Abstract
Chicken liver is a main protein source to prepare attractant for dog food. However, animal proteins are costly. Seeking high quality and low-cost protein sources has been a goal for the industry. Mushroom Lentinus edodes (L. edodes) and Mealworm Tenebrio molitor (T. molitor) are novel protein sources, showing high potential as raw material of attractants. In this paper, chicken liver, L. edodes, and T. molitor were used as three different protein sources to prepare attractants. Their palatability to dogs were then compared. Firstly, the enzymatic hydrolysis process of three proteins was optimized, with a degree of hydrolysis of 54.82%, 36.10% and 30.14% for chicken liver, L. edodes, and T. molitor respectively. Secondly, volatile compounds of three attractants were identified by HS-SPME/GC-MS and SDE/GC-MS. Using OAV and PLRS method, it was found that bis(2-methyl-3-furyl) disulfide, indole, methional, 2-(methyl thio) phenol, γ-butyrolacton, furfuryl alcohol, acetic acid and isovaleraldehyde were the key components. Although both T. molitor and L. edodes attractant showed less palatability than that of chicken liver, they could be readily improved via adding key palatable volatile compounds. The ingestion rate of dog food with attractant showed a similar trend and was higher than that of food without attractant.
Keywords: Food analysis, Attractant, Palatability, Flavour analysis, Different protein sources
Food analysis; Attractant; Palatability; Flavour analysis; Different protein sources.
1. Introduction
The palatability of pet food has been a major concern for consumers when making choice. It can be improved by adding attractants, a type of feed additives [1]. These attractants are able to stimulate pet appetite, thus increase pet food intake [2]. Fournier M. found a flavor with an added amount of less than 0.02% could effectively mask the unpleasant odor in dog food and make them more favorable to dogs without reducing palatability [3]. Catnip and its derivates have been used as attractants for cat food [4]. Arulvictor S. et al. pointed out that attractants of animal protein source can improve the palatability of pet food [5]. At present, most pet food attractants are prepared with animal proteins. Compared with animal proteins, edible fungi and insects are more novel and more prevalent sources for proteins. Lentinus edodes (L. edodes) is a typical mushroom containing high proteins, which has been added to pet food as supplements [6]. Insects are the most abundant and environment-friendly source of proteins in the world, and have been approved by European Union as protein sources in feed industry. Tenebrio molitor (T. molitor) is suitable for highly dense and large-scaled production due to its biological habits, and it is rich in specific hormones to improve immunity [7]. Also, cost of T. molitor is very low. Because of these advantages, T. molitor has been widely used in feed fields, especially in pet feed of birds and fish, which can effectively improve the immunity and reproductive rate of pets [8]. Enzymatic hydrolysis is usually the first step to prepare dog food attractants, and the quality of hydrolysates has a significant impact on the quality of attractants [9]. Therefore, it is essential to select the right enzymes and optimization of enzymatic hydrolysis.
Although chicken liver is among the most commonly used attractant ingredients today, it is expensive and has relatively high levels of heavy metals [10, 11]. Thus, a lot of pet nutritionists are choosing alternative food proteins as raw material of attractants. Attractants prepared from different proteins may possess various functions. And the relationships between the aroma characteristics of these three attractants and dogs’ food preference remains unclear.
Therefore, this study can be summarized to: 1) optimized the enzymatic hydrolysis process of chicken liver, L. edodes, and T. molitor, 2) identification of aroma substances in three attractants, 3) palatability evaluation study with dogs. This study provided some practical guidance for application of dog food attractants from fungi and insect proteins.
2. Material and methods
2.1. Reagents and materials
Raw chicken liver and L. edodes were purchased from Shandong Liuhe Group. T. molitor were bought from Xuchang Tenebrio molitor breeding base in Henan Province. Dog food was obtained from Shanghai Shilin Biotechnology Co., Ltd. Annzyme Complex protease PF116 and complex protease FF104 were purchased from Angel Yeast Co., Ltd. Edible fungal hydrolase and trypsin were purchased from Nanning Pangbo Biological Engineering Co., Ltd, Guangxi province. Xylose, cysteine hydrochloride, glycine, glutamic acid, thiamine, phosphoric acid, potassium sorbate, and tert-butylhydroquinone (food grade) were purchased from Huaheng Biotechnology Co., Ltd, Anhui province. O-dichlorobenzene and C5–C22 n-alkanes were purchased from Shanghai Anpu Experimental Technology Co., Ltd. Dichloromethane, anhydrous sodium sulfate, hydrochloric acid, sulfuric acid, methanol, and glacial acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai. Tween 80 was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. All chemical reagents used were analytical grade.
2.2. Optimization of enzymatic hydrolysis process
Chicken liver, L. edodes, and T. molitor were grounded by HC-400Y grinder (Damai, China), and then single-factor experiments were conducted. The experimental design is shown in Table 1. After enzymatic hydrolysis, the enzyme hydrolysate was heated at 90 °C for 10 min to inactivate enzyme. The hydrolysate was centrifuged (8000 rpm, 5 min) and the supernatant was taken to determine the degree of hydrolysis (DH/%). The measurement formula of DH was shown in Eq. (1).
| DH (%) = (free amino nitrogen content (g/mL) / total nitrogen content (g/mL)) ∗ 100 % | (1) |
Table 1.
Single-factor experiment design of enzymolysis.
| Protein | Level | Factors |
||||
|---|---|---|---|---|---|---|
| T (°C) | S: L1 | E/S2 (%) | t (h) | pH | ||
| Chicken liver | 1 | 45 | 4:1 | 0.4 | 1 | 6 |
| 2 | 50 | 3:1 | 0.6 | 2 | 6.5 | |
| 3 | 55 | 2:1 | 0.8 | 3 | 7 | |
| 4 | 60 | 1:1 | 1 | 4 | 7.5 | |
| 5 | 65 | 1:2 | 1.2 | 5 | 8 | |
| L. edodes | 1 | 40 | 1:2 | 0.2 | 1 | 4 |
| 2 | 45 | 1:4 | 0.4 | 2 | 4.5 | |
| 3 | 50 | 1:6 | 0.6 | 3 | 5 | |
| 4 | 55 | 1:8 | 0.8 | 4 | 5.5 | |
| 5 | 60 | 1:10 | 1 | 5 | 6 | |
| T. molitor | 1 | 45 | 1:3 | 0.4 | 2 | 7 |
| 2 | 50 | 1:6 | 0.8 | 3 | 7.5 | |
| 3 | 55 | 1:9 | 1.2 | 4 | 8 | |
| 4 | 60 | 1:12 | 1.6 | 5 | 8.5 | |
| 5 | 65 | 1:15 | 2 | 6 | 9 | |
Free amino nitrogen was determined by formaldehyde titration and total nitrogen content was determined by Kjeldahl apparatus.
On the basis of single-factor experiments, Plackett-Burman design was applied to screen out the factors that have significant effects on DH. According to the results of Plackett-Burman experiment, the Box-Behnken design of response surface analysis was applied to optimize the conditions of enzymatic hydrolysis.
2.3. Preparation of attractants by Maillard reaction
The optimized enzymatic hydrolysate was used to prepare attractants. The Maillard reaction formula of chicken liver/L. edodes/T. molitor attractant is shown in Table 2. Reaction conditions were as the following: temperature was held at 100 °C, for 90 min. pH was adjusted to neutral with food grade phosphoric acid before reaction, and finally adjusted to 3.3 with phosphoric acid after reaction.
Table 2.
Attractants formula of three different protein sources.
| Materials | Component (%) |
||
|---|---|---|---|
| Chicken liver | L. edodes | T. molitor | |
| Enzymatic hydrolysate | 80.00 | 91.00 | 48.00 |
| Water |
11.00 |
0.00 |
43.00 |
| D-Xylose | 4.70 | ||
| Cysteine hydrochloride | 1.00 | ||
| Glycine | 1.00 | ||
| Glutamic acid | 1.00 | ||
| Thiamine | 1.00 | ||
| Potassium sorbate | 0.28 | ||
| TBHQ | 0.02 | ||
2.4. HS-SPME-GC-MS analysis of volatile compounds in attractants
Attractants (4.0 g) were mixed with 50 μL 1, 2-Dichlorobenzene (internal standard, 100 μL/L) (Supelco, Bellefonte, PA, USA) solution in vials. They were kept at 50 °C for 30 min. Then 50HS-SPME with stable flex fiber coated by 50/30 μm layer of DVB/CAR/PDMS (Supelco, Bellefonte, PA, USA) was added in the space of the vial for 40 min at 50 °C to absorb the volatile compounds. Volatile compounds were released by desorption for 5 min at 250 °C in GC.
