Summary
Mounting an effective immune response is crucial for the host to protect itself against invading pathogens. It is now well appreciated that reprogramming of core metabolic pathways in immune cells is a key requirement for their activation and function during infections. The role of several ancillary metabolic pathways in shaping immune cell function is less well understood. One such pathway, for which interest has recently been growing, is the hexosamine biosynthesis pathway (HBP) that generates uridine diphosphate N‐acetylglucosamine (UDP‐GlcNAc), the donor substrate for a specific form of glycosylation termed O‐GlcNAcylation. O‐GlcNAc is an intracellular post‐translational modification that alters the functional properties of the modified proteins, in particular transcription factors and epigenetic regulators. An increasing number of studies suggest a central role for the HBP and O‐GlcNAcylation in dictating immune cell function, including the response to different pathogens. We here discuss the most recent insights regarding O‐GlcNAcylation and immunity, and explore whether targeting of O‐GlcNAcylation could hold promise as a therapeutic approach to modulate immune responses to infections.
Keywords: immunity, infection, O‐GlcNAc hydrolase, O‐GlcNAc transferase, O‐GlcNAcylation
An increasing number of studies suggest a central role for O‐GlcNAcylation – a specific post‐translational modification – in dictating immune cell function and immunity against different pathogens. In this review, we will discuss the most recent insights regarding the role of O‐GlcNAcylation in immunity and explore whether targeting of O‐GlcNAcylation could hold promise as a therapeutic approach to modulate immune responses to infections.
Abbreviations
- BCR
B‐cell receptor
- BLT1
leukotriene B4 receptor 1
- BMDM
bone‐marrow‐derived macrophages
- CTL
cytotoxic T lymphocyte
- EZH2
enhancer of zeste homolog 2
- fMLP
formyl‐methionyl‐leucyl‐phenylalanine
- GFAT1
glutamine‐fructose‐6‐phosphate amidotransferase
- GlcN
glucosamine
- GlcN‐6p
glucosamine‐6p
- HBP
hexosamine biosynthesis pathway
- HTLV‐1
human T‐cell lymphotropic virus type 1
- IAV
influenza A virus
- IL‐2
interleukin‐2
- ILC3
innate lymphoid cell 3
- iNOS
inducible NO synthase
- IRF3
interferon regulatory factor 3
- LPS
lipopolysaccharide
- Lsp‐1
lymphocyte‐specific protein 1
- LTB4
leukotriene B4
- MAVS
mitochondrial antiviral‐signaling protein
- mSin3A
mammalian Sin3A
- NK cell
natural killer cell
- NKG2D
natural killer group 2D
- NO
nitric oxide
- OGA
O‐GlcNAc hydrolase
- OGT
O‐GlcNAc transferase
- PTM
post‐translational modification
- RIPK3
receptor‐interacting serine/threonine‐protein kinase 3
- RLR
retinoic‐acid inducible gene I‐like receptor
- RORγT
retinoic acid‐related orphan receptor
γ
t
- STAT3
signal transducer and activator of transcription 3
- TCR
T‐cell receptor
- TET2
ten‐eleven‐translocation 2
- Th1
T helper 1
- TLR
Toll‐like receptor
- UDP‐GlcNAc
uridine diphosphate N‐acetylglucosamine
- VSV
vesicular stomatitis virus
Introduction
Immune responses are crucial for host defense against a wide range of pathogenic viruses, bacteria, fungi and parasites. 1 In recent years it has become increasingly clear that initiation, polarization, maintenance and resolution of immune responses against these different pathogens are accompanied by, and dependent on, metabolic reprogramming of the immune cells involved. The role of core metabolic pathways such as glycolysis and fatty acid metabolism in controlling the function and fate of various immune cells has been extensively studied. 2 More recently however, the importance of ancillary metabolic pathways in the shaping of immune cell function is becoming appreciated. One such metabolic pathway is the hexosamine biosynthesis pathway (HBP).
The HBP uses substrates from several other metabolic pathways to form its end product uridine diphosphate N‐acetylglucosamine (UDP‐GlcNAc) (Fig. 1). 3 UDP‐GlcNAc is a key building block for both N‐linked and O‐linked glycosylation, which are post‐translational modifications (PTM) whereby carbohydrates or carbohydrate chains (glycans) are covalently linked to a protein. 4 A particular type of glycosylation that is directly linked to HBP activity and that is receiving increasing interest in the field of immunology, is O‐GlcNAcylation. During O‐GlcNAcylation a single GlcNAc moiety is covalently linked to the hydroxyl group of a serine or threonine residue. 5 In contrast to classical forms of protein glycosylation, O‐GlcNAcylation is a dynamic PTM analogous to phosphorylation. O‐GlcNAcylation of a protein can affect its stability, localization and activity. Interestingly, although phosphorylation is regulated by a complex network of multiple phosphotransferases and phosphatases, O‐GlcNAcylation is regulated by only two enzymes. O‐GlcNAc transferase (OGT) catalyzes the transfer of GlcNAc from the donor substrate UDP‐GlcNAc onto acceptor proteins, 6 and O‐GlcNAc hydrolase (OGA) removes the O‐linked GlcNAc from a protein. 7 A distinguishing feature of O‐GlcNAcylation from other types of glycosylation is that it is strongly influenced by nutrient availability. The rates of O‐GlcNAcylation by OGT are highly dependent on the availability of donor substrate UDP‐GlcNAc, which is a product of the HBP, which activity is directly correlated to glucose and/or glutamine availability. 8 , 9 Hence, O‐GlcNAcylation is a striking example of how nutritional cues and cellular metabolic pathways can control protein modification.
Figure 1.
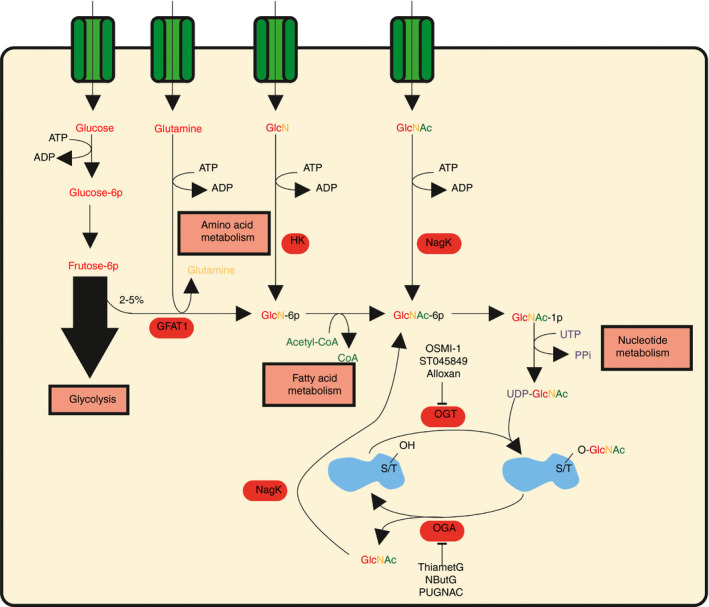
The hexosamine biosynthesis pathway (HBP) fuels O‐GlcNAcylation. Between 2% and 5% of fructose‐6p derived from glycolysis feeds into the HBP. The rate‐limiting enzyme glutamine‐fructose‐6‐phosphate amidotransferase (GFAT1) converts fructose‐6p together with glutamine into glucosamine‐6p (GlcN‐6p). Fatty acid metabolism donates the acetyl‐group to form GlcNAc‐6p and in the final step of the HBP nucleotide metabolism supplies UTP to generate the end product uridine diphosphate N‐acetylglucosamine (UDP‐GlcNAc). UDP‐GlcNAc is the key substrate for O‐GlcNAc transferase (OGT), which catalyzes O‐GlcNAcylation of proteins. O‐GlcNAc hydrolase (OGA) removes O‐GlcNAc from proteins. GlcNAc can then recycle back into the HBP by conversion into GlcNAc‐6p by N‐acetylglucosamine kinase to form UDP‐GlcNAc again. GlcN is an intermediate, which can also fuel the HBP through conversion into GlcN‐6p by hexokinase (HK). OGT can be inhibited by OSMI‐1, ST045849 and alloxan. OGA can be inhibited by ThiametG, NButG and PUGNAC.
