Abstract
Captive rearing of monarch butterflies is a commercial and personal pursuit enjoyed by many different groups and individuals. However, the practice remains controversial, especially after new evidence showed that both a group of commercially derived monarchs reared outdoors and a group of wild-derived but indoor-reared monarchs failed to orient south, unlike wild-derived monarchs reared outdoors. To more fully characterize the mechanisms responsible for the loss of orientation in both commercial and indoor-reared monarchs, we performed flight simulator experiments to determine (i) whether any fraction of commercial monarchs maintains a southern heading over multiple tests, and (ii) whether indoor conditions with the addition of sunlight can induce southern flight in wild-derived monarchs. Commercial monarchs changed their flight direction more often over the course of multiple tests than wild-derived monarchs. While as a group the commercial monarchs did not fly south on average, a subset of individuals did orient south over multiple tests, potentially explaining the discordance between flight simulator assays and the recovery of tagged commercial monarchs at overwintering locations. We also show that even when raised indoors with sunlight, wild-derived monarchs did not consistently orient south in the flight simulator, though wild-derived monarchs reared outdoors did orient south.
Keywords: Danaus plexippus, migration, captive rearing, commercial breeding, monarch flight behaviour, directional orientation
1. Introduction
Captive-reared monarch butterflies are reared and released at schools, weddings, conservation events, fairs and by individual enthusiasts. However, the term ‘captive reared’ represents a spectrum of practices, including (i) raising wild-collected eggs and caterpillars in non-natural environments for eventual release, (ii) breeding wild-collected individuals for a few generations and releasing them into the wild, and (iii) raising eggs and caterpillars bought from a commercial source for release. Captive breeding can affect reared individuals' behaviour, morphology and physiology in two distinct ways: changes to the genetic background of the population through inbreeding and adaptation to captive environments and exposure to and development in non-natural conditions [1,2].
Long-term breeding in captivity is known to alter behaviour in fishes, mice, Drosophila and toads [3–7]. In monarchs, we have previously identified a population of commercially bred individuals that are genetically divergent from North American wild-type monarchs that no longer orient south as a group even when reared in conditions known to induce directional orientation in wild-derived individuals [8]. While the orientation of the commercial monarchs was non-directional as a group [8], other tagged commercial monarchs have been found at Mexican overwintering sites, prompting the question of whether some fraction of the commercial individuals can, in fact, migrate. To assess the individual directionality of commercial monarchs, we assessed directional orientation of individuals from a known ‘non-directional’ North American commercial population [8] and North American wild-derived population (from here on referred to as commercial and wild type, respectively) multiple times to establish whether an individual would repeatedly fly south. Previous work, including our own, concluded testing after a single, successful orientation flight trial per individual [8–11]. By testing an individual repeatedly, we aim to determine whether specific individuals within the larger population exhibit directional orientation.
While rearing wild-collected monarch eggs will not change the genetic background of the individuals, artificial captive environments induce fitness differences in numerous fish species and monarch butterflies reared captively for a single generation [12–18]. In general, artificial rearing environments produce individuals that fare worse than wild individuals when released [2]. In both migratory fish and monarchs, changes to rearing environment affect migratory behaviour [8,17]. Specifically, rearing migratory wild-type North American monarchs in an autumn-like environmental chamber (short day length and cool temperature) resulted in a group that oriented in random directions, while rearing wild type outdoors resulted in a group that oriented south [8].
Since the environmental chamber does not replicate natural sunlight, we reared wild-type monarchs indoors with access to sunlight as filtered through glass windows during autumn and tested their directional orientation. Changes in photoperiod and declination of the sun during the transition between summer and autumn are hypothesized to be important environmental cues to induce migratory monarch development [19–21]. Monarchs are known to use a time-compensated sun compass to navigate; in fact, shifting their circadian clock with different light entrainment shifts orientation in migrating individuals [9–11]. While the position of the sun throughout the day plus light entrainment is critical for navigation, we do not know how important natural sunlight is for the development and triggering of directional orientation.
2. Methods
(a). Animal husbandry
In late July 2019, we caught approximately 20 wild monarchs in Hyde Park, Chicago, Illinois and ordered 20 commercial monarchs from the same source of commercial monarchs documented in Tenger-Trolander et al. [8]. We then checked the abdomens of each monarch for signs of Ophryocystis elektroscirrha (OE) spores and froze individuals with apparent infection. We housed the uninfected male and female monarchs from their respective populations in medium-size (91.5 cm × 30.5 cm2) mesh pop-up cages outdoors with access to their host plant, Asclepias syriaca. Once females laid eggs, we washed and transferred the eggs to small (30.5 cm3) mesh pop-up cages. We reared caterpillars in groups rather than individually. We fed caterpillars a diet of wild-collected Asclepias syriaca that we replenished daily. We washed the milkweeds in a 1% bleach solution and then in water before offering them to the larvae. Upon emergence, we labelled each adult with a unique identification number in permanent marker on the hindwing. Adults were housed in medium-size (91.5 cm × 30.5 cm2) mesh pop-up cages before directional orientation testing and fed a diet of Birds Choice butterfly nectar.
(b). Treatments
We housed the developing wild-type monarchs in one of three treatment groups: (i) outdoors, (ii) indoors in a glass-top greenhouse and (iii) indoors in our laboratory (lab) next to a south-facing window. The commercial monarchs developed outdoors and had no other treatments. For the outdoor treatment, pop-up cages were contained within a large outdoor 1.83 cm3 mesh cage. The greenhouse received only natural light, and we kept the temperature at 23°C during the day and 18°C at night. Temperatures in the laboratory remained fairly consistent between 22 and 23°C, 24 h a day. Both indoor groups emerged between 24 September and 28 October 2019. Commercial monarchs reared outdoors emerged between 12 September and 1 October 2019, and wild-type monarchs reared outdoors emerged between 8 September and 16 September 2019.
