Abstract
Lytic enzymes are novel antimicrobial agents that degrade bacterial cell walls, resulting in cell rupture and death. We tested one enzyme, the bacteriocin lysostaphin, for treatment of nonhuman primates (Macaca mulatta) with persistent methicillin-resistant Staphylococcus aureus (MRSA) infection of their cranial implant margins. The goal of this study was to determine if topical lysostaphin, either alone or as an adjunct therapy, could eliminate MRSA. Lysostaphin had in vitro lytic activity against all 4 previously identified NHP MRSA clones, as well as against 12 MRSA isolates of the same clonal type (MLST ST3862 and spa type t4167) before and after treatment, with no resistance discovered. In an in vivo pilot study, a 2-d application of lysostaphin alone reduced MRSA in the implant margins by 3-logs during treatment of one animal; however, MRSA titers had returned to control levels by 1 wk after treatment. In the main study, all animals (n = 4) received 10 d of systemic antibiotic treatment and both the animals and their environment (cages, equipment, room) underwent 5-d of decontamination. The experimental animals (n = 2) received 5 doses of topical lysostaphin (15 mg, every other day) applied onto their implant margins. Daily cultures showed that MRSA counts decreased significantly (≤ 25 colony-forming units/mL; P < 0.05). However, sampling of the cranial implant margin 7 d after last treatment showed that MRSA counts had returned to control levels. Our study suggests that lysostaphin, coupled with other treatment modalities, can decrease MRSA infection short-term but do not completely eradicate MRSA in the long-term. This reappearance of MRSA may be due to cross-contamination or reinfection from other infected areas, an inability of the treatment to reach all colonized areas, or insufficient dosing or length of treatment. Topical lysostaphin may be more useful clinically for superficial nonimplant associated wounds in which the lytic enzyme has better access to the infected tissue.
Abbreviations: DPBS, Dulbeccoʹs phosphate-buffered saline; MLST, Multilocus sequence typing; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; spa, staphylococcal protein A; TOD50, time to reach half starting absorbance
Antibiotic-resistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), can cause both superficial and invasive disease and are responsible for millions of serious and sometimes fatal infections worldwide.11,17,27,33,48 Staphylococcus aureus can produce severe secondary infections in immunocompromised individuals and cause disease in immunocompetent people.27 Besides skin and soft tissue infections, S. aureus can cause a large spectrum of diseases, including foodborne toxin-infection, urinary tract infections, pneumonia, pyomyositis, necrotizing fasciitis, endocarditis, toxic shock syndrome, sepsis, and meningitis. MRSA is increasingly prevalent in community settings, while remaining a major nosocomial pathogen.16,28,32,34,43 Besides being resistant to β-lactam antibiotics (penicillins, cephalosporins, carbapenems), MRSA strains are increasingly resistant to mupirocin, the key topical agent used for the decolonization of staphylococci in the nares, oropharynx, and on the skin.35,38 MRSA is also known to colonize and cause infections in livestock, pets, zoo animals, and marine mammals. Despite few reports of MRSA infection or colonization (carriage) in laboratory animals, interactions with humans, cohousing of many different species of animals, and the use (and potential overuse) of antimicrobials all create opportunities for the introduction and transmission of MRSA in animal facilities.17,33,43,47,48 Furthermore, the application of experimental invasive devices and implants, such as cranial implants in nonhuman primates (NHPs), pose a higher risk of MRSA infection, similar to what is seen in human patients.52 The implants prevent complete wound healing, which can serve as an entry point for bacteria to grow.17,46,48,52 These types of implant infections may affect overall animal health, necessitate device or implant removal, raise total animal husbandry costs, and increase the potential for zoonotic transmission of MRSA.
During routine care and infection surveillance in the Comparative Bioscience Center at The Rockefeller University in New York City, MRSA was isolated from cranial implant margin interfaces for 9 of 14 male rhesus macaques (Macaca mulatta).33 Further screening of the animals (head, nares, and rectum) and the environment (40 environmental surfaces) over 1 year recovered 114 S. aureus isolates. These isolates have been previously characterized by antibiograms, spa typing, multilocus sequence typing, pulsed-field gel electrophoresis [PFGE], mecA, Panton–Valentine Leukocidin, and arginine catabolic mobile element; these MRSA isolates were grouped into 4 clones: MA-II (t4167/ST3862/PFGE-B), MA-III (t4167/ST3862/PFGE-C), MA-IV (t16708/ST3862/PFGEC), and MA-V (t16709/ST3862/PFGE-C).33 Due to the high risk of MRSA-related complications in these animals and the potential for the spread of infection to other animals or laboratory personnel, decontamination procedures were undertaken. The affected NHPs received full-body bathing with chlorhexidine, application of mupirocin antibiotic ointment to the implant margins, and, in the case of the most affected animal, systemic antibiotics (that animal showed purulent discharge from the cranial implant margin). The immediate environment of the NHPs was also decontaminated.33 Unfortunately, posttreatment sampling showed that 6 of 9 animals were still positive for MRSA, based on cultures from the cranial implant margin.33
Given the potential for the MRSA to spread and the failure of aggressive cleaning in combination with topical and systemic antibiotics to eradicate the infection, we sought alternative treatments. As such, we studied the topical use of lytic enzymes as an adjunctive treatment for MRSA skin and wound infections in NHPs with permanent cranial implants.
Lytic enzymes or cell wall hydrolases are a unique class of antimicrobials that have been used to control drug-resistant bacterial pathogens in humans and experimental animals. These enzymes include lysins and bacteriocins. Lysins are peptidoglycan hydrolases whose genes are carried by bacteriophages.12-14,35,40,41 They have been effective and safe in several animal models of sepsis, endocarditis, pharyngitis, pneumonia, meningitis, and mucosal and skin decolonization.12-14,35,40,41 Furthermore, a lysin against S. aureus recently completed a successful phase 2 clinical trial for use in human medicine.12,35,36 Bacteriocins are similar to lysins, but are produced by bacteria instead of bacteriophages.50 Bacteriocins act as antibacterial substances against other bacterial strains or species. Lysostaphin is a well-studied bacteriocin that is a staphylococcal peptidoglycan hydrolase, similar to a bacteriophage lysin in terms of its modular architecture and mechanism of action.49 Lysostaphin is a zinc metalloenzyme originally isolated from a bacterial culture of Staphylococcus simulans.8 It has specific lytic action against many Staphylococcus species, including S. aureus. Lysostaphin has hexosaminidase, amidase, and endopeptidase activities. It cleaves polyglycine cross-bridge in the cell wall of Staphylococcus species, leading to cell lysis. Lysostaphin has been used safely in topical and systemic infection models in laboratory animals such as mice, rats, and rabbits and larger animals such as cattle.4,5,7-10,15,19,22-24,29,39,42 Furthermore, lysostaphin treatment is effective in treating systemic disease models of endocarditis in rabbits and neonatal sepsis in suckling rats.31,37,47
We selected lysostaphin as the lytic enzyme candidate for this study due to its commercial availability in a lyophilized, purified form for research use (Sigma–Aldrich St Louis, MO) and its efficacy and safety results in other animal models.4-10,15,19,22-24,29,31,37,39,42 The first aim of our current study was to determine if lysostaphin showed effective in vitro lytic activity against NHP MRSA isolates obtained from our study animals. The second aim was to conduct treatment trials to determine if the topical use of lysostaphin (alone or in combination with systemic antibiotics) could effectively reduce or eliminate MRSA colonization and infection in rhesus macaques with cranial implants. Our hypothesis was that lysostaphin would show lytic activity against NHP MRSA isolates, and that this treatment modality would be effective at reducing or eradicating topical NHP MRSA colonization/infection.
Materials and Methods
Study animals.
Animal experiments were performed in the Comparative Bioscience Center at The Rockefeller University, an AAALAC-accredited facility. All animal procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the Public Health Service Policy, the Guide for the Care and Use of Laboratory Animals and with the Animal Welfare Act and Regulations.1,2,18,30 The study animals were 4 male rhesus macaques (Macaca mulatta, 7 to 8 y) that cultured positive for MRSA despite previous standard decontamination and antibiotic treatments.33 All animals were part of the same neuroimaging experimental protocol and were housed in the same quarantined isolation room to prevent the spread of MRSA to the rest of the NHP colony. Two of the animals were pair-housed. The remaining 2 animals were socially incompatible, and thus were single-housed.
