Abstract
A central challenge in tissue engineering is obtaining a suitable cell type with a capable delivery vehicle to replace or repair damaged or diseased tissues with tissue mimics. Notably, for skeletal muscle tissue engineering, given the inadequate availability and regenerative capability of endogenous myogenic progenitor cells as well as the tumorigenic risks presented by the currently available pluri- and multipotent stem cells, seeking a safe regenerative cell source is urgently demanded. To conquer this problem, we previously established a novel reprogramming technology that can generate multipotent cells from dermal fibroblasts using a single protein, fibromodulin (FMOD). The yield FMOD-reprogrammed (FReP) cells exhibit exceeding myogenic capability without tumorigenic risk, making them a promising and safe cell source for skeletal muscle establishment. In addition to using the optimal cell for implantation, it is equally essential to maintain cellular localization and retention in the recipient tissue environment for critical-sized muscle tissue establishment. In this study, we demonstrate that the photopolymerizable methacrylated glycol chitosan (MeGC)/type I collagen (ColI)-hydrogel provides a desirable microenvironment for encapsulated FReP cell survival, spreading, extension, and formation of myotubes in the hydrogel three-dimensionally in vitro, without undesired osteogenic, chondrogenic, or tenogenic differentiation. Furthermore, gene profiling revealed a paired box 7 (PAX7) → myogenic factor 5 (MYF5) → myogenic determination 1 (MYOD1) → myogenin (MYOG) → myosin cassette elevation in the encapsulated FReP cells during myogenic differentiation, which is similar to that of the predominant driver of endogenous skeletal muscle regeneration, satellite cells. These findings constitute the evidence that the FReP cell-MeGC/ColI-hydrogel construct is a promising tissue engineering mimic for skeletal muscle generation in vitro, and thus possesses the extraordinary potential for further in vivo validation.
Impact statement
The present study revealed the promising potential of methacrylated glycol chitosan (MeGC)/ColI-hydrogel-encapsulated fibromodulin-reprogrammed (FReP) cells for skeletal muscle tissue engineering. Moreover, the excellent biocompatibility of MeGC/ColI-hydrogel with FReP cells—a novel induced multipotent cell type that is generated through innovative protein-based technology—makes it a suitable cell delivery vehicle for FReP cell-based tissue engineering. The development of various photopolymerizable MeGC/ColI-hydrogel/FReP cell constructs may also shift the paradigm of regenerative medicine into a feasible, safe, and efficient procedure with controllable in situ cell delivery.
Keywords: fibromodulin, fibromodulin-reprogrammed cells, photopolymerizable hydrogel, myogenesis
Introduction
Skeletal muscle comprises 40–50% of human body mass, and an adequate quantity is essential for its function.1,2 For instance, patients with congenital musculoskeletal deformities such as cleft lip/palate and hemifacial microsomia experience difficulties in eating, breathing, and speaking due to skeletal muscle deficiency.3,4 In addition, car accidents, natural disasters, electrical/physical/mechanical trauma, and oncological salvage surgeries constitute skeletal muscle as the most commonly injured tissue in the human body.5–10 More importantly, loss of skeletal muscle mass greatly diminishes the repair capacity: if more than 20% of the muscle is lost, the natural healing process will fail to reconstruct the defect due to lack of sufficient endogenous myogenic progenitor cells (MPCs).11–13 For instance, the predominant driver of endogenous skeletal muscle regeneration, satellite cells (SCs), only constitutes ∼1–5% of all nuclei in human myofiber.14 More importantly, as a consequence of the strong dependence on their complex endogenous microenvironment, the regenerative function of SCs is progressively diminished due to the disruption of the niches during cell isolation.15–21 Meanwhile, the contribution of other cells in myofibers, such as pericytes that often are adjacent to SCs, to skeletal muscle regeneration in vivo is negligible.17,22 Indeed, a recent study that tracked the fate of adult mouse skeletal muscle pericytes in an injury setting suggests that pericytes and vascular smooth muscle cells retain their identity instead of differentiating into skeletal myocytes in vivo.23 Furthermore, the skeletal muscle repair process is often blocked by fibrosis, an overgrowth of the extracellular matrix (ECM).24,25 Thus, in cases of extensive traumatic injury, amputation represents the standard of care, which has resulted in millions of patients with a physical handicap.2 Moreover, the MPCs of patients with congenital musculoskeletal deformities may function abnormally, and therefore cannot self-correct the anomalous skeletal muscle development.3,4 In these circumstances, skeletal muscle regeneration using autologous or allogeneic pluripotent or multipotent cells is an alternative strategy that has become a hot topic for investigation.
To conquer this difficulty, we recently developed a fibromodulin (FMOD)-based reprogramming approach to directly convert dermal fibroblasts, which can be easily isolated from a skin biopsy and expanded in culture,26 into the multipotent stage.27 Excitingly, when compared with the present intensively investigated induced pluripotent stem cells (iPSCs), FMOD ReProgrammed (FReP) cells exhibited a superior capability for bone and skeletal muscle regeneration with markedly less tumorigenic risk.27–29 Thus, FReP cells should be considered an alternative cell source for skeletal muscle regeneration, especially for patients suffering from critical-sized muscle defects.
Defined by Langer and Vacanti in 1993,30 tissue engineering aims to induce tissue-specific regeneration with a combination of the principles of materials and cell transplantation. A fundamental requirement of any scaffolds of cell delivery systems in tissue engineering and regenerative medicine is to maintain cellular viability during the regeneration process. To date, hyaluronan- and polyethylene glycol/fibrinogen-based photopolymerizable hydrogels have been used to manage skeletal muscle damages, in which 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propan-1-one (Irgacure® 2959) is used in combination with ultraviolet (UV) light.31,32 Unfortunately, several drawbacks hindered their further application. For example, even at a low concentration, Irgacure 2959 decreases cell viability,33,34 while UV radiation is always accompanied by the generation of ozone and potential mutations.34,35 To surmount these potential risks, methacrylated glycol chitosan (MeGC)-based hydrogels whose crosslinking could be induced by visible blue light (VBL) with riboflavin (also known as vitamin B2) as an initiator have been developed.36 These hydrogels are polymerized under mild conditions and have been successfully used to encapsulate mesenchymal stem cells (MSCs) for bone and cartilage regeneration.37–39 It is worth noting that the ECM only accounts for 1–10% of the muscle mass,40 far less than its contents in bone and cartilage.41,42 Besides, bone and cartilage possess the mechanical properties for load bearing, while skeletal muscle and other soft tissues are amenable to mechanical conditioning regimens. Thus, the scaffold properties desired for myogenesis may be significantly different from those needed for osteogenesis and chondrogenesis. Moreover, FReP cells may also require a different encapsulating milieu than MSCs for proliferation and differentiation. Therefore, it is necessary to evaluate the potential of VBL-induced photopolymerized MeGC-based hydrogel as a FReP cell encapsulating vehicle for skeletal muscle tissue engineering. By doing so, the present study aims to combine the recent advances in material engineering (the VBL-induced photopolymerized MeGC-based hydrogel) and the novel multipotent cell source (FReP cells) to develop innovative biological substitutes that are capable of replacing diseased or damaged skeletal muscle in humans.