Unfortunately, we experienced a suspected outbreak of nuclear polyhedrosis virus (NPV) which reduced expected sample sizes and pushed back the dates of emergence of our wild-type reared indoors as we attempted to control spread of the virus. We found the wild type population was particularly susceptible; however, final sample sizes were sufficient to determine directional orientation.
(c). Flight simulator and testing
After 4 days in their respective rearing conditions (outdoor, indoor greenhouse or indoor laboratory), we tethered the monarch adults following the protocol outlined in Tenger-Trolander et al. [8]. All tethered monarchs then spent 5 days recovering in glassine envelopes stored in a 12 : 12 h light–dark cycle, 21°C environmental chamber before testing. Directly before testing, all monarchs spent at least a full hour in an outdoor cage free to move.
We tested all individuals in the monarch flight simulator developed by Mouritsen & Frost [11] (figure 1a; see Tenger-Trolander et al. [8] for description of modified flight simulator). All testing occurred outdoors in sunny conditions between the hours of 10.00 and 14.30. We counted the orientation test as successful if the individual flew continuously for 10 min as confirmed by video recording (see electronic supplementary material, TengerTrolander_Video_S1.mp4 for an example of a non-directional monarch and TengerTrolander_Video_S2.mp4 for a southern-oriented monarch). We only tested individuals once per day whether the test was successful or not. Due to changing weather conditions, time restrictions on testing and variability in emergence dates, every tethered monarch could not be tested each day of testing. We focused testing on the outdoor wild-type and commercial individuals to determine individual preferences in directional orientation in these groups. Electronic supplementary material, table S1 details the number of orientation tests and successful tests of each individual by treatment and population.
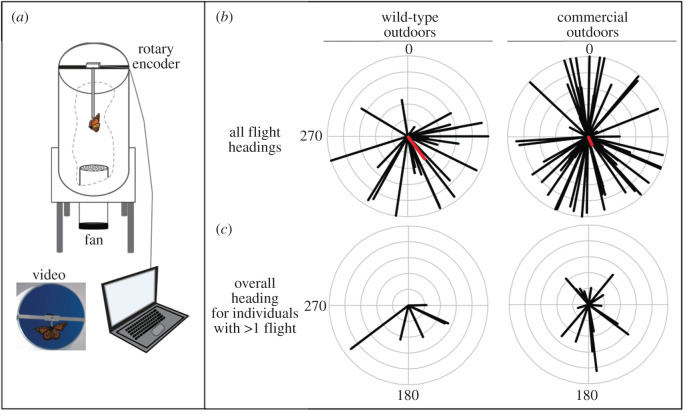
Figure 1.
(a) Flight simulator schematic. (b) Orientation plots of wild-type and commercial monarch butterflies reared outdoors in autumn in Chicago, IL. Black lines indicate the vector direction (0–359°) and the length of that line is the vector magnitude, indicating consistency of flight (0 to 1). 0° is North. All flight tests for eight wild-type monarchs with 24 total flights and 27 commercial monarchs with 61 total flights. Group mean direction and magnitude are highlighted. (c) Overall mean directions for six wild-type and 16 commercial monarchs with at least two flight tests. (Online version in colour.)
In total, we tethered 83 monarchs, of which 74 survived long enough to be tested, including 15 wild types reared outdoors, 18 wild types reared in the greenhouse, 4 wild types reared in the laboratory and 37 commercials reared outdoors. Of these 74 tested, 65% (n = 48) flew at least once, including 8 wild types reared outdoors, 12 wild types reared in the greenhouse, 3 wild types reared in the laboratory and 25 commercials reared outdoors. Of the 48 individuals that flew at least once, 56% (n = 27) completed at least one additional test, including 6 wild type reared outdoors, 4 wild types reared in the greenhouse, 1 wild type reared in the laboratory and 16 commercials reared outdoors. The number of repeated tests in the indoor-reared group was small (n = 5); however, we were not attempting to determine whether a portion of these individuals was migratory, but rather if the group as a whole (n = 15) headed south on average. The number of successful tests per individual ranged between one and seven (electronic supplementary material, table S1). Flight headings were recorded using a US Digital optical rotary encoder and captured on video (figure 1a). Since orientation data and video were recorded autonomously, testing was not conducted blind.
(d). Data analysis and circular statistics
In our flight simulator assays, tethered monarchs were attached to a rotary encoder and placed inside the simulator. As the monarch changed position, the rotary encoder recorded the new position (in degrees, 0–359°) and the amount of time elapsed (in milliseconds) between each change. We used circular statistics packages, Circular and Plotrix in R, to analyse and plot flight simulator data [22–24]. After converting degrees to cartesian coordinates, we found the mean vector direction (σ = 0–359°) and mean resultant vector magnitude (r = 0–1) of each test. The mean vector direction is the average heading and the vector magnitude is a measure of consistency of the heading (r = 1 − variance). We also calculated a weighted group mean vector and magnitude and used the Rayleigh test to determine whether the group mean was directional.
Additionally, we calculated overall vector mean direction and vector magnitude for each monarch with between two and seven independent orientation tests. For example, an individual monarch with three tests with strong vector magnitudes (r > 0.5) could still have a weak overall vector magnitude if it headed in vastly different directions (e.g. 0°, 90°, 180°) for each test. The weak overall vector magnitude indicates the monarch chose a different direction for each of the three tests. We then took each individual's overall heading and subtracted that from the individual's first heading. We compared the difference in degrees between commercial and wild type with a Welch's t-test. We also calculated each individual's vector magnitude variance for all tests and compared commercial and wild-type means with a Welch's t-test.
