Abstract
Cancers develop by sustained growth, migration and invasion properties of tumour cells, supported by complex interactions with stromal cells within the tumour micro-environment.
This review is focused on the latest discoveries regarding the highlighted role of angiogenesis and tumour micro-environment in ovarian cancer. This cancer milieu encompasses non-cancerous cells present in the tumour or nearby, including vessel-forming cells, fibroblasts and immune cells amongst others that work in a cooperative way with cancer cells, impacting tumour behaviour. Angiogenesis, migration and invasion, and more recently immune evasion, are cancer hallmarks clearly dependent on these supporting cells. Moreover, these stromal cells are more genetically stable than tumour cells and thus represent an attractive therapeutic target. A better understanding of the stromal cells function, and their complex interplay with cancer cells, will open additional areas to target, as the tumour–host interface.
Keywords: Ovarian cancer, Angiogenesis, Microenvironment, Pre-clinical models
Highlights
-
•
Cancer micro-environment is composed of cells that work cooperatively with tumour cells.
-
•
These supporting cells include vessel-forming cells, fibroblasts and immune cells, amongst others.
-
•
Angiogenesis, migration, invasion and immune evasion clearly impact on tumour behaviour.
-
•
Currently pre-clinical models allow the study of tumour and stromal cells interplay.
-
•
The better knowledge of stromal cells function will open additional areas to target tumours.
1. Introduction
For years, tumour development was considered to be a consequence of the accumulation of alterations in tumour cells that modify their growth properties. The unexpected finding that immortal cells with high accumulation of mutations were unable to develop tumours in mice was the evidence to change that belief. Paget was the first to propose that tumour development is governed by the interaction of tumour cells (the seed) and a permissive micro-environment (the soil). Ewing developed that idea and suggested that the metastatic process is influenced by a characteristic regional vasculature, describing the importance of this process in tumour development [1,2]. The research on molecular mechanisms responsible for progression and metastasis was first focused on the tumour genetic background and, to date, is not yet perfectly understood. However, in recent years, the impact of the tumour micro-environment and its relation with the tumour itself has gained more attention, and it is now recognised as a therapeutic target [3,4].
Ovarian carcinoma (OC) is the most lethal gynaecological tumour. Specifically, high-grade serous carcinomas are usually diagnosed at late stages of disease, with little chance of cure, due to the lack of symptoms until the disease obviously manifests [5]. Additionally, even though these patients will respond to initial treatment based on cytoreductive surgery and carboplatin/paclitaxel chemotherapy, the vast majority of them will recur, with a fatal impact on patient prognosis.
The Cancer Genome Atlas (TCGA) network described gene expression profiles associated with four high-grade serous ovarian carcinoma (HGSOC) molecular subtypes: differentiated, mesenchymal, immunoreactive and proliferative, which were later validated by other groups [6,7]. Tumour-infiltrating stromal cells significantly contribute to the assignments of two of these subtypes, mesenchymal and immunoreactive [8,9]. Additionally, the comparison of gene expression profiles from matched primary and recurrent tumours shows differences in their micro-environments, mainly in the major pathways of immune activation and matrix remodelling [10].
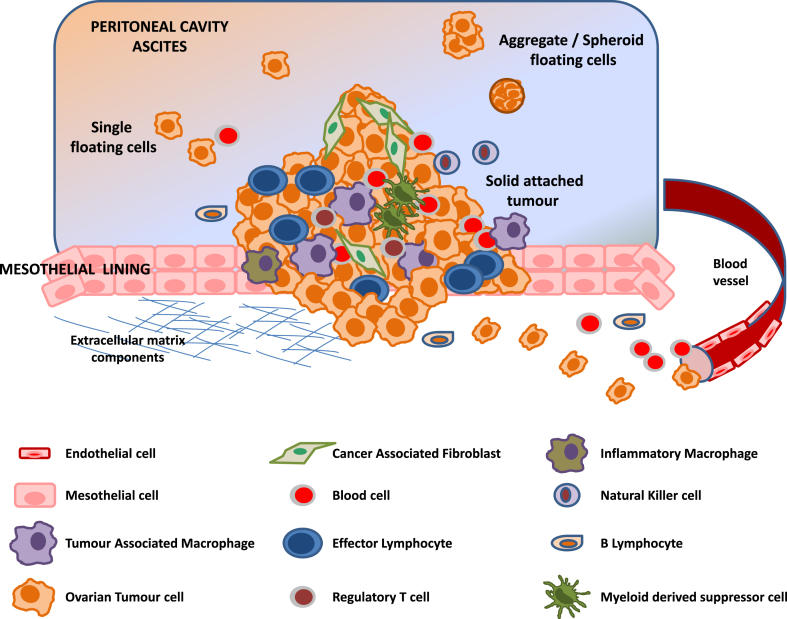
In this review, we provide a compendium of published data related to an important process, angiogenesis and new findings about the unique OC stroma (Fig. 1), including forming vessels (endothelial cells and pericytes), fibroblasts, adipocytes, mesothelial and immune cells (macrophages, regulatory T cells, myeloid-derived suppressor cells, natural killer (NK) cells and B cells).