Materials and Methods
Preparation of photopolymerizable chitosan hydrogel
To obtain photopolymerizable hydrogels, chitosan was first converted to a water-soluble derivative MeGC to increase its solubility in physiological solvents. In brief, glycidyl methacrylate (Sigma-Aldrich, St Louis, MO) was added to a 2% (w/v) glycol chitosan (MW: ∼500 kDa; Sigma-Aldrich) aqueous solution at 1:1 ratio. The mixture was adjusted to pH 9.0 and gently shaken at room temperature for 36 h to yield photopolymerizable MeGC.36 The reaction mixture was then neutralized to pH 7.0 and dialyzed against deionized water for 24 h (MW cutting off 1 kDa). The purified MeGC solution was lyophilized for 48 h and stored at −20°C. 1H NMR spectrum analysis43 revealed that the degree of substitution of methacrylate groups onto glycol chitosan was 39.5% ± 3.5%.
FMOD reprogramming
Confluent human newborn foreskin fibroblast BJ cells (CRL-2522, ATCC, Manassas, VA) were treated with DMEM (Life Technologies, Grand Island, NY) supplied with 0.4 mg/mL recombinant human FMOD daily for 21 days.27–29 After 3 weeks of continuous FMOD treatment, the yield FReP cells were isolated by the ReLeSR™ reagent and transferred onto Matrigel™ hESC-qualified Matrix (BD Biosciences, San Jose, CA) precoated plate and maintained in mTESR®1 medium (StemCell Technologies, Vancouver, Canada).28,29
Cell encapsulation
Four percent (w/v) MeGC was mixed with 1% (w/v) type I collagen (ColI; BD Biosciences) at a ratio of 50:50 (v/v). FReP cells were suspended in 40 μL MeGC/ColI-solution at a concentration of 1.25 × 107 cell/mL (5 × 105 cells/construct) before exposure to VBL (400–500 nm, 300–500 mW/cm2; Bisco, Inc., Schaumburg, IL) for 40 s in the presence of 6 μM riboflavin (Sigma-Aldrich). The final concentration of the MeGC is 2% (w/v) and the final concentration of the ColI is 0.5% (w/v). In another group, FReP cells were suspended in 2% (w/v)-MeGC without ColI for hydrogel encapsulation. Meanwhile, the parental BJ fibroblasts without FMOD reprogramming were also encapsulated in the MeGC/ColI-hydrogel as a control.
Cell viability
Immediately after encapsulating, the viability of cells was detected by the LIVE/DEAD® Cell Imaging kit (Life Technologies; Cat. #R37601) with a fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan) following the manufacturer's instruction. A fluorescence microscope (Olympus IX71; Olympus) was used to observe the cells immediately after the staining. Sixteen cell/hydrogel constructs per group were examined, and the numbers of green (live) and red (dead) cells were counted in each hydrogel/cell construct for the percentage calculation of live cells. Cell viability was re-evaluated in an additional 10 cell/hydrogel constructs per group after 1-day cultivation.
In vitro myogenic differentiation
Cell/hydrogel constructs were cultured in myogenic medium I [DMEM supplied with 10% fetal bovine serum (FBS), 10% horse serum (HS), 1% chicken embryo extract (CEE), and 1% penicillin/streptomycin (PS)] for 1 week and then transferred to myogenic medium II [DMEM supplied with 1% FBS, 1% HS, 0.5% CEE, and 1% PS] for another 2-week cultivation.27,29 All media were purchased from Life Technologies.
Apoptosis
After 1, 2, and 3 weeks of cultivation, four cell/hydrogel constructs per group were directly used for terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) analysis with the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer's instruction. Nuclei were counterstained by 4,6-diamidino-2-phenylindole (DAPI). Photos were documented by a confocal laser scanning microscope (CLSM; Leica Microsystems, Inc., Buffalo Grove, IL).
PCR array
Since PCR array is one of the most reliable tools for analyzing the expression of a focused panel of genes, RT2 Profiler™ PCR array for Human Skeletal Muscle (Qiagen, Frederick, MD) was used to profile myogenic-related gene expression according to the manufacturer's instruction. Briefly, 1.0 μg RNA isolated from the encapsulated cells by the RNeasy® Mini Kit (Qiagen) with DNase (Qiagen) treatment was used as a template for PCR array. Three different cDNA templates were tested on a 7300 Real-Time PCR system (Life Technologies). Concomitant glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping standard since its CT values are more consistent among the testing groups than other potential housekeeping genes suggested by the manufacturer (Supplementary Table S1). Hierarchical clustering analysis (HCA) and principal component analysis (PCA) were conducted in RStudio (version 1.2.1335; RStudio, Inc., Boston, MA) coupled R (version 3.6.0) with packages pheatmap (version 1.0.12) and stats/prcomp (version 3.6.0), respectively.
Immunofluorescence staining
The cell/hydrogel constructs were fixed in 4% paraformaldehyde at room temperature for one h and then directly used for myogenic differentiation confirmation by CLSM. It is worth noting that since the cell/hydrogel constructs cannot survive from heat-, enzyme-, or even acid-based retrieval processes, only the antibodies that do not require an antigen retrieval process were applied in the present study. In particular, antibodies against α-sarcomeric actin (ACTA; Cat. # ab28052, clone Alpha Sr-1; Abcam, Cambridge, MA), sarcomeric α-actinin (ACTN; Cat. # ab9465, clone EA-53; Abcam), and desmin (Cat. # ab8592; Abcam) were used to validate the myogenic differentiation according to the instructions of the manufacturer. On the contrary, antibodies against osteocalcin (OCN; Cat. # ab198228, Abcam), type II collagen [COLII; Cat. # II-II6B3, Developmental Studies Hybridoma Bank (DSHB), Iowa Citi, IA],28,44 and tenomodulin (TNMD; Cat. # ab203676, Abcam) were used to assess the osteogenic, chondrogenic, and tenogenic differentiation of encapsulated cells, respectively. DAPI was used for counterstaining. CLSM documented photos.
