Abstract
Here we review the impact of obstructive sleep apnea (OSA) on biomarkers of Alzheimer’s disease (AD) pathogenesis, neuroanatomy, cognition and neurophysiology, and present the research investigating the effects of continuous positive airway pressure (CPAP) therapy. OSA is associated with an increase in AD markers amyloid-β and tau measured in cerebrospinal fluid (CSF), by Positron Emission Tomography (PET) and in blood serum. There is some evidence suggesting CPAP therapy normalizes AD biomarkers in CSF but since mechanisms for amyloid-β and tau production/clearance in humans are not completely understood, these findings remain preliminary. Deficits in the cognitive domains of attention, vigilance, memory and executive functioning are observed in OSA patients with the magnitude of impairment appearing stronger in younger people from clinical settings than in older community samples. Cognition improves with varying degrees after CPAP use, with the greatest effect seen for attention in middle age adults with more severe OSA and sleepiness. Paradigms in which encoding and retrieval of information are separated by periods of sleep with or without OSA have been done only rarely, but perhaps offer a better chance to understand cognitive effects of OSA than isolated daytime testing. In cognitively normal individuals, changes in EEG microstructure during sleep, particularly slow oscillations and spindles, are associated with biomarkers of AD, and measures of cognition and memory. Similar changes in EEG activity are reported in AD and OSA, such as “EEG slowing” during wake and REM sleep, and a degradation of NREM EEG microstructure. There is evidence that CPAP therapy partially reverses these changes but large longitudinal studies demonstrating this are lacking. A diagnostic definition of OSA relying solely on the Apnea Hypopnea Index (AHI) does not assist in understanding the high degree of inter-individual variation in daytime impairments related to OSA or response to CPAP therapy. We conclude by discussing conceptual challenges to a clinical trial of OSA treatment for AD prevention, including inclusion criteria for age, OSA severity, and associated symptoms, the need for a potentially long trial, defining relevant primary outcomes, and which treatments to target to optimize treatment adherence.
Introduction
Obstructive sleep apnea (OSA) is a type of sleep-disordered breathing (SDB) (ICSD-3 2014) characterized by recurrent complete or partial closure of the upper airway followed by blood oxygen desaturation and/or arousal from sleep. Untreated, these repetitive respiratory events can lead to fragmented, disrupted sleep and chronic nocturnal intermittent hypoxia, re-oxygenation, and hypercapnia or hypocapnia. OSA diagnosis is made using assessment of physiological signals from oronasal airflow, respiratory effort and pulse oximetry during sleep. Polysomnography (PSG), including EEG, is considered the gold standard for investigation of suspected SDB, however recordings with only airflow and/or pulse oximetry are commonly used for diagnosis (Woods et al. 2014). OSA severity is predominantly classified with reference to the apnea-hypopnea index (AHI), defined as the number of respiratory events (apneas and hypopneas) per hour of sleep. Critically, there are multiple definitions of “hypopnea,” leading to multiple AHI’s, and no one definition of AHI is used consistently in clinical research investigating OSA and its effect on health outcomes.
A review of eleven population-based epidemiological studies from around the world between 1993 and 2013 found an average prevalence rate of 22% in men and 17% in women for at least mild OSA, and 6% in men and 4% in women for the OSA syndrome (OSAS), also known as OSA with excessive daytime sleepiness (EDS) (Franklin and Lindberg 2015). One of the more recent large studies of community-recruited individuals, the HypnoLaus study, used the AASM 2012 hypopnea definition ≥30% drop of airflow lasting at least 10 s with either an arousal or ≥3% oxygen desaturation (AHI3A). In this study, when subjects were dichotomized by age (ages 40–60 years and ages 60–85 years), the proportion of subjects with AHI3A ≥ 15/hour was 26.8% in the younger group (39.6% in men, 13.9% in women) and 48.7% in the older group (64.7% in med, 35.2% in women), highlighting the extraordinary prevalence of OSA in older individuals (Heinzer et al. 2015). Treatment options for OSA include positive airway pressure devices, oral appliances, behavioral/lifestyle modification, surgery and/or a combination of approaches. Deciding on the most effective treatment for an individual diagnosed with sleep OSA depends on disease severity, presenting symptoms and contributing causes. Continuous positive airway pressure (CPAP) is the most commonly prescribed treatment and works by the delivery of positive pressure via a mask worn over the nose (and/or mouth), functioning to splint the upper airway open during sleep (Sullivan et al. 1981).
Over the last few years, the literature has expanded regarding the links between sleep disruption, markers of Alzheimer’s disease (AD) pathogenesis, and the development of cognitive impairment and/or dementia. A recent meta-analysis showed SDB was a risk factor of all-cause dementia, AD, and vascular dementia (Shi et al. 2018), however many studies used self-reported measures of OSA, and few longitudinal studies were included. In older women, those diagnosed with OSA via PSG had increased risk of developing mild cognitive impairment (MCI) or dementia over a 5-year follow-up (Yaffe et al. 2011). In Asian populations, there are two large longitudinal community-based studies finding OSA/SDB is associated with increased risk of developing dementia and AD (Chang et al. 2013; Lee, Yang, et al. 2019). Analysis of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort found the presence of OSA was associated with an earlier age of cognitive decline and suggests CPAP treatment may delay the progression of cognitive impairment (Osorio et al. 2015). Here we present a narrative review of the literature investigating how sleep and sleep-disordered breathing influences markers of increasing risk of neurodegeneration through measures of amyloid-β and tau, neuroanatomy, cognition and neurophysiology. Lastly, we review the evidence concerning modification of these various markers with CPAP treatment. The literature included was selected using SCOPUS, searching with terms “OSA” and “Alzheimer’s disease”, “neuroimaging”, “cognition” and “EEG”, and with “CPAP” subsequently added to each pair of search terms. In addition, meta-analysis and systematic reviews focusing sleep and Alzheimer’s disease were reviewed along with their bibliographies for associated cited work.
OSA impacts markers associated with increasing AD risk
Amyloid/tau biomarkers
There is mounting evidence that OSA is associated with an increase in markers associated with AD, specifically amyloid-β and tau measured in cerebrospinal fluid (CSF), by Positron Emission Tomography (PET) and in blood serum. In several studies where OSA was diagnosed using PSG, reduced CSF amyloid-β levels compared to age-matched controls without OSA have been demonstrated (Ju et al. 2016; Liguori et al. 2017; Liguori et al. 2019). When groups were stratified by ApoE allele status, OSA severity positively correlated with amyloid-β42, phosphorylated tau (P-tau) and total tau (T-tau) in ApoE3 carriers and negatively correlated with amyloid-β42 levels in those with ApoE2 (Osorio et al. 2014). Interpreting AD risk based solely on CSF amyloid-β in cognitively normal older individuals at cross section is not straightforward. While there is consensus that CSF amyloid-β is lower in individuals with bona fide AD dementia versus age matched controls, there is evidence for elevated CSF amyloid-β with a steeper subsequent decline in individuals with genetic risk for AD (Bateman et al. 2012; Fagan et al. 2014). Additionally, an analysis of interrelationships between CSF amyloid-β and tau across a wide variety of ages showed that there was an overall positive correlation between CSF amyloid-β and tau between the ages of 45 and 70, whereas there was an overall negative correlation between CSF amyloid-β and tau between the ages of 71 and 90 (de Leon et al. 2018).
PET amyloid tracer uptake levels are a robust predictor of amyloid burden as well as of future development of AD (Waragai et al. 2009). The first PET study to show greater amyloid deposition with increasing OSA severity was a small pilot study of 5 people with MCI (Spira et al. 2014). However, the association between amyloid and OSA was not observed among the 8 cognitively normal participants. A more recent analysis in 14 OSAS patients with normal cognition also found no association between SDB and amyloid uptake (Handa et al. 2019). In contrast, a case control study of 19 participants with SDB found higher amyloid deposition in the right posterior cingulate gyrus and right temporal cortex compared to controls (Yun et al. 2017). Also, a cross sectional study of 117 cognitively normal Australian veterans (42 with OSA diagnosed by PSG) showed cortical uptake of 18F-florbetaben (ligand binding to amyloid aggregates) was higher in OSA group than in those without symptoms of OSA. In this study, a regression analysis revealed the relationship between amyloid deposition and OSA was independent of age and vascular risk factors, but not BMI or ApoE4 status, where the presence of ApoE4 amyloid burden was increased to a greater degree in those with OSA (Elias et al. 2018). Another cross-sectional analysis found 96 of 127 community dwelling, cognitively normal individuals with OSA showed greater amyloid burden via PET in the posterior cingulate cortex and precuneus areas which overlapped with increased grey matter volumes, perfusion and glucose metabolism (Andre et al. 2020).
Tau has been less studied overall with relation to OSA. Individuals with OSA have been shown to have elevated plasma concentrations of T-tau (Motamedi et al. 2018) and P-tau (Bu et al. 2015) in comparison to individuals without OSA. Although there is poor correlation between serum and CSF tau concentrations, serum tau may nonetheless be useful as a predictive marker for dementia (Pase et al. 2019). Relatedly, individuals with OSA were shown to have elevated concentrations of T-tau/Aβ42 in the CSF in comparison to either individuals without OSA or with PAP-treated OSA (Liguori et al. 2017), although no differences in individual T-tau or P-tau in the CSF were observed in another cross-sectional study (Ju et al. 2016). Tau PET imaging was employed in a cross-sectional analysis of 292 cognitively unimpaired adults who, when their bed-partners positively reported “witnessed apnea,” had more elevated tau-PET levels in the entorhinal cortex and inferior temporal cortex (Carvalho et al. 2020).
Longitudinal studies are therefore useful in clarifying some of the cross-sectional inconsistencies. Our group has reported two longitudinal studies using both CSF and PET imaging markers of Alzheimer’s disease in OSA. In Sharma et al. (2018), baseline severity of OSA was associated with a longitudinal decrease in CSF amyloid-β42 and longitudinal increase in cortical amyloid deposits by PET imaging. In Bubu et al. (2019), self-reported OSA in cognitively normal and MCI individuals was associated with increases in amyloid burden by both CSF and PET measures, and in CSF concentration of T-tau and P-tau over 2.5 years.
Changes in markers of disease with CPAP treatment
There is a paucity of literature demonstrating CPAP treatment effects on amyloid and tau biomarkers of AD. In the only study to examine a CPAP treatment effect that incorporated in-lab PSG, CSF sampling and neurocognitive testing, Liguori et al. (2017) compared 10 OSA positive patients treated with CPAP (for <1 year with good compliance), 25 untreated OSA patients, and 15 controls. Untreated OSA patients showed lower CSF amyloid-β42 concentrations, and higher T-tau/ amyloid-β42 ratio compared to both CPAP-treated individuals and controls. Furthermore, lower CSF amyloid-β42 levels correlated with lower average nocturnal oxygen saturation and cognition in OSA patients. In contrast, an observational study by Elias et al. (2018) found self-reported CPAP use in 24/42 veterans with OSA had no significant impact on amyloid deposition or cognition. In another study using a within participant design, Ju et al. (2019) measured CSF biomarkers in 18 adherent CPAP users before and after 1–4 months of CPAP therapy and found that greater improvement of OSA as a result of CPAP therapy (reduction of AHI and arousals) positively correlated with greater changes in amyloid and tau levels in a direction of reduced AD pathology. There were no significant changes in CSF biomarkers at the group level, suggesting a potential need to focus on individuals with more moderate to severe OSA.
Summary
In cognitively normal older subjects, OSA appears to be associated with an increase CSF measures of AD pathology at cross section, with heterogeneity potentially stemming from differences in OSA severity inclusion/exclusion criteria. CPAP reduces these markers perhaps more profoundly with increasing OSA severity, but the evidence is scant. A better understanding of the mechanisms by which OSA influences CSF amyloid-β and tau concentrations could stem from evaluating the independent contributions of sleep fragmentation and hypoxia within individuals in paradigms using withdrawal of therapeutic CPAP. Large cross-sectional and longitudinal studies indicate OSA increases AD biomarker burden measured using PET imaging, but it is still not clear if treatment with CPAP can reverse these effects long-term. Table 1 summarizes the OSA and AD biomarker research referenced in this section.
Table 1:
Research reviewed involving OSA and AD biomarkers
| Authors | Study design and recruitment | Population | Age (years) | Sex (% female) | AD Biomarkers |
|---|---|---|---|---|---|
| Spira et al. 2014 | Cross-sectional, community volunteers | 5 MCI, 8 controls | MCI 75±11, controls 69±6 | MCI 20%, controls 62.5% | Aβ deposition (PET-PiB) |
| Bu et al. 2015 | Cross-sectional, sleep center | 45 OSAS, 49 controls | OSAS 44±10, controls 43±10, | OSAS 27%, controls 31% | Serum Aβ40/Aβ42 |
| Ju et al. 2016 | Cross-sectional, community volunteers | 10 OSA, 31 controls | OSA 56±4, control 53±6 | OSA 30%, controls 48% | CSF Aβ40, Aβ42, tau, P-tau181 |
| Liguori et al. 2017 | Cross-sectional, sleep center | 25 OSA, 10 OSA-CPAP, 15 controls | OSA 68±8, OSA-CPAP 67±7, controls 66±9 | OSA 32%, OSA-CPAP 30%, controls 40% | CSF Aβ42, P-tau, T-tau |
| Yun et al. 2017 | Cross-sectional, community volunteers | 19 OSA, 19 controls | OSA 57±4, controls 57±4 | OSA 53%, controls 53% | Aβ deposition (PET-PiB) |
| Elias et al. 2018 | Cross-sectional, war veterans | 42 OSA, 77 non-OSA | OSA 68±5, non-OSA 68±4 | No females | Aβ deposition (PET 18F-florbetaben, 18F-AV1451) |
| Motamedi et al. 2018 | Cross-sectional, military personnel | 28 mod-severe OSA, 22 mild OSA, 24 controls | Mod-severe OSA 36±8, mild OSA 34±8, controls 31±8 | Mod-severe OSA 0%, mild OSA 4% controls 8% | Serum Aβ40, Aβ42, Tau |
| Sharma et al. 2018 | Longitudinal, community volunteers | 35 mod-severe OSA, 76 mild OSA, 97 controls | Mod-severe OSA 71±8, mild OSA 69±7, controls 68±7 | Mod-severe OSA 51%, mild OSA 58%, controls 69% | CSF Aβ42, P-tau181, T-tau, Aβ deposition (PET-PiB) |
| Liguori et al. 2019 | Cross-sectional, community volunteers | 20 OSA, 20 AD, 15 controls | OSA 59±4, AD 66±4, controls 64±8 | OSA 30%, AD 65%, controls 47% | CSF Aβ40, Aβ42, P-tau, T-tau, |
| Handa et al. 2019 | Cross-sectional, tertiary hospital | 14 OSA | 65±10 | 29% | Aβ deposition (PET-PiB) |
| Bubu et al. 2019 | Longitudinal, community volunteers | 798 (103 OSA+) MCI, 325 (22 OSA+) AD, 516 (29 OSA+) cognitively normal | MCI 74(68,79) AD 76(71,80) cognitively normal 74(71,78) | MCI 40% (25% OSA+), AD 37% (27% OSA+), cognitively normal 49% (27% OSA+) | CSF Aβ42, P-tau181, T-tau, Florbetapir PET uptake |
| Ju et al. 2019 | Interventional, community volunteers | 18 (14) OSA-CPAP | 57±8 | 23% | CSF Aβ40, Aβ42, T-tau |
| Carvalho et al. 2020 | Cross-sectional, population based | 292 cognitively unimpaired (43 with “witnessed apneas”) | WA+ 73 (range 69–80), WA− 75 (range 69–82) | WA+ 23% WA− 75 38% | AV-1451 Tau-PET, Aβ deposition (PET-PiB) |
| André et al. 2020 | Cross-sectional, community volunteers | 96 SDB+, 31 SDB− | SDB+ 69±4, SDB− 69±4 | SDB+ 58%, SDB− 77% | Florbetapir PET uptake |
Aβ; amyloid-β; AD: Alzheimer’s disease; CPAP: continuous positive airway pressure; CSF: cerebrospinal fluid; OSA: obstructive sleep apnea; OSAS: obstructive sleep apnea syndrome; PET: positron emission tomography; WA: witnessed apnea. Median (SEM), Median [IQR]. Ages and percentages values are rounded from the source manuscript. Studies involving CPAP are highlighted in green.
Cognition
Deficits in the cognitive domains of attention, vigilance, memory and executive functioning during daytime testing are observed in those suffering from OSA (Bucks, Olaithe, and Eastwood 2013; Leng et al. 2017). However, evidence from longitudinal studies show disparate finding with some linking OSA to cognitive decline (Yaffe et al. 2011; Spira et al. 2014; Osorio et al. 2015; Blackwell et al. 2015) whilst others produce weak or null findings in this regard (Boland et al. 2002; Martin et al. 2015; Lutsey et al. 2016). Overall, the magnitude of impairment emerges to a greater degree in younger and middle aged OSA patients from clinical settings than in older healthy community samples (Cross et al. 2017).
Cross-sectional studies investigating middle age and older adults with OSA have demonstrated cognitive impairments in executive function (Bawden, Oliveira, and Caramelli 2011; Mathieu et al. 2008; Quan et al. 2006; Hrubos-Strom et al. 2012; Blackwell et al. 2011; Nikodemova et al. 2013; Salorio et al. 2002; Spira et al. 2008; Yesavage et al. 1985; Sharma et al. 2010), attention (Bawden, Oliveira, and Caramelli 2011; Mathieu et al. 2008; Naegele et al. 1995; Quan et al. 2006; Yesavage et al. 1985), reaction time and psychomotor vigilance testing (PVT) (Sharma et al. 2010; Kim, Dinges, and Young 2007; Alchanatis et al. 2008) and memory (Bawden, Oliveira, and Caramelli 2011; Kloepfer et al. 2009; Naëgelé et al. 2006; Nikodemova et al. 2013; Salorio et al. 2002; Sharma et al. 2010; Ju et al. 2012; Yesavage et al. 1985; Berry et al. 1990). In contrast other groups have found no significant association between OSA and measures of executive function, attention or memory (Hayward et al. 1992; Phillips et al. 1992; Foley et al. 2003; Sforza et al. 2010).
Proposed explanatory mechanisms for findings relating OSA to cognitive impairment include daytime sleepiness from sleep fragmentation and/or neurovascular damage as a result of intermittent hypoxia, although the contributions of each are not well established (Cedernaes et al. 2017). Indeed, some of the aforementioned studies find the severity of disease corresponds to degree of cognitive impairment, though invariably it depends on not only on the measure investigated but the method of measurement of both OSA and cognition (Gagnon et al. 2019; Gosselin et al. 2019). Some earlier research suggests the degree of sleep fragmentation results in deficits of attention and memory (Naegele et al. 1995; Roehrs et al. 1989) whilst severity of hypoxemia influences motor function, vigilance and executive function (Bliwise 1993; Bedard et al. 1991). In a large study of 743 people undergoing a clinical PSG study who had an AHI ≥ 5 and completed a pre-sleep PVT assessment, Kainulainen et al. (2020) demonstrated novel measures of hypoxemia (median saturation of peripheral oxygen (SpO2) dip and desaturation area) were significantly associated with increased risk of impaired evening PVT performance whilst all other measures of OSA severity and sleep fragmentation did not. However, there have been no recent or sufficiently large studies corroborating such delineations with memory or executive function.
A complicating factor in the assessment of cognitive outcomes in people with OSA is the issue of sleepiness which is purported to worsen cognitive performance (Gagnon et al. 2014; Steiropoulos, Galbiati, and Ferini-Strambi 2019). Mathieu et al. (2008) found sleepiness contributed to poorer cognitive performance only in younger (< 50 years) patients with OSA. A review of the literature (Zhou et al. 2016) concluded that EDS contributes to greater neurocognitive dysfunction in OSA but highlights the need to sufficiently examine neurocognitive impairments in OSA without sleepiness.
Overnight memory
Most studies investigating cognitive function in OSA assess general cognitive abilities during daytime neuropsychological test batteries or neurobehavioral vigilance rather than sleep related memory processing which involves encoding and recall separated by a period of sleep (Ahuja et al. 2018). In the small number of studies incorporating overnight memory assessments in OSA patients, there is demonstrated impairment in processing of declarative and procedural memory tasks compared to healthy controls (Kloepfer et al. 2009; Landry et al. 2014), but memory encoding and implicit motor memory formation appear intact (Cellini 2017). In a series of studies showing impaired overnight motor sequence task learning in moderately severe OSA, a negative relationship was observed between task performance and a polysomnographic (PSG) arousal index (Djonlagic et al. 2015, 2014), with the later study showing increasing age to be associated with less overnight improvements.
CPAP withdrawal is a way to observe the deleterious effect of OSA on overnight memory task. A study by our group found the benefits of overnight sleep for improvements on a spatial navigation task are attenuated when OSA is induced by selective CPAP withdrawal during REM sleep (Varga et al. 2014).
