Abstract
Morphogenesis is a physical process that requires the generation of mechanical forces to achieve dynamic changes in cell position, tissue shape, and size as well as biochemical signals to coordinate these events. Mechanical forces are also employed by the embryo to transmit detailed information across space and detected by target cells leading to downstream changes in cellular properties and behaviors. Indeed, forces provide signaling information of complementary quality that can both synergize and diversify the functional outputs of biochemical signaling. Here we discuss recent findings that reveal how mechanical and biochemical signaling are integrated during morphogenesis and the possible context-specific advantages conferred by the interactions between these signaling mechanisms.
Introduction: force generation and detection in morphogenesis
Morphogenesis occurs across a range of time scales and physical space, requiring the coordinated interplay of a host of different cell behaviors. Although ligand-based biochemical signaling elicits cellular responses during tissue morphogenesis, the mechanical forces generated by cells downstream of this signaling ultimately mold tissues. However, these forces can also be detected by cells leading to biochemical and mechanical signal propagation within and between cells, that not only regulate cellular behavior and fate changes, but also coordinate and diversify the functional outputs of biochemical signaling to propel morphogenesis. Here we focus on this particular form of mechanical signaling in development and examine in vivo examples where forces are utilized by cells to transmit information to other cells with unique advantages.
Cells and tissues can generate and transmit forces by several general mechanisms, but all of these begin with the cytoskeleton [1]. Actin polymerization generates pushing force during the establishment of cellular protrusions, and tension is generated when non-muscle myosin II (MyoII) binds to filamentous actin (F-actin) and hydrolyzes ATP to convert chemical energy into mechanical movement [2]. Forces generated by actomyosin contractility are transmitted across tissues through adhesion molecules that allow individual cellular forces to be translated into global changes in tissue shape. Adherens junctions (AJs) vary in their size and composition, but are mediated by classic cadherins that connect to the actin cytoskeleton intracellularly through binding to β-catenin, which in turn binds α-catenin (Fig. 1A). Under contractility-generated tension, α-catenin undergoes conformational changes to recruit vinculin, which connects to F-actin [3], resulting in maturation and growth of the AJ and recruitment of additional F-actin (Fig. 1A’) [3,4,5]. This mechanosensory function allows the AJ to react dynamically to other cells and actomyosin contractility while mechanically coupling the intracellularly-generated force with surrounding cells. Whereas most of the force generation for morphogenesis has been thought to derive from the actomyosin cytoskeleton, microtubules can also generate forces within cells, and this is coordinated by cell-signaling to regulate cell shape and epithelial morphogenesis in Drosophila [6–8].
Figure 1. Mechanisms of mechanosensation.
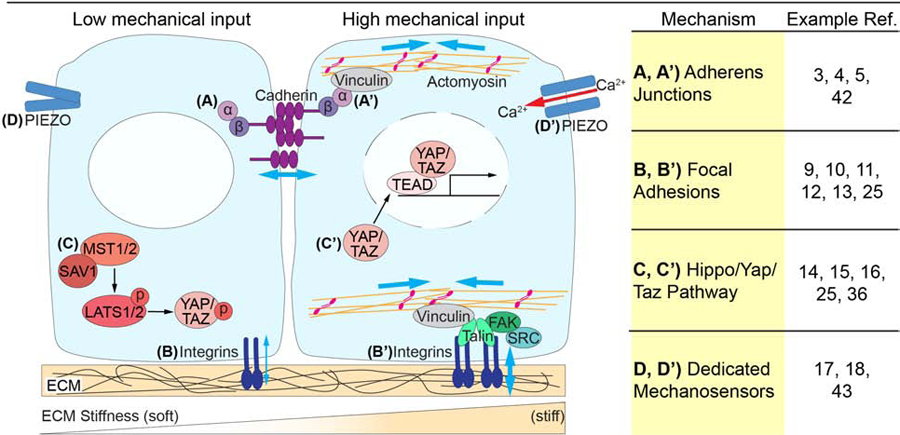
Mechanical forces are sensed and transmitted across cells and tissues through a variety of mechanisms. (A) Cadherins, bound intracellularly to β-catenin, which in turn binds α-catenin, make up adherens junctions. (A’) Under tension, generated by actomyosin contractility, α-catenin recruits the actin binding protein vinculin. The mechanosensory function of adherens junctions allows the mechanical coupling of adjacent cells. (B) Focal adhesions, composed of Integrins, couple cells to the ECM providing cells with both mechanical and biochemical information. (B’) Under tension a series of intracellular adaptors are recruited to focal adhesions, including FAK, SRC, Talin and Vinculin, linking the focal adhesions to actomyosin. (C) The Hippo/YAP/TAZ pathway is a critical mechanosensitive signaling pathway. When there is low mechanical input MST1/2 kinases bind SAV1, phosphorylating LATS1/2 kinases which in turn phosphorylate YAP/TAZ, preventing them from entering the nucleus. (C’) When there is high mechanical input Hippo signaling is inactive, allowing YAP/TAZ to translocate to the nucleus, where they bind to TEAD transcription factors, driving target gene expression. (D) Dedicated mechanosensors, such as PIEZO proteins, can also detect forces within a tissue. (D’) When sensing crowding forces, PEIZO channels undergo a conformational change, enabling a calcium influx into the cell to impact downstream signaling.
