Highlights
-
•
Maturity at harvest and after storage plus genotype impact melon fruit flavor.
-
•
Volatiles increased in storage for all melon genotypes with esters being dominant.
-
•
Short shelf-life melons associated with esters, sulphur compounds and a terpenoid.
-
•
Long shelf-life melons related with green/grassy aroma/flavor, firmness, aldehydes.
Keywords: Cucumis melo L., Fruit quality, Flavor, Volatiles, Sensory descriptive analysis, Postharvest storage, HS-SPME-GC–MS
Abstract
Flavor is a key attribute defining melon fruit quality and driving consumer preferences. We characterized and compared fruit ripening patterns (ethylene, respiration), physicochemical properties (rind/flesh color, firmness, soluble solids, acidity), aroma volatiles, and flavor-related sensory attributes in seven melon genotypes differing in shelf life capacity. Fruits were evaluated at optimal maturity and after storage for six days at 5 °C plus one day at room temperature. Total volatile content increased after storage in all genotypes, with esters being dominant. Shorter shelf-life genotypes, displaying a sharper climacteric phase, correlated with fruity/floral/sweet flavor-related descriptors, and with esters, sulfur-containing compounds and a terpenoid. Longer shelf-life types were associated with firmness, green and grassy aroma/flavor and aldehydes. Multivariate regression identified key volatiles that predict flavor sensory perception, which could accelerate breeding of longer shelf-life melons with improved flavor characteristics.
1. Introduction
Melons (Cucumis melo L.) belong to the Cucurbitaceae family and are an important food crop worldwide. Melons present extensive genotypic and phenotypic variation, categorized into several intraspecific classifications (Pitrat, 2013). Here we focus on the cantaloupensis (cantaloupes) and reticulatus (muskmelon) groups. Together with maturity at harvest and environmental factors, differences in genetic makeup have been shown to play a key role in dictating overall melon fruit quality and hence influencing melon commercial acceptability and market type (Kourkoutas et al., 2006, Nuñez-Palenius et al., 2008, Vallone et al., 2013).
Fruit quality is determined by multiple irreversible physiological and biochemical modifications that take place during fruit ripening (Klee & Giovannoni, 2011). These involve changes in fruit coloration, texture, and flavor (increase in sugar contents, decrease in organic acids, and changes in aroma (volatile compounds)) (Guis et al., 1997). The seven melon genotypes investigated in this study have been previously examined for texture properties (Farcuh et al., 2020), and represent pre-commercial and commercial germplasm. However, consumer surveys conducted across different melon cultivars indicated that besides texture, flavor is significantly correlated to overall consumer liking (Lester, 2006).
Although there is extensive flavor variation among melons (Beaulieu et al., 2003, Menezes et al., 2019, Obando-Ulloa et al., 2008, Shi et al., 2020), many breeding programs have focused on improving shelf-life capacity for increased marketability at the expense of flavor, due to the latter’s complexity and phenotyping costs (Beaulieu et al., 2004, Farcuh et al., 2020, Klee and Tieman, 2018). However, longer shelf-life type melons are perceived by consumers as having poor fruit quality, with resulting low acceptability ratings (Lignou, Parker, Baxter, & Mottram, 2014).
Flavor is the multidimensional set of taste, aroma and chemesthetic attributes perceived by the chemical senses from a fruit in the mouth, including non-volatile (sugars, acids) and volatile compounds released by the fruits (Klee et al., 2018). Over 240 volatile compounds have been identified in climacteric melons such as cantaloupes, approximately half of them being esters, while non-climacteric types have lower levels of esters (Kourkoutas et al., 2006, Obando-Ulloa et al., 2008, Shi et al., 2020). Numerous volatile compounds have been shown to play crucial roles in determining consumers’ overall liking (Klee et al., 2018), making volatiles key breeding targets to enhance fruit flavor perception.
Due to the intrinsic complexity of melon fruit flavor quality, we used an integrated approach, including the analysis of fruit ripening patterns (ethylene and respiration production rates), physicochemical properties (rind and flesh color, flesh firmness, soluble solids contents, and titratable acidity), untargeted aroma volatile profiling, and descriptive sensory analysis (aroma, taste and flavor-related attributes), at commercial harvest maturity and after postharvest ripening. To assess the role of genetic differences, we investigated a set of seven melon genotypes with short and long shelf-life capacities (Farcuh et al., 2020) that have not been described in the literature for their flavor-related components. We hypothesize that differences between melons at harvest and after postharvest ripening, as well as genotypic differences, impact melon fruit flavor quality and sensory perception. In addition to characterizing each genotype for the above features, we also identified correlations among all the evaluated parameters after storage. This enabled the identification of key volatile compounds that predict the sensory perception of melon flavor after ripening in storage.
2. Materials and methods
2.1. Plant material
Fruit from seven melon genotypes were harvested from HM.Clause Seed Company located in Davis, CA, USA during the 2019 season (Supplementary Fig. S1). All genotypes belong to subspecies melo, and included: Genotype #1: CM2190 (cantalupensis × reticulatus), Genotype #2: La Jolla (cantalupensis), Genotype #3: CM2327 (reticulatus), Genotype #4: Tacana (reticulatus), Genotype #5: Tonga (reticulatus), Genotype #6: Fiji (reticulatus), Genotype #7: Fiji (reticulatus).
Four plots of each melon genotype (40 plants/plot;) were planted in the field (land area ~1100 m2) during the spring season in a completely randomized design and grown on raised beds using drip irrigation and standard commercial cultivation practices.
Fruit maturities were monitored throughout the season and fruit were harvested at the optimal commercial maturity stage based on harvest indices during July and August. For genotypes with shorter shelf-life capacity (#1,#2), harvest indices included: (1) presence of a fully developed abscission zone (crack around the peduncle) or ¾- to full-slip stage; (2) change in rind color under the net from green to yellow; and (3) detectable aroma (Farcuh et al., 2020, Nuñez-Palenius et al., 2008). For the genotypes with longer shelf-life capacity (#3, #4, #5, #6, #7), harvest indices included: (1) visible cracking at the base of the peduncle; (2) change in rind color towards a lighter color; and (3) aroma at the blossom end (none to detectable) (Farcuh et al., 2020, Portela and Cantwell, 1998). Fruits with uniform size, absence of visual blemishes and/or diseases were harvested and immediately transported to the laboratory.
2.2. Fruit postharvest storage
A total of 96 fruits were harvested for each genotype, which were divided into two groups of 48 fruits each. Each group was composed of four biological replications of 12 fruits each. One group was evaluated at harvest and the other group was evaluated after storage at 5 °C and 90% relative humidity for six days plus one day at room temperature (20 °C). Both groups were evaluated for ripening patterns, physicochemical properties, and aroma volatile profiling, and for the second group sensory descriptive analyses were also conducted.
2.3. Fruit ripening patterns
For each genotype and evaluation stage, fruit ethylene (μL C2H4 kg–1h−1) and carbon dioxide (mL CO2 kg–1h−1) production rates were measured using a static system as previously described (Farcuh et al., 2018, Kim et al., 2015).
2.4. Melon sample preparation
Each fruit was cut to discard the stem and blossom ends, as only the equatorial region was used for analyses. The equatorial region of each fruit was cut longitudinally into four wedges and seed and cavity tissue were removed. Two wedges were used for physicochemical and volatile compound analyses, while the other two wedges were used for sensory analysis conducted on the same day (only for stored fruit).
2.5. Physicochemical evaluations
Physicochemical properties, including rind and flesh color, flesh firmness, soluble solids content (SSC) and titratable acidity (TA), were measured for each genotype and evaluation stage (Farcuh et al., 2017, Kim et al., 2015). Rind and flesh color were assayed on the two opposite paired wedges obtained from the equatorial region from each fruit using a colorimeter (Konica Minolta CR400 Chroma Meter, Konica Minolta Sensing, Inc., Osaka, Japan). Hue angle (hue°), representing changes of primary colors, was calculated as h = arctan(a*/b*). Flesh firmness was measured at the same locations as flesh color by using a Guss FTA penetrometer with an 8-mm tip (Guss, Strand, Western Cape, South Africa). Melon balls (2 cm in diameter) were cut from wedges representing one biological replication and pooled together to form a composite sample. SSC and TA were measured on juice extracted from composite samples with a hand press and filtered through cheesecloth. SSC was determined by using a digital refractometer (AR6 Series; Reichert Technologies, Reichert, Inc., NY, USA), and TA was computed by automatic titration (TIM 850; TitraLab, Radiometer Analytical SAS, Lyon, France) with a 0.1 N sodium hydroxide solution to an end point of pH 8.2, expressed as % citric acid.
2.6. Volatile compound analyses
Melon balls (2 cm in diameter) were pooled from the same two wedges used for physicochemical evaluations from the twelve fruits composing each biological replicate. Sample preparation for volatile profiling was performed as previously described (Vallone et al., 2013). Samples were stored at −80 °C until further analysis.
