Abstract
BACKGROUND:
The idea that memory is stored as enduring changes in the brain dates back at least to the time of Plato and Aristotle (circa 350 BCE), but its scientific articulation emerged in the 20th century when Richard Semon introduced the term “engram” to describe the neural substrate for storing and recalling memories. Essentially, Semon proposed that an experience activates a population of neurons that undergo persistent chemical and/or physical changes to become an engram. Subsequent reactivation of the engram by cues available at the time of the experience induces memory retrieval. After Karl Lashley failed to find the engram in a rat brain, studies attempting to localize an engram were largely abandoned. Spurred by Donald O. Hebb’s theory that augmented synaptic strength and neuronal connectivity are critical for memory formation, many researchers showed that enhanced synaptic strength was correlated with memory. Nonetheless, the causal relationship between these enduring changes in synaptic connectivity with a specific, behaviorally identifiable memory at the level of the cell ensemble (an engram) awaited further advances in experimental technologies.
ADVANCES:
The resurgence in research examining engrams may be linked to two complementary studies that applied intervention strategies to target individual neurons in an engram supporting a specific memory in mice. One study showed that ablating the subset of lateral amygdala neurons allocated to a putative engram disrupted subsequent memory retrieval (loss of function). The second study showed that artificially reactivating a subset of hippocampal dentate gyrus neurons that were active during a fearful experience (and, therefore, part of a putative engram) induced memory retrieval in the absence of external retrieval cues (gain of function). Subsequent findings from many labs used similar strategies to identify engrams in other brain regions supporting different types of memory.
There are several recent advances in engram research. First, eligible neurons within a given brain region were shown to compete for allocation to an engram, and relative neuronal excitability determines the outcome of this competition. Excitability-based competition also guides the organization of multiple engrams in the brain and determines how these engrams interact. Second, research examining the nature of the off-line, enduring changes in engram cells (neurons that are critical components of an engram) found increased synaptic strength and spine density in these neurons as well as preferential connectivity to other downstream engram cells. Therefore, both increased intrinsic excitability and synaptic plasticity work hand in hand to form engrams, and these mechanisms are also implicated in memory consolidation and retrieval processes. Third, it is now possible to artificially manipulate memory encoding and retrieval processes to generate false memories, or even create a memory in mice without any natural sensory experience (implantation of a memory for an experience that did not occur). Fourth, “silent” engrams were discovered in amnesic mice; artificial reactivation of silent engrams induces memory retrieval, whereas natural cues cannot. Endogenous engram silencing may contribute to the change in memory over time (e.g., systems memory consolidation) or in different circumstances (e.g., fear memory extinction). These findings suggest that once formed, an engram may exist in different states (from silent to active) on the basis of their retrievability. Although initial engram studies focused on single brain regions, an emerging concept is that a given memory is supported by an engram complex, composed of functionally connected engram cell ensembles dispersed across multiple brain regions, with each ensemble supporting a component of the overall memory.
OUTLOOK:
The ability to identify and manipulate engram cells and brainwide engram complexes has introduced an exciting new era of memory research. The findings from many labs are beginning to define an engram as the basic unit of memory. However, many questions remain. In the short term, it is critical to characterize how information is stored in an engram, including how engram architecture affects memory quality, strength, and precision; how multiple engrams interact; how engrams change over time; and the role of engram silencing in these processes. The long-term goal of engram research is to leverage the fundamental findings from rodent engram studies to understand how information is acquired, stored, and used in humans and facilitate the treatment of human memory, or other information-processing, disorders. The development of low- to noninvasive technology may enable new human therapies based on the growing knowledge of engrams in rodents.
Graphical Abstract
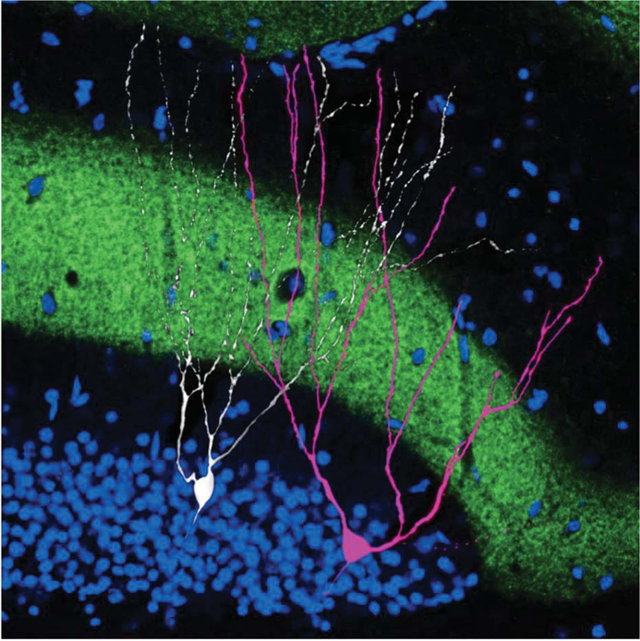
An engram cell alongside a nonengram cell. Within the hippocampus, dentate gyrus cells were filled with biocytin (white) to examine morphology. Engram cells active during context fear conditioning were engineered to express the red fluorescent protein mCherry, which appears pink owing to overlap with biocytin signals. Axons of the perforant path (green) express the excitatory opsin channelrhodopsin 2 and a fluorescent marker (enhanced yellow fluorescent protein). The upper blade of the dentate gyrus granule cell layer is revealed by the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI, blue).
In 1904, Richard Semon introduced the term “engram” to describe the neural substrate for storing memories. An experience, Semon proposed, activates a subset of cells that undergo off-line, persistent chemical and/or physical changes to become an engram. Subsequent reactivation of this engram induces memory retrieval. Although Semon’s contributions were largely ignored in his lifetime, new technologies that allow researchers to image and manipulate the brain at the level of individual neurons has reinvigorated engram research. We review recent progress in studying engrams, including an evaluation of evidence for the existence of engrams, the importance of intrinsic excitability and synaptic plasticity in engrams, and the lifetime of an engram. Together, these findings are beginning to define an engram as the basic unit of memory.
Memory is the ability to use the past in service of the present or future (1, 2). Memory is central to our everyday lives and defines who we are. Without it, we are condemned to an eternal present. That memory persists after an experience suggests that an internal representation of this experience is stored in the brain and that later this representation can be reconstructed and used. In 1904, Richard Semon, an evolutionary zoologist turned memory theorist, introduced the term “engram” to describe such memory representations (3, 4). Semon defined an engram as “…the enduring though primarily latent modifications in the irritable sub-stance produced by a stimulus…” (5, p. 12; 6). He postulated a fundamental “law of engraphy” in which “all simultaneous excitations… form a connected simultaneous complex of excitations which, as such, act engraphically, that is to say leaves behind it a connected, and to that extent, unified engram-complex” (7, p. 159–160). An engram, therefore, is roughly equivalent to a “memory trace.”
Semon’s innovative ideas were largely over-looked or dismissed during his lifetime. However, his theories foreshadowed many prominent contemporary memory concepts (8–11). Semon defined an engram as an off-line, physical change in some aspect of brain state but was suitably cautious when asked to speculate on the precise neural mechanisms underlying an engram, “To follow this into the molecular field seems to me…a hopeless undertaking at the present stage of our knowledge and for my part, I renounce the task” (7, p. 154).
A few years later, though, Karl Lashley, a geneticist turned psychologist, took up this challenge by systematically attempting to localize an engram in a mammalian brain (12–14). In a typical study, Lashley trained rats over many days to solve a maze by running a distinct route to collect a reward. Hypothesizing that some critical component of the engram supporting this maze-route memory is localized in the cortex, Lashley removed cortical tissue of varying sizes from varying locations and then tested the rats’ memory for the maze route. Although the amount of cortical tissue removed correlated with overall memory impairment, the location of the lesion did not. After more than 30 years of searching, Lashley failed to find an engram, declaring it “elusive.”
The next leap in engram-related research came when Donald O. Hebb, a psychologist, memory theorist, and student of Lashley, developed a cell assembly theory (similar to Semon’s engram complex) (15). Hebb hypothesized that a cell assembly is formed between reciprocally interconnected cells that are simultaneously active during an experience. Sufficient activity within the cell assembly induces growth and/or metabolic changes that strengthen the connections between these cells [a concept distilled in the phrase “neurons that fire together, wire together” (16)]. These synaptic and metabolic changes (perhaps including changes in intrinsic neuronal excitability) have implications for the function of a cell assembly. For instance, reactivation of only a fraction of assembly cells was hypothesized to produce reactivation of the entire assembly (15) [a process similar to pattern completion (17–19)]. By contrast, destruction of a fraction of assembly cells would not necessarily produce catastrophic failure of the entire representation (but rather gracefully degrade the representation). Interestingly, Semon also proposed similar types of properties for an engram (5).
Together, these (and other) scientists helped define and describe an engram. However, there was a paucity of studies examining the biological basis of engrams. More than 100 years ago, Semon wrote that to examine the neurobiological basis of an engram represented a “hopeless undertaking.” This may no longer be true. Recent excitement surrounding engram research may stem directly from the development of new tools allowing cell ensembles to be imaged and manipulated at the level of the individual cell. We begin by briefly reviewing the neurobiological evidence supporting the existence of engrams in the rodent brain and our collective ability to not only find but also manipulate engrams to better understand memory. Then, we discuss the current state of engram research by examining the results of explicit engram studies and previous memory and plasticity findings from an engram point of view. Guided by Semon, we define an engram as an enduring off-line representation of a past experience (Box 1). It is important to note that an engram is not yet a memory but rather provides the necessary physical conditions for a memory to emerge (20). Memories are retrieved when appropriate retrieval cues successfully reactivate an engram in a process Semon dubbed “ecphory.”
Box 1. Engram definitions.
An “engram” refers to the enduring offline physical and/or chemical changes that were elicited by learning and underlie the newly formed memory associations.
“Engram cells” are populations of cells that constitute critical cellular components of a given engram. These cells may (or may not) also be critical components of engrams supporting other memories. Engram cells are (i) activated by a learning experience, (ii) physically or chemically modified by the learning experience, and (iii) reactivated by subsequent presentation of the stimuli present at the learning experience (or some portion thereof), resulting in memory retrieval.
An “engram cell ensemble” refers to the collection of engram cells localized within a brain region. Engram cell ensembles in each brain region are connected, forming an “engram complex,” which is the entire brainwide engram supporting a memory that is stored in sets of engram cell ensembles in different brain regions connected via an engram cell pathway.
Experimental strategies to evaluate engrams
To evaluate the existence of engrams, we adapt the criteria and experimental strategies discussed by Morris and colleagues (21, 22) in their landmark papers evaluating the importance of synaptic plasticity in memory. Specifically, we discuss evidence from four types of studies. First, observational studies supporting the existence of engrams in the rodent brain should show that the same (or overlapping) cell populations are activated both by an experience and by retrieval of that experience and that, furthermore, learning should induce long-lasting cellular and/or synaptic modifications in these cells. Second, loss-of-function studies should show that impairing engram cell function after an experience impairs subsequent memory retrieval. Third, gain-of-function studies should show that artificially activating engram cells induces memory retrieval, in the absence of any natural sensory retrieval cues. Fourth, mimicry studies should artificially introduce an engram of an experience that never happened into the brain and show that rodents use the information of an artificial engram to guide behavior.
Memory traces, or at least physiological correlates of memory, have been examined in invertebrate species, such as flies (23–27), octopus (28, 29), Aplysia sea slugs (30, 31), honey bee (32), and Hermissenda sea slugs (33). Moreover, pioneering studies in mammals (34–36) greatly informed our current understanding of the neural basis of memory but did not examine memory at the cell ensemble level. The discussion here is limited primarily to rodent experiments examining memory of an explicit experience that probe memory at the level of an engram.
Observational studies
Typically, observational studies take advantage of immediate early genes (IEGs) such as c-Fos, Arc (activity-regulated cytoskeleton-associated protein), or Zif268 (zinc finger protein 225) (37–39) to visualize active neurons. Cells active during a memory test are marked using IEG immunohistochemistry, whereas cells active during a training experience are “tagged” through the use of temporally inducible IEG promoters that drive the expression of more enduring fluorescent (or other) reporter proteins (40–43). Above-chance overlap between these two cell populations (“active during training” and “active during test”) within a brain region (or throughout the brain) is suggestive of an engram.
In an initial observational study designed to examine a memory at the level of a cell ensemble, Mayford and colleagues (41) tagged neurons active during auditory fear conditioning. In this commonly used memory task, an initially innocuous tone (a conditioned stimulus) is paired with an aversive footshock (an unconditioned stimulus) in a conditioning context. When subsequently reexposed to the tone or conditioning context, rodents freeze (the active, learned conditioned response), showing memory of the training experience (44). In this experiment, mice were replaced in the conditioning context 3 days after training, and active neurons were marked with zif268 immunohistochemistry. Consistent with the existence of an engram supporting this conditioned fear memory, the overlap of neurons active during training (tagged) and testing (zif268+) in the basal amygdala nucleus exceeded chance (~11% total cells) (41).
Similar results, using different tagging methods, across multiple brain regions [including dorsal hippocampus (40, 45–55), amygdala (41, 45, 49, 51, 55, 56), and cortex (42, 45, 55, 57)] were reported for a variety of different memory tasks (including contextual fear conditioning, auditory fear conditioning, and novel object exploration). Control studies revealed that tagged cells were only reactivated by the corresponding conditioned stimulus and not by stimuli unrelated to the training experience (45). Although most observational studies did not address directly the enduring, learning-induced changes hypothesized by Semon, overall, these results (and their notable consistency across methods, tasks, and labs) provide broad support for the existence of engrams. However, causal studies are necessary to show that these reactivated putative engram cells indeed function as part of the internal representation of an experience.
Loss-of-function studies
Loss-of-function studies attempt to “capture” engram cells and specifically disrupt their function before a memory test. Josselyn and colleagues (58) performed the first loss-of-function memory study at the level of a cell ensemble. An allocation strategy was used to capture putative engram cells in the amygdala lateral nucleus (LA) supporting an auditory fear conditioned memory in mice. That is, a small, random population of LA neurons was biased for inclusion (or allocation) into a putative engram using a neurotropic virus expressing CREB (Ca++/cyclic AMP–responsive element-binding protein). CREB is a transcription factor that increases both neuronal excitability (59–64) and dendritic spine density (60, 65). Therefore, neurons infected with this CREB vector were hypothesized to be biased for inclusion into an engram. A virus expressing both CREB (to allocate neurons) and an inducible construct that produces cell-autonomous ablation was used to specifically kill allocated neurons after training (58). Ablating CREB-overexpressing neurons disrupted freezing to subsequent tone presentation, as if the memory was erased (Fig. 1). Importantly, mice were capable of learning a new fear conditioning task (showing overall LA function was not compromised), and ablating a similar number of non–CREB-overexpressing cells (nonengram cells) did not disrupt memory (showing specificity of the memory disruption at the cellular level).
