Abstract
BACKGROUND
This study examined the relationship between serum glutathione peroxidase 1 (GPx-1) activity and endothelial dysfunction in the subjects with and without metabolic syndrome (MetS).
METHODS
This case-control study was conducted on 76 subjects, 38 were patients with MetS and 38 were without MetS. The demographic, clinical, and laboratory features of the subjects were measured and then compared. The MetS was diagnosed according to the definitions of the National Cholesterol Education Program (NCEP) and International Diabetes Federation (IDF). Serum GPx-1 activity was measured by standard methods. Endothelial dysfunction was assessed with flow-mediated dilation (FMD) technique.
RESULTS
In case-control study of 76 subjects, all of MetS risk factors including abdominal obesity, triglyceride (TG), low serum level of high-density lipoprotein cholesterol (HDL-C), hypertension (HTN), and fasting plasma glucose (FPG) were significantly higher than healthy individuals (P < 0.050). FMD was significantly lower than normal subjects (P < 0.050). Serum GP-1 activity was significantly lower in patients with MetS compared to normal subjects (21.7 ± 13.5 vs. 79.0 ± 38.6, respectively) (P = 0.001). The value of GPx-1 was significantly correlated with diastolic blood pressure (DBP) (r = -0.249, P = 0.040), C-reactive protein (CRP) (r = -0.409, P = 0.014), and FMD (r = 0.293, P = 0.050) in patients with MetS. The results of logistic regression showed that a unite increase in CRP (mg/dl), FMD (%), and endothelin-1 (ET-1) (pg/ml) and a unit decrease in GPx significantly increased the odds ratio (OR) of MetS; after adjusting for age and sex the results remained significant except for FMD (P < 0.050)
CONCLUSION
Endothelial dysfunction is related to serum GPx-1 activity in patients with MetS. GPX-1 activity is associated with risk of cardiovascular diseases (CVDs) and peripheral vascular diseases (PVDs) in patients with MetS.
Keywords: Glutathione Peroxidase-1, Endothelium, Enzyme Activity, Metabolic Syndrome
Introduction
The metabolic syndrome (MetS) is currently characterized by a bunch of risk factors mainly for atherosclerosis and type-2 diabetes mellitus (T2DM).1,2 Typical features of MetS are low level of high-density lipoprotein cholesterol (HDL-C), high level of hyperglycemia, hypertriglyceridemia, hypertension (HTN), and abdominal obesity.3 The incidence of MetS is epidemiologically due to complex interactions between genetic and environmental factors, as well as predominant sedentary lifestyles and unhealthy dietary habits.4 Recent experiments have proposed that MetS may be the result of different but interrelated pathophysiological mechanisms, such as endothelial dysfunction, inflammatory process, visceral obesity, oxidative stress (OxS), and genetic factors.5 Different studies have revealed that subjects with MetS have altered antioxidant protection as well as elevated oxidative damage.6 Although insulin resistance is regarded as a core of the MetS, one of the main mechanisms underlying this pathology is OxS.7
OxS occurred as a result of dysregulation in the production and degradation of reactive oxygen species (ROS).8 Anti-oxidative enzymes including glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase inactivate ROS. In mammalian cells, glutathione (GSH) and the GPx form the principal antioxidant defense system.9
The GPx is a selenocysteine-containing protein that acts against OxS via utilizing reduced GSH to reduce hydrogen peroxide (H2O2) and lipid peroxides to their corresponding alcohols.10
Different reports have shown that upon the increase of adipose tissue, the activity of antioxidant enzymes such as GPx is significantly attenuated. This leads to various abnormalities, among which endothelial dysfunction was found.11 It was reported that individuals with T2DM showed significantly diminished plasma GSH in comparison with the control group, which is associated with ROS markers.12,13 In addition, subjects with MetS have decreased antioxidant enzymes activity levels such as catalase, SOD, and GPx.14,15 Controversially, other studies found no differences in the levels of GPx activity in subjects with MetS.16
The term endothelial dysfunction refers to the loss of a range of normal homeostatic functions of the endothelium such as vasodilation, inhibition of platelet aggregation, and leukocyte adhesion.17 Vascular cells are exclusively sensitive to plasma glucose levels fluctuations because glucose uptake by these cells is mainly insulin-independent. Thus, higher plasma glucose concentrations tend to enter into endothelial cells and cause glucose-mediated injury. Indeed, endothelial dysfunction as a result of glucose-mediated injury is suggested to accelerate atherosclerosis. So, there is a progressive interest to find a first line of defense against vascular complications.18
Endothelin-1 (ET-1) is a powerful endogenous vasoconstricting peptide that is produced and released by the vascular endothelium,19 and it has been linked to the pathogenesis of HTN, heart failure (HF), and atherosclerotic vascular disease.20,21
Endothelial function is achieved in vivo by measuring flow-mediated dilation (FMD) in the brachial artery. FMD has been proven to be a strong predictor of cardiovascular events.22
Following these assumptions, the purpose of the current study was to examine the relationship between serum GPx-1 activity and different factors related to endothelial dysfunction such as FMD, ET-1, and C-reactive protein (CRP) in subjects with MetS compared to healthy subjects.
