Abstract
Calcium (Ca2+) signals initiate egg activation across the animal kingdom and in at least some plants. These signals are crucial for the success of development and, in the case of mammals, health of the offspring. The mechanisms associated with fertilization that trigger these signals and the molecules that regulate their characteristic patterns vary widely. With few exceptions, a major contributor to fertilization-induced elevation in cytoplasmic Ca2+ is release from endoplasmic reticulum stores through the IP3 receptor. In some cases, Ca2+ influx from the extracellular space and/or release from alternative intracellular stores contribute to the rise in cytoplasmic Ca2+. Following the Ca2+ rise, the reuptake of Ca2+ into intracellular stores or efflux of Ca2+ out of the egg drive the return of cytoplasmic Ca2+ back to baseline levels. The molecular mediators of these Ca2+ fluxes in different organisms include Ca2+ release channels, uptake channels, exchangers and pumps. The functions of these mediators are regulated by their particular activating mechanisms but also by alterations in their expression and spatial organization. We discuss here the molecular basis for modulation of Ca2+ signalling at fertilization, highlighting differences across several animal phyla, and we mention key areas where questions remain.
Keywords: fertilization, calcium signalling, calcium channels, egg activation, oocyte
1. Introduction
How the terminally differentiated gametes interact with each other at fertilization to initiate the development of a new organism is a question that has fascinated scientists for over a century. The earliest studies of fertilization, largely performed in marine animals, were summarized in 1919 by Frank Lillie in Problems of Fertilization. In this treatise, he states regarding fertilization:
It [fertilization] is the central decisive event in the genesis of all sexually produced animals and plants. Thus from one point of view it envisages the entire problem of sex; from another point of view it constitutes the basis of all development and inheritance. The elements that unite are single cells, each usually incapable, under natural conditions, of continued existence or development—on the point of death; but by their union a rejuvenated individual is formed which constitutes a link in the eternal procession of life by virtue of its power of reproduction. [1]
Despite this early scientific interest in fertilization, it was not until almost 60 years later that Lionel Jaffe and co-workers discovered that the sperm initiates a wave of calcium (Ca2+) across the egg (as initially proposed by Dalcq in 1928 [2]) and that this signal triggers the formation of a ‘rejuvenated individual’—the newly developing embryo [3,4]. These first experiments documenting that Ca2+ initiates embryonic development at fertilization were performed in eggs of medaka, a freshwater fish, and were made possible by the identification of jellyfish aequorin as a bioluminescent Ca2+-sensing protein [5]. Similar experiments performed shortly thereafter showed that Ca2+ was released following the fertilization of sea urchin eggs [6]. With the development of new chemical and genetically encoded Ca2+ sensors and advances in experimental techniques of in vitro fertilization (IVF) and Ca2+ imaging, we now know that a Ca2+ rise signals the initiation of animal development with no exceptions with cross-phyla sampling and even in several species of flowering plants [7,8]. However, the pattern of Ca2+ rises at fertilization varies widely. Fertilization-induced Ca2+ changes depend on a large number of variables including exactly how the Ca2+ rise is initiated and how the newly formed zygote responds to this Ca2+ rise and perhaps to other signalling pathways triggered at fertilization. The collective set of responses, including the Ca2+ rise, that is required for the oocyte to begin development into an embryo is termed ‘egg activation’. Here, we will review the generation and modulation of Ca2+ signals during egg activation, focusing mainly on knowledge gained from intensive studies of mammalian fertilization over the past 30 years. However, we will also draw from studies of non-mammalian animals to illustrate additional mechanisms of generating and modulating the Ca2+ signals that initiate embryonic development.
Ca2+ is a ubiquitous second messenger responsible for numerous cellular responses to external stimuli. Its ubiquitous nature implies that Ca2+ signals must be tightly regulated and that additional information must be encoded in the localization, amplitude and timing of Ca2+ release events. In this way, different stimuli can be properly interpreted by the cell and result in the necessary downstream responses. For example, basal cytoplasmic Ca2+ levels maintained by constitutive Ca2+ release from the endoplasmic reticulum (ER) suppress autophagy whereas higher levels of cytoplasmic Ca2+ promote autophagy [9]. In cardiac muscle cells, cytoplasmic Ca2+ signals promote contraction whereas nuclear Ca2+ pulses activate transcription of specific genes [10]. In mast cells, localized Ca2+ influx across the plasma membrane drives specific transcriptional responses not induced by global cytoplasmic Ca2+ rises [11]. Cells release and regulate Ca2+ under the control of a ‘Ca2+ signalling toolkit’ composed of Ca2+-mobilizing signals, channels that regulate Ca2+ influx into the cytoplasm or release from intracellular stores, and pumps and exchangers that remove Ca2+ from the cytoplasm [12]. In the next section, we will provide an overview of the major molecular mechanisms used by somatic cells as their toolkit to regulate cellular Ca2+ signals. (For more detailed descriptions of the Ca2+ signalling toolkit, see excellent recent reviews [12–15].) This will be followed by an in-depth review of how the Ca2+ signalling toolkit is used at fertilization to generate the signals that drive embryo development.
2. Modulators of Ca2+ signals and homeostasis—an overview
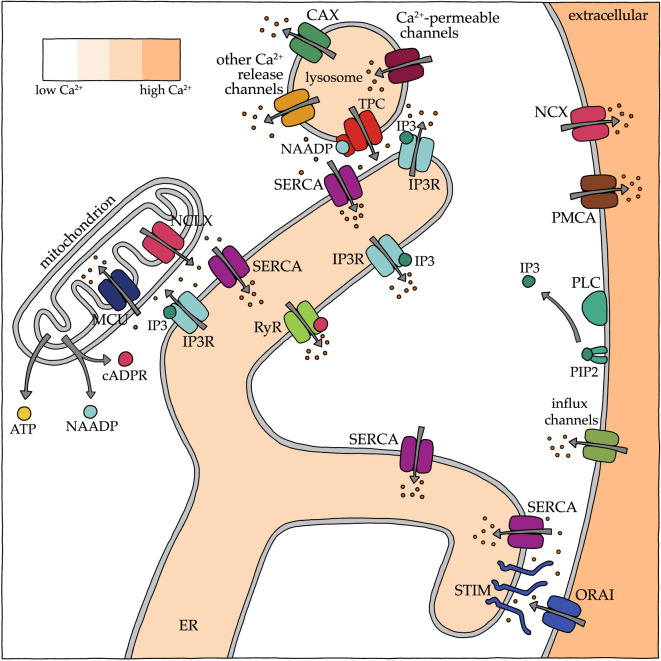
Ca2+ signalling is feasible due to the presence of tightly regulated and localized Ca2+ gradients in the cell. A simplified schematic showing the relationships between the main modulators of cellular Ca2+ signalling is shown in figure 1. Ca2+ is available to cells from the extracellular environment, which has very high (approx. 2 mM) Ca2+ levels relative to cytoplasmic Ca2+ that is normally in the 100 nM range. The entry of extracellular Ca2+ into the cell is tightly regulated by Ca2+-permeable channels on the plasma membrane that are sensitive to a variety of stimuli. For example, voltage-gated Ca2+ channels open in response to changes in membrane potential, whereas the large family of transient receptor potential (TRP) channels responds to diverse stimuli including temperature, stretch and osmolarity [16,17]. ‘Store-operated Ca2+ entry’ (SOCE) is a cellular mechanism to replenish depleted ER Ca2+ stores with Ca2+ from the extracellular environment. When ER stores are depleted, ER-resident STIM proteins oligomerize and interact with ORAI plasma membrane Ca2+ channels to stimulate Ca2+ influx [18]. When open, all of these plasma membrane channels allow rapid entry of Ca2+ into the cytoplasm due to the large concentration gradient from extracellular to intracellular cytoplasmic compartments.
Figure 1.
Elements of Ca2+ toolbox in endoplasmic reticulum, mitochondria, lysosomes and plasma membrane of somatic cells. Orange dots indicate Ca2+; grey arrows show direction of Ca2+ flow. cADPR, cyclic ADP ribose; CAX, Ca2+/proton exchanger; IP3, inositol trisphosphate; IP3R, IP3 receptor; MCU, mitochondrial uniporter; NCLX, mitochondrial sodium–Ca2+ exchanger; NAADP, nicotinic acid adenine dinucleotide phosphate; NCX, sodium/Ca2+ exchanger; ORAI, Ca2+ release-activated Ca2+ channel protein; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; PMCA, plasma membrane Ca2+ ATPase; RyR, ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase pump; STIM, stromal interaction molecule; TPC, two-pore channel.
Intracellular Ca2+ is largely sequestered within the ER (or sarcoplasmic reticulum of muscle cells), but additional intracellular Ca2+ stores include endolysosomes and mitochondria. Peripheral tubular regions of the ER contain membrane contact sites with the plasma membrane and many organelles including lysosomes and mitochondria [19]. At these sites, apposing membranes do not merge but come into very close proximity, promoting the efficient transfer of high local concentrations of Ca2+ and other molecules between the two compartments. Ca2+ is released from ER stores mainly through two channels: the inositol-1,4,5-trisphosphate (IP3) receptor and the ryanodine receptor. Both of these channels are activated by Ca2+ itself, which can induce an autocatalytic process termed ‘Ca2+-induced Ca2+ release’ and lead to the propagation of waves of Ca2+ across a cell. IP3 and sn-1,2 diacylglycerol (DAG) are generated by the action of phosphoinositide-specific phospholipase C (PLC) enzymes on phosphatidyl inositol-4,5-bisphosphate (PIP2), often in response to ligand–receptor interactions at the plasma membrane. IP3 binding to IP3 receptors leads to Ca2+ release from ER stores in part by sensitizing the IP3 receptor to Ca2+. DAG can directly impact canonical TRP channel activity and indirectly influence Ca2+ signals by altering protein kinase C activity [20,21]. Two additional Ca2+ mobilizing signals, independent of IP3-mediated signalling, are generated from nicotinamide-adenine dinucleotide (NAD) and NADP: cyclic ADP ribose (cADPR) and nicotinic acid dinucleotide phosphate (NAADP) [22]. cADPR sensitizes the ER-associated ryanodine receptor to Ca2+, resulting in Ca2+ release from ER stores. NAADP, in contrast, signals to two-pore channels in lysosomes and other acidic organelles, resulting in Ca2+ release from those membranous stores. Mitochondria, which normally have relatively low Ca2+ stores, release Ca2+ through the sodium–Ca2+ exchanger, NCLX [23,24].
Once released into the cytoplasm, Ca2+ must be removed rapidly to terminate signalling and return cytoplasmic levels to baseline. Cytoplasmic Ca2+ removal is accomplished through the combination of extrusion from the cell and reuptake into intracellular stores. Extrusion across the plasma membrane is mediated by plasma membrane Ca2+ ATPase (PMCA) pumps and sodium–Ca2+ exchangers (NCX). Perhaps the most important mechanism of reuptake into intracellular stores is through sarco-ER Ca2+ ATPase (SERCA) pumps, which actively transport Ca2+ across a steep concentration gradient into the ER. Lysosomes probably also have Ca2+ uptake mechanisms, but the molecular basis for this function is not known [14]. Mitochondria take up Ca2+ through the mitochondrial Ca2+ uniporter and, under conditions of high cytoplasmic Ca2+, through the voltage-dependent anion channel [15]. Alterations in the expression, localization and activity of these modulators of Ca2+ signals determine the timing and localization of Ca2+ release, reuptake and extrusion. The end result is extraordinary variability in the final Ca2+ signals experienced by different regions of the cell, explaining the wide variety of distinct signalling pathways that use Ca2+ as a second messenger.
3. Preparation for Ca2+ signalling during oocyte maturation
In all animals, oocytes are arrested in prophase of meiosis I while they undergo growth and differentiation. In response to hormonal or developmental signals, oocytes undergo nuclear maturation, which entails completion of two rounds of chromosome segregation, and cytoplasmic maturation, which is a general term for the changes in cytoplasmic contents and organization necessary to support fertilization and embryo development. The oocyte's prophase I nucleus is termed the ‘germinal vesicle’ (GV) and we refer to oocytes in this meiotic stage as GV oocytes. Following the maturation signal, the GV breaks down (GVBD) and meiosis resumes. Subsequent stages of meiosis may continue without interruption, such as in Caenorhabditis elegans and some molluscs, or meiosis may arrest again at various stages to coordinate with the timing of fertilization [25]. In most insects, the second arrest is at metaphase I, whereas in most vertebrates the second arrest is at metaphase II. For the purposes of this review, we will refer to oocytes that have resumed meiosis and then secondarily arrested at another meiotic stage in preparation for fertilization as ‘eggs’.
Even though GV oocytes possess a Ca2+ signalling toolkit, their capacity to elicit Ca2+ responses is reduced compared with eggs, indicating that the ability to mount a robust Ca2+ response is acquired during oocyte maturation. This concept was first discovered in starfish oocytes, in which Ca2+ release in response to fertilization is far lower in immature oocytes relative to mature eggs, despite having similar Ca2+ stores [26]. In the mouse, GV oocytes fertilized in vitro display Ca2+ oscillations of lower amplitude, frequency and persistence than those observed in eggs [27–29]. Similarly, Ca2+ release in response to IP3 or PLCζ injection is reduced in GV oocytes compared with eggs [28,30,31]. Several processes that take place during mouse oocyte maturation probably contribute to this difference in Ca2+ response: increase in Ca2+ stores, ER redistribution and increase in sensitivity to IP3, probably due to upregulation and phosphorylation of IP3 receptors. Ca2+ stores increase about four-fold during mouse meiotic maturation [29,32]. Interestingly, the smaller store size in GV oocytes appears to be due to a constant Ca2+ leak out of the ER through the IP3 receptor [33]. This leak ends around the time of GVBD, which is when the expansion of the ER Ca2+ store commences.
An increase in sensitivity to IP3 during oocyte maturation was first described in starfish oocytes and later demonstrated in hamster and mouse oocytes [26–28]. In the mouse, this finding is explained in part by a doubling of protein levels of the major IP3 receptor isoform, IP3R1, because inhibiting this maturation-associated increase in IP3R1 reduces IP3 sensitivity and alters Ca2+ oscillatory behaviour following fertilization [30,34–36]. During oocyte maturation, a redistribution of IP3R occurs, which mirrors ER redistribution (see below) in terms of localization and mechanisms [30,31,37]. However, altering IP3R1 redistribution using actin filament and microtubule depolymerizing agents does not change the sensitivity to IP3 [31]. As a general rule, IP3R1 phosphorylation enhances the conductivity of the channel. IP3R1 possesses phosphorylation consensus sites for numerous kinases, including M-phase kinases [such as polo-like kinase 1 (Plk1), mitogen-activated protein kinase (MAPK), cyclin-dependent kinase 1 (CDK1)], Ca2+/calmodulin-dependent protein kinase II (CaMKII), protein kinase A (PKA) and protein kinase C (PKC) [38]. Using the MPM-2 antibody, which recognizes an epitope present in proteins phosphorylated in the M-phase of the cell cycle, Lee et al. showed that MPM2 immunoreactivity of IP3R1 was barely detectable at the GV stage, increased sharply around GVBD, reached peak levels at MI and MII, and decreased again after fertilization [38]. This time-course of IP3R1 phosphorylation coincides with the activity of CDK1 and MAPK. Consistent with this finding, oocyte maturation is accompanied by an increase in IP3R1 phosphorylation at the CDK1 consensus sites S421 and T799 [31]. Furthermore, overexpression in eggs of a phosphomimetic IP3R1 protein in which the two CDK1 consensus sites (S421 and T799) and the ERK consensus site (S436) were mutated to aspartic acid led to increased sensitivity of the receptor to PLCζ injection or caged IP3 expression [39]. These findings strongly suggest that CDK1- and MAPK-mediated phosphorylation of IP3R1 explains at least part of the increase in IP3 sensitivity that occurs during maturation. Phosphorylation of IP3R1 by PKA occurs in oocytes but this phosphorylation is maximal at the GV stage and decreases around GVBD, so it is unlikely to contribute to the increase in IP3 sensitivity with maturation [31]. Whether or not additional kinases and/or phosphatases impact IP3R1 sensitivity is unknown.
The IP3R is not the only Ca2+ regulatory protein whose amount increases during maturation and has an important function in eggs. Regulator of G-protein signalling 2 (RGS2) is a protein that blocks the activity of both Gαs and Gαq proteins, thus inhibiting heterotrimeric G-protein-coupled receptor pathways that frequently trigger Ca2+ release. RGS2 protein levels are extremely low in GV oocytes, but the mRNA is recruited for translation during oocyte maturation, such that RGS2 levels increase approximately 20-fold [40]. RGS2 prevents premature Ca2+ release in eggs, which helps to prevent spontaneous egg activation events from beginning inappropriately prior to fertilization.
During the course of oocyte maturation, there is a reorganization of several organelles, including the ER, Golgi apparatus and mitochondria. Because the ER is the main cellular Ca2+ store, ER redistribution during oocyte maturation has been extensively studied and is correlated with the acquisition of a robust Ca2+ response. Using the fluorescent lipophilic dye DiI to label ER membranes, studies in starfish, marine nemertean worm, frog, hamster, human, mouse and plant oocytes demonstrated changes in ER organization during oocyte maturation [41–47]. In mouse, the ER is evenly distributed throughout the cytoplasm of GV oocytes with small accumulations in the interior, but not the cortical area, of the cell [46]. During GVBD, the ER becomes denser and envelops the meiotic spindle as it migrates towards the cortex [48]. The egg exhibits bright cortical clusters of ER, about 1–2 µm in diameter, that are absent from the region overlying the meiotic spindle [46,48]. The localization of both ER and IP3 receptors to cortical clusters in the egg situates the egg's main Ca2+ store and its releasing channel in proximity to the site of sperm–egg fusion, which is where the propagating Ca2+ wave starts at fertilization. The mechanism of this ER redistribution is a multi-step process driven by both microtubules and microfilaments [48]. Cytoplasmic lattices, unique to mammalian oocytes and preimplantation embryos, appear to have a role in ER redistribution during oocyte maturation because both Padi6- and Nlrp5-deficient oocytes, which lack these structures, fail to form cortical clusters [49,50]. Furthermore, Ca2+ oscillations are impaired in Nlrp5 null eggs [50]. In Xenopus oocytes, the ER-rich structures found in immature oocytes have been characterized as annulate lamellae (AL). AL are rich in IP3 receptor content, but paradoxically show minimal Ca2+ release activity, as opposed to ER patches in eggs, from which most Ca2+ is released in response to IP3. This finding suggests that AL are a functional Ca2+ store with attenuated IP3 receptor activity [51]. Whether this structure plays a similar role in mammalian oocytes is unknown. Although the pattern of ER organization varies in different species, the fact that all species examined change this pattern during oocyte maturation suggests a conserved and functional role for ER redistribution to prepare the egg for fertilization [52].