Fig. 1.
Schematic representation of the ovarian cancer micro-environment. Malignant cells establish as a tumour bulk in the peritoneal cavity, where stromal cells are also present. Once the tumour becomes more aggressive, cancer cells can migrate and invade the surrounding stroma or detach from the tumour and shed into ascitic fluid as single cells, aggregates or spheroids.
2. Ovarian angiogenesis
Tumour development must be accompanied by the supply of oxygen and nutrients supplied by the vasculature. Angiogenesis, defined as the formation of new vessels from pre-existing vasculature, is one of the hallmarks of cancer [11]. Tumour cells (TCs) turn on a misbalance in the factors that regulate this process, stimulating endothelial cell (EC) properties as proliferation, migration, invasion and adhesion that lead to vascular sprouting.
Vascular endothelial growth factor (VEGF) is a powerful pro-angiogenic molecule. Specifically in OC, despite early contradictory results, different authors have described high levels of VEGF in ascites, suggesting a more intense angiogenic activity in the peritoneal cavity, and it has also been related to a poor prognosis [12].
The inhibition of angiogenesis seems to be a rational front-line treatment, but also as maintenance therapy, since its fundamental role in tumour development was explored in two large prospective randomised trials, GOG-218 and ICON-7 [13,14]. Currently, bevacizumab is approved in Europe for first line and relapse settings [15], and other agents, including VEGF-trap, tyrosine kinase inhibitors or angiopoietin inhibitors have also been investigated, some of them with positive results [16,17]. These strategies seemed promising at the beginning and, although we know now that not all patients will respond to these therapies, the inhibition of angiogenesis showed that the action of micro-environment-targeting agents could be worth exploring, due to the important crosstalk between tumour and stromal cells, as stated in the next section.
Additionally, the factors that influence platinum resistance are complex and not perfectly understood, but there is evidence of the implication of angiogenesis in this process. It was reported that there is a differential expression of angiogenic factors between platinum-sensitive and -resistant patients [18]. It has also been described that anti-tumour response and angiogenesis are both modulated by extracellular vesicle secretion, and it seems that there is a modification of the extracellular vesicles. Interestingly, soluble E-cadherin levels have been recently found in conditioned media from ovarian cancer cells with highly metastatic properties and pointed as a new pro-angiogenic molecule [19].
3. Stromal ovarian compartment
3.1. Endothelial cells and pericytes
Blood vessels are composed of endothelial cells (ECs), located at the inner lining of the vascular tube wall. Pericytes are mural smooth muscle cells located at the vessel surface that provide structural support and regulate vascular tone. These two cellular components have reciprocal complex crosstalks to regulate EC proliferation and pericyte recruitment during angiogenesis [20]. Evidence suggests that ECs proliferate and migrate in response to cooperative signals sent by tumour cells as well as macrophages. There are many soluble factors that promote neovascularisation, including VEGFs, fibroblasts growth factors (FGFs) and platelet-derived growth factors (PDGFs) [21]. In vitro co-culture of macrophages with OC cells up-regulated interleukin 8 (CXCL8/IL-8) levels, which are responsible for ECs migration [22]. Perivascular stromal cells provide mature structural support to blood vessels, once the wall is formed by ECs. Although pericytes are less characterised than ECs, it has been described that exert tumour homing function and the co-injection of pericytes in a xenograft model of ovarian cancer increase tumour growth rate and metastasis [23].
