 Virulence attenuating combination therapy may be a promising approach to treating chronic P. aeruginosa infections in patients with cystic fibrosis.
Virulence attenuating combination therapy may be a promising approach to treating chronic P. aeruginosa infections in patients with cystic fibrosis.
Abstract
The World Health Organization considers the discovery of new treatments for P. aeruginosa a top priority. Virulence attenuating combination therapy (VACT) is a pragmatic strategy to improve bacterial clearance, repurpose outmoded antibiotics, improve drug efficacy at lower doses, and reduce the evolution of resistance. In vitro and in vivo studies have shown that adding a quorum sensing inhibitor or an extracellular polymeric substance repressor to classical antibiotics synergistically improves antipseudomonal activity. This review highlights why VACT could specifically benefit cystic fibrosis patients harboring chronic P. aeruginosa infections, outlines the current landscape of synergistic combinations between virulence-targeting small-molecules and anti-pseudomonal drugs, and suggests future directions for VACT research.
Introduction
Cystic fibrosis and P. aeruginosa: a deadly combination
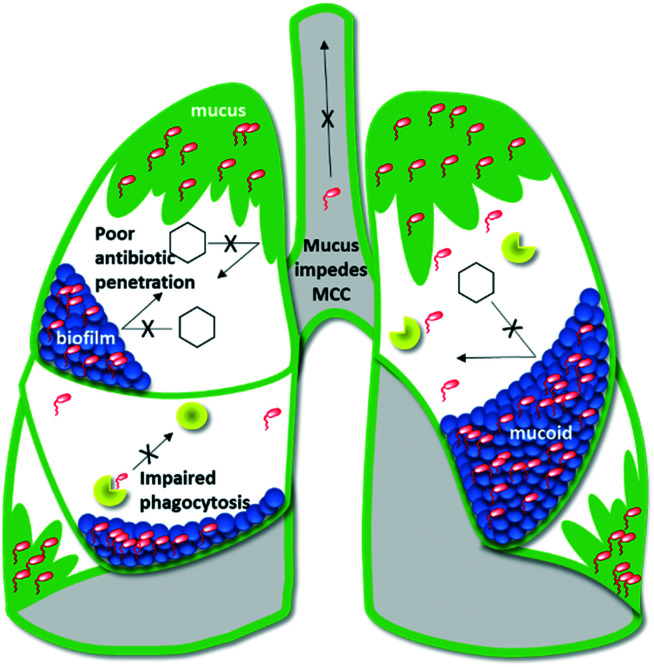
Individuals with cystic fibrosis (CF) carry a genetic mutation that causes defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The clinical result is an overproduction of thick, viscous mucus in the organ systems, notably the lungs.1–10 Airway mucus promotes chronic respiratory infections by physically shielding bacteria from antibiotics and impeding mucociliary clearance (MCC) of inhaled pathogens.8,11 To make matters worse, CFTR-dependent disruptions to complement-mediated immunity impair the ability of monocytes to phagocytose and kill bacteria.12,13 Consequently, bacteria thrive in CF lungs and decimate lung function (Fig. 1).14 A CFTR-mediated metabolic defect that results in excessive succinate release particularly favors colonization by succinate-metabolizing Pseudomonas aeruginosa.15 Chronic infection by this opportunistic Gram-negative rod is the leading cause of morbidity and mortality for CF patients.6,7,15–18
Fig. 1. Depiction of a CF lung.
Treating P. aeruginosa with antibiotics is exceptionally difficult:19–24 the bacteria's outer membrane under expresses OprD porins by which antibiotics can enter the cell and over expresses RND (resistance-nodulation-division) efflux pumps which expel antibiotics. In addition, P. aeruginosa has an inducible gene that codes for AmpC cephalosporinase, a potent beta-lactamase.24,25 The bacteria can also mutate to overproduce a protective, charged, alginate-filled biofilm.9,26–30 Bacteria in biofilms are up to 1000 times more resistant to antibiotic treatment than their planktonic counterparts, making this mucoidal phenotype infamously difficult to treat.31–37 To make matters worse, CF patients require frequent antibiotics, placing near-constant pressure on P. aeruginosa to evolve new resistance traits.38
On average, CF patients initially contract P. aeruginosa at age 2.6. By adolescence, 85% of CF patients are actively harboring the bacteria in their lungs.5,14,31,33 To treat the infection, CF patients cycle through bactericidal monotherapies (including colistin (COL), meropenem (MER), tobramycin (TOB), ceftazidime (CFT), gentamycin (GEN), and azithromycin (AZM)).23–25,39–42 Though these monotherapies may dampen P. aeruginosa flare-ups, they often fail to achieve full bacterial clearance due to the pathophysiology of CF (Fig. 1). Bacteria that persist in mucus or biofilms may select for resistance, repopulate the lung, and evolve mucoidy. Each failed treatment attempt increases the likelihood of eventually colonizing a mucoidal multidrug resistant (MDR) or extensively drug-resistant (XDR) strain.43–50 As such, this current treatment paradigm promotes heinous chronic infections and the World Health Organization (WHO) has assigned top priority to discovering novel therapies for treating P. aeruginosa.17
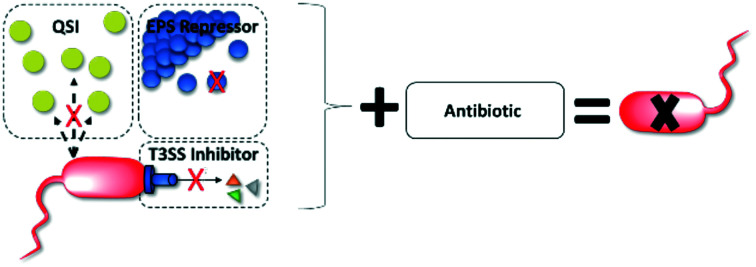
Virulence attenuating combination therapy (VACT)
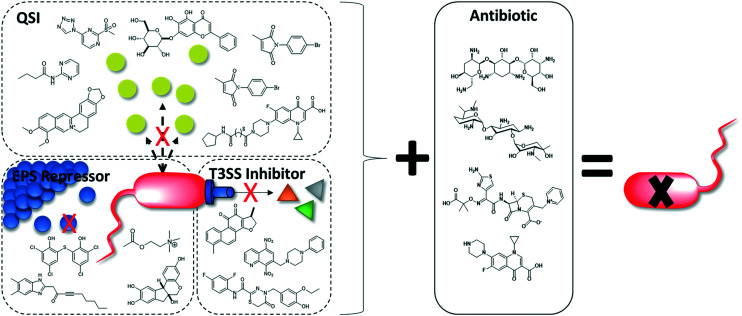
Modern insights into how bacteria specifically interact with the body to cause disease have shed light on new potential drug targets: virulence factors.44,51 Virulence inhibitors specifically interfere with the disease process, thereby preserving the host microbiome and minimizing the risk of selecting for resistance.44,52 VACT couples virulence-targeting small-molecules (to disarm pathogens) with a bactericidal antibiotic (to kill pathogens).