The Golgi apparatus of mouse, rhesus monkey and bovine GV oocytes comprises a series of cytoplasmic stacks, or ‘mini-Golgis’, more abundant in the interior than in the cortical region of the cell [53,54]. Despite their unusual appearance, these mini-Golgis are perturbed by the membrane trafficking inhibitor brefeldin A, indicating that they are functional. This experiment also demonstrated a role for membrane trafficking during oocyte maturation because brefeldin A-treated oocytes fail to complete meiosis I and arrest after GVBD [53]. During GVBD, these mini-Golgis fragment and become dispersed more homogeneously throughout the cytoplasm for the remainder of maturation. This pattern is reminiscent of what takes place in somatic cells entering mitosis [55]. The Golgi apparatus is estimated to provide about 5% of the total cellular Ca2+ store. Golgi membranes contain functional IP3 receptors [56], but the possible contributions of this organelle to Ca2+ release during egg activation is unknown. It is also unclear if there is an important role for the reorganization of the Golgi apparatus during oocyte maturation, or whether it is simply a response to major ER rearrangements or changing trafficking demands. One possible role for the Golgi fragments is serving as an intracellular source of PIP2 for hydrolysis by PLCζ at fertilization. The amount of vesicular PIP2 increases during oocyte maturation and, interestingly, the localization pattern of both PIP2 and PLCζ suggests that the vesicles could be part of the Golgi apparatus [57].
Mitochondria also undergo redistribution during oocyte maturation. At GVBD, they form a dense ring in the perinuclear area, followed by dispersion throughout the cytoplasm and formation of a new mitochondrial ring around the meiotic spindle [58–60]. By the metaphase II stage, mitochondria become homogeneously distributed throughout the cytoplasm, with very few localized in the polar body [59]. There is also a change in mitochondrial density or clustering during oocyte maturation that correlates with changes in ATP production (i.e. ATP production is high when mitochondria are found in large clusters [60]). Because mitochondria are essential for sustaining Ca2+ oscillations, at least in part by providing the ATP required for Ca2+ pumps, these changes in mitochondrial localization, clustering and ATP output during oocyte maturation may be necessary to elicit a robust Ca2+ response at fertilization.
4. Shapes and consequences of fertilization-associated Ca2+ signals
As mentioned previously, Ca2+ signalling is a universal hallmark of egg activation in all sexually reproducing species studied thus far. However, sperm-induced Ca2+ signals are significantly different across multiple phyla [61]. The diversity of Ca2+ dynamics during egg activation is schematized in figure 2. In most non-mammalian species, the fertilization-induced Ca2+ pattern takes the form of a single or a few waves. One exception is the marine worm Urechis caupo, in which the sperm triggers entry of extracellular Ca2+ uniformly around the egg cortex and no Ca2+ waves occur [69]. In medaka, sea urchins, frogs and C. elegans, a local cytoplasmic Ca2+ increase occurs immediately after sperm–egg fusion, and a single Ca2+ wave propagates from the sperm entry site across the entire egg [3,62,64,66,70]. The Ca2+ wave generally lasts from one to several minutes. Drosophila eggs also have a single Ca2+ wave; however, it is initiated from the egg pole through mechanical pressure and moves towards the centre of the oocyte and finally to the entire egg [63]. In species that undergo physiological polyspermy, such as newts and domestic fowl, each of the fertilizing sperm evokes a single slow Ca2+ wave that spreads across a small portion of the surface of the egg. Collectively, the multiple Ca2+ waves triggered around the whole egg ensure a global and long-lasting intracellular Ca2+ increase necessary for egg activation [71,72]. Another type of fertilization-induced Ca2+ pattern is a series of oscillations that travel in a wave-like fashion across the egg and last for minutes to hours. For example, fertilized marine nemertean worm and marine bivalve eggs display multiple oscillations that can persist for 30–60 min [73–75]. Similarly, mammalian eggs display a series of Ca2+ oscillations after sperm–egg fusion, but these persist for several hours [76,77].
Figure 2.
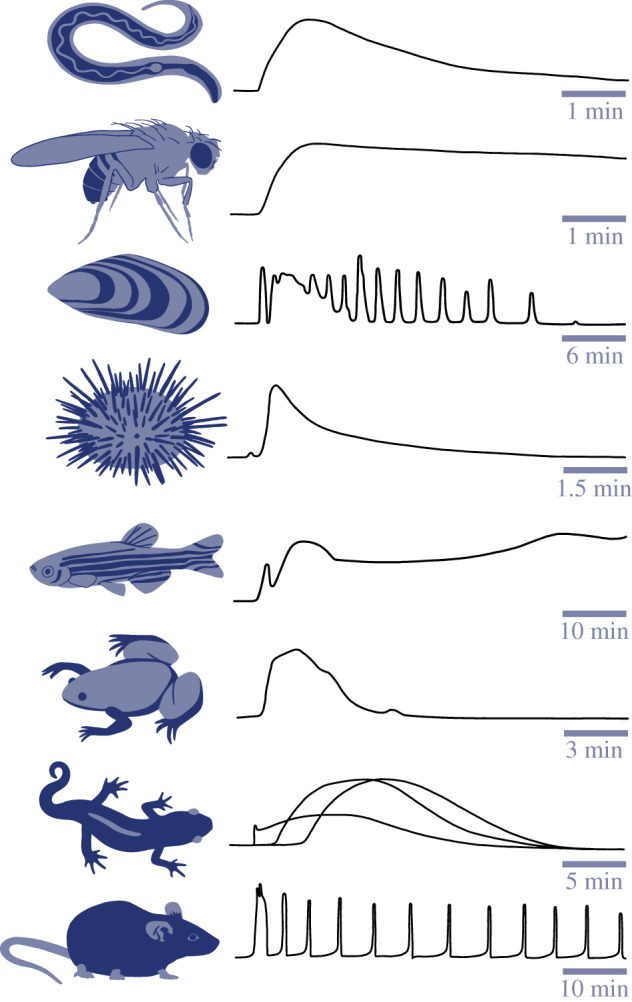
Diagrams of typical cytoplasmic Ca2+ changes at fertilization across various species. From top to bottom: nematode (Caenorhabditis elegans) [62], fruit fly (Drosophila melanogaster) [63], purple mussel (Septifer virgatus) [7], sea urchin (Paracentrotus lividus) [64], zebrafish (Danio rerio) [65], frog (Xenopus laevis) [66], newt (Cynops pyrrhogaster) [67] and mouse (Mus musculus) [68]. Time indicated on scale bar for each Ca2+ trace. The three lines on the newt trace represent spatially distinct signals induced by different sperm during physiological polyspermy. Traces adapted from indicated references.
Although Ca2+ dynamics vary widely among species, the Ca2+ oscillatory pattern is stereotypical for mammals. Shortly after sperm–egg fusion, the egg intracellular Ca2+ concentration rises more than 10-fold and persists close to this level for several minutes before returning to baseline. This initial relatively long Ca2+ transient is considered the main Ca2+ signal responsible for egg activation. The characteristic shape of this first Ca2+ transient has been well documented in different mammalian species using ratiometric Ca2+ imaging with epifluorescence signal detection [76,78–80]. Ca2+ concentration rises slowly at first and then rises rapidly to a peak, frequently followed by several short low-amplitude increases from the peak. The Ca2+ level then gradually falls over the course of several minutes before dropping rapidly back to baseline as cytoplasmic Ca2+ is cleared. The first transient is followed by a series of much shorter Ca2+ transients that are also characterized by an initial slow rise in Ca2+, a rapid rise to a peak that is usually lower than that of the first transient, and then rapid return to baseline [81]. In mammals, sperm-induced Ca2+ oscillations persist for several hours, until around the time of pronucleus formation. The frequency of Ca2+ oscillations appears to be a species-specific characteristic; however, it is possible that differences in media composition rather than inherent species differences influence oscillation frequency.
Long-lasting fertilization-associated changes in Ca2+ homeostasis orchestrate early and late events of egg activation. Artificial modulation of the Ca2+ oscillatory pattern by electropermeabilization revealed a correlation between the total number of Ca2+ transients applied and distinct cellular events of mouse egg activation [82]. Thus, cortical granule exocytosis is triggered by a single Ca2+ transient, whereas more than four transients are necessary to induce cell cycle resumption and polar body emission. Furthermore, robust pronuclear development, which is a later event of egg activation, requires additional electropermeabilization-induced Ca2+ transients. Studies in rabbit and mouse eggs concluded that the different egg activation events rely on a cumulative effect of Ca2+ input over downstream effectors, rather than a specific number or frequency of Ca2+ signals [83–85]. Therefore, eggs seem to be flexible regarding the Ca2+ pattern for activation, if the total summation of Ca2+ stimuli is sufficient to trigger egg activation.
Interestingly, experimental manipulation of Ca2+ signalling during or immediately after fertilization of mouse eggs results in alterations in the pre- and post-implantation developmental programs and even postnatal growth rate. (Note that the molecular mechanisms immediately downstream of Ca2+ signalling that promote egg activation are outside the scope of this review; for more information on this topic see [86,87].) In the mouse, experimental Ca2+ modulation following IVF causes alterations in the blastocyst transcriptome [84]. This finding is similar to observations in somatic cells, in which experimental modulation of Ca2+ signalling causes alterations in gene expression patterns [88]. Moreover, premature termination of the Ca2+ oscillatory pattern following fertilization results in lower implantation rates but no subsequent differences in development to term. By contrast, hyperstimulation of Ca2+ signalling following fertilization does not affect implantation but impairs development to term [84]. Similar experiments using rabbit eggs demonstrated that artificial modulation of Ca2+ dynamics during egg activation significantly affects implantation rates [83]. It is notable that the modulation of Ca2+ signalling during the relatively short time of egg activation also has long-term effects on offspring growth. Mouse offspring derived from hyperstimulated eggs gained less weight after weaning and the variation in body weight was much greater than that of controls [84]. These findings regarding abnormal growth trajectory and weight variance were also observed in two different mouse models of decreased Ca2+ signalling following fertilization in vivo [89]. Therefore, fine regulation of Ca2+ signalling following fertilization is critical to obtain developmentally competent embryos and healthy offspring. A link between abnormal patterns of Ca2+ signalling after IVF and the long-term effects on growth trajectory could be the impact of Ca2+ signalling on egg redox state and mitochondrial activity [90].
Despite advances in our understanding, it remains unclear whether these findings reported mainly from in vitro studies recapitulate the complex regulation of in vivo fertilization, in terms of the need for a specific Ca2+ oscillatory pattern for developmental success. Doubts arose after it was reported that several different genetic mouse models in which eggs show a very abnormal pattern of sperm-induced Ca2+ oscillations after IVF were still capable of producing offspring when fertilization occurred in vivo [89,91,92]. These reports suggest that there may not be a need for a prolonged series of Ca2+ oscillations to activate eggs effectively in vivo. Further studies are needed to define the fertilization-associated Ca2+ oscillatory patterns in vivo, within the highly specialized environment of the oviduct, and to determine whether alterations in Ca2+ signalling in vivo have similar detrimental effects on offspring health as artificial disruption in vitro.
5. Modulators of Ca2+ signals at fertilization
5.1. Ca2+-mobilizing signals
IP3 is the major Ca2+ mobilizing signal at fertilization and was the first identified. Early biochemical studies in sea urchins documented a rapid rise in both triphosphoinositides and diphosphoinositides that occurred after insemination but prior to evidence of Ca2+ release, suggesting that IP3 mediates Ca2+ release as had been shown previously in somatic cells [93]. This idea was tested by microinjection of IP3 into sea urchin eggs, which resulted in exocytosis of cortical granules and elevation of the fertilization envelope, both indicators of an increase in cytoplasmic Ca2+ concentration [94]. Subsequent studies in frog, hamster, mouse, marine worms and tunicates documented that IP3-induced Ca2+ release occurs at fertilization across multiple phyla [95–98].
Besides IP3-induced Ca2+ release, some species use alternative or additional mechanisms. In sea urchins, cADPR serves as a redundant mechanism for Ca2+ release at fertilization, presumably by activating the ryanodine receptor [70,99]. There is also evidence in sea urchin and starfish that NAADP stimulates Ca2+ release at fertilization [100–102]. Ca2+ entry from the extracellular milieu serves as a mechanism to increase cytoplasmic Ca2+ in echinoderms, molluscs and worms. In starfish, Ca2+ entry is a response to sperm-induced activation of a voltage-gated Ca2+ channel; this interaction results in a ‘cortical flash’ of Ca2+ [103,104]. In sea urchins, both voltage-gated channels and NAADP-induced Ca2+ release contribute to the cortical flash [105]. Limpets are exceptional in that they depend exclusively on Ca2+ influx, and not intracellular stores, to provide the fertilization Ca2+ signal [106]. In C. elegans, Ca2+ enters the egg through TRP channels located in the sperm plasma membrane following sperm–egg fusion [62]. In this way, the sperm membrane becomes a ‘conduit’ for Ca2+ entry as was first proposed by Jaffe [107]. This mode of Ca2+ entry is responsible for the rapid local rise in Ca2+ that precedes the global wave that crosses the egg, analogous to the cortical flash in starfish, but is not essential for the occurrence of the later global Ca2+ wave or for embryogenesis.
Some species undergo physiological egg activation in the absence of sperm. For example, the Ca2+ wave responsible for activating Drosophila eggs is initiated from extracellular Ca2+ sources in response to the mechanical stimulation of egg plasma membrane TRP channels during ovulation [63,108]. The propagation of the wave, however, depends on IP3 receptor-mediated Ca2+ release [108]. Similarly, spawning alone in species such as zebrafish and Sicyonia shrimp induces a Ca2+ wave in the absence of sperm [109,110]. In shrimp, the Ca2+ wave is initiated downstream of magnesium ions in seawater, but the molecular mechanism underlying Ca2+ entry is unknown [110]. Of note, separation of the initiation of the Ca2+ wave from the fertilizing sperm creates a requirement for highly efficient single sperm entry soon after egg activation to ensure that diploid embryonic development begins synchronously.
5.2. Phosphoinositide-specific phospholipase C
Generation of IP3 by PLC-mediated hydrolysis of PIP2 is essential for Ca2+ release at fertilization in most species studied, but the specific PLC used varies. There are six families of PLC enzymes in animals: PLCβ, PLCγ, PLCδ, PLCε, PLCζ and PLCη [111]. Each PLC family is activated in distinct ways, but all PLC isoforms have a conserved catalytic domain and Ca2+-binding EF hand motifs. Hence, they all carry out PIP2 hydrolysis to generate IP3 and DAG and can be modulated by changes in Ca2+ levels.
The first experiments to identify a specific PLC that was activated at fertilization to generate the IP3 responsible for Ca2+ release were performed in the starfish Asterina miniata. Carroll and colleagues demonstrated that src homology-2 (SH2) domain-mediated inhibition of PLCγ activity prevents the sperm-induced Ca2+ wave that follows the cortical flash [112]. Similar experiments performed in the ascidian Ciona intestinalis revealed that PLCγ mediates Ca2+ release in these marine invertebrates as well [113]. These findings provided support for the idea that IP3 is the major downstream mediator of the Ca2+ wave. In addition, these findings suggested that a tyrosine kinase functions upstream of PLCγ activity at fertilization given that this is the most common mechanism of activating PLCγ [111]. Further studies in sea urchin and Xenopus demonstrated that src family tyrosine kinases stimulate Ca2+ release at fertilization by activating PLCγ [66,114]. Hence, a soluble tyrosine kinase/PLCγ/IP3 pathway to initiate Ca2+ release appears to be a common mechanism among external fertilizing species. However, in mouse, SH2-domain-mediated inhibition of PLCγ activity has no impact on sperm-induced Ca2+ oscillations, indicating that either PLCγ is not involved or that it must be activated by an alternative mechanism [115].
The hypothesis that sperm introduce a PLC activity into the egg that is responsible for PIP2 hydrolysis and Ca2+ release following sperm–egg plasma membrane fusion at fertilization was first proposed by Whitaker & Irvine [94]. This idea was based on studies in sea urchins presented earlier that year demonstrating that injection of sperm extracts results in the formation of the fertilization envelope, which occurs downstream of Ca2+-induced exocytosis of cortical granules [116]. Similar experiments using sperm extract injections in ascidian, rabbit, mouse and hamster eggs demonstrated that a factor present in sperm extracts could generate Ca2+-dependent egg activation events in distinct phyla [117–119]. Consistent with the idea that a soluble sperm factor could be a common physiological inducer of Ca2+ release at fertilization, sperm–egg fusion precedes the increase in cytoplasmic Ca2+ in sea urchin, frog and mouse [95,120–122]. However, it was not until 2002 that a novel sperm-specific PLC, PLCζ, was cloned from a mouse spermatid cDNA library and identified convincingly as the protein in sperm extracts capable of activating eggs [123]. Microinjection of cRNA encoding PLCζ caused Ca2+ oscillatory patterns very similar to those observed at fertilization. Importantly, removal of PLCζ from hamster sperm extracts by an immunodepletion strategy caused the loss of the extract's Ca2+ releasing activity following microinjection into mouse eggs. Finally, mutation of a critical catalytic site residue abrogated this activity, indicating that it was the PLCζ enzymatic activity that was responsible for Ca2+ release. These studies effectively demonstrated that PLCζ was the critical component in microinjected sperm extracts that induces Ca2+ release, but left open the question of whether or not PLCζ was important for fertilization-induced Ca2+ release.
The first in vivo model to describe a role for PLCζ at fertilization was generated using a mouse transgenic RNA interference (RNAi) knockdown approach that resulted in an approximately 40% reduction in sperm PLCζ protein levels [124]. Eggs fertilized in vitro by these sperm had abnormal patterns of Ca2+ oscillations that terminated prematurely. Subsequent experiments using human sperm lacking PLCζ obtained from infertile males demonstrated that the sperm failed to induce Ca2+ release when injected into mouse eggs [125]. This finding was consistent with the clinical failure of human egg activation following intracytoplasmic sperm injection (ICSI) observed in these patients. Later, two different point mutations in the catalytic region of human PLCZ1 were identified that were associated with a case of male infertility refractory to ICSI [126,127]. Both of these mutations were associated with deficient Ca2+ oscillation-inducing ability in mouse eggs [127,128]. Additional novel PLCZ1 mutations associated with human egg activation failure have since been identified [129–132]. The recent development of three different mutant mouse Plcz1 models confirmed that PLCζ is essential for normal Ca2+ oscillations following fertilization [91,92]. Surprisingly, in all three mutant models, the males were not sterile but instead had very small litters. Both failure of egg activation (failure to form pronuclei) and polyspermy contributed to the subfertility. Careful evaluation of the Ca2+ oscillation phenotype in one of these models revealed that eggs fertilized in vitro by a single sperm exhibited approximately 3 Ca2+ transients on average, but that the onset of these transients was delayed by approximately 45–60 min relative to eggs fertilized with wild-type sperm [92]. Interestingly, sperm lacking PLCζ did not induce any Ca2+ transients following ICSI, suggesting that events associated with sperm–egg plasma membrane fusion were essential for this PLCζ-independent Ca2+-releasing activity. The molecular basis for this additional egg activation mechanism is unknown.