Materials and Methods
Subjects and design: By using a modified version of the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII), subject’s eligibility was determined according to published criteria.23 From 80 participants chosen in the study, 4 withdrew due to personal reasons. Participants were divided into two groups, either with or without MetS and were required to meet at least three of the following five criteria: (a) abdominal obesity, defined as waist circumference (WC) > 102 cm for men or > 88 cm for women, (b) elevated serum triglyceride (TG) (≥ 150 mg/dl), (c) low serum HDL-C (< 40 mg/dl for men and < 50 mg/dl for women), (d) HTN [blood pressure (BP) ≥ 130/85 mmHg] or current treatment for HTN, and (e) impaired fasting plasma glucose (FPG) ≥ 110 mg/dl); age > 18 years; free of diseases affecting serum lipids (e.g., thyroid disorders and pancreatitis); free of liver or kidney disease; not being substance abuser (including alcohol) or smoker; and (6) not being pregnant or lactating (for women).
Thus, 38 patients with MetS and 38 control patients without MetS (and nonsmoker) formed the basis for all subsequent analyses.
The Medical Ethics Committee of the Isfahan Cardiovascular Research Institute, Isfahan, Iran, under the approval no. 91115 approved the study protocol. The collected anthropometric data for all subjects were evaluated in Isfahan Cardiovascular Research Institute. Blood samples (5 ml) were collected in vacutainer tubes (after 12 hours of fasting) without anticoagulant; samples were stored on dry ice and centrifuged within first 2-3 hours (10000 g, 10 minutes) to obtain serum. Fresh serum samples were used for the measurement of FPG, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), and HDL-C. Remaining serum samples were kept at -70 °C until laboratory analyses. Enzymatic methods with commercial kits were used for the measurement of lipid profile parameters in all subjects [enzyme-linked immunosorbent assay (ELISA) kits read by Stat Fax 2100 Auto Microplate Reader].
Since patients were referred to the laboratory for initial control and were not aware of their MetS, they did not take medication. After the diagnosis, patients were referred to the physician.
Determination of GPx activity: GPx activity was evaluated based on Wendel study24 by ELISA kits (ZellBio GmbH, Commercial ELISA kit, Ulm, Germany). Biocore Diagnostika GPx Assay Kit provides a simple, reproducible, and standardized tool for assessment of GPx activity in biological sample e.g., plasma, serum, tissue homogenates, and cell lysates. The GPx activity was determined colorimetrically at 412 nm. The reaction was carried out at 25 °C in 600 𝜇l of solution containing 100 mM (pH: 7.7) potassium phosphate buffer, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.4 mM sodium azide, 2 mM GSH, 0.1 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), and 0.62 U of GSH reductase (GSSG-R). The activity of GPx was measured taking tert-butyl hydroperoxide (tBuOOH) as a substrate at 340 nm. The contribution of spontaneous NADPH oxidation was always subtracted from the overall reaction rate. GPx activity was expressed as nmol NADPH oxidized per minute per mg protein.
Quantitation of plasma ET-1: For plasma ET-1, 10 ml of venous blood was collected into an EDTA tube and centrifuged immediately at 2500 g for 20 minutes at 4 °C. ET-1 was quantitated using commercially available ELISA kits (Morinaga and R&D System).25 Standards, reagents, and test samples were prepared and assayed according to the instructions of the manufacturer.
