Abstract
Aim:
Marinobufagenin (MBG), a cardiotonic steroid and a natriuretic hormone, is elevated in response to high salt diet consumption. In animal models salt intake stimulates adrenocortical MBG secretion via increased angiotensin II, sympathetic activity and aldosterone. No evidence in humans exists to suggest the involvement of the angiotensinergic-sympatho-excitatory pathway in MBG production. We investigated whether MBG is related to indices of autonomic activity in men and women.
Methods:
This cross-sectional study included 680 black and white, men and women from the African-PREDICT study (aged 20–30 years). Continuous 24 hr ECG recordings were used to obtain low and high frequency (LF, HF) heart rate variability (HRV). We measured 24 hr urinary MBG excretion and serum aldosterone.
Results:
We found a positive association of MBG excretion with estimated salt intake (P < 0.001) and aldosterone (P < 0.001) in women and men. In women only, a positive relationship was evident between MBG excretion and LF HRV in multivariate adjusted regression analyses (Adj. R2 = 0.33; β = 0.11; P = 0.030). In men, MBG excretion associated positively with HF HRV in similar regression analyses (R2 = 0.36; β = 0.12; P = 0.034). Sex-specific results were corroborated only in blacks, namely, a positive association of MBG excretion with LF HRV in black women (R2 = 0.38; β = 0.13; P = 0.036), and negative association with HF HRV in black men (R2 = 0.40; β = 0.18; P = 0.045). No relationships were evident in white women (P = 0.58) or men (P = 0.27).
Conclusion:
Our findings in this human cohort support suggested mechanisms whereby MBG is elevated as a result of increased salt intake, including autonomic activity, previously demonstrated in Dahl salt-sensitive hypertension.
Keywords: Autonomic activity, human, marinobufagenin, salt intake, women
Introduction
It is well known that excessive salt intake is associated with increased cardiovascular risk [1]. In response to a high salt intake, endogenous cardiotonic steroids are released – with reports indicating clear adverse effects of these steroids [2,3]. This includes, amongst others, marinobufagenin (MBG), a circulating bioactive steroid, synthesized in the adrenal cortex by means of CYP27A1 enzymatic activity [4]. We have previously confirmed this significant positive relationship between salt intake and MBG in this study cohort [5]. While MBG has been linked to several pathological states [3], we have recently demonstrated in healthy young adults that MBG excretion is associated with increased blood pressure [6], arterial stiffness [5] and left ventricular mass [7].
Fedorova et al demonstrated that salt intake stimulates adrenocortical MBG secretion in Dahl salt-sensitive rats [2,8]. Acute salt loading promoted short-term increases in pituitary endogenous ouabain and angiotensin II (AT II), along with sustained increases in adrenocortical AT II, plasma norepinephrine and MBG. It was proposed that MBG production was increased in response to salt loading via increased brain ouabain, and concurrent renin-angiotensin-aldosterone-system and sympathetic activity [8]. Intrahippocampal administration of a low-dose ouabain mimics the effects of salt loading and stimulates marinobufagenin production in Dahl salt-sensitive rats [9]. Administration of anti-ouabain antibody inhibited the abovementioned brain ouabain-angiotensinergic-sympatho-excitatory pathway and resulted in lower circulating MBG and urinary MBG excretion [8] In addition, the administration of losartan decreased AT II, norepinephrine and MBG excretion but not endogenous ouabain [8].
Although MBG levels are elevated in young adults on a habitual high salt diet [5–7], no evidence in humans exists to suggest the involvement of the angiotensinergic-sympatho-excitatory pathway. We, therefore, investigated whether MBG is related to indices of autonomic activity in young healthy men and women on a habitual sodium chloride intake, without detected cardiovascular disease. Heart rate variability (HRV) is one of several methods used as an indirect measure of autonomic activity [10]. Whilst the heart has an intrinsic pacemaker [11], HRV is mainly controlled by the complex inter-regulation of the sympathetic and parasympathetic branches of the autonomic nervous system. Based on the results from Fedorova et al. [8] we hypothesize that MBG excretion will be positively associated with indices of sympathetic autonomic activity. We will also explore the relationship between MBG excretion and aldosterone, taking into account the possible role thereof as a stimulatory factor in MBG synthesis and excretion.
We previously indicated that men had higher levels of MBG excretion than women, but that women may paradoxically be more sensitive to the effects thereof [5,6], Therefore, we investigated the association between autonomic activity and MBG excretion separately in men and women. This may provide useful insights into to the unparalleled observation of higher sensitization to MBG despite lower levels, in accordance with reported increased salt sensitivity in women.
Methods
This cross-sectional study included the first consecutive 680 participants with complete 24 hr urinary data, from the African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension (African-PREDICT). Protocols of the African-PREDICT study conform to Institutional guidelines and the declaration of Helsinki, and were approved by the Institutional Health Research Ethics Committee. The study is registered at ClinicalTrails.gov (Nr. NCT03292094).
Participant recruitment
A fieldwork team recruited black and white adults between the ages of 20 and 30 from communities in and around Potchefstroom in the North West province of South Africa. Recruited volunteers were screened to determine eligibility for inclusion into the African-PREDICT study. Inclusion criteria were office blood pressure <140/90 mmHg [12], HIV uninfected, no previous diagnosis or medication use for chronic illnesses (self-reported), not pregnant or lactating. Eligible participants were invited to the Hypertension Research Clinic. All participants gave written informed consent before health screening and study measurements. General health and demographic questionnaires were completed by participants to obtain data on demographics (age, sex, ethnicity and socio-economic status) and lifestyle habits (e.g. contraceptive use).
Anthropometry
We measured height (SECA 213 Portable Stadiometer) (SECA, Hamburg, Germany), weight (SECA 813 Electronic Scales) and waist circumference (Lufkin Steel Anthropometric Tape; W606PM; Lufkin, Apex, USA) using standard methods, as reported earlier [7], and calculated body mass index (BMI)(kg/m2).