Fig. 1. Engram loss-of-function studies disrupt subsequent memory retrieval.
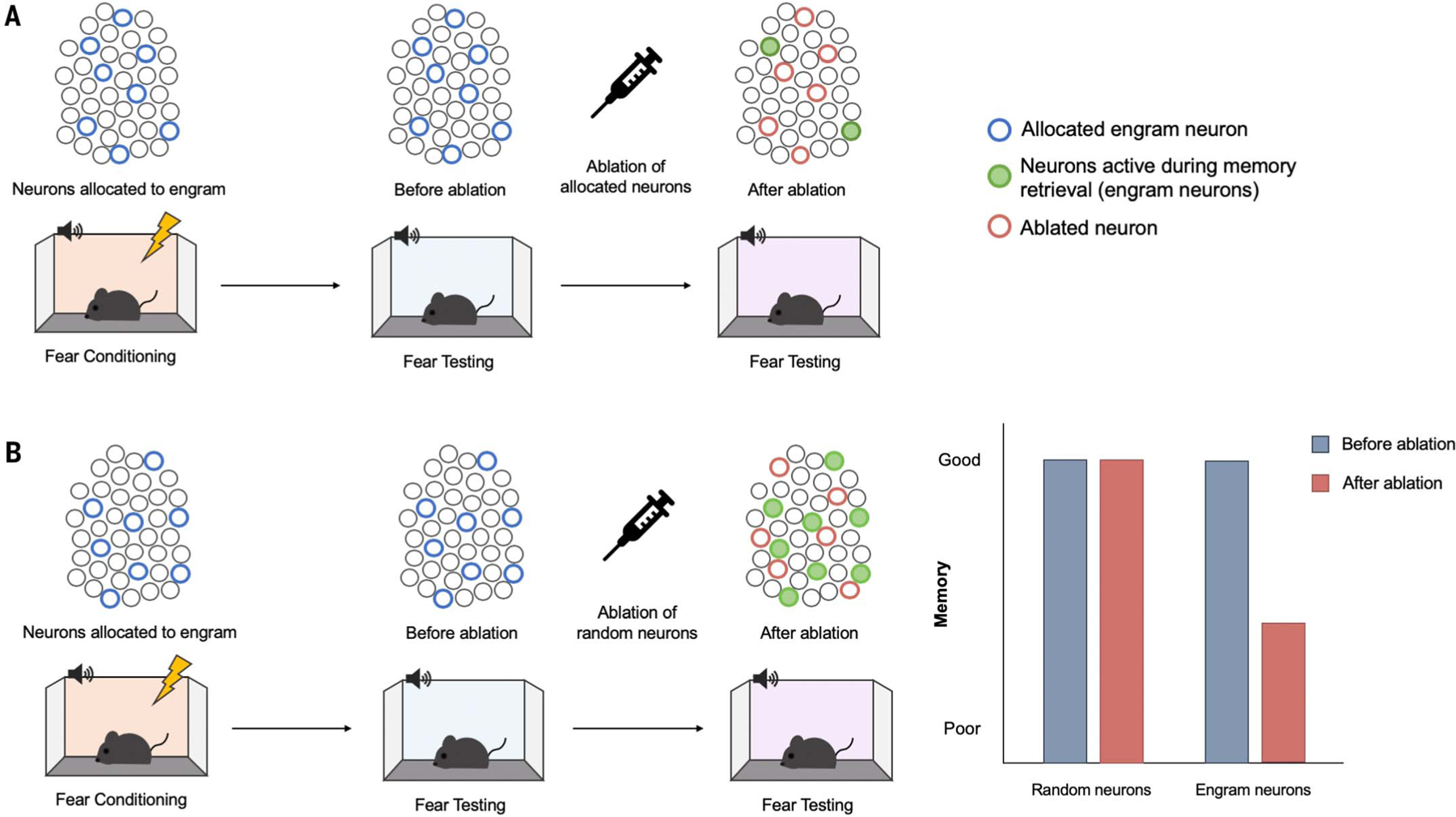
(A) Ablating allocated neurons. Lateral amygdala principal (excitatory) neurons were experimentally allocated to an engram (blue circles) by means of overexpression of the transcription factor CREB (122). Mice received auditory fear conditioning during which a tone (conditioned stimulus) was paired with a footshock (unconditioned stimulus). The majority of allocated neurons are active during the fear memory test (green filled circles), suggesting that allocated neurons are preferentially recruited to an engram supporting this conditioned fear memory. Specifically ablating experimentally allocated neurons (red circles) before a second memory test disrupts memory retrieval. (B) Ablating a similar number of random, nonallocated neurons does not disrupt memory retrieval. [Images: Adapted from (122)].
Subsequent studies using diverse methods to permanently or reversibly inactivate allocated or tagged neurons across several brain areas hypothesized to be part of an engram, in many memory tasks, produced comparable results (40, 48, 53, 63, 66, 67). Together, these findings suggest that neurons active during an experience become engram cells that are indispensable (or somehow necessary) for successful subsequent memory expression.
Why were these loss-of-function studies perhaps successful in “finding an engram” when Lashley was not? First, Lashley may have used an inappropriate behavioral test to probe an engram. The well-learned maze task Lashley typically used could be solved using different strategies and, therefore, may have been in-sensitive to damaging a distinct brain region. Second, Lashley may have targeted the wrong brain region for this type of spatial memory task (68).
Gain-of-function studies
Gain-of-function studies attempt to induce memory retrieval in the absence of natural retrieval cues by artificially reactivating engram cells. Tonegawa and colleagues (69) provided the first gain-of-function evidence for the existence of an engram. Hippocampal dentate gyrus (DG) neurons active during contextual fear conditioning (in which a context was paired with a footshock) were tagged (41) to express the excitatory opsin channelrhodopsin 2 (ChR2) (70). When tested in a nontraining context, mice did not freeze. However, photo-stimulation of tagged engram cells was sufficient to induce freezing, the learning-specific conditioned response (44), even though mice had never been shocked in this nontraining context (Fig. 2). Importantly, light-induced freezing was not due to activation of pre-wired learning-independent neural circuits or a simple reflex response, because similar photo-stimulation of tagged DG neurons failed to induce freezing if downstream CA1 neurons were silenced during training (thereby preventing learning) (71).
Fig. 2. Gain-of-function method for engram identification and distributed engram ensembles.
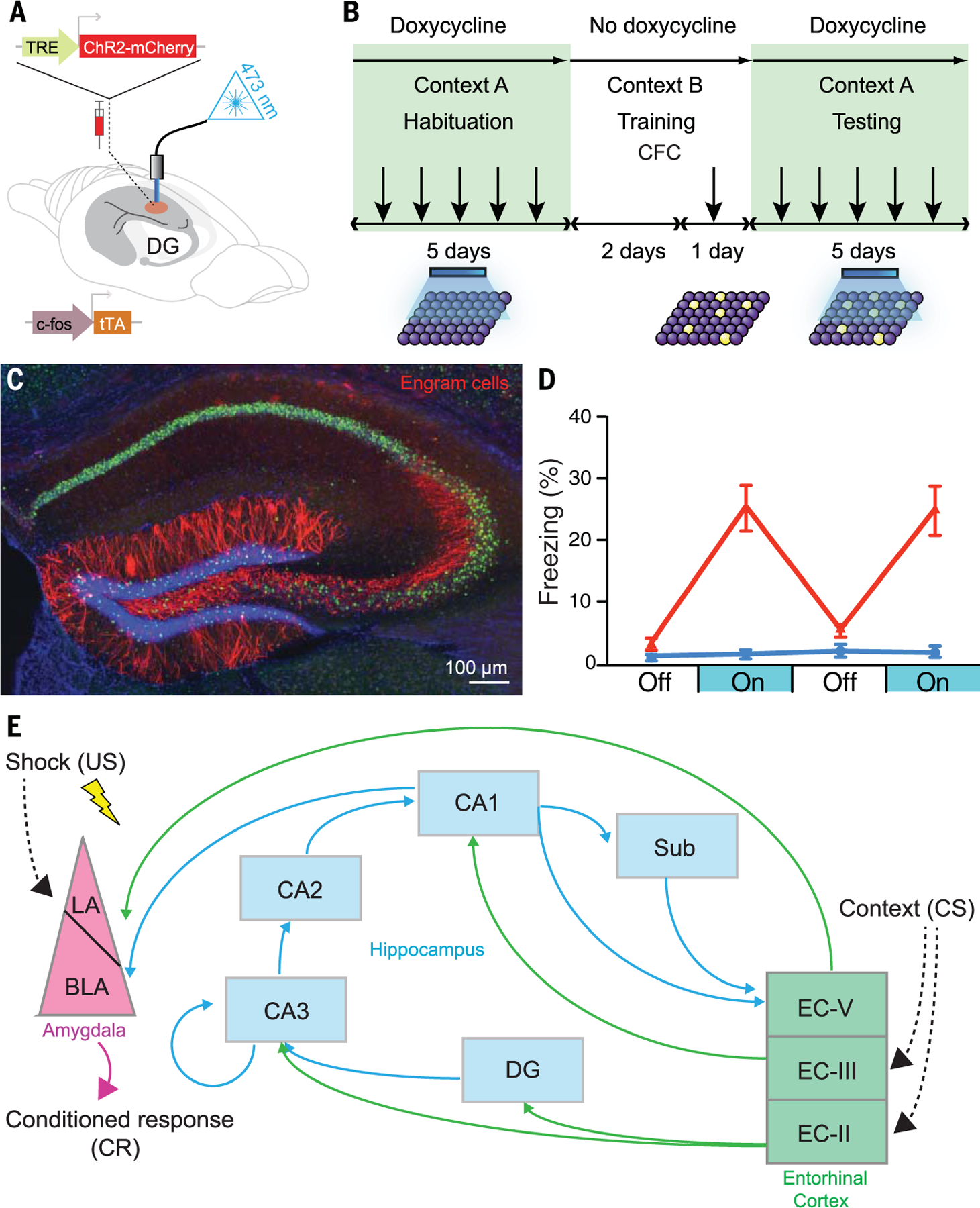
(A) A c-fos–tTA transgenic mouse is injected with AAV9-TRE-ChR2-mCherry (allowing active neurons in the absence of doxycycline to express the excitatory opsin ChR2) and implanted with an optical fiber to target blue light to activate ChR2-expressing neurons in the DG. (B) Basic experimental scheme. Mice are habituated to context A with light stimulation while on doxycycline for 5 days and are then taken off doxycycline for 2 days (to open the tagging window) and exposed to contextual fear conditioning (CFC) in context B. Mice are put back on doxycycline (to close the tagging window) and tested for 5 days in context A with light stimulation. (C) Representative image showing the expression of ChR2-mCherry–positive (red) engram cells in a mouse that was taken off doxycycline for 2 days and underwent CFC training. [Image credit: X. Liu and S. Ramirez (Tonegawa lab)] (D) Mice expressing ChR2 in engram cells from CFC in context B (red) show greater freezing during test light-on epochs in context A than a control group expressing mCherry only. Error bars indicate standard error of the mean. [Graph: Adapted from Liu et al. (69)] (E) A part of the engram cell ensemble complex for contextual fear memory. It is generally thought that the engram for a specific memory is distributed in more than one brain region. For instance, for contextual fear memory, the engram cell ensemble in the entorhinal cortex layer II (EC-II) as well as hippocampal subfields [DG, CA3, CA2, CA1, and subiculum (Sub)] may represent context, whereas amygdala engram cell ensembles represent fear information. These engram cell ensembles are functionally connected to form an engram cell ensemble complex. Thus, a concept has emerged that a specific pattern of cellular connectivity within an engram cell ensemble complex serves as the substrate for a specific memory. US, unconditioned stimulus; LA, lateral nucleus of the amygdala; BLA, basolateral nucleus of the amygdala; CS, conditioned stimulus.
Artificial optogenetic or chemogenetic (72, 73) reactivation of tagged or allocated engram cells across several brain regions similarly induced memory expression without external sensory retrieval cues in a variety of tasks (42, 53, 74–81). Therefore, artificial engram cell reactivation serves as a sufficient retrieval cue to “reawaken” a dormant engram to induce memory expression, similar to Semon’s original definition of ecphory [“the influences which awaken the mnemic trace or engram out of its latent state into one of manifested activity” (5, p. 12)].
Mimicry experiments
During natural memory retrieval, the sensory conditioned stimulus (e.g., the training context) is thought to reactivate engram cells to induce memory retrieval. The first gain-of-function study (69) was designed to mimic this retrieval process by directly reactivating engram cells by means of optogenetic stimulation, thus circumventing the need for the conditioned stimulus. That is, artificial stimulation replaced the natural conditioned stimulus to induce memory retrieval. Optogenetic stimulation of engram cells has also been used to artificially retrieve a previously experienced sensory stimulus during the formation of a new memory. For instance, DG neurons active during exploration of a new context (context A) were photostimulated when mice later received footshocks in a different context (context B). During a memory test, mice replaced in context A froze, even though they had never been shocked in this context. That is, mice retrieved an artificial memory. Mice also froze in context B (showing natural memory retrieval), but not in a third distinct context (context C), indicating freezing was a context-specific, and not a generalized, response (46). Both memories produced by “natural” and “artificial” means could only be retrieved by their respective conditioned stimuli, indicating both memories retained their identities. Similar to a compound conditioned stimulus in which both a tone and light predict footshock, the strength of the natural and artificial memories were roughly 50% of a single “normally induced” memory, suggesting cue competition between the natural and artificial conditioned stimuli [as originally described by (82)]. Therefore, when a biologically important event (e.g., footshock) occurs while an animal is retrieving a previously formed but perhaps unrelated memory, the two stimuli can be associated to form a new but false episodic memory. An analogous mechanism may underlie human false memories, except that in humans, the previously acquired memory would be retrieved by natural processes (83).
Mayford and colleagues (84) used a similar approach but tagged active neurons across the brain as mice explored a new context (context A). Chemogenetically reactivating these neurons while mice were fear conditioned in context B produced a “hybrid or synthetic” context representation that was not retrievable by either context alone [unlike (46), above]. However, mice froze in a test session that more closely matched the training conditions (placement in context B while chemogenetically activating context A engram cells), suggesting that this hybrid memory incorporated both natural and artificial cues. Differences in the spatial and temporal properties of artificial engram reactivation (more acute optogenetic activation of localized tagged DG neurons versus longer-term chemogenetic activation of nonlocalized tagged neurons across the brain) may account for the discrepant outcomes of these two artificial conditioned stimuli studies.
Neurons active during presentation of an unconditioned stimulus have also been tagged and artificially reactivated (85). Neuronal ensembles active during context exploration (the conditioned stimulus) and footshock (the unconditioned stimulus) were tagged separately in the CA1 subfield of the hippocampus and the basolateral complex of the amygdala, respectively. Synchronous optogenetic activation of these ensembles while mice were in the homecage was sufficient to induce a false memory; mice froze in the tagged (but nonshocked) context, as if the conditioned stimulus and unconditioned stimulus had been paired.