The macaques originated from 2 breeding colonies: Covance, Alice, TX, USA and Three Springs Scientific, (Perkasie, PA). Macaques were housed in stainless steel mobile caging (Apartment Module with conversion floor, 3m2, Primate Products, Immokalee, FL). Sanitization of cage components was performed with the use of a mechanical washer (Better Built, Northwest Systems, Delta, British Columbia) that provided an 82 °C final rinse water and used a combination of a chlorinated cleaning compound (ENVIRO-KLEEN 900S, Quip Laboratories, Wilmington, DE) and organic acid detergent (Acidulate 28, Quip Laboratories, Wilmington, DE) during the wash cycle. The macaques were fed a commercial diet (Monkey Diet Jumbo 5037, LabDiet, St Louis, MO) and were maintained on municipal water. They were housed at 22 ± 1 °C at 30% to 70% relative humidity, with a 12:12 h light:dark cycle (0700 to 1900 h). Daily environmental enrichment included manipulanda and structural enrichment; novel food items (nuts, dried pasta, fruits, vegetables, yogurt, etc.); occupational devices, and sensory enrichment (alternating between auditory and visual). All macaques were seronegative for simian immunodeficiency virus, simian T-cell leukemia virus type 1, simian type D retrovirus, and macacine alphaherpesvirus 1 as determined by ELISA (VRL Laboratories, San Antonio, TX). They were also negative for Mycobacterium tuberculosis based on an intradermal tuberculin skin test (Tuberculin Mammalian, Human Isolates Intradermic, Zoetis, San Diego, CA) and were vaccinated against measles.
All macaques were trained to enter a primate chair (closed-chair design) via positive reinforcement training for sample collection and treatment. Afterward, they received further positive reinforcement training to voluntarily cooperate with sample collections, such as presenting their peri-rectal area for culture swab access. No anesthetics or tranquilizing drugs were used during sample collections and treatment. The objective of the in vivo study was to conduct treatment trials to determine if topically applied lysostaphin coupled with standard decontamination alone (pilot study) or in addition to systemic antibiotics (main study) effectively decreased or eliminated MRSA colonization/infection in NHPs. As discussed below, all animals were confirmed MRSA positive on at least one site (for example, cranial implant margin, peri-rectal, nostril) through bacterial culture identification and antibiotic resistance to oxacillin (growth on Remel Mannitol Salt Agar with oxacillin (4 μg/mL) and/or growth on Remel MRSA selective media (Thermo Scientific, Lenexa, KS).
Lysostaphin formulations.
Vials with 15 mg of lyophilized lysostaphin (≥500 units/mg protein; Sigma–Aldrich St Louis, MO) from Staphylococcus staphylolyticus were used for all in vitro and in vivo experiments. The lysostaphin was freshly reconstituted on each treatment day with 3 mL of sterile Dulbecco phosphate-buffered saline (DPBS) with no calcium or magnesium (Gibco, Thermo Fisher Scientific, Waltham, MA) to achieve a 5 mg/mL concentration for treatment of the cranial implant margins.
In an effort to reduce cross-contamination, the nostrils and perirectal area as well as the cranial implant region were all treated during the main study based on previous detection of MRSA in those areas.33 Topical treatment of the nostrils and perirectal area required formulation of the lysostaphin in an ointment to ensure the adequate contact time for the enzyme on the treated area. The ideal ointment base to incorporate lyophilized lysostaphin had to be hydrophilic enough to allow the lyophilized lysostaphin to go into solution and be hydrophobic enough for adequate absorption into the skin and mucous membranes.35 A previous study found that Aquaphor (Beiersdorf, Wilton, CT) had these characteristics.35 Aquaphor is an emollient ointment whose active ingredient is petrolatum (41%); inactive ingredients include mineral oil, ceresin, lanolin alcohol, panthenol, glycerin, and bisabolol. A 5 mg/mL concentration of lysostaphin in Aquaphor was used for treatment of the nostrils and perirectal areas. This was formulated by adding 3 mL of Aquaphor into a 15 mg vial containing the same lots of lyophilized lysostaphin above. This lysostaphin ointment formulation was termed lysostaphin-A.
In vitro lysostaphin activity assays.
A turbidity reduction assay was used to determine the in vitro lytic activity of lysostaphin against NHP MRSA strains. These assays encompassed at least 2 isolates from all 4 of the MRSA clones (MA-II, MA-III, MA-IV, and MA-V) recovered from the NHPs in the prior year,33 as well as current isolates obtained from the treatment and control animals using a previously described methodology.13,14,25,40 Overnight cultures of MRSA isolates were used to start fresh cultures the following day (1:50 dilution). These cultures were grown to log-phase (OD600 = 0.5) at 37 °C, sedimented by centrifugation and the bacterial pellet brought to an optical density (OD600) of approximately 1.0 with 20 mM DPBS (Gibco, Thermo Fisher Scientific, Grand Island, NY), pH 8.0 (buffer A) as measured in 96-well microtiter plates (Falcon, Fisher Scientific, PA). From these bacterial stocks, 245 μL were added for every isolate into triplicate wells of a 96-well flat-bottomed microtiter plate. Each well received 5 μL of lysostaphin at various concentrations (0 to 83 µg/mL) to determine the concentrations that showed effective lytic activity against MRSA. Corresponding triplicate wells received 5 μL of 20 mM DPBS, pH 7.2 (buffer B) control vehicle. A Spectramax Plus 384 (Molecular Devices, San Jose, CA) was used to obtain spectrophotometric readings (at λ = 600 nm, that is, OD600) of each well, every minute, over an hour. The degree of reduction of turbidity in the test wells indicated the amount of lysin activity. To normalize and combine values from multiple tests, the final OD600 of the treated samples was divided by the final OD600 of the untreated samples. An OD600 ratio of 1.0 indicated no lysis, while an OD600 ratio of approximately 0.02 indicated complete lysis.13
To confirm the in vitro efficacy of lysostaphin-A ointment on the staphylococcal cell wall, we followed a previously published protocol in which a 2 L culture of MRSA strain MW2 in Mueller Hinton Broth was centrifuged, and the bacterial pellet was resuspended in 100 mL DPBS.35 Agar was added to this solution to achieve a 1.5% concentration. This mixture was then autoclaved to produce nonviable cells. The autoclaved Staphylococcus-agar suspension was placed in empty 150-mm petri plates and allowed to solidify. A 5mg/mL dose of lysostaphin-A formulation, along with Aquaphor alone and our lysostaphin solution (5 mg/mL DPBS) was streaked onto the surface of the Staphylococcus-impregnated agar using a 10 µL inoculation loop. A filter-paper disk containing 20 µg of lysostaphin (Hardy Diagnostics, Santa Maria, CA) was also added to the plate as a positive control. Agar plates were examined after 12 h at 37 °C for clearing zones to qualitatively assess the lytic activity of the lysostaphin formulations.
In vitro resistance testing.
In addition to determining MRSA isolate susceptibility to lysostaphin, we also tested a subset of 12 isolates (6 pretreatment isolates with their corresponding 6 posttreatment isolates from the same animal and sample location) for the development of resistance to lysostaphin after animal treatment. We followed a previously reported methodology in which 2 MRSA colonies obtained before (baseline) and after treatment from each of the treated animals and a control animal were randomly selected from Remel Spectra MRSA plates (selective and differential chromogenic medium for the detection of MRSA colonies; Thermo Scientific, Lenexa, KS).25,51 Two control S. aureus strains were used for this in vitro resistance testing: MSSA 8325, representing a standard methicillin-sensitive S. aureus strain from the National Collection of Type Cultures (NCTC, Public Health England) and MRSA MW2, which was a community acquired methicillin-resistant S. aureus from the American Type Culture Collection (ATCC). Two strains were used in case there were lysostaphin susceptibility differences between the MSSA and MRSA controls. Both the isolate and control strains were incubated overnight in 5 mL of tryptic soy broth (Difco, BBL, Becton Dickinson, Franklin Lakes, NJ) at 37 °C and shaken at 200 revolutions per minute. The next day the cultures were centrifuged to pellet the bacteria (4,000 revolutions per minute, 4 to 6 °C, 10 min) and washed once with 5 mL DPBS. After centrifugation, the cells were resuspended in a volume of DPBS to obtain an OD600 of 1.17 ± 0.12, as determined a Spectramax Plus 384. The turbidity reduction assay was performed in triplicate for every isolate by adding 195 µL of the bacterial suspensions into 3 wells of a 96-well flat-bottomed microtiter plate, each well then received either 5 µL of a 5 µg/mL or 10 µg/mL solution of lysostaphin in DPBS or DPBS alone (negative control). The OD600 was determined at 30-s intervals for 30 min. The time to reach half starting absorbance (TOD50) of the bacterial suspension was then determined for each isolate. The MRSA control strains were used as a reference to provide comparability between assays. The mean ratio (sample TOD50/reference TOD50 strains) and standard deviation were calculated for each isolate. These same 12 isolates were submitted to Cornell University's Animal Health Diagnostic Center for multilocus sequence typing (MLST) to determine if these isolates were clones of the same MRSA strain. Briefly, genomic libraries were prepared using Nextera DNA Flex protocol and sequenced on a MiSeq using 250 bp paired-end reads. De novo assemblies were generated using SKESA v.2.3.0.44 Multilocus sequence typing profiles were determined using MLST v 2.16.1 and spa types were identified using spaTyper 1.0.3,20
Animal selection for pilot and main studies.