Changes in cognition with CPAP treatment
CPAP treatment results in variable changes to cognition (Kielb et al. 2012), with the greatest effect seen for attention (Kylstra et al. 2013; Pan et al. 2015) in younger and middle age adults with more severe SDB and sleepiness (Zhou et al. 2016).
In the largest randomized controlled trial (RCT) to date, 6 months of CPAP treatment was associated with transient improvements (at 2 months only) in attention and executive functioning, with greater effects on a sustained working memory task at 6 months in those with higher baseline daytime sleepiness (ESS > 10) (Kushida et al. 2012). A summary of the existing RCTs shows both short and long-term CPAP treatment improves some of the deficits associated with OSA in middle-aged adults (Canessa et al. 2011; Castronovo et al. 2014; Ferini-Strambi et al. 2003). Alternatively, Saunamaki et al. (2010) did not observe any improvements in executive or visuospatial functioning, even after long-term CPAP treatment in a group of 17 men. In older adults, similar observations are made with large multi-center trials (Martinez-Garcia et al. 2015; McMillan et al. 2014) showing minimal or null effects of CPAP on cognition. However, a lack of treatment related outcomes could be explained by low CPAP adherence levels at least in the study by McMillan et al. (2014). In smaller, single center trials, improvements in attention, psychomotor speed, memory and executive function are observed particularly in those with higher CPAP usage (Aloia et al. 2003; Dalmases et al. 2015).
In an observational study of moderate- severe OSA, Antic et al. (2011) found a treatment dose-response effect for CPAP in terms of Epworth Sleepiness Scale (ESS) scores. Moreover, whilst a greater number of patients showed a normalization of sleepiness and neurobehavioral function with longer nightly durations of CPAP, a substantial number did not. In a large cross-sectional study, patients with mild-moderate OSA, Jackson et al. (2018) did not observe a reversal of neurocognitive impairments after 3 months of CPAP therapy, even with adequate CPAP use. Bhat et al. (2018) assessed changes in subjective sleepiness (via ESS) and objective vigilance (via PVT) after at least 1 month of CPAP therapy and found that although significantly less sleepiness was reported irrespective of OSA severity, improvements in vigilance measured using PVT was only observed in those with severe OSA before treatment.
Studies investigating OSA treatment and overnight memory consolidation find longer-term use, but not a single night of CPAP therapy, augment offline sleep-mediated motor memory consolidation (Djonlagic et al. 2015; Landry et al. 2016).
By looking at age of onset of MCI in subjects with self-reported OSA and use of CPAP for treatment in the Alzheimer’s Disease Neuroimaging Initiative, we observed that cognitively normal older individuals with self-reported untreated OSA had an average age of onset of MCI that was 11 years earlier than those subjects without OSA (72 year vs 83 years), while those individuals with self-reported CPAP treatment had an average age of MCI onset that was similar to those without OSA at all (82 years) (Osorio et al. 2015).
Treatment in MCI/AD with OSA
There is some evidence that CPAP treatment in those already suffering from cognitive impairments can improve symptoms or delay further cognitive decline. Chong et al. (2006: author) saw a reduction in sleepiness after 3 and 6 weeks of treatment in patients with mild to moderate AD after randomization to CPAP therapy but not after using a sham device. A pilot study investigating rate of cognitive decline in patients with moderate AD and severe OSA found CPAP-treated individuals had a significantly slower cognitive decline (Mini Mental State Exam (MMSE) score changes) compared to untreated individuals over three years (Troussiere et al. 2014). A recent quasi- experimental study investigating MCI and OSA found significant improvements in psychomotor/cognitive processing speed in CPAP adherent patients after 1 year compared to a non-adherent control group (Richards et al. 2019). They also observed improvements in memory, sleepiness and daily functioning suggesting CPAP may slow the progression of dementia.
Summary
Overall, more consistent findings of cognitive impairment are seen in young and middle-aged patients with OSA rather than in elderly community dwelling cohorts with findings likely driven by treatment-seeking individuals (Bubu et al. 2020). CPAP may be effective in improving cognition, particularly in the presence of sleepiness and given good treatment adherence. CPAP treatment also shows promise for delaying further cognitive decline in those with MCI and AD, but more cognitive assessments where encoding and recall are separated by a period of sleep are needed to understand OSA-related impaired cognition within individuals. Table 2 summarizes the OSA and cognition research referenced in this section.
Table 2:
Research reviewed involved OSA and cognition.
| Authors | Study design and recruitment | Population | Age (years) | Sex (% female) | Cognitive domain or task |
|---|---|---|---|---|---|
| Yesavage et al. 1985 | Cross sectional, sleep clinic | 41 OSA | 70±7 | No females | Attention, executive function, memory |
| Berry et al. 1990 | Cross sectional, sleep clinic | 8 OSAS, 12 controls | OSAS 69±5, controls 68±2 | No females | Memory |
| Bedard et al. 1991 | Cross sectional, sleep clinic | 20 mod-severe OSAS | 52±9 | Not reported | Reaction time, vigilance |
| Hayward et al. 1992 | Cross sectional, retirement village | 96 non-demented elderly | 78±4 | 22% | Attention, executive function, memory |
| Phillips et al. 1992 | Cross sectional, community volunteer | 92 healthy adults | 64±9 | 52% | Attention, executive function, memory |
| Naëgelé et al. 1995 | Cross sectional, clinic & community volunteers | 17 OSA, 17 controls | OSA 49±3, controls 49±3 | No females | Attention, memory |
| Boland et al. 2002 | Cross sectional, population based | 1700 SHHS cohort | 62 (range 52–75) | 54% | Attention, executive function, memory |
| Salorio et al. 2002 | Cross sectional, clinic & community volunteers | 28 OSA, 24 controls | OSA 44±8, controls 44±9 | OSA 29%, controls 42% | Memory, executive function |
| Foley et al. 2003 | Cross sectional, community volunteer | 718 OSA | Range 79–97 | No females | Attention, executive function, memory |
| Ferini-Strambi et al. 2003 | RCT, sleep clinic | 23 OSA (CPAP+ 15 days), 23 controls | OSA 57±6, controls 56±5 | OSA 9%, controls 17% | Processing speed, executive function |
| Aloia et al. 2003 | RCT, sleep clinic | 12 OSA (6 CPAP+) | 65±6 | Not reported | Attention, executive function, memory |
| Quan et al. 2006 | Cross sectional, population based | 67 OSA, 74 controls | OSA 59±9, controls 57±9 | OSA 33%, controls 47% | Attention, vigilance, executive function, memory |
| Naëgelé et al. 2006 | Cross sectional, clinic & community volunteers | 95 OSA, 95 controls | OSA 46±11, controls 46±8 | OSA 21%, controls 27% | Memory |
| Chong et al. 2006 | RCT, clinic | AD with OSA: 19 (CPAP+ 3 weeks), 20 (Sham CPAP) | 78±7 | CPAP 76%, Sham 75% | Sleepiness |
| Kim et al. 2007 | Cross sectional, population based | 611 Wisconsin cohort | 53±8 | 43% | Vigilance |
| Mathieu et al. 2008 | Cross sectional, sleep clinic | 14 young & 14 older OSAS, 12 young & 18 older controls | Young 38±2 & older 62±2 OSAS, young 39±2 & older 63±2 controls | Young 7% & older 7% OSAS, young 17% & older 11% controls | Attention, executive function, memory |
| Spira et al. 2008 | Cross sectional, community volunteer | 57 OSA, 391 controls | OSA 84±4, controls 83±3 | 100% | Executive function |
| Alchanatis et al. 2008 | Cross sectional, sleep clinic | 58 OSAS, 41 controls | OSA 49 (range 32–65), controls 49 (range 33–63) | Not reported | Attention/alertness |
| Kloepfer et al. 2009 | Cross sectional, clinic & community volunteers | 15 OSA, 20 controls | OSA 46±6, controls 47±6 | OSA 33%, controls 40% | Memory |
| Sharma et al. 2010 | Cross sectional, clinic & community volunteers | 50 severe OSA, 25 controls | OSA 43±8, controls 46±6 | OSA 16%, controls 16% | Alertness, memory executive function |
| Sforza et al. 2010 | Cross sectional, community volunteer | 445 OSA, 382 controls | 68±2 | OSA 49%, controls 69% | Attention, executive function, memory |
| Saunamäki et al. 2010 | RCT, sleep clinic | 20 OSA, 17 controls | OSA 50 (range 37–65), controls 44 (range 30–63) | No females | Memory, verbal fluency |
| Bawden et al 2011 | Cross sectional, clinic & community volunteers | 17 OSA, 20 controls | OSA 41±13, controls 28±9 | Not reported | Attention, memory executive function |
| Blackwell et al. 2011 | Cross sectional, population based | 2909 older men | 76±6 | No females | Executive function |
| Antic et al. 2011 | Cross sectional, sleep clinic | 174 mod-severe OSA | 50±12 | 25% | Sleepiness |
| Hrubos-Strom et al. 2012 | Cross sectional, community volunteer | 290 mod-severe OSA | 48±11 | 44% | Memory, executive function |
| Ju et al. 2012 | Cross sectional, sleep clinic | 42 OSA, 21 controls | OSA 68±4, controls 69±6 | OSA 38%, controls 48% | Executive function |
| Kushida et al. 2012 | RCT, sleep clinic | 556 OSA (CPAP+ 6 months), 542 OSA (Sham CPAP) | CPAP 52±12, Sham 51±12 | CPAP 35%, Sham 34% | Attention, vigilance, memory |
| Nikodemova et al. 2013 | Cross sectional, community volunteer | 755 Wisconsin cohort | 54 (range 30–81) | 38% | Memory, executive function |
| Landry et al. 2014 | Cross sectional, sleep clinic | 12 OSA, 12 controls | OSA 53±2, controls 53±2 | OSA 25%, controls 25% | Overnight motor memory, sleepiness |
| Djonlagic et al. 2014 | Cross sectional, sleep clinic | 20 OSA, 20 controls | OSA 41±1, controls 35±3 | Not reported | Overnight motor memory, vigilance |
| Varga et al. 2014 | Interventional, sleep clinic | 18 severe OSA (CPAP compliant 2+ months) | 54 | 22% | Overnight spatial memory, vigilance |
| McMillan et al. 2014 | RCT, sleep clinic | 278 OSA (140 CPAP+ 3,12 months) | OSA-CPAP 71±5, OSA 71±5 | OSA-CPAP 14%, OSA 21% | Sleepiness, global cognition |
| Troussière et al. 2014 | Cross sectional, memory clinic | AD with severe OSA 14(CPAP+ >3 months), 9 (no CPAP) | CPAP+ 73 (range 68–80) CPAP− 78 (range 75–79) | CPAP+ 73 (range 68–80) CPAP− 78 (range 75–79) | Global cognition |
| Blackwell et al. 2015 | Longitudinal, community volunteers | 1132 OSA, 1504 controls | OSA76±5, controls 76±5 | No females | Global cognition |
| Martin et al. 2015 | Longitudinal, population based | 599 OSA | 67±1 | 56% | Global cognition |
| Djonlagic et al. 2015 | Cross sectional, sleep clinic | 29 OSA (14 CPAP+ 1 night), 15 controls | OSA 43±13, CPAP 44±11, controls 37±11 | Not reported | Overnight motor memory, vigilance |
| Martínez-García et al. 2015 | RCT, sleep clinic | 115 (CPAP+ 3 months), 109 (no CPAP) | OSA-CPAP 75±4, OSA 76±4 | OSA-CPAP 36%, OSA 27% | Sleepiness, executive function |
| Dalmases et al. 2015 | RCT, sleep clinic | Severe OSA 17 CPAP+ 3 months, 16 no CPAP | OSA-CPAP 71±5, OSA 72±6 | OSA-CPAP 35%, OSA 25% | Memory, executive function |
| Lutsey et al. 2016 | Longitudinal, population based | 445 OSA, 521 controls | OSA 62±5, controls 61±5 | OSA 39%, controls 67% | Global cognition |
| Landry et al 2016 | Cross sectional, sleep clinics | 24 OSA, 13 CPAP+ 1night, 17 CPAP+ >6 weeks, 14 controls | OSA 47±2, CPAP 1night 48±2, CPAP compliant 49±2, Controls 47±3 | OSA 17%, CPAP 1night 38%, CPAP compliant 24%, Controls 57% | Overnight motor memory, sleepiness, vigilance |
| Jackson et al. 2018 | Cross sectional, clinic & community volunteers | 110 OSA (88 CPAP+ 3 months), 31 controls | OSA 47±1, CPAP 46±1, controls 48±2 | OSA 20%, CPAP 18%, controls 26% | Sleepiness, vigilance, executive function |
| Bhat et al. 2018 | Cross sectional, sleep clinic | 92 mild-mod & 90 severe OSA (CPAP+ >1 month) | Mild-mod OSA 50±12, severe 53±13 | 27% | Sleepiness, fatigue, vigilance |
| Gagnon et al. 2019 | Observational, community volunteers | 57 OSA, 54 mild/non OSA | OSA 63±6, non OSA 65±7 | OSA 55%, non OSA 62% | Subjective and objective cognition |
| Richards et al. 2019 | RCT, clinic & community volunteers | 54 MCI with OSA (29 CPAP+ 6,12 months) | OSA-CPAP 67±7, OSA 73±9 | OSA-CPAP 31%, OSA 60% | Global cognition, vigilance, memory |
| Kainulainen et al. 2020 | Observational, sleep clinic | 734 OSA | 57 (IQR 46–67) | 41% | Vigilance |
AD: Alzheimer’s disease; CPAP: continuous positive airway pressure; OSA: obstructive sleep apnea; OSAS: obstructive sleep apnea syndrome; RCT: randomized controlled trial; SHHS: sleep heart health study. Median (SEM), Median [IQR]. Ages and percentages values are rounded from the source manuscript. Studies involving CPAP are highlighted in green.
Neuroanatomical changes in OSA
OSA is clinically characterized by intermittent hypoxia and chronic sleep fragmentation, and both are proposed to alter normal brain morphology. OSA-related hypoxemia has been thought to be associated with an increase in sympathetic vasoconstriction and a concurrent decrease in vascular protective mechanisms thereby leading to changes in structure and function of blood vessels (Zimmerman and Aloia 2006). In addition, effects of OSA-related sleep fragmentation on brain structure and function can be inferred from studies on chronic and acute sleep deprivation. It has been suggested that sleep loss, either independently or as part of a comorbid condition such as OSA or other neurological disorders, is associated with increased atrophy within the frontal, temporal and parietal regions (Sexton et al. 2014). While delineating the relative contribution of intermittent hypoxia and sleep fragmentation to the neuroanatomical changes seen in OSA is challenging, neuroimaging tools can nevertheless provide a global picture of abnormal brain structure in OSA patients.
A growing body of studies utilizing neuroimaging tools have identified neuroanatomical abnormalities that coexist with OSA. Using voxel based morphometry (VBM), several studies identified gray matter (GM) density reduction in frontal, parietal, temporal, and hippocampal regions in patients with OSA (Macey et al. 2002; Canessa et al. 2011; Morrell et al. 2003; Gale and Hopkins 2004; Yaouhi et al. 2009). In these studies, the hippocampus and fronto-parietal cortices are noted to be the most sensitive to GM loss in OSA (Canessa et al. 2011). Care must be taken however, when interpreting observed changes in gray matter volume based on T1-weighted MR measurements. It has been observed that myelin or iron content rather than neurodegeneration (Lambert et al. 2013) may result in GM changes. As a result, it is likely that these GM changes are explained in part due to the tissue’s physical properties rather than neuronal loss. Either acquisition methods that go beyond T1-weighted imaging or analyses methods that supplement the T1-weighted imaging modality would help to better assess gray matter changes in OSA.
In addition to GM, white matter (WM) abnormalities are also seen in patients with OSA. Cross et al. (2018) and colleagues observed that reduced cortical thickness in the bilateral temporal lobes, evaluated using surface-based techniques as opposed to voxel-based, was associated with oxygen desaturation in OSA patients. Diffusion Tensor Imaging (DTI) studies revealed WM lesions in OSA to be more pronounced in the cortices, limbic system, pons and cerebellum tracts (Macey et al. 2008). Fractional anisotropy (FA) measures are routinely measured in DTI studies. The FA values measure directionality of water movement in brain tissue and can be used to infer the integrity of brain tissue. Using this technique, a recent study revealed a loss of WM integrity and structural connectivity in a largely mild-moderate OSA population (Lee, Yun, et al. 2019). Of note, at least some studies have suggested OSA might be associated with gray matter hypertrophy, rather than atrophy (Rosenzweig et al. 2013; Baril et al. 2017; Andre et al. 2020), a phenomenon that could represent underlying inflammation/edema early in the development of OSA before atrophic changes take place.
In addition to MRI and DTI studies, other modalities of neuroimaging have also been used to study the neuroanatomical changes seen in OSA. Magnetic resonance spectroscopy (MRS) has been widely used to non-invasively study biochemical changes in brain. MRS studies in OSA suggest metabolite profiles in the hippocampus (Bartlett et al. 2004) and anterior cingulate gyrus (Duffy et al. 2016) are related to disease severity and neurocognitive impairment. Other observations include significantly decreased levels of N-acetylaspartate (NAA), a marker or neuronal injury, and increased levels of the excitatory neurotransmitter glutamate in limbic regions including the hippocampus, thalamus, putamen and midbrain (Macey et al. 2017).
Changes in brain structure with CPAP treatment
In moderate to severe OSA, Rosenzweig et al. (2016) found improvements in sleepiness after 1 month of CPAP treatment that correlated with neuroplastic brain changes. These changes corresponded to improvement in attentional regulation and working memory capacity compared to a ‘wait list’ control condition (patients randomly allocated to group, stratified for OSA severity, age and level of education).
Treatment of OSA with CPAP is known to improve intermittent hypoxia and sleep fragmentation. As such it is plausible to hypothesize that CPAP treatment would also result in neuroanatomical changes that may reach levels seen in non-OSA subjects. Rosenzweig et al. (2015) found that one month of CPAP along with lifestyle modifications and psychoeducation induced hypertrophic changes in the bilateral thalamus. Canessa et al. (2011) showed that three month of CPAP treatment was associated with increases in GM volume within the hippocampus and frontal structures. On the other hand, O’Donoghue et al. (2005) did not observe any changes in GM density or regional volumes after 6 months of CPAP treatment. Notably, they also did not observe any changes in GM density or volumes at baseline, i.e., before treatment when compared to healthy controls. Subsequent commentaries reveal that the source of the discrepancies between results of the study by O’Donoghue et al. (2005) and that of other groups might be due to the methodologic differences and small sample sizes (Zimmerman and Aloia 2006). However, average nightly CPAP use is equally likely to be the source of the discrepancies between the results of these studies.
In addition to GM volume changes seen with CPAP treatment, normalization of WM changes seen in OSA has also been reported. Castronovo et al. (2014) evaluated a group of seventeen treated OSA subjects at baseline and after treatment at 3- and 12-month follow-ups. They reported a normalization of WM fiber integrity using DTI based FA measures after 12 months, with the 3-month follow up showing only limited changes. This study and those by others highlight another important aspect that is often observed in OSA studies looking at treatment effects, i.e. 3 months of CPAP treatment may not be enough to reverse presumed adverse effects of OSA. Using dynamic susceptibility contrast imaging (a sub-type of DTI), Maresky et al. (2019) observed changes in whole brain FA, mean diffusivity and brain perfusion. The FA and mean diffusivity changes imply improvements in the brain’s microstructural integrity. Moreover, CPAP treatment was observed to improve brain perfusion measured by quantifying cerebral blood flow and cerebral blood volume.
Summary
Advances in neuroimaging have enabled observation of significant neuroanatomical changes in OSA patients. Notably, these changes parallel those seen in healthy aging (Veasey 2012). Whether the progression of these neuroanatomical changes in healthy elderly is accelerated in presence of OSA or other comorbid neurological disorders remains to be tested. CPAP treatment is shown to induce neuroanatomical changes in treatment naïve OSA patients with indication of a presumed cumulative benefit from long-term CPAP use. However, several questions remain to be addressed given that there are various sources of variability that could influence the results namely methodology, severity and chronicity of disease, as well as nightly CPAP adherence. Table 3 summarizes the OSA and neuroanatomy research referenced in this section.
