Abstract
Pharmacological activation of integrin CD11b/CD18 (αMβ2, Mac-1, CR3) shows anti-inflammatory benefits in a variety of animal models of human disease, and it is a novel therapeutic strategy. Reasoning that genetic models can provide an orthogonal and direct system for the mechanistic study of CD11b agonism, we present here a novel knock-in (KI) model of constitutive active CD11b in mice. We genetically targeted the Itgam gene (which codes for CD11b) to introduce a point mutation that results in the I332G substitution in the protein. The I332G mutation in CD11b promotes an active, higher affinity conformation of the ligand-binding I/A-domain (CD11b αA-domain). In vitro, this mutation increased adhesion of KI neutrophils to fibrinogen, and decreased neutrophil chemotaxis to a formyl-Met-Leu-Phe (fMLF) gradient. In vivo, CD11bI332G animals showed a reduction in recruitment of neutrophils and macrophages in a model of sterile peritonitis. This genetic activation of CD11b also protected against development of atherosclerosis in the setting of hyperlipidemia via reduction of macrophage recruitment into atherosclerotic lesions. Thus, our animal model of constitutive genetic activation of CD11b can be a useful tool for the study of integrin activation and its potential contribution to modulating leukocyte recruitment and alleviating different inflammatory diseases.
INTRODUCTION
CD11b/CD18, also known as αMβ2, Mac-1, and CR3, is the predominant β2 integrin in polymorphonuclear leukocytes, and is abundantly expressed in monocytes, macrophages, and dendritic cells (1, 2). A heterodimer of the CD11b (αM) and CD18 (β2) subunits encoded by the ITGAM and ITGB2 genes, respectively, it exists on the cell surface in two key conformational states: 1) an inactive, low-affinity, closed conformation, and 2) an active, high-affinity, open conformation (3). CD11b/CD18 is among the most versatile of all integrins with more than 40 reported ligands (4). As a result, it plays an important role in a diversity of immunological processes, from leukocyte recruitment to the sites of tissue injury to the resolution of inflammation (5-7).
The affinity of CD11b/CD18 to multiple endothelial ligands (e.g., intercellular adhesion molecule 1 [ICAM-1], fibrinogen, endothelial protein C receptor [EPCR]) (8-10) has made it a promising target for the treatment of inflammatory diseases. Pharmacological blockade or genetic deletion of CD11b improved restenosis (11), cerebral and renal ischemic injury (12, 13), glomerulonephritis (14, 15), and thrombosis (16) in animal models. Based on these findings, it was initially thought that inhibition of ligand binding to CD11b/CD18 would be a potential therapeutic strategy for vascular inflammation. However, several such anti-integrin therapeutic antagonists failed to meet efficacy endpoints in clinical trials (17).
Similarly, large scale genome-wide association studies (GWAS) have shown that mutations that reduce activity of CD11b or render it inactive are pathogenic (18-23). These studies have identified single nucleotide polymorphisms (SNPs) in the human ITGAM gene that result in the production of functionally deficient CD11b/CD18 but without change in the level of surface expression (22, 24). The detected variants are associated with increased susceptibility to systemic lupus erythematosus (SLE) and lupus nephritis (LN) (18, 20, 21, 25). Animal models of autoimmunity also confirmed the relationship between Mac-1 deficiency and end-organ damage (26, 27). Together, these studies suggested that integrin activation may be an alternate and highly promising therapeutic approach for these pathologies.
Towards that, we were among the first to develop novel small molecule agonists of CD11b, and have shown that such agents reduce recruitment of CD11b+ cells and have therapeutic efficacy in a variety of disease models (28-30). These include experimental models of vascular injury (28, 30), nephritis (28), organ allograft vasculopathy (31), liver fibrosis (32), hyperoxic lung injury (33), autoimmunity (23), and pancreatic cancer (34). Small molecule CD11b agonists bind to an allosteric site in the αA-domain (also known as αI-domain) of CD11b, where they stabilize a more active conformation of the receptor and prime it for ligand binding (23). We and others have also shown that CD11b activation can inhibit pro-inflammatory TLR pathways (23, 35).
To fully understand the therapeutic potential of CD11b activation, as well as to elucidate the molecular pathways involved, alternative, orthologous approaches are needed. Here, we present a novel knock-in (KI) mouse model expressing a constitutively active mutant CD11b. In this model, the Itgam gene was genetically targeted to introduce a mutation (I332G) that promotes a higher affinity conformation of this integrin subunit. The ligand binding αA-domain of CD11b contains an allosteric site, known as SILEN (socket for isoleucine) (36) or IDAS (I domain allosteric site) (37), that modulates its conformation and binding affinity. Mutagenesis of isoleucine 332 to glycine (I332G) shifts the αA-domain into its open, ligand competent conformation. We introduced this mutation in murine CD11b to generate CD11bI332G mice (KI mice). Our investigations show that this activating mutation decreases inflammatory cell recruitment and vascular inflammation in models of sterile peritonitis and atherosclerosis, respectively. We believe that this novel experimental model can be used to gain deeper insight into mechanisms that can be further therapeutically targeted in inflammation and atherosclerosis.
MATERIALS AND METHODS
Construction of the CD11bI332G targeting vector
The CD11bI332G targeting vector was engineered by Cyagen (Santa Clara, CA) as portrayed in Figure 1A. Briefly, the 5’ and 3’ homology arms including exons 7 to 12 of the Itgam gene were amplified by PCR from bacterial artificial chromosome (BAC) clones RP23-343J4 and RP23-323J3 from the C57BL/6J library. The I332G mutation (ATC to GGC) was introduced by site-directed mutagenesis into exon 9 in the 3’ homology arm. The homology arms were cloned into the targeting vector using the NotI and BsiWI (5’ arm) and XhoI and NruI (3’ arm) restriction sites. A Neo cassette flanked by two Frt sites was inserted between the homology arms. A diphtheria toxin A cassette cloned downstream of the 3’ homology arm was used for negative selection. The above cloning strategy was confirmed by PCR, restriction digestion, and sequencing.