(e). Random re-sampling of migratory flight data
To determine whether any commercial monarchs with multiple orientation tests were likely migrators, we assessed the probability of finding each individual's multiple flight headings within a distribution of known migrators using a random re-sampling approach. Including data from the autumns of 2016, 2018 and 2019, we have orientation data for 55 wild-type North American monarchs raised outdoors. Directional orientation data from the outdoor-reared wild types tested in 2016 and 2018 are available in Dataset_S01.xlsx file of Tenger-Trolander et al. [8]. Data from 2019 are available in the electronic supplementary material, TengerTrolander_Data_S1.xlsx, of this paper. Monarchs from 2016, 2018 and 2019 were all reared outdoors in the same conditions, but with variability in eclosion dates. Monarchs reared in 2016 eclosed between 7 and 20 October, those reared in 2018 eclosed between 7 and 18 September, and those reared in 2019 eclosed between 8 and 16 September.
We binned the 55 orientation tests into either north (270–89°) or south (90–269°) bins, resulting in 51 southern binned and four northern binned tests. From those 55 binned wild-type migratory tests, we randomly sampled, with replacement, the number of tests an individual completed (between 2 and 7) 5000 times. Each random sample had several possible orientation patterns going north and south. For instance, in the case of 5000 random samples of three tests, the possible patterns encountered are SSS, SSN, SNN or NNN (where S was south and N was north and order was not considered). We then counted how many of those 5000 random patterns were SSS, SSN, SNN and NNN. In the case of SSN, we found it appeared 350 times out of 5000 trials or 7% of the time in the known migratory group. An individual with three orientation tests that oriented south twice and north once has a 7% probability of being a migrator.
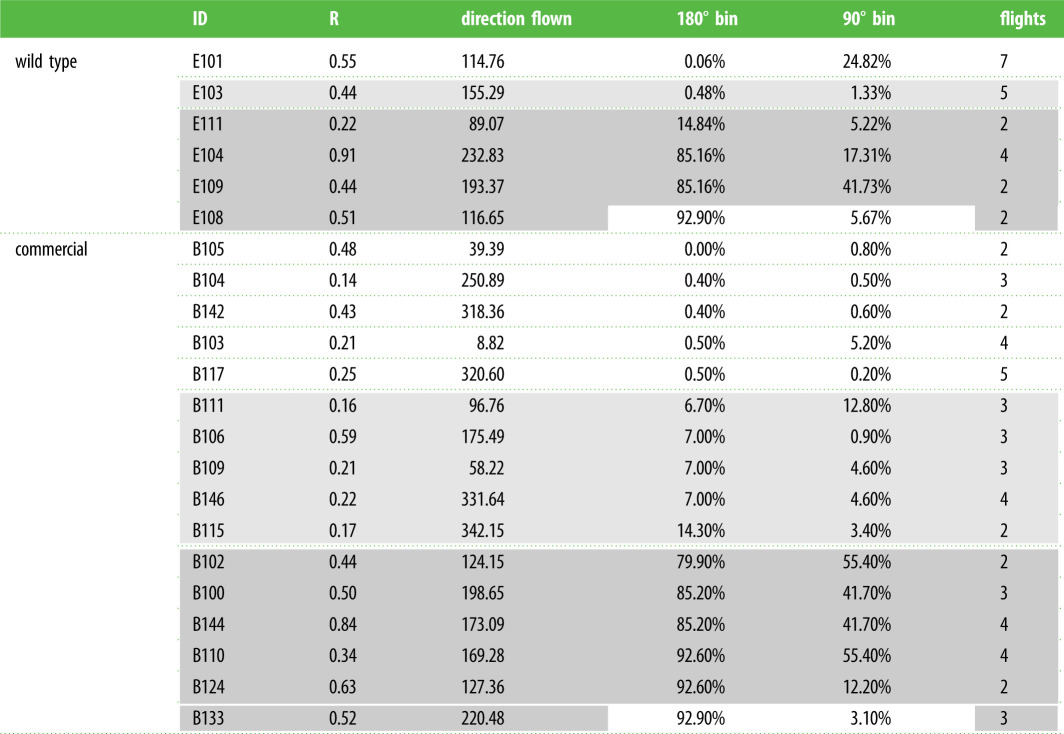
The number of bins and degree cutoffs for each bin was arbitrary and could be changed. We also analysed the data in 90° bins with the following degree cutoffs: northeast (0–89°), southeast (90–179°), southwest (180–269°) and northwest (270–359°). While the specific probabilities changed, which individuals are least and most likely to be migratory did not; exceptions are highlighted in white in table 1. In our dataset, degree cutoffs affected the probabilities of two individuals (E101 & E103) described in detail in the results.
Table 1.
Data for all individuals with multiple flight tests from wild-type and commercial groups. Identification number (ID), mean vector strength (R), overall mean vector (Direction Flown), the probability that individual's flight pattern is part of the migratory distribution using 180° binning (180° bin), the probability that individual's flight pattern is part of the migratory distribution using 90° binning (90° bin), and the total number of successful flight tests per individual (Flights) are reported. Data are organized from lowest to highest probability using 180° bin. Shading indicates the probability of the individual being part of the migratory flight distribution. Dark grey denotes those monarchs with very clear migratory results, light grey those monarchs with unclear results and white highlights either discrepancies between 90° and 180° binning probabilities (E101, E108, B133) or a low probability of being part of the migratory distribution.
|
(f). Outdoor exposure and southern orientation
After the conclusion of our study, new work suggested that indoor-reared monarchs could re-orient when released outdoors [25]. We were interested in whether increasing outdoor exposure would potentially correlate with southern orientation. Since tethered monarchs were brought outdoors during each testing session and remained outdoors for the full testing period, which lasts several hours, most individuals spent many hours outside over the course of days. Using flight records from each test day, we calculated the minimum time spent outdoors by each of the indoor-reared monarchs. We tested whether there was any correlation with directional orientation south using a non-parametric Kruskal–Wallis H test.
