Abstract
Visualizing and perturbing neural activity on a brain-wide scale in model animals and humans is a major goal of neuroscience technology development. Established electrical and optical techniques typically break down at this scale due to inherent physical limitations. In contrast, ultrasound readily permeates the brain, and in some cases the skull, and interacts with tissue with a fundamental resolution on the order of 100 μm and 1 ms. This basic ability has motivated major efforts to harness ultrasound as a modality for large-scale brain imaging and modulation. These efforts have resulted in already-useful neuroscience tools, including high-resolution hemodynamic functional imaging, focused ultrasound neuromodulation and local drug delivery. Furthermore, recent breakthroughs promise to connect ultrasound to neurons at the genetic level for biomolecular imaging and sonogenetic control. In this article, we review the state of the art and ongoing developments in ultrasonic neurotechnology, building from fundamental principles to current utility, open questions and future potential.
Keywords: ultrasound, focused ultrasound, neuroscience, functional imaging, molecular imaging, neuromodulation, sonogenetics
In Brief
The physics of ultrasound provides non-invasive access to the intact brain and the potential for large-scale imaging and control of neural activity. This article reviews the current state of ultrasound applications in neuroscience, building from fundamental principles to established techniques for functional imaging and neuromodulation, and highlighting ongoing technology development to connect ultrasound to neural activity at the molecular level.
Introduction: the fortuitous physics of ultrasound
Breakthroughs in neuroscience can often be traced to new experimental methods. Diverse techniques ranging from electrophysiology and histology to optical imaging, magnetic resonance, optogenetics and chemogenetics have provided new ways to study the structure and function of neural circuits. However, the established neuroscience toolkit does not yet satisfy a critical experimental need: the ability to observe and perturb neural circuit dynamics on a brain-wide scale in behaving mammals. Moreover, most neuroscience techniques are impossible to apply in humans without unacceptable invasiveness.
These limitations arise primarily from the physics connecting the forms of energy underlying each method with biological materials (Marblestone et al., 2013; Piraner et al., 2017a). Electrical recording of spiking neurons requires probes to be located within approximately 200 μm of the cell, which, together with finite probe size, limits the density and coverage of in vivo electrophysiology (Marblestone et al., 2013). Visible light is typically scattered within 1 mm of tissue, making it difficult to precisely resolve or target optical signals beyond this depth (Ntziachristos, 2010). The weak polarization of nuclear spins limits the signal and thereby the spatial resolution of functional magnetic resonance imaging (fMRI). The difficulty of focusing and localizing electromagnetic fields at depth hinders the precision of electrical or magnetic stimulation and source identification from outside the brain. Radioactive probes are limited by their pharmacokinetics and the spatial delocalization of their emissions. Chemogenetic tools typically require invasive delivery to operate at anatomically defined sites.
In contrast, ultrasound (US) is a form of energy that easily penetrates soft tissues at wavelengths on the order of 100 μm (Maresca et al., 2018a). This allows ultrasound-based methods to generate images or deliver focused energy several cm into tissue with spatial precision corresponding to this wavelength. Moreover, the speed of sound wave propagation – approximately 1.5 km/s in soft tissue – allows ultrasound to operate with temporal precision below 1 ms. These fortuitous properties have made ultrasound imaging one of the most widely used technologies in clinical medicine, facilitated by its relatively low cost, high portability and safety. At the same time, as the only form of non-ionizing energy that can be focused in deep tissues, focused ultrasound (FUS) has become a rapidly-growing modality for non-invasive therapy, used in the ablative treatment of cancer and neurological dysfunction , facilitated by advances in US hardware and image guidance (Escoffre and Bouakaz, 2016).
In the last 10 years, ultrasound has burst onto the scene of neuroscience research as the basis for several breakthrough tools, ranging from high-resolution hemodynamic and molecular imaging to non-invasive neuromodulation. This seemingly sudden emergence is, in fact, underpinned by decades of progress in basic ultrasound technology, which continue to create new possibilities as the first wave of neuroscience applications becomes established.
In this review, we spotlight the ultrasound-based techniques available for neuroscience research, describing their principles, applications, future potential and pitfalls. We start with ultrasound imaging, focusing on the already-useful hemodynamic functional ultrasound imaging (fUSI) and the emerging capabilities of biomolecular ultrasound. Next, we cover several ways in which FUS can perturb neural activity, including direct FUS neuromodulation, FUS-based drug and gene delivery and emerging “sonogenetic” control. Finally, we mention how ultrasound can be used to power and communicate with inorganic devices. Operating in diverse acoustic regimes (Fig. 1), each of these methods allows ultrasound to probe neural function through unique biophysical interactions. Our goal in this article is to introduce neuroscientists to these regimes and the imaging and perturbation methods they enable, and answer the question we often encounter from our neuroscience colleagues: “what can these technologies do for me?”.
Figure 1 -. Acoustic Regimes.
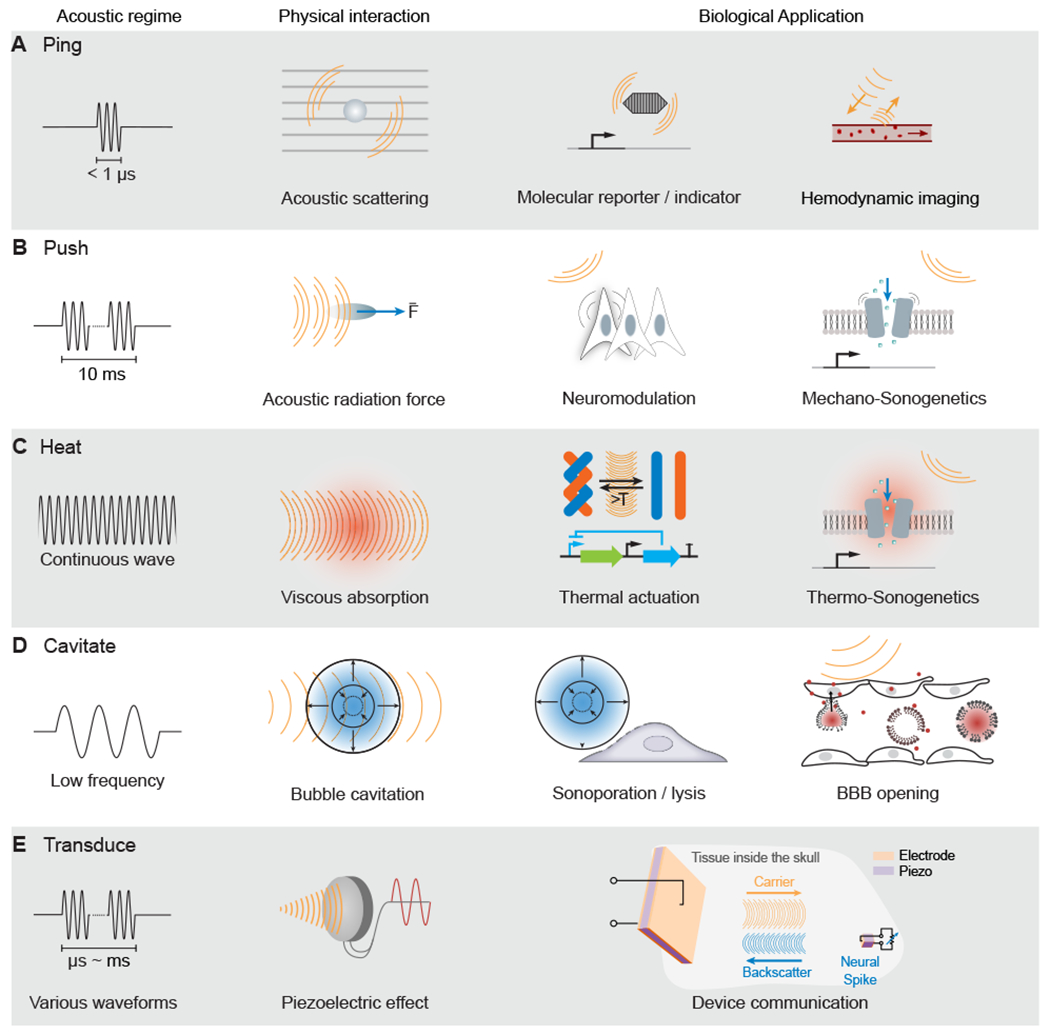
(A) Ultrasound imaging involves the emission of brief pulses of sound and recording of backscattered echoes from materials such as molecular reporters and blood cells.
(B) Sustained ultrasound application on the scale of milliseconds can generate mechanical forces, leading to cellular or molecular actuation.
(C) Extended ultrasound can deposit thermal energy in tissues, which can activate temperature-dependent molecular function.
(D) Ultrasound at lower frequencies can interact with bubbles to produce cavitation, leading to mechanical effects, such as blood-brain barrier (BBB) opening (A–D adapted from Maresca et al., 2018a).
(E) Ultrasound can communicate wirelessly with inorganic materials, such as millimeter-scale piezoelectric neural sensors (adapted from Seo et al., 2016).
Ultrasonic neuroimaging
Ultrasound (US) imaging is a pulse-echo technique involving the transmission of brief pulses (“pings” in Fig. 1a) of ultrasound into tissue and the recording of backscattered echoes from objects and interfaces within the tissue. The relative timing of the transmitted pulses and received signals, transduced by arrays of piezoelectric elements, is used to locate objects in space and form an image. Scattering arises from materials with different density and/or compressibility relative to their surrounding medium, including tissue interfaces, blood cells and contrast agents (Maresca et al., 2018a). In soft tissue, the frequencies typically used in ultrasound imaging (3-25 MHz) correspond to wavelengths of 500-60 μm and penetration depths of 10-1 cm, respectively. Penetration depth is determined by a combination of scattering and viscous energy dissipation. Acquiring a single pulse-echo image can take as little as tens of μs. This performance provides favorable trade-offs relative to electrical, optical and magnetic resonance techniques (Fig.2a). Although US is strongly attenuated and refracted by bone, imaging is possible through intact skull in mice. In other species, ultrasound imaging of the brain is typically performed through defined acoustic windows. Two recent fundamental advances have created the potential for ultrasound to play a major role in neuroscience. The first, already materialized, is the use of ultrafast ultrasound to visualize hemodynamic correlates of neural activity in behaving animals with brain-wide coverage and unprecedented spatiotemporal resolution. The second, still in development, is the use of genetically encodable acoustic reporter genes and biosensors to directly monitor the function of genetically defined cells.
Figure 2 -. Functional Ultrasound Imaging.
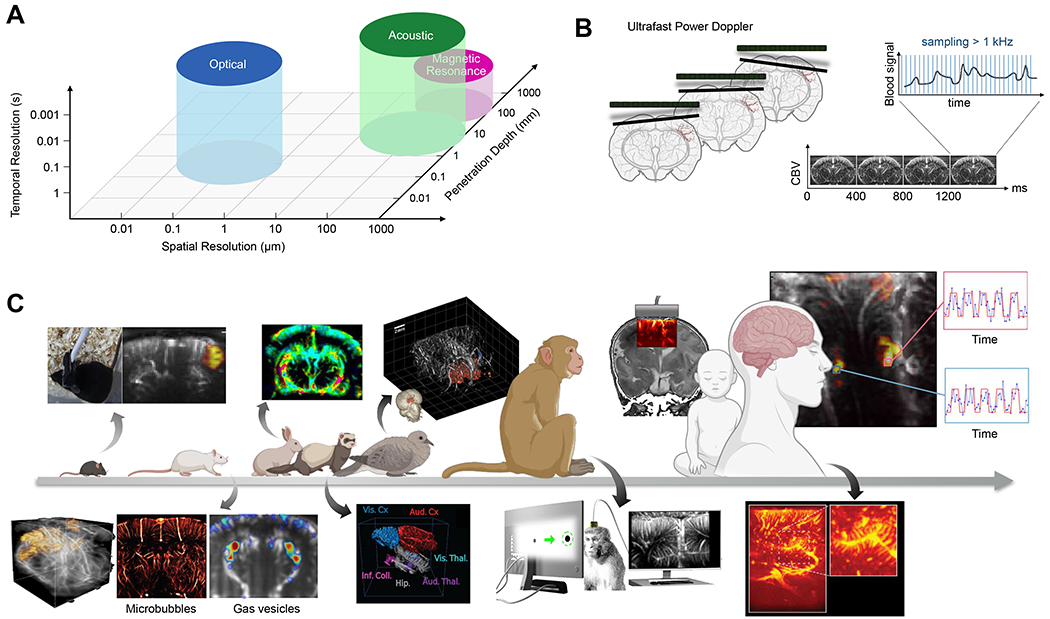
(A) Approximate performance characteristics of common brain-imaging techniques. (B) The transmission of ultrasound plane wave in the brain allows a fast temporal sampling of the Doppler signal for highly sensitive measures of CBV variations (adapted from Deffieux et al., 2018). (C) Functional ultrasound imaging has been applied in many animal models and humans. Above the arrow: from left to right: functional imaging in awake mice (Tiran et al., 2017), dynamic changes in cerebral blood flow in rabbits undergoing cardiac arrest (Demené et al., 2018), 3D functional imaging of a pigeon (Rau et al., 2018), monitoring of cerebral activity through the ultrasound permeable anterior fontanel window in newborns (Demene et al., 2017), and intraoperative functional mapping to maximize tumor removal while preserving functional brain areas (Imbault et al., 2017) are shown. Below the arrow: from left to right: 3D functional imaging of rats using a matrix array probe (Rabut et at., 2019), ultrafast ultrasound localization microscopy allows sub-wavelength structural imaging of cerebral microvessels (Errico et al., 2015), enhancement of hemodynamic signal using gas vesicles (Maresca et al., 2020), 3D tonotopic mapping of the auditory pathway of awake ferrets (Bimbard et al.), detection of functional activation in awake monkeys during visual tasks (Dizeux et at, 2019), and rich vascular characteristics found in pre-resection tumors during intraoperative fUSI acquisition (Soloukey et al., 2020) are shown.