CF patients suffering from intractable mucoid P. aeruginosa are a strong example of a population that could benefit from VACT. In CF lungs, VACT may improve antibiotic efficacy by attenuating biofilm and reducing P. aeruginosa virulence factor production to improve the antipseudomonal activity of antibiotics. Substantial in vitro and in vivo P. aeruginosa VACT studies support this theory. This review summarizes these synergistic combinations in accordance with their virulence target, including 1) quorum sensing (QS) systems and 2) biofilm extracellular polymeric substance (EPS) as well as advocates for future VACT studies that target the type 3 secretion system (T3SS) (Fig. 2).
Fig. 2. Adding a virulence inhibitor to antibiotic therapy improves P. aeruginosa killing.
Discussion
Quorum sensing
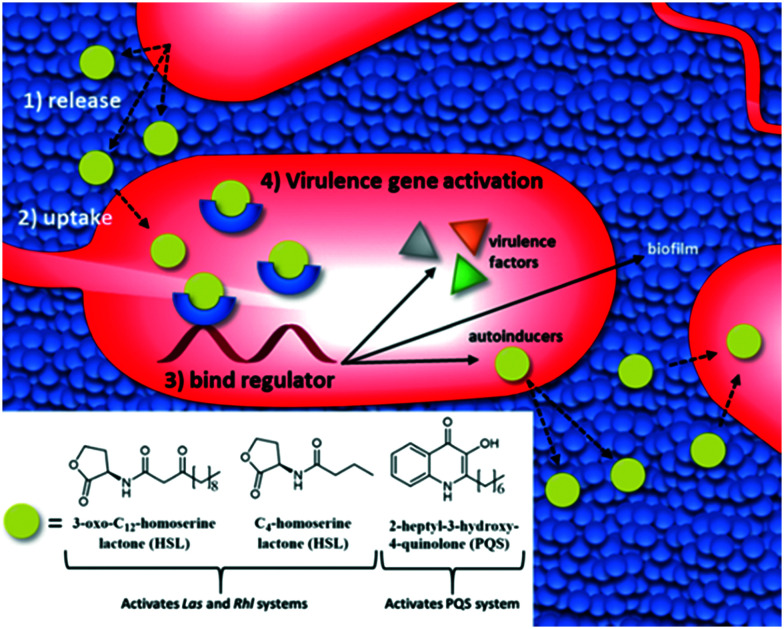
QS describes the process by which bacteria communicate with one another by synthesizing, releasing, and responding to the population-dependent concentration of small molecules known as autoinducers (Fig. 3).53–55 P. aeruginosa secretes two main classes of autoinducer: acyl-homoserine lactones (HSLs) and 2-heptyl-3-hydroxy-4-quinolone (PQS) (Fig. 3).56 When the environmental concentration of autoinducers reaches a threshold, transcriptional regulators alter gene expression to promote survival.55P. aeruginosa's three QS systems, (Las, Rhl, and PQS), work together to control over 300 genes.57–60 Many of those genes code for potent virulence factors such as LasB elastase, LasA protease, the T3SS, exotoxin A, and pyocyanin.60–62
Fig. 3. The QS systems of P. aeruginosa are activated when autoinducers bind to transcriptional regulators upregulating autoinducers, virulence factor production, and biofilm formation.
Quorum signaling also allows individual planktonic bacteria to make group-behavioral decisions, notably the choice to form a biofilm (Fig. 3).63 During biofilm formation, bacterial cells aggregate together within a self-produced matrix of EPS.64–66 Inside of the EPS, P. aeruginosa can persist, shielded from the host immune system, environmental stresses, and many antibiotics.37,63 Additionally, biofilms facilitate horizontal gene transfer, which can lead to the development of resistance.67
Functional QS systems are vital for P. aeruginosa pathogenesis.56,68 In mouse and rat models, P. aeruginosa mutants that lacked QS genes caused less lung pathology, suggesting that cell–cell signaling plays a key role in acute virulence.69,70 In addition, sputum cultures from CF patients infected with chronic P. aeruginosa were discovered to contain significant amounts of HSLs and PQS, indicating that all three QS systems are deeply involved in human infection.56,71,72 Thus, selectively perturbing P. aeruginosa's QS systems with small molecules is an extremely promising strategy for curtailing pathogen virulence in both acute and chronic infections.
Quorum sensing inhibitors and VACT
Small molecule quorum sensing inhibitors (QSIs) have demonstrated therapeutic anti-pseudomonal potential.73–77 Alone, they have been found to reduce virulence factor production, biofilm formation, bacterial motility, and pathogen virulence.78–81 In mouse and rat infection models, QSIs alone induced immunogenicity, improved bacterial clearance, decreased lung pathology, and improved survival.69,70,78,82
The addition of a QSI to antibiotic therapy generally attenuates biofilm to improve antibiotic penetration into the cell while also reducing virulence factor production. Strong QSI candidates for VACT must be non-cytotoxic to human cells and synthetically accessible (<5 steps) or commercially available (for feasible large-scale testing). Laboratories have been exploring VACT in vitro and in vivo with promising results as discussed herein.