Volatile compounds in attractants were analyzed by using 7890 GC with 5975 MSD (Agilent Technologies, CA, USA). The electron ionization energy of MSD was 70 eV. The EI ion source temperature was set up at 230 °C. The chromatograms were recorded via monitoring the total ion currents from m/z = 20–350. Volatile compounds were separated by using HP-INNOWAX column (60 m × 0.25 mm × 0.25 μm) (Agilent Technologies, CA, USA). Helium was used as the carrier gas at a flow rate of 3 mL/min in a splitless mode. The quadruple mass filter, transfer line temperature and inlet temperature was running at 150 °C, 280 °C, and 250 °C, respectively. The GC oven temperature was held at 40 °C for 1 min, ramped to 230 °C at 4 °C/min and maintained for 15 min. The compounds were identified by matching the retention time of standards, retention indices (RIs), and mass spectra in the NIST 11 database. The RIs of unknown compounds were determined by alkanes C4–C30.
Qualitative and semi-quantitative analyses of volatile compounds detected by GC-MS refer to Eqs. (2) and (3) [9].
| (2) |
Where t(x) is the retention time of volatile compounds. T(z) is the retention time of n-alkanes before and after the peak of the compound (x). z is the carbon number of n-alkanes.
| (3) |
Where wi is the concentration of volatile compounds (μg/g). ms is the internal standard content (μg). Ai is the peak area of volatile compounds. As is the peak area of internal standard and m0 is the weight of samples (g).
The odorous contributions of volatile compounds to attractants were evaluated by the odor activity value (OAV), which was measured as the ratio of the concentration of single compound to its detection threshold in water [12].
2.5. SDE-GC-MS analysis of volatile compounds in attractants
Attractants (25.0 g) were dissolved in water (250 mL). Dichloromethane (60 mL) was then added to the solution. They were stirred at 55 °C for 3 h in a sealed container. The extract was refrigerated for 24 h with a small amount of anhydrous sodium sulfate for dehydration. Filtrate was concentrated into 1 mL with a rotary evaporator (MLG3, Heidolph, Germany), and mixed with 20 μL 1, 2-Dichlorobenzene (100 μL/L) (internal standard). 0.2 μL mixture was then injected into GC-MS. Except for split ratio setting 10:1, other conditions were the same as used in 2.4.
2.6. Palatability, preferred food selection (PFS) and validation of key volatile compounds on attractants palatability experiment
PFS is the tendency of animals to choose a certain food first when there are two or more food choices. This experiment was performed at the School of Zoology, Shanghai Jiao Tong University. All dogs were healthy and did not receive antibiotic treatment for at least six months before the samples were collected. This experiment received ethical approval from the Animal Research Ethics Committee of Shanghai Jiao Tong University. Samples were collected according to the Animal Research Ethics Committee of Shanghai Jiao Tong University. The palatability of three attractants was evaluated by two-bowl test [16]. The amounts of PFS of three attractants were evaluated by single-bowl test [16]. Finally, the key aroma substances were added to the attractants, and the two-bowl test method was used to verify the effect [1]. Food without attractants was used as a blank control. The panel of two-bowl tests consisted of 20 pet dogs of various breeds (10 Beagles, 4–5 years old, female: male = 1:1; 10 Border Collie dogs, 4–5 years old, female: male = 1:1) and similar sizes (height: 36–38cm, weight: 9–10 kg). The basic foods of dogs were used in this study. They were dry and complete foods. The basic food formula was as the following: ground whole corn, animal fat, beet pulp, ground whole wheat, meat and bone meal, vegetable oil, brewers' rice, corn, and corn gluten meal. All dogs received the same food except the three different attractants. After extrusion and sterilization of the dogs’ basic food, the attractants were then sprayed onto to the food and allowed the foods to be evenly coated by attractants. The dogs received the same food without attractants before the tests for 7 days. Each two-bowl test lasted for 2 days, served 2 meals per day. The panel of single-bowl test lasted 14 days and consisted of 8 beagle dogs and 8 Border collie dogs, which were equally divided into 4 groups. Ingestion rate (IR) and first preference (FP) were recorded as the index of palatability and PFS during the test. It is notable that palatability and PFS of three attractants were tested through dog food with 5% addition of attractant. The measurement formula of ingestion rate (IR) was was shown in Eq. (4).
| IR = (consumption of dog feed weight (g)) / (total dog feed weight (g)) ∗ 100%, | (4) |
2.7. Statistical analysis
Duncan's multiple comparison tests at the level of 0.05 (P < 0.05) were applied to determine significant differences by SPSS Statistic 19. Each experiment was done in triplicates. Key volatile compounds (OAVs >1) data set was assigned as the X-variables and palatability index was designated as the Y-variables in PLSR analysis by Unscrambler 9.7 (CAMOAnalytics, Montclair, NJ, USA).
3. Results and discussion
3.1. Response surface mathematic model and ANOVA analysis of enzymatic hydrolysis process
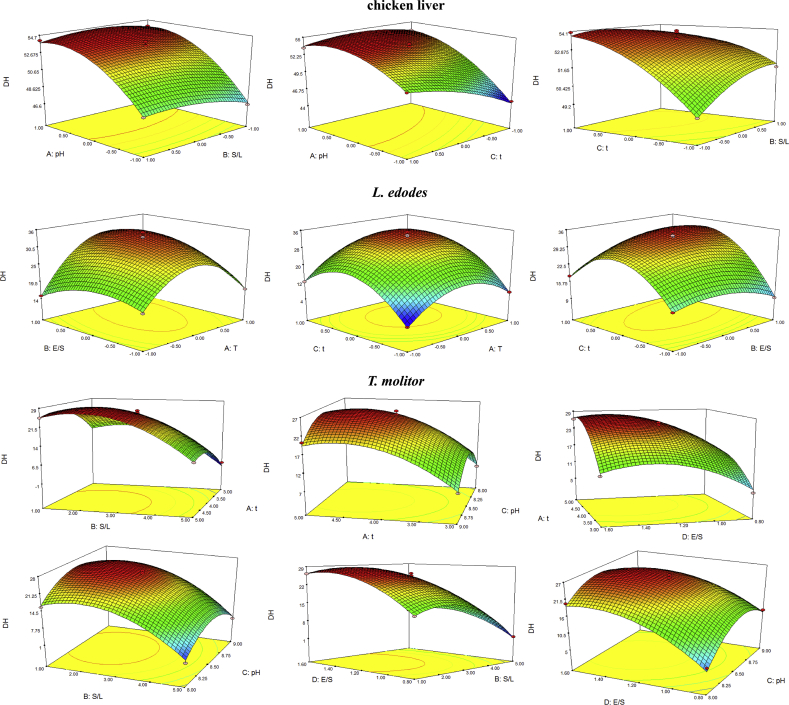
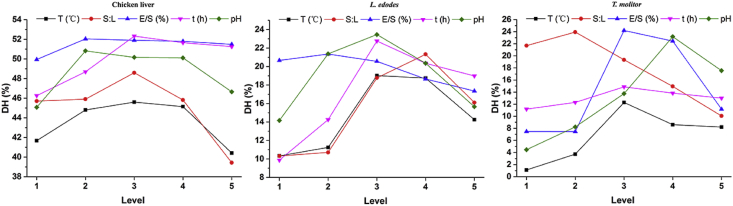
Based on single-factor experiments, Plackett-Burman design (Table 3) with 5 factors and 2 levels were established and significant factors were screened out. After that, the levels and factors of Box-Behnken design (Table 4 and Table 5) were conducted to establish the response surface analysis (Figure 1), which exhibited obvious paraboloids with the highest point on the response surface. It means that the highest response value can be obtained through the regression equation of the model.
Table 3.