3. Results
(a). Multiple directional orientation tests in wild-type and commercial monarchs reared outdoors
For the orientation tests comparing wild type and commercial, we reared monarchs outdoors during autumn. We tested eight wild-type and 27 commercial monarchs. Six of the wild types and 16 of the commercial yielded multiple orientation tests (figure 1b; wild type: 24 total flights = 8 first flights + 16 additional flights from the six individuals with multiple tests, commercial: 61 total flights = 27 first flights + 34 additional flights from the 16 individuals with multiple tests). Wild-type monarchs flew with an average heading south (σ = 143°) and a vector magnitude of r = 0.35 (figure 1b; Rayleigh test, z-score = 2.88, 0.05 < p < 0.10). Commercial monarchs' average heading was also south (σ = 155°), but with a much weaker magnitude, r = 0.11 (figure 1b; Rayleigh test, z-score = 0.68, p > 0.50).
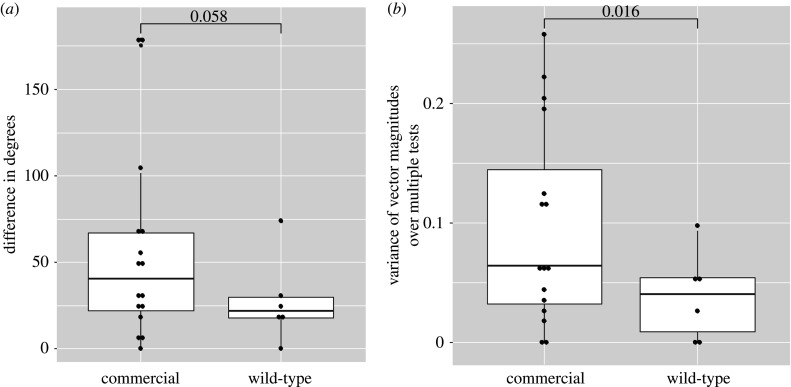
We then determined overall orientation headings for each of the monarchs with multiple orientation tests (figure 1c). Five of the six (83.33%) wild types had overall vector magnitudes greater than 0.4 with overall headings south (90–270°), while the 6th individual's overall direction was 89° with a relatively weak vector magnitude, 0.22 (figure 1c; table 1, wild type). Six of the 16 (37.5%) commercial individuals had overall headings south with vector magnitudes greater than 0.4 while the remaining 10 individuals' overall headings were north and/or with magnitudes less than 0.4 (figure 1c; table 1, commercial). The difference in degrees between an individual's first flight and the mean of all their flights showed wild-type monarchs chose more similar headings over multiple tests than commercial (t-test, t = 1.64, d.f. = 18.88, p-value = 0.058; figure 2a). Additionally, we compared the variance of vector strengths in each individual's multiple tests between the two groups. Commercial monarch vector magnitudes varied significantly more around an individual's mean than wild type (t-test, t = 2.29, d.f. = 19.33, p-value = 0.016; figure 2b), indicating that commercial monarchs were sometimes very directional during a test and then much less directional for the subsequent test whereas the wild types maintained similar vector strengths over multiple tests.
Figure 2.
Wild-type monarchs are more directional over multiple tests than commercial monarchs. (a) The difference (0–180°) between an individual's first vector heading and overall vector heading is nearly significant between commercial and wild-type monarchs. (b) Individual wild-type monarchs' vector magnitudes vary less around the individual's overall mean than commercial. (Online version in colour.)
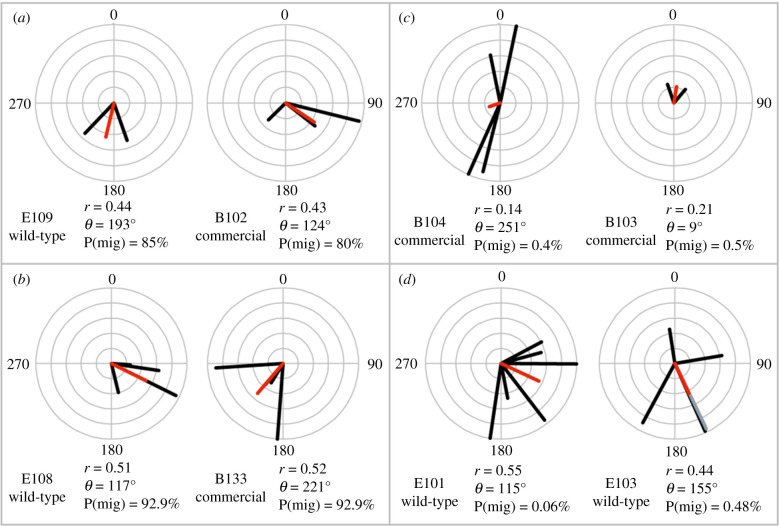
We next determined whether commercial monarchs with multiple orientation tests were possible migrators, by assessing the probability of finding each individual's multiple flight headings within a distribution of known migrators. 37.5% (six of 16) of commercial and 83.33% (five of six) of wild-type monarchs had orientation test patterns consistent with the known migratory distribution of orientations (table 1). Figure 3a,b are examples of four individuals whose test patterns suggest a strong probability of southern orientation. Figure 3c shows the patterns of two individuals with low probability.
Figure 3.