The volatile profiles of the melon samples were assessed using an automated headspace solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME–GC–MS) method, as previously described (Obando-Ulloa et al., 2008). Analyses were performed with a MPS2 Gerstel Multipurpose sampler (Gerstel US, Linthicum Heights, MD) coupled to an Agilent 6890N GC with a 5973 MS (Agilent Technologies, Wilmington, DE). Melon samples were thawed at room temperature (1 h) and placed in the heated tray of the GC at 35 °C for 2 h for the headspace to form. A 2-cm mixed-phase SPME fiber (50/30 μm DVB/Carboxen/PDMS; Supelco, St. Louis, MO), previously preconditioned in the injection port at 250 °C for 1 h, was employed for extraction and concentration of the volatile compounds. The needle entered 32 mm into the vial headspace and remained 45 min at 35 °C adsorbing volatiles. After extraction, volatiles were desorbed from the SPME fiber into the GC injection port set at 270 °C for 3 min. The injection port was operated at 270 °C in splitless mode and subjected to a pressure of 80 psi. Volatiles were separated on a 30 m × 0.25 mm i.d. × 0.25 µm thickness capillary column (HP-5MS, Agilent Technologies) that contained 5% phenyl-methyl silicone as a stationary phase. The carrier gas was helium (99.99% purity) with a flow rate of 1 mL/min. The initial oven temperature was 35 °C for 1 min, followed by a ramp of 5 °C/min up to 150 °C, and then at 30 °C/min to reach a final temperature of 280 °C, which was held for 10 min. Mass spectrometer parameters were as follows: MS source 230 °C, MS quadrupole 150 °C, MS transfer line 280 °C. Mass spectra were obtained by electron ionization (EI) at 70 eV with a scan range of m/z 35–350.
Detected compounds were analyzed and areas integrated in MSD Chemstation (version E.02.02, Agilent Technologies). Compounds were identified by matching their linear retention indices (RIs), calculated from a series of n-alkanes (C6–C20) (Sigma, St. Louis, MO), that had the same GC–MS analysis program as that applied to the sample, to mass spectral libraries (NIST/EPA/NIH Mass Spectral Library NIST, 2017), and to those reported in literature (Beaulieu, & Grimm, 2001). Any identified volatile that could not be matched by RI was removed. Volatile compounds with a signal-to-noise ratio greater than 5 were kept. The relative content of each volatile was calculated as 2-methylbutyl isovalerate (internal standard, 100 mM) equivalent by the GC peak area. All samples were analyzed in triplicate.
2.7. Sensory descriptive analysis
A hybrid descriptive analysis method (Frost, Ristenpart, & Guinard, 2020) that combines elements of quantitative descriptive analysis and spectrum methods (Lawless & Heymann, 1998) was used to describe and quantify the flavor, aroma and taste-related attributes of the melon genotypes. Nine panelists (ages between 25 and 40 years) were recruited via emails from the UC Davis campus community and were consumers and likers of melons. Panelists underwent nine one-hour training sessions over three weeks. During the initial training sessions and after tasting diverse commercial melon samples, panelists agreed on a list of attributes to evaluate (from the initial eleven aroma, three taste and ten flavor-related descriptors, panelists refined the list to nine, three, and eight descriptors, respectively; Table 1). Subsequently, panelists defined, evaluated and discussed different reference products to help achieve concept alignment and build a consensus reference scale per attribute. During the last training sessions, panelists assessed melon samples in separate booths, using the developed descriptors and reference scales, and received feedback on their performance.
Table 1.
Sensory attributes, descriptions and reference standards for descriptive sensory analysis.
| Attribute | Attribute description | References |
|---|---|---|
| Aroma-related | ||
| Overall aroma | The intensity of aroma of any type when opening the lid, ranging from low to high | None |
| Melon aroma | The intensity of overall fresh melon aroma, such as cantaloupes, when opening the lid, ranging from low to high | Cantaloupe melons |
| Fruity aroma | The intensity of fruity aroma like mixed fruit juice, a fresh fruit aroma, ranging from low to high | Mixed fruit juice (Dole, West Lake Village, USA) |
| Grassy aroma | The intensity of the fresh cut grass aroma, ranging from low to high | Fresh cut grass |
| Green aroma | The intensity of green, fresh aroma, like cucumber aroma, ranging from low to high | English cucumber peeled and cut into 1-cm slices |
| Sweet aroma | The intensity of sweet aroma, including the sweet sensation of marshmallows and honey, ranging from low to high | Marshmallows small size and honey |
| Melon rind aroma | The intensity of cantaloupe melon skin aroma, plant-like, slightly earthy, ranging from low to high | Cantaloupe skin |
| Floral aroma | The intensity of fresh flower smell, such as rose, ranging from low to high | Roses |
| Fermented/overripe aroma | The intensity of smell of overripe and alcoholic fruit, ranging from low to high | Melon left at room temperature for 2–3 days |
| Taste-related | ||
| Sweet taste | The intensity of sweet taste like sugar, candy, ranging from low to high | Marshmallows small size and honey |
| Sour/acid taste | The intensity of fresh sour taste like Granny Smith apple, ranging from low to high | Granny Smith apple |
| Bitter taste | The intensity of bitter taste like the peel of cucumber, ranging from low to high | Taste of peel of English cucumber |
| Flavor-related | ||
| Overall flavor | The first instant perception of intensity of overall flavor perceived in-mouth, ranging from low to high | None |
| Fruity flavor | The intensity of the flavor of fruit juice of mixed fruits perceived in-mouth, ranging from low to high | Mixed fruit juice (Dole, West Lake Village, USA) |
| Sweet flavor | The intensity of sweet flavor perceived in-mouth, ranging from low to high | Marshmallows small size and Honey |
| Fermented/overripe flavor | The intensity of flavor of overripe and alcoholic fruit, ranging from low to high | Melon left at room temperature for 2–3 days |
| Buttery flavor | The intensity of the flavor of fat/cream, ranging from low to high | Cream |
| Grassy flavor | The intensity of the fresh cut grass flavor, ranging from low to high | Fresh cut grass |
| Green flavor | The intensity of cucumber flavor, ranging from low to high | English cucumber peeled and cut into 1-cm slices |
| After flavor | The length of time flavors last in the mouth after swallowing, ranging from a few seconds to longer than 10 s | None |
From each biological replication of stored fruit, flesh melon balls (2 cm in diameter) were carved from each wedge and mixed in a bowl. A set of two sensory replications, defined as “samples” for the panelists, were presented to each panelist. Each sensory replication (or “sample”) consisted of a set of four melon balls, placed into one 120-mL plastic soufflé cup with lids and labeled with a random three-digit code, generated by Red Jade sensory science software (2019; RedJade Sensory Solutions LLC, Redwood City, CA). Individual samples were served in a randomized order, as established by the Red Jade program. Panelists were seated in isolated, temperature-controlled, and red-lit sensory booths. In each one-hour session, each panelist received a total of eight samples for assessment, served in sequential monadic fashion. Panelists recorded attributes via an iPad logged into Red Jade, on a 15-cm unstructured scale anchored at the ends with “low” and “high” (lowest and highest levels for a specific attribute, respectively). Water and unsalted crackers were provided for palate cleansing between samples. This study with human participants was approved by the institutional Review Board (IRB) of the University of California, Davis.
2.8. Statistical analyses
For ripening patterns, physicochemical evaluations and volatile analysis, sample means for each of the four biological replications of each genotype were submitted to two-way analysis of variance using Tukey’s test to compare between genotypes and stages of evaluation for the assessed variables (p ≤ 0.05).
The descriptive analysis data were exported from Red Jade, converting positions on the 15-cm unstructured scale into scores on a scale from 0 to 100 for each attribute. Sample means for each of the four biological replications of each genotype were submitted to one-way analysis of variance using Tukey’s test to compare between genotypes after storage, for the different attributes (p ≤ 0.05). The interaction of “genotype by assessor” was not significant (p > 0.05) for any of the attributes in this study.
Principal component analysis (PCA) was performed to analyze and visualize the relationships among all the analyzed variables postharvest. Pearson’s correlation coefficients (r), at a significance level of = 0.05, using log transformed and mean-centered data, were calculated for each pairwise-combination. PCA produced a ‘biplot’ graph, representing the relationships among the variables and the evaluated melon genotypes. Scree test was used to determine the number of principal components required to capture most of the relevant variation in the data. Subsequently, partial least square (PLS) regression analysis combined with variable importance in projection (VIP) was conducted to identify the key volatile compounds for prediction of melon flavor sensory perception. PLS was performed by using selected volatile compounds (predictors) (chosen based on their importance to explain the variance among samples), and the sensory attributes (response). The predictive power of the model was evaluated by performing the leave-one-out cross-validation test. VIP values exceeding 1.0 were selected as cut-off. Software package JMP (ver. 14.0; SAS Institute, Cary, NC) was used for statistical analyses.
