The severe acute respiratory syndrome novel coronavirus-2 (SARS-CoV-2) virus pandemic and related coronavirus disease (COVID-19), have dramatically altered health care delivery, worsened non–virus-related health outcomes, and increased the potential for disparities. As COVID-19 infections increased, public health and professional organizations issued guidance that all nonurgent surgeries and procedures, including cancer screening, should be delayed.1 Not surprisingly, early data suggest that these restrictions drastically impacted preventive care that requires direct patient–provider contact. Even for conditions requiring urgent intervention, such as myocardial infarctions,2 there is evidence that patients recently decreased health care use. An online evaluation by the EPIC health research network suggested fewer cancer screening encounters during the pandemic; however, these analyses did not directly measure recommended cancer screening tests within age-eligible populations and did not examine disparities over time.3 Thus, the pandemic’s broader impact on commonly performed cancer prevention and control measures remains largely unknown.
Decreases in cancer screening are particularly alarming because routinely screening asymptomatic people decreases morbidity and mortality related to breast, cervical, colorectal, and lung cancers.4 The current US Preventive Services Task Force recommendations include: biennial breast cancer screening with mammography in women ages 50–74 years; annual lung screening with low-dose computed tomography in adults aged 55–80 years with a >30 pack-year smoking history and a quit date within 15 years; colorectal cancer screening, most commonly completed using annual fecal immunochemical testing (FIT) or colonoscopy every 10 years among average risk adults aged 50–75 years; and periodic cervical screening with cytology with or without human papillomavirus testing in women ages 21–65 years.4 Thus, almost every adult is recommended to receive multiple cancer screening tests during their lifetime. At present, minimal data are available regarding the pandemic’s impact on cancer screening between diverse health care settings, among different cancer types, by various screening tests, and across disparate demographic groups.
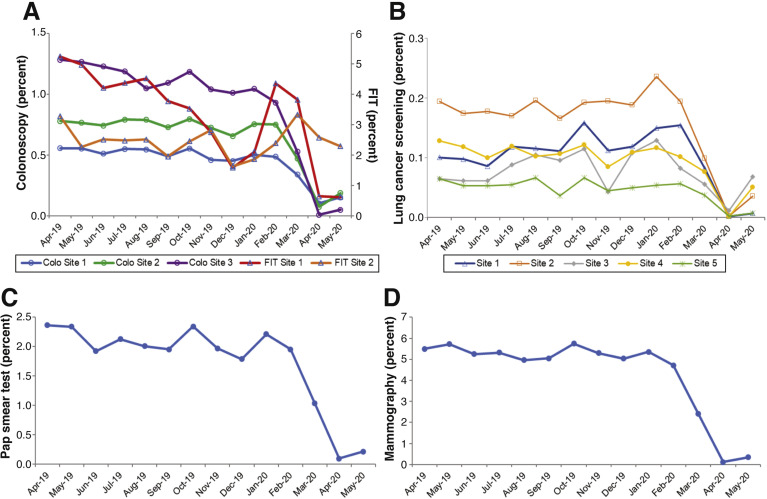
To address these knowledge gaps and to formulate a roadmap for resuming cancer screening, the National Cancer Institute’s Population-based Research to Optimize the Screening Process (PROSPR) consortium compared breast, cervical, colorectal, and lung cancer screening rates before and after the pandemic and developed pragmatic recommendations. The PROSPR consortium is designed to evaluate and improve cancer screening processes and outcomes. Data were available from eight large health care systems in seven states, covering >11 million individuals (approximately 1 of every 30 people in the United States). Most sites studied rapidly approached zero screening among target-age populations during the early phase of the pandemic, across diverse types of health care delivery systems (Figure 1 ). Breast cancer screening had the largest decrease (a 96% decrease), from 5.3% of age-eligible persons screened per month in April to September 2019 to 0.23% in April and May 2020 (P < .01). Screening for lung, cervical, and colorectal cancers at most sites had similar declines with 62%, 92%, and 82% decreases, respectively.