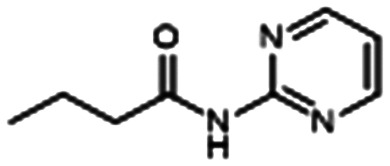
Furiga and colleagues took inspiration from the structure of C4-HSL (Fig. 3), a key signaling molecule in CF lung infections, to develop N-(2-pyrimidyl)butanamide (C11) (Table 1, entry A). C11 downregulates Las and Rhl QS systems, decreasing virulence factors LasB and RhlA. C11 also notably attenuates both aerobic biofilms and the more robust anaerobic biofilms that predominate in CF lung infection. When combined with CIP, TOB, and COL, C11 inhibited biofilm in a dose-dependent manner to improve antibiotic efficacy. They hypothesize that C11 tampers with QS, causing the bacteria to transition from a biofilm to a planktonic state where antibiotics have easier access for killing. C11 is a strong target for in vivo studies because it is stable, not cytotoxic to human cells, and synthetically accessible.83
Table 1. QSI chemical structures and VACT testing summary.
| Entry | Structure | Synergy with | Tested in vitro? | Tested in vivo? |
| A |
|
CIP, TOB, COL | Yes | No |
| B |
|
CIP | Yes | No |
| C |
|
TOB | Yes | No |
| D |
|
TOB | Yes | Yes |
| E |
|
AZM | Yes | Yes |
| F |
|
TOB | Yes | No |
| G |
|
CIP | Yes | Yes |
| H |
|
TOB, MER | Yes | No |
Similarly inspired by the structure of HSLs, Bortolotti and colleagues conjugated an antagonistic C12-HSL analog to CIP. They observed that their hybrid molecule, ET37, reduced biofilm formation and the development of CIP tolerance in clinical strains of P. aeruginosa (Table 1, entry B).84
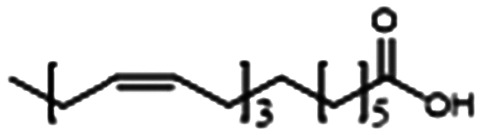
Aware that linolenic acid (LNA), an essential fatty acid, has antimicrobial properties, Chanda et al. added LNA to TOB therapy and found that the combination synergistically attenuated biofilm and virulence factor production by interfering with all three QS systems (Table 1, entry C).85 The LNA and TOB combination is promising for future in vivo exploration and eventual studies in CF patients for two reasons: 1) the regiment is less toxic than TOB alone because the addition of LNA allowed TOB efficacy at lower doses and 2) the VACT has been found to disrupt the production of alginate, a key contributor to chronicity in P. aeruginosa lung infections.
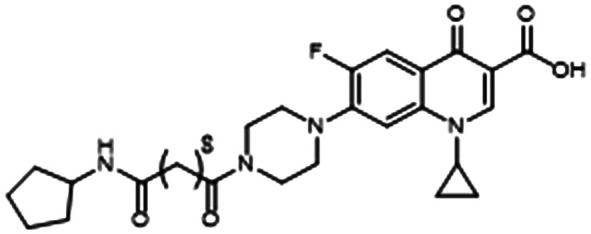
Work by Brackman and colleagues showed the addition of baicalin hydrate (BH) to TOB significantly increased biofilm killing in vitro (Table 1, entry D). They also found that a VACT regiment of BH and TOB improved survival in a C. elegans infection model.86 BH is commercially available, making larger-scale VACT studies (for example, in conditions that mimic the CF lung environment) very feasible.
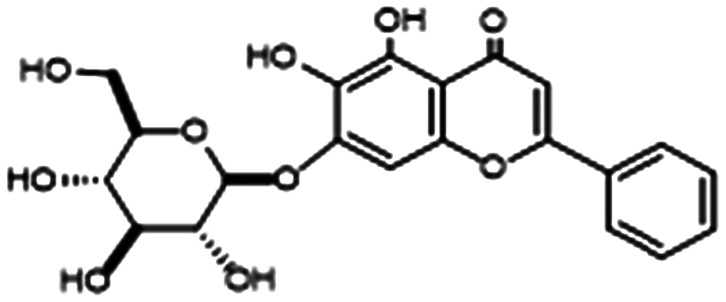
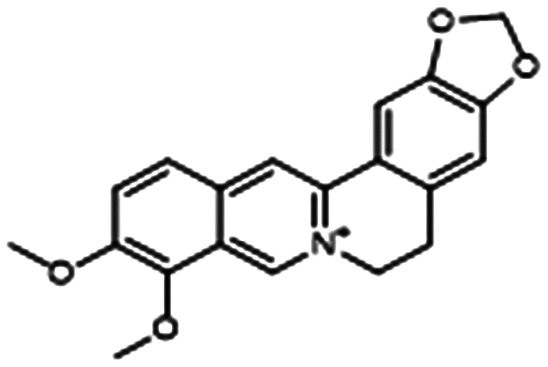
Berberine (BER) is an isoquinolone alkaloid that has been approved for over-the-counter use in China to treat gastrointestinal infections and is being studied as an anti-diabetic and antimicrobial.87,88 Li and coworkers found that sub-MIC regimens of BER and AZM interfered with the Las and Rhl QS systems to inhibit biofilm and virulence factor production (notably alginate) (Table 1, entry E). The VACT also proved effective against 10 clinical P. aeruginosa isolates cultured from the sputum of CF patients. Excitingly, infected mice treated with a regimen of 0.8 mg kg–1 of AZM combined with 3.2 mg kg–1 of BER showed markedly increased survival and decreased lung inflammation.89
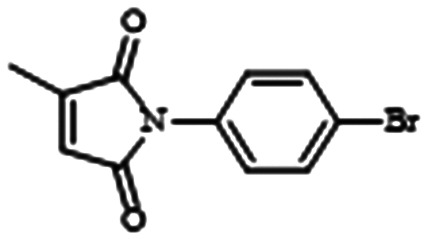
Intrigued by the antibacterial properties of itaconimides, Fong and colleagues synthesized an analog library based on structure–activity relationships (SAR) and found that 10 μM (non-cytotoxic up to 40 μM) of itaconimide 12a in combination with TOB completely eradicated an entire population of 72 hour old P. aeruginosa biofilm (Table 1, entry F). They hypothesize that 12a works by inhibiting the PQS system via the Las system.90 The VACT's ability to entirely remove preformed biofilms may suggest exciting utility against chronic mucoid infection. However, before initiating further clinically oriented studies, it is necessary to test 12a for reactivity with thiol containing compounds such as glutathione. Such reactivity has been linked to cell-death.91
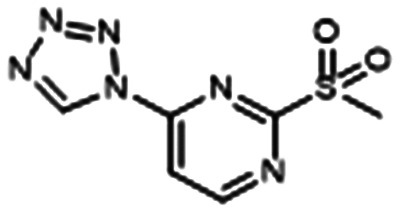
Using bioisosteric modification of known, single-target inhibitors, Thomann et al. developed compound 6, which is a multi-target drug that simultaneously inhibits the transcriptional regulator, PqsR, and a key enzyme for PQS production, PqsD (Table 1, entry G).92 Alone, 6 interfered with iron metabolism, decreased virulence factor production, and inhibited biofilm formation (IC50 = 100 μM) and eDNA production. It was also efficacious in vivo, increasing the survival of Galleria mellonella (low toxicity observed). The VACT combination of 1 μM CIP + 50 μM 6 restored CIP activity against P. aeruginosa biofilm. They hypothesize that this synergy is due to the ability of 6 to inhibit eDNA, which hinders CIP.
