Abstract
Efficient inactivation of Escherichia coli (E. coli) under visible (532 nm) pulsed light irradiation was achieved by fusion of a visible light-absorbing dye with E. coli. Inactivation experiments showed that 3-log inactivation of E. coli was obtained within 20 min under a 50 kJ/cm2 dose. This treatment time and dose magnitude were 10 times faster and 100 times lower, respectively, than the values previously obtained by using a visible femtosecond laser. The mechanism of bacterial death was modeled based on a transient photothermal evaporation effect, where a quantitative evaluation of the temperature increase was given based on the heat transfer equation. As a result of this theoretical analysis, the maximum temperature of the bacteria was correlated with the absorption ratio, pulse energy, and surface-to-volume ratio. An increase in the surface-to-volume ratio with the decreasing size of organic structures leads to the possibility of efficient inactivation of viruses and bacteria under low-dose and non-harmful-visible pulsed light irradiation. Hence, this method can be applied in many fields, such as the instantaneous inactivation of pathogenic viruses and bacteria in a safe and simple manner without damaging large organic structures.
Subject terms: Biophysics, Microbiology
Introduction
Inactivation with ultraviolet (UV) radiation is a well-established technique that has been used widely, including in the purification of water1–5, room decontamination6,7, and air purification. The wavelength of UV radiation used is generally shorter than 280 nm, which places it in the UVC region (the wavelength region from 250 to 280 nm is typically used); this wavelength is selected because UVC radiation inactivates pathogenic bacteria, viruses and other microorganisms8–10. Inactivation is believed to occur via the formation of thymine dimers in deoxyribonucleic acid (DNA) by the absorption of UVC photons; the dimers prevent further replication of the DNA strains11–13. However, it is generally known that many types of viruses and bacteria are resistant to UVC radiation, for example, blood-borne pathogens such as human immunodeficiency virus (HIV)14–16. Moreover, UVC is strongly absorbed by human cells and protein components; therefore, it raises concerns about damaging plasma components17 and causing platelet aggregation18. Thus, inactivation with UVC radiation lacks safety when applied in irradiation to the human body to inactivate pathogenic bacteria, viruses and other microorganisms attached to the skin or inside the human body.
To avoid the above problems related to the human body, many alternative methods have been studied such as inactivation by using cold plasma19,20, far-UVC light (200–220 nm region)21–23, and plasmonic effects24,25. However, these methods are still based on high-energy photons or plasma jets, and their effects on the human body have not yet been clarified. On the other hand, inactivation using continuous wave (CW) mode-locked femtosecond (fs: 10−15 s) lasers has attracted special interest as a potential alternative to UV irradiation26–28 because this method is based on low-energy photons in the visible or near-infrared region (400–800 nm). The inactivation mechanism is reported as impulsive stimulated Raman scattering of an ultrashort fs visible/near-infrared (NIR) laser pulse. The fs laser pulse coherently excites the mechanical vibrations29,30 of the protein capsid of target viral particles, leading to damage and inactivation of a broad spectrum of viruses and bacteria26–28 without using toxic or carcinogenic chemicals. This method seems to offer minimal concern of adverse effects to the human body31. However, fs laser inactivation methods have the following disadvantages: (1) a fs laser system is very expensive and cannot be easily obtained by everybody, (2) the inactivation efficiency is low thus it requires a long treatment time of more than 1 h26–28 for inactivation, and (3) it requires an extremely high peak power of the fs pulse on the order of 100 MW/cm2 for the inactivation of micrometer-sized bacteria32. These features impede the scalability and practical implementation of this photonic inactivation process.
In this work, we demonstrated the efficient inactivation of micrometer-sized bacteria fused with a dye by using a low-power and easily available nanosecond (ns) visible pulse laser (532 nm). We obtained 3-log inactivation of Escherichia coli (E. coli) bacteria in a short period of treatment time, i.e., on the order of 10 min. The inactivation mechanism obtained here is based on a transient photothermal evaporation effect of E. coli bacteria. For example, stained E. coli bacteria instantaneously absorbing 10 ns pulse irradiation were photothermally evaporated within 10 ms. This inactivation occurred under transient nonequilibrium states, which is entirely different from the usual photothermal inactivation performed under equilibrium states using CW lasers33–40. The combination of transient pulse irradiation and chromophore-fused target viruses and/or bacteria might not damage or heat large organic structures, such as human blood cells and stem cells. Hence, this method can be applied in many fields, such as the instantaneous inactivation of pathogenic viruses and bacteria in a safe and simple manner.
Materials and methods
Culturing, staining and enumeration of microorganisms
A pure culture of E. coli strain DH5α was incubated in nutrient broth (E-MC63; EIKEN Chemical Co., Tokyo, Japan) at 37 °C for 20 h. A concentration of 109–1011 colony forming units (CFU)/mL was achieved and used for the experiments. As shown in Fig. 1a, 0.5 mL of E. coli suspension was centrifuged at 4000 rpm for 5 min to separate the solution and bacterial cells. The supernatant was removed, and 0.5 mL normal saline solution was added to the cells (Fig. 1b). Then, the cells in saline solution were stained with a droplet of safranin dye solution (30 µL, Hayashi Pure Chemical Industry Limited Corporation, Japan), as shown in Fig. 1c. The stained cells and safranin dye solution were then separated by centrifugation (Fig. 1d). The stained bacterial cells were dissolved in saline solution at a density of 104 CFU/mL, as shown in Fig. 1e. E. coli stained with rhodamine B dye (Hayashi Pure Chemical Industry Limited Corporation, Japan) were also produced in the same manner. It should be noted that the reduction behavior (aging) of stained E. coli is almost the same as that of unstained E. coli; for example, both stained and unstained E. coli showed an approximately 10% reduction in CFU after one hour of experiments.
