Abstract
Innate and adaptive immune cell activation and infiltration is the key characteristic of tissue inflammation. The innate immune system is the front line of host defense in which innate immune cells are activated by danger signals, including pathogen- and danger-associated molecular pattern, and metabolite-associated danger signal. Innate immunity activation can directly contribute to tissue inflammation or immune resolution by phagocytosis and secretion of biologically active molecules, or indirectly via antigen-presenting cell (APC) activation-mediated adaptive immune responses. This review article describes the cellular and molecular interplay of innate-adaptive immune systems. Three major mechanisms are emphasized in this article for their role in facilitating innate-adaptive immunity interplay. 1) APC can be formed from classical and conditional innate immune cells to bridge innate-adaptive immune response. 2) Immune checkpoint molecular pairs connect innate and adaptive immune cells to direct one-way and two-way immune checkpoint reactions. 3) Metabolic reprogramming during immune responses leads to excessive cytosolic and mitochondrial reactive oxygen species (ROS) production. Increased NADPH oxidase-derived extracellular and intracellular ROS are mostly responsible for oxidative stress, which contributes to functional changes in immune cells. Further understanding of innate-adaptive immunity interplay and its underlying molecular basis would lead to the identification of therapeutic targets for immunological and inflammatory disease.
Keywords: Innate immunity, Adaptive immunity, Immune interplay, Immune checkpoint, Reactive oxygen species
1. Introduction
Innate and adaptive immunity are two branches of the immune system that are essential for the initiation and progression of chronic inflammatory diseases, including atherosclerosis [1,2]. Innate immunity serves as the first line of host defense against danger signals in a quick and non-specific manner. For example, a wide range of exogenous and endogenous danger signals, such as oxidized low density lipoprotein (ox-LDL), could activate endothelial cells (ECs) and classical innate immune cells, such as monocytes, macrophages, and dendritic cells (DCs), by binding to toll-like receptors (TLR) [3]. Activated innate immune cells contribute to tissue inflammation by secreting pro-inflammatory mediators, such as tumor necrosis factor α (TNFα), interleukine-6 (IL-6), and reactive oxygen species (ROS) [4]. Along with the innate immune responses, tissue inflammation is also accompanied by the infiltration of adaptive immune cells which convey antigen-specific immune responses by T cells that differentiate into effector T cells with pro- or anti-inflammatory cytokine production, and B cells that differentiate into plasma cells with specific antibody production. Numerous studies highlighted the important role of T and B cell responses in atherosclerosis [5,6].
Three signals were recognized as the mechanism of innate control of the adaptive immune responses, consisting of signal 1 engagement of the T/B cell receptor (TCR/BCR) by antigen peptide on major histocompatibility complex (MHC), signal 2 ligation of immune checkpoint molecular pairs (co-stimulation and co-inhibition), and signal 3 cytokine stimulation [[7], [8], [9]]. We recently proposed signal 4, metabolite-associated danger signals (MADS) recognition by metabolic sensor (MS), as a novel mechanism to connect innate and adaptive immune responses [7]. This signal 4 theory is mostly based on the discovery of homocysteine-mediated CD40+ monocyte activation and CD40L production in chronic kidney disease (CKD) patients [10]. As CD40/CD40L is a co-stimulatory molecular pair, essential for both T and B cell activation, uremic toxin and Hcy-induced CD40/CD40L production/ligation plays a critical role in activating both innate and adaptive immune cells. These findings sparked the hypothesis that a two-way immune checkpoint may be an important modulating machinery for the crosstalk between innate and adaptive immunity [7]. Numerous evidence support the notion that MADS, such as hyperhomocysteinemia (HHcy), urimic toxin, hyperglycemia, and hyperlipidemia, stimulate tissue inflammation by activating the innate and adaptive immune system [[10], [11], [12], [13], [14]].
We recently summarized major features of metabolic reprogramming in innate and adaptive immune cells and established models of metabolic interplay in anti- and pro-inflammatory responses [15]. During pro-inflammatory immune responses, most immune cells switch towards enhanced glycolysis and pentose phosphate pathway to prompt an activation and proliferation status and adopt pro-inflammatory effector functions. This metabolic reprogramming is associated with increased cytosolic and mitochondrial ROS production [15]. ROS has been increasingly recognized not only as a by-product of metabolic reprogramming but also play an important coordinated role in mediating cell-cell interaction and promoting immune cell activation and differentiation [16]. However, it is challenging to describe the molecular basis and to define the role of ROS in immune cell activation.
This review provides a comprehensive overview for molecular and redox regulation in innate-adaptive immunity interplay and tissue inflammation.
2. Myeloid cells are the major populations in tissue inflammation
Inflammation is a tissue pathological process with the major purpose of resolving infection and tissue repair. Numerous innate and adaptive immune cells reside in inflammatory tissue where they contribute to immune defense and tissue homeostasis in metabolic diseases [17,18]. Recently, single-cell RNA sequencing is appreciated to be an unbiased strategy to explore cell clusters with shared transcriptional signatures and to identify specific molecular signatures that are unique to different cell population. Data from several single-cell RNA sequencing studies [19,20] of aortic leukocytes (CD45+) from healthy and atherosclerotic mice have been reorganized and presented in Table 1. These information indicated that in the healthy aortic vessel, lymphocytes, especially T cells, were the most abundant leukocyte population in Ldlr−/- and Apoe−/- mice. In the atherosclerotic aorta, myeloid cell clusters, including monocyte, macrophage and dendritic cells, were increased in both mice fed a western/high-fat diet (WD/HFD) for 11–12 weeks. With disease progression, myeloid cells were further increased to 63.8% of total immune cells in advanced atherosclerotic aorta of Ldlr−/- mice fed a HFD for 20 weeks, which is similar to the composition in human carotic plaque (65%). Myeloid cells have also been reported as the major immune cell populations in aortic lesion in hyperlipidemia and HHcy mouse model (Tg-hCBS ApoE−/− Cbs−/− and Ldlr−/− Cbs−/+) [12,21]. Therefore, it is recognized that myeloid cells are the major populations in advanced atherosclerotic lesion.
Table1.
Immune cell population in atherosclerotic aorta. Immune cell population were characterized and presented as percentage of CD45+ leukocytes. *Cells were grouped in “other cells”.
| Cell type | Condition |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aorta (Ldlr-/-) |
Aorta (Apoe-/-) |
Carotic plaque (Human, deconvolve)19 | ||||||
| 12W CD19 | 12W WD19 | 11W HFD20 | 20W HFD20 | 12W CD19 | 12W WD19 | 12W WD20 | ||
| Macrophage | 13.6 | 27.0 | 28.9 | 49.6 | 4.9 | 9.6 | 9.6 | 51.1 |
| Monocyte | 6.2 | 21.1 | 12.3 | * | 10.3 | 12.6 | 3.9 | 13.9 |
| MoDC/DC | - | - | 14.9 | 14.2 | - | - | 9.7 | - |
| NKC | 1.7 | 2.1 | * | * | 2.4 | 1.6 | 1.9 | ∼4.17 |
| B cell | 24.4 | 4.0 | * | * | 21.9 | 27.2 | 27.0 | ∼11.3 |
| T cell | 54.1 | 45.8 | 28.3 | 25.3 | 60.6 | 49.0 | 38.2 | 19.3 |
| Other cell | - | - | 15.7 | 11.0 | - | - | 9.6 | - |
3. Innate immune response
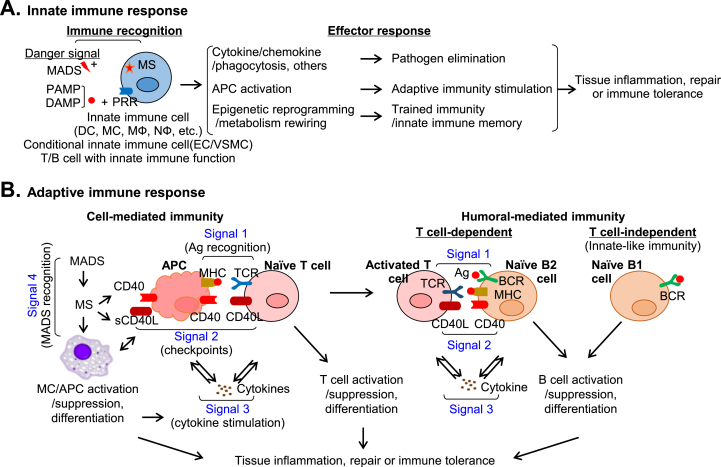
Conditional innate immune cell and metabolic sensor system — Innate immunity is the first line of host defense, providing initial non-specific defense against danger signals. Professional innate immune cells include DC, monocyte, macrophage, and B cell. Recent studies recognized EC and smooth muscle cell as conditional innate immune cells that share most features of professional innate immune cells in response to extracellular environmental changes [[22], [23], [24]]. As described in Fig. 1A, professional and conditional innate immune cells rely on pattern-recognition receptors (PRRs) or metabolic sensor (MS) to rapidly recognize and respond to danger signals derived from invading pathogens, injured self-cells or metabolic stresses. Pathogen-associated molecular patterns (PAMPs) are danger signals produced by microorganisms which active innate immune cells by PRR (PAMP:PRR recognition), such as TLR, nod-like receptors, and C-lectin receptors, with high affinity to facilitate the downstream signaling cascade to initiate immune responses. It was thought that immune system functioned by making a distinction between self- and non-self-constituents until Dr. Matzinger proposed the “danger model” claiming that “danger/alarm signals” from injured cells could initiate immune response in the 1990s[25]. The danger theory gives us a different view that self-constituents can trigger an immune response if they are dangerous, and non-self-constituents can be tolerated if they are not dangerous. Given these, many kinds of endogenous molecules were identified to be danger signals under pathological conditions. Danger-associated molecular patterns (DAMPs) are molecules released under stress or tissue damage condition and initiate immune response by DAMP:PRR recognition.
