-lasting neurobehavioral alterations, particularly when consumed in a binge-like fashion [15–17]. Moreover, preterm neonates can also be exposed during this period of brain development when they receive intravenous medications containing ethanol as an excipient or are handled in isolette incubators after the use of ethanol-containing hand sanitizers [18–20]. Exposure of rat pups during this period of development (postnatal day (P) 4-P7) caused deficits in the air righting reflex and negative geotaxis tests during the second week of life [21]. Performance impairments in the rotarod, inclined runway, rope climbing, and/or parallel bar tests have been demonstrated in adolescent or adult rats exposed to a variety of ethanol exposure paradigms (intragastric delivery via gastrostomy or oral intubation; intraperitoneal injection) during one (P6) or multiple days (e.g., P4 to P7, P9 or P10) of neonatal life [22–31]. Exposure of rat pups to ethanol in vapor chambers (peak BEC = 270 mg/dl) during P2-P12 caused a developmental delay in the acquisition of the air-righting reflex (measured at P13-P19) but did not cause gait abnormalities or affect performance in the balance beam or rotarod tests at P22-P24 [32]. These findings indicate that exposure of rat pups to ethanol causes age-dependent alterations in motor function.
Comparatively fewer studies have used mice to characterize the effects of ethanol exposure on motor coordination during the brain growth spurt. Wozniak et al. [33] administered alcohol to C57BL/6 mice via a subcutaneous injection at P7 and tested sensorimotor function at P25 or P72; these investigators found no effect of ethanol on the platform, ledge, walking initiation, vertical pole, and inclined/inverted screen tests. Susick et al. [34] tested the effect of ethanol (via a single subcutaneous injection at P6) on sensorimotor development (P7-P30) in wild-type C57Bl/6 mice and adenylyl cyclase 1/8 knockout mice and found no effects of ethanol treatment on the wire suspension and grid hang tests but did detect an increase in the negative geotaxis test time-to-turn in ethanol-treated knockout mice. Using the rotarod test, two studies did not detect motor coordination deficits in adolescent or adult mice exposed to a single injection (at P6; [34]) or multiple injections (at P5, P7, and P9; [35]) of ethanol administered subcutaneously. However, another study did find that exposure to alcohol of wild type (129SVJ × C57B6 background) and neuronal nitric oxide synthase knockout mice during P4-P9 (via intraperitoneal injection) causes performance deficits in both the rotarod and balance beam tests during adulthood (P85–90) [36]. Balance alterations (in the dowel crossing test) were also demonstrated in P30 offspring (exposed to ethanol at P5 via intragastric gavage) of dams maintained on a choline-deficient diet during gestation; this effect was mitigated by neonatal choline supplementation. Based on the results of these studies, it is unclear how consistently third trimester-equivalent ethanol exposure causes motor coordination impairments in mice. Moreover, these findings suggest that, in some cases, an interaction between alcohol and additional insults (e.g., choline deficiency or deletion of adenylyl cyclases 1/8) is required to produce motor deficits.
Another possibility is that the tests that have been used to assess motor coordination in mice developmentally exposed to ethanol do not have enough sensitivity to detect subtle alterations. The Catwalk is a computerized, highly sensitive system that has been used for the quantitative analysis of gait and locomotion in rodents [37]. The footprints of the animal are captured while it crosses an illuminated glass plate towards a goal box. We hypothesized that the Catwalk test would detect gait abnormalities in mice exposed to ethanol during the third trimester equivalent. For comparison, we assessed motor coordination and strength in these mice using the rotarod and triple horizontal bar tests. Motor function was assessed during adolescence (P30–50) in animals exposed to ethanol during the third trimester equivalent (P4–9) using vapor inhalation chambers.
2. Materials and methods
All experimental procedures described in this manuscript adhered to the US Public Health Service policy on human care and use of laboratory animals and were approved by the institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center. For all the experiments described below, the experimenters were blinded to the identity of the experimental groups.
2.1. Subjects
Venus-vesicular GABA transporter (VGAT) mice were generated as described by Wang et al. [38]. These mice express the Venus protein in GABAergic and glycinergic neurons. A total of 13 male and 18 female mice from 7 liters were exposed to air and a total of 14 males and 19 females from 7 litters were exposed to ethanol. We used these mice because they have previously been shown to develop structural cerebellar alterations in response to ethanol vapor exposure involving the third trimester-equivalent of human pregnancy [39]. Moreover, we plan to characterize the effect of developmental ethanol exposure on the function of different cerebellar neuron populations using these reporter mice. Mice were maintained as heterozygous for the Venus-VGAT transgene. At P2, pups were screened for the presence of the Venus-VGAT transgene by exposing them to 460–495 nm wavelength light and observing yellow fluorescence emitted by the brain with a 520- to 550-nm filter using a “miner’s lamp” (Biological Laboratory Equipment Maintenance and Service LTD, Budapest, Hungary). Mice that did not express the Venus-VGAT transgene were euthanized by decapitation under isoflurane anesthesia. Mice were maintained at 22° C on a reverse 12-hour light/dark cycle (lights on at 8 pm) with food and water available ad libitum. Mice were weaned on P21, housed with same sex littermates, and allowed to acclimate to new environment for approximately a week before performing adolescence behavioral tests (P30-P50).
2.3. Ethanol vapor exposure and blood ethanol concentration measurement.
Both pups and dams were exposed to either air or ethanol (95%, Koptec, King of Prussia, PA) in custom-built vapor chambers [40]. Mice were exposed for 3 h (10 am–1 pm) on P4 and for 4 h (10 am–2 pm) on P5-P9. Ethanol vapor concentrations measured with a Breathalyzer (Intoximeters, St. Louis, MO) at the end of exposure were 8–9 g/dl. In a group of mice, trunk blood samples were collected under isoflurane anesthesia and processed as described in [41]. Blood ethanol concentrations (BECs) were measured using an alcohol dehydrogenase-based kit, following the manufacturer instructions (Product Code: K-ETOH, Megazyme Inc, Chicago, IL). The main determinant of pup BECs is the ethanol vapor inhalation; however, ingestion of ethanol via maternal milk can also have a small contribution, particularly during the initial phases of the paradigm before the dam develops metabolic tolerance to ethanol [42]. Nursing from an ethanol intoxicated dam has been shown to produce short- and long-term disruptions in motor function [43]; however, this effect may not be due cerebellar damage by ethanol since pup blood ethanol levels achieved via breastfeeding are typically low (<20 mg/dl), which is due to the fact that ethanol concentrations in breastmilk are a fraction of those in blood [44].