Figure 1. Generation of CD11bI332G knock-in (KI) mice.

A) Schematic of the targeting strategy. From top to bottom, the diagrams indicate the genomic structure of the wild type (WT) allele, the targeting vector, and the targeted KI allele. Confirmatory Southern blots used a probe complementary to the Neo cassette and DNA digested with EcoRI or KpnI (see Supplementary Figure 1). B) Genotyping of the WT and KI alleles in mice by PCR followed by BglII digestion. The I332G mutation disrupts a BglII recognition site in exon 9 of the Itgam gene, generating a 269 bp band in KI homozygotes, and 107 and 162 bp bands for the WT allele. C) Partial amino acid alignment of the human, wild type (WT) mouse, and mutant (I332G) mouse CD11b subunits of integrin CD11b/CD18. The mutant position is highlighted in black. The boxed sequence delineates the CD11b A-domain, with asterisks indicating amino acid residues that form a hydrophobic coordination socket (known as SILEN) for wild type Ile332. Amino acid numbering is based on the full-length proteins in GenBank accession numbers NP_001139280.1 (https://www.ncbi.nlm.nih.gov/protein/NP_001139280.1) and NP_001076429.1 (https://www.ncbi.nlm.nih.gov/protein/NP_001076429.1) for human and mouse, respectively.
Generation of CD11bI332G knock-in (KI) mice
CD11bI332G KI mice were generated by Cyagen. Briefly, the targeting vector was linearized with NotI and electroporated into C57BL/6 embryonic stem (ES) cells. Ninety-three G418-resistant clones were screened by PCR, of which 23 positive clones were sent to sequencing. Fourteen ES clones were positive by sequencing, six of which were expanded for Southern blot confirmation using KpnI and EcoRI digestion, followed by hybridization with a probe complementary to the Neo cassette (Supplementary Figure 1). Clones 1B6 and 1C9 were selected for blastocyst injection and chimera production. Chimeras were bred with C57BL/6 females to test germline transmission. Three pups from clone 1B6 and three from clone 1C9 were identified as positive germline F1 founders by PCR and sequencing. F1 founders were crossed with homozygous ROSA26::FLPe KI mice (38) (stock #003946; Jackson Laboratory, Bar Harbor, ME) at the University of Miami to excise the Neo cassette. Mac-1I332G heterozygotes and negative for the Neo insertion were then backcrossed with C57BL/6 mice to remove the ROSA26::FLPe allele. Once achieved, mice were further backcrossed into the C57BL/6 background for 6-8 generations. Primers 5’-AAGGAATATCTTTTGCTGGACGTACG-3’ and 5’-CAGAAGGCAGCTTAGAACAGGGC-3’ were used to screen for the excision of Neo, while 5’-CACTGATATTGTAAGTAGTTTGC-3’ and 5’-CTAGTGCGAAGTAGTGATCAGG-3’ were used to detect the ROSA26::FLPe allele. The Mac-1I332G colony is maintained as heterozygotes, from which Mac-1I332G homozygotes and Mac-1 wild type (Mac-1WT) littermates are obtained for experiments. Genotyping of the Itgam WT and I332G alleles is performed with primers 5’-ATTTAGCTTTGGCTCCTTGGCAAC-3’ and 5’-TGGAGCAAGTCAGACCCAAATGTC-3’, followed by enzymatic digestion with BglII. This strategy results in 107- and 162-bp bands for the WT allele and the undigested 269-bp band for the I332G variant (Figure 1B). All animal procedures were performed according to the National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals) and approved by the respective Institutional Animal Care and Use Committees at the University of Miami Miller School of Medicine and Rush University Medical Center.
Peritonitis model
Peritonitis was induced in 14-16 weeks old Mac-1WT and Mac-1I332G mice of both sexes by injecting 3 mL of a sterile 3% Brewer’s thioglycollate broth (Sigma-Aldrich, St. Louis, MO). To analyze cell recruitment, leukocyte cell suspensions were obtained by lavage of the peritoneal cavity with 10 mL of phosphate buffered saline (PBS) at 4 hours and 48 hours after injection. Cells were collected by centrifugation for 10 min at 250 g, and washed twice with PBS before analysis.
Atherosclerosis model
Hypercholesterolemia was induced in 12 weeks old Mac-1WT and Mac-1I332G mice of both sexes after administration of a single tail-vein injection of the recombinant rAAV8-D377Y-mPCSK9 virus (UNC Vector Core, Chapel Hill, NC) (2.0×1010 copies/mouse) according to the published protocol (39). The exogenous expression of PCSK9 enhances internalization and degradation of hepatic LDL receptors. One week after the virus injection, mice started feeding a cocoa butter Teklad research custom diet (catalog TD.88051; Envigo, Somerset, NJ) for 12 weeks ad libitum. Cholesterol levels were measured one week after diet initiation and at the end of the 12-week diet using cholesterol test strips by PTS Panels (Indianapolis, IN). After completion of the diet, non-fasting blood was submitted to the University of Miami Division of Comparative Pathology for complete blood count (CBC) and blood chemistry, and whole aortas were submitted to Eehscience LLC (Pickerington, OH) for independent determination of atherosclerotic burden by Oil Red O staining. Serial cross-sections of the brachiocephalic trunk were obtained for plaque characterization. Lesion size and macrophage infiltration were quantified at three positions along the brachiocephalic artery (~550, ~450, and ~350 μm from the aortic arch).