Placket-Burman design and statistical analysis of three attractants.
| Protein | Source | Level |
Coefficient estimate1 | F Value | Prob > F | Significant2 | |
|---|---|---|---|---|---|---|---|
| -1 | 1 | ||||||
| Chicken liver | Model | 6.57 | 0.0201 | ∗∗ | |||
| T (°C) | 50 | 60 | -0.80 | 1.23 | 0.31 | ||
| S/L | 3:1 | 1:1 | 1.58 | 4.82 | 0.0705 | ∗ | |
| E/S (%) | 0.4 | 0.8 | 0.18 | 0.06 | 0.8106 | ||
| t (h) | 2 | 4 | 2.45 | 11.55 | 0.0145 | ∗∗ | |
| pH | 6 | 7 | 2.81 | 15.19 | 0.008 | ∗∗ | |
| L. edodes | Model | 6.07 | 0.0242 | ∗∗ | |||
| T (°C) | 45 | 55 | 1.28 | 5.01 | 0.0665 | ∗ | |
| S/L | 1:6 | 1:10 | -0.32 | 0.32 | 0.5945 | ||
| E/S (%) | 0.2 | 0.6 | -1.59 | 7.70 | 0.0322 | ∗∗ | |
| t (h) | 2 | 4 | 2.38 | 17.33 | 0.0059 | ∗∗ | |
| pH | 4.5 | 5.5 | -0.02 | 0.01 | 0.8954 | ||
| T. molitor | Model | 44.37 | 0.0001 | ∗∗ | |||
| T (°C) | 50 | 60 | 0.66 | 1.83 | 0.2249 | ||
| S/L | 1:1 | 1:5 | -5.84 | 141.43 | <0.0001 | ∗∗ | |
| E/S (%) | 0.8 | 1.6 | 3.07 | 39.12 | 0.0008 | ∗∗ | |
| t (h) | 3 | 5 | 2.26 | 21.07 | 0.0037 | ∗∗ | |
| pH | 8 | 9 | 2.11 | 18.41 | 0.0051 | ∗∗ | |
If the coefficient estimate was a positive number, the level of factor was positively related to DH (%). And if the coefficient estimate was a negative number, the level of factor was negatively related to DH (%).
‘∗’ stands for P < 0.1, and ‘∗∗’ stands for P < 0.05.
Table 4.
The levels and factors of Box-Behnken design.
| Protein | Code | Factor | Level |
||
|---|---|---|---|---|---|
| -1 | 0 | 1 | |||
| Chicken liver 1 | A | pH | 6 | 6.5 | 7 |
| B | S/L | 3:1 | 2:1 | 1:1 | |
| C | t (h) | 2 | 3 | 4 | |
| L. edodes2 | A | T (°C) | 45 | 50 | 55 |
| B | E/S (%) | 0.2 | 0.4 | 0.6 | |
| C | t (h) | 2 | 3 | 4 | |
| T. molitor3 | A | t (h) | 3 | 4 | 5 |
| B | S/L | 1:1 | 1:3 | 1:5 | |
| C | pH | 8 | 8.5 | 9 | |
| D | E/S (%) | 0.8 | 1.2 | 1.6 | |
The level of other insignificant factors based on the results of single-factor experiments (Figure 5), choosing the level of highest DH, which were T = 55 °C, E/S = 0.6 %.
pH = 5, E/S = 1:8.
T = 55 °C.
Table 5.
Box-Behnken design arrangement and experimental results.
| Run | Chicken liver |
L. edodes |
T. molitor |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | DH (%) | A | B | C | DH (%) | A | B | C | D | DH (%) | |
| 1 | -1 | -1 | 0 | 46.614 | -1 | -1 | 0 | 18.070 | -1 | -1 | 0 | 0 | 16.754 |
| 2 | 1 | -1 | 0 | 53.898 | 1 | -1 | 0 | 17.093 | - | -1 | 0 | 0 | 25.215 |
| 3 | -1 | 1 | 0 | 48.310 | -1 | 1 | 0 | 14.835 | -1 | 1 | 0 | 0 | 1.461 |
| 4 | 1 | 1 | 0 | 54.103 | 1 | 1 | 0 | 27.363 | 1 | 1 | 0 | 0 | 10.115 |
| 5 | -1 | 0 | -1 | 44.878 | -1 | 0 | -1 | 4.384 | 0 | 0 | 0 | -1 | 6.590 |
| 6 | 1 | 0 | -1 | 53.508 | 1 | 0 | -1 | 7.293 | 0 | 0 | 1 | -1 | 11.667 |
| 7 | -1 | 0 | 1 | 50.210 | -1 | 0 | 1 | 12.228 | 0 | 0 | -1 | 1 | 20.320 |
| 8 | 1 | 0 | 1 | 53.334 | 1 | 0 | 1 | 19.883 | 0 | 0 | 1 | 1 | 17.366 |
| 9 | 0 | -1 | -1 | 49.255 | 0 | -1 | -1 | 14.306 | -1 | 0 | 0 | -1 | 5.447 |
| 10 | 0 | 1 | -1 | 51.492 | 0 | 1 | -1 | 9.323 | 1 | 0 | 0 | -1 | 12.843 |
| 11 | 0 | -1 | 1 | 54.038 | 0 | -1 | 1 | 18.073 | -1 | 0 | 0 | 1 | 12.699 |
| 12 | 0 | 1 | 1 | 52.790 | 0 | 1 | 1 | 27.943 | 1 | 0 | 0 | 1 | 26.232 |
| 13 | 0 | 0 | 0 | 53.319 | 0 | 0 | 0 | 33.444 | 0 | -1 | -1 | 0 | 16.251 |
| 14 | 0 | 0 | 0 | 54.075 | 0 | 0 | 0 | 33.209 | 0 | 1 | -1 | 0 | 1.861 |
| 15 | 0 | 0 | 0 | 52.642 | 0 | 0 | 0 | 35.183 | 0 | -1 | 1 | 0 | 19.157 |
| 16 | 0 | 0 | 0 | 53.975 | 0 | 0 | 0 | 33.704 | 0 | 1 | 1 | 0 | 2.458 |
| 17 | 0 | 0 | 0 | 53.533 | 0 | 0 | 0 | 33.018 | -1 | 0 | -1 | 0 | 7.298 |
| 18 | 1 | 0 | -1 | 0 | 18.376 | ||||||||
| 19 | -1 | 0 | 1 | 0 | 8.761 | ||||||||
| 20 | 1 | 0 | 1 | 0 | 20.369 | ||||||||
| 21 | 0 | -1 | 0 | -1 | 13.497 | ||||||||
| 22 | 0 | 1 | 0 | -1 | 1.462 | ||||||||
| 23 | 0 | -1 | 0 | 1 | 26.209 | ||||||||
| 24 | 0 | 1 | 0 | 1 | 8.745 | ||||||||
| 25 | 0 | 0 | 0 | 0 | 24.001 | ||||||||
| 26 | 0 | 0 | 0 | 0 | 25.491 | ||||||||
| 27 | 0 | 0 | 0 | 0 | 25.502 | ||||||||
| 28 | 0 | 0 | 0 | 0 | 26.443 | ||||||||
| 29 | 0 | 0 | 0 | 0 | 25.488 | ||||||||
Figure 1.
Response surface analysis of hydrolysis of chicken liver, L. edodes and T. molitor.
According to Table 5, ANOVA of response surface analysis designed by Box-Behnken of chicken liver, L. edodes, and T. molitor hydrolysis was shown in Table 6.
Table 6.
ANOVA for response surface regression model.