In addition to cell-cell adhesion, cell-extracellular matrix (ECM) adhesion is critical to convey or buffer the transmission of forces across tissues during morphogenesis [9,10]. Physical coupling of cells to the ECM at focal adhesions (FA) is critical for cellular reorganization and movement, but the ECM is also an instructional biochemical signal received through integrin receptors to modulate downstream signaling cascades and control a variety of cell behaviors during development [9]. FAs are large multiprotein signaling hubs that include heterodimeric integrin receptors, which recruit intracellular adaptors including talin and vinculin, linking the FA to F-actin, and focal adhesion kinase (FAK) and SRC kinase, which can activate numerous downstream pathways (Fig. 1B, B’). Similar to α-catenin in AJs, physical force exerted by actomyosin contractility mechanically induces a conformational change in TALIN, allowing increased actin binding and greater FA stability [11]. The strength of cell-ECM adhesions is also modulated by the stiffness of the ECM wherein stiffer substrates allow cells to adhere more strongly and exert higher tension. Integrin/ECM binding therefore allows the detection of distinct types and compositions of ECM and organizes the formation of signaling complexes with the actomyosin cytoskeleton [10]. In this way cells effectively convert mechanical information into biochemical signals and through changes in actomyosin contractility, can reciprocally remodel ECM, resulting in a host of cellular and tissue-level changes; this form of mechanosensation has been reviewed extensively elsewhere [9,12], and will not be the focus of this review, which will instead focus on the ways that cells utilize force to communicate with other cells. In addition to providing a biochemical ligand for physical or chemical signaling into cells, ECM may generate forces signaling to cells; for example, hydration of chondroitin sulfate proteoglycan in the vegetal epithelium of sea urchins results in differential expansion and bending of a bilayered cell sheet [13].
One important consequence of mechanically sensitive signaling at FAs and AJs is the biochemical activation of the Yes Associated Protein (YAP) and WW Domain Containing Transcription Regulator 1 (TAZ), leading to changes in transcription that impact cell proliferation and differentiation. YAP and TAZ, initially identified as Yorkie in Drosophila (Yki/YAP/TAZ), are transcriptional effectors of the Hippo (Hpo/MST1/2) kinase signaling cascade [14]. When this pathway is active, Hpo/MST1/2 kinases bind to the Sav/SAV1 adapter protein and phosphorylate Wts/LATS1/2 kinases to activate them. In turn, the Wts/LATS1/2 serine/threonine kinases phosphorylate Yki/YAP/TAZ and prevent them from entering the nucleus and activating transcription (Fig. 1C). Inactivation of the Hippo pathway results in Yki/YAP/TAZ accumulation within the nucleus, where they bind to TEAD transcription factors to drive target gene expression and promote cell proliferation and survival (Fig. 1C’). Mechanical change detected by FAs and AJs under actomyosin-generated contractility is a key force-sensing signaling mechanism that leads to activation of Yki/YAP/TAZ and transcriptional changes. Cell junctions serve as a site of assembly for Hippo pathway members and loss of AJ components can lead to increased YAP nuclear localization in different contexts [14]. In contrast, activation of YAP at FAs involves FAK and SRC activation of PI3K, leading to inhibition of LATS1/2 and nuclear accumulation of YAP [15]. Forces transmitted through FAs can also mechanically alter nuclear shapes and stretch nuclear pores to allow active nuclear YAP import [16]. Generally, the ability of Hippo signaling to measure junctional changes depends on actomyosin contractility, and thereby provides a central pathway for translating physical information into biochemical information in the form of gene expression changes.
In addition to the described mechanisms of force detection at cell junctions, dedicated mechanosensors can detect forces within a tissue. For example, PIEZO proteins are mechanically sensitive ion channels that are critical for mechanosensation in multiple contexts in development by sensing crowding forces to induce cell extrusion and control cell density [17], and to regulate stem cell proliferation and differentiation [18,19]. Although the mechanisms by which PIEZO transduces a biochemical signal are still under active investigation, it is clear that they can function through Ca2+ signaling [18], or by impacting YAP/TAZ function (Fig. 1D, D’) [19,20].
While mechanical forces can be detected and translated into biochemical signaling, it is also the case that biochemical signaling pathways that regulate morphogenesis have outcomes that generate forces. For example, EPH/EPHRIN signaling often regulates actomyosin contractility [21,22]; mitogenic signals such as WNT and SHH increase cell number, and chemoattractant pathways such as FGF increase cellular aggregation, both of which lead to increased cell density to generate compression forces. That these forces are transmitted throughout a tissue with both directional and magnitude information and detected by other cells suggests that these forces may be utilized as signal transducers downstream of biochemical signals. Here we review recent discoveries that connect biochemical signaling with mechanical signaling, focusing particularly on those cases where mechanical forces mediate biochemical signaling to regulate morphogenesis. These studies support the idea that force is not only detected during development, but that it is actively employed to transmit and convey biochemical signaling information in a manner that provides unique advantages.
Mechanical signals coordinate physical information with cellular differentiation and proliferation
Several recent papers have demonstrated that forces can signal to couple cell position within a tissue with cell fate specification, thereby coordinating physical information and cellular differentiation (Fig. 2A). While it is known that the stemness of epidermal progenitors can be manipulated by altering cell shape or ECM stiffness [23,24], how mechanical changes are employed to enable specific cell fate decisions has remained unclear. Totaro et al. recently demonstrated that cell shape and ECM rigidity regulates YAP/TAZ, which in turn regulate Notch signaling and downstream differentiation within the epidermis. In epidermal stem cells experiencing higher mechanical forces from either cytoskeletal or ECM rigidity, YAP nuclear localization inhibits Notch signaling, promoting epidermal stemness [25]. Conversely, low mechanical force inhibits YAP/TAZ, thus releasing Notch signaling, promoting differentiation [25]. Interestingly, YAP/TAZ increase expression of several Notch ligands, including DLL1 and DLL3, which stimulate Notch activity in neighboring cells [25,26]. Importantly, these same ligands likely inhibit differentiation of basal cells through their cis-regulation, thus maintaining a layer of basal progenitors, and efficient differentiation of suprabasal cells [25,26].