Statistical analysis
Raw data are presented with mean ± standard deviation (SD). Data were assessed by one-way ANOVA and two-sample t-tests (one-tailed) with OriginPro 8 (Origin Lab Corp., Northampton, MA). p < 0.05 (*) was considered a suggestive difference, while p < 0.005 (**) was recognized as a statistically significant difference.45
Results
Viability and apoptosis of encapsulated FReP cells
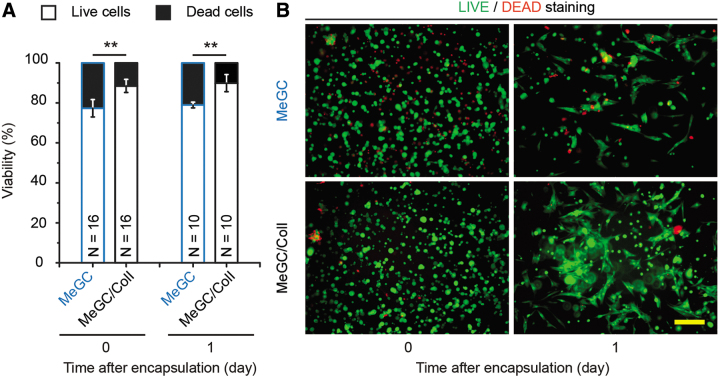
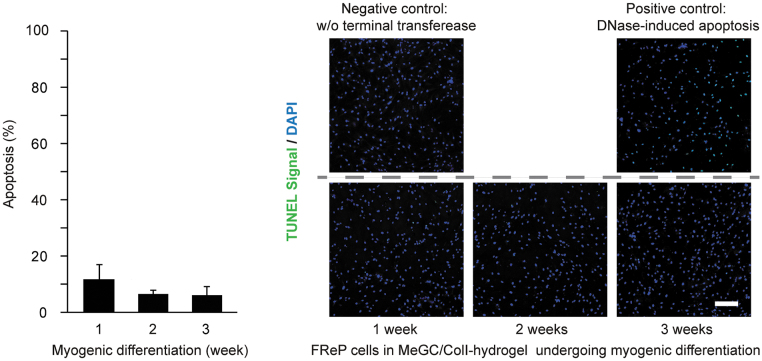
Immediately after the encapsulation (Day 0), live/dead staining revealed that more than 75% of FReP cells encapsulated in MeGC-hydrogel survived through the photocrosslinking procedure. At the same time, the incorporation of ColI into the hydrogel significantly increased the viability of the encapsulated FReP cells (Fig. 1A). Notably, the viability of FReP cells in both MeGC- and MeGC/ColI-hydrogel was not significantly altered after 1 day of myogenic cultivation (Fig. 1A). Meanwhile, the elongated morphology demonstrates that the encapsulated FReP cells already expanded and engrafted in these hydrogels at this early time (Fig. 1B). Considering ColI is the most abundant ECM in skeletal muscle and that TUNEL analysis revealed the apoptosis of the FReP cells remained at a minimal level throughout the entire 3-week myogenic differentiation when encapsulated in the MeGC/ColI hydrogel (Fig. 2), the MeGC/ColI-FReP cell construct was further investigated.
FIG. 1.
Viability of FReP cells encapsulated in the MeGC- and MeGC/ColI-hydrogels. (A) The viability of encapsulated FReP cells was significantly enhanced in the presence of ColI immediately after (Day 0) and 1-day post-photocrosslinking encapsulation (Day 1). (B) After 1-day cultivation, encapsulated FReP cells expanded in the hydrogel constructs. N = 16 (Day 0) or 10 (Day 1), respectively; **p < 0.005. Scale bar = 100 μm. MeGC, methacrylated glycol chitosan; FReP, fibromodulin-reprogrammed.
FIG. 2.
Apoptosis of FReP cells encapsulated in the MeGC/ColI-hydrogel. Apoptosis of MeGC/ColI-hydrogel-encapsulated FReP cells maintained at a relatively low level during the entire 3-week cultivation period. According to the manufacturer's instruction, MeGC/ColI-hydrogel-encapsulated FReP cells after 1-week cultivation were used as a negative control of TUNEL assay by omitting the terminal transferase in the reaction system, while MeGC/ColI-hydrogel-encapsulated FReP cells after 3-week cultivation with DNase treatment were used as a positive control. N = 4. Scale bar = 200 μm.