An in vivo pilot study was conducted to determine the preliminary efficacy of lysostaphin used alone. Two MRSA infected animals (one treatment animal and one control animal) were topically treated only at the cranial implant margins with lysostaphin or DPBS control buffer (further description below). The animals selected were single-housed to decrease the risk of cross-contamination between animals. For the subsequent main study (described below), the effectiveness of lysostaphin applied topically to 3 areas (that is, cranial implant margin, nostrils, and peri-rectal area) was coupled with systemic clindamycin. The main study was performed 9 mo later, with 2 singly housed NHPs (previously used for the pilot study) selected for treatment and the remaining 2 pair-housed NHPs selected as controls). The same animals were used in both studies due to limited number of MRSA colonized animals, and the fact that MRSA colonization in these animals had returned to CFU counts similar to their original MRSA colonization status (that is, before the pilot study) 9 mo prior. Treatment and control animals were kept on opposite sides of separate rooms to prevent animals or their biofluids from reaching other conspecifics, to decrease the chance of cross-contamination.
Pilot study.
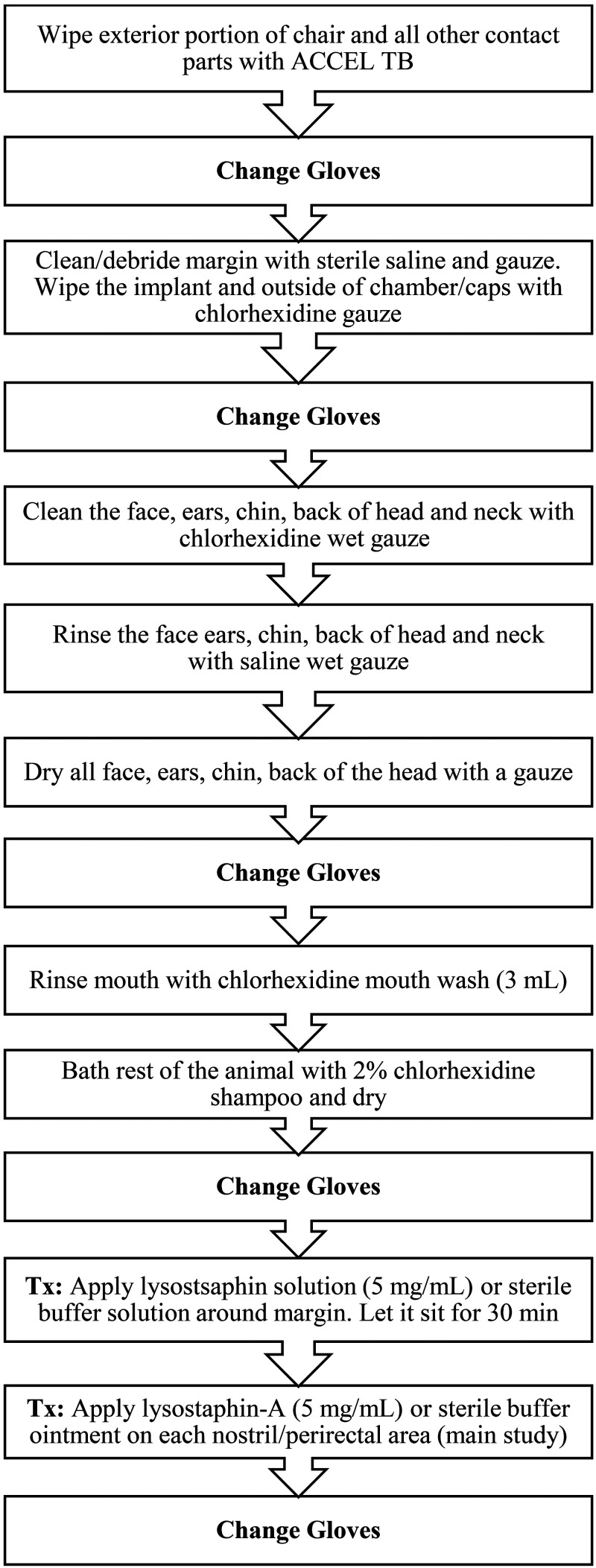
For the pilot study, both animals and their environment (cages, equipment, and room) underwent a 3-d (Day -2 to Day 0) decontamination protocol to reduce the risk of cross-contamination of MRSA from other nontreated areas on their bodies (Figures 1 and 2). In short, animals were placed in a chair (equipment was decontaminated with ACCEL TB [Virox Technologies, Oakville, ON, Canada] prior to animal placement) and moved to a separate bathing room. The head of the animals was fixed to the chair using their previously implanted head post to allow for safe head cleaning and treatment. The implant margin was cleaned/debrided with sterile sodium chloride 0.9% solution, gauze and swabs. The animal's head and face were also wiped down with gauze saturated in a chlorhexidine solution 2% (First Priority, Elgin, IL) with a contact time of approximately 3 to 5 min, followed by saline saturated wipes as a rinse. The mouth was treated with 3 mL chlorhexidine gluconate 0.12% oral rinse (Best Pet Rx, NY, NY). The rest of the animal's bodies were then completely bathed with a chlorhexidine scrub 2%. The head, face, and implant were dried with sterile gauze and the rest of the body with a hairdryer. No equipment was shared between treatment and control animals and personnel changed personal protective equipment between animals.
Figure 1.
Decontamination and treatment protocol for the topical treatment of MRSA in a group of NHPs.
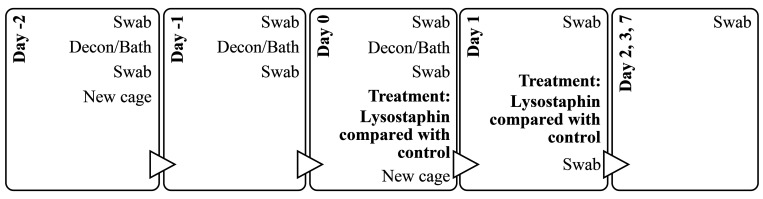
Figure 2.
Sampling and treatment timeline with the sequence of procedures for the pilot study to test the effectiveness of lysostaphin alone against topical MRSA infection in NHPs.
During animal treatment, the caging, equipment, and homerooms also underwent a decontamination procedure. Each day of the decontamination period, the walls of rooms and any exposed equipment were cleaned with ACCEL TB (Virox Technologies) while the animals were in the bathing room. This animal and room decontamination procedure was repeated once daily for 3 consecutive days. In addition, on Day -2 and Day 0 (then biweekly thereafter; Figure 2), the animals were placed in new decontaminated cages (mechanical cage washed with exposure to 82 °C and detergent) after bathing. After the initial decontamination day, the control animal was placed in a separate room from the treated animal to decrease the risk of cross-contamination.
Lysostaphin treatment consisted of 2 consecutive daily doses of liquid lysostaphin (5 mg/mL; total 3 mL/dose) applied topically around the cranial implant margin of the treatment animal using a 3 mL syringe and a 16G x 21/2’’ catheter tip. This concentration of lysostaphin (5 mg/mL) was considered to have high lytic effectiveness, based on the results of in vitro turbidity reduction assay. The control animal received 3 mL of sterile DPBS, delivered in the same way. After administration of the treatment, the animals were left in the chair for 30 min to reduce animal movement and allow the topical treatment to stay in place. To determine bacterial load, both animals were sampled using sterile Culturette Specimen Transport Amies swabs (PulmoLab, Porter Ranch, CA) during the pre, peri-, and post-decontamination and treatment period (Figure 2). During bacterial sampling, the circumference of the cranial implant margin was divided into 4 quadrants using the head rostro-caudal midline and ears as landmarks. One new culture swab was used for each quadrant to determine the exact location of MRSA colonization and to decrease the risk of cross-contamination between different areas of the margin during sampling. Bacterial load was assessed by determining the colony-forming units (CFUs) of MRSA for each of the sampling points (described below).
Main study.
Based on results from the pilot study, modifications were made to the main study that consisted of an increase in the number of decontamination procedures, more lysostaphin treatments, and the inclusion of systemic antibiotic treatment, with clindamycin given to both the control and lysostaphin treatment animals. All 4 animals received a 10-d course of systemic clindamycin (10 mg/kg, twice per day, intramuscularly) treatment (Day -2 to Day 7) (Alvogen Pine Brook, NJ) to decrease the possibility of systemic and fecal shedding of the bacteria, as well as potentially aid in the decrease of colonization at sites where lysostaphin would not make contact. As is the standard of care for any NHP receiving systemic antibiotics, all 4 animals received probiotics (PrimiOtic, BioServ, Flemington, NJ) 7 d before, during, and 14 d after systemic antibiotic administration to protect the animals from dysbiosis and diarrhea caused by the systemic antibiotic.26 In addition, all animals received bismuth subsalicylate 40 mg/kg (Pepto-Bismol, Procter and Gamble, Cincinnati, OH) once a day to treat clinical signs of mild diarrhea observed during the administration of the clindamycin. All animals (both control and treatment animals) received this same regime (that is, clindamycin, probiotics, bismuth subsalicylate) with the expectation that any observed differences in MRSA colonization between the control and treatment animals would be associated with the lysostaphin treatment.
