Abstract
A hallmark of aging is the progressive accumulation of cellular damage. Age-induced damage arises due to a decrease in organelle function along with a decline in protein quality control. Although somatic tissues deteriorate with age, the germline must maintain cellular homeostasis in order to ensure the production of healthy progeny. While germline quality control has been primarily studied in multicellular organisms, recent evidence suggests the existence of gametogenesis-specific quality control mechanisms in unicellular eukaryotes, highlighting the evolutionary conservation of meiotic events beyond chromosome morphogenesis. Notably, budding yeast eliminates age-induced damage during meiotic differentiation, employing novel organelle and protein quality control mechanisms to produce young and healthy gametes. Similarly, organelle and protein quality control is present in metazoan gametogenesis, however, whether and how these mechanisms contribute to cellular rejuvenation requires further investigation. Here, we summarize recent findings that describe organelle and protein quality control in budding yeast gametogenesis, examine similar quality control mechanisms in metazoan development, and identify research directions that will improve our understanding of meiotic cellular rejuvenation.
Introduction
Somatic tissues are highly susceptible to damage due to cellular aging. Age-induced damage arises as a result of organelle dysfunction, which includes perturbed nuclear envelope integrity (Scaffidi and Misteli, 2006; reviewed in Sakuma and D’Angelo, 2017), enlarged nucleoli (Tiku et al., 2017), decreased mitochondrial membrane potential (reviewed in Sun et al., 2016), and decreased lysosomal acidity (reviewed in Colacurcio and Nixon, 2016). Concomitant with organelle damage, cellular aging leads to the accumulation of misfolded proteins (Huang et al., 2019; Saarikangas et al., 2017; David et al., 2010; Demontis and Perrimon, 2010; Erjavec et al., 2007). Protein homeostasis (proteostasis) pathways, which are mediated by chaperones and protein degradative machinery, decline during somatic aging (Labbadia and Morimoto, 2015; Taylor and Dillin, 2013; Vilchez et al., 2012; Ben-Zvi et al., 2009; Hsu et al, 2003; reviewed in Verbeke, 2001). The underlying reasons behind the loss in organelle function and protein quality control in somatic tissues during aging, however, remain poorly understood.
Previous studies in metazoans have suggested that the germline can influence somatic aging (Berman and Kenyon, 2006; Hsin and Kenyon, 1999). Germ cells, which give rise to egg and sperm upon meiotic differentiation, transduce signals to transcriptionally repress proteostasis pathways in somatic tissues, highlighting an evolutionary trade-off between survival and reproduction (Sala et al., 2020; Labbadia and Morimoto, 2015, Vilchez et al., 2012). Notably, germ cells accumulate age-induced damage in metazoans but are able to generate healthy gametes (Bohnert and Kenyon 2017; David et al., 2010). It is unclear if gametes generated from aged germ cells are completely devoid of damage in metazoans. However, recent work in budding yeast has intriguingly suggested that gametes become devoid of age-induced damage during the course of meiotic differentiation and reset their aging clock (King and Goodman et al., 2019; Ünal et al., 2011; reviewed in King and Ünal, 2020).
Long used as a model organism to study aging, budding yeast undergo asymmetric cell divisions to generate rejuvenated daughter cells, resulting in the accumulation of age-induced damage in mother cells and eventually senescence (Hughes et al. 2019; Rempel et al., 2019; Saarikangas et al., 2017; Hughes and Gottschling, 2012; Erjavec et al., 2007; Sinclair and Guarente, 1997; reviewed in Longo et al., 2012). This cell division is akin to stem cell renewal, with mother cells being subject to organelle dysfunction and proteotoxic stress similar to that seen in non-stem daughter cells (Katajisto et al., 2015; Moore et al., 2015; Erjavec et al., 2007 Rujano et al., 2006; Aguilaniu et al., 2003). Under starvation conditions, diploid yeast cells also act as progenitor cells during meiotic differentiation, exhibiting unique modes of organelle and protein quality control to promote cellular rejuvenation of the nascent haploid gametes (King and Goodman, 2019; Brar et al., 2012; Ünal et al., 2011). However, little is known about how perturbing quality control in gametogenesis affects cellular rejuvenation. Furthermore, while similar aspects of organelle and protein quality control exist in metazoans, it remains unclear whether cellular rejuvenation is a widely conserved feature of gametogenesis.
In this review, we first examine the similarities of organelle quality control mechanisms in budding yeast and metazoan meiotic differentiation. Next, we discuss how proteostasis pathways that are activated by external stress operate endogenously in both budding yeast and metazoan developmental programs. Finally, we suggest future directions to determine if elevated organelle and protein quality control contribute to cellular rejuvenation in metazoans.
Organelle quality control during gametogenesis and development
Nuclear quality control
Across multiple organisms, the nucleus has emerged as a major hub for age-induced damage. In budding yeast, aged mother cells accumulate diverse types of damage at the nuclear periphery including extrachromosomal rDNA circles, abnormal nucleolar material, misorganized nuclear pore complexes (NPCs), and protein aggregates (Denoth-Lippuner et al, 2014; Lord et al., 2015; Morlot et al. 2019; Rempel et al., 2019; Saarikangas et al., 2017; Sinclair et al., 1997; Sinclair and Guarente, 1997). Similarly, in metazoans, both natural aging and Hutchinson-Gilford progeria syndrome result in defective nuclear architecture and organization including lamin abnormalities, DNA damage, and changes in epigenetic marks (Eriksson et al., 2003; Scaffidi and Misteli, 2006). Moreover, aged post-mitotic cells exhibit defects in nucleocytoplasmic transport due to NPC deterioration (D’Angelo et al., 2009). Pathological decline in nucleocytoplasmic transport has recently been implicated in several age-associated neurodegenerative diseases, including Huntington’s disease and amyotrophic lateral sclerosis (Chou et al., 2018; Freibaum et al., 2015; Gasset-Rosa et al., 2017; Grima et al., 2017; Zhang et al., 2015). As such, eliminating nuclear damage must be an integral facet of any cellular rejuvenation program.
Since the nuclear envelope remains intact throughout its lifecycle, budding yeast has evolved specialized processes during mitosis and meiosis to avoid the propagation of nuclear damage to future generations. During mitosis, nuclear damage is preferentially retained in the mother cell by at least two distinct mechanisms. Soluble aging factors such as extrachromosomal rDNA circles exhibit limited diffusion into daughter cells due to the geometric constraints imposed by a dumbbell-shaped anaphase nucleus (Boettcher et al., 2012; Gehlen et al, 2011). NPCs with attached aging factors are simultaneously constrained by a septin-mediated outer nuclear envelope diffusion barrier that regulates passage through the bud neck (Clay et al., 2014; Denoth-Lippuner et al., 2014). During meiosis, nuclear damage is eliminated from gamete nuclei symmetrically via the formation of a gamete-excluded nuclear envelope-bound compartment (Figure 1; King and Goodman et al., 2019; reviewed in King and Ünal, 2020). This compartment, known as the GUNC (for Gametogenesis Uninherited Nuclear Compartment), is subsequently eliminated during gamete development. Interestingly, a subset of Nups and nucleolar proteins are sequestered into the GUNC in young cells. The same nuclear proteins are largely re-generated de novo in the developing gametes, though whether or not their re-synthesis requires the formation of GUNC remains to be determined. A similar sequestration of NPCs has been observed during metazoan spermiogenesis, including in humans, raising the intriguing possibility that some aspects of gametogenesis-associated nuclear rejuvenation may be broadly conserved (Ho, 2010; Russel et al., 1991; Troyer and Schwager, 1982; Fawcett et al., 1979). Further research is required to determine how damage is compartmentalized away from gamete nuclei during budding yeast meiosis and whether similar metazoan mechanisms exist.
Figure 1: Organelle and protein quality control in budding yeast gametogenesis.