Table 3:
Research reviewed involving OSA and neuroanatomy.
| Authors | Study design and recruitment | Population | Age (years) | Sex (% female) | Neuroimaging approach |
|---|---|---|---|---|---|
| Macey et al. 2002 | Cross-sectional, sleep clinic | 21 OSA, 21 controls | OSA 49±11, controls 47±11 | No females | T1w-VBM |
| Morrell et al. 2003 | Cross-sectional, sleep clinic | 7 OSA, 7 controls | 50 (range 28–65) | No females | T1w-VBM |
| Bartlett et al. 2004 | Cross-sectional, sleep clinic | 8 OSA, 5 controls | OSA 47[16], controls 52[16] | No females | MRS |
| Gale & Hopkins 2004 | Cross-sectional, tertiary hospital | 14 OSA, 20 carbon monoxide (CO) poisoning | OSA 52±11, CO controls 38±7 | OSA 14%, CO controls 45% | T1w-VBM |
| O’Donoghue et al. 2005 | Interventional, sleep clinic | 27 severe OSA (23 CPAP+ 6 months), 24 controls | OSA 46±10, controls 43±9 | No females | T1w-VBM |
| Macey et al. 2008 | Cross-sectional, sleep clinic | 41 OSA, 69 controls | OSA 46±9, controls 48±9 | OSA 21%, controls 57% | DTI |
| Yaouhi et al. 2009 | Cross-sectional, sleep clinic | 16 OSA, 14 controls | OSA 55±6, controls 53±7 | OSA 6%, controls 7% | T1w-VBM, 18FDG-PET |
| Canessa et al. 2011 | Interventional, sleep clinic | 17 severe OSA (16 CPAP+ 3 months), 15 controls | Severe OSA 44±8, controls 42±7 | No females | T1w-VBM |
| Rosenzweig et al. 2013 | Cross-sectional, sleep clinic | 32 OSA, 32 controls | OSA 49±13, controls 50±12 | OSA=controls | T1w-VBM |
| Castronovo et al. 2014 | Interventional, sleep clinic | 13 OSA (CPAP+ 3 & 12 months), 15 controls | OSA 43±8, controls 42±7 | No females | DTI |
| Rosenzweig et al. 2016 | Cross-sectional, sleep clinic | 55 mod-severe OSA (28 CPAP+ 1 month), 35 controls | OSA 48±11, controls 42(2.1) | OSA 7%, Controls 20% | T1w-VBM |
| Duffy et al. 2016 | Cross-sectional, aging clinic | 24 at-risk dementia | 68±6 | 71% | MRS |
| Baril et al. 2017 | Cross-sectional, sleep clinic | 18 severe, 11 moderate, 30 mild OSA, 12 controls | Severe 66±6, moderate 66±5, mild OSA 66±6, controls 62±5 | Severe 17%, moderate 9%, mild OSA 33%, controls 18% | Volumes (voxel & surface based) |
| Macey et al. 2017 | Cross-sectional, sleep clinic | 14 OSA, 26 controls | OSA 47±10, controls 50±9 | OSA 36% Controls 46% | MRS |
| Cross et al. 2018 | Cross-sectional, aging clinic | 83 at-risk dementia | 67±8 | 64% | Volumes (voxel & surface based) |
| Lee et al. 2019 | Cross-sectional, community volunteers | 135 OSA, 165 controls | OSA 59±6, controls 58±6 | OSA 71% Controls 72% | DTI |
| Maresky et al 2019 | Interventional, sleep clinic | 7 OSA | 51±13 | 14% | DTI |
| André et al. 2020 | Cross-sectional, community volunteers | 96 SDB+, 31 SDB− | SDB+ 69±4, SDB− 69±4 | SDB+ 58%, SDB− 775 | T1w-VBM |
CO- carbon monoxide; CPAP: continuous positive airway pressure; CSF- cerebrospinal fluid; DTI- diffusion tensor imaging; FA - fractional anisotropy; OSA: obstructive sleep apnea; T1w-VBM - voxel-based morphometry; 18FDG-PET – Fluorodeoxyglucose Positron emission tomography. Mean ± standard dev. Median (SEM), Median [IQR]. Ages and percentages values are rounded from the source manuscript. Studies involving CPAP are highlighted in green.
Sleep disruption and markers of AD
Sleep disruption can be described in many ways including poor-quality, short duration and/or fragmented sleep. Varied approaches to sleep measurement, such as subjective reporting via questionnaires (Sprecher et al. 2015) and objective assessment via Actigraphy, where sleep is inferred by an absence of movement, are shown to be associated with CSF markers of AD (Ju et al. 2013; Lim et al. 2013; Musiek et al. 2018).
EEG is a powerful tool to investigate intra-individual stability and inter-individual variation in sleep/wake biology and behavior (Buckelmuller et al. 2006) as it displays fingerprint-like trait characteristics (De Gennaro et al. 2008). Scalp EEG is a low cost, non-invasive measure of brain activity with high temporal resolution widely used in diverse settings to determine sleep/wake state, diagnose disease and assess response to treatment (Jobert et al. 2012).
EEG measures as correlates of AD
Quantitative electroencephalographic (qEEG) measures during wakefulness in AD show a slowing of the dominant occipital rhythms compared to age-matched controls (Petit et al. 2004; Dauwels, Vialatte, and Cichocki 2010), and a combination of EEG markers have been shown to predict conversion from MCI to AD (Poil et al. 2013). Specifically, EEG theta activity during wakefulness has been observed to correlate positively with CSF T-tau, P-tau and cognitive decline (Stomrud et al. 2010). Some researchers suggest that EEG measures during sleep, particularly REM sleep, are more sensitive than wake measures in detecting EEG slowing indicative of progressing dementia (Petit et al. 1993; Brayet et al. 2016). One explanation offered is that the cholinergic neurons of the basal forebrain, known to degenerate early in AD, are more active during REM than during wake (Petit et al. 2017).
During PSG analysis, EEG components are visually inspected to determine sleep architecture measures in terms of sleep macrostructure, i.e. duration of non-REM stages N1, N2 and N3 (Slow Wave Sleep), rapid eye movement (REM) sleep and wake. Sleep EEG microstructure refers to the background rhythms and transient events that comprise the EEG signal. Slow waves (SWs), K-complexes (KCs) and sleep spindles are key EEG microstructure events used in visual sleep scoring to define and differentiate the three stages of NREM sleep. EEG microstructure oscillations can be automatically quantified using spectral and/or event-based analysis techniques and provide a more nuanced understanding of sleep neurophysiology (Younes 2017). SWs and slow wave activity (SWA) are in the frequency range of ~0.3 to 4 Hz and encapsulate both slow oscillations (SOs) of < 1 Hz and delta band activity between ~1–4 Hz (Achermann and Borbely 1997; Rasch and Born 2013). It may be important to functionally differentiate slow oscillations from delta activity as they have been shown to have differing impacts on both memory (in rodents) (Kim, Gulati, and Ganguly 2019) and cortical amyloid deposition (Mander et al. 2015). Slow wave sleep (SWS) and SWA during NREM sleep are putative markers of sleep homeostasis (Borbely 1982; Borbely et al. 1981) and are observed to attenuate with age (Carrier et al. 2001; Ohayon et al. 2004) but less so in women (Carrier et al. 2011).
High-density EEG and neuroimaging studies investigating the spatiotemporal dynamics of the slow oscillation find each wave originates at a definite (mostly prefrontal) site and travels in an anteroposterior direction across the scalp (Massimini et al. 2004; Nir et al. 2011), overlapping with neuroanatomical structures of the default mode network (DMN) (Dang-Vu et al. 2008; Murphy et al. 2009). Slow oscillations, and associated cortical neuronal activities, cycle between depolarization (active state of intense firing) and hyperpolarization (silent state), and are thought to group or coordinate other NREM oscillations such as delta waves and spindles (Steriade 2006; Molle et al. 2002; Staresina et al. 2015; Mak-McCully et al. 2017).
In cognitively normal individuals, EEG microstructure changes during sleep are associated with biomarkers of AD and measures of cognition and memory. NREM SWA has been shown to negatively correlate with CSF amyloid-β levels (Varga, Wohlleber, et al. 2016; Ju et al. 2017), amyloid-β burden within medial prefrontal cortex (mPFC) (Mander et al. 2015) and prefrontal cortical atrophy (Mander et al. 2013; Varga, Ducca, et al. 2016). Recently Lucey et al. (2019) found SWA, particularly in the < 2 Hz spectral frequency range, to be negatively associated with AD pathology according to both PET imaging and CSF measures. Moverover, impairments in declarative (Mander et al. 2013; Mander et al. 2015) and spatial memory transformation (Varga, Ducca, et al. 2016) have been shown to negatively correlate with SWA in older adults.
Sleep spindles are defined as waxing-and-waning oscillations of sigma frequency (~11–16 Hz) activity lasting around 0.5–3 seconds (De Gennaro and Ferrara 2003) that have a proposed role in intelligence (Fogel and Smith 2011), learning (Gais et al. 2002; Kam, Pettibone, et al. 2019), memory (Schabus et al. 2004) and sleep stability (Dang-Vu et al. 2010). Along with SOs and K-complexes they are also involved in cortical activity related to arousal stimuli (Halasz et al. 2014) from both the external environment and within the central nervous system. Using intra-cranial EEG and single unit neuronal recordings during preoperative localization of epileptic foci in humans, Andrillon et al. (2011) recorded the presence of two distinct spindles: slow (9–12 Hz) and fast (12–15 Hz) spindles with different topographical distributions over the scalp. Fast spindles dominated centro-parietal regions, while slow spindles dominated frontal areas during SWS. Neuroimaging, pharmacological and memory studies also support the existence of two topographically and functionally distinct spindles (Ayoub et al. 2013; Schabus et al. 2007; Barakat et al. 2011; Molle et al. 2011). Furthermore, spindle frequency activity (SFA) is reduced in middle-aged and older adults, relative to younger adults (Dijk, Beersma, and van den Hoofdakker 1989; Landolt et al. 1996; De Gennaro and Ferrara 2003). This age-related reduction in SFA is related to impairments in the number of sleep spindles generated (density per unit time) and other morphological features such as amplitude and duration. Spindle density shows more marked reductions frontally with age (De Gennaro and Ferrara 2003) whilst spindle durations become shorter, most noticeably in parietal regions (Martin et al. 2013). Evidence of sexual dimorphism in spindle activity is widely reported with females showing higher sleep spindle densities compared to men (Gaillard and Blois 1981; Huupponen et al. 2002; Purcell et al. 2017).
There is evidence from scalp EEG that fast spindles are synchronized to the depolarizing SO up-state and slow spindles to the transition towards the down-state (Molle and Born 2011; Klinzing et al. 2016). A recent study demonstrates less slow oscillation-spindle coupling to be associated with greater temporal lobe tau burden but not with amyloid-β burden, which was independently associated with < 1 Hz SWA (Winer et al. 2019). This is interesting in the context of recent work from our group showing fast spindle density and duration are inversely related to T-tau levels. In this work, spindle activity from a central EEG derivation was negatively correlated with both CSF measures of amyloid-β and tau, and with cognition (Kam, Parekh, et al. 2019).
Inventive applications of quantitative EEG analysis methodologies are being used to interpret sleep EEG changes in the context of brain health. For example, Latreille et al. (2019) demonstrate age-related reductions of EEG delta power during REM and NREM are mediated by thinning of the medial frontal and anterior cingulate cortices. Sun et al. (2019) demonstrate that sleep EEG has extractable features that can be identified by machine learning to reasonably predict brain age.
EEG features characteristic of non-REM sleep are seen to degrade with progressing MCI/AD beyond that of normal aging. Spontaneous K-complexes (De Gennaro et al. 2017) and fast sleep spindles show a gradually decreasing density in MCI and AD (Rauchs et al. 2008; Gorgoni et al. 2016). Furthermore, a study using auditory evoked K-complexes during sleep showed a decreased density and amplitude in AD, whilst greater dementia severity was associated with a lower probability of eliciting a K-complex (Crowley et al. 2005). A recent study demonstrated age-related cognitive impairment was related to a reduction in delta, theta and sigma power during NREM sleep in patients with subjective cognitive complaints or MCI (N=29) compared to controls (N=29). One-year follow-up of these patients showed changes in spindle characteristics (amplitude, duration, density or frequency) to be associated most strongly with cognitive decline (Taillard et al. 2019). In contrast, a larger analysis of older women found those who developed mild cognitive impairment or dementia (N=85) after 5 years had increased theta and sigma EEG power density during both REM and NREM sleep at baseline compared to those women who did not deteriorate (N=85) (Djonlagic et al. 2019). Possible reasons for the differing direction of findings relating to theta and sigma frequencies in these two studies include the differences between groups regarding medication usage and inclusion of males and females in the Taillard et al. (2019) study compared to the balanced group medication usage and older, exclusively women studied in the analysis by Djonlagic et al. (2019). It is worth noting that in the study by Taillard et al. (2019) approximately two thirds of participants met diagnostic criteria for OSA which may influence EEG measures as discussed in the next section.
Cyclic alternating pattern (CAP) analysis is a useful way to quantify EEG arousal and sleep instability phenomena. Briefly, the analysis identifies alternating sequences of cortical activation (phase A) followed by periods of deactivation (phase B). The phase A subtype A1-A3 events are described according to the proportion of event duration dominated by either high-voltage slow waves (EEG synchrony) or low-amplitude fast rhythms (EEG desynchrony) and include characteristic sleep EEG oscillations and transient events (Terzano et al. 1996; Parrino and Terzano 2017). CAP phases subtype A1 often contain SWs, K-complexes and spindles, and subtypes A2 and A3 are akin to AASM defined microarousal events (Parrino et al. 2012). Using CAP analysis, (Carnicelli et al. 2019) observed a decrease in A1 (SWs and K-complexes) and A2 events (starting with a SW and finishing in arousal) during NREM 2 sleep in those with MCI who converted to AD.
EEG in OSA, correlates of AD markers?
Changes in EEG activity are reported in OSA during both wake and sleep states, and are reviewed comprehensively in D’Rozario et al. (2017). In a similar way to findings reported in those with cognitive decline, EEG slowing during wakefulness is observed in OSA compared to controls (Morisson et al. 1998; Morisson et al. 2001; Mathieu et al. 2007; Greneche, Krieger, et al. 2008; Xiromeritis et al. 2011). Furthermore, increased EEG slowing is related to worse daytime symptoms, such as sleepiness (Lee et al. 2012), abnormal event related potentials (ERPs) during attention (Baril et al. 2013), and impaired PVT and driving simulator performance (D’Rozario et al. 2013; Wang et al. 2015). Whilst some investigations have found EEG slowing during wakefulness is associated with worsening OSA severity (increased arousal index, AHI or hypoxia) (Mathieu et al. 2007; Greneche, Saremi, et al. 2008), others have not observed the magnitude of EEG slowing to correspond to OSA severity using current diagnostic measures (Sforza et al. 2002).
Early investigations using qEEG analysis in OSA also found increased EEG slowing during REM sleep compared to controls (Morisson et al. 1998; Morisson et al. 2001) but these included a small number of individuals and only short segments of EEG. However, these original findings have since been replicated in a large study examining ambulatory qEEG measures in 664 men (> 40 years), demonstrating a positive association between OSA severity and both the EEG slowing ratio and EEG power in all frequency bands during REM (Appleton et al. 2019). Importantly, an earlier study by the same authors found greater EEG slowing (delta power) in REM sleep to predict worse simulated driving performance in 76 people with a range of OSA severity (Vakulin et al. 2016). In a small study of 8 males with moderate-severe OSA undergoing an extended wakefulness protocol, greater EEG slowing during REM sleep at baseline was associated with slower PVT reaction times, more PVT lapses, and more AusEd crashes after 24hrs awake. Across the same time period, decreased spindle density in NREM sleep was also associated with slower PVT reaction times (Mullins et al. 2020).
Most research quantifying EEG microstructure during NREM sleep in those with OSA shows reduced SWA compared to controls (Guilleminault et al. 2001; Saunamaki et al. 2009; Jones et al. 2014; Ju et al. 2016), or a slower dissipation of SWA across the sleep period (Ondze et al. 2003; Himanen, Joutsen, and Virkkala 2004). Given more SWS/SWA is associated with better next-day performance both in healthy younger (Jurado, Luna-Villegas, and Buela-Casal 1989) and older subjects (Anderson and Horne 2003) it is not surprising SWS duration and delta power during NREM sleep also predicts poorer driving simulator performance in OSA (Vakulin et al. 2016). Of note, only one small study has examined the relationship between SWA and CSF amyloid-β in OSA (Ju et al. 2016). In this study, NREM SWA was negatively correlated with CSF amyloid-β levels in controls but not in those with OSA.
Studies using CAP analysis in people with sleep-disordered breathing find CAP subtype A2 and A3 rates and durations are higher compared to controls in: OSA (Terzano et al. 1996), upper airway resistance syndrome (Guilleminault et al. 2007), and in OSA with excessive daytime sleepiness (EDS) compared to those without sleepiness (Korkmaz et al. 2018). The extent to which any changes in respiratory-related EEG microstructure events could influence measures of spectral power during NREM is not often acknowledged in investigations reporting EEG changes in those with AD pathology. This is pertinent given the partial spectral frequency overlap between slow wave oscillations and K-complexes (De Gennaro et al. 2017), and particularly salient considering that the specifics of how sleep EEG microstructure is altered in individuals with OSA is not well characterized. In respiratory failure patients with sleep-disordered breathing, a positive association between the partial pressure of carbon dioxide (PCO2) and percentage of SWS was observed, indicating abnormal blood gas exchange at night appears to influence the appearance of slow waves (Wang et al. 2011). Furthermore, there is evidence that OSA impairs the both the normal homeostatic reduction in SWA and K-complex amplitude across the sleep cycle (Parapatics et al. 2015), and whilst K-complexes are frequently elicited by respiratory events during sleep (Nguyen et al. 2016; Parekh et al. 2019), respiratory elicited K-complexes are morphologically different in OSA compared to spontaneous K-complexes (Sun et al. 2019). Some work by Chervin, Burns, and Ruzicka (2005) demonstrate that respiratory cycle related EEG changes, particularly sigma power variations, are predictive of sleepiness in those with SDB and likely reflect increased sigma (arousal activity) during inspiration.
Although findings are inconsistent, a systematic review of spindle oscillations in sleep disorders (Weiner and Dang-Vu 2016) shows that overall OSA patients have less spindle activity (using spectral, visual and automatic event analysis methods) and slower spindle frequencies (Schonwald et al. 2012) than those without OSA. Additionally, different spindle dynamics throughout the nocturnal sleep period are observed in OSA compared to controls (Ondze et al. 2003; Huupponen et al. 2008; Carvalho et al. 2014). Interestingly, work quantifying spindles in overweight adolescents with OSAS found increased spindle activity (number and density) compared controls matched for age and BMI (Madaeva et al. 2017), and highlights the potential influence of an adaptive cortical response to OSA in young adults. A study of EEG spectral power differences between young (~24 years) and older patients (~59 years) with OSAS, not taking medications, found both slow (<13 Hz) and fast (>13 Hz) sigma activity during NREM were higher in the older group and a ratio of slow/fast sigma activity was positively correlated with OSA severity measures in younger participants only (Lee et al. 2016).
Despite the well documented association between spindle activity and cognitive functioning (De Gennaro and Ferrara 2003; Schabus et al. 2004; Schabus et al. 2006), few have investigated the relationship between sleep spindle activity and cognition in OSA. A moderate positive correlation (not statistically significant) between spindles and overnight task improvement has been observed in OSA participants but not controls (Landry et al. 2014). Additionally, an enhancement of spindles after learning in OSA patients was not observed by Barner et al. (2016) as is seen in healthy young adults (Gais et al. 2002). However, the older age of the participants may explain the lack of effect (average age 52 years in Barner et al. (2016) compared to 24 years in Gais et al. (2002)). To date, no studies have been published investigating the effect of OSA on slow oscillation-spindle coupling.
Effects of CPAP treatment on EEG
Adequate CPAP treatment restores sleep macrostructure measures towards that of individuals without OSA, most notably SWS and REM durations increase and sleep latencies to these stages decrease (Parrino et al. 2000; McArdle and Douglas 2001; Brillante et al. 2012). Beyond these changes in sleep macrostructure and a reduction in respiratory-related arousals (Fietze et al. 1997), a modest amount of literature examines changes to EEG microstructure during wake and sleep EEG recordings after CPAP treatment.
Early studies examined small numbers (N~10), of mostly male patients (~80%) and conducted comprehensive EEG analysis on features such as CAP (Parrino et al. 2000; Thomas 2002; Parrino et al. 2005) and spectral power calculations (Morisson et al. 2001; Heinzer et al. 2001), comparing controls and OSA patients before, during and after various lengths of CPAP treatment. Parrino et al. (2000) noted a significant reduction in measures of arousal and CAP during the first night of CPAP below the levels of control participants, of which a reduced CAP rate showed significant correlation with improvements in sleepiness. Later the same group observed progressive normalization of CAP across a month of CPAP with the number of A1 subtype events remaining below the levels of control patients at the end of 30 days consecutive treatment (Parrino et al. 2005). In this study they also found Multiple Sleep Latency Test (MSLT) scores negatively correlated with arousals and CAP rate which was not the case in their earlier study. Initial studies using spectral power analysis before and after CPAP treatment observed a correction of EEG slowing in wake and REM but not complete resolution of sleepiness (Morisson et al. 2001). Furthermore, in a similar group of patients the same research group observed increased SWA during the first part of the night after 9 months of CPAP compared to during the untreated baseline sleep (Heinzer et al. 2001). A number of later studies also show several months CPAP treatment partially reverses waking EEG abnormalities (EEG slowing) and improves some daytime functioning (Greneche et al. 2011; Xiromeritis et al. 2011; Lee et al. 2012; Wang et al. 2014), see (D’Rozario et al. 2017) for detailed review.