3. Results and discussion
3.1. Ripening patterns and evaluation of physicochemical properties
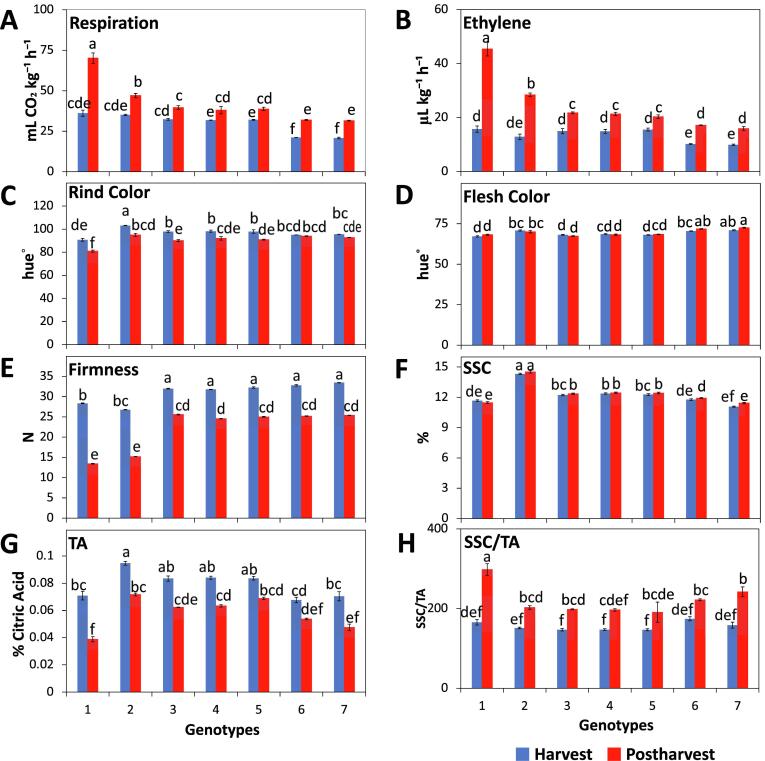
Fruit ripening behavior has been defined as climacteric or non-climacteric, characterized by an upsurge in respiration rates and autocatalytic ethylene production in the former but not in the latter (Biale & Young, 1981). Both ripening patterns can be found in the same species, as shown in melons (Obando-Ulloa et al., 2008) and Japanese plums (Farcuh et al., 2017). All genotypes in this study displayed a significant increase in respiration and ethylene production rates after storage (Fig. 1A,B), thus exhibiting climacteric behavior. Variations in ethylene production rates could result in differences in shelf-life capacity (Farcuh et al., 2020). Genotype #1, followed by genotype #2 presented the highest ethylene and respiration production rates after storage, supporting a higher breakdown of carbon compounds and a shorter shelf-life capacity due to a sharper climacteric phase (Obando-Ulloa et al., 2008). The lower increases in ethylene production rates displayed by genotypes #3 through #7 after storage support their lower climacteric expression and longer shelf-life capacity (Aubert and Bourger, 2004, Farcuh et al., 2020).
Fig. 1.
Ripening patterns, and physicochemical properties of seven melon genotypes at harvest and postharvest. (A) Respiration rates; (B) ethylene rates; (C) rind coloration; (D) flesh coloration; (E) flesh firmness; (F) soluble solid contents (SSC); (G) titratable acidity (TA); (H) SSC/TA ratio. Values are means ± SE (n = 4). Different letters indicate significant differences (p ≤ 0.05). Genotype #1 (CM2190), #2 (La Jolla), #3 (CM2327), #4 (Tacana), #5 (Tonga), #6 (Fiji), #7 (MegaPac).
Melon rind and flesh coloration play important roles in consumer choice at purchase and overall fruit quality (Park et al., 2018). Changes in rind color are also an important harvest index in melon fruit (Nuñez-Palenius et al., 2008, Portela and Cantwell, 1998). Rind color hue values ranged between 90° and 100° at harvest and significantly decreased after storage towards the 80° to 90° range, except for genotypes #6 and #7 (Fig. 1C). The decrease in hue values was previously reported (Nguyen, Zsom, Dam, Baranyai, & Hitka, 2019) and indicates a change from a green (180°)/yellow (90°) towards a yellow (90°)/ orange (45°) rind color, related to chlorophyll loss and increased carotenoids contents (Guis et al., 1997). Blocking ethylene production and perception delays the decrease in rind color hue values, due to a decrease in chlorophyll degradation (Nguyen et al., 2019), consistent with the constant hue values displayed by genotypes #6 and #7 (lowest ethylene rates; Fig. 1B) and the large decrease in rind hue values after storage exhibited by genotype #1 (highest ethylene rates). Flesh coloration of genotypes #1 and #3 displayed significantly lower hue values (indicative of a more orange flesh coloration) as compared to genotypes #2, #6, and #7 (Fig. 1D), consistent with studies indicating that carotenoid synthesis, mainly responsible for flesh coloration, is ethylene independent (Miccolis & Saltveit, 1995).
Flesh firmness significantly decreased in all genotypes after storage (Fig. 1E). Genotypes #1 and #2 presented a 50% decrease in firmness after postharvest storage, exhibiting the significantly lowest values; while genotypes #3 through #7 did not differ. Differences in the extent of softening may be associated with ethylene production rates as fruit cell wall disassembly is an ethylene-dependent mechanism in melons from the cantaloupensis group (Guis et al., 1997, Nishiyama et al., 2007). These results are consistent with our previous study (Farcuh et al., 2020) in which genotypes #1 and #2 also displayed lower values for puncture and compression testing as compared to genotypes #4, #5 and #6.
Soluble sugars content, generally used to estimate the sugar content in fruit and to evaluate maturity in melon (Park et al., 2018), did not change significantly after storage (Fig. 1F), but the highest values were displayed by genotype #2 (~14% SSC). Other studies also found no changes in SSC throughout storage (Miccolis et al., 1995), but did find significant differences among melon cultivars (Beaulieu et al., 2003).
Although melons (C. melo) are unique due to their unusual low acidity levels in the ripe fruit (Cohen et al., 2012), a significant decrease in TA values, resulting from the use of acids as a substrate for respiration during ripening (Guis et al., 1997), was observed in all genotypes after storage (Fig. 1G). TA results followed the same pattern as SSC, thus displaying differences among genotypes for the SSC/TA ratio (Fig. 1H).
3.2. Volatile compound analyses
A total of 59 volatile compounds were detected in this study (Table 2) including 35 esters, 9 aldehydes, 4 sulfur-containing compounds, 6 terpenoids, 2 ketones, 1 alcohol and 2 alkanes. Significant differences in most volatile compounds were found across genotypes and stages of evaluation. Significant interactions were also observed for several volatiles (Table 2), indicating that the main effects of genotype and stage cannot be interpreted independently, as significant differences between stages vary depending on the assessed genotype. Total volatile content increased by 17% from harvest to postharvest (Supplementary Table S1) consistent with studies reporting that the total volatile content in melons significantly increases during ripening (Lignou et al., 2014, Senesi et al., 2005, Vallone et al., 2013). Within genotypes, melons #1 and #2 presented the highest volatile contents (38 μg/kg FW and 33 μg/kg FW, respectively), followed by #3, #4 and #6 (ranging from 21 to 23 μg/kg FW) while #5 and #7 had the lowest contents (13 μg/kg FW and 10 μg/kg FW, respectively) (Supplementary Table S1). These differences may be associated with the significantly lower ethylene production rates of genotypes #5 and #7 as compared to genotypes #1 and #2 (Fig. 1B), as reduced ethylene production decreases overall volatile content (Flores et al., 2001). Furthermore, genotype-specific differences in volatiles have been reported (Senesi et al., 2005, Shi et al., 2020), indicating that aroma volatiles are under strong genetic control.
Table 2.