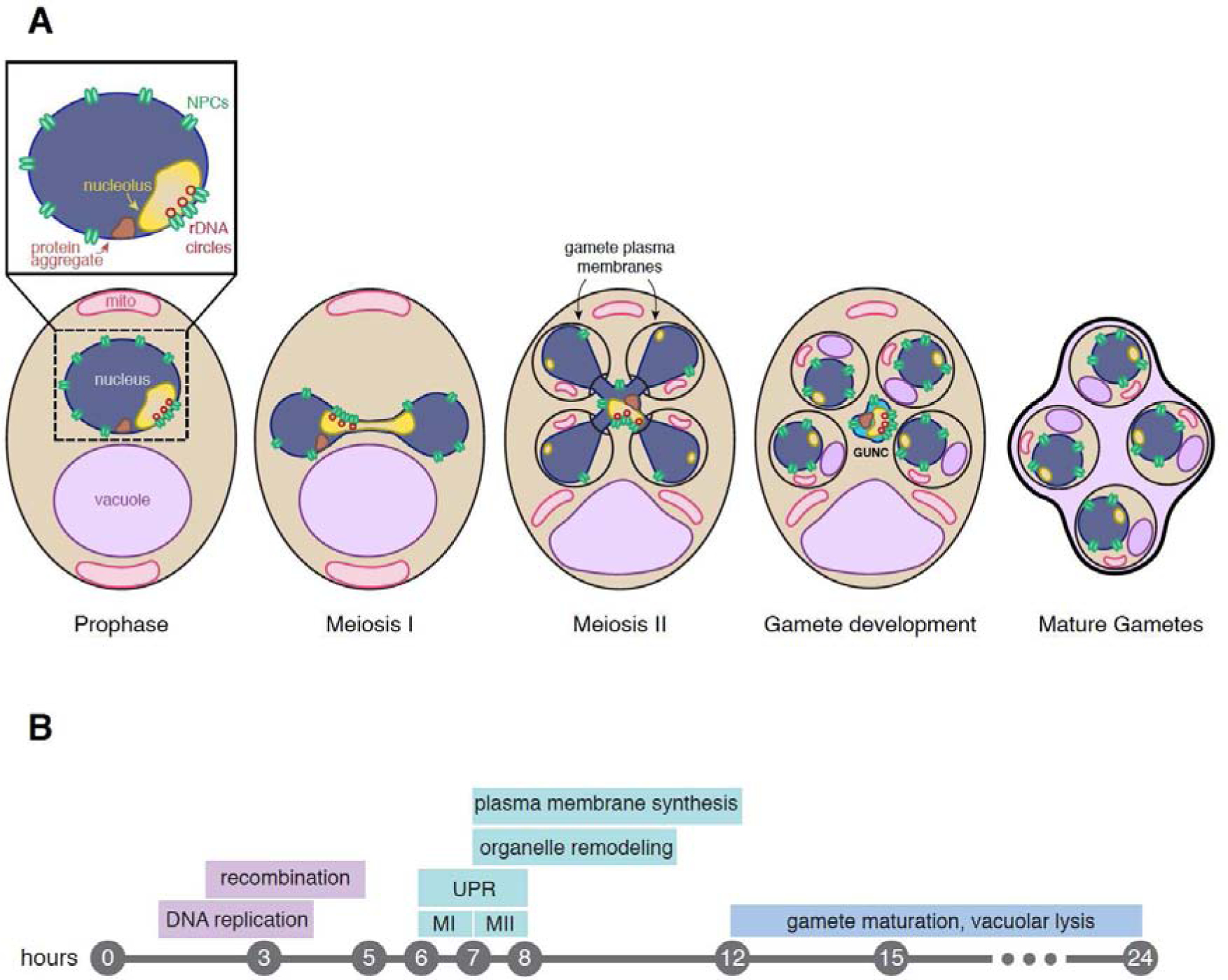
(A) Protein aggregates, rDNA circles, abnormal nucleolar material, and misorganized nuclear pore complexes (NPCs) accumulate in mother cells during replicative aging (Rempel et al., 2019; Saarikangas et al., 2017; Denoth-Lippuner et al., 2014; Erjavec et al., 2007; Sinclair et al., 1997; Sinclair and Guarente, 1997). Nuclear damage along with a subpopulation of mitochondria is not inherited by the nascent gametes during anaphase II. This process is coupled to a nuclear remodeling event that generates the GUNC (Gametogenesis Uninherited Nuclear Compartment), which is subsequently eliminated late in gametogenesis via vacuolar lysis (Eastwood ane Meneghini, 2015; Eastwood et al., 2012). Adapted from King and Goodman et al., 2019. (B) Summary of quality control events that take place hours post yeast gametogenesis induction. Timing of UPR induction was estimated by assessing ribosome footprint levels of HAC1 (Brar et al., 2012).
Interestingly, certain metazoan stem cell lineages utilize nuclear proteins to sequester protein damage away from stem daughter cells. In mice neuronal stem cells, the asymmetric inheritance of damaged proteins by non-stem daughters is established by an endoplasmic reticulum (ER) diffusion barrier mediated by lamins, key structural components of the nuclear envelope during interphase (Moore et al., 2015). This mechanism is strikingly similar to the septin-dependent mitotic nuclear envelope diffusion barrier in budding yeast, even though the nuclear envelope is disassembled during metazoan mitosis. Whether a similar diffusion barrier is present in other stem cell contexts remains to be determined. Strikingly, in male germline stem cells of Drosophila melanogaster, chromatin itself undergoes asymmetric segregation, with the sister chromatids of sex chromosomes being differentiated from one another in a manner dependent on histone tail phosphorylation and nuclear envelope SUN-KASH proteins (Xie et al., 2015; Yadlapalli and Yamashita, 2013; Tran et al., 2012). It is intriguing to speculate that mechanisms might exist in some stem cell contexts to recognize and asymmetrically segregate chromatin-associated nuclear damage to non-stem daughter cells.
As in budding yeast, reproduction in metazoans involves nuclear rejuvenation. During cellular reprogramming to a pluripotent state as occurs in fertilization and embryogenesis, aging is reset to enable the formation of young offspring (Rando and Chang, 2012). Transient expression of pluripotency factors in aged mice and human cells is sufficient to reset nuclear dysfunction, including epigenetic defects and lamin abnormalities (Ocampo et al, 2016; Sarkar et al., 2020). Notably, nuclear age resetting takes place before the reprogramming of cell fate (Ocampo et al., 2016; Sarkar et al., 2020), suggesting that co-opting this natural age-resetting pathway may have therapeutic potential. It remains to be determined, however, whether eliminating nuclear damage directly contributes to cellular rejuvenation.
Reproduction-associated nuclear quality control events have also been observed by regulating potentially harmful genetic elements. With age, rDNA copy number decreases in Drosophila melanogaster male germline stem cells, but is able to get recovered in their offspring (Lu et al., 2018). Other types of toxic DNA, such as viruses, are repressed in budding yeast gametogenesis. During gamete maturation, mitochondrial endonuclease NUC1 localizes to cytoplasm and is capable of protecting gametes from accumulating the L-A and killer viruses (Gao et al., 2019). Additionally, the activity of mobile genetic elements, including retrotransposons has been implicated in oocyte nuclear quality control. Recent studies in female mice have illustrated that LINE-1 (LI) retrotransposons can instigate fetal oocyte attrition (FOA), which leads to the apoptotic destruction of oocytes during prophase I (Tharp et al., 2020; Malki et al., 2014). Perturbing L1 retrotransposon activity with a reverse transcriptase inhibitor reduces FOA (Tharp et al., 2020; Malki et al., 2014). This suggests that oocytes are selected based on their L1 activity. Whether or not L1 induced FOA plays any physiological benefit or detriment to fertility, however, remains to be determined. Together, these diverse quality control mechanisms emphasize a common requirement that nuclear damage be eliminated during reproduction and development.
Mitochondrial quality control
Mitochondrial dysfunction is a hallmark of aging, leading to an increase in reactive oxygen species (ROS) and a decline in membrane potential (Hughes et al., 2019; Higuchi, et al., 2013; Hughes and Gottschling 2012; reviewed in Sun et al., 2016). Remarkably, budding yeast is able to partition damaged mitochondria and ROS to mother cells during mitosis in an actin-dependent manner (Higuchi et al., 2013). Additionally, daughter cells maintain their youth by expressing cytosolic catalases to combat elevated ROS levels originating from damaged mitochondria (Erjavec and Nystrom, 2007). Analogous studies suggest that epithelial cells with stem cell-like behavior also asymmetrically partition mitochondria during each division and that daughters containing younger mitochondria exhibit greater stem cell-like behavior (Katajisto et al., 2015). Other metrics of mitochondrial damage, such as elevated ROS, may exhibit an asymmetric pattern of inheritance in these stem-like cells and require further investigation.