2.4. Behavioral testing
Assessments for motor coordination and balance were used to test the function of the motor and vestibular systems. Mice were tested during their active (dark) cycle under red light illumination. All behavioral testing was conducted in presence of white noise to reduce extraneous auditory stimuli.
2.4.1. Catwalk
Gait analysis in adult mice was performed using the Catwalk XT system (Noldus, Leesburg, VA, USA). Animals were allowed to walk freely across a glass runway to reach goal box containing an empty cage with bedding. A compliant run consisted of a mouse moving across the runway without stopping or changing directions within a time frame of 0.5–5 s. Three compliant runs made up the testing trial. The ceiling of the runway was illuminated by red light to allow for the silhouette of the mice to be captured. Green light enters at the edge of the glass runway and is internally reflected; light escapes each time a paw makes contact with the glass. Paw prints and silhouettes were recorded by a digital high-speed video camera positioned under the Catwalk. Catwalk XT 8.1 software (Noldus) was used to analyze the data. The Catwalk data were systematically analyzed using a multi-step approach that avoids biased parameter selection [45].
2.4.2. Triple-horizontal bar
This test was adapted from Deacon et al [46]. Metal bars of varying diameters (2, 4, and 6 mm) were raised 49 cm above the bench by two plexiglass columns. A cage with bedding was placed underneath the bars to soften the mice’s fall. Mice were raised by the base of their tail above the center of the rod and allowed to grasp it with only their forepaws. Mice were given 30 s on each rod, where they could hold on for the entire 30 s, fall, or transverse the rod and touch the vertical plexiglass columns. A fall that occurs in the first 5 s is considered a result of poor placement by the experimenter and the mouse is retested after 30 s of rest. Mice were first tested on the 2 mm diameter rod, if it held on for 30 s or touched the plexiglass columns mice were then tested on the thicker diameter rod after a period of rest. The time the mice remained on each of the bars was quantified. The average of three trials was calculated and the times spent on the three bars were added to generate cumulative times. Tests were video-recorded and later analyzed using the open source Simple Video Coder [47].
2.4.3. Rotarod
An accelerating rotarod was used (Panlab, Barcelona, Spain). On day 1 of the test (training day), mice were placed on the rotarod for 3 consecutive trials, after each trial the mice were returned to their home cage while the rest of their littermates were tested. On day 2 of the test (test day), mice were also placed on the rotarod for 3 consecutive trials, as described above. Each trial consisted of placing the mice on the rod rotating at an initial speed of 4 revolutions per minute (RPM). After 10 s, when mice were facing away from the direction of the rotation, the rotating speed was gradually increased to 20 RPM. The time and the RPM at which each mouse fell was recorded. If mice grabbed the rod and somersaulted around it, the time at which this happened was recorded and considered the fall time. If a mouse fell during the first 10 s of trial, it was retested up to three times before given a score of 4 RPM.
2.5. Statistical analyses
Statistical analyses were performed using Prism Version 8.4.3 (GraphPad Software, San Diego, CA) and SPSS version 26 (IBM, Armonk, NY). Pup weights and litter sizes at P2 were analyzed using an unpaired t-test with effect sizes reported as Hedges’ g. Animal weights during adolescence were analyzed using a two-way ANOVA, with sex and exposure condition as the fixed factors. ANOVA effect sizes are reported as partial eta squared (ηp2). The Catwalk data were systematically analyzed using a multi-step approach. Prior to statistical analysis of treatment, raw data output from 186 Catwalk variables were first transformed. Values for each variable were z-scored, and then the absolute value of the minimum z-score from the global set of z-scores was added to each value in the entire dataset, plus 1. This transformation 1) allowed for the direct comparison between variables originally on different scales while maintaining the original data distributions and 2) avoided mathematical issues that would have arisen in subsequent analyses (i.e. divide by zero errors). Outliers were then detected using hierarchical clustering (HC), principle component analysis (PCA), and interquartile range (IQR). A total of 4 subjects were removed whose mean values were outside of the 1.5*IQR range; these same 4 subjects were also identified as outliers in either/or both of the other methods (PCA/HC). Because the distribution of many of the traits was not normal, non-parametric rank-sum tests were performed between ethanol exposed and air exposed groups for each variable. Variables with significant effects of treatment were then determined as those that survived Benjamini & Hochberg false discovery rate threshold [48]. For visualizing data, effect sizes and significance thresholds were determined by computing the log2 fold-change of differences in the sum of ranks between groups and the –log10 of the rank-sum p values, respectively. Paw coupling was calculated with the following formula: Coupling = (Initial contact time of target paw - Initial contact time of anchor paw) / (step cycle of anchor paw) ×100. Given that the contact time of a target paw can never precede the contact time of an anchor paw, values range between 0 and 100%, where the lower the value, the closer in time the two paws make contact with the runaway. The Catwalk results for coupling between the left front paw and right hind paw (LF/RH coupling) were subsequently analyzed using a linear mixed regression model (LMM) to determine if including random effects associated with the intercept for each litter significantly improves statistical modeling. Sex and treatment were included as fixed effects [49, 50]. The results of the triple horizontal bar and rotarod tests were also analyzed using a LMM. The LMM for each dependent variable was first fit including random intercepts associated with each litter, and then a model was fit excluding random intercepts for each litter. The −2 restricted maximum log-likelihood values for each model fit were then analyzed using a likelihood ratio chi-square test. If the p-value from this chi-square test was < 0.05, including random effects significantly improved the LMM and were thus retained in further models. The subsequent iteration of the LMM was then fit using heterogenous error variances for treatment groups, and a second likelihood ratio chi-square test was performed. If the p-value from this second test was < 0.05, inclusion of heterogenous error variances significantly improved the LMM and were therefore used in the final model. F-ratios from Type III F-tests using Satterthwaite approximated degrees of freedom and p-values from the ultimate LMM are reported in the text. For the triple horizontal bar and rotarod tests, outliers were identified via ROUT test (coefficient q = 1.0%) and excluded from analysis. Effect sizes for main effects of sex and exposure are reported as Hedges’ g. Detailed statistics from LMM tests appear in Supplemental Table 1. LMMs results are strong even when violating assumptions of normality, because normality of residuals has no effect on parameter estimates in multilevel models [51]. However, due to the fact that residuals from LMMs for rotarod measures failed Shapiro-Wilkes normality tests with a p-value of < 0.05, we present non-parametric Mann-Whitney U tests for main effects of sex and treatment for these LMMs (with effect size as r) in Supplemental Table 1. Data are presented as group means and standard error of the means. We report precise p-values, as recommended in a recent article [52].