Figure 1.
Staining of E. coli with a safranin dye solution. (a) An E. coli suspension (0.5 mL) separated into the supernatant and bacterial cells by centrifugation. (b) E. coli cells were taken, and 0.5 mL normal saline solution was added to the cells. (c) E. coli cells in saline solution were stained with a droplet of safranin dye solution. (d) Stained cells and safranin dye solution were separated by centrifugation. (e) The stained bacterial cells were dissolved in saline solution at a density of 104 CFU/mL. (f) Optical absorption spectrum (absorbance) of an unstained E. coli solution (blue line), a safranin dye-stained E. coli solution (red line), and a rhodamine B dye-stained E. coli solution (orange line). The absorbance of E. coli at 532 nm was 0.084 for an unstained sample, 0.2 for a safranin-stained sample, and 0.38 for a rhodamine B-stained sample. (g) Theoretical plot of a temperature rise of a dye-stained E. coli as a function of time for pulsed (blue line) or CW (red line) laser irradiation. Inset shows schematic diagram of the thermal inactivation of E. coli by laser irradiation (green arrows), where the laser beam is efficiently absorbed by safranin- or rhodamine B dye-stained E. coli. Energy dissipation due to thermal convection is indicated by dashed arrows.
Figure 1f shows the optical absorption spectrum (absorbance) of the E. coli solutions stained with safranin dye (red line), rhodamine B dye (orange line) and without any dye (blue line). The absorbance of E. coli at the laser excitation wavelength (532 nm) was 0.084 for the unstained sample, 0.2 for the safranin-stained sample, and 0.38 for the rhodamine B-stained sample. Therefore, as shown in Fig. 1g, E. coli stained with safranin or rhodamine B dye efficiently absorbed 532 nm laser radiation from the second harmonics (SH) of the yttrium aluminum garnet (YAG) laser, increasing the temperature of E. coli. In this case, when a high-intensity laser pulse with the duration of 10 ns irradiates the E. coli, the temperature instantaneously increases beyond the evaporation point as shown by the blue line (theoretical plot) of Fig. 1g, leading to destruction of cell structure. On the other hand, with the same dose using CW laser irradiation, instantaneous thermal heating does not occur as shown by the red line (theoretical plot) of Fig. 1g. Thus, pulse irradiation seems to be promising for achieving a much higher inactivation rate than that obtained by CW irradiation at the same dose. We note here that the temperature increase of E. coli solution at steady state is the same magnitude between CW and pulsed laser irradiations, because we put the same energy into the solution. The detail of the thermal heating mechanism is described in the discussion section. To perform the inactivation experiments by using the SH of the YAG laser, 600 µL of the stained bacterial cells was taken. Colonies were counted after incubation for 24 h at 37 °C. Plates yielding 1–1000 CFU were considered for analysis. All experiments were performed at least three times independently.
Inactivation by SH of CW or Q-switched pulsed YAG laser
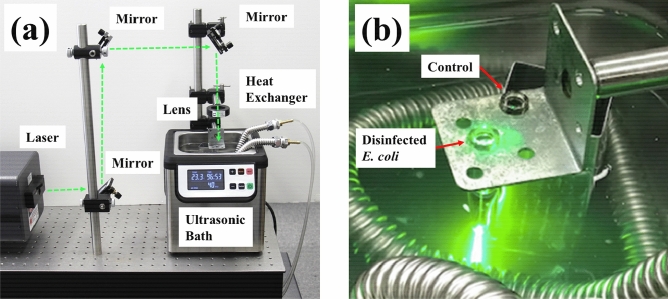
Figure 2a shows the optical setup for the inactivation system by using the SH of a CW (100 mW) or pulsed YAG laser (pulse duration: 10 ns, repetition rate: 10 Hz, pulse energy: 10 mJ). A laser beam was guided to a microtube made of borosilicate glass with 5.7 mm φ × 50 mm, which contained a suspension of E. coli (600 µL). The focusing beam was made by using a convex lens with a focal length of 200 mm, and the center of the suspension was irradiated by the SH of the YAG laser, as shown in Fig. 2b. The power of both the CW and pulsed YAG laser beams was maintained at 100 mW, and the corresponding power density was 50 W/cm2. The inactivation reaction occurred at the central spot of the glass microtube, where the diameter of the focused beam was approximately 0.5 mm, and the irradiated region was approximately 4 µL (0.5φ × 20 mm). The suspension in the tube was homogeneously diffused by using an ultrasonic bath with a frequency of 46 kHz. The temperature of the ultrasonic bath was maintained at 23 °C by using a heat exchanger, where the heat exchanger played a role in inhibiting a temperature increase due to 60 min of ultrasonic operation. (We note here that the temperature of the microtube will rise to approximately 50 °C for 60 min of ultrasonic operation without the heat exchanger). The control suspension, which was not subjected to laser irradiation, was also placed in the ultrasonic bath to precisely distinguish the inactivation caused by the ultrasonic effect from that of laser irradiation. However, it should be noted that the CFU reduction by the ultrasonic treatment was less than 10% of the CFU of the initial control sample; therefore, we used the E. coli CFU from the suspension in the ultrasonic bath as the control sample.
Figure 2.