Cardiovascular measurements
Participants were fitted with a 24 hr ambulatory blood pressure (ABPM) and electrocardiogram (ECG) apparatus (Card(X)plore, Meditech, Budapest, Hungary). An appropriately size brachial blood pressure cuff was attached to the non-dominant arm of participants with the apparatus attached to their waist. Daytime blood pressure recordings were taken in 30 min intervals (6 AM – 10 PM) and nighttime blood pressure every hour (10 PM – 6 AM). Continuous 24 hr ECG recordings were analyzed using Cardio Visions 1.15.2 Personal Edition software (Meditech, Budapest, Hungary) to obtain HRV data and provide information on the time- and frequency domains. Although the time domain (including the standard deviation of normal to normal interval (SDNN)) provides information on changes in total HRV, this technique is limited in providing information on specific components contributing to the variance [11,13,14]. The frequency domain, however, reflects different components of the autonomic nervous system with the low frequency (LF) HRV band being an index of sympathetic tone, with a parasympathetic component, and the high frequency (HF) HRV band a reflector of parasympathetic tone, with a sympathetic component [13]. Power spectral density analyses were used to calculate the LF HRV (0.04–0.15 Hz) and HF HRV (0.15–0.40 Hz), expressed in normalized units [14], as well as the LF/HF ratio.
Biological sampling and biochemical analyses
Participants fasted from 10 PM the evening prior to study measurements. A registered nurse took early morning blood samples using a sterile winged infusion set and syringes, in a private room. Thorough instructions were provided to each participant on how to collect 24 hr urine after they discarded the first passed urine of the day.
24 hr urinary MBG was analyzed using a solid-phase Dissociation-Enhanced Lanthanide Fluorescent Immunoassay (DELFIA), based on a 4G4 anti-MBG mouse monoclonal antibody [15], which demonstrates a low cross-immunoreactivity with contraceptive hormones [5] and aldosterone [15]. We previously indicated that MBG from nonextracted urine samples, in the presence of other hormones and steroids, is measured reliably [5]. When comparing MBG levels from extracted urine samples on C18 columns versus nonextracted urine samples, the highly specific 4G4 anti-MBG mouse monoclonal antibody consistently detected similar levels of MBG in both samples.
The Cobas Integra 400plus (Roche, Basel Switzerland) was used to determine 24 hr urinary sodium and potassium, as well as serum concentrations of high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, C-reactive protein (CRP), γ-glutamyltransferase (GGT) and glucose. We measured serum cotinine using the chemiluminescence method on the Immulite (Siemens, Erlangen, Germany), and serum aldosterone using the RIA Aldosterone Kit (Beckman Coulter, Immunotech, Radiova, Czech Republic).
Estimated salt intake for this study was calculated as:
Statistical analyses
We made use of Statistica version 13.2 (TIBCO Software Inc., Tulsa, Oklahoma, USA) to perform statistical analyses and GraphPad Prism version 5.0 (GraphPad Software Inc., California, USA) to draw figures. Skewed data were logarithmically transformed, and presented as the geometric mean; 5th and 95th percentile intervals. We performed independent t-tests to compare the basic characteristics (continuous data) of men and women and Chi-square tests for categorical data. Pearson, partial and multiple regression analyses were done in the total group, men and women to investigate the relationship between indirect indices of autonomic activity and MBG excretion. We considered several covariates for inclusion as possible independent variables based on the strongest bivariate associations with HRV variables and MBG excretion. In order to explore the potential influence of covariates on the relationship of LF HRV and HF HRV with MBG excretion we assessed the unadjusted, partially adjusted and fully adjusted β-values as part of regression analyses with MBG excretion as dependent variable. Pearson, partial and multiple regression analyses were repeated in subgroup analyses of black and white, men and women. We performed sensitivity analyses for hormonal contraceptive use in black and white women.
Results
Table 1 outlines the basic characteristics of the young men (aged 24.9 ± 2.98 years) and women (aged 24.8 ± 3.08 years) from this study. The groups had equal distribution of black and white ethnicity (P = 0.42). Men had higher blood pressure, LF HRV, LF/HF and SDNN (P < 0.001), while women demonstrated elevated 24 hr HR and HF HRV (P < 0.001). In agreement with what we have previously published, men compared to women had a greater estimated salt intake (P = 0.004), and in accordance, demonstrated higher levels of MBG excretion (P < 0.001) [5]. Additionally, in this study population 77% (n = 303) of women and 81% (n = 229) of men consumed more than 5 grams of salt per day. The women and men from this study respectively consumed approximately 7.24 (95% C.I. 7.99; 9.11) and 8.29 (95% C.I. 9.17; 10.5) grams of salt per day, which are higher than the Worlds Health Organizations recommended amount for adults (5 g salt per day) [16]. While estimated salt intake did not differ between black and white adults in the total group (P = 0.21) or in men (P = 0.34), black women consumed significantly more salt compared to white women (P = 0.007).
Table 1.
Characteristics of men and women from the African-PREDICT study.
