Abstract
In recent years, controlled drug delivery has become an important area of research. Nano-biocomposites can fulfil the necessary requirements of a targeted drug delivery device. This review describes use of polymeric nano-biocomposites in controlled drug delivery devices. Selection of suitable biopolymer and methods of preparation are discussed.
Keywords: Biopolymers, Controlled drug delivery, Nano-biocomposites, Nano-materials
Introduction
Nanotechnology offers new ideas for the development of improved diagnostic and therapeutic tools in surgical and medical treatment (Broichsitter et al. 2010). Nanotechnology is set to play an important role in sustained and targeted drug delivery applications due to its ability to provide tailored active site chemistry and drastically increase the surface area of nano-scale particles (Liu and Webster 2010; Shi et al. 2010). Nano-biocomposites and nanoparticles have already been used for controlled and targeted drug delivery (Steichen et al. 2013; Alba et al. 2019). Polymeric nano-biocomposites (PNBs) are materials derived from the combination of polymer-polymer or nano-scale fillers and polymers whereby the fillers may be organic or inorganic clays, metal nanoparticles and hydroxyapatite (Armentano et al. 2010). Nano-composites constitute a fascinating multidisciplinary area which brings together material science, biological science and nanotechnology and have significant impact in the area of medical science (Liu and Webster 2010).
Biodegradable polymers have attracted much attention in recent years due to reasons associated with the environment and minimization of natural fossil resources (Rahim and Mas Haris 2015; Rahim and Mas Haris 2016; Gao et al. 2019; Rahim and Mas Haris 2019). In a similar fashion, researchers have been inspired to produce environment-friendly advance nano-composite materials (Bordes et al. 2009). Biopolymers and clay minerals represent interesting constituents in agricultural and pharmaceutical products (Aguzzi et al. 2010).
In recent years, such biopolymers and clay nano-biocomposites have attracted much attention for therapeutic and medical applications (Sasmal et al. 2009). Typical applications of biopolymers in pharmaceutics are prosthesis for tissue replacement, artificial organs and sustained drug and vaccine release devices (Chen et al. 2007a, b; Liu and Webster 2010). Nano-biocomposites can also be used for the treatment of certain tissue and bone diseases (Basha et al. 2015).
Nano-structure biocomposites
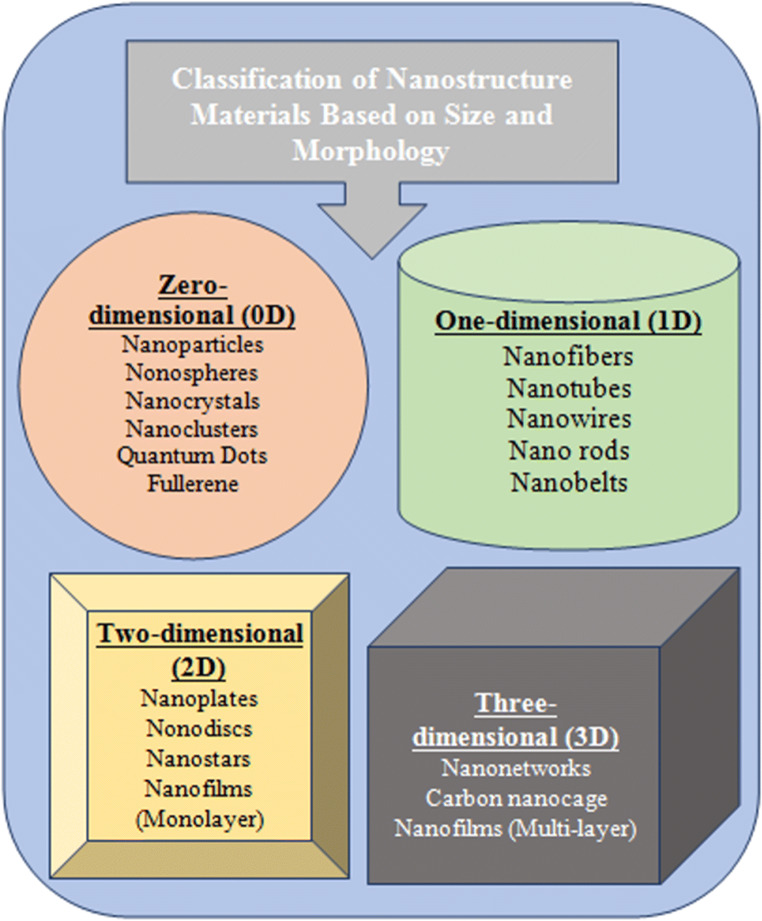
Nano-structured materials have been categorized into four groups on the basis of three-dimensional geometry, i.e. zero- (0D), one- (1D), two- (2D), and three-dimensional (3D) structures (Lu et al. 2011a, b), as shown in Fig. 1. The spherical nano-biocomposites are considered as 0D structures also known as nano-cluster materials or nano-dispersion. The biocomposites of 1D structure are of nano-scale at one dimension, while the remaining two dimensions may be of larger scale. The biocomposites of 1D structure are of tube shape which is a few nanometres thick and 100–1000 nm long. The biocomposites of 2D structure are of nano-scale at two dimensions, while the third dimension may be long and are known as nano-sheets. The 3D structure nanocomposites are of nano-scale at three dimensions; such biocomposites are known as iso-dimensional nano-biocomposites which can also form polycrystalline systems (Korotcenkov 2010; Kaushika et al. 2013).