Figure 2. Integration of mechanical and biochemical signaling during morphogenesis.
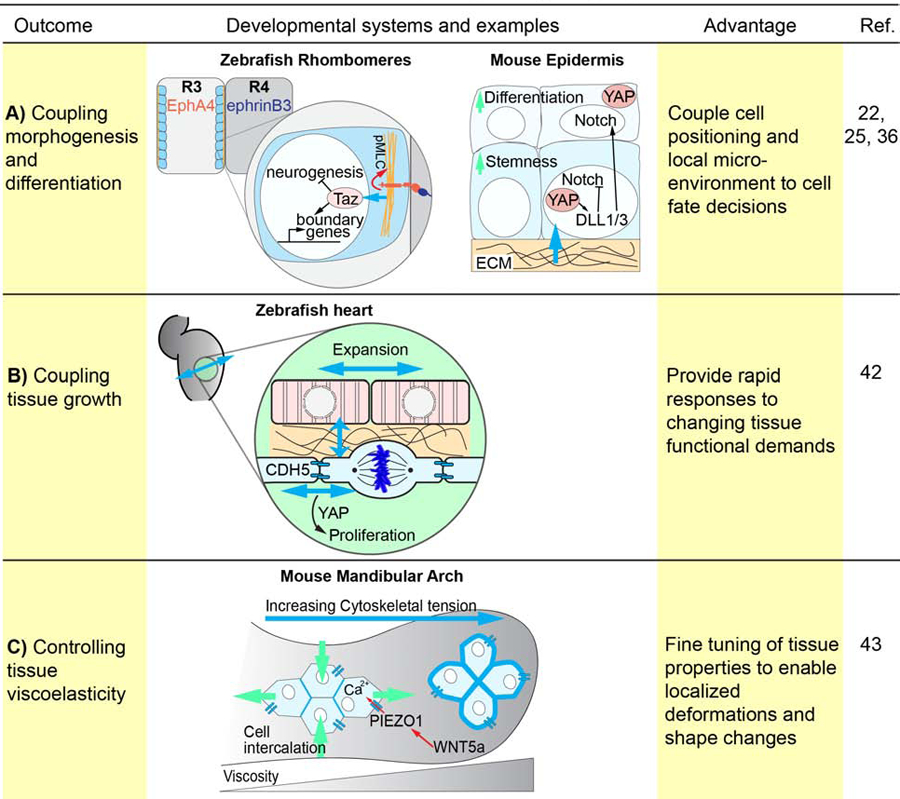
Mechanical and biochemical signaling can be integrated to affect various morphogenetic outcomes including tissue growth and cellular differentiation. (A) In developing zebrafish rhombomeres morphogenesis is coupled to cellular differentiation through Eph/Ephrin signaling generated actomyosin contractility, which in turn activates Yap/Taz signaling in boundary cells. Additionally, YAP/TAZ mechanotransduction inhibits NOTCH signaling in the developing mouse epidermis to maintain epidermal stemness in basal cells, while promoting differentiation of the suprabasal layer. (B) In the zebrafish heart myocardial and endocardial growth are coupled through tension sensing via VE-cadherin and Yap1 to regulate cell proliferation. (C) Mechanical and biochemical signaling can also be integrated to modulate tissue viscoelasticity as demonstrated in the developing mandibular arch, where WNT5a acts upstream of YAP and Piezo1 to coordinate cellular polarity and force oscillations in the middle arch to diminish tissue rigidity, enabling cell intercalations.
Mechanical signals are integrated to coordinate boundary formation and cell differentiation during rhombomere formation in the developing hindbrain. Rhombomeres are developmentally transient blocks of neuroepithelial cells that give rise to distinct structures in the vertebrate hindbrain. Boundaries between rhombomeres are formed as a result of signaling between Eph receptor tyrosine kinases and their signaling partner, the Ephrins; in zebrafish, alternating rhombomeres express EphA4 and Ephrin-B3 such that EphA4/Ephrin-B3 signaling only occurs at the rhombomere boundary [27–30]. Boundary cells express molecular markers that distinguish them from non-boundary cells, provide proliferating progenitors and organize spatially-restricted neurogenesis within segments [31–33], and are involved in boundary straightening through the formation of actomyosin-cable like structures at rhombomeric boundaries [34]. Disruption of either actomyosin contractility or Eph/Ephrin signaling disrupts boundary sharpness [34,35]. Interestingly, increased tension from actomyosin contractility at rhombomere boundaries creates this positional information, which impacts boundary cell identity [22,36]. EphA4 loss of function results in reduced actomyosin contractility at rhombomere boundaries and a loss of boundary cells in EphA4-expressing rhombomere segments, indicating that EphA4 signaling generates positionally-specific tension to simultaneously specify rhombomere separation and cell identity specification [22]. This increased tension at rhombomere boundaries promotes Taz nuclear localization and downstream activation of boundary markers [22,36] in EphA4-expressing boundary cells. When Yap/Taz pathway components are disrupted, border cell marker expression is lost [22]. Yap also maintains the proliferative capacity and the progenitor potential in boundary cells, with neurogenesis coinciding with Yap downregulation as daughter cells exit the boundary domain [36]. Together these data elucidate a complete pathway linking boundary formation and maintenance through Eph/Ephrin signaling to downstream cell fate decisions by a mechanical signaling intermediary. Interestingly, as Notch pathway components are also expressed in boundary cells and have been shown to regulate neurogenesis in rhombomeres, it is intriguing to postulate that integration of mechanical signals and Notch activation may similarly exist here as in the example above to preserve progenitor state in boundary cells.