Gene profiles of encapsulated FReP cells and parental fibroblasts in the MeGC/ColI-hydrogel with the myogenic stimulating condition in vitro
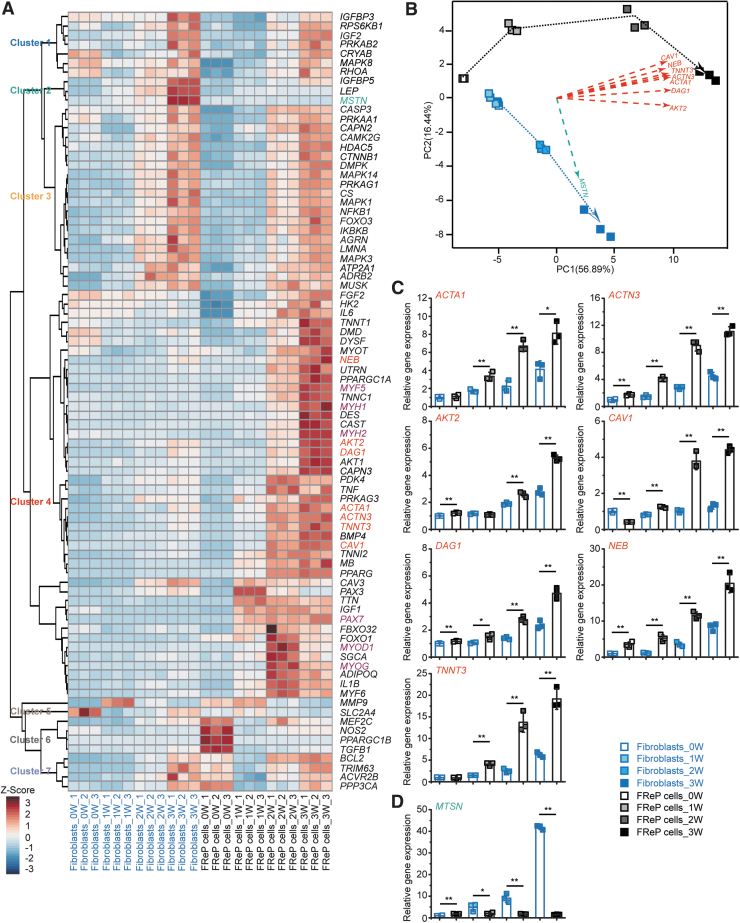
To obtain comprehensive expression patterns of the encapsulated cells during the myogenic differentiation in vitro, 84 myogenic-related genes were organized by HCA to identify distinctive cluster markers (Fig. 3A). PCA revealed that six key principal components (PC1-PC6) explain more than 95% of the total variance (Supplementary Table S2). Next, a visual plot was developed by comparing with two principal components explaining 73.3% of the overall variance of the gene profiles: 56.89% for PC1 and 16.44% for PC2, respectively (Fig. 3B). PCA also demonstrated an apparent separation in gene expression between MeGC/ColI-hydrogel-encapsulated fibroblasts and FReP cells, although they were cultured in the same myogenic situation (Fig. 3B, blue and black dotted lines, respectively). It is worth noting that the major PC1 contributors (Fig. 3B, red dashed lines), including ACTA1, ACTN3, AKT2 (encoding AKT serine/threonine kinase 2), CAV1 (encoding caveolin 1), DAG1 (encoding dystroglycan 1), NEB (encoding nebulin), and TNNT3 (encoding troponin T3, fast skeletal type), were all grouped in Cluster 4 of HCA (Fig. 3A). The expression of these PC1 major contributors significantly increased in the MeGC/ColI-hydrogel-encapsulated FReP cells instead of the encapsulated fibroblasts during the myogenic cultivation (Fig. 3C). On the other hand, myostatin (MSTN), the predominant contributor for PC2 (Fig. 3A, green dashed line), was upregulated in the MeGC/ColI-hydrogel-encapsulated fibroblasts, instead of the encapsulated FReP cells, during the myogenic cultivation (Fig. 3D).
FIG. 3.
HCA and PCA of myogenic-related genes in MeGC/ColI-hydrogel-encapsulated BJ fibroblasts and FReP cells during myogenic differentiation in vitro. (A) HCA identified distinctive clusters of genes, suggesting encapsulated FReP cells presented a significantly higher expression of muscle-specific genes than encapsulated BJ fibroblasts. (B) PCA plot comparing with two principal components, PC1 and PC2, demonstrated the distinct differentiation path of BJ fibroblasts (blue dotted line) and FReP cells (black dotted line). The major contributors for PC1 (red dashed lines) and PC2 (green dashed line) were shown. Gene expression of major contributing genes of PC1 (C) and PC2 (D) was tracked during the 3-week cultivation, respectively. Data normalized to undifferentiated encapsulated BJ fibroblasts. N = 3; *p < 0.05; **p < 0.005. HCA, hierarchical clustering analysis; PCA, principal component analysis.
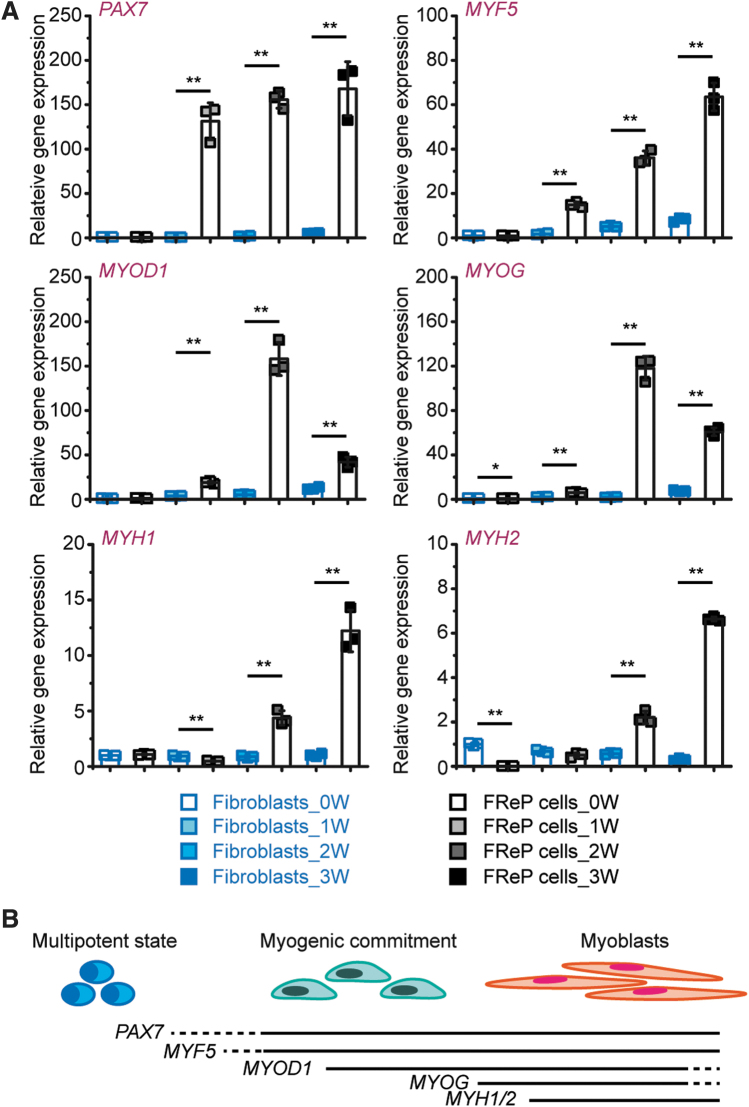
In addition, gene profiling also revealed that paired box 7 (PAX7), a vital early myogenesis marker,20 and the transcription of PAX7's downstream muscle-specific transcription factor cassette, myogenic factor 5 (MYF5) → myogenic determination 1 (MYOD1) → myogenin (MYOG),20,46 were also distinguished between fibroblasts and FReP cells in the Cluster 4 of HCA (Fig. 3A). The expression of PAX7 and MYF5 was constitutively upregulated in the encapsulated FReP cells, while levels of MYOD1 and MYOG reached their peaks at week 2, and dropped at week 3 (Fig. 4A). Subsequently, expression of muscle-specific cytoskeletons, such as myosin [as presented by genes myosin heavy chain 1 (MYH1) and MYH2 (Fig. 4A)], was also significantly induced in encapsulated FReP cells by the 3-week myogenic stimulation in vitro. The expression pattern of these genes in encapsulated FReP cells during myogenic cultivation in vitro (Fig. 4B) was similar to that of SCs,47,48 whereas none of these genes was noticeably upregulated in the encapsulated fibroblasts during the entire period (Fig. 4A).