Hemodynamic functional ultrasound imaging (fUSI)
Hemodynamic functional ultrasound imaging (fUSI) visualizes neural activity by mapping local changes in blood flow. The ability of ultrasound to quantify this flow is classically based on the Doppler effect – the shift in frequency of an emitted wave due to the motion of the emitter (in this case, a sound-scattering red blood cell) relative to the detector. Doppler ultrasound is commonly used in medical cardiology, and transcranial Doppler applied through the temporal bone can monitor blood flow in the basal intracerebral arteries (Aaslid et al., 1982). However, the speed and signal-to-noise ratio (SNR) of conventional Doppler ultrasound was insufficient to monitor the subtle blood flow changes caused by neurovascular coupling until the development of ultrafast ultrasound imaging (Tanter and Fink, 2014). Ultrafast ultrasound can produce thousands of images per second, compared to the typical 50 frames per second of conventional ultrasound scanners. The key idea behind ultrafast ultrasound is to use plane-wave transmissions instead of sequential focused beams to cover an entire field of view at a frame rate of up to 20 kHz. Enabled by the computational power to solve a ~ 100,000-element linear algebra problem for each frame, this method allowed US for the first time to image transient phenomena occurring in the millisecond range deep inside organs and greatly increased the signal-to-noise ratio (SNR).
fUSI was born when ultrafast ultrasound was applied to imaging cerebral blood flow (Bercoff et al., 2011; Mace et al., 2011). In this paradigm, plane waves return echoes scattered from red blood cells, whose motion within each voxel changes the complex amplitude of the echo arising from that voxel between successive frames. The rate of phase change in this signal corresponds to a Doppler velocity, while the absolute magnitude of the time-varying echoes after filtering out static and slow-moving tissue signals represents the Doppler power (Mace et al., 2013). This Power Doppler (PD) signal is proportional to cerebral blood volume (CBV) and relatively robust to noise, making it the primary signal used in fUSI neuroimaging. To attain sufficient SNR for robust neurovascular contrast, plane waves are typically transmitted at a series of angles (e.g., ± 10°) and their echoes are coherently summed before PD processing (Fig. 2b). At present, a common fUSI implementation uses a pulse repetition frequency of 5 kHz, 10 angles and 200 compounded frames (each acquired at 500 Hz) to produce one PD image in 400 ms. At these parameters fUSI is sensitive to CBV changes from large cerebral vessels, with blood flow velocity >10 mm/s, to the smallest microvessels, with velocity of 0.5-1.5 mm/s (Boido et al., 2019).
The first in vivo proof of concept for fUSI was established in 2011 by imaging the rat brain during whisker stimulation and epileptic seizures (Mace et al., 2011), and proved sufficient sensitivity to detect single-trial CBV changes of just 2%. The spatial resolution of fUSI at 15 MHz transmit frequency is estimated as approximately 100×100×300 μm3 based on measurement of the point spread function of the flow of particles in a sub-wavelength channel (Macé et al., 2018). The precise quantitative relationship between the fUSI signal and underlying neural activity is under active investigation. A recent study demonstrated a linear correspondence between local dendritic calcium, fUSI signal and optically measured CBV in the mouse olfactory bulb (Boido et al., 2019).
Following its pioneering development in 2011, fUSI has been used in over 100 studies in a variety of animal models and contexts, including rodents, birds, ferrets, monkeys and humans (Fig. 2c), as reviewed extensively by (Deffieux et al., 2018). For example, this technique has been applied to functional connectivity (Osmanski et al., 2014a), mapping of sensory cortical regions (Bimbard et al.; Osmanski et al., 2014b; Rau et al., 2018), tracking of spreading depression waves (Demene et al., 2017; Macé et al., 2011; Rabut et al., 2019) and planning of movement (Norman et al., 2020).
Since bones attenuate and aberrate acoustic waves at high frequencies (Pinton et al., 2012), most of these applications require circumventing the skull. This is not necessary in mice and young rats, where fUSI can be performed through intact skull (Tiran et al., 2017). In rats, open craniotomy has been mostly chosen for terminal acquisition sessions (Gesnik et al., 2017; Mace et al., 2013; Osmanski et al., 2014a, 2014b), and chronic imaging has relied on thinned-skull procedures (Urban et al., 2014) or the installation of polymeric acoustically transparent cranial windows (Bergel et al., 2018; Sieu et al., 2015; Urban et al., 2015). Similar methods have been implemented in ferrets (Bimbard et al.), pigeons (Rau et al., 2018) and rabbits (Demené et al., 2018). In non-human primates, fUSI has been performed through a cranial window (Blaize et al., 2020; Dizeux et al., 2019; Kévin et al., 2019; Norman et al., 2020). In humans, fUSI has been applied in scenarios where the skull is absent, including during intra-operative craniotomy procedures (Imbault et al., 2017; Soloukey et al., 2020) and though the anterior fontanelle window of newborns (Demene et al., 2017).
One of the biggest advantages of fUSI compared to fMRI is its ability to image awake, behaving subjects, including during locomotion. Ambulatory imaging is accomplished by reversibly fixing a miniaturized ultrasonic probe on the skull around the cranial window. Awake imaging makes possible the monitoring of cerebral functions without the potential confounds of anesthesia and also enables investigating brain functions in behaving rodents during operant tasks (Urban et al., 2015) or during sleep (Bergel et al., 2018; Sieu et al., 2015). Recently, pharmaco-fUSI was introduced to monitor drug effects on perfusion and functional connectivity in awake, feely moving mice (Rabut et al., 2020). Another major advantage of fUSI is its spatiotemporal resolution, with studies routinely achieving ~100 μm in-plane resolution and 400 ms sampling. In principle, the temporal resolution can be pushed to < 200 ms using existing methods, making it much faster than the underlying hemodynamic transfer function (Aydin et al., 2020), while even faster dynamics can potentially be inferred from the relative timing of response initiation between regions (Dizeux et al., 2019). Finally, fUSI can be combined with other techniques (Fig. 2a), including electrophysiology (Bergel et al., 2018, 2020; Sieu et al., 2015) (Demene et al., 2017), two-photon microscopy (Boido et al., 2019; Rungta et al., 2017) and optogenetic stimulation (Brunner et al., 2020). fMRI has also been used to assist fUSI acquisitions in clinical applications (Demene et al., 2017; Imbault et al., 2017; Soloukey et al., 2020).
How difficult is it for neuroscientists to start using fUSI? Today, it is relatively straightforward thanks to the commercialization of turn-key systems to perform non-clinical realtime fUSI and analyze the results. Alternatively, it is possible to implement fUSI on a general-purpose programmable ultrasound engine with home-made code, provided sufficient expertise and time to implement and optimize computationally-demanding routines for image and signal analysis.
As a new technology, fUSI has considerable scope for improvement and expansion. Major areas of ongoing research include the extension of fUSI into simultaneous 3D imaging of whole brains, the use of contrast agents to increase fUSI sensitivity and enable imaging through intact skull in larger species, and the development of chronic fUSI-based brain-machine interfaces (BMI). The original implementation of fUSI visualized one 2D plane at a time, and 3D imaging required physically translating the transducer between scans (Demené et al., 2016; Gesnik et al., 2017; Macé et al., 2018; Rau et al., 2018). This approach is effective in mapping neural activity in 3D by repeating a task while acquiring different planes. However, this increases experiment duration, and makes it impossible to cross-correlate activity between planes as needed, for example, in connectivity analysis. Last year, it was demonstrated for the first time that simultaneous 3D volumetric fUSI of nearly the whole brain in craniotomized rodents can be performed using a planar matrix array transducer -as opposed to the typical linear array- (Rabut et al., 2019). This required a substantial increase in the number of acquisition channels (1024 compared to the typical 128 or 256), the use of advanced multi-plane wave pulse sequences to compensate for a loss of SNR due to smaller transducer elements, and computationally-intensive image processing. A second study demonstrating volumetric fUSI recently appeared as a pre-print, using matrix array multiplexing to enable 3D scanning with only 256 channels (Brunner et al., 2020). With these impressive demonstrations, it is clear that 3D imaging is the future of fUSI, especially when it becomes implemented on turn-key commercial systems.
A second area for technology development is imaging through intact skull in a broader set of animals and humans. One solution to compensate for skull attenuation involves enhancing blood flow signal using intravenous ultrasound contrast agents. The most common type of micrometric bubbles of perfluorocarbon gas encapsulated by lipid shells (Frinking et al., 2020). Owing to the large density and compressibility difference between the surrounding liquid and the gaseous core, microbubbles strongly scatter sound waves, and their presence in the blood enables transcranial blood flow imaging in adult rats (Errico et al., 2016). However, because microbubble formulations are typically polydisperse in size and acoustic properties, they add not just signal, but also noise, diminishing their utility for visualizing temporally varying hemodynamic signals. To overcome this challenge, it helps to use microfluidically-sorted monodisperse bubbles (Segers et al., 2018) or nanometric biosynthetic agents (gas vesicles, described in the following section), which provide a “smooth” enhancement of signal and increase fUSI sensitivity (Maresca et al., 2020).
Contrast agents also offer a powerful complement to fUSI: super-resolution imaging, or ultrasound localization microscopy reviewed in (Christensen-Jeffries et al., 2020) and (Couture et al., 2018). Inspired by optical super-resolution techniques, ultrasound localization microscopy exploits the spatiotemporal sparsity of microbubble-generated echoes to precisely map vasculature, enabling transcranial reconstruction of cortical vasculature in rat brains at a sparial resolution below 10 μm (Errico et al., 2015). Over the past few years, other significant advances in super-resolution imaging have leveraged sparsity to enhance temporal resolution (Bar-Zion et al., 2018), used phase-correction and focusing for transcranial imaging in humans (Soulioti et al., 2020), and adapted matrix arrays for volumetric imaging (Harput et al., 2019; Heiles et al., 2019). However, there is much room for improvement. For instance, the persistence of currently available contrast agents in vivo is limited; development of agents with enhanced circulation times would facilitate studies in awake animals, where repeated injections are impractical. Nevertheless, the combination of fUSI and ultrasound localization microscopy will provide valuable insight into fundamental questions such as the functional and anatomical changes underlying neurodevelopment and neurodegeneration, as reviewed in (Iadecola, 2017; Sweeney et al., 2018).
Finally, given the high performance of fUSI, one could ask whether this technique could serve as a method for chronic neural recording or a brain-machine interface. Both electrophysiology and optical apparatus have been miniaturized and affixed to the skull of animals or humans. Likewise, the piezoelectric transducer arrays used for fUSI could be chronically mounted to the skull for sustained long-term recording, as they have already been acutely (Bergel et al., 2018; Sieu et al., 2015; Urban et al., 2015). A major motivation is provided by the possibility of developing minimally invasive clinical brain-machine interfaces, wherein an ultrasound transducer is implanted in place of a section of skull, but performs imaging through an intact protective dura, thereby bypassing potential complications from transdural surgery. A recent study recording fUSI from the posterior parietal cortex of macaques during movement planning demonstrated decoding of movement intentions before their execution with greater than 85% accuracy, motivating future work in this direction (Norman et al., 2020).