Since the discovery of O‐GlcNAcylation, many proteins that can be modified by O‐GlcNAc have been identified, including transcription factors, kinases and epigenetic regulators. 3 Furthermore, O‐GlcNAcylation has been implicated in cross‐talk with various other PTMs such as ubiquitination and phosphorylation, of which the latter – just like O‐GlcNAcylation – occurs on serine and threonine residues. 10 , 11 The diverse targets of O‐GlcNAcylation and its cross‐talk with other PTMs, make O‐GlcNAcylation a highly complex PTM to study; many of its effects at the molecular and cellular level remain to be discovered. Nonetheless, it is clear that proper control of O‐GlcNAcylation is involved in maintaining health, as dysregulation of O‐GlcNAcylation has already been implicated in various diseases such as cancer and neurodegenerative disorders. 12 More recently, there has been a growing interest in exploring the role of O‐GlcNAcylation in regulation of the immune system; during homeostasis as well as infection. We here aim to provide the latest insights into the role of O‐GlcNAcylation in regulating immune responses against viral, bacterial, fungal and parasitic infections, address the outstanding questions in this field, and discuss whether O‐GlcNAcylation may hold promise as therapeutic target.
O‐GlcNAcylation in viral infections
Innate immune cells that play a central role in early detection of, and response against, viral infections are macrophages. 1 Several studies have implicated a key role for O‐GlcNAcylation in macrophages in recognition of RNA viruses (Fig. 2a). Detection of RNA viruses occurs through the cytosolic retinoic acid inducible gene I‐like receptors (RLRs). 13 Central players are retinoic acid inducible gene I and melanoma differentiation‐associated gene 5, which recruit mitochondrial antiviral‐signaling protein (MAVS) for interferon regulatory factor 3 (IRF3) activation and ultimately interferon type 1 production. When OGT was deleted in murine bone‐marrow‐derived macrophages (BMDM), these cells demonstrated impaired MAVS‐dependent signaling downstream of RLRs and reduced inflammatory cytokine expression, following a challenge with vesicular stomatitis virus (VSV). 14 Similar observations were made for BMDM challenged with influenza viruses (IAVs), which also showed greatly diminished anti‐viral downstream signaling when OGT was deleted. 15 O‐GlcNAcylation of serine 366 on MAVS was determined to be critical for RLR signaling in one study, 14 but not in the other, which found multiple O‐GlcNAcylation sites within amino acids 324–347 to be essential. 15 Despite these disparities, both groups found that O‐GlcNAcylation was required for MAVS to be targeted for lysine 63 ubiquitination and downstream immune activation. 14 , 15 Moreover, both studies used mice in which OGT was specifically deleted in myeloid cells to demonstrate that susceptibility to VSV 14 and IAV 15 infection was increased, highlighting the importance of O‐GlcNAcylation in macrophages for generating immunity against RNA viruses. However, a more recent study demonstrated that enhanced O‐GlcNAcylation in macrophages can also have deleterious effects on immunity against IAV infection as a consequence of promoting a virus‐induced cytokine storm that compromises viral clearance. Mechanistically, strong inflammatory cytokine expression by macrophages in response to IAV infection was found to be dependent on O‐GlcNAcylation of IRF5 at serine 430, which allowed for its activation following ubiquitination at lysine 63. 16 As a consequence, mice deficient for OGT or IRF5 in myeloid cells displayed less body weight loss and systemic cytokine production following IAV infection and were better able to control viral infection compared with their wild‐type littermates.
Figure 2.
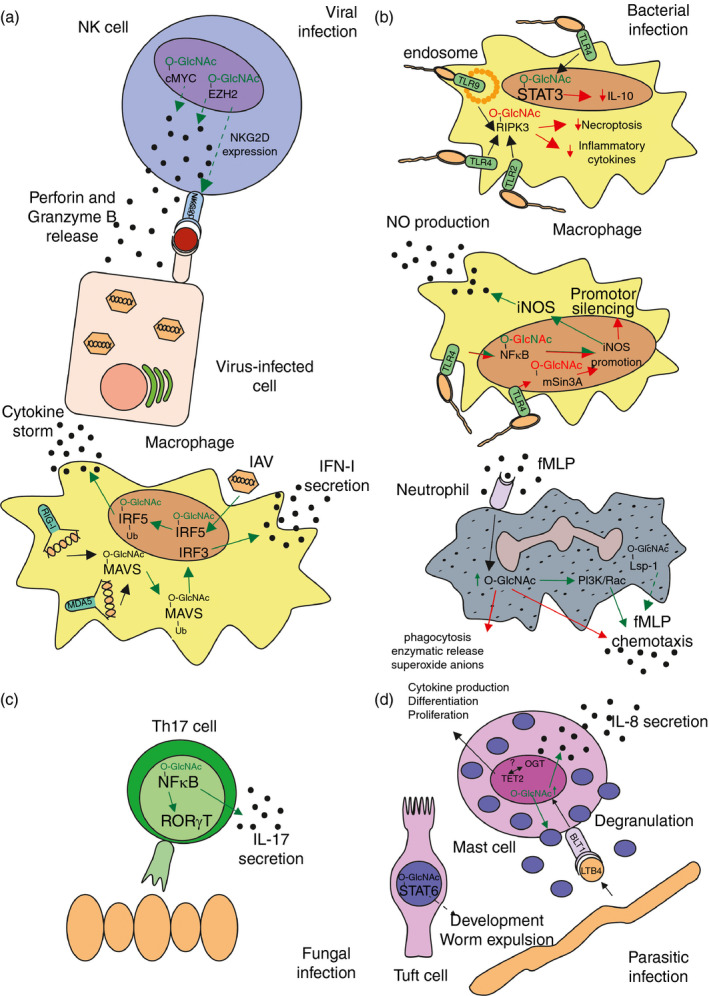
Role of O‐GlcNAcylation in regulation of immune cell responses against viral (a), bacterial (b), fungal (c) or extracellular parasitic (d) infection. Red O‐GlcNAc or arrows indicate a negative or inhibitory effect, green O‐GlcNAc or arrows indicate a positive or inducing effect. Dotted arrows present a possible, but not yet established effect. (a) Natural killer (NK) cells require O‐GlcNAcylation for NKG2D‐expression and perforin and granzyme B release against virus‐infected cells. Macrophages depend on O‐GlcNAcylation for type I interferon secretion. Influenza virus (IAV) infection triggers a cytokine storm in an O‐GlcNAcylation‐dependent manner in macrophages. (b) Inducible nitric oxide synthase (iNOS) expression in macrophages in response to bacterial infections is controlled by O‐GlcNAcylation, as are interleukin‐10 (IL‐10) secretion, necroptosis and inflammatory cytokine secretion. Neutrophil chemotaxis is influenced by O‐GlcNAcylation. (c) T helper type 17 (Th17) ‐specific gene expression relies on O‐GlcNAcylation. (d) Mast cells require O‐GlcNAcylation for cytokine production, differentiation and proliferation in parasitic infections. Moreover, tuft cells aid in worm expulsion in an O‐GlcNAcylation‐dependent manner.