Analysis of variance of volatile compounds: significance levels of main effects and interactions.
| Codea | Volatile compound | CAS # | RIb | RIc | Genotype | Stage | Genotype × Stage |
|---|---|---|---|---|---|---|---|
| Esters | |||||||
| E1 | Methyl acetate | 79-20-9 | 555 | 559 | <0.0001 | 0.7921 | 0.0076 |
| E2 | Ethyl acetate | 141-78-6 | 605 | 605 | <0.0001 | 0.0278 | 0.455 |
| E3 | Methyl propanoate | 554-12-1 | 625 | 621 | <0.0001 | 0.0605 | 0.0435 |
| E4 | Isopropyl acetate | 108-21-4 | 655 | 648 | 0.0003 | 0.0531 | <0.0001 |
| E5 | Methyl isobutyrate | 547-63-7 | 685 | 690 | <0.0001 | 0.7914 | 0.9174 |
| E6 | Ethyl propanoate | 105-37-3 | 710 | 708 | <0.0001 | 0.4105 | <0.0001 |
| E7 | Propyl acetate | 109-60-4 | 706 | 707 | <0.0001 | <0.0001 | 0.4756 |
| E8 | Methyl butanoate | 623-42-7 | 717 | 717 | <0.0001 | 0.3588 | 0.4305 |
| E9 | Ethyl isobutyrate | 97-62-1 | 761 | 751 | 0.0001 | 0.4036 | 0.6237 |
| E10 | Isobutyl acetate | 110-19-0 | 764 | 768 | <0.0001 | 0.0066 | 0.1016 |
| E11 | Methyl 2-methylbutanoate | 868-57-5 | 772 | 772 | <0.0001 | 0.0084 | 0.9024 |
| E12 | Ethyl butanoate | 105-54-4 | 804 | 803 | <0.0001 | 0.0139 | 0.0025 |
| E13 | Propyl propanoate | 106-36-5 | 807 | 807 | <0.0001 | 0.6079 | 0.8905 |
| E14 | Butyl acetate | 123-86-4 | 812 | 812 | <0.0001 | <0.0001 | 0.4945 |
| E15 | Isopropyl butanoate | 638-11-9 | 837 | 837 | <0.0001 | 0.062 | <0.0001 |
| E16 | Ethyl 2-methylbutyrate | 7452-79-1 | 849 | 846 | 0.0023 | 0.0283 | 0.0039 |
| E17 | 2-Methylpropyl propanoate | 540-42-1 | 862 | 863 | <0.0001 | 0.3087 | 0.0334 |
| E18 | 2-Methylbutyl acetate | 624-41-9 | 876 | 877 | <0.0001 | <0.0001 | 0.3621 |
| E19 | Propyl butanoate | 105-66-8 | 896 | 897 | <0.0001 | 0.1208 | 0.2919 |
| E20 | Butyl propanoate | 590-01-2 | 908 | 907 | <0.0001 | 0.0569 | 0.0018 |
| E21 | Pentyl acetate | 628-63-7 | 911 | 912 | <0.0001 | 0.2106 | 0.0668 |
| E22 | 3-Methyl-2-butenyl acetate | 1191-16-8 | 920 | 918 | <0.0001 | 0.2384 | 0.3024 |
| E23 | Methyl hexanoate | 106-70-7 | 925 | 922 | 0.0003 | 0.3634 | 0.0022 |
| E24 | Propyl 2-methylbutanoate | 37064-20-3 | 943 | 943 | <0.0001 | 0.1102 | 0.4075 |
| E25 | 2-Methylpropyl butanoate | 539-90-2 | 955 | 953 | <0.0001 | 0.1484 | 0.0154 |
| E26 | Pentyl propanoate | 624-54-4 | 969 | 968 | <0.0001 | 0.0357 | <0.0001 |
| E27 | Ethyl hexanoate | 123-66-0 | 1000 | 999 | 0.0858 | 0.0215 | 0.0184 |
| E28 | (Z)-3-Hexenyl acetate | 3681-71-8 | 1007 | 1004 | <0.0001 | <0.0001 | 0.2486 |
| E29 | Hexyl acetate | 142-92-7 | 1012 | 1011 | <0.0001 | 0.0095 | 0.4239 |
| E30 | 2,3-Butanediol diacetate | 1114-92-7 | 1054 | 1064 | <0.0001 | 0.0262 | 0.3695 |
| E31 | Methyl benzoate | 93-58-3 | 1093 | 1091d | 0.0378 | 0.4329 | 0.0932 |
| 2-Methylbutyl isovalerate (IS) | 2445-77-4 | 1108 | 1107 | ||||
| E32 | Benzyl acetate | 140-11-4 | 1164 | 1164 | <0.0001 | 0.4713 | 0.1974 |
| E33 | Octyl acetate | 112-14-1 | 1210 | 1213 | <0.0001 | 0.4501 | 0.2671 |
| E34 | Phenethyl acetate | 103-45-7 | 1258 | 1255 | <0.0001 | 0.0338 | 0.0871 |
| E35 | 3-Phenylpropyl acetate | 122-72-5 | 1373 | 1373 | <0.0001 | 0.6506 | 0.0258 |
| Aldehydes | |||||||
| A1 | Acetaldehyde | 75-07-0 | 500 | 528 | 0.0052 | 0.0621 | 0.0002 |
| A2 | Propanal | 123-38-6 | 552 | 554 | <0.0001 | 0.0699 | 0.2725 |
| A3 | Pentanal | 110-62-3 | 699 | 699 | <0.0001 | 0.0943 | 0.4171 |
| A4 | Hexanal | 66-25-1 | 801 | 801 | <0.0001 | 0.0195 | 0.7514 |
| A5 | 2-Butylfuran | 4466-24-4 | 893 | 894 d | 0.0044 | 0.3395 | 0.0172 |
| A6 | Heptanal | 111-71-7 | 901 | 902 | 0.0026 | 0.3608 | 0.0107 |
| A7 | Benzaldehyde | 100-52-7 | 960 | 962 | <0.0001 | 0.7105 | 0.0007 |
| A8 | 2-Pentylfuran | 3777-69-3 | 993 | 989 | <0.0001 | 0.3456 | 0.9622 |
| A9 | Decanal | 112-31-2 | 1206 | 1205 | <0.0001 | 0.3111 | <0.0001 |
| Sulphur-compounds | |||||||
| S1 | S-Methyl ethanethionate | 1534-08-3 | 700 | 701 | <0.0001 | 0.482 | 0.4988 |
| S2 | Dimethyl disulfide | 624-92-0 | 738 | 748 d | <0.0001 | 0.0003 | 0.0016 |
| S3 | S-Methyl 3-methylbutanethioate | 23747-45-7 | 938 | 938 | <0.0001 | 0.003 | 0.0002 |
| S4 | Dimethyl trisulfide | 3658-80-8 | 972 | 981 d | <0.0001 | 0.0002 | 0.0014 |
| Terpenoids | |||||||
| T1 | Limonene | 138-86-3 | 1030 | 1029 | <0.0001 | 0.3252 | 0.3159 |
| T2 | beta-Cytocitral | 432-25-7 | 1220 | 1220 | <0.0001 | 0.0571 | 0.7275 |
| T3 | alpha-Terpinyl acetate | 80-26-2 | 1342 | 1350 d | <0.0001 | 0.0089 | 0.0994 |
| T4 | beta-Ionone | 79-77-6 | 1486 | 1484 | <0.0001 | 0.0699 | 0.5269 |
| T5 | Dihydroactinidiolide | 17092-92-1 | 1537 | 1539 d | <0.0001 | 0.1329 | 0.7597 |
| T6 | 1,8-Cineole | 470-82-6 | 1032 | 1032 | <0.0001 | 0.1154 | <0.0001 |
| Ketones | |||||||
| K1 | Acetone | 67-64-1 | 510 | 503 d | <0.0001 | 0.2922 | 0.7313 |
| K2 | Acetophenone | 98-86-2 | 1065 | 1065 d | 0.5156 | 0.0006 | 0.0141 |
| Alkanes | |||||||
| L1 | Dodecane | 112-40-3 | 1200 | 1200 | 0.0301 | 0.4994 | 0.1801 |
| L2 | Tetradecane | 629-59-4 | 1400 | 1400 | 0.0049 | 0.0519 | 0.1076 |
| Alcohols | |||||||
| C1 | Benzyl alcohol | 100-51-6 | 1036 | 1033 | <0.0001 | 0.7548 | 0.2827 |
Bold p-values indicate statistical significance (p ≤ 0.05).
IS: Internal standard.
Code used for labels in Fig. 2
RI: Retention indices calculated from the RT of a series of n-alkanes (C6–C20).
RI: Retention indices reported in the literature for DB-5MS capillary GC columns (Beaulieu & Grimm, 2001).
RI: Retention indices reported in Flavornet and NIST Library 14 for DB-5MS capillary GC column.
Esters comprised 94% of the total volatiles at harvest, a percentage which increased to 96% after storage (Supplementary Table S1); thus, esters were predominant in melon flesh aroma profile, as widely reported (Amaro, Fundo, Oliveira, Beaulieu, Fernández-Trujillo, & Almeida, 2013; Lignou et al., 2014). Differences in contents of total esters among genotypes followed the same trend as observed for total volatile contents (Supplementary Table S1), consistent with reports indicating that longer shelf-life genotypes produce lower levels of esters (Lignou et al., 2014). These differences may be related to alterations in alcohol acyl-transferase (AAT) activity, one of the main enzymes involved in ester biosynthesis (El-Sharkawy et al., 2005, Gonda et al., 2016). Several esters identified as the most abundant in our study are key components of characteristic melon aroma, contributing to the sensory perception of floral, sweet and fruity notes (Senesi et al., 2005, Verzera et al., 2011), e.g., 2-methylbutyl acetate (E18), hexyl acetate (E29) (fruity), isobutyl acetate (E10) (floral), (Z)-3-hexenyl acetate (E28) (green/herbal/banana), ethyl butanoate (E12) (fruity/candy) and ethyl 2-methylbutyrate (E16) (cantaloupe-like, fruity) (Table 2; Supplementary Table S1). Consistently, these ester compounds have been reported as the most abundant in melons from the reticulatus and cantaloupensis groups (Aubert and Bourger, 2004, Shi et al., 2020).