(a) Optical setup of the visible laser inactivation system. (b) Photograph of an E. coli bacterial sample irradiated by the second harmonics of a YAG laser in an ultrasonic bath. An E. coli bacterial sample without laser irradiation was also placed in the ultrasonic bath as a control sample to take into account the inactivation caused by ultrasonic vibrations.
Results
Inactivation of unstained E. coli by CW or pulsed laser treatments
The results of the efficacy of inactivation by using the SH of the CW- or Q-switched pulsed YAG laser system are shown in Fig. 3a–f, where Fig. 3a is the control plate (without CW laser irradiation) of the CW YAG laser experiments and Fig. 3d is the control plate (without pulsed laser irradiation) of the pulsed YAG laser experiments. Figure 3b is the inactivated plate subjected to a 180 kJ/cm2 dose (50 W/cm2 and 1 h irradiation) from the SH of the CW YAG laser, and Fig. 3e is the inactivated plate subjected to the same dose but using the SH of the pulsed YAG laser. Despite the use of an identical dose, inactivation by pulse irradiation was considerably higher compared to that observed for CW operation. For example, the number of colonies was not reduced by using the CW laser, which was 192 ± 11 CFU (Fig. 3a) for the control plate and 184 ± 13 CFU (Fig. 3b) for that subjected to the 180 kJ/cm2 dose of the CW YAG laser. However, when the same dose was implemented with a pulsed laser, the number of colonies was reduced from 182 ± 12 CFU (Fig. 3d) for the control plate to 13 ± 2 CFU (Fig. 3e). The CFU reductions for the CW or pulsed laser experiments are shown in the bar graph of Fig. 3c,f, respectively. Here, error bars for the graphs were calculated and plotted based on the standard deviation. These results clearly showed that a transient heat reaction occurred in E. coli due to instantaneous pulse irradiation, whereas thermal heating did not occur with CW irradiation. Thus, pulse irradiation seems to be promising for achieving a much higher inactivation rate than that obtained by CW irradiation at the same dose. However, inactivation caused by visible pulse laser irradiation was not sufficient or effective. This was because of the low absorbance of the bacteria, as shown in Fig. 1f.
Figure 3.
The results of the efficacy of inactivation by using the SH of a CW or Q-switched pulsed YAG laser; (a) control plate for the CW YAG laser experiment, (b) inactivated plate subjected to 180 kJ/cm2 from a 532 nm CW YAG laser, and (c) the number of CFU on the control plate (192 ± 11 CFU) and treated plate (184 ± 13 CFU) after treatment with a 532 nm CW YAG laser. (d) Control plate of the 532 nm pulsed YAG laser, (e) inactivated plate subjected to 180 kJ/cm2 from a 532 nm pulsed YAG laser, and (f) the number of CFU on the control plate (182 ± 12 CFU) and treated plate (13 ± 2 CFU) after treatment with a 532 nm pulsed YAG laser.
Inactivation of stained E. coli by CW or pulsed laser treatments
The results of the efficacy of inactivation by using the SH of a CW or Q-switched pulsed YAG laser for E. coli stained with safranin dye (Fig. 4a–f) and rhodamine B dye (Fig. 4g–i) are shown in Fig. 4, where Fig. 4a–c were obtained with the CW YAG laser, and Fig. 4d–i were obtained with the pulsed YAG laser. When we used the CW laser, we did not observe a significant reduction in the CFU after staining treatment when comparing Fig. 4a with Fig. 4b; for example, the number of colonies was not reduced by using the CW laser, 845 ± 61 CFU for the control plate and 530 ± 116 CFU for the 180 kJ/cm2 dose given by a CW YAG laser, as shown in the bar graph of Fig. 4c. However, by applying the same dose with a pulsed laser, reductions in CFU were clearly observed for both safranin- (Fig. 4d,e) and rhodamine B-dyed E. coli (Fig. 4g,h). Furthermore, it was clearly observed that the reduction behaviors were strongly correlated with the magnitude of absorbance. For example, the number of colonies was reduced from 446 ± 30 CFU to 2 ± 1 CFU for safranin-dyed E. coli (OD: 0.2) with a 180 kJ/cm2 dose, as shown in the bar graph of Fig. 4f, and the relatively large absorbance of the rhodamine B-dyed E. coli (OD: 0.38) showed a relatively large inactivation rate. For example, almost the same CFU reduction was obtained with the relatively low dose of 45 kJ/cm2 for the rhodamine B-dyed sample, such as the reduction from 831 ± 39 CFU to 5 ± 1.1 CFU, as shown in the bar graph of Fig. 4i. Thus, it was clearly shown that the inactivation rate was correlated with the magnitudes of both the absorbance and the irradiation dose.
Figure 4.
The results of the efficacy of inactivation for safranin-stained E. coli obtained by CW YAG laser irradiation: (a) control plate, (b) inactivated plate subjected to 180 kJ/cm2 irradiation, and (c) a bar graph showing the number of CFU for the control plate (845 ± 61 CFU) and inactivated plate (530 ± 116 CFU). The results of the efficacy of inactivation for safranin-stained E. coli obtained by pulsed YAG laser irradiation; (d) control plate, (e) inactivated plate subjected to 180 kJ/cm2 irradiation, and (f) a bar graph showing the number of CFU on the control plate (446 ± 30 CFU) and inactivated plate (2 ± 1 CFU). The results of the efficacy of inactivation for rhodamine B dye-stained E. coli obtained by pulsed YAG laser; (g) control plate, (h) inactivated plate subjected to 45 kJ/cm2 irradiation, and (i) a bar graph showing the number of CFU for the control plate (831 ± 39 CFU) and inactivated plate (5 ± 1.1 CFU).