In many contexts, changes in force can be interpreted as morphogenetic signals to rapidly remodel and differentiate specialized cell types that further contribute to organ development and function (Fig. 2B). Shear force due to blood flow is detected during outflow tract (OFT) valve development in zebrafish, coupling the positions of highest shear force due to blood flow with positional specification of smooth muscle differentiation that results in valve morphogenesis [20]. In the regions of the OFT with the smallest diameter, where shear stress is highest, Piezo mechanosensitive channels detect this shear force, resulting in spatially-restricted expression of Klf2 and Notch signaling within the valve endothelium, and Yap1 activation and differentiation of the underlying smooth muscle. In the atrioventricular heart valve, Klf2a and Notch signaling activity are also high in regions experiencing high blood flow [37,38], though the structure of this valve, and the forces it experiences are somewhat different. Indeed, it is notable that Klf2 expression and Notch signaling are commonly mechanosensitive to blood flow during development [39,40], supporting them as common nodes in pathways converting mechanical to biochemical signaling information in a spatially-restricted manner.
Intra-organ communication is necessary to coordinate the growth and position of discrete, but interdependent structures (Fig. 2C). In zebrafish heart development, Wnt8a signaling is critical for promoting cardiomyocyte formation and its overexpression results in increased atrial and decreased ventricle myocardial size [41]. Interestingly, this effect is mirrored by changes in the size of the underlying atrial and ventricular endocardium [42]. Expansion of the myocardium places the endocardial cells under tension, which is sensed by junctional Cadherin-5 (VE-cadherin), resulting in nuclear Yap1 localization and increased proliferation of endocardial cells to compensate for myocardial overgrowth. These data reveal that tension generated by tissue growth can signal to neighboring tissues allowing the coordination of tissue-intrinsic growth rates.
Chemical signals modulate cell polarity, adhesion, and tissue deformability to signal mechanically
The emergence of coordinated collective cell behaviors requires the detection, coupling, and propagation of forces across groups of cells. Tissue rheology, or the way in which tissues mechanically react, arises from the contractility of the cells composing the tissue, the ECM, and the strength of the cell-cell contacts within a tissue. Viscoelasticity determines the deformability of the tissue and permissibility for cellular arrangement in response to inductive signals. Modulation of these properties within a tissue allows for regulated deformation and shaping of a tissue.
Chemical signals can guide morphogenesis by tuning tissue mechanics and viscoelasticity through control of adhesion, cortical contractility, and associated cell polarity. This was recently demonstrated in the developing mouse pharyngeal arch, which is composed of a mesenchymal core surrounded by a single layer of epithelium, and undergoes extensive outgrowth and shape changes throughout development. Tao et al. demonstrated that, in the mesenchyme, WNT5a activates PIEZO1 to induce oscillations in cortical tension in the middle portion of the developing arch, resulting in reduced tissue viscoelasticity and increased cell intercalation to drive arch elongation [43]. In Wnt5a mutant mice the shape of the mandibular arch is disrupted with diminished cortical oscillations and a decrease in oriented cell intercalation, suggesting that WNT5a coordinates mandibular cell behaviors through control of cell polarity and cytoskeleton tension [43]. This study therefore demonstrates a mechanism by which chemical signals impact tissue mechanics to enable proper morphogenesis.
Signaling by WNT5a through the ROR2 receptor is also critical during angiogenesis, where it coordinates endothelial cell behavior by activating CDC42 and stabilizing vinculin at the AJ [44]. This results in mechanocoupling between endothelial cells and their collective polarization, which is necessary for their proper migration. Therefore, non-canonical WNT signaling tunes the sensitivity of endothelial cells to junctional force to modulate their behavior. Detection and sensitivity of cells to forces is often tuned by biochemical signaling pathways, thereby allowing these pathways to influence the cellular outcomes upon experiencing a given force. As in heart development, shear force from blood flow is critical for vessel reorganization during angiogenesis, such that the direction and strength of flow dictates endothelial cell polarization and migration. Endothelial non-canonical WNT signaling is required for the detection of shear force, and modulates sensitivity to this force in order to select which vessels undergo normal pruning [45]. Interestingly, VEGFR3 signaling also influences sensitivity of endothelial cells to shear stress from flow, indicating that in this context multiple biochemical pathways converge to regulate sensitivity to a force-based signal [46]. Differences in VEGFR3 levels may be a major determinant of differences in sensitivity to shear stress by vascular endothelial cells, compared with lymphatic endothelial cells, which have a higher sensitivity to shear stress allowing detection of lower flow rates and therefore the remodeling of these different vascular cell types at different force reception set-points [46].
Mechanical modulation of chemical signaling by cell density and crowding forces
As morphogenesis progresses, changes in tissue shape and cell organization can concurrently reshape the spatial distribution of signaling molecules (Fig. 3A). For instance, villi formation in the developing chick gut as a result of mechanical buckling of the endodermal epithelium distorts the SHH signaling gradient from the epithelium, concentrating the signal at the tip of each villus to activate high threshold response genes in the mesenchyme that ultimately determine the location of intestinal stem cells [47]. This suggests that tissue mechanical forces can actively modulate signaling pattern via emerging cellular organization. This idea is consistent with recent findings in developing chick feather buds, which arise in the midline of the dorsal skin as regularly spaced mesenchymal aggregates beneath epidermal placodes, with subsequent new buds formed laterally in a spatiotemporal manner. Feather bud development is initiated as a result of MyoII-dependent mesenchymal contraction that amplifies randomly formed small cell clusters into larger aggregates [48]. Condensed mesenchyme in turn compresses the overlying epithelium and mechanically induces nuclear accumulation of β-catenin to initiate the follicle genetic program [48,49]. This mesenchymal contraction also concentrates and upregulates local FGF20 signaling from the epithelium to further promote mesenchymal condensation [50,51]. Simultaneously, condensed mesenchyme begins to express BMP4, which diffuses and inhibits epithelial Fgf20 expression neighboring the condensate [50]. Tissue mechanical forces thus help shape FGF20 and BMP4 expression pattern with altering peaks and troughs of FGF20 and BMP4 signaling activities, which function as the activator and inhibitor respectively in a Turing reaction-diffusion system [52] to establish the formation of feather buds repetitively at a regular interval.