Natural killer (NK) cells play an important role in defense against viral infections by recognizing and killing virus‐infected cells (Fig. 2a). Although no studies have directly interrogated the role of O‐GlcNAcylation in NK cell function, there is some indirect evidence. Natural killer group 2D (NKG2D) is one receptor through which NK cells recognize and subsequently kill virus‐infected cells expressing stress‐induced peptide–major histocompatibility complex class I complexes. 17 Expression of NKG2D and NKG2D‐mediated NK cell cytotoxicity against cancer cell lines partially relies on the transcription factor enhancer of zeste homolog 2 (EZH2), 18 which in human breast and colorectal cancer cells has been shown to depend on O‐GlcNAcylation at multiple sites for its stability. 19 , 20 , 21 This makes it conceivable that NK cells require O‐GlcNAcylated EZH2 for NKG2D‐mediated cytotoxicity against virus‐infected cells. Another transcription factor through which O‐GlcNAcylation may regulate NK cell function is c‐Myc. NK cells require c‐Myc for granzyme B expression and cytotoxic activity against virus‐infected cells. 22 c‐Myc is a known OGT target; O‐GlcNAcylation at threonine 58 is required in various cancer cells, 23 , 24 as well as B cells 25 to prevent cMyc from being targeted for degradation. In keeping with a key role for O‐GlcNAcylation in preventing cMyc clearance, lowering of UDP‐GlcNAc levels due to depletion of glutamine resulted in rapid reduction of cMyc levels in NK cells. 22 Further studies are required to fully delineate the role of O‐GlcNAcylation of EZH2 and cMyc in regulating NK cell function in general, and in the context of viral infection in particular.
Following innate responses to viral infection, adaptive immune responses mediated by B and T cells are activated (Fig. 3). B‐cell activation occurs through the B‐cell receptor (BCR), which involves signaling by Src‐family kinase member Lyn. B‐cell activation and expansion depends on O‐GlcNAcylation of Lyn at serine 19 and its subsequent interactions with Syk. 26 BCR‐mediated B‐cell activation also causes O‐GlcNAcylation of the transcription factors nuclear factor‐κB (NF‐κB) and nuclear factor of activated T cells, 27 resulting in their activation and accompanying CD69 expression. Furthermore, upon BCR cross‐linking, apoptosis programs are triggered to prevent survival of autoreactive B cells; whether these programs are ultimately executed depends on the balance between apoptotic and pro‐survival signals downstream of BCR signaling. 28 Key in the regulation of these signals is lymphocyte‐specific protein 1 (Lsp‐1), which requires O‐GlcNAcylation at S209 for phosphorylation of S243, which enables Lsp‐1 binding to F‐actin. This binding subsequently results in apoptosis as a consequence of reduced Bcl‐2 and Bcl‐XL expression. 29 Hence, O‐GlcNAcylation is also involved in apoptosis of activated B cells. Lastly, the O‐GlcNAcylation of EZH2 may also be of importance in humoral immunity against viral infections. V(D)J recombination, central in the generation of antibodies with unique variable chains, is impaired if EZH2‐driven methylation is hindered, an activity that – as alluded to before – requires O‐GlcNAcylation. 30 Consequently, antibody production in mice was greatly reduced in response to IAV infection when EZH2 was knocked down in B cells. 31 Hence, O‐GlcNAcylation‐based control of EZH2 function may impact antibody production of B cells. Consistent with this idea is that loss of OGT in murine B cells resulted in reduced general antibody levels as a result of decreased survival of germinal center B cells. 26 However, this study did not address how O‐GlcNAcylation controls antibody levels, but it is tempting to speculate that EZH2 is involved.
Figure 3.
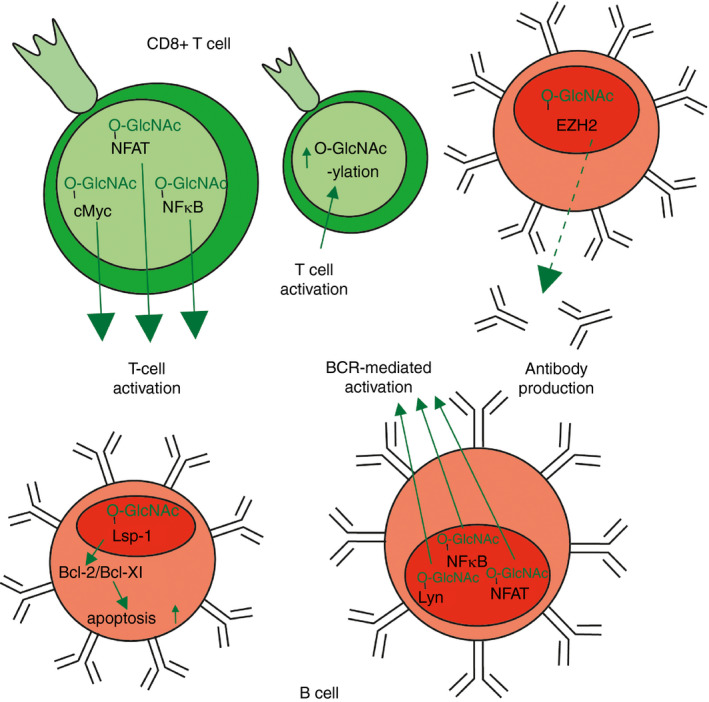
Overview of T‐cell and B‐cell responses that are influenced by O‐GlcNAcylation. Red O‐GlcNAc or arrows indicate a negative or inhibitory effect, green O‐GlcNAc or arrows indicate a positive or inducing effect. Dotted arrows present a possible, but not yet established, effect. B‐cell receptor (BCR) ‐mediated activation, antibody production and apoptosis to remove autoreactive B cells are B‐cell processes that depend on O‐GlcNAcylation. Activation of CD8+ T cells is also affected by O‐GlcNAcylation.
The other main cell type in the adaptive arm of the immune response against viral infections is the CD8+ cytotoxic T lymphocyte (CTL), which can recognize and kill virus‐infected cells (Fig. 3). The role of O‐GlcNAcylation specifically in anti‐viral CTL responses remains to be elucidated. However, several studies have interrogated the role of O‐GlcNAcylation in T‐cell receptor (TCR) ‐mediated CD8+ T‐cell activation following polyclonal stimulation. Descriptive studies using O‐GlcNAc‐targeted proteomics of TCR‐stimulated T cells have identified around 300 proteins that were O‐GlcNAcylated. 32 , 33 , 34 One study specifically identified 227 proteins that had increased or decreased their O‐GlcNAcylation in response to TCR stimulation. 32 Functionally, OGT down‐regulation by short interfering RNA impaired interleukin‐2 (IL‐2) and CD69 expression by T cells, suggesting an important role for enhanced O‐GlcNAcylation in CD8+ T‐cell activation. A possible mechanistic basis for these findings comes from studies that have shown that NF‐κB and c‐Myc, transcription factors that are key to CD8+ T‐cell activation, are O‐GlcNAcylated upon TCR‐mediated T‐cell stimulation. 27 Specifically, c‐Rel, a subunit of NF‐κB, was O‐GlcNAcylated at serine 350. 35 This site is located in the inhibitory domain and an S350A mutant showed greatly diminished transcriptional activity because of reduced DNA‐binding ability. As a result, IL‐2 secretion was impaired, as was the transcription of other TCR‐induced genes. Another NF‐κB subunit is RelA (p65) of which the O‐GlcNAcylation status following TCR stimulation is still disputed. Several non‐T‐cell studies have found O‐GlcNAcylation sites on p65. 36 , 37 , 38 However, one study with a T‐cell line suggested that c‐Rel in complex with p65 is often O‐GlcNAcylated, but not p65 itself, 35 but another study did report p65 to be O‐GlcNAcylated. 27 In addition, transcription factor c‐Myc, which is induced by IL‐2 to sustain activated T‐cell proliferation, was found to require to be O‐GlcNAcylated at T58 to prevent its phosphorylation and subsequent degradation. Correspondingly, c‐Myc levels were decreased in activated CTL when OGT was inhibited. 39 Hence, O‐GlcNAcylation controls factors both upstream and downstream of IL‐2 signaling in activated T cells to support activation and proliferation. However, these insights were primarily gained from in vitro T‐cell activation studies, so their relevance for regulation of CTL responses to viral infections in vivo remains to be determined.