| Men N = 284 | Women N = 396 | P | |
|---|---|---|---|
| Ethnicity, Black, N (%) | 136 (40.2) | 202 (59.8) | 0.42 |
| Age (years) | 24.9 ± 2.98 | 24.8 ± 3.08 | 0.59 |
| Socio economic status, N (%) | 0.28 | ||
| Low | 105 (37.0) | 149 (37.6) | |
| Middle | 61 (21.5) | 103 (26.0) | |
| High | 118 (41.5) | 144 (36.4) | |
| Anthropometric measurements | |||
| BMI (kg/m2) | 24.9 ± 5.32 | 25.8 ± 5.93 | 0.047 |
| WC (cm) | 83.7 ± 13.2 | 78.3 ± 12.7 | <0.001 |
| Cardiovascular profile | |||
| 24 hr SBP (mmHg) | 121 ± 8.18 | 113 ± 8.12 | <0.001 |
| 24 hr DBP (mmHg) | 69.7 ± 5.98 | 68.0 ± 5.27 | <0.001 |
| 24 hr Heart rate (bpm) | 69.5 ± 9.22 | 78.7 ± 9.76 | <0.001 |
| 24 hr LF HRV (n.u.) | 64.9 (46.0; 83.0) | 57.9 (37.0; 78.0) | <0.001 |
| 24 hr HF HRV (n.u.) | 29.7 (16.0; 49.0) | 35.9 (20.0; 60.0) | <0.001 |
| 24 hr LF/HF HRV | 2.17 (0.90; 5.10) | 1.60 (0.60; 3.70) | <0.001 |
| 24 hr HRV SDNN (ms) | 167 (107; 262) | 131 (85.0; 206) | <0.001 |
| 24 hr Urinary profile | |||
| Volume (L/24 hr) | 1.42 ± 0.76 | 1.36 ± 0.79 | 0.35 |
| MBG cone. (nmol/L) | 3.26 (1.26; 7.16) | 2.26 (0.72; 5.75) | <0.001 |
| MBG exc. (nmol/day) | 4.11 (1.59; 10.2) | 2.69 (0.93; 8.01) | <0.001 |
| Na+ exc. (mmol/day) | 141 (41.1; 360) | 123 (45.8; 326) | 0.004 |
| NaCI intake (g/day) | 8.29 (2.42; 21.2) | 7.24(2.70; 19.2) | 0.004 |
| Black | 7.98 (2.18; 23.7) | 7.83 (2.94; 20.5)† | |
| White | 8.57 (3.22; 17.2) | 6.68 (2.52; 15.4)† | |
| K+ exc. (mmol/day) | 41.8 (13.2, 103) | 39.3 (15.7; 103) | 0.18 |
| Na+:K+ ratio | 3.41 (1.36; 7.14) | 3.22 (1.40; 6.78) | 0.17 |
| Biochemical profile | |||
| Aldosterone (pg/ml) | 64.1 (16.8; 210) | 74.7 (17.1; 423) | 0.034 |
| Glucose (mmol/L) | 4.85 ± 0.75 | 4.67 ± 0.63 | 0.001 |
| HDL-C (mmol/L) | 1.15 (0.75; 1.73) | 1.34 (0.81; 2.14) | <0.001 |
| LDL-C (mmol/L) | 2.76 (1.54; 4.71) | 2.62 (1.50; 4.23) | 0.054 |
| Triglycerides (mmol/L) | 0.90 (0.45; 2.06) | 0.76 (0.39; 1.67) | <0.001 |
| C-reactive protein (mg/L) | 0.72 (0.10; 6.37) | 1.34 (0.12; 12.0) | <0.001 |
| Lifestyle measures | |||
| Smoking, N (%) | 96 (33.9) | 54 (13.6) | <0.001 |
| Cotinine >10 (ng/ml) | 82 (35.5) | 52 (16.6) | <0.001 |
| GGT (U/L) | 26.2 (12.8; 66.2) | 18.0 (7.80; 54.6) | <0.001 |
| Hormonal contraception, W (%) | - | 155 (40.2) |
Note: Arithmetic mean ± standard deviation; geometric mean (5th percentile; 95th percentile intervals).
BMI, Body mass index; DBP, Diastolic blood pressure; GGT, γ-glutamyl transferase; HDL-C, High density lipoprotein cholesterol; K+, Potassium; LDL-C, Low density lipoprotein cholesterol; MBG, Marinobufagenin; Na+, Sodium; NaCl, Estimated salt intake; SBP, Systolic blood pressure; SDNN, Standard deviation of normal R-R intervals; WC, waist circumference; 24 hr LF HRV, Normalized low frequency power heart rate variability; 24 hr HF HRV, Normalized high frequency power heart rate variability, 24 hr LF/HF HRV, Ratio of low-to high frequency power.
P = 0.007.
Regression analyses
We firstly performed single, partial and multiple regression analyses in the total group which indicated a significant contribution of sex on the relationship between MBG excretion and HRV autonomic activity parameters (Supplementary Table 1 & Supplementary Figure 1). Subsequent single, partial and multiple regression analyses were thus performed according to sex stratification (Table 2). In women only, a positive relationship was evident between MBG excretion and LF HRV in single (r = 0.11; P = 0.033), partial (r = 0.10; P = 0.055) (Table 2) and multivariate adjusted regression analyses (Adj. R2 = 0.33; β = 0.11; P = 0.031) (Table 3 & Figure 1). Partially adjusted β-values of LF HRV in regression analyses with MBG excretion as dependent variable (Figure 1), indicated that the inclusion of estimated salt intake (Adj. R2 = 0.23; P = 0.006) or aldosterone (Adj. R2 = 0.005; P = 0.12) significantly adjusted the relationship between MBG excretion and LF HRV.
Table 2.
Pearson and partial correlations between indices of autonomic activity and MBG excretion.
| MBG excretion (nmol/day) | ||
|---|---|---|
| Men N = 284 | Women N=396 | |
| 24 hr Heart rate (bpm) | r< 0.001; p = 0.99 | r =−0.06; p = 0.20 |
| 24 hr LF HRV (n.u.) | r = 0.07; p = 0.27 | r = 0.11;p = 0.033 |
| 24 hr HF HRV (n.u.) | r = −0.05; p = 0.39 | r = −0.06; p = 0.21 |
| 24 hr LF/HF HRV | r = 0.06; p = 0.31 | r = 0.08;p = 0.13 |
| SDNN | r = −0.02; p = 0.70 | r = 0.004; p = 0.94 |
| Adjusted for age, ethnicity and body mass index | ||
| 24 hr Heart rate (bpm) | r = −0.04; p = 0.51 | r = −0.06; p = 0.26 |
| 24 hr LF HRV (n.u.) | r = 0.021; p = 0.73 | r = 0.10; p = 0.055 |
| 24 hr HF HRV (n.u.) | r = −0.01; p = 0.93 | r = −0.05; p = 0.34 |
| 24 hr LF/HF HRV | r = 0.01; p = 0.81 | r = 0.06; p = 0.21 |
| SDNN | r<−0.001; p = 0.99 | r = −0.02; p = 0.68 |
Note: 24 hr LF HRV, Normalized low frequency power heart rate variability; 24 hr HF HRV, Normalized high frequency power heart rate variability, 24 hr LF/HF HRV, Ratio of low-to high frequency power; SDNN, Standard deviation of normal R-R intervals.
Table 3.