PLCζ, the smallest PLC identified to date, is unique when compared with previously identified PLCs [123]. It lacks a pleckstrin homology (PH) domain present in all other PLCs and lacks coiled coil, src homology, ras-association and RAS-GEF domains that are present in some but not all other PLCs [111]. Because PH domains mediate the association of PLCs with the plasma membrane through an interaction with their substrate PIP2, the lack of this domain in PLCζ explains its ability to diffuse freely through the egg cytoplasm. Indeed, there is evidence that plasma membrane PIP2 does not serve as a substrate for PLCζ at fertilization [57,133]. In mouse eggs, plasma membrane PIP2 levels do not decrease at fertilization. Additional PIP2 is localized to intracellular vesicles, and depletion of intracellular vesicle-associated PIP2 impairs Ca2+ oscillations induced by the fertilizing sperm [57,134]. The nature of the PIP2-associated vesicles is not known, but they are distinct from ER clusters. The interaction of PLCζ with these vesicles appears to be mediated by basic residues in the XY-linker, EF-hand and C2 domains [129,135–137]. The catalytic activity of most PLC isoforms is autoinhibited by negatively charged residues in the XY-linker region; the enzymes are activated by various mechanisms of releasing autoinhibition [111]. This is not the case for PLCζ. The XY-linker in PLCζ is positively charged and its deletion causes a loss of enzymatic activity, suggesting that PLCζ is constitutively active [138]. Ca2+ is the only known activator of PLCζ, with an EC50 of approximately 50 nM and 70% maximal activity at 100 nM [139]. Hence, PLCζ is almost maximally active at the basal intracellular Ca2+ levels present in the egg cytoplasm. By contrast, other PLCs are activated by approximately 10-fold higher Ca2+ levels [111].
Although PLCζ is responsible for the PLC activity that initiates Ca2+ release during egg activation, an open question is whether or not endogenous egg PLCs contribute to the Ca2+ oscillatory pattern by generating additional IP3-mediated Ca2+ release. On somatic cell membranes, ligand-G-protein-coupled receptor interactions frequently stimulate PLCβ isoform-mediated generation of IP3 and DAG through the activity of heterotrimeric G-protein α subunits in the Gαq family [111]. By analogy, one proposed mechanism for sperm-mediated egg activation was through a sperm ligand–egg receptor interaction that could activate PLCβ. Support for this mechanism came from the observation that overexpression of the Gαq-coupled m1 muscarinic receptor in mouse eggs followed by exposure to its cognate ligand, acetylcholine, caused egg activation events downstream of cytoplasmic Ca2+ elevation [140,141]. However, subsequent experiments demonstrated that although stimulation of Gαq could cause complete egg activation, sperm did not require this activity to successfully activate eggs, indicating that Gαq-mediated PLCβ stimulation was not required for Ca2+ release at fertilization [142]. The role of PLCβ was also tested using a knockdown strategy to generate mice carrying eggs with decreased levels of PLCβ1 [143]. Reduction in PLCβ1 caused a significant decrease in the amplitude of sperm-induced Ca2+ transients, but did not impact other characteristics of the oscillatory pattern, suggesting a contributory but not major role for PLCβ1. An interesting recent study took advantage of the development of a new fluorescent IP3 sensor to monitor both Ca2+ and IP3 concurrently in fertilized mouse eggs [144]. Using this tool, it was shown that during later phases of the Ca2+ oscillations following fertilization, Ca2+ activates PLC to generate IP3 peaks that follow each Ca2+ transient. These findings further support the idea that endogenous egg PLCs are active at fertilization and impact the overall Ca2+ signals experienced by the early embryo, but which PLC, if any, has yet to be determined.
5.3. IP3 receptor
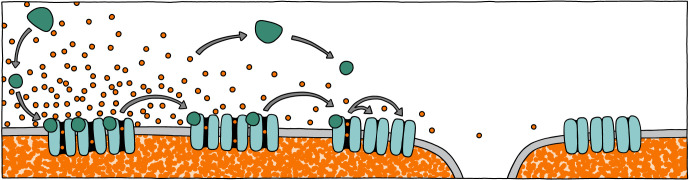
The IP3 receptor is an ion channel that releases Ca2+ from the ER in response to IP3 and Ca2+ binding. It is composed of large subunits that assemble as tetramers to generate a greater than 1 MDa Ca2+ channel mainly present in ER membranes. Only a small portion of the IP3 receptor extends into the ER lumen; the majority extends into the cytoplasm, giving it a square mushroom-shaped structure that is evident by cryo-electron microscopy [145]. Each subunit has a single IP3-binding site. IP3 receptor opening requires binding of IP3 to each of the four subunits along with Ca2+ binding, which has a biphasic impact on channel opening [146–148]. Small increases in cytoplasmic Ca2+ levels promote channel opening, whereas high (greater than 300 nM) Ca2+ concentrations inhibit channel opening. Hence, Ca2+ released by channel opening feeds back on the IP3 receptor to promote closing, resulting in intermittent Ca2+ release events from single receptors. When IP3 receptors are enriched in specific locations, this feedback system leads to localized Ca2+-induced Ca2+ release as nearby receptors open in response to Ca2+ released by others. Finally, as sufficient Ca2+ is released to enable diffusion to more distant sites, distal IP3 receptors open. When supported by Ca2+-induced activation of PLC to generate additional IP3, this process can eventually lead to the formation of regenerative Ca2+ waves that travel across the cell (figure 3) [94,134,149]. Generation of these Ca2+ waves depends on the absolute number, spacing and localization of the IP3 receptors as well as the localization and intensity of the signals generating the necessary IP3 and Ca2+ inputs.
Figure 3.
Generation of a Ca2+ wave. Localized production of IP3 (teal circles) by PLC (teal semicircles) leads to IP3-mediated Ca2+ release from clusters of IP3 receptors in endoplasmic reticulum (ER) membranes. The released Ca2+ promotes Ca2+-induced Ca2+ release from nearby clusters, increasing the cytoplasmic Ca2+ gradient. In addition, the released Ca2+ stimulates PLC to generate additional IP3 in a positive feedback loop. Continued IP3 production and Ca2+ diffusion lead eventually to Ca2+ release from distal IP3 receptor clusters in distinct ER regions and a wave of Ca2+ release across the cell. Orange dots indicate Ca2+; grey arrows show the direction of IP3 or Ca2+ flow.
The large cytoplasmic portion of the receptor supports the modulation of IP3 receptor function by numerous agonists and antagonists in addition to IP3 and Ca2+ (reviewed in [150,151]). These modulators include other small molecules such as ATP and NADH, reactive oxygen species, and Ca2+-binding proteins such as calmodulin. IP3 receptor opening is also modulated by phosphorylation/dephosphorylation and other covalent modifications including ubiquitination, cross-linking and limited proteolysis. For example, PKA, PKC and CaMKII all phosphorylate the IP3 receptor to regulate channel opening [152–154]. Hence, the IP3 receptor serves as a signalling hub that integrates inputs from many different signalling pathways to drive the resulting Ca2+ signals received by the cell.
The release of Ca2+ through the IP3 receptor is essential at fertilization for most animals studied. This was first demonstrated in hamster eggs, in which microinjection of a function-blocking monoclonal antibody that inhibits Ca2+ release from the IP3 receptor prevented sperm-induced Ca2+ oscillations following fertilization [155]. Similar studies in mouse eggs demonstrated that the antibody blockade of IP3 receptor function completely prevented egg activation [156]. In starfish, the critical role of IP3-receptor-mediated Ca2+ release at fertilization was demonstrated using an ‘IP3 sponge’ that prevents receptor activation by absorbing IP3 [37]. Although IP3 receptor activity is essential for sea urchin fertilization, the release of Ca2+ through the related ER-resident ryanodine receptor is also necessary for a full Ca2+ release response [99,104]. In ascidians, the IP3 receptor generates Ca2+ release responsible for the fertilization-associated Ca2+ wave, but Ca2+ released through the ryanodine receptor promotes post-fertilization plasma membrane insertion events that do not occur when only the IP3 receptor is active [157]. Although the ryanodine receptor can be detected in mammalian eggs, it does not appear to contribute significantly to Ca2+ release or other egg activation events at fertilization in mammals [158–161].
Many animals, including Xenopus, Drosophila, C. elegans and starfish, appear to have a single IP3 receptor isoform [37,162–164]. However, three highly homologous IP3 receptor subunits (IP3R1, IP3R2 and IP3R3) are found in mammals; several splice variants also have been identified [165]. The IP3 receptor can be composed of either homotetramers or heterotetramers of distinct subunit types [166]. Although all subunit types carry out similar Ca2+ release functions in response to IP3 and Ca2+, there are differences in their regulatory properties. For example, IP3R2 has a higher affinity for IP3 than IP3R1, and IP3R1 and IP3R2 are activated when phosphorylated by PKA, but IP3R3 is not [167–169]. IP3R1 has been detected in mouse and bovine eggs where it is enriched in the cortical region [30,170]. In mouse and bovine eggs, IP3R2 and IP3R3 also can be detected; however, IP3R1 is far more abundant [34,36,170]. Hence, it is likely that IP3R1 carries out the majority of the fertilization-induced Ca2+ release activity in mammalian eggs as first demonstrated in hamster eggs by Miyazaki et al. [155].
An important consideration regarding the regulation of IP3 receptor activity is the particular ER subdomains where the receptor is localized. As mentioned above, IP3 receptor quantity, localization and phosphorylation status change during oocyte maturation in mammals and these changes probably impact Ca2+ signalling. In Xenopus oocytes, IP3 receptor quantity changes minimally during maturation but there are still significant differences in IP3 receptor-mediated Ca2+ release events between oocytes and eggs [51,163]. It turns out that these differences are modulated in part by the association of IP3 receptors with distinct ER subdomains such as annulate lamellae, which are remodelled during oocyte maturation [51]. In mammalian eggs, specific differences in IP3 receptor behaviour based on localization to ER subdomains have not yet been demonstrated.
5.4. Two-pore channels
Two-pore channels, which localize to acidic organelles of the endolysosomal system, release Ca2+ in response to stimulation by NAADP. These channels were first identified based on their similarities to voltage-gated Ca2+ channels and TRP channels [171]. Their identification provided a possible molecular basis for the observation originally made in sea urchin egg homogenates that NAADP releases Ca2+ from reserve (yolk) granules, a cellular compartment distinct from the ER and a functional equivalent of lysosomes [172]. The reserve granules mediate the contribution of NAADP to the generation of the cortical flash during sea urchin fertilization, probably through one of the sea urchin TPC isoforms, though a direct connection has not yet been documented [105]. More recent studies indicate that NAADP interacts with an accessory protein that modulates TPC opening rather than directly with the TPC [173]. It is likely that only small amounts of Ca2+ are released from endolysosomal stores in response to NAADP; however, these Ca2+ release events can then trigger Ca2+-induced Ca2+ release mediated by ER-associated IP3 and/or ryanodine receptors [22,174]. Likewise, IP3 receptor-mediated Ca2+ release from ER stores can promote Ca2+ release from acidic stores through retrograde signalling [175]. This crosstalk appears to depend on the close apposition of ER and acidic stores at membrane contact sites [19,176].
5.5. Sarco/endoplamic reticulum Ca2+-ATPase pumps
The sarco-ER Ca2+-ATPase (SERCA) is a P-type Ca2+ pump located in ER membranes that transports Ca2+ against a concentration gradient from the cytoplasm into the ER lumen. In mammals, it is encoded by three genes (Atp2a1–3) that generate the three main isoforms (SERCA1–3), but because the transcripts are subject to alternative splicing, they give rise to 11 SERCA isoforms [177]. These isoforms differ in their tissue and developmental expression patterns, affinity for Ca2+, and regulation. SERCA1a and 1b are expressed in adult and foetal fast-twitch skeletal muscle, respectively. SERCA2a is expressed in cardiac and slow-twitch skeletal muscle, whereas SERCA2b is ubiquitously expressed and considered the housekeeping SERCA isoform. SERCA3 isoforms are found in certain non-muscle cells, usually co-expressed with SERCA2b. The functional organization of SERCA proteins, like all Ca2+-ATPases, is composed of a transmembrane domain containing 10 membrane-spanning helices and three cytoplasmic domains: the actuator, phosphorylation and nucleotide-binding domains. The SERCA pump contains two Ca2+-binding sites in the transmembrane domain and hence transports two Ca2+ ions per ATP hydrolyzed. SERCA pumps can be inhibited by general P-type ATPase inhibitors, such as La3+ and orthovanadate, and by the specific inhibitor thapsigargin [178].
SERCA2 isoforms are the main variants present during fertilization. In Xenopus oocytes, endogenous SERCA2 is found in cortical clusters [179]. Exogenous expression of avian SERCA1 results in IP3-induced Ca2+ oscillations of higher frequency and shorter duration, indicating that SERCA isoforms probably are important in Ca2+ regulation at fertilization in frogs [180]. In mouse eggs, SERCA2B is the major isoform expressed [181]. A role for SERCA in sustaining Ca2+ oscillations following fertilization was demonstrated by showing that thapsigargin treatment severely reduces the magnitude and duration of the first Ca2+ transient and decreases persistence of oscillations [76]. Whereas SERCA2B protein levels remain relatively constant during oocyte maturation, a spatial redistribution of the protein occurs, which mimics ER redistribution from a more diffuse pattern in GV oocytes to cortical clusters in eggs [181]. This localization positions the SERCA pump in close proximity to the IP3 receptor, which probably facilitates refilling ER Ca2+ stores after depletion following fertilization.
5.6. Plasma membrane Ca2+-ATPase pumps and sodium–Ca2+ exchangers
The PMCA is a P-type Ca2+ pump that transports excess intracellular Ca2+ outside of the cell to maintain homeostasis. It has high affinity for Ca2+ and low transport capacity, and it exchanges Ca2+ for H+. Its domain structure is similar to other P-type pumps (one transmembrane and three cytoplasmic domains), but it also contains a calmodulin (CaM)-binding site in its C-terminus that functions as an auto-inhibitory domain. Under resting conditions, this domain folds into the ATP-binding site, inhibiting the pump. When intracellular Ca2+ increases, Ca2+-bound CaM interacts with the CaM-binding site, releasing the auto-inhibitory conformation of the pump and restoring activity [177,178]. PMCA can also be activated by acidic phospholipids through interaction with two binding sites, one in the transmembrane domain, one in the carboxy terminus of the pump. Therefore, membrane composition can affect both activity and localization of PMCA [178,182,183]. Among acidic phospholipids, PIP2 is the most potent activator of PMCA. Moreover, the interaction between PMCA and PIP2 not only activates PMCA enzymatic activity, but also protects PIP2 from hydrolysis by PLC enzymes, thus modulating Ca2+ responses [184]. It remains to be determined if this modulatory role of PMCA is relevant in mouse eggs, where the pool of PIP2 hydrolyzed by PLCζ is located in intracellular vesicles rather than in the plasma membrane.
Mammalian PMCA is encoded by four genes (Atp2b1–4) that are alternatively spliced to generate around 30 isoforms. PMCA1 and PMCA4 are ubiquitously expressed and considered the housekeeping PMCAs. PMCA2 is expressed in the nervous system and mammary gland, whereas PMCA3 is expressed in the nervous system [178]. In Xenopus oocytes and eggs, the presence and localization of PMCA was demonstrated using an antibody that recognizes all four mammalian PMCA isoforms [179]. This study demonstrated a functional role for PMCA in frog oocytes but not eggs. Inhibition of PMCA in fertilized mouse eggs results in Ca2+ oscillations in which the first transient has higher amplitude and longer duration, indicating a role for PMCA in extruding excess Ca2+ after fertilization [185]. Similarly, Ca2+ oscillations triggered by PLCζ injection can be sustained in the absence of extracellular Ca2+ if Ca2+ efflux through PMCA is blocked by 5 mM Gd3+ [181]. Microarray data from mouse oocytes, eggs and preimplantation embryos indicates that Atp2b1 is the most abundant isoform, followed by Atp2b3, while Atp2b2 and Atp2b4 are nearly undetectable [186–188]. However, information about the presence and localization of PMCA at the protein level in mouse eggs and its potential role during fertilization is lacking.
Ca2+ efflux can occur through PMCA, but also through the Na+–Ca2+ exchanger (NCX). This transporter, present in the plasma membrane of most cells, exchanges three Na+ for one Ca2+ and the directionality of the fluxes depends on the membrane potential and the concentration gradients of these cations [189]. NCX contains 10 transmembrane helices, where the ion transport sites and two Ca2+-binding regulatory sites reside. In mammals, NCX is encoded by three genes, Ncx1–3, with Ncx1 and 3 undergoing alternative splicing. NCX1 is ubiquitously expressed, NCX2 is expressed in the nervous system, and NCX3 is expressed in the brain and skeletal muscle [189]. The relative importance of NCX activity to overall Ca2+ efflux varies depending on the tissue; it is essential in the heart to extrude Ca2+ from myocytes during each cardiac cycle, whereas it does not play a major role in the liver [190].
Removal of extracellular Na+ causes the NCX to function in the reverse mode, leading to Ca2+ influx. This approach has been used to infer the presence of NCX in hamster, mouse, rat and Xenopus oocytes [191–195]. In Xenopus oocytes, further characterization demonstrated that both NCX1 and NCX3 are expressed in the plasma membrane and are more abundant in the animal hemisphere [195]. In the mouse, experiments using extracellular Na+ depletion, as well as Na+ ionophore and Ca2+ addition after incubation in Ca2+-free medium, demonstrated that NCX is present in oocytes and eggs. However, the changes in Ca2+ fluxes observed after manipulating Na+ gradients are modest, and oocytes can recover from these perturbations, suggesting that NCX does not play an important role under physiological conditions [193].
Both PMCA and NCX undergo changes during oocyte maturation that suggest a decrease in Ca2+ efflux in metaphase II eggs compared with GV oocytes. Mouse oocytes respond to an increase in intracellular Na+ or a depletion of extracellular Na+ by increasing Ca2+ influx, due to the NCX ‘reverse mode’ activity. Mouse eggs, on the other hand, fail to respond to these changes in Na+ gradients [193]. The reason for maturation-associated downregulation of NCX activity is unknown. In Xenopus, PMCA is endocytosed during oocyte maturation, leading to depletion of this ATPase from the plasma membrane and a reduction in Ca2+ efflux [196]. Whether or not internalization of PMCA also takes place in mouse oocytes has not been assessed. A decrease in Ca2+ efflux during oocyte maturation is another mechanism to maximize Ca2+ responses at fertilization.
5.7. Ca2+ influx channels
We have long been aware that Ca2+ influx occurs at fertilization across numerous species. For example, fertilization of sea urchin and marine worm eggs results in a dramatic increase in Ca2+ influx within a few minutes as indicated by radiolabeled Ca2+ uptake [197,198]. In fertilized hamster and mouse eggs, early electrical recording studies demonstrated that alterations in extracellular Ca2+ concentrations change the frequency of periodic hyperpolarizing responses, suggesting that Ca2+ influx modulates the frequency of the intracellular Ca2+ release events responsible for hyperpolarization [77,191]. In the complete absence of extracellular Ca2+, fertilization-induced Ca2+ oscillations rapidly cease due to the depletion of ER Ca2+ stores [76,191]. In addition to the refilling of ER stores, Ca2+ influx in mouse eggs is required for second polar body emission, suggesting that cortical Ca2+ signals downstream of Ca2+ influx are essential for complete egg activation [185].