Regulated mitochondrial inheritance during meiosis may also contribute to gamete fitness in budding yeast. As yeast cells progress through the meiotic divisions, mitochondria detach from the plasma membrane and collapse prior to their inheritance (Figure 1; Sawyer et al., 2019). Mitochondrial collapse is dependent on a meiosis-specific kinase, Ime2, which is analogous to cyclin-dependent kinases (Benjamin et al., 2003). Ime2 phosphorylates mitochondrial-plasma membrane tethers, resulting in their degradation (Sawyer et al., 2019). Following mitochondrial collapse, a subset of mitochondria is inherited by the nascent gametes, while the remaining pool is degraded with other cellular components present in the GUNC (King and Goodman et al, 2019; Gorsich et al., 2004; reviewed in Neiman, 2011). This raises the interesting possibility that gametes may preferentially inherit healthy mitochondria over damaged mitochondria to maximize their longevity. Indeed, mitochondria that are not partitioned into the gametes are more depolarized, but it remains unclear if depolarization is a consequence of pre-existing damage in the mother cell or if mitochondria become depolarized during the meiotic program (Eastwood and Meneghini, 2015). More thorough studies examining mitochondrial dynamics in aged meiotic cells will shed light on whether mitochondrial inheritance strongly influences the cellular rejuvenation process.
Several studies have illustrated that mitochondria undergo a unique mode of inheritance in metazoan oogenesis. Female germline stem cells of most animals differentiate into cystocytes, which in turn act as precursor cells that generate oocytes (reviewed in Bastock and Johnson 2008). Cystocytes undergo incomplete cytokinesis and remain interconnected to supply the developing oocyte with a portion of their cellular contents. In D. melanogaster, mitochondria derived from cystocytes are transported to the oocyte by the fusome, which is a germline specific organelle that establishes germ cell polarity in a microtubule-dependent manner (Cox and Spradling, 2006; Cox and Spradling 2003; Grieder et al., 2000). Mitochondria in cystocytes condense with the Golgi apparatus, ER membranes, and RNA granules to form what is known as the Balbiani body (Figure 2; Lei and Spradling, 2016; Pepling et al., 2007; Cox and Spradling, 2003). The oocyte eventually receives Balbiani bodies to inherit organelle contents from nurse cells. In mouse oocytes, mitochondria initially exhibit perinuclear localization before they disperse throughout the entire cell (Lei and Spradling, 2016). Intriguingly, mitochondria in budding yeast gametes establish transient contact with the nuclear envelope prior to gamete maturation (Sawyer et al., 2019; Miyakawa et al., 1984), suggesting that perinuclear localization may be a conserved feature of mitochondrial inheritance.
Figure 2: The Balbiani body facilitates mitochondrial inheritance during D. melanogaster oogenesis.
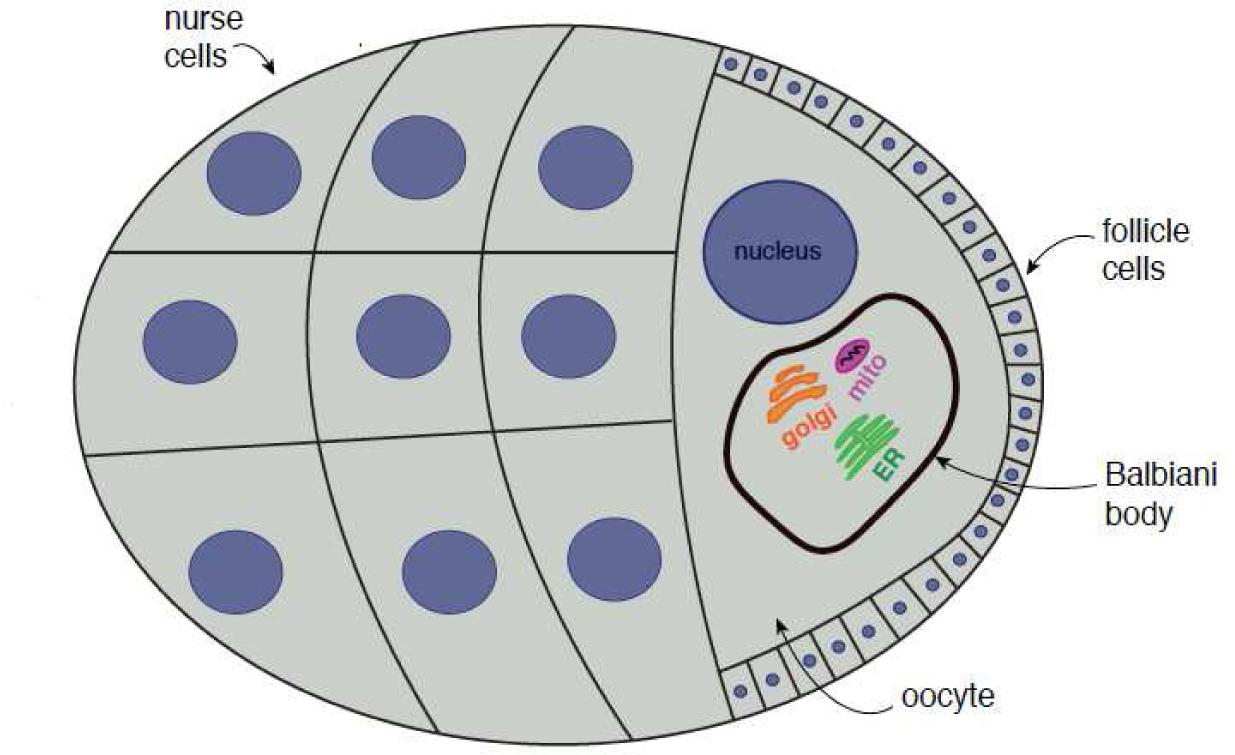
Nurse cells, which are derived from cystoblasts, transfer cytoplasmic material to the developing oocyte. Mitochondria, along with other organelle components, are encapsulated by Balbiani bodies and are transferred to the oocyte along the fusomes of nurse cells (Cox and Spradling, 2006; Cox and Spradling, 2003). Balbiani bodies initially localize to the perinuclear region before distributing organelle contents throughout the oocyte cytoplasm (Lei and Spradling, 2016).
Evidence suggests that healthy mitochondria are preferentially inherited over damaged mitochondria during metazoan oogenesis. Recent electron microscopy work revealed oocytes preferentially inherit healthy mitochondria over damaged mitochondria in the insect Thermobia domestica. In this study, Balbiani bodies were found to contain mitochondria with a high membrane potential but exclude mitochondria with a low potential and damaged morphology (Tworzydlo et al., 2016). Additionally, oocytes have quality control mechanisms to distinguish between wild type and mutant mitochondrial DNA (mtDNA) in the germarium (Lieber et al., 2019; Hill et al., 2014). This occurs through the downregulation of Mitofusin, which results in the physical separation of mitochondrial genomes into individual mitochondrial fragments. If a mitochondrion carries mutations in its mtDNA, this manifests itself in reduced ATP production and elimination by mitophagy (Lieber et al., 2019). Importantly, inhibiting mitochondrial fragmentation in the germline prevents oocytes from selecting against mutant mtDNA, while inducing mitochondrial fragmentation in the soma is sufficient to block amplification of mutant mtDNA (Lieber et al., 2019). Further elucidating the consequences of blocking mtDNA selection during oogenesis will reveal the importance of mitochondrial quality control in gamete fitness.
Regulated mitochondrial inheritance occurs in other metazoan developmental contexts, most notably the elimination of paternal mitochondria during fertilization to ensure that new progeny only receive maternal mitochondria. In D. melanogaster, maternal mitochondrial inheritance depends on Oskar, a gene required for germ plasm assembly (Hurd et al., 2016; Ephrussi and Lehmann, 1992). Specifically, the long isoform of Oskar, which is dispensable for generating the germ plasm, facilitates maternal mitochondrial localization to the posterior region of the embryo in an actin-dependent fashion (Hurd et al., 2016). However, it remains to be determined how the long isoform of Oskar promotes actin-mediated partitioning of mitochondria to the posterior region of the embryo and if Oskar-mediated mitochondrial inheritance affects the development of ensuing germ cells.