Fig. 1.
Innate-adaptive immunity interplay in immune response.A. Innate immune response. Immune recognition (MADS:MS and PAMP/DAMP:PRR) initiates innate immune response leading to pathogen elimination, adaptive immunity stimulation, and trained immunity via cytokine, chemokine, phagocytosis response, APC activation, and epigenetic reprogramming/metabolism rewiring, and contributes to tissue inflammation or repair. B. Adaptive immune response. Adaptive immunity determines tissue inflammation and repair, consisting of cell- and humoral-mediated immunity. Four signals are involved in cell-mediated immunity (Ag recognition, checkpoint, cytokine stimulation, and MADS recognition). CD40:CD40L is a representative immune checkpoint molecular pair. There are 2 types of humoral immune response (T cell-dependent and -independent). Three signals are described for T cell-dependent B cell immune response (Ag recognition, immune checkpoint and cytokine stimulation). B cell can also respond to Ag without the participation of Th cell in T cell-independent immunity.
However, current knowledge could not support the idea that all pro-inflammatory endogenous metabolites can bind to PRRs. MADS are proposed as a novel category of danger signals comprising small molecular metabolites which trigger immune responses mostly by MADS:MS recognition [7]. Using HHcy as a prototype disease model, we found that the Hcy-methionine (HM) cycle functioned as a MS system determining methylation-regulated pathogenic signaling [12,26]. This hypothesis is proved in CKD patients in which Hcy mediates inflammatory CD40+ monocyte differentiation, a biomarker for sever CKD [10]. The HM cycle determines production of S-adenosylmethionine and S-adenosylhomocysteine, a universal donor and competitive inhibitor of cellular methylation, respectively. Unlike PAMP/DAMP:PRR recognition, MADS:MS recognition is mostly receptor-independent, and largely depends on metabolic reprograming and metabolic modification on targeted molecules and pathogenic signaling [12,26].
Effector response and trained immunity — Immune recognition triggers innate immune cell activation and a series of effector responses (Fig. 1A). One of the effector responses is pathogen elimination by secreting cytokines and chemokines, phagocytosis, and others. In addition, immune recognition induces phenotypic and functional maturation of innate immune cells into potent antigen-presenting cells (APC) which initiate adaptive immune responses [30,31]. Trained immunity or innate immune memory is a novel feature of innate immune cells and conditional innate immune cells [32]. These cells mount protection against secondary infection and respond in a non-specific sensitized manner by undergoing epigenetic reprogramming and metabolism rewiring [32]. Evidence for trained immunity first emerged in humans showing vaccination-induced protections did not only work against the targeted diseases, but also other pathogens [33]. β-glucan, lipopolysaccharides, ox-LDL, uric acid and some pro-inflammatory cytokines (IL-8 and IL-12) induced trained immunity in monocytes [[34], [35], [36]]. Conditional innate immune cells can also be trained to confer long-lasting immunological memories [37]. For example, lysophosphatidylcholine has been shown to elicit trained immunity in ECs by up-regulating glycolysis, mevalonate, and acetyl-CoA generating pathways [38].
4. Adaptive immune response
The adaptive immune response is antigen-specific and encompasses cell-mediated (T cell) and humoral-mediated (B cell) immunities which are both critical to drive tissue inflammation or repair (Fig. 1B). T cell response involves 4 distinct regulatory signals [7,39]. Signal 1 (antigen recognition) determines the specificity of T cell response. Antigen peptide is presented by MHCI/II on APC and engaged with antigen-specific TCRs on naïve T cell. Signal 2 (immune checkpoint) is marked by the ligation of stimulatory/inhibitory molecular pairs [7]. Signal 3 (cytokine stimulation) strengthens immune cell activation. Activated APCs produce various cytokines that enhance T cell clonal expansion and differentiation [40,41]. Signal 4 (MADS recognition) is a novel signal we proposed to describe small molecular metabolite-induced MADS:MS recognition [7,10].
B cell response is marked by antibody production that play an important role in both innate and adaptive immunity. There are 2 types of B cell immunity, T cell-dependent and -independent B cell responses [42]. T cell-dependent B cell response describes B2 cells mount antibody responses with the help of follicular helper T (Tfh) cells. Three signals are involved in T cell-dependent B cell response. Signal 1 (antigen recognition) determines the specificity of B cell response in which B cell receptor recognizes specific antigens. Signal 2 (immune checkpoints) is featured by CD40/CD40L ligation contributing to B cell activation, isotype switching and affinity maturation. Signal 3 (cytokine stimulation) strengthens B cell immune response. T cell-independent B cell response refers to the innate defense of B1 cells against a wide range of PAMP/DAMPs. In the absence of stimulation, B1 cells spontaneously secrete natural IgM antibodies to maintain resting immunoglobulin levels in the body. In the presence of stimulation, B1 cells produce both natural antibodies and immunomodulatory molecules, such as IL-10, IL35, and granulocyte-macrophage colony-stimulating factor, to regulate acute and chronic inflammatory diseases. The natural antibodies produced by B1 cells differ from B2 cell adaptive antibodies by their low affinity and polyreactivity. Current evidence supports that the IgM antibody generated by B1 cells are atheroprotective [43,44].
5. Innate-adaptive immunity interplay
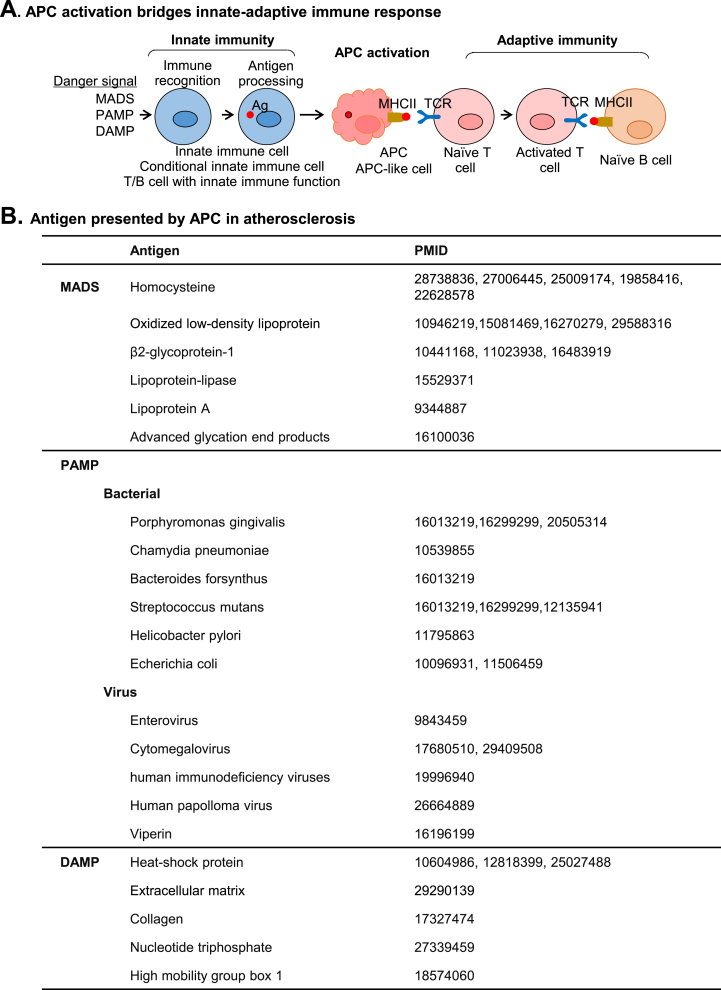
APC activation bridges innate-adaptive immunity — APC plays a central role in initiating adaptive immune responses and bridging innate-adaptive immunity through their capacity to present antigen to T cells (Fig. 2A). Upon rapid activation by PAMP/DAMP:PRR or MADS:MS recognition, innate immune cells acquire enhanced capacity to process and present antigen by upregulating MHCII and immune checkpoint molecules, such as CD80, CD86 and CD40, which allow them to form APC and efficiently activate CD4 and CD8 T cells [4,7,[45], [46], [47]]. DC is one of the professional APCs which link the innate and adaptive response [48,49]. In response to ox-LDL, human peripheral monocytes can differentiate into DCs which further induce Th1 and Th17 activation and proliferation [50]. Of note, ECs have also been shown to effectively take up, process, and present antigens, with the expression of MHCI/II, immune checkpoint molecules and cytokines, to prime adaptive immunity [51].