Fig. 1.
Classification of nano-biocomposites based on a three-dimensional structure
Kaushika et al. (2013) and Lu et al. (2011a, b) have focused on one-dimensional nano-structures because of their unique chemical and physical properties. Such 1D materials have very high aspect ratios which are effective in transporting carrier particles (atoms, ions and molecules) along a controlled direction. One-dimensional nano-biocomposites are very effective for moving charged ions and active drugs along the long dimension. A feature of the nano aspect of such devices is that particle size greatly affects the properties of nano-structured materials such as structure, spectroscopic, electronic, thermodynamic, electromagnetic and interfacial chemistry (Ansari et al. 2010).
Selection of biopolymer
Polymers can be broadly classified as either natural or synthetic. Biopolymers are naturally occurring materials having a well-defined covalent structure, arranged sequence of monomers and exact chemical composition (Nitta and Numata 2013). Prior to use, all polymers (synthetic or natural) must pass through various processes of purification, modification and derivatization in order to ensure human health suitability/safety when used for controlled drug delivery (Yang et al. 2020). Commonly used polymers in pharmaceutical applications have been categorized by Raizada et al. into six groups on the basis of their origin and chemical composition (Raizada et al. 2010): (a) water-soluble synthetic polymers, (b) water-insoluble biodegradable polymers, (c) cellulose-based polymers, (d) starch-based polymers, (e) plastics and rubber based and (f) hydrocolloids.
Among the first group (water-soluble synthetic polymers), polylactic acid (PLA), polyglycolic acid (PGA), poly (DL-lactide-co-glycolide) (PLG), polyanhydrides, carbomer and polymethyl acrylates are the most common synthetic polymers used for controlled drug delivery (Van and Kiekens 2002; Pandey and Khuller 2004; Singh 2011). Outside of this group, we make particular note of starch-based and hydrocolloid-based polymer groupings. Starch-based polymers have been used extensively in pharmaceutical and medical applications because of their biodegradability, bio-safety and biocompatibility (Lu et al. 2009). Starch-based biopolymers are the most frequently used biopolymers for the preparation of nano-biocomposites for controlled and targeted drug delivery. Among the hydrocolloids, alginate and chitosan are cost-effective and environmentally friendly (Alba et al. 2019, b) as well as being biodegradable (Termsarasab et al. 2013; Tiyaboonchai 2013). Alginate is popular because of its ability to be employed as a base material in grafting, blending, derivatization and copolymerization reactions (Lee and Mooney 2012). Alginate is a natural water-soluble linear polysaccharide obtained from seaweed and is composed of alternating blocks of (1→4)-linked α-L-gluluronic (G) and α-D-mannuronic acid (M) units, whereas chitosan is a water-insoluble copolymer of β-(1 → 4)-lined D-glucosamine and N-acetyl-D-glucosamine units obtained from chitin via N-deacetylation; the chemical structure of alginate and chitosan is shown in Fig. 2 (Tsigos et al. 2000; Basu et al. 2011).
Fig. 2.
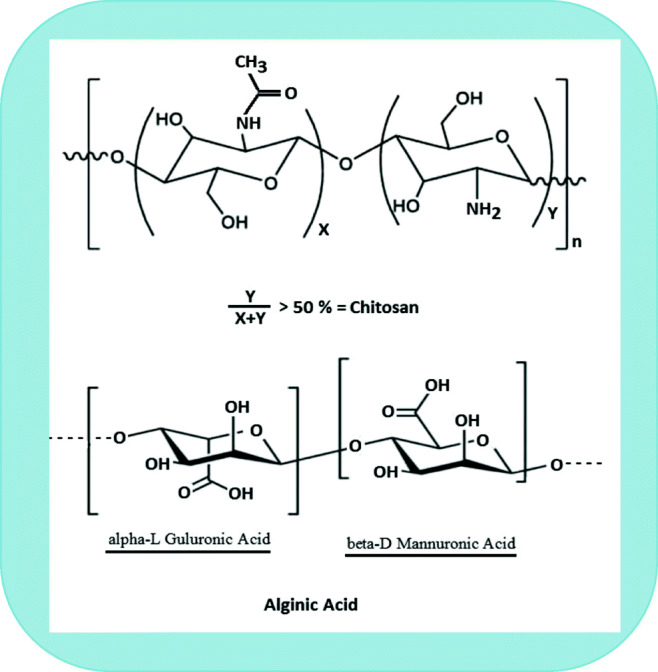
Chemical structure of chitosan and alginate
Types of nano-biocomposites
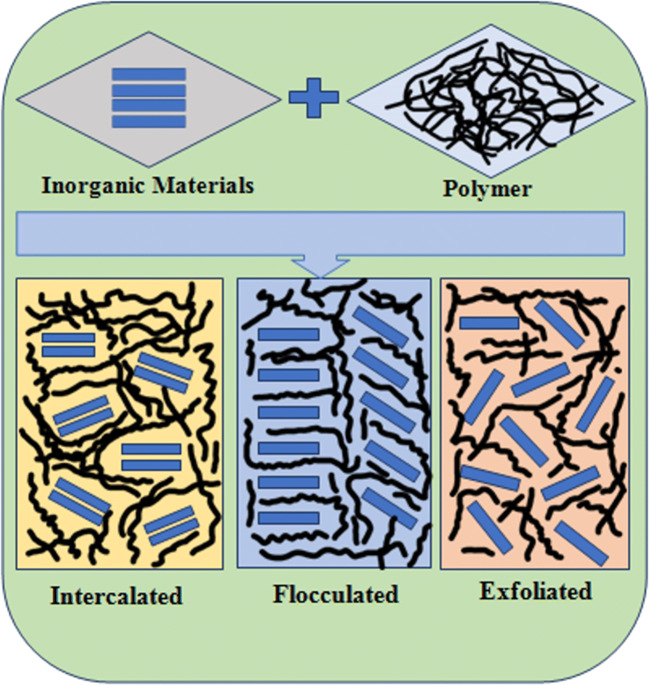
According to the literature, nano-biocomposites have been categorized into two groups, i.e. intercalated and delaminated (Gilman 1999). The intercalated or layered nano-biocomposites are those composites in which the extended polymer has been intercalated between the host polymers in well-ordered multilayers; in delaminated nano-biocomposites, the host polymer may be of nano-scale which should be dispersed in continuous polymer matrix (Burnside and Giannelis 1995; Weimer et al. 1999). Within the literature, some consider the delaminated group as being split into two further groups, namely, flocculated and exfoliated (Fig. 3). The categorization is based on the interfacial interactions between extended and host polymers (Sinha and Okamoto 2003; Chen et al. 2008). With regard to these three groups, we note the following:
Intercalated nano-composites: are composites in which the insertion of inorganic materials or polymer (extended polymer or inorganic material) into another polymer (host polymer) occurs in a regular manner irrespective of the polymer-polymer or polymer-inorganic material ratio. The composites are regularly inter-layered with properties similar to ceramic materials.