Aldehydes constituted 3.6% of the total volatiles at harvest and decreased to 2.1% after storage, in agreement with previous studies (Amaro et al., 2013, Vallone et al., 2013). Within genotypes, the aldehyde fraction of genotypes #5 and #7 constituted 11% and 7% of their total volatiles, respectively, while for all the other genotypes aldehydes comprised less than 2.5% of their total volatile profile. These differences can be attributed to variation among genotypes in the activities of lipoxygenase and hydroperoxide lyase, which catabolize fatty acids to synthesize aldehydes; and to alterations among genotypes in the activity of alcohol dehydrogenase, which converts aldehyde substrates to their respective alcohols (and subsequently to esters, through AAT) (Gonda et al., 2016). Hexanal (A4), particularly known to impart green and grassy notes in melon aroma (Verzera et al., 2011), was the most abundant aldehyde (Supplementary Table S1).
Sulfur-containing compounds, which have a major impact on the musky notes of cantaloupes and consumer preference (Kourkoutas et al., 2006, Wyllie and Leach, 1992), comprised 2.2% of the total volatile profile of genotype #2, while for all the other genotypes these compounds constituted less than 1%. Significantly higher contents of the thioester S-methyl ethanethionate (S1) were observed at harvest and postharvest for genotype #2. Our results agree with previous studies reporting that the formation of these compounds is under genetic control due to the cultivar-dependent nature of their occurrence (Wyllie et al., 1992). Differences in the enzymatic activity of l-methionine--lyase, which catabolizes l-methionine (precursor to sulfur-containing volatiles) have been associated to different levels of sulfur-containing volatiles in melon (Gonda et al., 2013, Gonda et al., 2016).
The remaining fraction of the volatile profile was constituted by terpenoids, ketones, alkanes and alcohols, displaying differences among genotypes (Table 2) as previously reported (Lignou et al., 2014).
3.3. Sensory evaluation
Twenty sensory descriptors, including nine aroma-, three taste-, and eight flavor-related attributes, were used to describe the sensory properties of the melon genotypes after storage (Table 3; Supplementary Fig. S2). Panelists detected significant differences amongst all melon genotypes in all evaluated sensory attributes, consistent with previous melon sensory panel studies (Lignou et al., 2014, Menezes et al., 2019, Vallone et al., 2013).
Table 3.
Differences in sensory attributes among seven melon genotypes after postharvest storage.
| Melon genotypes | #1 CM2190 | #2 LaJolla | #3 CM2327 | #4 Tacana | #5 Tonga | #6 Fiji | #7 MegaPac |
|---|---|---|---|---|---|---|---|
| Aroma-related attributes | |||||||
| Overall aroma | 51.10a ± 0.65 | 53.56a ± 0.75 | 47.13b ± 0.60 | 46.75b ± 0.99 | 37.46c ± 0.97 | 47.75b ± 0.30 | 34.74c ± 0.41 |
| Melon aroma | 40.08ab ± 1.50 | 42.82a ± 1.12 | 35.40b ± 0.75 | 37.64b ± 0.53 | 28.24c ± 0.30 | 37.08b ± 1.44 | 26.43c ± 1.14 |
| Fruity aroma | 25.92a ± 0.42 | 26.76a ± 1.09 | 20.38bc ± 0.77 | 24.03ab ± 1.49 | 19.40 cd ± 0.83 | 25.85a ± 0.38 | 15.75d ± 0.44 |
| Grassy aroma | 18.35ab ± 0.32 | 16.85b ± 0.89 | 18.83ab ± 0.61 | 18.39ab ± 0.11 | 19.69ab ± 0.30 | 18.50ab ± 0.72 | 20.42a ± 1.05 |
| Green aroma | 17.08bc ± 0.35 | 16.24c ± 0.11 | 18.47a ± 0.11 | 17.08bc ± 0.18 | 17.72ab ± 0.25 | 17.11bc ± 0.44 | 18.76a ± 0.19 |
| Sweet aroma | 24.54c ± 0.67 | 34.82a ± 0.98 | 29.81b ± 0.90 | 32.86ab ± 0.96 | 29.50b ± 1.01 | 21.43 cd ± 0.43 | 19.32d ± 0.56 |
| Melon rind aroma | 30.31a ± 1.60 | 31.06a ± 0.65 | 29.53ab ± 0.29 | 27.14abc ± 0.33 | 24.67c ± 0.73 | 25.47c ± 0.73 | 25.69bc ± 1.05 |
| Floral aroma | 22.10a ± 0.76 | 22.89a ± 0.82 | 17.76 cd ± 0.63 | 20.39abc ± 0.72 | 19.44bcd ± 0.45 | 20.71ab ± 0.54 | 16.89d ± 0.46 |
| Fermented/overripe aroma | 19.68a ± 0.65 | 19.79a ± 0.28 | 14.67bc ± 0.54 | 14.67bc ± 0.96 | 12.74c ± 0.49 | 15.93b ± 0.47 | 13.25bc ± 0.47 |
| Taste-related attributes | |||||||
| Sweet taste | 42.64bc ± 1.66 | 66.29a ± 0.51 | 45.79b ± 0.50 | 45.44b ± 0.99 | 45.35b ± 0.30 | 45.31b ± 0.51 | 40.57c ± 0.79 |
| Sour/acid taste | 12.24b ± 0.19 | 12.94ab ± 0.23 | 12.67ab ± 0.28 | 13.32a ± 0.27 | 12.96ab ± 0.33 | 12.51ab ± 0.16 | 12.18b ± 0.09 |
| Bitter taste | 15.18a ± 0.55 | 12.54b ± 0.13 | 15.00a ± 0.28 | 14.71a ± 0.31 | 15.58a ± 0.30 | 14.60a ± 0.32 | 14.65a ± 0.38 |
| Flavor-related attributes | |||||||
| Overall flavor | 51.43b ± 1.68 | 59.60a ± 0.55 | 47.89bc ± 0.25 | 47.57bc ± 0.56 | 41.25d ± 1.55 | 46.64c ± 0.40 | 34.14d ± 1.10 |
| Fruity flavor | 35.00b ± 0.99 | 41.88a ± 1.11 | 30.64c ± 0.93 | 32.67bc ± 0.30 | 24.79d ± 0.48 | 33.49bc ± 0.41 | 19.57e ± 0.42 |
| Sweet flavor | 40.50bc ± 0.49 | 59.97a ± 0.53 | 42.26b ± 1.61 | 41.94b ± 0.37 | 41.72b ± 0.29 | 35.97c ± 1.49 | 29.56d ± 1.17 |
| Fermented/ overripe flavor | 24.96a ± 0.51 | 25.25a ± 0.47 | 20.46b ± 0.13 | 21.46b ± 0.56 | 14.04c ± 0.32 | 21.89b ± 0.36 | 12.67c ± 0.48 |
| Buttery flavor | 14.72b ± 0.45 | 18.61a ± 0.23 | 14.40bc ± 0.53 | 13.92bc ± 0.07 | 12.71c ± 0.68 | 14.11bc ± 0.38 | 12.63c ± 0.14 |
| Grassy flavor | 15.07ab ± 0.53 | 14.19b ± 0.13 | 15.54ab ± 0.37 | 15.78ab ± 0.27 | 15.22ab ± 0.51 | 15.71ab ± 0.44 | 15.99a ± 0.12 |
| Green flavor | 17.46b ± 0.41 | 14.90c ± 0.38 | 20.82a ± 0.23 | 17.39b ± 0.18 | 19.69a ± 0.43 | 17.46b ± 0.17 | 21.18a ± 0.39 |
| After flavor/aftertaste | 37.74b ± 0.70 | 43.64a ± 0.62 | 33.50 cd ± 0.90 | 34.10c ± 0.42 | 30.57d ± 0.34 | 35.24bc ± 0.42 | 21.51e ± 0.98 |
Values are means ± standard error (n = 4). Different letters indicate significant differences among genotypes (p ≤ 0.05) according to Tukey’s test.