Figure 1.
Changes in cancer screening rates among screening eligible ages (by cancer type) within 8 PROSPR sites. Five PROSPR sites contributed to lung cancer data for low-dose computerized tomography (LDCT), 3 sites to colorectal cancer data, 1 to breast cancer data, and 1 to cervical cancer data. Two colorectal cancer sites provided data for both total colonoscopies performed and fecal immunochemical testing (FIT) completed. Variations in FIT-based screening also relate to site-specific variations in outreach during holiday periods (less outreach) and colon cancer awareness month. Lung cancer data include screening eligible ages, but do not include smoking history criteria.
Two important findings may inform future actions. First, 1 large site in the Western United States continued mailing FIT for colorectal screening, which does not require a face-to-face interaction, and maintained high screening test returns during April and May 2020 (Figure 1; tests are sent steadily over the year related to birthday, for members not up to date with screening. Typical FIT return rates are >50% and the net screening up-to-date proportion for this site, using all modalities, is >80% (this includes people with a prior colonoscopy for FIT positive). This notable exception strongly suggests that remote cancer screening methods can be successful during the pandemic. Although remote sampling methods, such as FIT or human papilloma virus testing, allow widespread testing without in-person contacts for initial screening, they still require follow-up in-person evaluations for positive tests. This point emphasizes the need for safe in-person testing environments. Second, screening decreases were uniform across sites, despite marked geographical variation in underlying SARS-CoV-2 infection rates: around May 10, for example, the 7-day average test positivity rates for states with PROSPR centers studied ranged from 0.3% to 16.8%, even though almost all sites approached zero screening. This discordance suggests the potential for closer alignment between local infection risk and concomitant reductions in preventive health care delivery, assuming ample SARS-CoV-2 monitoring is available. Positive testing rates for SARS-CoV-2 are difficult to interpret, given variation in testing criteria during the pandemic (eg, symptomatic vs asymptomatic). The infection rates for preprocedure testing of asymptomatic people are likely far lower.5
The costs of decreasing cancer screening are likely to include delayed cancer detection, more advanced stages of malignancy at diagnosis, and loss of life-years among those with cancer. A modeling study from the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network suggested that even moderately longer times between a positive screening test and follow-up diagnostic testing could significantly reduce the life-years gained from cancer screening.6 Significantly decreased benefit was suggested with even a few weeks delay for breast cancer and within months for colorectal cancer.6 The decreases in screening across the United States demonstrated by PROSPR data predict substantial increases in cancer morbidity and mortality in the coming years. Although detailed cancer diagnoses, staging, and survival during the pandemic await verification from cancer registries, 1 PROSPR site with such real-time pathology data demonstrated that the monthly average colorectal cancer diagnoses decreased by 31% between April and September 2019 and April and May 2020 (P < .01).
Still unknown is whether pandemic-related health care changes will induce disparities in other health outcomes, including cancers amenable to screening.7 This uncertainty is despite well-documented racial, ethnic, and socioeconomic disparities for COVID-related diagnoses and deaths.8 , 9 Among persons completing screening within the PROSPR populations studied, screening rates decreased markedly across all PROSPR sites, for all cancer types, independent of race/ethnicity (P > .10 for all comparisons). Among persons screened, the demographic proportions completing a test were similar before versus during the COVID-19 pandemic by race/ethnicity (eg, non-Hispanic Whites 50% vs 46%; Hispanic 22% vs 29%; Asian 16% vs 13%; and African Americans 7% vs 8%, respectively).
The reintroduction of cancer screening during the pandemic, however, poses a large risk for enhancing or introducing new disparities. Will the COVID-19–related financial stresses on health care systems allow equal resumption of robust screening programs across the population? Will shifts to telemedicine generate differences in who will request or be referred for screening, who will receive and complete active outreach, or who will schedule in-person follow-up testing? Will the pandemic’s economic ramifications (which impact job and insurance status) exacerbate existing national sociodemographic differences in health care access and outcomes? The resumption of routine health care practices, including cancer screening, must incorporate intentional strategies to minimize the introduction of health disparities.