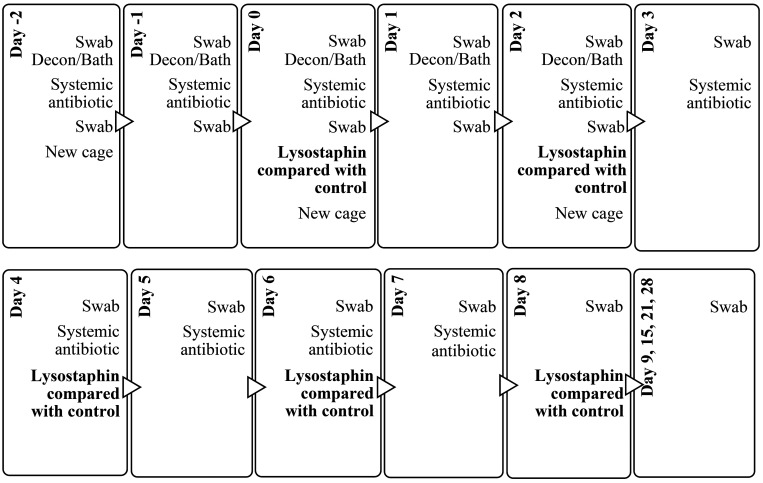
All 4 animals underwent a 5-d (Day -2 to Day 2) decontamination protocol using the same methodology as described for the pilot study (Figures 1 and 3). During animal treatment, the caging, equipment, and homerooms also underwent a similar decontamination procedure as the pilot study, but this time it was repeated once daily for 5 consecutive d. On Day -2, 0, and 2 (biweekly thereafter) the animals were also placed in new decontaminated cages after bathing. Control and treatment animals were maintained in separate rooms, and the treatment animals, which were single-housed and always kept separated to avoid physical contact between them. In addition, we also increased the number of areas on the animals that were sampled and treated (that is, nostrils and peri-rectal area in addition to the cranial regions). A total of 5 lysostaphin treatments were administered every other day starting on Day 0 (last treatment on Day 8; Figure 3). For the treatment animals, 3 mL of lysostaphin solution (5 mg/mL) was applied on the cranial implant margin using a 16G x 21/2’’ catheter tip. For the control animals, DPBS without lysostaphin was applied to the cranial implant margin. Approximately 1 mL per site of lysostaphin-A ointment (5mg/mL) or sterile DPBS-A ointment was applied to both nostrils and the perirectal area using sterile tip cotton applicators.
Figure 3.
Sampling and treatment timeline with the sequence of procedures for the main study, to test the effectiveness of lysostaphin coupled with systemic antibiotics against topical MRSA infection in NHPs.
Bacterial sampling, isolation and enumeration of MRSA.
For initial confirmation of MRSA infection or colonization, culture samples from cranial implant margins, nostrils (main study), and perirectal areas (main study) from all study animals were obtained before the decontamination and treatment procedures. All samples were taken using Culturette Specimen Transport Amies swabs (PulmoLab) and were immediately transported to the laboratory for processing. Each swab was used to inoculate 6 mL of tryptic soy broth (Difco, BBL, Becton Dickinson), by vortexing for 10 s with the swab in the culture tube, followed by incubation of the tube at 37 °C with continuous shaking overnight. The next day 800 µL of the culture was mixed with equal parts 60% glycerol and stored at -80 °C for storage. Isolation of S. aureus was accomplished by streaking the overnight cultures to mannitol salt agar plates (Remel, Thermo Fisher Scientific, Lenexa, KS) using an inoculation loop, which were then incubated at 37 °C for 48 h. This was followed by coagulase agglutination testing of the mannitol salt agar positive colonies (Staphaurex, Thermo Fisher Scientific, Lenexa, KS) to confirm S. aureus.32 Further detection of MRSA isolates was accomplished by inoculating both the tryptic soy broth (Franklin Lakes, NJ) culture and mannitol salt agar positive colonies to Remel Mannitol Salt Agar with oxacillin (4μg/mL) and Remel Spectra MRSA culture plates (Thermo Scientific). Following the manufactures instructions, all plates were incubated for 48 h at 37 °C and then were assessed for colony morphology, changes in media color, and changes in colony color to indicate the presence of MRSA.
For the pilot study, MRSA colonization/infection was determined by obtaining culture swabs from each quadrant of the cranial implant margins and for the main study MRSA colonization/infection was determined by obtaining culture swabs from the cranial implant margin quadrants, the nostrils, and peri-rectal area. Samples were obtained immediately before and after each decontamination and treatment timepoint, and subsequently days after the treatment regime ended (Figures 2 and 3). To enumerate the MRSA CFU of each sample, the tips of the culture swabs were cut into a 1.5 mL microfuge tube filled with 1 mL of sterile DPBS and vortexed for 30 seconds. Each sample was serially diluted 10-fold (100 - 10−5) in a 96-well plate. An aliquot of each dilution (10 µL) was inoculated onto the Remel Spectra MRSA culture plates (Thermo Scientific) and incubated at 37 °C for 48h. Colonies for each sample and dilution were counted 48 h post incubation to determine the number of CFUs.
Statistical analysis.
Statistical analyses used Microsoft Excel and GraphPad Prism software packages (La Jolla, CA). Calculations included F-test 2-sample for variances to determine equal or unequal variances and t-Test for differences in 2 sample populations. Differences were considered significant when the P value was less than 0.05. Results are shown as mean ± SD.
Results
Isolation and confirmation of S. aureus and MRSA.
For the pilot study, samples obtained from the cranial implant margins of both animals after the 3-d decontamination period, before lysostaphin treatment, showed the presence of MRSA colonization. The selected treatment and control animals had an average MRSA count (avg. of 4 cranial quadrants/NHP) of 9.53 × 104 CFUs/mL and 3.14 × 103 CFUs/mL, respectively. For the main study, culture swabs from 3 areas (cranial implant margin, nares, and peri-rectal area) were collected from the study cohort (n = 4) at Day -2 (before decontamination and treatment) confirmed that all 4 animals were positive for MRSA in all tested locations, despite prior standard decontamination protocols and antibiotic treatments (Table 1).33
Table 1.
Number of initial MRSA CFU/mL counts by sampling location on rhesus macaques (Macaca mulatta) in the main study before experiment. 1.0 × 102was the minimum detectable CFU count.
| Macaque no. | Avg. Cranial implant margina | Nasal | Perirectal |
| 1 (Treatment) | 2.17 × 106 | 1.0 × 102 | 1.0 × 102 |
| 2 (Treatment) | 5.98 × 105 | 2.0 × 105 | 1.0 × 102 |
| 3 (Control) | 2.10 × 106 | 2.0 × 102 | 4.0 × 102 |
| 4 (Control) | 8.74 X106 | 9.5 × 103 | 1.0 × 102 |
Cranial implant margin CFU count shown is the average of 4 margin quadrants sampled separately.
Turbidity reduction assay.
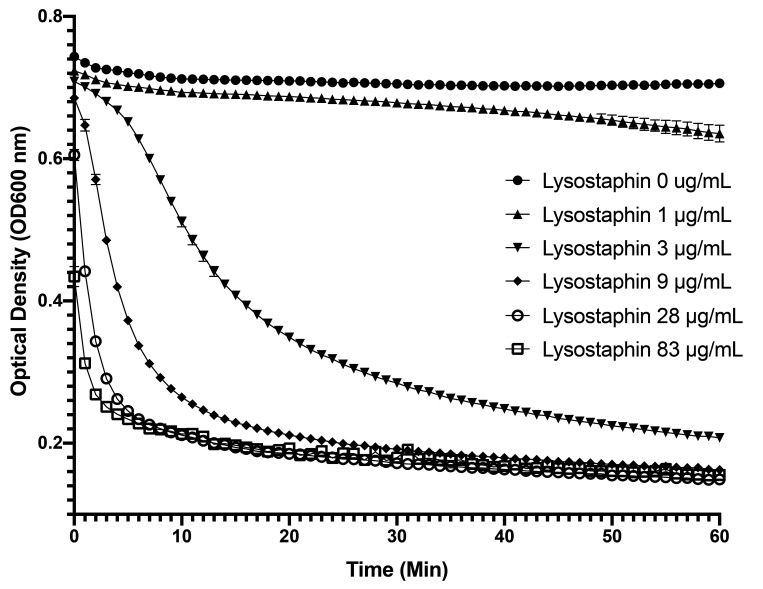
To confirm that our liquid lysostaphin formulation had lytic activity against MRSA isolates derived from the NHPs, the activity of lysostaphin was first tested at multiple concentrations against one isolate from the pilot study animal (Macaque no.1 MRSA, cranial margin). Starting at an OD600 around 0.8, all samples showed a reduction in their optical density over time (60 min) using lysostaphin concentrations between 3 and 83 µg/mL (Figure 4), with maximum activity seen at concentrations above 28 µg/mL.
Figure 4.
Turbidity reduction assay of lysostaphin against a NHP MRSA isolate. Log-phase cultures were exposed to various concentrations of lysostaphin (0 – 83 µg/mL) for 60 min in phosphate buffer and the reduction in OD600nm measured over time.
We then repeated these experiments against 7 more MRSA isolates identified via Spectra MRSA plates from the cranial margins of the 4 different experimental animals (1 from pilot study and 3 from main study), and additional isolates representing the 4 different MRSA clones (MA-II, MA-III, MA-IV, and MA-V) recovered from the NHPs and their environment in the prior year.33 All samples were sensitive to lysostaphin at 10 µg/mL and showed a similar reduction in their optical density over time (60 min) (data not shown).