FMD measure: FMD was measured by ultrasonography with an automated edge tracking system (UNEX 18G, UNEX Co., Nagoya, Japan) as previously described.26
Results
Data are presented as mean ± standard deviation (SD) for quantitative variables and frequency and percentage for qualitative variables in table 1. Two independent samples t-test was used to compare mean of study variables between study groups and Mann-Whitney test was used if normality assumption not hold. Frequency of sex was compared between study groups using chi-square test.
Table 1.
Anthropometric, cardiac, and biochemical parameters of each group
| Parameters | Normal group | MetS group | P |
|---|---|---|---|
| Sex (men) | 20 (52.6) | 20 (52.6) | 0.999**> |
| Age (year) | 34.24 ± 10.50 | 44.02 ± 11.01 | < 0.001* |
| BMI (kg/m2) | 22.26 ± 4.33 | 28.89 ± 4.78 | < 0.001* |
| WC (cm) | 85.76 ± 10.20 | 101.42 ± 9.48 | < 0.001* |
| Hip circumference (cm) | 100.45 ± 6.09 | 111.16 ± 8.67 | < 0.001* |
| WHR (cm) | 0.88 ± 0.07 | 0.92 ± 0.05 | < 0.001 |
| SBP (mmHg) | 106.89 ± 9.62 | 123.92 ± 13.43 | < 0.001* |
| DBP (mmHg) | 68.66 ± 6.94 | 77.76 ± 9.56 | < 0.001* |
| FMD (%) | 4.17 ± 0.69 | 3.84 ± 0.55 | 0.024* |
| FBG (mmol/l) | 82.39 ± 6.04 | 96.26 ± 18.53 | < 0.001* |
| TG (mmol/l) | 113.71 ± 32.29 | 208.47 ± 69.29 | < 0.001* |
| TC (mmol/l) | 176.03 ± 40.32 | 208.58 ± 41.47 | < 0.001* |
| HDL-C (mmol/l) | 44.89 ± 7.51 | 38.45 ± 6.36 | < 0.001* |
| LDL-C (mmol/l) | 89.24 ± 23.92 | 97.82 ± 22.51 | < 0.001* |
| CRP (mg/dl) | 2.85 ± 1.05 | 4.11 ± 1.58 | < 0.001* |
| GPx-1 | 79.00 ± 38.60 | 21.75 ± 13.55 | < 0.001* |
| ET-1 (pg/ml) | 51.25 ± 8.95 | 97.30 ± 90.03 | < 0.001* |
Data are shown as mean ± standard deviation (SD) or frequancy and percentage
Two independent samples t-test or Mann-whitney test was used
Chi-square test was used
MetS: Metabolic syndrome; BMI: Body mass index; WC: Waist circumference; WHR: Waist-to-hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FMD: Flow-mediated dilation; FBG: Fasting blood glucose; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; CRP: C-reactive protein; GPx-1: Glutathione peroxidase-1; ET-1: Endothelin-1
Crude and adjusted relationships between the levels of CRP, FMD, ET-1, GPx-1, and the components of MetS were evaluated using Pearson’s correlation and multiple linear regression analyses, respectively; age and sex were used as adjustment in regression model.
Crude and adjusted coefficients were presented in table 2. Logistic regression models also were used for examining effects of a unite increase in CRP, FMD, ET-1, and GPx-1 on odds of MetS. Crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for MetS were presented in table 3. Age and sex were used as adjustments. Statistical analysis was performed using SPSS software (version 15, SPSS Inc., Chicago, IL, USA). The P < 0.050 was considered as statistically significant.
Table 2.