3. Results
At P2, litters were randomly assigned to the air or ethanol groups. The average pup weights per litter at P2 were 1.79 ± 0.04 g (n = 5) and 1.814 ± 0.13 g (n = 6) for the air and ethanol groups, respectively (t(9) = 0.1597; p = 0.88, g = 0.091). The average numbers of pups per litter at P2 were 8 ± 0.68 g (n = 6) and 7.43 ± 0.52 g (n = 7) for the air and ethanol groups, respectively (t(11) = 0.6718; p = 0.52, g = 0.345). The average numbers of Venus positive pups per litter at P2 were 4.85 ± 0.5 g (n = 7) and 4.71 ± 0.64 g (n = 7) for the air and ethanol groups, respectively (t(12) = 0.1741; p = 0.86, g = 0.087). Exposure to ethanol from P4-P9 vapor produced peak BECs of 37 ± 10 mM (170 ± 45 mg/dl; n = 4) at the end of the 3 h ethanol exposure at P4 and 6.4 ± 2.6 mM (29 ± 12 mg/dl; n = 4) 7 h after the end of the exposure. At P7, peak BECs were 55 ± 12 mM (252 ± 55 mg/dl; n = 5) at the end of the 4 h ethanol exposure at P7 and 28.9 ± 5.6 mM (130 ± 25 mg/dl; n = 4) 7 h after the end of the exposure. During adolescence (P30–50), the weights for air and ethanol exposed female mice were 15.7 ± 0.36 g (n = 16) and 14.07 ± 0.21 g (n = 19), respectively. The weights for air and ethanol exposed male mice were 19.25 ± 0.82 g (n = 12) and 17.53 ± 0.29 g (n = 14), respectively. Air-exposed mice weighed more than ethanol-exposed mice (exposure: F(1,57) = 15.958, p = 0.0002, ηp2 = 0.219), and male mice weighed more than female mice (sex: F(1,57) = 67.218, p < 0.0001, ηp2 = 0.541). There was no interaction between sex and exposure condition for pup weights during adolescence (sex*exposure: F(1,57) = 0.004, p = 0.95, ηp2 = < 0.001).
We assessed the effect of neonatal ethanol exposure on motor function during adolescence with the Catwalk test. Results were analyzed using a multistep approach as described above. Of 186 parameters analyzed, only one was found to be significantly affected by ethanol exposure following FDR correction. Specifically, ethanol exposure decreased LF/RH coupling (Figure 1A; false discovery rate-corrected rank-sum p = 0.0423 [uncorrected rank-sum p = 0.00023384]. The distribution of individual values (ranks) used for statistical evaluation of group differences in the coordination of this paw pair is presented in Figure 1B. It shows the difference in time between the LF and RH runaway contact times, which was reduced in ethanol-exposed mice (i.e., suggesting altered coupling between paws). Linear mixed regression model analysis showed that including random intercepts associated with litter (the random