| Protein | Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F |
|
|---|---|---|---|---|---|---|---|
| Chicken liver |
Model | 131.195 | 9 | 14.577 | 58.145 | <0.0001 | significant |
| A-pH | 77.072 | 1 | 77.072 | 307.423 | <0.0001 | ||
| B–S/L | 1.044 | 1 | 1.044 | 4.165 | 0.0806 | ||
| C-t | 15.787 | 1 | 15.787 | 62.969 | <0.0001 | ||
| AB | 0.555 | 1 | 0.555 | 2.216 | 0.1802 | ||
| AC | 7.577 | 1 | 7.577 | 30.221 | 0.0009 | ||
| BC | 3.036 | 1 | 3.036 | 12.109 | 0.0103 | ||
| A2 | 18.472 | 1 | 18.472 | 73.679 | <0.0001 | ||
| B2 | 1.964 | 1 | 1.964 | 7.833 | 0.0266 | ||
| C2 | 3.656 | 1 | 3.656 | 14.584 | 0.0066 | ||
| Residual | 1.755 | 7 | 0.251 | ||||
| Lack of Fit | 0.430 | 3 | 0.143 | 0.432 | 0.7416 | not significant | |
| Pure Error | 1.325 | 4 | 0.331 | ||||
| Cor Total | 132.950 | 16 | |||||
| R2 | 0.987 | ||||||
| RAdj2 | 0.970 | ||||||
| RPred2 | 0.933 | ||||||
| Adeq Precision |
24.085 > 4 |
||||||
|
L. edodes |
Model | 1688.862 | 9 | 187.651 | 316.884 | <0.0001 | significant |
| A-T | 61.127 | 1 | 61.127 | 103.225 | <0.0001 | ||
| B-E/S | 17.765 | 1 | 17.765 | 29.999 | 0.0009 | ||
| C-t | 229.194 | 1 | 229.194 | 387.036 | <0.0001 | ||
| AB | 45.596 | 1 | 45.596 | 76.998 | <0.0001 | ||
| AC | 5.632 | 1 | 5.632 | 9.510 | 0.0177 | ||
| BC | 55.158 | 1 | 55.158 | 93.145 | <0.0001 | ||
| A2 | 456.972 | 1 | 456.972 | 771.680 | <0.0001 | ||
| B2 | 65.809 | 1 | 65.809 | 111.131 | <0.0001 | ||
| C2 | 641.875 | 1 | 641.875 | 1083.923 | <0.0001 | ||
| Residual | 4.145 | 7 | 0.592 | ||||
| Lack of Fit | 1.175 | 3 | 0.392 | 0.528 | 0.6868 | not significant | |
| Pure Error | 2.970 | 4 | 0.742 | ||||
| Cor Total | 1693.007 | 16 | |||||
| R2 | 0.998 | ||||||
| RAdj2 | 0.994 | ||||||
| RPred2 | 0.986 | ||||||
| Adeq Precision |
50.313 > 4 |
||||||
| T. molitor | Model | 2018.567 | 14 | 144.183 | 113.041 | <0.0001 | significant |
| A-t | 307.336 | 1 | 307.336 | 240.954 | <0.0001 | ||
| B–S/L | 689.763 | 1 | 689.763 | 540.780 | <0.0001 | ||
| C-pH | 6.875 | 1 | 6.875 | 5.390 | 0.0358 | ||
| D-E/S | 300.661 | 1 | 300.661 | 235.721 | <0.0001 | ||
| AB | 0.009 | 1 | 0.009 | 0.007 | 0.9329 | ||
| AC | 0.070 | 1 | 0.070 | 0.055 | 0.8181 | ||
| AD | 9.413 | 1 | 9.413 | 7.380 | 0.0167 | ||
| BC | 1.331 | 1 | 1.331 | 1.044 | 0.3243 | ||
| BD | 7.368 | 1 | 7.368 | 5.777 | 0.0307 | ||
| CD | 16.128 | 1 | 16.128 | 12.645 | 0.0032 | ||
| A2 | 159.432 | 1 | 159.432 | 124.996 | <0.0001 | ||
| B2 | 390.170 | 1 | 390.170 | 305.896 | <0.0001 | ||
| C2 | 303.886 | 1 | 303.886 | 238.249 | <0.0001 | ||
| D2 | 180.093 | 1 | 180.093 | 141.194 | <0.0001 | ||
| Residual | 17.857 | 14 | 1.275 | ||||
| Lack of Fit | 14.873 | 10 | 1.487 | 1.994 | 0.2642 | not significant | |
| Pure Error | 2.984 | 4 | 0.746 | ||||
| Cor Total | 2036.424 | 28 | |||||
| R2 | 0.991 | ||||||
| RAdj2 | 0.982 | ||||||
| RPred2 | 0.956 | ||||||
| Adeq Precision | 32.967 > 4 |
For chicken liver experiment, model p-value less than 0.0001 indicated that the model was significant. The A, C, AC, BC, A2, B2, C2 p-value less than 0.05 indicated model terms were significant. P-value of the Lack of Fit just about 0.7416 implied the Lack of Fit was not significant relative to the pure error. Non-significant lack of fit was good for the model to fit.
For L. edodes experiment, model p-value less than 0.0001 implied the model was significant. The A, B, C, AB, AC, BC, A2, B2, C2 p-value less than 0.05 indicated model terms were significant. Non-significant lack of fit was good for the model to fit. The RPred2 of 0.986 was in reasonable agreement with the RAdj2 of 0.994, indicating that less than 1 % variables could not be explained by this model.
For T. molitor experiment, model p-value less than 0.0001 implied the model was significant. The A, B, C, D, AD, BD, CD, A2, B2, C2, D2 p-value less than 0.05 indicated model terms were significant. P-value of Lack of Fit just about 0.2642 implied that the result can be raised by 26.42 % chance. The RPred2 of 0.956 was in reasonable agreement with the RAdj2 of 0.982, indicating that less than 2 % variables could not be explained by this model.
The above ANOVA results were similar with some literatures. Wang et al. [17] used response surface method to optimize enzymolysis of pine seed protein. They got the ANOVA results after regression and variance analysis. It was found that the regression model was significant (p < 0.001), lack of fit was insignificant, R2 = 94.85%, R2Adj = 85.48%, which indicated that the model fits well with the experimental data and the model can be used to analysis and predict the results of enzymolysis of pine kernel protein. Pan et al. [18] optimized the enzymolysis of the lotus seed protein by response surface method. According to ANOVA, the model was highly significant with a p-value (p < 0.0001) to predict the response values. T, TE, and E2 were significant term (p < 0.05). The predicated R2 and adjusted R2 were both close to 1, which indicated the adequacy of the model. Adequate precision value was found to be 38.878 (greater than 4), revealing that the model was sufficient for discrimination. The non-significant value of lack of fit (p > 0.05) also showed that the quadratic model was valid for the present study. Value of the coefficient of variation (4.61%) demonstrated a good precision and reliability of the experiments.
3.2. Odorous compounds in three kinds of attractants
There were 66 odorous compounds detected from attractants of chicken liver, L. edodes, and T. molitor attractant (Table 7), and 47 flavor compounds had OAV more than 1 (Table 8). The OAVs of methyl mercaptan, furfuryl mercaptan, acetic acid, hexanoic acid, hexanal, octanal, nonanal, furfural, methional, bread thiophene, methyl 2-methyl-3-furyl disulfide, and sulfurol were larger than 100, indicating they were the main aroma components of attractants. The majority of these compounds contained sulfur and nitrogen with low threshold, and they are commonly used in meat flavor. The content of sulfur compounds in chicken liver attractant was higher than those of other two. In a recent study, Chen et al. [19] investigated the volatile compounds of chicken liver Maillard reaction product. They found that aldehydes, especially furfural, nonanal, 3-methyl-butanal, benzaldehyde, were the main volatile compounds in the chicken liver Maillard reaction products. Meantime, they reported heterocyclic compound such as furfural contributed toasted, nutty, sweet, and caramel-like aroma. They used xylose as a reduced sugar might cause the volatile compounds difference from this study. The most characteristic volatile compound in L. edodes attractant was 1-octen-3-ol with a mushroom flavor. In Li et al. [20] literature, they also found that 1-octen-3-ol was a typical flavor component in L. edodes. The content of pyrazine in the attractant of T. molitor was relatively high and makes the roast note of the attractant. In Seo's study, they reported that pyrazines, pyrrolidines and carbonyls increased or appeared in roasted and fried mealworms (Tenebrio molitor Larvae) [21].
Table 7.
Compositions and OAV of odorous compounds in three different attractants.