Elimination of paternal mitochondria can occur through multiple degradation mechanisms. In a variety of metazoans, paternal mitochondrial proteins are sequestered into autophagosomes and are degraded in the lysosome shortly after fertilization (Rojansky et al., 2016; Politi et al., 2014; Al Rawi et al., 2011; Sato and Sato, 2011). In contrast, paternal mitochondrial nucleoids in metazoans are eliminated by endonuclease G. While paternal nucleoid destruction in D. melanogaster occurs prior to fertilization, Caenorhabditis elegans degrade their paternal nucleoids after fertilization (Zhou et al., 2016; DeLuca and O’Farrell, 2012). Strikingly, inhibiting paternal nucleoid destruction in C. elegans causes minor embryonic lethality, though it is unclear if this is similarly observed in other metazoans (Zhou et al., 2016). Current evidence suggests endonucleases are also activated in yeast during meiotic differentiation to cleave and eliminate uninherited nuclear DNA (Nishimura et al., 2020; Eastwood and Meneghini, 2015; Eastwood et al., 2012). Whether or not these endonucleases are able to prevent deleterious mtDNA from being inherited into budding yeast gametes remains to be determined. Overall, mitochondria exhibit unique modes of quality control and inheritance during gametogenesis, though how this influences ensuing gamete fitness is still a largely unexplored question.
Lysosomal quality control
Lysosomes are acidic organelles that are largely responsible for eliminating cellular damage during stress (reviewed in Settembre et al., 2013). With age, lysosomal function declines due to increased pH and a decline in autophagy (reviewed in Colacurcio and Nixon, 2016; reviewed in Cuervo, 2008). Similarly, budding yeast vacuoles, which are functionally equivalent to lysosomes, become increasingly dysfunctional with age, contributing to the accumulation of protein and organelle damage (Hughes et al., 2020; Hughes and Gottschling, 2012; reviewed in Colacurcio and Nixon, 2016; reviewed in Cuervo, 2008). Strikingly, vacuoles clear age-induced damage during meiotic differentiation (King and Goodman et al., 2019). Vacuoles in the progenitor mother cell are not inherited by the gametes and subsequently lyse, leading to degradation of age-induced damage and uninherited cellular material that is left outside of the gametes (Figure1; Eastwood and Meneghini, 2015; Eastwood et al., 2012). Elucidating the steps involved in vacuolar lysis will allow analysis of whether it has significant roles in promoting gamete fitness and cellular rejuvenation.
Interestingly, C. elegans accumulates protein aggregates in oocytes during aging and employs a lysosomal acidification process, which depends on the vacuolar-type H+-ATPase (V-ATPase) proton pump, to clear age-induced damage following fertilization (Figure 3; Goudeau et al., 2020; Bohnert and Kenyon, 2017; David et al., 2010; Goudeau and Aguilaniu, 2010). Surprisingly, lysosome-mediated aggregate clearance does not appear to depend on canonical autophagy machinery but depends on male sperm protein (MSP) signaling. Identifying factors that act downstream of the MSP signal to stimulate V-ATPase activity will provide increased insight into how fertilization leads to the elimination of age-induced damage.
Figure 3: Age-induced protein aggregates are eliminated during C. elegans oogenesis.
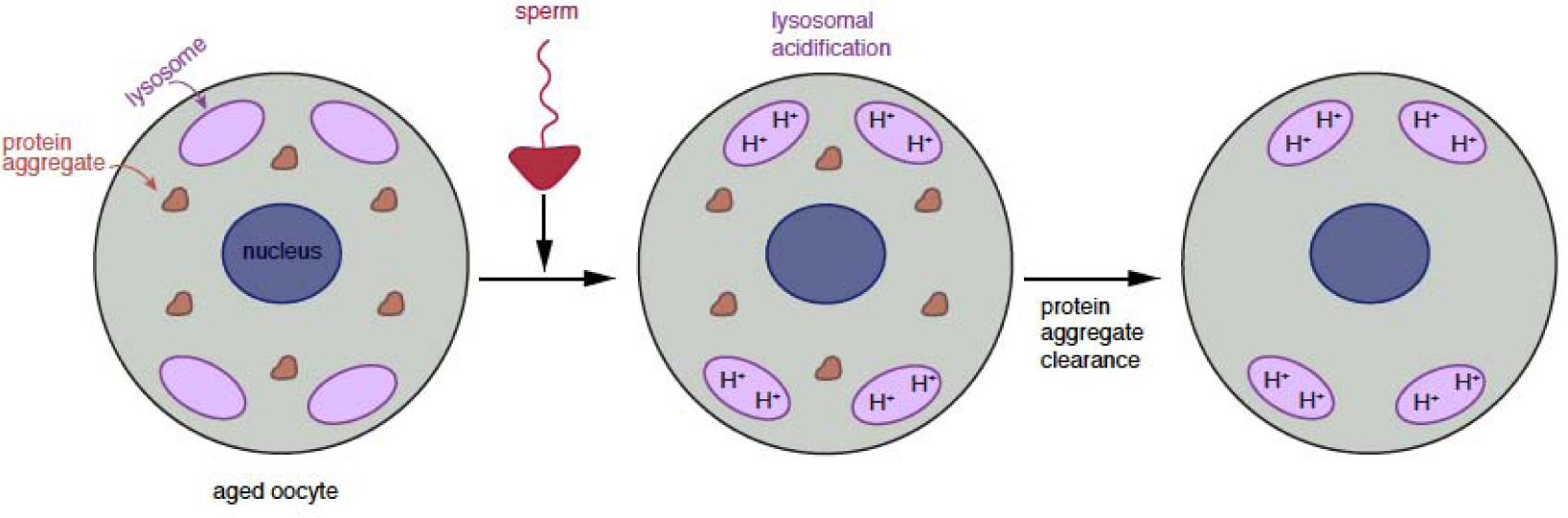
Protein aggregates accumulate in oocytes prior to fertilization (David et al., 2010; Goudeau and Aguilaniu, 2010). Male sperm protein (MSP) signaling enhances Vacuolar-type H+ ATPase (V-ATPase) activity in oocyte lysosomes, leading to protein aggregate clearance (Goudeau et al., 2020; Bohnert and Kenyon, 2017).
Somatic cells in the germline can utilize lysosomal machinery to facilitate oogenesis in an unconventional matter. Recent studies in D. melanogaster have revealed that follicle stem cells (FSCs), which are derived from the epithelium to provide maintenance to germ cells, recruit V-ATPase machinery to the plasma membrane to acidify adjacent nurse cells (Mondragon et al., 2019). Additionally, FSCs are able to export lysosomal proteases in an exocytosis-dependent manner to fragment nuclear DNA that is not partitioned into the developing oocyte. Indeed, reducing the expression of lysosomal protease and V-ATPase subunits in FSCs is sufficient to prolong nuclear fragmentation in nurse cells during oocyte maturation. How FSCs are triggered to stimulate nurse cell acidification, what additional factors are degraded in nurse cells, and the functional significance it plays in oogenesis all remain to be determined.
Other metazoan cell types eliminate age-induced damage using similar lysosomal quality control mechanisms. In particular, lysosomes are more abundant in mouse quiescent neural stem cells (qNSCs) compared to activated neural stem cells (Kobayashi et al., 2019; Leeman et al., 2018). As such, protein aggregates accumulate in lysosomes with age in mouse qNSCs, making the NSCs less able to proliferate (Leeman et al., 2018). Enhancing lysosomal activity in aged qNCSs, both by TFEB overexpression or nutrient deprivation, promotes protein aggregate clearance and restores NSC activation. However, it remains unclear if the driving force behind NSC activation is the reduction of age-induced protein aggregates or simply the increased function of lysosomes. Nevertheless, the degradative machinery of lysosomes is integral in eliminating age-induced damage during gametogenesis and development.