In addition, for the main study, a similar turbidity reduction assay was conducted on 12 MRSA isolates obtained pre- and post-lysostaphin treatment, from the same animals, to determine MRSA susceptibility to lysostaphin and to assess the potential development of resistance (Table 2). Overall, the results showed that the MRSA isolates from the treatment and control animals were similar in sensitivity, both to each other and to the reference strains (MSSA-8325 and MRSA-MW2) at a lysostaphin concentration of 10 µg/mL; a 500× lower concentration than used for the in vivo treatment (5 mg/mL). Pretreatment groups had mean TOD50 ratios of sample to reference MSSA 8325 and MRSA MW2 of 1.33 to 1.40 ± 0.09 and 1.06 to 1.12 ± 0.07, respectively. Isolates obtained 2 wk after the treatment regime also showed susceptibility to lysostaphin, with no significant difference in the mean TOD50 ratio of sample to reference. Both lysostaphin and buffer control treated isolates had the same TOD50 ratios; 1.63 ± 0.03 and 1.31 ± 0.03 for MSSA 8325 and MRSA MW2 respectively (Table 2), suggesting that the isolates did not acquire, nor were selected for, resistance by the lysostaphin treatments (P = 0.12).25 The MLST results for these 12 MRSA isolates showed they all belonged to the same sequence type ST3862 and spa type t4167, thus were similar to the MRSA strains (MA-II or MA-III) identified in the NHP colony previously.33
Table 2.
Lysostaphin susceptibility testing using the turbidity reduction assay of various MRSA isolates obtained from the cranial implant margins of 2 treatment and one control animals before and after lysostaphin (Tx) or buffer (control) treatment.
| S. aureus isolate designation | TOD50 ratio of sample to reference (MSSA 8325) | TOD50 ratio of sample to reference (MRSA MW2) |
| Pre-Lysostaphin Treatment | ||
| Tx sample no.1 (Animal 1) | 1.25 ± 0.22 | 1.00 ± 0.18 |
| Tx sample no.2 (Animal 1) | 1.54 ± 0.04 | 1.23 ± 0.03 |
| Tx sample no.8 (Animal 2) | 1.69 ± 0.09 | 1.36 ± 0.07 |
| Tx sample no.9 (Animal 2) | 1.13 ± 0.00 | 0.90 ± 0.00 |
| Pre-Tx Mean | 1.40 ± 0.09 | 1.12 ± 0.07 |
| Control sample no.18 (Animal 3) | 1.26 ± 0.02 | 1.01 ± 0.02 |
| Control sample no.19 (Animal 3) | 1.39 ± 0.02 | 1.11 ± 0.02 |
| Pre-Control Mean | 1.3 ± 0.02 | 1.06 ± 0.02 |
| Post-Lysostaphin Treatment | ||
| Tx sample no.6 (Animal 1) | 1.85 ± 0.06 | 1.48 ± 0.05 |
| Tx sample no.7 (Animal 1) | 2.19 ± 0.02 | 1.76 ± 0.02 |
| Tx sample no.13 (Animal 2) | 1.17 ± 0.00 | 0.93 ± 0.00 |
| Tx sample no.14 (Animal 2) | 1.32 ± 0.05 | 1.06 ± 0.04 |
| Post-Tx Mean | 1.63 ± 0.03 | 1.31 ± 0.03 |
| Control sample no.23 (Animal 3) | 0.76 ± 0.02 | 0.61 ± 0.02 |
| Control sample no.24 (Animal 3) | 2.50 ± 0.00 | 2.00 ± 0.00 |
| Post-Control Mean | 1.63 ± 0.01 | 1.31 ± 0.01 |
| References | ||
| MSSA 8325 | 12 ± 3.1 min to TOD50 | N/A |
| MRSA MW2 | N/A | 15 ± 2.2 min to TOD50 |
The concentration of lysostaphin used for this assay was 10 µg/mL. All isolates were tested 3 times and compared with the reference strains MSSA 8325 and MRSA MW2. N/A = not applicable.
In vitro testing of lysostaphin-A ointment.
Topical treatment for the nostrils and perirectal area required the formulation of the lysostaphin in an ointment (Aquaphor) to retain the enzyme on the treated area for longer periods. The lytic activity of lysostaphin-A was qualitatively tested by applying the preparation on S. aureus bacteria suspended in an agar matrix, similar to previously described work (data not shown).35 Lysostaphin was released from the Aquaphor and penetrated the agar, lysing S. aureus cell walls to create an area of clearance. Aquaphor alone did not show lytic activity.
Lysostaphin treatment of animals.
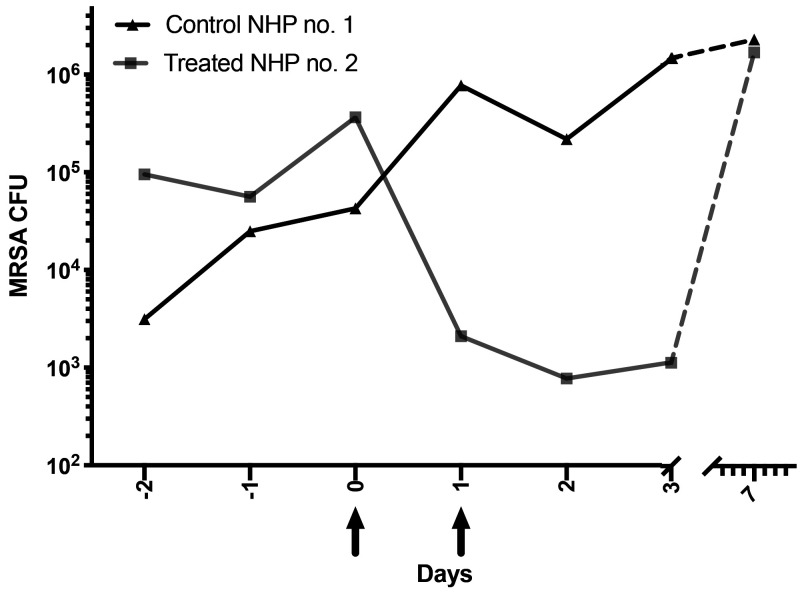
For the pilot study we wanted to assess the direct effect of lytic enzyme treatment on MRSA colonized animals. Besides animal and environmental cleaning and decontamination, respectively, no other treatments (that is, antibiotics) were implemented. One experimental animal received 2 consecutive daily doses of lysostaphin (3 mL of 5 mg/mL) but only to the cranial implant margin, while the other control animal received sterile buffer instead. Before treatment (Day 0), the control animal had one-log lower CFU of MRSA than the experimental treatment animal (Figure 5). Twenty-four hours after the first lysostaphin treatment, MRSA CFUs were reduced more than 2-logs, compared with the previous day and the control at the same time point. While MRSA increased in the control animal samples over time, the treated experimental animal's CFUs continued to decrease after the second treatment and remained low for at least 48 h after the last treatment. Thus, lysostaphin treatment produced a 3-log reduction in the CFU counts compared with control during our initial treatment study. In addition, no gross adverse effects were observed at the treatment margins or in the over-all health of the animals. The daily sampling of the margins was halted after day 3 to prevent possible cross-contamination. Unfortunately, at 7 d after the initial treatment, the CFU count of the treatment animal returned to levels comparable to the nontreated control.
Figure 5.
Pilot study: Colony-forming units per average culture swab (4 quadrants/swabs per animal) during the decontamination (days -2 – 0), treatment (days 0 and 1), and posttreatment (days 2 – 7) periods of lysostaphin (5 mg/mL) treatment compared with sterile buffer control animals. Arrows symbolize treatment time points.
Considering the results of the pilot study, for the main study we strengthened the treatment protocol to hopefully produce a greater reduction in MRSA for an extended time period. Thus, the decontamination period was extended to 5 d, and the period of lysostaphin administration was lengthened to 5 every-other-day treatments and combined with a 10-d course of systemic clindamycin. The MRSA isolates we used had previously been documented as sensitive to clindamycin in antibiograms (data not shown).33 Also, 2 additional sites on the NHPs (nostrils and peri-rectal areas), known to previously harbor MRSA that could possibly cross-contaminate the cranial implant margins, were also sampled and treated with lysostaphin in Aquaphor (lysostaphin-A) on treatment days. At the start of the main study, the nostrils and peri-rectal area initially had low average CFU counts of 3.6 × 103 ± 2.3 × 104 CFU/mL and 119 ± 68 CFU/mL, respectively, but ranged from a high of 2.0 × 105 CFU/mL to the lowest detectable CFU count of 100 CFU/mL (data not shown). The lowest detectable CFU count was observed in all animals after initiating decontamination procedures and administration of systemic antibiotics (that is, Day -2 to Day 0), before lysostaphin-A treatment in the nostril and peri-rectal area. With a few exceptions, throughout the treatment with lysostaphin-A and clindamycin, most samples from the treatment and control animals remained at or close to the lowest detectable CFU count (≤ 100 CFU/mL). Furthermore, 3 wk after the last treatment, all animals had no detectable MRSA CFU in either the nostrils or peri-rectal area (data not shown).