Biomolecular ultrasound
Neural activity is mediated by molecules such neurotransmitters, second messengers, enzymes, ion channels and transcription factors. As a result, tools for visualizing the concentrations and activities of these molecules play major roles in neuroscience research. Today, most molecular neuroimaging is performed using optical methods and relies on genetically encoded fluorescent indicators such as GCaMP for calcium, iGluSnFR for glutamate, ASAP for voltage or activity-dependent promoters for transcriptional activation (Andreoni et al., 2019; Lin and Schnitzer, 2016); (Kavalali and Jorgensen, 2014; Knöpfel, 2012; Knöpfel et al., 2015). These indicators provide excellent sensitivity, specificity and kinetics, which have been extensively optimized over multiple generations. In addition, genetic encoding allows these indicators to be expressed in specific subsets of neurons using a variety of genetic approaches (Luo et al., 2008, 2018), aiding in the interpretation of their signals. Because intracellular calcium is intrinsically linked to neuronal excitation and neurotransmission, GCaMP has become a mainstay of in vitro and in vivo tracking of neural activity. The major limitation of these optical tools is the limited penetration of visible light into tissue, making it extremely challenging to image neural activity in vivo at depths beyond approximately 1 mm from the optical access point. Non-optical indicators have been developed for MRI and radionuclear imaging (Bartelle et al., 2016; Ghosh et al., 2018; Hammoud et al., 2007; Le Roux and Schellingerhout, 2014; Ghosh et al., 2018; Westmeyer and Jasanoff, 2007), but have not yet received wide adoption due to their limited performance (sensitivity, kinetics, genetic encodability) and demanding technical requirements such as expensive scanners or radiochemical synthesis.
Could ultrasound fulfill the need for genetically targeted biomolecular imaging that can access the entire brain? Until recently, this idea considered very unlikely, since only synthetic contrast agents were available for ultrasound imaging (Ferrara et al., 2007; Paefgen et al., 2015; Sheeran et al., 2012; Unnikrishnan and Klibanov, 2012), and it was not clear how they could be targeted to neurons or be connected to gene expression or specific molecular activity. All the optical indicators mentioned above were enabled by the discovery of the green fluorescent protein (GFP) as a genetically encodable and engineerable fluorophore. Before any analogous indicators for ultrasound can be developed, there first needs to be a protein that can produce ultrasound contrast. Fortunately, an acoustic biomolecule of this type was identified in 2014 (Fig. 3, a–b) (Shapiro et al., 2014) and recently adapted as a reporter gene (Bourdeau et al., 2018; Farhadi et al., 2019) and biosensor (Lakshmanan et al., 2020) for US. While this technology has not yet been applied to neural imaging, its rapid development over the last few years gives it strong potential to impact this field.
Figure 3 -. Biomolecular Ultrasound.
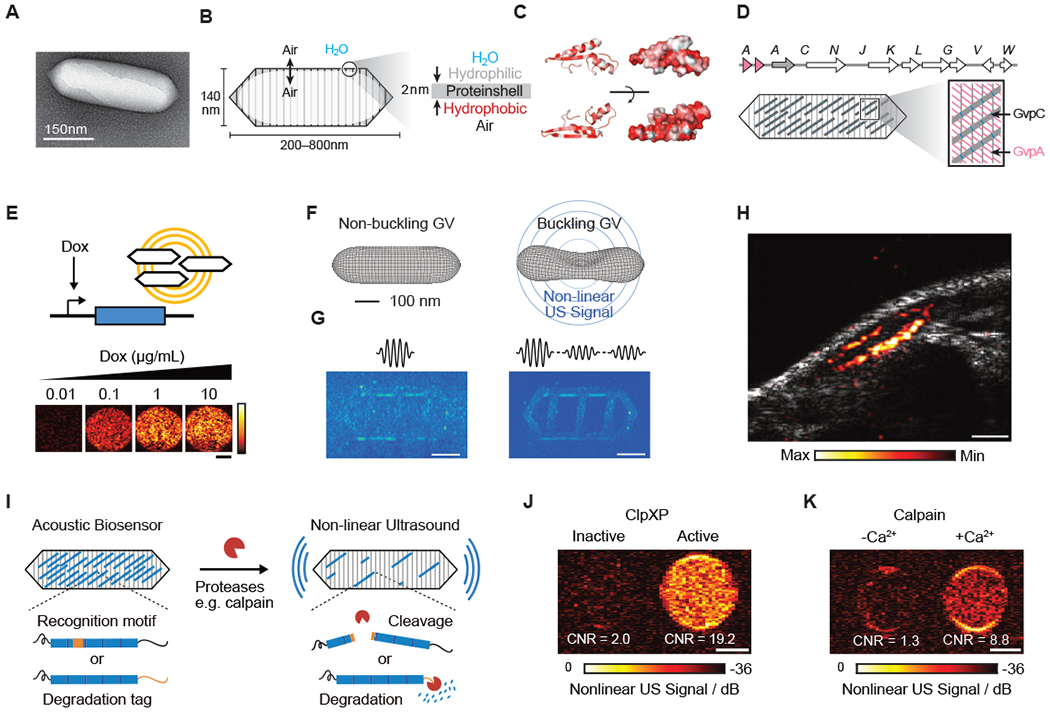
(A) Transmission electron microscopy (TEM) image of a gas vesicle (GV). (B) Diagram of a GV, showing the ability of gas to cross the shell, and the exclusion of water (left); physiochemical properties of the GV shell, namely the hydrophilic exterior that helps to solubilize the GV in the aqueous environment of the cell and the hydrophobic interior that prevents water from forming a liquid phase inside the GV (right). (C) Structural models of GvpA, the primary structural protein of the GV. Hydrophilic residues are colored white and hydrophobic residues red. The former can be seen to cluster on the convex side and the latter on the concave side. (D) (Top) Gene cluster from Anabaena flos-aquae encoding the formation of GVs, with each Gvp gene labeled. (Bottom) Diagram of a GV showing the relative contributions of the two main structural proteins to the structure of the GV (A-D from Maresca et al., 2018a). (E) Ultrasound images of human HEK293T cells in agarose gel expressing GVs under the control of a doxycycline (Dox)-inducible promoter. Scale bar, 1 mm (adapted from Farhadi et al., 2019). (F) Diagram showing the linear and nonlinear responses of GVs to ultrasound. (G) (Left) Ultrasound pulse sequence and corresponding image for a sample in the linear imaging mode. Tissue-mimicking linearly scattering particles and GVs are indistinguishable. (Right) The same sample imaged in nonlinear mode is shown, which detects nonlinearly scattering GVs, but not linearly scattering particles (F and G adapted with permission from Maresca et al., 2017) . (H) Ultrasound image of a GV-expressing tumor growing directly underneath the skin of a mouse. Scale bar, 1 mm (adapted from Farhadi et al., 2019). (I) Diagram of GV-based acoustic biosensor of protease activitiy (J) Representative ultrasound images of agarose phantoms containing acoustic biosensors sensing ClpXP activity. (K) Representative ultrasound images of agarose phantoms containing acoustic biosensor of calpain incubated with calpain and Ca2+ or calpain without Ca2+. (I-K adapted with permission from Lakshmanan et al., 2020). Scale bars for (J) and (K), 1 mm.
The “GFP for ultrasound” is based on a unique class of genetically encoded air-filled protein nanostructures known as gas vesicles or GVs, which evolved in aquatic photosynthetic microbes as a means to achieve buoyancy (Pfeifer, 2012; Walsby, 1994). GVs comprise a 2-nm thick protein shell, with a typical diameter of ~140 nm and length of 500 nm, enclosing a compartment of gas which is at equilibrium with the surrounding media (Fig. 3, a–b) (Maresca et al., 2018a; Pfeifer, 2012; Walsby, 1994). Their protein shell mostly comprises a crystalline 2D arrangement of a single protein, GvpA, reinforced by an optional external scaffolding protein, GvpC (Fig. 3, c–d). These proteins are included in gene clusters of 8 or more genes, with the other genes encoding minor structural components, chaperones, or other essential “assembly factors”.
When imaged in purified form (Shapiro et al., 2014b) or expressed in bacteria (Bourdeau et al., 2018b) or mammalian cells (Farhadi et al., 2019), GVs produce bright backscattered ultrasound contrast (Fig. 3e), and specialized ultrasound imaging paradigms have been developed to detect GVs with maximal sensitivity and specificity (Farhadi et al., 2019; Maresca et al., 2017, 2018b). These paradigms take advantage of the unique, engineerable nonlinear mechanical properties of GVs (Fig. 3, f–g) (Cherin et al., 2017; Lakshmanan et al., 2016; Zhang et al.). To date, GV expression has been imaged using ultrasound in tumors (Fig. 3h) and GI-resident bacteria, driven in both cases by chemically inducible promoters. Both the expression of GVs and their imaging with ultrasound is well-tolerated by the cell types tested. In mammalian cells, GV are detectable by ultrasound when they occupy < 0.1% of the cytoplasm. One major potential application of GVs in neuroscience is as transcriptional reporters, for example driven by activity-dependent promoters (Guzowski et al., 2005; Kawashima et al., 2013, 2014). This would allow patterns of activation arising from a certain sensory or behavioral task to be visualized non-invasively and repeatedly throughout the entire brain, giving more dynamic information than the current method of euthanizing animals and visualizing activation with immunofluorescence. To make this possible, GV genes must be packaged into viral vectors for convenient delivery to the brain and expression in neurons, which are topics of ongoing technology development. The tolerability of GV expression in neurons will also need to be evaluated, as with any new genetically encoded tool.
Could GVs be engineered as dynamic acoustic biosensors of intracellular or extracellular signals beyond gene expression? Indeed, this possibility was very recently demonstrated with the development of GV-based biosensors of protease activity (Lakshmanan et al., In press.). Proteases are involved in many aspects of cellular signaling, homeostasis, disease and therapy (Patron et al., 2018; Saita et al., 2016), and were among the first targets for the development of dynamic fluorescent indicators (Heim and Tsien, 1996; Mitra et al., 1996). In neuroscience, proteases are involved in both intracellular signaling (e.g. calpain) and extracellular interactions (e.g., matrix metalloproteinases). Acoustic biosensors of protease activity were developed by engineering the GV shell-stiffening protein GvpC to contain protease-recognition sequences for human calpain, the model endopeptidase TEV or the model proteasome ClpXP. In the presence of active enzyme, the GvpC was cleaved or degraded off the GV surface, resulting in GVs with greater deformability and the production of nonlinear ultrasound contrast (Fig. 3, i–k). The basic acoustic biosensor design demonstrated in this study should be amenable to the sensing of additional proteases, and could be applied in neurons with mammalian genetic encoding (Farhadi et al., 2019). A major open question is whether GV-based acoustic biosensors could go beyond cleavage-based, irreversible sensors, to allosteric conformational change-based sensors of molecules such as calcium or neurotransmitters. If so, these molecular signals could be imaged brain-wide with US.
While promising, acoustic reporter genes and biosensors are still in the early stages of development, with open questions remaining with regard to their expression and tolerability in the brain and their molecular sensitivity. In the meantime, one neuroscience application of GVs has already been implemented: when injected into the blood stream, purified GVs provide smoother enhancement of the Doppler signal than that created by microbubbles, boosting the SNR of fUSI imaging (Maresca et al., 2020). This smooth enhancement arises from GVs’ relatively monodisperse acoustic properties and their greater number per given gas volume (each microbubble is the size of 100-1000 GVs), reducing stochastic variation in acoustic scattering.
An alternative mechanism by which ultrasound can help visualize molecular dynamics is through photoacoustic imaging, a technique wherein optical excitation is absorbed by chromophores and converted into thermoelastic pressure waves that can be detected by ultrasound transducers, enabling visualization of optical molecules in tissues with the resolution of ultrasound (Wang and Hu, 2012). A big potential advantage of this approach is its compatibility with existing fluorescent indicators, but this requires overcoming the strong background absorbance of hemoglobin by engineering reporters that work in the infrared, and efforts to do so are still in the relatively early stages (Deán-Ben et al., 2016; Yao et al., 2016; Ovsepian et al., 2017).
Ultrasonic control of neural activity
The same properties that make ultrasound attractive for neural imaging make it appealing for neuromodulation, including the ability of sound waves to penetrate deep into tissues and achieve high spatiotemporal precision. In addition, the fact that ultrasound only needs to travel one-way for neuromodulation, and is typically used at lower frequencies, means that focus ultrasound (FUS) can be applied transcranially, including in humans. Furthermore, FUS has several modes of physical interaction with constituents of biological tissue, depending on their composition and ultrasound pulse parameters, including local heating, mechanical force and bubble cavitation (Fig. 1, b–d). These interactions are already used in clinical medicine to destroy tumors, ablate brain regions and break up kidney stones. In their gentler form, they provide several avenues for neuromodulation, including direct activation or inhibition of unmodified neurons, sonogenetic control of genetically modified cells, local delivery of pharmacological agents, and acoustic targeting of chemogenetic agents.