Protein quality control during gametogenesis and development
The Heat Shock Response
The Heat Shock Response (HSR) is the most well characterized pathway that alleviates proteotoxic stress in eukaryotes. The HSR relies on the conserved transcription factor HSF11 to promote the expression of heat shock proteins (Hsps). Hsps, which include chaperones, predominantly act to reduce the level of proteotoxicity either by refolding or degrading misfolded proteins that accumulate in the cytosol (reviewed in Craig et al., 1994). Activation of HSF1 can be induced by a variety of external stressors including heat shock and oxidative stress (reviewed in Morimoto, 1998). Since most studies of the HSR in eukaryotes have been studied after exposure to these stressors, less is known about when and how it operates in endogenous physiological contexts.
Notably, the HSR declines with age in metazoans, leading to an accumulation of misfolded proteins in the cytosol (Labbadia and Morimoto, 2015; Ben-Zvi et al., 2009; Hsu et al, 2003; reviewed in Verbeke, 2001). Interestingly, the somatic decline of the HSR in C. elegans coincides with the time of egg laying, implying that the germline is involved in regulating the HSR. Hsf1 down regulation in the soma results from increased H3K27 trimethylation (H3K27me3; Labbadia and Morimoto, 2015). In young worms, H3K27me3 is counteracted by the histone demethylase Jmjd3.1; with age, Jmjd3.1 levels decrease in somatic tissues, leading to increased repression of Hsf1 and a decline in the HSR (Labbadia and Morimoto, 2015). Worms that are devoid of a germline show higher levels of Jmjd3.1 and lower levels of H3K27me3 in older animals compared to wild type worms, suggesting that the germline represses the HSR by increasing H3K27me3 in the soma. This highlights the evolutionary trade-off between adult health and the ability to generate fit progeny.
A recent study revealed additional germline factors that repress the HSR in the soma. Genetic knockdown of factors that contribute to the vitelline layer of eggshells, including Cbd1, suppresses protein aggregation in muscle tissues and restores the HSR in older animals (Sala et al., 2020). Restoration of the HSR in animals producing damaged eggs is dependent on other quality control genes that promote longevity in metazoans, including the Forkhead box protein O (FOXO) transcription factor Daf16 (Ben-Zvi et al., 2009; Hsu et al, 2003, Lin et al., 2001). It remains unclear, however, whether eggshell formation directly leads to a decrease in Jmjd3.1 expression and an increase in H3K27me3 at the Hsf1 locus. Furthermore, while there is a somatic decline in the HSR with age, it is not yet understood whether this allows the germline to better combat proteotoxic stress in C. elegans gametogenesis.
The HSR may also play a role in protein quality control during gametogenesis in other model organisms. In mice, HSF1 induces transcription of Hsp90 along with other chaperones during oogenesis (Metchat et al., 2009). Inhibiting HSP90 causes oocytes to exhibit delays in the G2/M transition and defects in cytokinesis during meiosis I. It has yet to be determined, however, if Hsf1 activation in mouse oogenesis is similar to its activation during heat shock-induced stress and if other chaperones contribute to these meiotic phenotypes. In budding yeast, some Hsps are upregulated during meiosis, though their functional roles in meiosis remain to be determined (Brar et al., 2012). In parallel Hsf1 synthesis increases during meiosis, though it remains unclear if its main function is to alleviate proteotoxic stress or to induce meiotic genes that are not part of the canonical HSR (Brar et al., 2012).
In other developmental contexts, HSF1 can transcriptionally target genes outside the HSR. Under stress conditions, HSF1 engages with cis-acting promoter motifs called heat shock elements (HSEs) to initiate the HSR (Liu et al., 1997). Interestingly, C. elegans larvae utilize HSF1 and HSEs to transcriptionally activate developmental genes (reviewed in Li et al., 2017). Unlike canonical HSR genes, these developmental genes contain other cis promoter elements adjacent to HSEs that facilitate the binding of additional co-activators, including the E2F/DP heterodimer (Li et al., 2016). How these co-activators engage with HSF1 to promote transcriptional specificity in larval development remains to be determined. In a similar manner, D. melanogaster expresses Hsf1 during oogenesis to activate genes that are implicated in meiotic progression, including factors required for DNA recombination, cohesin assembly, and synaptonemal complex formation (Le Masson et al., 2011). The nature by which HSF1 can differentially regulate canonical HSR genes and developmental genes requires further investigation.
The Unfolded Protein Response
The Unfolded Protein Response (UPR) is another conserved stress response pathway that regulates proteotoxic stress in eukaryotes. In metazoan model systems, the UPR has three distinct branches operating in the ER: the PERK pathway, the ATF6 pathway, and the IRE1/XBP1 pathway (reviewed in Hetz, 2012). The IRE1/XBP1 pathway, which is also conserved in budding yeast, relies on the alternative splicing of Xbp1 to transcriptionally activate UPR genes that alleviate the burden of misfolded proteins in the ER. With age, the ER UPR declines and overexpressing Xbp1 results in an increase in longevity (Taylor and Dillin, 2013). Like the HSR, the ER UPR has mainly been studied upon exposure to external stressors and its function under normal physiological conditions remains poorly understood.
Ribosome footprinting data suggests that the XBP1 branch of the ER UPR is upregulated during budding yeast gametogenesis: the ortholog of Xbp1 in budding yeast, HAC1, exhibits two periods of activity in meiosis, with one occurring during the meiotic divisions (Figure 1; Brar et al., 2012). Interestingly, haploid gametes lacking HAC1 undergo autodiploidization after resuming mitotic growth, suggesting that it regulates genome content during germination (Lee at al., 2003). It remains unclear if HAC1 is able to facilitate meiotic cellular rejuvenation by combating proteotoxic stress.
Although HAC1 is predominately known for inducing ER UPR genes, it may exhibit unique transcriptional features in budding yeast gametogenesis to repress genes that are not part of the canonical ER UPR. Recent studies have shown that treating mitotic yeast cells with the ER-stress inducing drugs DTT or tunicamycin causes HAC1 to produce long undecoded transcript isoforms (LUTIs) of genes that inhibit the transcription and translation of their canonical protein-coding mRNA isoforms (Van Dalfsen et al., 2018). Notably, the PERK branch of the UPR in metazoans alleviates ER stress by attenuating translation. Therefore, it is possible that LUTI induction by HAC1 is compensating for the lack of additional ER UPR branches. LUTIs are also present during gametogenesis (Tresenrider et al., 2019; Cheng and Otto et al., 2018; Chen et al., 2017), so it is possible that HAC1 may stimulate LUTI induction as a quality control measure during gametogenesis to combat proteotoxic stress.
Outside of budding yeast, little work has been done in studying the ER UPR in meiosis and other developmental contexts. C. elegans larvae arrested in the quiescent L1 stage acquire damage similar to that observed during aging, such as fragmented mitochondria and protein aggregation, but revert back to their youthful state when exposed to bacterial nutrients (Roux et al., 2016). The ability to restore youthfulness in developmentally arrested animals requires the IRE1/XBP1 pathway of the ER UPR. Though Ire1 null animals are unable to recover after quiescence, Xbp1 null animals show only a modest defect in recovery, suggesting that Ire1 activates other genes outside of its canonical UPR regulon to promote cellular rejuvenation. In addition, Xbp1 can actively regulate genes without Ire1 during development. An Xbp1 homologue in Xenopus laevis has been shown to play a role in regulating mesodermal and neural development during gastrulation (Cao et al., 2006). Remarkably, the Xbp1 homologue is alternatively spliced by an unconventional mechanism independent of IRE1 function. Additional in vitro and in vivo studies indicated that the XBP1 homologue protein associates with transcriptional co-activators implicated in BMP4 and TGFβ signaling. It will therefore be intriguing to investigate how the UPR utilizes transcriptional machinery not implicated with canonical UPR induction to regulate other aspects of development.
Conclusions
Organelle quality control is an integral feature of cellular homeostasis. While most tissues gradually lose the ability of combat cellular damage with age, the germline has developed robust strategies to prevent damage from being inherited during reproduction. It has recently been shown that meiotic budding yeast cells sequester and eliminate certain types of nuclear damage to ensure that nascent gametes are born young (King and Goodman et al., 2019; Unal et al., 2011). It remains unknown how nuclear damage is sequestered to the eliminated compartment and whether other types of organelle damage are preferentially eliminated to promote rejuvenation.