Work investigating the effect of CPAP treatment on sleep spindle characteristics shows increased spindle density during a CPAP titration compared to a diagnostic night PSG (Chokroverty et al. 2015; Yetkin and Aydogan 2018). A study of spindle characteristics finds CPAP treatment partially normalizes spindle features of the group towards those seen in healthy controls (Saunamaki et al. 2017).
There is evidence that K-complexes also change as a result of CPAP treatment. Parapatics et al. (2015) found KC densities and homeostatic amplitude dissipation are returned to levels of normal controls after 3–6 months of CPAP treatment. Work from our group indicates CPAP treatment results in a decrease in KC density and an increase in SWA and slow wave activity surrounding K-complexes (ΔSWAK) (Parekh et al. 2019). Furthermore, in this analysis ΔSWAK was a significant predictor of vigilance (PVT lapses), where less slow wave activity elicited by a KC was associated with greater performance impairments. This relationship between sleep EEG and daytime performance was observed not only cross-sectionally in both chronic and acute OSA conditions, but also longitudinally with CPAP treatment.
Summary
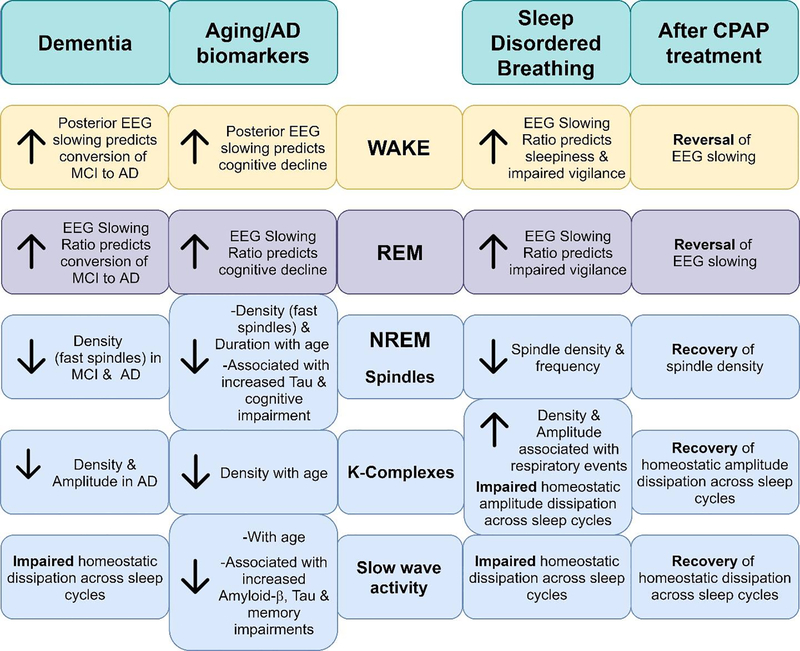
There are well documented EEG changes with age and dementia during both wake and sleep states that differ in males and females. Preliminary findings indicate that in OSA sleep is disrupted such that EEG microstructure is altered in similar ways to those during aging and dementia, but large-scale studies are mostly lacking (Figure 1). Additionally, high levels of inter-individual variability in neurophysiological responses to SDB and CPAP treatment indicate complex relationships between the severity and chronicity of sleep fragmentation and intermittent hypoxia that are difficult to disentangle. Table 4 summarizes the OSA and EEG research referenced in this section.
Figure 1:
Illustration summarizing EEG features seen in dementia, aging, with AD biomarkers, sleep-disordered breathing and CPAP treatment.
Table 4:
Research reviewed involving OSA and quantitative EEG measures.
| Authors | Study design and recruitment | Population | Age (years) | Sex (% female) | EEG Measure |
|---|---|---|---|---|---|
| Terzano et al. 1996 | Cross-sectional, clinic & community volunteers | 12 OSAS, 12 controls | OSAS 50±8, controls 49±6 | OSAS 33%, controls 33% | CAP |
| Fietze et al. 1997 | Interventional, sleep clinic | 38 OSAS (CPAP+ 3 nights) | OSAS 49±21 | No females | Arousals (visually identified) |
| Morisson et al. 1998 | Cross-sectional, sleep clinic | 21 OSAS, 10 controls | OSAS 44±7, controls 44±6 | OSAS 10%, controls 10% | Spectral activity (sleep) |
| Parrino et al. 2000 | Interventional, sleep clinic | 10 OSAS (CPAP+ 1 night), 10 controls | OSAS 47±5, controls 48±6 | OSAS 20%, controls 20% | CAP |
| Morisson et al. 2001 | Interventional, sleep clinic | 14 mod-severe OSAS (CPAP+ 6 months), 10 controls | OSAS 45±6, controls 44±6 | OSAS 7%, controls 10% | Spectral activity (sleep) |
| Guilleminault et al. 2001 | Cross-sectional, sleep clinic | 12 OSHS, 12 OSAS, 12 UARS, 12 controls | OSHS 44±7, OSAS 42±7, UARS 42±7, controls 42±7 | No females | Spectral activity (sleep) |
| Heinzer et al. 2001 | Interventional, sleep clinic | 12 severe OSA (CPAP+ 9 months), 10 controls | OSA 43±2, controls 44±2 | No females | Spectral activity (SWA) |
| Sforza et al. 2002 | Cross-sectional, sleep clinic | 48 OSA | OSA 49±2 | 41% | Spectral activity (wake) |
| Thomas 2002 | Cross-sectional, sleep clinic | 12 OSA (CPAP+ 1 night) | OSA 44 (range: 22–64) | Not reported | CAP |
| Ondze et al. 2003 | Cross-sectional, sleep clinic | 18 mild SDB, 18 controls | SDB 36±11, controls 33±12 | SDB 50%, controls 64% | Spectral activity (SWA, spindle) |
| Himanen et al. 2004 | Cross-sectional, clinical | 8 OSAS, 8 controls | OSAS 43(range 31–59), controls 45 (range 34–54) | OSAS 50%, controls 50% | SWs (visually identified) |
| Chervin et al. 2005 | Cross-sectional, sleep clinic | 27 SDB, 11 controls | SDB 45±14, controls 33±10 | SDB 33%, controls 82% | Spectral activity (sleep) |
| Parrino et al. 2005 | Interventional, sleep clinic | 10 OSAS (CPAP+ night 1,2,3 & 30), 10 controls | OSAS 48±4, controls age-matched | No females | CAP |
| Mathieu et al. 2007 | Cross-sectional, clinic & community volunteers | 12 young & 13 older OSAS, 13 young & 14 older controls | Young 38±2 & older OSAS 62±2, Young 36±2 & older controls 60±2 | Young 20% & older OSAS 0%, Young 8% & older controls 0% | EEG slowing |
| Guilleminault et al. 2007 | Cross-sectional, clinic & community | 30 UARS, 30 controls | Not reported | UARS 50%, controls 50% | CAP |
| Greneche et al. 2008a | Cross-sectional, clinical | 12 OSA, 8 controls | OSA 51±3, controls 49±3 | Not reported (No females inferred) | Spectral activity (wake) |
| Greneche et al. 2008b | Cross-sectional, clinical | 12 mod-severe OSAHS | OSAHS 51±3 | No females | Spectral activity (wake) |
| Saunamaki et al. 2009 | Interventional, sleep clinic | 15 OSAS (CPAP+ 6–12 months), 15 controls | OSAS 50 (range 37–59), controls 44 (range 30–63) | No females | Spectral activity (SWA) |
| Xiromeritis et al. 2011 | Interventional, sleep clinic | 131 OSA (29 CPAP+ 6 months), 30 controls | OSA 49±10, control 47±12 | OSA 7%, control 7% | Spectral activity (wake) |
| Greneche et al. 2011 | Interventional, sleep clinic | 12 OSAHS (10 CPAP+ 3 & 6 months) | OSAHS 51±3 | Not reported (No females inferred) | Spectral activity (wake) |
| Lee et al 2012 | Interventional, clinic | 13 OSAS (CPAP+ 3 months) | OSAS range: 37–59 | No females | Spectral activity (wake) |
| Schonwald et al. 2012 | Cross-sectional, sleep clinic | 11 mild & 10 mod OSA, 7 non-OSA | Mild 51±7 & mod 52±9 OSA, controls 46±6 | Mild 55% & mod 20% OSA, controls 43% | Spindle characteristics |
| Baril et al. 2013 | Cross-sectional, clinic | 12 OSA, 12 controls | OSA 48±14, controls 44±10 | OSA 25%, controls 8% | Spectral activity (wake), ERP |
| D’Rozario et al. 2013 | Cross-sectional, clinic | 8 OSA, 9 non-OSA | OSA 45±8, non-OSA 29±4 | OSA 0%, non-OSA 11% | DFA |
| Jones et al. 2014 | Cross-sectional, community volunteers | 9 OSA, 9 controls | OSA 53±10, controls 52±9 | OSA 33%, controls 33% | Spectral activity (sleep) |
| Carvalho et al. 2014 | Cross-sectional, sleep clinic | 11 mild & 10 mod OSA, 7 non-OSA | Mild 51±7 & mod 52±9 OSA, controls 46±6 | Mild 55% & mod 20% OSA, controls 43% | Spindle characteristics |
| Parapatics et al. 2015 | Cross-sectional, clinic & community volunteers | 22 OSA (CPAP+ 3–6 months), 22 controls | OSA 59, controls 59 | OSA 9%, controls 9% | K-complexes |
| Chokroverty et al. 2015 | Interventional, sleep clinic | 9 severe OSA (CPAP+ split night) | Range: 28–80 | 11% | Spindle density |
| Vakulin et al. 2016 | Cross-sectional, sleep clinic | 76 OSA | 43 (range 23–65) | 16% | Spectral activity |
| Nguyen et al. 2016 | Cross-sectional, sleep clinic | 10 severe & 10 mod OSA, 10 controls | Severe 48±12 & mod OSA 46±14, controls 39±12 | Severe 30% & mod OSA 30%, controls 30% | K-complexes, spectral activity |
| Lee et al. 2016 | Cross-sectional, sleep clinic | 40 young OSAS & 36 older OSAS | Young OSAS 24±5, Older OSAS 59±5 | Young OSAS 13%, Older OSAS 22% | Spectral activity |
| Barner et al. 2016 | Cross-sectional, clinic | 21 OSA | 53±2 | 11% | Spindle density |
| Madaeva et al. 2017 | Cross-sectional, sleep clinic | 18 overweight OSAS, 12 overweight controls, 15 normal weight controls | Overweight OSAS 17, Overweight controls 16, Normal weight controls 16 | No females | Spindle characteristics |
| Saunamaki et al. 2017 | Interventional, clinic & community volunteers | 20 OSA (CPAP+ 6 months), 20 controls | OSA 50 (range 37–65), controls 49 (range 34–60) | No females | Spindle characteristics |
| Korkmaz et al. 2018 | Cross-sectional, sleep clinic | 10 OSA with ES, 28 OSA without ES | OSA with ES 56±4, OSA without ES 54±5 | No females | CAP |
| Yetkin & Aydogan 2018 | Interventional, sleep clinic | 73 consecutive patients | 50±12 | 16% | Spindle density |
| Appleton et al. 2019 | Cross-sectional, community volunteers | 84 severe, 97 mod, 195 mild, 358 non-OSA | Severe 64±12, mod 63±10, mild 62±11, non-OSA 59±11 | No females | Spectral activity |
| Parekh et al. 2019 | Interventional, sleep clinic | 28 OSA (CPAP+), 19 controls | OSA 47±11, controls 34±2 | OSA 25%, controls 37% | K-complexes, spectral activity |
| Mullins et al. 2020 | Cross-sectional, sleep clinic | 8 mod-severe OSA | OSA 45±8 | No females | Spectral activity, spindle density |
CAP: cyclic alternating pattern; CPAP: continuous positive airway pressure; DFA: detrended fluctuation analysis; ERP: event related potential; ES: excessive sleepiness; OSA: obstructive sleep apnea; OSAS: obstructive sleep apnea syndrome; OSHS: obstructive sleep hypopnea syndrome; SDB: sleep-disordered breathing; SW: slow wave; SWA: slow wave activity; UARS: upper airway resistance. Median (SEM), Median [IQR]. Ages and percentages values are rounded from the source manuscript. Studies involving CPAP are highlighted in green.
Factors beyond Amyloid and Tau
OSA and ApoE
The ApoE gene has 3 common alleles, and carrying one or more copies of ApoE4 is the greatest known genetic risk factor for late onset Alzheimer disease (Corder et al. 1993). In the brain, ApoE protein is produced by astrocytes to transport cholesterol to neurons, a process essential to neuronal membrane function as well as neuronal integrity (such as neurite extension among others)(Mahley and Rall Jr 2000). While some early studies suggested having a single ApoE4 allele increases the odds of having moderate-severe OSA (Gottlieb et al. 2004; Kadotani et al. 2001), several subsequent studies have suggested no relationship between ApoE genotype and OSA (Saarelainen et al. 1998; Thakre, Mamtani, and Kulkarni 2009; Xu et al. 2015; Lu et al. 2016). Work by our group found ApoE genotype can influence the relationship between OSA severity and CSF amyloid-β42 or tau, with positive associations between OSA severity and both CSF amyloid-β42 or tau seen in ApoE3 carriers, but either no association (tau) or negative association (amyloid-β42) observed in ApoE4 carriers (Osorio et al. 2014). However, the number of ApoE4 carriers was very small in comparison to ApoE3 carriers, so additional work is needed to ascertain the influence of ApoE genotype on this relationship. The effects of ApoE genotype on amyloid burden in general appear to extend to individuals with OSA, as higher cortical amyloid load by PET imaging in participants with OSA carrying the ApoE e4 allele was observed in a cross-sectional study (Elias et al. 2018). Negative effects of OSA and ApoE4 carrier status may be synergistic as cognitively normal middle-aged adult ApoE4 carriers had impaired spatial working memory versus non-carriers, only in subjects with comorbid OSA, but not in normally-breathing controls (Cosentino et al. 2008). These studies highlight the complex interaction of OSA severity and ApoE and suggest that untreated apnea can affect biomarkers and subtle cognitive deficits which precede clinical manifestation of AD by a decade.
OSA and Immune factors
Research in AD and sleep fields has identified an important crosstalk between the brain and immune system. Microglia are the primary immune cells of the brain with a diverse set of functions in neurodevelopment, infection response, and neuronal synaptic homeostasis. These resident immune cells can also be activated by astrocytes, another glial cell type in the brain. Exactly how OSA affects this crosstalk is largely unknown due to the available methods to measure the immune system. While microglia secrete cytokines as a response to clear pathogens in the brain, much of the findings from the OSA field come from systemic immune sources measured in the blood. For example, in moderate to severe OSA, blood derived IL6 and tau were greater than controls without OSA (Motamedi et al. 2018). Other circulating factors like CRP, IL-1Ra, IL-8 and TNF-α show elevation in patients with OSA and an acute cardiovascular event (Testelmans et al. 2013). Nevertheless, missing from the field is widespread adoption of biomarkers of microglial activation from the brain in vivo. One candidate is YKL-40, an astroglial secreted protein that activates microglia and is shown to track with early cognitive decline in MCI when measured from the CSF (Craig-Schapiro et al. 2010). In sleep apnea, serum-derived YKL-40 offers a less invasive biomarker but at the cost of still being of systemic immune origin. For example, elevated levels of serum YKL-40 were found in patients with OSA and coronary artery disease (Sui and Gao 2013), OSA and diabetes (Sun, Liu, and Li 2015), as well as OSA and hypertension measured cross-sectionally (Jafari and Mohsenin 2016). When measured from the CSF, cognitively normal elderly with subjectively defined poor sleep show detectably higher YKL-40 levels (Sprecher et al. 2017). In order to dissect the role of SWS disruption on multiple CSF derived proteins including YKL-40 Ju et al. (2017) conducted a single night SWS disruption paradigm and CSF YKL-40 did not correlate with the change in delta power compared to sham. These results indicate microglial activation likely requires chronic sleep fragmentation with intermittent hypoxia seen with OSA (Ju et al. 2017). Soluble CSF TREM2 is a microglial protein product of a genetic risk factor for AD (Jonsson et al. 2013; Guerreiro et al. 2013) and is found to be higher in AD compared to cognitively normal elderly (Heslegrave et al. 2016; Ewers et al. 2019). Development of PET tracers to monitor microglial activation in vivo and in specific brain areas is actively being investigated in AD and warrants future consideration in OSA (Edison, Donat, and Sastre 2018). In an animal model of sleep restriction without intermittent hypoxia, morphological evidence for both microglial and astrocytic phagocytosis of synapses suggests one way that the brain responds to prolonged wakefulness in order to maintain synaptic homeostasis (Bellesi et al. 2017). However, dissecting the pathological crosstalk between microglia and astrocytes in OSA will require specific biomarkers that can be assayed both simultaneously and longitudinally in vivo.
OSA and Blood-Brain barrier/Glymphatic system
The blood-brain-barrier (BBB) functionally separates the brain from circulating blood. Hypotheses have been put forth regarding how OSA may disrupt BBB permeability (Lim and Pack 2014) but only recently have imaging methods been developed to measure BBB permeability in vivo in humans (Raja, Rosenberg, and Caprihan 2018). Pseudo-continuous arterial spin labeling MRI indicates patients with OSA have reduced water diffusion across the BBB compared to non-OSA controls (Palomares et al. 2015). Once the BBB breaks down, circulating cells could be allowed to infiltrate into the brain. Using perfusion imaging, reduced global cerebral blood flow and increased leucocyte apoptosis, primarily of granulocytes, was found in the OSA group compared to controls (Chen et al. 2017). When combined with the finding of regional cerebral blood hypoperfusion, particularly in elderly severe OSA patients (Baril et al. 2015), it appears that OSA contributes to region-specific BBB breakdown. However, this same study found that other brain regions such as the hippocampus, amygdala and caudate had paradoxically hyperperfusion which correlated positively with AHI, suggesting region-specific hyperactivity as a result of frequent apneas and hypopneas (Baril et al. 2015).
Work in animals identified a glymphatic system that clears amyloid-β and other brain metabolites from the interstitial space into the CSF (Xie et al. 2013). However, the field is contentious due to lack of evidence for interstitial fluid (ISF) clearance of solutes (Abbott et al. 2018). Methods to directly measure the exchange of solutes from the ISF to the CSF in vivo in humans are lacking, but one method uses ultrafast magnetic resonance encephalography to identify cardiac, respiratory, and very low frequency pulse-mediated flow in the brain (Kiviniemi et al. 2016). The temporal relationship between sleep stages and CSF flow has remained an enigma of the sleeping human brain. Using simultaneous EEG and ultrafast MRI, delta waves were found to precede BOLD and CSF rhythms during slow wave sleep (Fultz et al. 2019). The authors propose CSF inflow from the 4th ventricle is a new pathway by which CSF and ISF mix during SWS (Fultz et al. 2019). Ideally, these advanced imaging methods will be applied in future studies to understand how OSA potentially impacts glymphatic clearance. The disruption of slow wave sleep from OSA and large intrathoracic pressures changes between apneas and recovery breaths could be acting at odds to impact cyclic CSF pulsations and potentially regulate glymphatic clearance.
OSA and Neuronal factors
Orexin (aka hypocretin) is a neuropeptide involved in wake maintenance, arousal and feeding, and is produced primarily in the lateral hypothalamus. In a mouse model of amyloid overproduction, orexin delivered into the brain increased ISF amyloid-β while a dual orexin receptor antagonist decreased ISF amyloid-β and decreased wake duration across 24 hours (Kang et al. 2009). In humans without OSA, an experimental 5 nights of sleep restriction led to increased CSF orexin without increases to amyloid-β, tau, or neuron-derived proteins (neurofilament light, neuron-specific enolase, and fatty acid–binding protein) (Olsson et al. 2018). In an older cohort of cognitively normal elderly, CSF orexin positively correlated with CSF AD biomarkers amyloid-β, P-tau, and T-tau which was not driven by AHI (Osorio et al. 2016). Orexin measures in patients with obstructive sleep apnea, however, are inconsistent with one study showing no change in CSF orexin between subject with and without OSA (Kanbayashi et al. 2003) and another showing elevated CSF orexin levels in untreated OSA subjects in comparison to both treated OSA subjects and normally-breathing subjects (Liguori et al. 2019). Additionally, plasma orexin levels in OSA appear tied to sleepiness with higher levels in sleepy subjects with OSA (ESS ≥ 13 vs ESS ≤ 9) (Sanchez-de-la-Torre et al. 2011). Other promising neuronally derived biomarkers are CSF VILIP-1 and neurogranin which are both shown to be lower in a moderately-severe OSA group compared to mild OSA (Ju et al. 2016). These heterogenous findings of neuronally derived biomarkers warrants a larger study of the effect of OSA on markers of neuronal integrity.