For aroma-related sensory attributes, defined as the sensory perception of volatile compounds sniffed through the nostrils (Lawless & Heymann, 1998), genotypes #1 and #2 received significantly higher scores for overall aroma, melon, melon skin, floral and overripe/fermented aromas, followed by genotypes #3, #4 and #6, and by genotypes #5 and #7 (Table 3; Supplementary Fig. S2). Fruity aroma perception was highest for genotypes #1, #2 and #6, in agreement with our expectations, as regulation of melon aroma, and particularly volatile esters, has been linked to ethylene production (Bauchot et al., 1998, Flores et al., 2001, Lignou et al., 2014), which were highest for genotypes #1 and #2 (Fig. 1B). Genotype #2 displayed the highest sweet aroma scores which were significantly lower for genotypes #1, #6 and #7 (Table 3). These differences could result from genetic variations in carbohydrate metabolic patterns (Kyriacou, Leskovar, Colla, & Rouphael, 2018). Additionally, green and grassy aroma ratings trended opposite to the previous descriptors, with genotype #7 displaying the highest values amongst all genotypes (Table 3), in agreement with the higher aldehyde contents of this genotype, which contribute to green and grassy aromas (Verzera et al., 2011) (Table 2; Supplementary Table S1).
Taste-related attributes indicated that Genotype #2 was rated as the sweetest, while genotypes #1 and #7 received the lowest ratings (Table 3; Supplementary Fig. S2). Sour/acid taste values were highest for genotype #4 and lowest for #1 and #7. These results are consistent with SSC and TA (Fig. 1F,G) and reported genetic variations in sugar and acid contents among melon cultivars (Beaulieu et al., 2003).
The flavor-related descriptors, which are perceived by the panelists after mastication and swallowing of the samples as volatile compounds travel retronasally from the oral to the nasal cavity (Lawless & Heymann, 1998), displayed similar trends to the aroma-related attributes (Table 3). This was expected, as in both cases the same volatile compounds are being perceived but what changes is whether it is via orthonasal (aroma) or retronasal (flavor) olfaction. Genotypes #2 and #7 received the highest and lowest ratings for most of the flavor-related descriptors, respectively, with the exception of grassy and green flavors, where the opposite was observed. Other melon studies have also reported similar trends for those attributes (Menezes et al., 2019, Vallone et al., 2013). For overall flavor, fruity, fermented/overripe, buttery flavors and after-flavor/ after-taste, genotype #2′s ratings were highest, followed by genotype #1, and the lowest ratings were for genotype #5 (Table 3). Genotype #2 received the highest ratings for sweet flavor, consistent with the sweet taste ratings and the measured SSC (Fig. 1F).
3.4. Relationships among ripening patterns, physicochemical parameters, volatile compounds and sensory attributes
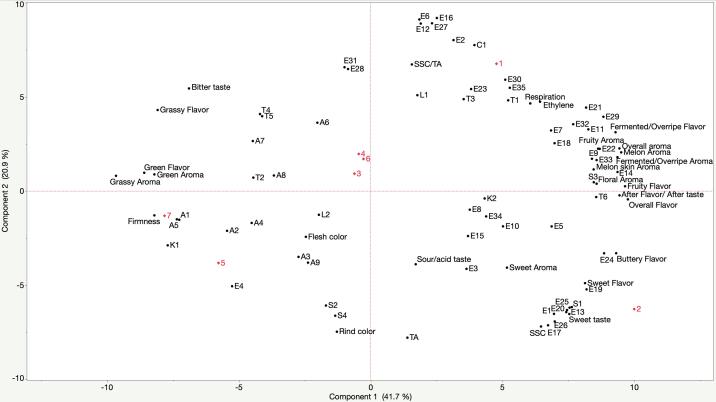
Correlation coefficients were calculated and a principal component analysis (PCA) was performed to visualize the relationships among the parameters described above (Fig. 2). Subsequently, volatile compounds that were important in explaining the variance among samples were used to make a PLS regression with all the evaluated sensory attributes to identify key volatiles for prediction of melon flavor sensory perception (Fig. 3). The PCA showed that the first and second principal components explained 41.7% (Component 1) and 20.9% (Component 2) of the observed variation (62.6% total). On the first principal component, genotype separation was driven by the sensory attributes of green and grassy aroma, green and grassy flavor as well as by flesh firmness on the negative side (associated with genotypes #5, #7 and #3, #4, #6) and by the sensory descriptors of fruity flavor and overall flavor on the positive side (associated with genotypes #1 and #2) (Fig. 2). The distribution of the melon genotypes along Component 1 of the PCA coincides with our previous study indicating that #1 and #2 display a shorter shelf-life capacity, while #3, #4, #5, #6 and #7 have a longer shelf-life (Farcuh et al., 2020). Additionally, this separation is supported by differences in their genetic background as genotype #2 is a Charentais melon (cantaloupensis group) and #1 is a Charentais × muskmelon hybrid (cantaloupensis × reticulatus groups), while the rest of the genotypes are muskmelons (reticulatus group).
Fig. 2.
Biplot from Principal Component Analysis of ripening patterns, physicochemical properties, volatile compounds and sensory attributes of seven melons genotypes. Numbers in red correspond to genotypes #1 (CM2190), #2 (La Jolla), #3 (CM2327), #4 (Tacana), #5 (Tonga), #6 (Fiji), #7 (MegaPac). Codes for volatiles correspond to codes in Table 2.
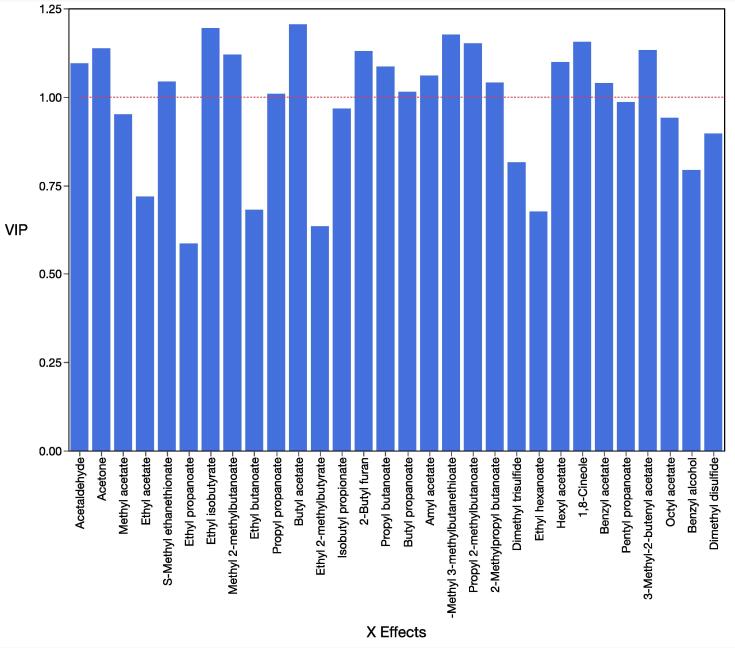
Fig. 3.
Variable importance in the projection (VIP) values of the selected predictor volatile compounds used in the PLS regression.
All the analyzed variables that presented statistically higher values in #1 and #2 were located on the positive (right) side of the biplot (Fig. 2). Ethylene and respiration production rates were positively correlated (r = 0.99), in agreement with previous reports (Biale et al., 1981; Farcuh et al., 2018) and supporting the climacteric behavior exhibited by all genotypes, particularly genotypes #1 and #2. We observed significantly positive correlations of ethylene production with attributes such as overall aroma and flavor, melon and floral aromas, fruity aroma and flavor, fermented/overripe aroma and flavor (r ≥ 0.59) (Fig. 2), consistent with reports that ethylene is involved in the regulation of melon volatile production and aroma profile development during ripening (Flores et al., 2001, Obando-Ulloa et al., 2008). Furthermore, ethylene production was highly correlated with several esters, e.g., 3-phenylpropyl acetate (E35) (r = 0.96), benzyl acetate (E32) (r = 0.87), 2,3-butanediol diacetate (E30) (r = 0.84), methyl 2-methylbutanoate (E11) (r = 0.82) and propyl acetate (E7) (r = 0.70). Similar results were obtained in a study of three muskmelon cultivars (reticulatus group) at different harvest maturities (Vallone et al., 2013) and in another where total esters concentrations were significantly affected when ethylene biosynthesis was downregulated in transformed Charentais melons (cantaloupensis group) (Bauchot et al., 1998).
Fruity aroma was significantly and positively correlated with fruity flavor (r = 0.93) and with several volatiles, including hexyl acetate (E29), amyl acetate (E21), butyl acetate (E14), 2-methylbutyl acetate (E18), methyl 2-methylbutanoate (E11) (r ≥ 0.75) (Fig. 2). Fruity aroma was also significantly correlated with floral aroma (r = 0.92) and both descriptors were highly correlated with volatiles S-methyl 3-methylbutanethionate (S3), 1,8-cineole (T6) (r ≥ 0.75) and isobutyl acetate (E10) (r = 0.60). These volatiles have previously been described to impart fruity, fresh and floral odor characters in various melon types (Kourkoutas et al., 2006, Verzera et al., 2011). Several of these volatiles were identified by PLS regression as highly influential in predicting perception of sensory attributes (Fig. 3).