Finally, a recent study investigated whether a memory could be implanted through artificial means in the total absence of natural stimuli (either conditioned stimulus or unconditioned stimulus). To be a true memory implantation, such an experiment should satisfy several criteria (86). First, the “learning experience” should occur entirely within the brain through, for example, direct stimulation of putative conditioned-stimulus and unconditioned-stimulus neural pathways. Second, the presence of the implanted memory should be probed through presentation of a “real” external retrieval cue (not just the internal neural cue). Finally, behavioral manifestation of this memory should reflect the predicted memory content and be retrieved only by the “trained” conditioned stimulus (not to similar cues). In this study, optogenetic stimulation of a genetically specific olfactory glomerulus (the conditioned stimulus) was paired with optogenetic stimulation of either appetitive or aversive neural pathways (the unconditioned stimuli) (86). After this entirely intracranial conditioning, mice showed either an attraction or aversion, respectively, to the real odor that activated this olfactory glomerulus. In short, a memory was made in the absence of experience. These results satisfy the mimicry criterion of experimental evidence outlined by Martin and colleagues (21, 22) and, as such, provide another line of persuasive evidence for the existence of engrams.
Understanding memory through engrams
The “enduring changes” of an engram
The ability to label in vivo engram cells supporting a specific memory provided an opportunity to investigate the nature of the “enduring changes” proposed by Semon. Guided by Hebb’s influential theory on the critical importance of synaptic plasticity (the increase in synaptic strength between neurons) in memory [e.g., (21, 22)], Tonegawa and colleagues showed that learning augmented synaptic strength, specifically in engram cells. First, 1 day after training, hippocampal DG granule engram cells tagged during contextual fear conditioning showed greater synaptic strength [higher AMPA/NMDA ratio, which is a means of assessing basal strength of excitatory synapses by examining the relative expression of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR)–mediated synaptic currents to N-methyl-D-aspartate receptor (NMDAR)–mediated synaptic currents of a population of stimulated synapses (87)] and increased spine density at entorhinal cortex junctions than nonengram DG cells (71). Second, compared to nonengram CA3 cells, downstream CA3 engram cells were more functionally connected with upstream DG engram cells (71). Moreover, Kaang and colleagues showed that the number and sizes of spines on CA1 engram cells tagged during contextual fear conditioning receiving input from CA3 engram cells was greater than on nonengram CA1 cells. This enhanced interregional connectivity between CA3 and CA1 engram cells correlated with memory strength and occluded long-term potentiation (LTP), suggesting a previous LTP-like phenomenon endogenously occurred (88). Similarly, LA engram cells tagged during auditory fear conditioning showed enhanced synaptic connectivity with presynaptic neurons (56, 89). Finally, shrinking potentiated synapses in primary motor cortex (M1) engram cells supporting a motor memory disrupted subsequent performance of this, and not a similar, motor memory (90). Together, these studies are beginning to integrate previous research on synaptic plasticity with engrams and suggest preferential engram cell–to–engram cell connectivity is a critical part of the enduring changes to an engram generated by learning. Overall these findings suggest an update of Hebb’s axiom: Engram cells that fire together, wire together.
Distributed engram ensembles
Although one specific brain region is often examined in engram studies, it is generally appreciated that an engram supporting a specific experience may be widely distributed throughout the brain. Engram cell ensembles in different brain regions may support distinct aspects of an experience. For instance, in contextual fear memory, hippocampal (DG, CA3, and CA1) engram cell ensembles may represent the context (40, 48, 91–93), whereas amygdala engram cell ensembles may represent valence information (69, 71, 75), and cortical engram cell ensembles may represent distinct sensory information (79, 94–96).
Several studies have examined potential engram cell ensembles supporting contextual fear memories across the brain (42, 97–99). For instance, Frankland and colleagues compared the brainwide (84 brain regions) distribution of active cells after retrieval of recent (1 day after training) versus remote (36 days after training) contextual fear memory. On the basis of coactivation, graph theory was used to construct functional connectome “memory maps” (97) and identify hub-like regions hypothesized to play privileged roles in memory retrieval. Subsequent chemogenetic inhibition confirmed that these identified hub regions were necessary for subsequent memory retrieval (98). Using a combination of engram tagging technology [targeted recombination in active populations 2 (TRAP2) transgenic mice] and IEG immunohistochemistry to examine overlap between neurons active at contextual fear training and testing, Luo and colleagues (42) showed that retrieval of a remote (14 day) contextual fear memory engaged more neurons in prelimbic cortex than retrieval of a recent (1 day) memory, suggesting that an engram changes over time [consistent with the findings of (100)]. Finally, a preliminary study (99) mapped candidate engram ensembles representing a contextual fear conditioning memory in 409 brain regions in mice. Roy and colleagues tagged cells active at training and those active at recall throughout the brain in the same mouse using a CLARITY-like tissue-clearing technique (101) dubbed SHIELD (stabilization under harsh conditions via intramolecular epoxide linkages to prevent degradation) (102), thereby permitting the entire intact brain to be imaged at once. From this activation data, these researchers developed an “engram index” (defined as the degree to which cells in a given brain region were active at memory encoding and retrieval) that allowed the rank ordering of different brain regions. Using optogenetic and chemogenetic methods to interrogate the effects of artificially activating regions with a high engram index, this study showed many of these engram ensembles are functionally connected and activated simultaneously by an experience. These findings suggest that an experience is represented in specifically connected multiple engram ensembles distributed across multiple brain regions and provide experimental support for Semon’s “unified engram complex” hypothesis.
Engrams, place cells, and sleep
Location-specific firing of CA1 place cells is well established (103). Stable place cells may be important in engrams supporting spatial or contextual memories (104–106). Recently, McHugh and colleagues (107) contrasted the roles of CA1 place cells and engram cells in memory. While mice explored a new context, engram cells were tagged and place cells identified using tetrode recordings. Most tagged engram cells were also place cells, but the majority of place cells were not tagged. Nontagged place cells behaved like traditional place cells (stable in the same context but re-mapping in a new context). By contrast, tagged place cells fired in a context-specific manner, albeit with imprecise spatial information, and were not active (did not remap) in a new context. Therefore, engram cells may provide general contextual information, with nontagged place cells providing precise spatial information.
Postencoding reactivation or replay of hippocampal place cell firing, especially during slow-wave sleep (SWS) (108, 109), is thought to be important for memory consolidation (110–113). During SWS, hippocampal neurons fire in an oscillatory rhythm (termed sharp-wave ripples), tending to co-occur with rhythmic firing of cortical neurons (termed spindles) (114). Disrupting either sharp-wave ripple–spindle coupling (115, 116) or sharp-wave ripple–associated replay of hippocampal place cells (104, 105, 117, 118) impairs memory recall. The precise role of these rhythmic oscillations with respect to engram cells is unclear. Sharp-wave ripples promote synaptic depression of CA1 hippocampal neurons (119, 120). A recent study suggests that CA1 engram cells tagged during context exploration are more likely than nonengram neurons to participate in sharp-wave ripple events, perhaps allowing these engram cells to escape this SWS-induced synaptic depression (120). In this way, postencoding reactivation of engram cells during oscillatory rhythms may help refine an engram by decreasing irrelevant “noise” of nonengram neuronal activity during memory consolidation.
Lifetime of an engram
Birth of an engram
Josselyn, Silva, and colleagues discovered that during engram formation, eligible neurons in a given brain region compete against each other for allocation (or recruitment) to an engram. Neurons with relatively increased intrinsic excitability win this allocation competition to become engram cells (58, 63, 66, 76, 77, 121–126) (Fig. 3). Competitive excitability-based allocation to an engram occurs in other brain regions and supports different types of memories [e.g., dorsal CA1 region of hippocampus (91–93) and prefrontal cortex (126) (for a contextual fear memory), insular cortex (127) (conditioned taste-aversion memory), and retrosplenial cortex (128) (spatial memory)].
Fig. 3. Neuronal allocation to an engram.
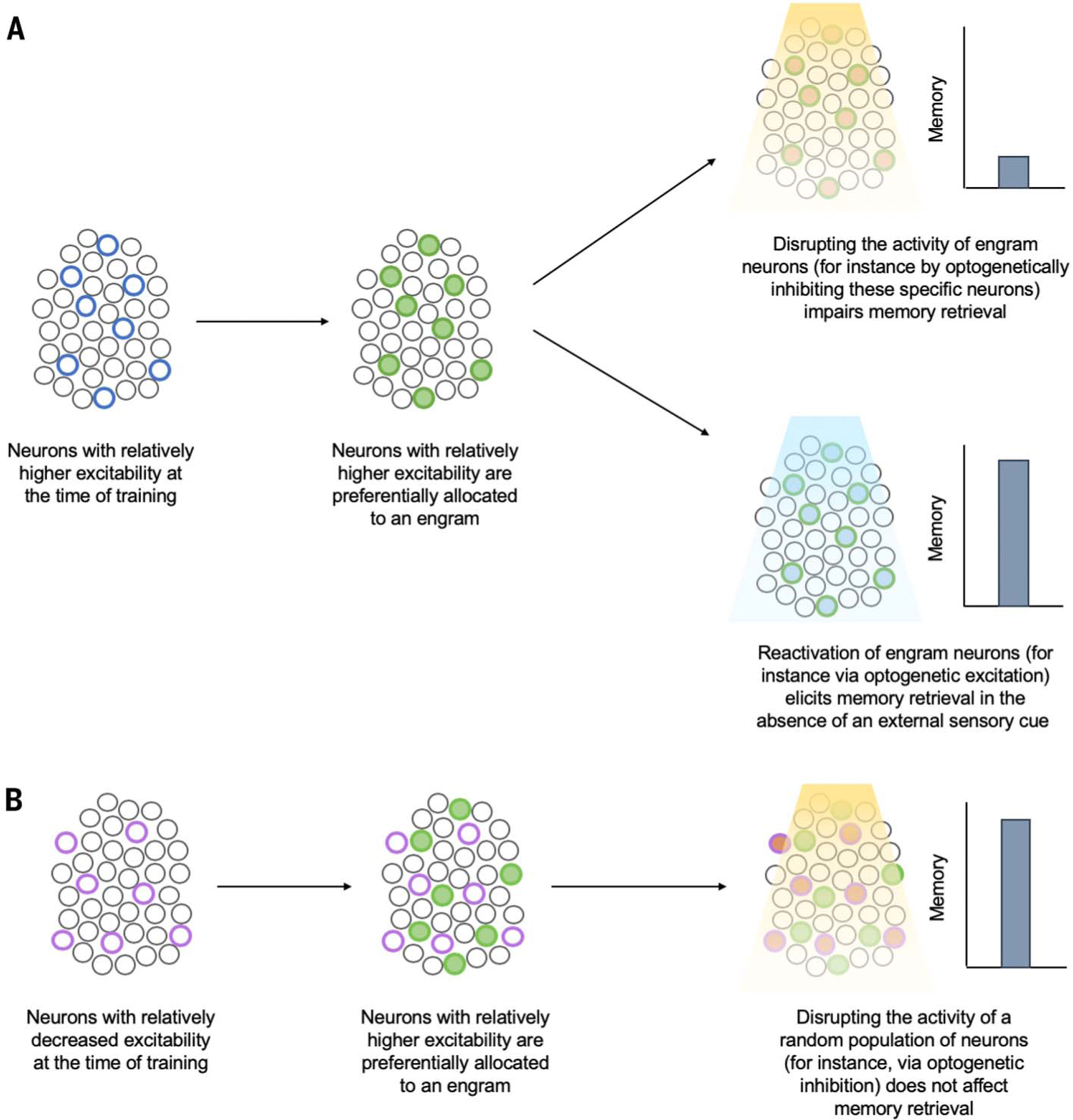
Eligible neurons compete for allocation to an engram supporting a memory, and neurons with increased relative excitability at the time of training “win” this competition for allocation. (A) Neurons that were endogenously more excitable than their neighbors at the time of training or were experimentally manipulated to become relatively more excitable (blue circles) are preferentially allocated to an engram (green filled circles). Subsequent disruption of these allocated or engram neurons disrupts memory retrieval (top right), whereas artificial reactivation of these neurons elicits memory retrieval in the absence of normal sensory retrieval cues (bottom right). (B) Neurons with relatively decreased excitability at the time of training (either endogenously or through experimental manipulation) (purple circles) are preferentially excluded from the engram (green filled circles). Subsequent disruption of nonallocated or nonengram neurons does not impact memory retrieval.
In addition to aversive memories, LA neurons experimentally made more excitable during training were also preferentially allocated to an engram supporting a cocaine-cue rewarding memory (66). Similarly, increasing the excitability of a small, random portion of piriform cortex principal neurons resulted in their allocation to an engram supporting either a rewarding or an aversive olfactory memory, depending on the nature of the training experience (129). Excitability-based neuronal allocation is predicted by computational modeling (130–132), occurs endogenously (56, 89), and is consistent with previous research implicating intrinsic excitability in the formation of invertebrate memory traces (33, 133–135). Together, these findings suggest that in some brain regions, at any given time, a small portion of eligible neurons are “primed” to become part of an engram (should an experience occur), regardless of experience valence.
Although stable place cells and engram cells in dorsal CA1 of the hippocampus differ (107), some mechanisms underlying their formation may be shared. In a given environment, only a small subset of CA1 neurons are place cells, because the majority of CA1 neurons are silent (136). Those neurons with relatively higher excitability immediately before placement in a novel environment are more likely to become place cells in that environment (137–139), and experimentally increasing the excitability of an initially silent cell biased this cell toward becoming a place cell (140, 141).
It is interesting to note the similarities between data from current allocation studies and the long-standing idea of selective stabilization (142, 143). Selective stabilization proposes that multiple prerepresentations are endogenously generated in the brain and only one or a few that fit the situation are selected at any given point of time to control behavior and/or persist. Both allocation and selective stabilization resonate with the conceptual framework of Darwinian competition.
Observational and tagging experimental studies agree with computational theories [e.g., (144)] that an engram is sparsely encoded. That is, not all neurons within a given brain region become an engram cell supporting a particular memory. The size of an engram within a given brain region (that is, the number of engram cells) is stable and invariant to memory strength. For instance, the size of an LA engram (number of LA engram cells) is similar for an auditory fear conditioned memory and a cocaine-cue memory (66, 122, 145), and memory strength does not affect engram size (89, 122) [for review, see (146)]. Rather, a stronger memory engages a greater number of synapses between engram cells (88).
Several lines of evidence suggest that one mechanism constraining engram size involves inhibitory neurons. Thus, inhibiting parvalbumin-containing interneurons in the basolateral amygdala complex increased the size of an engram in the LA supporting an auditory fear memory through a process involving di-synaptic inhibition (145), in which an excitatory neuron inhibits another excitatory neuron via an intervening inhibitory neuron. Moreover, inhibiting somatostatin-containing interneurons increased the size of a DG contextual fear memory engram through a lateral-inhibition like process (147). The importance of inhibitory neurons in engrams has also been highlighted in human studies. For instance, evidence suggests that in the cortex, associative memories are represented in excitatory engrams and matched (equal and opposite) inhibitory engrams. Memories are expressed upon disinhibition of the excitatory engram (148–150). Further exploration of excitatory-inhibitory balance in engram formation, storage, and retrieval is necessary to understand how these opposing forces interact to support memory function.