Fig. 2.
APC bridges innate-adaptive immune responses. A. APC bridges innate-adaptive immune responses. The initial process of immune response is immune recognition of innate immune cell or innate immune-like cell-mediated by MADS:MS or DAMP/PAMP:PRR recognition. Activated innate immune cells process antigens intracellularly and present them via MHCII molecules. This process is called APC formation and serves as a bridge between innate and adaptive immunity. APC presents antigen to T cell leading to T cell activation. B. Antigen presented by APC in atherosclerosis. Antigens presented by APC in atherogenesis are summarized based on literature searching.
Considering the important roles of T and B cells in chronic inflammatory diseases, identification of specific antigens driving adaptive immunity is critical to advance therapeutic strategies. In Fig. 2B, we summarized a series of antigens which have been associated with the pathogenesis of atherosclerosis. Lipoprotein a, lipoprotein-lipase and advanced glycation end products are established MADS relevant to atherosclerosis development. They can induce the expression of MHC and immune checkpoint molecules on DC to activate T cell [[52], [53], [54]]. Scavenger receptors such as CD36, CD68 and SRA are PRRs responsible for LDL uptake, which then presented by MHCII on the surface of monocytes, macrophage and vascular cells [55,56]. In the PAMP system, several bacteria and viruses have been described. For instance, C. pneumoniae is a gram-negative bacterium which may be causative for atherosclerosis [57]. Knockout of TLR2/4 attenuated C. pneumonia-accelerated atherosclerosis and reduced foam cell formation in Apoe−/- mice. Several DAMPs, including heat shock proteins, extracellular matrix components and some cell death associated products, are described in atherogenesis. Heat shock proteins may be engaged in humoral immune response as the level of plasma heat shock proteins and their autoantibodies positively correlated with atherosclerosis in human [58]. T cells specifically responding to heat shock proteins was also found in atherosclerotic plaques [59].
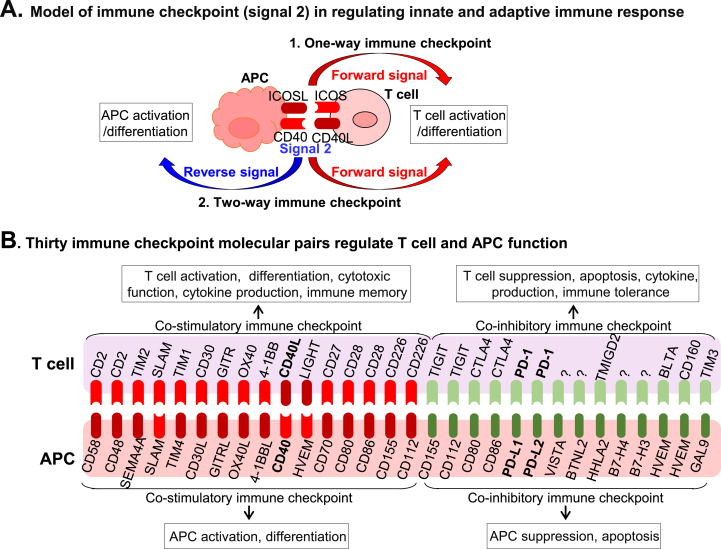
Immune checkpoint connects innate and adaptive immune cell response — As one of the mechanisms for innate control of adaptive immunity, signal 2 has been well-studied about its function to direct T cell function. Based on the findings over the past few years, we classified signal 2 immune checkpoint into one-way and two-way immune checkpoints (Fig. 3A) [7]. The one-way immune checkpoint involves only forward signaling and directs T cell activation. ICOS/ICOSL is one of the well-described one-way immune checkpoints, which are essential for T cell expansion and differentiation [60]. However, the two-way immune checkpoint is bi-directional involving forward and reverse signaling, which modulates both innate and adaptive immunity by inducing T cell and monocyte/APC differentiation/suppression. CD40/CD40L is the best representative of two-way co-stimulatory immune checkpoints, which modulate effector functions of both T cell [61] and monocytes [10,62]. Immune checkpoints can be further divided into two groups by their co-stimulatory or co-inhibitory function (Fig. 3B) [[63], [64], [65]]. The stimulatory immune checkpoint molecules deliver positive signals leading to APC/T cell activation, differentiation and immune memory. The inhibitory immune checkpoint molecules deliver negative signals resulting in APC/T cell suppression, apoptosis and immune tolerance. Thirty immune checkpoint molecular pairs, consisting of 16 co-stimulatory and 14 co-inhibitory immune checkpoints are summarized in Fig. 3B. This list is based on our recent publication [63] and included updated information by literature search.
Fig. 3.
Immune checkpoint molecules in innate-adaptive immunity interplay. A. Immune checkpoint (signal 2) in regulating innate-adaptive immune response. We defined two types of immune checkpoints, one-way and two-way, based on signal 2 direction. The one-way immune checkpoint only involves forward signaling and directs to T cell activation or suppression. The two-way immune checkpoint involves forward and reverse signaling which modulates both innate and adaptive immunity by inducing TC activation/suppression and innate immune cell/APC activation/suppression. B. Thirty immune checkpoint molecular pairs regulate T cell and APC function. Thirty immune checkpoint molecular pairs and their functions are illustrated. Red color symbol describes the stimulatory molecular pair and green color symbol describes inhibitory molecular pairs. Stimulatory immune checkpoint molecular pair ligation leads to T cell/APC activation and differentiation, whereas inhibitory immune checkpoint molecular pair ligation leads to T cell/APC suppression and apoptosis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Redox regulation in immune responses — ROS are generated by the partial reduction of oxygen to form chemically reactive species, such as superoxide (O2•-), hydrogen peroxide (H2O2), hydroxyl radical (OH•), peroxynitrite (ONOO−), and hypochlorous acid (HOCl), which are also natural byproducts of metabolic reactions [66,67]. Recently, we summarized current understanding on immune cell metabolic reprogramming and established models of metabolic switch in the progress of immune cell activation [15]. Briefly, increased glycolysis and impaired oxidative phosphorylation are two major metabolic changes in proinflammatory innate and adaptive immune cells. These metabolic changes are associated with excessive ROS production which play critical roles in physiological and pathological processes. Recent redox biology researches in immune cells have generated important information regarding to redox signaling and regulation in immune responses (Fig. 4A).
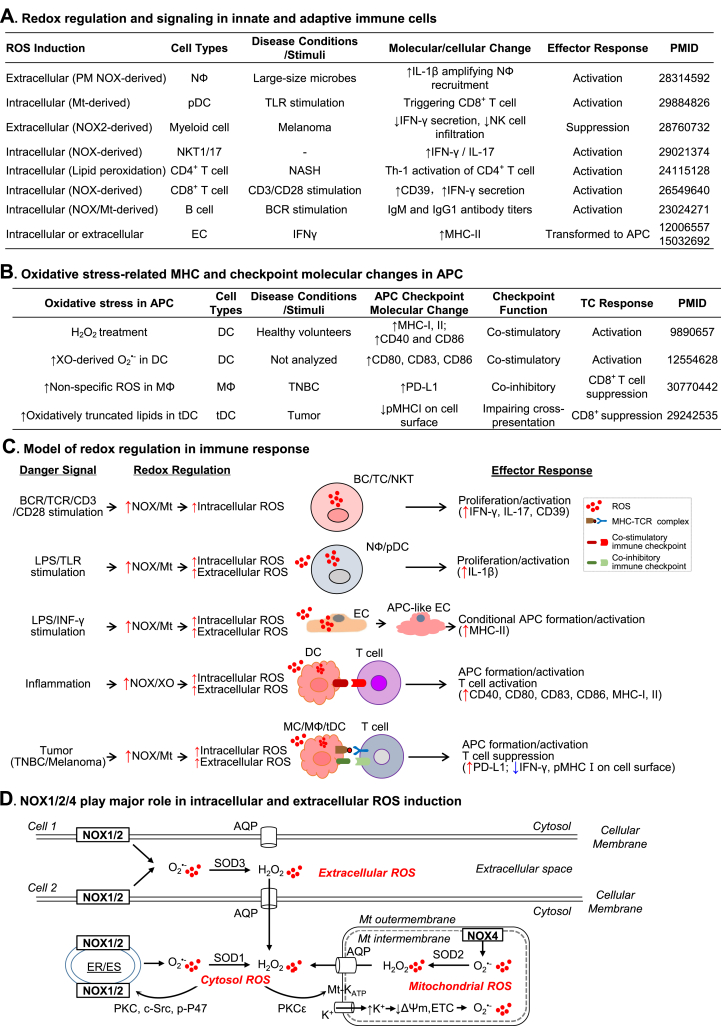
Fig. 4.