Inactivation rate constants of stained or unstained E. coli by CW or pulsed laser treatments
To quantitatively investigate the reduction in inactivation rates as a function of the 532 nm laser irradiation dose, we plotted the CFU response to the 532 nm laser irradiation dose (inactivated CFU by 532 nm irradiation, N(D)), divided by the control CFU (N0), caused by the CW laser or pulsed laser treatments, as shown in Fig. 5. Here, D is the magnitude of the dose (kJ/cm2), N0 is the number of CFU in the unirradiated control (CFU/mL), and N(D) is the number of CFU at a given irradiation dose D. Blue squares are the inactivation rates of unstained E. coli obtained with CW laser treatment, red squares are the rates of inactivation of safranin dye-stained E. coli obtained with CW laser treatment, blue circles are the rates of inactivation of unstained E. coli obtained with pulsed laser treatment, red circles are the rates of inactivation of safranin dye-stained E. coli obtained with pulsed laser treatment, and orange circles are the rates of inactivation of rhodamine B dye-stained E. coli obtained with pulsed laser treatment. It was clearly observed that CW laser irradiation did not reduce the CFU; that is, the inactivation rate constant was almost equal to 0 for both unstained- or stained E. coli, which are denoted by dashed blue or red lines shown in Fig. 5, respectively, while pulsed laser irradiation at the same dose showed a significant reduction in the CFU. Furthermore, an increase in the absorbance (from 0.1 for unstained E. coli to 0.38 for rhodamine B-stained E. coli) by staining exhibited an additional reduction rate of CFU to the pulsed laser inactivation rates. Based on the above experimentally observed inactivation rates as a function of dose D (kJ/cm2), the dose-based inactivation rate constant κ (cm2/kJ) determined by − κD = log10[N(D)/N0] for each sample was obtained by the least square fitting method. The experimentally observed rates were fitted by κ = 6.95 × 10−3 for unstained E. coli (blue line), κ = 1.17 × 10−2 for safranin-stained E. coli (red line), and κ = 4.91 × 10−2 for rhodamine B-stained E. coli (orange line), as shown by the solid lines in Fig. 5. These results are summarized in Table 1. Based on the obtained rate constant κ (cm2/kJ), an inactivation efficiency of the pulsed laser irradiation region (the inactivation efficiency inside the beam passing region) η (s−1) can be estimated using the following relation, η = κI0(V0/V), and the efficiency becomes approximately 40%/s for E. coli stained rhodamine B dye, 9%/s for safranin-stained E. coli, and 5%/s for unstained E. coli, where we use I0 = 50 W/cm2, V0 = 4 µL, and V = 600 µL, respectively. The detail of the estimation is given in the following discussion section.
Figure 5.

532 nm dose response of stained or unstained E. coli inactivated by CW or pulsed YAG laser treatment. Blue squares are the inactivation rates of unstained E. coli obtained by CW laser treatment, red squares are the inactivation rates of safranin dye-stained E. coli obtained with CW laser treatment, blue circles are the inactivation rates of unstained E. coli obtained with pulsed laser treatment, red circles are the inactivation rates of safranin dye-stained E. coli obtained with pulsed laser treatment, and orange circles are the inactivation rates of rhodamine B dye-stained E. coli obtained with pulsed laser treatment. The dose (D)-based inactivation rate constant κ (cm2/kJ) obtained for the pulsed laser was determined by − κD = log10[N(D)/N0], and κ = 6.95 × 10−3 for unstained E. coli (solid blue line), κ = 1.17 × 10−2 for safranin-stained E. coli (solid red line), and κ = 4.91 × 10−2 for rhodamine B-stained E. coli (solid orange line).
Table 1.
Inactivation rate constants κ (cm2/kJ) obtained experimentally (first line) or theoretically (second line) for unstained, safranin-stained, and rhodamine B-stained E. coli irradiated by a CW or pulsed YAG laser.
| Inactivation rate constant | Non-stained and pulse | Safranin and pulse | Rhodamine B and pulse | Non-stained and CW | Safranin and CW |
|---|---|---|---|---|---|
| κ (Experiment) | 6.95 × 10−3 | 1.17 × 10−2 | 4.91 × 10−2 | 0 | 0 |
| κ (Theory) | 6.51 × 10−3 | 1.30 × 10−2 | 5.65 × 10−2 | 3.42 × 10−5 | 6.84 × 10−5 |
Discussion
Based on the model described in Fig. 1g, we evaluated the temperature increase of a single E. coli cell caused by CW or pulsed laser irradiation based on the following heat transfer equation41:
| 1 |
where σ = 5.67 × 10−12 (J/s cm2 K4) is the Stefan-Boltzmann constant41, ρ is the density (g/cm3), c is the specific heat (J/g K), v is the volume (cm3), T is the time-dependent temperature due to the absorption of CW or pulsed laser radiation (K), T0 is the temperature before irradiation (K), α is the absorption ratio of laser radiation (dimensionless), S is the surface (cm2), γ is the convective heat transfer coefficient (J/s cm2 K), and ε is the emissivity (dimensionless), of E. coli, respectively. In Eq. (1), I(t) is the laser-intensity-pulse-profile as a function of time (J/s cm2); for CW excitation, I(t) takes a constant value as I(t) = I0, and for pulse excitation, I(t) is expressed, using the Dirac delta function, as I(t) = Epδ(t), where Ep is the pulse energy (J/cm2) and the delta function has the dimension of s−1. As a form of energy dissipation from E. coli, we ignored the thermal radiation heat transfer component [εσS(T4 − T04)] due to its contribution being more than 10 times smaller than that of convection loss [γS(T − T0)] at the considered temperature (T = 1000 K, and T0 = 300 K). Because the energy dissipation terms can be calculated as (i) the convection term, γS(T − T0) = 5.6 × 10−6 W and (ii) the thermal radiation term, εσS(T4 − T04) = 4.5 × 10−7 W, where we uses S = 8 × 10−8 cm2, γ = 0.1 (J/s cm2 K) obtained in the CW laser experiments [see Eq. (2)], and the maximum emissivity ε = 1 is assumed.