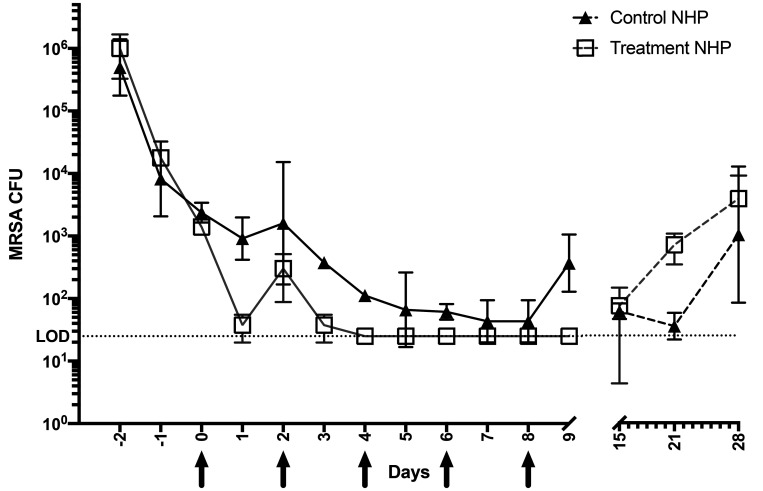
During the first 5 d of animal bathing and decontamination, no statistical reduction was found between an individual animal's MRSA CFU before and immediately after the bathing/decontamination procedures on the same day (that is, comparison of pre- and post-bathing samples). Thus, only post-bathing CFU counts were used for subsequent statistical analyses to determine the contribution of antibiotic and lysostaphin treatments (Figure 6). Notably, during the first 3 d of the decontamination and antibiotic treatment period (Day -2 to Day 0), MRSA CFU decreased by 2 to 3 logs in all animals, from an average of 8.2 × 105 ± 5.5 × 105 CFU/mL to 1.9 × 103 ± 8.1 × 102 CFU/mL (P < 0.05; Figure 6), with no statistical difference observed in the CFU counts between the animals previously designated as lysostaphin treatment and controls before lysostaphin treatment. At 24 h after the first administration of lysostaphin, CFU counts fell an additional 1 to 2 logs in the treatment animals, compared with the previous day (Day 0 compared with Day 1) and the controls. Within 48 h after the second dose of lysostaphin, the treatment animals had CFU at or below the minimum detectable CFU count (≤ 25 CFU/mL) and CFU counts remained at this level throughout the 9-d experimental treatment period. These MRSA counts were significantly lower overall in treated as compared with control animals for most of the lysostaphin treatment period (P < 0.05; Figure 6). However, one control animals (Control NHP no.1) showed highly variable CFU counts throughout the study; in some cases, no CFU were detected (for example, Day 7, 8, and 21) and thus this animal was not significantly different from the treatment animals. Nevertheless, on the day after the end of the treatment period (Day 9), the animals that received topical lysostaphin plus clindamycin had significantly lower CFU counts (below the minimum detectable CFU) than did control animals that received antibiotic alone (P < 0.05; Figure 6). At one week after the last treatment all animals had similar low CFU counts (25 to 125 CFU/mL). Unfortunately, similar to the pilot study and prior antibiotic treatments regimes (clindamycin plus mupirocin, data not shown), the MRSA CFU count on all animals increased (3.5 × 103 ± 7.5 × 103 CFU/mL) between 2 to 3 wk after the treatment study ended (Figure 6).33
Figure 6.
Main study: colony forming units per average culture swab (4 quadrants/swabs per animal) of the cranial implant margin during the decontamination period (days 1–5), treatment (Tx NHP no. 1 and no. 2 (black arrows), and posttreatment (days 12–31) periods using lysostaphin (5 mg/mL) treatment) and control animals (sterile PBS; Control NHP no. 1 and no. 2).
Discussion
A rise in MRSA infections and colonization in animals including captive NHPs has been observed across the world, highlighting the need for new therapeutic methods to combat this pathogen.17,43,48 During routine screening of NHPs in the vivarium of The Rockefeller University, NY, an outbreak of MRSA infection/colonization of experimental macaques with cranial implants was discovered. As we have described, previous attempts to decolonize all of the NHPs of MRSA were unsuccessful, despite a regimen of environmental decontamination combined with cleaning of the cranial implant margins and bathing the animals with a solution of 2% chlorhexidine (First Priority, Elgin, IL) for 5 consecutive days, followed by application of 2% topical mupirocin ointment (NYCOMED US, Melville, NY), applied twice daily into the nostrils and implant margins for 2 wk, with systemic antibiotics based on antibiogram sensitivity profiles.33 When these rigorous decontamination and treatments procedures failed, we sought to determine if topically applied lysostaphin, coupled with decontamination, cleaning, and bathing (pilot study) or in addition to decontamination and systemic antibiotics (main study) could more effectively decrease or eliminate MRSA colonization/infection in NHPs.
We first confirmed MRSA infection or colonization in these animals. The degree of colonization varied per site, with the cranial implant margin having the highest CFU count (8.74 × 106 – 2.10 × 106 CFU/mL), whereas lower counts were seen in the nasal and perirectal area (9.5 × 103 – 1.0 × 102 CFU/mL). These counts were determined using Spectra MRSA chromogenic selective media. The use of MRSA selective media was successfully implemented in a previous study to establish a simple way to detect MRSA colonization in cynomolgus macaques (Macaca fascicularis), using CHROMagar MRSA plates (CHROMagar, Paris, France) as a rapid screening tool.21 This study found 100% sensitivity when comparing MRSA positive results from chromogenic agar plates with the standard protocol of culture and antibiotic sensitivity testing, with all 3 animals that were MRSA positive by the standard protocol were found to be positive by culture on selective media.16
Next, we tested the activity of the lysostaphin against MRSA isolates obtained from the NHPs. Our in vitro results showed effective lytic activity by lysostaphin against our initial MRSA isolate obtained in the pilot study over a wide concentration range (3 to 83 µg/mL). In addition, all subsequent MRSA isolates tested before and after this treatment study were highly susceptible to lysis by lysostaphin suspended in solution at a concentration of 10 µg/mL. These results coincide with previously reported work describing lysostaphin activity in other animal species experimentally infected with MRSA.6,8,22 None of the isolates initially sampled from the NHPs were found to be resistant to lysostaphin. Furthermore, the topical treatments with lysostaphin did not select for MRSA isolates that were significantly more resistant to lysostaphin compared with the previous and untreated control samples. We were also able to successfully formulate an ointment containing lysostaphin (5 mg/mL) which used Aquaphor as the vehicle and showed effective in vitro lytic activity against S. aureus. This ointment formulation may potentially be used in the future to treat other mucocutaneous areas or wounds that require longer contact time to be effective.
In addition to previously implemented decontamination and bathing procedures, our preliminary in vivo pilot study used a liquid solution of lysostaphin for topical application, in hopes that the fluid would penetrate from the cranial implant margin into deeper areas of the implant-skull interface. After just one topical treatment with 3mL of lysostaphin solution (5 mg/mL), the MRSA CFU count from the margin was significantly reduced (2-log CFU) compared with the CFU counts from control animal and the previous day's measurements. Moreover, a second treatment 24 h later also further reduced MRSA colonization of the margin and prevented the further expansion of MRSA CFU seen in the control animal. Thus, lysostaphin treatment resulted in a greater than 3 log difference between the control and treated animal, for at least 3 d after initiation of the treatment period. Unfortunately, upon margin sampling at 7 d after the initial treatment, the CFUs of the treatment animal had returned to levels comparable to the nontreated control. Thus, MRSA colonization increased in a period between 3 to 7 d posttreatment. We hypothesized that the increase in MRSA in the margin after a week may have been caused by various factors, such as cross-contamination from untreated areas, the inability of lysostaphin to reach all of the infected/colonized areas in the implant margin, and/or insufficient number of treatments to reduce the MRSA to a CFU count in which the NHPs immune defenses could clear the infection.