3.2. Fibroblasts and cancer-associated fibroblasts
Fibroblasts are supporting cells representing the majority of stromal cells. They form the submesothelial stroma in the peritoneal cavity and secrete structural proteins such as fibronectin or vitronectin [24]. It is now accepted that normal fibroblasts in contact with tumour cells can differentiate into a myofibroblast-activated state, known as cancer-associated fibroblasts (CAFs). These CAFs contribute to the creation of a reactive stroma and tumour desmoplasia and constitute the predominant cells of the tumour-associated micro-environment [25]. Co-culture of OC cell lines with fibroblasts produces an increase of α-smooth muscle actin (αSMA), a myofibroblastic marker, and the promotion of cancer cells invasion and migration [26,27]. Moreover, OC spheroids injected subcutaneously in mice promote a vascular stabilisation of tumours when they are infiltrated by CAFs [28].
A positive correlation between CAF infiltration of tumours and aggressive features such as tumour grade has been demonstrated, suggesting that this stromal component is important for metastasis development [26]. The crucial role of these CAFs in ovarian tumour progression has been stated by the TCGA data, which pointed out the mesenchymal subtype as the one with the poorest outcome [29].
Although the relation of cancer cells and fibroblasts has been extensively studied, it is not well established how quiescent fibroblasts become activated. Snail Family Transcriptional Repressor 2 (SNAI2) has been recently described as an important transcription factor in the reprogramming of normal fibroblasts to their activated state in OC [30].
CAFs are able to promote mitogenic and survival signals by secreting growth factors (HGF, FGFs and insulin-like growth factor 1 (IGF1)) and chemokines (C-X-C motif chemokine ligand 12 (CXCL12)), to induce epithelial to mesenchymal transition (EMT) of tumour cells and to generate an immune-suppressive state via TGF-β secretion. They are also pointed to promote angiogenesis by interleukin 6 (IL6), cyclooxygenase 2b (PTGS2/COX-2) and C-X-C motif chemokine ligand 1 (CXCL1) secretion and lymphangiogenesis via Sonic Hedgehog (SHH) – VEGF-C signalling in ovarian cancer [31,32].
3.3. Adipocytes and mesothelial cells
There is evidence of the role of omental fat in intraperitoneal carcinogenesis, including OC. In vitro studies have shown that adipocytes stimulate homing, migration and invasion of OC cells, and this effect is mediated by IL8. Additionally, fatty acid binding protein 4 (FABP4) has been reported to be increased in omental metastases compared to primary tumours [33].
The peritoneal surface is covered by a layer of mesothelial cells that have both mesenchymal and epithelial properties and play a prominent role in peritoneal homeostasis. Some authors think that these cells exert a passive role in OC as energy storing cells. Others propose that they have alternative roles such as promoting adhesion of OC cells (by binding fibronectin and hyaluronan from the mesothelial cell surface to α5β1 integrin and CD44 on cancer cells, respectively) or the stimulation of cancer cell progression and angiogenesis [34,35].
Mesothelial cells have recently been implicated in the promotion of tumour neovascularisation and vascular permeability by VEGF production. These cells change their morphology in contact with malignant ascites, showing a mesenchymal pattern, and are able to induce migration and tube formation of endothelial cells in vitro [36].
3.4. Tumour-associated macrophages
Tumour-associated macrophages (TAMs) play a well-defined role in tumour progression, but their phenotype is influenced by location, since macrophages from different anatomical sites show changes in their gene expression profiles. They are abundant in most cancers, usually at the tumour boundaries, but in OC they can be found inside the tumour bulk and also in ascitic fluid [37]. The recruitment of macrophages to the tumour is mediated by cytokines and growth factors secreted by themselves and other stromal cells that determine their phenotype. It is thought that hypoxic environments attract macrophages by secretion of VEGF [38].
Exposure to different molecular signalling can induce TAM differentiation to M1 (classically activated pro-inflammatory macrophages) or M2 (alternatively activated pro-tumour macrophages). In OC, TAMs display a mixed phenotype, with CD86 and TNF-α, typical of M1, and IL-10 and CD163 markers, characteristic of M2 phenotype [39]. Secretions of IL-6 and IL-10 promote the transformation to the M2 pro-cancerous macrophage subtype, which are also involved in the formation of floating spheroids in the peritoneal cavity and are implicated in the early steps of the metastatic process [40]. In a comprehensive study of ovarian tumour spheroids and TAMs, the expression of TAMs from patients with a worse outcome was mainly related to pro-metastatic events, including matrix remodelling, angiogenesis, immunosuppression and invasion. Conversely, TAMs from longer survivors expressed cytokine networks linked to effector T-cells chemoattraction and activation [41].