Fully adjusted multiple regression models with MBG excretion as dependent variable in men and women.
| MBG excretion (nmol/day) | ||||
|---|---|---|---|---|
| Men N = 243 | Women N = 349 | |||
| R2 | R2 | |||
| 0.36 | 0.33 | |||
| β (S.E.) | P | β (S.E.) | P | |
| LF HRV(n.u.) | −0.078 (0.057) | 0.17 | 0.108 (0.050) | 0.031 |
| Ethnicity (black/white) | 0.164 (0.067) | 0.016 | 0.045 (0.056) | 0.42 |
| Age (years) | −0.024 (0.056) | 0.67 | −0.106 (0.046) | 0.023 |
| BMI (kg/m2) | −0.023 (0.074) | 0.75 | 0.004 (0.061) | 0.95 |
| 24 hr SBP (mmHg) | −0.025 (0.061) | 0.68 | 0.070 (0.054) | 0.20 |
| NaCI intake (g/day) | 0.567 (0.055) | <0.001 | 0.542 (0.046) | <0.001 |
| HDL-C (mmol/l) | −0.068 (0.057) | 0.23 | −0.210 (0.049) | <0.001 |
| CRP (mg/l) | −0.064 (0.059) | 0.28 | −0.213 (0.051) | <0.001 |
| Glucose (mmol/l) | −0.085 (0.062) | 0.17 | −0.084 (0.047) | 0.073 |
| Aldosterone (pg/ml) | 0.203 (0.059) | <0.001 | 0.165 (0.049) | <0.001 |
| MBG Excretion (nmol/day) | ||||
| R2 | R2 | |||
| 0.36 | 0.32 | |||
| β (S.E.) | P | β (S.E.) | P | |
| HF HRV (n.u.) | 0.120 (0.056) | 0.034 | −0.031 (0.051) | 0.55 |
| Ethnicity (black/white) | 0.173 (0.068) | 0.011 | 0.076 (0.057) | 0.18 |
| Age (years) | −0.014 (0.056) | 0.80 | −0.095 (0.047) | 0.045 |
| BMI (kg/m2) | −0.022 (0.075) | 0.76 | 0.011 (0.062) | 0.88 |
| 24 hr SBP (mmHg) | −0.021 (0.061) | 0.74 | 0.076 (0.055) | 0.17 |
| NaCI intake (g/day) | 0.566 (0.055) | <0.001 | 0.546 (0.046) | <0.001 |
| HDL-C (mmol/l) | −0.068 (0.059) | 0.24 | −0.197 (0.049) | <0.001 |
| CRP (mg/l) | −0.063 (0.059) | 0.29 | −0.209 (0.052) | <0.001 |
| Glucose (mmol/l) | −0.086 (0.062) | 0.16 | −0.093 (0.047) | 0.049 |
| Aldosterone (pg/ml) | 0.207 (0.059) | <0.001 | 0.163 (0.050) | 0.001 |
Note: BMI, Body mass index; CRP, C-reactive protein; HDL-C, High density lipo-protein cholesterol; 24 hr LF HRV, Normalized low frequency power heart rate variability; 24 hr HF HRV, Normalized high frequency power heart rate variability, NaCl intake, Estimated salt intake; SBP, Systolic blood pressure.
Figure 1.
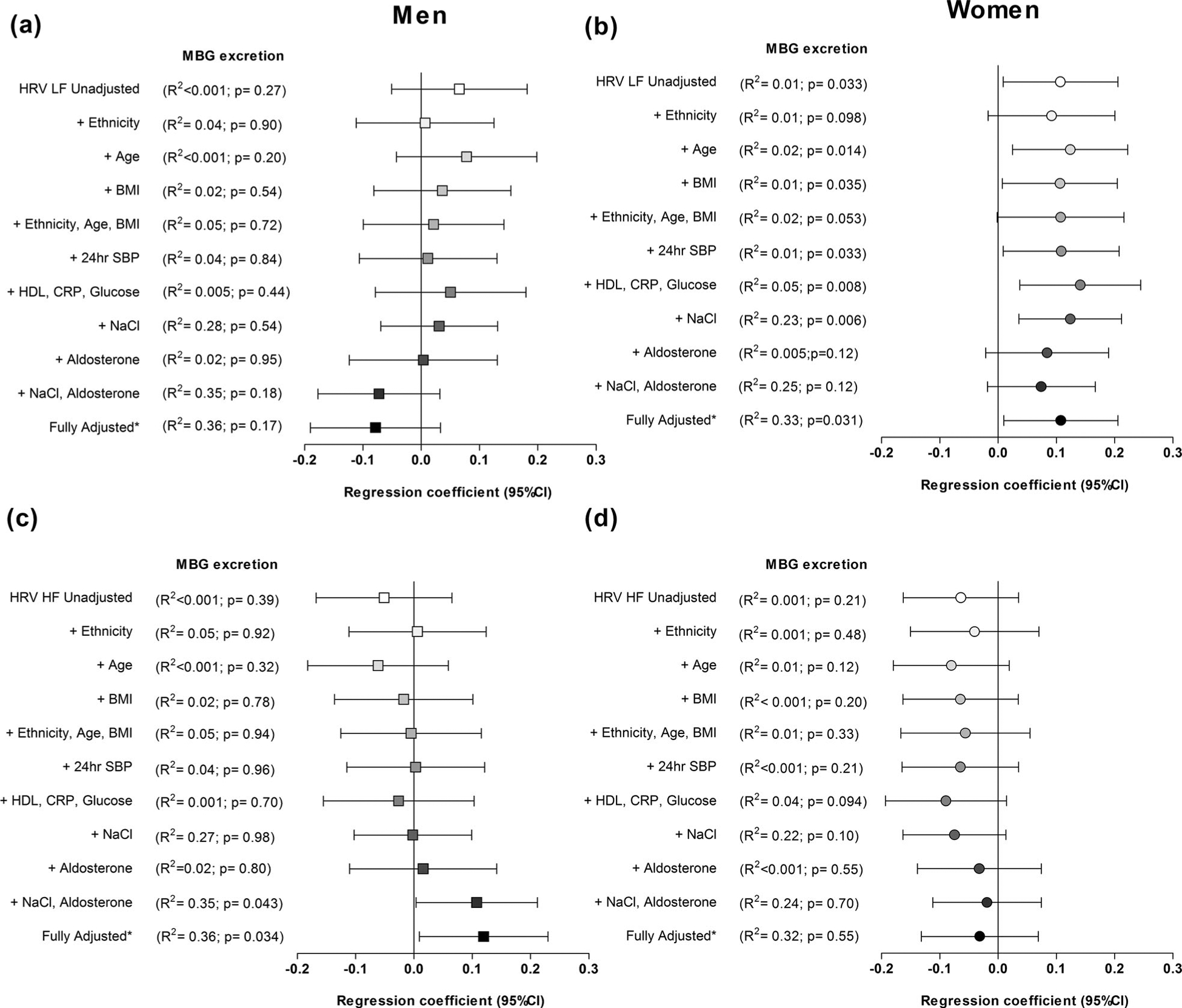
Unadjusted, partially adjusted and fully adjusted β-values of heart rate variability estimates (LF HRV ((a) men; (b) women) or HF HRV ((c) men; (d) women)) as part of regression analyses with MBG excretion as dependent variable. *In fully adjusted multiple regression models HRV estimates were adjusted for age, ethnicity, body mass index (BMI), 24 hr systolic blood pressure (24 hr SBP), high density lipoprotein cholesterol (HDL-C), C- reactive protein (CRP), glucose, estimated NaCl intake, aldosterone.