The relationship between glutathione peroxidase-1 (GPx-1) and study variables in each study group
| GPx-1 | Normal group |
MetS group |
|||
|---|---|---|---|---|---|
| Crude** | Adjusted* | Crude** | Adjusted* | ||
| BMI (kg/m2) | r | 0.139 | 0.095 | -0.008 | -0.010 |
| P | 0.450 | 0.531 | 0.975 | 0.051 | |
| SBP (mmHg) | r | 0.039 | 0.032 | -0.249 | -0.234 |
| P | 0.821 | 0.853 | 0.040 | 0.171 | |
| DBP (mmHg) | r | 0.109 | 0.110 | -0.193 | -0.165 |
| P | 0.524 | 0.526 | 0.282 | 0.312 | |
| FBG (mg/dl) | r | 0.157 | 0.149 | 0.085 | -0.006 |
| P | 0.370 | 0.373 | 0.623 | 0.971 | |
| TG (mg/dl) | r | 0.206 | -0.419 | -0.170 | 0.268 |
| P | 0.132 | 0.008 | 0.380 | 0.103 | |
| TC (mg/dl) | r | -0.282 | -0.305 | -0.113 | 0.139 |
| P | 0.105 | 0.068 | 0.490 | 0.429 | |
| HDL-C (mg/dl) | r | 0.308 | 0.241 | 0.142 | 0.095 |
| P | 0.109 | 0.110 | 0.502 | 0.501 | |
| LDL-C (mg/dl) | r | -0.129 | -0.131 | -0.111 | 0.011 |
| P | 0.450 | 0.450 | 0.940 | 0.948 | |
| CRP (mg/dl) | r | 0.245 | -0.247 | -0.409 | -0.402 |
| P | 0.142 | 0.148 | 0.014 | 0.014 | |
| FMD (%) | r | 0.207 | 0.296 | 0.293 | -0.155 |
| P | 0.293 | 0.014 | 0.050 | 0.296 | |
| ET-1 (pg/ml) | r | 0.215 | 0.203 | 0.130 | 0.099 |
| P | 0.197 | 0.237 | 0.130 | 0.573 | |
Data are shown as crude correlation coefficient or adjusted standardized beta regression
Coefficients are adjusted for age and sex using multiple linear regression;
Crude correlation coefficient computed using Pearson’s correlation analysis
GPx-1: Glutathione peroxidase-1; MetS: Metabolic syndrome; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; CRP: C-reactive protein; FMD: Flow-mediated dilation; ET-1: Endothelin-1
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of metabolic syndrome (MetS) by serum glutathione peroxidase-1 (GPx-1) activity using a logistic regression model
| MetS | Factors | OR | (95% CI) | P |
|---|---|---|---|---|
| Crude model | CRP (mg/dl) | 2.17 | (1.37-3.44) | 0.001 |
| FMD (%) | 2.42 | (1.09-5.35) | 0.030 | |
| ET-1 (pg/ml) | 1.01 | (1.00-1.02) | 0.019 | |
| GPx | 0.83 | (0.75-0.92) | < 0.001 | |
| Adjusted model* | CRP (mg/dl) | 2.25 | (1.33-3.80) | 0.002 |
| FMD (%) | 1.85 | (0.62-5.52) | 0.264 | |
| ET-1 (pg/ml) | 1.01 | (1.00-1.01) | 0.019 | |
| GPx | 0.79 | (0.67-0.93) | 0.004 |
Data are shown as crude or adjusted odds ratio (OR) and 95% confidence interval (CI)
Adjusted for age and sex
CRP: C-reactive protein; FMD: Flow-mediated dilation; ET-1: Endothelin-1; GPx: Glutathione peroxidase; OR: Odds ratio; CI: Confidence interval; MetS: Metabolic syndrome
A unit increase in CRP (mg/dl), FMD (%), ET-1 (pg/ml) increases OR of MetS and a unit increase in GPx decreases the OR of MetS.
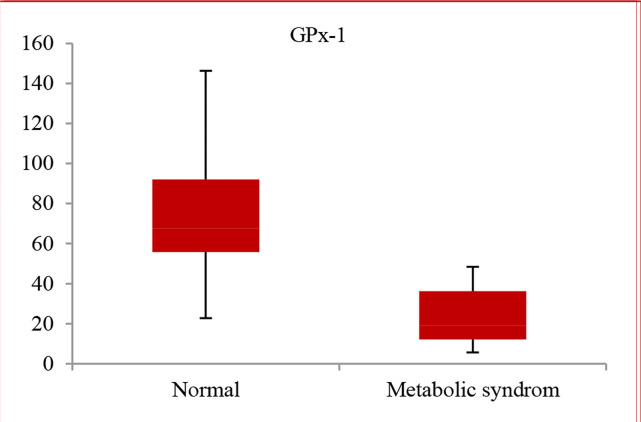
Anthropometric and cardiac variables as well as biochemical parameters of the study participants are summarized in table 1. Except sex, there were significant differences between anthropometric, cardiac, and biochemical parameters of the study groups. Data from the study revealed that in patients with MetS, serum GPx-1 activity was significantly lower than healthy individuals (21.7 ± 13.5 vs. 79.0 ± 38.6, respectively) (P = 0.001) (Figure 1).
Figure 1.