There are numerous Ca2+-permeable channels on the plasma membrane that when open allow Ca2+ to transit from the extracellular milieu down an approximately 10 000-fold concentration gradient into the cytosol. These channels are activated by a wide variety of signals. The voltage-gated Ca2+ channels open in response to changes in membrane potential and are best known for their roles in excitable cells such as neurons and cardiomyocytes. The TRP channels are ubiquitously expressed, and most are involved in transmitting sensory inputs or environmental signals. The TRP channels are permeable to multiple cations in addition to Ca2+, with variable selectivity toward different cations, and are functional at resting membrane potentials in non-excitable cells [16]. Ubiquitous store-operated Ca2+ channels on the plasma membrane are activated in response to depletion of intracellular Ca2+ stores, often following PLC-mediated activation of Ca2+ release through the IP3 receptor. There is experimental evidence for a role for each of these three channel classes in supporting Ca2+ influx necessary for successful egg activation, though the evidence for SOCE is very limited.
SOCE is well established as a major mechanism in somatic cells whereby ER stores are replenished following depletion [199,200]. This mechanism functions across the animal kingdom, including in insects, birds, amphibians and mammals. The major molecular basis for SOCE is that the ER has two molecular sensors of Ca2+ store depletion, STIM1 and STIM2, which are ubiquitously expressed single transmembrane proteins with Ca2+-binding EF hand domains in the ER lumen. In response to ER store depletion, Ca2+ dissociates from the STIM proteins, which then oligomerize and move toward ER-plasma membrane contact sites. STIM oligomers form large clusters, or punctae, that directly interact with plasma membrane ORAI channels, stimulating Ca2+ entry. Because depletion of ER Ca2+ stores occurs in the many species that depend on IP3-mediated Ca2+ release at fertilization, SOCE appears to be an obvious mechanism to replenish ER stores. However, SOCE is inactivated in mammalian cells during mitosis [201] and in Xenopus it is inactive during meiosis [202], suggesting that it is unlikely to have a role at fertilization. Both STIM1 and ORAI1 proteins are expressed in mouse eggs [203,204], but two different studies using chemical SOCE inhibitors concluded that SOCE was not required at fertilization in the mouse [185,205]. Other groups used exogenously expressed, fluorescently tagged STIM and/or ORAI proteins to draw conclusions regarding SOCE function in mouse oocytes [204,206]. Both of these studies concluded that SOCE was disabled or minimally active in metaphase II-arrested eggs, consistent with the inhibitor studies. However, conflicting studies in pig oocytes reported that STIM1 and ORAI1 were necessary for egg activation; these studies used knockdown approaches whereby siRNAs were microinjected into immature oocytes to deplete protein levels [207,208]. Unfortunately, neither of these studies tested the impact of protein knockdown on IP3-sensitive ER Ca2+ stores in mature oocytes prior to fertilization. Because low ER Ca2+ stores before fertilization would be anticipated to result in inhibition of Ca2+ oscillatory behaviour following fertilization, these studies were not conclusive. In an effort to definitively determine whether SOCE was required to replenish ER stores at fertilization, a mouse knockout approach was used to generate eggs lacking both STIM1 and STIM2; Orai1-null eggs were also tested [68]. Stim1/Stim2 double knockout eggs had normal ER Ca2+ stores and no alterations in Ca2+ influx or Ca2+ oscillatory behaviour at fertilization relative to controls. Furthermore, eggs lacking ORAI1, the only ORAI channel expressed in mouse eggs, exhibit normal Ca2+ handling and homeostasis at fertilization. These findings clearly indicate that in the mouse, SOCE is not the Ca2+ influx mechanism responsible for refilling ER stores following fertilization. It remains possible that in other animals SOCE could be used.
Voltage-gated Ca2+ channels (CaV) are also important mediators of calcium influx. They are composed of a pore-forming α1 subunit associated with an intracellular β subunit, and extracellular α2 subunit bound by disulfide linkage to a transmembrane δ subunit, and sometimes a transmembrane γ subunit [17]. Their differential responses to alterations in membrane potential are largely driven by the α1 subunits, which are encoded by ten distinct genes in mammals. The voltage-gated Ca2+ channels are divided into three families based on their structure and function. The CaV1 channels are sensitive to large changes in membrane potential and tend to support long-lasting, large-conductance Ca2+ currents; hence, they are known as ‘L-type’ channels. The CaV2 channels are largely expressed only in brain regions and will not be discussed further here. The CaV3 channels are sensitive to small changes in membrane potential at negative voltages, are rapidly inactivated, and have transient kinetics leading to their characterization as ‘T-type’ channels. An interesting feature of T-type channels is that they can be open even in resting cells at a window of membrane potential between the activating and inactivating potentials; the resulting inward Ca2+ current is called ‘window’ current [209].
There is evidence for both L-type and T-type Ca2+ channels in supporting Ca2+ influx following fertilization in different species. Many marine animals and amphibians use L-type Ca2+ channels for this purpose; their opening is triggered by a large change in membrane potential (known as the ‘fertilization potential’) that occurs in response to either sperm–egg binding or fusion [210]. For example, oocytes of the marine worm Pseudopotamilla occelata use L-type channels to generate the global Ca2+ wave responsible for resumption of meiosis, but not the initial sperm-induced Ca2+ increase [211]. In the marine bivalve Mytilus edulis, the initial rise in Ca2+ following fertilization is dependent on Ca2+ influx through L-type channels, whereas internal Ca2+ stores provide later Ca2+ signals [75]. Limpet oocytes depend entirely on Ca2+ influx through L-type channels to provide the egg activation signal [106,212]. Mammalian eggs do not have a positive shift in membrane potential at fertilization, so would not have a mechanism to open L-type channels [213,214]. However, mouse eggs do have a classical T-type current that can be activated under physiological conditions [215,216]. The channel supporting this current is CaV3.2, which is likely to be active through a window current mechanism [217]. Mouse eggs lacking CaV3.2 have lower levels of Ca2+ influx during oocyte maturation and following fertilization. Furthermore, female mice whose eggs lack CaV3.2 have reduced litter sizes, suggesting that this channel is critical for efficient egg activation and development to term [217]. However, CaV3.2 cannot be the only Ca2+ influx channel active at fertilization in the mouse because in at least some eggs lacking CaV3.2, Ca2+ oscillations can persist long-term following fertilization.
In the past few years, attention has turned to the possible roles of TRP channels in supporting Ca2+ influx at fertilization. The TRP superfamily is encoded by a large number of distinct protein-coding genes: 28 in mice, 17 in C. elegans, and 13 in Drosophila [16]. TRP channels are divided into subfamilies based on sequence and topological homology, but the channels within each subfamily are not always activated by similar mechanisms. The canonical TRPs (TRPC1–7 in mammals) are activated downstream of PLC activation and there is some evidence for their involvement in SOCE in somatic cells [218]. It is unlikely that any of these TRPs function to support Ca2+ influx at fertilization, at least in the mouse, because mice globally lacking all seven TRPC channels are fully fertile [219]. TRPV3 is a warm temperature-dependent TRP channel whose activity is potentiated by PLC activation [220], suggesting it could function at fertilization. TRPV3 is expressed and functional in mouse eggs, and when stimulated using chemical activators supports Ca2+ influx and egg activation [221]. However, Trpv3-null mice are fertile, and their eggs have no alterations in sperm-induced Ca2+ oscillatory patterns, indicating that this channel is dispensable, though it could contribute to supporting Ca2+ influx at fertilization. TRPM7 is a constitutively active ion channel permeable to divalent cations including magnesium and Ca2+, but it can be activated by mechanical signals and inhibited by low millimolar magnesium levels [222,223]. TRPM7 is unusual in that it functions both as a channel and a serine/threonine kinase due to the presence of an intracellular kinase domain at the C-terminus [224]. Functional ion channels with characteristics of TRPM7 are expressed on mouse eggs and can support Ca2+ influx. Chemical inhibition of these channels in fertilized one-cell embryos impairs development beyond the two-cell stage [225]. To definitively test whether TRPM7 is required to support Ca2+ influx at fertilization, Trpm7 was conditionally deleted from mouse eggs using the Gdf9-cre transgene [89]. Eggs lacking TRPM7 did not have classical TRPM7 ion currents and in addition, their ability to support both spontaneous and ER store depletion-induced Ca2+ influx was lost. Furthermore, they had significantly blunted responses to alterations in extracellular Ca2+ and magnesium, and reduced Ca2+ oscillation frequency and persistence following fertilization. Although female mice carrying Trpm7-null eggs were fertile, their heterozygous offspring had abnormalities in weight variance and growth trajectories. These findings indicate that TRPM7 mediates spontaneous and SOCE-like Ca2+ influx, is largely responsible for the impact of extracellular Ca2+ and magnesium concentrations on Ca2+ influx, and is required to support Ca2+ influx following fertilization.
Given the previously established role of CaV3.2 in supporting Ca2+ influx at fertilization, a double knockout mouse model was established to generate eggs lacking both CaV3.2 and TRPM7 [89]. These eggs had reduced ER Ca2+ stores and minimal Ca2+ influx following ER store depletion. During approximately the first hour following fertilization, the double knockout eggs had only one or two Ca2+ transients, a finding very similar to the oscillatory pattern observed in wild-type eggs cultured after fertilization in Ca2+-free medium. These findings indicate that CaV3.2 and TRPM7 are largely responsible for the Ca2+ influx needed to replenish ER stores and to support persistent sperm-induced oscillations. However, many of the double knockout eggs restarted their oscillations again after about an hour, suggesting that a new Ca2+ influx mechanism becomes active over time. The restart pattern did not occur when the double knockout eggs were fertilized by ICSI, indicating that the new influx mechanism depends on either sperm–egg plasma membrane interaction or fusion. Female mice carrying CaV3.2/TRPM7 double knockout eggs had reduced litter sizes explained by differences in implantation or post-implantation development and the offspring had increased weight variability relative to controls. These findings indicate that together, CaV3.2 and TRPM7 serve as essential mediators of Ca2+ influx following fertilization in mice, though additional channels such as TRPV3 could also contribute. Whether or not they serve a similar function in eggs of other mammals is not known.
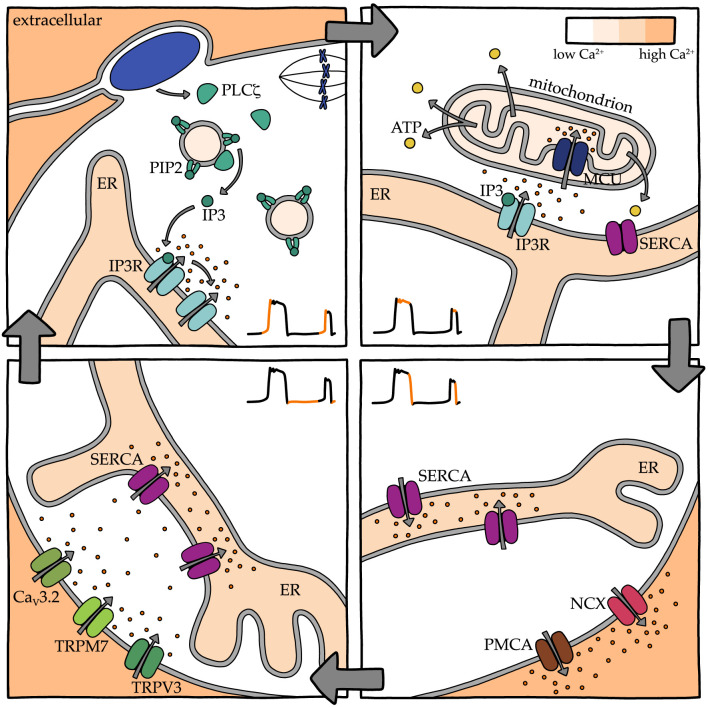
There is new evidence that TRP channel function in Ca2+ influx at fertilization is conserved in insects and worms. As mentioned above, Drosophila egg activation is initiated by Ca2+ influx that occurs in response to mechanical pressure during ovulation, though propagation of the Ca2+ wave depends on IP3-mediated Ca2+ release [63,226]. It turns out that the pressure-induced Ca2+ influx is mediated by Trpm, the only Drosophila orthologue of mammalian TRPM7 [108]. In C. elegans, a sperm plasma membrane TRP channel, TRP-3, serves as a conduit for localized Ca2+ influx into the egg following sperm–egg fusion [62]. In the absence of sperm TRP-3, eggs still activate following sperm–egg fusion but their global Ca2+ wave is delayed and abnormally shaped. Taken together, these findings indicate that TRP channels are essential modulators of Ca2+ signals required for the activation of development in both protostomes and deuterostomes. A schematic illustrating the Ca2+ release, reuptake and efflux mechanisms active in mouse eggs following fertilization is shown in figure 4.
Figure 4.
Cycle of Ca2+ transient generation in mammalian eggs at fertilization. Starting at the top left, the large grey arrows show temporal order. For each panel, the cytoplasmic Ca2+ trace is coloured orange at the portions of the trace that are generated mainly due to the steps illustrated in that panel. Top left: Sperm PLCζ acts on PIP2 in intracellular vesicles to generate IP3, which stimulates IP3R-mediated Ca2+ release and subsequent Ca2+-induced Ca2+ release. Top right: Ca2+ stimulates mitochondrial ATP production; ATP is required for SERCA pump activity. Bottom right: Ca2+ is pumped back into the ER through SERCA pumps and out of the egg through PMCA pumps and NCX. Bottom left: Ca2+ flows into the cytoplasm through TRMP7, CaV3.2 and TRPV3 channels and is then available for SERCA pumps to replenish ER Ca2+ stores in preparation for the next Ca2+ release event. Orange dots indicate Ca2+ at its destination; small grey arrows show the direction of flow. CaV3.2, T-type voltage-dependent Ca2+ channel; IP3, inositol trisphosphate; IP3R, IP3 receptor; MCU, mitochondrial uniporter; NCX, sodium/Ca2+ exchanger; PIP2, phosphatidylinositol 4,5-bisphosphate; PLCζ, phospholipase C zeta; PMCA, plasma membrane Ca2+ ATPase; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase pump; TRPM7, transient receptor potential cation channel subfamily M member 7; TRPV3, transient receptor potential cation channel subfamily V member 3.
6. Artificial activation
Eggs of numerous species can be activated in the absence of sperm, known as parthenogenetic activation, by artificially increasing cytoplasmic Ca2+ levels. Although the importance of Ca2+ was not known at the time, Jacques Loeb found during a series of studies beginning in the late 1800's that placement of sea urchin eggs into hypertonic solutions resulted in the elevation of the fertilization envelope, an initial step of fertilization [227]. It was later discovered that many different stimuli could be used to induce ‘artificial parthenogenesis' in marine animals, including electrical currents, various acids and ultraviolet light [228–230]. Based on these studies, Gregory Pincus found that mammalian eggs could undergo parthenogenetic activation when exposed to hypertonic solutions, acid or heat [231]. This body of work paved the way for numerous subsequent experimental studies using parthenogenetic activation to answer basic questions in developmental biology, including the finding that artificially activated mammalian eggs only develop part way due to imprinting [232,233]. More recently, parthenogenetic activation has been used for clinical applications during assisted reproductive technologies (ART) in humans (ICSI failure) and domestic animals [to improve egg activation following ICSI or during cloning (somatic cell nuclear transfer, SCNT)]. The present section aims to review the different stimuli used for egg activation in mammals, with emphasis on the latest methods reported and the role of Ca2+ in these artificial processes.
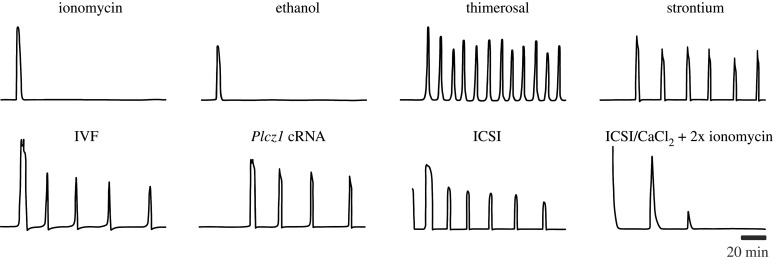
Many different methods are used to induce the rise in intracellular Ca2+ levels that artificially triggers cell cycle resumption and embryo development. We refer to these methods as ‘artificial egg activation’ (AEA) because they are often used in the presence of the paternal genome and therefore are not the same as parthenogenetic activation. Whereas some of these methods mimic the physiological repetitive Ca2+ increases observed in mammals, others simply induce a single large rise in Ca2+ that is sufficient to activate the downstream effectors necessary to complete egg activation. Finally, alternative methods completely bypass the Ca2+-dependent steps to induce AEA by activating directly the necessary downstream signalling pathways; these methods will not be discussed here as they are outside the scope of this review. Typical Ca2+ traces observed in mammalian eggs following the various methods of egg activation are shown in figure 5.
Figure 5.
Representative Ca2+ traces resulting from egg activation by various methods. Top row, chemical inducers of artificial egg activation (AEA) in bovine eggs [234]. Bottom row, in vitro fertilization (IVF) [68], Plcz1 cRNA injection [123] and intracytoplasmic sperm injection (ICSI) [235] in mouse eggs; ICSI using sperm lacking PLCζ activity, in medium containing CaCl2, followed by two treatments with ionomycin in human egg [235]. Scale bar applies to all traces. Traces adapted from indicated references.
6.1. Physical stimuli
Mechanical activation can be achieved efficiently in a small number of species. Under physiological conditions, Drosophila egg activation is initiated by mechanosensitive ion channels. These eggs can be artificially activated by osmotic swelling after exposure to hypotonic water or by applying hydrostatic pressure to mature eggs [226]. Ca2+ entry provoked after mechanical membrane disruption of frog eggs also results in AEA.
Electrostimulation has been used in several species to trigger egg activation [82,83,236]. This strategy involves the use of high-voltage electrical pulses to induce transitory pores in cellular membranes. The resulting influx of Ca2+ from the extracellular space causes a transient rise in intracellular Ca2+. The advantage of this method is that the Ca2+ rises can be controlled completely based on the equipment settings. Traditionally, electrical pulse length was from milliseconds to microseconds, but these relatively long-duration electric pulses can open large pores that are irreversible and lead to cell death [237]. New technology allows the use of electrical pulses with nanosecond duration. The main advantage to this approach is that nanosecond pulses preferentially affect intracellular membranes with almost no effect on the plasma membrane [238]. In mouse eggs, nanosecond pulsed electric fields (nsPEF) support Ca2+ efflux from the ER and even induce spontaneous Ca2+ oscillations while maintaining the integrity of the plasma membrane [239]. This procedure results in a high activation rate and a significant improvement in parthenogenetic embryo development. The use of nsPEF for egg activation in species other than mouse has not been tested.
6.2. Chemical stimuli
Chemicals are the most popular stimuli used for egg activation across most species. Different responses are registered after egg exposure to chemical agents, varying from a single Ca2+ transient to spontaneous Ca2+ oscillations. The characteristic responses serve as a reference for their classification (figure 5).