Although we found no correlation between MBG excretion and autonomic parameters in single or partial regression analyses in men (Table 2), MBG excretion associated positively with HF HRV in fully adjusted multiple regression analyses (R2 = 0.36; β = 0.12; P = 0.034) (Table 3). Notably, the relationship of MBG excretion with HF HRV is altered by the parallel inclusion of estimated salt intake and aldosterone, as indicated by partially adjusted and fully adjusted β-values of HF HRV (Figure 1).
As expected we found a significant positive association of MBG excretion with estimated salt intake (P < 0.001) and aldosterone (P < 0.001) in women and men.
Ethnicity
Due to known salt-sensitivity in black populations [17], we additionally performed subgroup analyses in black and white men and women. Our sex-specific results were corroborated only in black women and men (Supplementary Table 2 & Table 4). We found a significant positive association of MBG excretion with LF HRV in black women (R2 = 0.38; β = 0.13; P = 0.036), and HF HRV in black men (R2 = 0.40; β = 0.18; P = 0.045), but not in their white counterparts (all P > 0.26).
Table 4.
Fully adjusted multiple regression models with MBG excretion as dependent variable in black and white men and women.
| MBG excretion (nmol/day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Black | White | |||||||
| Men N = 102 | Women N = 168 | Men N = 141 | Women N = 181 | |||||
| R2 | R2 | R2 | R2 | |||||
| 0.39 | 0.38 | 0.25 | 0.32 | |||||
| β (S.E.) | P | β (S.E.) | P | β (S.E.) | P | β (S.E.) | P | |
| LF HRV (n.u.) | −0.158 (0.086) | 0.071 | 0.134 (0.063) | 0.036 | −0.005 (0.076) | 0.94 | 0.036 (0.064) | 0.58 |
| Age (years) | −0.002 (0.087) | 0.98 | −0.154 (0.066) | 0.021 | −0.047 (0.077) | 0.54 | −0.063 (0.064) | 0.33 |
| BMI (kg/m2) | −0.009 (0.095) | 0.93 | −0.018 (0.084) | 0.83 | −0.024 (0.099) | 0.81 | 0.016 (0.088) | 0.85 |
| 24 hr SBP (mmHg) | −0.027 (0.089) | 0.76 | 0.138 (0.070) | 0.058 | −0.013 (0.084) | 0.88 | 0.027 (0.084) | 0.75 |
| NaCI intake (g/day) | 0.618 (0.084) | <0.001 | 0.609 (0.066) | <0.001 | 0.517 (0.077) | <0.001 | 0.502 (0.063) | <0.001 |
| HDL-C (mmol/l) | −0.142 (0.085) | 0.10 | −0.087 (0.064) | 0.18 | 0.012 (0.079) | 0.88 | −0.261 (0.067) | <0.001 |
| CRP (mg/l) | −0.076 (0.086) | 0.38 | −0.102 (0.074) | 0.17 | −0.072 (0.089) | 0.42 | −0.282 (0.071) | <0.001 |
| Glucose (mmol/l) | −0.019 (0.085) | 0.82 | −0.150 (0.065) | 0.023 | −0.160 (0.075) | 0.034 | 0.019 (0.064) | 0.77 |
| Aldosterone (pg/ml) | 0.260 (0.087) | 0.004 | 0.192 (0.065) | 0.004 | 0.148 (0.077) | 0.056 | 0.138 (0.064) | 0.031 |
| Sensitivity analysis for hormonal contraceptive use | ||||||||
| MBG excretion (nmol/day) | ||||||||
| R2 | R2 | |||||||
| 0.36 | 0.38 | |||||||
| β (S.E.) | P | P (S.E.) | P | β (S.E.) | P | β (S.E.) | P | |
| LF HRV (n.u.) | - | 0.138 (0.067) | 0.040 | - | 0.017 (0.062) | 0.79 | ||
| Contraceptive use (no/yes) | - | 0.040 (0.067) | 0.55 | - | −0.252 (0.066) | <0.001 | ||
| MBG excretion (nmol/day) | ||||||||
| R2 | R2 | R2 | R2 | |||||
| 0.40 | 0.37 | 0.24 | 0.32 | |||||
| β (S.E.) | P | β (S.E.) | P | β (S.E.) | P | β (S.E.) | P | |
| HF HRV (n.u.) | 0.175 (0.086) | 0.045 | −0.101 (0.063) | 0.11 | 0.085 (0.076) | 0.27 | 0.024 (0.064) | 0.71 |
| Age (years) | 0.002 (0.086) | 0.98 | −0.145 (0.067) | 0.031 | −0.028 (0.077) | 0.71 | −0.057 (0.064) | 0.38 |
| BMI (kg/m2) | −0.001 (0.095) | 0.99 | −0.011 (0.085) | 0.90 | −0.035 (0.100) | 0.72 | 0.022 (0.088) | 0.80 |
| 24 hr SBP (mmHg) | −0.027 (0.089) | 0.76 | 0.140 (0.073) | 0.056 | 0.002 (0.086) | 0.98 | 0.037 (0.083) | 0.66 |
| NaCI intake (g/day) | 0.630 (0.084) | <0.001 | 0.607 (0.066) | <0.001 | 0.502 (0.077) | <0.001 | 0.507 (0.063) | <0.001 |
| HDL-C (mmol/l) | −0.147 (0.085) | 0.087 | −0.067 (0.065) | 0.30 | 0.020 (0.080) | 0.81 | −0.255 (0.067) | <0.001 |
| CRP (mg/l) | −0.067 (0.085) | 0.43 | −0.088 (0.075) | 0.24 | −0.072 (0.090) | 0.43 | −0.287 (0.070) | <0.001 |
| Glucose (mmol/l) | −0.021 (0.085) | 0.80 | −0.163 (0.065) | 0.014 | −0.167 (0.075) | 0.028 | 0.017 (0.065) | 0.79 |
| Aldosterone (pg/ml) | 0.261 (0.086) | 0.003 | 0.188 (0.066) | 0.005 | 0.152 (0.078) | 0.052 | 0.141 (0.063) | 0.028 |
Note: BMI, Body mass index; CRP, C-reactive protein; HDL-C, High density lipoprotein cholesterol; 24 hr LF HRV, Normalized low frequency power heart rate variability; 24 hr HF HRV, Normalized high frequency power heart rate variability, NaCl intake, Estimated salt intake; SBP, Systolic blood pressure.