Orientation plots of eight monarchs reared outdoors. The overall mean of orientation tests is highlighted. Line direction indicates the vector heading (θ = 0–359°) and the length of that line is vector magnitude (r = 0 to 1). 0° is North. P(mig) is the probability the individual's pattern of orientation tests is migratory given 180° bins. (a) E109 and B102 are wild-type and commercial monarchs, respectively, with all tests heading south, strong overall vector magnitudes and strong probabilities of being migratory. (b) E108 and B133 are wild-type and commercial monarchs, respectively, with all tests heading south and strong probabilities of being migratory when binned by 180°, but significantly lower when binned by 90°. (c) B104 and B103 are both commercial monarchs with low probabilities of being migratory. (d) E101 and E103, wild types reared outdoors, have lower than expected probabilities of being migratory due to the constraints imposed by strict binning cutoffs (i.e. all flights must be exactly between 90° and 269° to count as part of the migratory distribution in 180° bins). For E103, note two flights are overlapping one shown in black and the other in grey. (Online version in colour.)
We noted that though E103 and E101 (wild-type reared outdoors) had low probabilities of being part of the migratory distribution in the 180° binning procedure, both have overall southern headings with strong vector magnitudes (table 1 and figure 3d). E103 headed north on two out of five tests, but one of those flights was within 10° of being binned as south (figure 3d). This is in contrast to the low probability commercial individuals, which all had northern mean headings or weak southern vector magnitudes (table 1). In total, only 6 of 16 commercial monarchs showed signs of directional orientation south (table 1).
(b). Directional orientation in wild-type monarchs reared indoors
We reared wild-type monarchs with natural light (as filtered through glass windows) during autumn in both a glass-top greenhouse and near a south-facing window in our laboratory and compared them to the outdoor-reared group. We tested 15 indoor-reared monarchs, five of which produced multiple orientation tests (figure 4; indoor wild type: 26 total flights = 15 first flights + 11 additional flights from five individuals with multiple tests). The mean heading for those reared indoors was west, σ = 259° with a weak vector magnitude, r = 0.12 (figure 4a; Rayleigh test, z-score = 0.40, p > 0.50). Eleven of the 26 flights (42.3%) (from nine distinct individuals) had northern headings (figure 4a) compared to six (from three distinct individuals) of 24 (25%) flights in wild-type reared outdoors (figure 1b). The five wild types reared indoors with multiple tests had overall means both south and north with strong and weak vector magnitudes (figure 4b). Even with the addition of autumnal sunlight through windows, we found outdoor wild-type flight behaviour was not completely recapitulated in the indoor-reared group.
Figure 4.
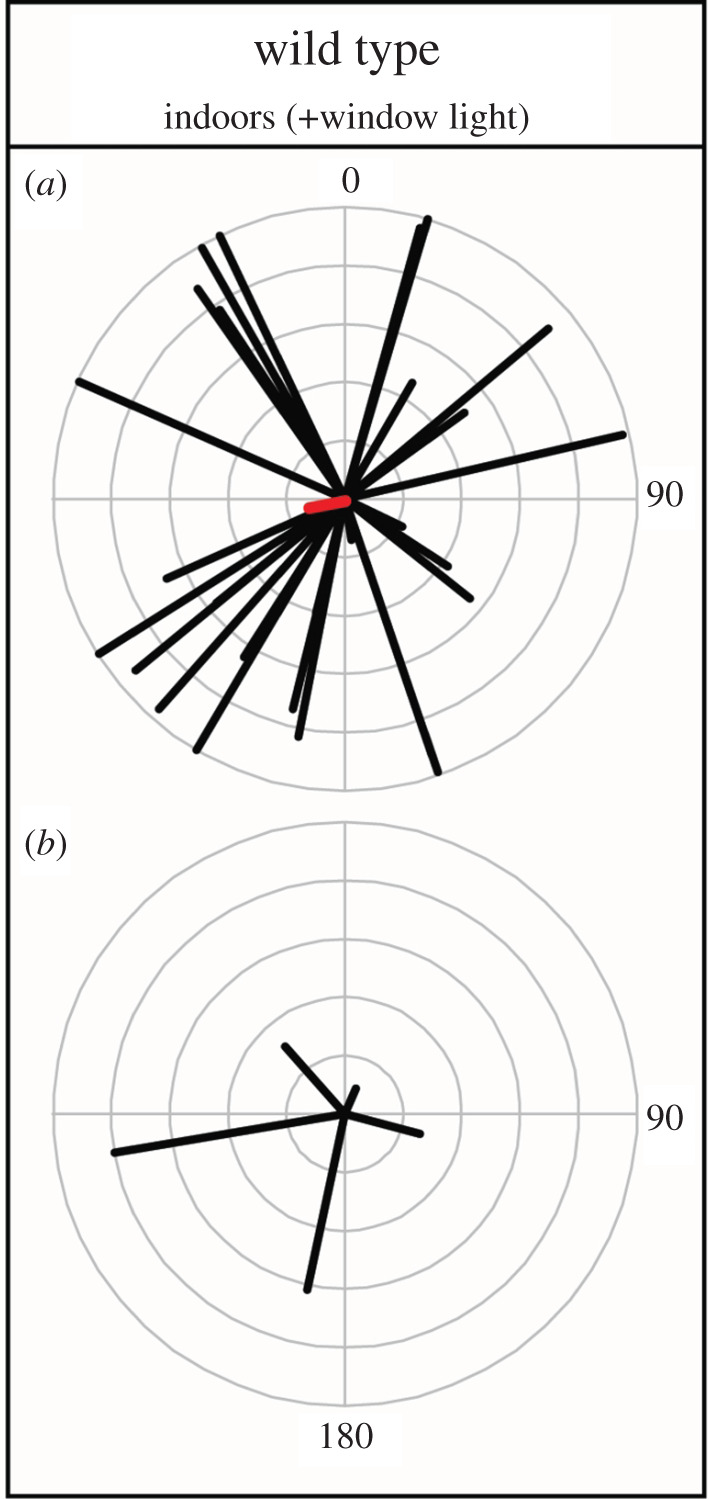
Wild-type monarchs raised indoors with window light. (a) all flight tests, for 15 individuals reared indoors with a total of 26 flights. Group mean direction and magnitude are highlighted. (b) The overall mean directions for five indoor-reared individuals with at least two flight tests. (Online version in colour.)