The exact mechanisms that drive this pro-tumour conversion of TAMs are not fully understood, but it has been recently described that ZEB1, one of the EMT drivers, is essential for TAM-mediated tumour promotion in a mouse model of ovarian cancer [42]. These pro-tumour TAMs exert their immunosuppressive properties by inhibiting the activation of T cells via the expression of PD-L1 and B7–H4 and through the mobilisation of regulatory T cells (Tregs) by secreting their chemoattractants CCL3, CCL20 and CCL2 [43].
3.5. Other immune cells in the ovarian tumour micro-environment
The interaction of immune cells and cancer cells was firstly described by Virchow [44]. Nowadays it is well established that there is an association between inflammation and cancer and that different immune subsets are recruited to tumour areas and invasive margins by different chemokine networks [45]. OC is considered in the middle of the immunogenic tumours ranking [46].
Lymphocyte effector subsets related to good prognosis include CD8+ T cells able to kill tumour cells, as well as CD4+ T helper cells, memory B cells and plasmatic cells that appear to work cooperatively [47].
Tumour infiltrating lymphocytes (TILs) are a heterogeneous population that can be found in the tumour stroma. TILs in ovarian cancer were described decades ago, and although their positive prognostic impact was suggested years later, nowadays, due to their heterogeneity regarding cell type and location, it is still under debate [48].
Another mechanism used by tumours to escape the immune response is by Tregs recruitment. The negative impact of these cells was demonstrated for the first time in ovarian tumours [49]. These CD4+ T cells are characterised by the expression of FoxP3 and CDC25 and exert the immune response suppression via secretion of IL-10 and TGF-β. They are recruited in response to CCL22, which is mainly produced by macrophages and tumour cells and is highly expressed in ovarian cancer and ascites [50].
HGSOC frequently express IDO-1 enzyme, implicated in tryptophan metabolism and inhibition of T-cell response [51]. Other regulatory axes in immune response are those including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death ligand 1 (PD-L1) molecules. The former is supposed to act earlier, in lymph nodes, whereas the latter exerts its suppressive role in peripheral tissues. PD-L1 is frequently expressed by TAMs, as mentioned before, but also by tumour cells, whilst PD-1 receptor is expressed by a proportion of CD4+ and CD8+ TILs. The combination of immune therapies could be potentially active in ovarian cancer and is currently under evaluation. B cells are usually found at the invasive margin of tumours and lymphoid structures. These cells are less characterised than effector T cells. They express CXCR4 and may be recruited by CXCL12. Their infiltration has been described as a good prognosis marker in ovarian cancer [52].
Innate cytotoxic lymphocytes, mainly natural killer (NK) cells, infiltrate tumour stroma and are also present in ascites, but the mechanisms involved in their regulation are not yet clarified. MHC Class I polypeptide-related sequence A (MICA) and UL16 binding protein 1 (ULBP) are implicated in the down-regulation of NKG2-D Type II integral membrane protein (NKG2D), expressed by NK cells. It has been recently published that the presence of these soluble ligands in ascites is correlated with poor survival [53]. Ovarian cancer cells are killed by cytokine-stimulated NK cells, but a decreased functionality and cytotoxic efficacy of ascites-derived NK cells have been observed. Although more research is required to decipher their role, it seems that enhancing their function could be a potential immunotherapeutic intervention [54].
Myeloid-derived suppressor cells (MDSCs) are other inhibitory immune cells with increased levels in human cancers, but are difficult to quantify because they present variable phenotypes, including myeloid progenitor cells, immature macrophages, granulocytes and dendritic cells, all sharing a granulocytic morphology. These cells contribute to a tumour permissive environment interacting with several components of the immune system. They inhibit the activation of CD8+ T cells expressing nitric oxide synthase (NOS2) and arginase 1 (ARG1), and they also induce Tregs and polarisation of macrophages [55]. NK cells show pro-angiogenic functions, and the cross-regulation between them and MDSCs that impacts on tumour development is still poorly understood [56]. VEGF expression in ovarian cancer seems to induce MDSCs by myeloid chemoattractants up-regulation, as seen in HGSOC samples. Additionally, MDSCs express both VEGFR1 and VEGFR2, and high MDSC infiltration correlates with shorter overall survival [57].