Adhesion assay
Peritoneal neutrophils were isolated 4 hours after sterile 3% Brewer’s thioglycollate injection. Cell purity was assessed by flow cytometry. Cell adhesion assays were performed as previously described (40) and used immobilized fibrinogen as Mac-1 ligand. Briefly, 384-well microplates (Corning, Corning, NY) were coated with 15 μg/mL fibrinogen in PBS containing 1 mM of each Ca2+ and Mg2+ by incubating overnight at 4°C. The nonspecific sites were blocked with 1% gelatin in Tris buffered saline (TBS), followed by washing three times with TBS. Cells were suspended in TBS containing 1mM of each Ca2+ and Mg2+ (TBS2+ buffer) or in TBS containing 1 mM Mn2+, in the presence or absence of an anti-CD11b blocking antibody (clone M1/70, 10 μg/mL). Cells were incubated in the ligand-coated wells (3,000 cells per well) for 10 minutes at 37°C. The assay plates were then gently inverted and kept in the inverted position for 30 minutes at room temperature to dislodge nonadherent cells. The remaining adherent cells were fixed using 4% formaldehyde and quantified by automated imaging microscopy as previously described (41). Assays were performed in triplicate wells and at least three independent times.
Chemotaxis assay
Peritoneal neutrophils were obtained as above. Neutrophil chemotaxis on planar surfaces was performed using 48-well Zigmond chambers (Neuro Probe, Gaithersburg, MD) as previously described (40). Briefly, cells were preincubated for 10 minutes on fibrinogen-coated (25 μg/mL) glass coverslips in a humidified chamber with RPMI 1640 supplemented with 1% FBS. The coverslips were then placed on top of Zigmond chambers, and a formyl-Met-Leu-Phe (fMLF; Sigma-Aldrich) gradient was created by placing the assay buffer (RPMI 1640 with 1% FBS) in one well of the chamber and 10 μM fMLF in assay buffer in the other well. The migration of neutrophils toward fMLF was recorded at 5- to 30-seconds intervals for a period of 25 minutes using a Nikon Eclipse 90i microscope, as previously described (40, 42). Images were acquired using a Nikon DS camera with a PLAN APO 20x differential interference contrast microscopy objective and the Nikon Imaging software. Analysis of neutrophil migration was performed with the motile population that had moved more than 10 μm using the ImageJ software (National Institutes of Health, Bethesda, MD) with manual cell tracking using the chemotaxis and migration tool plugins (Ibidi) for ImageJ. Data from 30 neutrophils per mouse strain were quantified in at least three independent experiments.
Enzyme-linked immunosorbent assay (ELISA)
Primary macrophages were collected by peritoneal lavage from 14-16 weeks old Mac-1WT and Mac-1I332G mice of both sexes, 4 days after injecting 3 mL of sterile 3% Brewer’s thioglycollate broth (Sigma-Aldrich). Cells were adherence-purified for 1 hour, followed by a wash with PBS to remove non-adherent cells. Adherent cells were suspended in DMEM supplemented with 10% FBS and counted. One million cells per well were plated in 12-well plates and allowed to settle down for two days before treatment. Cells were stimulated with 50 ng/mL of LPS-EK (InvivoGen, San Diego, CA), and supernatants collected at baseline and at 2, 4, and 8 hours after treatment. Levels of secreted IL-1β, IL-6, and TNF-α were quantified by ELISA following the manufacturer’s protocols (R&D Systems, Minneapolis, MN).
Immunofluorescence (IF)
Three brachiocephalic artery cross-sections per animal, corresponding to positions ~550, ~450, and ~350 μm from the aortic arch were co-stained with anti-galectin-3 (Mac2) and smooth muscle actin (SMA) antibodies to identify macrophages and smooth muscle cells (SMC), respectively. Briefly, antigen retrieval was performed in 10 mM sodium citrate, 0.05% Tween20, pH 6 solution for 30 minutes in 95°C water, followed by treatment with 3% hydrogen peroxide and TNB blocking solution (#FP1020, Perkin Elmer, Waltham, MA). Then, slides were incubated with rat anti-mouse Mac2 antibody (1:50; #125402, BioLegend, San Diego, CA) and mouse anti-human SMA antibody (1:200; #M0851, Dako) overnight at 4οC. The next day, the slides were incubated with Alexa Fluor 546 goat anti-rat antibody (1:1000; #A11081, Thermo Fisher Scientific, Waltham, MA) and Alexa Fluor 488 goat anti-mouse antibody (1:1000; #A11029, Thermo Fisher Scientific) for 45 minutes. Sections were counter stained with 300 nM DAPI solution (#D1306, Thermo Fisher Scientific) in PBS for 3 minutes, and mounted in DABCO antifading polyvinyl alcohol mounting medium (#10981, Sigma-Aldrich). Sections were examined in an Olympus Ix71 inverted microscope and photographed using the Olympus cellSens Standard software. Image analysis was performed with ImageJ (National Institutes of Health).
Flow cytometry
All antibodies and reagents were purchased from BioLegend (San Diego, CA) except when indicated otherwise. Quantification of integrin subunit expression used the antibodies CD11a PE (#101107), CD11b PerCP (#101230), CD11c BV650 (#564079, BD Biosciences), CD18 APC (#562828, BD Biosciences), CD115 BV605 (catalog #135577), and Gr-1 PE/Dazzle 594 (#108452). Briefly, cells were washed with PBS twice, counted, and Fc receptors were blocked with anti-mouse CD16/32 antibody for 10 minutes at 4°C. Cells were washed with PBS, and True-Stain Monocyte Blocker was added before labeling with eBioscience Fixable Viability Dye eFluor 660 (Thermo Scientific) for 30 minutes. Cells were washed with FACS buffer with 0.1% sodium azide (flow buffer), and True-Stain Monocyte Blocker was added again followed by 30-minute incubation with the remaining labeling antibodies. Excess antibodies were washed and cells were fixed with flow cytometry buffer supplemented with 1% paraformaldehyde prior to flow cytometric analyses. Flow cytometry data was read using a BD LSR-Fortessa-HTS analyzer (BD Biosciences) and processed with the FlowJo software (Ashland, OR).
Statistics
Statistical analyses were performed in GraphPad Prism 5 (San Diego, CA). Normally distributed values are presented as mean ± standard error of the mean (SEM), and compared using a two-tailed Student’s t-test or one-way ANOVA. Non-normally distributed data are presented as median ± interquartile range (IQR) and compared using the Mann-Whitney test. A p-value <0.05 was considered significant.