Flocculated nano-composites: are almost similar to the intercalated nano-composites; the only difference is the arrangement of the extended polymer within host polymer.
Exfoliated nano-composites: in exfoliated nano-composites, extended polymer layer should be separated in a continuous polymer matrix with a mean space that depends on the filler’s loading. Generally, the filler amount has been observed lower than that of the other types of nano-biocomposites.
Fig. 3.
Types of nano-biocomposites based on interfacial interactions between monomers of the extended and host polymer
Colloidal nano-composites
Colloidal nano-composites are the dispersion of blobs (blob’s particle size of 100–1000 nm) in a non-reactive solvent generally water. Recently, attention has been focused on polymer/silica nano-composites with specific morphology, characteristic and structure. Colloidal nano-composites are the new class of hybrid materials that frequently exhibit remarkable optical, electrical, mechanical, chemical and rheological properties (Balmer et al. 2008; Pyun 2012; Hill and Pyun 2014). Disadvantages of the colloidal nano-composites are—the reaction mixture should be proceeded in moderate acidic mefium. The reaction mixture is highly exothermic; they may be limited to rather low concentration of monomers. The reaction solutions often contain significant amounts of contaminants, and therefore subsequent purification can be a big issue (Balmer et al. 2008). Aside from the colloidal preparation method, other types of polymerizations are based on the creation of suspensions (Mayes and Mosbach 1996; Jayaratne and Sita 2000; Liu et al. 2011), mini-emulsions, dispersions and supercritical fluid polymerizations (Bourgeat and Lang 1998; Asua 2002; Ferguson et al. 2005; Lin et al. 2005).
Methods for biocomposite preparation
Blending
Blending is one of the traditional, simple and easy methods to prepare biocomposites. Polymers can be directly mixed with each other mainly by two methods, i.e. melt blending and solution blending. As the melt blending method is both efficient and environmentally friendly, researchers have preferentially focused on the melt blending as compared with the solution blending method. As the particle load increases, the polymer does not melt or the melt polymer is overly viscous meaning that both the melt and solution blend methods become infeasible. To overcome this situation, a solid blend method has been developed which avoids thermal and solvent problems (Johnston 1992; Prut and Zelenetskii 2001).
General polymerization technique
The general polymerization method for preparation of nano-biocomposites involves three main steps—the preparation of additives and surface modification, the dispersion of additives into monomer, and then solution or bulk polymerization. This method has advantages over other techniques such as better efficiency, higher speed and better performance of the product (Zou et al. 2008).
Photopolymerization
Photopolymerization is the process where liquid monomer has been transferred very fast into solid film under the influence of UV light. The process involves the production of and capture of radical species by the interaction of UV light using a suitable initiator. Similarly, electron beam–induced polymerization has attracted much attention because the process is solvent-free which is significantly green and environment-friendly (Yokoyama et al. 2003; Zhou et al. 2008; Zou et al. 2008). However, UV light can deactivate and degrade bioactive molecules such as drugs and therefore sometimes cause bioactive ingredients to lose their activity.
Solution polymerization
Solution polymerization is a method of industrial polymerization whereby the monomers can be dissolved in a non-reactive solvent. Sometimes a catalyst is incorporated in order to initiate polymerization. The main disadvantage of this type of polymerization is the presence of solvent when high concentration of the polymer is required. Oftentimes, removal of excess solvent is also difficult on industrial scale. The advantage of this method is that the heat produced by the reaction can be absorbed by the solvent, thereby modifying the rate of reaction. Overheating can be controlled which disturbs the activity of bioactive molecules (Desai and Hubbell 1991; Seidel and Malmonge 2000; Edmondson et al. 2004).