While several types of damage can arise in middle-aged yeast cells, (less than 10 doublings; King and Goodman et al., 2019; Saarikangas et al., 2017), other types of damage, including mitochondrial dysfunction are only observed in much older populations (greater than 10 doublings; Hughes and Gottschling, 2012). It would therefore be intriguing to determine if other types of damage in older populations are eliminated during gametogenesis. However, older populations (greater than 15 doublings) have impaired meiotic entry, due to the loss of IME1 expression, (Boselli et al., 2009; reviewed in Vershon and Pierce, 2000). Akin to aged human oocytes, older populations of yeast exhibit chromosome segregation defects, suggesting that age-induced damage can no longer be sequestered or eliminated (Gruhn et al., 2019; Boselli et al., 2009). It remains unclear why older populations are unable to enter or complete gametogenesis. Interestingly, reducing the propensity of rDNA circles in older yeast cells, improves their ability to undergo gametogenesis (Boselli et al., 2009). This raises the interesting possibility that defects in older populations are attributed to the enhanced level of age-induced damage, or a decline in meiotic quality control. Further characterizing quality control in yeast meiosis will help determine ways to combat segregation abnormalities similarly observed in aged metazoans, and if cellular rejuvenation is a conserved feature of gametogenesis.
Proteotoxicity is another major source of cellular damage that is combated with conserved stress response pathways. Genes that control the HSR and the ER UPR, HSF1 and HAC1 respectively, are activated in budding yeast gametogenesis (Brar et al., 2012), yet it is unknown how these pathways are endogenously activated and whether these transcription factors play specific roles that are distinguishable from their functions in response to external stressors. It will be intriguing to assess whether these stress response pathways affect meiotic organelle quality control. Consistent with this notion, mouse oocytes utilize Hsps activated by HSF1 to reduce oxidative damage generated by mitochondria. While HSF1-deficient oocytes eventually progress through meiosis, they fail to progress past the 2-cell stage in embryogenesis due to increased mitochondrial dysfunction and apoptosis (Bierkamp et al., 2010). This raises the interesting possibility that HSF1 can influence maternal mitochondrial health prior to embryogenesis. Perhaps protein quality control pathways are able to influence organelle quality control in gametogenesis and other developmental contexts.
A decline in quality control during somatic aging may be strongly influenced by the development of the germline. For example, in C. elegans, the HSR in somatic tissues is compromised due to a germline-dependent signal that represses HSF1 (Labbadia and Morimoto, 2015). In germline-deficient worms, much of the proteostasis machinery, including Hsps and the proteasome, are upregulated, resulting in extended longevity (Vilchez et al., 2012). This suggests there is an evolutionary trade-off for animals between producing healthy progeny and maintaining somatic tissue health. Understanding how quality control operates in the germline and how it outcompetes or antagonizes other tissues for metabolic resources may reveal therapeutic strategies to combat cellular aging in the soma.
Acknowledgments
We would like to thank Tina Sing and Cyrus Reudiger for helpful suggestions regarding this review. JSG is supported by a National Institutes of Health F31 Fellowship (F31AG060656) and a National Institutes of Health Traineeship (T32 GM007127-40S1). GAK is supported by a National Science Foundation Graduate Research Fellowship (DGE 1752814) and a National Institutes of Health Traineeship (T32 GM007232). EÜ is supported by funds from the Pew Charitable Trusts (00027344), Damon Runyon Cancer Research Foundation (35-15), National Institutes of Health (DP2 AG055946-01), Glenn Foundation for Medical Research, and Shurl & Kay Curci Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For consistency, all metazoan gene nomenclature is referred as “Hsf1” while metazoan protein nomenclature is referred as “HSF1.”
References
- 1.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299(5613):1751–1753. doi: 10.1126/science.1080418 [DOI] [PubMed] [Google Scholar]
- 2.Al Rawi S, Louvet-Vallee S, Djeddi A, et al. Postfertilization Autophagy of Sperm Organelles Prevents Paternal Mitochondrial DNA Transmission. Science (80- ). 2011;334(6059):1144–1147. doi: 10.1126/science.1211878 [DOI] [PubMed] [Google Scholar]
- 3.Bastock R, St Johnston D. Drosophila oogenesis. Curr Biol. 2008;18(23):R1082–R1087. doi: 10.1016/j.cub.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106(35):14914–14919. doi: 10.1073/pnas.0902882106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin KR. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17(12):1524–1539. doi: 10.1101/gad.1101503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman JR, Kenyon C. Germ-Cell Loss Extends C. elegans Life Span through Regulation of DAF-16 by kri-1 and Lipophilic-Hormone Signaling. Cell. 2006;124(5):1055–1068. doi: 10.1016/j.cell.2006.01.039 [DOI] [PubMed] [Google Scholar]
- 7.Bierkamp C, Luxey M, Metchat A, Audouard C, Dumollard R, Christians E. Lack of maternal Heat Shock Factor 1 results in multiple cellular and developmental defects, including mitochondrial damage and altered redox homeostasis, and leads to reduced survival of mammalian oocytes and embryos. Dev Biol. 2010;339(2):338–353. doi: 10.1016/j.ydbio.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 8.Boettcher B, Marquez-Lago TT, Bayer M, Weiss EL, Barral Y. Nuclear envelope morphology constrains diffusion and promotes asymmetric protein segregation in closed mitosis. J Cell Biol. 2012;197(7):921–937. doi: 10.1083/jcb.201112117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnert KA, Kenyon C. A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature. 2017;551(7682):629–633. doi: 10.1038/nature24620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boselli M, Rock J, Ünal E, Levine SS, Amon A. Effects of Age on Meiosis in Budding Yeast. Dev Cell. 2009;16(6):844–855. doi: 10.1016/j.devcel.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. High-resolution view of the yeast meiotic program revealed by ribosome profiling. Science. 2012;335(6068):552–557. doi: 10.1126/science.1215110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Knöchel S, Oswald F, Donow C, Zhao H, Knöchel W. XBP1 forms a regulatory loop with BMP-4 and suppresses mesodermal and neural differentiation in Xenopus embryos. Mech Dev. 2006;123(1):84–96. doi: 10.1016/j.mod.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Tresenrider A, Chia M, et al. Kinetochore inactivation by expression of a repressive mRNA. Elife. 2017;6:1–31. doi: 10.7554/eLife.27417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Otto GM, Powers EN, et al. Pervasive, Coordinated Protein-Level Changes Driven by Transcript Isoform Switching during Meiosis. Cell. 2018;172(5):910–923.e16. doi: 10.1016/j.cell.2018.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou C-C, Zhang Y, Umoh ME, et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018;21(2):228–239. doi: 10.1038/s41593-017-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clay L, Caudron F, Denoth-Lippuner A, et al. A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. Elife. 2014;3:e01883. doi: 10.7554/eLife.01883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification—The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox RT, Spradling AC. A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development. 2003;130(8):1579–1590. doi: 10.1242/dev.00365 [DOI] [PubMed] [Google Scholar]
- 19.Cox RT, Spradling AC. Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development. 2006;133(17):3371–3377. doi: 10.1242/dev.02514 [DOI] [PubMed] [Google Scholar]
- 20.Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: Mediators of protein conformation and turnover in the cell. Cell. 1994;78(3):365–372. doi: 10.1016/0092-8674(94)90416-2 [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24(12):604–612. doi: 10.1016/j.tig.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-Dependent Deterioration of Nuclear Pore Complexes Causes a Loss of Nuclear Integrity in Postmitotic Cells. Cell. 2009;136(2):284–295. doi: 10.1016/j.cell.2008.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLOS Biol. 2010;8(8):e1000450 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLuca SZ, O’Farrell PH. Barriers to Male Transmission of Mitochondrial DNA in Sperm Development. Dev Cell. 2012;22(3):660–668. doi: 10.1016/j.devcel.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–825. doi: 10.1016/j.cell.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denoth-Lippuner A, Krzyzanowski MK, Stober C, Barral Y. Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. Elife. 2014;3. doi: 10.7554/eLife.03790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastwood MD, Cheung SWT, Lee KY, Moffat J, Meneghini MD. Developmentally Programmed Nuclear Destruction during Yeast Gametogenesis. Dev Cell. 2012;23(1):35–44. doi: 10.1016/j.devcel.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 28.Eastwood MD, Meneghini MD. Developmental coordination of gamete differentiation with programmed cell death in sporulating yeast. Eukaryot Cell. 2015;14(9):858–867. doi: 10.1128/EC.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358(6385):387–392. doi: 10.1038/358387a0 [DOI] [PubMed] [Google Scholar]
