Key Points
Question
How does higher-calorie refeeding compare with the standard of care, lower-calorie refeeding, for malnourished patients with anorexia nervosa?
Findings
In this randomized clinical trial enrolling 120 adolescents and young adults with anorexia nervosa and atypical anorexia nervosa and 60% or more of median body mass index for age and sex, higher-calorie refeeding restored medical stability faster, with no increase in electrolyte abnormalities and marked cost savings.
Meaning
Higher-calorie refeeding is more efficacious and less costly than lower-calorie refeeding, with no increase in safety events among hospitalized patients with malnutrition due to restrictive eating disorders.
Abstract
Importance
The standard of care for refeeding inpatients with anorexia nervosa, starting with low calories and advancing cautiously, is associated with slow weight gain and protracted hospital stay. Limited data suggest that higher-calorie refeeding improves these outcomes with no increased risk of refeeding syndrome.
Objective
To compare the short-term efficacy, safety, and cost of lower-calorie vs higher-calorie refeeding for malnourished adolescents and young adults with anorexia nervosa.
Design, Setting, and Participants
In this multicenter randomized clinical trial with prospective follow-up conducted at 2 inpatient eating disorder programs at large tertiary care hospitals, 120 adolescents and young adults aged 12 to 24 years hospitalized with anorexia nervosa or atypical anorexia nervosa and 60% or more of median body mass index were enrolled from February 8, 2016, to March 7, 2019. The primary analysis was a modified intent-to-treat approach.
Interventions
Higher-calorie refeeding, beginning at 2000 kcal/d and increasing by 200 kcal/d vs lower-calorie refeeding, beginning at 1400 k/cal and increasing by 200 kcal every other day.
Main Outcomes and Measures
Main outcomes were end-of-treatment outcomes; the primary end point of this trial will be clinical remission over 12 months. Short-term efficacy was defined a priori as time to restore medical stability in the hospital, measured by the following 6 indices: 24-hour heart rate of 45 beats/min or more, systolic blood pressure of 90 mm Hg or more, temperature of 35.6 °C or more, orthostatic increase in heart rate of 35 beats/min or less, orthostatic decrease in systolic blood pressure of 20 mm Hg or less, and 75% or more of median body mass index for age and sex. The prespecified safety outcome was incidence of electrolyte abnormalities; cost efficacy was defined as savings associated with length of stay.
Results
Because 9 participants withdrew prior to treatment, the modified intention-to-treat analyses included 111 participants (93%; 101 females [91%]; mean [SD] age, 16.4 [2.5] years). Higher-calorie refeeding restored medical stability significantly earlier than lower-calorie refeeding (hazard ratio, 1.67 [95% CI, 1.10-2.53]; P = .01). Electrolyte abnormalities and other adverse events did not differ by group. Hospital stay was 4.0 days shorter (95% CI, −6.1 to −1.9 days) among the group receiving higher-calorie refeeding, which was associated with a savings of $19 056 (95% CI, −$28 819 to −$9293) in hospital charges per participant.
Conclusions and Relevance
In the first randomized clinical trial in the US to compare refeeding approaches in patients with anorexia nervosa and atypical anorexia nervosa, higher-calorie refeeding demonstrated short-term efficacy with no increase in safety events during hospitalization.
Trial Registration
ClinicalTrials.gov Identifier: NCT02488109
This randomized clinical trial compares the short-term efficacy, safety, and cost of lower-calorie vs higher-calorie refeeding for malnourished adolescents and young adults with anorexia nervosa.
Introduction
Malnutrition can develop rapidly in adolescents with anorexia nervosa (AN) owing to caloric restriction, overexercising, and other behaviors during a critical period of growth and development. Patients who become medically unstable secondary to malnutrition are hospitalized for short-term nutritional rehabilitation, or “refeeding,” to restore vital sign stability.1,2 Despite lengthy hospitalizations, 45% of patients are readmitted, and only 18% to 55% meet criteria for clinical remission at 1 year.3,4 Furthermore, AN has the highest mortality of all psychiatric disorders (5.1%).5 Together, these challenges make eating disorders a common and costly pediatric diagnosis.6 The standard of care for in-hospital refeeding in the US has been lower-calorie refeeding (LCR), beginning at approximately 1200 kcal/d and increasing slowly.7,8,9 This cautious approach was intended to minimize risk for refeeding syndrome, which begins with rapid electrolyte and fluid shifts and can progress to delirium, cardiac arrest, and death.10,11,12,13,14 Although LCR is generally considered safe, it is associated with slow weight gain15 and protracted hospital stays.16,17
Clinical practice is moving toward higher-calorie refeeding (HCR), loosely defined as starting at 1400 kcal/d or more.18 Studies have established the feasibility of HCR in the hospital and provided hopes for improved outcomes15,17,19,20,21,22,23,24,25; however, interpretation of these mostly retrospective studies is limited. Among the few studies including a comparison group, selection for LCR was biased toward lower-weight patients,15,17,26 likely owing to evidence that these patients are at higher risk for refeeding syndrome.2,27,28,29 This limitation has been heightened by recognition of atypical AN (AAN),30 a new diagnosis describing AN psychopathology and significant weight loss yet “normal” weight. Patients with AAN and medical instability requiring hospitalization now comprise nearly one-third of the inpatient population of patients with eating disorders,31,32,33 with no consensus on whether and how much weight gain is needed during refeeding.34,35,36 The greatest gap in the evidence, however, is safety. Wide variations in electrolyte monitoring and correction impede comparison across studies.37 In a 2013 systematic review of 17 refeeding studies in 1039 participants, rates of refeeding hypophosphatemia (the hallmark indicator of risk for the refeeding syndrome) ranged from 0% to 48%.27 Finally, while the prospect of a shorter hospital stay may appeal to insurers and patients alike, potential cost savings of HCR have not been assessed.
The Study of Refeeding to Optimize Inpatient Gains was a multicenter randomized clinical trial (RCT) comparing the efficacy, safety, and cost of HCR vs LCR in hospitalized adolescents with AN and AAN. In this article, we examine our short-term hypothesis, that medical stability would be restored faster among patients receiving HCR without increased incidence of electrolyte abnormalities and with significant cost savings associated with shorter length of stay. Evaluation of long-term outcomes is ongoing (NCT02488109).