6.2.1. Single peak
Ca2+-selective ionophores are the most commonly used chemicals for egg activation. Ionophores are natural lipid-soluble agents that reversibly bind ions. Ca2+ ionophores such as ionomycin and calcimycin (A23187) bind Ca2+ in a 1 : 1 ratio and facilitate its transport across the cell membrane. In somatic cells, exposure to calcimycin promotes Ca2+ efflux from intracellular stores as well as Ca2+ influx from the extracellular space [76], whereas ionomycin at low concentrations acts primarily on intracellular membranes but also induces extracellular Ca2+ influx [240]. In mouse, human and bovine eggs, treatment with either ionophore leads to a rapid rise in intracellular Ca2+ followed by a slow decline over the course of several minutes. Ca2+ mobilization stops after drug washout, resulting in a transient effect, though additional ionophore treatments can result in additional Ca2+ increases in the same egg. Ca2+ ionophores are used in some human ART clinics to artificially activate eggs that fail to activate following ICSI. Although both ionophores function similarly, a direct comparison between ionomycin and commercially available ‘ready to use’ A23187 revealed that ionomycin is more efficient in activating both mouse and human eggs [241]. However, even an ICSI protocol in which the sperm is injected in medium containing CaCl2 followed by two treatments with ionomycin does not truly mimic a physiological Ca2+ oscillatory pattern (figure 5).
Eggs also can be artificially activated with 7% ethanol, which results in a dramatic rise in intracellular Ca2+, mainly due to IP3-mediated Ca2+ release from intracellular stores [234,242]. Ethanol activation has been successfully achieved in mouse, bovine and porcine; however, ethanol was not effective for human egg activation [234,242–244]. In bovine, the Ca2+ rise induced by ethanol is longer than that observed following ionophore-mediated activation and is associated with a higher activation rate [234]. Ethanol effects cease upon drug washout, comparable to ionophore treatment.
A single increase in the egg intracellular Ca2+ level results in relatively low rates of egg activation in mammals. Presumably, a transient rise in Ca2+ levels leads to inactivation of maturation promoting factor, a key Ca2+ target whose inactivation is essential for resumption of meiosis, but is not sufficient to sustain late events of egg activation that require additional Ca2+ signals [82,245,246]. For this reason, ionophore- or ethanol-mediated activation are usually followed by a treatment with protein synthesis or protein kinase inhibitors to improve activation rates [247].
6.2.2. Multiple peaks
In mammals, the information to elicit egg activation is encoded in sperm-induced repetitive Ca2+ signals. Therefore, chemicals that trigger Ca2+ oscillations activate eggs more efficiently than chemicals that induce a single rise, even though the eggs are flexible regarding the exact Ca2+ oscillatory pattern for activation. Strontium is the compound of choice for egg activation in rodents, but its efficacy in other species is controversial. The discovery that strontium activates mouse eggs was made inadvertently during experiments designed to examine the ability of various divalent cations to substitute for Ca2+ during sperm capacitation [248]. In the mouse egg, strontium induces prolonged Ca2+ oscillations and supports preimplantation embryo development. Strontium-induced activation is mediated by the IP3 receptor, probably through its sensitization to Ca2+, and depends on the presence of TRPV3 in the egg [221]. A long first Ca2+ transient is observed immediately after eggs are exposed to strontium, which is followed by repetitive smaller spikes resembling the oscillatory pattern observed after IVF [249]. In bovine, pig and horse, strontium can induce egg activation; however, the activation rate and embryo development are lower relative to other activation methods [247,250–252]. In large animals, Ca2+ dynamics after strontium-mediated activation remain unexplored, but it is likely that the activation failure is related to inefficient induction of Ca2+ oscillations. Strontium does not induce Ca2+ oscillations in human eggs and, consequently, fails to trigger egg activation [253,254].
Thimerosal is a mercury-containing organic compound that induces Ca2+ release in several cell types. It evokes repetitive Ca2+ oscillations in eggs, as was first observed in experiments with golden hamster eggs [255]. Thimerosal increases the sensitivity of the IP3 receptor to Ca2+ by oxidation of sulfhydryl groups, resulting in multiple Ca2+ oscillations with similar dynamics to sperm-induced Ca2+ signalling, but egg activation is not observed with use of thimerosal alone [256]. Thimerosal has a deleterious effect on eggs due to tubulin oxidation, which prevents its polymerization and impairs spindle formation [257]. However, a sequential treatment of thimerosal followed by dithiothreitol (DTT), a reducing reagent, prevents tubulin oxidation and results in a single intracellular Ca2+ rise that leads to egg activation in pigs [258]. Surprisingly, although the use of DTT stops thimerosal induced Ca2+ signalling in the egg, it enhances Ca2+ response after IVF or other activators of Ca2+-spiking, which highlights a difference in the mechanism of Ca2+ release [256]. In cow and human eggs, thimerosal also evokes Ca2+ oscillations, whereas in sea urchin, thimerosal induces a single Ca2+ wave that closely resembles fertilization signalling [234,259,260]. The use of thimerosal for egg activation is not widespread, probably due to both safety concerns about mercury and the need for a reducing reagent that results in the premature termination of the Ca2+ oscillations.
6.2.3. ‘Physiological’ stimuli
Even though we do not fully understand the long-term effects of AEA, methods that best mimic sperm-induced Ca2+ responses are likely to be more effective than those that induce non-physiological patterns. Therefore, microinjection of eggs with sperm extracts, PLCζ or IP3 to trigger egg activation is more attractive for clinical applications. Sperm extracts were first proposed for egg activation after it was shown that they had this activity. Activation rates of cloned horse embryos using stallion sperm extracts were significantly improved relative to other methods [261]. Recently, the use of sperm extracts for activation of SCNT reconstructed embryos in zebrafish was reported to improve the early development of parthenogenetic and cloned embryos; however, it remains uncertain if the Ca2+ response is similar to that induced by sperm [262].
To avoid using complex biological extracts, the use of cRNA encoding PLCζ emerged as a suitable solution. Bovine SCNT embryos activated by PLCZ1 cRNA microinjection exhibited not only a similar Ca2+ oscillatory pattern but also a similar gene expression profile when compared with IVF controls [263]. Moreover, ICSI and parthenogenetic activation rates, as well as embryo development, were improved with PLCZ1 cRNA microinjection in human eggs as compared with ionomycin-mediated activation [264]. Similar results were reported for mouse eggs [265].
There are several concerns regarding the use of PLCZ1 cRNA and sperm extracts for clinical use. First, it is not possible to accurately control the expression of PLCζ protein from microinjected cRNA [264]. In addition, governmental policies closely regulate the use of biological extracts and nucleic acids for clinical treatments because of the potential for infectious or hereditary long-term outcomes. Both considerations can be bypassed by the use of recombinant PLCζ protein. In this regard, microinjection of human eggs with recombinant human PLCζ protein produced in E. coli overcomes clinical ICSI failure and supports embryo development to the blastocyst stage [266]. Despite these promising results, recombinant PLCζ is not yet commercially available.
The increasing use of artificial reproductive technologies has sparked interest among researchers and human as well as animal clinics about AEA. However, special caution needs to be taken due to the concerns regarding possible long-term effects on offspring health of abnormal Ca2+ signalling after IVF. Further investigation is required to design accurate methods for egg activation that mimic physiological events, sustain embryonic development and ensure offspring health.
7. Post-ovulatory ageing
In mammals, after ovulation there is a defined time window for successful fertilization and development after which deterioration of the egg takes place, known as post-ovulatory ageing. Post-ovulatory aged eggs display increased fragmentation, reduced fertilization and developmental potential and, when successfully fertilized, abnormalities in the offspring [267]. Eggs that undergo post-ovulatory ageing in vitro or in vivo have altered Ca2+ responses triggered by many stimuli including sperm, IP3 and PLCζ. Both the amplitude and the rate of rise of Ca2+ transients are lower in post-ovulatory aged eggs [268–271]. The likely reasons for these changes are that the size of ER Ca2+ stores is smaller and that the IP3 receptor sensitivity and level of phosphorylation are decreased in aged eggs [271,272]. Also, the spatial organization of the IP3 receptor is altered with ageing in that the cortical IP3R clusters normally present in freshly ovulated MII eggs are dispersed [272]. IP3 receptor sensitivity, phosphorylation and cortical clustering are all associated with efficient Ca2+ release [33]. Inhibition of IP3R1 phosphorylation in mouse eggs results in a decrease in both amplitude and persistence of Ca2+ oscillations [38]. Consistent with this finding, the total duration of Ca2+ oscillations is also shorter in post-ovulatory aged eggs [269,270,273]. Post-ovulatory ageing also results in higher frequency of Ca2+ oscillations [269,271,273]. An increase in Ca2+ influx triggers more frequent Ca2+ oscillations [205]. Interestingly, in bovine eggs, increased Ca2+ influx occurs during post-ovulatory ageing; this influx seems to be responsible for the lower developmental potential of aged eggs [274]. Whether Ca2+ influx is altered in post-ovulatory aged eggs of other mammalian species is currently unknown. The rate of decrease of Ca2+ transients is also smaller in post-ovulatory aged eggs, probably because SERCA2 mRNA and protein levels are reduced [269,271,273]. Mitochondrial function is hampered in post-ovulatory aged eggs, which can affect Ca2+ oscillations in at least two ways [272,273]. ATP production, necessary for SERCA and PMCA pump activity, is reduced and Ca2+ uptake into the mitochondria is probably diminished. Alterations in these two processes would result in a decline in the eggs' ability to buffer cytosolic Ca2+ and could explain why the decrease in Ca2+ levels after each transient is slower in aged eggs.
An IP3R1 protein fragment generated by caspase-mediated cleavage may also cause altered Ca2+ signalling during post-ovulatory ageing. In Jurkat cells undergoing apoptosis, caspase-3 cleaves IP3R1, generating a 95 kDa C-terminal fragment that contains all transmembrane domains and the channel pore [275]. In vitro aged eggs contain this 95 kDa fragment, which is not present in freshly ovulated eggs. Injection of mRNA encoding this fragment into fresh eggs results in fragmentation and impaired Ca2+ oscillations, suggesting that processing of the IP3 receptor is responsible, at least in part, for altered Ca2+ signalling during ageing [276]. Interestingly, whereas Ca2+ oscillations trigger egg activation in freshly ovulated eggs, they cause apoptosis in post-ovulatory aged eggs [277]. The fusion of an in vitro aged, activated egg with a freshly ovulated egg decreases fragmentation and improves developmental potential, indicating that some cytoplasmic factors are lost during ageing. One such molecule is the anti-apoptotic factor Bcl-2, whose mRNA and protein levels are reduced in aged eggs [270].
The process of post-ovulatory ageing and its detrimental effects on fertilization and embryonic development are particularly relevant for ART. An extended period of time can occur between egg retrieval and insemination, especially in ‘rescue ICSI’ cases, in which eggs that failed to fertilize following insemination are later injected with sperm. Further elucidation of the mechanisms involved in post-ovulatory ageing is key to developing protocols to alleviate this age-dependent deterioration of fertilization and developmental potential during ART procedures.
8. Some remaining questions
Throughout the text, we pointed out areas of uncertainty or controversy regarding how Ca2+ signals are modulated at fertilization. Here, we will highlight a few new areas that could have significant impacts on these Ca2+ signals but have not been studied in depth. First, altered metabolic states have broad-ranging impacts on cellular physiology through changed dynamics of reactive oxygen species. These impacts probably include Ca2+ handling, for example, by affecting the amount of Ca2+ in intracellular stores, production of ATP necessary for active Ca2+ transport or changing downstream intracellular signalling pathways. What are the impacts of altered adult metabolic status on fertilization-induced Ca2+ signals, and do these changes impact offspring health? Second, there is voluminous support in somatic cells for the idea that membrane contact sites are regions critical for Ca2+ exchange between organelles and/or the extracellular space. Furthermore, lipid-transport proteins at membrane contact sites support the rapid transport of phospholipids, perhaps including PIP2, between the ER and other membrane-bound compartments. Both of these functions suggest a role for membrane contact sites and their associated proteins in regulating Ca2+ signals at fertilization, but to date there is no such evidence. Third, to what extent do organelles besides the ER function in regulating Ca2+ signals at fertilization? There is good evidence that Ca2+ released at fertilization impacts mitochondrial ATP production [278], but are mitochondria and/or vesicles of the endolysosomal system important for Ca2+ regulation as well? For mammals, what is the nature of the intracellular vesicles where the PIP2 substrate for IP3 synthesis is found?
Finally, although the wealth of information we have regarding Ca2+ signalling in external fertilizers was derived from generally physiological experimental conditions, almost all that we know regarding internal fertilizers, particularly mammals, has been obtained from experiments performed in culture dishes. Two exceptions to this statement are C. elegans and Drosophila in which genetic tools, combined with the relative transparency of the relevant organs, allowed in vivo observations of Ca2+ signals at fertilization [62,63]. We have long assumed that findings from in vitro studies reflect what occurs in vivo, but this assumption was strongly challenged by the recent findings that mouse sperm lacking PLCζ produce offspring despite highly abnormal patterns of Ca2+ signalling when studied in vitro [91,92]. These findings suggested that either the in vivo patterns were different from in vitro or that studies defining the necessity for a prolonged series of Ca2+ oscillations to support full-term development were only applicable in vitro. This question could be resolved with appropriate genetically encoded Ca2+ indicators combined with intravital imaging. These and other questions will keep scientists interested in understanding the Ca2+ signals responsible for the initiation of development busy for the foreseeable future.
Acknowledgements
We thank Laurinda Jaffe and Gary Bird for the critical review of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
P.S., V.S. and C.J.W. wrote the manuscript. A.M.W. created the figures and provided editorial input on the manuscript.
Competing Interests
We declare we have no competing interests.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, 1ZIAES102985. A.M.W. was supported by the University of Chicago Training Program in Developmental Biology, NIH T32 HD055164, and NIH R01GM126047 to Sally Horne-Badovinac (University of Chicago).