Silent engrams in memory loss
Engrams may become damaged, such that a memory becomes forever unavailable. However, engrams may also be temporarily inaccessible, such that the engram still exists but cannot be retrieved by natural means. Silent engrams, engrams that cannot be retrieved by natural retrieval cues but can be retrieved with direct optogenetic stimulation, were first revealed in an experiment in which the protein synthesis inhibitor, anisomycin, was administered immediately after contextual fear conditioning in mice (71). Inhibiting protein synthesis before or immediately after an experience is known to induce amnesia (151, 152) and block cellular consolidation (153–155). Cellular consolidation refers to the relatively fast process of memory stabilization thought to involve the expression of genes necessary to strengthen synapses. By contrast, systems consolidation (discussed below) refers to the slower, time-dependent reorganization of memories over distributed brain circuits (156–159).
In this study, mice administered anisomycin immediately after training showed little freezing when replaced in the training context 1 day later (71). Therefore, as expected, disrupting protein synthesis induced retrograde amnesia by blocking cellular consolidation. However, optogenetic reactivation of DG engram neurons tagged during contextual fear training was sufficient for memory recovery, even 8 days after training (Fig. 4). These results indicate that the engram was formed and persisted for several days but that this engram could not be retrieved by natural means. Silent DG engram cells showed weaker physiological (increased synaptic strength) and structural (increased dendritic spine density) alterations than normal engram cells (in control mice), suggesting that a silent engram may be the result of disrupting the synaptic strengthening normally induced by training. That optogenetic activation of DG engram cells was able to induce memory retrieval suggests that direct optogenetic activation was able to circumvent this requirement for synaptic and structural plasticity within engram cells. Consistent with this, genetic restoration of spine density [targeted overexpression of p-21 activated kinase (PAK 1)] also allowed a silent engram to be reactivated and memory expressed by natural retrieval cues (160).
Fig. 4. Active and silent engram cells in amnesia and during memory systems consolidation.
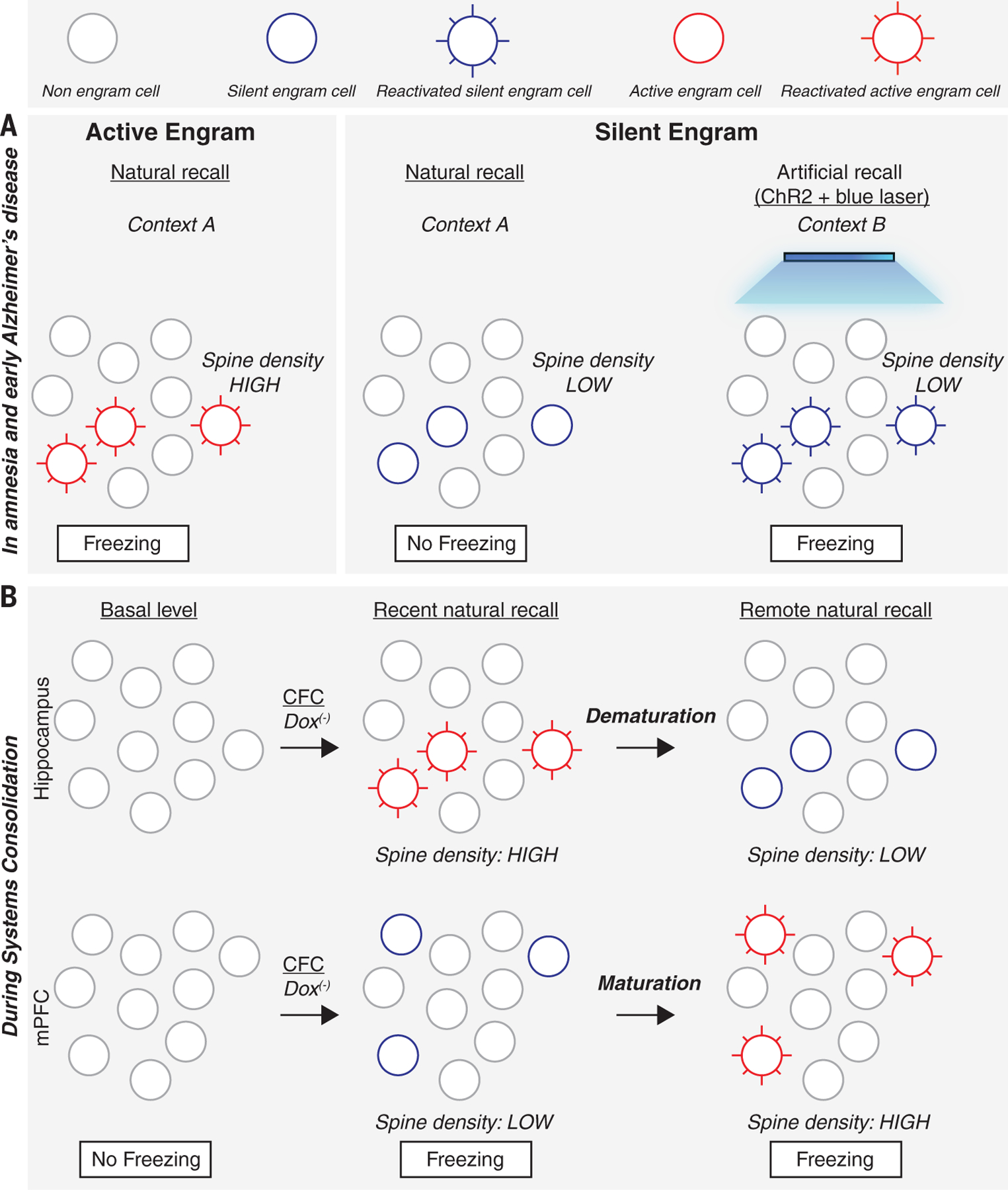
(A) Active engram cells have higher spine density and are activated in the conditioned context A to produce the conditioned response, freezing. Silent engram cells generated in amnesia and in a mouse designed to model early Alzheimer’s disease show lower spine density and cannot be activated in the conditioned context A to produce a conditioned response but can be activated by blue light in an unconditioned context B if they were tagged with ChR2 during encoding. (B) During memory systems consolidation, active engram cells with high spine density are formed in the hippocampus during contextual fear conditioning and for several days, the conditioned context can evoke a conditioned response. However, by two weeks (remote recall), these hippocampal engram cells demature to become silent, with reduced spine density. In the mPFC, engram cells are formed during CFC but are silent with low spine density. During the following 2 weeks, these mPFC silent engram cells acquire higher spine density and become active engram cells.
The idea that engrams may be silenced by disrupting synaptic efficacy and spine density and reawakened by enhancing synaptic plasticity is consistent with findings from a nonengram study examining auditory fear conditioning (161). Rats were trained in a variant of an auditory fear conditioning task in which the tone conditioned stimulus was replaced by optogenetic activation of LA axon terminals from neurons originating in the medial geniculate nucleus and auditory cortex. Immediately after conditioning, long-term depression (LTD)–like optogenetic stimulation was administered. LTD is thought to weaken synaptic efficacy and decrease spine density (162–165). Consistent with the interpretation that LTD-like stimulation silenced the engram, this opto-LTD stimulation im-paired subsequent memory recall. However, LTP-like optogenetic stimulation allowed the memory to be retrieved (consistent with the interpretation that the engram was “unsilenced”). Again, subsequent LTD-like optogenetic stimulation silenced this memory, whereas LTP-like optogenetic stimulation allowed recovery of this memory.
These findings raise the question of whether engrams (and the memories they support) in other amnesic conditions are truly “lost” or are simply inaccessible such that they cannot be retrieved under natural conditions. Silent engrams were reactivated by artificially stimulating engram cells in amnestic mice used to study the early stages of Alzheimer’s disease (AD) (166, 167). These transgenic mice [APP/PS1 mice containing human transgenes with the familial AD mutation in both amyloid precursor protein (APP) and presenilin 1 (PSEN1)] showed contextual fear memory deficits (166). However, optogenetic reactivation of ChR2-labeled DG engram cells induced robust freezing comparable to control mice (166). Consistent with other examples of silent engram cells, DG engram cells in these mice used to study AD showed decreased spine density. However, LTP-like optogenetic stimulation at entorhinal cortex engram cell inputs onto DG engram cells restored not only spine density in DG engram cells but also the ability of natural retrieval cues to elicit memory retrieval (thereby unsilencing the engram) (166). These findings in mice are consistent with reports that memory retrieval in people with early-stage AD may be enhanced by particular retrieval cues (168, 169). Therefore, under certain conditions, a previously inaccessible memory may be retrieved in human AD, consistent with the interpretation that some engrams in early-AD brains may be silent rather than lost.
Apart from clinical implications, the finding of silent engrams is relevant to discussions on the role of protein synthesis–dependent cellular consolidation in terms of memory storage versus retrieval. There has been persistent debate on this issue (170–173). The majority of neuroscientists examining cellular memory consolidation may favor the view that disrupting protein synthesis disrupts memory storage. However, in many amnesia experiments, memory storage is conflated with memory retrieval. The finding that optogenetically stimulating a silent engram in an otherwise amnestic mouse, even 1 week after training, induces memory retrieval challenges the view that protein synthesis–dependent cellular consolidation is important for memory storage. Instead, these findings suggest that the role of cellular consolidation is to enhance subsequent retrievability of an engram, consistent with the idea of engram “retrieval handles” that are established after memory formation and may be remodeled after memory retrieval (1). Importantly, silent engrams are consistent with the pioneering cognitive psychologist Endel Tulving’s (174) conceptual distinction between memory availability and accessibility, in which memory failure may reflect the absence of the information or difficulties accessing the information [see (175) for review].
Silent engrams in normal memory
Memory may change with time and circumstance. Might these changes in memory be mediated by endogenous engram silencing? This was explored in a social discrimination task in which mice interact more with a new, rather than a familiar, mouse. This social discrimination memory lasts roughly an hour after exposure to a familiar mouse (the training experience) and is absent 24 hours after training (176). The dorsal CA2 to ventral CA1 (vCA1) hippocampal circuit plays a pivotal role in social discrimination (177), with a vCA1 engram representing the familiar mouse (178). Consistent with the time course of social discrimination memory, the familiar mouse engram in vCA1 becomes silent an hour after training. However, artificially reactivating this engram 24 hours after training (when the social discrimination memory normally has dissipated) reinstates social discrimination memory, as if the trained-but-forgotten familiar mouse is being remembered. Besides artificial engram reactivation, the accessibility of vCA1 engram (and social discrimination memory) is prolonged by interventions such as group housing. These findings provide a hint that engram silencing may be one way in which the brain normally regulates mnemonic processes.
Additional evidence comes from memory extinction studies. After conditioning, repeated presentation of the conditioned stimuli alone (in the absence of the unconditioned stimulus) produces a gradual decrease of the conditioned response (82)—a phenomenon called extinction. Therefore, after extinction training, the ability of the conditioned stimulus to induce memory retrieval is diminished, an outcome that is similar phenomenologically to engram silencing. Might engram silencing account for extinction? Consistent with this general idea, some auditory fear extinction protocols induce synaptic depotentiation of LA neurons, that is, the reversal of synaptic potentiation induced by fear conditioning (179, 180). Moreover, after fear conditioning, LTD-like electrical stimulation of external capsule inputs to the LA induces synaptic depotentiation and decreases fear behavior (181), resembling both extinction and engram silencing. Finally, shortly after extinction training, the chemogenetic artificial activation of cells tagged brainwide during context fear training (the putative fear engram) was reported to increase freezing levels (182), suggesting that the original fear engram was silenced during extinction. The similarities between engram silencing and extinction are consistent with theoretical views that during extinction, the conditioned stimulus–unconditioned stimulus contingency is “unlearned” (183, 184).
However, other accounts stress that extinction does not reflect unlearning the original association (perhaps by silencing the original engram) but rather reflects learning a new “conditioned stimulus–no unconditioned stimulus” association (185, 186) with a corresponding new extinction engram. That the original memory is not “erased” by extinction is suggested by findings that after extinction training, the conditioned response may return if the conditioned stimulus is presented (i) in a new nonextinction context (renewal), (ii) after a stressor (reinstatement), or (iii) after the passage of time (spontaneous recovery) (187–192). A recent study concluded that contextual fear extinction may be supported by a novel fear extinction engram in the DG that is distinct from and suppresses the contextual fear DG engram with a time course that corresponds to the emergence of spontaneous recovery (53). In this experiment, spontaneous recovery was observed remotely (29 days), but not recently (6 days), after extinction training. Moreover, the original fear engram was reactivated at the remote, but not recent, memory test after extinction training. The opposite pattern of results was observed for active cells tagged after extinction training (the presumed fear extinction engram). Interestingly, artificial reactivation of the fear extinction engram prevented spontaneous recovery of the original fear memory, even at remote times. These results suggest that the original fear engram and the extinction engram compete for control over behavior; the extinction engram first suppressed or silenced the original fear engram, but, with time, the fear extinction engram was itself silenced. Conversely, activation of a remote DG contextual fear engram (labeled 25 days after contextual fear conditioning) itself may also be important for subsequent fear memory extinction (52), perhaps similar to a process referred to as reconsolidation-updating (193, 194). However, the extent to which DG neurons that were activated 25 days after contextual fear conditioning overlap with DG neurons active during training remains an open question (40, 51).
Finally, a recent study examined fear extinction engrams in the amygdala and found that extinction engram cells were formed in a genetically distinct and “reward-responsive” subpopulation of basal amygdala neurons. These fear extinction engram cells suppressed the fear engram neurons that were also present in basal amygdala and, furthermore, induced appetitive behavior when optogenetically stimulated (195). These findings in mice are consistent with the results of a recent study in fruit flies (26) and highlight the similarities between fear extinction and reward processes across species. Moreover, these results are consistent with the general idea of competition between memory traces in the control of behavior.