| Compounds | RI1 | KI2 | Content (mg/kg) |
Threshold3 (mg/kg) | OAV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken liver |
L. edodes |
T. molitor |
Chicken liver |
L. edodes |
T. molitor |
||||||||||
| SPME | SDE | SPME | SDE | SPME | SDE | SPME | SDE | SPME | SDE | SPME | SDE | ||||
| Alcohols | |||||||||||||||
| methyl mercaptan | 680 | 675 | 0.036 | -4 | 0.040 | - | - | - | 0.00024 | 151.42 | - | 165.72 | - | - | - |
| amyl alcohol | 1253 | 1255 | - | - | Tr5 | 2.710 | Tr | 1.656 | 0.14 | - | - | - | 19.26 | - | 11.82 |
| 3-octanol | 1388 | 1394 | 0.034 | - | 0.037 | - | Tr | - | 0.02 | 1.69 | - | 1.85 | - | - | - |
| furfuryl mercaptan | 1419 | 1430 | - | 16.985 | - | - | Tr | 18.898 | 0.00002 | - | 8.49×105 | - | - | - | 9.45×105 |
| 1-octen-3-ol | 1457 | 1456 | 0.156 | - | 0.355 | 20.948 | 0.016 | - | 0.026 | 6.02 | - | 13.66 | 805.71 | 0.63 | - |
| 2-ethyl-1-hexanol | 1473 | 1484 | 0.023 | - | Tr | - | Tr | - | 0.26 | 0.09 | - | - | - | - | - |
| Octanol | 1532 | 1564 | 0.024 | - | 0.013 | - | Tr | - | 0.037 | 0.65 | - | 0.36 | - | - | - |
| Furfuryl alcohol | 1625 | 1660 | 0.144 | 71.027 | - | - | Tr | 7.993 | 2 | 0.07 | 35.51 | - | - | - | 3.99 |
| phenethyl alcohol | 1918 | 1921 | - | - | 0.022 | 5.671 | - | - | 0.07 | - | - | 0.32 | 81.01 | - | - |
| Esters | |||||||||||||||
| dextro-bornyl acetate | 1576 | 1582 | - | - | Tr | 4.877 | - | - | 0.44 | - | - | - | 11.08 | - | - |
| gamma-butyrolactone | 1640 | 1643 | 0.029 | 6.095 | Tr | - | Tr | - | 0.0008 | 36.63 | 7618.69 | - | - | - | - |
| ethyl myristate | 2089 | 2070 | Tr | 4.243 | Tr | - | Tr | - | 0.18 | - | 23.57 | - | - | - | - |
| ethyl palmitate | 2221 | 2250 | - | 4.665 | - | 10.402 | - | - | 2 | - | 2.33 | - | 5.20 | - | - |
| Acids | |||||||||||||||
| acetic acid | 1455 | 1460 | 0.1528 | 44.671 | 0.031 | 16.348 | 0.047 | 18.898 | 0.025 | 6.11 | 1786.83 | 1.24 | 653.93 | 1.89 | 755.93 |
| Formic acid | 1461 | 1470 | Tr | - | Tr | - | Tr | - | 14.5 | - | - | - | - | - | - |
| Valeric acid | 1721 | 1734 | 0.014 | - | - | - | - | 0.864 | 0.001 | 13.85 | - | - | - | - | 864.41 |
| hexanoic acid | 1853 | 1831 | 0.031 | 3.460 | 0.052 | 8.948 | 0.101 | 36.051 | 0.012 | 2.53 | 288.33 | 4.34 | 745.70 | 8.43 | 3004.24 |
| octanoic acid | 2020 | 2039 | 0.039 | - | 0.044 | - | 0.067 | 2.695 | 0.037 | 1.06 | - | 1.2 | - | 1.82 | 72.84 |
| lauric acid | 2458 | 2493 | - | - | - | 43.555 | - | - | 0.1 | - | - | 0.04 | 435.55 | 0.02 | - |
| myristic acid | 2715 | 2716 | 0.011 | 79.365 | Tr | - | - | - | 10 | 0.001 | 7.94 | - | - | - | - |
| palmitic acid | 2901 | 2890 | - | 107.851 | - | - | - | 147.947 | 10 | - | 10.78 | - | - | - | 14.79 |
| Aldehydes | |||||||||||||||
| isovaleraldehyde | 918 | 924 | 0.082 | - | 0.040 | - | - | - | 0.003 | 27.31 | - | 13.26 | - | - | - |
| hexanal | 1077 | 1083 | Tr | 14.243 | Tr | 19.226 | Tr | 13.429 | 0.03 | - | 474.77 | - | 640.86 | - | 447.65 |
| (E)-2-pentenal | 1149 | 1134 | - | 0.914 | 0.012 | 18.213 | - | 1.893 | 1.4 | - | 0.65 | 0.01 | 13.01 | - | 1.35 |
| heptanal | 1187 | 1186 | - | - | - | 1.013 | - | 0.819 | 0.14 | - | - | - | 7.24 | - | 5.85 |
| 3-methyl-2-butenal | 1191 | 1206 | - | 14.601 | - | 14.393 | - | 14.402 | 0.5 | - | 29.2 | - | 28.77 | - | 28.8 |
| Octanal | 1295 | 1290 | - | - | Tr | 2.722 | Tr | 2.384 | 0.021 | - | - | - | 129.58 | - | 113.53 |
| nonanal | 1393 | 1396 | 0.072 | 2.291 | 0.019 | 2.226 | 0.020 | 1.875 | 0.02 | 3.58 | 114.54 | 0.96 | 111.29 | 0.98 | 93.73 |
| furfural | 1468 | 1476 | 0.253 | 1714.472 | 0.114 | 524.964 | 0.181 | 1337.541 | 0.25 | 1.01 | 6857.87 | 0.46 | 2099.87 | 0.72 | 5350.17 |
| methional | 1480 | 1480 | Tr | 31.691 | - | 10.639 | - | 2.311 | 0.06 | 0.12 | 528.19 | - | 177.31 | - | 38.51 |
| benzaldehyde | 1519 | 1530 | 0.295 | 4.801 | 0.395 | 5.897 | 0.611 | 11.667 | 0.61 | 0.48 | 7.87 | 0.65 | 9.67 | 1.01 | 19.13 |
| 5-methyl furfural | 1549 | 1558 | - | 1.045 | - | - | - | 1.559 | 1 | - | 1.04 | - | - | - | 1.56 |
| para-tolualdehyde | 1622 | 1638 | 0.020 | - | Tr | - | Tr | - | 0.0012 | 16.57 | - | - | - | - | - |
| phenyl acetaldehyde | 1660 | 1650 | 0.053 | 65.81 | - | - | 0.015 | 4.864 | 0.007 | 7.60 | 9401.44 | - | - | 2.12 | 694.92 |
| bread thiophene | 1745 | 1755 | 0.150 | 14.362 | Tr | 3.155 | 0.013 | 7.226 | 0.0045 | 33.32 | 3191.56 | 0.79 | 701.07 | 2.87 | 1605.78 |
| 2-undecenal | 1761 | 1759 | - | - | - | 19.516 | - | - | 0.1 | - | - | - | 195.16 | - | - |
| 2,4-decadienal | 1785 | 1797 | - | - | - | 9.129 | - | - | 0.0001 | - | - | - | 9.13×104 | - | - |
| 2-dodecenal | 1850 | 1842 | - | - | - | 20.019 | - | - | 0.0014 | - | - | - | 1.43×104 | - | - |
| cinnamaldehyde | 2058 | 2043 | 0.032 | - | Tr | - | Tr | - | 0.14 | 0.23 | - | - | - | - | - |
| Ketones | |||||||||||||||
| 3-hexanone | 1040 | 1050 | - | 7.395 | - | - | - | 6.056 | 0.06 | - | 123.24 | - | - | - | 100.94 |
| 3-penten-2-one | 1119 | 1126 | - | - | - | 4.916 | Tr | 3.463 | 0.07 | - | - | - | 70.23 | - | 49.47 |
| 2-heptanone | 1190 | 1189 | - | - | - | - | Tr | - | 0.045 | - | - | - | - | - | - |
| coffee furanone | 1240 | 1242 | - | 3.317 | - | 3.052 | Tr | 3.271 | 0.25 | - | 13.27 | - | 12.21 | - | 13.08 |
| 1-octen-3-one | 1314 | 1305 | 0.098 | - | 0.043 | - | - | - | 0.004 | 24.39 | - | 10.66 | - | - | - |
| 3-octen-2-one | 1425 | 1408 | - | - | - | - | Tr | - | 0.02 | - | - | - | - | - | - |
| 3-mercapto-2-pentanone | 1350 | 1343 | 0.010 | - | - | - | 0.010 | 1.689 | 0.0007 | 8.86 | - | - | - | 10.11 | 2413.24 |
| acetophenone | 1634 | 1645 | 0.054 | - | 0.010 | - | 0.014 | - | 0.065 | 0.84 | - | 0.16 | - | 0.21 | - |
| para-methylacetophenone | 1800 | 1797 | 0.012 | - | Tr | - | Tr | - | 0.01 | 1.25 | - | - | - | - | - |
| (E)-geranyl acetone | 1846 | 1858 | 0.023 | - | Tr | - | - | - | 0.186 | 0.13 | - | - | - | - | - |
| alpha-ionone | 1861 | 1860 | - | - | 0.028 | - | 0.010 | - | 0.0016 | - | - | 17.61 | - | 4.43 | - |
| beta-ionone | 1931 | 1947 | 0.024 | - | 0.019 | - | - | - | 0.0012 | 19.81 | - | 16.26 | - | - | - |
| Heterocyclics | |||||||||||||||
| thiophene | 1015 | 1021 | - | - | - | 14.864 | - | - | 0.006 | - | - | - | 2477.42 | - | - |
| 2-methyl thiophene | 1121 | 1100 | 0.044 | 5.780 | 0.017 | 8.961 | Tr | 4.278 | 0.08 | 0.55 | 72.25 | 0.21 | 112.02 | - | 53.46 |
| 2-pentyl furan | 1241 | 1231 | 0.010 | 0.392 | Tr | - | 0.018 | 3.017 | 0.27 | 0.04 | 1.45 | - | - | 0.07 | 11.17 |
| 3,4-dimethyl thiophene | 1255 | 1250 | - | - | Tr | 5.903 | - | - | 0.008 | - | - | - | 737.90 | - | - |
| fish thiol | 1306 | 1305 | - | - | - | - | 0.065 | - | 0.00007 | - | - | - | - | 927.97 | - |
| 2,5-dimethyl pyrazine | 1357 | 1348 | - | - | - | - | Tr | - | 0.87 | - | - | - | - | - | - |
| 2,3,5-trimethyl pyrazine | 1426 | 1422 | - | - | - | - | Tr | - | 0.05 | - | - | - | - | - | - |
| 2-acetyl furan | 1495 | 1488 | - | 3.472 | - | - | - | 2.842 | 10 | - | 0.35 | - | - | - | 0.28 |
| 1-furfuryl pyrrole | 1829 | 1820 | Tr | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 2-acetyl pyrrole | 1989 | 1974 | Tr | 1.733 | - | - | Tr | 2.848 | 2 | - | 0.87 | - | - | - | 1.42 |
| methyl 2-methyl-3-furyl disulfide | 2105 | 2110 | 0.855 | - | 0.012 | - | 0.063 | - | 0.00001 | 8.55×104 | - | 1188.58 | - | 6319.92 | - |
| sulfurol | 2305 | 2302 | 0.474 | 15.157 | 0.094 | - | 0.120 | 37.575 | 0.03 | 15.80 | 505.24 | 3.13 | - | 4 | 1252.17 |
| indole | 2469 | 2453 | 0.017 | - | - | - | - | - | 0.0004 | 43.25 | - | - | - | - | - |
| Phenols | |||||||||||||||
| 2-(methyl thio) phenol | 1870 | 1873 | - | 3.911 | - | - | - | - | 0.8 | - | 4.90 | - | - | - | - |
| phenol | 1994 | 1989 | 0.037 | - | Tr | - | 0.013 | - | 0.046 | 0.80 | - | - | - | 0.29 | - |
Table 8.