References
- 1.Lillie F. 1919. Problems of fertilization. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Dalcq A. 1928. Le rôle du calcium et du potassium dans l'entrée en maturation de l'oeuf de pholade (Barnea candida). Protoplasma 4, 18–44. ( 10.1007/BF01607955) [DOI] [Google Scholar]
- 3.Ridgway EB, Gilkey JC, Jaffe LF. 1977. Free calcium increases explosively in activating medaka eggs. Proc. Natl Acad. Sci. USA 74, 623–627. ( 10.1073/pnas.74.2.623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT. 1978. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J. Cell Biol. 76, 448–466. ( 10.1083/jcb.76.2.448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimomura O, Johnson FH, Saiga Y. 1962. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 59, 223–239. ( 10.1002/jcp.1030590302) [DOI] [PubMed] [Google Scholar]
- 6.Steinhardt R, Zucker R, Schatten G. 1977. Intracellular calcium release at fertilization in the sea urchin egg. Dev. Biol. 58, 185–196. ( 10.1016/0012-1606(77)90084-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. 2013. Comparative biology of sperm factors and fertilization-induced calcium signals across the animal kingdom. Mol. Reprod. Dev. 80, 787–815. ( 10.1002/mrd.22222) [DOI] [PubMed] [Google Scholar]
- 8.Dresselhaus T, Sprunck S, Wessel GM. 2016. Fertilization mechanisms in flowering plants. Curr. Biol. 26, R125–R139. ( 10.1016/j.cub.2015.12.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decuypere JP, Bultynck G, Parys JB. 2011. A dual role for Ca2+ in autophagy regulation. Cell Calcium 50, 242–250. ( 10.1016/j.ceca.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 10.Ljubojevic S, Bers DM. 2015. Nuclear calcium in cardiac myocytes. J. Cardiovasc. Pharmacol. 65, 211–217. ( 10.1097/FJC.0000000000000174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Capite J, Ng SW, Parekh AB.. 2009. Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr. Biol. 19, 853–858. ( 10.1016/j.cub.2009.03.063) [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. ( 10.1038/35036035) [DOI] [PubMed] [Google Scholar]
- 13.Krebs J, Agellon LB, Michalak M. 2015. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 460, 114–121. ( 10.1016/j.bbrc.2015.02.004) [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Evans E, Waller-Evans H. 2019. Lysosomal Ca2+ homeostasis and signaling in health and disease. Cold Spring Harb. Perspect. Biol. 12, a035311 ( 10.1101/cshperspect.a035311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Garcia S, Prado-Garcia H. 2019. Mitochondrial calcium: transport and modulation of cellular processes in homeostasis and cancer. Int. J. Oncol. 54, 1155–1167. ( 10.3892/ijo.2019.4696) [DOI] [PubMed] [Google Scholar]
- 16.Venkatachalam K, Montell C. 2007. TRP channels. Annu. Rev. Biochem. 76, 387–417. ( 10.1146/annurev.biochem.75.103004.142819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catterall W. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 555 ( 10.1146/annurev.cellbio.16.1.521) [DOI] [PubMed] [Google Scholar]
- 18.Smyth JT, DeHaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW. 2006. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim. Biophys. 1763, 1147–1160. ( 10.1016/j.bbamcr.2006.08.050) [DOI] [PubMed] [Google Scholar]
- 19.Phillips MJ, Voeltz GK. 2016. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82. ( 10.1038/nrm.2015.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soboloff J, et al. 2007. TRPC channels: integrators of multiple cellular signals. In Transient receptor potential (TRP) channels (eds Flockerzi V, Nilius B), pp. 575–591. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 21.Eichmann TO, Lass A. 2015. DAG tales: the multiple faces of diacylglycerol—stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 72, 3931–3952. ( 10.1007/s00018-015-1982-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan AJ. 2016. Ca2+ dialogue between acidic vesicles and ER. Biochem. Soc. Trans. 44, 546–553. ( 10.1042/BST20150290) [DOI] [PubMed] [Google Scholar]
- 23.Palty R, et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441. ( 10.1073/pnas.0908099107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luongo TS, et al. 2017. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 545, 93–97. ( 10.1038/nature22082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Stetina JR, Orr-Weaver TL.. 2011. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb. Perspect. Biol. 3, 1–19. ( 10.1101/cshperspect.a005553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba K, Kado RT, Jaffe LA. 1990. Development of calcium release mechanisms during starfish oocyte maturation. Dev. Biol. 140, 300–306. ( 10.1016/0012-1606(90)90080-3) [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. 1993. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev. Biol. 156, 69–79. ( 10.1006/dbio.1993.1059) [DOI] [PubMed] [Google Scholar]
- 28.Mehlmann LM, Kline D. 1994. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol. Reprod. 51, 1088–1098. ( 10.1095/biolreprod51.6.1088) [DOI] [PubMed] [Google Scholar]
- 29.Jones KT, Carroll J, Whittingham DG. 1995. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J. Biol. Chem. 270, 6671–6677. ( 10.1074/jbc.270.12.6671) [DOI] [PubMed] [Google Scholar]
- 30.Mehlmann LM, Mikoshiba K, Kline D. 1996. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev. Biol. 180, 489–498. ( 10.1006/dbio.1996.0322) [DOI] [PubMed] [Google Scholar]
- 31.Wakai T, Vanderheyden V, Yoon SY, Cheon B, Zhang N, Parys JB, Fissore RA. 2012. Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. J. Cell. Physiol. 227, 705–717. ( 10.1002/jcp.22778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tombes RM, Simerly C, Borisy GG, Schatten G. 1992. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J. Cell Biol. 117, 799–811. ( 10.1083/jcb.117.4.799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakai T, Fissore RA. 2019. Constitutive IP3R1-mediated Ca2+ release reduces Ca2+ store content and stimulates mitochondrial metabolism in mouse GV oocytes. J. Cell Sci. 132, jcs225441 ( 10.1242/jcs.225441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parrington J, Brind S, De Smedt H, Gangeswaran R, Anthony Lai F, Wojcikiewicz R, Carroll J. 1998. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: the type I isoform is upregulated in oocytes and downregulated after fertilization. Dev. Biol. 203, 451–461. ( 10.1006/dbio.1998.9071) [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Williams CJ, Kopf GS, Schultz RM. 2003. Maturation-associated increase in IP3 receptor type 1: role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev. Biol. 254, 163–171. ( 10.1016/s0012-1606(02)00049-0) [DOI] [PubMed] [Google Scholar]
- 36.Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. 1999. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol. Reprod. 60, 49–57. ( 10.1095/biolreprod60.1.49) [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki H, Chiba K, Uchiyama T, Yoshikawa F, Suzuki F, Ikeda M, Furuichi T, Mikoshiba K. 2002. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J. Biol. Chem. 277, 2763–2772. ( 10.1074/jbc.M108839200) [DOI] [PubMed] [Google Scholar]
- 38.Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. 2006. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development 133, 4355–4365. ( 10.1242/dev.02624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N, Yoon SY, Parys JB, Fissore RA. 2015. Effect of M-phase kinase phosphorylations on type 1 inositol 1,4,5-trisphosphate receptor-mediated Ca2+ responses in mouse eggs. Cell Calcium 58, 476–488. ( 10.1016/j.ceca.2015.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernhardt ML, et al. 2015. Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development 142, 2633–2640. ( 10.1242/dev.121707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaffe LA, Terasaki M. 1994. Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev. Biol. 164, 579–587. ( 10.1006/dbio.1994.1225) [DOI] [PubMed] [Google Scholar]
- 42.Stricker SA, Silva R, Smythe T. 1998. Calcium and endoplasmic reticulum dynamics during oocyte maturation and fertilization in the marine worm Cerebratulus lacteus. Dev. Biol. 203, 305–322. ( 10.1006/dbio.1998.9058) [DOI] [PubMed] [Google Scholar]
- 43.Kume S, Yamamoto A, Inoue T, Muto A, Okano H, Mikoshiba K. 1997. Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis. Dev. Biol. 182, 228–239. ( 10.1006/dbio.1996.8479) [DOI] [PubMed] [Google Scholar]
- 44.Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S. 1995. Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev. Biol. 170, 594–606. ( 10.1006/dbio.1995.1239) [DOI] [PubMed] [Google Scholar]
- 45.Mann JS, Lowther KM, Mehlmann LM. 2010. Reorganization of the endoplasmic reticulum and development of Ca2+ release mechanisms during meiotic maturation of human oocytes1. Biol. Reprod. 83, 578–583. ( 10.1095/biolreprod.110.085985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehlmann L, Terasaki M, Jaffe L, Kline D. 1995. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev. Biol. 170, 607–615. ( 10.1006/dbio.1995.1240) [DOI] [PubMed] [Google Scholar]
- 47.Ponya Z, Kistof Z, Ciampolini F, Faleri C, Cresti M. 2004. Structural change in the endoplasmic reticulum during the in situ development and in vitro fertilisation of wheat egg cells. Sex Plant Reprod. 17, 177–188. ( 10.1007/s00497-004-0226-8) [DOI] [Google Scholar]
- 48.FitzHarris G, Marangos P, Carroll J. 2007. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev. Biol. 305, 133–144. ( 10.1016/j.ydbio.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 49.Kan R, Yurttas P, Kim B, Jin M, Wo L, Lee B, Gosden R, Coonrod SA. 2011. Regulation of mouse oocyte microtubule and organelle dynamics by PADI6 and the cytoplasmic lattices. Dev. Biol. 350, 311–322. ( 10.1016/j.ydbio.2010.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim B, Zhang X, Kan R, Cohen R, Mukai C, Travis AJ, Coonrod SA. 2014. The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes. Dev. Biol. 386, 331–339. ( 10.1016/j.ydbio.2013.12.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulware MJ, Marchant JS. 2005. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr. Biol. 15, 765–770. ( 10.1016/j.cub.2005.02.065) [DOI] [PubMed] [Google Scholar]
- 52.Stricker SA. 2006. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Semin. Cell Dev. Biol. 17, 303–313. ( 10.1016/j.semcdb.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 53.Moreno RD, Schatten G, Ramalho-Santos J. 2002. Golgi apparatus dynamics during mouse oocyte in vitro maturation: effect of the membrane trafficking inhibitor brefeldin A. Biol. Reprod. 66, 1259–1266. ( 10.1095/biolreprod66.5.1259) [DOI] [PubMed] [Google Scholar]
- 54.Payne C, Schatten G. 2003. Golgi dynamics during meiosis are distinct from mitosis and are coupled to endoplasmic reticulum dynamics until fertilization. Dev. Biol. 264, 50–63. ( 10.1016/j.ydbio.2003.08.004) [DOI] [PubMed] [Google Scholar]
- 55.Roth MG. 1999. Inheriting the Golgi. Cell 99, 559–562. ( 10.1016/s0092-8674(00)81544-5) [DOI] [PubMed] [Google Scholar]
- 56.Wang WA, Agellon LB. 2019. Organellar calcium handling in the cellular reticular network. Cold Spring Harb. Perspect. Biol. 11, 1–20. ( 10.1101/cshperspect.a038265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. 2012. PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P 2. Mol. Biol. Cell 23, 371–380. ( 10.1091/mbc.E11-08-0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Blerkom J. 1991. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc. Natl Acad. Sci. USA 88, 5031–5035. ( 10.1073/pnas.88.11.5031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalton CM, Carroll J. 2013. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J. Cell Sci. 126, 2955–2964. ( 10.1242/jcs.128744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. 2010. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J. Cell. Physiol. 224, 672–680. ( 10.1002/jcp.22171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stricker SA. 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 211, 157–176. ( 10.1006/dbio.1999.9340) [DOI] [PubMed] [Google Scholar]
- 62.Takayama J, Onami S. 2016. The sperm TRP-3 channel mediates the onset of a Ca2+ wave in the fertilized C. elegans oocyte. Cell Rep. 15, 625–637. ( 10.1016/j.celrep.2016.03.040) [DOI] [PubMed] [Google Scholar]
- 63.Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF. 2015. Calcium waves occur as Drosophila oocytes activate. Proc. Natl Acad. Sci. USA 112, 791–796. ( 10.1073/pnas.1420589112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tosca L, Glass R, Bronchain O, Philippe L, Ciapa B. 2012. PLCγ, G-protein of the Gαq type and cADPr pathway are associated to trigger the fertilization Ca2+ signal in the sea urchin egg. Cell Calcium 52, 388–396. ( 10.1016/j.ceca.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 65.Sharma D, Kinsey WH. 2008. Regionalized calcium signaling in zebrafish fertilization. Int. J. Dev. Biol. 52, 561–570. ( 10.1387/ijdb.072523ds) [DOI] [PubMed] [Google Scholar]
- 66.Sato KI, Tokmakov AA, Iwasaki T, Fukami Y. 2000. Tyrosine kinase-dependent activation of phospholipase Cγ is required for calcium transient in Xenopus egg fertilization. Dev. Biol. 224, 453–469. ( 10.1006/dbio.2000.9782) [DOI] [PubMed] [Google Scholar]
- 67.Harada Y, Kawazoe M, Eto Y, Ueno S, Iwao Y. 2011. The Ca2+ increase by the sperm factor in physiologically polyspermic newt fertilization: its signaling mechanism in egg cytoplasm and the species-specificity. Dev. Biol. 351, 266–276. ( 10.1016/j.ydbio.2011.01.003) [DOI] [PubMed] [Google Scholar]
- 68.Bernhardt ML, Padilla-Banks E, Stein P, Zhang Y, Williams CJ. 2017. Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs. Cell Calcium 65, 63–72. ( 10.1016/j.ceca.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephano JL, Gould MC. 1997. The intracellular calcium increase at fertilization in Urechis caupo oocytes: activation without waves. Dev. Biol. 191, 53–68. ( 10.1006/dbio.1997.8709) [DOI] [PubMed] [Google Scholar]
- 70.Galione A, McDougall A, Busa WB, Willmott N, Gillot I, Whitaker M. 1993. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science 261, 348–352. ( 10.1126/science.8392748) [DOI] [PubMed] [Google Scholar]
- 71.Iwao Y. 2012. Egg activation in physiological polyspermy. Reproduction 144, 11–22. ( 10.1530/REP-12-0104) [DOI] [PubMed] [Google Scholar]
- 72.Ueno T, Ohgami T, Harada Y, Ueno S, Iwao Y. 2014. Egg activation in physiologically polyspermic newt eggs: involvement of IP3 receptor, PLCγ, and microtubules in calcium wave induction. Int. J. Dev. Biol. 58, 315–323. ( 10.1387/ijdb.130333yi) [DOI] [PubMed] [Google Scholar]
- 73.Stricker SA. 1996. Repetitive calcium waves induced by fertilization in the nemertean worm Cerebratulus lacteus. Dev. Biol. 176, 243–263. ( 10.1006/dbio.1996.0131) [DOI] [PubMed] [Google Scholar]
- 74.Stricker SA, Cline C, Goodrich D. 2013. Oocyte maturation and fertilization in marine nemertean worms: using similar sorts of signaling pathways as in mammals, but often with differing results. Biol. Bull. 224, 137–155. ( 10.1086/BBLv224n3p137) [DOI] [PubMed] [Google Scholar]
- 75.Deguchi R, Osanai K, Morisawa M. 1996. Extracellular Ca2+ entry and Ca2+ release from inositol 1,4,5-trisphosphate-sensitive stores function at fertilization in oocytes of the marine bivalve Mytilus edulis. Development 122, 3651–3660. [DOI] [PubMed] [Google Scholar]
- 76.Kline D, Kline JT. 1992. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J. Biol. Chem. 267, 17 624–17 630. [PubMed] [Google Scholar]
- 77.Igusa Y, Miyazaki S-I, Yamashita N. 1983. Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J. Physiol. 340, 633–647. ( 10.1113/jphysiol.1983.sp014784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun FZ, Hoyland J, Huang X, Mason W, Moor RM. 1992. A comparison of intracellular changes in porcine eggs after fertilization and electroactivation. Development 115, 947–956. [DOI] [PubMed] [Google Scholar]
- 79.Nakada K, Shiraishi K, Miyazaki S, Mizuno J, Endo K. 1995. Initiation, persistence, and cessation of the series of intracellular Ca2+ responses during fertilization of bovine eggs. J. Reprod. Dev. 41, 77–84. ( 10.1262/jrd.41.77) [DOI] [Google Scholar]
- 80.Bedford SJ, Kurokawa M, Hinrichs K, Fissore RA. 2004. Patterns of intracellular calcium oscillations in horse oocytes fertilized by intracytoplasmic sperm injection: possible explanations for the low success of this assisted reproduction technique in the horse. Biol. Reprod. 70, 936–944. ( 10.1095/biolreprod.103.021485) [DOI] [PubMed] [Google Scholar]
- 81.Swann K, Ozil JP. 1994. Dynamics of the calcium signal that triggers mammalian egg activation. Int. Rev. Cytol. 152, 183–222. ( 10.1016/S0074-7696(08)62557-7) [DOI] [PubMed] [Google Scholar]
- 82.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. 2002. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev. Biol. 250, 280–291. ( 10.1016/S0012-1606(02)90788-8) [DOI] [PubMed] [Google Scholar]
- 83.Ozil JP, Huneau D. 2001. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development 128, 917–928. [DOI] [PubMed] [Google Scholar]
- 84.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. 2005. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev. Biol. 282, 39–54. ( 10.1016/j.ydbio.2005.02.035) [DOI] [PubMed] [Google Scholar]
- 85.Tóth S, Huneau D, Banrezes B, Ozil JP. 2006. Egg activation is the result of calcium signal summation in the mouse. Reproduction 131, 27–34. ( 10.1530/rep.1.00764) [DOI] [PubMed] [Google Scholar]
- 86.Miao YL, Williams CJ. 2012. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol. Reprod. Dev. 79, 742–756. ( 10.1002/mrd.22078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders JR, Swann K. 2016. Molecular triggers of egg activation at fertilization in mammals. Reproduction 152, R41–R50. ( 10.1530/REP-16-0123) [DOI] [PubMed] [Google Scholar]
- 88.Slusarski DC, Pelegri F. 2007. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev. Biol. 307, 1–13. ( 10.1016/j.ydbio.2007.04.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernhardt ML, et al. 2018. TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proc. Natl Acad. Sci. USA 115, E10370–E10378. ( 10.1073/pnas.1810422115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP. 2011. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS ONE 6, e20388 ( 10.1371/journal.pone.0029388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hachem A, et al. 2017. Plcζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 144, 2914–2924. ( 10.1242/dev.150227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. 2018. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 8, 1–10. ( 10.1038/s41598-018-19497-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner PR, Sheetz MP, Jaffe LA. 1984. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature 310, 414–415. ( 10.1038/310414a0) [DOI] [PubMed] [Google Scholar]
- 94.Whitaker M, Irvine R.. 1984. Inositol 1,4,5-trisphosphate microinjection activates sea urchin eggs. Nature 312, 636–639. ( 10.1083/jcb.106.2.345) [DOI] [Google Scholar]
- 95.Busa WB, Nuccitelli R. 1985. An elevated free cytosolic Ca2+ wave follows fertilization in eggs of the frog, Xenopus laevis. J. Cell Biol. 100, 1325–1329. ( 10.1083/jcb.100.4.1325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyazaki S. 1988. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J. Cell Biol. 106, 345–353. ( 10.1083/jcb.106.2.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurasawa S, Schultz RM, Kopf GS. 1989. Egg-induced modifications of the zona pellucida of mouse eggs: effects of microinjected inositol 1,4,5-trisphosphate. Dev. Biol. 133, 295–304. ( 10.1016/0012-1606(89)90320-5) [DOI] [PubMed] [Google Scholar]