O‐GlcNAcylation in bacterial infections
Neutrophils are among the first leukocytes to migrate to a site of a bacterial infection where they phagocytose bacteria, release reactive oxygen species and undergo necrosis, leading to bacterial entrapment and destruction (Fig. 2b). The chemoattractant formyl‐methionyl‐leucyl‐phenylalanine (fMLP), produced by for example Escherichia coli, is commonly used as an in vitro proxy for studying neutrophil responses to a bacterial infection. 40 In vitro fMLP‐mediated chemotaxis of neutrophils was previously shown to be enhanced by augmented O‐GlcNAcylation through glucosamine (GlcN) treatment. 41 , 42 GlcN supplements the HBP by bypassing the rate‐limiting enzyme glutamine‐fructose‐6‐phosphate amidotransferase (GFAT1) and results in increased synthesis of UDP‐GlcNAc to promote O‐GlcNAcylation (Fig. 1). Mechanistically, an overall increase of O‐GlcNAcylation levels in neutrophils through exposure to GlcN or treatment with the OGA inhibitor PUGNAC appeared to promote Rac‐ and phosphoinositide 3‐kinase‐dependent chemotaxis. 41 , 42 This effect might stem from enhanced O‐GlcNAcylation of targets downstream of Rac signaling in fMLP‐induced neutrophils. Rac is responsible for the phosphorylation of mitogen‐activated protein kinase p38, 43 which in turn activates mitogen‐activated protein kinase‐activated protein kinase 2, which phosphorylates serine 243 on Lsp‐1. 44 Lsp‐1 was recently shown to co‐localize with F‐actin in murine neutrophils, following phosphorylation at serine 243, driving fMLP‐mediated chemotaxis. 45 It is not known if Lsp‐1 in neutrophils is influenced by O‐GlcNAcylation, but this protein, as alluded to before, requires O‐GlcNAcylation at serine 209 for serine 243 phosphorylation and subsequent F‐actin interactions in murine B cells. 29 A more extensive analysis of overall O‐GlcNAcylation levels in human neutrophils by flow cytometry and fluorescence microscopy also showed increased O‐GlcNAcylation following fMLP stimulation when compared with unstimulated controls. 46 Although these results indicate that neutrophil chemotaxis is positively dependent on O‐GlcNAcylation, there also is a study which found an inhibitory effect of GlcN treatment on human neutrophil chemotaxis and fMLP‐driven F‐actin interactions. 47 This discrepancy could be explained by the 10‐fold lower concentration of GlcN used in this study, 47 when compared with the aforementioned studies. Furthermore, in this latter work neutrophil phagocytosis, enzymatic release, superoxide anion production and p38/mitogen‐activated protein kinase phosphorylation were impaired after GlcN but not after GlcNAc treatment. 47 The authors hypothesized that GlcN blocks receptor interactions, which caused the inhibitory effects on neutrophil effector functions. Although GlcNAc was thought to exert a similar influence, this effect was not observed, which was attributed to GlcNAc concentrations being too low. These contradictory results illustrate the need for further research to better define the role of O‐GlcNAcylation in chemotaxis and effector function of neutrophils.
Macrophages also play key roles in containment of bacterial infections by their ability to phagocytose and kill bacteria, the latter primarily by means of nitric oxide (NO) production by inducible NO synthase (iNOS). A first hint for a role of O‐GlcNAcylation in regulating macrophage responses against bacteria comes from the observation that stimulation of murine macrophages with toll‐like receptor 4 (TLR4) ligand lipopolysaccharide (LPS) leads to a ∼60% increase in the molar abundance of the total UDP‐GlcNAc pool. 48 Indeed, several functional studies have revealed that O‐GlcNAcylation in macrophages in response to bacterial products affects both their pro‐ and anti‐inflammatory responses (Fig. 2b). A recent study found that receptor‐interacting serine/threonine‐protein kinase 3 (RIPK3) is O‐GlcNAcylated at threonine 467 in macrophages in response to bacterial TLR2, TLR4 or TLR9 agonists. 49 Together with RIPK1, RIPK3 forms the necrosome complex that induces necroptosis, a form of programmed necrosis in infected cells. 50 Moreover, RIPK3 promotes inflammatory cytokine expression. O‐GlcNAcylation of RIPK3 was found to suppress its kinase activity and its interactions with RIPK1. 49 This prevented induction of necroptosis as well as pro‐inflammatory cytokine expression, resulting in impaired anti‐bacterial responses. Likewise, several studies indicate a suppressive role for O‐GlcNAcylation in regulation of iNOS expression and NO production by macrophages. iNOS expression is induced by NF‐κB activation in macrophages following stimulation with LPS. 51 NF‐κB subunits c‐Rel, p50 and p65 are known to bind to the nitric oxide synthase 2 promotor, which encodes iNOS. 52 Various studies have shown that GlcN treatment suppresses iNOS expression in LPS‐treated macrophages, although O‐GlcNAcylation increases were not evaluated. 53 , 54 Furthermore, both macrophages as well as microglia (macrophages of the brain) treated with GlcN displayed attenuated LPS‐mediated p50 and c‐Rel DNA‐binding. 55 Likewise, LPS treatment of OGT‐overexpressing macrophages resulted in inhibition of NF‐κB activity. 56 Mechanistically, using a Gal4‐dependent reporter assay it was demonstrated that this inhibition was due to O‐GlcNAcylation of p65 and c‐Rel resulting in reduced transcriptional activation. Conversely, OGA inhibition in LPS‐stimulated macrophage‐like RAW264.7 cells resulted in increased iNOS/NO expression. 51 These results together suggest that O‐GlcNAcylation limits LPS‐induced iNOS expression by impairing NF‐κB activity. However, there are also studies showing that LPS stimulation directly enhanced O‐GlcNAcylation of c‐Rel, which led to increased transcriptional activation through p50 interactions. 55 Concordantly, knockdown of OGT in these studies reduced the association of c‐Rel with p50. It is currently unclear what lies behind these discrepancies. However, some of these observations might be explained by the effects that O‐GlcNAcylation has on other regulators of iNOS expression. For instance, iNOS/NO expression is also regulated by the transcriptional repressor mammalian Sin3A (mSin3A). LPS treatment of macrophages resulted in reduced O‐GlcNAcylation of mSin3A, which in turn allowed for its binding to the nitric oxide synthase 2 promotor and to repress transcriptional activity. 56 Hence, contrary to NF‐κB, reduction of O‐GlcNAcylation of mSin3A is associated with repression of iNOS expression. Clearly, O‐GlcNAcylation has both inhibitory and activating effects on iNOS/NO expression depending on the transcription factor or repressor that is targeted, and illustrates the complex role of O‐GlcNAcylation in controlling iNOS expression and NO production by macrophages.