FIG. 4.
Profile of myogenic transcription factor cassette and myosin encoding genes in MeGC/ColI-hydrogel-encapsulated BJ fibroblasts and FReP cells during myogenic differentiation in vitro. (A) Gene expression of PAX7→MYF5→MYOD1→MYOG myogenic transcription factor cassette and myosin encoding genes, MYH1 and MYH2, was dissected during the 3-week cultivation. N = 3; *p < 0.05; **p < 0.005. (B) Schematic representation of the myogenic differentiation and maturation of multipotent cells. Data normalized to undifferentiated encapsulated BJ fibroblasts.
Immunofluorescence staining of encapsulated FReP cells in the MeGC/ColI-hydrogel with the myogenic stimulating condition in vitro
The myogenic marker expression was further validated in the protein level with immunofluorescence staining. For instance, after cultivation with myogenic stimulation for 3 weeks, the vigorous staining intensity of ACTA1, ACTN, and desmin was observed in MeGC/ColI-hydrogel-encapsulated FReP cells, while the signal was barely detectable in the encapsulated fibroblasts in the same condition (Fig. 5A). Moreover, FReP cells fused in the MeGC/ColI-hydrogel and formed myotubes three dimensionally (Fig. 5B), further representing the myogenic differentiation of FReP cells in vitro. On the contrary, MeGC/ColI-hydrogel-encapsulated FReP cells expressed neither OCN nor COLII (Supplementary Fig. S1A, B), while only a minimal amount of cells displayed TNMD signaling (Supplementary Fig. S1C), demonstrating that the encapsulated FReP cells did not considerably undergo osteogenic, chondrogenic, or tenogenic differentiation in this previously established myogenic condition. Therefore, present data validate the specific myogenic differentiation of MeGC/ColI-hydrogel-encapsulated FReP cells in vitro at both transcriptional and translational levels.
FIG. 5.
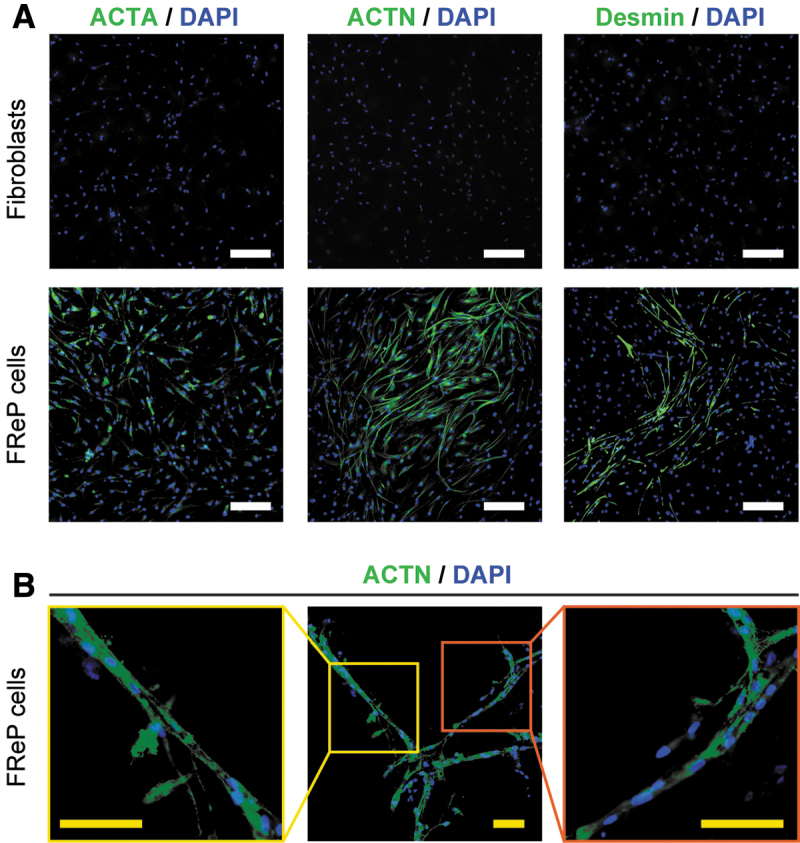
IF staining of muscle-specific cytoskeletons in MeGC/ColI-hydrogel-encapsulated fibroblasts and FReP cells after a 3-week myogenic differentiation in vitro. (A) After 3-week myogenic differentiation, MeGC/ColI-hydrogel-encapsulated FReP cells displayed remarkable staining of skeletal muscle-specific cytoskeletons. On the contrary, none of these markers was detectable on MeGC/ColI-hydrogel-encapsulated fibroblasts at the same time. (B) With the IF staining of ACTN, the fusion of MeGC/ColI-hydrogel-encapsulated FReP cells, as well as the subsequent myotube formation, was visualized. Scale bar = 200 μm (white) or 100 μm (yellow), respectively. IF, immunofluorescence.