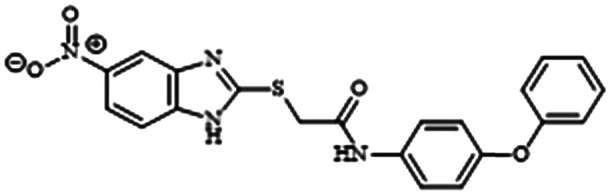
Excitingly, Maura and colleagues found that another PqsR inhibitor, M64, is one hundred times more potent at inhibiting biofilm than compound 6 (Table 1, entry H).93 Pre-treatment with M64 restored TOB and MER efficacy against antibiotic-tolerant biofilms. Concurrent treatment with M64 and MER or TOB decreased biofilm CFU by 178 and 17 times respectively.
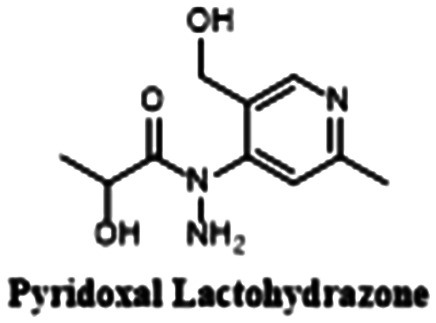
In recent years, a plethora of auspicious QSIs have been discovered. Many of these inhibitors are commercially available, have low toxicity, or inhibit alginate (the main component of mucoid P. aeruginosa biofilm). For these reasons, such compounds and their synthetic analogs merit VACT studies in the context of CF. A summary of promising QSIs for VACT studies can be found in Table 2.78,79,81,94–97
Table 2. Summary and evaluation of promising QSIs not tested for synergy.
| Compound | Reduces virulence factors | Inhibits biofilm? | Commercially available? |
|
Alginate, pyocyanin | Yes | No (2 synthetic steps) |
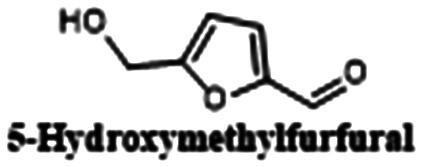
|
Alginate, rhamnolipid (sub-MIC) | Yes | Yes |
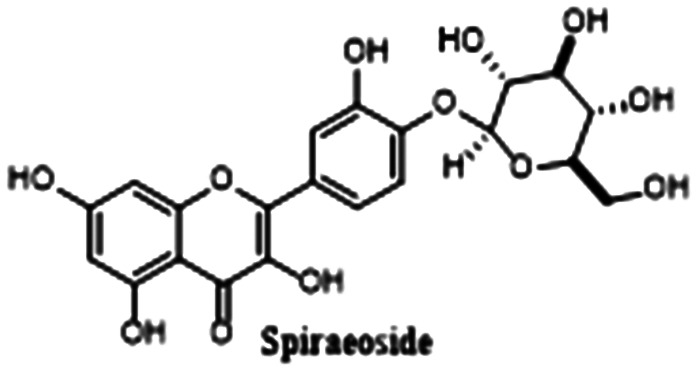
|
Elastase, pyocyanin, violacetin | Yes | Yes |
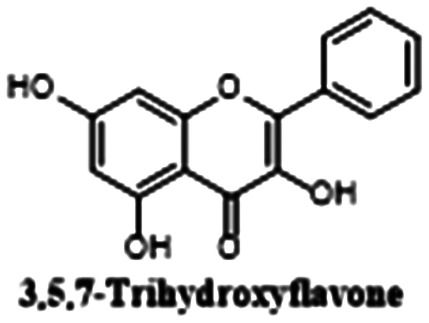
|
Pyocyanin | Yes (IC85 = 100 μM) | Yes |
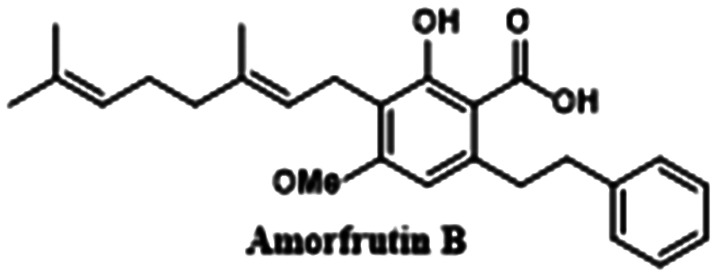
|
Elastase, pyocyanin (IC50 = 10 μM) | Yes (IC50 = 50 μM) | No |
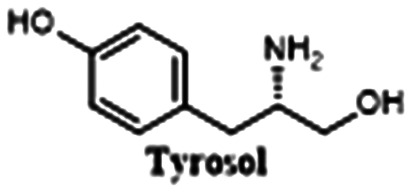
|
Elastase, pyocyanin | Yes | Yes |
Extracellular polymeric substance repressors
During the early stages of biofilm formation, bacteria generate a matrix made up of extracellular polymeric substances (EPSs).98P. aeruginosa produces three EPSs: Pel, Psl, and alginate. Pel is believed to function as an eDNA cross-linker and has been implicated in the development of antimicrobial resistance.99,100 Psl may act as a signal to initiate biofilm formation and is a critical structural element of biofilms during the microcolony formation stage.101,102
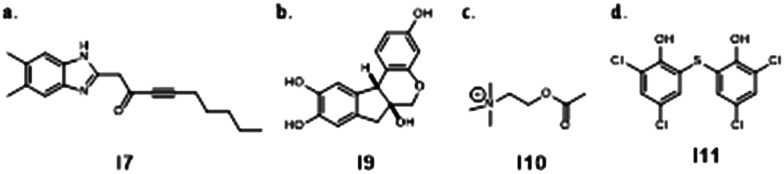
Pel and Psl are exciting targets for VACT because inhibiting EPSs reduces or eliminates biofilm, making bacteria more susceptible to antimicrobials.100 The Lewenza Lab used a high-throughput gene expression screen to find compounds that repress Pel.103 When studied in combination with COL, polymyxin B (PB), TOB, GEN, and CIP, EPS inhibitors I7-I11 attenuated mucoidal biofilms to improve antibiotic penetration and efficacy (Fig. 4). Notably, compound I7 also demonstrated ability to attenuate biofilm in mucoidal P. aeruginosa, making it a prime candidate for in vivo exploration in the context of CF.
Fig. 4. Chemical structures of EPS inhibitors by the Lewenza Lab used in VACT.