The sensory attributes of fermented/overripe aroma and flavor were significantly correlated (r = 0.88), as well as with benzyl alcohol (C1) (r ≥ 0.65). Increased alcohol production in melons is often associated with advanced ripeness and/or senescence in fruit (Senesi et al., 2005). Nevertheless, benzyl alcohol was not identified as a significant predictor of melon sensory perception (Fig. 3). The positive correlation exhibited by benzyl alcohol with ethylene and respiration levels (r ≥ 0.88) is consistent with studies showing that benzyl alcohol can be significantly reduced in melons by 1-methylcyclopropane, an inhibitor of ethylene perception (Amaro et al., 2013). This supports the strong association between benzyl alcohol and genotype #1, which displayed the highest ethylene and respiration levels (Fig. 1A.B).
Sweet taste was positively correlated with SSC (r = 0.97) and with sweet flavor (r = 0.93) and sweet aroma (r = 0.70), which were also significantly correlated (r = 0.84) (Fig. 2). Genotype #2 displayed the highest association with all the sweet-related attributes. Furthermore, in strawberry and tomato fruits, volatile compounds have been shown to contribute to the perception of sweetness beyond the effects of sugars (Klee et al., 2018). In our study, the concentration of numerous volatiles was also highly correlated (r ≥ 0.60) with the sensory attributes of sweet aroma, sweet flavor and sweet taste (Fig. 2), and these were identified as important predictors of sensory attribute perception in melons by PLS regression (Fig. 3); e.g. propyl butanoate (E19), 2-methylpropyl butanoate (E25), propyl 2-methylbutanoate (E24), butyl propanoate (E20), and propyl propanoate (E13) among the esters, as well as S-methyl ethanethioate (S1), a sulfur-containing compound (Fig. 2). Several of these esters have been associated with sweetness perception in melons (Senesi et al., 2005). Sulfur-containing compounds were significantly higher in melons from the cantaloupensis group, as compared to the reticulatus and inodorus groups (Kourkoutas et al., 2006), and considerably reduced in longer versus shorter shelf-life cultivars (Aubert et al., 2004), supporting their association with genotype #2. Trace amounts of sulfur-containing compounds may contribute to the musky notes in melon aroma and in determining consumer preference (Bauchot et al., 1998; Wyllie et al., 1992). In terms of SSC/TA, highly significant correlations (r ≥ 0.70) were observed with volatile esters such as 2,3-butanediol diacetate (E30), methyl benzoate (E31), and 3-phenylpropyl acetate (E35) and with benzyl alcohol (C1) (r = 0.78).
Flesh firmness was negatively correlated to ethylene production (r = –0.89), as ethylene has been shown to play an important role in fruit softening through cell wall disassembly in melon (Guis et al., 1997, Nishiyama et al., 2007). Consistently, the lowest sensory and instrumental firmness values were associated with shorter shelf-life genotypes, such as #1 and #2, while longer shelf-life genotypes #4, #5 and #6 were firmer (Farcuh et al., 2020), consistent with their positioning in the PCA (Fig. 2). Several reports have shown a negative relation between firmness and consumer preference and acceptability of melons (Park et al., 2018, Vallone et al., 2013). Flesh firmness influenced the perception of buttery flavor, as both variables were negatively correlated (r = –0.70). Flesh firmness was positively correlated to green and grassy aroma and flavor (r > 0.70), which were also correlated (r ≥ 0.75). Green and grassy aroma attributes were significantly correlated to volatile compounds from the aldehyde class (r ≥ 0.59), such as 2-butylfuran (A5), acetaldehyde (A1), propanal (A2), and hexanal (A4). These volatiles have been reported to contribute to the green and grassy notes of melon aroma (Amaro et al., 2013, Verzera et al., 2011), and particularly in this study, acetaldehyde (A1) and 2-butylfuran (A5) were identified as key predictors of melon sensory perception (Fig. 3). Other studies also found a positive correlation between firmness and cucumber/cucurbit aroma (likely equivalent to our green aroma) and aldehyde concentrations when evaluating different cultivars from the reticulatus group (Beaulieu and Lancaster, 2007, Vallone et al., 2013).
4. Conclusions
Sensory, physicochemical and volatile compound analysis identified significant differences in a set of seven short and long shelf-life melon genotypes, which can be attributed to either the maturity stage at harvest and after storage or the genotype. After storage, genotypes #1 and #2 display a sharper climacteric phase, supporting their shorter shelf-life capacity, as compared to the rest of the genotypes, which present a longer-shelf life capacity. Sensory descriptors of fruity and floral aroma and flavor were associated with genotypes #1 and #2, and particularly with genotype #2 for sweet-related attributes. Esters such as butyl acetate, ethyl isobutyrate, hexyl acetate, propyl 2-methylbutanoate, 3-methyl-2-butenyl acetate, among others, together with sulfur-containing compounds, including S-methyl ethanethioate and S-methyl 3-methylbutanethionate, and terpenoid 1,8-cineole were identified as important predictors of melon flavor sensory perception and significantly correlate with genotypes #1 and #2 and the above attributes. Longer shelf-life genotypes are associated with green and grassy aroma and flavor attributes, fruit firmness, and display positive correlations to volatiles from the aldehyde class, including acetaldehyde and 2-butylfuran, also identified as key predictors of melon flavor sensory perception. This work could be applied to predict the sensory perception of flavor during melon ripening in storage. Phenotyping based on these parameters is likely to accelerate the breeding of melons with improved flavor characteristics.
CRediT authorship contribution statement
Macarena Farcuh: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Bill Copes: Conceptualization, Writing - review & editing. Gaelle Le-Navenec: Conceptualization, Writing - review & editing. Juan Marroquin: Conceptualization, Writing - review & editing. Dario Cantu: Conceptualization, Methodology, Writing - review & editing. Kent J. Bradford: Conceptualization, Methodology, Writing - review & editing. Jean-Xavier Guinard: Conceptualization, Methodology, Writing - review & editing. Allen Van Deynze: Conceptualization, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by HM.Clause grant #201604289. We are thankful to Mackenzie Batali and Larry Lerno for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100107.
Contributor Information
Macarena Farcuh, Email: mfarcuh@umd.edu.
Bill Copes, Email: b.copes@hmclause.com.
Gaelle Le-Navenec, Email: gaelle.lenavenec@hmclause.com.
Juan Marroquin, Email: Juan.Marroquin@hmclause.com.
Dario Cantu, Email: dacantu@ucdavis.edu.
Kent J. Bradford, Email: kjbradford@ucdavis.edu.
Jean-Xavier Guinard, Email: jxguinard@ucdavis.edu.
Allen Van Deynze, Email: avandeynze@ucdavis.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Amaro A.L., Fundo J.F., Oliveira A., Beaulieu J.C., Fernández-Trujillo J.P., Almeida D.P. 1-Methylcyclopropene effects on temporal changes of aroma volatiles and phytochemicals of fresh-cut cantaloupe. Journal of the Science of Food and Agriculture. 2013;93(4):828–837. doi: 10.1002/jsfa.5804. [DOI] [PubMed] [Google Scholar]
- Aubert C., Bourger N. Investigation of volatiles in Charentais cantaloupe melons (Cucumis melo var. cantalupensis). Characterization of aroma constituents in some cultivars. Journal of Agricultural and Food Chemistry. 2004;52(14):4522–4528. doi: 10.1021/jf049777s. [DOI] [PubMed] [Google Scholar]
- Bauchot A.D., Mottram D.S., Dodson A.T., John P. Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe Charentais melon (Cv. Védrandais) Journal of Agricultural and Food Chemistry. 1998;46(11):4787–4792. doi: 10.1021/jf980692z. [DOI] [Google Scholar]
- Beaulieu J.C., Grimm C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. Journal of Agricultural and Food Chemistry. 2001;49(3):1345–1352. doi: 10.1021/jf0005768. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.C., Ingram D.A., Lea J.M., Bett-Garber K.L. Effect of harvest maturity on the sensory characteristics of fresh-cut cantaloupe. Journal of Food Science. 2004;69(7):250–258. [Google Scholar]
- Beaulieu J.C., Lancaster V.A. Correlating volatile compounds, sensory attributes, and quality parameters in stored fresh-cut cantaloupe. Journal of Agricultural and Food Chemistry. 2007;55(23):9503–9513. doi: 10.1021/jf070282n. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.C., Lea J.M., Eggleston G., Peralta-Inga Z. Sugar and organic acid variations in commercial cantaloupes and their inbred parents. Journal of the American Society for Horticultural Science. 2003;128(4):531–536. doi: 10.21273/jashs.128.4.0531. [DOI] [Google Scholar]
- Biale, J. B., & Young, R. E. (1981). Respiration and ripening in fruits–retrospect and prospect. In J. Friend, & M.J.C Rhosed (Eds.), Recent advances in the biochemistry of fruits and vegetables (pp. 1–39). London: Academic Press.