Silent engrams and time
The representation of a memory in the brain may change with time. For instance, dorsal hippocampal lesions in rodents disrupt expression of contextual fear memories in the days, but not weeks after training (196–198). At more remote times, cortical areas, including anterior cingulate cortex or medial prefrontal cortex (mPFC), become preferentially engaged (100). The time-dependent reorganization of memory reflects systems consolidation, a process that typically refers to initially hippocampal-based episodic-like memories (158, 159). Systems consolidation was recently examined at the level of the engram in the hippocampus and mPFC, where findings indicate time-dependent silencing of active engrams and conversions of silent engrams to active engrams (51, 199). During contextual fear conditioning, active mPFC neurons were labeled to express ChR2. When placed in the conditioning context, mice showed robust freezing when tested either 2 days or 13 days after training. However, the engram ensemble components supporting memory retrieval differed with test time. Tagged mPFC neurons were reactivated 13 days, but not 2 days, after training, suggesting that the mPFC engram was silent shortly after training but active after longer delays. DG engram cells showed an opposite pattern; DG engram cells were reactivated shortly after training but silenced more remotely. Similar to other instances of silent engrams discussed above, the mPFC engram cells shortly after training and the DG engram cells at longer delays after training showed reduced spine density, and, furthermore, optogenetic activation of these silent engrams was sufficient to induce memory retrieval. Interestingly, posttraining tetanus toxin–induced inhibition of the input from DG engram cells to mPFC engram cells blocked the maturation of the silent mPFC engram cells to an accessible state, suggesting coordinated network function between different engram ensemble components is important in systems consolidation.
Memories may also become less precise and more generalized with time (200–202). According to memory transformation theory, changes in the nature and quality of memories correspond to changes in neural representations, with hippocampal-dependent context-specific detailed memories transforming into gist-like schematic memories represented in cortical structures over time (201, 203, 204). The neural processes governing remote memory generalization at the engram level suggest that the availability of the DG engram is critical for memory specificity (205). In this experiment, shortly after contextual fear conditioning (1 day), mice froze in the training context only, whereas at more remote time points (16 days after training), mice also froze in a nonshocked context. This finding is consistent with previous reports of contextual fear memory generalizing over time (51, 201). At the recent, but not remote, time, DG engram cells showed greater connectivity to parvalbumin-expressing CA3 basket cells (thereby inhibiting CA3 pyramidal neurons through feedforward inhibition) than nonengram DG cells, suggesting that greater feedforward inhibition in DG-CA3 circuits helps maintain memory precision. Interestingly, optogenetic activation of DG engram cells 10 days after training did not induce memory retrieval (suggesting that this engram had become unavailable), except if feedforward inhibition of CA3 pyramidal neurons was genetically enhanced. Moreover, mice with genetically enhanced feedforward inhibition also showed precise memory, even when tested at more remote times. Together, these data suggest that enhanced feedforward inhibition onto CA3 neurons maintains DG engram cell availability and delays the loss of context specificity associated with remote memories.
These findings suggest that engram silencing may represent a continuum of a natural state of an engram. That is, an engram may be (i) unavailable (neither natural conditioned stimuli nor artificial reactivation induces memory expression), (ii) silenced (only artificial reactivation is sufficient to induce memory expression), (iii) dormant or latent, as initially named by Semon (natural conditioned stimuli may induce memory retrieval), or (iv) active (currently being retrieved). Different processes may mediate these distinct engram states. For example, similar to silencing a DG engram, posttraining anisomycin administration silenced an LA engram supporting an auditory fear memory (79). However, if in addition to anisomycin, a peptide to induce autophagy (a mechanism of protein degradation) was administered after training, then optogenetic reactivation of inputs to the LA was no longer sufficient to induce memory retrieval (206), suggesting that autophagy made the engram unavailable rather than simply inaccessible.
From engrams to knowledge
Thus far, we have discussed engrams supporting a single memory. Of course, animals (including humans) learn and remember many things. Some of these experiences may be best remembered as distinct episodes, rich with episodic details (207–209). However, in other circumstances, it may be advantageous to link related experiences, thereby creating a general concept or principle (210–214). This raises the question of how engrams representing different experiences interact. The mechanisms governing neuronal allocation to an engram supporting a single experience also serve to either coallocate neurons to overlapping engrams (thereby linking experiences) or disallocate neurons to nonoverlapping engrams (thereby disambiguating experiences) (121, 215–217) (Fig. 5). In this way, relative neuronal excitability is critical not only for initial engram formation but also in organizing different memory representations across the brain.
Fig. 5. Neuronal allocation and memory linking.
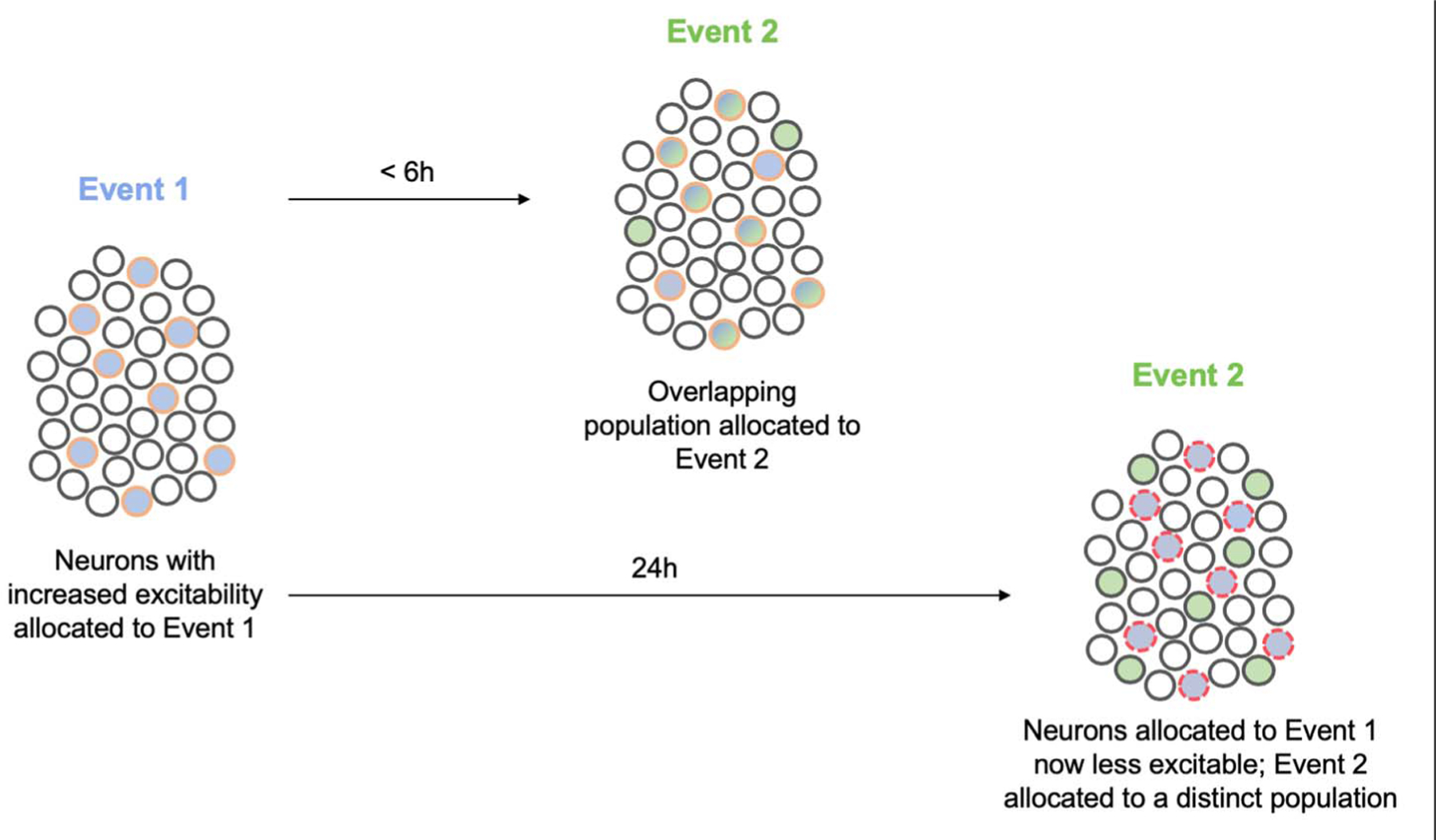
Neurons with increased excitability at the time of event 1 (blue) are allocated to the engram supporting this memory (blue filled circles outlined in orange). These allocated engram neurons remain more excitable than their neighbors for several hours after event 1. If a similar event 2 (green) occurs during this time, neurons allocated to the engram supporting event 1 are more excitable and, therefore, also allocated to the engram supporting event 2 (blue and green filled circles outlined in orange). In this way, neurons are coallocated to events 1 and 2. By virtue of coallocation, these two memories become linked. After some time, neurons allocated to the engram supporting event 1 become less excitable than their neighbors (“refractory”), and if event 2 occurs in this time window, a new population of more excitable neurons wins the competition for allocation to the engram supporting event 2. This disallocation allows the two memories to be remembered separately. Circles with red dashed outlines represent less excitable neurons.
Neurons that are relatively more excitable than their neighbors at the time of an experience are more likely to be allocated to the engram supporting the memory of that experience (121). Increased excitability in engram cells is also maintained for several hours after an experience (215, 218, 219). Therefore, if a related experience occurs in this time window, these same (or overlapping) engram cells are more excitable than their neighbors and thus coallocated to the engram supporting the memory of the second experience. Because the memories of the two experiences are coallocated to overlapping engram cells, these two memories become linked (or integrated); thinking of one experience automatically makes one think of the second. For example, LA neurons allocated to one fear memory were coallocated to a second fear memory if the second event occurred minutes to hours (30 min to 6 hours), but not 24 hours, after the first (215). This linking occurred even if the conditioned stimuli used in the two training sessions were of different modalities (e.g., a light and a tone or a context and a tone). Similarly, coallocation of CA1 engram cells supporting memories of two distinct contexts was observed if exposure to the contexts was separated by a short time interval (216). Behaviorally extinguishing one memory produced extinction for the second memory, even though the second memory was not behaviorally extinguished, indicating that the two memories were functionally linked (215). Coallocated memories may maintain their distinct identity by engaging specific synapses within shared engram cells (79). Moreover, in addition to integrating two similar memories (two fear memories or two contextual memories), two aversive, but otherwise dissimilar memories (a conditioned fear and a conditioned taste aversion memory), were integrated by repeated coretrieval of these memories (220). Overall, these data from rodent experiments agree with results from human memory experiments showing that the representations of memories for events experienced close in time or with related content overlap may be integrated or linked, thus enabling generalization and flexible use of this shared information [e.g., (212, 221–224)].
Memory retrieval also transiently reactivates engram cells (89, 215, 219). This increase in excitability both enhances the precision and efficiency of memory retrieval (219) and opens a new “coallocation window” (215), perhaps explaining how new information is integrated into preexisting knowledge.
Conclusions and perspectives
Overall, these studies provide persuasive evidence for the existence of engrams in rodent brains. We agree with Endel Tulving who stated “As a scientist I am compelled to the conclusion—not postulation, not assumption, but conclusion—that there must exist certain physical-chemical changes in the nervous tissue that correspond to the storage of information, or to the engram, changes that constitute the necessary conditions of remembering. (The alternative stance, that it may be possible for any behavior or any thought to occur independently of physical changes in the nervous system, as all your good readers know, is sheer mysticism)” (225). The findings from many labs using different methods to examine many types of memory converge to support the idea that complex information may not be represented in single cells [e.g., a “grandmother cell” (226, 227)]; instead, these findings suggest that the basic unit of computation in the brain is an engram (228, 229).
To understand a complex, multilayered system such as the brain, it is crucial to causally link a process or phenomenon occurring at a lower level of complexity to those at higher levels. Traditionally, such studies have been carried out using interventions such as tissue lesion or pharmacological disruption. Many of the studies discussed in this review took advantage of state-of-the-art intervention techniques and their combinations, including temporally inducible targeted transgenics and optogenetics, that may generally permit the identification of more precise cause-consequence relationships. Nevertheless, even advanced interventions inevitably artificially manipulate the brain and therefore provide information as to what an engram can do, but not necessarily what it does do (physiologically). This point has been articulated in several other reviews on memory research [e.g., (230)]. However, the results of these intervention studies provide direction as to which processes we should focus our efforts to understand how the brain actually forms and retrieves memory. Furthermore, the high specificity of the state-of-the-art intervention methods, spanning from the molecular level up to the behavioral level, have already revealed mechanisms that would have been difficult to study using other techniques. For instance, these artificial intervention studies allowed the field to identify the silent state of an engram and the mechanism underlying memory allocation.
More than 100 years ago, Semon put forth a law of engraphy. Combining these theoretical ideas with the new tools that allow researchers to image and manipulate engrams at the level of cell ensembles facilitated many important insights into memory function. For instance, evidence indicates that both increased intrinsic excitability and synaptic plasticity work hand in hand to form engrams and that these processes may also be important in memory linking, memory retrieval, and memory consolidation. Interestingly, disrupting synaptic plasticity in engram cells either by disease processes (as in mice used to study AD) or amnestic drugs (such as protein synthesis inhibitors) or during some natural behaviors (housing condition in social discrimination memory, memory systems consolidation, and perhaps fear extinction training) silences engrams such that they can no longer be accessed by normal sensory cues. However, these studies show that silent engrams still exist in the brain and that the information they represent may not be forever lost. The pioneering psychologist and behaviorist Edward Tolman (231) advanced the concept of latent learning and latent memory: learning that occurs without reinforcement, the memory of which is not revealed or expressed until the need or motivation for the acquired knowledge arises (232, 233). It would be interesting to determine whether at least some latent memories are based on silent engrams and, if so, use the conversion of silent engram to active engram as a means of identifying and characterizing the brain circuits mediating the relevant motivation.
A continuum of engram accessibility states may exist. Engrams may be entirely unavailable and not retrievable, even through artificial means (the memory would be forgotten). Or, engrams may be silenced such that memories may be retrieved by artificially reactivating engram cells. The processes that silence or erase an engram, as well as strategies for unsilencing engrams, are a subject for further investigation. That it was possible to artificially reactivate silent engrams in mice designed to study the memory deficits of AD hint at the extraordinary translational potential of this line of research.
Some additional general themes emerge from the results of engram studies. The first theme is that findings from engram studies are reminiscent of reconsolidation studies. Upon retrieval, a memory may enter a labile and modifiable state that lasts for several hours. The process of restabilizing this memory is referred to as reconsolidation. Although reconsolidation has a longer history (234), the modern reawakening of this phenomenon stems from a finding by Nader, LeDoux, and Schafe (235). At the time that this ground-breaking study was conducted, the general thinking was that memories become stabilized in a process of cellular consolidation that occurs once, shortly after a learning experience. However, Nader, LeDoux, and Schafe challenged this view by showing that memory retrieval opens a several-hour “reconsolidation window” during which different interventions may weaken or strengthen the original memory. For instance, disrupting protein synthesis during the reconsolidation window of a conditioned fear memory produced apparent amnesia for this memory. This result was replicated and generalized to several types of memory (156, 236, 237). There are many similarities between this reconsolidation blockade and engram silencing. For instance, reconsolidation blockade is only observed when a memory is being actively retrieved, because administering anisomycin (or another similar intervention) in the absence of memory reactivation does not impair its subsequent retrieval. Viewed from an “engram conceptual framework,” retrieval of a specific memory would activate the underlying engram, and disrupting protein synthesis shortly after this activation might silence this engram. The function of reconsolidation may be to update a memory (1, 211, 238–240). That the reconsolidation window is not unlike the coallocation window suggests that these two processes might be similar ways of explaining the same (or similar) phenomenon at different levels of analysis.