Direct ultrasonic neuromodulation (UNM)
Non-invasive neuromodulation technologies are valuable tools for both basic neuroscience and the clinic. The most widely used technique – transcranial magnetic stimulation – has yielded important scientific insights and medical treatments, but is difficult to target with high spatial precision or focus at depth. In contrast, FUS can deliver mechanical energy to neurons deep within the brain in the form of an acoustic pressure wave (Fig. 4a), which results in thermal or mechanical effects, depending on the pulsing parameters (frequency, pressure, pulse duration and pulse energy) (O’Brien, 2007).
Figure 4 -. Ultrasonic Neuromodulation and Sonogenetics.
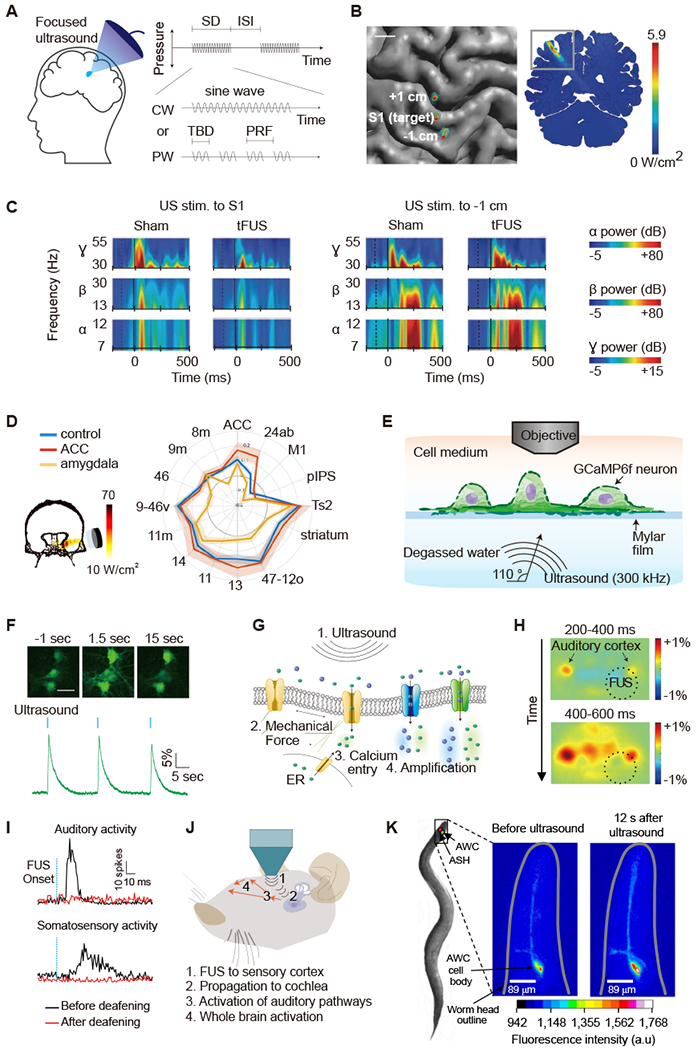
(A) Illustration of focused ultrasound application to the brain and acoustic pulse parameters. CW, continuous wave; ISI, inter stimulus interval; PRF, pulse repetition frequency; PW, pulsed wave; SD, sonication duration; TBD, tone burst duration. (B) Top-down view of the brain showing acoustic intensity field of ultrasound beam targeting the S1 and sites 1 cm anterior (+1 cm) and posterior (−1 cm). (C) Time-frequency plots showing the power of evoked neural oscillations in the α, β, γ frequency bands in relation to the onset of ultrasound (dashed vertical line) and median nerve stimulation (solid vertical line) for sham and ultrasound treatment condition (B and C adapted with permission from Legon et al., 2014). (D) Left: acoustic intensity field targeting amygdala area of the primate brain. Right: functional connectivity fingerprint shows the strength of activity coupling between amygdala and other areas in control (blue), after ultrasound to amygdala (yellow), and after ultrasound to anterior cingulate cortex (ACC, red; adapted with permission from Folloni et al., 2019). (E) Diagram of experimental setup for in vitro neuronal ultrasound stimulation under acoustically realistic conditions. (F) GCaMP6f calcium signals in cultured neurons in response to ultrasound stimulation. Scale bar, 30 μm. (G) Biomolecular mechanisms of ultrasonic neuromodulation (E-G adapted with permission from Yoo et al., 2020). (H) Indirect excitation of mouse auditory cortex by FUS application to the visual cortex followed by widespread cortical response, as observed with wide-field calcium imaging (adapted with permission from Sato et al., 2018). (I) Neuronal responses to FUS recorded from guinea pig auditory or somatosensory cortex before and after deafening. (J) Mechanisms of indirect ultrasonic neuromodulation, where propagating ultrasound waves vibrate the cochlea, activating both auditory and non-auditory ascending pathways, leading to widespread activation. Deafening eliminates ultrasound-evoked multiunit sensory activity (I and J adapted from with permission from Guo et al., 2018). (K) Ultrasound activation of calcium signal in a C. elegans neuron engineered to express TRP-4 (adapted with permission from Ibsen et al., 2015).
The concept of using ultrasound to modulate brain function arose in the 1950s, with a seminal study demonstrating the suppression of visual-evoked potentials in cats (Fry et al., 1958). This concept re-emerged 10 years ago when a study demonstrated the use of low-intensity FUS to excite neurons in mice, resulting in electrical and motor responses (Tufail et al., 2010). Following this pioneering work, a number of studies applying ultrasonic neuromodulation (UNM) emerged in various animal models (Supplementary Table 1), evaluating the dependence of UNM on acoustic parameters and FUS targeting. Concurrently, UNM was tested in human subjects, eliciting somatosensory and visual evoked potentials and tactile sensations of individual fingers, or enhancing performance on sensory discrimination tasks (Lee et al., 2015, 2016; Legon et al., 2012a, 2014). (Fig. 4, b–c).
At low ultrasound intensities (spatial peak time-averaged intensity, Ispta < 10 W cm−2), most studies have reported motor or sensory effects consistent with neural excitation. However, inhibitory effects have also been reported on visual-evoked potentials in rabbits (Yoo et al., 2011b) and rats (Kim et al., 2015), and somatosensory evoked potentials in humans (Legon et al., 2014b). In NHPs, UNM has been shown to affect ipsilateral antisaccade latencies (Deffieux et al., 2013), the firing rates of single neurons in the frontal eye field (Wattiez et al., 2017), fMRI signals in primary sensory cortex (Yang et al., 2018) and decision bias toward the contralateral direction during visuomotor decision tasks (Kubanek et al., 2020).
The safety of UNM applied at typical low-intensity UNM parameters has been evaluated in humans, demonstrating no significant adverse effects (Legon et al., 2020). A careful histological analysis in sheep confirmed that FUS applied at similar parameters does not cause tissue damage or open the blood-brain barrier (Gaur et al., 2020). Most UNM studies today operate at ultrasound frequencies of 250-1000 kHz, Ispta of 3 to 30 W cm−2, with either pulsed (~1 kHz) or continuous stimuli lasting hundreds of ms each.
The application of FUS over longer periods (0.5–5 min) has been shown to produce persistent neuromodulatory effects, lasting from a few minutes to several hours. In an acute epileptic animal model, low-intensity FUS applied to the thalamic area for 3 min led to suppression of hyperactivity for tens of minutes (Min et al., 2011). Similar treatments could suppress tremor in mouse models of Parkinson’s disease (Dallapiazza et al., 2018; Sharabi et al., 2018; Wang et al., 2020). Prolonged FUS was also reported to induce excitation-like effects and changes in connectivity in NHPs and human subjects lasting for over an hour. Stimulation of the amygdala and supplementary motor area in NHPs affected circuit connectivity, resulting in a change in the coupling of fMRI-observed activity between the FUS targets and other brain areas (Folloni et al., 2019; Verhagen et al., 2019) (Fig. 4d). Very recently, the application of FUS to the frontal eye field of macaques for 100 ms immediately before a visual cue shifted the bias of a choice task in favor of the corresponding contralateral side (Kubanek et al., 2020). Human UNM positively shifted mood by targeting the right inferior frontal gyrus (rIFG) and modulating functional connectivity (Sanguinetti et al., 2020). Some experiments applying UNM to patients in a vegetative state even suggested that this form of stimulation could facilitate consciousness, although sham controls were not included (Monti et al., 2016).
The biggest question concerning UNM concerns its underlying mechanisms. Given the multiple ways ultrasound can interact with tissues (Fig. 1), biophysical mechanisms involving mechanical force, cavitation and heating have been considered. Thermal effects are unlikely under most UNM parameters, which result in too little energy deposition to generate appreciable heat (Constans et al., 2018; Lee et al., 2016), with the exception of inhibitory effects from very high intensity FUS (Kim et al., 2020). Cavitation of bubbles nucleated in the neuronal membrane has been advocated on a theoretical basis (Krasovitski et al., 2011; Plaksin et al., 2016) and appears to be consistent with the parameters effective in UNM, but has not been confirmed experimentally. Most commonly it has been hypothesized that UNM operates via mechanical force on neurons, transduced by mechanosensitive ion channels (Tyler, 2011).
A major challenge in studying the biophysical mechanisms of UNM arises from the fact that conventional in vitro models – involving the growth of neurons or other cells on hard substrates and/or recording of their activation with stiff electrodes (Han et al., 2018; Kim et al., 2017; Oh et al., 2020; Prieto et al., 2013; Tufail et al., 2010; Tyler et al., 2008) – comprise acoustic and mechanical conditions far removed from the those experienced by neurons in vivo. At the same time, as discussed further below, the presence of auditory side effects in small animals constrains mechanistic studies of UNM in vivo. A recent study addressed these challenges by examining the mechanisms of UNM in mouse neurons cultured under acoustically realistic conditions comprising an acoustically transparent platform and using all-optical readouts to avoid mechanical confounds (Yoo et al., 2020) (Fig. 4e). This study demonstrated that FUS parameters common in animal studies (> 100 ms, > 3 W/cm2) elicit direct and reversible neural responses (Fig. 4f), and that these responses do not involve significant cavitation, heating, large-scale mechanical deformation or synaptic transmission. Instead, FUS was found to induce calcium accumulation through specific mechanosensitive ion channels, leading to activation of calcium-dependent sodium channels and low-threshold calcium channels, resulting in substantial excitation (Fig. 4g). As demonstrated with pharmacological and genetic perturbations, the ion channels involved in responding to FUS included TRPP1/2, TRPC1 and TRPM4. Overexpression of these channels increased the ultrasound responsiveness of the neurons. While these results provide evidence that neurons themselves can respond to FUS, other work points to astrocytes as an additional or alternative cell type involved in UNM (Oh et al., 2020).
Another important question concerning UNM is its specificity and potential side-effects. Early studies in small animals showed that motor responses did not follow the expected spatial targeting of FUS, for example with bilateral body movements actuated when stimulating only one side of the motor cortex (Ye et al., 2016), or maximal motor responses elicited when targeting non-motor cortical regions. Given the small size of rodent heads relative to the FUS beam and the ms-scale pulse durations used in UNM, some lack of spatial specificity could arise from the formation of standing waves due to multiple reflections of ultrasound inside the skull (O’Reilly et al., 2010; Pulkkinen et al., 2014). In addition, given the exceptionally light anesthesia used in most experiments (King et al., 2013, 2014; Tufail et al., 2010; Ye et al., 2016; Yoo et al., 2011; Younan et al., 2013), there was a possibility that indirect sensory effects of UNM could be involved in eliciting animal movement.
Indeed, two side-by-side studies published in 2018 showed that FUS, applied with widely used UNM parameters, produced significant auditory activation (Guo et al., 2018; Sato et al., 2018). Using wide-filed calcium imaging in transgenic mice, one of the studies showed robust FUS activation of the contralateral auditory cortex, even when visual cortex was targeted, followed by broader cortical activation (Sato et al., 2018). In addition, mice responded with movement not only in response to US, but also in response to audible stimuli applied at the same pulse repetition frequency, and to air puffs, suggesting the involvement of startle. Chemical deafening strongly attenuated the response to ultrasound and audible sound, but not to air puffs. In the second study, electrical recordings from the auditory cortex and inferior colliculus in wild-type guinea pigs revealed responses to FUS applied anywhere in the brain and even to other parts of the animal (Guo et al., 2018). These responses were eliminated with surgical deafening. Together, these studies suggested that FUS can vibrate the inner ears to activate auditory pathways (Fig.4 h–j), in addition to any direct neuromodulatory effects on the targeted brain region. Audible percepts have recently been reported in human volunteers treated with FUS (Braun et al., 2020; Sanguinetti et al., 2020), demonstrating their relevance in larger crania.