For CW irradiation, Eq. (1) can be solved as a steady-state equation. In this case, the temperature increase of the E. coli by laser irradiation was given by the following simple equation:
| 2 |
Here, α could be determined from the absorbance spectrum shown in Fig. 1f and the Beer–Lambert law42, and the result was α = 2.0 × 10−3 for an unstained E. coli, α = 4.0 × 10−3 for a safranin-stained E. coli, and α = 1.8 × 10−2 for a rhodamine B-stained E. coli. When the irradiation intensity of the CW laser was 50 W/cm2, the temperature of the safranin-stained E. coli solution was measured by a thermocouple and increased to 2 °C from the base temperature (T0). Therefore, by using Eq. (2), the corresponding convective heat transfer coefficient γ was determined to be 0.1 (J/s cm2 K). The magnitude of this convective heat transfer coefficient was large; therefore, the temperature of the bacterial cell could not be increased by CW excitation.
On the other hand, for pulse irradiation, the transient response of Eq. (1) can be solved by putting I(t) = Epδ(t) into it as:
| 3 |
and the maximum temperature of E. coli can be determined by the absorption ratio (α), pulse energy (Ep), and surface-to-volume ratio (S/v). By utilizing the parameters of E. coli, such as ρ = 1.0 g/cm338, c = 4.2 J/g K (Here we assume that c is equal to the value of water because almost 80% of cells are water43), v = 1 × 10−12 cm3, S = 8 × 10−8 cm2, and Ep = 5 J/cm2, the theoretically calculated maximum temperature (T − T0)M and the time constant of heat dissipation could be obtained. The temperatures [(T − T0)M] were 190 K for unstained E. coli, 380 Κ for safranin-stained E. coli, and 1710 K for rhodamine B-stained E. coli. The time constant γS/ρcv for these samples was 1.9 × 103 Hz. These results are summarized in Table 2. (We note here that the theoretical plot of the temperature rise by pulsed irradiation (blue line) shown in Fig. 1g was obtained by α = 2.1 × 10−2).
Table 2.
Theoretically calculated values of the surface-to-volume ratio (S/v), absorption ratio (α), temperature increase (T − T0), and time constant (γS/ρcv) for viruses, bacteria (unstained-, safranin-stained and rhodamine B-stained E. coli), and human red blood cells.
| Virus | E. coli unstained | E. coli safranin | E. coli rhodamine | Red blood cells | |
|---|---|---|---|---|---|
| S/v (cm−1) | 1.5 × 106 | 8 × 104 | 8 × 104 | 8 × 104 | 2 × 104 |
| α | 0.001 (650 nm) | 0.002 | 0.004 | 0.018 | 0.001 (650 nm) |
| Temp. increase (K) | 1780 | 190 | 380 | 1710 | 24 |
| Time constant (Hz) | 36,000 | 1900 | 1900 | 1900 | 475 |
To obtain a quantitative comparison, the laser intensity was assumed to be 50 W/cm2; repetition rate, 10 Hz; density 1.0 g/cm3; and specific heat, 4.2 J/g K, for all the organic structures.
It was clearly seen that the inactivation rate constant κ (cm2/kJ) obtained with the results shown in Fig. 5 exhibited a correlation with the maximum temperature (T − T0)M, with the linearly proportional relation κ = (T − T0)M/ξ, where the parameter ξ (kJ K/cm2) is characterized by the temperature and dose of inactivation. When we assumed the parameter ξ = 12.7 × 103 (kJ K/cm2), we could evaluate the theoretical values of κ. The calculated values were κ = 6.51 × 10−3 for unstained, κ = 1.30 × 10−2 for safranin-stained, and κ = 5.65 × 10−2 for rhodamine B-stained E. coli. The results are summarized in Table 1. The theoretically obtained inactivation rate constants for unstained, safranin dye-stained, and rhodamine B dye-stained E. coli agreed well with the experimentally obtained constants. This agreement strongly suggested that the mechanism of inactivation obtained by visible pulse laser irradiation originated from the transient photothermal evaporation effect, which is quantitatively described by κ = (T − T0)M/ξ.
It should be noted that due to the factor of the surface-to-volume ratio (S/v) in Eq. (3), it is possible for relatively small organic structures, such as viruses, to undergo transient photothermal inactivation without any staining treatments. For example, when we irradiated human red blood cells (HRBCs) and viruses at the same time, the wavelength of the pulsed laser was selected to be in a low-absorption region for HRBCs, such as 600 nm or more44,45, and the other conditions, such as the density and the specific heat, were assumed to be the same as described above (almost 80% of HRBCs are water46,47), the temperature of the HRBCs did not increase and remained at approximately 50 K. On the other hand, the temperature of viruses with structures on the order of 10–100 nm significantly increased up to 1800 K.