- Cohen S., Tzuri G., Harel-Beja R., Itkin M., Portnoy V., Sa’ar U. Co-mapping studies of QTLs for fruit acidity and candidate genes of organic acid metabolism and proton transport in sweet melon (Cucumis melo L.) Theoretical and Applied Genetics. 2012;125(2):343–353. doi: 10.1007/s00122-012-1837-3. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy, I., Manriquez, D., Flores, F., Regad, F., Bouzayen, M., Latche, A., & Pech, J.C. (2005). Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Molecular Biology, 59, 345–362. doi: 10.1007/s11103-005-8884-y. [DOI] [PubMed]
- Farcuh M., Copes B., Le-Navenec G., Marroquin J., Jaunet T., Chi-Ham C. Texture diversity in melon (Cucumis melo L.): Sensory and physical assessments. Postharvest Biology and Technology. 2020;159 doi: 10.1016/j.postharvbio.2019.111024. [DOI] [Google Scholar]
- Farcuh M., Li B., Rivero R.M., Shlizerman L., Sadka A., Blumwald E. Sugar metabolism reprogramming in a non-climacteric bud mutant of a climacteric plum fruit during development on the tree. Journal of Experimental Botany. 2017;68(21–22) doi: 10.1093/jxb/erx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcuh M., Rivero R.M., Sadka A., Blumwald E. Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums. Postharvest Biology and Technology. 2018;139 doi: 10.1016/j.postharvbio.2018.01.012. [DOI] [Google Scholar]
- Flores F., Amor M. Ben, Jones B., Pech J.C., Bouzayen M., Latché A., Romojaro F. The use of ethylene-suppressed lines to assess differential sensitivity to ethylene of the various ripening pathways in Cantaloupe melons. Physiologia Plantarum. 2001;113(1):128–133. doi: 10.1034/j.1399-3054.2001.1130117.x. [DOI] [Google Scholar]
- Frost S., Ristenpart W., Guinard J.X. Effects of brew strenth, brew yield, and roast on the sensory quality of drip brewed coffee. Journal of Food Science. 2020;85(8):2530–2543. doi: 10.1111/1750-3841.15326. [DOI] [PubMed] [Google Scholar]
- Gonda I., Burger Y., Schaffer A.A., Ibdah M., Tadmor Y.A., Katzir N. Biosynthesis and perception of melon aroma. In: Frenkel D.H., Dudai N., editors. Biotechnology in flavor production. Wiley Blackwell; New Jersey: 2016. pp. 281–305. [Google Scholar]
- Gonda I., Lev S., Bar E., Sikron N., Portnoy V., Davidovich-Rikanati R. Catabolism of L-methionine in the formation of sulfur and other volatiles in melon (Cucumis melo L.) fruit. The Plant Journal. 2013;74(3):458–472. doi: 10.1111/tpj.12149. [DOI] [PubMed] [Google Scholar]
- Guis M., Botondi R., Ben-Amor M., Ayub R., Bouzayen M., Pech J.C., Latché A. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. Journal of the American Society for Horticultural Science. 1997;122(6):748–751. doi: 10.21273/jashs.122.6.748. [DOI] [Google Scholar]
- Kim H.-Y., Farcuh M., Cohen Y., Crisosto C., Sadka A., Blumwald E. Non-climacteric ripening and sorbitol homeostasis in plum fruits. Plant Science. 2015;231 doi: 10.1016/j.plantsci.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Klee H.J., Giovannoni J.J. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- Klee H.J., Tieman D.M. The genetics of fruit flavour preferences. Nature Reviews Genetics. 2018;19(6):347–356. doi: 10.1038/s41576-018-0002-5. [DOI] [PubMed] [Google Scholar]
- Kourkoutas D., Elmore J.S., Mottram D.S. Comparison of the volatile compositions and flavour properties of cantaloupe, Galia and honeydew muskmelons. Food Chemistry. 2006;97(1):95–102. doi: 10.1016/j.foodchem.2005.03.026. [DOI] [Google Scholar]
- Kyriacou M.C., Leskovar D.I., Colla G., Rouphael Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Scientia Horticulturae. 2018;234:393–408. doi: 10.1016/j.scienta.2018.01.032. [DOI] [Google Scholar]
- Lawless H.T., Heymann H. Springer; New York: 1998. Sensory evaluation of food: Principles and practices. [Google Scholar]
- Lester, G. (2006). Consumer preference quality attributes of melon fruits. IV International Conference on Managing Quality in Chains-The Integrated View on Fruits and Vegetables Quality 712, 175–182.
- Lignou S., Parker J.K., Baxter C., Mottram D.S. Sensory and instrumental analysis of medium and long shelf-life Charentais cantaloupe melons (Cucumis melo L.) harvested at different maturities. Food Chemistry. 2014;148:218–229. doi: 10.1016/j.foodchem.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes E.M., Lee S.M., Boyden L., Guinard J. Sensory properties and consumer acceptance of cantaloupe melon cultivars. Journal of Food Science. 2019;84(8):2278–2288. doi: 10.1111/1750-3841.14724. [DOI] [PubMed] [Google Scholar]
- Miccolis, V., & Saltveit, M. E. (1995). Influence of storage period and temperature on the postharvest characteristics of six melon (Cucumis melo L., Inodorus Group) cultivars. In Postharvest Biology and Technology Postharvest Biology and Technology (Vol. 5).
- Le Nguyen L.P., Zsom T., Dam M.S., Baranyai L., Hitka G. Evaluation of the 1-MCP microbubbles treatment for shelf-life extension for melons. Postharvest Biology and Technology. 2019;150:89–94. doi: 10.1016/j.postharvbio.2018.12.017. [DOI] [Google Scholar]
- Nishiyama K., Guis M., Rose J.K.C., Kubo Y., Bennett K.A., Wangjin L. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. Journal of Experimental Botany. 2007;58(6):1281–1290. doi: 10.1093/jxb/erl283. [DOI] [PubMed] [Google Scholar]
- Nuñez-Palenius H.G., Gomez-Lim M., Ochoa-Alejo N., Grumet R., Lester G., Cantliffe D.J. Melon fruits: Genetic diversity, physiology, and biotechnology features. Critical Reviews in Biotechnology. 2008;28(1):13–55. doi: 10.1080/07388550801891111. [DOI] [PubMed] [Google Scholar]
- Obando-Ulloa J.M., Moreno E., García-Mas J., Nicolai B., Lammertyn J., Monforte A.J., Fernández-Trujillo J.P. Climacteric or non-climacteric behavior in melon fruit. 1. Aroma volatiles. Postharvest Biology and Technology. 2008;49(1):27–37. doi: 10.1016/j.postharvbio.2007.11.004. [DOI] [Google Scholar]
- Park E., Luo Y., Marine S.C., Everts K.L., Micallef S.A., Bolten S., Stommel J. Consumer preference and physicochemical evaluation of organically grown melons. Postharvest Biology and Technology. 2018;141:77–85. doi: 10.1016/j.postharvbio.2018.03.001. [DOI] [Google Scholar]
- Pitrat M. Phenotypic diversity in wild and cultivated melons (Cucumis melo) Plant Biotechnology. 2013;30(3):273–278. doi: 10.5511/plantbiotechnology.13.0813a. [DOI] [Google Scholar]
- Portela S.I., Cantwell M.I. Quality changes of minimally processed honeydew melons stored in air or controlled atmosphere. Postharvest Biology and Technology. 1998;14(3):351–357. doi: 10.1016/S0925-5214(98)00052-0. [DOI] [Google Scholar]
- Senesi E., Di Cesare L.F., Prinzivalli C., Scalzo R. Lo. Influence of ripening stage on volatiles composition, physicochemical indexes and sensory evaluation in two varieties of muskmelon (Cucumis melo L reticulatus var Naud) Journal of the Science of Food and Agriculture. 2005;85(8):1241–1251. doi: 10.1002/jsfa.2094. [DOI] [Google Scholar]
- Shi J., Wu H., Xiong M., Chen Y., Chen J., Zhou B. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chemistry. 2020;316:126–342. doi: 10.1016/j.foodchem.2020.126342. [DOI] [PubMed] [Google Scholar]
- Vallone S., Sivertsen H., Anthon G.E., Barrett D.M., Mitcham E.J., Ebeler S.E., Zakharov F. An integrated approach for flavour quality evaluation in muskmelon (Cucumis melo L. reticulatus group) during ripening. Food Chemistry. 2013;139(1–4):171–183. doi: 10.1016/j.foodchem.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Verzera A., Dima G., Tripodi G., Ziino M., Lanza C.M., Mazzaglia A. Fast quantitative determination of aroma volatile constituents in melon fruits by headspace-solid-phase microextraction and gas chromatography-mass spectrometry. Food Analytical Methods. 2011;4(2):141–149. doi: 10.1007/s12161-010-9159-z. [DOI] [Google Scholar]
- Wyllie S.G., Leach D.N. Sulfur-containing compounds in the aroma volatiles of melons (Cucumis melo) Journal of Agricultural and Food Chemistry. 1992;40(2):253–256. doi: 10.1021/jf00014a017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.