RESULTS
Generation of CD11bI332G KI mice
Isoleucine 332 in CD11b, corresponding to residue 316 after processing the N-terminal signal peptide, has been identified as a key residue in stabilizing the inactive low-affinity conformation of the CD11b/CD18 integrin receptor (36). The regulatory role of this residue relies on its hydrophobic interactions with the socket for isoleucine (SILEN; Figure 1C). The I332G substitution weakens these interactions and favors the active, high-affinity, open conformation of the CD11b αA-domain (36). However, consequences of such activation of CD11b in animals are currently not fully understood. Therefore, to investigate the role of active CD11b in vivo, we generated mice harboring the I332G activating CD11b mutation following the strategy delineated in Figure 1. Briefly, a genetic construct was designed to replace the wild type exon 9 in the Itgam gene with the mutant exon 9 downstream of a Frt-flanked Neo cassette (Figure 1A). The construct was inserted by homologous recombination in ES cells, followed by antibiotic selection, PCR screening, and Southern blot confirmation (Supplementary Figure 1). After blastocyst injection and chimera production, heterozygous founders were crossed with ROSA26::FLPe mice to generate Neo-deleted CD11bI332G KI animals (KI animals), and backcrossed successively into the C57BL/6 background. Figure 1B presents the genotyping analysis of CD11bI332G KI and littermate WT mice.
CD11bI332G KI mice are normal in size and in reproductive and social behavior. Their complete blood count (CBC) at 16 weeks showed no significant differences from littermate WT mice (Table I). Surface expression of the integrin subunits CD11b and CD18, and of other α subunits (CD11a and CD11c) from the β2 integrin family, in peripheral blood neutrophils and monocytes was also similar between KI and WT animals (Supplementary Figure 2).
Table I.
Complete blood count analysis of peripheral blood collected from 16-week-old wild type and CD11bI332G knock-in mice
| Wild type (n=8) |
CD11bI332G Knock-in (n=8) |
P value | |
|---|---|---|---|
| Complete Blood Count | |||
| WBC (x103/uL) | 3.2 ± 1.5 | 2.2 ± 0.5 | 0.09 |
| RBC (x106/uL) | 7.8 ± 0.5 | 7.4 ± 0.6 | 0.11 |
| Hemoglobin (g/dL) | 10.9 ± 0.5 | 10.5 ± 0.5 | 0.16 |
| Hematocrit (%) | 37.3 ± 2.3 | 35.3 ± 2.5 | 0.12 |
| MCV (fL) | 47.5 ± 1.2 | 47.9 ± 1.6 | 0.61 |
| MCH (pg) | 14.0 (13.3-14.0) | 14.0 (14.0-14.8) | 0.50 |
| MCHC (%) | 29.5 ± 1.6 | 30.0 ± 0.8 | 0.44 |
| Segs (x103/uL) | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 0.24 |
| Bands (x103/uL) | N.D. | N.D. | - |
| Lymphocytes (x103/uL) | 2.4 ± 1.0 | 1.7 ± 0.4 | 0.09 |
| Monocytes (x103/uL) | 0.4 ± 0.3 | 0.3 ± 0.1 | 0.09 |
| Eosinophils (x103/uL) | 0.03 (0.0-0.04) | 0.02 (0.0-0.03) | 0.41 |
| Basophils (x103/uL) | N.D. | N.D. | - |
| NRBC | N.D. | N.D. | - |
Values are presented as mean ± standard deviation or median (interquartile range). N.D., not detected.
Adhesion and chemotaxis of CD11bI332G neutrophils in vitro
The functional effect of the I332G mutation was tested in peritoneal neutrophils using adhesion and chemotaxis assays (Figures 2-4). In agreement with the activating role of this mutation (36), neutrophils from CD11bI332G mice showed increased static adhesion to fibrinogen than those from WT controls under physiological conditions (1 mM Ca2+, 1 mM Mg2+; Figure 2). The enhanced adhesion of KI neutrophils was CD11b-dependent and could be neutralized with an anti-CD11b blocking antibody (M1/70). Both WT and CD11bI332G neutrophils reached similar levels of adhesion in the presence of the activating cation Mn2+ (Figure 2) (43).
Figure 2. Adhesion of neutrophils from CD11b wild type (WT) and knock-in (I332G) mice.

Adhesion of peritoneal neutrophils to fibrinogen in the presence of physiological divalent cations (1 mM Ca2+, 1 mM Mg2+), the activating cation Mn2+ (1 mM), and the anti-CD11b blocking antibody clone M1/70. Bars represent the mean ± SEM, n=4 per group in three independent experiments. N.S., not significant.
Figure 4. Time-lapse video and photography of chemotaxing neutrophils from CD11b wild type (WT) and knock-in (I332G) mice.

A) Time-lapse videos of representative peritoneal neutrophils during chemotaxis towards a formyl-Met-Leu-Phe (fMLF) gradient. B) Microphotographs of representative chemotaxing neutrophils from CD11bWT and CD11bI332G mice in response to an fMLF stimulus.
Firmer adhesion of neutrophils is expected to result in impaired chemotaxis in response to a formyl-Met-Leu-Phe (fMLF) gradient (28), and indeed, we observed that KI neutrophils showed significantly reduced chemotaxis compared to WT cells (Figures 3 and 4A). As with chemotaxis of neutrophils upon pharmacologic CD11b activation (28), the chemotaxing KI neutrophils showed prevalence of elongated, trailing uropods compared to those from WT animals (Figure 4B). These results suggest increased adhesion and reduced detachment from the substrate as the reasons for their impaired motility.
Figure 3. Chemotaxis of neutrophils from CD11b wild type (WT) and knock-in (I332G) mice.

A) Spider plots delineating the chemotaxis of peritoneal neutrophils in response to a formyl-Met-Leu-Phe (fMLF) gradient. B-D) Quantification of Euclidean distance (B), accumulated distance (C) and cell velocity (D) in 30 migration tracks from CD11bWT and CD11bI332G neutrophils as shown in A. Bars represent the mean ± SEM, n=30 per group in three independent experiments.