Methods
Design
This is a multicenter RCT comparing HCR vs LCR in adolescents and young adults hospitalized with medical instability and malnutrition secondary to AN or AAN. Written informed consent was obtained from young adults and parents of minors, who provided written assent. The study included 2 clinical sites, large tertiary care children’s hospitals with eating disorder inpatient programs attended by interdisciplinary adolescent medicine care teams at the University of California San Francisco and Stanford University, and a separate data coordination center. Participants were assigned to a study group stratified by site in random blocks of 2 to 6 generated by the data coordination center. This procedure and all other research protocols were developed centrally and disseminated to study sites; research staff and clinicians were trained on the protocol prior to the start of enrollment and refreshed yearly or as needed with new personnel. The institutional review board at both sites approved the study; an independent data safety monitoring board ensured the safety of participants, validity of the trial, and quality of the data (trial protocol in Supplement 1). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Patients aged 12 to 24 years with malnutrition secondary to AN or AAN and hospitalized with medical instability per Society for Adolescent Health and Medicine criteria38 were enrolled from February 8, 2016, to March 7, 2019. Patients were excluded if their body mass index (BMI) was less than 60% of the median BMI (mBMI), defined as the 50th percentile BMI for age and sex39 (owing to concerns for medical fragility) or if they had been admitted to the hospital within the previous 6 months, were currently pregnant, or had a chronic disease, active suicidality, or psychosis.
Intervention and Baseline Evaluation
Lower-calorie refeeding began with 1400 kcal/d and increased by 200 kcal every other day. This level, which was slightly higher than the currently recommended 1200 kcal,7,8,9 was intended to avoid the initial weight loss documented in our preliminary studies.15,16,17 Higher-calorie refeeding began at 2000 kcal/d and increased by 200 kcal/d. Diets were prescribed by study physicians and increased per protocol until individual caloric goals were met based on Estimated Energy Requirement equations40 using height, age, and treatment goal weight. As previously described,16 our refeeding approach included 3 meals and 2 to 3 snacks per day (approximately 30%-40% fat, 15%-25% protein, and 35%-55% carbohydrate), with a person present to monitor the patient during meals and 30 to 45 minutes afterward. At every meal, the percentage of every item consumed (eg, percentage of sandwich and percentage of milk) was evaluated. Calories refused in food were replaced by a high-energy oral liquid formula providing 1.50 kcal/mL; no participants received nasogastric feeding. All participants received a daily adult multivitamin with minerals once per day and 500 mg of elemental calcium carbonate twice per day.
Baseline measures were assessed within 24 hours of admission. Daytime heart rate was measured every 4 to 8 hours. Orthostatic changes were defined as the difference between supine measurements after 5 minutes of rest followed by standing measurements taken after 2 minutes. Night heart rate was measured with continuous cardiac monitoring. Temperature was taken orally. Weight was measured in the morning on an electronic mobile stand-on scale, after voiding and in gown only; height was measured on a stadiometer. Baseline electrolyte levels were evaluated within 24 hours of admission; abnormalities were corrected per protocol (eTable in Supplement 2) prior to the initiation of the intervention. The Eating Disorder Examination questionnaire,41 a demographic survey including a socioeconomic status score derived from the Hollingshead occupational index for mother or primary parent,42 and history of weight and family eating disorder were self-reported or parent-reported by proctored questionnaire.
Short-term Outcome Measures
Efficacy
Short-term efficacy was specified a priori as time to restore medical stability, adjudicated by a 6-point clinical index: (1) 24-hour heart rate of 45 beats/min or more, (2) systolic blood pressure of 90 mm Hg or more, (3) temperature of 35.6 °C or more, (4) orthostatic increase in heart rate of 35 beats/min or less, (5) orthostatic decrease in systolic blood pressure of 20 mm Hg or less, and (6) 75% or more of mBMI for age and sex. Thresholds for these indicators represent the reversal of published vital sign instabilities supporting hospitalization in adolescents with eating disorders,43 except for orthostatic change in heart rate. Whereas a heart rate change of more than 20 beats/min indicates hospitalization, a heart rate change of 35 beats/min or less was considered restored for this trial, consistent with clinical practice and based on evidence that orthostatic changes in heart rate take 3 weeks or more to resolve.1 Criteria were assessed daily; for vital signs with multiple daily measures, the most deviant value was recorded (eg, lowest heart rate). Each criterion was scored as “1” if met, “0” if unmet, and missing (not scored) if not measured. Medical stability was considered restored when all measured criteria were stable for 24 hours, allowing a maximum of 2 missing values. Percentage mBMI was calculated as BMI (calculated as weight in kilograms divided by height in meters squared, using daily weight and baseline height) divided by mBMI and multiplied by 100. Vital signs were measured every 24 hours as described. Additional efficacy outcomes were time to restore heart rate to 45 beats/min or more (among those with bradycardia at baseline) and weight gain (change in percentage mBMI).
Safety
The prespecified safety outcome was incidence of electrolyte abnormalities, defined as the occurrence of hypophosphatemia (phosphorus, <3.0 mg/dL [to convert to millimoles per liter, multiply by 0.323]), hypomagnesemia (magnesium, <1.8 mg/dL [to convert to millimoles per liter, multiply by 0.4114]), and hypokalemia (potassium, <3.5 mEq/L [to convert to millimoles per liter, multiply by 1.0]) during treatment (after 1 full day of refeeding). Blood was drawn daily between 5 am and 7 am for the first 7 days, and then every other day unless greater frequency was needed; serum was analyzed in hospital laboratories. Electrolyte abnormalities were corrected with oral supplementation per a standardized protocol embedded in the electronic medical record and automated to populate physician orders (eTable in Supplement 2). Supplementation was given only if serum levels decreased below the specified thresholds. Additional safety outcomes were the proportion of participants in each group requiring electrolyte supplementation and time to reach electrolyte nadir. Other adverse events (eg, suicidality) were tracked and recorded per a National Institutes of Health clinical trial data safety monitoring plan.
Cost
The cost efficacy outcome was estimated cost associated with length of hospital stay, starting with the first full day in the hospital until the day of discharge. Cost was assessed using age-adjusted national Healthcare Cost and Utilization Project (HCUP) mean cost and charges for patients with AN. Mean adult or child HCUP hospital costs per day were multiplied by study length of stay to obtain total hospital cost per participant.