The presence of auditory side effects or cutaneous perception (Gavrilov et al., 1977a; Legon et al., 2012b) is by no means an insurmountable problem for UNM. As has been done with transcranial magnetic stimulation (Foysal and Baker, 2020; Tringali et al., 2012) and transcranial direct current stimulation (tDCS) (Matsumoto and Ugawa, 2016), sensory side effects must be comprehensively studied and understood to enable the design of adequate sham controls. Recent work has shown that auditory side-effects, while present, are not the primary source of the neuromodulatory responses of animals to FUS. For example, it was shown that FUS still elicits motor responses in chemically or genetically deafened rodents (Mohammadjavadi et al., 2019; Xiaodan Niu, 2018; Yu et al., 2019). Additional work is needed to examine the role of other potential sensory confounds such as tactile or pain sensation (Gavrilov et al., 1977b; Legon et al., 2012a). At the same time, the source of the auditory side-effect of FUS is becoming better understood. A recent computational model showed how FUS can create skull-conducted shear waves, which propagate well outside the insonated region to reach the cochlea within 10 ps of stimulus initiation (Salahshoor et al., 2020). It has also been recognized that auditory responses can be exacerbated by applying FUS with sharp pulsing (creating broadband frequencies) or using repetition rates in the audible frequency range (often ~1 kHz). Auditory effects can be minimized by applying longer, continuous pulses and smoothing their application with ramps. Most UNM studies published in the last 2 years have included controls to demonstrate that their observed responses are not caused by auditory side-effects (Folloni et al., 2019; Mohammadjavadi et al., 2019; Verhagen et al., 2019; Wang et al., 2020; Xiaodan Niu, 2018). In humans, auditory side-effects were successfully masked using coapplied audible stimuli (Braun et al., 2020).
Is UNM ready for “prime time” use by neuroscientists? Yes, but with caution. One of the primary selling points of UNM is its applicability to NHPs and human subjects, facilitated by the design of improved and simplified methods to target FUS to desired brain regions with steerable transducer arrays (Chaplin et al., 2018) and 3D-printed acoustic lenses (Brown et al., 2017; Ferri et al., 2018; Jiménez-Gambín et al., 2019; Maimbourg et al., 2018). However, the modulatory effects observed in these species have been relatively modest and divergent in terms of their polarity and duration, making it challenging to predict the outcome of any new application. In addition, the presence of potential auditory or other sensory side-effects requires the design of adequate sham controls and masking stimuli to make the results of UNM experiments convincing. We are hopeful that recent and ongoing work on the mechanisms and safety limits of UNM will reveal FUS parameters with larger neuromodulatory effects and facilitate the design and interpretation of future UNM experiments.
Sonogenetics
One of the biggest advantages of optogenetic and chemogenetic techniques is their ability to target genetically defined neuronal populations. Given the unique spatial focusing and tissue penetration capabilities of FUS, there has been considerable interest in the development of equivalent “sonogenetic” approaches. In fact, sonogenetic methods for applications outside the nervous system have a long history, starting with the use of thermal FUS (Fig. 1c) to activate heat-sensitive transcription via mammalian heat shock promoters or bacterial thermal bioswitches (Deckers et al., 2009; Guilhon et al., 2003; Kruse et al., 2008; Piraner et al., 2017b). In these applications, brief non-toxic temperature elevations (e.g. 42°C for 1 hour) drove spatially selective expression of a desired gene. More recently, mechanical sonogenetics was demonstrated when heterologous expression of large conductance mechanosensitive ion channel (MscL) sensitized mammalian cells to ultrasound-driven mechanical perturbation mediated by synthetic microbubbles (Heureaux et al., 2014).
The first study to apply sonogenetics to neuroscience, which also coined the name for this class of tools, used overexpression of the TRP-4 mechanosensitive ion channel (MSC) in C. elegans, in conjunction with microbubbles, to elicit reversible behavioral responses in transgenic worms (Ibsen et al., 2015) (Fig. 4k). Since then, several other established or putative MSCs, including MscL (Ye et al., 2018), Piezo1 (Pan et al., 2018; Prieto et al., 2018), MEC-4, DEG/ENaC/ASIC (Kubanek et al., 2018), Prestin (Huang et al., 2020), TREK-1/2, TRAAK (Kubanek et al., 2016), TRPP1/2 and TRPC1 (Yoo et al., 2020) have been reported to sensitize cells to acoustic stimuli in vitro. Because most of these studies employed synthetic microbubbles or used acoustic and mechanical conditions unrepresentative of in vivo brains, it is not yet certain which of these channels will be useful in vivo. Relatively preliminary in vivo experiments suggest that some of these channels can be activated with ultrasound in the mouse brain (Huang et al., 2020; Ye et al., 2018), but rigorous performance characterization and demonstration of a significant behavioral effect are still lacking. In addition to mechanical sonogenetics, efforts are underway to make use of thermal mechanisms to sensitize neurons to ultrasound, for example by overexpressing the temperature-sensitive ion channel TRPV1 (Yang et al., 2020).
Before sonogenetic techniques become widely useful to neuroscientists, more extensive in vivo characterization must be performed, including a demonstration of robust behavioral effects. In addition, there needs to be a better understanding of the mechanical conditions required for activation, for example the importance of microbubbles or other ultrasound-responsive actuators. Potential cross-talk between sonogenetic activation and UNM must also be considered, along with the possibility of sensory side-effects. For thermal sonogenetics, it is important to consider the temperature senitivity of endogenous ion channels and conditions under which certain receptors may need to be knocked out (Güler et al., 2012).
Acoustically targeted pharmacology and chemogenetics
Pharmacological perturbation is an established means to modulate neural activity based on the selective inhibition or agonism of specific neuronal receptors. However, in vivo neuropharmacology is greatly limited by the restrictive transport of molecules across the blood brain barrier (BBB) from systemic circulation (Misra et al., 2003) and the requirement of invasive injections to obtain spatial specificity. Both of these challenges can be addressed using the ability of FUS to locally open the BBB and actuate the release or entry of pharmaceutical compounds.
FUS opening of the BBB is an established technique that relies on the stable cavitation (expansion and contraction) of circulating microbubbles (Fig. 1d and Fig. 5a) to mechanically open the tight junctions forming the BBB (Hynynen et al., 2001). This opening enables the permeation of particles up to a few tens of nm in size, including small molecules, proteins, nanoparticles and viral vectors (Hynynen et al., 2001; Kinoshita et al., 2006; Samiotaki et al., 2015; Thevenot et al., 2012; Wang et al., 2015). BBB opening requires a few seconds or minutes of FUS, and closes within a few hours. The safety of FUS-BBB opening performed at appropriate acoustic parameters is well-established (Downs et al., 2015; Kobus et al., 2016), and recent studies have demonstrated safe BBB opening in human patients (Carpentier et al., 2016; Lipsman et al., 2018).
Figure 5 -. Acoustically Targeted Pharmacology and Chemogenetics.
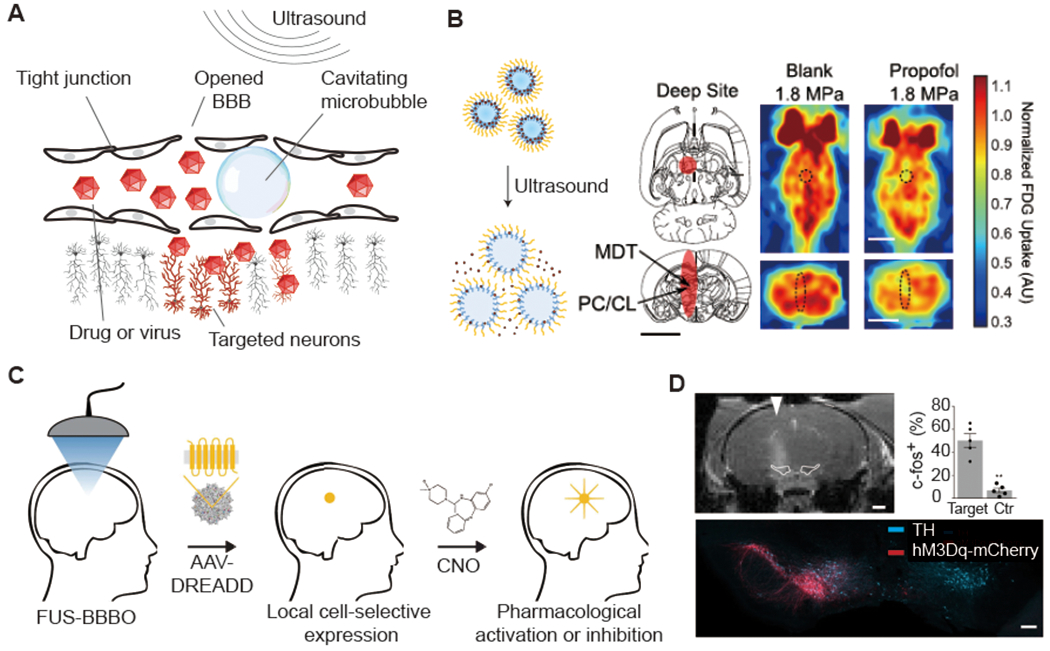
(A) Illustration of microbubble-mediated blood brain barrier (BBB) opening by focused ultrasound. (B) Left: illustration of drug release from propofol-loaded nanoemulsions (adapted from Airan et al., 2017). Middle: illustration of ultrasound focal zone. Right: Fluorodeoxyglucose Positron emission tomography (FDG PET) images captured during sonication with or without propofol-loaded nanoparticle administration are shown. Scale bar, 5 mm (adapted from Wang et al., 2018). (C) Schematic of acoustically targeted chemogenetics (ATAC) paradigm. (D) Representative MRI scan indicating the site of the BBB opening (top, scale bar: 1 mm) and immunostaining imaging (bottom, scale bar: 200 μm). AAVs encoding hMsDq-mCherry were selectively delivered to the left SNc/VTA. The targeted neurons were excited by clozapine-N-oxide (CNO) ( C and D adapted with permission from Szablowski et al., 2018).
As applied to neuroscience research, one use of FUS-BBB opening involves the local administration of systemically circulating BBB-impermeable drugs, such as the inhibitory neurotransmitter GABA (Constans et al., 2020; McDannold et al., 2015; Todd et al., 2019). Since the BBB remains permeable for several hours after opening, it is possible to open a targeted brain region in anesthetized animals under MRI guidance, then move them to a behavioral setup for simple systemic administrations of the drug during a sensory or behavioral task. It is also possible to accomplish local pharmacological neuromodulation without BBB opening by applying FUS in conjunction with circulating drug-loaded perfluorocarbon nanodroplets loaded with a BBB-permeable drug (Airan et al., 2017). Using this approach, local delivery of the anesthetic propofol demonstrated effective functional inhibition in rats (Wang et al., 2018) (Fig. 5b). The advantages of both of these pharmacological approaches are that animals are not permanently modified, and different brain regions can in theory be targeted with different drugs on different experimental days. Their completely noninvasive nature and use of FDA-approved substances further enhances potential translation to humans. However, the need to perform FUS BBB opening for each experiment or treatment can be logistically challenging, and the pharmaceutical agents administered to date lack cell type specificity.
An alternative approach enables a single FUS treatment to provide repeatable, long-term pharmacological control of spatially and genetically defined neurons (Szablowski et al., 2018). In acoustically targeted chemogenetics (ATAC), FUS BBB opening is used to transduce neurons at specific locations in the brain with virally-encoded engineered chemogenetic receptors (Sternson and Roth, 2014). These receptors can then respond to systemically administered designer compounds to activate or inhibit the activity of these neurons as needed for experimental perturbation or treatment (Fig. 5c). In the first implementation of ATAC, FUS-BBB opening was used to target adeno-associated viral vectors (AAV9) encoding inhibitory designer receptors exclusively activated by designer drugs (DREADDs) to excitatory neurons in the hippocampus of mice, showing robust and specific pharmacological inhibition of memory formation (Szablowski et al., 2018). Neuronal excitation and intersectional genetic targeting were also demonstrated (Fig. 5d). A major advantage of the ATAC approach is that FUS is applied only once, and subsequent pharmacological perturbation happens like any normal pharmacological treatment. Another significant advantage compared to local drug release is that the use of a genetic component enables cell type-specific targeting. Furthermore, compared to conventional surgically-delivered chemogenetic approaches, ATAC can cover larger brain regions – by sweeping the ultrasound beam – than accessible with injections, making it particularly attractive for use in larger animals and human patients.
The primary challenge of ATAC as currently implemented is the requirement of a fairly high dose of systemic virus to achieve efficient transduction at the ultrasound focus, resulting in co-transduction of peripheral organs. The use of cell-type-specific promoters is largely effective in confining expression of chemogenetic receptors to neurons, but nevertheless it would be preferable to develop viral vectors with higher efficiency for this specific application. In addition, there is substantial scope to optimize the chemogenetic receptors and ligands used in a given application. Finally, the requirement of a genetic perturbation lengthens the timeframe on which ATAC could be translated to humans.