Figure 3. Mechanical modulation of chemical signaling.
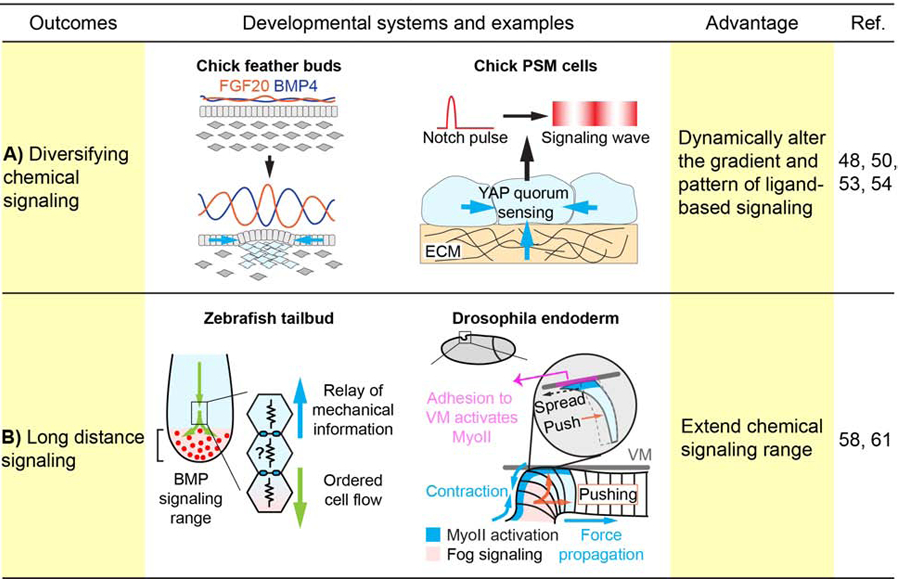
Tissue mechanical forces can modulate the gradient and pattern of biochemical signaling. (A) In the chick feather buds, condensing mesenchyme contracts overlying epithelium to concentrate local FGF20, and at the same time secrets BMP4 that diffuses and inhibits neighboring FGF20 expression, resulting in a Turing-like pattern. In the chick presomitic mesoderm (PSM), YAP integrates mechanical information from the substrate and cell density to transform signaling pulses into oscillations and waves. (B) Tissue forces can also function as a second messenger downstream of a biochemical source to relay its instructive signal across space. For instance, in the zebrafish tailbud, the anterior-to-posterior cell flow is modulated by mechanical signals transmitted from cell to cell and thus beyond the range of Bmp signaling in the tail organizer. Similarly, during Drosophila endoderm invagination, although the initial MyoII activation is initiated by Fog signaling, the subsequent traveling wave of MyoII activation and apical contraction is independent from Fog signaling and is induced by cyclic forward pushing of buckling cells and apical spreading of edge cells along the vitelline membrane (VM).
Formation of repetitive structures can also be achieved through molecular oscillators, such as in the vertebrate presomitic mesoderm (PSM). In this model, cyclic activation of Notch and WNT pathways and corresponding signaling responsive genes generate periodic travelling waves of signaling activation and instruct the formation of segmented structures called somites [53]. Interestingly, when PSM cells are dissociated and scrambled in primary cell culture, they continue to oscillate and produce waves of Notch signaling [54]. However, this phenomenon is only maintained when the cell density is above certain threshold. The system exhibits a quorum sensing behavior involving YAP, which functions as a checkpoint to only allow full Notch signaling when a certain cell crowding threshold is reached. Intriguingly, quorum sensing via YAP can be modulated by cell shapes and actin-dependent mechanical forces, raising the possibility that signaling oscillation during somite formation is regulated by mechanical inputs associated with changes in crowding-force [54]. It will be interesting to determine if such an excitable density detection system similarly functions in other developmental contexts involving cell condensation, such as in the feather bud example above, to govern local activation of specific signaling cascades and generation of signaling waves.
Mechanical force as a long-range intermediary signal to regulate morphogenesis
While paracrine signaling is only effective over a relatively short distance of 50–100 μm (spanning 5–10 cells) due to rapid signal dilution and decay in its intensity [55–57], mechanical forces can be directionally transmitted over a longer distance and function as a long-range morphogenetic signal downstream of a localized biochemical stimulus (Fig. 3B). One example demonstrating mechanical signaling over distance is the regulation of zebrafish body elongation by the tail organizer [58]. Bmp signaling from the tail organizer is postulated to promote an ordered anterior-to-posterior cell flow in the tail bud that contributes to body elongation [59,60]. When Bmp signaling is perturbed, a cell-to-cell relay of disturbed cell motion in the tail bud results in a mechanical transmission of cellular jamming that travels posterior-anteriorly, resulting in disorganized cell motion outside the Bmp signaling range [58]. This hints at a mechanism whereby signaling ligands may induce directional movement of cells outside the signaling range by propagating mechanical signals through neighboring cells.