Statistical Analysis
With 60 participants per group, this trial was powered at 0.80 to detect a 12% to 20% difference in restored medical stability at 0.05 type I error and correlation between time points from 0.1 to 1. Distributions of patient covariates at baseline by group were summarized and assessed with boxplots to ensure that the 2 groups were comparable (Table 1).
Table 1. Participant Demographic Characteristics and Baseline Clinical Data in the Modified Intention-to-Treat Population.
| Demographic characteristic | HCR (n = 60) | LCR (n = 51) |
|---|---|---|
| Age, mean (SD), y | 16.6 (2.5) | 16.2 (2.4) |
| Female, No. (%) | 53 (88) | 48 (94) |
| Race/ethnicity, No. (%) | ||
| Non-Hispanic White | 36 (60) | 34 (67) |
| Asian | 7 (12) | 6 (11.8) |
| Hispanic or Latino | 15 (25) | 9 (17.6) |
| Other or >1 race/ethnicity reported | 2 (3) | 2 (4) |
| Family history of eating disorder | 19 (32) | 16 (31.3) |
| Socioeconomic score, mean (SD)a | 5.1 (3.1) | 5.3 (3.2) |
| Weight and menstrual history | ||
| Weight loss, mean (SD), kg | 13.4 (10.8) | 13.6 (7.8) |
| Percentage of body weight lost, mean (SD) | 21.1 (9.5) | 21.5 (9.5) |
| Percentage of mBMI lost, mean (SD) | 29.5 (20.6) | 31.9 (21.7) |
| Duration of weight loss, mean (SD), mo | 16.9 (17.6) | 14.1 (16.6) |
| Time since last menstrual period, mean (SD), mob | 8.2 (7.6) | 5.9 (4.7) |
| No menses in 3 mo, No. (%) | 23/38 (61) | 25/37 (68) |
| Eating disorder diagnosis and psychopathology | ||
| Atypical anorexia nervosa, No. (%)c | 21 (35) | 27 (53) |
| EDE-Q global score, mean (SD)d | 3.32 (1.68) | 3.45 (1.71) |
| Admission weight and vital signs, mean (SD) | ||
| Admission percentage mBMI | 83.3 (11.1) | 86.6 (12.2) |
| Lowest 24-hour heart rate, beats/min | 41.5 (6.9) | 40.7 (5.9) |
| Lowest SBP, mm Hg | 94.2 (8.0) | 93.3 (8.3) |
| Lowest temperature, °C | 36.6 (0.2) | 36.6 (0.2) |
| Orthostatic change, mean (SD) | ||
| Heart rate, beats/min | 26.8 (13.0) | 29.1 (14.2) |
| SBP, mm Hg | 0.6 (6.7) | 1.0 (7.7) |
| Instabilities, No. (%) | ||
| Bradycardia (heart rate <45 beats/min) | 46 (77) | 42 (82) |
| Hypotension (SBP <90 mm Hg) | 17 (28) | 17 (33) |
| Hypothermia (temperature <35.6 °C) | 1 (2) | 0 |
| Orthostatic heart rate increase ≥35 beats/min | 13 (22) | 14 (28) |
| Orthostatic SBP decrease ≥10 mm Hg | 5 (8) | 6 (11) |
| Admission laboratory values, mean (SD) (reference range)d | ||
| Phosphorus (3.0-5.1 mg/dL) | 3.83 (0.52) | 3.72 (0.58) |
| Potassium (3.5-5.1 mEq/L) | 3.83 (0.35) | 3.780 (0.38) |
| Magnesium (1.8-2.4 mg/dL) | 2.14 (0.14) | 2.15 (0.19) |
| Electrolyte abnormalities, No. (%) | ||
| Hypophosphatemia (<3.0 mg/dL)e | 2 (3) | 3 (6) |
| Hypokalemia (<3.5 mEq/L) | 6 (10) | 9 (18) |
| Hypomagnesemia (<1.8 mg/dL) | 0 | 3 (6) |
Abbreviations: EDE-Q, Eating Disorder Examination questionnaire; HCR, higher-calorie refeeding; LCR, lower-calorie refeeding; mBMI, 50th percentile body mass index for age and sex; SBP, systolic blood pressure.
SI conversion factors: To convert phosphorus to millimoles per liter, multiply by 0.323; potassium to millimoles per liter, multiply by 1.0; and magnesium to millimoles per liter, multiply by 0.4114.
Socioeconomic score based on the Hollingshead index of social status.
Among 75 postmenarcheal girls not taking hormonal contraception.
The remainder of patients received a diagnosis of anorexia nervosa.
Reference range for hypophosphatemia taken from Ornstein et al2; all other reference ranges from clinical laboratories in accordance with Clinical Laboratory Improvement Amendments.
Among 95 participants.
The primary analysis was a modified intent-to-treat (mITT) approach including all randomized participants who received treatment for at least 1 day.44 A total of 9 participants were excluded: 3 were found to be ineligible after randomization and 6 withdrew prior to receiving treatment. Sensitivity analyses showed that (1) patients who withdrew prior to treatment did not differ from those included in the mITT analysis by baseline mBMI, lowest heart rate, temperature, or systolic blood pressure; however, they were slightly older (mean [SD] age, 19.0 [2.7] vs 16.4 [2.5] years); and (2) outcome analyses that were performed including the patients who withdrew led to similar results as the mITT analysis. Survival analysis of time to medical stability used a log-rank test, which does not assume proportional hazards. Those who did not reach medical stability before hospital discharge were censored; analyses accounted for the site effect by stratification.
Additional short-term efficacy outcomes of group differences and 95% CIs in the proportion of each group achieving medical stability, restored heart rate, and change in percentage mBMI from day 1 to day 2 were estimated with exact or asymptotic CIs depending on whether or not an expected cell number was smaller than 5. Safety outcomes of group differences and their 95% CIs in incidence of electrolyte abnormalities, proportion receiving electrolyte supplementation, and time to electrolyte nadir during hospitalization were estimated similarly. Cost outcomes of group differences and their 95% CIs in length of stay and associated costs were estimated.