Type 3 secretion system inhibitors and VACT
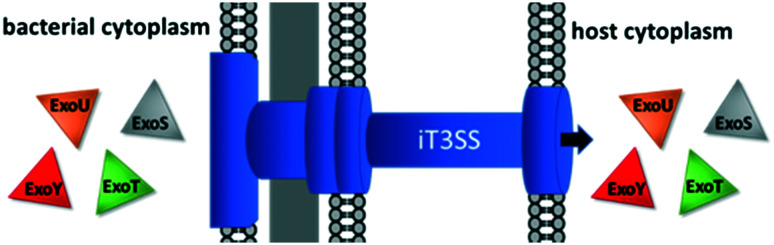
Type 3 secretion systems (T3SSs) play leading roles in P. aeruginosa virulence and are a strong yet unexplored candidate for VACT.104,105 The T3SS is broadly responsible for transferring proteins out of the bacterial cell and highly conserved amongst Gram-negative pathogens.105,106 P. aeruginosa expresses two distinct T3SSs: the fT3SS and the iT3SS.107 The fT3SS expels flagellar proteins, enabling chemotaxis and biofilm formation and secreting effector toxins.107–109 The iT3SS, or injectosome, ejaculates cytotoxic effector toxins (ETA, ExoS, ExoT, ExoU, ExoY) directly into the host cell's cytoplasm (Fig. 5).80,110,111
Fig. 5. The T3SS secretes effector toxins into the host cytoplasm.
The most potent effector toxin is ExoU.18,22 ExoU has been found to lyse lung cells and destroy the alveolar epithelia, leading to septic shock and severe acute lung damage.112,113 ExoS prevents human neutrophils from producing reactive oxygen species (ROS), which are vital for killing phagocytosed bacteria.114 ExoT inhibits mammalian cytokinesis, causing slowed wound healing.9,115 ExoY causes apoptosis.113,116 In addition to their individual functions, the four major effector toxins collude to kill and impair alveolar neutrophils and macrophages, severely handicapping the phagocytotic immune response to bacteria.117
Inhibiting the T3SS with small-molecules is a highly appealing anti virulence strategy: the T3SS is specific to pathogenic bacteria, limiting the chance of off-target effects.18,80,118–121 In addition, because the T3SS is not vital for the bacteria's survival, disrupting it should not place evolutionary pressure on P. aeruginosa to evolve new resistance mechanisms.122 Ideally, a selective T3SS inhibitor would stop P. aeruginosa from impairing phagocytosis, empowering the host immune system to kill the bacteria.118 However, T3SS inhibitors have never been studied for synergy with bactericidal antibiotics. Several promising T3SS inhibitors and VACT candidates are featured in Fig. 6 and discussed herein. However, for a thorough review of targeting the T3SS to fight P. aeruginosa, see Anantharajah et al.123 and Duncan et al.124
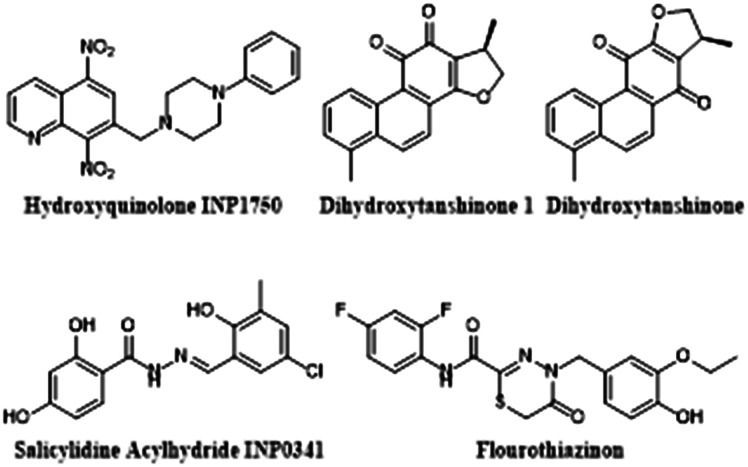
Fig. 6. Promising T3SS inhibitors for future VACT study.
Promising T3SS inhibitors for future VACT studies
Sheremet and colleagues studied the activity of flourothiazinon (FT), a 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-one, against P. aeruginosa infections in a mouse model.122,125 They found that mice dosed with 50 mg kg–1 of FT twice daily for 4 days after being infected with various lethal doses of multi-drug resistant clinical isolates of P. aeruginosa often survived the infection and displayed less lung pathology, lower levels of systemic inflammation, increased clearance of bacteria in their lungs and spleen, and no bacteremia. This suggests that at both a systemic level and in the lungs, FT intercepts P. aeruginosa's characteristic attempt to suppress host immunity, improving phagocytotic clearance of pathogen from the cells to allow survival. In vitro experiments showed that FT specifically inhibited secretion of ExoT and ExoY and significantly decreased bacterial cytotoxicity. They also found that FT restored the ability HeLa cells to phagocytose bacteria in a dose dependent manner. When plated with FT, P. aeruginosa growth was not affected. The efficacy, specificity, and documented low toxicity104 of FT make it a highly promising candidate for VACT.
Anantharajah and colleagues explored two classes of T3SS inhibitors for antibiotic potential: salicylidine acylhydrides and hydroxyquinolones.107 These molecular classes were promising as they had previously been found to inhibit transcription of the iT3SS in other gram negative pathogens including Y. pseudotuberculosis, Shigella, Salmonella, and Chlamydia.119–121 The team compared the anti-pseudomonal activity of the two chemical classes by selecting a non-cytotoxic model molecule from each and testing it in a series of in vitro experiments. From the salicylidine acylhydrides, they selected INP0341 (SA INP0341), and from the hydroxyquinolines, they selected INP1750 (HINP1750). They found that both INPs reduced P. aeruginosa's cytotoxicity in phagocytotic and epithelial eukaryotic cells. Further experimentation revealed that SA INP0341 interferes with iT3SS gene transcription while HINP1750 mediates cytotoxicity by inhibiting a key ATPase homologous to the iT3SS and the fT3SS to decrease ExoS secretion and flagellar motility. HINP1750 had a more robust effect on T3SS inhibition as it was active against both T3SS systems, making it a stronger candidate for future study in acute animal infection models and VACT. However, before such studies take place, it is necessary to test the mutagenicity of HINP1750 with an Ames test or its equivalent—nitroaromatic groups have been linked to mutagenicity and HINP1750 contains 2 nitro groups attached to an aromatic ring.126
The iT3SS injectosome needle is composed of repeated subunits of a single protein termed PscF. Before PscF is secreted to form the needle, it is protected by two chaperonins, PscE, and PscG. Without protection from the chaperonins, PscF will degrade in the cytosol, and the injectosome needle will not form. Thus, inhibiting the interactions between PscF and the chaperonins will prevent biogenesis of the iT3SS and its resulting cytotoxicity.80,111 This effect is confirmed by PscE/PscG knockout studies.111 In July 2019, Feng and colleagues made a serendipitous discovery that non-bactericidal tanshinones compete with PscF for PscE-PscG binding.80 They found that dihydrotanshinone 1 (dHTSN1) demonstrated an IC50 of 0.68 μM, and dihydrotanshinone (dHTSN) demonstrated an IC50 of 1.5 μM in a fluorescence polarization (FP) assay. In addition, the compounds were found by Western Blot to decrease ExoS secretion at concentrations of 100 μM in Ca2+ depleted (T3SS stimulating) conditions. Western blot also revealed that the tanshinones repressed P. aeruginosa-induced macrophage lysis by reducing bacterial caspase-1 release. Caspase-1 is normally released by the T3SS, further suggesting that the tanshinones inhibit T3SS biogenesis.127 In mouse models, 90% of mice challenged with LD70 of PA01 survived when dosed with either dHTSN or dHTSN1. These mice showed less bacteria in their lungs, lower levels of inflammation, and less alveolar damage. The impressive anti virulence properties of tanshinones demonstrated in vitro and in vivo make them prime candidates for VACT exploration.