Emulsion polymerization
Emulsion polymerization is the most widely used method for fabricating polymeric particles (Fan et al. 2017). Emulsion polymerization starts with the formation of an emulsion of monomers, surfactant and water. Oil-in-water is the most common type of emulsion polymerization due to its ease of production (Feast and Moore 1981; Burguière et al. 2001; Chen et al. 2007a, b). However, the emulsion polymerization reaction also has many disadvantages, such as the situation where the adsorptive bonded emulsifier desorbs upon which it can then immediately migrate to the surface along with the polymer bulk. Similarly, hydrophilicity of the polymer surface increases as the hydrophilicity of the end product decreases (Tauer et al. 1990).
Surface-induced polymerization
Interfacial interactions play a key role in the construction of nano-composites. Two routes have been developed for the grafting of polymer chains at the particle surface that are known as grafting-to and grafting-from techniques.
Grafting-to method: This method involves chemical reaction between the reactive groups on the substrate surface and the (end-)functionalized polymer. Such polymer grafting-to methods have been widely used for solid surface modification. A thin polymer layer (end-)grafted to a solid substrate can significantly affect the properties of the polymer surface such as lubrication, friction, wettability, adhesion and biocompatibility (Minko et al. 2002; Zdyrko and Luzinov 2011).
Grafting-from method: The grafting-from polymerization procedure is dramatically effective for preparing nano-composites. Solid surfaces can be modified directly using an immobilized initiator. On the solid substrate the initiator is immobilized and the polymer layer is produced via in situ polymerization (Choi et al. 2005; Goda et al. 2006).
Selection of preparative method
Research has been focused on the development of drug carriers in which the drugs should be active only in the targeted area (Kim et al. 2005; Cao et al. 2010). Therefore, methods of preparation should be selective and specific in order to prepare the nano-composites. Each method has several advantages and disadvantages; the majority of published literature survey reveals that most researchers tend to use the solution polymerization method. Solution polymerization is (as mentioned) better than other methods because the heat produced during the process can be absorbed by the solvent, and therefore overheating can be controlled, thereby helping to secure the bioactivity of active ingredients. Additionally the by-products produced during the reaction can be washed away easily (Lai et al. 2003; Wang et al. 2007; Sun et al. 2008; Abdeen and Salahuddin 2013).
Drug loading and release
The most effective nano-composite system is one which has a high capacity for drug loading in order to minimize the amount of carrier. Drug loading can be achieved by two methods either the incorporation or the impregnation method. The incorporation method involves the drug being entrapped by nano-composites at the time of preparation. The impregnation method involves drug entrapment by incubation of the nano-composites in a solution. Higher entrapment efficiencies of nano-composites can be achieved by the incorporation method rather than impregnation technique (Soppimath et al. 2001; Braga et al. 2008). However most of the published drug loading studies have been carried out using the impregnation method. The impregnation method is somewhat superior to the incorporation method because the activity of active materials may be lost during the incorporation process (Yong et al. 1994; Ray et al. 2003; Charnay et al. 2004; Chen et al. 2010; Ke et al. 2011). Braga et al. 2008 reported that the recording of sorption isotherms provides valuable information for designing the best formulation of targeted drug delivery. Similarly, sorption isotherms also provide the sorption capacity of the nano-composites and the final actual amount of sorbed drug (Soppimath et al. 2001). It has been suggested that sorption and release kinetic data should be analysed using different isotherms (such as linear, Freundlich, Langmuir isotherms and BET model) to discern the mechanism of drug binding associated with the impregnation method (Chung et al. 2015).
Drug release from nano-biocomposites and subsequent biodegradation are also important factors for determination of the most effective formulations. The drug release rate depends on the following factors; surface-bound release, diffusion through nano-composite matrix, erosion of nano-composite matrix and combined release due to diffusion-erosion (Lu et al. 2011a, b; Kaczmarek and Sionkowska 2017). Therefore, drug release studies based on the determination of the release mechanism should be conducted using the following methods; dialysis bag, side-by-side diffusion (using biological membrane), reverse dialysis sac and ultrafiltration techniques (Gross et al. 1973; Stringer and Peppas 1996; Heiati et al. 1997; Boyd 2003).
Conclusion
The use of biopolymers for sustained drug delivery is a well-established method. Use of nano-biocomposites as sustained or targeted drug delivery devices have attracted much attention because of their potential advantages in the areas of biodegradation and biocompatibility, safe-use, environment-friendly and cost-effectiveness. In recent years, a number of nano-biocomposite-based drug delivery devices have become commercially available. Starch-based biopolymers particularly alginate and chitosan are presently the most suitable materials for nano-biocomposites preparation. The in situ solution and emulsion polymerization methods have been shown to be highly suitable for nano-biocomposite preparation. Novel methods for nano-biocomposites preparation with specific arrangement of monomer units in three-dimensional space represent the next direction for improvement of nano-composites in targeted and controlled drug delivery processes. Our research group, along with others, are attempting to prepare such materials for the controlled release of drugs.
Future of nano-biocomposites for targeted and controlled-release drugs
Nano-biocomposites have a wide range of applications in agriculture and biomedical sciences (Oyen 2008). Tissue engineering represents one important application in biomedical science. Tissue engineering is the development of three-dimensional structure that can serve to support the regeneration and replacement of tissues in a natural way. Biopolymer scaffolds and nano-biocomposites potentially offer themselves as key materials in tissue engineering (Rajzer et al. 2014; Shahini et al. 2014; Wu et al. 2014).