New work has suggested that monarchs reared indoors, but then released are capable of re-orienting outdoors [25]. In the light of this work, we used our testing records to calculate the total amount of time that each indoor-reared monarch spent outdoors prior to their test and found no correlation with directional orientation—more outdoor time did not increase the likelihood of southern orientation (Kruskal–Wallis chi-squared = 25, d.f. = 25, p-value = 0.4624; electronic supplementary material, table S2).
4. Discussion
While a great deal is known about inducing diapause [19,26,27] as well as how the monarch uses its circadian clock to navigate [9–11], how monarchs develop and maintain directional orientation is less clear. The southern directional orientation phenotype requires a yet unknown combination of environmental conditions and genetics. Our earlier work suggested changes in long-term selection pressures and short-term developmental conditions can affect whether monarchs orient south in a flight simulator [8]. Here, we looked more closely at the behaviour of individual commercially sourced monarchs and investigated the effects of indoor rearing conditions with sunlight exposure on directional orientation.
We found that the commercial monarchs are a mix of southern-orienting and non-southern-orienting individuals, suggesting that the directional orientation phenotype is not fixed in this population. Migration imposes a strong selective pressure on migratory monarchs as only successful migrators will pass on their genes in the coming spring. In commercial facilities, the difficulties of flying thousands of kilometres, finding the overwintering ground and surviving till spring are no longer barriers to successful breeding. Add to that small population sizes inherent to commercial breeding and long-term captivity could lead to stochastic increase in the frequency of non-migratory alleles that do not respond to the correct environmental cues or alter the reaction norm of the population making responses to the environment more variable. While this study is limited to a single population of commercial monarchs, the mechanism of loss may be relevant to all long-term captive breeding populations.
While the effect of commercial releases on the North American monarch population is currently unknown, it may be ultimately inconsequential if natural selection purges the wild population of non-migratory individuals. After all, any non-migratory individuals would simply die in winter, their alleles never passed on to the next generation. However, this argument ignores two things, (i) the presence of new resident populations in the southern USA that can offer refuge to poor migrators and (ii) the likely recessive [8] and polygenic nature of migration genetics. In fact, crosses of the commercial and wild-type monarchs resulted in offspring that oriented south in autumn [8]. Non-migratory alleles could persist in the genetic background of a migratory individual. Releasing these commercial individuals may result in more monarchs in Mexican overwintering grounds in the short term, but have unintended consequences on their genetics in the long term. Additionally, the introduction of non-migratory alleles into the wild population may actually increase the number of individuals that breed year-round in the southern USA [28–30], which has implications for the increased transmission of the monarch parasite OE. Resident populations have higher rates of OE infections [31], and having more resident populations could lead to increased infection in the migratory population as it travels between the overwintering grounds and summer habitat [30]. Beneficial, neutral or detrimental, the release of non-migratory alleles into a wild migratory population is worth discussing critically.
The effect of rearing environment should also be considered. Wild-type monarchs reared indoors with full exposure to natural autumn sun did not consistently orient south, though their genetic background is identical to the wild types reared outdoors. That being said, our results do not fully answer the question of what degree of ‘naturalness’ is required to rear a directional adult. As we have only five indoor-reared individuals with multiple tests, we do not know if some proportion of the indoor-reared individuals is directional. However, placing captive-reared monarch larvae/pupae near a window does not result in as many directionally oriented monarchs as full outdoor exposure. Scientists have long speculated about the potential environmental variables that ‘turn on’ the migration developmental program including photoperiod changes, temperature variation, sun declination and host plant quality [19–21,32,33]. While we do not know which cue or combination of factors is responsible or the critical development times, we do know that the following conditions did not result in adults with consistent southern orientation: (i) rearing in an autumn-like environmental chamber (ii) rearing in a room with sunlight and autumn-like temperatures during autumn and (iii) eclosing in an environmental chamber after almost complete juvenile development outdoors. So far, in our flight simulator experiments, only wild adult monarchs caught in autumn and wild-type monarchs reared outdoors in autumn fly consistently south. And once oriented south, storing monarchs in an environmental chamber does not affect their southern orientation [34] unless the temperature is dropped. Exposure to very cool temperatures in an environmental chamber causes re-orientation north [35] in preparation for the spring re-migration.
New work from Wilcox et al. [25] suggests that monarchs reared indoors may recover southern orientation after release. In their study, Wilcox et al. [25] used a flight simulator to find the headings of a group of indoor-reared monarchs and found they did not orient south, consistent with our flight results of monarchs reared indoors. They also released groups of radio transmitter tagged monarchs reared indoors and found that the individuals flew an average of 37.4 km south [25]. These results imply that regardless of rearing conditions experienced during development, adults given sufficient time outdoors in autumn would eventually fly south, suggesting monarchs are capable of re-orienting.
Currently, we cannot directly compare the flight simulator or radio-tracking data from Wilcox et al. [25] to wild-caught or wild-type monarchs reared outdoors, which are known to fly south in autumn, because Wilcox et al. [25] did not employ positive or negative controls. While our results and those of Wilcox et al. [25] do not give us a completely clear understanding of the development of southern orientation in autumn in monarchs, together they suggest southern directional flight behaviour could be engaged in adulthood. In light of this possibility, we calculated the amount of time each indoor-reared monarch spent outdoors prior to each test but found no correlation between increased time spent outdoors and propensity to fly south.