Summary
The immune system in particular has gained increasing attention as playing an important role in the pathogenesis and progression of AD. OSA likely has direct impact on cytokines and immune cells, as well as access of immune cells to the brain via influences on the blood brain barrier, but an understanding of to what extent these effects impact risk for and progression of AD is in its nascency. Table 5 summarizes the OSA biomarker research referenced in this section.
Table 5:
Research reviewed involving OSA and biomarkers beyond amyloid and tau.
| Authors | Study design and recruitment | Population | Age (years) | Sex (% female) | Biomarkers |
|---|---|---|---|---|---|
| Saarelainen et al. 1998 | Cross-sectional, sleep clinic and population based | 291 OSA, 728 controls | OSA 53 (range 26–75), controls 54 (range 39–70) | OSA 9%, Controls 22% | ApoE genotype |
| Kadotani et al. 2001 | Cross-sectional, population based | 791 middle-aged adults | 49±8 | 40% | ApoE genotype |
| Kanbayashi et al. 2003 | Cross-sectional, clinic | 16 narcolepsy with OSA, 12 controls | 55±15 | No females | CSF hypocretin 1 (Orexin A) |
| Gottlieb et al. 2004 | Cross-sectional, population based | 1,775 SHHS cohort | 71±10 | 55% | ApoE genotype |
| Cosentino et al. 2008 | Cross-sectional, sleep clinic & community volunteers | 123 OSA, 121 controls | OSA 59±9, controls 58±10 | OSA 33%, controls 36% | ApoE genotype |
| Sanchez-de-la-Torre et al. 2011 | Cross-sectional, sleep clinic | 132 OSA with EDS, 132 OSA w/out EDS | OSA EDS+ 50±11, OSA EDS− 51±10 | OSA EDS+ 17%, OSA EDS− 14% | Neuropeptides, metabolic hormones |
| Testelmans et al. 2013 | Cross-sectional, sleep clinic | 15 obese OSA, 15 non-obese OSA, 15 OSA+CV, 12 CV, 15 controls | OSA obese 52±10, non-obese OSA 52±8, OSA+CV 53±9, CV 53±9, controls 48±6 | OSA obese 77%, non-obese OSA 20%, OSA+CV 7%, CV 17%, controls 47% | Cytokines |
| Sui & Gao 2013 | Cross-sectional, Army hospital | 134 OSAS with CAD, 112 OSAS without CAD | CAD+ 57±8, CAD− 56±9 | CAD+ 28%, CAD− 26% | Serum YKL-40 |
| Palomares et al. 2015 | Cross-sectional, sleep clinic | 9 OSA, 9 controls | OSA 47±11, controls 39±7 | OSA 56%, controls 44% | BBB function |
| Baril et al. 2015 | Cross-sectional, sleep clinic | 50 OSA, 20 controls | OSA 64±6, controls 64±7 | OSA 14%, controls 40% | Cerebral perfusion |
| Sun et al. 2015 | Cross-sectional, population based | 448 older women | 83±3 | 100% | ApoE genotype |
| Jafari & Mohsenin 2016 | Cross-sectional, sleep clinic | 36 OSA+HT, 27 OSA-HT, 13 Non-OSA+HT, 19 Non-OSA-HT | OSA+HT 56±1, OSA-HT 48±2, Non-OSA+HT 46±2, Non-OSA-HT 48±2, | OSA+HT 19%, OSA-HT 26%, Non-OSA+HT 38%, Non-OSA-HT 68% | Serum YKL-40 |
| Ju et al. 2016 | Cross-sectional, community volunteers | 10 OSA, 31 controls | OSA 56±4, control 53±6 | OSA 30%, controls 48% | CSF VILIP-1, neurogranin |
| Osorio et al. 2016 | Cross-sectional, community volunteers | 63 cognitively normal individuals | 70±9 | 57% | CSF Orexin-A, ApoE genotype |
| Chen et al. 2017 | Cross-sectional, sleep clinic | 20 mod-severe OSA, 16 controls | OSA 40±8, controls 38±9 | No females | Cerebral perfusion, leucocyte apoptosis |
| Liguori et al. 2017 | Cross-sectional, sleep center | 25 OSA, 10 OSA-CPAP, 15 controls | OSA 68±8, OSA-CPAP 67±7, controls 66±9 | OSA 32%, OSA-CPAP 30%, controls 40% | CSF Lactate |
| Liguori et al. 2019 | Cross-sectional, clinic | 20 OSA, 20 AD, 15 controls | OSA 59±4, AD 66±4, controls 64±8 | OSA 30%, AD 65%, controls 47% | Orexin-A |
ApoE: Apolipoprotein E; BBB: blood brain barrier; CV: cardiovascular event; EDS excessive daytime sleepiness; HT: hypertension; OSA: obstructive sleep apnea; SHHS: sleep heart health study; YKL-40: 40 kDa heparin- and chitin-binding glycoprotein. Median (SEM), Median [IQR]. Ages and percentages values are rounded from the source manuscript. Studies involving CPAP are highlighted in green.
Challenges and Future Directions
Difficulties with definitive trials investigating the effect of CPAP treatment on biomarkers of AD and dementia – Future directions
One fundamental challenge to the intersecting research fields of AD and sleep-disordered breathing appears to be one of measurement, in relation to both defining sleep apnea severity and adequate treatment response.
According to The AASM manual for the scoring of sleep and associated events: Rules, Terminology and Technical Specifications (Iber et al., 2007), there are four types of respiratory events contributing to an AHI score: obstructive, central, and mixed apnea events (complete cessation of airflow), and the hypopnea event (reduction in airflow). The hypopnea lacks a single definition and is categorized according to event sequelae (EEG micro-arousal and/or oxygen desaturation events classically, but for example events associated with change in heart rate have been used in research studies). This heterogeneity results in several fundamental barriers to understanding the negative consequences of OSA, as does the reliance purely on frequency of events. For example, is someone with an AHI of 10/hour where each event lasts 120 seconds and is associated with significant oxygen desaturation into the 70’s better or worse than someone with an AHI of 30/hour where each event lasts 10 seconds and is associated purely with arousal and no change in oxygen? It is impossible to disentangle sleep fragmentation (due to arousals) and hypoxemia (due to oxygen desaturation) within a single AHI measure. Furthermore, if large epidemiological or clinical trials are conducted using one definition, it is difficult to integrate research using an alternative definition into existing knowledge. Perhaps to understand the contribution of fragmentation and hypoxia to negative outcomes in OSA, the field may need to move beyond the AHI for OSA diagnosis.
This could possibly be achieved with more detailed characterization of sleep physiology in OSA during both diagnosis and treatment using novel analysis of existing measures of sleep physiology in PSG. Novel quantification of the rich oscillatory activity collected from multiple organ systems during PSG creates possibilities for explaining individual variation in vulnerability to the negative consequences of sleep disorders. This possibility, however, is hampered by the strong push to complete sleep testing at home, where home sleep tests increasingly focus on collecting just enough signals to provide an AHI. There is a high degree of inter-individual variability inherent to human physiology which likely contributes to our limited understanding about how OSA exerts its negative health effects within an individual (Davies and Harrington 2016) or the inability to predict who will benefit from CPAP treatment. Despite being the most effective and frequently recommended treatment for OSA, CPAP acceptance and compliance rates are variable and can be particularly poor in individuals who are not sleepy or other otherwise symptomatic from their OSA (McArdle et al. 1999; Weaver and Grunstein 2008). Furthermore, the benchmark used in clinical and research settings for adequate CPAP usage is greater than 4 hours for at least 70% of nights but this recommendation was selected virtually at random (Weaver et al. 2007; Sawyer et al. 2011) and ignores any individual’s habitual sleep duration, the fraction of untreated sleep, and the severity of apnea during untreated sleep. In partial users of CPAP, the untreated portion of sleep is most typically the latter half, which is rich in REM, a stage of sleep in which OSA is often at its worst due to REM muscle atonia. Research highlighting the detrimental outcomes of REM related OSA could inform the research agenda concerning AD and sleep (Varga and Mokhlesi 2019). In a secondary analysis of the Sleep Heart Health Study, Pase et al. (2017) observed a reduced REM sleep duration and an increased latency to REM were associated with incident AD dementia. Furthermore, REM sleep is reported to be reduced in MCI (Maestri et al. 2015; Liguori et al. 2016). In an interesting study focusing on REM related OSA, Baril et al. (2020) found more SDB during REM sleep was associated with reduced daytime regional cerebral blood flow in frontal areas.
While there can be inter-individual variability in OSA, there may also be features common to subsets of individuals that allow for common grouping (i.e. OSA driven by high upper airway collapsibility vs low arousal threshold) (Seely and Macklem 2004; Buckelmuller et al. 2006), and encouragingly there is much focused research investigating endophenotypes (Eckert et al. 2013) and personalized medicine approaches (Pack 2016; Zinchuk et al. 2018) in OSA. One area historically neglected in OSA research is sexual dimorphism. Emerging evidence of significant differences between males and females with respect to OSA phenotypes (Won et al. 2020) and the impact of CPAP therapy on health outcomes (Gagnadoux et al. 2017) necessitate the consideration of sex in study design rather than in post-hoc analysis. Understanding individual rather than group changes in response to treatment with respect to sleepiness, vigilance and cognition will likely yield a better indication on treatment modality or required usage to achieve improvements in daytime functioning for the individual.
Another difficulty with using the AHI as a method of diagnosis and disease severity is the insensitivity to chronicity of disease. Implicit in understanding the effect of OSA on health is the propensity for adaptive processes in response to stressors to the system, also understood as resilience. The high level of heritability of sleep EEG (Ambrosius et al. 2008), the intra-individual stability (Lewandowski, Rosipal, and Dorffner 2013; Poon et al. 2019), plus the known influence of genes on EEG patterns and sleep/wake regulation (Shi et al. 2017), could be leveraged to understanding sleep disorder heterogeneity and risk. The concept of a resilience brain in the face of perturbations to sleep during SDB offers a framework with which to use sleep EEG microstructure identify biomarkers of neurodegeneration (Melpignano et al. 2019) or strategies to achieve healthy sleep for the individual (Parrino and Vaudano 2018). In a similar vein, non-linear methods of signal analysis (Ma et al. 2018) to assess biological complexity (Goldberger 2006) and fractal properties (Franca et al. 2018) are interesting approaches to characterize sleep disruption and physiological changes with age (Goldberger et al. 2002) and AD (Li et al. 2018). For example, Zhang et al. (2009) showed severity of OSA could be assessed using a fractal dimension sequence analysis of sleep EEG which stabilized with CPAP treatment (Zhang et al. 2015). Perhaps such methods could be applied to sleep EEG quantitatively distinguish ‘healthy’ homeostatic slow waves from ‘detrimental’ pathologic slow waves or EEG slowing (Petit et al. 2017). Despite the potential for novel measures of sleep to better characterize individual vulnerability to negative consequences of sleep disruption, it also adds to the multitude of sleep features currently tested when investigating the link between sleep, OSA and AD. To this end, the literature may have considerable publication bias with many measures tested, only significant associations reported and without adequate correction for multiple comparisons.
Based on the available evidence, many would argue that a clinical trial of OSA treatment for AD prevention is warranted. But there are several fundamental issues in conceptualizing such a trial, many of which are intrinsic to any intervention designed to slow or prevent AD. The AD field recognizes that there is a long preclinical phase, lasting years to possibly decades, during which changes to the brain occur, including dysfunction of synapses and the accumulation of amyloid and tau before any hint of cognitive change is apparent. Therefore, an idealized clinical trial for AD prevention would target recruitment of participants who are relatively young and such preclinical brain changes are just beginning to occur. But this presents the obvious question of how long such a trial would need to last to determine an impact on AD development. The answer is likely to be many years – 10 to 20 would not be out of the question – which negatively impacts the feasibility (and fundability) of such a proposal. Instead of the development of frank dementia as the primary outcome, intermediate surrogate outcomes could be assessed (e.g. brain amyloid and/or tau load by PET imaging), but the field still lacks consensus on whether such outcomes ultimately would or would not impact the development of dementia. While recruitment age, duration of follow-up, and defining primary outcome are problems in designing any AD treatment trial, there are additional issues specific to OSA. In an idealized trial, one would likely want to recruit individuals who stand the most to gain from treatment – individuals with severe OSA and daytime sleepiness, particularly in light of the observation that all cause sleepiness was shown to be associated with longitudinal accumulation of brain amyloid in older subjects (Carvalho et al. 2018). However, randomizing individuals with severe OSA and daytime sleepiness to a control arm lacking treatment is probably not ethically justifiable, particularly if it would need to occur over many years. Therefore, one could focus on individuals from the community with incidentally identified severe OSA who lack daytime symptoms. However, in such individuals, adherence to CPAP in the treatment arm is likely to be quite low, as was observed in a recent clinical trial of CPAP for cardiovascular endpoints (McEvoy et al. 2016), a study in which mean CPAP use was just 3.3 hours per night over a mean follow-up duration of 3.7 years. The adherence issue could be minimized by liberalizing the treatment arm to “any intervention that lowers the AHI” below some threshold value, a concept championed by our colleague Dr. David Rapoport, which could include use of mandibular advancement devices, nasal EPAP, positional therapy, or other interventions. While appealing, the feasibility of using multiple interventions in a trial and tracking adherence as can easily be done with CPAP, are factors that remain to be worked out.
Acknowledgments
We thank Drs. David Rapoport and Indu Ayappa, for their continuous mentorship, support, and insights. This work was supported by Alzheimer’s Association grant 2018-AARG-589632, Merck Investigator Studies Program, and by NIA grants AG 056031 (R.S.O.), AG056531 (R.S.O.), AG056682 (A.W.V.) and AG059179 (A.W.V.).
Abbreviations
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- AHI
Apnea hypopnea index
- AHI3A
Apnea hypopnea index - hypopnea criteria of ≥3% oxygen desaturation or arousal
- AHI4%
Apnea hypopnea index - hypopnea criteria of ≥4% oxygen desaturation only
- ApoE
Apolipoprotein E
- CSF
Cerebrospinal fluid
- CPAP
Continuous positive airway pressure
- EDS
Excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- ERPs
Event related potentials
- ISF
Interstitial Fluid
- KC
K-comple
- mPFC
Medial prefrontal cortex
- MCI
Mild cognitive impairment
- MMSE
Mini mental state exam
- MSLT
Multiple Sleep Latency Test
- OSA
Obstructive sleep apnea
- OSAS
Obstructive sleep apnea syndrome
- PCO2
Partial pressure of carbon dioxide
- P-tau
Phosphorylated tau
- PPG
Photoplethysmography
- PiB
[11C] Pittsburgh compound B
- PSG
Polysomnography
- PET
Positron Emission Tomography
- PVT
Psychomotor vigilance test
- qEEG
Quantitative electroencephalographic
- SDB
Sleep-disordered breathing
- SWA
Slow wave activity
- SpO2
Saturation of peripheral oxygen
- T-tau
Total tau
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, and Thorne RG. 2018. ‘The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system?’, Acta Neuropathologica, 135: 387–407. [DOI] [PubMed] [Google Scholar]
- Achermann P, and Borbely AA. 1997. ‘Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram’, Neuroscience, 81: 213–22. [DOI] [PubMed] [Google Scholar]
- Ahuja S, Chen RK, Kam K, Pettibone WD, Osorio RS, and Varga AW. 2018. ‘Role of normal sleep and sleep apnea in human memory processing’, Nat Sci Sleep, 10: 255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchanatis M, Zias N, Deligiorgis N, Liappas I, Chroneou A, Soldatos C, and Roussos C. 2008. ‘Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea’, Sleep Breath, 12: 17–24. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, and Giles DE. 2003. ‘Neuropsychological changes and treatment compliance in older adults with sleep apnea’, Journal of Psychosomatic Research, 54: 71–6. [DOI] [PubMed] [Google Scholar]
- Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, Bettecken T, Holsboer F, Yassouridis A, and Friess E. 2008. ‘Heritability of sleep electroencephalogram’, Biological Psychiatry, 64: 344–8. [DOI] [PubMed] [Google Scholar]
- Anderson C, and Horne JA. 2003. ‘Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people’, Psychophysiology, 40: 349–57. [DOI] [PubMed] [Google Scholar]
- Andre C, Rehel S, Kuhn E, Landeau B, Moulinet I, Touron E, Ourry V, Le Du G, Mezenge F, Tomadesso C, de Flores R, Bejanin A, Sherif S, Delcroix N, Manrique A, Abbas A, Marchant NL, Lutz A, Klimecki OM, Collette F, Arenaza-Urquijo EM, Poisnel G, Vivien D, Bertran F, de la Sayette V, Chetelat G, Rauchs G, and Group Medit-Ageing Research. 2020. ‘Association of Sleep-Disordered Breathing With Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial’, JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, and Fried I. 2011. ‘Sleep spindles in humans: insights from intracranial EEG and unit recordings’, Journal of Neuroscience, 31: 17821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, Williamson B, Windler S, and McEvoy RD. 2011. ‘The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA’, Sleep, 34: 111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton SL, Vakulin A, D’Rozario A, Vincent AD, Teare A, Martin SA, Wittert GA, McEvoy RD, Catcheside PG, and Adams RJ. 2019. ‘Quantitative electroencephalography measures in rapid eye movement and nonrapid eye movement sleep are associated with apnea-hypopnea index and nocturnal hypoxemia in men’, Sleep, 42. [DOI] [PubMed] [Google Scholar]
- Ayoub A, Aumann D, Horschelmann A, Kouchekmanesch A, Paul P, Born J, and Marshall L. 2013. ‘Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity’, Sleep, 36: 905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, and Carrier J. 2011. ‘Fast and slow spindle involvement in the consolidation of a new motor sequence’, Behavioural Brain Research, 217: 117–21. [DOI] [PubMed] [Google Scholar]
- Baril AA, Gagnon K, Arbour C, Soucy JP, Montplaisir J, Gagnon JF, and Gosselin N. 2015. ‘Regional Cerebral Blood Flow during Wakeful Rest in Older Subjects with Mild to Severe Obstructive Sleep Apnea’, Sleep, 38: 1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril AA, Gagnon K, Brayet P, Montplaisir J, Carrier J, Soucy JP, Lafond C, Blais H, d’Aragon C, Gagnon JF, and Gosselin N. 2020. ‘Obstructive sleep apnea during REM sleep and daytime cerebral functioning: A regional cerebral blood flow study using high-resolution SPECT’, Journal of Cerebral Blood Flow and Metabolism, 40: 1230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, Lafond C, L’Heureux F, Gagnon JF, and Gosselin N. 2017. ‘Gray Matter Hypertrophy and Thickening with Obstructive Sleep Apnea in Middle-aged and Older Adults’, American Journal of Respiratory and Critical Care Medicine, 195: 1509–18. [DOI] [PubMed] [Google Scholar]
- Baril AA, Gagnon K, Gagnon JF, Montplaisir J, and Gosselin N. 2013. ‘Association between waking electroencephalography and cognitive event-related potentials in patients with obstructive sleep apnea’, Sleep Medicine, 14: 685–7. [DOI] [PubMed] [Google Scholar]
- Barner Christine, Ngo Hong-Viet V., Diekelmann Susanne, Weeß Hans-Günther, and Schlarb Angelika A.. 2016. ‘Memory consolidation in fragmented sleep’, Somnologie, 20: 37–46. [Google Scholar]
- Bartlett DJ, Rae C, Thompson CH, Byth K, Joffe DA, Enright T, and Grunstein RR. 2004. ‘Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea’, Sleep Medicine, 5: 593–6. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC, and Network Dominantly Inherited Alzheimer. 2012. ‘Clinical and biomarker changes in dominantly inherited Alzheimer’s disease’, New England Journal of Medicine, 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawden FC, Oliveira CA, and Caramelli P. 2011. ‘Impact of obstructive sleep apnea on cognitive performance’, Arquivos de Neuro-Psiquiatria, 69: 585–9. [DOI] [PubMed] [Google Scholar]
- Bedard MA, Montplaisir J, Richer F, and Malo J. 1991. ‘Nocturnal hypoxemia as a determinant of vigilance impairment in sleep apnea syndrome’, Chest, 100: 367–70. [DOI] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, and Cirelli C. 2017. ‘Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex’, Journal of Neuroscience, 37: 5263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DT, Phillips BA, Cook YR, Schmitt FA, Honeycutt NA, Arita AA, and Allen RS. 1990. ‘Geriatric sleep apnea syndrome: a preliminary description’, Journal of Gerontology, 45: M169–74. [DOI] [PubMed] [Google Scholar]