effect) did not improve the model and, therefore, were not included in the final LMM. However, including heterogeneous error variances for treatment groups significantly improved the model and were included (exposure: F(1,45.903) = 18.822, p = 0.000054, g = 1.127). The model did not reveal an effect of sex or an exposure*sex interaction (sex: F(1,45.903) = 1.039, p = 0.3134, g = 0.279; exposure*sex: F(1,45.903) = 2.208, p = 0.1442). Thus, ethanol exposure had an impact on the coordination between the LF/RH paw pair that was not attributable to influences of sex or litter.
Fig 1.
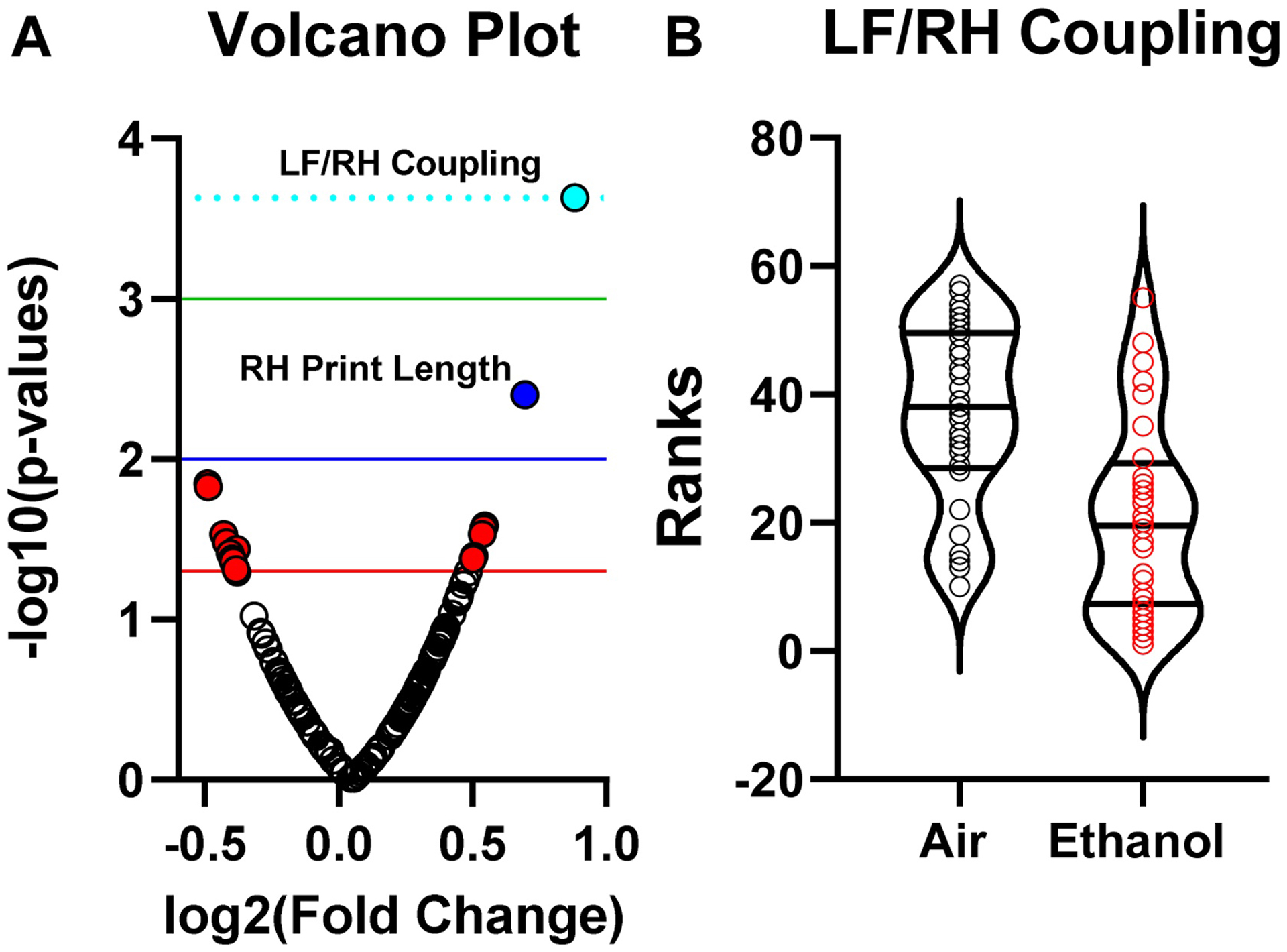
Effect of ethanol exposure on the Catwalk test. (A) Volcano plot for rank sum test. Of 186 parameters analyzed, only left front paw/right hind paw (LF/RH) coupling was found to be affected by ethanol exposure following false discovery rate correction. (B) Distribution of ranks for LF/RH Coupling. Linear mixed regression model revealed a significant effect of ethanol exposure on LF/RH coupling (see text for details). n = 30 mice from 7 litters (air) and n = 32 mice from 7 litters (ethanol).
Mice were also tested in the triple horizontal bar test (Fig 2). Linear mixed model analyses did not reveal random litter effects in this test; therefore, litter was not included in subsequent analyses. Ethanol exposure reduced the cumulative time spent on the triple horizontal bars (exposure: F(1,60) = 4.805, p = 0.0323, g = 0.505) and no effect of sex (sex: F(1,60) = 0.674, p = 0.4151, g = 0.209) or an interaction between sex and exposure (sex*exposure: F(1,60 = 1.280, p = 0.2624) were detected. Neither random effect of litter nor heterogenous error variance significantly improved the LMM for time spent on the triple horizontal bars and were not included in the final model.
Fig 2.
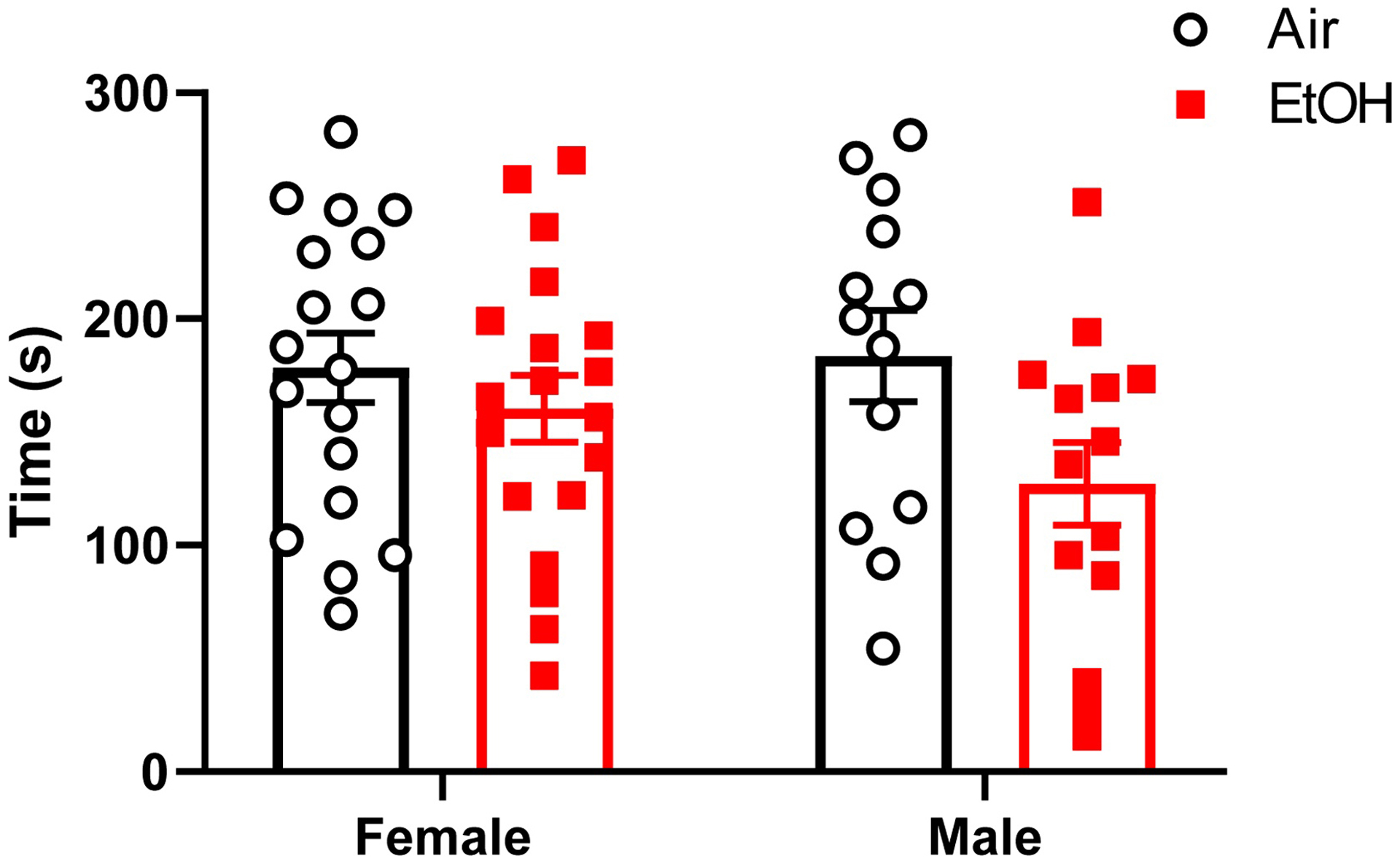
Effect of ethanol exposure on the triple horizontal bar. Cumulative time spent in the triple horizontal bars by adolescent mice (postnatal days (P) 31–50) that were vapor chamber-exposed to air (white circles) or ethanol (EtOH; red squares) as neonates (P4–9). Mice were allowed to grasp metal bars of increasing diameter (2, 4, and 6 mm) with their forepaws (30 s per bar for 3 trials). Cumulative times spent by each time holding onto the bar were calculated as described in the Materials and Methods section. Mean ± SEM of 18 female and 13 male mice from 6–7 litters (air), and 19 female and 14 male mice from 7 litters (ethanol). Linear mixed model revealed a significant effect of treatment (see text for details).