Of all the available strategies for FUS neuromodulation, direct UNM is the easiest for neuroscience laboratories to try, and can be used in human experiments, but comes with several open questions and caveats. Ultrasound-targeted pharmacology and ATAC operate through well-understood mechanisms, but for now are limited to applications in animals. Sonogenetic approaches still require in-depth validation in vivo.
Ultrasound-interacting inorganic materials
In addition to its interactions with molecules and tissues, ultrasound can also be used to actuate or communicate with inorganic materials and devices based on piezoelectricity. In this application, the ability of ultrasound to focally concentrate energy allows it to efficiently deliver signals and power to mm-sized devices that would otherwise have poor resonance with radiofrequency electromagnetic transmission. In addition, devices containing piezoelectric materials can be engineered to modulate their backscattered ultrasound intensity depending on a signal of interest. For example “neural dust” was introduced as a 3 mm3 implantable device to measure compound action potentials from peripheral nerves, providing a detection noise floor of 160 μVrms (Seo et al., 2016). Piezoelectric materials can also convert acoustic pressure into electric current, which, combined with appropriate circuits, can be used to stimulate neurons (Charthad et al., 2018; Piech et al., 2020).
Piezoelectric materials can also be used at the nano-to-micro-scale. For example, barium titanate nanoparticles have been used to stimulate neurons in culture based on the conversion of ultrasound to electrical current (Marino et al., 2015). Alternatively, mechanoluminescent materials such as doped ZnS nanoparticles can convert ultrasound to light, which is then connected to neural stimulation through optogenetics (Wu et al., 2019).
The advantages of ultrasound-based implantable devices and materials relative to more established radiofrequency and magnetic tools for neuroscience applications have yet to be conclusively demonstrated. However, the fundamental advantages of spatial focusing and penetration provided by ultrasound make this a promising area of technology development.
Discussion
In this review, we have attempted to describe the primary concepts and developments underlying the recent emergence of ultrasound as a tool for neuroscience research, and accurately convey its present and potential future utility. Ultrasound has unique advantages in terms of its biophysical interactions with tissue, which have been leveraged to both image and modulate neural activity. Some ultrasound neurotechnologies have immediate uses, including fUSI, direct UNM (with some caveats) and ultrasound-targeted gene and drug delivery. Others, including biomolecular ultrasound, sonogenetics and ultrasound-interacting devices, show promise but need further development.
How difficult is it for a neuroscientist interested in these techniques to pick up ultrasound? In short, it is no more difficult than optics. Both modalities rely on a similar core set of physical concepts, the basics of which can be learned without much complication. Like optical tools, the recent commercialization of ultrasound neurotechnologies will make working with them easier, while ongoing collaborations between neuroscientists and ultrasound engineers will push the boundaries of what ultrasound can do in this biological arena.
Supplementary Material
Acknowledgements
The authors thank members of the Shapiro, Andersen, Tsao, Tanter and Caskey laboratories for helpful discussions. C.R. is supported by the Human Frontier Science Program Cross-Disciplinary Fellowship. S.Y. is supported by the Brain and Behavior Research Foundation NARSAD Young Investigator Award. Related research in the Shapiro laboratory was supported by the National Institutes of Health (R01EB018975, UG3MH120102, U01NS099724, RF1MH117080), the Defense Advanced Research Projects Agency (W911NF-14-1-0111, D14AP00050), the David and Lucille Packard Foundation, the Sontag Foundation, the Burroughs Wellcome Fund, the Jacobs Institute of Molecular Engineering in Medicine, the Rosen Center for Bioengineering, the Tianqiao and Chrissy Chen Institute for Neuroscience, and the Heritage Medical Research Institute.
Table of Acronyms
- US
Ultrasound
- FUS
Focused ultrasound
- fUSI
Functional ultrasound imaging
- GV
Gas vesicles
- UNM
Ultrasonic neuromodulation
- BBB
Blood brain barrier
- MSC
Mechanosensitive Ion Channel
- ATAC
Acoustically targeted chemogenetics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The California Institute of Technology own patents and patent applications related to some of the technologies described in this article.
References
- Aaslid R, Markwalder T-M, and Nornes H (1982). Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg 57, 769–774. [DOI] [PubMed] [Google Scholar]
- Airan RD, Meyer RA, Ellens NPK, Rhodes KR, Farahani K, Pomper MG, Kadam SD, and Green JJ (2017). Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions. Nano Lett. 17, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoni A, Davis CMO, and Tian L (2019). Measuring brain chemistry using genetically encoded fluorescent sensors. Curr. Opin. Biomed. Eng 12, 59–67. [Google Scholar]
- Aydin A-K, Haselden WD, Goulam Houssen Y, Pouzat C, Rungta RL, Demené C, Tanter M, Drew PJ, Charpak S, and Boido D (2020). Transfer functions linking neural calcium to single voxel functional ultrasound signal. Nat. Commun 11, 2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelle BB, Barandov A, and Jasanoff A (2016). Molecular fMRI. J. Neurosci 36, 4139–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Zion A, Solomon O, Tremblay-Darveau C, Adam D, and Eldar YC (2018). SUSHI: Sparsity-Based Ultrasound Super-Resolution Hemodynamic Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 2365–2380. [DOI] [PubMed] [Google Scholar]
- Bercoff J, Montaldo G, Loupas T, Savery D, Mézière F, Fink M, and Tanter M (2011). Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 134–147. [DOI] [PubMed] [Google Scholar]
- Bergel A, Deffieux T, Demené C, Tanter M, and Cohen I (2018). Local hippocampal fast gamma rhythms precede brain-wide hyperemic patterns during spontaneous rodent REM sleep. Nat. Commun 9, 5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergel A, Tiran E, Deffieux T, Demené C, Tanter M, and Cohen I (2020). “Online” modulation of brain hemodynamics despite stereotyped running (Neuroscience). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimbard C, Demene C, Girard C, Radtke-Schuller S, Shamma S, Tanter M, and Boubenec Y Multi-scale mapping along the auditory hierarchy using high-resolution functional UltraSound in the awake ferret. ELife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaize K, Arcizet F, Gesnik M, Ahnine H, Ferrari U, Deffieux T, Pouget P, Chavane F, Fink M, Sahel J-A, et al. (2020). Functional ultrasound imaging of deep visual cortex in awake nonhuman primates. Proc. Natl. Acad. Sci 201916787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boido D, Rungta RL, Osmanski B-F, Roche M, Tsurugizawa T, Bihan DL, Ciobanu L, and Charpak S (2019). Mesoscopic and microscopic imaging of sensory responses in the same animal. Nat. Commun 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau RW, Lee-Gosselin A, Lakshmanan A, Farhadi A, Nety S, Kumar SR, and Shapiro MG (2018). Acoustic reporter genes for non-invasive imaging of microorganisms in mammalian hosts. Nature 553, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Blackmore J, Cleveland RO, and Butler CR (2020). Transcranial ultrasound stimulation in humans is associated with an auditory confound that can be effectively masked. BioRxiv 2020.03.07.982033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Nikitichev DI, Treeby BE, and Cox BT (2017). Generating arbitrary ultrasound fields with tailored optoacoustic surface profiles. Appl. Phys. Lett 110, 094102. [Google Scholar]
- Brunner C, Grillet M, Sans-Dublanc A, Farrow K, Lambert T, Macé É, Montaldo G, and Urban A (2020). A platform for brain-wide functional ultrasound imaging and analysis of circuit dynamics in behaving mice (Neuroscience). [DOI] [PubMed] [Google Scholar]
- Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, et al. (2016). Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8, 343re2. [DOI] [PubMed] [Google Scholar]
- Chaplin V, Phipps MA, and Caskey CF (2018). A random phased-array for MR-guided transcranial ultrasound neuromodulation in non-human primates. Phys. Med. Biol 63, 105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charthad J, Chang TC, Liu Z, Sawaby A, Weber MJ, Baker S, Gore F, Felt SA, and Arbabian A (2018). A mm-Sized Wireless Implantable Device for Electrical Stimulation of Peripheral Nerves. IEEE Trans. Biomed. Circuits Syst 12, 257–270. [DOI] [PubMed] [Google Scholar]
- Cherin M, Melis JS, Bourdeau RW, Yin M, Kochmann DM, Foster FS, and Shapiro MG (2017). Acoustic behavior of Halobacterium salinarum gas vesicles in the high frequency range: experiments and modeling. Ultrasound Med. Biol 43, 1016–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Jeffries K, Couture O, Dayton PA, Eldar YC, Hynynen K, Kiessling F, O’Reilly M, Pinton GF, Schmitz G, Tang M-X, et al. (2020). Super-resolution Ultrasound Imaging. Ultrasound Med. Biol 46, 865–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans C, Mateo P, Tanter M, and Aubry J-F (2018). Potential impact of thermal effects during ultrasonic neurostimulation: retrospective numerical estimation of temperature elevation in seven rodent setups. Phys. Med. Biol 63, 025003. [DOI] [PubMed] [Google Scholar]
- Constans C, Ahnine H, Santin M, Lehericy S, Tanter M, Pouget P, and Aubry J-F (2020). Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Controlled Release 318, 223–231. [DOI] [PubMed] [Google Scholar]
- Couture O, Hingot V, Heiles B, Muleki-Seya P, and Tanter M (2018). Ultrasound Localization Microscopy and Super-Resolution: A State of the Art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 65, 1304–1320. [DOI] [PubMed] [Google Scholar]
- Dallapiazza RF, Timbie KF, Holmberg S, Gatesman J, Lopes MB, Price RJ, Miller GW, and Elias WJ (2018). Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J Neurosurg 128, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deán-Ben XL, Sela G, Lauri A, Kneipp M, Ntziachristos V, Westmeyer GG, Shoham S and Razansky D (2016). Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci Appl 5, e16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, and Moonen CT (2009). Image-guided, noninvasive, spatiotemporal control of gene expression. Proc Natl Acad Sci U A 106, 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieux T, Demene C, Pernot M, and Tanter M (2018). Functional ultrasound neuroimaging: a review of the preclinical and clinical state of the art. Curr. Opin. Neurobiol 50, 128–135. [DOI] [PubMed] [Google Scholar]
- Demené C, Tiran E, Sieu L-A, Bergel A, Gennisson JL, Pernot M, Deffieux T, Cohen, and Tanter M (2016). 4D microvascular imaging based on ultrafast Doppler tomography. Neuroimage 127, 472–483. [DOI] [PubMed] [Google Scholar]
- Demene C, Baranger J, Bernal M, Delanoe C, Auvin S, Biran V, Alison M, Mairesse J, Harribaud E, Pernot M, et al. (2017). Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med 9, eaah6756. [DOI] [PubMed] [Google Scholar]
- Demené C, Maresca D, Kohlhauer M, Lidouren F, Micheau P, Ghaleh B, Pernot M, Tissier R, and Tanter M (2018). Multi-parametric functional ultrasound imaging of cerebral hemodynamics in a cardiopulmonary resuscitation model. Sci. Rep 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizeux A, Gesnik M, Ahnine H, Blaize K, Arcizet F, Picaud S, Sahel J-A, Deffieux T, Pouget P, and Tanter M (2019). Functional ultrasound imaging of the brain reveals propagation of task-related brain activity in behaving primates. Nat. Commun 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs ME, Buch A, Sierra C, Karakatsani ME, Teichert T, Chen SS, Konofagou EE, and Ferrera VP (2015). Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task (vol 10, e0125911, 2015). PLoS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, and Tanter M (2015). Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502. [DOI] [PubMed] [Google Scholar]
- Errico C, Osmanski B-F, Pezet S, Couture O, Lenkei Z, and Tanter M (2016). Transcranial functional ultrasound imaging of the brain using microbubble-enhanced ultrasensitive Doppler. Neuroimage 124, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffre J-M, Bouakaz A (2016). Therapeutic Ultrasound (Switzerland: Springer International Publishing; ). [Google Scholar]
- Farhadi A, Ho GH, Sawyer DP, Bourdeau RW, and Shapiro MG (2019). Ultrasound imaging of gene expression in mammalian cells. Science 365, 1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara K, Pollard R, and Borden M (2007). Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng 9. [DOI] [PubMed] [Google Scholar]
- Ferri M, Bravo JM, Sánchez-Pérez JV, and Redondo J Enhanced 3D-printed holographic acoustic lens for aberration correction of single-element transcranial focused ultrasound. 34. [DOI] [PubMed] [Google Scholar]
- Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry JF, Rushworth MFS, and Sallet J (2019). Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron 101, 1109–1116 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foysal KMR, and Baker SN (2020). Induction of plasticity in the human motor system by motor imagery and transcranial magnetic stimulation. J. Physiol 598, 2385–2396. [DOI] [PubMed] [Google Scholar]
- Frinking P, Segers T, Luan Y, and Tranquart F (2020). Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol 46, 892–908. [DOI] [PubMed] [Google Scholar]