Redox regulation in innate-adaptive immunity interplay. Elevated Intracellular or extracellular ROS production is a critical metabolic change in immune responses. A. Redox regulation and signaling in innate and adaptive immune cells. In response to various stimuli or disease condition, immune cells display increased extracellular and intracellular ROS induction which are associated with inflammatory molecular-cellular changes and effector responses. B. Oxidative stress-related MHC and checkpoint molecular changes in APC. APC activation is associated with increased ROS or ROS-derived products which leads to immune checkpoint activation and T cell response. C. Model of redox regulation in immune response. Danger signals activate NOX which is responsible for increased intracellular and extracellular ROS production in immune or immune-like cells. The redox regulation mediates innate and adaptive immune cell activation, inflammatory response, APC formation/activation, and TC response via modulating the expression of major histocompatibility complex and immune checkpoint molecules. D. NOX1/2/4 play major role in intracellular and extracellular ROS induction. Plasma membrane-located NOX1/2 activation results in extracellular ROS production which can be dismutated by SOD3 to form H2O2 and then be diffused into the cytosol via water channel AQP. NOX1/2 located in PM/ER/ES can be activated via PKC-induced P47phox phosphorylation, resulting in intracellular O2•- generation which can be dismutated by SOD1 to form H2O2. NOX1/2-derived H2O2 can activate Mt-KATP via PKC-ε signaling, leading to the K+ influx, decrease of Mt membrane potential (ΔΨm), and ETC-derived ROS production. O2•- generated from Mt-located NOX4 is released into Mt matrix and dismutated by SOD2 to H2O2 which then be transported into cytosol via AQP.
Redox signaling and regulation in innate immunity — Innate immune response is initiated by immune recognition through PRR or MS which can induce ROS production. Increased ROS is the main antimicrobial response in phagocytes and has been known as respiratory/oxidative burst [68]. In response to large microbes, neutrophil produced an excess amount of extracellular ROS by plasma membrane NADPH oxidase (NOX) activation which caused selective oxidation of NF-кB leading to increased IL1β expression and amplified neutrophil recruitment [69]. Moreover, mitochondrial has been shown to contribute to ROS production and antimicrobial responses in macrophages in response to E. coli or TLR1,4 and 6 agonist [70]. Reducing mtROS by using mitochondrial electron transport chain complex II SDH-specifice inhibitor (3-nitropropionic acid) or overexpressing mitochondrial catalase resulted in defective bacterial killing in macrophages [70,71]. ROS also induces dendritic cell differentiation and their antigen representing functions [72].
It is established that NF-кB activation mediates ROS-induced innate immune response. For example, mtROS-mediated formation of disulfide bond in the NF-кB essential modulator (NEMO) is essential for the activation of ERK1/2 and NF-кB signaling in infected macrophages [73]. Innate immune activation induced by asbestos inhalation was revealed to be mediated by NOX-derived ROS-NLPR3 inflammasome signaling [74]. In response to TLR agonist R848, the increased mtROS production was associated with increased cross-presentation capacity of plasmacytoid DC [75]. However, high ROS is not always associated with NF-кB activation, as LPS-treated ROS-high DC had low NF-кB activity compared with ROS-low DC [76].
Natural killer T (NKT) cells play critical roles in killing infected and malignant cells and has been shown to produce higher levels of NOX1/2-related ROS, especially NKT1/17, compared to CD4+/CD8+ T cells [77]. However, in myeloid specific NOX2-deficient mice or by using NOX2-inhibitor histamine dihydrochloride, myeloid cell-derived ROS has been demonstrated to facilitate metastasis of melanoma cells by suppressing IFNγ-producing NKT cell function [78]. EC has been proposed as one of the conditional innate immune cells which can be activated to form APC and to initiate adaptive immune responses [22,79]. Consistently, IFNγ induced the expression of MHCII in EC partially mediated by ROS-mediated signaling [80,81].
Taken together, NOX and mitochondria are two major sources of increased ROS production in innate immune cells, which play critical roles in regulating innate immunity via modulating mostly ERK1/2, NF-кB, TLR and NLPR3 signalings [74].
Redox signaling and regulation in adaptive immunity — Excessive ROS production is associated with the activation, differentiation and survival in T and B cells. Indeed, moderate ROS production is essential for T cell activation whereas excessive ROS can inhibit NF-кB phosphorylation and T cell activities [82]. NOX2-derived ROS was responsible for CD3/CD28 stimulation-mediated CD8+ T cell activation [83]. MtROS production was also induced after TCR activation and promoted T cell activation by regulating IL2 and IL4 expression [84]. Hepatic oxidative stress induced humoral and cellular immune responses in methionine‐choline deficient model of non-alcoholic steatohepatitis [85]. Upon activation, T cells differentiate to distinct subsets with different cytokine production. ROS can modulate both T cell differentiation and cytokine production by polarized T cell subsets. For instance, H2O2 reduced IFNγ production of activated Th1 and increased IL4 secretion of activated Th2 in vitro which was associated with bronchial hyper-responsiveness and airway remodeling [86]. NOX-derived ROS was also important for Treg differentiation and function since mice with mutated p45phox or gp91phox displayed hampered Treg induction and T cell suppression [87]. Moreover, ROS was involved in activation-induced cell death via Fas/FasL pathway to maintain T cell homeostasis [88].
Similarly, BCR stimulation induced rapid ROS production in primary resting murine B cells [89]. Two distinct sources of ROS were revealed downstream of the BCR signaling, Nox2 in the erary stage of B cell activation and mitochondrial respiration at later stage of B cell activation [89]. However, the role of ROS in B cell subsets differentiation remain elusive. Collectively, ROS produced by NOX and mitochondrial are involved in adaptive immune responses which potentially involve in NF-кB activation.
Redox regulation in innate-adaptive immunity interplay — Considering that MHC and immune checkpoint molecules are two key molecular features of innate-adaptive immunity activation, we paid special attention on the role of ROS-modulated MHC and immune checkpoint molecules expression in APC (Fig. 4B).
H2O2-treated DC has been shown to be more efficient in promoting T cell proliferation compared with normal DC due to increased expression of MHCI, MHCII, and the co-stimulatory molecules CD40 and CD86 [90]. Moreover, xanthine oxidase-derived O2•- induced phenotypic and functional maturation of DC, partly through an NF-кB-dependent mechanism [91]. However, in response to ROS inducer, including glutathione synthesis inhibitor, buthionine sulphoximine, and paclitaxel, human and mouse tumor-associated macrophages acquired immunosuppressive phenotype by upregulating the expression of PD-L1 and immunosuppressive cytokines, including IL-4, IL-10 and IL17 [92]. Oxidatively truncated lipid species formed via oxidative degradation of triacylglyceride and cholesterol ester decreased trafficking of peptide-MHCI complexes to the cell surface in tumor-associated DCs, consequently impaired their ability to initiate and sustain adequate CD8+ T cells responses to tumor cells [93]. Anti-oxidant α-tocopherol vitamin E abrogated this defect by preventing the generation of peroxidized lipids [93].
Overall, ROS production can be induced in both activated innate and adaptive immune cells, and in turn regulate their activities as well as innate-adaptive immunity interplay. However, further mechanistic studies are needed to translate this knowledge into therapeutic opportunities for metabolic and inflammatory diseases.
6. Model of redox regulation in immune response
In Fig. 4C, we presented a model to characterize redox regulation in different immune cells during immune responses. Various danger signals led to increased intracellular and extracellular ROS production in immune cells. Elevated NOX- and mitochondria-derived intracellular ROS is associated with B and T cells activation and proliferation in response to BCR/TCR/CD3/CD28 stimulation. Under TLR stimulation, the production of NOX- and mitochondria-derived intracellular and extracellular ROS in neutrophil and pDC are increased and can further promote their activation and proliferation. Moreover, in innate immune-like cell EC, pro-inflammation cytokine IFN-γ/LPS induce NOX- and mitochondria-derived intracellular and extracellular ROS production, which contribute to the conversion and activation of ECs to APC by upregulating MHCII molecules. The increased NOX/xanthine oxidase-derived intracellular and extracellular ROS in innate immune cells under inflammatory danger signal stimulation played a critical role in mediating APC and T cell activation by modulating the expression of MHC and immune checkpoint molecules. Differently, in the tumor microenvironment, excessive intracellular or extracellular ROS production in innate immune cells, including monocyte, macrophage and DC, is associated with APC formation and activation but leads to T cell suppression partially mediated by increased co-inhibitory immune checkpoint molecule PD-L1.