4. New models for the study of ovarian cancer complexity
Over the past decade, our knowledge of OC has dramatically shifted. It has become clear now that OC is a heterogeneous disease comprised by different entities with particular molecular and clinical features.
Experimental models that faithfully capture the hallmarks of tumour development and their complexity are limited. Cell lines and patient-derived xenografts (PDXs) are the most common models used for pre-clinical disease characterisation. Despite their unquestionable contribution to cancer research, these models have specific advantages and drawbacks. Two-dimensional cultures, although easy to handle, lack the architecture present in the tumour, which is an important factor for the study of some features. 3D cultures overcome this pitfall, and the technical evolution permits an approach for high-throughput testing techniques [58,59].
Xenografts require more expertise and an elevated investment, but are suitable to study the interaction of tumour cells and the components of tumour micro-environment, such as blood vessels, keeping in mind that they are murine stromal cells. Recently, a syngeneic model of OC has been developed with success, being appropriate for metastases development studies and for drug testing under an immune competent micro-environment [60].
Some in vitro co-culture studies have been proposed to overcome all these inconvenients with success [[61], [62], [63]]. Another important issue is that cell lines are subjected to a strong in vitro selection to be established, accumulating molecular alterations [64]. Primary culture seems to be more similar to the original tumour, so it seems that the best model, to date, could be patient-derived 3D culture (PD3D), also named organoids. It has been recently published that the efficient derivation and long-term expansion of OC organoids, which recapitulate histological and genomic features of the original tumour from which they are derived, can be used for drug-screening purposes. To date, this seems a very interesting approach for OC research [65,66].
5. Concluding remarks
It is now well established that the micro-environment's influence on tumour development is crucial to understand disease evolution. The interrelation of tumour cells with stromal components as endothelial cells, fibroblasts and immune cells has an influence on tumour development. The exhaustive characterisation of the specific role of each cellular component and their interactions has become a priority challenge, in order to design effective combination therapies in OC.
Conflict of interest statement
The authors Victoria Heredia-Soto, José Antonio López-Guerrero and Marta Mendiola have no disclosures to state. Andrés Redondo has received research funding from Pharmamar, Roche and Eisai and has an advisory role relationship with Pharmamar, Lilly, Novartis, Amgen, Astra Zeneca, Tesaro and Roche.
Footnotes
This paper is part of a supplement supported by Pharma Mar S.A.
Contributor Information
A. Redondo, Email: andres.redondos@uam.es.
M. Mendiola, Email: marta.mendiola@gmail.com.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. The Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 2.Ewing J. 3rd ed. W. B. Saunders Company; Philadelphia: 1928. Neoplastic diseases: a treatise on tumors. [Google Scholar]
- 3.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Kosary C.L., editors. SEER cancer statistics review, 1975-2014. National Cancer Institute; Bethesda, MD: 2017. https://seer.cancer.gov/csr/1975_2014/ based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 6.Cancer Genome Atlas Research N Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konecny G.E., Wang C., Hamidi H., Winterhoff B., Kalli K.R., Dering J. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tothill R.W., Tinker A.V., George J., Brown R., Fox S.B., Lade S. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res : Off J Am Assoc Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 9.Crijns A.P., Fehrmann R.S., de Jong S., Gerbens F., Meersma G.J., Klip H.G. Survival-related profile, pathways, and transcription factors in ovarian cancer. PLoS Med. 2009;6:e24. doi: 10.1371/journal.pmed.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuzinger C., Geroldinger A., Smeets D., Braicu E.I., Sehouli J., Koller J. A complex network of tumor microenvironment in human high-grade serous ovarian cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-1159. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 12.Bamias A., Pignata S., Pujade-Lauraine E. Angiogenesis: a promising therapeutic target for ovarian cancer. Crit Rev Oncol-Hematol. 2012;84:314–326. doi: 10.1016/j.critrevonc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Burger R.A., Brady M.F., Bookman M.A., Fleming G.F., Monk B.J., Huang H. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 14.Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 15.http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000582/human_med_000663.jsp&mid=WC0b01ac058001d124, December 2017.