- 30.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. doi: 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erjavec N, Larsson L, Grantham J, Nystrom T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes & Dev. 2007;21(19):2410–2421. doi: 10.1101/gad.439307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erjavec N, Nystrom T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae. Proc Natl Acad Sci. 2007;104(26):10877–10881. doi: 10.1073/pnas.0701634104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fawcett DW, Chemes HE. Changes in distribution of nuclear pores during differentiation of the male germ cells. Tissue Cell. 1979;11(1):147–162. doi: 10.1016/0040-8166(79)90015-6 [DOI] [PubMed] [Google Scholar]
- 34.Freibaum BD, Lu Y, Lopez-Gonzalez R, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525(7567):129–133. doi: 10.1038/nature14974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Chau S, Chowdhury F, et al. Meiotic viral attenuation through an ancestral apoptotic pathway. Proc Natl Acad Sci. 2019;116(33):16454–16462. doi: 10.1073/pnas.1900751116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasset-Rosa F, Chillon-Marinas C, Goginashvili A, et al. Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron. 2017;94(1):48–57.e4. doi: 10.1016/j.neuron.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehlen LR, Nagai S, Shimada K, Meister P, Taddei A, Gasser SM. Nuclear geometry and rapid mitosis ensure asymmetric episome segregation in yeast. Curr Biol. 2011;21(1):25–33. doi: 10.1016/j.cub.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 38.Gorsich SW, Shaw JM. Importance of Mitochondrial Dynamics During Meiosis and Sporulation. Mol Biol Cell. 2004;15(10):4369–4381. doi: 10.1091/mbc.e03-12-0875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goudeau J, Aguilaniu H. Carbonylated proteins are eliminated during reproduction in C. elegans. Aging Cell. 2010;9(6):991–1003. doi: 10.1111/j.1474-9726.2010.00625.x [DOI] [PubMed] [Google Scholar]
- 40.Goudeau J, Samaddar M, Bohnert KA, Kenyon C. Addendum: A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature. 2020;580(7802):E5–E5. doi: 10.1038/s41586-020-2108-0 [DOI] [PubMed] [Google Scholar]
- 41.Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127(19):4253–4264. [DOI] [PubMed] [Google Scholar]
- 42.Grima JC, Daigle JG, Arbez N, et al. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017;94(1):93–107.e6. doi: 10.1016/j.neuron.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruhn JR, Zielinska AP, Shukla V, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science (80- ). 2019;365(6460):1466–1469. doi: 10.1126/science.aav7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hetz C The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- 45.Higuchi R, Vevea JD, Swayne TC, et al. Actin Dynamics Affect Mitochondrial Quality Control and Aging in Budding Yeast. Curr Biol. 2013;23(23):2417–2422. doi: 10.1016/j.cub.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill JH, Chen Z, Xu H. Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nat Genet. 2014;46(4):389–392. doi: 10.1038/ng.2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho H-C. Redistribution of nuclear pores during formation of the redundant nuclear envelope in mouse spermatids. J Anat. 2010;216(4):525–532. doi: 10.1111/j.1469-7580.2009.01204.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399(6734):362–366. doi: 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- 49.Hsu A-L, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Wagner-Valladolid S, Stephens AD, et al. Intrinsically aggregation-prone proteins form amyloid-like aggregates and contribute to tissue aging in Caenorhabditis elegans. Elife. 2019;8:1–22. doi: 10.7554/eLife.43059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492(7428):261–265. doi: 10.1038/nature11654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes CE, Coody TK, Jeong M, Berg JA, Winge DR, Hughes AL. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell. 2020;180(2):296–310.e18. doi: 10.1016/j.cell.2019.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurd TR, Herrmann B, Sauerwald J, et al. Long Oskar Controls Mitochondrial Inheritance in Drosophila melanogaster Article Long Oskar Controls Mitochondrial Inheritance in Drosophila melanogaster. Dev Cell. 2016;39(5):560–571. doi: 10.1016/j.devcel.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katajisto P, Döhla J, Chaffer CL, et al. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348(6232):340–343. doi: 10.1126/science.1260384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King GA, Ünal E. The dynamic nuclear periphery as a facilitator of gamete health and rejuvenation. Curr Genet. January 2020. doi: 10.1007/s00294-019-01050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King GA, Goodman JS, Schick JG, et al. Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. Elife. 2019;8:1–32. doi: 10.7554/eLife.47156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi T, Piao W, Takamura T, et al. Enhanced lysosomal degradation maintains the quiescent state of neural stem cells. Nat Commun. 2019;10(1):5446. doi: 10.1038/s41467-019-13203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Labbadia J, Morimoto RI. Repression of the Heat Shock Response Is a Programmed Event at the Onset of Reproduction. Mol Cell. 2015;59(4):639–650. doi: 10.1016/j.molcel.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Masson F, Razak Z, Kaigo M, et al. Identification of Heat Shock Factor 1 Molecular and Cellular Targets during Embryonic and Adult Female Meiosis. Mol Cell Biol. 2011;31(16):3410–3423. doi: 10.1128/MCB.05237-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K, Neigeborn L, Kaufman RJ. The unfolded protein response is required for haploid tolerance in yeast. J Biol Chem. 2003;278(14):11818–11827. doi: 10.1074/jbc.M210475200 [DOI] [PubMed] [Google Scholar]
- 61.Leeman DS, Hebestreit K, Ruetz T, et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science. 2018;359(6381):1277–1283. doi: 10.1126/science.aag3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science (80- ). 2016;352(6281):95–99. doi: 10.1126/science.aad2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Chauve L, Phelps G, Brielmann RM, Morimoto RI. E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev. 2016;30(18):2062–2075. doi: 10.1101/gad.283317.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Labbadia J, Morimoto RI. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017;27(12):895–905. doi: 10.1016/j.tcb.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lieber T, Jeedigunta SP, Palozzi JM, Lehmann R, Hurd TR. Mitochondrial fragmentation drives selective removal of deleterious mtDNA in the germline. Nature. 2019;570(7761):380–384. doi: 10.1038/s41586-019-1213-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850 [DOI] [PubMed] [Google Scholar]
- 67.Liu XD, Liu PC, Santoro N, Thiele DJ. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997;16(21):6466–6477. doi: 10.1093/emboj/16.21.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16(1):18–31. doi: 10.1016/j.cmet.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lord CL, Timney BL, Rout MP, Wente SR. Altering nuclear pore complex function impacts longevity and mitochondrial function in S. cerevisiae. J Cell Biol. 2015;208(6):729–744. doi: 10.1083/jcb.201412024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu KL, Nelson JO, Watase GJ, Warsinger-Pepe N, Yamashita YM. Transgenerational dynamics of rDNA copy number in Drosophila male germline stem cells. Elife. 2018;7. doi: 10.7554/eLife.32421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A. A Role for Retrotransposon LINE-1 in Fetal Oocyte Attrition in Mice. Dev Cell. 2014;29(5):521–533. doi: 10.1016/j.devcel.2014.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Metchat A, Akerfelt M, Bierkamp C, et al. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90alpha expression. J Biol Chem. 2009;284(14):9521–9528. doi: 10.1074/jbc.M808819200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyakawa I, Aoi H, Sando N, Kuroiwa T. Fluorescence microscopic studies of mitochondrial nucleoids during meiosis and sporulation in the yeast, Saccharomyces cerevisiae. J Cell Sci. 1984;66:21–38. [DOI] [PubMed] [Google Scholar]