Protein O‐GlcNAcylation in macrophages shapes not only their pro‐inflammatory responses to bacterial exposure, but also their anti‐inflammatory responses, as illustrated by the importance of O‐GlcNAcylation in expression of the anti‐inflammatory cytokine IL‐10. Treatment of BMDM with LPS was found to induce O‐GlcNAcylation of signal transducer and activator of transcription 3 (STAT3) at threonine 717, which inhibited its phosphorylation at tyrosine 705. 57 As a result, STAT3 could not bind to the IL‐10 promotor to induce its expression. In addition to TLR4 stimulation, bacterial TLR2 and TLR9 stimulation of macrophages deficient for Cullin3, a protein that is known to suppress OGT expression, also resulted in lower IL‐10 expression when compared with wild‐type macrophages. Taken together, this suggests a largely suppressive role for O‐GlcNAcylation in macrophage activation, both pro‐inflammatory and anti‐inflammatory, in response to bacterial exposure. 57
Cells that play a key role in the adaptive arm of the immune response against bacterial infections are B cells, T helper 1 (Th1) cells, and particularly in the case of intracellular bacteria, CTL. 1 Although there are few data on the role of O‐GlcNAcylation in the regulation of B‐cell responses, other than a similar role in antibody production as described in viral infections, some have looked into O‐GlcNAcylation in T cells during bacterial infections (Fig. 3). Mice infected with the obligate intracellular bacterium Listeria monocytogenes displayed increased O‐GlcNAcylation in their CD8+ T cells, as determined by flow cytometry as well as chemo‐enzymatic glycan labeling. 34 , 39 In general, both CTL and Th1 cells were noted to increase their glucose and glutamine uptake upon TCR triggering by anti‐CD3/CD28 antibodies, which would be consistent with enhanced fueling of the HBP to support O‐GlcNAcylation. 39 The functional relevance of increased O‐GlcNAcylation in Th1 cell or CTL biology in bacterial infections remains to be determined, but based on the importance of proper O‐GlcNAcylation in polyclonal TCR‐mediated T‐cell activation, as discussed before in the section on viral infections, it is to be expected that O‐GlcNAcylation is critical in supporting Th1 cell or CTL responses in bacterial infections.
O‐GlcNAcylation in fungal infections
Fungal infections generally elicit type 17 immune responses in which neutrophils, macrophages, type 3 innate lymphoid cells (ILC3) and Th17 cells play important roles. 58 Information on the role of O‐GlcNAcylation in shaping Th17 immune responses following fungal infections is still scant. However, there are some indications that Th17 responses in other non‐infectious diseases are regulated by O‐GlcNAcylation (Fig. 2c). For example, Th17 differentiation is inhibited in vitro and in vivo in multiple sclerosis models by miR‐15b. 59 miR‐15b inhibits OGT, resulting in decreased p65 and c‐Rel O‐GlcNAcylation and subsequent expression of several genes including retinoic acid‐related orphan receptor γt (RORγT), a master regulator of Th17 differentiation. Further support for the importance of O‐GlcNAcylation in Th17 responses comes from observations with CD4+ T cells from obese mice as well as those of human origin. Treatment with the OGA inhibitor ThiametG increased overall O‐GlcNAcylation levels as evaluated by immunoblot, which coincided with expression of IL‐17A, a canonical Th17 cytokine. 60 Again, RORγT was involved, although now activating ligands in the form of fatty acids were noted to be increased upon treatment with ThiametG. Hence, O‐GlcNAcylation appears to be required for Th17 differentiation by regulating RORγT, although it remains to be determined whether a similar dependency can be observed in fungal infections.
O‐GlcNAcylation in parasitic infections
Infections with multicellular parasites classically promote a type 2 immune response of the host. This response is characterized by the production of type 2 cytokines, such as IL‐4, IL‐5 and IL‐13 by type 2 innate lymphoid cells (ILC2) and Th2 cells, which collectively promote recruitment and activation of effector cells such as eosinophils, mast cells and alternatively activated macrophages as well as intestinal mucus production and peristalsis, to eradicate the worms and enhance tissue repair. 1 There is currently a clear paucity in our understanding of the role of O‐GlcNAcylation in the regulation of immune cells involved in type 2 immune responses. Nonetheless, there are indications for a role of O‐GlcNAcylation in anti‐helminth type 2 immunity, through control of tuft cells (Fig. 2d). Tuft cells are specialized cells that line the intestinal mucosa and aid in the expulsion of parasites through activation of ILC2 and Th2 responses. 61 STAT6 is essential for tuft cell development and it was found that O‐GlcNAcylation of STAT6 is a prerequisite for its phosphorylation and initiation of its downstream signaling. 62 , 63 Correspondingly, OGT expression was found to be crucial for intestinal tuft cell development and OGT‐deficient mice displayed impaired type 2 immune responses against infections with the intestinal nematodes Heligomosomoides polygyrus and Nippostrongylus brasiliensis. 63 The fact that STAT6 is also essential for macrophages to become alternatively activated in response to IL‐4 stimulation may also point towards a role for O‐GlcNAcylation in the regulation of anti‐helminth responses by immune cells, such as by macrophages. In keeping with this notion would be the finding that IL‐4 treatment leads to the accumulation of intracellular UDP‐GlcNAc in macrophages. 48 , 64 Clearly, this warrants specific studies interrogating the functional role of O‐GlcNAcylation in shaping macrophage as well as other immune cell responses against helminth infections. 65
Furthermore, one study has directly linked mast cell function to O‐GlcNAcylation in an infection with the protozoan parasite Trichomonas vaginalis (Fig. 2d). 66 Contrary to helminths, T. vaginalis is a unicellular parasite that induces IL‐8 secretion to initiate an immune response involving neutrophil‐ and mast cell‐mediated killing. 67 Leukotriene B4 (LTB4), a component of T. vaginalis‐derived secretory products, interacts with mast cell LTB4 receptor 1 (BLT1), resulting in an increase in overall O‐GlcNAcylation of proteins as determined by immunoblot. 66 Pharmacological inhibition or genetic silencing of OGT reduced mast cell migration, and mast cell‐induced IL‐8 production and exocytotic degranulation, in a BLT1‐dependent manner. LTB4 is more commonly known as a product of macrophages and is known to induce chemotaxis of neutrophils and mobilize leukocytes through interaction with BLT1. 68 , 69 Therefore, the role of O‐GlcNAcylation in the LTB4/BLT1 context is likely to extend beyond its role in this specific parasitic infection. Apart from this direct evidence for involvement in mast cell responses, O‐GlcNAcylation may also regulate mast cells at other levels. Mast cells rely on ten‐eleven‐translocation 2 (TET2) for correct differentiation and proliferation as well as cytokine production in response to IgE stimulation. 70 Several studies have demonstrated an interplay between TET2 and OGT enzymatic activity, although not specifically in mast cells (Fig. 2d). 71 , 72 A preliminary analysis of the O‐GlcNAcylation pattern in TET2‐deficient mast cells did not show gross alterations. Nonetheless, more detailed studies will be needed to delineate the molecular mechanisms, involving TET2 and beyond, through which O‐GlcNAcylation regulates mast cell responses to parasitic infections. 70 Moreover, further exploration of the O‐GlcNAcylation requirements in parasite infection is imperative, such as its role in eosinophils, basophils and Th2 cells.