It is difficult to directly measure the temperature of the above small organic structures. However, this analysis can be applied not only to small organic structures but also to inorganic nanoparticles whose thermodynamic and thermophysical properties are well established. Here, we quantitatively analyse the temperature of gold (Au) nanoparticles by using Eq. (3) and compare the results with those obtained in previous studies48–51. The red and blue lines in Fig. 6 show the theoretically calculated maximum temperatures of Au nanoparticles with diameters of 60 nm (red line) and 100 nm (blue line) as a function of absorbed laser fluence (mJ/cm2). To obtain these lines, we used the density of Au nanoparticles of ρ = 19.3 g/cm352 and the specific heat of c = 0.13 J/g K52. The theoretically calculated maximum temperatures obtained here coincide with the previously reported values of temperature48–52; therefore, we consider that the temperature behaviour of the small organic structures also obeys Eq. (3).
Figure 6.

Maximum temperature of a single Au nanoparticle versus absorbed fluence of a pulsed laser. Red and blue lines are the theoretically calculated temperatures of Au nanoparticles with diameters of 60 nm (red line) and 100 nm (blue line). The solid red triangle is the temperature obtained by Ref.48, the solid red rhombus is the temperature obtained by Ref.49, the solid red circles are the temperatures obtained by Ref.50, the solid blue circles are the temperatures obtained by Ref.49, and the solid blue rhombi are the temperatures obtained by Ref.51. The theoretically calculated maximum temperatures obtained by Eq. (3) agree well with these previously reported temperature values.
The theoretically calculated values of the surface-to-volume ratio, maximum temperature, absorption ratio and time constant as a function of the size of organic structures are given and compared in Table 2. We considered that short-pulse irradiation yielded an effective and simple method for the inactivation of small pathogens, such as viruses and bacteria.
Conclusions
In this study, we demonstrated the efficient inactivation of E. coli stained with safranin or rhodamine B dyes by using a low-power and easily available nanosecond visible pulse laser and obtained 3-log inactivation of E. coli in a short period of treatment time, on the order of 10 min, with a relatively low irradiation dose on the order of 50 kJ/cm2. The treatment time and dose magnitude was much faster and much lower, respectively, than those obtained with a fs laser31,32.
We used a staining treatment for the inactivation of E. coli because, as shown in Fig. 1f, E. coli has no absorption band in the visible region. However, there are many bacteria that have inherent absorption bands in the visible region, such as Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus), Micrococcus luteus (M. luteus), and Kocuria oceani (K. oceani)53–56. We consider that by choosing the wavelength of pulsed laser to match the absorption bands, the transient photothermal inactivation of these pathogenic bacteria can be made without the staining treatment. We have some promising results confirming the excitation wavelength dependence of the inactivation efficacy for M. luteus and K. oceani. These results will be reported elsewhere.
A qualitative model based on the transient photothermal evaporation effect was discussed, and a quantitative evaluation of the temperature increase based on the heat transfer equation was made. As a result of this theoretical analysis, the maximum temperature of bacteria or viruses was correlated with the absorption ratio (α), pulse energy (Ep), and surface-to-volume ratio (S/v). The importance of the surface-to-volume ratio leads to the selectivity of inactivation of viruses and bacteria without damaging or heating large organic structures, such as human blood cells and stem cells. We consider that the proposed transient photothermal evaporation method can be applied to many fields such as a sterilization technique of skin and intraoral organ in a convenient manner. To confirm the validity of this method to these practical applications, it is necessary to evaluate the threshold temperature to induce the photothermal evaporation effect for various bacteria and viruses under practical conditions as well as to reduce the irradiation pulse energy.
Acknowledgements
The authors would like to thank Tomomi Fukuoka, Rika Kikojima, and Yuya Sugiura for their help with the laser inactivation experiments. The authors would also like to thank Dr. Masanori Isaka and Dr. Hideyuki Matsui for information about the handling techniques for bacteria.
Author contributions
Y.K. is the first author. Y.K., T.H., and T.M. contributed to the design of the CW and pulsed laser inactivation system, and Y.K., N.I., and I.T. completed all of the experiments. I.T. and T.H. provided technical support and bacterial expertise for the bacterial growth, dye fusion techniques, and colony-forming experiments. I.T., T.H., and T.M. constructed a quantitative model of the transient photothermal evaporation effect based on the heat transfer equation. Y.K. and N.I. performed statistical analyses of the inactivation experiments. All authors read and approved this submitted manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pirnie, M., Linden, K. G. & Malley. J. P. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule. UV Disinfection Guidance Manual 2006, LT2ESWTR.
- 2.Crawford, M. H., Banas, M. A., Ross, M. P., Ruby, D. S., Nelson, J. S., Boucher, R. & Allerman, A. A. Final LDRD Report: Ultraviolet Water Purification Systems for Rural Environments and Mobile Applications. Sandia Report 2005, SAND2005-7245.