Discussion
The goal of tissue engineering is to assemble functional constructs that can replace or repair damaged or diseased tissues. Obtaining a sufficient cell source that either has the full functions or is capable of growing into the required functionality is an essential objective for tissue engineering approaches. For skeletal muscle regeneration, in particular, the major challenge to date is isolation and generation of readily available and safe regenerative cell sources with myogenic potency since direct transplantation of committed myoblasts or MPCs is hindered by inadequate availability, limited spreading, and poor survivability.2,31,49 To date, multiple cell types, including SCs (the quiescent canonical myogenic precursors) and MSCs, have been used to alleviate the severity of skeletal muscle injuries or diseases.50,51 However, the application of SCs was held up due to their limited availability source-specific predetermination nature, and unstable regenerative capacity.2,51 Meanwhile, previous studies suggested that MSCs transplanted in severely traumatized muscle predominantly went through osteogenesis rather than myogenesis.2,52 In addition, traditional avenues of MSC derivation, including bone marrow aspiration and liposuction, along with less common avenues such as muscle biopsy, are invasive and potentially entail pain and medical or surgical risks (e.g., risks of bleeding and anesthesia).53–55 More importantly, recent studies have found that MSCs can develop chromosomal aberrations during cultivation,56 undergo spontaneous tumorigenic transformation,57 and promote glioblastoma and sarcoma formation directly or indirectly,58,59 particularly with inflammatory stimulation60 that typically arises in the wound scenario. Considering that the highly vascularized microenvironment in skeletal muscle can be especially conducive to tumor formation,61 these observations question the safety of MSC application in vivo. Embryonic stem cells (ESCs) are not a practical option because of the moral dilemmas, the potential risk of rejection, and the possibility of tumorigenesis.62–64 Undoubtedly, within regenerative medicine, the most exciting paradigm is the generation of iPSCs. However, iPSCs likely carry an even higher tumorigenic risk than ESCs because of oncogene activation or interruption from viral integration.65–68 To conquer this difficulty, we recently developed an FMOD-based reprogramming approach to directly convert dermal fibroblasts, which can be easily isolated from a skin biopsy and expanded in culture, into the multipotent stage.27 The yield FReP cells shared multiple similarities with iPSCs, such as the triploblastic differentiation capability.27–29 Excitingly, when compared with iPSCs, FReP cells exhibited a superior capacity for bone and skeletal muscle regeneration with markedly less tumorigenic risk.27–29 Therefore, FReP cells could be considered an alternative cell source for skeletal muscle regeneration, especially for patients suffering from critical-sized muscle defects.
Another principal challenge of tissue engineering is introducing a suitable cell type with a capable delivery vehicle since maintaining cellular aggregation of implanted cells is equally critical for successful tissue regeneration. The cell-to-cell interaction directly regulates cell differentiation in the recipient tissue environment.51,69,70 In particular, in cases of large-volume muscle implantations for treatment of critical-sized defects (such as those from severe trauma or large tumor resections) and aesthetic purposes, preserving an appropriate cellular localization and retention is difficult due to the absence of a facial soft tissue envelope. To address these issues, a diversity of hydrogels have been used as encapsulating cell delivery vehicles since hydrogels can homogeneously incorporate cells and provide a highly hydrated environment that is amenable to the rapid diffusion of nutrients and metabolites.34,51 Due to the development of photopolymerization techniques, we now can spatially and temporally control the polymerization process to generate stable and mechanically secure photopolymerizable hydrogels in the presence of photoinitiators using visible or UV light.34,51 However, for photopolymerizable hydrogels, the free radicals produced by photoinitiators are highly reactive and can react with not only the polymerizable monomers but also the cells and thus cause cellular damage during the encapsulation.71,72 Here, by demonstrating that more than 75% of FReP cells encapsulated in MeGC- and MeGC/ColI hydrogels survived through the photocrosslinking procedure, we are convinced that the toxicity of these photopolymerizable hydrogels is tolerable. Moreover, agreed with previous studies on MSCs,37–39 our present study confirms that the incorporation of native ECM components of the target tissue in the engineered hydrogel/cell construct will significantly improve the survival and differentiation of encapsulated FReP cells. Our data also constitute the evidence that the photopolymerized MeGC/ColI-hydrogel allows nutrients, oxygen, and metabolic products to diffuse easily through its matrices and thus supports the survival of encapsulated FReP cells without inducing meaningfully cellular damage.
In addition, gene profiling of MeGC/ColI-hydrogel-encapsulated FReP cells revealed a trend of myogenic-related gene upregulation, which is in agreement with the current understanding of the path that multipotent progenitor cells differentiate and mature into myoblasts.15 In agreement with the gene profiling data, the MeGC/ColI-hydrogel-encapsulated FReP cells displayed the expression of muscle-specific cytoskeletons at the protein level. Furthermore, CLSM documented the spreading, extending, and myotube formation of the encapsulated FReP cells in the MeGC/ColI-hydrogel three dimensionally, further verifying their myogenic differentiation in vitro. These findings constitute the evidence that photopolymerized MeGC/ColI-hydrogel provided a desirable microenvironment for FReP cell survival and myogenic differentiation. Thus, the FReP cell-MeGC/ColI-hydrogel construct could be considered a promising tissue engineering mimic for skeletal muscle generation.
On the contrary, it is known that overexpression of MSTN inhibits the myogenic process by downregulating the muscle regulatory factors MYOD1 and MYOG.73 Thus, the upregulation of MSTN in MeGC/ColI-hydrogel-encapsulated fibroblasts but not FReP cells may explain the nonmyogenic differentiation of the encapsulated fibroblasts during the cultivation. Moreover, unlike the encapsulated FReP cells, the encapsulated parental fibroblasts presented significantly lower levels of muscle-specific transcription factors and cytoskeletons, confirming the myogenic potential of FReP cells, but not their parental fibroblasts, in MeGC/ColI-hydrogel.
Taken together, by combining the current breakthroughs in the fields of biomaterials and cellular reprogramming, we introduce a photopolymerizable MeGC/ColI-hydrogel/FReP cell construct that possesses a promising capability for skeletal muscle regeneration in vitro, while further in vivo validation is warranted for its clinical application. The excellent biocompatibility of MeGC/ColI-hydrogel with FReP cells will significantly enhance the development of various photopolymerizable MeGC/ColI-hydrogel/FReP cell constructs and shift the paradigm of regenerative medicine into a feasible, safe, and efficient procedure with controllable in situ cell delivery. Since ColI is the predominant type of collagen (accounting for 90% of total body collagen) and generally distributes in most connective tissues, MeGC/ColI-hydrogel may also provide a friendly matrix for multipotent FReP cells differentiating into a wide range of tissues under diverse microenvironments. However, we should note that the ratio of MeGC and ColI, as well as the encapsulated density of FReP cells, should be further optimized for different regenerative scenarios in vivo, including for aesthetic and functional muscle reconstruction in a diversity of undamaged and injured situations.
Supplementary Material
Acknowledgment
We thank Ms. Soyon Kim from the Department of Bioengineering, UCLA, for her assistance in chitosan hydrogel construction.