- Fry FJ, Ades HW, and Fry WJ (1958). Production of Reversible Changes in the Central Nervous System by Ultrasound. Science 127, 83–84. [DOI] [PubMed] [Google Scholar]
- Gaur P, Casey KM, Kubanek J, Li N, Mohammadjavadi M, Saenz Y, Glover GH, Bouley DM, and Pauly KB (2020). Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep. Brain Stimulat. 13, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinski OB, Tsirulnikov EM, and Shchekanov EE (1977a). A study of reception with the use of focused ultrasound. I. Effects on the skin and deep receptor structures in man. Brain Res. 135, 265–277. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinski OB, Tsirulnikov EM, and Shchekanov EE (1977b). A study of reception with the use of focused ultrasound. I. Effects on the skin and deep receptor structures in man. Brain Res. 135, 265–277. [DOI] [PubMed] [Google Scholar]
- Gesnik M, Blaize K, Deffieux T, Gennisson J-L, Sahel J-A, Fink M, Picaud S, and Tanter M (2017). 3D functional ultrasound imaging of the cerebral visual system in rodents. NeuroImage 149, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Harvey P, Simon JC, and Jasanoff A (2018). Probing the brain with molecular fMRI. Curr. Opin. Neurobiol 50, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhon E, Voisin P, de Zwart JA, Quesson B, Salomir R, Maurange C, Bouchaud V, Smirnov P, de Verneuil H, Vekris A, et al. (2003). Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J Gene Med 5, 333–342. [DOI] [PubMed] [Google Scholar]
- Güler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, and Palmiter RD (2012). Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun 3, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Hamilton li M, Offutt SJ, Gloeckner CD, Li T, Kim Y, Legon W, Alford JK, and Lim HH (2018). Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 99, 866. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, and Barnes CA (2005). Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol 15, 599–606. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Hoffman JM, and Pomper MG (2007). Molecular Neuroimaging: From Conventional to Emerging Techniques. Radiology 245, 21–42. [DOI] [PubMed] [Google Scholar]
- Han S, Kim M, Kim H, Shin H, and Youn I (2018). Ketamine Inhibits Ultrasound Stimulation-Induced Neuromodulation by Blocking Cortical Neuron Activity. Ultrasound Med. Biol 44, 635–646. [DOI] [PubMed] [Google Scholar]
- Harput S, Christensen-Jeffries K, Ramalli A, Brown J, Zhu J, Zhang G, Leow CH, Toulemonde M, Boni E, Tortoli P, et al. (2019). 3-D Super-Resolution Ultrasound (SR-US) Imaging with a 2-D Sparse Array. ArXiv190201608 Phys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiles B, Correia M, Hingot V, Pernot M, Provost J, Tanter M, and Couture O (2019). Ultrafast 3D Ultrasound Localization Microscopy Using a 32 $\times$ 32 Matrix Array. IEEE Trans. Med. Imaging 38, 2005–2015. [DOI] [PubMed] [Google Scholar]
- Heim R, and Tsien RY (1996). Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol 6, 178–182. [DOI] [PubMed] [Google Scholar]
- Heureaux J, Chen D, Murray VL, Deng CX, and Liu AP (2014). Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell. Mol. Bioeng 7, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-S, Fan C-H, Hsu N, Chiu N-H, Wu C-Y, Chang C-Y, Wu B-H, Hong S-R, Chang Y-C, Yan-Tang Wu A, et al. (2020). Sonogenetic Modulation of Cellular Activities Using an Engineered Auditory-Sensing Protein. Nano Lett. 20, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, and Jolesz FA (2001). Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 220, 640–646. [DOI] [PubMed] [Google Scholar]
- Iadecola C (2017). The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen S, Tong A, Schutt C, Esener S, and Chalasani SH (2015). Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun 6, 8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbault M, Chauvet D, Gennisson J-L, Capelle L, and Tanter M (2017). Intraoperative Functional Ultrasound Imaging of Human Brain Activity. Sci. Rep. 7, 7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Gambín S, Jiménez N, Benlloch J, and Camarena F (2019). Holograms to Focus Arbitrary Ultrasonic Fields through the Skull. Phys. Rev. Appl. 12. [Google Scholar]
- Kavalali ET, and Jorgensen EM (2014). Visualizing presynaptic function. Nat. Neurosci. 17, 10–16. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, Kano M, Okuno H, Ohki K, and Bito H (2013). Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat. Methods 10, 889–895. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, and Bito H (2014). A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front. Neural Circuits 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kévin B, Marc G, Fabrice A, Harry A, Ulisse F, Thomas D, Pierre P, Frédéric C, Mathias F, José-Alain S, et al. (2019). Functional ultrasound imaging of deep visual cortex in awake non-human primates (Neuroscience). [Google Scholar]
- Kim H-B, Swanberg KM, Han H-S, Kim J-C, Kim J-W, Lee S, Lee CJ, Maeng S, Kim T-S, and Park J-H (2017). Prolonged stimulation with low-intensity ultrasound induces delayed increases in spontaneous hippocampal culture spiking activity. J. Neurosci. Res 95, 885–896. [DOI] [PubMed] [Google Scholar]
- Kim MG, Kamimura HAS, Lee SA, Aurup C, Kwon N, and Konofagou EE (2020). Image-guided focused ultrasound modulates electrically evoked motor neuronal activity in the mouse peripheral nervous system in vivo. J. Neural Eng 17, 026026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RL, Brown JR, Newsome WT, and Pauly KB (2013). Effective Parameters for Ultrasound-Induced In Vivo Neurostimulation. Ultrasound Med. Biol 39, 312–331. [DOI] [PubMed] [Google Scholar]
- King RL, Brown JR, and Pauly KB (2014). Localization of Ultrasound-Induced In Vivo Neurostimulation in the Mouse Model. Ultrasound Med. Biol 40, 1512–1522. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, and Hynynen K (2006). Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl. Acad. Sci 103, 11719–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöpfel T (2012). Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci 13, 687–700. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Gallero-Salas Y, and Song C (2015). Genetically encoded voltage indicators for large scale cortical imaging come of age. Curr. Opin. Chem. Biol 27, 75–83. [DOI] [PubMed] [Google Scholar]
- Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, and McDannold N (2016). Safety Validation of Repeated Blood–Brain Barrier Disruption Using Focused Ultrasound. Ultrasound Med. Biol 42, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasovitski B, Frenkel V, Shoham S, and Kimmel E (2011). Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U A 108, 3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse DE, Mackanos MA, O’Connell-Rodwell CE, Contag CH, and Ferrara KW (2008). Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys. Med. Biol 53, 3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Shi J, Marsh J, Chen D, Deng C, and Cui J (2016). Ultrasound modulates ion channel currents. Sci Rep 6, 24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Shukla P, Das A, Baccus SA, and Goodman MB (2018). Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J Neurosci 38, 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Brown J, Ye P, Pauly KB, Moore T, and Newsome W (2020). Remote, brain region–specific control of choice behavior with ultrasonic waves. Sci. Adv 6, eaaz4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Jin Z, Nety S, Sawyer DP, Lee-Gosselin A, Malounda D, Maresca D, Swift M, and Shapiro MG (In press). Acoustic biosensors for ultrasound imaging of enzyme activity. Nat. Chem. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Farhadi A, Nety SP, Lee-Gosselin A, Bourdeau RW, Maresca D, and Shapiro MG (2016). Molecular Engineering of Acoustic Protein Nanostructures. ACS Nano 10, 7314–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux LG, and Schellingerhout D (2014). Molecular Neuroimaging: The Basics. Semin. Roentgenol 49, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim H, Jung Y, Song I-U, Chung YA, and Yoo S-S (2015). Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep 5, 8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim HC, Jung Y, Chung YA, Song IU, Lee JH, and Yoo SS (2016). Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep 6, 34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Rowlands A, Opitz A, Sato TF, and Tyler WJ (2012a). Pulsed Ultrasound Differentially Stimulates Somatosensory Circuits in Humans as Indicated by EEG and fMRI. PLOS ONE 7, e51177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Rowlands A, Opitz A, Sato TF, and Tyler WJ (2012b). Pulsed Ultrasound Differentially Stimulates Somatosensory Circuits in Humans as Indicated by EEG and fMRI. PLoS ONE 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, and Tyler WJ (2014). Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 17, 322–329. [DOI] [PubMed] [Google Scholar]
- Legon W, Adams S, Bansal P, Patel PD, Hobbs L, Ai L, Mueller JK, Meekins G, and Gillick BT (2020). A retrospective qualitative report of symptoms and safety from transcranial focused ultrasound for neuromodulation in humans. Sci. Rep 10, 5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MZ, and Schnitzer MJ (2016). Genetically encoded indicators of neuronal activity. Nat. Neurosci 19, 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, et al. (2018). Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun 9, 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2008). Genetic Dissection of Neural Circuits. Neuron 57, 634–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2018). Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macé E, Montaldo G, Cohen I, Baulac M, Fink M, and Tanter M (2011). Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664. [DOI] [PubMed] [Google Scholar]
- Mace E, Montaldo G, Osmanski B-F, Cohen I, Fink M, and Tanter M (2013). Functional ultrasound imaging of the brain: theory and basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 60, 492–506. [DOI] [PubMed] [Google Scholar]
- Macé É, Montaldo G, Trenholm S, Cowan C, Brignall A, Urban A, and Roska B (2018). Whole-Brain Functional Ultrasound Imaging Reveals Brain Modules for Visuomotor Integration. Neuron 100, 1241–1251.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimbourg G, Houdouin A, Deffieux T, Tanter M, and Aubry J-F (2018). 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys. Med. Biol 63, 025026. [DOI] [PubMed] [Google Scholar]
- Marblestone AH, Zamft BM, Maguire YG, Shapiro MG, Cybulski TR, Glaser JI, Amodei D, Stranges PB, Kalhor R, Dalrymple DA, et al. (2013). Physical principles for scalable neural recording. Front. Comput. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Lakshmanan A, Lee-Gosselin A, Melis JM, Ni Y-L, Bourdeau RW, Kochmann DM, and Shapiro MG (2017). Nonlinear ultrasound imaging of nanoscale acoustic biomolecules. Appl. Phys. Lett 110, 073704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Lakshmanan A, Abedi M, Bar-Zion A, Farhadi A, Lu GJ, Szablowski JO, Wu D, Yoo S, and Shapiro MG (2018a). Biomolecular Ultrasound and Sonogenetics. Annu. Rev. Chem. Biomol. Eng 9, 229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Sawyer DP, Renaud G, Lee-Gosselin A, and Shapiro MG (2018b). Nonlinear X-Wave Ultrasound Imaging of Acoustic Biomolecules. Phys. Rev. X 8, 041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Payen T, Lee-Gosselin A, Ling B, Malounda D, Demené C, Tanter M, and Shapiro MG (2020). Acoustic biomolecules enhance hemodynamic functional ultrasound imaging of neural activity. NeuroImage 209, 116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino A, Arai S, Hou Y, Sinibaldi E, Pellegrino M, Chang YT, Mazzolai B, Mattoli V, Suzuki M, and Ciofani G (2015). Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 9, 7678–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, and Ugawa Y (2016). Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Zhang Y, Power C, Arvanitis CD, Vykhodtseva N, and Livingstone M (2015). Targeted, noninvasive blockade of cortical neuronal activity. Sci. Rep 5, 16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B-K, Bystritsky A, Jung K-I, Fischer K, Zhang Y, Maeng L-S, In Park S, Chung Y-A, Jolesz FA, and Yoo S-S (2011). Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Ganesh S, Shahiwala A, and Shah SP (2003). Drug delivery to the central nervous system: a review. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm 6, 252–273. [PubMed] [Google Scholar]
- Mitra RD, Silva CM, and Youvan DC (1996). Fluorescence resonance energy transfer between blue-emitting and red-shifted excitation derivatives of the green fluorescent protein. Gene 173, 13–17. [DOI] [PubMed] [Google Scholar]
- Mohammadjavadi M, Ye PP, Xia A, Brown J, Popelka G, and Pauly KB (2019). Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain Stimulat. 12, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Schnakers C, Korb AS, Bystritsky A, and Vespa PM (2016). Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury: A First-in-Man Report. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 9, 940–941. [DOI] [PubMed] [Google Scholar]
- Norman SL, Maresca D, Christopoulos VN, Griggs WS, Demene C, Tanter M, Shapiro MG, and Andersen RA (2020). Single Trial Decoding of Movement Intentions Using Functional Ultrasound Neuroimaging (Neuroscience). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos V (2010). Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods 7, 603–614. [DOI] [PubMed] [Google Scholar]