Nano-biocomposites can be used in combination with imaging agent to exploit magnetic resonance imaging (MRI) for the diagnosis and detection of various diseases such as cancer, tissue and bone injury and infections (An et al. 2014; Wang et al. 2014). Biopolymer nano-composites can be used to support permanent or temporary prostheses of tissue replacements, surgical operation and artificial organs. The materials can also be used for targeted delivery of hormones, vaccines, specific targeted delivery of insulin and as anti-cancer drug controlled-release devices.
Biosensors are electrochemical devices that are capable to provide quantitative information using receptor (biological recognition element) directly connected with a transduction element (Motahare et al. 2012; Swain et al. 2014). Tsai (Tsai et al. 2007) reported nano-biocomposites as surface coatings in biosensor devices and therefore may have potential significance in this area (Sharma et al. 2009; Sanchez et al. 2010).
Heavy metals have received extensive attention due to their toxic effects even at low concentration. Various methods (solvent extraction, ion-exchange, precipitation, reduction and membrane-process) have been applied to remove heavy metals from water. Recently, one of the efficient and cost-effective methods is the sorption of heavy metals via nano-biocomposite (Masoumi et al. 2014). However, the mentioned applications require further improvement before their effective use with nano-biocomposites for sorbents of heavy metals.
Acknowledgement
The authors would like to acknowledge Universiti Sains Malaysia (USM) for providing financial support (grant no. 1001/PKIMIA/814124).
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdeen R, Salahuddin N. Modified chitosan-clay nanocomposite as a drug delivery system intercalation and in vitro release of ibuprofen. J Chem. 2013;2013:576370. [Google Scholar]
- Aguzzi C, Capra P, Bonferoni C, Cerezo P, Salcedo I, Sánchez R, Caramella C, Viseras C. Chitosan–silicate biocomposites to be used in modified drug release of 5-aminosalicylic acid (5-ASA) Appl Clay Sci. 2010;50(1):106–111. [Google Scholar]
- Alba CMD, Cota Reguero A, Osuna Barroso FJ, Pavón González E, Perdigón Aller AC, Raffin F. Bionanocomposites based on chitosan intercalation in designed swelling high-charged micas. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-46495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Hu H, Du J, Wei J, Wang L, Yang H, Wu D, Shi H, Li F, Yang S. Paramagnetic hollow silica nanospheres for in vivo targeted ultrasound and magnetic resonance imaging. Biomaterials. 2014;35(20):5381–5392. doi: 10.1016/j.biomaterials.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Ansari AA, Alhoshan M, Alsalhi MS, Aldwayyan AS. Prospects of nanotechnology in clinical immunodiagnostics. Sensors. 2010;10(7):6535–6581. doi: 10.3390/s100706535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM. Biodegradable polymer matrix nanocomposites for tissue engineering: a review. Polym Degrad Stab. 2010;95(11):2126–2146. [Google Scholar]
- Asua JM. Miniemulsion polymerization. Prog Polym Sci. 2002;27(7):1283–1346. [Google Scholar]
- Balmer JA, Schmid A, Armes SP. Colloidal nanocomposite particles: quo vadis? J Mater Chem. 2008;18(47):5722–5730. [Google Scholar]
- Basha RY, Sampath Kumar TS, Doble M. Design of biocomposite materials for bone tissue regeneration. Mater Sci Eng C. 2015;57:452–463. doi: 10.1016/j.msec.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Basu S, Jana S, Gandhi A, Sen K. Natural polymers and their application in drug delivery and biomedical field. J Pharma Sci Tech. 2011;1(1):16–27. [Google Scholar]
- Bordes P, Pollet E, Averous L. Nano-biocomposites: biodegradable polyester/nanoclay systems. Prog Polym Sci. 2009;34(2):125–155. [Google Scholar]
- Bourgeat LE, Lang J. Encapsulation of inorganic particles by dispersion polymerization in polar media: 1. Silica Nanoparticles Encapsulated by Polystyrene. J Colloid Interface Sci. 1998;197(2):293–308. doi: 10.1006/jcis.1997.5265. [DOI] [PubMed] [Google Scholar]
- Boyd BJ. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260(2):239–247. doi: 10.1016/s0378-5173(03)00262-x. [DOI] [PubMed] [Google Scholar]
- Braga ME, Pato MTV, Silva HS, Ferreira EI, Gil MH, Duarte CM, de Sousa HC. Supercritical solvent impregnation of ophthalmic drugs on chitosan derivatives. J Supercrit Fluids. 2008;44(2):245–257. [Google Scholar]
- Broichsitter BM, Thieme M, Nguyen J, Schmehl T, Gessler T, Seeger W, Agarwal S, Greiner A, Kissel T. Novel “nano in nano” composites for sustained drug delivery: biodegradable nanoparticles encapsulated into nanofiber non-wovens. Macromol Biosci. 2010;10(12):1527–1535. doi: 10.1002/mabi.201000100. [DOI] [PubMed] [Google Scholar]