Disclosure Statement
Drs. Kang Ting, Chia Soo, and Zhong Zheng are inventors on the fibromodulin-related patents filed from UCLA. Drs. Kang Ting, Chia Soo, and Zhong Zheng are founders of Scarless Laboratories, Inc., which sublicenses fibromodulin-related patents from the US Regents. Drs. Chia Soo and Zhong Zheng are also officers of Scarless Laboratories, Inc., respectively.
Funding Information
This study was supported by the Plastic Surgery Foundation® (2013 National Endowment for Plastic Surgery 269698) and the National Institutes of Health (NIH)/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant UL1TR0001881. CLSM was performed at the Advanced Light Microscopy/Spectroscopy Laboratory and the Leica Microsystems Center of Excellence at the California NanoSystems Institute at UCLA with funding support from NIH Shared Instrumentation Grant S10OD025017 and NSF Major Research Instrumentation grant CHE-0722519.
Supplementary Material
References
- 1. Huard J., Li Y., and Fu F.H.. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am Vol 84, 822, 2002 [PubMed] [Google Scholar]
- 2. Turner N.J., and Badylak S.F.. Regeneration of skeletal muscle. Cell Tissue Res 347, 759, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Ven Bvd, Defrancq J., and Defrancq E.. Cleft Lip Surgery-a Practical Guide. Zgiera, Poland: Drukarna WIST, 2008 [Google Scholar]
- 4. Takushima A., Harii K., Asato H., and Yamada A.. Neurovascular free-muscle transfer to treat facial paralysis associated with hemifacial microsomia. Plast Reconstr Surg 109, 1219, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bhatt D.L., Gaylor D.C., and Lee R.C.. Rhabdomyolysis due to pulsed electric fields. Plast Reconstr Surg 86, 1, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Knottenbelt J.D. Traumatic Rhabdomyolysis from Severe Beating - Experience of Volume Diuresis in 200 Patients. J Trauma 37, 214, 1994 [PubMed] [Google Scholar]
- 7. Oda J., Tanaka H., Yoshioka T., et al. Analysis of 372 patients with crush syndrome caused by the Hanshin-Awaji earthquake. J Trauma 42, 470, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Minard G., Sugerman H.J., Klein S.R., Atweh N.A., Betts J.M., and Oda J.. Analysis of 372 patients with crush syndrome caused by the Hanshin-Awaji earthquake - Discussion. J Trauma 42, 475, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Hawkes D.H., Alizadehkhaiyat O., Kemp G.J., Fisher A.C., Roebuck M.M., and Frostick S.P.. Shoulder muscle activation and coordination in patients with a massive rotator cuff tear: an electromyographic study. J Orthop Res 30, 1140, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Albashir S., Olansky L., and Sasidhar M.. Progressive muscle weakness: more there than meets the eye. Cleve Clin J Med 78, 385, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Aarimaa V., Kaariainen M., Vaittinen S., et al. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromusc Disord 14, 421, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Crow B.D., Haltom J.D., Carson W.L., Greene W.B., and Cook J.L.. Evaluation of a novel biomaterial for intrasubstance muscle laceration repair. J Orthop Res 25, 396, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Menetrey J., Kasemkijwattana C., Fu F.H., Moreland M.S., and Huard J.. Suturing versus immobilization of a muscle laceration - A morphological and functional study in a mouse model. Am J Sport Med 27, 222, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Marg A., Escobar H., Gloy S., et al. Human satellite cells have regenerative capacity and are genetically manipulable. J Clin Invest 124, 4257, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almada A.E., and Wagers A.J.. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol 17, 267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shadrin I.Y., Khodabukus A., and Bursac N.. Striated muscle function, regeneration, and repair. Cell Mol Life Sci 73, 4175, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sirabella D., De Angelis L., and Berghella L.. Sources for skeletal muscle repair: from satellite cells to reprogramming. J Cachexia Sarcopeni 4, 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almeida C.F., Fernandes S.A., Ribeiro A.F., Okamoto O.K., and Vainzof M.. Muscle satellite cells: exploring the basic biology to rule them. Stem Cells Int 2016, 1078686, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bentzinger C.F., Wang Y.X., von Maltzahn J., and Rudnicki M.A.. The emerging biology of muscle stem cells: implications for cell-based therapies. Bioessays 35, 231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentzinger C.F., Wang Y.X., and Rudnicki M.A.. Building Muscle: molecular regulation of myogenesis. Csh Perspect Biol 4, a008342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mann C.J., Perdiguero E., Kharraz Y., et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1, 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dellavalle A., Maroli G., Covarello D., et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2, 499, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Guimaraes-Camboa N., Cattaneo P., Sun Y., et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345 e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sumner-Smith G. Bone in Clinical Orthopedics. 2nd, enhanced edition. New York: AO Publishing, 2002 [Google Scholar]
- 25. Gates C., and Huard J.. Management of skeletal muscle injuries in military personnel. Oper Techn Sport Med 13, 247, 2005 [Google Scholar]
- 26. Vangipuram M., Ting D., Kim S., Diaz R., and Schule B.. Skin punch biopsy explant culture for derivation of primary human fibroblasts. J Vis Exp 77, e3779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng Z., Jian J., Zhang X., et al. Reprogramming of human fibroblasts into multipotent cells with a single ECM proteoglycan, fibromodulin. Biomaterials 33, 5821, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Li C.S., Yang P., Ting K., et al. Fibromodulin reprogrammed cells: a novel cell source for bone regeneration. Biomaterials 83, 194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng Z., Li C., Ha P., et al. CDKN2B upregulation prevents teratoma formation in multipotent fibromodulin reprogrammed cells. J Clin Invest 129, 3236, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langer R., and Vacanti J.P.. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Rossi C.A., Flaibani M., Blaauw B., et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J 25, 2296, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Fuoco C., Salvatori M.L., Biondo A., et al. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skelet Muscle 2, 24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bryant S.J., Nuttelman C.R., and Anseth K.S.. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomat Sci Polym Ed 11, 439, 2000 [DOI] [PubMed] [Google Scholar]
- 34. El-Sherbiny I.M., and Yacoub M.H.. Hydrogel scaffolds for tissue engineering: progress and challenges. Global Cardiol Sci Pract 2013, 316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shao J.Z., Huang Y., and Fan Q.U.. Visible light initiating systems for photopolymerization: status, development and challenges. Polym Chem 5, 4195, 2014 [Google Scholar]