How then do cells propagate mechanical signals over distance without dampening force transmission? A recent paper addressed this question by studying Drosophila endoderm morphogenesis, a MyoII-dependent process involving invagination of endoderm primordium moving posterior-anteriorly [61]. Importantly, while Rho1/MyoII activation is initiated by secreted Fog/GPCR signaling, a wave of MyoII actomyosin contractility continually propagates endoderm invagination anteriorly along the dorsal epithelium without Fog signal propagation. As MyoII can be activated in response to mechanical stimuli, such as increased cellular tension [62–64], cellular forces associated with epithelial buckling trigger apical spreading in unbuckled cells at the anterior edge of the furrow and activate MyoII in these cells and their subsequent buckling, thus cyclically amplifying the travelling mechanical wave. Interestingly, sequential activation of MyoII is also observed in other developmental contexts, such as the mechanical interaction between the invaginating endoderm and extending germband in Drosophila and the zippering process during neural tube closure in Ciona intestinalis [65,66], all suggesting that mechanical forces can act as a long-range signal and as a second messenger to regulate morphogenesis at a distance. Future work will determine whether such mechanisms can also control other cell behaviors, such as differentiation, proliferation, and polarity in this and other developmental processes.
Conclusions and perspectives
Cells may have evolved to actively utilize force as an extracellular second messenger to transduce information between cells with several advantages. The recent studies that we describe above give insight into this idea, and present explanations of the advantages that might be achieved by employing mechanical signals: these signals can coordinate growth of organs, specify cell fate with respect to tissue architecture, modulate chemical signals, and act over longer distances than biochemical signals. Importantly, mechanical force is a multiparameter signal; whereas a biochemical signal detected at a single point has only a magnitude value, mechanical force is a vector quantity, encoding both magnitude and directional information. This property makes mechanical forces particularly compelling for providing information to organize directed cell behaviors such as cell polarity and cell migration. Multiple biochemical signals may therefore converge to collate and convert information from multiple cellular inputs into a mechanical force signal that can be transmitted in a coordinated fashion. As mechanical signals have been historically challenging to observe, increasingly integrating techniques such as atomic force microscopy and laser ablation with genetic and biochemical approaches as well as the application of new techniques such as the application of oil droplets, or magnetic beads to measure and apply forces, will be transformative in further understanding the interplay between mechanical and biochemical signals during development [67,68].
Acknowledgements
This is a broad and active area of investigation, and we apologize to those colleagues whose work we could not discuss due to space limitations. A.K. was supported by F31DE028175 from NIH/NIDCR, J.H. was supported by R00DE025874 from the NIH/NIDCR. J.O.B was supported by R01DE023337 and R01DE025877 from the NIH/NIDCR, and R01HL144785 from NIH/NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Notable, recently-published papers have been highlighted in references as being of *special interest or **outstanding interest
References
- 1.Heisenberg C-P, Bellaïche Y: Forces in Tissue Morphogenesis and Patterning. Cell 2013, 153:948–962. [DOI] [PubMed] [Google Scholar]
- 2.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR: Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009, 10:778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris TJC, Tepass U: Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 2010, 11:502–514. [DOI] [PubMed] [Google Scholar]
- 4.Kale GR, Yang X, Philippe J-M, Mani M, Lenne P-F, Lecuit T: Distinct contributions of tensile and shear stress on E-cadherin levels during morphogenesis. Nat Commun 2018, 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladoux B, Nelson WJ, Yan J, Mège RM: The mechanotransduction machinery at work at adherens junctions. Int Bio (Cam) 2015, 7:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda M, Sami MM, Wang Y-C: A homeostatic apical microtubule network shortens cells for epithelial folding via a basal polarity shift. Nat Cell Biol 2018, 20:36–45. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Saha T, Begemann I, Ricker A, Nüsse H, Thorn-Seshold O, Klingauf J, Galic M, Matis M: Polarized microtubule dynamics directs cell mechanics and coordinates forces during epithelial morphogenesis. Nat Cell Biol 2018, 20:1126–1133. [DOI] [PubMed] [Google Scholar]
- 8.Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD: Microtubules provide directional information for core PCP function. eLife 2014, 3:e02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzamba BJ, DeSimone DW: Extracellular Matrix (ECM) and the Sculpting of Embryonic Tissues. Curr Top Dev Biol 2018, 130:245–274. [DOI] [PubMed] [Google Scholar]
- 10.Bachir AI, Horwitz AR, Nelson WJ, Bianchini JM: Actin-Based Adhesion Modules Mediate Cell Interactions with the Extracellular Matrix and Neighboring Cells. Cold Spring Harb Perspect Biol 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP: Stretching single talin rod molecules activates vinculin binding. Science 2009, 323:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muncie JM, Weaver VM: The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr Top Dev Biol 2018, 130:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane MC, Koehl MA, Wilt F, Keller R: A role for regulated secretion of apical extracellular matrix during epithelial invagination in the sea urchin. Development 1993, 117:1049–1060. [DOI] [PubMed] [Google Scholar]
- 14.Karaman R, Halder G: Cell Junctions in Hippo Signaling. Cold Spring Harb Perspect Biol 2018, 10:a028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N-G, Gumbiner BM: Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol 2015, 210:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux A-L, et al. : Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171:1397–1410.e14. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien C-B, Morcos PA, Rosenblatt J: Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484:546–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, Si G, Huang J, Samuel ADT, Perrimon N: Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 2018, 555:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, Bernardis E, Flanagan LA, Tombola F: Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA 2014, 111:16148–16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duchemin A-L, Vignes H, Vermot J: Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. Elife 2019, 8.*This study of zebrafish outflow tract valve development demonstrates that mechanosensitive pathways are observed at sites of high shear stress from blood flow. This shear force is detected by Piezo and Trp mechanosensitive channels which are critical for the proper differentiation of both endocardial and smooth muscle cell types in the OFT valve, and for valve morphogenesis. Together these data provide a new understanding of how heart valve morphogenesis is specified by mechanical forces.