- 16.du Bois A., Floquet A., Kim J.W., Rau J., del Campo J.M., Friedlander M. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2014;32:3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 17.du Bois A., Kristensen G., Ray-Coquard I., Reuss A., Pignata S., Colombo N. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78–89. doi: 10.1016/S1470-2045(15)00366-6. [DOI] [PubMed] [Google Scholar]
- 18.Trachana S.P., Pilalis E., Gavalas N.G., Tzannis K., Papadodima O., Liontos M. The development of an angiogenic protein "signature" in ovarian cancer ascites as a tool for biologic and prognostic profiling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156403. e0156403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang M.K.S., Yue P.Y.K., Ip P.P., Huang R.L., Lai H.C., Cheung A.N.Y. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9:2270. doi: 10.1038/s41467-018-04695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durham J.T., Surks H.K., Dulmovits B.M., Herman I.M. Pericyte contractility controls endothelial cell cycle progression and sprouting: insights into angiogenic switch mechanics. Am J Physiol Cell Physiol. 2014;307:C878–C892. doi: 10.1152/ajpcell.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Zhao X., Wang K., Wu L., Duan T. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci. 2013;104:516–523. doi: 10.1111/cas.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha D., Chong L., George J., Schluter H., Monchgesang S., Mills S. Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin Cancer Res : Off J Am Assoc Cancer Res. 2016;22:1813–1824. doi: 10.1158/1078-0432.CCR-15-1931. [DOI] [PubMed] [Google Scholar]
- 24.Tracy L.E., Minasian R.A., Caterson E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5:119–136. doi: 10.1089/wound.2014.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Tang H., Cai J., Zhang T., Guo J., Feng D. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Yao Q., Qu X., Yang Q., Wei M., Kong B. CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009;22:541–548. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 28.Gilead A., Meir G., Neeman M. The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Canc Journal international du cancer. 2004;108:524–531. doi: 10.1002/ijc.11583. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q., Burdette J.E., Wang J.P. Integrative network analysis of TCGA data for ovarian cancer. BMC Syst Biol. 2014;8:1338. doi: 10.1186/s12918-014-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z., Yang X., Xu S., Jin P., Li X., Wei X. Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression. Mol Cancer. 2017;16:163. doi: 10.1186/s12943-017-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erez N., Glanz S., Raz Y., Avivi C., Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun. 2013;437:397–402. doi: 10.1016/j.bbrc.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 32.Wei R., Lv M., Li F., Cheng T., Zhang Z., Jiang G. Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling axis. Oncotarget. 2017;8:67315–67328. doi: 10.18632/oncotarget.18621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lessan K., Aguiar D.J., Oegema T., Siebenson L., Skubitz A.P. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sako A., Kitayama J., Yamaguchi H., Kaisaki S., Suzuki H., Fukatsu K. Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: possible role of mesothelial cell on the development of peritoneal metastasis. J Surg Res. 2003;115:113–120. doi: 10.1016/s0022-4804(03)00307-x. [DOI] [PubMed] [Google Scholar]
- 36.Fujikake K., Kajiyama H., Yoshihara M., Nishino K., Yoshikawa N., Utsumi F. A novel mechanism of neovascularization in peritoneal dissemination via cancer-associated mesothelial cells affected by TGF-beta derived from ovarian cancer. Oncol Rep. 2018;39:193–200. doi: 10.3892/or.2017.6104. [DOI] [PubMed] [Google Scholar]
- 37.Takaishi K., Komohara Y., Tashiro H., Ohtake H., Nakagawa T., Katabuchi H. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101:2128–2136. doi: 10.1111/j.1349-7006.2010.01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colvin E.K. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi: 10.3389/fonc.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard J.W. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin M., Li X., Tan S., Zhou H.J., Ji W., Bellone S. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Investig. 2016;126:4157–4173. doi: 10.1172/JCI87252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worzfeld T., Finkernagel F., Reinartz S., Konzer A., Adhikary T., Nist A. Proteotranscriptomics reveal signaling networks in the ovarian cancer microenvironment. Mol Cell Proteom: MCP. 2017 doi: 10.1074/mcp.RA117.000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes M., Sanchez-Moral L., de Barrios O., Fernandez-Acenero M.J., Martinez-Campanario M.C., Esteve-Codina A. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J. 2017;36:3336–3355. doi: 10.15252/embj.201797345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb C.E., Mills A.M., Cross J.V., Ring K.L. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: a comparison of matched primary and metastatic tumors. Gynecol Oncol. 2017;144:607–612. doi: 10.1016/j.ygyno.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 45.Nagarsheth N., Wicha M.S., Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandalaft L.E., Powell D.J., Jr., Singh N., Coukos G. Immunotherapy for ovarian cancer: what's next? J Clin Oncol : Off J Am Soc Clin Oncol. 2011;29:925–933. doi: 10.1200/JCO.2009.27.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo Z., Wang Q., Lau W.B., Lau B., Xu L., Zhao L. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377:174–182. doi: 10.1016/j.canlet.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Ovarian Tumor Tissue Analysis C., Goode E.L., Block M.S., Kalli K.R., Vierkant R.A., Chen W. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3(12) doi: 10.1001/jamaoncol.2017.3290. e173290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 50.Campbell D.J., Koch M.A. Treg cells: patrolling a dangerous neighborhood. Nat Med. 2011;17:929–930. doi: 10.1038/nm.2433. [DOI] [PubMed] [Google Scholar]
- 51.Inaba T., Ino K., Kajiyama H., Yamamoto E., Shibata K., Nawa A. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Milne K., Kobel M., Kalloger S.E., Barnes R.O., Gao D., Gilks C.B. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006412. e6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas M., Reinartz S., Hoffmann N., Reiners K.S., Lieber S., Jansen J.M. Soluble NKG2D ligands in the ovarian cancer microenvironment are associated with an adverse clinical outcome and decreased memory effector T cells independent of NKG2D downregulation. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1339854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felices M., Chu S., Kodal B., Bendzick L., Ryan C., Lenvik A.J. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145:453–461. doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okla K., Wertel I., Polak G., Surowka J., Wawruszak A., Kotarski J. Tumor-associated macrophages and myeloid-derived suppressor cells as immunosuppressive mechanism in ovarian cancer patients: progress and challenges. Int Rev Immunol. 2016;35:372–385. doi: 10.1080/08830185.2016.1206097. [DOI] [PubMed] [Google Scholar]
- 56.Bruno A., Mortara L., Baci D., Noonan D.M., Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771. doi: 10.3389/fimmu.2019.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horikawa N., Abiko K., Matsumura N., Hamanishi J., Baba T., Yamaguchi K. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23:587–599. doi: 10.1158/1078-0432.CCR-16-0387. [DOI] [PubMed] [Google Scholar]
- 58.Vinci M., Gowan S., Boxall F., Patterson L., Zimmermann M., Court W. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heredia-Soto V., Redondo A., Berjon A., Miguel-Martin M., Diaz E., Crespo R. High-throughput 3-dimensional culture of epithelial ovarian cancer cells as preclinical model of disease. Oncotarget. 2018;9:21893–21903. doi: 10.18632/oncotarget.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson-Ryan I., Pham M.M., Sergent P., Tafe L.J., Berwin B.L. A syngeneic mouse model of epithelial ovarian cancer port site metastases. Transl Oncol. 2019;12:62–68. doi: 10.1016/j.tranon.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flores-Perez A., Rincon D.G., Ruiz-Garcia E., Echavarria R., Marchat L.A., Alvarez-Sanchez E. Angiogenesis analysis by in vitro coculture assays in transwell chambers in ovarian cancer. Methods Mol Biol. 2018;1699:179–186. doi: 10.1007/978-1-4939-7435-1_13. [DOI] [PubMed] [Google Scholar]
- 62.Long L., Yin M., Min W. 3D Co-culture system of tumor-associated macrophages and ovarian cancer cells. Bio-protocol. 2018;8 doi: 10.21769/BioProtoc.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao T., Yu Y., Cong Q., Wang Y., Sun M., Yao L. Human mesenchymal stem cells in the tumour microenvironment promote ovarian cancer progression: the role of platelet-activating factor. BMC Canc. 2018;18:999. doi: 10.1186/s12885-018-4918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beaufort C.M., Helmijr J.C., Piskorz A.M., Hoogstraat M., Ruigrok-Ritstier K., Besselink N. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103988. e103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kopper O., de Witte C.J., Lohmussaar K., Valle-Inclan J.E., Hami N., Kester L. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 66.Hill S.J., Decker B., Roberts E.A., Horowitz N.S., Muto M.G., Worley M.J., Jr. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–1421. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]