7. Intracellular ROS and ROS compartmentalization
ROS generation within specific subcellular compartments is mostly related with spatial metabolic processes, differential thiol concentration and specific redox enzyme distribution. This compartmentalization provides local control of ROS regulation and signaling between compartments. We modeled and discussed biochemical and molecular basis underlying ROS compartmentalization in our recent publications [15,67]. For instance, glycolysis and pentose phosphate pathway contribute to cytosolic ROS (cROS) production by generating reducing equivalents NADPH catalyzed by NOX to produce O2•- or H2O2. As a consequence of electron leakage, the mitochondrial electron transport chain continuously generates mitochondrial ROS (mtROS) using NADH and FADH as reductants [15]. Mitochondria are a major source of intracellular ROS and especially susceptible to oxidative stress. This is most likely due to high levels of ROS generated from lipid peroxidation, protein oxidation and mitochondrial DNA mutations. However, it was found that mitochondria have higher concentration of glutathione (GSH) which is a major hydrophilic antioxidant and most reactive electron donor [94]. About 10–15% of the total cell GSH content was found in mitochondria which play a critical role in the maintenance of mitochondrial function and cell survival [95].
Moreover, redox enzyme subcellular localization is one of the major mechanisms defining ROS compartmentalization. As indicated in Fig. 4D, ROS clearance enzyme superoxide dismutase (SOD) has 3 family members in mammal, of which SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular [96]. NOX, a membrane-bound multi-enzyme complex, is the major producer of ROS with various tissue distribution and subcellular localization. NOX family is responsible for transporting electrons across biological membranes and the reduction of oxygen into superoxide. NOX family consists of seven members, NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX267. Localization of NOX play critical roles in regulating ROS production and the pathways they control. NOX1 and NOX2 has been found in plasma membrane, endosome, phagosome, and endoplasmic reticulum (ER). The NOX4 has been identified in focal adhesions, nucleus, ER and mitochondrial intermembrane. The DUOX1 and DUOX2 were localized to the plasma membrane. However, little information is available about the subcellular localization of NOX3 and NOX5. As NOX and NOX-derived ROS have been implicated in regulating immune responses and the pathologies of various diseases, we further illustrated the biochemical processes of how NOX contributes to the generation of intracellular and extracellular ROS in immune cells in Fig. 4D.
NOX1/2/4 play major role in intracellular and extracellular ROS induction (Fig. 4D) — Plasma membrane-located NOX1/2 is the major producer of extracellular ROS which may serve as communication molecules in immune responses. The activity of NOX is dependent on subunit interaction [97]. In response to danger signals, including PAMP, DAMP and MADS, plasma membrane located NOX1/2 are activated [98]. Activated NOX1/2 produce and release O2•- directly into the extracellular spaces which are dismutated into H2O2 by superoxide dismutase (SOD) 3. Different from O2•-, H2O2 is relatively permeable and may be diffused into cell via aquaporins which are initially defined as membrane-intrinsic proteins water channel and involved in osmotic volume regulation. Recently, increasing studies have demonstrated that some aquaporin isotypes from mammals and plants can transport H2O2 across organelle and plasma membranes and play a role in oxidative stresses [[99], [100], [101], [102]]. However, the efficiency of H2O2 transport varies with different aquaporin homologs. For instance, two plant aquaporins, AtPIP2; 4 and SoPIP2; 1, and a human aquaporin AQP1 were all transporters of both H2O and H2O2, but the plant aquaporins were more permeable to H2O2 than the human homologue [100]. Controversially, by using improved H2O2 imaging, another study demonstrated that H2O2 produced in HeLa cells upon EGFR activation can not be diffused out across the cytoplasm [103]. Further studies are needed to address how aquaporins regulate H2O2 transport across organelle and plasma membranes, and whether H2O2 molecules produced outside or inside the cells function differently.
NOX located in ER, endosome, and mitochondrial play major roles in intracellular ROS production. The compartmentation of NOX-derived ROS may contribute to its functional specificity [102]. Excessive cROS in immune cells is mostly produced by NOX1/2 located in ER and endosome, which can be activated via protein kinase C (PKC)-induced P47phox phosphorylation. Intracellular O2•- can be dismutated by SOD1 to form H2O2. Further, cytosolic H2O2 can activate Mt-KATP via PKC-ε signaling. Increased K+ influx reduces the mitochondrial membrane potential (ΔΨm) leading to electron transport chain-derived O2•- production. NOX4 is located in the mitochondrial intermembrane, which is the key enzyme facilitating mitochondria-derived O2•- production. NOX4-derived O2•- is released into mitochondrial matrix and dismutated by SOD2 to H2O2, which can then be transported into the cytosol via AQP. Therefore, NOX1/2/4-located in cytoplasm and ER/endosome membranes and mitochondrial intermembrane play major roles in intracellular and extracellular ROS induction in immune response.
The general structure of different NOX isotypes is similar, consisting of two transmembrane subunits, three cytosolic subunits, and G protein Rac. The membrane subunits form the catalytic core of NOX, such as gp91phox (also known as NOX2, or its homologs) and p22phox. The cytosolic subunits, including p47phox, p67phox, p40phox, and G protein Rac, are required for assembly and activation of NOX complex [104]. The regulation of NOX activity varies depending on the isoform [105]. Generally, NOX activation requires the translocation of the cytosolic subunits and Rac to the transmembrane subunits to activate the catalytic core of NOX which produces O2•- by transferring electrons from NADPH to molecular oxygen [104]. Phosphorylation of p47phox, p67phox and Rac by kinases, such as PKC, PKA, mitogen activated protein kinase, cAMP dependent kinase, and p21 activated kinase, plays critical roles in facilitating these process [[106], [107], [108]]. Overall, NOX activity is mostly regulated by the activation and interaction of its subunits.
8. Therapeutic potential of antioxidant
Reodx regulation is implicated in modulating innate and adaptive immunity and pathophysilology of many diseases, such as atherosclerosis, diabetes, rheumatoid arthritis, cancer, and neurodegenerative diseases [98,109]. Increasing efforts have been made to study the effects of antioxidants on the prevention and treatment of these diseases [110]. There are two major category of antioxidants, enzymatic and non-enzymatic antioxidants [111]. Enzymatic antioxidants are endogenous enzymes catalyzing the neutralization of ROS and reactive nitrogen species, including SOD, catalase, glutathione peroxidase, and thioredoxin reductase. Whereas non-enzymatic antioxidants are small molecules produced endogenously by metabolism, such as GSH, coenzyme W, and bilirubin, or provided by food or supplements, such as vitamin E, vitamin C, and carotenoids [111]. By neutralizing the excess of free radicals, antioxidant has been shown to be protective in vitro and contribute to disease prevention in several mouse models [[112], [113], [114]]. However, the results from clinical trails are controversial. For instance, although the most well studied antioxidants, vitamin C and E, have been shown to be atheroprotective in mouse [115], randomized controlled trial revealed that neither vitamin C nor vitamin E supplementation reduced the risk of major cardiovascular events in middle-aged and older men after a mean of 8 years of treatment and follow-up [116]. A systematic review and meta-analysis included 68 randomized trials with 232,606 participants revealed that treatment with beta carotene, vitamin A, and vitamin E may increase mortality [117]. In fact, although redox homeostasis is essential to life, the application of antioxidant supplement or therapy should undergo further evaluation. Disease site, cell type and compartment-specific control of ROS by agents with stable antioxidant activities may be potential targets for future investigation and treatment.
9. Summary
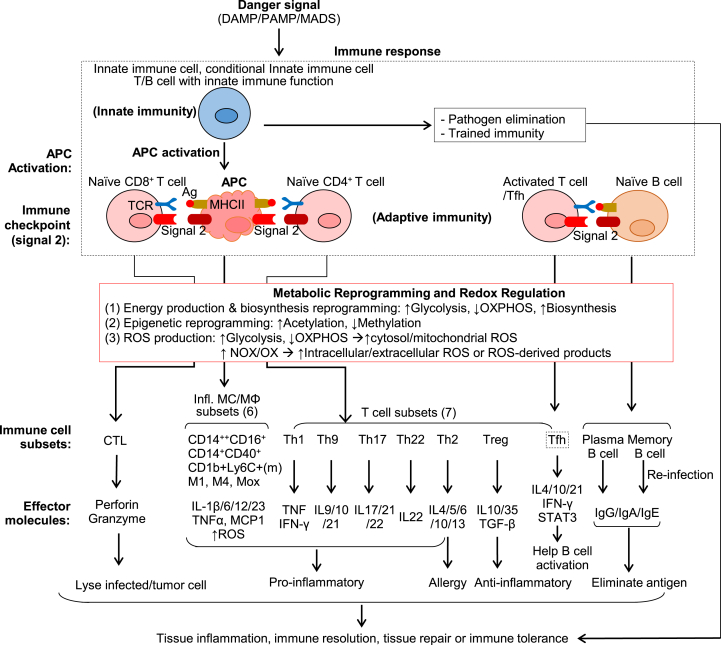
Three mechanisms are highlighted in this review to illustrate the innate-adaptive immunity interplay in tissue immune response (Fig. 5). 1) APC activation bridges innate-adaptive immune responses. 2) Immune checkpoint molecular reaction directs innate and adaptive immune cell responses. 3) Metabolic reprogramming leads to excessive intracellular and extracellular ROS production, mostly related to NOX activation in immune cells.