Perspectives and outlook
There is an increasing appreciation that O‐GlcNAcylation is a process with key regulatory effects on immune responses against infections. So far, the importance for O‐GlcNAcylation in immune cell activation and function is best characterized in macrophages, B cells and T cells. Studies on these cells reveal that O‐GlcNAcylation controls various aspects of cellular activation and effector function. Sometimes this involves responses irrespective of the type of infection, such as BCR‐mediated and TCR‐mediated activation. However, these responses may also be infection specific, as illustrated by studies with macrophages showing an important stimulatory role for O‐GlcNAcylation in anti‐viral responses, while having generally inhibitory effects when it comes to anti‐bacterial responses. Despite these interesting insights, it is also clear that information about the role of O‐GlcNAcylation in immune responses in fungal and parasitic infection is still scarce. The same holds true for specific immune cells such as dendritic cells, eosinophils, basophils, and different ILC and Th cells. This lack of knowledge illustrates that this is a still novel and developing field in which many questions remain to be addressed, some of which are discussed below.
Many studies demonstrate the importance of the process of O‐GlcNAcylation in the regulation of immune cell function, but the exact mechanisms by which O‐GlcNAcylation exerts its effect on immune cells are in most cases still unknown. To further the field, it will be crucial not only to identify the proteins that are O‐GlcNAcylated in a certain context, such as following activation, but also the exact O‐GlcNAcylation sites on those proteins, to be able to start delineating the consequences for protein function of certain O‐GlcNAcylated sites. In this context, it will be important to also elucidate the intricate interplay between O‐GlcNAcylation and other PTMs near to the O‐GlcNAc modified sites. Proteins for which the O‐GlcNAcylation sites have been identified have illustrated that specifically this interplay is an important mechanism through which O‐GlcNAcylation can mediate its biological effects. O‐GlcNAcylation can promote phosphorylation of proteins such as Lsp‐1 in B cells, which is required for its interactions with F‐actin. 29 Furthermore, O‐GlcNAcylation can target proteins for ubiquitination, such as the macrophage proteins MAVS and IRF5, which require ubiquitination for their downstream signaling. 14 , 15 , 16 However, this is likely only the tip of the iceberg. An analysis of the phospho‐proteome of murine splenic B cells that were activated in the presence or absence of the OGA inhibitor ThiametG revealed 313 phosphorylation sites on 224 phosphoproteins that required O‐GlcNAcylation for their phosphorylation to occur. 29 All sites were modified after B‐cell activation, so were likely important for this process. An in‐depth, site‐specific O‐GlcNAcylation analysis of proteins in activated T cells revealed 1045 proteins, of which 45% of O‐GlcNAcylation sites were within 10 amino acids of a phosphorylation site and 15% coincided completely. 32 Studies like these affirm the intricate relationship between O‐GlcNAcylation and other PTMs in infection. Further research into O‐GlcNAcylation sites will definitely deepen the understanding of interactions with other crucial PTMs such as phosphorylation and ubiquitination and will help in deciphering the mechanism by which O‐GlcNAcylation affects immune cell biology under homeostasis as well as during infection.
Given the importance of O‐GlcNAcylation in immune responses against infections, an interesting question is whether the host O‐GlcNAcylation machinery is used or even manipulated by certain pathogens for their own advantage. Indeed several studies have been hinting at this, mostly in viral infections. For example, studies with hepatitis C virus revealed an increased size of virions and increased ability to infect liver cells following OGT knockdown in these host cells. 73 These studies indicate an effect of O‐GlcNAcylation on pathogen virulence and morphology. Moreover, human T‐cell lymphotropic virus type 1 (HTLV‐1) requires O‐GlcNAcylation of the host transcription factor cAMP‐response element‐binding protein for transcription from its long terminal repeat. 74 Notably, HTLV‐1‐infected T cells express a splice variant of OGA with decreased activity, which ultimately leads to increased O‐GlcNAcylation levels. Hence, this virus specifically exploits host O‐GlcNAcylation machinery to enhance its virulence. Lastly, hepatitis B virus replication depends on host HBP, as GFAT1 inhibition led to a decrease in viral copy numbers in liver cells. 75 Not only viruses, but also some parasites exploit host O‐GlcNAcylation. Entamoeba histolytica is a protozoan parasite that evades immune responses by inducing host cell apoptosis through intracellular caspase activation. 76 Distinct features of E. histolytica‐induced apoptosis, DNA fragmentation and lactate dehydrogenase release, were reduced in HepG2 host cells following treatment with OGA inhibitors. Moreover, E. histolytica infection was shown to down‐regulate OGT protein levels. These studies highlight the complexity of studying the role of O‐GlcNAcylation in infections as both host and pathogen may depend on and utilize it for their own benefit,.
As we decipher the precise role of O‐GlcNAcylation in various processes in immune responses against infections, another question that arises is if and how to apply this knowledge to steer immune responses in a therapeutic context. Several animal models have illustrated the potential of harnessing O‐GlcNAcylation for controlling immune responses against infections. For example, rats suffering from LPS‐induced septic shock treated with the OGA inhibitor NButGT displayed reduced mortality. 77 Likewise, mice with sepsis caused by cecal ligation and puncture had increased survival when treated with GlcN. 51 In this study, LPS‐induced sepsis did not cause mortality, but GlcN administration did alleviate several inflammatory symptoms and markers. Mice with LPS‐induced systemic inflammatory response also displayed increased survival when treated with either ThiametG or GlcN. 78 These studies illustrate the potential of targeting O‐GlcNAcylation to dampen often life‐threatening overzealous immune reactions in sepsis. Moreover, in the light of the observations with macrophages showing opposing roles for O‐GlcNAcylation in responses against bacteria versus viruses, it will be very interesting to determine whether analogous differences in dependency on O‐GlcNAcylation for their function exist between distinct Th cell or ILC subsets. Potentially, this could provide a therapeutic window that could be exploited to steer immune responses/polarization by either boosting or interfering with O‐GlcNAcylation. Clearly, given the pleiotropic effects of O‐GlcNAcylation, careful monitoring of the consequences of systemically interfering with general O‐GlcNAcylation will be paramount, before targeting of O‐GlcNAcylation could be considered as a viable therapeutic option to treat infectious diseases such as sepsis.
Although many questions remain, there is a growing body of literature suggesting a key role for O‐GlcNAcylation in shaping immune responses during infections. Most studies have used in vitro models or murine models to explore this, and currently little is known about its importance for human infections. However, recent work, that has linked infection susceptibility to single nucleotide polymorphisms in OGT, 79 may provide a first indication that O‐GlcNAcylation is also a crucial regulator of human immune responses against infections. As illustrated by this review, O‐GlcNAcylation constitutes an exciting field, in which new discoveries are continuously made on the diverse ways in which O‐GlcNAcylation is involved in immunity against infectious diseases. This will be further fueled by recent advancements in proteomic and metabolomic techniques that are making detailed characterization of O‐GlcNAcylation patterns in immune cells more straightforward and will be important to further disentangle the complex role of O‐GlcNAcylation in regulation of the immune system.
Author contributions
MQ wrote the review; BE and CH critically reviewed the manuscript.
Disclosures
The authors declare no competing interests.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Metabolic mediators: how immunometabolism directs the immune response to infection. Immunology 2020, 161: 163‐164.