Finally, motor function was assessed in the rotarod test (Fig 3). For the time spent on the rotarod during training day, both litter effect and heterogeneous error variance significantly improved the model; therefore, these parameters were included in the analysis. Ethanol exposure did not affect the time spent on the rotarod during training day (exposure: F(1,10.277) = 0.723, p = 0.4145, g = 0.263). There were no sex (sex: F(1,43.097) = 0.886, p = 0.3519, g = 0.033) or sex*exposure (sex*exposure: F(1,43.097) = 0.244, p = 0.6241) effects for time spent on the rotarod during the training day. For the time spent on the rotarod during test day, neither litter effect nor heterogeneous error variance significantly improved the model; therefore, these parameters were not included in the analysis. Ethanol exposure increased the time spent on the rotarod during test day (exposure: F(1,58) = 4.875, p = 0.0312, g = 0.619). There were no sex (sex: F(1,58) = 1.724, p = 0.1944, g = 0.333) or sex*exposure (sex*exposure: F(1,58) = 1.474, p = 0.2297) effects for time spent on the rotarod during the test day. For the RPMs tolerated by the mice before falling during training day, both litter effect and heterogeneous error variance significantly improved the model; therefore, these parameters were included in the analysis. Ethanol exposure did not affect the RPMs tolerated before falling from the rotarod during training day (Exposure F(1,10.396) = 0.926, p = 0.3578). There were no sex (sex: F(1,43.678) = 1.200, p = 0.2794, g = 0.002) or sex*exposure (sex*exposure: F(1,43.678) = 0.344, p = 0.5607) effects for RPMs on the training day. For the RPMs tolerated by the mice before falling during test day, neither litter effect nor heterogeneous error variance significantly improved the model; therefore, these parameters were not included in the analysis. Ethanol exposure increased the RPMs tolerated before falling from the rotarod during test day (exposure: F(1,58) = 5.797, p = 0.0193, g = 0.674). There were no sex (sex: F(1,58) = 1.334, p = 0.2528, g = 0.292) or sex*exposure (sex*exposure: F(1,58) = 1.501, p = 0.2255) effects for RPMs tolerated on the test day.
Fig 3.
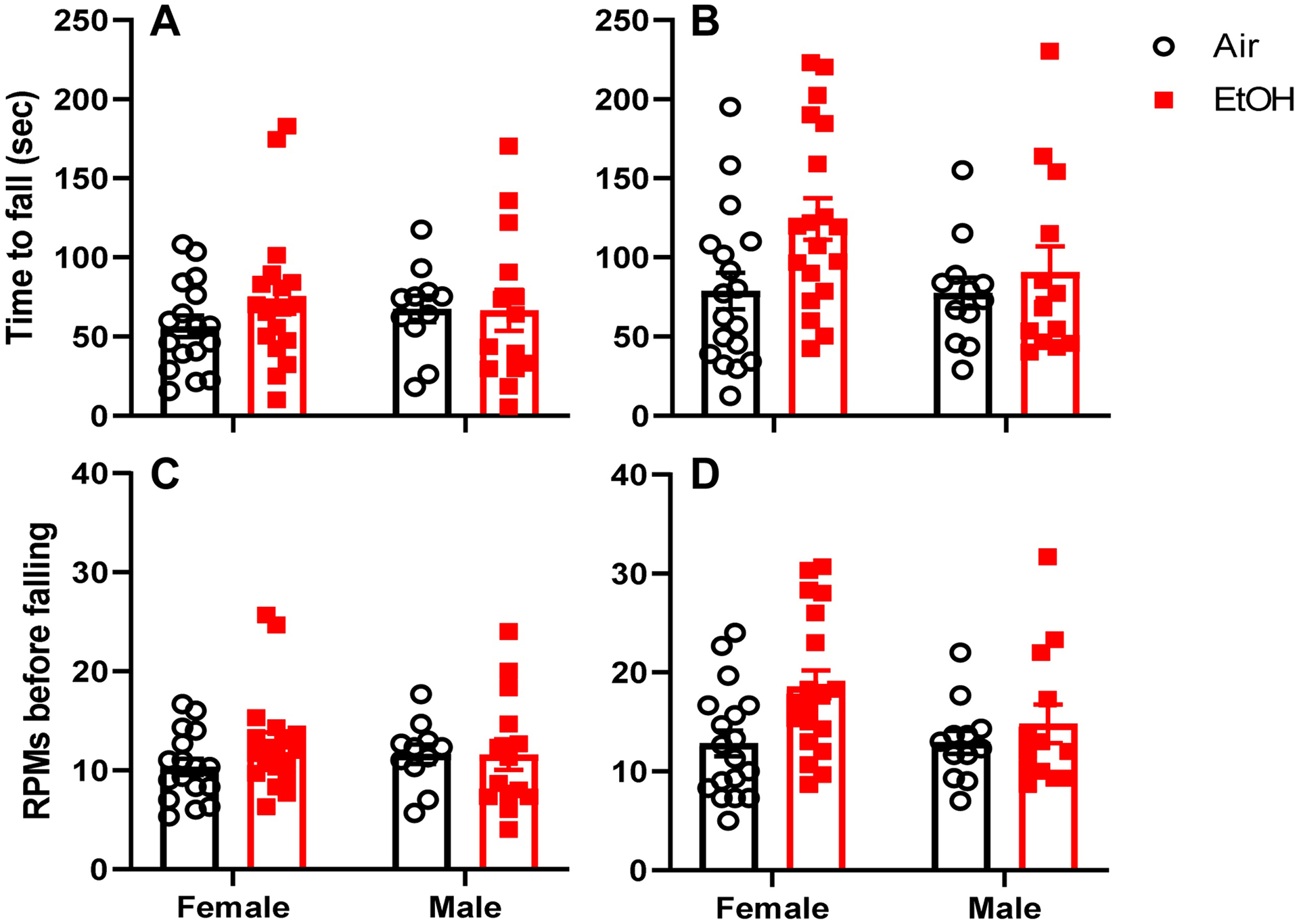
Effect of ethanol exposure on the rotarod test. Time to fall from the accelerating rotarod during training day (A) and test day (B) for adolescent mice (postnatal days (P) 40–50) that were vapor chamber-exposed to air (white circles) or ethanol (EtOH; red squares) as neonates (P4–9). The revolutions per minute (RPM) at which the mice fell down from the accelerating rotarod are shown in (C) for training day and (D) for test day. Mean ± SEM of 17–18 female and 11–12 male mice from 6–7 litters (air) and 19 female and 13–14 male mice from 7 litters (ethanol). Linear mixed model analysis revealed an effect of ethanol exposure on both time to fall and RPMs tolerated before falling during test day (see text for details).