Fig. 5.
Innate-adaptive immunity interplay and redox regulation in immune response. Danger signals (DAMP/PAMP/MADS) induce immune response firstly via innate immune cell activation which leads to cytokine/chemokine production, trained immunity, pathogen elimination, and APC activation. The activated APC interacts with Naïve CD8+ and Naïve CD4+ T cell and function as a bridge to connect the innate and adaptive immune systems. Immune checkpoint molecule pairs (signal 2) determine the stimulatory or inhibitory immune response on T cell and APC. In the course of immune cell activation, active metabolic reprogramming leads to redox regulation and increased intracellular and extracellular ROS production. This results in immune subset differentiation. Naïve CD8+ T cell differentiates into CTL which lyse infected and tumor cells. Naïve CD4+ T cell differentiates into inflammatory subsets Th1/2/9/17/22, anti-inflammatory subsets Treg, and Tfh subset which helps Naïve B cell/B2 to differentiate into a plasma cell and the memory cell. MC/MΦ are classical APC and can be differentiated into anti-inflammatory subsets (not listed) and pro-inflammatory subsets (CD14++CD16+, CD14+CD40+, CD1b + Ly6C+(m), M1, M4, Mox). Immune cell subsets produce distinguish effector molecules which contribute to tissue inflammation or repair.
Immune recognition via DAMP/PAMP:PRR or MADS:MS in innate immune cells triggers APC activation, which bridges the innate and adaptive immune systems by interacting with Naïve CD4+/CD8+ T cells. As mentioned, four signals are initiated by APC to elicit adaptive immune responses. Immune checkpoint molecule pairs (signal 2) play a critical role in connecting innate and adaptive immune cells for stimulatory or inhibitory immune response, and direct immune response towards either cell by one-way or two-way immune checkpoint reactions. Importantly, as detailed in our recent publication, immune cells undergo metabolic reprogramming during immune responses, which is characterized as altered energy production, biosynthesis and epigenetic reprogramming [15]. To fulfill increased energetic and biosynthetic demands for defense response and damage repair, activated immune cells tend to increased glycolysis activity but decrease oxidative phosphorylation for prompt ATP production to adopt proliferative status and pro-inflammatory effector functions. As one of the consequences of cellular metabolism changes, distinct epigenetic reprogramming has also been found in activated immune cells due to altered accessibility of acetyl/methyl group donor and metabolite-modulated activity of epigenetic enzymes [15]. Elevated acetylation but suppressed methylation is often associated with a pro-inflammatory status in immune cells in many cases [15].
In activated immune cells, metabolic reprogramming directly leads to increased ROS production. For example, activated glycolysis and pentose phosphate pathway lead to increased NADPH and cROS production. Impaired mitochondrial respiratory chain leads to mtROS production [118]. Elevated levels of mtROS can also be attributed to mitochondrial intermembrane-located NOX4 complex activation. In addition, plasma membrane bounded-NOX1/2 complexes activation results in extracellular ROS production. ER and endosome membrane bound- NOX1/2 complex activation leads to cROS production. Excessive ROS production in immune cells leads to oxidative stress, which causes molecular damage, disrupted biochemical and cellular functions.
The regulation of innate-adaptive immunity interplay by APC activation, immune checkpoint molecular reactions and metabolic reprogramming determines distinct immune cell subsets differentiation and direct the immune responses to tissue inflammation, immune resolution, tissue repair or immune tolerance. In response to danger signals, monocyte can be differentiated into anti-inflammatory subsets (CD14++CD16− monocyte in human, CD1b+Ly6C− monocyte in mice, CD14+CD40− monocyte and M2/Mhem macrophage) and pro-inflammatory subsets (CD14++CD16+ monocyte in human, CD1b+Ly6C+ monocyte in mice, CD14+CD40+ monocyte, M1 and M4 macrophage) [4,119]. The pro-inflammatory monocytes selectively traffic to the sites of inflammation and are most likely to be differentiated to pro-inflammatory macrophages which contribute to tissue inflammation by producing TNFα, IL-1β, ROS and other inflammatory mediators [4]. However, anti-inflammatory MCs are prone to differentiate to anti-inflammatory macrophages and secrete anti-inflammatory cytokines (IL-10), resulting in tissue repair. Naïve CD8+ T cells can be activated to cytotoxic T lymphocyte to destroy infected cells and tumor cells. In contrast, naïve CD4+ T cell can be differentiated into different effector T cells, including the pro-inflammatory subsets Th1/2/9/17/22, anti-inflammatory regulatory T cells and Tfh cell. Here, we refer the details about the functions of different immune cells subsets in tissue inflammation and diseases to another review article we recently published [119].
This article comprehensively illustrated the updated knowledge of innate-adaptive immunity interplay with a focus on the bridging role of APC formation, immune checkpoint, and redox regulation. Further understanding the molecular basis of innate-adaptive immunity interplay and how they contribute to metabolic and inflammatory diseases could lead to the identification of novel therapeutic targets.
Source of funding
This work was supported in part by the National Institutes of Health (NIH) grants HL82774, HL-110764, HL130233, HL131460, DK104114, DK113775 and HL131460 to HW.
Declaration of competing interest
None.
Nonstandard abbreviations and acronyms.
| Ag | Antigen |
| APC | Antigen-presenting cell |
| AQP | Aquaporin |
| BCR | B cell receptor |
| CKD | Chronic kidney disease |
| cROS | Cytosolic reactive oxygen species |
| DC | Dendritic cell |
| DAMP | Danger-associated molecular pattern |
| EC | Endothelial cell |
| ER | Endoplasmic reticulum |
| ES | Endsome |
| HHcy | Hyperhomocysteinemia |
| IFNγ | Interferonγ |
| IL6 | Interleukin6 |
| MADS | Metabolite-associated danger signal |
| MHC | Major histocompatibility complex |
| MtROS | Mitochondrial reactive oxygen species |
| MS | Metabolic sensor |
| MФ | Macrophage |
| NKT | Natural killer T cell |
| NOX | NADPH oxidase |
| NФ | Neutrophil |
| PAMP | Pathogen-associated molecular pattern |
| pDC | Plasmacytoid dendritic cell |
| PKC | Protein kinase C |
| PRR | Pattern recognition receptor |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TCR | Toll cell receptor |
| Tfh | Follicular helper T cell |
| TNFα | Tumor necrosis factor α |
| TLR | Toll-like receptor |
| TNBC | Triple negative breast cancer |
| XO | Xanthine oxidase |
| VSMC | Vascular smooth muscle cell |
References
- 1.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witztum J.L., Lichtman A.H. The influence of innate and adaptive immune responses on atherosclerosis. Annual review of pathology. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulopoulou S., McCarthy C.G., Webb R.C. Toll-like receptors in the vascular system: sensing the dangers within. Pharmacol. Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J., Zhang L., Yu C., Yang X.F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker research. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigusa R., Winkels H., Ley K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020;17:387–401. doi: 10.1038/s41569-020-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sage A.P., Tsiantoulas D., Binder C.J., Mallat Z. The role of b cells in atherosclerosis. Nat. Rev. Cardiol. 2019;16:180–196. doi: 10.1038/s41569-018-0106-9. [DOI] [PubMed] [Google Scholar]
- 7.Dai J., Fang P., Saredy J., Xi H., Ramon C., Yang W. Metabolism-associated danger signal-induced immune response and reverse immune checkpoint-activated cd40(+) monocyte differentiation. J. Hematol. Oncol. 2017;10:141. doi: 10.1186/s13045-017-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain A., Pasare C. Innate control of adaptive immunity: beyond the three-signal paradigm. J. Immunol. 1950;198:3791–3800. doi: 10.4049/jimmunol.1602000. Baltimore, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J., Fang P., Yu D., Zhang L., Zhang D., Jiang X. Chronic kidney disease induces inflammatory cd40+ monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circ. Res. 2016;119:1226–1241. doi: 10.1161/CIRCRESAHA.116.308750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J., Lu S., Ding Y., Zheng M., Wang X. Homocysteine activates t cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein & cell. 2016;7:391–402. doi: 10.1007/s13238-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D., Jiang X., Fang P., Yan Y., Song J., Gupta S. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang P., Zhang D., Cheng Z., Yan C., Jiang X., Kruger W.D. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia C., Rao X., Zhong J. Role of t lymphocytes in type 2 diabetes and diabetes-associated inflammation. Journal of diabetes research. 2017;2017:6494795. doi: 10.1155/2017/6494795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L., Yang X., Yuan Z., Wang H. Metabolic reprogramming in immune response and tissue inflammation. Arterioscler. Thromb. Vasc. Biol. 2020;40:1990–2001. doi: 10.1161/ATVBAHA.120.314037. ATVBAHA120314037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchina D.G., Dostert C., Brenner D. Reactive oxygen species: involvement in t cell signaling and metabolism. Trends Immunol. 2018;39:489–502. doi: 10.1016/j.it.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Zmora N., Bashiardes S., Levy M., Elinav E. The role of the immune system in metabolic health and disease. Cell Metabol. 