- Burguière C, Pascual S, Bui C, Vairon J-P, Charleux B, Davis KA, Matyjaszewski K, Bétremieux I. Block Copolymers of poly(styrene) and poly(acrylic acid) of various molar masses, topologies, and compositions prepared via controlled/living radical polymerization. Application as Stabilizers in Emulsion Polymerization. Macromolecules. 2001;34(13):4439–4450. [Google Scholar]
- Burnside SD, Giannelis EP. Synthesis and properties of new poly (dimethylsiloxane) nanocomposites. Chem Mater. 1995;7(9):1597–1600. [Google Scholar]
- Cao SW, Zhu Y-J, Wu J, Wang K-W, Tang Q-L. Preparation and sustained-release property of triblock copolymer/calcium phosphate nanocomposite as nanocarrier for hydrophobic drug. Nanoscale Res Lett. 2010;5(4):781–785. doi: 10.1007/s11671-010-9558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay C, Bégu S, Tourné-Péteilh C, Nicole L, Lerner DA, Devoisselle J-M. Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur J Pharm Biopharm. 2004;57(3):533–540. doi: 10.1016/j.ejpb.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Chen C, Lv G, Pan C, Song M, Wu C, Guo D, Wang X, Chen B, Gu Z. Poly(lactic acid) (PLA) based nanocomposites--a novel way of drug-releasing. Biomed Mater. 2007;2(4):L1–L4. doi: 10.1088/1748-6041/2/4/L01. [DOI] [PubMed] [Google Scholar]
- Chen T, Colver PJ, Bon SAF. Organic–inorganic hybrid hollow spheres prepared from TiO2-stabilized pickering emulsion polymerization. Adv Mater. 2007;19(17):2286–2289. [Google Scholar]
- Chen B, Evans JR, Greenwell HC, Boulet P, Coveney PV, Bowden AA, Whiting A. A critical appraisal of polymer–clay nanocomposites. Chem Soc Rev. 2008;37(3):568–594. doi: 10.1039/b702653f. [DOI] [PubMed] [Google Scholar]
- Chen D, Jiang M, Li N, Gu H, Xu Q, Ge J, Xia X, Lu J. Modification of magnetic silica/iron oxide nanocomposites with fluorescent polymethacrylic acid for cancer targeting and drug delivery. J Mater Chem. 2010;20(31):6422–6429. [Google Scholar]
- Choi WS, Park JH, Koo HY, Kim JY, Cho B, Kim DY. “Grafting-from” polymerization inside a polyelectrolyte hollow-capsule microreactor. Angew Chem Int Ed. 2005;44(7):1096–1101. doi: 10.1002/anie.200460971. [DOI] [PubMed] [Google Scholar]
- Chung H-K, Kim W-H, Park J, Cho J, Jeong T-Y, Park P-K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J Ind Eng Chem. 2015;28:241–246. [Google Scholar]
- Desai NP, Hubbell JA. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials. 1991;12(2):144–153. doi: 10.1016/0142-9612(91)90193-e. [DOI] [PubMed] [Google Scholar]
- Edmondson S, Osborne VL, Huck WT. Polymer brushes via surface-initiated polymerizations. Chem Soc Rev. 2004;33(1):14–22. doi: 10.1039/b210143m. [DOI] [PubMed] [Google Scholar]
- Fan J-B, Song Y, Liu H, Lu Z, Zhang F, Liu H, Meng J, Gu L, Wang S, Jiang L. A general strategy to synthesize chemically and topologically anisotropic Janus particles. Sci Adv. 2017;3(6):e1603203. doi: 10.1126/sciadv.1603203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feast AA, Moore JD (1981) Emulsion polymerization process, Google Patents.
- Ferguson CJ, Hughes RJ, Nguyen D, Pham BT, Gilbert RG, Serelis AK, Such CH, Hawkett BS. Ab initio emulsion polymerization by RAFT-controlled self-assembly §. Macromolecules. 2005;38(6):2191–2204. [Google Scholar]
- Gao M, Li J, Bao Z, Hu M, Nian R, Feng D, An D, Li X, Xian M, Zhang H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat Commun. 2019;10(1):1–10. doi: 10.1038/s41467-018-07879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JW. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites1 This work was carried out by the National Institute of Standards and Technology (NIST), an agency of the U. S. government, and by statute is not subject to copyright in the United States.1. Appl Clay Sci. 1999;15(1):31–49. [Google Scholar]
- Goda T, Konno T, Takai M, Moro T, Ishihara K. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials. 2006;27(30):5151–5160. doi: 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Gross BM, Oudet P, Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Heiati H, Tawashi R, Shivers RR, Phillips NC. Solid lipid nanoparticles as drug carriers. I. Incorporation and retention of the lipophilic prodrug 3′-azido-3′-deoxythymidine palmitate. Int J Pharm. 1997;146(1):123–131. [Google Scholar]
- Hill L, Pyun J (2014) Colloidal polymers via dipolar assembly of magnetic nanoparticle monomers. ACS Appl Mater Interfac 6(9):6022–6032 [DOI] [PubMed]
- Jayaratne KC, Sita LR. Stereospecific living Ziegler-Natta polymerization of 1-hexene. J Am Chem Soc. 2000;122(5):958–959. [Google Scholar]
- Johnston RL (1992) Process and apparatus for blending viscous polymers in solvent, Google Patents.