A second emerging theme is that of competition. Allocation to an engram involves competition between eligible neurons within a given brain region at the time of memory encoding. Competition represents a fundamental property of many biological systems and has been previously shown to be important in other mnemonic phenomena. For instance, memory traces may compete for control of behavior at the time of retrieval (241). In addition, human studies reveal that memories may compete if they are linked to a common retrieval cue. Retrieval of a target memory may lead to retrieval-induced forgetting of currently irrelevant competing memories (242).
Although recent engram studies have offered important insights into memory, several key questions remain. First, although the majority of observational studies reveal that the overlap between populations of neurons active during training and testing exceed chance levels, the overall correspondence between these two populations is relatively low (roughly 10 to 40%, depending on the study). That this overlap does not approach 100% suggests a number of possibilities. First, the methods to label active neurons using IEG promoters may be imprecise (either “overtagging” or “under-tagging” the “real” engram at training and/or testing). Alternatively, engrams may be dynamic, even over relatively short (days) periods of time, with cells “dropping into” or “dropping out of” the engram as it is refined or consolidated (243, 244). It will be interesting to determine how the mechanisms of engram silencing contribute to and/or interact with this refinement process and the implications this may have on memory quality, precision, or strength. Moreover, it will be important to determine how engrams change over more prolonged periods of time. For example, do all engrams (engrams representing different types of memories such as episodic, semantic, or even procedural or motor memories, with different valence) change over time, gradually engaging more cortical regions? Is there a role for top-down (mPFC to hippocampal) processing in the dematuration of hippocampal engrams and a possible role of silent hippocampal engrams in remote memory recall?
Second, how can we leverage our knowledge of engrams in rodents to better understand human memory? There is good evidence for general engram-like memory representations in humans [e.g., (245)], but, to date, there are no compelling findings at the cellular ensemble level. To extend the findings from rodent engram studies to humans, it may be necessary to develop non- to low-invasive methods to image and manipulate engrams at the single-cell or specific ensemble level in humans. Progress in this general area of human “artificial memory manipulation” has been made by harnessing the power of reconsolidation (194, 235, 237, 246, 247) in which engram cells are thought to be specifically reactivated by memory retrieval. Pharmacological blockade of reconsolidation and noninvasive techniques that “update” memory during reconsolidation have shown some success in manipulating human memories (248, 249).
Finally, it is important that the links between neuroscience and artificial intelligence (AI) are leveraged to inform both fields. Understanding how the brain encodes, stores, and uses information, especially at the level of the engram, can help inspire the development of more intelligent machines. For instance, engrams and how engrams serve to link memories and organize information in the brain may motivate the development of new algorithms and AI architectures to better allow these agents to form generalizations and schema. In addition, machine learning and deep neural networks may inspire or generate testable theories at the level of the engram for neuroscientists to investigate. In this way, uniting the foundational theories of AI pioneer Alan Turing with those of Endel Tulving could benefit both AI and memory research.
ACKNOWLEDGMENTS
We thank our many colleagues for interesting conversations that shaped this review. In particular, we would like to acknowledge the contributions of Y. Dudai, P. Frankland, S. Köhler, M. Pignatelli, and S. Waddell, as well as J. Lau (for figure preparation) and D. Roy and J. Yu (for a sorted publication list); and the members of the Josselyn, Tonegawa, and Frankland labs for helpful discussions.
Funding: Supported by the Canadian Institute of Health Research (CIHR, FDN-388455), the Natural Science and Engineering Research Council (NSERC) Discovery Grant, the Canadian Institute for Advanced Studies (CiFAR) Grant, and the NIH (NIMH, 1 R01 MH119421-01) (to S.A.J); and by RIKEN’s Center for Brain Science, Howard Hughes Medical Institute (HHMI), and JPB Foundation (to S.T.).
Footnotes
Competing interests:
The authors declare no competing interests.
REFERENCES AND NOTES
- 1.Dudai Y, The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol 55, 51–86 (2004). doi: 10.1146/annurev.psych.55.090902.142050 [DOI] [PubMed] [Google Scholar]
- 2.Schacter DL, Constructive memory: Past and future. Dialogues Clin. Neurosci 14, 7–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schacter DL, Stranger Behind the Engram: Theories of Memory and the Psychology of Science (Erlbaum Associates, 1982). [Google Scholar]
- 4.Schacter DL, Eich JE, Tulving E, Richard Semon’s theory of memory. J. Verbal Learn. Verbal Behav 17, 721–743 (1978). doi: 10.1016/S0022-5371(78)90443-7 [DOI] [Google Scholar]
- 5.Semon R, The Mneme (G. Allen & Unwin, 1921). [Google Scholar]
- 6.Semon R, Die Mneme als erhaltendes Prinzip im Wechsel des organischen Geschehens, Engelmann W, Ed. (Leipzig, 1904). [Google Scholar]
- 7.Semon RW, Mnemic Psychology (G. Allen & Unwin, 1923). [Google Scholar]
- 8.Josselyn SA, Köhler S, Frankland PW, Finding the engram. Nat. Rev. Neurosci 16, 521–534 (2015). doi: 10.1038/nrn4000 [DOI] [PubMed] [Google Scholar]
- 9.Josselyn SA, Köhler S, Frankland PW, Heroes of the engram. J. Neurosci 37, 4647–4657 (2017). doi: 10.1523/JNEUROSCI.0056-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonegawa S, Liu X, Ramirez S, Redondo R, Memory engram cells have come of age. Neuron 87, 918–931 (2015). doi: 10.1016/j.neuron.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 11.Schacter DL, Forgotten Ideas, Neglected Pioneers: Richard Semon and the Story of Memory (Psychology Press, 2001). [Google Scholar]
- 12.Lashley KS, in Society of Experimental Biology Symposium, No. 4: Psychological Mechanisms in Animal Behavior, Danielli JF, Brown R, Eds. (Academic Press, 1950), pp. 454–482. [Google Scholar]
- 13.Lashley KS, Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain (Dover Books on Psychology, vol. T1038, Dover Publications, 1963). [Google Scholar]
- 14.Lashley KS, Mass action in cerebral function. Science 73, 245–254 (1931). doi: 10.1126/science.73.1888.245 [DOI] [PubMed] [Google Scholar]
- 15.Hebb DO, The Organization of Behavior: A Neuropsychological Theory (Wiley, 1949). [Google Scholar]
- 16.Shatz CJ, The developing brain. Sci. Am 267, 60–67 (1992). doi: 10.1038/scientificamerican0992-60 [DOI] [PubMed] [Google Scholar]
- 17.Marr D, Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. Ser. B 262, 23–81 (1971). doi: 10.1098/rstb.1971.0078 [DOI] [PubMed] [Google Scholar]
- 18.Hunsaker MR, Kesner RP, The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci. Biobehav. Rev 37, 36–58 (2013). doi: 10.1016/j.neubiorev.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 19.Knierim JJ, Neunuebel JP, Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem 129, 38–49 (2016). doi: 10.1016/j.nlm.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscovitch M, in Science of Memory: Concepts, Roediger HLI, Dudai Y, Fitzpatrick SM, Eds. (Oxford Univ. Press, 2007), pp. 17–29. [Google Scholar]
- 21.Martin SJ, Grimwood PD, Morris RG, Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci 23, 649–711 (2000). doi: 10.1146/annurev.neuro.23.1.649 [DOI] [PubMed] [Google Scholar]
- 22.Martin SJ, Morris RG, New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus 12, 609–636 (2002). doi: 10.1002/hipo.10107 [DOI] [PubMed] [Google Scholar]
- 23.Yu D, Tan Y, Chakraborty M, Tomchik S, Davis RL, Elongator complex is required for long-term olfactory memory formation in Drosophila. Learn. Mem 25, 183–196 (2018). doi: 10.1101/lm.046557.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owald D et al. , Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86, 417–427 (2015). doi: 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perisse E et al. , Aversive learning and appetitive motivation toggle feed-forward inhibition in the Drosophila mushroom body. Neuron 90, 1086–1099 (2016). doi: 10.1016/j.neuron.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felsenberg J et al. , Integration of parallel opposing memories underlies memory extinction. Cell 175, 709–722.e15 (2018). doi: 10.1016/j.cell.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyashita T, Kikuchi E, Horiuchi J, Saitoe M, Long-term memory engram cells are established by c-Fos/CREB transcriptional cycling. Cell Rep. 25, 2716–2728.e3 (2018). doi: 10.1016/j.celrep.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 28.Young JZ, Computation in the learning system of cephalopods. Biol. Bull 180, 200–208 (1991). doi: 10.2307/1542389 [DOI] [PubMed] [Google Scholar]
- 29.Young JZ, in Cephalopod Neurobiology: Neuroscience Studies in Squid, Octopus and Cuttlefish, Abbott NJ, Williamson R, Maddock L, Eds. (Oxford Univ. Press, 1995), pp. 431–443. [Google Scholar]
- 30.Bédécarrats A, Chen S, Pearce K, Cai D, Glanzman DL, RNA from trained Aplysia can induce an epigenetic engram for long-term sensitization in untrained Aplysia. eNeuro 5, ENEURO.0038–0018.2018 (2018). doi: 10.1523/ENEURO.0038-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carew TJ, Hawkins RD, Abrams TW, Kandel ER, A test of Hebb’s postulate at identified synapses which mediate classical conditioning in Aplysia. J. Neurosci 4, 1217–1224 (1984). doi: 10.1523/JNEUROSCI.04-05-01217.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzel R, Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem 8, 53–62 (2001). doi: 10.1101/lm.38801 [DOI] [PubMed] [Google Scholar]
- 33.Alkon DL, Calcium-mediated reduction of ionic currents: A biophysical memory trace. Science 226, 1037–1045 (1984). doi: 10.1126/science.6093258 [DOI] [PubMed] [Google Scholar]
- 34.Mccormick DA et al. , The engram found? Role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses. Bull. Psychon. Soc 18, 103–105 (1981). doi: 10.3758/BF03333573 [DOI] [Google Scholar]
- 35.Fuster JM, Jervey JP, Inferotemporal neurons distinguish and retain behaviorally relevant features of visual stimuli. Science 212, 952–955 (1981). doi: 10.1126/science.7233192 [DOI] [PubMed] [Google Scholar]
- 36.Miyashita Y, Neuronal correlate of visual associative long-term memory in the primate temporal cortex. Nature 335, 817–820 (1988). doi: 10.1038/335817a0 [DOI] [PubMed] [Google Scholar]
- 37.Greenberg ME, Ziff EB, Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311, 433–438 (1984). doi: 10.1038/311433a0 [DOI] [PubMed] [Google Scholar]
- 38.Curran T, Morgan JI, Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science 229, 1265–1268 (1985). doi: 10.1126/science.4035354 [DOI] [PubMed] [Google Scholar]
- 39.Guzowski JF, McNaughton BL, Barnes CA, Worley PF, Imaging neural activity with temporal and cellular resolution using FISH. Curr. Opin. Neurobiol 11, 579–584 (2001). doi: 10.1016/S0959-4388(00)00252-X [DOI] [PubMed] [Google Scholar]
- 40.Denny CA et al. , Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014). doi: 10.1016/j.neuron.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reijmers LG, Perkins BL, Matsuo N, Mayford M, Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007). doi: 10.1126/science.1143839 [DOI] [PubMed] [Google Scholar]
- 42.DeNardo LA et al. , Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci 22, 460–469 (2019). doi: 10.1038/s41593-018-0318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sørensen AT et al. , A robust activity marking system for exploring active neuronal ensembles. eLife 5, e13918 (2016). doi: 10.7554/eLife.13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanselow MS, Lester LS, in Evolution and Learning, Bolles RC, Beecher MD, Eds. (Lawrence Erlbaum Associates, Inc., 1988), pp. 185–212. [Google Scholar]
- 45.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ, Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol 23, 99–106 (2013). doi: 10.1016/j.cub.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 46.Ramirez S et al. , Creating a false memory in the hippocampus. Science 341, 387–391 (2013). doi: 10.1126/science.1239073 [DOI] [PubMed] [Google Scholar]
- 47.Deng W, Mayford M, Gage FH, Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312 (2013). doi: 10.7554/eLife.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka KZ et al. , Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014). doi: 10.1016/j.neuron.2014.09.037 [DOI] [PubMed] [Google Scholar]
- 49.Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS, Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J. Neurosci 34, 8462–8466 (2014). doi: 10.1523/JNEUROSCI.3624-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakazawa Y, Pevzner A, Tanaka KZ, Wiltgen BJ, Memory retrieval along the proximodistal axis of CA1. Hippocampus 26, 1140–1148 (2016). doi: 10.1002/hipo.22596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitamura T et al. , Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017). doi: 10.1126/science.aam6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalaf O et al. , Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242 (2018). doi: 10.1126/science.aas9875 [DOI] [PubMed] [Google Scholar]
- 53.Lacagnina AF et al. , Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci 22, 753–761 (2019). doi: 10.1038/s41593-019-0361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura H, Teshirogi C, Nakayama D, Minami M, Ikegaya Y, Prior observation of fear learning enhances subsequent self-experienced fear learning with an overlapping neuronal ensemble in the dorsal hippocampus. Mol. Brain 12, 21 (2019). doi: 10.1186/s13041-019-0443-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trouche S, Sasaki JM, Tu T, Reijmers LG, Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013). doi: 10.1016/j.neuron.2013.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonaka A et al. , Synaptic plasticity associated with a memory engram in the basolateral amygdala. J. Neurosci 34, 9305–9309 (2014). doi: 10.1523/JNEUROSCI.4233-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie H et al. , In vivo imaging of immediate early gene expression reveals layer-specific memory traces in the mammalian brain. Proc. Natl. Acad. Sci. U.S.A 111, 2788–2793 (2014). doi: 10.1073/pnas.1316808111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han JH et al. , Selective erasure of a fear memory. Science 323, 1492–1496 (2009). doi: 10.1126/science.1164139 [DOI] [PubMed] [Google Scholar]
- 59.Dong Y et al. , CREB modulates excitability of nucleus accumbens neurons. Nat. Neurosci 9, 475–477 (2006). doi: 10.1038/nn1661 [DOI] [PubMed] [Google Scholar]
- 60.Marie H, Morishita W, Yu X, Calakos N, Malenka RC, Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 45, 741–752 (2005). doi: 10.1016/j.neuron.2005.01.039 [DOI] [PubMed] [Google Scholar]
- 61.Han MH et al. , Role of cAMP response element-binding protein in the rat locus ceruleus: Regulation of neuronal activity and opiate withdrawal behaviors. J. Neurosci 26, 4624–4629 (2006). doi: 10.1523/JNEUROSCI.4701-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez de Armentia M et al. , cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J. Neurosci 27, 13909–13918 (2016). doi: 10.1523/JNEUROSCI.3850-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Y et al. , CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci 12, 1438–1443 (2009). doi: 10.1038/nn.2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benito E, Barco A, CREB’s control of intrinsic and synaptic plasticity: Implications for CREB-dependent memory models. Trends Neurosci. 33, 230–240 (2010). doi: 10.1016/j.tins.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 65.Sargin D et al. , CREB regulates spine density of lateral amygdala neurons: Implications for memory allocation. Front. Behav. Neurosci 7, 209 (2013). doi: 10.3389/fnbeh.2013.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsiang HL et al. , Manipulating a “cocaine engram” in mice. J. Neurosci 34, 14115–14127 (2014). doi: 10.1523/JNEUROSCI.3327-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koya E et al. , Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci 12, 1069–1073 (2009). doi: 10.1038/nn.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milner PM, Cell assemblies: Whose idea? Psycholoquy 10, 1 (1999). [Google Scholar]