Conclusions
Despite a cornucopia of promising academic work in the development of virulence inhibitors such as QSIs, EPS repressors, and T3SS inhibitors, these compounds have yet to progress to clinical trials. One potential explanation for this disconnect is that large pharmaceutical companies are likely deterred by the financial risk of such an endeavor for two reasons. 1) The path by which such a compound would pass phase 3 is unclear and has yet to be evaluated and 2) testing molecules that specifically inhibit virulence and not bacterial growth in humans might raise some ethical questions. However, with antibiotic resistance on the rise, failing to pursue non-bactericidal options, even in combination with approved drugs, is potentially both dangerous and a missed opportunity to revolutionize our antiquated approach to treating infectious disease.
Virulence inhibitors (notably QSIs and EPS repressors) have demonstrated a strong ability to potentiate antipseudomonal antibiotics in both in vitro and animal models. There is a long road from in vitro and murine model experimentation to clinical testing. However, the early promising results of VACT merit further exploration: first, synergy testing of known and novel QSIs and EPS inhibitors is needed to identify new and potent VACT combinations. Computational screening methods can be used to identify highly specific, multi-target virulence inhibitors for such testing. Second, labs must begin testing VACT regimens in conditions that replicate the CF lung. For example, in the presence of alginate, CF sputum,128 or in genetically engineered mouse models (GEMM) with CFTR defects. Third, VACT studies with T3SS (and other virulence) inhibitors demand exploration. It is our hope that the next generation of chemists will use virulence-inhibiting small-molecules to resuscitate antibiotics and annihilate resistant bacteria.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The laboratory is supported by the National Institute of General Medical Sciences (GM119426) and the National Science Foundation (CHE1755698). We thank Alexander T. Kim, Amy Solinski, Amber Scharnow, Anna Kaplan, and Ingrid Wilt for valuable suggestions and feedback.
Biographies

Elana Shaw
Elana Shaw earned her BS in Chemistry at Emory University in Dec 2019 where she worked on the synthesis of novel precision antibiotic candidates in the Wuest Lab. She is currently a post-baccalaureate fellow at the National Institutes of Health studying the biomolecular underpinnings of rare immunological diseases.

William M. Wuest
Bill Wuest received his B.S. in Chemistry/Business (Notre Dame), his Ph.D. in organic chemistry (UPENN) and was a NIH Postdoctoral Fellow at Harvard Medical School before beginning his independent career at Temple University in 2011. In 2017, he moved to Emory University where he is currently a Georgia Research Alliance Distinguished Investigator and Associate Professor of Chemistry. His research focuses on the diverted total synthesis of pathogen-specific antibiotics. Bill is the recipient of a number of awards including the NIH ESI MIRA, NSF CAREER Award, ACS Medicinal Chemistry David W. Robertson Award, and the ACS Infectious Diseases Young Investigator Award.
References
- Grossman S., Grossman L. C. Crit. Care Nurse. 2005;25(4):46–51. [PubMed] [Google Scholar]
- Haack A., Aragão G. G., Novaes M. R. C. G. World J. Gastroenterol. 2013;19(46):8552–8561. doi: 10.3748/wjg.v19.i46.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Physiol. Rev. 1999;79(1 Suppl):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Schram C. A. Can. Fam. Physician. 2012;58(12):e699–e704. [PMC free article] [PubMed] [Google Scholar]
- Davies J. C. Paediatr. Respir. Rev. 2002;3(2):128–134. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R. L. Pediatr. Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Kaplan A. R., Wuest W. M. ChemBioChem. 2019;20(1):34–39. doi: 10.1002/cbic.201800383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagirath A. Y., Li Y., Somayajula D., Dadashi M., Badr S., Duan K. BMC Pulm. Med. 2016;16(1):174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. R., Kulasekara H. D., Emerson J., Houston L. S., Burns J. L., Ramsey B. W., Miller S. I. J. Cystic Fibrosis. 2009;8(1):66–70. doi: 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D., Gaggar A., Bruscia E., Hector A., Marcos V., Jung A., Greene C., McElvaney G., Mall M., Döring G. J. Cystic Fibrosis. 2012;11(5):363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Livraghi A., Randell S. H. Toxicol. Pathol. 2007;35(1):116–129. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- Lovewell R. R., Patankar Y. R., Berwin B. Am. J. Physiol. 2014;306(7):L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Weert-van Leeuwen P. B., Van Meegen M. A., Speirs J. J., Pals D. J., Rooijakkers S. H., Van der Ent C. K., Terheggen-Lagro S. W., Arets H. G., Beekman J. M. Am. J. Respir. Cell Mol. Biol. 2013;49(3):463–470. doi: 10.1165/rcmb.2012-0502OC. [DOI] [PubMed] [Google Scholar]
- Lyczak J. B., Cannon C. L., Pier G. B. Clin. Microbiol. Rev. 2002;15(2):194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme S. A., Lozano C., Moustafa A. M., Liimatta K., Tomlinson K. L., Britto C., Khanal S., Gill S. K., Narechania A., Azcona-Gutiérrez J. M., DiMango E., Saénz Y., Planet P., Prince A. Sci. Transl. Med. 2019;11(499):eaav4634. doi: 10.1126/scitranslmed.aav4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pseudomonas, https://www.cff.org/Life-With-CF/Daily-Life/Germs-and-Staying-Healthy/What-Are-Germs/Pseudomonas/.