- Kaczmarek B, Sionkowska A. Drug release from porous matrixes based on natural polymers. Curr Pharm Biotechnol. 2017;18(9):721–729. doi: 10.2174/1389201018666171103141347. [DOI] [PubMed] [Google Scholar]
- Kaushika A, Aryab SK, Vasudevc A, Bhansalia S. Nanocomposites based on chitosan-metal/metal oxides hybrids for biosensors applications. J Nanosci Lett. 2013;3:32. [Google Scholar]
- Ke F, Yuan Y-P, Qiu L-G, Shen Y-H, Xie A-J, Zhu J-F, Tian X-Y, Zhang L-D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J Mater Chem. 2011;21(11):3843–3848. [Google Scholar]
- Kim HW, Knowles JC, Kim HE. Porous scaffolds of gelatin–hydroxyapatite nanocomposites obtained by biomimetic approach: characterization and antibiotic drug release. J Biomed Mater Res B Appl Biomater. 2005;74(2):686–698. doi: 10.1002/jbm.b.30236. [DOI] [PubMed] [Google Scholar]
- Korotcenkov G (2010) Chemical sensors: fundamentals of sensing materials. In: Nanostructured Materials, vol 2. Momentum Press, New York, pp 113–187
- Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VS-Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125(15):4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Cui X, Yen C, Wai CM. Platinum/carbon nanotube nanocomposite synthesized in supercritical fluid as electrocatalysts for low-temperature fuel cells. J Phys Chem B. 2005;109(30):14410–14415. doi: 10.1021/jp0514675. [DOI] [PubMed] [Google Scholar]
- Liu H, Webster TJ. Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites. J Biomed Mater Res A. 2010;93(3):1180–1192. doi: 10.1002/jbm.a.32614. [DOI] [PubMed] [Google Scholar]
- Liu K, Chen L, Chen Y, Wu J, Zhang W, Chen F, Fu Q. Preparation of polyester/reduced graphene oxide composites via in situ melt polycondensation and simultaneous thermo-reduction of graphene oxide. J Mater Chem. 2011;21(24):8612–8617. [Google Scholar]
- Lu D, Xiao C, Xu S. Starch-based completely biodegradable polymer materials. Express Polym Lett. 2009;3(6):366–375. [Google Scholar]
- Lu X, Zhang W, Wang C, Wen T-C, Wei Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog Polym Sci. 2011;36(5):671–712. [Google Scholar]
- Lu XY, Wu D-C, Li Z-J, Chen G-Q (2011b) Polymer Nanoparticles. In: Progress in Molecular Biology and Translational Science, 104th edn. Academic Press, Massachusetts, pp 299–323 [DOI] [PubMed]
- Masoumi A, Ghaemy M, Bakht AN (2014) Removal of metal ions from water using poly (MMA-co-MA)/modified-Fe3O4 magnetic nnano-composite: isotherm and kinetic study. Ind Eng Chem Res 53(19):81888–8197
- Mayes AG, Mosbach K. Molecularly imprinted polymer beads: suspension polymerization using a liquid perfluorocarbon as the dispersing phase. Anal Chem. 1996;68(21):3769–3774. doi: 10.1021/ac960363a. [DOI] [PubMed] [Google Scholar]
- Minko S, Patil S, Datsyuk V, Simon F, Eichhorn K-J, Motornov M, Usov D, Tokarev I, Stamm M. Synthesis of adaptive polymer brushes via “grafting to” approach from melt. Langmuir. 2002;18(1):289–296. [Google Scholar]
- Motahare SH, Mohammad T-S, Issa A, Nooshin H, MohammadAli S, Mehri GB. Relationship between cell compatibility and elastic modulus of silicone rubber/organoclay nanobiocomposites. Jundishapur J Nat Pharmaceut Prod. 2012;02(Spring):65–70. [PMC free article] [PubMed] [Google Scholar]
- Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14(1):1629–1654. doi: 10.3390/ijms14011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen ML. The materials science of bone: lessons from nature for biomimetic materials synthesis. MRS Bull. 2008;33(01):49–55. [Google Scholar]
- Pandey R, Khuller GK. Polymer based drug delivery systems for mycobacterial infections. Curr Drug Deliv. 2004;1(3):195–201. doi: 10.2174/1567201043334669. [DOI] [PubMed] [Google Scholar]
- Prut EV, Zelenetskii AN. Chemical modification and blending of polymers in an extruder reactor. Russ Chem Rev. 2001;70(1):65–79. [Google Scholar]
- Pyun J. Self-assembly and colloidal polymerization of polymer–nanoparticle hybrids into mesoscopic chains. Angew Chem Int Ed. 2012;51(50):12408–12409. doi: 10.1002/anie.201206245. [DOI] [PubMed] [Google Scholar]
- Rahim M, Mas Haris MRH. Application of biopolymer composites in arsenic removal from aqueous medium: a review. J Radiat Res Appl Sci. 2015;8(2):255–263. [Google Scholar]
- Rahim M, Mas Haris MRH. Application of advanced polymeric materials for controlled release pesticides. IOP Conf Ser Mater Sci Eng. 2016;146(1):012020. [Google Scholar]
- Rahim M, Mas Haris MRH (2019) Banana trunk fibers (BF) immobilized in chitosan (CS) natural composites (BF-i-CS), and its application in controlled-release of pesticides. J Nat Fib. 10.1080/15440478.15442019.11691119
- Raizada A, Bandari A, Kumar B. Polymers in drug delivery: a review. Int J Pharmaceut Res Dev. 2010;2:9–20. [Google Scholar]
- Rajzer I, Menaszek E, Kwiatkowski R, Chrzanowski W (2014) Bioactive nanocomposite PLDL/nano-hydroxyapatite electrospun membranes for bone tissue engineering. J Mater Sci: Mater Medicine 25(5):1239–1247 [DOI] [PMC free article] [PubMed]