Results
Participant Characteristics
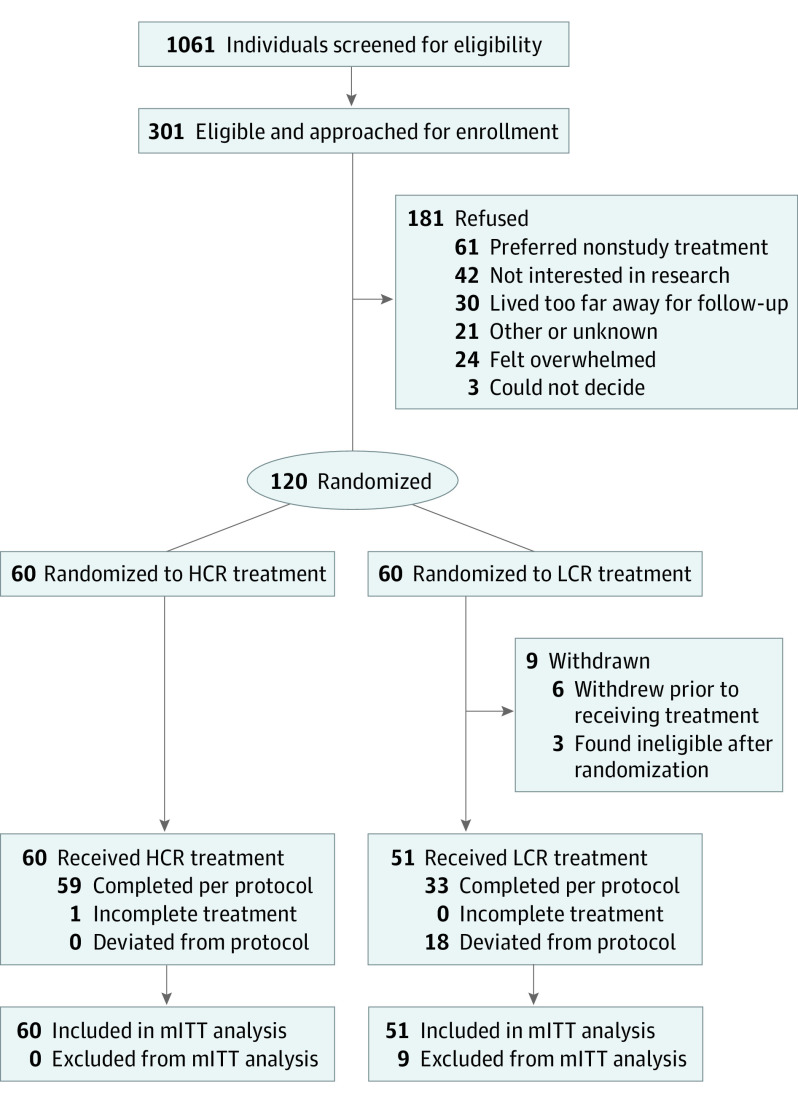
Figure 1 shows participant flow through the trial. A total of 9 participants withdrew from the LCR group, leaving a final study population of 111 (93%; 101 females [91%]; 70 non-Hispanic White participants [63%]; mean [SD] age, 16.4 [2.5] years). Only 1 patient did not complete the refeeding treatment, a young adult in the HCR group who left the hospital against medical advice. Among the 6515 study meals and snacks prescribed during treatment, fidelity was 94%: 6131 meals were within 200 kcal of protocol. All deviations of 200 kcal or more were in the LCR group and served to provide more calories than protocol. Consumption of the high-energy oral liquid formula was approximately 250 to 350 kcal/d during week 1 and did not differ by group, consistent with a prior study showing that patients could consume HCR meals without additional reliance on drinking a high-energy formula.15 Groups were balanced by baseline demographics and characteristics (Table 1). The most common medical instabilities on admission were bradycardia (88 [79%]), hypotension (34 [31%]), and orthostatic increase in heart rate (27 [24%]). Mean electrolyte levels were within normal limits at baseline, with no differences between groups. Among those with electrolyte abnormalities, hypokalemia was most common (15 [14%]); 5 participants (5%) presented with hypophosphatemia.
Figure 1. Flow of Participants Through STRONG (Study of Refeeding to Optimize Inpatient Gains) Trial.
HCR indicates higher-calorie refeeding; LCR, lower-calorie refeeding; and mITT, modified intent-to-treat.
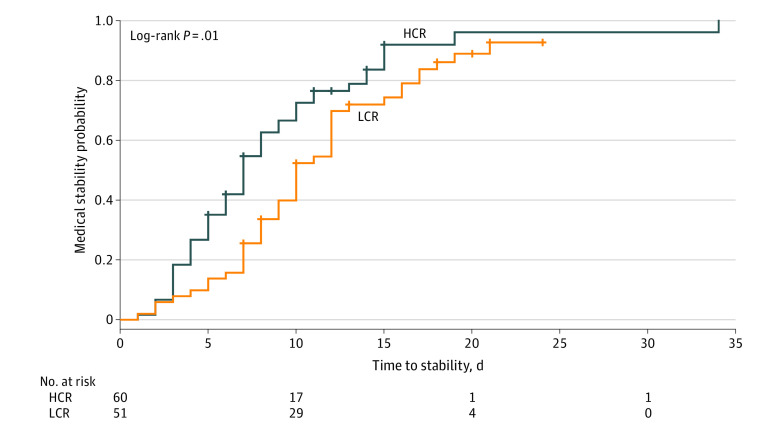
As shown in Figure 2, medical stability was restored significantly faster with HCR vs LCR treatment (hazard ratio, 1.67 [95% CI, 1.10-2.53]; P = .01). The proportion of participants meeting medical stability criteria (87% [52 of 60] vs 84% [43 of 51]; difference, 3% [95% CI, −16% to 11%]) or among those admitted with bradycardia, restored heart rate (83% [38 of 46] vs 79% [33 of 42]; difference, 4% [95% CI, −13% to 21%]) during hospitalization did not differ between HCR and LCR. However, as shown in Table 2, HCR restored medical stability 3.0 days earlier (95% CI, –4.9 to –1.2 days) and restored heart rate among those admitted with bradycardia 4.0 days earlier (95% CI, –6.5 to –1.5 days). In addition, overall weight gain was 0.79 kg (2.3% mBMI) greater in the HCR group than the LCR group. Neither the incidence of electrolyte disturbances during treatment nor the proportion of participants requiring supplementation differed by group; however, electrolyte nadirs occurred earlier in the HCR group for phosphorus and magnesium, but not for potassium. In terms of cost, the significantly shorter length of stay in the HCR group than the LCR group (–4.0 days; 95% CI, –6.1 to –1.9 days) was associated with an estimated savings of $5518 (95% CI, –$8266 to –$2770) per participant stay in cost and $19 056 (95% CI, −$28 819 to −$9293) per participant stay in hospital charges. Daily changes in heart rate and weight are illustrated in Figure 3: whereas those in the LCR group initially lost weight, those in the HCR group gained weight by day 2. Mean daily electrolyte levels were normal during the course of refeeding (eFigure in Supplement 2). Groups did not differ by any other adverse events and no cases of clinical refeeding syndrome occurred.