Odorous compounds with an OAV >1.
| Code | Compound | Code | Compound | Code | Compound |
|---|---|---|---|---|---|
| 1 | methyl mercaptan | 17 | (E)-2-pentenal | 33 | 3-penten-2-one |
| 2 | amyl alcohol | 18 | heptanal | 34 | coffee furanone |
| 3 | 3-octanol | 19 | 3-methyl-2-butenal | 35 | 1-octen-3-one |
| 4 | furfuryl mercaptan | 20 | octanal | 36 | 3-mercapto-2-pentanone |
| 5 | 1-octen-3-ol | 21 | nonanal | 37 | alpha-ionone |
| 6 | furfuryl alcohol | 22 | furfural | 38 | beta-ionone |
| 7 | phenethyl alcohol | 23 | methional | 39 | thiophene |
| 8 | dextro-bornyl acetate | 24 | benzaldehyde | 40 | 2-methyl thiophene |
| 9 | gamma-butyrolactone | 25 | 5-methyl furfural | 41 | 2-pentyl furan |
| 10 | ethyl palmitate | 26 | para-tolualdehyde | 42 | 3,4-dimethyl thiophene |
| 11 | acetic acid | 27 | phenyl acetaldehyde | 43 | fish thiol |
| 12 | hexanoic acid | 28 | bread thiophene | 44 | sulfurol |
| 13 | octanoic acid | 29 | 2-undecenal | 45 | methyl 2-methyl-3-furyl disulfide |
| 14 | palmitic acid | 30 | 2,4-decadienal | 46 | indole |
| 15 | isovaleraldehyde | 31 | 2-dodecenal | 47 | 2-(methyl thio) phenol |
| 16 | hexanal | 32 | 3-hexanone |
3.3. Correlation between odorous compounds and palatability
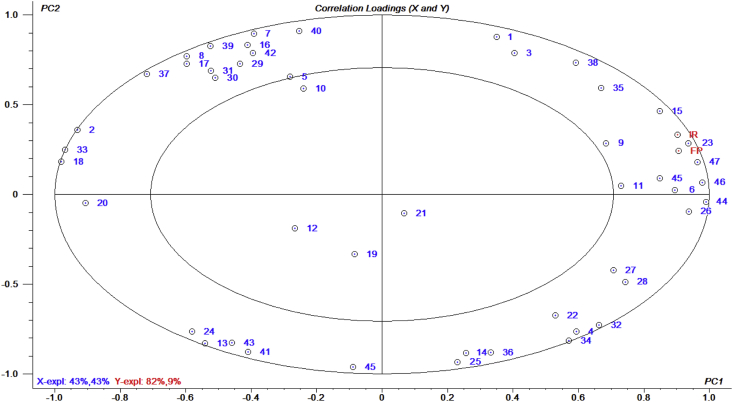
The correlation of PLSR was analyzed by taking OAV of odorous compounds (Table 8) as X variable and palatability index (IR and FP) as Y variable (Figure 2). The derived PLSR model showed that PC1 and PC2 explained 86 % and 91 % respectively, which demonstrated that optimal number of components in model was determined by two principal components (PC). The variance contribution rates of two ellipses in the model were r2 = 0.5 (medial ellipse) and r2 = 1 (lateral ellipse). The variables dispersed between two ellipses indicated that they contributed more to flavor, while the variables in medial ellipse indicated that contribution of flavor was less than 50%. The two Y variables were very close, indicating that the effects of IR and FP are similar, which accurately reflected the results of palatability experiments. Chen et al. [24] also used such a method to find the correlation between volatile compounds and palatability value of seven dog food attractants.
Figure 2.
An overview of the variation found in the mean data from the partial least squares regression (PLSR) correlation loading plot. The model was derived from odorous compounds (OAV >1) as the X-axis and palatability index (ingestion rate and first preference) as the Y-axis. The concentric circles represent r2 = 0.5 and r2 = 1.0, respectively.
The X variables around the palatability index indicated that volatile compounds were highly correlated with palatability. Among them, methyl 2-methyl-3-furyl disulfide (45) and 2-(methyl thio) phenol (47) has strong meat and garlic flavor, which commonly exists in chicken flavor. Methional (23) has the typical flavor of baked potatoes, which can contribute attractant the sense of roasted. Although indole (46) has an unpleasant odor of fecal, its combination with some sulfur-containing substances at low concentrations can enhance the authenticity of chicken flavor. Both ϒ-butyrolactone (9) and isovaleraldehyde (15) have favorable fat flavor, which could be combined with other aldehydes and esters to form a round fat flavor. Acetic acid (11) has a pungent acidity, which satisfies dog's preference for sour taste, and its aroma is more penetrating than other short chain fatty acids. In Chen's study, they reported that 23 aroma compounds such as hexanoic acid, acetaldehyde, heptanone, butyl hexanoate, heptyl formate, methyl pyrazine, 2,5-dimethyl pyrazine, 2-heptanone, pentanal, ethyl decanoate, heptanal, octanal, pentanol, acetone, ethyl caprylate, 3-methyl butanal, anisole, 2-ethyl hexanol, 2-pentyl furan, 2,3-butanediol, benzaldehyde, ethyl vanillin, and vanillin were related to the palatability of dry dog foods [24]. Three aroma compounds (benzaldehyde, vanillin, and 2,5-dimethyl pyrazine) were selected and added to dry dog food to validate the PLSR results [24]. These results are mostly inconsistent with our findings. The reason might be related to the great difference of the technical conditions of Maillard product reaction. Furfuryl alcohol (6) has a typical caramel odor, which exists in Maillard reaction products. This caramel-flavored compound combined with sulfur compounds can bring roasted meat flavor. In Chen's finding, they also reported that furfuryl alcohol was detected in all samples and was known to play an important role in the flavor of dry dog foods [24]. Chicken liver attractant is better than both L. edodes attractant and T. molitor attractant in terms of palatability. It is proved that attractant with combined flavor of meat, roast, fat, caramel, and sour taste can effectively improve the palatability of dog food, which meets dogs' feeding preference [25].