- Bhat Sushanth, Gupta Divya, Akel Omar, Polos Peter G, DeBari Vincent A, Akhtar Shaista, McIntyre Anna, Ming Sue X, Upadhyay Hinesh, and Chokroverty Sudhansu. 2018. ‘The relationships between improvements in daytime sleepiness, fatigue and depression and psychomotor vigilance task testing with CPAP use in patients with obstructive sleep apnea’, Sleep Medicine, 49: 81–89. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL, and Group Osteoporotic Fractures in Men Study. 2011. ‘Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study’, Sleep, 34: 1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, Song Y, Stone KL, and Group Osteoporotic Fractures in Men Study. 2015. ‘Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study’, Journal of the American Geriatrics Society, 63: 453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise Donald L. 1993. ‘Sleep apnea and cognitive function: where do we stand now?’, Sleep: Journal of Sleep Research & Sleep Medicine. [DOI] [PubMed] [Google Scholar]
- Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ, and Investigators Sleep Heart Health Study. 2002. ‘Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study’, Journal of Sleep Research, 11: 265–72. [DOI] [PubMed] [Google Scholar]
- Borbely AA 1982. ‘A two process model of sleep regulation’, Human Neurobiology, 1: 195–204. [PubMed] [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, and Lehmann D. 1981. ‘Sleep deprivation: effect on sleep stages and EEG power density in man’, Electroencephalography and Clinical Neurophysiology, 51: 483–95. [DOI] [PubMed] [Google Scholar]
- Brayet P, Petit D, Frauscher B, Gagnon JF, Gosselin N, Gagnon K, Rouleau I, and Montplaisir J. 2016. ‘Quantitative EEG of Rapid-Eye-Movement Sleep: A Marker of Amnestic Mild Cognitive Impairment’, Clinical EEG and Neuroscience, 47: 134–41. [DOI] [PubMed] [Google Scholar]
- Brillante R, Cossa G, Liu PY, and Laks L. 2012. ‘Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea’, Respirology, 17: 547–53. [DOI] [PubMed] [Google Scholar]
- Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, Gao D, Chen JC, and Wang YJ. 2015. ‘Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome’, Scientific Reports, 5: 13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, Hogan MM, Shim AM, Mukhtar F, Sharma N, Mbah AK, Seixas AA, Kam K, Zizi F, Borenstein AR, Mortimer JA, Kip KE, Morgan D, Rosenzweig I, Ayappa I, Rapoport DM, Jean-Louis G, Varga AW, Osorio RS, and Initiative Alzheimer’s Disease Neuroimaging. 2019. ‘Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes’, Sleep, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu Omonigho M., Andrade Andreia G., Umasabor-Bubu Ogie Q., Hogan Megan M., Turner Arlener D., de Leon Mony J., Ogedegbe Gbenga, Ayappa Indu, Girardin Jean-Louis G, Jackson Melinda L., Varga Andrew W., and Osorio Ricardo S.. 2020. ‘Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research’, Sleep Medicine Reviews, 50: 101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckelmuller J, Landolt HP, Stassen HH, and Achermann P. 2006. ‘Trait-like individual differences in the human sleep electroencephalogram’, Neuroscience, 138: 351–6. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Olaithe M, and Eastwood P. 2013. ‘Neurocognitive function in obstructive sleep apnoea: a meta-review’, Respirology, 18: 61–70. [DOI] [PubMed] [Google Scholar]
- Canessa Nicola, Castronovo Vincenza, Cappa Stefano F, Aloia Mark S, Marelli Sara, Falini Andrea, Alemanno Federica, and Ferini-Strambi Luigi. 2011. ‘Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment’, American Journal of Respiratory and Critical Care Medicine, 183: 1419–26. [DOI] [PubMed] [Google Scholar]
- Carnicelli L, Maestri M, Di Coscio E, Tognoni G, Fabbrini M, Schirru A, Giorgi FS, Siciliano G, Bonuccelli U, and Bonanni E. 2019. ‘A longitudinal study of polysomnographic variables in patients with mild cognitive impairment converting to Alzheimer’s disease’, Journal of Sleep Research, 28: e12821. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, and Monk TH. 2001. ‘The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old)’, Psychophysiology, 38: 232–42. [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, and Filipini D. 2011. ‘Sleep slow wave changes during the middle years of life’, European Journal of Neuroscience, 33: 758–66. [DOI] [PubMed] [Google Scholar]
- Carvalho DZ, Gerhardt GJ, Dellagustin G, de Santa-Helena EL, Lemke N, Segal AZ, and Schonwald SV. 2014. ‘Loss of sleep spindle frequency deceleration in Obstructive Sleep Apnea’, Clinical Neurophysiology, 125: 306–12. [DOI] [PubMed] [Google Scholar]
- Carvalho DZ, St Louis EK, Knopman DS, Boeve BF, Lowe VJ, Roberts RO, Mielke MM, Przybelski SA, Machulda MM, Petersen RC, Jack CR Jr., and Vemuri P. 2018. ‘Association of Excessive Daytime Sleepiness With Longitudinal beta-Amyloid Accumulation in Elderly Persons Without Dementia’, JAMA Neurol, 75: 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DZ, St Louis EK, Schwarz CG, Lowe VJ, Boeve BF, Przybelski SA, Reddy A, Mielke MM, Knopman DS, Petersen RC, Jack CR Jr., and Vemuri P. 2020. ‘Witnessed apneas are associated with elevated tau-PET levels in cognitively unimpaired elderly’, Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, Cappa SF, Strambi LF, and Falini A. 2014. ‘White matter integrity in obstructive sleep apnea before and after treatment’, Sleep, 37: 1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, and Benedict C. 2017. ‘Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease’, Sleep Medicine Reviews, 31: 102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N 2017. ‘Memory consolidation in sleep disorders’, Sleep Medicine Reviews, 35: 101–12. [DOI] [PubMed] [Google Scholar]
- Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, Huang HL, and Tsai SJ. 2013. ‘Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan’, PloS One, 8: e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, Lin HC, Lu CH, Chen PC, Huang CC, Chou KH, Su MC, Friedman M, Chen YW, and Lin WC. 2017. ‘Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea’, Journal of Sleep Research, 26: 789–98. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Burns JW, and Ruzicka DL. 2005. ‘Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea’, American Journal of Respiratory and Critical Care Medicine, 171: 652–8. [DOI] [PubMed] [Google Scholar]
- Chokroverty S, Bhat S, Donnelly D, Gupta D, Rubinstein M, and DeBari VA. 2015. ‘Sleep spindle density increases after continuous positive airway pressure titration in severe obstructive sleep apnea: a preliminary study’, Sleep Medicine, 16: 1029. [DOI] [PubMed] [Google Scholar]
- Chong MS, Ayalon L, Marler M, Loredo JS, Corey-Bloom J, Palmer BW, Liu L, and Ancoli-Israel S. 2006. ‘Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathing’, Journal of the American Geriatrics Society, 54: 777–81. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, and Pericak-Vance MA. 1993. ‘Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families’, Science, 261: 921–3. [DOI] [PubMed] [Google Scholar]
- Cosentino FI, Bosco P, Drago V, Prestianni G, Lanuzza B, Iero I, Tripodi M, Spada RS, Toscano G, Caraci F, and Ferri R. 2008. ‘The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea’, Sleep Medicine, 9: 831–9. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D’Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, and Holtzman DM. 2010. ‘YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease’, Biological Psychiatry, 68: 903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, and Naismith SL. 2017. ‘Is Obstructive Sleep Apnoea Related to Neuropsychological Function in Healthy Older Adults? A Systematic Review and Meta-Analysis’, Neuropsychology Review, 27: 389–402. [DOI] [PubMed] [Google Scholar]
- Cross Nathan E, Memarian Negar, Duffy Shantel L, Paquola Casey, LaMonica Haley, D’Rozario Angela, Lewis Simon JG, Hickie Ian B, Grunstein Ronald R, and Naismith Sharon L. 2018. ‘Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia’, European Respiratory Journal, 52: 1800740. [DOI] [PubMed] [Google Scholar]
- Crowley K, Sullivan EV, Adalsteinsson E, Pfefferbaum A, and Colrain IM. 2005. ‘Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease’, Sleep, 28: 865–70. [DOI] [PubMed] [Google Scholar]
- D’Rozario AL, Kim JW, Wong KK, Bartlett DJ, Marshall NS, Dijk DJ, Robinson PA, and Grunstein RR. 2013. ‘A new EEG biomarker of neurobehavioural impairment and sleepiness in sleep apnea patients and controls during extended wakefulness’, Clinical Neurophysiology, 124: 1605–14. [DOI] [PubMed] [Google Scholar]
- D’Rozario Angela L, Cross Nathan E, Vakulin Andrew, Bartlett Delwyn J, Wong Keith KH, Wang David, and Grunstein Ronald R. 2017. ‘Quantitative electroencephalogram measures in adult obstructive sleep apnea–potential biomarkers of neurobehavioural functioning’, Sleep Medicine Reviews, 36: 29–42. [DOI] [PubMed] [Google Scholar]
- Dalmases M, Sole-Padulles C, Torres M, Embid C, Nunez MD, Martinez-Garcia MA, Farre R, Bargallo N, Bartres-Faz D, and Montserrat JM. 2015. ‘Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study’, Chest, 148: 1214–23. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, and Ellenbogen JM. 2010. ‘Spontaneous brain rhythms predict sleep stability in the face of noise’, Current Biology, 20: R626–7. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, and Maquet P. 2008. ‘Spontaneous neural activity during human slow wave sleep’, Proceedings of the National Academy of Sciences of the United States of America, 105: 15160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, and Cichocki A. 2010. ‘Diagnosis of Alzheimer’s disease from EEG signals: where are we standing?’, Curr Alzheimer Res, 7: 487–505. [DOI] [PubMed] [Google Scholar]
- Davies CR, and Harrington JJ. 2016. ‘Impact of Obstructive Sleep Apnea on Neurocognitive Function and Impact of Continuous Positive Air Pressure’, Sleep Medicine Clinics, 11: 287–98. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, and Ferrara M. 2003. ‘Sleep spindles: an overview’, Sleep Medicine Reviews, 7: 423–40. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Gorgoni M, Reda F, Lauri G, Truglia I, Cordone S, Scarpelli S, Mangiaruga A, D’Atri A, Lacidogna G, Ferrara M, Marra C, and Rossini PM. 2017. ‘The Fall of Sleep K-Complex in Alzheimer Disease’, Scientific Reports, 7: 39688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro De, Luigi Cristina Marzano, Fratello Fabiana, Moroni Fabio, Maria Concetta Pellicciari Fabio Ferlazzo, Costa Stefania, Couyoumdjian Alessandro, Curcio Giuseppe, and Sforza Emilia. 2008. ‘The electroencephalographic fingerprint of sleep is genetically determined: a twin study’, Annals of Neurology, 64: 455–60. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim HJ, Fortea J, Fossati S, Laska E, Siegel C, Butler T, Li Y, Rusinek H, Zetterberg H, Blennow K, Initiative Alzheimer’s Disease Neuroimaging, and Center National Alzheimer’s Coordinating. 2018. ‘The nonlinear relationship between cerebrospinal fluid Abeta42 and tau in preclinical Alzheimer’s disease’, PloS One, 13: e0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, and van den Hoofdakker RH. 1989. ‘All night spectral analysis of EEG sleep in young adult and middle-aged male subjects’, Neurobiology of Aging, 10: 677–82. [DOI] [PubMed] [Google Scholar]
- Djonlagic I, Aeschbach D, Harrison SL, Dean D, Yaffe K, Ancoli-Israel S, Stone K, and Redline S. 2019. ‘Associations between quantitative sleep EEG and subsequent cognitive decline in older women’, Journal of Sleep Research, 28: e12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonlagic I, Guo M, Matteis P, Carusona A, Stickgold R, and Malhotra A. 2014. ‘Untreated sleep-disordered breathing: links to aging-related decline in sleep-dependent memory consolidation’, PloS One, 9: e85918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2015. ‘First night of CPAP: impact on memory consolidation attention and subjective experience’, Sleep Medicine, 16: 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy Shantel L., Lagopoulos Jim, Terpening Zoe, Lewis Simon J.G., Grunstein Ron, Mowszowski Loren, Cross Nathan, Hermens Daniel F., Hickie Ian B., and Naismith Sharon L.. 2016. ‘Association of Anterior Cingulate Glutathione with Sleep Apnea in Older Adults At-Risk for Dementia’, Sleep, 39: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A, and Wellman A. 2013. ‘Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets’, American Journal of Respiratory and Critical Care Medicine, 188: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Donat CK, and Sastre M. 2018. ‘In vivo Imaging of Glial Activation in Alzheimer’s Disease’, Frontiers in Neurology, 9: 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias A, Cummins T, Tyrrell R, Lamb F, Dore V, Williams R, Rosenfeld JV, Hopwood M, Villemagne VL, and Rowe CC. 2018. ‘Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-beta and Tau Imaging’, Journal of Alzheimer’s Disease, 66: 733–41. [DOI] [PubMed] [Google Scholar]
- Ewers M, Franzmeier N, Suarez-Calvet M, Morenas-Rodriguez E, Caballero MAA, Kleinberger G, Piccio L, Cruchaga C, Deming Y, Dichgans M, Trojanowski JQ, Shaw LM, Weiner MW, Haass C, and Initiative Alzheimer’s Disease Neuroimaging. 2019. ‘Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease’, Science Translational Medicine, 11: eaav6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, Morris JC, Holtzman DM, and Network Dominantly Inherited Alzheimer. 2014. ‘Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease’, Science Translational Medicine, 6: 226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, and Cappa SF. 2003. ‘Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP)’, Brain Research Bulletin, 61: 87–92. [DOI] [PubMed] [Google Scholar]
- Fietze I, Quispe-Bravo S, Hansch T, Rottig J, Baumann G, and Witt C. 1997. ‘Arousals and sleep stages in patients with obstructive sleep apnoea syndrome: Changes under nCPAP treatment’, Journal of Sleep Research, 6: 128–33. [DOI] [PubMed] [Google Scholar]
- Fogel SM, and Smith CT. 2011. ‘The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation’, Neuroscience & Biobehavioral Reviews, 35: 1154–65. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Masaki K, White L, Larkin EK, Monjan A, and Redline S. 2003. ‘Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men’, Sleep, 26: 596–9. [DOI] [PubMed] [Google Scholar]
- Franca LGS, Miranda JGV, Leite M, Sharma NK, Walker MC, Lemieux L, and Wang Y. 2018. ‘Fractal and Multifractal Properties of Electrographic Recordings of Human Brain Activity: Toward Its Use as a Signal Feature for Machine Learning in Clinical Applications’, Frontiers in Physiology, 9: 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin Karl A, and Lindberg Eva. 2015. ‘Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea’, Journal of Thoracic Disease, 7: 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, and Lewis LD. 2019. ‘Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep’, Science, 366: 628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnadoux F, Priou P, Meslier N, and Trzepizur W. 2017. ‘Effects of sleep apnoea therapy on blood pressure and metabolism: a CPAP sex gap?’, Eur Respir J, 50. [DOI] [PubMed] [Google Scholar]
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Decary A, Lafond C, Desautels A, Montplaisir J, and Gosselin N. 2014. ‘Cognitive impairment in obstructive sleep apnea’, Pathologie Biologie, 62: 233–40. [DOI] [PubMed] [Google Scholar]
- Gagnon Katia, Baril Andrée-Ann, Montplaisir Jacques, Carrier Julie, Louis De Beaumont Caroline D Aragon, Chami Sirin, Pelleieux Sandra, Poirier Judes, and Gauthier Serge. 2019. ‘Disconnection Between Self-Reported and Objective Cognitive Impairment in Obstructive Sleep Apnea’, Journal of Clinical Sleep Medicine, 15: 409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard JM, and Blois R. 1981. ‘Spindle density in sleep of normal subjects’, Sleep, 4: 385–91. [DOI] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, and Born J. 2002. ‘Learning-dependent increases in sleep spindle density’, Journal of Neuroscience, 22: 6830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, and Hopkins RO. 2004. ‘Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea’, Journal of the International Neuropsychological Society, 10: 60–71. [DOI] [PubMed] [Google Scholar]
- Goldberger AL 2006. ‘Giles f. Filley lecture. Complex systems’, Proceedings of the American Thoracic Society, 3: 467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PCh, Peng CK, and Stanley HE. 2002. ‘Fractal dynamics in physiology: alterations with disease and aging’, Proceedings of the National Academy of Sciences of the United States of America, 99 Suppl 1: 2466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, D’Atri A, Tempesta D, Ferrara M, Marra C, Rossini PM, and De Gennaro L. 2016. ‘Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment’, Neural Plasticity, 2016: 8376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N, Baril AA, Osorio RS, Kaminska M, and Carrier J. 2019. ‘Obstructive Sleep Apnea and the Risk of Cognitive Decline in Older Adults’, American Journal of Respiratory and Critical Care Medicine, 199: 142–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, and Young T. 2004. ‘APOE4 is associated with obstructive sleep apnea/hypopnea’, The Sleep Heart Health Study, 63: 664–68. [DOI] [PubMed] [Google Scholar]
- Greneche J, J Krieger, Erhardt C, Bonnefond A, Eschenlauer A, Muzet A, and Tassi P. 2008. ‘EEG spectral power and sleepiness during 24h of sustained wakefulness in patients with obstructive sleep apnea syndrome’, Clinical Neurophysiology, 119: 418–28. [DOI] [PubMed] [Google Scholar]
- Greneche J, Krieger J, Bertrand F, Erhardt C, Muzet A, and Tassi P. 2011. ‘Effect of continuous positive airway pressure treatment on the subsequent EEG spectral power and sleepiness over sustained wakefulness in patients with obstructive sleep apnea-hypopnea syndrome’, Clinical Neurophysiology, 122: 958–65. [DOI] [PubMed] [Google Scholar]
- Greneche J, Saremi M, Erhardt C, Hoeft A, Eschenlauer A, Muzet A, and Tassi P. 2008. ‘Severity of obstructive sleep apnoea/hypopnoea syndrome and subsequent waking EEG spectral power’, European Respiratory Journal, 32: 705–9. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, and Group Alzheimer Genetic Analysis. 2013. ‘TREM2 variants in Alzheimer’s disease’, New England Journal of Medicine, 368: 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Do Kim Y, Chowdhuri S, Horita M, Ohayon M, and Kushida C. 2001. ‘Sleep and daytime sleepiness in upper airway resistance syndrome compared to obstructive sleep apnoea syndrome’, European Respiratory Journal, 17: 838–47. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Lopes MC, Hagen CC, and da Rosa A. 2007. ‘The cyclic alternating pattern demonstrates increased sleep instability and correlates with fatigue and sleepiness in adults with upper airway resistance syndrome’, Sleep, 30: 641–7. [DOI] [PubMed] [Google Scholar]
- Halasz P, Bodizs R, Parrino L, and Terzano M. 2014. ‘Two features of sleep slow waves: homeostatic and reactive aspects--from long term to instant sleep homeostasis’, Sleep Medicine, 15: 1184–95. [DOI] [PubMed] [Google Scholar]
- Handa SS, Baba S, Yamashita K, Nishizaka M, and Ando S. 2019. ‘The severity of obstructive sleep apnea syndrome cannot predict the accumulation of brain amyloid by imaging with [11C]-Pittsburgh compound B PET computed tomography in patients with a normal cognitive function’, Annals of Nuclear Medicine, 33: 541–44. [DOI] [PubMed] [Google Scholar]
- Hayward L, Mant A, Eyland A, Hewitt H, Purcell C, Turner J, Goode E, Le Count A, Pond D, and Saunders N. 1992. ‘Sleep disordered breathing and cognitive function in a retirement village population’, Age and Ageing, 21: 121–8. [DOI] [PubMed] [Google Scholar]
- Heinzer R, Gaudreau H, Decary A, Sforza E, Petit D, Morisson F, and Montplaisir J. 2001. ‘Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness’, Chest, 119: 1807–13. [DOI] [PubMed] [Google Scholar]
- Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, and Haba-Rubio J. 2015. ‘Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study’, Lancet Respiratory Medicine, 3: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave A, Heywood W, Paterson R, Magdalinou N, Svensson J, Johansson P, Ohrfelt A, Blennow K, Hardy J, Schott J, Mills K, and Zetterberg H. 2016. ‘Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease’, Molecular Neurodegeneration, 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen SL, Joutsen A, and Virkkala J. 2004. ‘Visual assessment of selected high amplitude frontopolar slow waves of sleep: differences between healthy subjects and apnea patients’, Clinical EEG and Neuroscience, 35: 125–31. [DOI] [PubMed] [Google Scholar]
- Hrubos-Strom H, Nordhus IH, Einvik G, Randby A, Omland T, Sundet K, Moum T, and Dammen T. 2012. ‘Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project’, Sleep Breath, 16: 223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huupponen E, Himanen SL, Varri A, Hasan J, Lehtokangas M, and Saarinen J. 2002. ‘A study on gender and age differences in sleep spindles’, Neuropsychobiology, 45: 99–105. [DOI] [PubMed] [Google Scholar]