- 98.Thomas TW, Eckberg WR, Dubé F, Galione A. 1998. Mechanisms of calcium release and sequestration in eggs of Chaetopterus pergamentaceus. Cell Calcium 24, 285–292. ( 10.1016/S0143-4160(98)90052-5) [DOI] [PubMed] [Google Scholar]
- 99.Lee HC, Aarhus R, Walseth TF. 1993. Calcium mobilization by dual receptors during fertilization of sea urchin eggs. Science 261, 352–355. ( 10.1126/science.8392749) [DOI] [PubMed] [Google Scholar]
- 100.Chini EN, Beers KW, Dousa TP. 1995. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J. Biol. Chem. 270, 3216–3223. ( 10.1074/jbc.270.7.3216) [DOI] [PubMed] [Google Scholar]
- 101.Lim D, Kyozuka K, Gragnaniello G, Carafoli E, Santella L. 2001. NAADP+ initiates the Ca2+ response during fertilization of starfish oocytes. FASEB J. 15, 2257–2267. ( 10.1096/fj.01-0157com) [DOI] [PubMed] [Google Scholar]
- 102.Moccia F, Lim D, Kyozuka K, Santella L. 2004. NAADP triggers the fertilization potential in starfish oocytes. Cell Calcium 36, 515–524. ( 10.1016/j.ceca.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki S, Ohmori H, Sasaki S. 1975. Action potential and non-linear current–voltage relation in starfish oocytes. J. Physiol. 246, 37–54. ( 10.1113/jphysiol.1975.sp010879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen SS, Buck WR. 1993. Sources of calcium in sea urchin eggs during the fertilization response. Dev. Biol. 157, 157–169. ( 10.1006/dbio.1993.1120) [DOI] [PubMed] [Google Scholar]
- 105.Vasilev F, Limatola N, Chun JT, Santella L. 2019. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 15, 757–775. ( 10.7150/ijbs.28461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deguchi R. 2007. Fertilization causes a single Ca2+ increase that fully depends on Ca2+ influx in oocytes of limpets (Phylum Mollusca, Class Gastropoda). Dev. Biol. 304, 652–663. ( 10.1016/j.ydbio.2007.01.017) [DOI] [PubMed] [Google Scholar]
- 107.Jaffe LF. 1990. The roles of intermembrane calcium in polarizing and activating eggs. In Mechanisms of fertilization: plants to humans (ed. Dale B.), pp. 389–417. Berlin, Germany: Springer. [Google Scholar]
- 108.Hu Q, Wolfner MF. 2019. The Drosophila Trpm channel mediates calcium influx during egg activation. Proc. Natl Acad. Sci. USA 116, 18 994–19 000. ( 10.1073/pnas.1906967116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee KW, Webb SE, Miller AL. 1999. A wave of free cytosolic calcium traverses zebrafish eggs on activation. Dev. Biol. 214, 168–180. ( 10.1006/dbio.1999.9396) [DOI] [PubMed] [Google Scholar]
- 110.Lindsay LAL, Hertzler PL, Clark WH. 1992. Extracellular Mg2+ induces an intracellular Ca2+ wave during oocyte activation in the marine shrimp Sicyonia ingentis. Dev. Biol. 152, 94–102. ( 10.1016/0012-1606(92)90159-E) [DOI] [PubMed] [Google Scholar]
- 111.Kadamur G, Ross EM. 2013. Mammalian phospholipase C. Annu. Rev. Physiol. 75, 127–154. ( 10.1146/annurev-physiol-030212-183750) [DOI] [PubMed] [Google Scholar]
- 112.Carroll DJ, Ramarao CS, Mehlmann LM, Roche S, Terasaki M, Jaffe LA. 1997. Calcium release at fertilization in starfish eggs is mediated by phospholipase Cγ. J. Cell Biol. 138, 1303–1311. ( 10.1083/jcb.138.6.1303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Runft LL, Jaffe LA. 2000. Sperm extract injection into ascidian eggs signals Ca2+ release by the same pathway as fertilization. Development 127, 3227–3236. [DOI] [PubMed] [Google Scholar]
- 114.Giusti AF, O'Neill FJ, Yamasu K, Foltz KR, Jaffe LA. 2003. Function of a sea urchin egg Src family kinase in initiating Ca2+ release at fertilization. Dev. Biol. 256, 367–378. ( 10.1016/S0012-1606(03)00043-5) [DOI] [PubMed] [Google Scholar]
- 115.Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. 1998. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 203, 221–232. ( 10.1006/dbio.1998.9051) [DOI] [PubMed] [Google Scholar]
- 116.Dale B, DeFelice L, Ehrenstein G. 1985. Injection of a soluble sperm fraction into sea-urchin eggs triggers the cortical reaction. Experientia 41, 1068–1070. ( 10.1007/BF01952148) [DOI] [PubMed] [Google Scholar]
- 117.Dale B. 1988. Primary and secondary messengers in the activation of ascidian eggs. Exp. Cell Res. 177, 205–211. ( 10.1080/03078698.1994.9674073) [DOI] [PubMed] [Google Scholar]
- 118.Swann K. 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110, 1295–1302. [DOI] [PubMed] [Google Scholar]
- 119.Stice SL, Robl JM. 1990. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol. Reprod. Dev. 25, 272–280. ( 10.1002/mrd.1080250309) [DOI] [PubMed] [Google Scholar]
- 120.Longo FJ, Lynn JW, McCulloh DH, Chambers EL. 1986. Correlative ultrastructural and electrophysiological studies of sperm–egg interactions of the sea urchin, Lytechinus variegatus. Dev. Biol. 118, 155–166. ( 10.1016/0012-1606(86)90083-7) [DOI] [PubMed] [Google Scholar]
- 121.McCulloh DH, Chambers EL. 1992. Fusion of membranes during fertilization: Increases of the sea urchin egg's membrane capacitance and membrane conductance at the site of contact with the sperm. J. Gen. Physiol. 99, 137–175. ( 10.1085/jgp.99.2.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawrence Y, Whitaker M, Swann K. 1997. Sperm–egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development 124, 233–241. [DOI] [PubMed] [Google Scholar]
- 123.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. 2002. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129, 3533–3544. [DOI] [PubMed] [Google Scholar]
- 124.Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. 2005. Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization1. Biol. Reprod. 72, 992–996. ( 10.1095/biolreprod.104.036244) [DOI] [PubMed] [Google Scholar]
- 125.Yoon SY, et al. 2008. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J. Clin. Invest. 118, 3671–3681. ( 10.1172/JCI36942.the) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heytens E, et al. 2009. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum. Reprod. 24, 2417–2428. ( 10.1093/humrep/dep207) [DOI] [PubMed] [Google Scholar]
- 127.Kashir J, et al. 2012. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum. Reprod. 27, 222–231. ( 10.1093/humrep/der384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nomikos M, Elgmati K, Theodoridou M, Calver BL, Cumbes B, Nounesis G, Swann K, Lai FA. 2011. Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP2 hydrolysis activity of sperm PLCζ. Biochem. J. 434, 211–217. ( 10.1042/BJ20101772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Escoffier J, et al. 2016. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum. Mol. Genet. 25, 878–891. ( 10.1093/hmg/ddv617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferrer-Vaquer A, Barragan M, Freour T, Vernaeve V, Vassena R. 2016. PLCζ sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J. Assist. Reprod. Genet. 33, 747–756. ( 10.1007/s10815-016-0718-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torra-Massana M, Cornet-Bartolomé D, Barragán M, Durban M, Ferrer-Vaquer A, Zambelli F, Rodriguez A, Oliva R, Vassena R. 2019. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum. Reprod. 34, 1494–1504. ( 10.1093/humrep/dez094) [DOI] [PubMed] [Google Scholar]
- 132.Dai J, et al. 2020. Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCζ in sperm. Clin. Genet. 97, 347–351. ( 10.1111/cge.13636) [DOI] [PubMed] [Google Scholar]
- 133.Halet G, Tunwell R, Balla T, Swann K, Carroll J. 2002. The dynamics of plasma membrane Ptdlns(4,5)P2 at fertilization of mouse eggs. J. Cell Sci. 115, 2139–2149. [DOI] [PubMed] [Google Scholar]
- 134.Sanders JR, Ashley B, Moon A, Woolley TE, Swann K. 2018. PLCζ induced Ca2+ oscillations in mouse eggs involve a positive feedback cycle of Ca2+ induced InsP3 formation from cytoplasmic PIP2. Front. Cell Dev. Biol. 6, 36 ( 10.3389/fcell.2018.00036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, Lai FA. 2011. Phospholipase Cζ binding to PtdIns(4,5)P 2 requires the XY-linker region. J. Cell Sci. 124, 2582–2590. ( 10.1242/jcs.083485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nomikos M, et al. 2015. Essential role of the EF-hand domain in targeting sperm phospholipase Cζ to membrane phosphatidylinositol 4,5-bisphosphate (PIP2). J. Biol. Chem. 290, 29 519–29 530. ( 10.1074/jbc.M115.658443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nomikos M, et al. 2017. Male infertility-linked point mutation reveals a vital binding role for the C2 domain of sperm PLCζ. Biochem. J. 474, 1003–1016. ( 10.1042/BCJ20161057) [DOI] [PubMed] [Google Scholar]
- 138.Nomikos M, Elgmati K, Theodoridou M, Georgilis A, Gonzalez-Garcia JR, Nounesis G, Swann K, Lai FA. 2011. Novel regulation of PLCζ activity via its XY-linker. Biochem. J. 438, 427–432. ( 10.1042/BJ20110953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. 2004. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 279, 10 408–10 412. ( 10.1074/jbc.M313801200) [DOI] [PubMed] [Google Scholar]
- 140.Williams CJ, Schultz RM, Kopf GS. 1992. Role of G proteins in mouse egg activation: stimulatory effects of acetylcholine on the ZP2 to ZP2f conversion and pronuclear formation in eggs expressing a functional m1 muscarinic receptor. Dev. Biol. 151, 288–296. ( 10.1016/0012-1606(92)90233-7) [DOI] [PubMed] [Google Scholar]
- 141.Moore G, Kopf G, Schultz R. 1993. Complete mouse egg activation in the absence of sperm by stimulation of an exogenous G protein-coupled receptor. Dev. Biol. 159, 669–678. ( 10.1006/dbio.1993.1273) [DOI] [PubMed] [Google Scholar]
- 142.Williams CJ, Mehlmann LM, Jaffe LA, Kopf GS, Schultz RM. 1998. Evidence that G(q) family G proteins do not function in mouse egg activation at fertilization. Dev. Biol. 198, 116–127. ( 10.1006/dbio.1998.8892) [DOI] [PubMed] [Google Scholar]
- 143.Igarashi H, Knott JG, Schultz RM, Williams CJ. 2007. Alterations of PLCβ1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev. Biol. 312, 321–330. ( 10.1016/j.ydbio.2007.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matsu-ura T, Shirakawa H, Suzuki KGN, Miyamoto A, Sugiura K, Michikawa T, Kusumi A, Mikoshiba K. 2019. Dual-FRET imaging of IP3 and Ca2+ revealed Ca2+ -induced IP3 production maintains long lasting Ca2+ oscillations in fertilized mouse eggs. Sci. Rep. 9, 1–11. ( 10.1038/s41598-019-40931-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paknejad N, Hite RK. 2018. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat. Struct. Mol. Biol. 25, 660–668. ( 10.1038/s41594-018-0089-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Finch EA, Turner TJ, Goldin SM. 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252, 443–446. ( 10.1126/science.2017683) [DOI] [PubMed] [Google Scholar]
- 147.Marchant JS, Taylor CW. 1997. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 7, 510–518. ( 10.1016/S0960-9822(06)00222-3) [DOI] [PubMed] [Google Scholar]
- 148.Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE, Van Petegem F, Yule DI. 2016. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 9, 1–13. ( 10.1126/scisignal.aad6281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dupont G, Dumollard R. 2004. Simulation of calcium waves in ascidian eggs: insights into the origin of the pacemaker sites and the possible nature of the sperm factor. J. Cell Sci. 117, 4313–4323. ( 10.1242/jcs.01278) [DOI] [PubMed] [Google Scholar]
- 150.Berridge MJ. 2016. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 96, 1261–1296. ( 10.1152/physrev.00006.2016) [DOI] [PubMed] [Google Scholar]
- 151.Prole DL, Taylor CW. 2019. Structure and function of IP3 receptors. Cold Spring Harb. Perspect. Biol. 11, a035063 ( 10.1101/cshperspect.a035063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Supattapone S, Danoff SK, Theibert A, Joseph SK, Steiner J, Snyder SH. 1988. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc. Natl Acad. Sci. USA 85, 8747–8750. ( 10.1073/pnas.85.22.8747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. 1991. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc. Natl Acad. Sci. USA 88, 2232–2235. ( 10.1073/pnas.88.6.2232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. 2009. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta 1793, 959–970. ( 10.1038/jid.2014.371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. 1992. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257, 251–255. ( 10.1126/science.1321497) [DOI] [PubMed] [Google Scholar]
- 156.Xu Z, Kopf GS, Schultz RM. 1994. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development 120, 1851–1859. [DOI] [PubMed] [Google Scholar]
- 157.Albrieux M, Sardet C, Villaz M. 1997. The two intracellular Ca2+ release channels, ryanodine receptor and inositol 1,4,5-trisphosphate receptor, play different roles during fertilization in ascidians. Dev. Biol. 189, 174–185. ( 10.1006/dbio.1997.8674) [DOI] [PubMed] [Google Scholar]
- 158.Yue C, White KL, Reed WA, Bunch TD. 1995. The existence of inositol 1,4,5-trisphosphate and ryanodine receptors in mature bovine oocytes. Development 121, 2645–2654. [DOI] [PubMed] [Google Scholar]
- 159.Ayabe T, Kopf GS, Schultz RM. 1995. Regulation of mouse egg activation: presence of ryanodine receptors and effects of microinjected ryanodine and cyclic ADP ribose on uninseminated and inseminated eggs. Development 121, 2233–2244. [DOI] [PubMed] [Google Scholar]
- 160.Macháty Z, Funahashi H, Day BN, Prather RS. 1997. Developmental changes in the intracellular Ca2+ release mechanisms in porcine oocytes. Biol. Reprod. 56, 921–930. ( 10.1095/biolreprod56.4.921) [DOI] [PubMed] [Google Scholar]
- 161.Sousa M, Barros A, Tesarik J. 1996. The role of ryanodine-sensitive Ca2+ stores in the Ca2+ oscillation machine of human oocytes. Mol. Hum. Reprod. 2, 265–272. ( 10.1093/molehr/2.4.265) [DOI] [PubMed] [Google Scholar]
- 162.Patel S, Joseph SK, Thomas AP. 1999. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 25, 247–264. ( 10.1054/ceca.1999.0021) [DOI] [PubMed] [Google Scholar]
- 163.Kume S, Muto A, Aruga J, Nakagawa T, Michikawa T, Furuichi T, Nakade S, Okano H, Mikoshiba K. 1993. The Xenopus IP3 receptor: structure, function, and localization in oocytes and eggs. Cell 73, 555–570. ( 10.1016/0092-8674(93)90142-D) [DOI] [PubMed] [Google Scholar]
- 164.Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB. 1999. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J. Mol. Biol. 294, 467–476. ( 10.1006/jmbi.1999.3229) [DOI] [PubMed] [Google Scholar]
- 165.Taylor CW, Genazzani AA, Morris SA. 1999. Expression of inositol trisphosphate receptors. Cell Calcium 26, 237–251. ( 10.1054/ceca.1999.0090) [DOI] [PubMed] [Google Scholar]
- 166.Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. 1995. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J. Biol. Chem. 270, 14 700–14 704. ( 10.1074/jbc.270.24.14700) [DOI] [PubMed] [Google Scholar]
- 167.Südhof TC, Newton CL, Archer BT, Ushkaryov YA, Mignery GA. 1991. Structure of a novel InsP3 receptor. EMBO J. 10, 3199–3206. ( 10.1002/j.1460-2075.1991.tb04882.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Taylor CW. 2017. Regulation of IP3 receptors by cyclic AMP. Cell Calcium 63, 48–52. ( 10.1016/j.ceca.2016.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Soulsby MD, Wojcikiewicz RJH. 2007. Calcium mobilization via type III inositol 1,4,5-trisphosphate receptors is not altered by PKA-mediated phosphorylation of serines 916, 934, and 1832. Cell Calcium 42, 261–270. ( 10.1016/j.ceca.2006.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He CL, Damiani P, Ducibella T, Takahashi M, Tanzawa K, Parys JB, Fissore RA. 1999. Isoforms of the inositol 1,4,5-trisphosphate receptor are expressed in bovine oocytes and ovaries: the type-1 isoform is down-regulated by fertilization and by injection of adenophostin A. Biol. Reprod. 61, 935–943. ( 10.1095/biolreprod61.4.935) [DOI] [PubMed] [Google Scholar]
- 171.Galione A. 2019. NAADP receptors. Cold Spring Harb. Perspect. Biol. 11, a035071 ( 10.1101/cshperspect.a035071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. 2002. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708. ( 10.1016/S0092-8674(02)01082-6) [DOI] [PubMed] [Google Scholar]
- 173.Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. 2012. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem. 287, 2308–2315. ( 10.1074/jbc.M111.306563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Churchill GC, Galione A. 2001. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 20, 2666–2671. ( 10.1093/emboj/20.11.2666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morgan AJ, Davis LC, Wagner SKTY, Lewis AM, Parrington J, Churchill GC, Galione A. 2013. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 200, 789–805. ( 10.1083/jcb.201204078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. 2010. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 285, 38 511–38 516. ( 10.1074/jbc.M110.162073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brini M, Carafoli E. 2009. Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378. ( 10.1152/physrev.00032.2008) [DOI] [PubMed] [Google Scholar]
- 178.Chen J, Sitsel A, Benoy V, Sepúlveda MR, Vangheluwe P. 2020. Primary active Ca2+ transport systems in health and disease. Cold Spring Harb. Perspect. Biol. 12, a035113 ( 10.1101/cshperspect.a035113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.El-Jouni W, Jang B, Haun S, Machaca K. 2005. Calcium signaling differentiation during Xenopus oocyte maturation. Dev. Biol. 288, 514–525. ( 10.1016/j.ydbio.2005.10.034) [DOI] [PubMed] [Google Scholar]
- 180.Camacho P, Lechleiter J. 1993. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260, 226–229. ( 10.1126/science.8385800) [DOI] [PubMed] [Google Scholar]
- 181.Wakai T, Zhang N, Vangheluwe P, Fissore RA. 2013. Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J. Cell Sci. 126, 5714–5724. ( 10.1242/jcs.136549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Choquette D, Hakim G, Filoteo AG, Plishker GA, Bostwick JR, Penniston JT. 1984. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem. Biophys. Res. Commun. 125, 908–915. ( 10.1016/0006-291X(84)91369-X) [DOI] [PubMed] [Google Scholar]
- 183.Missiaen L, Raeymaekers L, Wuytack F, Vrolix M, De Smedt H, Casteels R.. 1989. Phospholipid-protein interactions of the plasma-membrane Ca2+-transporting ATPase. Biochem. J. 263, 687–694. ( 10.1042/bj2630687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Penniston JT, Padányi R, Pászty K, Varga K, Enyedi L, Enyedi A. 2014. Apart from its known function, the plasma membrane Ca2+ atpase can regulate Ca2+ signaling by controlling phosphatidylinositol 4,5-bisphosphate levels. J. Cell Sci. 127, 72–84. ( 10.1242/jcs.132548) [DOI] [PubMed] [Google Scholar]
- 185.Miao Y-L, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. 2012. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc. Natl Acad. Sci. USA 109, 4169–5174. ( 10.1073/pnas.1112333109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zeng F, Baldwin DA, Schultz RM. 2004. Transcript profiling during preimplantation mouse development. Dev. Biol. 272, 483–496. ( 10.1016/j.ydbio.2004.05.018) [DOI] [PubMed] [Google Scholar]
- 187.Pan H, O'Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. 2005. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev. Biol. 286, 493–506. ( 10.1016/j.ydbio.2005.08.023) [DOI] [PubMed] [Google Scholar]
- 188.Pan H, Ma P, Zhu W, Schultz RM. 2008. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 316, 397–407. ( 10.1016/j.ydbio.2008.01.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Verkhratsky A, Trebak M, Perocchi F, Khananshvili D, Sekler I. 2018. Crosslink between calcium and sodium signalling. Exp. Physiol. 103, 157–169. ( 10.1113/EP086534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Philipson KD, Nicoll DA. 2000. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62, 111–133. ( 10.1146/annurev.physiol.62.1.111) [DOI] [PubMed] [Google Scholar]