Effect of integrin activation in acute inflammation
The CD11b/CD18 integrin plays a fundamental role in the recruitment of neutrophils and monocytes to sites of inflammation (5, 44, 45). Therefore, we tested the effects of the CD11bI332G mutation in inflammatory cell recruitment in vivo in the setting of thioglycollate-induced peritonitis. The number of infiltrated neutrophils and macrophages in the peritoneal cavity was measured at two time points after thioglycollate injection (Figure 5A-B). Neutrophil counts were significantly lower in KI vs. WT mice 4 hours after injection, but equivalent at 48 hours in both animal groups (Figure 5A). Similarly, there was a significant decrease in peritoneal macrophage infiltration in CD11bI332G mice compared to WT animals at the 48-hour time point (Figure 5B). These results indicate a reduction and/or delay in leukocyte recruitment in KI mice in the setting of acute sterile inflammation.
Figure 5. Inflammatory cell recruitment and cytokine secretion in thioglycollate-induced peritonitis.

A-B) Cell counts of neutrophils (A) and macrophages (B) in the peritoneum of CD11bWT and CD11bI332G mice by flow cytometry at 4 and 48 hours after sterile thioglycollate injection. Neutrophils were selected as Gr-1hi, while macrophages were identified as F4/80+. Bars represent the mean ± SEM, n=7-9 mice per group. C-E) Secretion of inflammatory cytokines by peritoneal macrophages isolated at 4 days after thioglycollate injection. Cells were count normalized and stimulated with LPS for increasing periods of time in vitro. Bars represent the mean ± SEM, n=4 mice per group in three independent experiments. N.S., not significant.
Cytokine secretion by CD11bI332G macrophages
Primary macrophages were obtained from the peritoneal cavity of CD11bI332G and WT animals at 4 days after thioglycollate-induced peritonitis. Count-normalized cells were plated and cultured for two days in the absence of CD11b-specific ligands. After this pre-treatment period, cells were stimulated with LPS and cytokine secretion was quantified at three time points (Figure 5C-E). CD11bI332G cells showed significantly lower IL-1β and TNF-α secretion starting at 2 hours after treatment (Figure 5C and E). Significantly reduced IL-6 levels were also observed starting at 4 hours post stimulation (Figure 5D). These results parallel published reports with pharmacological CD11b-activating agents (23, 46), and are further supported by the opposite pattern in CD11b knockout (KO) animals (47).
Effect of genetic activation of CD11b in atherosclerosis
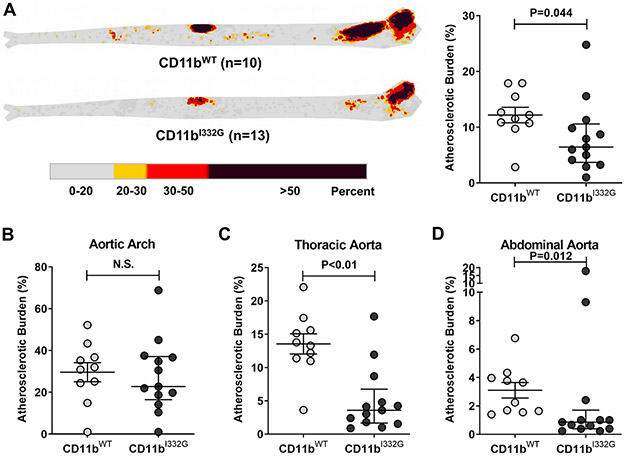
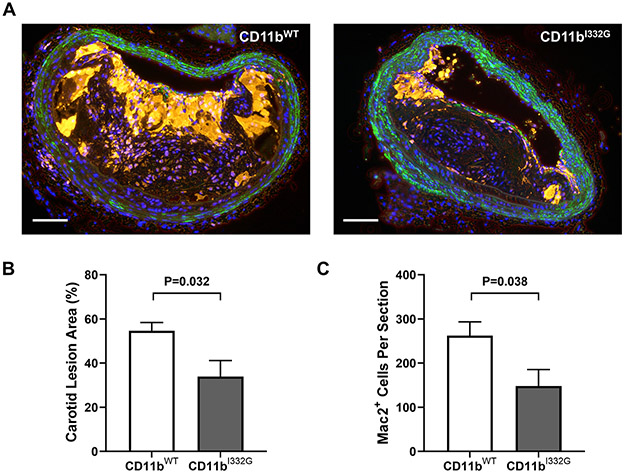
CD11b is a key mediator of vascular inflammation and atherosclerosis by regulating leukocyte adhesion and extravasation across the endothelium via its ligands ICAM-1, lipoprotein(a), and others (5, 7, 48). We previously showed that pharmacologic activation of CD11b reduces influx of macrophages after arterial injury (28). To investigate whether activation of CD11b is also atheroprotective, we used KI animals in a model of atherosclerosis. Hyperlipidemia was induced in cohorts of CD11bI332G and WT mice via injection of the recombinant rAAV8-D377Y-mPCSK9 virus followed by 12 weeks of high fat diet (HFD), a recently established model of atherosclerosis (39). Both experimental groups had overt hypercholesterolemia at the end point (Supplementary Figure 3A-B). However, CD11bI332G mice showed significantly lower atherosclerotic burden than WT animals, particularly in the thoracic and abdominal aorta (Figure 6). These results were confirmed in cross-sections of the brachio-cephalic artery (Figure 7). Lesion size (as % of total wall area) was significantly smaller in CD11bI332G mice compared to their WT counterparts (Figure 7B). In addition, the number of infiltrated macrophages per section was significantly lower in the former (Figure 7C). This suggests that CD11b activation has a protective role in atherosclerosis, in great part due to a reduction of macrophage recruitment to atherosclerotic lesions.
Figure 6. Atherosclerosis development in the aorta of CD11b wild type (WT) and knock-in (I332G) mice.