- 36. Amsden B.G., Sukarto A., Knight D.K., and Shapka S.N.. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules 8, 3758, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Choi B., Kim S., Lin B., et al. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater 12, 30, 2015 [DOI] [PubMed] [Google Scholar]
- 38. Kim S., Cui Z.K., Fan J., Fartash A., Aghaloo T.L., and Lee M.. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J Mater Chem B 4, 5289, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arakawa C., Ng R., Tan S., Kim S., Wu B., and Lee M.. Photopolymerizable chitosan-collagen hydrogels for bone tissue engineering. J Tissue Eng Regen Med 11, 164, 2014 [DOI] [PubMed] [Google Scholar]
- 40. Gillies A.R., and Lieber R.L.. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox A.J.S., Bedi A., and Rodeo S.A.. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fuchs R.K., Thompson W.R., and Warden S.J.. Bone biology. In: Pawelec KM, Planell JA, eds. Bone Repair Biomaterials Regeneration and Clinical Applications. 2nd Edition. Cambridge, United Kingdom: Woodhead Publishing, 2019. pp. 15–52 [Google Scholar]
- 43. Hu J.L., Hou Y.P., Park H., et al. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater 8, 1730, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Li C.S., Zheng Z., Ha P., et al. Neural EGFL like 1 as a potential pro-chondrogenic, anti-inflammatory dual-functional disease-modifying osteoarthritis drug. Biomaterials 226, 119541, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benjamin D.J., Berger J.O., Johannesson M., et al. Redefine statistical significance. We propose to change the default P-value threshold for statistical significance from 0.05 to 0.005 for claims of new discoveries. Nat Hum Behav 1, 0189, 2017 [Google Scholar]
- 46. Dubinska-Magiera M., Zaremba-Czogalla M., and Rzepecki R.. Muscle development, regeneration and laminopathies: how lamins or lamina-associated proteins can contribute to muscle development, regeneration and disease. Cell Mol Life Sci 70, 2713, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crist C.G., Montarras D., and Buckingham M.. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell 11, 118, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Rudnicki M.A., Le Grand F., McKinnell I., and Kuang S.. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73, 323, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Beauchamp J.R., Morgan J.E., Pagel C.N., and Partridge T.A.. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 144, 1113, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vilquin J.T., Catelain C., and Vauchez K.. Cell therapy for muscular dystrophies: advances and challenges. Curr Opin Organ Tran 16, 640, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Lev R., and Seliktar D.. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J R Soc Interface 15, 20170380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jackson W.M., Aragon A.B., Bulken-Hoover J.D., Nesti L.J., and Tuan R.S.. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res 27, 1645, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu S.H., Wei C.F., Yang A.H., Chancellor M.B., Wang L.S., and Chen K.K.. Isolation and characterization of human muscle-derived cells. Urology 74, 440, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Kim Y.H., Cha S.M., Naidu S., and Hwang W.J.. Analysis of postoperative complications for superficial liposuction: a review of 2398 cases. Plast Reconstr Surg 127, 863, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Hjortholm N., Jaddini E., Halaburda K., and Snarski E.. Strategies of pain reduction during the bone marrow biopsy. Ann Hematol 92, 145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Omelchenko D.O., Rzhaninova A.A., and Gol'dshtein D.V.. Comparative transcriptome pairwise analysis of spontaneously transformed multipotent stromal cells from human adipose tissue. Russ J Genet 50, 96, 2014 [PubMed] [Google Scholar]
- 57. Pan Q., Fouraschen S.M., de Ruiter P.E., et al. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med 239, 105, 2014 [DOI] [PubMed] [Google Scholar]
- 58. Akimoto K., Kimura K., Nagano M., et al. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev 22, 1370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lye K.L., Nordin N., Vidyadaran S., and Thilakavathy K.. Mesenchymal stem cells: from stem cells to sarcomas. Cell Biol Int 40, 610, 2016 [DOI] [PubMed] [Google Scholar]
- 60. Ren G.W., Zhao X., Wang Y., et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNF alpha. Cell Stem Cell 11, 812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lensch M.W., and Ince T.A.. Assaying Pluripotency via Teratoma Formation. In: Stein GS, Borowski M, Luong MX, Shi MJ, Smith KP, Vazquez P, eds. Human Stem Cell Technology and Biology: A Research Guide and Laboratory Manual. Hoboken, NJ: Wiley, 2011 [Google Scholar]
- 62. Manzar N., Manzar B., Hussain N., Hussain M.F., and Raza S.. The Ethical Dilemma of embryonic stem cell research. Sci Eng Ethics 19, 97, 2013 [DOI] [PubMed] [Google Scholar]
- 63. de Rham C., and Villard J.. How to cross immunogenetic hurdles to human embryonic stem cell transplantation. Semin Immunopathol 33, 525, 2011 [DOI] [PubMed] [Google Scholar]
- 64. Knoepfler P.S. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27, 1050, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takahashi K., and Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Yu J., Vodyanik M.A., Smuga-Otto K., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Okita K., and Yamanaka S.. Induced pluripotent stem cells: opportunities and challenges. Philos T R Soc B 366, 2198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walia B., Satija N., Tripathi R.P., and Gangenahalli G.U.. Induced pluripotent stem cells: fundamentals and applications of the reprogramming process and its ramifications on regenerative medicine. Stem Cell Rev 8, 100, 2011 [DOI] [PubMed] [Google Scholar]
- 69. Tono-Okada K., Okada Y., Masuda M., et al. Micro 3D culture system using hyaluronan-collagen capsule for skeletal muscle-derived stem cells. Open Tissue Eng Regen Med J 3, 18, 2010 [Google Scholar]
- 70. Tang Y.L., Zhao Q., Qin X., Shen L, Cheng L, Ge J, and Phillips M.I.. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80, 229; discussion 36–7, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Fedorovich N.E., Oudshoorn M.H., van Geemen D., Hennink W.E., Alblas J, and Dhert W.J.A.. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 30, 344, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Atsumi T., Iwakura I., Fujisawa S., and Ueha T.. The production of reactive oxygen species by irradiated camphorquinone-related photosensitizers and their effect on cytotoxicity. Arch Oral Biol 46, 391, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Rios R., Carneiro I., Arce V.M., and Devesa J.. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282, C993, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.