- O’Brien WD (2007). Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol 93, 212–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, Hong GS, Koh W, Kwon J, Hwang ES, et al. (2020). Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr Biol 30, 948. [DOI] [PubMed] [Google Scholar]
- O’Reilly MA, Huang Y, and Hynynen K (2010). The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys Med Biol 55, 5251–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanski B-F, Pezet S, Ricobaraza A, Lenkei Z, and Tanter M (2014a). Functional ultrasound imaging of intrinsic connectivity in the living rat brain with high spatiotemporal resolution. Nat. Commun 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanski BF, Martin C, Montaldo G, Laniece P, Pain F, Tanter M, and Gurden H (2014b). Functional ultrasound imaging reveals different odor-evoked patterns of vascular activity in the main olfactory bulb and the anterior piriform cortex. Neuroimage 95, 176–184. [DOI] [PubMed] [Google Scholar]
- Paefgen V, Doleschel D, and Kiessling F (2015). Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front Pharmacol 6, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang YJ, Sadelain M, Shung KK, et al. (2018). Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc Natl Acad Sci U A 115, 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Sprenger H-G, and Langer T (2018). m -AAA proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res. 28, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F (2012). Distribution, formation and regulation of gas vesicles. Nat Rev Micro 10, 705–715. [DOI] [PubMed] [Google Scholar]
- Piech DK, Johnson BC, Shen K, Ghanbari MM, Li KY, Neely RM, Kay JE, Carmena JM, Maharbiz MM, and Muller R (2020). A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng 4, 207–222. [DOI] [PubMed] [Google Scholar]
- Pinton G, Aubry J-F, Bossy E, Muller M, Pernot M, and Tanter M (2012). Attenuation, scattering, and absorption of ultrasound in the skull bone. Med. Phys 39, 299–307. [DOI] [PubMed] [Google Scholar]
- Piraner DI, Farhadi A, Davis HC, Wu D, Maresca D, Szablowski JO, and Shapiro MG (2017a). Going Deeper: Biomolecular Tools for Acoustic and Magnetic Imaging and Control of Cellular Function. Biochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraner DI, Abedi MH, Moser BA, Lee-Gosselin A, and Shapiro MG (2017b). Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem Biol 13, 75–80. [DOI] [PubMed] [Google Scholar]
- Plaksin M, Kimmel E, and Shoham S (2016). Cell-Type-Selective Effects of Intramembrane Cavitation as a Unifying Theoretical Framework for Ultrasonic Neuromodulation. ENeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto ML, Oralkan Ö, Khuri-Yakub BT, and Maduke MC (2013). Dynamic Response of Model Lipid Membranes to Ultrasonic Radiation Force. PLOS oNe 8, e77115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto ML, Firouzi K, Khuri-Yakub BT, and Maduke M (2018). Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med. Biol. 44, 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen A, Werner B, Martin E, and Hynynen K (2014). Numerical simulations of clinical focused ultrasound functional neurosurgery. Phys. Med. Biol 59, 1679–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut C, Correia M, Finel V, Pezet S, Pernot M, Deffieux T, and Tanter M (2019). 4D functional ultrasound imaging of whole-brain activity in rodents. Nat. Methods 16, 994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut C, Ferrier J, Bertolo A, Osmanski B, Mousset X, Pezet S, Deffieux T, Lenkei Z, and Tanter M (2020). Pharmaco-fUS: Quantification of pharmacologically-induced dynamic changes in brain perfusion and connectivity by functional ultrasound imaging in awake mice. NeuroImage 222, 117231. [DOI] [PubMed] [Google Scholar]
- Rau R, Kruizinga P, Mastik F, Belau M, de Jong N, Bosch JG, Scheffer W, and Maret G (2018). 3D functional ultrasound imaging of pigeons. NeuroImage 183, 469–477. [DOI] [PubMed] [Google Scholar]
- Rungta RL, Osmanski B-F, Boido D, Tanter M, and Charpak S (2017). Light controls cerebral blood flow in naive animals. Nat. Commun 8, 14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saita S, Ishihara T, Maeda M, lemura S-I, Natsume T, Mihara K, and Ishihara N (2016). Distinct types of protease systems are involved in homeostasis regulation of mitochondrial morphology via balanced fusion and fission. Genes Cells Devoted Mol. Cell. Mech 21, 408–424. [DOI] [PubMed] [Google Scholar]
- Salahshoor H, Shapiro MG, and Ortiz M (2020). Transcranial Focused Ultrasound Generates Skull-Conducted Shear Waves: Computational Model and Implications for Neuromodulation. BioRxiv 2020.04.16.045237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiotaki G, Acosta C, Wang S, and Konofagou EE (2015). Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood--brain barrier opening in vivo. J Cereb Blood Flow Metab 35, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti JL, Hameroff S, Smith EE, Sato T, Daft CMW, Tyler WJ, and Allen JJB (2020). Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Shapiro MG, and Tsao DY (2018). Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron 98, 1031–1041.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers T, Lassus A, Bussat P, Gaud E, and Frinking P (2018). Improved coalescence stability of monodisperse phospholipid-coated microbubbles formed by flow-focusing at elevated temperatures. Lab. Chip 19, 158–167. [DOI] [PubMed] [Google Scholar]
- Seo D, Neely RM, Shen K, Singhal U, Alon E, Rabaey JM, Carmena JM, and Maharbiz MM (2016). Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron 91, 529–539. [DOI] [PubMed] [Google Scholar]
- Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, and Conolly SM (2014). Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol 9, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi S, Daniels D, Last D, Guez D, Zivli Z, Castel D, Levy Y, Volovick A, Grinfeld J, Rachmilevich I, et al. (2018). Non-thermal focused ultrasound induced reversible reduction of essential tremor in a rat model. Brain Stimul. [DOI] [PubMed] [Google Scholar]
- Sheeran PS, Luois SH, Mullin LB, Matsunaga TO, and Dayton PA (2012). Design of ultrasonically-activatable nanoparticles using low boiling point perfluorocarbons. Biomaterials 33, 3262–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieu L-A, Bergel A, Tiran E, Deffieux T, Pernot M, Gennisson J-L, Tanter M, and Cohen I (2015). EEG and functional ultrasound imaging in mobile rats. Nat. Methods 12, 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloukey S, Vincent AJPE, Satoer DD, Mastik F, Smits M, Dirven CMF, Strydis C, Bosch JG, van der Steen AFW, De Zeeuw CI, et al. (2020). Functional Ultrasound (fUS) During Awake Brain Surgery: The Clinical Potential of Intra-Operative Functional and Vascular Brain Mapping. Front. Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulioti DE, Espindola D, Dayton PA, and Pinton GF (2020). Super-Resolution Imaging Through the Human Skull. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 67, 25–36. [DOI] [PubMed] [Google Scholar]
- Sternson SM, and Roth BL (2014). Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37, 387–407. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, and Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci 21, 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szablowski JO, Lee-Gosselin A, Lue B, Malounda D, and Shapiro MG (2018). Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng 2, 475–484. [DOI] [PubMed] [Google Scholar]
- Szablowski JO, Bar-Zion A, and Shapiro MG (2019). Achieving Spatial and Molecular Specificity with Ultrasound-Targeted Biomolecular Nanotherapeutics. Acc. Chem. Res 52, 2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanter M, and Fink M (2014). Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 102–119. [DOI] [PubMed] [Google Scholar]
- Thevenot E, Jordao JF, O’Reilly MA, Markham K, Weng YQ, Foust KD, Kaspar BK, Hynynen K, and Aubert I (2012). Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther 23, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiran E, Ferrier J, Deffieux T, Gennisson J-L, Pezet S, Lenkei Z, and Tanter M (2017). Transcranial Functional Ultrasound Imaging in Freely Moving Awake Mice and Anesthetized Young Rats without Contrast Agent. Ultrasound Med. Biol 43, 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd N, Zhang Y, Power C, Becerra L, Borsook D, Livingstone M, and McDannold N (2019). Modulation of brain function by targeted delivery of GABA through the disrupted blood-brain barrier. NeuroImage 189, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringali S, Perrot X, Collet L, and Moulin A (2012). Repetitive transcranial magnetic stimulation: Hearing safety considerations. Brain Stimulat. 5, 354–363. [DOI] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, and Tyler WJ (2010). Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 66, 681–694. [DOI] [PubMed] [Google Scholar]
- Tyler WJ (2011). Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist 17, 25–36. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, and Majestic C (2008). Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One 3, e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unekawa M, Tomita M, Tomita Y, Toriumi H, Miyaki K, and Suzuki N (2010). RBC velocities in single capillaries of mouse and rat brains are the same, despite 10-fold difference in body size. Brain Res. 1320, 69–73. [DOI] [PubMed] [Google Scholar]
- Unnikrishnan S, and Klibanov AL (2012). Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. Am. J. Roentgenol 199, 292–299. [DOI] [PubMed] [Google Scholar]
- Urban A, Mace E, Brunner C, Heidmann M, Rossier J, and Montaldo G (2014). Chronic assessment of cerebral hemodynamics during rat forepaw electrical stimulation using functional ultrasound imaging. Neuroimage 101, 138–149. [DOI] [PubMed] [Google Scholar]
- Urban A, Dussaux C, Martel G, Brunner C, Mace E, and Montaldo G (2015). Real-time imaging of brain activity in freely moving rats using functional ultrasound. Nat. Methods 12, 873–878. [DOI] [PubMed] [Google Scholar]
- Verhagen L, Gallea C, Folloni D, Constans C, Jensen DE, Ahnine H, Roumazeilles L, Santin M, Ahmed B, Lehericy S, et al. (2019). Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE (1994). Gas vesicles. Microbiol. Rev 58, 94–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LV, and Hu S (2012). Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Aryal M, Zhong Q, Vyas DB, and Airan RD (2018). Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks. Neuron 100, 728–738.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Olumolade OO, Sun T, Samiotaki G, and Konofagou EE (2015). Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 22, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan J, Wang X, Yuan Y, and Li X (2020). Transcranial Ultrasound Stimulation Directly Influences the Cortical Excitability of the Motor Cortex in Parkinsonian Mice. Mov. Disord 35, 693–698. [DOI] [PubMed] [Google Scholar]
- Westmeyer GG, and Jasanoff A (2007). Genetically controlled MRI contrast mechanisms and their prospects in systems neuroscience research. Magn. Reson. Imaging 25, 1004–1010. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhu X, Chong P, Liu J, Andre LN, Ong KS, Brinson K, Mahdi AI, Li J, Fenno LE, et al. (2019). Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl. Acad. Sci 116, 26332–26342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaodan Niu KY (2018). On the neuromodulatory pathways of the in vivo brain by means of transcranial focused ultrasound. Curr. Opin. Biomed. Eng. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P-F, Phipps MA, Newton AT, Chaplin V, Gore JC, Caskey CF, and Chen LM (2018). Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection. Sci. Rep 8, 7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Pacia CP, Ye D, Zhu L, Baek H, Yue Y, Yuan J, Miller MJ, Cui J, Culver JP, et al. (2020). Sonogenetics for noninvasive and cellular-level neuromodulation in rodent brain. BioRxiv 2020.01.28.919910. [Google Scholar]
- Yao J, Kaberniuk AA, Li L, Shcherbakova DM, Zhang R, Wang L, Li G, Verkhusha VV, and Wang LV (2016). Multi-scale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat. Methods 13, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Tang S, Meng L, Li X, Wen X, Chen S, Niu L, Li X, Qiu W, Hu H, et al. (2018). Ultrasonic Control of Neural Activity through Activation of the Mechanosensitive Channel MscL. Nano Lett. 18, 4148–4155. [DOI] [PubMed] [Google Scholar]
- Ye PP, Brown JR, and Pauly KB (2016). Frequency Dependence of Ultrasound Neurostimulation in the Mouse Brain. Ultrasound Med. Biol 42, 1512–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, Mittelstein DR, Hurt R, Lacroix J, and Shapiro MG (2020). Focused ultrasound excites neurons via mechanosensitive calcium accumulation and ion channel amplification. BioRxiv 2020.05.19.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Bystritsky A, Lee J-H, Zhang Y, Fischer K, Min B-K, McDannold NJ, Pascual-Leone A, and Jolesz FA (2011). Focused ultrasound modulates region-specific brain activity. Neuroimage 56, 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, and Aubry J-F (2013). Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med. Phys 40, 082902. [DOI] [PubMed] [Google Scholar]
- Yu K, Niu X, Krook-Magnuson E, and He B (2019). Intrinsic Cell-type Selectivity and Interneuronal Connectivity Alteration by Transcranial Focused Ultrasound. BioRxiv 576066. [Google Scholar]
- Zhang S, Huang A, Bar-Zion A, Wang J, Mena OV, Shapiro MG, and Friend J The Vibration Behavior of Sub-Micrometer Gas Vesicles in Response to Acoustic Excitation Determined via Laser Doppler Vibrometry. Adv. Funct. Mater n/a, 2000239. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