- 74.Mondragon AA, Yalonetskaya A, Ortega AJ, et al. Lysosomal Machinery Drives Extracellular Acidification to Direct Non-apoptotic Cell Death. Cell Rep. 2019;27(1):11–19.e3. doi: 10.1016/j.celrep.2019.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore DL, Pilz GA, Araúzo-Bravo MJ, Barral Y, Jessberger S. A mechanism for the segregation of age in mammalian neural stem cells. Science (80- ). 2015;349(6254):1334–1338. doi: 10.1126/science.aac9868 [DOI] [PubMed] [Google Scholar]
- 76.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796. doi: 10.1101/gad.12.24.3788 [DOI] [PubMed] [Google Scholar]
- 77.Morlot S, Song J, Léger-Silvestre I, Matifas A, Gadal O, Charvin G. Excessive rDNA Transcription Drives the Disruption in Nuclear Homeostasis during Entry into Senescence in Budding Yeast. Cell Rep. 2019;28(2):408–422.e4. doi: 10.1016/j.celrep.2019.06.032 [DOI] [PubMed] [Google Scholar]
- 78.Neiman AM. Sporulation in the Budding Yeast Saccharomyces cerevisiae. Genetics. 2011;189(3):737–765. doi: 10.1534/genetics.111.127126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimura Y, Shikanai T, Kawamoto S, Toh-e A. Step-wise elimination of α-mitochondrial nucleoids and mitochondrial structure as a basis for the strict uniparental inheritance in Cryptococcus neoformans. Sci Rep. 2020;10(1):2468. doi: 10.1038/s41598-020-59277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ocampo A, Reddy P, Martinez-Redondo P, et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016;167(7):1719–1733.e12. doi: 10.1016/j.cell.2016.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci U S A. 2007;104(1):187–192. doi: 10.1073/pnas.0609923104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Politi Y, Gal L, Kalifa Y, Ravid L, Elazar Z, Arama E. Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila. Dev Cell. 2014;29(3):305–320. doi: 10.1016/j.devcel.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 83.Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148(1–2):46–57. doi: 10.1016/j.cell.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rempel IL, Crane MM, Thaller DJ, et al. Age-dependent deterioration of nuclear pore assembly in mitotic cells decreases transport dynamics. Elife. 2019;8. doi: 10.7554/eLife.48186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rojansky R, Cha M-Y, Chan DC. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife. 2016;5. doi: 10.7554/eLife.17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roux AE, Langhans K, Huynh W, Kenyon C. Reversible Age-Related Phenotypes Induced during Larval Quiescence in C. elegans. Cell Metab. 2016;23(6):1113–1126. doi: 10.1016/j.cmet.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rujano MA, Bosveld F, Salomons FA, et al. Polarised Asymmetric Inheritance of Accumulated Protein Damage in Higher Eukaryotes Walter P, ed. PLoS Biol. 2006;4(12):e417. doi: 10.1371/journal.pbio.0040417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell LD, Russell JA, MacGregor GR, Meistrich ML. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat. 1991;192(2):97–120. doi: 10.1002/aja.1001920202 [DOI] [PubMed] [Google Scholar]
- 89.Saarikangas J, Caudron F, Prasad R, et al. Compartmentalization of ER-Bound Chaperone Confines Protein Deposit Formation to the Aging Yeast Cell. Curr Biol. 2017;27(6):773–783. doi: 10.1016/j.cub.2017.01.069 [DOI] [PubMed] [Google Scholar]
- 90.Sakuma S, D’Angelo MA. The roles of the nuclear pore complex in cellular dysfunction, aging and disease. Semin Cell Dev Biol. 2017;68:72–84. doi: 10.1016/j.semcdb.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sala AJ, Bott LC, Brielmann RM, Morimoto RI. Embryo integrity regulates maternal proteostasis and stress resilience. Genes Dev. March 2020. doi: 10.1101/gad.335422.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarkar TJ, Quarta M, Mukherjee S, et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020;11(1):1545. doi: 10.1038/s41467-020-15174-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato M, Sato K. Degradation of Paternal Mitochondria by Fertilization-Triggered Autophagy in C. elegans Embryos. Science (80- ). 2011;334(6059):1141–1144. doi: 10.1126/science.1210333 [DOI] [PubMed] [Google Scholar]
- 94.Sawyer EM, Joshi PR, Jorgensen V, Yunus J, Berchowitz LE, Ünal E. Developmental regulation of an organelle tether coordinates mitochondrial remodeling in meiosis. J Cell Biol. 2019;218(2):559–579. doi: 10.1083/jcb.201807097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–1063. doi: 10.1126/science.1127168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14(5):283–296. doi: 10.1038/nrm3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sinclair DA, Mills K, Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277(5330):1313–1316. doi: 10.1126/science.277.5330.1313 [DOI] [PubMed] [Google Scholar]
- 98.Sinclair DA, Guarente L. Extrachromosomal rDNA Circles— A Cause of Aging in Yeast. Cell. 1997;91(7):1033–1042. doi: 10.1016/S0092-8674(00)80493-6 [DOI] [PubMed] [Google Scholar]
- 99.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61(5):654–666. doi: 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153(7):1435–1447. doi: 10.1016/j.cell.2013.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tharp ME, Malki S, Bortvin A. Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity. Nat Commun. 2020;11(1):330. doi: 10.1038/s41467-019-14055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiku V, Jain C, Raz Y, et al. Small nucleoli are a cellular hallmark of longevity. Nat Commun. 2017;8(1):16083. doi: 10.1038/ncomms16083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tran V, Lim C, Xie J, Chen X. Asymmetric Division of Drosophila Male Germline Stem Cell Shows Asymmetric Histone Distribution. Science (80- ). 2012;338(6107):679–682. doi: 10.1126/science.1226028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tresenrider A, Jorgensen V, Chia M, Liao H, van Werven FJ, Ünal E. Integrated Genomic Analysis Reveals Key Features of Long Undecoded Transcript Isoform (LUTI)-based Gene Repression. bioRxiv. January 2019. doi: 10.1101/843458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Troyer D, Schwager P. Evidence for nuclear membrane fluidity: Proacrosome migration and nuclear pore redistribution during grasshopper spermiogenesis. Cell Motil. 1982;2(4):355–367. doi: 10.1002/cm.970020405 [DOI] [Google Scholar]
- 106.Tworzydlo W, Kisiel E, Jankowska W, Witwicka A, Bilinski SM. Exclusion of dysfunctional mitochondria from Balbiani body during early oogenesis of Thermobia. Cell Tissue Res. 2016;366(1):191–201. doi: 10.1007/s00441-016-2414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Unal E, Kinde B, Amon A. Gametogenesis Eliminates Age-Induced Cellular Damage and Resets Life Span in Yeast. Science (80- ). 2011;332(6037):1554–1557. doi: 10.1126/science.1204349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Dalfsen KM, Hodapp S, Keskin A, et al. Global Proteome Remodeling during ER Stress Involves Hac1-Driven Expression of Long Undecoded Transcript Isoforms. Dev Cell. 2018;46(2):219–235.e8. doi: 10.1016/j.devcel.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verbeke P, Fonager J, Clark BF, Rattan SI. Heat shock response and ageing: mechanisms and applications. Cell Biol Int. 2001;25(9):845–857. doi: 10.1006/cbir.2001.0789 [DOI] [PubMed] [Google Scholar]
- 110.Vershon AK, Pierce M. Transcriptional regulation of meiosis in yeast. Curr Opin Cell Biol. 2000;12(3):334–339. doi: 10.1016/S0955-0674(00)00104-6 [DOI] [PubMed] [Google Scholar]
- 111.Vilchez D, Morantte I, Liu Z, et al. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489(7415):263–268. doi: 10.1038/nature11315 [DOI] [PubMed] [Google Scholar]
- 112.Xie J, Wooten M, Tran V, et al. Histone H3 Threonine Phosphorylation Regulates Asymmetric Histone Inheritance in the Drosophila Male Germline. Cell. 2015;163(4):920–933. doi: 10.1016/j.cell.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yadlapalli S, Yamashita YM. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature. 2013;498(7453):251–254. doi: 10.1038/nature12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang K, Donnelly CJ, Haeusler AR, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525(7567):56–61. doi: 10.1038/nature14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Q, Li H, Li H, et al. Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science (80- ). 2016;353(6297):394–399. doi: 10.1126/science.aaf4777 [DOI] [PMC free article] [PubMed] [Google Scholar]