- Govindaraj Vaithinathan A., Vanitha A. Perspect. Public Health. 2018;138(2):87–88. doi: 10.1177/1757913917743881. [DOI] [PubMed] [Google Scholar]
- Galle M., Carpentier I., Beyaert R. Curr. Protein Pept. Sci. 2012;13(8):831–842. doi: 10.2174/138920312804871210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Kulasekara B. R., Lory S. Genome Dyn. 2009;6:126–139. doi: 10.1159/000235767. [DOI] [PubMed] [Google Scholar]
- Labaer J., Qiu Q., Anumanthan A., Mar W., Zuo D., Murthy T. V., Taycher H., Halleck A., Hainsworth E., Lory S., Brizuela L. Genome Res. 2004;14(10b):2190–2200. doi: 10.1101/gr.2482804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saderi H., Owlia P. Iran. J. Pathol. 2015;10(4):265–271. [PMC free article] [PubMed] [Google Scholar]
- Ader F., Le Berre R., Faure K., Gosset P., Epaulard O., Toussaint B., Polack B., Nowak E., Viget N. B., Kipnis E., Guery B. P. Infect. Immun. 2005;73(7):4263–4271. doi: 10.1128/IAI.73.7.4263-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todar K., Todar's Online Textbook of Bacteriology, in Pseudomonas aeruginosa, 2012, (accessed 6/10/19). [Google Scholar]
- Lister P. D., Wolter D. J., Hanson N. D. Clin. Microbiol. Rev. 2009;22(4):582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. D., Bardin E., Cameron L., Edmondson C. L., Farrant K. V., Martin I., Murphy R. A., Soren O., Turnbull A. R., Wierre-Gore N., Alton E. W., Bundy J. G., Bush A., Connett G. J., Faust S. N., Filloux A., Freemont P. S., Jones A. L., Takats Z., Webb J. S., Williams H. D., Davies J. C. FEMS Microbiol. Lett. 2017;364(14) doi: 10.1093/femsle/fnx121. [DOI] [PubMed] [Google Scholar]
- Boyd A., Chakrabarty A. M. J. Ind. Microbiol. 1995;15(3):162–168. doi: 10.1007/BF01569821. [DOI] [PubMed] [Google Scholar]
- Li Z., Kosorok M. R., Farrell P. M., Laxova A., West S. E., Green C. G., Collins J., Rock M. J., Splaingard M. L. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- Ryall B., Carrara M., Zlosnik J. E. A., Behrends V., Lee X., Wong Z., Lougheed K. E., Williams H. D. PLoS One. 2014;9(5):e96166. doi: 10.1371/journal.pone.0096166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. W., Schurr M. J., Mudd M. H., Govan J. R., Holloway B. W., Deretic V. Proc. Natl. Acad. Sci. U. S. A. 1993;90(18):8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Deretic V. Microbiol. Rev. 1996;60(3):539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T., Jensen P. Ø., Fiandaca M. J., Pedersen J., Hansen C. R., Andersen C. B., Pressler T., Givskov M., Høiby N. Pediatr. Pulmonol. 2009;44(6):547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- Høiby N. BMC Med. 2011;9:32–32. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltshe S. L., Khan U., Beckett V., Baines A., Emerson J., Sanders D. B., Gibson R. L., Morgan W., Rosenfeld M. J. Cystic Fibrosis. 2018;17(3):341–347. doi: 10.1016/j.jcf.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt B., O'Brien L., Winn W. Am. J. Clin. Pathol. 2007;128(1):32–34. doi: 10.1309/KJRPC7DD5TR9NTDM. [DOI] [PubMed] [Google Scholar]
- Pressler T., Frederiksen B., Skov M., Garred P., Koch C., Høiby N. J. Cystic Fibrosis. 2006;5(1):9–15. doi: 10.1016/j.jcf.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Potera C. Environ. Health Perspect. 2010;118(7):A288. doi: 10.1289/ehp.119-a288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T. APMIS, Suppl. 2013;(136):1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- Lucca F., Guarnieri M., Ros M., Muffato G., Rigoli R., Da Dalt L. Clin. Respir. J. 2018;12(7):2189–2196. doi: 10.1111/crj.12787. [DOI] [PubMed] [Google Scholar]
- Manno G., Cruciani M., Romano L., Scapolan S., Mentasti M., Lorini R., Minicucci L. Int. J. Antimicrob. Agents. 2005;25(3):193–197. doi: 10.1016/j.ijantimicag.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Pang Z., Raudonis R., Glick B. R., Lin T.-J., Cheng Z. Biotechnol. Adv. 2019;37(1):177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Yayan J., Ghebremedhin B., Rasche K. PLoS One. 2015;10(10):e0139836. doi: 10.1371/journal.pone.0139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. W., Speert D. P. Drug Resist. Updates. 2000;3(4):247–255. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- Kohanski M. A., Dwyer D. J., Collins J. J. Nat. Rev. Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy A. E., Pierson E., Hung D. T. Nat. Chem. Biol. 2007;3(9):541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- Ventola C. L. P. T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- Tan S. Y., Tatsumura Y. Singapore Med. J. 2015;56(7):366–367. doi: 10.11622/smedj.2015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R. M., Halls G., Hu Y. Br. J. Pharmacol. 2011;163(1):184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Pediatr. Pulmonol. 2002;34(3):232–236. doi: 10.1002/ppul.10135. [DOI] [PubMed] [Google Scholar]
- Fauvart M., De Groote V. N., Michiels J. J. Med. Microbiol. 2011;60(Pt 6):699–709. doi: 10.1099/jmm.0.030932-0. [DOI] [PubMed] [Google Scholar]
- Ferroni A., Guillemot D., Moumile K., Bernede C., Le Bourgeois M., Waernessyckle S., Descamps P., Sermet-Gaudelus I., Lenoir G., Berche P., Taddei F. Pediatr. Pulmonol. 2009;44(8):820–825. doi: 10.1002/ppul.21076. [DOI] [PubMed] [Google Scholar]