- 3.Gray NF. Ultraviolet Disinfection, in Microbiology of Waterborne Diseases Microbiological Aspects and Risks. Waltham: Academic Press; 2014. [Google Scholar]
- 4.Matsumoto T, Kikojima R, Fukuoka T, Tatsuno I, Hasegawa T. Total internal reflection of deep-ultraviolet light in a water waveguide and its application to water disinfection technologies. Water. 2019;11:294. doi: 10.3390/w11020294. [DOI] [Google Scholar]
- 5.Matsumoto T, Tatsuno I, Hasegawa T. Instantaneous water purification by deep ultraviolet light in water waveguide: Escherichia coli bacteria disinfection. Water. 2019;11:968. doi: 10.3390/w11050968. [DOI] [Google Scholar]
- 6.Havill NL, Moore BA, Boyce JM. Comparison of the microbiological efficacy of hydrogen peroxide vapor and ultraviolet light process for room decontamination. Infect. Control Hosp. Epidemiol. 2012;33:508–512. doi: 10.1086/665326. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DJ, Gergen MF, Smather E, Sexton DJ, Chen LF, Weber DJ, Rutala WA. Decontamination of targeted pathogens from patient rooms using automated ultraviolet-c-emitting device. Infect. Control Hosp. Epidemiol. 2013;34:466–471. doi: 10.1086/670215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harm W. Biological Effects of Ultraviolet Irradiation. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- 9.Jagger J. Yearly review: near-UV radiation effects on microorganisms. Photochem. Photobiol. 1981;34:761–768. doi: 10.1111/j.1751-1097.1981.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 10.Jagger J. Physiological effects of near-UV radiation effects on bacteria. Photochem. Photobiol. Rev. 1983;7:2–65. [Google Scholar]
- 11.Setlow JK. The effects of ultraviolet radiation and photoreactivation. Compr. Biochem. 1967;27:157–209. doi: 10.1016/B978-1-4831-9716-6.50013-6. [DOI] [Google Scholar]
- 12.Sutherland BM, Shih AG. Quantitation of pyrimidine dimer contents of nonradioactive deoxyribonucleic acid by electrophoresis in alkaline agarose gels. Biochemistry. 1983;22:745–749. doi: 10.1021/bi00273a006. [DOI] [PubMed] [Google Scholar]
- 13.Sinha RP, Häder D-P. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 14.Mohr H, Gravemann U, Bayer A, Müller TH. Sterilization of platelet concentrates at production scale by irradiation with short wave ultraviolet light. Transfusion. 2009;49:1956–1963. doi: 10.1111/j.1537-2995.2009.02228.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohr H, Steil L, Gravemann U, Thiele T, Hammer E, Greinacher A, Müller TH, Völker U. Blood components: a novel approach to pathogen reduction in platelet concentrates using short wave ultraviolet light. Transfusion. 2009;49:2612–2624. doi: 10.1111/j.1537-2995.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 16.Terpstra FG, Van’t Wout AB, Schuitemaker H, Van Engelenburg FAC, Dekkers DWC, Verhaar R, Korte DD, Verhoeven AJ. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion. 2008;48:304–313. doi: 10.1111/j.1537-2995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan HL, Gaffney PR, Waterfield MD, Anderle H, Matthiessen P, Schwarz HP, Turecek PL, Timms JF. Proteomic analysis of UVC irradiation-induced damage of plasma proteins: serum amyloid P component as a major target of photolysis. FEBS Lett. 2006;580:3229–3236. doi: 10.1016/j.febslet.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Verhaar R, Dekkers DWC, De Cuyper IM, Ginsberg MH, de Korte D, Verhoeven AJ. UV-C irradiation disrupts platelet surface disulfide bonds and activates the platelet integrin αIIbβ3. Blood. 2008;112:4935–4939. doi: 10.1182/blood-2008-04-151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lackmann J-W, Bandow JE. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014;98:6205–6213. doi: 10.1007/s00253-014-5781-9. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor N, Cahill O, Daniels S, Galvin S, Humphreys H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J. Hosp. Infect. 2014;88:59–65. doi: 10.1016/j.jhin.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Welch D, Buonanno M, Grilj V, Shuryak I, Crickmore C, Bigelow AW, Randers-Pehrson G, Johnson GW, Brenner DJ. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8:2752. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narita K, Asano K, Naito K, Ohashi H, Sasaki M, Morimoto Y, Igarashid T, Nakane A. 222-nm UVC inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 2020;20:030. doi: 10.1016/j.jhin.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Narita K, Asano M, Morimoto Y, Igarashid T, Nakane A. Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PLoS ONE. 2018;13:e0201259. doi: 10.1371/journal.pone.0201259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazari M, Xi M, Lerch S, Alizadeh MH, Ettinger C, Akiyama H, Gillespie C, Gummuluru S, Erramilli S, Reinhard BM. Plasmonic enhancement of selective photonic virus inactivation. Sci. Rep. 2017;7:11951. doi: 10.1038/s41598-017-12377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunz JN, Voronine DV, Lu W, Liege Z, Lee HWH, Zhang Z, Scully MO. Aluminum plasmonic nanoshielding in ultraviolet inactivation of bacteria. Sci. Rep. 2017;7:9026. doi: 10.1038/s41598-017-08593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsen KT, Tsen SD, Chang C, Hung C, Wu TC, Kiang JG. Inactivation of viruses with a very low power visible femtosecond laser. J. Phys. Condens. Matter. 2007;19:322102. doi: 10.1088/0953-8984/19/32/322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsen SD, Wu TC, Kiang JG, Tsen KT. Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation. J. Biomed. Sci. 2012;19:62. doi: 10.1186/1423-0127-19-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsen SD, Kingsley DH, Poweleit C, Achilefu S, Soroka DS, Wu TC, Tsen KT. Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser. Virology J. 2014;11:20. doi: 10.1186/1743-422X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson KA, Dwayne Miller RJ, Lutz DR, Fayer MD. Optical generation of tunable ultrasonic waves. J. Appl. Phys. 1982;53:1144–1151. doi: 10.1063/1.329864. [DOI] [Google Scholar]