A) Heatmap and percentage of plaque burden by area in whole aortas from CD11bWT (n=10) and CD11bI332G hyperlipidemic mice (n=13). B-D) Quantification of plaque burden in the aortic arch (B), the thoracic aorta (C), and the abdominal aorta (D) of hyperlipidemic mice. Error bars represent the median and interquartile range, n=10-13 per group. N.S., not significant.
Figure 7. Atherosclerosis development in the brachiocephalic trunk of CD11b wild type (WT) and knock-in (I332G) mice.
A) Representative cross-sections of advanced atherosclerotic lesions in the brachiocephalic artery of CD11bWT and CD11bI332G hyperlipidemic mice. Galectin-3 (Mac2)+ positive macrophages/foam cells are stained in yellow, smooth muscle actin (SMA)+ cells are in green, and nuclei are counter-stained in blue (DAPI). Scale bar = 100 μm. B-C) Quantification of lesion size as % of total wall area (B) and Mac2+ macrophage counts per section (C) in brachiocephalic artery sections of CD11bWT and CD11bI332G mice. Values represent the average of three positions in the artery (at ~550, ~450, and ~350 μm from the aortic arch). Error bars represent the median and interquartile range, n=7 per group.
DISCUSSION
Integrin CD11b/CD18 is essential for a diversity of functions in immune cells (2, 4). In addition to regulating cell adhesion, migration, and phagocytosis, CD11b/CD18 modulates pro-and anti-inflammatory signaling (23, 49-51). Our recent studies show that CD11b acts as a negative regulator of pro-inflammatory TLR signaling and of B-cell autoreactivity (23), and that pharmacologic activation of CD11b reduces inflammation and injury (28-31, 33). However, our current knowledge of CD11b functions and downstream signaling pathways is largely based on pharmacological agents and CD11b KO models, while a genetic activation model was missing. Here, we present a novel, genetic model of constitutive CD11b activation to study the role of active integrin CD11b/CD18 in inflammatory injury in vivo.
Our results show that global, constitutive CD11bI332G KI animals developed reduced inflammatory injury in two different models of inflammation. In vitro studies with KI neutrophils showed impaired chemotaxis and pronounced uropod elongation in response to an fMLF gradient. Directional motility of neutrophils requires a tightly coordinated and self-organizing cell polarity. Re-distribution of β2 integrins and regulation of their ligand-binding affinity along the cell body is one of the mechanisms by which neutrophils regulate polarized adhesion (52). Ultimately, after initial adhesion and cell elongation, the trailing edge of the cell (uropod) needs to be able to detach and retract to promote forward protrusion of the leading edge (pseudopod) in the direction of the chemoattracting agent (52, 53). In migrating neutrophils, CD11b/CD18 redistributes to the uropod, where it stabilizes microtubules and regulates myosin light chain mediated uropod contractility (54-57). The morphology of chemotaxing neutrophils from CD11bI332G KI mice is indicative of impaired uropod detachment as a result of CD11b/CD18 integrin activation. In vivo, both CD11b+ neutrophils and monocytes from CD11bI332G mice showed a significant reduction in inflammatory cell recruitment in the setting of peritonitis. These data confirm our previous findings that CD11b activation can efficiently regulate neutrophil and monocyte infiltration (28, 29).
Lastly, using a model of chronic vascular inflammation, we show that genetic activation of CD11b results in a significant decrease in aortic and brachiocephalic plaque burden in hyperlipidemic KI mice compared to WT controls. Our results suggest that a reduction in macrophage infiltration is one of the mechanisms behind CD11b-mediated atheroprotection. Nonetheless, future studies will help determine whether other mechanisms contribute to this effect, including lower macrophage retention or proliferation in plaques, and/or a regulatory role in macrophage activation and polarization. Previous studies have associated CD11b/CD18 surface upregulation and activation in macrophages with increased macrophage egression from atherosclerotic plaques (58) and the peritoneum (6). Others have proposed that active CD11b suppresses the alternative activation of macrophages, and decreases foam cell formation in vitro through the downregulation of lipid peroxidation mechanisms and scavenger receptors (49, 59). However, a clear understanding of these mechanisms is lacking due to the use on non-selective integrin activating agents, and the absence of a murine model of CD11b activation in which to test these findings in vivo. Similarly, CD11b KO mice have provided conflicting data (60, 61), likely due to compensatory effects by other integrins, among other factors. Despite the previously reported atheroprotective role of CD11b in females but not males (61), we observed a similar trend of decreased plaque burden in both sexes. Unfortunately, our study was not adequately powered for gender effects. Therefore, this is a question that deserves further attention. Future investigations on the role of activated CD11b on controlling atherogenesis and/or enhancing atherosclerosis regression are warranted.
In conclusion, our work presents a novel murine model that constitutively expresses an active variant of the CD11b/CD18 integrin. Given the recent interest in integrin activation as a potential therapeutic approach, we expect that this model will not only provide a unique tool for future studies on the effects of CD11b activation in a variety of disease settings, but will also be extremely useful in fully elucidating underlying mechanisms of action that can support therapeutic drug development.
Supplementary Material
Key Points.
Genetic activation of CD11b reduces leukocyte recruitment in acute peritonitis.
Genetic activation of CD11b decreases atherosclerosis in hyperlipidemic animals.
CD11bI332G mice are a novel system to study the pathways of active Mac-1 in vivo.
Acknowledgments
FINANCIAL SUPPORT
This study was supported by the National Institutes of Health grants R01-HL125672 to RIVP and OCV, R01-DK121227 to RIVP, K08-HL151747 to LM, and the American Heart Association Predoctoral Fellowship 18PRE34030314 to ZMZ. This project was also supported in part by support from Bears Care, the Department of Internal Medicine at Rush University Medical Center, and the National Institutes of Health grants R01-DK107984, R01-DK084195, and R01-CA244938 to VG.