In addition to radio-tracking data, mark-recapture studies of indoor-reared monarchs do recover a number of individuals at overwintering sites [36,37]. However, a study that tagged and released groups of both wild-caught and captive-reared eastern monarchs showed the recovery rate of captive-reared monarchs was significantly lower than that of the wild monarchs [37]. Fifty-six of 11 333 wild-caught monarchs were recovered in Mexico whereas only two of 3056 captive-reared monarchs were recovered (χ2 = 10.96, p = 0.00093) [37]. The same study also re-captured monarchs as they travelled south. While only three reared and five wild recoveries are reported in the paper [37], a total of 10 indoor-reared and 6 wild-caught individuals were eventually recovered (G. Steffy, 2020, personal communication). The captive-reared travelled an average distance of 120 km and the wild caught an average of 560 km (Mann–Whitney U, p-value = 0.002997) [37] (G. Steffy, 2020, personal communication). Even in the case that monarchs reared indoors re-orient upon release, captive-reared monarchs were less successful in reaching Mexico than wild monarchs [37].
While many people hope that captive rearing is helping a declining population, the cumulative data available suggest that captive breeding of monarchs has negative consequences for migration behaviour and that monarchs reared indoors are not as well equipped to survive migration as those left in the wild [8,13,37]. We also know that rearing monarchs at home and in educational settings inspires new generations of conservationists, nature-lovers and scientists. For those who love rearing monarchs, we advise the following: rear caterpillars individually in clean enclosures, rear outdoors when possible (especially in late summer and autumn), limit the total number reared, avoid purchasing and participate in citizen science projects. The non-profit Monarch Joint Venture (monarchjointventure.org/get-involved/study-monarchs-citizen-science-opportunities) lists links to many on-going studies which have contributed vastly to our understanding of monarch biology. Finally, if we want to ensure the future of migratory monarch populations, we must promote longer-term solutions, such as protecting and restoring habitat and addressing climate change.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Jack Chaillet for help with catching and rearing the monarch butterflies used in this study. We thank the many monarch enthusiasts and biologists with whom we have discussed these and previous results. Their passion and insight have helped fuel this research. We also thank our reviewers, who provided valuable feedback which greatly improved the paper.
Data accessibility
All directional orientation data are included in the electronic supplementary material.
Funding
This work was supported by the Graduate Research Fellowship Program, National Institute of Health Genetics and Regulation Training Grant (grant no. T32 GM07197), US Fish and Wildlife Service Award (award no. F17AC01222), National Science Foundation Grant (grant nos. IOS-1452648 and IOS-1922624) and National Institute of Health Grant (grant nos. GM131828 and GM108626).
Competing interests
We declare we have no competing interests.
References
- 1.Frankham R. 2008. Genetic adaptation to captivity in species conservation programs. Mol. Ecol. 17, 325–333. ( 10.1111/j.1365-294X.2007.03399.x) [DOI] [PubMed] [Google Scholar]
- 2.Jonsson B, Jonsson N. 2006. Cultured Atlantic salmon in nature: a review of their ecology and interaction with wild fish. ICES J. Mar. Sci. 63, 1162–1181. ( 10.1016/j.icesjms.2006.03.004) [DOI] [Google Scholar]
- 3.Jonsson B, Jonsson N, Jonsson M. 2019. Supportive breeders of Atlantic salmon have reduced fitness in nature. Conserv. Sci. Pract. 1, e85 ( 10.1111/csp2.85) [DOI] [Google Scholar]
- 4.Courtney JSK, Munn AJ, Byrne PG. 2017. Effects of captivity on house mice behaviour in a novel environment: implications for conservation practices. App. Ani. Behav. Sci. 189, 98–106. ( 10.1016/j.applanim.2017.01.007) [DOI] [Google Scholar]
- 5.Gilligan DM, Frankham R. 2003. Dynamics of genetic adaptation to captivity. Conserv. Genet. 4, 189–197. [Google Scholar]
- 6.Kraaijeveld-Smit FJL, Griffiths RA, Moore RD, Beebee TJC. 2006. Captive breeding and the fitness of reintroduced species: a test of the responses to predators in a threatened amphibian. J. App. Ecol. 43, 360–365. ( 10.1111/j.1365-2664.2006.01137.x) [DOI] [Google Scholar]
- 7.McPhee EM. 2004. Generations in captivity increases behavioral variance: considerations for captive breeding and reintroduction programs. Biol. Conserv. 115, 71–77. ( 10.1016/S0006-3207(03)00095-8) [DOI] [Google Scholar]
- 8.Tenger-Trolander A, Lu W, Noyes M, Kronforst MR. 2019. Contemporary loss of migration in monarch butterflies. Proc. Natl Acad. Sci. USA 116, 14 671–14 677. ( 10.1073/pnas.1904690116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froy O. 2003. Illuminating the circadian clock in monarch butterfly migration. Science. 300, 1303–1305. ( 10.1126/science.1084874) [DOI] [PubMed] [Google Scholar]
- 10.Merlin C, Gegear RJ, Reppert SM. 2009. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700–1704. ( 10.1126/science.1176221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouritsen H, Frost BJ. 2002. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc. Natl Acad. Sci. USA 99, 10 162–10 166. ( 10.1073/pnas.152137299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr JW, Whoriskey F, O'reilly P. 2004. Efficacy of releasing captive reared broodstock into an imperilled wild Atlantic salmon population as a recovery strategy. J. Fish Biol. 65, 38–54. ( 10.1111/j.0022-1112.2004.00546.x) [DOI] [Google Scholar]