Circles of Life: linking metabolic and epigenetic cycles to immunity. Immunology 2020, 161: 165‐174.
The battle for iron in enteric infections. Immunology 2020, 161: 186‐199.
Implications of cellular metabolism for immune cell migration. Immunology 2020, 161: 200‐208.
Data availability statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 2010; 125(Suppl 2):S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016; 16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Qian K. Protein O‐GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol 2017; 18:452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol 2019; 15:346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O‐GlcNAc. J Cell Biol 2015; 208:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of Nuclear and Cytoplasmic proteins – purification and characterisation of a uridine diphospho‐n‐acetylglucosamine polypeptide B‐N‐acetylglucosaminyltransferase. J Biol Chem 1992; 267:9. [PubMed] [Google Scholar]
- 7. Wells L, Gao Y, Mahoney JA, Vosseller K, Chen CH, Rosen A, et al Dynamic O‐Glycosylation of Nuclear and Cytosolic Proteins – further characterisation of the nucleocytoplasmic B‐N‐acetylglucosaminidase, O‐GlcNAcase. J Biol Chem 2001; 277:8. [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Marchase RB, Chatham JC. Glutamine‐induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O‐GlcNAc levels. J Mol Cell Cardiol 2007; 42:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teo CF, Wollaston‐Hayden EE, Wells L. Hexosamine flux, the O‐GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol 2010; 318:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hart GW, Slawson C, Ramirez‐Correa G, Lagerlof O. Cross talk between O‐GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 2011; 80:825–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruan HB, Nie Y, Yang X. Regulation of protein degradation by O‐GlcNAcylation: crosstalk with ubiquitination. Mol Cell Proteomics 2013; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banerjee PS, Lagerlof O, Hart GW. Roles of O‐GlcNAc in chronic diseases of aging. Mol Aspects Med 2016; 51:1–15. [DOI] [PubMed] [Google Scholar]
- 13. Loo YM, Gale M Jr. Immune signaling by RIG‐I‐like receptors. Immunity 2011; 34:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li T, Li X, Attri KS, Liu C, Li L, Herring LE, et al O‐GlcNAc transferase links glucose metabolism to MAVS‐mediated antiviral innate immunity. Cell Host Microbe 2018; 24:791–803. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song N, Qi Q, Cao R, Qin B, Wang B, Wang Y, et al MAVS O‐GlcNAcylation is essential for host antiviral immunity against lethal RNA viruses. Cell Rep 2019; 28:2386–96. e5. [DOI] [PubMed] [Google Scholar]
- 16. Wang Q, Fang P, He R, Li M, Yu H, Zhou L, et al O‐GlcNAc transferase promotes influenza A virus‐induced cytokine storm by targeting interferon regulatory factor‐5. Sci Adv 2020; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002; 17:11. [DOI] [PubMed] [Google Scholar]
- 18. Yin J, Leavenworth JW, Li Y, Luo Q, Xie H, Liu X, et al Ezh2 regulates differentiation and function of natural killer cells through histone methyltransferase activity. Proc Natl Acad Sci USA 2015; 112:15988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu CS, Lo PW, Yeh YH, Hsu PH, Peng SH, Teng YC, et al O‐GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA 2014; 111:1355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo PW, Shie JJ, Chen CH, Wu CY, Hsu TL, Wong CH. O‐GlcNAcylation regulates the stability and enzymatic activity of the histone methyltransferase EZH2. Proc Natl Acad Sci USA 2018; 115:7302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang W, et al O‐GlcNAcylation promotes colorectal cancer metastasis via the miR‐101‐O‐GlcNAc/EZH2 regulatory feedback circuit. Oncogene 2019; 38:301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loftus RM, Assmann N, Kedia‐Mehta N, O'Brien KL, Garcia A, Gillespie C, et al Amino acid‐dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun 2018; 9:2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chou T, Hart GW, Dang CV. cMyc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 1995; 270:5. [DOI] [PubMed] [Google Scholar]
- 24. Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, et al O‐GlcNAc transferase integrates metabolic pathways to regulate the stability of c‐MYC in human prostate cancer cells. Cancer Res 2013; 73:5277–87. [DOI] [PubMed] [Google Scholar]
- 25. Lee DH, Kwon NE, Lee WJ, Lee MS, Kim DJ, Kim JH, et al Increased O‐GlcNAcylation of c‐Myc promotes pre‐B cell proliferation. Cells 2020; 9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu JL, Chiang MF, Hsu PH, Tsai DY, Hung KH, Wang YH, et al O‐GlcNAcylation is required for B cell homeostasis and antibody responses. Nat Commun 2017; 8:1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golks A, Tran TT, Goetschy JF, Guerini D. Requirement for O‐linked N‐acetylglucosaminyltransferase in lymphocytes activation. EMBO J 2007; 26:4368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J Immunol 2005; 174:1775–81. [DOI] [PubMed] [Google Scholar]
- 29. Wu JL, Wu HY, Tsai DY, Chiang MF, Chen YJ, Gao S, et al Temporal regulation of Lsp1 O‐GlcNAcylation and phosphorylation during apoptosis of activated B cells. Nat Commun 2016; 7:12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, et al Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol 2003; 4:124–31. [DOI] [PubMed] [Google Scholar]
- 31. Guo M, Price MJ, Patterson DG, Barwick BG, Haines RR, Kania AK, et al EZH2 represses the B cell transcriptional program and regulates antibody‐secreting cell metabolism and antibody production. J Immunol 2018; 200:1039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woo CM, Lund PJ, Huang AC, Davis MM, Bertozzi CR, Pitteri SJ. Mapping and quantification of over 2000 O‐linked glycopeptides in activated human T cells with isotope‐targeted glycoproteomics (Isotag). Mol Cell Proteomics 2018; 17:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lund PJ, Elias JE, Davis MM. Global analysis of O‐GlcNAc glycoproteins in activated human T cells. J Immunol 2016; 197:3086–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aguilar AL, Gao Y, Hou X, Lauvau G, Yates JR, Wu P. Profiling of protein O‐GlcNAcylation in murine CD8+ effector‐ and memory‐like T cells. ACS Chem Biol 2017; 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh‐Wilson LC, Baltimore D. Activation of the transcriptional function of the NF‐κB protein c‐Rel by O‐GlcNAc glycosylation. Sci Signal 2013; 6:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, et al O‐GlcNAc modification of NFκB p65 inhibits TNF‐α‐induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One 2011; 6:e24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang WH, Park SY, Nam HW, Kim DH, Kan JG, Kang ES, et al NFκB activation is associated with its O‐GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA 2008; 105:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma Z, Chalkley RJ, Vosseller K. Hyper‐O‐GlcNAcylation activates nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) signaling through interplay with phosphorylation and acetylation. J Biol Chem 2017; 292:9150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, et al Glucose and glutamine fuel protein O‐GlcNAcylation to control T cell self‐renewal and malignancy. Nat Immunol 2016; 17:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, Nairn R, et al Purification and identification of formyl‐methionyl‐leucyl‐phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli . J Biol Chem 1984; 259:10. [PubMed] [Google Scholar]