Comparing glutathione peroxidase-1 (GPx-1) level in metabolic syndrome (MetS) and normal groups [patients with MetS were determined using a modified version of the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII) criteria for MetS, according to published criteria]23
The correlations between the levels of GPx-1 with variables studied in the MetS group and normal group are shown in table 2.
The values of GPx-1 were significantly correlated with diastolic blood pressure (DBP) (r = -0.249, P = 0.040), CRP (r = -0.409, P = 0.014), and FMD (r = 0.293, P = 0.050).
OR and 95% CI of Mets based on a unit change in CRP (mg/dl), FMD (%), ET-1 (pg/ml), and GPx are 2.17 (1.37-3.44), 2.42 (1.09-5.35), 1.01 (1.00-1.02), and 0.81 (0.75-0.92), respectively and were significant (P < 0.050). After adjusting for age and sex, the results remained significant except for FMD (P < 0.050).
Discussion
This study revealed some significant associations of serum GPx-1 concentration activity with some cardiometabolic risk factors, notably components of MetS, among patients with MetS.27
OxS plays important roles in the pathogenesis of different diseases.28 In the condition of patients with DM, OxS impairs glucose uptake in muscle and fat tissues,29 and it has a negative effect on insulin secretion from pancreatic β cells.30 Increased OxS also underlies the pathophysiology of HTN31 and atherosclerosis,32 by directly affecting vascular wall cells.
We have analyzed the GPx-1 activity in the patients with MetS and without MetS and found a level of OxS in MetS group (Figure 1). In another study, results showed that in > 45-year-old subjects in the MetS group, the peroxidases activity was significantly decreased.33 Previous studies indicated that development of MetS was associated with OxS.34
GPx removes H2O2 by coupling the oxidation of GSH, an abundant thiol-containing tripeptide. GPx-1 activity, a major intracellular and extracellular enzymatic defense system against superoxides, was significantly lower in subjects with MetS, and there was a negative correlation between GPx-1 levels with systolic blood pressure (SBP) and CRP.
In coronary artery disease (CAD), OxS plays an important role. GPx is the most important part of the antioxidant defense system,35 and in the eukaryotic cells, GPx-1 is among the most abundant isoforms. Modulatory role of GPx-1 in the vascular function was reported in the in vivo studies in knockout and transgenic mice.36 In this study a positive correlation was found between GPx-1 levels and FMD. In addition, in this study the ORs of MetS were calculated based on the levels of CRP, ET-1, and GPx-1. Subjects with higher levels of CRP and ET-1 had a significantly greater risk of MetS after adjusting for age and gender.
OxS is associated with many components of MetS. Significant decrease in GPx-1 activity in MetS group compared to nonMetS group indicates a higher decrease, leading to the concept of amelioration of risk factors comprising MetS, including insulin resistance, elevated BP, elevated lipid levels, inflammation, and endothelial dysfunction may ameliorate OxS and thus, curtail the progression of metabolic disease complications.37
While the association between OxS and the development of both endothelial dysfunction and coronary arteriosclerosis was investigated before,38,39 the role of oxidative damage markers received comparatively little attention as a prognostic factor, and the results have not been clear so far. Indeed, in the particular case of GPx-1, few study reports have examined its association with the onset of cardiovascular events, and the results (an inverse association between higher GPx-1 and the rate of adverse events during follow-up) were in disagreement with those of our study.40,41 Admittedly, those study populations comprised mostly persons with stable ischemic heart disease (IHD), whereas in this study all patients were admitted with acute coronary syndrome (ACS). Another factor to consider is that the method by which GPx-1 was determined in our study was different from that used in previous studies, which might also yield different results.
We calculated the ORs of MetS according to the levels of GPx-1 (Table 2). Reducing GPx and increasing the amount of CRP and ET-1 increase the chance of MetS. Our results could be interpreted such that GPx-1 can protect patients against OxS in MetS.
Our results are similar to those of Sutipornpalangkul et al. who reported that increased lipid peroxidation occurred along with elevated GPx-1 activity and lower concentrations of total GSH, an indication of increased production of ROS in patients with osteoarthritis (OA).42 As suggested by the authors, the increased activity of the antioxidant enzyme GPx-1 “may be a compensatory regulation in response to increased OxS”.42
Previous studies found a lower activity of GPx-1 in a group of subjects with hypertriglyceridemia, a part of MetS presence, and the drop of its activity was almost to 75% of that of the control group.43,44 Bougoulia et al. showed a decreased activity of GPx-1 in obese subjects as well as an increase after weight reduction.45
Because of its high reactivity with GPx-1 and thiols,9 it is extremely difficult to detect, in vivo, a physiological modification of H2O2 concentration. However, the activity of GPx-1 and GSSG-R as well as the level of total GSH are accessible especially in erythrocytes, cells without nuclear capacity to restore homeostasis. Therefore, a modification in the blood GSH level can be an early biomarker of chronic OxS; then, can be an early step in the development of cardiometabolic complications.