4. Discussion
Using the Catwalk system, we demonstrate here that binge-like neonatal ethanol exposure causes subtle alterations in gait. Rather than arbitrarily selecting a subset of the large number of parameters generated by this test, we used an innovative multistep process to systematically analyze all of them in an unbiased fashion. The importance of analyzing all gait parameters in Catwalk experiments was recently emphasized by Timotius et al. [45] who used an Initial Data Analysis procedure to clean and screen the data, as well as heat-maps for reporting the results. We used HC, PCA, and IQR calculation to identify outliers and performed normality tests. Since the data from many Catwalk variables were not normally distributed, non-parametric rank-sum tests were used to analyze the data, followed by calculation of false discovery. Instead of using heat maps, we chose volcano plots to illustrate the data. Using this rigorous approach, we found that only one Catwalk parameter was affected by ethanol exposure; namely, there was a reduction in the coupling between LF and RH paws. This finding indicates that the coordination of position between these two paws was impaired by neonatal ethanol exposure. Interestingly, an earlier study from our laboratory also detected similar deficits in adolescent rats exposed to ethanol between P3-P5 in vapor chambers [53]. The regions of the cerebellum and other parts of the brain that are involved in the control of inter-paw coordination in mice are not fully understood. Therefore, future studies will be required to determine the mechanism responsible for this effect. Alterations in GABAergic synaptic plasticity at Purkinje cells have been shown to alter inter-paw coordination [54] and it will be interesting to determine if this mechanism contributes to the effect of neonatal ethanol exposure that we report here.
We also detected ethanol exposure-induced deficits in the triple horizontal bar test, which measures both muscle strength and coordination in the forelimbs [46]. Specifically, mice exposed to ethanol held on to the bars for less time than control mice. This test is a refinement of the classical horizontal bar test (performed with a single bar of 2 mm in diameter) that has been used to show motor deficiencies in scrapie-infected mice [55]. The horizontal bar test has also used to demonstrate differences in motor abilities between C57BL/6 and C57BL/10 mice [56], as well as motor deficits in a mouse model of Friedreich ataxia [57] and mutant mice lacking ATP-sensitive potassium channels formed by the Kir6.2 subunit [58]. It has been used to characterize motor function in a mouse model of mucopolysaccharidosis type II [59]. Our results suggest that another application of this test is the characterization of the effects of developmental ethanol exposure in mice.
In contrast, the accelerated rotarod test, which is widely used to evaluate motor coordination and balance, showed a paradoxical increase in performance in ethanol exposed mice. In the context of neurodevelopmental disorders, improved performance in the rotarod test has been observed in mouse models of autism spectrum disorders, which may indicate a greater ability to acquire a repetitive motor sequence in these animals [60, 61]. Improved performance in the rotarod was also observed in a mouse model of Alzheimer’s disease that may be a result of differences in gait length and/or balance ability [62]. It is also possible that ethanol-exposed mice are less anxious and this improves their performance in the rotarod test [63]. Alternatively, studies using Sprague-Dawley rats have shown that third-trimester equivalent ethanol exposure can increase anxiety like behavior [64], which may lead to increased performance on the rotarod task as animals may expend more effort to stay on the rod if they determine the situation to be more dangerous than air-exposed controls do. Future studies will be required to determine the mechanisms by which neonatal ethanol vapor exposure produces this increase in performance in the rotarod test.
There are several limitations in this study. First, we only tested the effect of one ethanol exposure paradigm. We have previously shown that vapor chamber exposure produces a gradual increase in BECs in Venus-VGAT mice [41]. It is possible that paradigms that produce more rapid increases in BECs and structural/functional cerebellar alterations (i.e., intragastric gavage; [65] cause greater alterations in motor function. Noteworthy, one of such paradigms, subcutaneous injection, had little effect on mouse performance in a battery of sensorimotor tasks [33]. Second, our findings could be specific to the Venus-VGAT mouse strain and it should be investigated if different strains of wild-type mice have different sensitivity to the effects of third trimester-equivalent ethanol exposure. Finally, it is important to assess the effect of ethanol exposure during the third trimester in a species in which it occurs in utero rather than early in postnatal life (e.g., guinea pigs, sheep, and primates) [15].
In conclusion, we demonstrate here that ethanol exposure of mice during the brain growth spurt causes alterations in motor function during adolescence that are more evident in behavioral tests that interrogate both motor strength and coordination, as well as balance. Gait appears to be generally unaffected with the exception of a single front-hind paw coupling parameter. Taken together, our findings underscore the importance of assessing the effect of developmental ethanol exposure on motor performance in both sexes and using a battery of tests that evaluate multiple domains (e.g., gait, strength, coordination).