Sensitivity analysis for contraceptive use
We have previously reported on the need to perform sensitivity analysis for hormonal contraceptive use when reporting associations of MBG with cardiovascular measures in women [5]. Forty percent of the young women in this study made use of hormonal contraceptives (41.8% of black and 38.5% of white women). Hormonal injection was more common in black women (black women n = 55 (28.4%); white women n = 6 (3.13%)) compare to oral contraceptive use in white women (white women n = 68 (35.4%); black women n = 23 (12%)). These differences in hormonal contraceptive use methods between ethnic groups are in line with a recently published study in the South African Medical Journal [18]. Taking this into account we performed sensitivity analyses for hormonal contraceptive use in black and white women respectively (Table 4). In black women the relationship between MBG and LF HRV remained robust (R2 = 0.36; β = 0.14; P = 0.040), and there was no relationship between MBG excretion and hormonal contraceptive use (R2 = 0.36; β = 0.040; P = 0.55). Interestingly, although there was no relationship between MBG and LF HRV in white women (R2 = 0.38; β = 0.17; P = 0.79), MBG excretion negatively associated with hormonal contraceptive use (R2 = 0.38; β = 0.25; P < 0.001).
Discussion
Main findings
For the first time in a human cohort, our study indicated a significant positive association between the endogenous cardiotonic steroidal inhibitor of Na+/K+-ATPase, MBG, and autonomic activity in young healthy men and women, supporting a previously demonstrated pathway in animal models [8]. In addition, our results were especially evident in black men and women, highlighting ethnic specific differences with regards to possible autonomic pathways whereby MBG excretion may be mediated.
Although the relationship between salt intake and MBG excretion is well established in humans [7], including this study population [5], the relevant stimulatory mechanisms whereby MBG is released have only been described in animal models [2,8]. Salt loading was shown to increase brain ouabain and concurrently AT II and sympathetic activity [8,19,20], thereby promoting MBG excretion via this ouabain-angiotensinergic-sympatho-excitatory pathway (Figure 2) [8]. Brain aldosterone has been also been suggested to stimulate the ouabain-angiotensinergic-sympatho-excitatory pathway via the neuromodulatory pathway [21].
Figure 2.
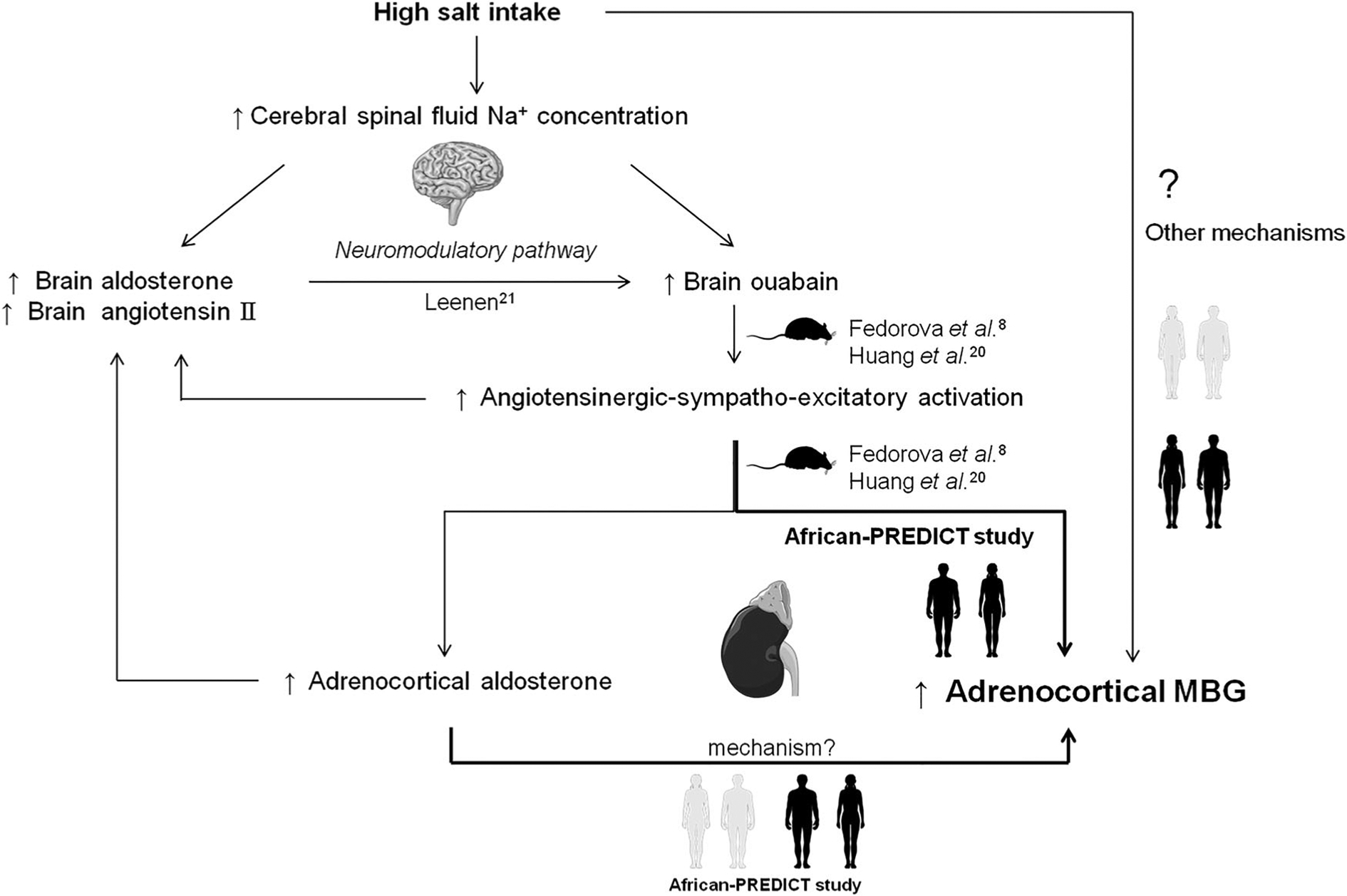
Suggested ouabain-angiotensinergic-sympatho-excitatory-MBG pathway.