We tried to reduce cross-contamination in our main study by: (1) increasing the number of animal bathing and environmental decontamination procedures to 5 d and overlapping these with the lysostaphin treatments; (2) topically treating other areas of potential cross-contamination (that is, nostrils and peri-rectal area); (3) increasing the number of lysostaphin treatments to 5 applications every other day; and (4) including systemic antibiotic treatment with clindamycin to reduce MRSA in areas that could not directly interact with lysostaphin. Analysis of MRSA CFUs from the samples over the first 5 d showed that cleaning of the margins with saline, and bathing of the other areas of the NHP with chlorohexidine, did not immediately reduce the number of MRSA CFU in the implant margins on the same day. MRSA CFU increased in some samples of the margin after bathing, perhaps due to the transfer of the bacteria during cleaning or swabbing/sampling of the margins. To reduce this form of cross-contamination, all bathing was stopped after the second day of lysostaphin treatment, and sampling was reduced after the last treatment day to just once a week. Overall, the decontamination/bathing procedures, combined with systemic antibiotic treatment alone, were effective at significantly reducing MRSA colonization in all animals in their cranial implant margins, nostrils, and perirectal areas, before the initiation of lysostaphin treatment. This was best observed in the controls, where the slow decline of CFU continued throughout the 10-d course of antibiotics. Likewise, we were not fully able to ascertain whether lysostaphin provided adjunctive therapy for the reduction of MRSA colonization in the nostrils and peri-rectal area, since both the treatment and control animals showed a decrease to the minimal detectable CFU counts in those areas over the course of our treatment regime, likely due to the use of the systemic antibiotic. With administration of lysostaphin to the treatment animals, MRSA CFU counts further decreased significantly to a level at or below the limit of detection in the cranial implant margin. Unfortunately, one of the control animals showed highly variable CFU counts throughout the treatment period and in some instances, the count was similar to the treatment animals. Nevertheless, after the second lysostaphin treatment, CFU counts at the cranial implant margins of the experimental animals remained lower than the controls throughout the treatment period. Furthermore, at the end of the treatment period (Day 9), the animals that received lysostaphin had significantly lower CFU counts than did control animals that received antibiotic alone (≤ 25 CFU/mL compared with 475 CFU/mL; P < 0.05). This suggests lysostaphin may also provide additional adjunctive therapy to systemic antibiotics alone. Upon follow-up sampling a week after all treatments ceased, MRSA CFUs were again present in the margins in all animals at similarly low CFUs. We hypothesized that this was most likely due to the continued effects of the systemic antibiotics that all animals received. Unfortunately, once again these effects were not permanent, as the decrease in MRSA colonization in the treatment animals was followed by variable increases in CFU counts that were observed in all animals during the 3 wk after the last treatment. This suggests that while the addition of lysostaphin appeared to provide efficacious adjunctive therapy short-term, the MRSA infection came back after therapy stopped, with all animals culturing positive for MRSA and the animal with a history of purulent discharge showing those clinical signs again. These results were reminiscent of all the previously unsuccessful treatments schedules (chlorohexidine baths, systemic clindamycin, mupirocin topical treatment, etc.) we had attempted on the NHPs before this trial33 and of accounts by others who attempted to use antibiotics to achieve long-term MRSA decolonization in NHPs with MRSA at other body sites.17,45,46
Although various factors (for example, cross-contamination from the environment or other untreated areas on the NHPs) could explain the unsuccessful long-term eradication of the MRSA in the cranial margins of the NHPs in the main experiment, we do not believe that the bacteria we selected were lysostaphin resistant. As discussed above, we tested the possibility of MRSA acquiring lysostaphin resistance by turbidity reduction and found no decrease in lytic activity on isolates obtained after treatment, indicating no apparent resistance had developed.25 We speculate that the regrowth of MRSA was most likely caused by the inability of lysostaphin to reach all of the colonized/infected tissues surrounding or deeper inside the cranial implant, and/or an insufficient period of treatment to allow the drugs and the innate immune defenses of the NHP to clear the infection around the margin. This speculation is further supported by the molecular typing of the 4 pre- and 4 post- lysostaphin treatment MRSA isolates from the treated NHPs. All 8 of the MRSA isolates had the same MLST and spa type, (ST3862 / t1467), suggesting they might be clones of the same MRSA strain and most likely, the original MRSA colonization was not completely cleared. It is important to note that similar isolates with the same clonal ST3862/ t1467 pattern (MA-II and MA-III) were also identified and characterized in this NHP colony a year before our study.33 Even though we did not presently sample the environment for MRSA colonization, this previous study found these MRSA isolates on cage surfaces, work chairs, and transport carts. Thus, the possibility of the animals being recolonized by MRSA from environmental contamination cannot be completely ruled out. These MRSA ST3862/ t1467 clones have not been described in humans, but are associated with primates in different locations, raising the possibility that these animals were already colonized with MRSA when they arrived in the facility.33 Overall, we suggest the most problematic factor for successful decolonization or wound resolution in our situation may be the cranial implant itself, which like most implanted devices, provides an ideal environment for bacteria such as MRSA to thrive.17 As such, in the past, some NHPs in our facility that did not have a cranial implant or in which the decontamination procedures were able to penetrate all implant surfaces colonized by MRSA have been successfully cleared of MRSA with the normal decontamination, bathing, and/or clindamycin treatment.33 Furthermore, we found that MRSA or other infections were more easily treated in some of these historical cases in which the implant was removed.
A unique aspect of this study was that the experimental NHPs were spontaneously exposed to MRSA; the margin wounds were colonized, and infections developed in a natural way, reminiscent of case studies in humans or other animal infections. This may be perceived as a weakness of this study in comparison to controlled experimental infections in laboratory animals. Instead of a uniformly infected population, with roughly the same size of lesion and bacterial counts, the NHPs in our study probably differed in the degree and duration of the infection, the degree of immune response and wound healing, and/or possible touching and cross-contaminating their wounds. Furthermore, because we were dealing with a naturally occurring infection in a subset of animals, we had a limited sample size and therefore could not perform some of the controls that would have been informative (for example, the lysostaphin treatment with and without antibiotic treatments) in statistically large enough numbers to accurately represent the general trend of a larger population. Thus, additional studies are needed that address these variables and to better determine if different lysostaphin treatment regimens (or other lytic enzymes) can provide effective longer lasting effects for the treatment of topical MRSA (or other target bacteria) colonization in instrumented NHPs.
In conclusion, the results of these studies show that lysostaphin, either alone or coupled with other adjunct treatments, did not eradicate topical MRSA from cranial implant margins in NHPs and thus was not effective long-term (at 7 d or longer after last treatment). However, during treatment, lysostaphin alone significantly reduced MRSA colonization, compared with pretreatment levels, and when coupled with systemic antibiotics, further reduced MRSA CFU counts below detectable limits during treatment. Thus, the potential of the lytic enzyme lysostaphin to completely eradicate MRSA in complex implant wound infections is still incompletely tested. Although not explored in this study, we speculate that the main reason for treatment failure in the cranial implant margin in these NHPs was the inability of the lysostaphin treatment to access every possible space colonized by MRSA in the skull-implant interface. Lysostaphin has been proven to effectively kill MRSA both in vitro and in vivo, but to exert its therapeutic function it needs physical contact with all target bacteria. Previous work has demonstrated topical lysostaphin effectiveness in superficial open areas such as the nasal mucosal surface.24 Thus, the clinical applicability of topical lysostaphin (and probably lytic enzymes in general) may be limited to superficial, unobstructed areas and simple wounds such as lacerations, cuts, and scrapes, where the lytic enzyme can have full access and interaction with the affected tissue.
Acknowledgments
We thank Dr. Vincent Fischetti for reagents, useful discussions about the experiments, and comments on the manuscript. We also thank the staff at the Comparative Bioscience Center of The Rockefeller University for their logistical assistance with environmental and animal decontamination procedures. The project described was sponsored by the Shapiro–Silverberg Fund for the Advancement of Translational Research. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Animal Welfare Act as Amended. 2013. 7 USC §2131–2156.
- 2.Animal Welfare Regulations. 2008. 9 CFR §3.129.