Footnotes
CONFLICT OF INTEREST
VG is founder of Adhaere Pharmaceuticals, Inc (now part of Gossamer Bio, Inc.), that is developing CD11b targeted therapeutics. VG has significant financial interest in it. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Arnaout MA 1990. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 75: 1037–1050. [PubMed] [Google Scholar]
- 2.Schittenhelm L, Hilkens CM, Morrison VL. 2017. beta2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front Immunol. 8: 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. 2006. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 25: 583–594. [DOI] [PubMed] [Google Scholar]
- 4.Podolnikova NP, Podolnikov AV, Haas TA, Lishko VK, Ugarova TP. 2015. Ligand recognition specificity of leukocyte integrin alphaMbeta2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry. 54: 1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. 2003. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J Immunol. 171: 6105–6111. [DOI] [PubMed] [Google Scholar]
- 6.Cao C, Lawrence DA, Strickland DK, Zhang L. 2005. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 106: 3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kourtzelis I, Mitroulis I, von Renesse J, Hajishengallis G, Chavakis T. 2017. From leukocyte recruitment to resolution of inflammation: the cardinal role of integrins. J. Leukoc. Biol 102: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MS, Staunton DE, Marlin SD, Springer TA. 1991. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 65: 961–971. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC, Agbanyo FR, Plescia J, Ginsberg MH, Edgington TS, Plow EF. 1990. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18). J Biol Chem. 265: 12119–12122. [PubMed] [Google Scholar]
- 10.Fink K, Busch HJ, Bourgeois N, Schwarz M, Wolf D, Zirlik A, Peter K, Bode C, von Zur Muhlen C. 2013. Mac-1 directly binds to the endothelial protein C-receptor: a link between the protein C anticoagulant pathway and inflammation? PLoS One. 8: e53103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers C, Edelman ER, Simon DI. 1998. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci U S A. 95: 10134–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. 1999. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 30: 134–139. [DOI] [PubMed] [Google Scholar]
- 13.Dehnadi A, Benedict Cosimi A, Neal Smith R, Li X, Alonso JL, Means TK, Arnaout MA. 2017. Prophylactic orthosteric inhibition of leukocyte integrin CD11b/CD18 prevents long-term fibrotic kidney failure in cynomolgus monkeys. Nat Commun. 8: 13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirahashi J, Hishikawa K, Kaname S, Tsuboi N, Wang Y, Simon DI, Stavrakis G, Shimosawa T, Xiao L, Nagahama Y, et al. 2009. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 120: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN. 1997. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 186: 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, A. S. D, Bledzka K, Ustinov V, Zhu L, Qin J, et al. 2017. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbalpha. Nat Commun. 8: 15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA, Investigators AS. 2003. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 34: 2543–2548. [DOI] [PubMed] [Google Scholar]
- 18.Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, Gilkeson GS, Chen W, Zhu C, McEver RP, Kimberly RP, et al. 2008. A nonsynonymous functional variant in integrin-alpha(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat Genet. 40: 152–154. [DOI] [PubMed] [Google Scholar]
- 19.International Consortium for Systemic Lupus Erythematosus, G., Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, et al. 2008. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 40: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Kim-Howard X, Deshmukh H, Kamatani Y, Viswanathan P, Guthridge JM, Thomas K, Kaufman KM, Ojwang J, Rojas-Villarraga A, et al. 2009. Evaluation of imputation-based association in and around the integrin-alpha-M (ITGAM) gene and replication of robust association between a non-synonymous functional variant within ITGAM and systemic lupus erythematosus (SLE). Hum Mol Genet. 18: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Zhao M, Hirankarn N, Lau CS, Mok CC, Chan TM, Wong RW, Lee KW, Mok MY, Wong SN, et al. 2009. ITGAM is associated with disease susceptibility and renal nephritis of systemic lupus erythematosus in Hong Kong Chinese and Thai. Hum Mol Genet. 18: 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Wu J, Kucik DF, White NB, Redden DT, Szalai AJ, Bullard DC, Edberg JC. 2013. Multiple lupus-associated ITGAM variants alter Mac-1 functions on neutrophils. Arthritis Rheum. 65: 2907–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faridi MH, Khan SQ, Zhao W, Lee HW, Altintas MM, Zhang K, Kumar V, Armstrong AR, Carmona-Rivera C, Dorschner JM, et al. 2017. CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus. J. Clin. Invest. 127: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM, Luscinskas FW, Cullere X, Zhu C, Mayadas TN. 2015. A Lupus-Associated Mac-1 Variant Has Defects in Integrin Allostery and Interaction with Ligands under Force. Cell Rep. 10: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YH, Bae SC. 2015. Association between the functional ITGAM rs1143679 G/A polymorphism and systemic lupus erythematosus/lupus nephritis or rheumatoid arthritis: an update meta-analysis (vol 35, pg 815, 2015). Rheumatol Int. 35: 825–827. [DOI] [PubMed] [Google Scholar]
- 26.Rosetti F, Tsuboi N, Chen K, Nishi H, Ernandez T, Sethi S, Croce K, Stavrakis G, Alcocer-Varela J, Gomez-Martin D, et al. 2012. Human lupus serum induces neutrophil-mediated organ damage in mice that is enabled by Mac-1 deficiency. J Immunol. 189: 3714–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaves LD, Bao L, Wang Y, Chang A, Haas M, Quigg RJ. 2014. Loss of CD11b exacerbates murine complement-mediated tubulointerstitial nephritis. PLoS One. 9: e92051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, et al. 2011. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 4: ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickinson CM, LeBlanc BW, Edhi MM, Heffernan DS, Faridi MH, Gupta V, Cioffi WG, O’Brien X, Reichner JS. 2018. Leukadherin-1 ameliorates endothelial barrier damage mediated by neutrophils from critically ill patients. J Intensive Care. 