- 21.O’Neill AK, Kindberg AA, Niethamer TK, Larson AR, Ho H-YH, Greenberg ME, Bush JO: Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. J Cell Biol 2016, 215:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cayuso J, Xu Q, Addison M, Wilkinson DG: Actomyosin regulation by Eph receptor signaling couples boundary cell formation to border sharpness. Elife 2019, 8.**This paper identifies a signaling cascade in which Yap/TAZ respond to actomyosin contractility generated at EphrinB3-EphA4 boundaries in the zebrafish hindbrain rhombomere boundaries to impact cell proliferation and differentiation
- 23.Connelly JT, Gautrot JE, Trappmann B, Tan DW-M, Donati G, Huck WTS, Watt FM: Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 2010, 12:711–718. [DOI] [PubMed] [Google Scholar]
- 24.Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, et al. : Extracellular-matrix tethering regulates stem-cell fate. Nature Materials 2012, 11:642–649. [DOI] [PubMed] [Google Scholar]
- 25.Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, Piccolo S: YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun 2017, 8:15206.*This paper identifies the integration of the YAP/TAZ mechanotransduction pathway with Notch signaling regulation to coordinate cell-cell communication, epidermal stemness, and differentiation.
- 26.Lowell S, Jones P, Le Roux I, Dunne J, Watt FM: Stimulation of human epidermal differentiation by Delta–Notch signalling at the boundaries of stem-cell clusters. Current Biology 2000, 10:491–500. [DOI] [PubMed] [Google Scholar]
- 27.Becker N, Seitanidou T, Murphy P, Mattéi M-G, Topilko P, Nieto MA, Wilkinson DG, Charnay P, Gilardi-Hebenstreit P: Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mechanisms of Development 1994, 47:3–17. [DOI] [PubMed] [Google Scholar]
- 28.Bergemann AD, Cheng HJ, Brambilla R, Klein R, Flanagan JG: ELF-2, a new member of the Eph ligand family, is segmentally expressed in mouse embryos in the region of the hindbrain and newly forming somites. Molecular and Cellular Biology 1995, 15:4921–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke J, Moens C, Roth L, Durbin L, Shiomi K, Brennan C, Kimmel C, Wilson S, Holder N: Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development 2001, 128:571–580. [DOI] [PubMed] [Google Scholar]
- 30.Xu Q, Mellitzer G, Robinson V, Wilkinson DG: In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 1999, 399:267–271. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Quevedo R, Lee Y, Poss KD, Wilkinson DG: Neuronal regulation of the spatial patterning of neurogenesis. Dev Cell 2010, 18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretz Y, Eren N, Kohl A, Hen G, Yaniv K, Weisinger K, Cinnamon Y, Sela-Donenfeld D: A new role of hindbrain boundaries as pools of neural stem/progenitor cells regulated by Sox2. BMC Biology 2016, 14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terriente J, Gerety SS, Watanabe-Asaka T, Gonzalez-Quevedo R, Wilkinson DG: Signalling from hindbrain boundaries regulates neuronal clustering that patterns neurogenesis. Development 2012, 139:2978–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calzolari S, Terriente J, Pujades C: Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J 2014, 33:686–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke JE, Kemp HA, Moens CB: EphA4 Is Required for Cell Adhesion and Rhombomere-Boundary Formation in the Zebrafish. Current Biology 2005, 15:536–542. [DOI] [PubMed] [Google Scholar]
- 36.Voltes A, Hevia CF, Engel-Pizcueta C, Dingare C, Calzolari S, Terriente J, Norden C, Lecaudey V, Pujades C: Yap/Taz-TEAD activity links mechanical cues to progenitor cell behavior during zebrafish hindbrain segmentation. Development 2019, 146.**This study describes how Yap/Taz-TEAD activity links mechanical cues to progenitor cell proliferation and behavior during zebrafish hindbrain segmentation.
- 37.Pestel J, Ramadass R, Gauvrit S, Helker C, Herzog W, Stainier DYR: Real-time 3D visualization of cellular rearrangements during cardiac valve formation. Development 2016, 143:2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steed E, Faggianelli N, Roth S, Ramspacher C, Concordet J-P, Vermot J: klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nat Commun 2016, 7:11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, et al. : Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 2006, 11:845–857. [DOI] [PubMed] [Google Scholar]
- 40.Groenendijk BCW, Hierck BP, Gittenberger-De Groot AC, Poelmann RE: Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn 2004, 230:57–68. [DOI] [PubMed] [Google Scholar]
- 41.Dohn TE, Waxman JS: Distinct phases of Wnt/β-catenin signaling direct cardiomyocyte formation in zebrafish. Developmental Biology 2012, 361:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bornhorst D, Xia P, Nakajima H, Dingare C, Herzog W, Lecaudey V, Mochizuki N, Heisenberg C-P, Yelon D, Abdelilah-Seyfried S: Biomechanical signaling within the developing zebrafish heart attunes endocardial growth to myocardial chamber dimensions. Nat Commun 2019, 10:1–10.**By utilizing zebrafish genetics, together with measures of junctional tension and cellular density and proliferation in the developing heart, the authors demonstrate that myocardial growth is coupled with endocardial growth through a pathway of tension-sensation that involves VE-cadherin and Yap1 regulation of cell proliferation. These data indicate that mechanical forces may be coupled between organs or tissues to ensure coordinated growth during development.