2017;25:506–521. doi: 10.1016/j.cmet.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Brestoff J.R., Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkels H., Ehinger E., Vassallo M., Buscher K., Dinh H.Q., Kobiyama K. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell rna-sequencing and mass cytometry. Circ. Res. 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochain C., Vafadarnejad E., Arampatzi P., Pelisek J., Winkels H., Ley K. Single-cell rna-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D., Fang P., Jiang X., Nelson J., Moore J.K., Kruger W.D. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in ldlr/cbs-deficient mice. Circ. Res. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mai J., Virtue A., Shen J., Wang H., Yang X.F. An evolving new paradigm: endothelial cells--conditional innate immune cells. J. Hematol. Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Wang L., Fang P., Sun Y., Jiang X., Wang H. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J. Biol. Chem. 2018;293:11033–11045. doi: 10.1074/jbc.RA118.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnack L., Sohrabi Y., Lagache S.M.M., Kahles F., Bruemmer D., Waltenberger J. Mechanisms of trained innate immunity in oxldl primed human coronary smooth muscle cells. Front. Immunol. 2019;10:13. doi: 10.3389/fimmu.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 26.Shen W., Gao C., Cueto R., Liu L., Fu H., Shao Y., Yang W.Y., Fang P., Choi E.T., Wu Q., Yang X.F., Wang H. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2019;28:101322. doi: 10.1016/j.redox.2019.101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi J., Jung J., Hong S.W., Lee J.Y., Han D., Kim K.S. Unregulated antigen-presenting cell activation by t cells breaks self tolerance. Proc. Natl. Acad. Sci. U.S.A. 2019;116:1007–1016. doi: 10.1073/pnas.1818624116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratky W., Reis e Sousa C., Oxenius A., Sporri R. Direct activation of antigen-presenting cells is required for cd8+ t-cell priming and tumor vaccination. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17414–17419. doi: 10.1073/pnas.1108945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Bekkering S., Quintin J., Joosten L.A., van der Meer J.W., Netea M.G., Riksen N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014;34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 35.Foster S.L., Hargreaves D.C., Medzhitov R. Gene-specific control of inflammation by tlr-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K., Maekawa T., Zhu Y., Renard-Guillet C., Chatton B., Inoue K. The transcription factor atf7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat. Immunol. 2015;16:1034–1043. doi: 10.1038/ni.3257. [DOI] [PubMed] [Google Scholar]
- 37.Hamada A., Torre C., Drancourt M., Ghigo E. Trained immunity carried by non-immune cells. Front. Microbiol. 2018;9:3225. doi: 10.3389/fmicb.2018.03225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Sun Y., Drummer Ct, Nanayakkara G.K., Shao Y., Saaoud F. Increased acetylation of h3k14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells - novel qualification markers for chronic disease risk factors and conditional damps. Redox biology. 2019;24:101221. doi: 10.1016/j.redox.2019.101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith-Garvin J.E., Koretzky G.A., Jordan M.S. T cell activation. Annu. Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerner M.Y., Heltemes-Harris L.M., Fife B.T., Mescher M.F. Cutting edge: il-12 and type i ifn differentially program cd8 t cells for programmed death 1 re-expression levels and tumor control. J. Immunol. 1950;191:1011–1015. doi: 10.4049/jimmunol.1300652. Baltimore, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Sasson S.Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I. Il-1 acts directly on cd4 t cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe M., Fujihara C., Radtke A.J., Chiang Y.J., Bhatia S., Germain R.N. Co-stimulatory function in primary germinal center responses: Cd40 and b7 are required on distinct antigen-presenting cells. J. Exp. Med. 2017;214:2795–2810. doi: 10.1084/jem.20161955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsiantoulas D., Diehl C.J., Witztum J.L., Binder C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin F., Oliver A.M., Kearney J.F. Marginal zone and b1 b cells unite in the early response against t-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 45.Roche P.A., Furuta K. The ins and outs of mhc class ii-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz D.R. Antigen presentation, antigen-presenting cells and antigen processing. Curr. Opin. Immunol. 1988;1:213–219. doi: 10.1016/0952-7915(88)90004-0. [DOI] [PubMed] [Google Scholar]
- 47.Pober J.S., Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thery C., Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 2001;13:45–51. doi: 10.1016/s0952-7915(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 49.Unanue E.R. Antigen-presenting function of the macrophage. Annu. Rev. Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 50.Liu A., Ming J.Y., Fiskesund R., Ninio E., Karabina S.A., Bergmark C. Induction of dendritic cell-mediated t-cell activation by modified but not native low-density lipoprotein in humans and inhibition by annexin a5: involvement of heat shock proteins. Arterioscler. Thromb. Vasc. Biol. 2015;35:197–205. doi: 10.1161/ATVBAHA.114.304342. [DOI] [PubMed] [Google Scholar]
- 51.Carman C.V., Martinelli R. T lymphocyte-endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front. Immunol. 2015;6:603. doi: 10.3389/fimmu.2015.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenna I.M., Waalkes M.P., Chen L.C., Gordon T. Comparison of inflammatory lung responses in wistar rats and c57 and dba mice following acute exposure to cadmium oxide fumes. Toxicol. Appl. Pharmacol. 1997;146:196–206. doi: 10.1006/taap.1997.8241. [DOI] [PubMed] [Google Scholar]
- 53.de Carvalho J.F., Borba E.F., Viana V.S., Bueno C., Leon E.P., Bonfa E. Anti-lipoprotein lipase antibodies: a new player in the complex atherosclerotic process in systemic lupus erythematosus? Arthritis Rheum. 2004;50:3610–3615. doi: 10.1002/art.20630. [DOI] [PubMed] [Google Scholar]
- 54.Ishizaka N., Saito K., Mori I., Matsuzaki G., Ohno M., Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin ii-infused rats. Arterioscler. Thromb. Vasc. Biol. 2005;25:2282–2288. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
- 55.Kimura T., Kobiyama K., Winkels H., Tse K., Miller J., Vassallo M. Regulatory cd4(+) t cells recognize major histocompatibility complex class ii molecule-restricted peptide epitopes of apolipoprotein b. Circulation. 2018;138:1130–1143. doi: 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kunjathoor V.V., Febbraio M., Podrez E.A., Moore K.J., Andersson L., Koehn S. Scavenger receptors class a-i/ii and cd36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld M.E., Campbell L.A. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemostasis. 2011;106:858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- 58.Wick G., Jakic B., Buszko M., Wick M.C., Grundtman C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 2014;11:516–529. doi: 10.1038/nrcardio.2014.91. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002;22:1547–1559. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 60.Esensten J.H., Helou Y.A., Chopra G., Weiss A., Bluestone J.A. Cd28 costimulation: from mechanism to therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elgueta R., Benson M.J., de Vries V.C., Wasiuk A., Guo Y., Noelle R.J. Molecular mechanism and function of cd40/cd40l engagement in the immune system. Immunol. Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molnar E., Swamy M., Holzer M., Beck-Garcia K., Worch R., Thiele C. Cholesterol and sphingomyelin drive ligand-independent t-cell antigen receptor nanoclustering. J. Biol. Chem. 2012;287:42664–42674. doi: 10.1074/jbc.M112.386045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen H., Wu N., Nanayakkara G., Fu H., Yang Q., Yang W.Y. Co-signaling receptors regulate t-cell plasticity and immune tolerance. Front. Biosci. 2019;24:96–132. doi: 10.2741/4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., Flies D.B. Molecular mechanisms of t cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schildberg F.A., Klein S.R., Freeman G.J., Sharpe A.H. Coinhibitory pathways in the b7-cd28 ligand-receptor family. Immunity. 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox biology. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L., Wang X., Cueto R., Effi C., Zhang Y., Tan H. Biochemical basis and metabolic interplay of redox regulation. Redox biology. 2019;26:101284. doi: 10.1016/j.redox.2019.101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babior B.M. The respiratory burst of phagocytes. J. Clin. Invest. 1984;73:599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warnatsch A., Tsourouktsoglou T.D., Branzk N., Wang Q., Reincke S., Herbst S. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity. 2017;46:421–432. doi: 10.1016/j.immuni.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garaude J., Acin-Perez R., Martinez-Cano S., Enamorado M., Ugolini M., Nistal-Villan E. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016;17:1037–1045. doi: 10.1038/ni.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P. Tlr signalling augments macrophage bactericidal activity through mitochondrial ros. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Prete A., Zaccagnino P., Di Paola M., Saltarella M., Oliveros Celis C., Nico B. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic. Biol. Med. 2008;44:1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 73.Herb M., Gluschko A., Wiegmann K., Farid A., Wolf A., Utermohlen O. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of nemo. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aar5926. [DOI] [PubMed] [Google Scholar]