- 191.Igusa Y, Miyazaki S. 1983. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J. Physiol. 340, 611–632. ( 10.1113/jphysiol.1983.sp014783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pepperell JR, Kommineni K, Buradagunta S, Smith PJS, Keefe DL. 1999. Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol. Reprod. 60, 1137–1143. ( 10.1095/biolreprod60.5.1137) [DOI] [PubMed] [Google Scholar]
- 193.Carroll J. 2000. Na+-Ca2+ exchange in mouse oocytes: modifications in the regulation of intracellular free Ca2+ during oocyte maturation. J. Reprod. Fertil. 118, 337–342. ( 10.1530/reprod/118.2.337) [DOI] [PubMed] [Google Scholar]
- 194.Cui W, Zhang J, Zhang C-X, Jiao G-Z, Zhang M, Wang T-Y, Luo M-J, Tan J-H. 2013. Control of spontaneous activation of rat oocytes by regulating plasma membrane Na+/Ca2+ exchanger activities. Biol. Reprod. 88, 160 ( 10.1095/biolreprod.113.108266) [DOI] [PubMed] [Google Scholar]
- 195.Solís-Garrido LM, Pintado AJ, Andrés-Mateos E, Figueroa M, Matute C, Montiel C. 2004. Cross-talk between native plasmalemmal Na+/Ca2+ exchanger and inositol 1,4,5-trisphosphate-sensitive Ca2+ internal store in Xenopus oocytes. J. Biol. Chem. 279, 52 414–52 424. ( 10.1074/jbc.M408872200) [DOI] [PubMed] [Google Scholar]
- 196.El-Jouni W, Haun S, Machaca K. 2008. Internalization of plasma membrane Ca2+-ATPase during Xenopus oocyte maturation. Dev. Biol. 324, 99–107. ( 10.1016/j.ydbio.2008.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nakazawa T, Asami K, Shoger R, Fujiwara A, Yasumasu I. 1970. Ca2+ uptake, H+ ejection and respiration in sea urchin eggs on fertilization. Exp. Cell Res. 63, 143–146. ( 10.1016/0014-4827(70)90342-3) [DOI] [PubMed] [Google Scholar]
- 198.Johnston RN, Paul M. 1977. Calcium influx following fertilization of Urechis caupo eggs. Dev. Biol. 57, 364–374. ( 10.1016/0012-1606(77)90221-4) [DOI] [PubMed] [Google Scholar]
- 199.Parekh AB, Putney JW. 2005. Store-operated calcium channels. Physiol. Rev. 85, 757–810. ( 10.1152/physrev.00057.2003) [DOI] [PubMed] [Google Scholar]
- 200.Feske S, Wulff H, Skolnik EY. 2015. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 33, 291–353. ( 10.1146/annurev-immunol-032414-112212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Smyth JT, Putney JW. 2012. Regulation of store-operated calcium entry during cell division. Biochem. Soc. Trans. 40, 119–123. ( 10.1042/BST20110612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yu F, Sun L, Machaca K. 2009. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc. Natl Acad. Sci. USA 106, 17 401–17 406. ( 10.1073/pnas.0904651106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gómez-Fernández C, Pozo-Guisado E, Gañán-Parra M, Perianes MJ, Alvarez IS, Martín-Romero FJ. 2009. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction 138, 211–221. ( 10.1530/REP-09-0126) [DOI] [PubMed] [Google Scholar]
- 204.Cheon B, Lee HC, Wakai T, Fissore RA. 2013. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol. Biol. Cell 24, 1396–1410. ( 10.1091/mbc.E13-01-0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. 2013. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem. Biophys. Res. Commun. 430, 60–65. ( 10.1016/j.bbrc.2012.11.024) [DOI] [PubMed] [Google Scholar]
- 206.Lee B, Palermo G, Machaca K. 2013. Downregulation of store-operated Ca2+ entry during mammalian meiosis is required for the egg-to-embryo transition. J. Cell Sci. 126, 1672–1681. ( 10.1242/jcs.121335) [DOI] [PubMed] [Google Scholar]
- 207.Lee K, Wang C, Machaty Z. 2012. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev. Biol. 367, 154–162. ( 10.1016/j.ydbio.2012.04.028) [DOI] [PubMed] [Google Scholar]
- 208.Wang C, Lee K, Gajdócsi E, Papp ÁB, Machaty Z. 2012. Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Dev. Biol. 365, 414–423. ( 10.1016/j.ydbio.2012.03.007) [DOI] [PubMed] [Google Scholar]
- 209.Capiod T. 2011. Cell proliferation, calcium influx and calcium channels. Biochimie 93, 2075–2079. ( 10.1016/j.biochi.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 210.Hagiwara S, Jaffe LA. 1979. Electrical properties of egg cell membranes. Annu. Rev. Biophys. Bioeng. 8, 385–416. ( 10.1146/annurev.bb.08.060179.002125) [DOI] [PubMed] [Google Scholar]
- 211.Nakano T, Kyozuka K, Deguchi R. 2008. Novel two-step Ca2+ increase and its mechanisms and functions at fertilization in oocytes of the annelidan worm Pseudopotamilla occelata. Dev. Growth Differ. 50, 365–379. ( 10.1111/j.1440-169X.2008.01022.x) [DOI] [PubMed] [Google Scholar]
- 212.Gould MC, Stephano JL, De Ortz-Barrn BJ, Prez-Quezada I.. 2001. Maturation and fertilization in Lottia gigantea oocytes: intracellular pH, Ca2+, and electrophysiology. J. Exp. Zool. 290, 411–420. ( 10.1002/jez.1082) [DOI] [PubMed] [Google Scholar]
- 213.Miyazaki S, Igusa Y. 1981. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290, 702–704. ( 10.1038/290702a0) [DOI] [PubMed] [Google Scholar]
- 214.Jaffe LA, Sharp AP, Wolf DP. 1983. Absence of an electrical polyspermy block in the mouse. Dev. Biol. 96, 317–323. ( 10.1016/0012-1606(83)90168-9) [DOI] [PubMed] [Google Scholar]
- 215.Peres A. 1987. The calcium current of mouse egg measured in physiological calcium and temperature conditions. J. Physiol. 391, 573–588. ( 10.1113/jphysiol.1987.sp016757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Day ML, Johnson MH, Cook DI. 1998. Cell cycle regulation of a T-type calcium current in early mouse embryos. Pflugers Arch. Eur. J. Physiol. 436, 834–842. ( 10.1007/s004240050712) [DOI] [PubMed] [Google Scholar]
- 217.Bernhardt ML, Zhang Y, Erxleben CF, Padilla-banks E, Mcdonough CE, Miao Y, Armstrong DL, Williams CJ. 2015. CaV3.2T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J. Cell Sci. 128, 4442–4452. ( 10.1242/jcs.180026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Saul S, Stanisz H, Backes CS, Schwarz EC, Hoth M. 2014. How ORAI and TRP channels interfere with each other: interaction models and examples from the immune system and the skin. Eur. J. Pharmacol. 739, 49–59. ( 10.1016/j.ejphar.2013.10.071) [DOI] [PubMed] [Google Scholar]
- 219.Birnbaumer L. 2015. From GTP and G proteins to TRPC channels: a personal account. J. Mol. Med. 93, 941–953. ( 10.1007/s00109-015-1328-5) [DOI] [PubMed] [Google Scholar]
- 220.Xu H, Delling M, Jun JC, Clapham DE. 2006. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 9, 628–635. ( 10.1038/nn1692) [DOI] [PubMed] [Google Scholar]
- 221.Carvacho I, Lee HC, Fissore RA, Clapham DE. 2013. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 5, 1375–1386. ( 10.1016/j.celrep.2013.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nadler MJS, et al. 2001. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595. ( 10.1038/35079092) [DOI] [PubMed] [Google Scholar]
- 223.Xiao E, Yang HQ, Gan YH, Duan DH, He LH, Guo Y, Wang SQ, Zhang Y. 2015. Brief reports: TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 33, 615–621. ( 10.1002/stem.1858) [DOI] [PubMed] [Google Scholar]
- 224.Runnels LW, Yue L, Clapham DE. 2001. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047. ( 10.1126/science.1058519) [DOI] [PubMed] [Google Scholar]
- 225.Carvacho I, Ardestani G, Lee HC, McGarvey K, Fissore RA, Lykke-Hartmann K. 2016. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci. Rep. 6, 1–12. ( 10.1038/srep34236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Horner VL, Wolfner MF. 2008. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev. Biol. 316, 100–109. ( 10.1016/j.ydbio.2008.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Loeb J. 1899. On the nature of the process of fertilization and the artificial production of normal larvæ (plutei) from the unfertilized eggs of the sea urchin. Am. J. Physiol. Content 3, 135–138. ( 10.1152/ajplegacy.1899.3.3.135) [DOI] [Google Scholar]
- 228.Loeb J. 1914. Activation of the unfertilized egg by ultra-violet rays. Science 40, 680–681. ( 10.1126/science.40.1036.680) [DOI] [PubMed] [Google Scholar]
- 229.Lillie R. 1926. The activation of starfish eggs by acids. J. Gen. Physiol. 8, 339–367. ( 10.1085/jgp.8.4.339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cattell W. 1926. Electrical activation of the nereis egg. Science 64, 558–560. ( 10.1126/science.64.1666.558-a) [DOI] [PubMed] [Google Scholar]
- 231.Pincus G, Enzmann E. 1936. The comparative behavior of mammalian eggs in vivo and in vitro. II. The activation of tubal eggs of the rabbit. J. Exp. Zool. 73, 195–208. ( 10.1084/jem.62.5.665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Surani MA, Barton SC, Norris ML. 1984. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308, 548–550. ( 10.1038/308548a0) [DOI] [PubMed] [Google Scholar]
- 233.McGrath J, Solter D. 1984. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37, 179–183. ( 10.1016/0092-8674(84)90313-1) [DOI] [PubMed] [Google Scholar]
- 234.Nakada K, Mizuno J. 1998. Intracellular calcium responses in bovine oocytes induced by spermatozoa and by reagents. Theriogenology 50, 269–282. ( 10.1016/s0093-691x(98)00135-6) [DOI] [PubMed] [Google Scholar]
- 235.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P.. 2014. Assisted oocyte activation following ICSI fertilization failure. Reprod. Biomed. Online 28, 560–571. ( 10.1016/j.rbmo.2014.01.008) [DOI] [PubMed] [Google Scholar]
- 236.Zhu J, Telfer EE, Fletcher J, Springbett A, Dobrinsky JR, De Sousa PA, Wilmut I.. 2002. Improvement of an electrical activation protocol for porcine oocytes. Biol. Reprod. 66, 635–641. ( 10.1095/biolreprod66.3.635) [DOI] [PubMed] [Google Scholar]
- 237.Joshi RP, Schoenbach KH. 2002. Mechanism for membrane electroporation irreversibility under high-intensity, ultrashort electrical pulse conditions. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 66, 52901 ( 10.1103/PhysRevE.66.052901) [DOI] [PubMed] [Google Scholar]
- 238.Batista Napotnik T, Reberšek M, Vernier PT, Mali B, Miklavčič D. 2016. Effects of high voltage nanosecond electric pulses on eucaryotic cells (in vitro): a systematic review. Bioelectrochemistry 110, 1–12. ( 10.1016/j.bioelechem.2016.02.011) [DOI] [PubMed] [Google Scholar]
- 239.Liu J, Lu Q, Liang R, Guo J, Wang K, Dong F, Wang J, Zhang J, Fang J. 2019. Communicating with mouse oocytes via regulating calcium oscillation patterns by nanosecond pulsed electric fields. Phys. Rev. Appl. 11, 024001 ( 10.1103/PhysRevApplied.11.024001) [DOI] [Google Scholar]
- 240.Morgan AJ, Jacob R. 1994. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 300, 665.–. ( 10.1042/bj3000665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nikiforaki D, Vanden Meerschaut F, De Roo C, Lu Y, Ferrer-Buitrago M, De Sutter P, Heindryckx B.. 2016. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil. Steril. 105, 798–806. ( 10.1016/j.fertnstert.2015.11.007) [DOI] [PubMed] [Google Scholar]
- 242.Rickords LF, White KL. 1993. Electroporation of inositol 1,4,5-triphosphate induces repetitive calcium oscillations in murine oocytes. J. Exp. Zool. 265, 178–184. ( 10.1002/jez.1402650209) [DOI] [PubMed] [Google Scholar]
- 243.Balakier H, Casper RF. 1993. Experimentally induced parthenogenetic activation of human oocytes. Hum. Reprod. 8, 740–743. ( 10.1093/oxfordjournals.humrep.a138132) [DOI] [PubMed] [Google Scholar]
- 244.Ruddock NT, Machaty Z, Cabot RA, Prather RS. 2001. Porcine oocyte activation: differing roles of calcium and pH. Mol. Reprod. Dev. 59, 227–234. ( 10.1002/mrd.1027) [DOI] [PubMed] [Google Scholar]
- 245.Presicce GA, Yang X. 1994. Parthenogenetic development of bovine oocytes matured in vitro for 24 hr and activated by ethanol and cycloheximide. Mol. Reprod. Dev. 38, 380–385. ( 10.1002/mrd.1080380405) [DOI] [PubMed] [Google Scholar]
- 246.Fujinami N, Hosoi Y, Kato H, Matsumoto K, Saeki K, Iritani A. 2004. Activation with ethanol improves embryo development of ICSI-derived oocytes by regulation of kinetics of MPF activity. J. Reprod. Dev. 50, 171–178. ( 10.1262/jrd.50.171) [DOI] [PubMed] [Google Scholar]
- 247.Wang Z, Wang W, Yu S, Xu Z. 2008. Effects of different activation protocols on preimplantation development, apoptosis and ploidy of bovine parthenogenetic embryos. Anim. Reprod. Sci. 105, 292–301. ( 10.1016/j.anireprosci.2007.03.017) [DOI] [PubMed] [Google Scholar]
- 248.Fraser LR. 1987. Strontium supports capacitation and the acrosome reaction in mouse sperm and rapidly activates mouse eggs. Gamete Res. 18, 363–374. ( 10.1002/mrd.1120180410) [DOI] [PubMed] [Google Scholar]
- 249.Zhang D, Pan L, Yang L-H, He X-K, Huang X-Y, Sun F-Z. 2005. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum. Reprod. 20, 3053–3061. ( 10.1093/humrep/dei215) [DOI] [PubMed] [Google Scholar]
- 250.Barbosa Fernandes C, Devito LG, Martins LR, Blanco IDP, De Lima Neto JF, Tsuribe PM, Gonçalves CGP, Da Cruz Landim-Alvarenga F.. 2014. Artificial activation of bovine and equine oocytes with cycloheximide, roscovitine, strontium, or 6-dimethylaminopurine in low or high calcium concentrations. Zygote 22, 387–394. ( 10.1017/S0967199412000627) [DOI] [PubMed] [Google Scholar]
- 251.Yamazaki W, Ferreira CR, Meo SC, Leal CLV, Meirelles FV, Garcia JM. 2005. Use of strontium in the activation of bovine oocytes reconstructed by somatic cell nuclear transfer. Zygote 13, 295–302. ( 10.1017/S0967199405003333) [DOI] [PubMed] [Google Scholar]
- 252.Che L, Lalonde A, Bordignon V. 2007. Chemical activation of parthenogenetic and nuclear transfer porcine oocytes using ionomycin and strontium chloride. Theriogenology 67, 1297–1304. ( 10.1016/j.theriogenology.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 253.Lu Y, et al. 2018. Strontium fails to induce Ca2+ release and activation in human oocytes despite the presence of functional TRPV3 channels. Hum. Reprod. Open 2018, 1–11. ( 10.1093/hropen/hoy005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Swann K. 2018. The role of Ca2+ in oocyte activation during in vitro fertilization: insights into potential therapies for rescuing failed fertilization. Biochim. Biophys. Acta—Mol. Cell Res. 1865, 1830–1837. ( 10.1016/j.bbamcr.2018.05.003) [DOI] [PubMed] [Google Scholar]
- 255.Swann K. 1991. Thimerosal causes calcium oscillations and sensitizes calcium-induced calcium release in unfertilized hamster eggs. FEBS Lett. 278, 175–178. ( 10.1016/0014-5793(91)80110-O) [DOI] [PubMed] [Google Scholar]
- 256.Cheek TR, McGuinness OM, Vincent C, Moreton RB, Berridge MJ, Johnson MH. 1993. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development 119, 179–189. [DOI] [PubMed] [Google Scholar]
- 257.Alexandre H, Delsinne V, Goval J-J. 2003. The thiol reagent, thimerosal, irreversibly inhibits meiosis reinitiation in mouse oocyte when applied during a very early and narrow temporal window: a pharmacological analysis. Mol. Reprod. Dev. 65, 454–461. ( 10.1002/mrd.10319) [DOI] [PubMed] [Google Scholar]
- 258.Machaty Z, Wang WH, Day BN, Prather RS. 1997. Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biol. Reprod. 57, 1123–1127. ( 10.1095/biolreprod57.5.1123) [DOI] [PubMed] [Google Scholar]
- 259.McDougall A, Gillot I, Whitaker M. 1993. Thimerosal reveals calcium-induced calcium release in unfertilised sea urchin eggs. Zygote 1, 35–42. ( 10.1017/s0967199400001271) [DOI] [PubMed] [Google Scholar]
- 260.Herbert M, Gillespie J, Murdoch A. 1997. Development of calcium signalling mechanisms during maturation of human oocytes. Mol. Hum. Reprod. 3, 965–973. ( 10.1093/molehr/3.11.965) [DOI] [PubMed] [Google Scholar]
- 261.Hinrichs K, Choi YH, Varner DD, Hartman DL. 2007. Production of cloned horse foals using roscovitine-treated donor cells and activation with sperm extract and/or ionomycin. Reproduction 134, 319–325. ( 10.1530/REP-07-0069) [DOI] [PubMed] [Google Scholar]
- 262.Prukudom S, Perez GI, Cibelli JB, Siripattarapravat K. 2019. Use of soluble sperm extract to improve cloning efficiency in zebrafish. Int. J. Dev. Biol. 63, 287–293. ( 10.1387/ijdb.180367ks) [DOI] [PubMed] [Google Scholar]
- 263.Ross PJ, Rodriguez RM, Iager AE, Beyhan Z, Wang K, Ragina N, Yoon SY, Fissore RA, Cibelli JB. 2009. Activation of bovine somatic cell nuclear transfer embryos by PLCZ cRNA injection. Reproduction 137, 427–437. ( 10.1530/REP-08-0419) [DOI] [PubMed] [Google Scholar]
- 264.Yamaguchi T, Ito M, Kuroda K, Takeda S, Tanaka A. 2017. The establishment of appropriate methods for egg-activation by human PLCZ1 RNA injection into human oocyte. Cell Calcium 65, 22–30. ( 10.1016/j.ceca.2017.03.002) [DOI] [PubMed] [Google Scholar]
- 265.Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K. 2015. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Cζ. Mol. Hum. Reprod. 21, 783–791. ( 10.1093/molehr/gav042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yoon SY, et al. 2012. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum. Reprod. 27, 1768–1780. ( 10.1093/humrep/des092) [DOI] [PubMed] [Google Scholar]
- 267.Tarín JJ, Pérez-Albalá S, Cano A. 2000. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update 6, 532–549. ( 10.1093/humupd/6.6.532) [DOI] [PubMed] [Google Scholar]
- 268.Jones KT, Whittingham DG. 1996. A comparison of sperm- and IP3-induced Ca2+ release in activated and aging mouse oocytes. Dev. Biol. 178, 229–237. ( 10.1006/dbio.1996.0214) [DOI] [PubMed] [Google Scholar]
- 269.Igarashi H, Takahashi E, Hiroi M, Doi K. 1997. Aging-related changes in calcium oscillations in fertilized mouse oocytes. Mol. Reprod. Dev. 48, 383–390. () [DOI] [PubMed] [Google Scholar]
- 270.Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. 2002. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol. Reprod. 66, 1828–1837. ( 10.1095/biolreprod66.6.1828) [DOI] [PubMed] [Google Scholar]
- 271.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. 2009. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol. Reprod. 80, 493–502. ( 10.1095/biolreprod.108.072017) [DOI] [PubMed] [Google Scholar]
- 272.Zhang N, Wakai T, Fissore RA. 2011. Caffeine alleviates the deterioration of Ca2+ release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol. Reprod. Dev. 78, 684–701. ( 10.1002/mrd.21366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Szpila M, Walewska A, Sabat-Pośpiech D, Strączyńska P, Ishikawa T, Milewski R, Szczepańska K, Ajduk A. 2019. Postovulatory ageing modifies sperm-induced Ca2+ oscillations in mouse oocytes through a conditions-dependent, multi-pathway mechanism. Sci. Rep. 9, 11859 ( 10.1038/s41598-019-48281-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhao S, Liu ZX, Bao ZJ, Wu Y, Wang K, Yu GM, Wang CM, Zeng SM. 2015. Age-associated potency decline in bovine oocytes is delayed by blocking extracellular Ca2+ influx. Theriogenology 83, 1493–1501. ( 10.1016/j.theriogenology.2015.01.034) [DOI] [PubMed] [Google Scholar]
- 275.Hirota J, Furuichi T, Mikoshiba K. 1999. Inositol 1,4,5-trisphosphate receptor type I is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J. Biol. Chem. 274, 34 433–34 437. ( 10.1074/jbc.274.48.34433) [DOI] [PubMed] [Google Scholar]
- 276.Verbert L, et al. 2008. Caspase-3-truncated type 1 inositol 1,4,5-trisphosphate receptor enhances intracellular Ca2+ leak and disturbs Ca2+ signalling. Biol. Cell 100, 39–49. ( 10.1042/bc20070086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gordo AC, Wu H, He CL, Fissore RA. 2000. Injection of sperm cytosolic factor into mouse metaphase II oocytes induces different developmental fates according to the frequency of [Ca2+]i oscillations and oocyte age. Biol. Reprod. 62, 1370–1379. ( 10.1095/biolreprod62.5.1370) [DOI] [PubMed] [Google Scholar]
- 278.Campbell K, Swann K. 2006. Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev. Biol. 298, 225–233. ( 10.1016/j.ydbio.2006.06.032) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.