Figure 2. Survival Analysis of Primary Outcome, Time to Medical Stability, in Participants Treated With Higher-Calorie Refeeding (HCR) vs Lower-Calorie Refeeding (LCR).
Crosses indicate censoring.
Table 2. Efficacy and Safety Outcomes by Group.
| Outcome | HCR (n = 60) | LCR (n = 51) | Difference (95% CI) |
|---|---|---|---|
| Efficacy outcomes, mean (SD) | |||
| Days to medical stability | 7.0 (7.0) | 10.0 (8.0) | −3.0 (−4.9 to −1.2)a |
| Days to restored heart rateb | 4.5 (6.0) | 8.0 (8.0) | −4.0 (−6.5 to −1.5)a |
| Weight gain, mean (SD), kg | 2.9 (1.6) | 2.2 (1.3) | 0.79 (0.2 to 1.3)a |
| Percentage of mBMI, mean (SD) | 5.4 (3.0) | 3.2 (5.7) | 2.3 (0.1 to 3.9)a |
| Weight change at day 1-2, mean (SD), percentage of mBMI | 0.38 (0.71) | −0.31 (0.77) | 0.7 (0.4 to 1.0)a |
| Safety indicators | |||
| Electrolyte abnormalities, No. (%)c | |||
| Hypophosphatemia (<3.0 mg/dL) | 4 (7) | 3 (6) | 1 (−8 to 10) |
| Hypomagnesemia (<3.5 mEq/L) | 7 (12) | 14 (28) | −16 (−31 to 1) |
| Hypokalemia (<1.8 mg/dL) | 3 (5) | 5 (10) | −5 (−15 to 5) |
| Electrolyte correction, No. (%)d | |||
| Phosphorus | 6 (10) | 2 (4) | 5 (−4 to 14) |
| Magnesium | 12 (20) | 10 (20) | 0.3 (−12 to 2) |
| Potassium | 5 (8) | 4 (8) | 0.4 (−9. to 10) |
| Day of electrolyte nadir, mean (SD) | |||
| Phosphorus | 4.6 (1.9) | 5.8 (2.5) | −1.2 (−2.0 to −0.4)a |
| Magnesium | 5.1 (2.5) | 6.2 (2.7) | −1.1 (−2.1 to −0.1)a |
| Potassium | 4.0 (2.7) | 4.8 (3.1) | −0.9 (−2.0 to 0.2) |
| Cost outcomes, mean (SD) | |||
| Length of stay, d | 8.0 (5.5) | 12.0 (6.0) | −4.0 (−6.1 to −1.9)a |
| Cost per participant, $ | 10 784 (7410) | 16 302 (8230) | −5518 (−8266 to −2770)a |
| Hospital charges per participant, $ | 38 112 (26 043) | 57 168 (30 486) | −19 056 (−28 819 to −9293)a |
Abbreviations: HCR, higher-calorie refeeding; LCR, lower-calorie refeeding; mBMI, 50th percentile body mass index for age and sex.
SI conversion factors: To convert phosphorus to millimoles per liter, multiply by 0.323; potassium to millimoles per liter, multiply by 1.0; and magnesium to millimoles per liter, multiply by 0.4114.
CI does not include zero indicating a difference between groups at significance level α = .05.
Heart rate restored to 45 beats/min or more for 24 hours in hospital, among 46 HCR and 42 LCR participants who presented with a heart rate of less than 45 beats/min (Table 1).
Electrolyte abnormalities that developed after initiation of refeeding treatment.
See eTable in Supplement 2 for electrolyte correction protocol, including dosage based on serum level.
Figure 3. Comparison of Daily Heart Rate and Weight Change in Participants Treated With Higher-Calorie Refeeding (HCR) vs Lower-Calorie Refeeding (LCR).
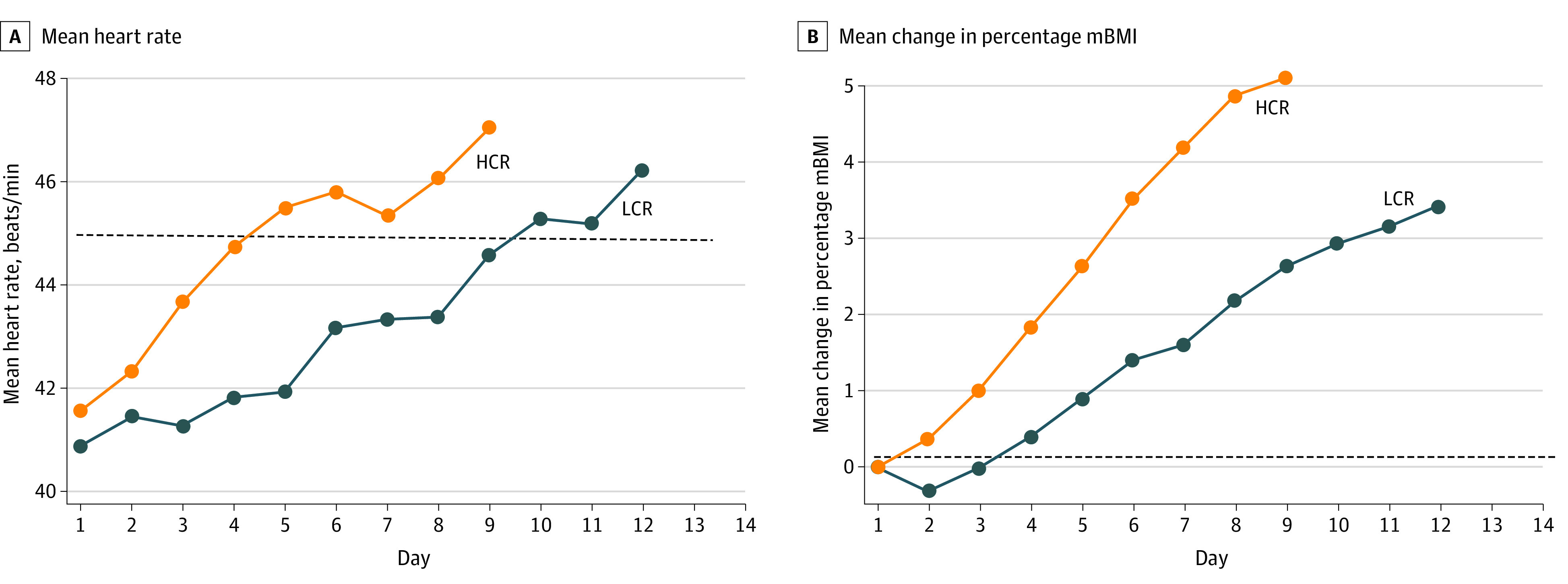
A, Mean heart rate. The dashed line indicates heart rate stability threshold (45 beats/min for 24 hours). B, Mean change in percentage mBMI (50th percentile body mass index for age and sex). The dashed line indicates baseline weight.