- 43.Tao H, Zhu M, Lau K, Whitley OKW, Samani M, Xiao X, Chen XX, Hahn NA, Liu W, Valencia M, et al. : Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat Commun 2019, 10:1703.*This study demonstrates how Wnt5a acts upstream of YAP and Piezo1 to coordinate cell polarity and cortical force oscillations to diminish tissue rigidity and enable cell intercalation.
- 44.Carvalho JR, Fortunato IC, Fonseca CG, Pezzarossa A, Barbacena P, Dominguez-Cejudo MA, Vasconcelos FF, Santos NC, Carvalho FA, Franco CA: Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franco CA, Jones ML, Bernabeu MO, Vion A-C, Barbacena P, Fan J, Mathivet T, Fonseca CG, Ragab A, Yamaguchi TP, et al. : Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. Elife 2016, 5:e07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas J-L, et al. : Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ: Bending gradients: How the intestinal stem cell gets its home. Cell 2015, 161:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM: Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 2017, 357:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W: beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105:533–545. [DOI] [PubMed] [Google Scholar]
- 50.Ho WKW, Freem L, Zhao D, Painter KJ, Woolley TE, Gaffney EA, McGrew MJ, Tzika A, Milinkovitch MC, Schneider P, et al. : Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol 2019, 17:e3000132.*Initiated by mesenchymal condensation, tissue contraction in chick feather primordium shapes the expression pattern of epithelial FGF20 and mesenchymal BMP4, which act as the activator and inhibitor respectively to generate Turing-like repetitive developmental patterns.
- 51.Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM: Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol 1998, 196:11–23. [DOI] [PubMed] [Google Scholar]
- 52.Turing AM: The chemical basis of morphogenesis. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 1952, 237:37–72. [Google Scholar]
- 53.Hubaud A, Pourquié O: Signalling dynamics in vertebrate segmentation. Nat Rev Mol Cell Biol 2014, 15:709–721. [DOI] [PubMed] [Google Scholar]
- 54.Hubaud A, Regev I, Mahadevan L, Pourquié O: Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell 2017, 171:668–682.e11.**In a presomitic mesoderm culture model, it was demonstrated that biochemical signaling oscillation is a collective cell behavior. Oscillation is triggered by a quorum sensing mechanism involving YAP and Notch and modulated by actin-dependent mechanical signals conveyed through cell shapes and substrate types.
- 55.Lee Y, McIntire LV, Zygourakis K: Analysis of endothelial cell locomotion: Differential effects of motility and contact inhibition. Biotechnol Bioeng 1994, 43:622–634. [DOI] [PubMed] [Google Scholar]
- 56.Francis K, Palsson BO: Effective intercellular communication distances are determined by the relative time constants for cyto/chemokine secretion and diffusion. Proc Natl Acad Sci U S A 1997, 94:12258–12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Handly LN, Pilko A, Wollman R: Paracrine communication maximizes cellular response fidelity in wound signaling. eLife [date unknown], 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das D, Jülich D, Schwendinger-Schreck J, Guillon E, Lawton AK, Dray N, Emonet T, O’Hern CS, Shattuck MD, Holley SA: Organization of Embryonic Morphogenesis via Mechanical Information. Dev Cell 2019, 49:829–839.e5.**Perturbation of BMP4 signaling in the zebrafish tailbud results in deregulated cell movement in regions beyond the BMP4 signaling range and combined imaging and in silico analyses suggest that the tail organizer integrates mechanical information relayed through the elongating tissue to regulate the coherence of posterior cell flow.
- 59.Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, Pontius W, Emonet T, Holley SA: Regulated tissue fluidity steers zebrafish body elongation. Development 2013, 140:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, Campàs O: A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 2018, 561:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailles A, Collinet C, Philippe J-M, Lenne P-F, Munro E, Lecuit T: Genetic induction and mechanochemical propagation of a morphogenetic wave. Nature 2019, doi: 10.1038/s41586-019-1492-9.**Invagination of Drosophila endoderm epithelium is initiated by Fog signaling and this is followed by a tissue-scale wave of Rho1/MyoII activation and epithelial invagination that is independent of sustained gene transcription and Fog signaling.
- 62.Heissler SM, Sellers JR: Kinetic Adaptations of Myosins for Their Diverse Cellular Functions. Traffic 2016, 17:839–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Gonzalez R, Simoes S de M, Röper J-C, Eaton S, Zallen JA: Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 2009, 17:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitrossilis D, Röper J-C, Le Roy D, Driquez B, Michel A, Ménager C, Shaw G, Le Denmat S, Ranno L, Dumas-Bouchiat F, et al. : Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat Commun 2017, 8:13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lye CM, Blanchard GB, Naylor HW, Muresan L, Huisken J, Adams RJ, Sanson B: Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila. PLOS Biology 2015, 13:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashimoto H, Robin FB, Sherrard KM, Munro EM: Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev Cell 2015, 32:241–255. [DOI] [PubMed] [Google Scholar]
- 67.Campàs O, Mammoto T, Hasso S, Sperling RA, O’Connell D, Bischof AG, Maas R, Weitz DA, Mahadevan L, Ingber DE: Quantifying cell-generated mechanical forces within living embryonic tissues. Nat Methods 2014, 11:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu M, Tao H, Samani M, Luo M, Wang X, Hopyan S, Sun Y: Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. PNAS 2020, doi: 10.1073/pnas.1912656117. [DOI] [PMC free article] [PubMed] [Google Scholar]