- 74.Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oberkampf M., Guillerey C., Mouries J., Rosenbaum P., Fayolle C., Bobard A. Mitochondrial reactive oxygen species regulate the induction of cd8(+) t cells by plasmacytoid dendritic cells. Nat. Commun. 2018;9:2241. doi: 10.1038/s41467-018-04686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheng K.C., Pietersz G.A., Tang C.K., Ramsland P.A., Apostolopoulos V. Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J. Immunol. 1950;184:2863–2872. doi: 10.4049/jimmunol.0903458. Baltimore, Md. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.H., Kumar A., Chang C.H., Pyaram K. Reactive oxygen species regulate the inflammatory function of nkt cells through promyelocytic leukemia zinc finger. J. Immunol. 1950;199:3478–3487. doi: 10.4049/jimmunol.1700567. Baltimore, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aydin E., Johansson J., Nazir F.H., Hellstrand K., Martner A. Role of nox2-derived reactive oxygen species in nk cell-mediated control of murine melanoma metastasis. Cancer immunology research. 2017;5:804–811. doi: 10.1158/2326-6066.CIR-16-0382. [DOI] [PubMed] [Google Scholar]
- 79.Shao Y., Saredy J., Yang W.Y., Sun Y., Lu Y., Saaoud F. Vascular endothelial cells and innate immunity. Arterioscler. Thromb. Vasc. Biol. 2020;40:e138–e152. doi: 10.1161/ATVBAHA.120.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grimm M., Spiecker M., De Caterina R., Shin W.S., Liao J.K. Inhibition of major histocompatibility complex class ii gene transcription by nitric oxide and antioxidants. J. Biol. Chem. 2002;277:26460–26467. doi: 10.1074/jbc.M110538200. [DOI] [PubMed] [Google Scholar]
- 81.Harari O., Liao J.K. Inhibition of mhc ii gene transcription by nitric oxide and antioxidants. Curr. Pharmaceut. Des. 2004;10:893–898. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X., Song M., Zhang B., Zhang Y. Vol. 2016. 2016. Reactive oxygen species regulate t cell immune response in the tumor microenvironment; p. 1580967. (Oxidative Medicine and Cellular Longevity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai A., Moss A., Rothweiler S., Serena Longhi M., Wu Y., Junger W.G. Nadh oxidase-dependent cd39 expression by cd8(+) t cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 2015;6:8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaminski M.M., Sauer S.W., Klemke C.D., Suss D., Okun J.G., Krammer P.H. Mitochondrial reactive oxygen species control t cell activation by regulating il-2 and il-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 1950;184:4827–4841. doi: 10.4049/jimmunol.0901662. Baltimore, Md. [DOI] [PubMed] [Google Scholar]
- 85.Sutti S., Jindal A., Locatelli I., Vacchiano M., Gigliotti L., Bozzola C. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in nash. Hepatology. 2014;59:886–897. doi: 10.1002/hep.26749. [DOI] [PubMed] [Google Scholar]
- 86.Frossi B., De Carli M., Piemonte M., Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by th1 and th2 cells. Mol. Immunol. 2008;45:58–64. doi: 10.1016/j.molimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Kraaij M.D., Savage N.D., van der Kooij S.W., Koekkoek K., Wang J., van den Berg J.M. Induction of regulatory t cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17686–17691. doi: 10.1073/pnas.1012016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green D.R., Droin N., Pinkoski M. Activation-induced cell death in t cells. Immunol. Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 89.Wheeler M.L., Defranco A.L. Prolonged production of reactive oxygen species in response to b cell receptor stimulation promotes b cell activation and proliferation. J. Immunol. 1950;189:4405–4416. doi: 10.4049/jimmunol.1201433. Baltimore, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rutault K., Alderman C., Chain B.M., Katz D.R. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic. Biol. Med. 1999;26:232–238. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 91.Kantengwa S., Jornot L., Devenoges C., Nicod L.P. Superoxide anions induce the maturation of human dendritic cells. Am. J. Respir. Crit. Care Med. 2003;167:431–437. doi: 10.1164/rccm.200205-425OC. [DOI] [PubMed] [Google Scholar]
- 92.Roux C., Jafari S.M., Shinde R., Duncan G., Cescon D.W., Silvester J. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of pd-l1. Proc. Natl. Acad. Sci. U.S.A. 2019;116:4326–4335. doi: 10.1073/pnas.1819473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veglia F., Tyurin V.A., Mohammadyani D., Blasi M., Duperret E.K., Donthireddy L. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017;8:2122. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ribas V., Garcia-Ruiz C., Fernandez-Checa J.C. Glutathione and mitochondria. Front. Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mari M., Morales A., Colell A., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxidants Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the cuzn-sod (sod1), mn-sod (sod2), and ec-sod (sod3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 97.Brandes R.P., Weissmann N., Schroder K. Nox family nadph oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y., Lu Y., Saredy J., Wang X., Drummer Iv C., Shao Y. Ros systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox biology. 2020:101696. doi: 10.1016/j.redox.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Almasalmeh A., Krenc D., Wu B., Beitz E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014;281:647–656. doi: 10.1111/febs.12653. [DOI] [PubMed] [Google Scholar]
- 100.Wang H., Schoebel S., Schmitz F., Dong H., Hedfalk K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020;1862:183065. doi: 10.1016/j.bbamem.2019.183065. [DOI] [PubMed] [Google Scholar]
- 101.Watanabe S., Moniaga C.S., Nielsen S., Hara-Chikuma M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016;471:191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 102.Laforenza U., Pellavio G., Marchetti A.L., Omes C., Todaro F., Gastaldi G. Aquaporin-mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int. J. Mol. Sci. 2016:18. doi: 10.3390/ijms18010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishina N.M., Tyurin-Kuzmin P.A., Markvicheva K.N., Vorotnikov A.V., Tkachuk V.A., Laketa V. Does cellular hydrogen peroxide diffuse or act locally? Antioxidants Redox Signal. 2011;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 104.Rastogi R., Geng X., Li F., Ding Y. Nox activation by subunit interaction and underlying mechanisms in disease. Front. Cell. Neurosci. 2016;10:301. doi: 10.3389/fncel.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson J.L., Park J.W., Benna J.E., Faust L.P., Inanami O., Babior B.M. Activation of p47(phox), a cytosolic subunit of the leukocyte nadph oxidase. Phosphorylation of ser-359 or ser-370 precedes phosphorylation at other sites and is required for activity. J. Biol. Chem. 1998;273:35147–35152. doi: 10.1074/jbc.273.52.35147. [DOI] [PubMed] [Google Scholar]
- 107.Diekmann D., Abo A., Johnston C., Segal A.W., Hall A. Interaction of rac with p67phox and regulation of phagocytic nadph oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 108.El Benna J., Faust R.P., Johnson J.L., Babior B.M. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase c, protein kinase a, and a mitogen-activated protein kinase. J. Biol. Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 109.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raghunath A., Sundarraj K., Nagarajan R., Arfuso F., Bian J., Kumar A.P. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox biology. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. International journal of biomedical science : IJBS. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 112.Kumari S., Deori M., Elancheran R., Kotoky J., Devi R. In vitro and in vivo antioxidant, anti-hyperlipidemic properties and chemical characterization of centella asiatica (l.) extract. Front. Pharmacol. 2016;7:400. doi: 10.3389/fphar.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobayashi N., DeLano F.A., Schmid-Schonbein G.W. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 2005;25:2114–2121. doi: 10.1161/01.ATV.0000178993.13222.f2. [DOI] [PubMed] [Google Scholar]
- 114.Prasanthi J.R., Dasari B., Marwarha G., Larson T., Chen X., Geiger J.D. Caffeine protects against oxidative stress and alzheimer's disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010;49:1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nespereira B., Perez-Ilzarbe M., Fernandez P., Fuentes A.M., Paramo J.A., Rodriguez J.A. Vitamins c and e downregulate vascular vegf and vegfr-2 expression in apolipoprotein-e-deficient mice. Atherosclerosis. 2003;171:67–73. doi: 10.1016/j.atherosclerosis.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 116.Sesso H.D., Buring J.E., Christen W.G., Kurth T., Belanger C., MacFadyen J. Vitamins e and c in the prevention of cardiovascular disease in men: the physicians' health study ii randomized controlled trial. Jama. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 118.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470 e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fang P., Li X., Dai J., Cole L., Camacho J.A., Zhang Y. Immune cell subset differentiation and tissue inflammation. J. Hematol. Oncol. 2018;11:97. doi: 10.1186/s13045-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]