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faridi MH, Altintas MM, Gomez C, Duque JC, Vazquez-Padron RI, Gupta V. 2013. Small molecule agonists of integrin CD11b/CD18 do not induce global conformational changes and are significantly better than activating antibodies in reducing vascular injury. Biochim. Biophys. Acta 1830: 3696–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan SQ, Guo L, Cimbaluk DJ, Elshabrawy H, Faridi MH, Jolly M, George JF, Agarwal A, Gupta V. 2014. A Small Molecule beta2 Integrin Agonist Improves Chronic Kidney Allograft Survival by Reducing Leukocyte Recruitment and Accompanying Vasculopathy. Front Med (Lausanne). 1: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Nawabi A, Schmitt T, Nault R, Zacharewski TR, Rockwell CE, Flick MJ, Luyendyk JP. 2016. Fibrin deposition following bile duct injury limits fibrosis through an alphaMbeta2-dependent mechanism. Blood. 127: 2751–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagarapu J, Kelchtermans J, Rong M, Chen S, Hehre D, Hummler S, Faridi MH, Gupta V, Wu S. 2015. Efficacy of Leukadherin-1 in the Prevention of Hyperoxia-Induced Lung Injury in Neonatal Rats. Am. J. Respir. Cell Mol. Biol 53: 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, et al. 2019. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Means TK, Luster AD. 2010. Integrins limit the Toll. Nat Immunol. 11: 691–693. [DOI] [PubMed] [Google Scholar]
- 36.Xiong JP, Li R, Essafi M, Stehle T, Arnaout MA. 2000. An isoleucine-based allosteric switch controls affinity and shape shifting in integrin CD11b A-domain. J Biol Chem. 275: 38762–38767. [DOI] [PubMed] [Google Scholar]
- 37.Huth JR, Olejniczak ET, Mendoza R, Liang H, Harris EA, Lupher ML Jr., Wilson AE, Fesik SW, Staunton DE. 2000. NMR and mutagenesis evidence for an I domain allosteric site that regulates lymphocyte function-associated antigen 1 ligand binding. Proc. Natl. Acad. Sci. U. S. A 97: 5231–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farley FW, Soriano P, Steffen LS, Dymecki SM. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 28: 106–110. [PubMed] [Google Scholar]
- 39.Bjorklund MM, Hollensen AK, Hagensen MK, Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG, Bentzon JF. 2014. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. 114: 1684–1689. [DOI] [PubMed] [Google Scholar]
- 40.Celik E, Faridi MH, Kumar V, Deep S, Moy VT, Gupta V. 2013. Agonist leukadherin-1 increases CD11b/CD18-dependent adhesion via membrane tethers. Biophys J. 105: 2517–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JY, Arnaout MA, Gupta V. 2007. A simple, no-wash cell adhesion-based high-throughput assay for the discovery of small-molecule regulators of the integrin CD11b/CD18. J Biomol Screen. 12: 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zigmond SH 1988. Orientation chamber in chemotaxis. Methods Enzymol. 162: 65–72. [DOI] [PubMed] [Google Scholar]
- 43.Altieri DC 1991. Occupancy of CD11b/CD18 (Mac-1) divalent ion binding site(s) induces leukocyte adhesion. J Immunol. 147: 1891–1898. [PubMed] [Google Scholar]
- 44.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. 2006. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med 203: 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. 2010. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol 185: 7057–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao X, Dong G, Zhu Y, Yan F, Zhang H, Ma Q, Fu X, Li X, Zhang Q, Zhang J, et al. 2019. Leukadherin-1-Mediated Activation of CD11b Inhibits LPS-Induced Pro-inflammatory Response in Macrophages and Protects Mice Against Endotoxic Shock by Blocking LPS-TLR4 Interaction. Front. Immunol 10: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M, Xu W, Wang Y, Jiang X, Li Y, Yang Y, Yuan H. 2018. CD11b-activated Src signal attenuates neuroinflammatory pain by orchestrating inflammatory and anti-inflammatory cytokines in microglia. Mol. Pain 14: 1744806918808150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, et al. 2006. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J. 20: 559–561. [DOI] [PubMed] [Google Scholar]
- 49.Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. 2011. alphaMbeta(2) integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ. Res 108: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stockl J, Majdic O, Pickl WF, Rosenkranz A, Prager E, Gschwantler E, Knapp W. 1995. Granulocyte activation via a binding site near the C-terminal region of complement receptor type 3 alpha-chain (CD11b) potentially involved in intramembrane complex formation with glycosylphosphatidylinositol-anchored Fc gamma RIIIB (CD16) molecules. J Immunol. 154: 5452–5463. [PubMed] [Google Scholar]
- 51.Lefort CT, Hyun YM, Schultz JB, Law FY, Waugh RE, Knauf PA, Kim M. 2009. Outside-in signal transmission by conformational changes in integrin Mac-1. J Immunol. 183: 6460–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hind LE, Vincent WJ, Huttenlocher A. 2016. Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration. Dev. Cell 38: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LA, Aranda-Espinoza H, Haun JB, Dembo M, Hammer DA. 2007. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 92: L58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. 2012. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 120: 3563–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szczur K, Zheng Y, Filippi MD. 2009. The small Rho GTPase Cdc42 regulates neutrophil polarity via CD11b integrin signaling. Blood. 114: 4527–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes BJ, Hollers JC, Crockett-Torabi E, Smith CW. 1992. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J Clin Invest. 90: 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hyun YM, Choe YH, Park SA, Kim M. 2019. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp Mol Med. 51: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray JL, Shankar R. 1995. Down regulation of CD11b and CD18 expression in atherosclerotic lesion-derived macrophages. Am. Surg 61: 674–679; discussion 679–680. [PubMed] [Google Scholar]
- 59.Yakubenko VP, Hsi LC, Cathcart MK, Bhattacharjee A. 2013. From macrophage interleukin-13 receptor to foam cell formation: mechanisms for alphaMbeta2 integrin interference. J. Biol. Chem 288: 2778–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubo N, Boisvert WA, Ballantyne CM, Curtiss LK. 2000. Leukocyte CD11b expression is not essential for the development of atherosclerosis in mice. J Lipid Res. 41: 1060–1066. [PubMed] [Google Scholar]
- 61.Szpak D, Izem L, Verbovetskiy D, Soloviev DA, Yakubenko VP, Pluskota E. 2018. alphaMbeta2 Is Antiatherogenic in Female but Not Male Mice. J. Immunol 200: 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.