- 30.Yan Y, Gamble EB, Jr, Nelson KA. Impulsive stimulated scattering: general importance in femtosecond laser pulse interactions with matter, and spectroscopic applications. J. Chem. Phys. 1985;83:5391–5399. doi: 10.1063/1.449708. [DOI] [Google Scholar]
- 31.Tsen KT, Tsen SD, Sankey OF, Kiang JGK. Selective inactivation of micro-organisms with nearinfrared femtosecond laser pulses. J. Phys. Condens. Matter. 2007;19:472201. doi: 10.1088/0953-8984/19/47/472201. [DOI] [Google Scholar]
- 32.Lu CH, Lin KH, Hsu YT, Tsen KT, Kuan YS. Inhibition of Escherichia coli respiratory enzymes by short visible femtosecond laser irradiation. J. Phys. D Appl. Phys. 2014;47:315402. doi: 10.1088/0022-3727/47/31/315402. [DOI] [Google Scholar]
- 33.Tanabe T, Oyamada M, Fujita K, Dai P, Tanaka H, Takamatsu T. Multiphoton excitation–evoked chromophore-assisted laser inactivation using green fluorescent protein. Nat. Methods. 2005;2:503–505. doi: 10.1038/nmeth770. [DOI] [PubMed] [Google Scholar]
- 34.He J, Wang Y, Missinato MA, Onuoha E, Perkins LA, Watkins SC, St. Croix CM, Tsang M, Bruchez MP. A genetically targetable near-infrared photosensitizer. Nat. Methods. 2016;13:263–268. doi: 10.1038/nmeth.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao JC, Roider J, Jay DG. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free radicals. Proc. Natl. Acad. Sci. USA. 1994;91:2659–2663. doi: 10.1073/pnas.91.7.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jay DG, Keshishian H. Laser inactivation of fasciclin I disrupts axon adhesion of grasshopper pioneer neurons. Nature. 1990;348:548–550. doi: 10.1038/348548a0. [DOI] [PubMed] [Google Scholar]
- 40.Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc. Natl. Acad. Sci. USA. 1988;85:5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holman JP. Heat Transfer. 10. New York: McGraw-Hill; 2010. [Google Scholar]
- 42.Mayerhöfer TG, Popp J. Beer's law–why absorbance depends linearly on concentration. ChemPhysChem. 2019;20:511–515. doi: 10.1002/cphc.201801073. [DOI] [PubMed] [Google Scholar]
- 43.Neidhardt FC. Escherichia coli and Salmonella. Cell Mol. Biol. (Noisy-le-grand) 1996;1:14. [Google Scholar]
- 44.Kunitsyn VG, Panin LE. Mechanism of erythrocyte deformation under the action of stress hormones. Int. J. Biophys. 2013;3:1–14. [Google Scholar]
- 45.Himbert S, Alsop RJ, Rose M, Hertz L, Dhaliwal A, Moran-Mirabal JM, Verschoor CP, Bowdish DME, Kaestner L, Wagner C, Rheinstädter MC. The molecular structure of human red blood cell membranes from highly oriented, solid supported multi-lamellar membranes. Sci. Rep. 2017;7:39661. doi: 10.1038/srep39661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013;40:463–471. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 47.Phillips KG, Jacques SL, McCarty OJT. Measurement of single cell refractive index, dry mass, volume, and density using a transillumination microscope. Phys. Rev. Lett. 2012;109:118105. doi: 10.1103/PhysRevLett.109.118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plech A, Kotaidis V, Grésillon S, Dahmen C, von Plessen G. Laser-induced heating and melting of gold nanoparticles studied by time-resolved x-ray scattering. Phys. Rev. B. 2004;70:195423. doi: 10.1103/PhysRevB.70.195423. [DOI] [Google Scholar]
- 49.Ananyeva MV, Zvekovb AA, Galkina EV, Kalenskii AV. The influence of optical properties of Au nanoparticles on their laser heating in an inert medium. Proc. Eng. 2017;201:603–611. doi: 10.1016/j.proeng.2017.09.650. [DOI] [Google Scholar]
- 50.Hashimoto S, Werner D, Uwada T. Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication. J. Photochem. Photobiol. C Rev. 2012;13:28–54. doi: 10.1016/j.jphotochemrev.2012.01.001. [DOI] [Google Scholar]
- 51.Nedyalkov NN, Imamova SE, Atanasov PA, Toshkova RA, Gardeva EG, Yossifova LS, Alexandrov MT. Nanosecond laser heating of gold nanoparticles. Application in photothermal cancer cell therapy. Comp. rend. Acad. Bulg. Sci. 2010;63:467–774. [Google Scholar]
- 52.Qin Z, Bishof JC. Thermophysical and biological responses of gold nanoparticle laser heating. Chem. Soc. Rev. 2012;41:1191–1217. doi: 10.1039/C1CS15184C. [DOI] [PubMed] [Google Scholar]
- 53.Wadday AK, Saleh ZA, Al-Marjani MF. Spectroscopic characteristics and energy transfer of bacterial pigment: (pyocyanin/curcumin) AIP Conf. Proc. 2019;2201:020014. doi: 10.1063/1.5141438. [DOI] [Google Scholar]
- 54.McBirney SE, Trinh K, Wong-Beringer A, Armani AM. Wavelength-normalized spectroscopic analysis of Staphylococcus aureus and Pseudomonas aeruginosa growth rates. Biomed. Opt. Exp. 2016;7:4034–4042. doi: 10.1364/BOE.7.004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurachi M. Studies on the biosynthesis of pyocyanine. II. Isolation and determination of pyocyanine. Bull. Inst. Chem. Res. Kyoto Univ. 1958;36:174–187. [Google Scholar]