Discussion
In this multicenter RCT comparing refeeding approaches in hospitalized patients with malnutrition secondary to AN and AAN, HCR restored medical stability 3 days faster than LCR, with no increase in safety events. By comparison, participants treated with LCR lost weight initially, gained less weight overall, and stayed in the hospital 4 additional days. Protracted hospitalizations contribute to the high health care costs of AN. Eating disorders are among the top 3 most expensive primary mental health diagnoses in pediatrics,6 with estimated charges of $46 130 per hospitalization. The shorter length of stay in participants treated with HCR was associated with $19 056 savings in hospital charges.
Refeeding has been approached with extreme caution since the refeeding syndrome was first described in conscientious objectors and prisoners of World War II.45,46 In the US, recommendations from the American Psychiatric Association7,8 and the Academy of Nutrition and Dietetics9 are still in place for LCR to start at approximately 1200 kcal/d. Over the decades that LCR has endured as the standard of care for AN, only a small number of cases documenting cardiac arrest, delirium,11,47 and death14,48 during refeeding were reported. Although LCR was generally considered safe, preliminary studies15,16,17 showed that it was associated with initial weight loss, slow weight gain, and protracted hospital stay. The present RCT confirms that LCR contributes to these poor outcomes, now recognized collectively as the “underfeeding syndrome.”14
Patients with AN are hypometabolic on admission,49 in a hibernation-like state of energy conservation, marked by bradycardia and other vital sign abnormalities45,46 that can be reversed with refeeding.49,50,51 Therefore, we created a multidimensional medical stability index to adjudicate the effect of refeeding. Preliminary work relied on length of hospital stay to indicate efficacy, showing 3-day to 6-day shorter stay with HCR vs LCR15,17; however, hospital stay can be lengthened by factors that do not reflect the effect of refeeding (eg, discharge planning). Overall weight gain was 2.3% mBMI (0.8 kg) greater in the HCR group. Prior studies examining weight gain as a predictive factor reported that greater weight gain in the hospital was associated with weight recovery at 12 months,52,53,54 and greater weight gain during the first 3 to 4 weeks of outpatient psychotherapy (0.8-0.9 kg/wk [1.7-1.9 lb/wk]) was associated with full remission at 12 months.55 We are currently following up with participants through 12 months to compare long-term clinical remission with HCR vs LCR.
Perhaps the most important goal of the present RCT was to examine indicators of safety, which have not been adequately studied, to our knowledge. Here we relied on electrolyte disturbances, most commonly occurring during the first 7 days of refeeding,2,28,56 to indicate risk for the refeeding syndrome. Hypophosphatemia is the hallmark abnormality; however, hypomagnesaemia and hypokalemia also occur during refeeding.17 Electrolyte data from the available retrospective and observational studies were obfuscated by wide variations in approaches to electrolyte correction.37 Although some programs initiated prophylactic supplementation when refeeding started, others used electrolyte replacement to treat low and/or declining serum phosphorus.37 Using a standardized electrolyte monitoring and replacement protocol, we found lower rates of hypophosphatemia than previously reported and no group differences in electrolyte disturbances or other adverse events. Furthermore, no unexpected serious adverse events occurred in either group. Thus we did not find evidence for increased risk or need for more intensive electrolyte management with HCR. However, we did find earlier electrolyte nadirs in the HCR group. This finding reflects the purported mechanism whereby electrolytes shift intracellularly during refeeding and suggests that the caloric load influences the timing of these shifts. This has not been previously reported, to our knowledge, and justifies the frequency of monitoring during the first week of refeeding with HCR.
Limitations
We recognize several limitations to the present study. First, this was a study of refeeding by meals, with food served on bedside trays and a high-energy formula given orally only as needed to replace refused foods. Meal-based approaches are preferred in the US,15,17 whereas enteral feeding is reported internationally.24,57,58 Second, treatments were not blinded because both the patients and clinicians who work with this population are highly skilled at estimating calories and could determine their group assignment by viewing meal trays. Third, all protocol deviations occurred in the LCR group and provided more calories than protocol, suggesting a bias toward HCR among the study physicians experienced with both treatments. Although this may have reduced our effect size, findings were nonetheless highly significant. Fourth, patients with 60% or lower mBMI were excluded owing to concerns for medical fragility; therefore, these results cannot be extrapolated to those with extreme malnutrition. Fifth, given the questionable feasibility and ethics of examining refeeding syndrome as an outcome, we used electrolyte disturbances and cannot make claims of safety; refeeding should be performed under close medical supervision.
Conclusions
Balancing the potential risks of the refeeding syndrome with the need to maximize weight gain during refeeding represents a fundamental paradox for the care of hospitalized patients with AN. Clinical practice has been moving toward HCR,19,20,21,22,23,24,25,59 with insufficient efficacy and safety data. Findings from this RCT support the development of evidence-based recommendations for HCR in adolescents and young adults hospitalized with moderate malnutrition secondary to AN and AAN. The primary end point of this trial, 12-month clinical remission, will be reported when participants complete long-term follow-up.