Conclusion
Data show that endothelial dysfunction is related to serum GPx-1 activity in patients with MetS. GPx-1 activity is associated with risk of cardiovascular diseases (CVDs) and peripheral vascular diseases (PVDs) in patients with MetS and it can be an early biomarker of chronic cardiometabolic disease.
Acknowledgments
The authors would like to thank the internship colleagues at Department of Clinical Biochemistry, Isfahan Pharmaceutical Sciences Research Center, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences for their wonderful collaboration. This work was supported by the Department of Clinical Biochemistry, Isfahan Pharmaceutical Sciences Research Center, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences.
Besides, we thanks Marzieh Taheri for doing statistical analysis.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Vavrova L, Kodydkova J, Zeman M, Dusejovska M, Macasek J, Stankova B, et al. Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes Facts. 2013;6(1):39–47. doi: 10.1159/000348569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaman A, Aydin H, Geckinli B, Cetinkaya A, Karaman S. DNA damage is increased in lymphocytes of patients with metabolic syndrome. Mutat Res Genet Toxicol Environ Mutagen. 2015;782:30–5. doi: 10.1016/j.mrgentox.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Yubero-Serrano EM, Delgado-Lista J, Pena-Orihuela P, Perez-Martinez P, Fuentes F, Marin C, et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp Mol Med. 2013;45:e28. doi: 10.1038/emm.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Baez-Duarte BG, Zamora-Ginez I, De Jesus KL, Torres-Rasgado E, Gonzalez-Mejia ME, Porchia L, et al. Association of the metabolic syndrome with antioxidant defense and outstanding superoxide dismutase activity in Mexican subjects. Metab Syndr Relat Disord. 2016;14(3):154–60. doi: 10.1089/met.2015.0088. [DOI] [PubMed] [Google Scholar]
- 6.Baez-Duarte BG, Mendoza-Carrera F, Garcia-Zapien A, Flores-Martinez SE, Sanchez-Corona J, Zamora-Ginez I, et al. Glutathione peroxidase 3 serum levels and GPX3 gene polymorphisms in subjects with metabolic syndrome. Arch Med Res. 2014;45(5):375–82. doi: 10.1016/j.arcmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Pena-Orihuela P, Camargo A, Rangel-Zuniga OA, Perez-Martinez P, Cruz-Teno C, Delgado-Lista J, et al. Antioxidant system response is modified by dietary fat in adipose tissue of metabolic syndrome patients. J Nutr Biochem. 2013;24(10):1717–23. doi: 10.1016/j.jnutbio.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostis P, Efstathiadou ZA, Gougoura S, Polyzos SA, Karathanasi E, Dritsa P, et al. Oxidative stress and reduced antioxidative status, along with endothelial dysfunction in acromegaly. Horm Metab Res. 2013;45(4):314–8. doi: 10.1055/s-0032-1323765. [DOI] [PubMed] [Google Scholar]
- 9.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184(3):455–65. doi: 10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- 10.Forgione MA, Weiss N, Heydrick S, Cap A, Klings ES, Bierl C, et al. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282(4):H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–32. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese V, Cornelius C, Leso V, Trovato-Salinaro A, Ventimiglia B, Cavallaro M, et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822(5):729–36. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, Micinski D, Huning L, Kahlon G, Bass PF, Levine SN. Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur J Clin Nutr. 2014;68(10):1148–53. doi: 10.1038/ejcn.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7(9):e45693. doi: 10.1371/journal.pone.0045693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota T, Kinugawa S, Yamato M, Hirabayashi K, Suga T, Takada S, et al. Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients with metabolic syndrome. Diabetes Care. 2013;36(5):1341–6. doi: 10.2337/dc12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collazo-Roman M, Munoz-Forti K, Gonzalez A, Jimenez G, Mangual R, Perez Y, et al. Levels of antioxidant activity and oxidative stress in metabolic syndrome Puerto Rican participants (1138.9). The FASEB Journal. 2014;28(1_supplement):1138–9. [Google Scholar]