3.4. Palatability and PFS of three attractants
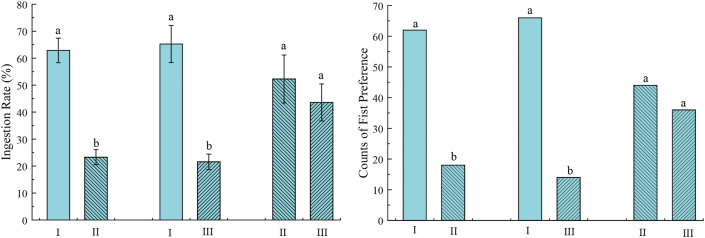
Two-bowl tests were used to evaluate palatability through recording ingestion rate (IR) and first preference (FP). As shown in Figure 3, the IR and FP of food with chicken liver attractant (I) were significantly higher than those of food with L. edodes attractant (II) or T. molitor attractant (III) (P < 0.05). Compared with II or III, the IR of I reached 62.85 %. While corresponding IR of II or III was less than 25 %. Chen et al. reported that the intake ratios (IR) of the seven basal dry dog foods ranged from 43.8 to 74.6, which indicated that dogs consumed most of samples and had a good acceptance towards these attractants [24]. These findings were similar with ours results. Moreover, the FP of I accounted for more than 75 % in 80 cases, indicating that the experimental dogs had better acceptance and preference for the flavor of chicken liver attractant. Although IR and FP of II were slightly higher than those of III, no significant difference was observed. It highlighted that the least preference of the experimental dogs to T. molitor attractant. The palatability test verified that chicken liver was the preferred protein source to prepare dog food attractant.
Figure 3.
Significant difference in ingestion rate and first preference between three kinds of attractants (P < 0.05). I means feed added chicken liver attractant; II means feed added L. edodes attractant; III means feed added T. molitor attractant.
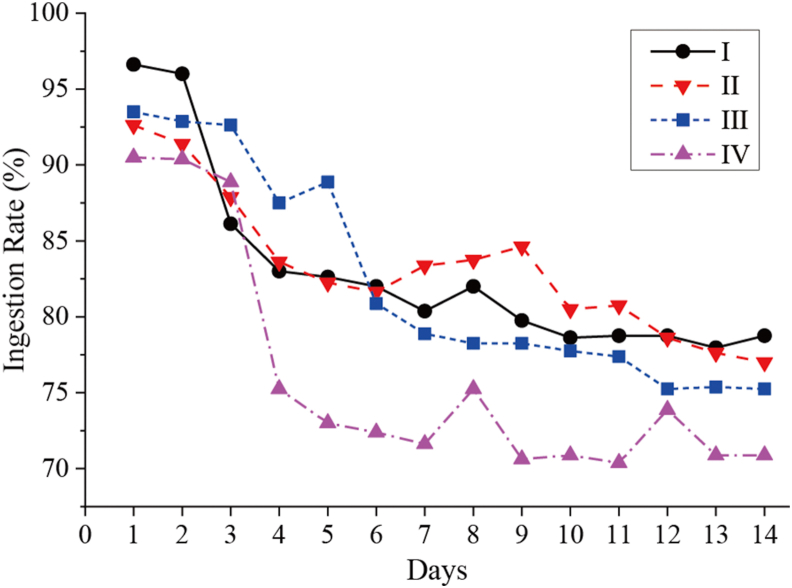
The IR of preferred food as index of three attractants was evaluated by a 14-day single-bowl test (Table 9). The results showed that IR declined for all four foods. As shown in Figure 4, the IR of I, II, and III fluctuated greatly in some specific days, but without significant difference. In summary, the IR of dog food added with attractant showed a similar trend, and was higher than that of food without attractant.
Table 9.
Ingestion rate of four types of dog food for 14 days.
| Sample | I1 | II | III | IV |
|---|---|---|---|---|
| IR (%) | ||||
| Day 1 | 96.62 ± 1.97% aA2 | 93.96 ± 1.25% aB | 93.50 ± 2.35% aB | 90.50 ± 0.91% aB |
| Day 2 | 96.05 ± 3.19% abA | 91.38 ± 1.11% abB | 92.88 ± 2.29% aAB | 90.38 ± 0.48% aB |
| Day 3 | 86.12 ± 12.12% abA | 87.88 ± 4.39% abcA | 92.63 ± 2.56% aA | 88.88 ± 0.85% aA |
| Day 4 | 83.33 ± 9.54% abA | 83.63 ± 5.78% abcA | 87.50 ± 7.90% abcA | 75.25 ± 0.65% bA |
| Day 5 | 82.62 ± 14.77% abA | 82.25 ± 5.39% abcA | 88.88 ± 3.01% abA | 73.00 ± 1.08% bA |
| Day 6 | 82.00 ± 7.82% abA | 81.63 ± 7.86% abcA | 80.88 ± 10.62% bcA | 72.38 ± 1.93% bA |
| Day 7 | 80.37 ± 7.69% abA | 83.38 ± 5.12% abcA | 78.88 ± 7.18% bcA | 71.63 ± 1.38% bA |
| Day 8 | 81.96 ± 5.99% abA | 83.75 ± 6.81% abcA | 78.25 ± 6.84% bcA | 75.25 ± 4.13% bA |
| Day 9 | 79.75 ± 3.48% abAB | 84.63 ± 6.38% abcA | 78.25 ± 7.10% bcAB | 70.63 ± 5.15% bB |
| Day 10 | 78.63 ± 1.89% abA | 80.50 ± 8.84% abcA | 77.75 ± 5.45% bcA | 70.88 ± 1.93% bA |
| Day 11 | 78.75 ± 1.76% abA | 80.75 ± 6.81% abcA | 77.38 ± 5.22% bcA | 70.38 ± 3.30% bB |
| Day 12 | 78.75 ± 1.32% abA | 78.63 ± 5.47% bcA | 75.25 ± 4.57% cA | 73.88 ± 1.44% bA |
| Day 13 | 77.96 ± 1.06% bA | 77.63 ± 4.99% cA | 75.38 ± 3.09% cAB | 70.88 ± 1.65% bB |
| Day 14 | 78.75 ± 1.10% abA | 77.00 ± 4.38% cA | 75.25 ± 2.72% cAB | 70.88 ± 1.55% bB |
I means feed added chicken liver attractant; II means feed added L. edodes attractant; III means feed added T. molitor attractant; IV means feed without attractant.
Means not sharing a common lower case letter in a column are significantly different at P < 0.05. Means not sharing a common upper case letter in a row are significantly different at P < 0.05.
Figure 4.
Variation trend of ingestion rate of four kinds of dog feed. I means feed added chicken liver attractant; II means feed added L. edodes attractant; III means feed added T. molitor attractant; IV means feed without attractant.
3.5. Validation of key volatile compounds on attractants palatability
It's widely known that the overall flavor of attractant is hard to be changed greatly via a single compound. The key odorous compounds should be formulated into flavor accordingly based on GC-MS determination ratio. Then the compensated flavor was added to L. edodes attractant and T. molitor attractant at 0.1 % concentration respectively. A two-bowl test was implemented to compare the palatability of non-flavored chicken liver attractant (A) with that of flavored L. edodes attractant (B/f) and flavored T. molitor attractant (C/f) (Table 10).
Table 10.
Validation of key volatile compounds on attractants palatability.
| Attractant | IR (%) | FP | |
|---|---|---|---|
| A1 | 49.23 ± 4.32 a | 42 a | |
| B/f2 | 48.67 ± 5.22 a | 38 a | |
| A | 50.89 ± 5.67 a | 43 a | |
| C/f3 | 49.25 ± 3.33 a | 37 a | |
|
Code |
Compound |
Content (%) | |
| Bb applied | Cc applied | ||
| 6 | furfuryl alcohol | 2.30 | 2.30 |
| 9 | gamma-butyrolactone | 0.34 | 0.33 |
| 11 | acetic acid | 1.98 | 1.81 |
| 15 | isovaleraldehyde | 0.67 | 1.30 |
| 23 | methional | 0.15 | 0.15 |
| 45 | methyl 2-methyl-3-furyl disulfide 10 % | 1.38 | 1.33 |
| 46 | indole | 0.26 | 0.27 |
| 47 | 2-(methyl thio) phenol | 0.55 | 0.57 |
| Salad oil | 99.45 | 99.43 | |
represented non-flavored chicken liver attractant.
represented flavored L. edodes attractant.
represented flavored T. molitor attractant.