We found that salt intake as well as aldosterone positively and independently associated with MBG excretion in both men and women, which is in accordance with previous studies [22,23]. Tomaschitz et al. found significantly elevated MBG levels in patients with primary aldosteronism, and demonstrated positive relationships between plasma aldosterone and MBG [22]. However, in women only, an independent positive relationship between MBG excretion and an indirect measure of sympathetic activity, LF HRV [24,25], was found. This relationship was strengthened when including estimated salt intake in the regression model, supporting the suggested mechanism whereby MBG excretion is stimulated via increased autonomic activity in response to salt intake. Intriguingly, we have previously demonstrated positive associations of MBG excretion with blood pressure [6], arterial stiffness [5] and left ventricular mass [7], predominantly in women. In support, other studies have found the effect of salt intake to more pronounced in women [26–28], and that women demonstrate a 15% greater reactivity of aldosterone levels in response to AT II infusion compared to men [28].
MBG may additionally play a role in the autonomic feedback regulation via its affect on Na+/K+-ATPase [29], implicated in cardiac sympathetic regulation [30]. There is some indication in the previous publications on a high sensitivity of human Na+/K+-ATPase to MBG. Accordingly, human skeletal muscle α−2 Na+/K+-ATPase exhibited a high affinity to MBG [31]. In addition, human α1-Na+/K+-ATPase with a high affinity for MBG is expressed in the brain and nerve endings, although it is not the primary isoform [29,32]. The effect of MBG on human neuronal Na+/K+-ATPase, and specifically α−3 subunit, abundant in the nerve endings [32], would merit future detailed investigations. This feedback mechanism may also exaggerate sympathetic activation in women. Our results in the women of this study population [5–7], along with the findings of other studies [26–28], may suggest that women are likely at a greater risk to the harmful effects of salt in the development of cardiovascular disease- where MBG may play an important role [5–7].
Conversely, in men a significant positive relationship between MBG excretion and a measure of parasympathetic activity [14], HF HRV, was only evident after parallel adjustment for both estimated salt intake and aldosterone. Although we anticipated relationships of salt intake and aldosterone with MBG excretion, the positive association between MBG excretion and HF HRV (potentially reflecting parasympathetic activity) was unexpected, as only indices of sympathetic activity have previously been implicated in increased MBG excretion [8].
The apparent sex-specific relationship of MBG excretion with LF HRV in women, and HF HRV in men warrants further investigation into sympathetic and parasympathetic modulation of MBG excretion. Although 24 hr LF HRV and HF HRV have been thought to reflect sympathetic and parasympathetic activity, respectivly [24], this assumption is controversial [13,33,34], While vagal activity has been recognized as a major component of HF HRV by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [14], the physiological interpretation of LF HRV has been debated [34].
Some have argued that that HRV does not reflect sympathetic activation based on the dissociation between HRV and norepinephrine spillover [33], peripheral muscle sympathetic nerve activity (MSNA) [35] and expected increased sympathetic activity with exercise [36]. However, other studies demonstrate contrasting results. Furlan et al. indicated a significant increase in LF HRV (n.u.) along with heart rate, MSNA, plasma epinephrine, norepinephrine, and a decrease in HF HRV during a tilt test in healthy adults [25]. In support, Sandrone et al. have found that beta blockers reduce LF HRV after myocardial infarction [37]. It has also been observed that the circadian variation of LF HRV corresponds with expected decreased sympathetic activity at night and early morning sympathetic surge [24]. We, therefore, recommend that our findings be repeated using more direct measures of sympathetic activity such as microneurography [38].
Apart from the sex differences, the relationship between MBG excretion and autonomic activity was particularly evident in black women and men, who are known to be more salt sensitive [17], and have increased autonomic activity [39]. While the young age of these participants might still be protective to prevent MBG over production, exaggerated sympathetic drive and aldosterone sensitivity [40] associated with black ethnicity could increase their cardiovascular risk over time.
Strengths and limitations
Our young study population allows us to investigate the possible relationship of MBG excretion with indices of autonomic activity in adults who are at their peak cardiovascular health, while also consuming a high salt diet (77% of the women and 81% of the men had salt intake > 5grams/day) [16]. This is the first study to investigate this relationship in a human cohort that supports mechanisms previously only demonstrated in animal model studies. The cross-sectional design limits the discussion of cause and effect, and therefore the observational results should be interpreted accordingly. For this study a single 24 hr urine sample was collected for each participant as opposed to the multiple samples for the estimation of salt intake. The World Health Organization, however, have indicated that the use of a single 24 hr urine sample, as the method for estimating population sodium intake, is sufficient [41]. The impact of age and cardiovascular diseases on the association of MBG and autonomic activity would merit further investigations.
Conclusion
Our study demonstrated a positive association between MBG excretion and autonomic activity in young healthy men and women, and particularly in black adults. Our findings in this human cohort support suggested mechanisms whereby MBG is elevated as a result of increased salt intake, including autonomic activity, previously demonstrated in Dahl salt-sensitive hypertension.
Supplementary Material
Acknowledgements
The authors of this study are grateful towards all individuals participating voluntarily in the study. The dedication of the support and research staff as well as students at the Hypertension Research and Training Clinic at the North-West University is also duly acknowledged.
Funding
The research funded in this manuscript is part of an ongoing research project financially supported by the South African Medical Research Council (SAMRC) with funds from National Treasury under its Economic Competitiveness and Support Package; the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and National Research Foundation (NRF) of South Africa [grant numbers: UID86895; 111862]; the SAMRC with funds received from the South African National Department of Health; GlaxoSmithKline R&D (Africa Non-Communicable Disease Open Lab grant), the UK Medical Research Council and with funds from the UK Government’s Newton Fund; as well as corporate social investment grants from Pfizer (SA), Boehringer Ingelheim (SA), Novartis (SA), the Medi Clinic Hospital Group (SA) and in kind contributions of Roche Diagnostics (SA). The study is also supported by the National Institute on Aging, NIH Intramural Research Program (USA). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in regard.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be accessed https://doi.org/10.1080/1028415X.2018.1564985.