Trial Protocol
eFigure. Mean Daily Serum Electrolytes in Participants Treated With Higher-Calorie vs Lower-Calorie Refeeding
eTable. StRONG Trial Electrolyte Replacement Protocol
Data Sharing Statement
References
- 1.Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health. 2003;32(1):73-77. doi: 10.1016/S1054-139X(02)00533-5 [DOI] [PubMed] [Google Scholar]
- 2.Ornstein RM, Golden NH, Jacobson MS, Shenker IR. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: implications for refeeding and monitoring. J Adolesc Health. 2003;32(1):83-88. doi: 10.1016/S1054-139X(02)00456-1 [DOI] [PubMed] [Google Scholar]
- 3.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284-1293. doi: 10.1176/appi.ajp.159.8.1284 [DOI] [PubMed] [Google Scholar]
- 4.Murray SB, Quintana DS, Loeb KL, Griffiths S, Le Grange D. Treatment outcomes for anorexia nervosa: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2019;49(4):535-544. doi: 10.1017/S0033291718002088 [DOI] [PubMed] [Google Scholar]
- 5.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68(7):724-731. doi: 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- 6.Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association Work Group on Eating Disorders Practice guideline for the treatment of patients with eating disorders (revision). Am J Psychiatry. 2000;157(1)(suppl):1-39. [PubMed] [Google Scholar]
- 8.American Psychiatric Association Treatment of patients with eating disorders,third edition. Am J Psychiatry. 2006;163(7)(suppl):4-54. [PubMed] [Google Scholar]
- 9.American Dietetic Association Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J Am Diet Assoc. 2006;106(12):2073-2082. doi: 10.1016/j.jada.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Fisher M, Simpser E, Schneider M. Hypophosphatemia secondary to oral refeeding in anorexia nervosa. Int J Eat Disord. 2000;28(2):181-187. doi: [DOI] [PubMed] [Google Scholar]
- 11.Kohn MR, Golden NH, Shenker IR. Cardiac arrest and delirium: presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health. 1998;22(3):239-243. doi: 10.1016/S1054-139X(97)00163-8 [DOI] [PubMed] [Google Scholar]
- 12.Beumont PJ, Large M. Hypophosphataemia, delirium and cardiac arrhythmia in anorexia nervosa. Med J Aust. 1991;155(8):519-522. doi: 10.5694/j.1326-5377.1991.tb93887.x [DOI] [PubMed] [Google Scholar]
- 13.Hall DE, Kahan B, Snitzer J. Delirium associated with hypophosphatemia in a patient with anorexia nervosa. J Adolesc Health. 1994;15(2):176-178. doi: 10.1016/1054-139X(94)90546-0 [DOI] [PubMed] [Google Scholar]
- 14.MARSIPAN Working Group Management of really sick patients with anorexia nervosa. 1st ed. CR 162. Royal College of Psychiatrists and Royal College of Physicians; October 2010.
- 15.Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer MA, Moscicki AB. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J Adolesc Health. 2013;53(5):579-584. doi: 10.1016/j.jadohealth.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health. 2012;50(1):24-29. doi: 10.1016/j.jadohealth.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden NH, Keane-Miller C, Sainani KL, Kapphahn CJ. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J Adolesc Health. 2013;53(5):573-578. doi: 10.1016/j.jadohealth.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 18.Garber AK, Sawyer SM, Golden NH, et al. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord. 2016;49(3):293-310. doi: 10.1002/eat.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health. 2010;46(6):577-582. doi: 10.1016/j.jadohealth.2009.11.207 [DOI] [PubMed] [Google Scholar]
- 20.Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585-589. doi: 10.1016/j.jadohealth.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 21.Peebles R, Lesser A, Park CC, et al. Outcomes of an inpatient medical nutritional rehabilitation protocol in children and adolescents with eating disorders. J Eat Disord. 2017;5:7. doi: 10.1186/s40337-017-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith K, Lesser J, Brandenburg B, et al. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa and atypical anorexia nervosa at children’s hospitals and clinics of Minnesota. J Eat Disord. 2016;4:35. doi: 10.1186/s40337-016-0124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maginot TR, Kumar MM, Shiels J, Kaye W, Rhee KE. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa: Rady Children’s Hospital San Diego/University of California, San Diego. J Eat Disord. 2017;5:1. doi: 10.1186/s40337-016-0132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden S, Miskovic-Wheatley J, Clarke S, Touyz S, Hay P, Kohn MR. Outcomes of a rapid refeeding protocol in adolescent anorexia nervosa. J Eat Disord. 2015;3:8. doi: 10.1186/s40337-015-0047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker EK, Faruquie SS, Anderson G, et al. Higher caloric refeeding is safe in hospitalised adolescent patients with restrictive eating disorders. J Nutr Metab. 2016;2016:5168978. doi: 10.1155/2016/5168978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor G, Nicholls D, Hudson L, Singhal A. Refeeding low weight hospitalized adolescents with anorexia nervosa: a multicenter randomized controlled trial. Nutr Clin Pract. 2016;31(5):681-689. doi: 10.1177/0884533615627267 [DOI] [PubMed] [Google Scholar]
- 27.O’Connor G, Nicholls D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: a systematic review. Nutr Clin Pract. 2013;28(3):358-364. doi: 10.1177/0884533613476892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Society for Adolescent Health and Medicine Refeeding hypophosphatemia in hospitalized adolescents with anorexia nervosa: a position statement of the Society for Adolescent Health and Medicine. J Adolesc Health. 2014;55(3):455-457. doi: 10.1016/j.jadohealth.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CA, Sabel AL, Gaudiani JL, Mehler PS. Predictors of hypophosphatemia during refeeding of patients with severe anorexia nervosa. Int J Eat Disord. 2015;48(7):898-904. doi: 10.1002/eat.22406 [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; 2013. [Google Scholar]