- 69.Liu X et al. , Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). doi: 10.1038/nature11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K, Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–1268 (2005). doi: 10.1038/nn1525 [DOI] [PubMed] [Google Scholar]
- 71.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S, Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015). doi: 10.1126/science.aaa5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U.S.A 104, 5163–5168 (2007). doi: 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nichols CD, Roth BL, Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front. Mol. Neurosci 2, 16 (2009). doi: 10.3389/neuro.02.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cowansage KK et al. , Direct reactivation of a coherent neocortical memory of context. Neuron 84, 432–441 (2014). doi: 10.1016/j.neuron.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Redondo RL et al. , Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014). doi: 10.1038/nature13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yiu AP et al. , Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron 83, 722–735 (2014). doi: 10.1016/j.neuron.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 77.Rogerson T et al. , Synaptic tagging during memory allocation. Nat. Rev. Neurosci 15, 157–169 (2014). doi: 10.1038/nrn3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH, Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci 17, 65–72 (2014). doi: 10.1038/nn.3592 [DOI] [PubMed] [Google Scholar]
- 79.Abdou K et al. , Synapse-specific representation of the identity of overlapping memory engrams. Science 360, 1227–1231 (2018). doi: 10.1126/science.aat3810 [DOI] [PubMed] [Google Scholar]
- 80.Guskjolen A et al. , Recovery of “lost” infant memories in mice. Curr. Biol 28, 2283–2290.e3 (2018). doi: 10.1016/j.cub.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 81.Ghandour K et al. , Orchestrated ensemble activities constitute a hippocampal memory engram. Nat. Commun 10, 2637 (2019). doi: 10.1038/s41467-019-10683-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pavlov I, Conditioned Reflexes (Oxford Univ. Press, 1927). [Google Scholar]
- 83.Schacter DL, Loftus EF, Memory and law: What can cognitive neuroscience contribute? Nat. Neurosci 16, 119–123 (2013). doi: 10.1038/nn.3294 [DOI] [PubMed] [Google Scholar]
- 84.Garner AR et al. , Generation of a synthetic memory trace. Science 335, 1513–1516 (2012). doi: 10.1126/science.1214985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohkawa N et al. , Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep. 11, 261–269 (2015). doi: 10.1016/j.celrep.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 86.Vetere G et al. , Memory formation in the absence of experience. Nat. Neurosci 22, 933–940 (2019). doi: 10.1038/s41593-019-0389-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kauer JA, Malenka RC, Synaptic plasticity and addiction. Nat. Rev. Neurosci 8, 844–858 (2007). doi: 10.1038/nrn2234 [DOI] [PubMed] [Google Scholar]
- 88.Choi JH et al. , Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435 (2018). doi: 10.1126/science.aas9204 [DOI] [PubMed] [Google Scholar]
- 89.Gouty-Colomer LA et al. , Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 21, 364–375 (2016). doi: 10.1038/mp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayashi-Takagi A et al. , Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338 (2015). doi: 10.1038/nature15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park S et al. , Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 41, 2987–2993 (2016). doi: 10.1038/npp.2016.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekeres MJ et al. , Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J. Neurosci 32, 17857–17868 (2012). doi: 10.1523/JNEUROSCI.1419-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sekeres MJ, Neve RL, Frankland PW, Josselyn SA, Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn. Mem 17, 280–283 (2010). doi: 10.1101/lm.1785510 [DOI] [PubMed] [Google Scholar]
- 94.Sacco T, Sacchetti B, Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329, 649–656 (2010). doi: 10.1126/science.1183165 [DOI] [PubMed] [Google Scholar]
- 95.Kim WB, Cho JH, Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J. Neurosci 37, 4868–4882 (2017). doi: 10.1523/JNEUROSCI.3579-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y et al. , Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat. Neurosci 19, 1348–1355 (2016). doi: 10.1038/nn.4370 [DOI] [PubMed] [Google Scholar]
- 97.Wheeler AL et al. , Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol 9, e1002853 (2013). doi: 10.1371/journal.pcbi.1002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vetere G et al. , Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94, 363–374.e4 (2017). doi: 10.1016/j.neuron.2017.03.037 [DOI] [PubMed] [Google Scholar]
- 99.Roy DS et al. , Brain-wide mapping of contextual fear memory engram ensembles supports the dispersed engram complex hypothesis. bioRxiv 668483 [Preprint]. 12 June 2019. .doi: 10.1101/668483 [DOI] [Google Scholar]
- 100.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ, The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304, 881–883 (2004). doi: 10.1126/science.1094804 [DOI] [PubMed] [Google Scholar]
- 101.Chung K et al. , Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013). doi: 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park YG et al. , Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O’Keefe J, Dostrovsky J, The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). doi: 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- 104.Ego-Stengel V, Wilson MA, Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB, Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci 12, 1222–1223 (2009). doi: 10.1038/nn.2384 [DOI] [PubMed] [Google Scholar]
- 106.McHugh TJ et al. , Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 (2007). doi: 10.1126/science.1140263 [DOI] [PubMed] [Google Scholar]
- 107.Tanaka KZ et al. , The hippocampal engram maps experience but not place. Science 361, 392–397 (2018). doi: 10.1126/science.aat5397 [DOI] [PubMed] [Google Scholar]
- 108.Wilson MA, McNaughton BL, Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994). doi: 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- 109.Diekelmann S, Born J, The memory function of sleep. Nat. Rev. Neurosci 11, 114–126 (2010). doi: 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- 110.Joo HR, Frank LM, The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci 19, 744–757 (2018). doi: 10.1038/s41583-018-0077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buzsáki G, Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). doi: 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carr MF, Jadhav SP, Frank LM, Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat. Neurosci 14, 147–153 (2011). doi: 10.1038/nn.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kudrimoti HS, Barnes CA, McNaughton BL, Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J. Neurosci 19, 4090–4101 (1999). doi: 10.1523/JNEUROSCI.19-10-04090.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siapas AG, Wilson MA, Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 (1998). doi: 10.1016/S0896-6273(00)80629-7 [DOI] [PubMed] [Google Scholar]
- 115.Xia F et al. , Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife 6, e27868 (2017). doi: 10.7554/eLife.27868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abbas AI et al. , Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100, 926–939.e3 (2018). doi: 10.1016/j.neuron.2018.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J, The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci 13, 995–1002 (2010). doi: 10.1038/nn.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jadhav SP, Kemere C, German PW, Frank LM, Awake hippocampal sharp-wave ripples support spatial memory. Science 336, 1454–1458 (2012). doi: 10.1126/science.1217230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewis S, Sleep: Ever-decreasing ripples. Nat. Rev. Neurosci 19, 184 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Norimoto H et al. , Hippocampal ripples down-regulate synapses. Science 359, 1524–1527 (2018). doi: 10.1126/science.aao0702 [DOI] [PubMed] [Google Scholar]
- 121.Josselyn SA, Frankland PW, Memory allocation: Mechanisms and function. Annu. Rev. Neurosci 41, 389–413 (2018). doi: 10.1146/annurev-neuro-080317-061956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han JH et al. , Neuronal competition and selection during memory formation. Science 316, 457–460 (2007). doi: 10.1126/science.1139438 [DOI] [PubMed] [Google Scholar]
- 123.Han JH et al. , Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn. Mem 15, 443–453 (2008). doi: 10.1101/lm.993608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Josselyn SA, Continuing the search for the engram: Examining the mechanism of fear memories. J. Psychiatry Neurosci 35, 221–228 (2010). doi: 10.1503/jpn.100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J, Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395 (2009). doi: 10.1126/science.1174519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matos MR et al. , Memory strength gates the involvement of a CREB-dependent cortical fear engram in remote memory. Nat. Commun 10, 2315 (2019). doi: 10.1038/s41467-019-10266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sano Y et al. , CREB regulates memory allocation in the insular cortex. Curr. Biol 24, 2833–2837 (2014). doi: 10.1016/j.cub.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Czajkowski R et al. , Encoding and storage of spatial information in the retrosplenial cortex. Proc. Natl. Acad. Sci. U.S.A 111, 8661–8666 (2014). doi: 10.1073/pnas.1313222111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi GB et al. , Driving opposing behaviors with ensembles of piriform neurons. Cell 146, 1004–1015 (2011). doi: 10.1016/j.cell.2011.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim D, Paré D, Nair SS, Assignment of model amygdala neurons to the fear memory trace depends on competitive synaptic interactions. J. Neurosci 33, 14354–14358 (2013). doi: 10.1523/JNEUROSCI.2430-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim D, Paré D, Nair SS, Mechanisms contributing to the induction and storage of Pavlovian fear memories in the lateral amygdala. Learn. Mem 20, 421–430 (2013). doi: 10.1101/lm.030262.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim D, Samarth P, Feng F, Pare D, Nair SS, Synaptic competition in the lateral amygdala and the stimulus specificity of conditioned fear: A biophysical modeling study. Brain Struct. Funct 221, 2163–2182 (2016). doi: 10.1007/s00429-015-1037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alkon DL, Changes of membrane currents during learning. J. Exp. Biol 112, 95–112 (1984). [DOI] [PubMed] [Google Scholar]
- 134.Alkon DL, Lederhendler I, Shoukimas JJ, Primary changes of membrane currents during retention of associative learning. Science 215, 693–695 (1982). doi: 10.1126/science.7058334 [DOI] [PubMed] [Google Scholar]
- 135.Scholz KP, Byrne JH, Long-term sensitization in Aplysia: Biophysical correlates in tail sensory neurons. Science 235, 685–687 (1987). doi: 10.1126/science.2433766 [DOI] [PubMed] [Google Scholar]
- 136.Wilson MA, McNaughton BL, Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 (1993). doi: 10.1126/science.8351520 [DOI] [PubMed] [Google Scholar]
- 137.Cohen JD, Bolstad M, Lee AK, Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. eLife 6, e23040 (2017). doi: 10.7554/eLife.23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Epsztein J, Brecht M, Lee AK, Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120 (2011). doi: 10.1016/j.neuron.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rich PD, Liaw HP, Lee AK, Large environments reveal the statistical structure governing hippocampal representations. Science 345, 814–817 (2014). doi: 10.1126/science.1255635 [DOI] [PubMed] [Google Scholar]
- 140.Lee D, Lin BJ, Lee AK, Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science 337, 849–853 (2012). doi: 10.1126/science.1221489 [DOI] [PubMed] [Google Scholar]
- 141.Rickgauer JP, Deisseroth K, Tank DW, Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci 17, 1816–1824 (2014). doi: 10.1038/nn.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Changeux J-P, Danchin A, Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature 264, 705–712 (1976). doi: 10.1038/264705a0 [DOI] [PubMed] [Google Scholar]
- 143.Young JZ, Learning as a process of selection and amplification. J. R. Soc. Med 72, 801–814 (1979). doi: 10.1177/014107687907201103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanerva P, Sparse Distributed Memory (MIT Press, 1988). [Google Scholar]
- 145.Morrison DJ et al. , Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol. Learn. Mem 135, 91–99 (2016). doi: 10.1016/j.nlm.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 146.Rao-Ruiz P, Yu J, Kushner SA, Josselyn SA, Neuronal competition: Microcircuit mechanisms define the sparsity of the engram. Curr. Opin. Neurobiol 54, 163–170 (2019). doi: 10.1016/j.conb.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P, Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron 89, 1074–1085 (2016). doi: 10.1016/j.neuron.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 148.Barron HC, Vogels TP, Behrens TE, Ramaswami M, Inhibitory engrams in perception and memory. Proc. Natl. Acad. Sci. U.S.A 114, 6666–6674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barron HC et al. , Unmasking latent inhibitory connections in human cortex to reveal dormant cortical memories. Neuron 90, 191–203 (2016). doi: 10.1016/j.neuron.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hennequin G, Agnes EJ, Vogels TP, Inhibitory plasticity: Balance, control, and codependence. Annu. Rev. Neurosci 40, 557–579 (2017). doi: 10.1146/annurev-neuro-072116-031005 [DOI] [PubMed] [Google Scholar]
- 151.Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA, Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur. J. Neurosci 18, 3080–3088 (2003). doi: 10.1111/j.1460-9568.2003.03063.x [DOI] [PubMed] [Google Scholar]
- 152.Schafe GE, LeDoux JE, Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci 20, RC96 (2000). doi: 10.1523/JNEUROSCI.20-18-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hernandez PJ, Abel T, The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol. Learn. Mem 89, 293–311 (2008). doi: 10.1016/j.nlm.2007.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE, Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn. Mem 6, 97–110 (1999). [PMC free article] [PubMed] [Google Scholar]
- 155.Josselyn SA, Kida S, Silva AJ, Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem 82, 159–163 (2004). doi: 10.1016/j.nlm.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 156.Dudai Y, Eisenberg M, Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron 44, 93–100 (2004). doi: 10.1016/j.neuron.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 157.Wang SH, Morris RG, Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu. Rev. Psychol 61, 49–79, C1–C4 (2010). doi: 10.1146/annurev.psych.093008.100523 [DOI] [PubMed] [Google Scholar]
- 158.Frankland PW, Bontempi B, The organization of recent and remote memories. Nat. Rev. Neurosci 6, 119–130 (2005). doi: 10.1038/nrn1607 [DOI] [PubMed] [Google Scholar]