- Ray LAM, Chiffoleau S, Iooss P, Grimandi G, Gouyette A, Daculsi G, Merle C. Vancomycin encapsulation in biodegradable poly (< i > ε</i > -caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials. 2003;24(3):443–449. doi: 10.1016/s0142-9612(02)00357-5. [DOI] [PubMed] [Google Scholar]
- Sanchez GMD, Hilliou L, Lagaron JM. Nanobiocomposites of carrageenan, zein, and mica of interest in food packaging and coating applications. J Agric Food Chem. 2010;58(11):6884–6894. doi: 10.1021/jf1007659. [DOI] [PubMed] [Google Scholar]
- Sasmal A, Nayak P, Nanda R, Nayak P, Sasmal S, Chang Y-W, Kang SC, Yoon J-Y. Soy protein isolate—furfural cross-linked nanocomposites for controlled release of cefadroxil. Int J Plast Technol. 2009;13(1):8–21. [Google Scholar]
- Seidel JM, Malmonge SM. Synthesis of PolyHEMA hydrogels for using as biomaterials. Bulk and solution radical-initiated polymerization techniques. Mater Res. 2000;3(3):79–83. [Google Scholar]
- Shahini A, Yazdimamaghani M, Walker KJ, Eastman MA, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV, Vashaee D, Tayebi L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int J Nanomedicine. 2014;9:167. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Soni VP, Bellare JR. Electrophoretic deposition of nanobiocomposites for orthopedic applications: influence of current density and coating duration. J Mater Sci Mater Med. 2009;20(1):93–100. doi: 10.1007/s10856-008-3490-6. [DOI] [PubMed] [Google Scholar]
- Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AV. Biopolymers in drug delivery: a review. Pharmacologyonline. 2011;1:62. [Google Scholar]
- Sinha RS, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28(11):1539–1641. [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JL, Peppas NA. Diffusion of small molecular weight drugs in radiation-crosslinked poly (ethylene oxide) hydrogels. J Control Release. 1996;42(2):195–202. [Google Scholar]
- Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SK, Kisku SK, Sahoo G (2014) Preparation of thermal resistant gas barrier chitosan nanobiocomposites. Polym Compos 35(12):2324–2328
- Tauer K, Goebel KH, Kosmella S, Stähler K, Neelsen J (1990). Emulsion polymerization in the presence of polymerizable emulsifiers and surface active initiators. Makromolekulare Chemie. Macromolecular Symposia, Wiley Online Library.
- Termsarasab U, Cho H-J, Kim DH, Chong S, Chung S-J, Shim C-K, Moon HT, Kim D-D. Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int J Pharm. 2013;441(1–2):373–380. doi: 10.1016/j.ijpharm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Tiyaboonchai W. Chitosan nanoparticles: a promising system for drug delivery. Naresuan Univ J. 2013;11(3):51–66. [Google Scholar]
- Tsai YC, Chen S-Y, Liaw H-W. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sensors Actuators B Chem. 2007;125(2):474–481. [Google Scholar]
- Tsigos I, Martinou A, Kafetzopoulos D, Bouriotis V. Chitin deacetylases: new, versatile tools in biotechnology. Trends Biotechnol. 2000;18(7):305–312. doi: 10.1016/s0167-7799(00)01462-1. [DOI] [PubMed] [Google Scholar]
- Van DVK, Kiekens P. Biopolymers: overview of several properties and consequences on their applications. Polym Test. 2002;21(4):433–442. [Google Scholar]
- Wang X, Du Y, Luo J, Lin B, Kennedy JF. Chitosan/organic rectorite nanocomposite films: structure, characteristic and drug delivery behaviour. Carbohydr Polym. 2007;69(1):41–49. [Google Scholar]
- Wang Z, Liu J, Li T, Wang B (2014) Controlled synthesis of MnFe2O4 nanoparticles and Gd complex-based nanocomposites as tunable and enhanced T1/T2-weighed MRI contrast agents. J Mater Chem B 2(29):4748–4753 [DOI] [PubMed]
- Weimer MW, Chen H, Giannelis EP, Sogah DY. Direct synthesis of dispersed nanocomposites by in situ living free radical polymerization using a silicate-anchored initiator. J Am Chem Soc. 1999;121(7):1615–1616. [Google Scholar]
- Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R Rep. 2014;80:1–36. [Google Scholar]
- Yang W, Qin R, Qin R, Zhang L, Qiu M. Removal of cadmium in aqueous solution by sulfidated nanoscale zero-valent iron. Nat Environ Pollut Technol. 2020;19(2):755–760. [Google Scholar]
- Yokoyama S, Nakahama T, Miki H, Mashiko S. Fabrication of three-dimensional microstructure in optical-gain medium using two-photon-induced photopolymerization technique. Thin Solid Films. 2003;438–439:452–456. [Google Scholar]
- Yong HK, Bae YH, Kim SW. pH/temperature-sensitive polymers for macromolecular drug loading and release. J Control Release. 1994;28(1):143–152. [Google Scholar]
- Zdyrko B, Luzinov I. Polymer brushes by the “grafting to” method. Macromol Rapid Commun. 2011;32(12):859–869. doi: 10.1002/marc.201100162. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang D, Ma G, Tan H, Jin Y, Nie J. A pH-sensitive water-soluble N-carboxyethyl chitosan/poly(hydroxyethyl methacrylate) hydrogel as a potential drug sustained release matrix prepared by photopolymerization technique. Polym Adv Technol. 2008;19(8):1133–1141. [Google Scholar]
- Zou H, Wu S, Shen J. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem Rev. 2008;108(9):3893–3957. doi: 10.1021/cr068035q. [DOI] [PubMed] [Google Scholar]