We urgently recommend several pragmatic steps to address the opportunities and challenges of resuming cancer screening services during the pandemic. Informed by the data presented, and with the goal of increasing effective, consistent, and universal delivery of safe screening services, the PROSPR consortium has developed and recommends the following urgent interventions:
-
•
Broader implementation of remote testing, to reduce the need for in person visits, such as increased use of established methods (eg, mailed FIT for colorectal cancer screening) and rapid evaluation of emerging strategies (eg, self-sampling for human papillomavirus for cervical cancer screening).
-
•
Screening outreach programs that intentionally target patients at highest social risk, including demographic groups who are less likely to spontaneously seek or complete screening.
-
•
Rapid implementation of risk stratification tools to identify those at highest medical risk of cancer by age and other risk factors (including lack of prior screening) and those at lowest risk, who are unlikely to benefit from screening.
-
•
Infection control measures to maximize patients and staff safety, such as pre-procedure testing. These measures should include effective communication to decrease patient concerns regarding screening. Test performance characteristics (ie, false positives and negatives) will influence the benefit of preprocedure testing at very low or moderately high background levels of disease prevalence.5
-
•
Customized cancer screening practices, coordinated with local SARS-CoV-2 risk, to maximize screening test completion in areas with low viral prevalence.
-
•
Real-time demographic data for early identification of screening service uptake disparities.
The COVID-19 pandemic has created unprecedented decreases in cancer screening services, which will likely have long-term deleterious effects on cancer morbidity and mortality. When to resume routine care remains uncertain and delivery patterns may change. Several pragmatic steps are urgently recommended to reduce potential cancer-related outcomes and to avoid exacerbating disparities. These steps can help to restart screening and thereby equitably decrease the noninfectious impacts of the SARS-CoV-2 pandemic.
Footnotes
This study was conducted as part of the NCI-funded consortium Population-based Research to Optimize the Screening Process (PROSPR) consortium. The overall aim of PROSPR is to conduct multi-site, coordinated, transdisciplinary research to evaluate and improve cervical, colorectal, and lung cancer screening processes. The PROSPR Research Centers and their associated sites reflect the diversity of US delivery system organizations. UM1CA222035, UM1CA221939, U24CA221936.
Contributor Information
The National Cancer Institute’s PROSPR Consortium:
Douglas A. Corley, Mai Sedki, Debra P. Ritzwoller, Robert T. Greenlee, Christine Neslund-Dudas, Katharine A. Rendle, Stacey A. Honda, Joanne E. Schottinger, Natalia Udaltsova, Anil Vachani, Sarah Kobrin, Christopher I. Li, and Jennifer S. Haas
References
- 1.Centers for Medicare & Medicaid Services CMS Releases Recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response 2020. www.cms.gov/newsroom/press-releases/cms-releases-recommendations-adult-elective-surgeries-non-essential-medical-surgical-and-dental Available:
- 2.Solomon M.D., McNulty E.J., Rana J.S., et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 3.EPIC Health Research Network Preventive cancer screenings during COVID-19 pandemic. www.ehrn.org/wp-content/uploads/Preventive-Cancer-Screenings-during-COVID-19-Pandemic.pdf 2020 Available: 2020 Accessed June 29, 2020.
- 4.US Preventative Services Task Force A and B recommendations. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/uspstf-and-b-recommendations
- 5.Sultan S., Lim J.K., Altayar O., et al. AGA rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020;159:739–758.e4. doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutter C.M., Kim J.J., Meester R.G.S., et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol Biomarkers Prev. 2018;27:158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman H.W., Chen Z., Niles J., et al. Changes in the number of US patients with newly identified cancer before and during the Coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yehia B.R., Winegar A., Fogel R., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rentsch C.T., Kidwai-Khan F., Tate J.P., et al. In: PLoS Med. Zelner J., editor. Vol. 17. 2020. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]