- 31.Whitelaw M, Gilbertson H, Lee KJ, Sawyer SM. Restrictive eating disorders among adolescent inpatients. Pediatrics. 2014;134(3):e758-e764. doi: 10.1542/peds.2014-0070 [DOI] [PubMed] [Google Scholar]
- 32.Whitelaw M, Lee KJ, Gilbertson H, Sawyer SM. Predictors of complications in anorexia nervosa and atypical anorexia nervosa: degree of underweight or extent and recency of weight loss? J Adolesc Health. 2018;63(6):717-723. doi: 10.1016/j.jadohealth.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 33.Sawyer SM, Whitelaw M, Le Grange D, Yeo M, Hughes EK. Physical and psychological morbidity in adolescents with atypical anorexia nervosa. Pediatrics. 2016;137(4):e20154080. doi: 10.1542/peds.2015-4080 [DOI] [PubMed] [Google Scholar]
- 34.Garber AK, Cheng J, Accurso EC, et al. Weight loss and illness severity in adolescents with atypical anorexia nervosa. Pediatrics. 2019;144(6):e20192339. doi: 10.1542/peds.2019-2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy GA, Forman SF, Woods ER, et al. History of overweight/obesity as predictor of care received at 1-year follow-up in adolescents with anorexia nervosa or atypical anorexia nervosa. J Adolesc Health. 2017;60(6):674-679. doi: 10.1016/j.jadohealth.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata JM, Garber AK, Buckelew SM. Weight restoration in atypical anorexia nervosa: a clinical conundrum. Int J Eat Disord. 2018;51(11):1290-1293. doi: 10.1002/eat.22953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz BI, Mansbach JM, Marion JG, Katzman DK, Forman SF. Variations in admission practices for adolescents with anorexia nervosa: a North American sample. J Adolesc Health. 2008;43(5):425-431. doi: 10.1016/j.jadohealth.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 38.Golden NH, Katzman DK, Sawyer SM, et al. ; Society for Adolescent Health and Medicine . Position paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121-125. doi: 10.1016/j.jadohealth.2014.10.259 [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Clinical growth charts. 2000. Accessed May 24, 2018. https://www.cdc.gov/growthcharts/clinical_charts.htm
- 40.Institute of Medicine; Food and Nutrition Board; Panel on Macronutrients; Panel on the Definition of Dietary Fiber; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. The National Academies Press, 2005. [Google Scholar]
- 41.Fairburn CG, Cooper Z, O’Connor ME Eating disorder examination (edition 16.0D). In: Fairburn CG, ed. Cognitive Behavior Therapy and Eating Disorders Guilford Press; 2008:265-308. [Google Scholar]
- 42.Hollingshead A. Four Factor Index of Social Status Department of Psychology, Yale University; 1975.
- 43.Golden NH, Katzman DK, Sawyer SM, et al. Update on the medical management of eating disorders in adolescents. J Adolesc Health. 2015;56(4):370-375. doi: 10.1016/j.jadohealth.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 44.Dossing A, Tarp S, Furst DE, et al. Modified intention-to-treat analysis did not bias trial results. J Clin Epidemiol. 2016;72:66-74. doi: 10.1016/j.jclinepi.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med. 1951;35(1):69-96. doi: 10.7326/0003-4819-35-1-69 [DOI] [PubMed] [Google Scholar]
- 46.Keys A, Brozek K, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. University of Minnesota Press; 1950. doi: 10.5749/j.ctv9b2tqv [DOI] [Google Scholar]
- 47.Norris ML, Pinhas L, Nadeau PO, Katzman DK. Delirium and refeeding syndrome in anorexia nervosa. Int J Eat Disord. 2012;45(3):439-442. doi: 10.1002/eat.20963 [DOI] [PubMed] [Google Scholar]
- 48.Weinsier RL, Krumdieck CL. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Am J Clin Nutr. 1981;34(3):393-399. doi: 10.1093/ajcn/34.3.393 [DOI] [PubMed] [Google Scholar]
- 49.Schebendach JE, Golden NH, Jacobson MS, Hertz S, Shenker IR. The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Ann N Y Acad Sci. 1997;817:110-119. doi: 10.1111/j.1749-6632.1997.tb48200.x [DOI] [PubMed] [Google Scholar]
- 50.Vaisman N, Rossi MF, Corey M, Clarke R, Goldberg E, Pencharz PB. Effect of refeeding on the energy metabolism of adolescent girls who have anorexia nervosa. Eur J Clin Nutr. 1991;45(11):527-537. [PubMed] [Google Scholar]
- 51.Rigaud D, Verges B, Colas-Linhart N, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92(5):1623-1629. doi: 10.1210/jc.2006-1319 [DOI] [PubMed] [Google Scholar]
- 52.Lock J, Litt I. What predicts maintenance of weight for adolescents medically hospitalized for anorexia nervosa? Eat Disord. 2003;11(1):1-7. doi: 10.1002/erv.496 [DOI] [PubMed] [Google Scholar]
- 53.Lund BC, Hernandez ER, Yates WR, Mitchell JR, McKee PA, Johnson CL. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. Int J Eat Disord. 2009;42(4):301-305. doi: 10.1002/eat.20634 [DOI] [PubMed] [Google Scholar]
- 54.Baran SA, Weltzin TE, Kaye WH. Low discharge weight and outcome in anorexia nervosa. Am J Psychiatry. 1995;152(7):1070-1072. doi: 10.1176/ajp.152.7.1070 [DOI] [PubMed] [Google Scholar]
- 55.Le Grange D, Accurso EC, Lock J, Agras S, Bryson SW. Early weight gain predicts outcome in two treatments for adolescent anorexia nervosa. Int J Eat Disord. 2014;47(2):124-129. doi: 10.1002/eat.22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedli N, Stanga Z, Sobotka L, et al. Revisiting the refeeding syndrome: results of a systematic review. Nutrition. 2017;35:151-160. doi: 10.1016/j.nut.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 57.Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29(5):627-632. doi: 10.1016/j.clnu.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 58.Rigaud D, Brondel L, Poupard AT, Talonneau I, Brun JM. A randomized trial on the efficacy of a 2-month tube feeding regimen in anorexia nervosa: a 1-year follow-up study. Clin Nutr. 2007;26(4):421-429. doi: 10.1016/j.clnu.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 59.Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr. 2011;23(4):390-394. doi: 10.1097/MOP.0b013e3283487591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Mean Daily Serum Electrolytes in Participants Treated With Higher-Calorie vs Lower-Calorie Refeeding
eTable. StRONG Trial Electrolyte Replacement Protocol
Data Sharing Statement