- 3.Bartels MD, Petersen A, Worning P, Nielsen JB, Larner-Svensson H, Johansen HK, Andersen LP, Jarløv JO, Boye K, Larsen AR, Westh H. 2014. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 52:4305–4308. 10.1128/JCM.01979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramley AJ, Foster R. 1990. Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res Vet Sci 49:120–121. 10.1016/S0034-5288(18)31061-0. [DOI] [PubMed] [Google Scholar]
- 5.Ceotto-Vigoder H, Marques SLS, Santos INS, Alves MDB, Barrias ES, Potter A, Alviano DS, Bastos MCF. 2016. Nisin and lysostaphin activity against preformed biofilm of Staphylococcus aureus involved in bovine mastitis. J Appl Microbiol 121:101–114. 10.1111/jam.13136. [DOI] [PubMed] [Google Scholar]
- 6.Climo MW, Ehlert K, Archer GL. 2001. Mechanism and suppression of lysostaphin-resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 45:1431–1437. 10.1128/AAC.45.5.1431-1437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Climo MW, Patron RL, Goldstein BP, Archer GL. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob Agents Chemother 42:1355–1360. 10.1128/AAC.42.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dajcs JJ, Hume EBH, Moreau JM, Caballero AR, Cannon BM, O'Callaghan RJ. 2000. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Invest Ophthalmol Vis Sci 41:1432–1437. [PubMed] [Google Scholar]
- 9.Daley MJ, Oldham ER. 2000. Lysostaphin: immunogenicity of locally administered recombinant protein used in mastitis therapy. Vet Immunol Immunopathol 31:301–312. 10.1016/0165-2427(92)90017-K. [DOI] [PubMed] [Google Scholar]
- 10.de Freire Bastos M, Coutinho BG, Varella Coelho ML. 2010. Lysostaphin: A staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals (Basel) 3:1139–1161. 10.3390/ph3041139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated methicillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischetti VA. 2003. Novel method to control pathogenic bacteria on human mucous membranes. Ann N Y Acad Sci 987:207–214. 10.1111/j.1749-6632.2003.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmer DB, Schmitz JE, Thandar M, Euler CW, Fischetti VA. 2017. The phage lysin PlySs2 decolonizes Streptococcus suis from murine intranasal mucosa. PLoS One 12:1–13. 10.1371/journal.pone.0169180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg LM, DeFranco JM, Watanakunakorn C, Hamburger M. 1967. Studies in experimental staphylococcal endocarditis in dogs. VI. Treatment with lysostaphin. Antimicrob Agents Chemothe (Bethesda) 7:45–53. [PubMed] [Google Scholar]
- 16.Gould IM, Reilly J, Bunyan D, Walker A. 2010. Costs of healthcare-associated methicillin-resistant Staphylococcus aureus and its control. Clin Microbiol Infect 16:1721–1728. 10.1111/j.1469-0691.2010.03365.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein AW, Boyle-Vavra S, Maddox CW, Tang X, Halliday LC, Fortman JD. 2019. Carriage of methicillin-resistant Staphylococcus aureus in a colony of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp Med 69:311–320. 10.30802/AALAS-CM-18000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Johnson CT, Wroe JA, Agarwal R, Martin KE, Guldberg RE, Donlan RM, Westblade LF, Garcíab AJ. 2018. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc Natl Acad Sci USA 115:E4960–E4969. 10.1073/pnas.1801013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolley KA, Maiden MC. 2010. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:1–11. 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TM, Park H, Lee KW, Choi EW, Moon SH, Lee YS, Cho K, Park WJ, Park JB, Kim SJ. 2017. A simple way to eradicate methicillin-resistant Staphylococcus aureus in cynomolgus macaques (Macaca fascicularis). Comp Med 67:356–359. [PMC free article] [PubMed] [Google Scholar]
- 22.Kiri N, Archer G, Climo M. 2002. Combinations of lysostaphin with beta-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob Agents Chemother 46:2017–2020. 10.1128/AAC.46.6.2017-2020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokai-Kun JF, Chanturiya T, Mond JJ. 2007. Lysostaphin as a treatment for systemic Staphylococcus aureus infections in a mouse model. J Antimicrob Chemother 60:1051–1059. 10.1093/jac/dkm347. [DOI] [PubMed] [Google Scholar]
- 24.Kokai-Kun JF, Walsh SM, Chanturiya T, Mond JJ. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob Agents Chemother 47:1589–1597. 10.1128/AAC.47.5.1589-1597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusuma CM, Kokai-Kun JF. 2005. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob Agents Chemother 49:3256–3263. 10.1128/AAC.49.8.3256-3263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecker JL, Froberg-Fejko K. 2015. PrimiOtic and PrimiOtic Plus: novel probiotic for primates suffering from idiopathic chronic diarrhea. Lab Anim (NY) 44:414–415. 10.1038/laban.844. [DOI] [PubMed] [Google Scholar]
- 27.Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. 2018. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers 4:1–23. 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 28.Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, Zimmer SM, Potter MA, Macal CM, Lauderdale DS, Miller LG, Daum RS. 2012. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 19:528–536. 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao J, Pangule RC, Paskaleva EE, Hwang E, Kane RS, Linhardt RJ, Dordick JS. 2011. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 32:9557–9567. 10.1016/j.biomaterials.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 30.Office of Laboratory Animal Welfare. [Internet]. 2015. PHS policy on humane care and use of laboratory animals. [Cited 22 October 2019]. Available at: https://olaw.nih.gov/policies-laws/phs-policy.htm.
- 31.Oluola O, Kong L, Fein M, Weisman LE. 2007. Lysostaphin in treatment of neonatal Staphylococcus aureus infection. Antimicrob Agents Chemother 51:2198–2200. 10.1128/AAC.00506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardos de la Gandara M, Curry M, Berger J, Burstein D, Della-Latta P, Kopetz V, Quale J, Spitzer E, Tan R, Urban C, Wang G, Whittier S, de Lencastre H, Tomasz A. 2016. MRSA causing infections in hospitals in greater metropolitan New York: Major shift in the dominant clonal type between 1996 and 2014. 2016. PLoS One 11:1–13. 10.1371/journal.pone.0156924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardos de la Gandara M, Diaz L, Euler CW, Chung M, Gonzalez A, Cheleuitte C, Freiwald W, Tomasz A, Fischetti VA, de Lencastre H. 2019. Staphylococcus aureus infecting and colonizing experimental animals, macaques, in a research animal facility. Microb Drug Resist 25:54–62. 10.1089/mdr.2018.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardos de la Gandara M, Raygoza Garay JA, Mwangi M, Tobin JN, Tsang A, Khalida C, D'Orazio B, Kost RG, Leinberger-Jabari A, Coffran C, Evering TH, Coller BS, Balachandra S, Urban T, Parola C, Salvato S, Jenks N, Wu D, Burgess R, Chung M, de Lencastre H, Tomasz A. 2015. Molecular types of methicillin-resistant Staphylococcus aureus and methicillin-sensitive S. aureus strains causing skin and soft tissue infections and nasal colonization, identified in community health centers in New York City. J Clin Microbiol 53:2648–2658. 10.1128/JCM.00591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. 2010. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and –sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. https://doi.org/10.1128/AAC. 00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastagia M, Schuch R, Fischetti VA, Huang DB. 2013. Lysins: the arrival of pathogen-directed anti-infectives. J Med Microbiol 62:1506–1516. 10.1099/jmm.0.061028-0. [DOI] [PubMed] [Google Scholar]
- 37.Patron RL, Climo MW, Goldstein BP, Arcer GL. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 43:1754–1755. 10.1128/AAC.43.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poovelikunnel T, Gethin G, Humphreys H. 2015. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother 70:2681–2692. 10.1093/jac/dkv169. [DOI] [PubMed] [Google Scholar]
- 39.Schaffner W, Melly MA, Koenig MG. 1967. Lysostaphin: an enzymatic approach to staphylococcal disease. II. In vivo studies. Yale J Biol Med 39:230–244. [PMC free article] [PubMed] [Google Scholar]
- 40.Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuch R, Pelzek AJ, Raz A, Euler CW, Ryan PA, Winer BY, Farnsworth A, Bhaskaran SS, Stebbins CE, Xu Y, Clifford A, Bearss DJ, Vankayalapati H, Goldberg AR, Fischetti VA. 2013. Use of bacteriophage lysin to identify a novel target for antimicrobial development. PLoS One 8:1–9. 10.1371/journal.pone.0060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuhardt VT, Schindler CA. 1964. Lysostaphin therapy in mice infected with Staphylococcus aureus. J Bacteriol 88:815–816. 10.1128/JB.88.3.815-816.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soge OO, No D, Michael KE, Dankoff J, Lane J, Vogel K, Smedley J, Roberts MC. 2016. Transmission of MDR MRSA between primates, their environment and personnel at a United States primate centre. J Antimicrob Chemother 71:2798–2803. 10.1093/jac/dkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souvorov A, Agarwala R, Lipman DJ. 2018. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol 19:1–13. 10.1186/s13059-018-1540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slingerland BCGC, Keehnen M, Ouwerling B, Tavakol M, Snijders SV, Verbrugh HA, Vos MC, Remarque EJ, Langermans JAM, van Wamel WJB. 2018. An experimental Staphylococcus aureus carriage and decolonization model in rhesus macaques (Macaca mulatta). PLoS One 13:1–11. 10.1371/journal.pone.0194718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor AR. 2013. Methicillin-resistant Staphylococcus aureus infection. Prim Care 40:637–654. 10.1016/j.pop.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 47.van den Berg S, van Wamel WJB, Snijders SV, Ouwerling B, de Vogel CP, Boelens HA, Willems RJ, Hujisdens XW, Verreck FA, Kondova I, Heidt PJ, Verbrugh HA, van Belkum A. 2011. Rhesus macaques (Macaca mulatta) are natural hosts of specific Staphylococcus aureus lineages. PLoS One 6:1–14. 10.1371/journal.pone.0026170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weese JS. 2010. Methicillin-resistant Staphylococcus aureus in animals. ILAR J 51:233–244. 10.1093/ilar.51.3.233. [DOI] [PubMed] [Google Scholar]
- 49.Windolf CD, Lögters T, Scholz M, Windolf J, Flohé S. 2014. Lysostaphin-coated titan-implants preventing localized osteitis by Staphylococcus aureus in a mouse model. PLoS One 9:1–16. 10.1371/journal.pone.0115940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang SC, Lin CH, Sung CT, Fang JY. 2014. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:1–10. 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young C, Lapsley L, Newton D. [Internet]. 2008. Comparison of four media for the detection of methicillin-resistant Staphylococcus aureus (MRSA) from nasal swabs. [Cited 22 October 2019]. Available at: https://www.thermofisher.com/order/catalog/product/R01821. [DOI] [PMC free article] [PubMed]
- 52.Zimmerli W, Widmer A, Blatter M, Frei R, Ochsner PE. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-body infection (FBI) study group. JAMA 279:1537–1541. 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]