- Huupponen E, Kulkas A, Tenhunen M, Saastamoinen A, Hasan J, and Himanen SL. 2008. ‘Diffuse sleep spindles show similar frequency in central and frontopolar positions’, Journal of Neuroscience Methods, 172: 54–9. [DOI] [PubMed] [Google Scholar]
- ICSD-3, American Academy of Sleep Medicine. 2014. ‘International classification of sleep disorders—third edition (ICSD-3)’, Diagnostic and coding manual, AASM Resource Library. [Google Scholar]
- Jackson ML, McEvoy RD, Banks S, and Barnes M. 2018. ‘Neurobehavioral Impairment and CPAP Treatment Response in Mild-Moderate Obstructive Sleep Apneas’, Journal of Clinical Sleep Medicine, 14: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari B, and Mohsenin V. 2016. ‘Chitinase-3-like protein-1 (YKL-40) as a marker of endothelial dysfunction in obstructive sleep apnea’, Sleep Medicine, 25: 87–92. [DOI] [PubMed] [Google Scholar]
- Jobert M, Wilson FJ, Ruigt GS, Brunovsky M, Prichep LS, Drinkenburg WH, and Ipeg Pharmaco-EEG Guidelines Committee. 2012. ‘Guidelines for the recording and evaluation of pharmaco-EEG data in man: the International Pharmaco-EEG Society (IPEG)’, Neuropsychobiology, 66: 201–20. [DOI] [PubMed] [Google Scholar]
- Jones SG, Riedner BA, Smith RF, Ferrarelli F, Tononi G, Davidson RJ, and Benca RM. 2014. ‘Regional reductions in sleep electroencephalography power in obstructive sleep apnea: a high-density EEG study’, Sleep, 37: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, and Stefansson K. 2013. ‘Variant of TREM2 associated with the risk of Alzheimer’s disease’, New England Journal of Medicine, 368: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Yoon IY, Lee SD, Kim TH, Choe JY, and Kim KW. 2012. ‘Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults’, Journal of the American Geriatrics Society, 60: 1099–103. [DOI] [PubMed] [Google Scholar]
- Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, Crimmins DL, Fagan AM, and Holtzman DM. 2016. ‘Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid’, Annals of Neurology, 80: 154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, and Holtzman DM. 2013. ‘Sleep quality and preclinical Alzheimer disease’, JAMA Neurol, 70: 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen Jahr, and Holtzman DM. 2017. ‘Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels’, Brain, 140: 2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Zangrilli MA, Finn MB, Fagan AM, and Holtzman DM. 2019. ‘Obstructive sleep apnea treatment, slow wave activity, and amyloid-beta’, Annals of Neurology, 85: 291–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado JL, Luna-Villegas G, and Buela-Casal G. 1989. ‘Normal human subjects with slow reaction times and larger time estimations after waking have diminished delta sleep’, Electroencephalography and Clinical Neurophysiology, 73: 124–8. [DOI] [PubMed] [Google Scholar]
- Kadotani Hiroshi, Kadotani Tomiko, Young Terry, Peppard Paul E., Finn Laurel, Colrain Ian M., Murphy Greer M. Jr, and Mignot Emmanuel. 2001. ‘Association Between Apolipoprotein E ∈4 and Sleep-Disordered Breathing in Adults’, JAMA, 285: 2888–90. [DOI] [PubMed] [Google Scholar]
- Kainulainen Samu, Duce Brett, Korkalainen Henri, Oksenberg Arie, Leino Akseli, Arnardottir Erna S., Kulkas Antti, Myllymaa Sami, Töyräs Juha, and Leppänen Timo. 2020. ‘Severe desaturations increase psychomotor vigilance task-based median reaction time and number of lapses in obstructive sleep apnoea patients’, European Respiratory Journal, 55: 1901849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Parekh A, Sharma RA, Andrade A, Lewin M, Castillo B, Bubu OM, Chua NJ, Miller MD, Mullins AE, Glodzik L, Mosconi L, Gosselin N, Prathamesh K, Chen Z, Blennow K, Zetterberg H, Bagchi N, Cavedoni B, Rapoport DM, Ayappa I, de Leon MJ, Petkova E, Varga AW, and Osorio RS. 2019. ‘Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: novel roles for sleep spindles and tau’, Molecular Neurodegeneration, 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Korey, Pettibone Ward D., Shim Kaitlyn, Chen Rebecca K., and Varga Andrew W.. 2019. ‘Dynamics of sleep spindles and coupling to slow oscillations following motor learning in adult mice’, Neurobiology of Learning and Memory, 166: 107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbayashi T, Inoue Y, Kawanishi K, Takasaki H, Aizawa R, Takahashi K, Ogawa Y, Abe M, Hishikawa Y, and Shimizu T. 2003. ‘CSF hypocretin measures in patients with obstructive sleep apnea’, Journal of Sleep Research, 12: 339–41. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, and Holtzman DM. 2009. ‘Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle’, Science, 326: 1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielb SA, Ancoli-Israel S, Rebok GW, and Spira AP. 2012. ‘Cognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment’, Neuromolecular Medicine, 14: 180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Dinges DF, and Young T. 2007. ‘Sleep-disordered breathing and psychomotor vigilance in a community-based sample’, Sleep, 30: 1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jaekyung, Gulati Tanuj, and Ganguly Karunesh. 2019. ‘Competing Roles of Slow Oscillations and Delta Waves in Memory Consolidation versus Forgetting’, Cell, 179: 514–26. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, and Nedergaard M. 2016. ‘Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms?’, Journal of Cerebral Blood Flow and Metabolism, 36: 1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing JG, Molle M, Weber F, Supp G, Hipp JF, Engel AK, and Born J. 2016. ‘Spindle activity phase-locked to sleep slow oscillations’, NeuroImage, 134: 607–16. [DOI] [PubMed] [Google Scholar]
- Kloepfer C, Riemann D, Nofzinger EA, Feige B, Unterrainer J, O’Hara R, Sorichter S, and Nissen C. 2009. ‘Memory Before and After Sleep in Patients with Moderate Obstructive Sleep Apnea’, Journal of Clinical Sleep Medicine, 5: 540–48. [PMC free article] [PubMed] [Google Scholar]
- Korkmaz Selda, Bilecenoglu Nedime Tugce, Aksu Murat, and Yoldas Tahir Kurtulus. 2018. ‘Cyclic Alternating Pattern in Obstructive Sleep Apnea Patients with versus without Excessive Sleepiness’, Sleep disorders, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, Simon RD Jr., Guilleminault C, White DP, Goodwin JL, Schweitzer PK, Leary EB, Hyde PR, Hirshkowitz M, Green S, McEvoy LK, Chan C, Gevins A, Kay GG, Bloch DA, Crabtree T, and Dement WC. 2012. ‘Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES)’, Sleep, 35: 1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylstra WA, Aaronson JA, Hofman WF, and Schmand BA. 2013. ‘Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis’, Sleep Medicine Reviews, 17: 341–7. [DOI] [PubMed] [Google Scholar]
- Lambert Christian, Chowdhury Rumana, FitzGerald Thomas HB, Fleming Stephen M, Lutti Antoine, Hutton Chloe, Draganski Bogdan, Frackowiak Richard, and Ashburner John. 2013. ‘Characterizing Aging in the Human Brainstem Using Quantitative Multimodal MRI Analysis’, Frontiers in Human Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, and Borbely AA. 1996. ‘Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men’, Brain Research, 738: 205–12. [DOI] [PubMed] [Google Scholar]
- Landry S, Anderson C, Andrewartha P, Sasse A, and Conduit R. 2014. ‘The impact of obstructive sleep apnea on motor skill acquisition and consolidation’, Journal of Clinical Sleep Medicine, 10: 491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry S, O’Driscoll DM, Hamilton GS, and Conduit R. 2016. ‘Overnight Motor Skill Learning Outcomes in Obstructive Sleep Apnea: Effect of Continuous Positive Airway Pressure’, Journal of Clinical Sleep Medicine, 12: 681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latreille V, Gaubert M, Dube J, Lina JM, Gagnon JF, and Carrier J. 2019. ‘Age-related cortical signatures of human sleep electroencephalography’, Neurobiology of Aging, 76: 106–14. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yang SW, Ju YJ, Ki SK, and Chun KH. 2019. ‘Sleep-disordered breathing and Alzheimer’s disease: A nationwide cohort study’, Psychiatry Research, 273: 624–30. [DOI] [PubMed] [Google Scholar]
- Lee MH, Yun CH, Min A, Hwang YH, Lee SK, Kim DY, Thomas RJ, Han BS, and Shin C. 2019. ‘Altered structural brain network resulting from white matter injury in obstructive sleep apnea’, Sleep, 42. [DOI] [PubMed] [Google Scholar]
- Lee SD, Ju G, Kim JW, and Yoon IY. 2012. ‘Improvement of EEG slowing in OSAS after CPAP treatment’, Journal of Psychosomatic Research, 73: 126–31. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim JW, Lee YJ, and Jeong DU. 2016. ‘Sleep EEG Characteristics in Young and Elderly Patients with Obstructive Sleep Apnea Syndrome’, Psychiatry Investigation, 13: 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, McEvoy CT, Allen IE, and Yaffe K. 2017. ‘Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis’, JAMA Neurol, 74: 1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski A, Rosipal R, and Dorffner G. 2013. ‘On the Individuality of Sleep EEG Spectra’, J Psychophysiol, 27: 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Yu L, Lim ASP, Buchman AS, Scheer Fajl, Shea SA, Schneider JA, Bennett DA, and Hu K. 2018. ‘Fractal regulation and incident Alzheimer’s disease in elderly individuals’, Alzheimers Dement, 14: 1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, and Placidi F. 2017. ‘Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes’, Sleep, 40. [DOI] [PubMed] [Google Scholar]
- Liguori C, Mercuri NB, Nuccetelli M, Izzi F, Cordella A, Bernardini S, and Placidi F. 2019. ‘Obstructive sleep apnea may induce orexinergic system and cerebral beta-amyloid metabolism dysregulation: is it a further proof for Alzheimer’s disease risk?’, Sleep Medicine, 56: 171–76. [DOI] [PubMed] [Google Scholar]
- Liguori C, Nuccetelli M, Izzi F, Sancesario G, Romigi A, Martorana A, Amoroso C, Bernardini S, Marciani MG, Mercuri NB, and Placidi F. 2016. ‘Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease’, Neurobiology of Aging, 40: 120–26. [DOI] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, and Bennett DA. 2013. ‘Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons’, Sleep, 36: 1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DC, and Pack AI. 2014. ‘Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier’, Sleep Medicine Reviews, 18: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Wu X, Jin X, Peng F, and Lin J. 2016. ‘Apolipoprotein E varepsilon2/varepsilon3/varepsilon4 variant in association with obstructive sleep apnoea and lipid profile: A meta-analysis’, Journal of International Medical Research, 44: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey Brendan P., McCullough Austin, Landsness Eric C., Toedebusch Cristina D., McLeland Jennifer S., Zaza Aiad M., Fagan Anne M., McCue Lena, Xiong Chengjie, Morris John C., Benzinger Tammie L. S., and Holtzman David M.. 2019. ‘Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease’, Science Translational Medicine, 11: eaau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, Wruck LM, MacLehose RF, and Alonso A. 2016. ‘Obstructive Sleep Apnea and 15-Year Cognitive Decline: The Atherosclerosis Risk in Communities (ARIC) Study’, Sleep, 39: 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Yan, Shi Wenbin, Peng Chung-Kang, and Yang Albert C. 2018. ‘Nonlinear dynamical analysis of sleep electroencephalography using fractal and entropy approaches’, Sleep Medicine Reviews, 37: 85–93. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, and Harper RM. 2002. ‘Brain morphology associated with obstructive sleep apnea’, American Journal of Respiratory and Critical Care Medicine, 166: 1382–7. [DOI] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, and Harper RM. 2008. ‘Brain structural changes in obstructive sleep apnea’, Sleep, 31: 967–77. [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Sarma MK, Prasad JP, Ogren JA, Aysola R, Harper RM, and Thomas MA. 2017. ‘Obstructive sleep apnea is associated with altered midbrain chemical concentrations’, Neuroscience, 363: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaeva Irina, Berdina Olga, Rychkova Liubov, and Bugun Olga. 2017. ‘Sleep spindle characteristics in overweight adolescents with obstructive sleep apnea syndrome’, Sleep and Biological Rhythms, 15: 251–57. [Google Scholar]
- Maestri M, Carnicelli L, Tognoni G, Di Coscio E, Giorgi FS, Volpi L, Economou NT, Ktonas P, Ferri R, Bonuccelli U, and Bonanni E. 2015. ‘Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study’, Sleep Medicine, 16: 1139–45. [DOI] [PubMed] [Google Scholar]
- Mahley Robert W, and Rall Stanley C Jr. 2000. ‘Apolipoprotein E: far more than a lipid transport protein’, Annual review of genomics human genetics, 1: 507–37. [DOI] [PubMed] [Google Scholar]
- Mak-McCully RA, Rolland M, Sargsyan A, Gonzalez C, Magnin M, Chauvel P, Rey M, Bastuji H, and Halgren E. 2017. ‘Coordination of cortical and thalamic activity during non-REM sleep in humans’, Nat Commun, 8: 15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, and Walker MP. 2015. ‘beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation’, Nature Neuroscience, 18: 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, and Walker MP. 2013. ‘Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging’, Nature Neuroscience, 16: 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresky HS, Shpirer I, Klar MM, Levitt M, Sasson E, and Tal S. 2019. ‘Continuous positive airway pressure alters brain microstructure and perfusion patterns in patients with obstructive sleep apnea’, Sleep Medicine, 57: 61–69. [DOI] [PubMed] [Google Scholar]
- Martin MS, Sforza E, Roche F, Barthelemy JC, Thomas-Anterion C, and Proof study group. 2015. ‘Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort’, Sleep, 38: 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, and Carrier J. 2013. ‘Topography of age-related changes in sleep spindles’, Neurobiology of Aging, 34: 468–76. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia MA, Chiner E, Hernandez L, Cortes JP, Catalan P, Ponce S, Diaz JR, Pastor E, Vigil L, Carmona C, Montserrat JM, Aizpuru F, Lloberes P, Mayos M, Selma MJ, Cifuentes JF, Munoz A, and Network Spanish Sleep. 2015. ‘Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment’, European Respiratory Journal, 46: 142–51. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, and Tononi G. 2004. ‘The sleep slow oscillation as a traveling wave’, Journal of Neuroscience, 24: 6862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu A, Mazza S, Decary A, Massicotte-Marquez J, Petit D, Gosselin N, Malo J, and Montplaisir J. 2008. ‘Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients’, Sleep Medicine, 9: 112–20. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Mazza S, Petit D, Décary A, Massicotte-Marquez J, Malo J, and Montplaisir J. 2007. ‘Does age worsen EEG slowing and attention deficits in obstructive sleep apnea syndrome?’, Clinical Neurophysiology, 118: 1538–44. [DOI] [PubMed] [Google Scholar]
- McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, and Douglas NJ. 1999. ‘Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome’, American Journal of Respiratory and Critical Care Medicine, 159: 1108–14. [DOI] [PubMed] [Google Scholar]
- McArdle N, and Douglas NJ. 2001. ‘Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial’, American Journal of Respiratory and Critical Care Medicine, 164: 1459–63. [DOI] [PubMed] [Google Scholar]
- McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Save Investigators, and Coordinators. 2016. ‘CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea’, New England Journal of Medicine, 375: 919–31. [DOI] [PubMed] [Google Scholar]
- McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, Nunn AJ, Stradling JR, Riha RL, Morrell MJ, and Predict Investigators. 2014. ‘Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial’, Lancet Respir Med, 2: 804–12. [DOI] [PubMed] [Google Scholar]
- Melpignano A, Parrino L, Santamaria J, Gaig C, Trippi I, Serradell M, Mutti C, Ricco M, and Iranzo A. 2019. ‘Isolated rapid eye movement sleep behavior disorder and cyclic alternating pattern: is sleep microstructure a predictive parameter of neurodegeneration?’, Sleep, 42. [DOI] [PubMed] [Google Scholar]
- Molle M, Bergmann TO, Marshall L, and Born J. 2011. ‘Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing’, Sleep, 34: 1411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle M, and Born J. 2011. ‘Slow oscillations orchestrating fast oscillations and memory consolidation’, Progress in Brain Research, 193: 93–110. [DOI] [PubMed] [Google Scholar]
- Molle M, Marshall L, Gais S, and Born J. 2002. ‘Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep’, Journal of Neuroscience, 22: 10941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisson F, Decary A, Petit D, Lavigne G, Malo J, and Montplaisir J. 2001. ‘Daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive airway pressure’, Chest, 119: 45–52. [DOI] [PubMed] [Google Scholar]
- Morisson F, Lavigne G, Petit D, Nielsen T, Malo J, and Montplaisir J. 1998. ‘Spectral analysis of wakefulness and REM sleep EEG in patients with sleep apnoea syndrome’, European Respiratory Journal, 11: 1135–40. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, and Corfield DR. 2003. ‘Changes in brain morphology associated with obstructive sleep apnea’, Sleep Medicine, 4: 451–4. [DOI] [PubMed] [Google Scholar]
- Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, Mysliwiec V, and Gill J. 2018. ‘Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea’, Sleep Medicine, 43: 71–76. [DOI] [PubMed] [Google Scholar]
- Mullins AE, Kim JW, Wong K, Bartlett D, Vakulin A, Dijk D, Marshall N, Grunstein R, and D’Rozario A. 2020. ‘Sleep EEG microstructure is associated with neurobehavioral impairment after extended wakefulness in obstructive sleep apnea’, Sleep and Breathing. [DOI] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, and Tononi G. 2009. ‘Source modeling sleep slow waves’, Proceedings of the National Academy of Sciences of the United States of America, 106: 1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, and Ju YS. 2018. ‘Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease’, JAMA Neurol, 75: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele B, Thouvard V, Pepin JL, Levy P, Bonnet C, Perret JE, Pellat J, and Feuerstein C. 1995. ‘Deficits of cognitive executive functions in patients with sleep apnea syndrome’, Sleep, 18: 43–52. [PubMed] [Google Scholar]
- Naëgelé Bernadette, Launois Sandrine H, Mazza Stéphanie, Feuerstein Claude, Pépin Jean-Louis, and Lévy Patrick. 2006. ‘Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory’, Sleep, 29: 533–44. [DOI] [PubMed] [Google Scholar]
- Nguyen CD, Wellman A, Jordan AS, and Eckert DJ. 2016. ‘Mild Airflow Limitation during N2 Sleep Increases K-complex Frequency and Slows Electroencephalographic Activity’, Sleep, 39: 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Finn L, Mignot E, Salzieder N, and Peppard PE. 2013. ‘Association of sleep disordered breathing and cognitive deficit in APOE epsilon4 carriers’, Sleep, 36: 873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, and Tononi G. 2011. ‘Regional slow waves and spindles in human sleep’, Neuron, 70: 153–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, Tarquinio N, Jackson GD, and Pierce RJ. 2005. ‘Cerebral structural changes in severe obstructive sleep apnea’, American Journal of Respiratory and Critical Care Medicine, 171: 1185–90. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, and Vitiello MV. 2004. ‘Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan’, Sleep, 27: 1255–73. [DOI] [PubMed] [Google Scholar]
- Olsson M, Arlig J, Hedner J, Blennow K, and Zetterberg H. 2018. ‘Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease’, Sleep, 41. [DOI] [PubMed] [Google Scholar]
- Ondze B, Espa F, Dauvilliers Y, Billiard M, and Besset A. 2003. ‘Sleep architecture, slow wave activity and sleep spindles in mild sleep disordered breathing’, Clinical Neurophysiology, 114: 867–74. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, Burschtin OE, Taxin Z, During E, Spector N, Biagioni M, Pirraglia E, Lau H, Zetterberg H, Blennow K, Lu SE, Mosconi L, Glodzik L, Rapoport DM, and de Leon MJ. 2014. ‘The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals’, Neurobiol Aging, 35: 1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Ducca EL, Wohlleber ME, Tanzi EB, Gumb T, Twumasi A, Tweardy S, Lewis C, Fischer E, Koushyk V, Cuartero-Toledo M, Sheikh MO, Pirraglia E, Zetterberg H, Blennow K, Lu SE, Mosconi L, Glodzik L, Schuetz S, Varga AW, Ayappa I, Rapoport DM, and de Leon MJ. 2016. ‘Orexin-A is Associated with Increases in Cerebrospinal Fluid Phosphorylated-Tau in Cognitively Normal Elderly Subjects’, Sleep, 39: 1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ, and Initiative Alzheimer’s Disease Neuroimaging. 2015. ‘Sleep-disordered breathing advances cognitive decline in the elderly’, Neurology, 84: 1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AI 2016. ‘Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care?’, Ann Am Thorac Soc, 13: 1456–67. [DOI] [PubMed] [Google Scholar]