- 41. Kneass ZT, Marchase RB. Neutrophils exhibit rapid agonist‐induced increases in protein‐associated O‐GlcNAc. J Biol Chem 2004; 279:8. [DOI] [PubMed] [Google Scholar]
- 42. Kneass ZT, Marchase RB. Protein O‐GlcNAc modulates motility‐associated signaling intermediates in neutrophils. J Biol Chem 2005; 280:14579–85. [DOI] [PubMed] [Google Scholar]
- 43. Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, et al Deficiency of the hematopoietic cell‐specific rho family GTPase Rac2 is characterised by abnormalities in neutrophil function and host defense. Immunity 1999; 10:14. [DOI] [PubMed] [Google Scholar]
- 44. Huang CK, Zhan L, Ai Y, Jongstra J. LSP1 is the major substrate for mitogen‐activated protein kinase‐activated protein kinase 2 in human neutrophils. J Biol Chem 1997; 272:4. [DOI] [PubMed] [Google Scholar]
- 45. Wu Y, Zhan L, Ai Y, Hannigan M, Gaestel M, Huang CK, et al MAPKAPK2‐mediated LSP1 phosphorylation and FMLP‐induced neutrophil polarization. Biochem Biophys Res Commun 2007; 358:170–5. [DOI] [PubMed] [Google Scholar]
- 46. Madsen‐Bouterse SA, Xu Y, Petty HR, Romero R. Quantification of O‐GlcNAc protein modification in neutrophils by flow cytometry. Cytometry A 2008; 73:667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hua J, Sakamoto K, Nagaoka I. Inhibitory actions of glucosamine, a therapeutic agent for osteoarthritis, on the functions of neutrophils. J Leukoc Biol 2002; 71:9. [PubMed] [Google Scholar]
- 48. Puchalska P, Huang X, Martin SE, Han X, Patti GJ, Crawford PA. Isotope tracing untargeted metabolomics reveals macrophage polarization‐state‐specific metabolic coordination across intracellular compartments. iScience 2018; 9:298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X, Gong W, Wang H, Li T, Attri KS, Lewis RE, et al O‐GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor‐interacting serine/threonine‐protein kinase 3. Immunity 2019; 50:576–90. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 2017; 18:127–36. [DOI] [PubMed] [Google Scholar]
- 51. Hwang JS, Kim KH, Park J, Kim SM, Cho H, Lee Y, et al Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis‐induced lung injury and inflammation. J Biol Chem 2019; 294:608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie Q, Kashiwabara Y, Nathan C. Role of transcription factor NF‐κB/Rel in induction of nitric oxide synthase. J Biol Chem 1994; 269:4. [PubMed] [Google Scholar]
- 53. Meininger CJ, Kelly KA, Li H, Haynes TE, Wu G. Glucosamine inhibits inducible nitric oxide synthesis. Biochem Biophys Res Commun 2000; 279:234–9. [DOI] [PubMed] [Google Scholar]
- 54. Hwang SY, Hwang JS, Kim SY, Han IO. Glucosamine inhibits lipopolysaccharide‐stimulated inducible nitric oxide synthase induction by inhibiting expression of NF‐κB/Rel proteins at the mRNA and protein levels. Nitric Oxide 2013; 31:1–8. [DOI] [PubMed] [Google Scholar]
- 55. Hwang SY, Hwang JS, Kim SY, Han IO. O‐GlcNAcylation and p50/p105 binding of c‐Rel are dynamically regulated by LPS and glucosamine in BV2 microglia cells. Br J Pharmacol 2013; 169:1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hwang SY, Hwang JS, Kim SY, Han IO. O‐GlcNAc transferase inhibits LPS‐mediated expression of inducible nitric oxide synthase through an increased interaction with mSin3A in RAW264.7 cells. Am J Physiol Cell Physiol 2013; 305:C601–C608. [DOI] [PubMed] [Google Scholar]
- 57. Wen H, Singer JD, Asara JM, Herring LE, Swanson BJ, Lazenby AJ, et al Myeloid‐derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. J Exp Med 2017; 214:1093–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol 2005; 129:569–82. [DOI] [PubMed] [Google Scholar]
- 59. Liu R, Ma X, Chen L, Yang Y, Zeng Y, Gao J, et al MicroRNA‐15b suppresses Th17 differentiation and is associated with pathogenesis of multiple sclerosis by targeting O‐GlcNAc transferase. J Immunol 2017; 198:2626–39. [DOI] [PubMed] [Google Scholar]
- 60. Machacek M, Saunders H, Zhang Z, Tan EP, Li J, Li T, et al Elevated O‐GlcNAcylation enhances pro‐inflammatory Th17 function by altering the intracellular lipid microenvironment. J Biol Chem 2019; 294:8973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016; 529:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gewinner C, Hart G, Zachara N, Cole R, Beisenherz‐Huss C, Groner B. The coactivator of transcription CREB‐binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem 2004; 279:3563–72. [DOI] [PubMed] [Google Scholar]
- 63. Zhao M, Ren K, Ruan HB. Intestinal epithelial O‐GlcNAc signaling is indispensable for anti‐helminth type 2 immunity. FASEB J 2019; 33:1.30593122 [Google Scholar]
- 64. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015; 42:419–30. [DOI] [PubMed] [Google Scholar]
- 65. Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, et al Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun 2019; 10:4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Min A, Lee YA, Kim KA, Shin MH. BLT1‐mediated O‐GlcNAcylation is required for NOX2‐dependent migration, exocytotic degranulation and IL‐8 release of human mast cell induced by Trichomonas vaginalis‐secreted LTB4. Microbes Infect 2018; 20:376–84. [DOI] [PubMed] [Google Scholar]
- 67. Mercer F, Johnson PJ. Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 2018; 34:683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol 1998; 30:6. [DOI] [PubMed] [Google Scholar]
- 69. Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat 2002; 68–69:11. [DOI] [PubMed] [Google Scholar]
- 70. Montagner S, Leoni C, Emming S, Della Chiara G, Balestrieri C, Barozzi I, et al TET2 regulates mast cell differentiation and proliferation through catalytic and non‐catalytic activities. Cell Rep 2016; 15:1566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O‐GlcNAcylation during gene transcription. Nature 2013; 493:561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, et al TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J 2013; 32:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herzog K, Bandiera S, Pernot S, Fauvelle C, Juhling F, Weiss A, et al Functional microRNA screen uncovers O‐linked N‐acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut 2020; 69:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Groussaud D, Khair M, Tollenaere AI, Waast L, Kuo MS, Mangeney M, et al Hijacking of the O‐GlcNAcZYME complex by the HTLV‐1 Tax oncoprotein facilitates viral transcription. PLoS Pathog 2017; 13:e1006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li H, Zhu W, Zhang L, Lei H, Wu X, Guo L, et al The metabolic responses to hepatitis B virus infection shed new light on pathogenesis and targets for treatment. Sci Rep 2015; 5:8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee YA, Min A, Shin MH. O‐deGlcNAcylation is required for Entamoeba histolytica‐induced HepG2 cell death. Microb Pathog 2018; 123:285–95. [DOI] [PubMed] [Google Scholar]
- 77. Ferron M, Cadiet J, Persello A, Prat V, Denis M, Erraud A, et al O‐GlcNAc stimulation: a new metabolic approach to treat septic shock. Sci Rep 2019; 9:18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Silva JF, Olivon VC, Mestriner F, Zanotto CZ, Ferreira RG, Ferreira NS, et al Acute increase in O‐GlcNAc improves survival in mice with LPS‐induced systemic inflammatory response syndrome. Front Physiol 2019; 10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Selvan N, George S, Serajee FJ, Shaw M, Hobson L, Kalscheuer V, et al O‐GlcNAc transferase missense mutations linked to X‐linked intellectual disability deregulate genes involved in cell fate determination and signaling. J Biol Chem 2018; 293:10810–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.