- 17.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10(10):1713–65. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 18.de Haan JB, Cooper ME. Targeted antioxidant therapies in hyperglycemia-mediated endothelial dysfunction. Front Biosci (Schol Ed) 2011;3:709–29. doi: 10.2741/s182. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZH, Richard V, von SL, Bauer E, Stulz P, Turina M, et al. Threshold concentrations of endothelin-1 potentiate contractions to norepinephrine and serotonin in human arteries. A new mechanism of vasospasm? Circulation. 1990;82(1):188–95. doi: 10.1161/01.cir.82.1.188. [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol. 2003;81(6):533–41. doi: 10.1139/y03-009. [DOI] [PubMed] [Google Scholar]
- 22.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51(10):997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–33. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- 25.Bondonno CP, Yang X, Croft KD, Considine MJ, Ward NC, Rich L, et al. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic Biol Med. 2012;52(1):95–102. doi: 10.1016/j.freeradbiomed.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Nitroglycerine-induced vasodilation for assessment of vascular function: A comparison with flow-mediated vasodilation. Arterioscler Thromb Vasc Biol. 2013;33(6):1401–8. doi: 10.1161/ATVBAHA.112.300934. [DOI] [PubMed] [Google Scholar]
- 27.Samsamshariat SZA, Sakhaei F, Salehizadeh L, Keshvari M, Asgary S. Relationship between resistin, endothelin-1, and flow-mediated dilation in patient with and without metabolic syndrome. Adv Biomed Res. 2019;8:16. doi: 10.4103/abr.abr_126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 29.Maddux BA, See W, Lawrence JC, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50(2):404–10. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 30.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88(22):10045–8. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91(6):2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samsam-Shariat SZ, Bolhasani M, Sarrafzadegan N, Najafi S, Asgary S. Relationship between blood peroxidases activity and visfatin levels in metabolic syndrome patients. ARYA Atheroscler. 2014;10(4):218–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27(9-10):916–21. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci U S A. 2001;98(22):12503–8. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiwanitkit V. Oxidative stress and metabolic syndrome. Korean J Fam Med. 2014;35(1):44. doi: 10.4082/kjfm.2014.35.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens JW, Gable DR, Hurel SJ, Miller GJ, Cooper JA, Humphries SE. Increased plasma markers of oxidative stress are associated with coronary heart disease in males with diabetes mellitus and with 10-year risk in a prospective sample of males. Clin Chem. 2006;52(3):446–52. doi: 10.1373/clinchem.2005.060194. [DOI] [PubMed] [Google Scholar]
- 39.Vassalle C, Boni C, Di Cecco P, Landi P. Elevated hydroperoxide levels as a prognostic predictor of mortality in a cohort of patients with cardiovascular disease. Int J Cardiol. 2006;110(3):415–6. doi: 10.1016/j.ijcard.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 40.Forsberg L, de Faire U, Morgenstern R. Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys. 2001;389(1):84–93. doi: 10.1006/abbi.2001.2295. [DOI] [PubMed] [Google Scholar]
- 41.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–13. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 42.Sutipornpalangkul W, Morales NP, Charoencholvanich K, Harnroongroj T. Lipid peroxidation, glutathione, vitamin E, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int J Rheum Dis. 2009;12(4):324–8. doi: 10.1111/j.1756-185X.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 43.Cardona F, Tunez I, Tasset I, Montilla P, Collantes E, Tinahones FJ. Fat overload aggravates oxidative stress in patients with the metabolic syndrome. Eur J Clin Invest. 2008;38(7):510–5. doi: 10.1111/j.1365-2362.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 44.Cardona F, Tunez I, Tasset I, Murri M, Tinahones FJ. Similar increase in oxidative stress after fat overload in persons with baseline hypertriglyceridemia with or without the metabolic syndrome. Clin Biochem. 2008;41(9):701–5. doi: 10.1016/j.clinbiochem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Bougoulia M, Triantos A, Koliakos G. Plasma interleukin-6 levels, glutathione peroxidase and isoprostane in obese women before and after weight loss. Association with cardiovascular risk factors. Hormones (Athens) 2006;5(3):192–9. doi: 10.14310/horm.2002.11182. [DOI] [PubMed] [Google Scholar]