- Palomares JA, Tummala S, Wang DJ, Park B, Woo MA, Kang DW, St Lawrence KS, Harper RM, and Kumar R. 2015. ‘Water Exchange across the Blood-Brain Barrier in Obstructive Sleep Apnea: An MRI Diffusion-Weighted Pseudo-Continuous Arterial Spin Labeling Study’, Journal of Neuroimaging, 25: 900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YY, Deng Y, Xu X, Liu YP, and Liu HG. 2015. ‘Effects of Continuous Positive Airway Pressure on Cognitive Deficits in Middle-aged Patients with Obstructive Sleep Apnea Syndrome: A Meta-analysis of Randomized Controlled Trials’, Chinese Medical Journal (Engl.), 128: 2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parapatics S, Anderer P, Gruber G, Saletu B, Saletu-Zyhlarz G, and Dorffner G. 2015. ‘K-complex amplitude as a marker of sleep homeostasis in obstructive sleep apnea syndrome and healthy controls’, Somnologie - Schlafforschung und Schlafmedizin, 19: 22–29. [Google Scholar]
- Parekh A, Mullins AE, Kam K, Varga AW, Rapoport DM, and Ayappa I. 2019. ‘Slow-wave activity surrounding stage N2 K-complexes and daytime function measured by psychomotor vigilance test in obstructive sleep apnea’, Sleep, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino L, Ferri R, Bruni O, and Terzano MG. 2012. ‘Cyclic alternating pattern (CAP): the marker of sleep instability’, Sleep Medicine Reviews, 16: 27–45. [DOI] [PubMed] [Google Scholar]
- Parrino L, Smerieri A, Boselli M, Spaggiari MC, and Terzano MG. 2000. ‘Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome’, Neurology, 54: 1633–40. [DOI] [PubMed] [Google Scholar]
- Parrino L, Thomas RJ, Smerieri A, Spaggiari MC, Del Felice A, and Terzano MG. 2005. ‘Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment’, Clinical Neurophysiology, 116: 2228–39. [DOI] [PubMed] [Google Scholar]
- Parrino L, and Vaudano AE. 2018. ‘The resilient brain and the guardians of sleep: New perspectives on old assumptions’, Sleep Medicine Reviews, 39: 98–107. [DOI] [PubMed] [Google Scholar]
- Parrino Liborio, and Terzano Mario Giovanni. 2017. ‘Central Nervous System Arousals and Cyclic Alternating Patterns’ in Roth Thomas and Dement William C. (eds.), Principles and Practice of Sleep Medicine (Elsevier; ). [Google Scholar]
- Pase MP, Beiser AS, Himali JJ, Satizabal CL, Aparicio HJ, DeCarli C, Chene G, Dufouil C, and Seshadri S. 2019. ‘Assessment of Plasma Total Tau Level as a Predictive Biomarker for Dementia and Related Endophenotypes’, JAMA Neurol, 76: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ, Thomas RJ, Gottlieb DJ, Auerbach SH, and Seshadri S. 2017. ‘Sleep architecture and the risk of incident dementia in the community’, Neurology, 89: 1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, and Montplaisir J. 2004. ‘Sleep and quantitative EEG in neurodegenerative disorders’, Journal of Psychosomatic Research, 56: 487–96. [DOI] [PubMed] [Google Scholar]
- Petit D, Lorrain D, Gauthier S, and Montplaisir J. 1993. ‘Regional spectral analysis of the REM sleep EEG in mild to moderate Alzheimer’s disease’, Neurobiology of Aging, 14: 141–5. [DOI] [PubMed] [Google Scholar]
- Petit Dominique, Montplaisir Jacques, St. Louis Erik K., and Boeve Bradley F.. 2017. ‘Alzheimer Disease and Other Dementias’ in Roth Thomas and Dement William C. (eds.), Principles and Practice of Sleep Medicine (Elsevier; ). [Google Scholar]
- Phillips BA, Berry DT, Schmitt FA, Magan LK, Gerhardstein DC, and Cook YR. 1992. ‘Sleep-disordered breathing in the healthy elderly. Clinically significant?’, Chest, 101: 345–9. [DOI] [PubMed] [Google Scholar]
- Poil SS, de Haan W, van der Flier WM, Mansvelder HD, Scheltens P, and Linkenkaer-Hansen K. 2013. ‘Integrative EEG biomarkers predict progression to Alzheimer’s disease at the MCI stage’, Frontiers in Aging Neuroscience, 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon JJY, Chapman JL, Wong KKH, Mullins AE, Cho G, Kim JW, Yee BJ, Grunstein RR, Marshall NS, and D’Rozario AL. 2019. ‘Intra-individual stability of NREM sleep quantitative EEG measures in obstructive sleep apnea’, Journal of Sleep Research, 28: e12838. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, Panagiotaropoulou G, Saxena R, Pan JQ, Smoller JW, Redline S, and Stickgold R. 2017. ‘Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource’, Nat Commun, 8: 15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, Kaszniak A, Boland LL, Caccappolo E, and Bootzin RR. 2006. ‘Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study’, Sleep Medicine, 7: 498–507. [DOI] [PubMed] [Google Scholar]
- Raja R, Rosenberg GA, and Caprihan A. 2018. ‘MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies’, Neuropharmacology, 134: 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, and Born J. 2013. ‘About sleep’s role in memory’, Physiological Reviews, 93: 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs Géraldine, Schabus Manuel, Parapatics Silvia, Bertran Françoise, Clochon Patrice, Hot Pascal, Denise Pierre, Desgranges Béatrice, Eustache Francis, and Gruber Georg. 2008. ‘Is there a link between sleep changes and memory in Alzheimer’s disease?’, Neuroreport, 19: 1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, Wang Y, Sawyer A, Weaver T, Lozano A, Carter P, and Johnson J. 2019. ‘CPAP Adherence May Slow 1-Year Cognitive Decline in Older Adults with Mild Cognitive Impairment and Apnea’, Journal of the American Geriatrics Society, 67: 558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Zorick F, Wittig R, Conway W, and Roth T. 1989. ‘Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders’, Chest, 95: 1202–6. [DOI] [PubMed] [Google Scholar]
- Rosenzweig I, Glasser M, Crum WR, Kempton MJ, Milosevic M, McMillan A, Leschziner GD, Kumari V, Goadsby P, Simonds AK, Williams SC, and Morrell MJ. 2016. ‘Changes in Neurocognitive Architecture in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure’, EBioMedicine, 7: 221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, and Morrell MJ. 2015. ‘Sleep apnoea and the brain: a complex relationship’, Lancet Respir Med, 3: 404–14. [DOI] [PubMed] [Google Scholar]
- Rosenzweig I, Kempton MJ, Crum WR, Glasser M, Milosevic M, Beniczky S, Corfield DR, Williams SC, and Morrell MJ. 2013. ‘Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning?’, PloS One, 8: e83173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen S, Lehtimaki T, Kallonen E, Laasonen K, Poussa T, and Nieminen MM. 1998. ‘No relation between apolipoprotein E alleles and obstructive sleep apnea’, Clinical Genetics, 53: 147–8. [DOI] [PubMed] [Google Scholar]
- Salorio CF, White DA, Piccirillo J, Duntley SP, and Uhles ML. 2002. ‘Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome’, Journal of Clinical and Experimental Neuropsychology, 24: 93–100. [DOI] [PubMed] [Google Scholar]
- Sanchez-de-la-Torre M, Barcelo A, Pierola J, Esquinas C, de la Pena M, Duran-Cantolla J, Capote F, Masa JF, Marin JM, Vila M, Cao G, Martinez M, de Lecea L, Gozal D, Montserrat JM, and Barbe F. 2011. ‘Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea’, Respiratory Medicine, 105: 1954–60. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Himanen SL, Polo O, and Jehkonen M. 2010. ‘Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome’, European Neurology, 63: 215–20. [DOI] [PubMed] [Google Scholar]
- Saunamaki T, Huupponen E, Loponen J, and Himanen SL. 2017. ‘CPAP Treatment Partly Normalizes Sleep Spindle Features in Obstructive Sleep Apnea’, Sleep Disord, 2017: 2962479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunamaki T, Jehkonen M, Huupponen E, Polo O, and Himanen SL. 2009. ‘Visual dysfunction and computational sleep depth changes in obstructive sleep apnea syndrome’, Clinical EEG and Neuroscience, 40: 162–7. [DOI] [PubMed] [Google Scholar]
- Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, and Weaver TE. 2011. ‘A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions’, Sleep Medicine Reviews, 15: 343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, and Maquet P. 2007. ‘Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep’, Proceedings of the National Academy of Sciences of the United States of America, 104: 13164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, Klimesch W, Saletu B, and Zeitlhofer J. 2004. ‘Sleep spindles and their significance for declarative memory consolidation’, Sleep, 27. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, Parapatics S, Saletu B, Klimesch W, and Zeitlhofer J. 2006. ‘Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities’, European Journal of Neuroscience, 23: 1738–46. [DOI] [PubMed] [Google Scholar]
- Schonwald SV, Carvalho DZ, de Santa-Helena EL, Lemke N, and Gerhardt GJ. 2012. ‘Topography-specific spindle frequency changes in obstructive sleep apnea’, BMC Neuroscience, 13: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely AJ, and Macklem PT. 2004. ‘Complex systems and the technology of variability analysis’, Critical Care (London, England), 8: R367–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, and Fjell AM. 2014. ‘Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults’, Neurology, 83: 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Grandin S, Jouny C, Rochat T, and Ibanez V. 2002. ‘Is waking electroencephalographic activity a predictor of daytime sleepiness in sleep-related breathing disorders?’, European Respiratory Journal, 19: 645–52. [DOI] [PubMed] [Google Scholar]
- Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, Maudoux D, Pichot V, Laurent B, and Barthelemy JC. 2010. ‘Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study’, Sleep, 33: 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Sharma SK, Kadhiravan T, Mehta M, Sreenivas V, Gulati V, and Sinha S. 2010. ‘Pattern & correlates of neurocognitive dysfunction in Asian Indian adults with severe obstructive sleep apnoea’, Indian Journal of Medical Research, 132: 409–14. [PubMed] [Google Scholar]
- Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, Wohlleber M, Miller MD, Andrade A, Lewis C, Tweardy S, Buj M, Yau PL, Sadda R, Mosconi L, Li Y, Butler T, Glodzik L, Fieremans E, Babb JS, Blennow K, Zetterberg H, Lu SE, Badia SG, Romero S, Rosenzweig I, Gosselin N, Jean-Louis G, Rapoport DM, de Leon MJ, Ayappa I, and Osorio RS. 2018. ‘Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study’, American Journal of Respiratory and Critical Care Medicine, 197: 933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Wu D, Ptacek LJ, and Fu YH. 2017. ‘Human genetics and sleep behavior’, Current Opinion in Neurobiology, 44: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, and Lu L. 2018. ‘Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis’, Sleep Medicine Reviews, 40: 4–16. [DOI] [PubMed] [Google Scholar]
- Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, and Yaffe K. 2008. ‘Sleep-disordered breathing and cognition in older women’, Journal of the American Geriatrics Society, 56: 45–50. [DOI] [PubMed] [Google Scholar]
- Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, Kumar A, Brasic JR, Wong DF, and Wu MN. 2014. ‘Objectively Measured Sleep and beta-amyloid Burden in Older Adults: A Pilot Study’, SAGE Open Med, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, and Benca RM. 2015. ‘Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults’, Neurobiology of Aging, 36: 2568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Sager MA, Asthana S, Johnson SC, Benca RM, and Bendlin BB. 2017. ‘Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults’, Neurology, 89: 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, and Fell J. 2015. ‘Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep’, Nature Neuroscience, 18: 1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiropoulos P, Galbiati A, and Ferini-Strambi L. 2019. ‘Detection of mild cognitive impairment in middle-aged and older adults with obstructive sleep apnoea: does excessive daytime sleepiness play a role?’, European Respiratory Journal, 53: 1801917. [DOI] [PubMed] [Google Scholar]
- Steriade M 2006. ‘Grouping of brain rhythms in corticothalamic systems’, Neuroscience, 137: 1087–106. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Minthon L, Blennow K, Rosen I, and Londos E. 2010. ‘Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years’, Neurobiology of Aging, 31: 215–23. [DOI] [PubMed] [Google Scholar]
- Sui X, and Gao C. 2013. ‘Association of serum YKL-40 levels with the presence and severity of coronary artery disease in patients with obstructive sleep apnea syndrome’, Clinical and Investigative Medicine. Medecine Clinique et Experimentale, 36: E306–11. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG, Berthon-Jones M, and Eves L. 1981. ‘Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares’, Lancet, 1: 862–5. [DOI] [PubMed] [Google Scholar]
- Sun H, Paixao L, Oliva JT, Goparaju B, Carvalho DZ, van Leeuwen KG, Akeju O, Thomas RJ, Cash SS, Bianchi MT, and Westover MB. 2019. ‘Brain age from the electroencephalogram of sleep’, Neurobiology of Aging, 74: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Liu JY, and Li LR. 2015. ‘Serum YKL-40 levels are associated with type 2 diabetes mellitus in patients with obstructive sleep apnea syndrome’, Genetics and Molecular Research, 14: 8919–25. [DOI] [PubMed] [Google Scholar]
- Taillard J, Sagaspe P, Berthomier C, Brandewinder M, Amieva H, Dartigues JF, Rainfray M, Harston S, Micoulaud-Franchi JA, and Philip P. 2019. ‘Non-REM Sleep Characteristics Predict Early Cognitive Impairment in an Aging Population’, Frontiers in Neurology, 10: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzano MG, Parrino L, Boselli M, Spaggiari MC, and Di Giovanni G. 1996. ‘Polysomnographic analysis of arousal responses in obstructive sleep apnea syndrome by means of the cyclic alternating pattern’, Journal of Clinical Neurophysiology, 13: 145–55. [DOI] [PubMed] [Google Scholar]
- Testelmans D, Tamisier R, Barone-Rochette G, Baguet JP, Roux-Lombard P, Pepin JL, and Levy P. 2013. ‘Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events’, Cytokine, 62: 210–6. [DOI] [PubMed] [Google Scholar]
- Thakre TP, Mamtani MR, and Kulkarni H. 2009. ‘Lack of association of the APOE epsilon 4 allele with the risk of obstructive sleep apnea: meta-analysis and meta-regression’, Sleep, 32: 1507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ 2002. ‘Cyclic alternating pattern and positive airway pressure titration’, Sleep Medicine, 3: 315–22. [DOI] [PubMed] [Google Scholar]
- Troussiere AC, Charley CM, Salleron J, Richard F, Delbeuck X, Derambure P, Pasquier F, and Bombois S. 2014. ‘Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease’, Journal of Neurology, Neurosurgery and Psychiatry, 85: 1405–8. [DOI] [PubMed] [Google Scholar]
- Vakulin A, D’Rozario A, Kim JW, Watson B, Cross N, Wang D, Coeytaux A, Bartlett D, Wong K, and Grunstein R. 2016. ‘Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea’, Clinical Neurophysiology, 127: 1428–35. [DOI] [PubMed] [Google Scholar]
- Varga AW, Ducca EL, Kishi A, Fischer E, Parekh A, Koushyk V, Yau PL, Gumb T, Leibert DP, Wohlleber ME, Burschtin OE, Convit A, Rapoport DM, Osorio RS, and Ayappa I. 2016. ‘Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation’, Neurobiology of Aging, 42: 142–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Kishi A, Mantua J, Lim J, Koushyk V, Leibert DP, Osorio RS, Rapoport DM, and Ayappa I. 2014. ‘Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory’, Journal of Neuroscience, 34: 14571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, and Mokhlesi B. 2019. ‘REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments’, Sleep Breath, 23: 413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, ME Wohlleber, Giménez S, Romero S, Alonso JF, Ducca EL, Kam K, Lewis C, Tanzi EB, and Tweardy S. 2016. ‘Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Aβ42 Levels in Cognitively Normal Elderly’, Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC 2012. ‘Piecing together phenotypes of brain injury and dysfunction in obstructive sleep apnea’, Frontiers in Neurology, 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Bai XX, Williams SC, Hua SC, Kim JW, Marshall NS, D’Rozario A, and Grunstein RR. 2015. ‘Modafinil Increases Awake EEG Activation and Improves Performance in Obstructive Sleep Apnea during Continuous Positive Airway Pressure Withdrawal’, Sleep, 38: 1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Piper AJ, Wong KK, Yee BJ, Marshall NS, Dijk DJ, and Grunstein RR. 2011. ‘Slow wave sleep in patients with respiratory failure’, Sleep Medicine, 12: 378–83. [DOI] [PubMed] [Google Scholar]
- Wang D, Piper AJ, Yee BJ, Wong KK, Kim JW, D’Rozario A, Rowsell L, Dijk DJ, and Grunstein RR. 2014. ‘Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic sleep disordered breathing patients’, Journal of Clinical Sleep Medicine, 10: 517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waragai M, Okamura N, Furukawa K, Tashiro M, Furumoto S, Funaki Y, Kato M, Iwata R, Yanai K, Kudo Y, and Arai H. 2009. ‘Comparison study of amyloid PET and voxel-based morphometry analysis in mild cognitive impairment and Alzheimer’s disease’, Journal of the Neurological Sciences, 285: 100–8. [DOI] [PubMed] [Google Scholar]
- Weaver TE, and Grunstein RR. 2008. ‘Adherence to continuous positive airway pressure therapy: the challenge to effective treatment’, Proceedings of the American Thoracic Society, 5: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, and Pack AI. 2007. ‘Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning’, Sleep, 30: 711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OM, and Dang-Vu TT. 2016. ‘Spindle Oscillations in Sleep Disorders: A Systematic Review’, Neural Plasticity, 2016: 7328725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, Knight RT, Jagust WJ, and Walker MP. 2019. ‘Sleep as a Potential Biomarker of Tau and beta-Amyloid Burden in the Human Brain’, Journal of Neuroscience, 39: 6315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, Wellman A, Sands S, and Redline S. 2020. ‘Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis’, Sleep, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods Cindy E., Usher Kim J., Jersmann Hubertus, and Maguire Graeme Paul. 2014. “Sleep disordered breathing and polysomnography in Australia: trends in provision from 2005 to 2012 and the impact of home-based diagnosis.” In Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, and Nedergaard M. 2013. ‘Sleep drives metabolite clearance from the adult brain’, Science, 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiromeritis AG, Hatziefthimiou AA, Hadjigeorgiou GM, Gourgoulianis KI, Anagnostopoulou DN, and Angelopoulos NV. 2011. ‘Quantitative spectral analysis of vigilance EEG in patients with obstructive sleep apnoea syndrome: EEG mapping in OSAS patients’, Sleep Breath, 15: 121–8. [DOI] [PubMed] [Google Scholar]
- Xu H, Qian Y, Guan J, Yi H, and Yin S. 2015. ‘No association between the ApoE epsilon2 and epsilon4 alleles and the risk of obstructive sleep apnea: A systematic review and meta-analysis’, Biomed Rep, 3: 313–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, and Stone KL. 2011. ‘Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women’, JAMA, 306: 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaouhi K, Bertran F, Clochon P, Mezenge F, Denise P, Foret J, Eustache F, and Desgranges B. 2009. ‘A combined neuropsychological and brain imaging study of obstructive sleep apnea’, Journal of Sleep Research, 18: 36–48. [DOI] [PubMed] [Google Scholar]
- Yesavage J, Bliwise D, Guilleminault C, Carskadon M, and Dement W. 1985. ‘Preliminary communication: intellectual deficit and sleep-related respiratory disturbance in the elderly’, Sleep, 8: 30–3. [DOI] [PubMed] [Google Scholar]
- Yetkin O, and Aydogan D. 2018. ‘Effect of CPAP on sleep spindles in patients with OSA’, Respiratory Physiology & Neurobiology, 247: 71–73. [DOI] [PubMed] [Google Scholar]
- Younes Magdy. 2017. ‘The case for using digital EEG analysis in clinical sleep medicine’, Sleep Science and Practice, 1: 2. [Google Scholar]
- Yun CH, Lee HY, Lee SK, Kim H, Seo HS, Bang SA, Kim SE, Greve DN, Au R, Shin C, and Thomas RJ. 2017. ‘Amyloid Burden in Obstructive Sleep Apnea’, Journal of Alzheimer’s Disease, 59: 21–29. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lv J, Zhou J, Su L, Feng L, Ma J, Wang G, and Zhang J. 2015. ‘The effect of CPAP treatment on EEG of OSAS patients’, Sleep Breath, 19: 1121–4. [DOI] [PubMed] [Google Scholar]
- Zhang J, XC Yang, Luo L, Shao J, Zhang C, Ma J, Wang GF, Liu Y, Peng C-K, and Fang J. 2009. ‘Assessing severity of obstructive sleep apnea by fractal dimension sequence analysis of sleep EEG’, Physica A: Statistical Mechanics and its Applications, 388: 4407–14. [Google Scholar]
- Zhou J, Camacho M, Tang X, and Kushida CA. 2016. ‘A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness’, Sleep Medicine, 23: 99–108. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, and Aloia MS. 2006. ‘A review of neuroimaging in obstructive sleep apnea’, Journal of Clinical Sleep Medicine, 2: 461–71. [PubMed] [Google Scholar]
- Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, Selim BJ, Strohl KP, Redeker NS, Concato J, and Yaggi HK. 2018. ‘Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea’, Thorax, 73: 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]