Supplementary Material
Highlights:
Neonatal Venus-VGAT mice were exposed to ethanol
Mice were left undisturbed until adolescence
Coupling of left front/right hind paw coupling was reduced in Catwalk test
Ethanol exposure impaired performance in the triple horizontal bar test
Ethanol-exposed mice performed better in the rotarod test
Acknowledgements
This work was supported by NIH grants R37 AA015614 and P50 AA022534 (CFV), R00 AA025120 (DNL), and the University of New Mexico HSC Undergraduate Pipeline Network Program. Rotarod and Catwalk tests were performed at the UNM Center for Brain Recovery and Repair supported by NIH grant P20 GM109089.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None.
References
- [1].Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 2016;138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–87. [DOI] [PubMed] [Google Scholar]
- [3].Valenzuela CF, Jotty K. Mini-Review: Effects of Ethanol on GABAA Receptor-Mediated Neurotransmission in the Cerebellar Cortex--Recent Advances. Cerebellum. 2015;14:438–46. [DOI] [PubMed] [Google Scholar]
- [4].Cheng DT, Jacobson SW, Jacobson JL, Molteno CD, Stanton ME, Desmond JE. Eyeblink Classical Conditioning in Alcoholism and Fetal Alcohol Spectrum Disorders. Front Psychiatry. 2015;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21:133–47. [DOI] [PubMed] [Google Scholar]
- [6].Gill JS, Sillitoe RV. Functional Outcomes of Cerebellar Malformations. Front Cell Neurosci. 2019;13:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nguyen VT, Chong S, Tieng QM, Mardon K, Galloway GJ, Kurniawan ND. Radiological studies of fetal alcohol spectrum disorders in humans and animal models: An updated comprehensive review. Magn Reson Imaging. 2017;43:10–26. [DOI] [PubMed] [Google Scholar]
- [8].Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN. Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2013;37:1499–507. [DOI] [PubMed] [Google Scholar]
- [9].du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, et al. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2014;38:1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dukes K, Tripp T, Willinger M, Odendaal H, Elliott AJ, Kinney HC, et al. Drinking and smoking patterns during pregnancy: Development of group-based trajectories in the Safe Passage Study. Alcohol. 2017;62:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, et al. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, et al. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clin Chem. 2015;61:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Murphy DJ, Dunney C, Mullally A, Adnan N, Fahey T, Barry J. A prospective cohort study of alcohol exposure in early and late pregnancy within an urban population in Ireland. Int J Environ Res Public Health. 2014;11:2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood). 2005;230:389–93. [DOI] [PubMed] [Google Scholar]
- [16].Kostović I, Jovanov-Milosević N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–22. [DOI] [PubMed] [Google Scholar]
- [17].Niclasen J, Andersen AM, Strandberg-Larsen K, Teasdale TW. Is alcohol binge drinking in early and late pregnancy associated with behavioural and emotional development at age 7 years? Eur Child Adolesc Psychiatry. 2014;23:1175–80. [DOI] [PubMed] [Google Scholar]
- [18].Akinmboni TO, Davis NL, Falck AJ, Bearer CF, Mooney SM. Excipient exposure in very low birth weight preterm neonates. J Perinatol. 2018;38:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hsieh S, Sapkota A, Wood R, Bearer C, Kapoor S. Neonatal ethanol exposure from ethanol-based hand sanitisers in isolettes. Arch Dis Child Fetal Neonatal Ed. 2018;103:F55–F8. [DOI] [PubMed] [Google Scholar]
- [20].Zuccotti GV, Fabiano V. Safety issues with ethanol as an excipient in drugs intended for pediatric use. Expert Opin Drug Saf. 2011;10:499–502. [DOI] [PubMed] [Google Scholar]
- [21].Diaz J, Samson HH. Impaired brain growth in neonatal rats exposed to ethanol. Science. 1980;208:751–3. [DOI] [PubMed] [Google Scholar]
- [22].Thomas JD, O’Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:223–9. [DOI] [PubMed] [Google Scholar]
- [23].Goodlett CR, Thomas JD, West JR. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicol Teratol. 1991;13:69–74. [DOI] [PubMed] [Google Scholar]
- [24].Goodlett CR, Lundahl KR. Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits. Pharmacol Biochem Behav. 1996;55:531–40. [DOI] [PubMed] [Google Scholar]
- [25].Melcer T, Gonzalez D, Somes C, Riley EP. Neonatal alcohol exposure and early development of motor skills in alcohol preferring and nonpreferring rats. Neurotoxicol Teratol. 1995;17:103–10. [DOI] [PubMed] [Google Scholar]
- [26].Klintsova AY, Cowell RM, Swain RA, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats . I. Behavioral results. Brain Res. 1998;800:48–61. [DOI] [PubMed] [Google Scholar]
- [27].Idrus NM, McGough NN, Riley EP, Thomas JD. Administration of memantine during ethanol withdrawal in neonatal rats: effects on long-term ethanol-induced motor incoordination and cerebellar Purkinje cell loss. Alcohol Clin Exp Res. 2011;35:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ewenczyk A, Ziplow J, Tong M, Le T, de la Monte SM. Sustained Impairments in Brain Insulin/IGF Signaling in Adolescent Rats Subjected to Binge Alcohol Exposures during Development. J Clin Exp Pathol. 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyer LS, Kotch LE, Riley EP. Alterations in gait following ethanol exposure during the brain growth spurt in rats. Alcohol Clin Exp Res. 1990;14:23–7. [DOI] [PubMed] [Google Scholar]
- [30].Thomas JD, Leany BD, Riley EP. Differential vulnerability to motor deficits in second replicate HAS and LAS rats following neonatal alcohol exposure. Pharmacol Biochem Behav. 2003;75:17–24. [DOI] [PubMed] [Google Scholar]
- [31].Thomas JD, Burchette TL, Dominguez HD, Riley EP. Neonatal alcohol exposure produces more severe motor coordination deficits in high alcohol sensitive rats compared to low alcohol sensitive rats. Alcohol. 2000;20:93–9. [DOI] [PubMed] [Google Scholar]
- [32].Diaz MR, Vollmer CC, Zamudio-Bulcock PA, Vollmer W, Blomquist SL, Morton RA, et al. Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor delta subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology. 2014;79:262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–14. [DOI] [PubMed] [Google Scholar]