- 13.Davis AK, Smith FM, Ballew AM. 2020. A poor substitute for the real thing: captive-reared monarch butterflies are weaker, paler and have less elongated wings than wild migrants. Biol. Lett. 16, 20190922 ( 10.1098/rsbl.2019.0922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalfe NB, Valdimarsson SK, Morgan IJ. 2003. The relative roles of domestication, rearing environment, prior residence and body size in deciding territorial contests between hatchery and wild juvenile salmon. J. App. Ecol. 40, 535–544. ( 10.1046/j.1365-2664.2003.00815.x) [DOI] [Google Scholar]
- 15.Milot E, Perrier C, Papillon L, Dodson JJ, Bernatchez L. 2013. Reduced fitness of Atlantic salmon released in the wild after one generation of captive breeding. Evol. App. 6, 472–485. ( 10.1111/eva.12028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosengren M, Kvingedal E, Näslund J, Johnsson JI, Sundell K. 2016. Born to be wild: effects of rearing density and environmental enrichment on stress, welfare, and smolt migration in hatchery-reared Atlantic salmon. Can. J. Fish. Aquat. Sci. 74, 396–405. ( 10.1139/cjfas-2015-0515) [DOI] [Google Scholar]
- 17.Putman NF, Meinke AM, Noakes DLG. 2014. Rearing in a distorted magnetic field disrupts the ‘map sense’ of juvenile steelhead trout. Biol. Lett. 10, 20140169 ( 10.1098/rsbl.2014.0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwinn M, Baktoft H, Aarestrup K, Koed A. 2017. A comparison of the survival and migration of wild and F1-hatchery-reared brown trout (Salmo trutta) smolts traversing an artificial lake. Fish. Res. 196, 47–55. ( 10.1016/j.fishres.2017.08.011) [DOI] [Google Scholar]
- 19.Goehring L, Oberhauser KS. 2002. Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol. Entomol. 6, 674–685. ( 10.1046/j.1365-2311.2002.00454.x) [DOI] [Google Scholar]
- 20.Oberhauser KS. 2019. Concerns that captive breeding affects the ability of monarch butterflies to migrate. Nature 573, 501–502. ( 10.1038/d41586-019-02644-y) [DOI] [PubMed] [Google Scholar]
- 21.Taylor ORJ, et al. 2019. Is the timing, pace, and success of the monarch migration associated with sun angle? Front. Ecol. Evol. 7, 442 ( 10.3389/fevo.2019.00442) [DOI] [Google Scholar]
- 22.Agostinelli C, Lund UR. 2017. Package ‘circular’. R package version 0.4–93. See https://r-forge.r-project.org/projects/circular/.
- 23.Lemon J. 2006. Plotrix: a package in the red light district of R. R News. 6, 8–12. [Google Scholar]
- 24.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www/R-project.org/. [Google Scholar]
- 25.Wilcox AAE, Newman AEM, Raine NE, Norris DR. 2020. Captive-reared migratory monarch butterflies show natural orientation when released in the wild. Bioarxiv ( 10.1101/2020.01.24.919027) [DOI]
- 26.Green DA, Kronforst MR. 2019. Monarch butterflies use an environmentally sensitive, internal timer to control overwintering dynamics. Mol. Ecol. 28, 3642–3655. ( 10.1111/mec.15178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman WS. 1981. Studies on the adult reproductive diapause of the monarch butterfly, Danaus plexippus . Biol. Bull. 160, 89–106. ( 10.2307/1540903) [DOI] [Google Scholar]
- 28.Howard E, Aschen H, Davis AK. 2010. Citizen science observations of monarch butterfly overwintering in the southern United States. Psyche: A J. Entomol. 2010, 1–6. ( 10.1155/2010/689301) [DOI] [Google Scholar]
- 29.Knight A, Brower LP. 2009. The influence of eastern North American autumnal migrant monarch butterflies (Danaus plexippus L.) on continuously breeding resident monarch populations in southern Florida. J. Chem. Ecol. 35, 816–823. ( 10.1007/s10886-009-9655-z) [DOI] [PubMed] [Google Scholar]
- 30.Satterfield DA, Maerz JC, Hunter MD, Flockhart DTT, Hobson KA, Norris DR, Streit H, de Roode JC, Altizer S. 2018. Migratory monarchs that encounter resident monarchs show life-history differences and higher rates of parasite infection. Ecol. Lett. 21, 1670–1680. ( 10.1111/ele.13144) [DOI] [PubMed] [Google Scholar]
- 31.Satterfield DA, Maerz JC, Altizer S. 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B 282, 20141734 ( 10.1098/rspb.2014.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brower L. 1996. Monarch butterfly orientation: missing pieces of a magnificent puzzle. J. Exp. Biol. 199, 93–103. [DOI] [PubMed] [Google Scholar]
- 33.Reppert SM, Gegear RJ, Merlin C. 2010. Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 33, 399–406. ( 10.1016/j.tins.2010.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez SM, Taylor OR. 2004. Monarch butterflies migratory behavior persists despite changes in environmental conditions. In Monarch butterfly biology & conservation (eds Oberhauser KS, Solensky MJ), pp. 85–88. Ithaca, NY: Cornell University Press. [Google Scholar]
- 35.Guerra PA, Reppert SM. 2013. Coldness triggers northward flight in remigrant monarch butterflies. Curr. Biol. 23, 419–423. ( 10.1016/j.cub.2013.01.052) [DOI] [PubMed] [Google Scholar]
- 36.James DG, James TS, Seymour L, Kappen L, Russell T, Harryman B, Bly C. 2018. Citizen scientist tagging reveals destinations of migrating monarch butterflies, Danaus plexippus (L.) from the Pacific Northwest. Lepi. 72, 127–144. ( 10.18473/lepi.v72i2.a5) [DOI] [Google Scholar]
- 37.Steffy G. 2015. Trends observed in fall migrant monarch butterflies (Lepidoptera: Nymphalidae) east of the Appalachian Mountains at an inland stopover in southern Pennsylvania over an eighteen-year period. Ann. Entomol. Soc. Am. 108, 718–728. ( 10.1093/aesa/sav046) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All directional orientation data are included in the electronic supplementary material.