- 159.Wiltgen BJ, Brown RAM, Talton LE, Silva AJ, New circuits for old memories: The role of the neocortex in consolidation. Neuron 44, 101–108 (2004). doi: 10.1016/j.neuron.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 160.Roy DS, Muralidhar S, Smith LM, Tonegawa S, Silent memory engrams as the basis for retrograde amnesia. Proc. Natl. Acad. Sci. U.S.A 114, E9972–E9979 (2017). doi: 10.1073/pnas.1714248114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nabavi S et al. , Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014). doi: 10.1038/nature13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Monfils MH, Teskey GC, Induction of long-term depression is associated with decreased dendritic length and spine density in layers III and V of sensorimotor neocortex. Synapse 53, 114–121 (2004). doi: 10.1002/syn.20039 [DOI] [PubMed] [Google Scholar]
- 163.Zhou Q, Homma KJ, Poo MM, Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004). doi: 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 164.Bourne JN, Harris KM, Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci 31, 47–67 (2008). doi: 10.1146/annurev.neuro.31.060407.125646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bear MF, Malenka RC, Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol 4, 389–399 (1994). doi: 10.1016/0959-4388(94)90101-5 [DOI] [PubMed] [Google Scholar]
- 166.Roy DS et al. , Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512 (2016). doi: 10.1038/nature17172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perusini JN et al. , Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus 27, 1110–1122 (2017). doi: 10.1002/hipo.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.El Haj M, Postal V, Allain P, Music enhances autobiographical memory in mild Alzheimer’s disease. Educ. Gerontol 38, 30–41 (2012). doi: 10.1080/03601277.2010.515897 [DOI] [Google Scholar]
- 169.Herlitz A, Adolfsson R, Bäckman L, Nilsson LG, Cue utilization following different forms of encoding in mildly, moderately, and severely demented patients with Alzheimer’s disease. Brain Cogn. 15, 119–130 (1991). doi: 10.1016/0278-2626(91)90020-9 [DOI] [PubMed] [Google Scholar]
- 170.Warrington EK, Weiskrantz L, Amnesic syndrome: Consolidation or retrieval? Nature 228, 628–630 (1970). doi: 10.1038/228628a0 [DOI] [PubMed] [Google Scholar]
- 171.Miller RR, Matzel LD, Retrieval failure versus memory loss in experimental amnesia: Definitions and processes. Learn. Mem 13, 491–497 (2006). doi: 10.1101/lm.241006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hardt O, Wang SH, Nader K, Storage or retrieval deficit: The yin and yang of amnesia. Learn. Mem 16, 224–230 (2009). doi: 10.1101/lm.1267409 [DOI] [PubMed] [Google Scholar]
- 173.Squire LR, Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learn. Mem 13, 522–529 (2006). doi: 10.1101/lm.310306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tulving E, Pearlstone Z, Availability versus accessibility of information in memory for words. J. Verbal Learn. Verbal Behav 5, 381–391 (1966). doi: 10.1016/S0022-5371(66)80048-8 [DOI] [Google Scholar]
- 175.Frankland PW, Josselyn SA, Köhler S, The neurobiological foundation of memory retrieval. Nat. Neurosci 22, 1576–1585 (2019). doi: 10.1038/s41593-019-0493-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kogan JH, Franklandand PW, Silva AJ, Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56 (2000). doi: [DOI] [PubMed] [Google Scholar]
- 177.Hitti FL, Siegelbaum SA, The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014). doi: 10.1038/nature13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S, Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). doi: 10.1126/science.aaf7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim J et al. , Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. U.S.A 104, 20955–20960 (2007). doi: 10.1073/pnas.0710548105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hong I et al. , Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur. J. Neurosci 30, 2089–2099 (2009). doi: 10.1111/j.1460-9568.2009.07004.x [DOI] [PubMed] [Google Scholar]
- 181.Lin C-H, Lee C-C, Gean P-W, Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol. Pharmacol 63, 44–52 (2003). doi: 10.1124/mol.63.1.44 [DOI] [PubMed] [Google Scholar]
- 182.Yoshii T, Hosokawa H, Matsuo N, Pharmacogenetic reactivation of the original engram evokes an extinguished fear memory. Neuropharmacology 113, 1–9 (2017). doi: 10.1016/j.neuropharm.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 183.Clem RL, Schiller D, New learning and unlearning: Strangers or accomplices in threat memory attenuation? Trends Neurosci. 39, 340–351 (2016). doi: 10.1016/j.tins.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Luchkina NV, Bolshakov VY, Mechanisms of fear learning and extinction: Synaptic plasticity-fear memory connection. Psychopharmacology (Berl.) 236, 163–182 (2019). doi: 10.1007/s00213-018-5104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Orsini CA, Maren S, Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev 36, 1773–1802 (2012). doi: 10.1016/j.neubiorev.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Myers KM, Davis M, Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007). doi: 10.1038/sj.mp.4001939 [DOI] [PubMed] [Google Scholar]
- 187.Bouton ME, Westbrook RF, Corcoran KA, Maren S, Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol. Psychiatry 60, 352–360 (2006). doi: 10.1016/j.biopsych.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 188.Ji J, Maren S, Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 17, 749–758 (2007). doi: 10.1002/hipo.20331 [DOI] [PubMed] [Google Scholar]
- 189.Bouton ME, Context and behavioral processes in extinction. Learn. Mem 11, 485–494 (2004). doi: 10.1101/lm.78804 [DOI] [PubMed] [Google Scholar]
- 190.Quirk GJ, Mueller D, Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 (2008). doi: 10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bouton ME, Nelson JB, Context-specificity of target versus feature inhibition in a feature-negative discrimination. J. Exp. Psychol. Anim. Behav. Process 20, 51–65 (1994). doi: 10.1037/0097-7403.20.1.51 [DOI] [PubMed] [Google Scholar]
- 192.Rescorla RA, Heth CD, Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process 1, 88–96 (1975). doi: 10.1037/0097-7403.1.1.88 [DOI] [PubMed] [Google Scholar]
- 193.Monfils MH, Cowansage KK, Klann E, LeDoux JE, Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science 324, 951–955 (2009). doi: 10.1126/science.1167975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schiller D et al. , Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53 (2010). doi: 10.1038/nature08637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang X, Kim J, Tonegawa S, Amygdala reward neurons form and store fear extinction memory. Neuron 10.1016/j.neuron.2019.12.025 (2020). [DOI] [PubMed] [Google Scholar]
- 196.Kim JJ, Fanselow MS, Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992). doi: 10.1126/science.1585183 [DOI] [PubMed] [Google Scholar]
- 197.Maren S, Aharonov G, Fanselow MS, Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res 88, 261–274 (1997). doi: 10.1016/S0166-4328(97)00088-0 [DOI] [PubMed] [Google Scholar]
- 198.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ, Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol 23, 99–106 (2013). doi: 10.1016/j.cub.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 199.Tonegawa S, Morrissey MD, Kitamura T, The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci 19, 485–498 (2018). doi: 10.1038/s41583-018-0031-2 [DOI] [PubMed] [Google Scholar]
- 200.Wiltgen BJ, Silva AJ, Memory for context becomes less specific with time. Learn. Mem 14, 313–317 (2007). doi: 10.1101/lm.430907 [DOI] [PubMed] [Google Scholar]
- 201.Wiltgen BJ et al. , The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol 20, 1336–1344 (2010). doi: 10.1016/j.cub.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang SH, Teixeira CM, Wheeler AL, Frankland PW, The precision of remote context memories does not require the hippocampus. Nat. Neurosci 12, 253–255 (2009). doi: 10.1038/nn.2263 [DOI] [PubMed] [Google Scholar]
- 203.Winocur G, Moscovitch M, Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc 17, 766–780 (2011). doi: 10.1017/S1355617711000683 [DOI] [PubMed] [Google Scholar]
- 204.Moscovitch M, Cabeza R, Winocur G, Nadel L, Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu. Rev. Psychol 67, 105–134 (2016). doi: 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guo N et al. , Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med 24, 438–449 (2018). doi: 10.1038/nm.4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shehata M et al. , Autophagy enhances memory erasure through synaptic destabilization. J. Neurosci 38, 3809–3822 (2018). doi: 10.1523/JNEUROSCI.3505-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kirwan CB, Stark CE, Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem 14, 625–633 (2007). doi: 10.1101/lm.663507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leutgeb JK, Leutgeb S, Moser MB, Moser EI, Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007). doi: 10.1126/science.1135801 [DOI] [PubMed] [Google Scholar]
- 209.Norman KA, O’Reilly RC, Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol. Rev 110, 611–646 (2003). doi: 10.1037/0033-295X.110.4.611 [DOI] [PubMed] [Google Scholar]
- 210.Gilboa A, Marlatte H, Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci 21, 618–631 (2017). doi: 10.1016/j.tics.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 211.McKenzie S, Eichenbaum H, Consolidation and reconsolidation: Two lives of memories? Neuron 71, 224–233 (2011). doi: 10.1016/j.neuron.2011.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schlichting ML, Preston AR, Memory integration: Neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci 1, 1–8 (2015). doi: 10.1016/j.cobeha.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tse D et al. , Schemas and memory consolidation. Science 316, 76–82 (2007). doi: 10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- 214.Zeithamova D, Preston AR, Temporal proximity promotes integration of overlapping events. J. Cogn. Neurosci 29, 1311–1323 (2017). doi: 10.1162/jocn_a_01116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rashid AJ et al. , Competition between engrams influences fear memory formation and recall. Science 353, 383–387 (2016). doi: 10.1126/science.aaf0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cai DJ et al. , A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016). doi: 10.1038/nature17955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sehgal M et al. , Memory allocation mechanisms underlie memory linking across time. Neurobiol. Learn. Mem 153, 21–25 (2018). doi: 10.1016/j.nlm.2018.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rao-Ruiz P et al. , Engram-specific transcriptome profiling of contextual memory consolidation. Nat. Commun 10, 2232 (2019). doi: 10.1038/s41467-019-09960-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pignatelli M et al. , Engram cell excitability state determines the efficacy of memory retrieval. Neuron 101, 274–284.e5 (2019). doi: 10.1016/j.neuron.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 220.Yokose J et al. , Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355, 398–403 (2017). doi: 10.1126/science.aal2690 [DOI] [PubMed] [Google Scholar]
- 221.Levy BJ, Wagner AD, Measuring memory reactivation with functional MRI: Implications for psychological theory. Perspect. Psychol. Sci 8, 72–78 (2013). doi: 10.1177/1745691612469031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mack ML, Love BC, Preston AR, Building concepts one episode at a time: The hippocampus and concept formation. Neurosci. Lett 680, 31–38 (2018). doi: 10.1016/j.neulet.2017.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schlichting ML, Preston AR, Memory reactivation during rest supports upcoming learning of related content. Proc. Natl. Acad. Sci. U.S.A 111, 15845–15850 (2014). doi: 10.1073/pnas.1404396111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zeithamova D, Schlichting ML, Preston AR, The hippocampus and inferential reasoning: Building memories to navigate future decisions. Front. Hum. Neurosci 6, 70 (2012). doi: 10.3389/fnhum.2012.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gazzaniga MS, Conversations in the Cognitive Neurosciences (MIT Press, 1997). [Google Scholar]
- 226.Barlow HB, in The Cognitive Neurosciences, Gazzaniga MS, Ed. (MIT Press, 1995), pp. 415–435. [Google Scholar]
- 227.Gross CG, Genealogy of the “grandmother cell”. Neuroscientist 8, 512–518 (2002). doi: 10.1177/107385802237175 [DOI] [PubMed] [Google Scholar]
- 228.Yuste R, From the neuron doctrine to neural networks. Nat. Rev. Neurosci 16, 487–497 (2015). doi: 10.1038/nrn3962 [DOI] [PubMed] [Google Scholar]
- 229.Eichenbaum H, Barlow versus Hebb: When is it time to abandon the notion of feature detectors and adopt the cell assembly as the unit of cognition? Neurosci. Lett 680, 88–93 (2018). doi: 10.1016/j.neulet.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Denny CA, Lebois E, Ramirez S, From engrams to pathologies of the brain. Front. Neural Circuits 11, 23 (2017). doi: 10.3389/fncir.2017.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tolman EC, Honzik CH, Introduction and removal of reward, and maze performance in rats. Univ. Calif. Publ. Psychol 4, 257–275 (1930). [Google Scholar]
- 232.Philips GT, Tzvetkova EI, Marinesco S, Carew TJ, Latent memory for sensitization in Aplysia. Learn. Mem 13, 224–229 (2006). doi: 10.1101/lm.111506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lubow RE, Moore AU, Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. J. Comp. Physiol. Psychol 52, 415–419 (1959). doi: 10.1037/h0046700 [DOI] [PubMed] [Google Scholar]
- 234.Lewis DJ, Psychobiology of active and inactive memory. Psychol. Bull 86, 1054–1083 (1979). doi: 10.1037/0033-2909.86.5.1054 [DOI] [PubMed] [Google Scholar]
- 235.Nader K, Schafe GE, Le Doux JE, Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). doi: 10.1038/35021052 [DOI] [PubMed] [Google Scholar]
- 236.Przybyslawski J, Sara SJ, Reconsolidation of memory after its reactivation. Behav. Brain Res 84, 241–246 (1997). doi: 10.1016/S0166-4328(96)00153-2 [DOI] [PubMed] [Google Scholar]
- 237.Kida S et al. , CREB required for the stability of new and reactivated fear memories. Nat. Neurosci 5, 348–355 (2002). doi: 10.1038/nn819 [DOI] [PubMed] [Google Scholar]
- 238.Sara SJ, Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem 7, 73–84 (2000). doi: 10.1101/lm.7.2.73 [DOI] [PubMed] [Google Scholar]
- 239.Morris RG et al. , Memory reconsolidation: Sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50, 479–489 (2006). doi: 10.1016/j.neuron.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 240.Lee JL, Memory reconsolidation mediates the updating of hippocampal memory content. Front. Behav. Neurosci 4, 168 (2010). doi: 10.3389/fnbeh.2010.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Eisenberg M, Kobilo T, Berman DE, Dudai Y, Stability of retrieved memory: Inverse correlation with trace dominance. Science 301, 1102–1104 (2003). doi: 10.1126/science.1086881 [DOI] [PubMed] [Google Scholar]
- 242.Anderson MC, Bjork RA, Bjork EL, Remembering can cause forgetting: Retrieval dynamics in long-term memory. J. Exp. Psychol. Learn. Mem. Cogn 20, 1063–1087 (1994). doi: 10.1037/0278-7393.20.5.1063 [DOI] [PubMed] [Google Scholar]
- 243.Richards BA, Frankland PW, The conjunctive trace. Hippocampus 23, 207–212 (2013). doi: 10.1002/hipo.22089 [DOI] [PubMed] [Google Scholar]
- 244.Rubin A, Geva N, Sheintuch L, Ziv Y, Hippocampal ensemble dynamics timestamp events in long-term memory. eLife 4, e12247 (2015). doi: 10.7554/eLife.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brodt S et al. , Fast track to the neocortex: A memory engram in the posterior parietal cortex. Science 362, 1045–1048 (2018). doi: 10.1126/science.aau2528 [DOI] [PubMed] [Google Scholar]
- 246.Nader K, Memory traces unbound. Trends Neurosci. 26, 65–72 (2003). doi: 10.1016/S0166-2236(02)00042-5 [DOI] [PubMed] [Google Scholar]
- 247.Dudai Y, The restless engram: Consolidations never end. Annu. Rev. Neurosci 35, 227–247 (2012). doi: 10.1146/annurev-neuro-062111-150500 [DOI] [PubMed] [Google Scholar]
- 248.Schiller D, Phelps EA, Does reconsolidation occur in humans? Front. Behav. Neurosci 5, 24 (2011). doi: 10.3389/fnbeh.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kindt M, Soeter M, Vervliet B, Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat. Neurosci 12, 256–258 (2009). doi: 10.1038/nn.2271 [DOI] [PubMed] [Google Scholar]