- [34].Susick LL, Lowing JL, Bosse KE, Hildebrandt CC, Chrumka AC, Conti AC. Adenylyl cylases 1 and 8 mediate select striatal-dependent behaviors and sensitivity to ethanol stimulation in the adolescent period following acute neonatal ethanol exposure. Behav Brain Res. 2014;269:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamilton GF, Hernandez IJ, Krebs CP, Bucko PJ, Rhodes JS. Neonatal alcohol exposure reduces number of parvalbumin-positive interneurons in the medial prefrontal cortex and impairs passive avoidance acquisition in mice deficits not rescued from exercise. Neuroscience. 2017;352:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bonthius DJ Jr., Winters Z, Karacay B, Bousquet SL, Bonthius DJ. Importance of genetics in fetal alcohol effects: null mutation of the nNOS gene worsens alcohol-induced cerebellar neuronal losses and behavioral deficits. Neurotoxicology. 2015;46:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K, et al. Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 2010;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Y, Kakizaki T, Sakagami H, Saito K, Ebihara S, Kato M, et al. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience. 2009;164:1031–43. [DOI] [PubMed] [Google Scholar]
- [39].Nirgudkar P, Taylor DH, Yanagawa Y, Valenzuela CF. Ethanol exposure during development reduces GABAergic/glycinergic neuron numbers and lobule volumes in the mouse cerebellar vermis. Neurosci Lett. 2016;632:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Morton RA, Diaz MR, Topper LA, Valenzuela CF. Construction of vapor chambers used to expose mice to alcohol during the equivalent of all three trimesters of human development. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bird CW, Barber MJ, Post HR, Jacquez B, Chavez GJ, Faturos NG, et al. Neonatal ethanol exposure triggers apoptosis in the murine retrosplenial cortex: Role of inhibition of NMDA receptor-driven action potential firing. Neuropharmacology. 2020;162:107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Morton RA, Diaz MR, Topper LA, Valenzuela CF. Construction of vapor chambers used to expose mice to alcohol during the equivalent of all three trimesters of human development. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–50. [DOI] [PubMed] [Google Scholar]
- [44].Guerri C, Sanchis R. Alcohol and acetaldehyde in rat’s milk following ethanol administration. Life Sci. 1986;38:1543–56. [DOI] [PubMed] [Google Scholar]
- [45].Timotius IK, Canneva F, Minakaki G, Moceri S, Plank AC, Casadei N, et al. Systematic data analysis and data mining in CatWalk gait analysis by heat mapping exemplified in rodent models for neurodegenerative diseases. J Neurosci Methods. 2019;326:108367. [DOI] [PubMed] [Google Scholar]
- [46].Deacon RM. Measuring motor coordination in mice. J Vis Exp. 2013:e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barto D, Bird CW, Hamilton DA, Fink BC. The Simple Video Coder: A free tool for efficiently coding social video data. Behav Res Methods. 2017;49:1563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Benjamini YH Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological). 1995;57:289–300. [Google Scholar]
- [49].West BT, Welch KB, Galecki AT Two-Level Models for Clustered Data: The Rat Pup Example Linear Mixed Models: A Practical Guide Using Statistical Software. Second edition ed. Boca Raton, Fl: CRC Press; 2015. p. 59–134. [Google Scholar]
- [50].Golub MS, Sobin CA. Statistical modeling with litter as a random effect in mixed models to manage “intralitter likeness”. Neurotoxicol Teratol. 2020;77:106841. [DOI] [PubMed] [Google Scholar]
- [51].Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge ; New York: Cambridge University Press; 2007. [Google Scholar]
- [52].Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–7. [DOI] [PubMed] [Google Scholar]
- [53].Welch JH, Mayfield JJ, Leibowitz AL, Baculis BC, Valenzuela CF. Third trimester-equivalent ethanol exposure causes micro-hemorrhages in the rat brain. Neuroscience. 2016;324:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Neureither F, Ziegler K, Pitzer C, Frings S, Mohrlen F. Impaired Motor Coordination and Learning in Mice Lacking Anoctamin 2 Calcium-Gated Chloride Channels. Cerebellum. 2017;16:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guenther K, Deacon RM, Perry VH, Rawlins JN. Early behavioural changes in scrapie-affected mice and the influence of dapsone. Eur J Neurosci. 2001;14:401–9. [DOI] [PubMed] [Google Scholar]
- [56].Deacon RM, Thomas CL, Rawlins JN, Morley BJ. A comparison of the behavior of C57BL/6 and C57BL/10 mice. Behav Brain Res. 2007;179:239–47. [DOI] [PubMed] [Google Scholar]
- [57].Filali M, Lalonde R, Gerard C, Coulombe Z, Tremblay JP. Sensorimotor skills in Fxn KO/Mck mutants deficient for frataxin in muscle. Brain Res. 2015;1608:91–6. [DOI] [PubMed] [Google Scholar]
- [58].Deacon RM, Brook RC, Meyer D, Haeckel O, Ashcroft FM, Miki T, et al. Behavioral phenotyping of mice lacking the K ATP channel subunit Kir6.2. Physiol Behav. 2006;87:723–33. [DOI] [PubMed] [Google Scholar]
- [59].Gleitz HF, O’Leary C, Holley RJ, Bigger BW. Identification of age-dependent motor and neuropsychological behavioural abnormalities in a mouse model of Mucopolysaccharidosis Type II. PLoS One. 2017;12:e0172435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome- engineered mouse model for human 15q11–13 duplication seen in autism. Cell. 2009;137:1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hisaoka T, Komori T, Kitamura T, Morikawa Y. Abnormal behaviours relevant to neurodevelopmental disorders in Kirrel3-knockout mice. Sci Rep. 2018;8:1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stover KR, Campbell MA, Van Winssen CM, Brown RE. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav Brain Res. 2015;281:16–23. [DOI] [PubMed] [Google Scholar]
- [63].Xu W, Hawkey AB, Li H, Dai L, Brim HH, Frank JA, et al. Neonatal Ethanol Exposure Causes Behavioral Deficits in Young Mice. Alcohol Clin Exp Res. 2018;42:743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Baculis BC, Diaz MR, Valenzuela CF. Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacol Biochem Behav. 2015;137:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-γ agonists. Brain Behav Immun. 2011;25 Suppl 1:S137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