References
- [1].O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. [DOI] [PubMed] [Google Scholar]
- [2].Fedorova OV, Shapiro JI, Bagrov AY. Endogenous cardiotonic steroids and salt-sensitive hypertension. BBA – Mol Bas Dis. 2010;1802:1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fedorova OV, Zernetkina VI, Shilova VY, Grigorova YN, Juhasz O, Wei W, et al. Synthesis of an endogenous steroidal Na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ: Cardiovasc Genet. 2015;8:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Strauss M, Smith W, Wei W, Bagrov AY, Fedorova OV, Schutte AE. Large artery stiffness is associated with marinobufagenin in young adults: the African-PREDICT study. J Hypertens. 2018;36:2333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Strauss M, Smith W, Wei W, Fedorova OV, Schutte AE. Marinobufagenin is related to elevated central and 24-h systolic blood pressures in young black women: the African-PREDICT study. Hypertens Res. 2018;41: 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Strauss M, Smith W, Kruger R, Wei W, Fedorova OV, Schutte AE. Marinobufagenin and left ventricular mass in young adults: the African-PREDICT study. Eur J Prev Cardiol. 2018;25:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marino-bufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23:1515–23. [DOI] [PubMed] [Google Scholar]
- [9].Fedorova OV, Zhuravin IA, Agalakova NI, Yamova LA, Talan MI, Lakatta EG, et al. Intrahippocampal microinjection of an exquisitely low dose of ouabain mimics NaCl loading and stimulates a bufadienolide Na/KATPase inhibitor. J Hypertens. 2007;25:1834–44. [DOI] [PubMed] [Google Scholar]
- [10].Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6 (1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- [13].Billman GE. Heart rate variability – a historical perspective. Front Physiol. 2011;2:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;93:1043–65. [PubMed] [Google Scholar]
- [15].Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26:2414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].World Health Organization. Guideline: sodium intake for adults and children. Geneva: WHO Press; 2012. [PubMed] [Google Scholar]
- [17].Falkner B, Kushner H. Effect of chronic sodium loading on cardiovascular response in young blacks and whites. Hypertension. 1990;15:36–43. [DOI] [PubMed] [Google Scholar]
- [18].Chersich MF, Wabiri N, Risher K, Shisana O, Celentano D, Rehle T, et al. Contraception coverage and methods used among women in South Africa: a national household survey. S Afr Med J. 2017;107:307–14. [DOI] [PubMed] [Google Scholar]
- [19].Guild SJ, McBryde FD, Malpas SC, Barrett CJ. High dietary salt and angiotensin II chronically increase renal sympathetic nerve activity: a direct telemetric study. Hypertension. 2012;59:614–20. [DOI] [PubMed] [Google Scholar]
- [20].Huang BS, Veerasingham SJ, Leenen FH. Brain “ouabain,” ANG II, and sympathoexcitation by chronic central sodium loading in rats. Am J Physiol. 1998;274:H1269–76. [DOI] [PubMed] [Google Scholar]
- [21].Leenen FH. The central role of the brain aldosterone–“ouabain” pathway in salt-sensitive hypertension. BBA-Mol Bas Dis. 2010;1802:1132–9. [DOI] [PubMed] [Google Scholar]
- [22].Tomaschitz A, Piecha G, Ritz E, Meinitzer A, Haas J, Pieske B, et al. Marinobufagenin in essential hypertension and primary aldosteronism: a cardiotonic steroid with clinical and diagnostic implications. Clin Exp Hypertens. 2015;37:108–15. [DOI] [PubMed] [Google Scholar]
- [23].Piecha G, Kujawa-Szewieczek A, Kuczera P, Skiba K, Sikora-Grabka E, Wiecek A. Plasma marinobufagenin immunoreactivity in patients with chronic kidney disease: a case control study. Am J Physiol Renal Physiol. 2018;315:F637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, et al. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–47. [DOI] [PubMed] [Google Scholar]
- [25].Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, et al. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–92. [DOI] [PubMed] [Google Scholar]
- [26].He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murao S, Takata Y, Yasuda M, Osawa H, Kohi F. The influence of sodium and potassium intake and insulin resistance on blood pressure in normotensive individuals is more evident in women. Am J Hypertens. 2018;31:876–85. [DOI] [PubMed] [Google Scholar]
- [28].Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, et al. Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension. 2018;71:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bagrov AY, Fedorova OV. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+,K+-pump in human mesenteric arteries. J Hypertens. 1998;16:1953–8. [DOI] [PubMed] [Google Scholar]
- [30].Bers DM, Despa S. Na/K-ATPase – an integral player in the adrenergic fight-or-flight response. Trends Cardiovasc Med. 2009;19:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kotova O, Al-Khalili L, Talia S, Hooke C, Fedorova OV, Bagrov AY, et al. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem. 2006;281: 20085–94. [DOI] [PubMed] [Google Scholar]
- [32].Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol. 2017;8:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, et al. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goldstein DS, Bentho O, Park M-Y, Sharabi Y. LF power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wakeham TR, Fonkoue IT, Durocher JJ, Cooke WH, Carter JR. Reliability of heart rate variability as an assessment of cardiac sympathetic activity in humans. FASEB. 2017;31:1071–6. [Google Scholar]
- [36].Pichon AP, de Bisschop C, Roulaud M, Denjean A, Papelier Y. Spectral analysis of heart rate variability during exercise in trained subjects. Med Sci Sports Exerc. 2004;36:1702–8. [DOI] [PubMed] [Google Scholar]
- [37].Sandrone G, Mortara A, Torzillo D, La Rovere MT, Malliani A, Lombardi F. Effects of beta blockers (atenolol or metoprolol) on heart rate variability after acute myocardial infarction. Am J Cardiol. 1994;74:340–5. [DOI] [PubMed] [Google Scholar]
- [38].Macefield VG. Sympathetic microneurography. Handb Clin Neurol. 2013;117:353–64. [DOI] [PubMed] [Google Scholar]
- [39].Reimann M, Hamer M, Schlaich M, Malan NT, Rudiger H, Ziemssen T, et al. Autonomic responses to stress in Black versus Caucasian Africans: the SABPA study. Psychophysiology. 2012;49:454–61. [DOI] [PubMed] [Google Scholar]
- [40].Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, et al. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Elliott P, Brown I. Sodium intakes around the world. Geneva: World Health Organization; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