There was no significant difference (p > 0.05) in IR and FP between B/f and C/f and A. The IR of B/f or C/f was not distinguished from that of A, which was about 49 %. Compared with the results in Figure 5, IR of B/f and C/f increased by 26 and 27% respectively, whereas that of A decreased by 14 %. In addition, FP of B/f and C/f accounted for 47 % of the total experimental times, which increased by 22 % compared with results shown in Figure 3. In Chen's study, they added the key aroma compounds to dry dog food to validate the PLSR results [24]. The addition of those compounds positively impacted on the flavour of the dry dog foods and their presence significantly increased the palatability of the all the samples [24]. This methodology was also used in our study. Therefore, animal experiments can be used to verify the practical effect of 8 key volatile compounds on attractant palatability. The characteristic flavor of chicken liver attractant with significant palatability was imitated by compensating with the addition of key odorous compounds, which effectively compensated for the insufficient key flavor of non-meat protein source, such as L. edodes and T. molitor, in the preparation of meat-flavor attractant.
Figure 5.
Effect of different factors on enzymatic hydrolysis of chicken liver, L. edodes and T. molitor (level setting refers to Table 1).
4. Conclusion
In summary, the disadvantage of L. edodes or T. molitor attractant palatability compared to chicken liver attractant could gradually be overcome by addition of key odorous compounds.
The key flavor of attractant were methyl mercaptan, furfuryl mercaptan, acetic acid, hexanoic acid, hexanal, octanal, nonanal, furfural, methional, bread thiophene, methyl 2-methyl-3-furyl disulfide, and sulfurol. The key volatile compounds affecting palatability were 2-methyl-3-furyl disulfide, indole, methional, 2-(methyl thio) phenol, gamma-butyrolactone, furfuryl alcohol, acetic acid, and isovaleraldehyde, which could improve palatability of L. edodes and T. molitor attractant.
Declarations
Author contribution statement
Tao Feng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zhongshan Hu, Yanzun Tong: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Lingyun Yao, Jun Lu: Conceived and designed the experiments; Wrote the paper.
Haining Zhuang, Xiao Zhu: Performed the experiments.
Shiqing Song: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by the National Natural Science Foundation of China (31771942), Natural Science Foundation of Shanghai (17ZR1429600), and Shanghai Local Capacity Building Projects (16090503800).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Lingyun Yao, Email: Lyyao@sit.edu.cn.
Jun Lu, Email: jun.lu@aut.ac.nz.
References
- 1.Aldrich G., Koppel K. Pet food palatability evaluation: a review of standard assay techniques and interpretation of results with a primary focus on limitations. Animals. 2015;5(1):43–55. doi: 10.3390/ani5010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobie C., Péron F., Larose C. Assessing food preferences in dogs and cats: a review of the current methods. Animals. 2015;5(1):126–137. doi: 10.3390/ani5010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier M. 2016. Method for Preparing Wet Pet Food Products Having an Improved Appeal to Pet Owners and at Least a Maintained Palatability to Pets. WO/2016/001323. [Google Scholar]
- 4.Chen T.K., Trivedi N.B. 2005. Animal Food Palatability Enhancer and Method of Use and Manufacture Thereof: US. US 20050276881 A1. [Google Scholar]
- 5.Suresh A.V., Vasagam K.P., Nates S. Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeusstylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture. 2011;319(1):132–140. [Google Scholar]
- 6.Nair V. 2011. Fermented Soy Nutritional Supplements Including Mushroom Components: US. US20150216918A1. [Google Scholar]
- 7.Tan X., Chen H., Huang R., Hao E., Zhang N., Jia S. Research progress of the insect protein feed development. Feed Rev. 2015;3:32–34. [Google Scholar]
- 8.Martin K., Krammer-Lukas S.J.M. 2011. Feed Composition for Companion Animals.https://patents.google.com/patent/US20110052751A1/en US Patent, US20110052751 A1. [Google Scholar]
- 9.Yi J., Huang X., Yang F., Nie Q., Dai J., Li B., Hu J. A review on the application and research progress of feed attractant in pet. Feed Industry. 2016;37:61–64. [Google Scholar]
- 10.Hussain R.T., Ebraheem M.K., Moker H.M. Assessment of heavy metals (Cd, Pb and Zn) contentsin livers of chicken available in the local marketsof Basrah city, Iraq. Basrah J. Vet. Res. 2012;1(11):43–51. [Google Scholar]
- 11.Zhuang P., Zou H., Shu W. Biotransfer of heavy metals along a soil-plant-insect-chicken food chain: field study. J. Environ. Sci. 2009;21(6):849–853. doi: 10.1016/s1001-0742(08)62351-7. [DOI] [PubMed] [Google Scholar]
- 12.Shui M., Feng T., Tong Y., Zhuang H., Lo C., Sun H., Chen L., Song S. Characterization of key aroma compounds and construction of flavor base module of Chinese sweet oranges. Molecules. 2019;24(13):2384–2396. doi: 10.3390/molecules24132384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolaev I.V., Sforza S., Lambertini F.D., Yu I., Khotchenkov V.P., Volik V.G., Dossena A., Popov V.O., Koroleva O.V. Biocatalytic conversion of poultry processing leftovers: optimization of hydrolytic conditions and peptide hydrolysate characterization. Food Chem. 2016;197:611–621. doi: 10.1016/j.foodchem.2015.10.114. [DOI] [PubMed] [Google Scholar]
- 14.Lotfy S.N., Fadel H.H.M., El-Ghorab A.H., Shaheen M.S. Stability of encapsulated beef-like flavourings prepared from enzymatically hydrolysed mushroom proteins with other precursors under conventional and microwave heating. Food Chem. 2015;187:7–13. doi: 10.1016/j.foodchem.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Lu W.J., Han T., Zhang H.Y., Li H.W., Lv Y. On enzymatic hydrolysis of protein in Tenebrio molitor with trypsin. J. Beijing Univ. Agri. 2012;27:77–80. [Google Scholar]
- 16.Ramírez J., Gilardoni G., Jácome M., Montesinos J., Rodolfi M., Guglielminetti M.L., Cagliero C., Bicchi C., Vidari G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant LepechiniamuticaBenth (Lamiaceae) Chem. Biodivers. 2017;14(12):1–11. doi: 10.1002/cbdv.201700292. [DOI] [PubMed] [Google Scholar]
- 17.Wang S.N., Jiang L.Z., Li Y., Li D.D., Sui X.N. Optimization on aqueous enzymatic extraction conditions of pine seed protein by response surface method. Proc. Eng. 2011;15:4956–4966. [Google Scholar]
- 18.Pan A.D., Zeng H.Y., Alain G.B.F.C., Feng B. Heat-pretreatment and enzymolysis behavior of the lotus seed protein. Food Chem. 2016;201:230–236. doi: 10.1016/j.foodchem.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 19.Chen X., Zou Y., Wang D.Y., Xiong G.Y., Xu W.M. Effects of ultrasound pretreatment on the extent of Maillard reaction and the structure, taste and volatile compounds of chicken liver protein. Food Chem. 2020;331:127369. doi: 10.1016/j.foodchem.2020.127369. [DOI] [PubMed] [Google Scholar]
- 20.Li B., Liu C.Y., Fang D.L., Yuan B., Hu Q.H., Zhao L.Y. Effect of boiling time on the contents of flavor and taste in Lentinus edodes. Flavour Fragrance J. 2019;34:506–513. [Google Scholar]
- 21.Seo H., Kim H.R., Cho I.H. Aroma characteristics of raw and cooked Tenebrio molitor larvae (mealworms) Food Sci. Animal Res. 2020;40(4):649–658. doi: 10.5851/kosfa.2020.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukahara T., Matsukawa N., Tomonaga S., Inoue R., Ushida K., Ochiai K. High-sensitivity detection of short-chain fatty acids in porcine ileal, cecal, portal and abdominal blood by gas chromatography-mass spectrometry. Anim. Sci. J. 2014;85:494–498. doi: 10.1111/asj.12188. [DOI] [PubMed] [Google Scholar]
- 23.Kovats E.S. Gas chromatographic characterization of organic substances in the retention index system. Adv. Chromatogr. 1965;1:229–247. [Google Scholar]
- 24.Chen M.S., Chen X.M., Nsor-Atindana J., Masamba K.G., Ma J.G., Zhong F. Optimization of key aroma compounds for dog food attractants. Anim. Feed Sci. Technol. 2017;225:173–181. [Google Scholar]
- 25.Hopkins M.J., Macfarlane G.T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]