- Brown S. P., Cornforth D. M., Mideo N. Trends Microbiol. 2012;20(7):336–342. doi: 10.1016/j.tim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Ren B., Tong Y., Dai H., Zhang L. Virulence. 2015;6(4):362–371. doi: 10.1080/21505594.2015.1039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C. M., Bassler B. L. Annu. Rev. Cell Dev. Biol. 2005;21(1):319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Papenfort K., Bassler B. L. Nat. Rev. Microbiol. 2016;14:576. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ma S. ChemMedChem. 2017;12(6):420–425. doi: 10.1002/cmdc.201700015. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Iglewski B. H. J. Clin. Invest. 2003;112(10):1460–1465. doi: 10.1172/JCI20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M., Wu H., Andersen J. B., Riedel K., Rasmussen T. B., Bagge N., Kumar N., Schembri M. A., Song Z., Kristoffersen P., Manefield M., Costerton J. W., Molin S., Eberl L., Steinberg P., Kjelleberg S., Høiby N., Givskov M. EMBO J. 2003;22(15):3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Schaefer A. L., Dandekar A. A., Greenberg E. P. Proc. Natl. Acad. Sci. U. S. A. 2015;112(7):2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostylev M., Kim D. Y., Smalley N. E., Salukhe I., Greenberg E. P., Dandekar A. A. Proc. Natl. Acad. Sci. U. S. A. 2019;116(14):7027–7032. doi: 10.1073/pnas.1819796116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Surette M. G. J. Bacteriol. 2007;189(13):4827–4836. doi: 10.1128/JB.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M., Lee K. M., Greenberg E. P. Proc. Natl. Acad. Sci. U. S. A. 1999;96(24):13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal Jimenez P., Koch G., Thompson J. A., Xavier K. B., Cool R. H., Quax W. J. Microbiol. Mol. Biol. Rev. 2012;76(1):46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C., Echeverz M., Lasa I. Curr. Opin. Microbiol. 2014;18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh Corehtash Z., Khorshidi A., Firoozeh F., Akbari H., Mahmoudi Aznaveh A. Jundishapur J. Microbiol. 2015;8(10):e22345. doi: 10.5812/jjm.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owlia P., Nosrati R., Alaghehbandan R., Lari A. R. GMS Hyg. Infect. Control. 2014;9(2):Doc13. doi: 10.3205/dgkh000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Infect. Immun. 1980;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H.-C., Neu T. R., Wozniak D. J. J. Bacteriol. 2007;189(22):7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler B. L. Cell. 2002;109(4):421–424. doi: 10.1016/s0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Feldman M., Iglewski B. H., Prince A. Infect. Immun. 2000;68(7):4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Song Z., Givskov M., Doring G., Worlitzsch D., Mathee K., Rygaard J., Hoiby N. Microbiology. 2001;147(Pt 5):1105–1113. doi: 10.1099/00221287-147-5-1105. [DOI] [PubMed] [Google Scholar]
- Erickson D. L., Endersby R., Kirkham A., Stuber K., Vollman D. D., Rabin H. R., Mitchell I., Storey D. G. Infect. Immun. 2002;70(4):1783–1790. doi: 10.1128/IAI.70.4.1783-1790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D. N., Anderson L., McKnight S. L., Noah T. L., Knowles M., Boucher R., Schwab U., Gilligan P., Pesci E. C. FEMS Microbiol. Lett. 2002;215(1):41–46. doi: 10.1111/j.1574-6968.2002.tb11367.x. [DOI] [PubMed] [Google Scholar]
- Gökalsın B., Berber D. and Sesal N. C., Pseudomonas aeruginosa Quorum Sensing and Biofilm Inhibition, in Quorum Sensing, ed. G. Tommonaro, Academic Press, 2019, ch. 9, pp. 227–256. [Google Scholar]
- Pérez-Pérez M., Jorge P., Pérez Rodríguez G., Pereira M. O., Lourenço A. Biofouling. 2017;33(2):128–142. doi: 10.1080/08927014.2016.1272104. [DOI] [PubMed] [Google Scholar]
- Müh U., Hare B. J., Duerkop B. A., Schuster M., Hanzelka B. L., Heim R., Olson E. R., Greenberg E. P. Proc. Natl. Acad. Sci. U. S. A. 2006;103(45):16948–16952. doi: 10.1073/pnas.0608348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Nair S. K. Chem. Biol. 2009;16(9):961–970. doi: 10.1016/j.chembiol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Muimhneacháin E., Reen F. J., O'Gara F., McGlacken G. P. Org. Biomol. Chem. 2018;16(2):169–179. doi: 10.1039/c7ob02395b. [DOI] [PubMed] [Google Scholar]
- Kim B., ParK J.-S., Choi H.-Y., Kwak J.-H., Kim W.-G. Sci. Rep. 2019;9(1):7741. doi: 10.1038/s41598-019-44236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Sun S., Li L., Dai X., Li H., He Q., Zhu H. Bioorg. Chem. 2019;91:103140. doi: 10.1016/j.bioorg.2019.103140. [DOI] [PubMed] [Google Scholar]
- Feng C., Huang Y., He W., Cheng X., Liu H., Huang Y., Ma B., Zhang W., Liao C., Wu W., Shao Y., Xu D., Su Z., Lu W. ACS Cent. Sci. 2019;5(7):1278–1288. doi: 10.1021/acscentsci.9b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abinaya M., Gayathri M. Bioorg. Chem. 2019;87:291–301. doi: 10.1016/j.bioorg.2019.03.050. [DOI] [PubMed] [Google Scholar]
- Vadakkan K., Hemapriya J., Selvaraj V. Clin. Phytosci. 2019;5(1):24. [Google Scholar]
- Furiga A., Lajoie B., El Hage S., Baziard G., Roques C. Antimicrob. Agents Chemother. 2015;60(3):1676–1686. doi: 10.1128/AAC.02533-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti D., Trapella C., Bragonzi A., Marchetti P., Zanirato V., Alogna A., Gentili V., Cervellati C., Valacchi G., Sicolo M., Turrin G., Fantinati A., Di Luca D., Rizzo R. J. Chem. 2019;2019:13. [Google Scholar]
- Chanda W., Joseph T. P., Padhiar A. A., Guo X., Min L., Wang W., Lolokote S., Ning A., Cao J., Huang M., Zhong M. Exp. Ther. Med. 2017;14(5):4328–4338. doi: 10.3892/etm.2017.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman G., Cos P., Maes L., Nelis H. J., Coenye T. Antimicrob. Agents Chemother. 2011;55(6):2655–2661. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Xing H., Ye J. Metabolism. 2008;57(5):712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. H., Kim K. J., Cha J. D., Kim H. K., Lee Y. E., Choi N. Y., You Y. O. J. Med. Food. 2005;8(4):454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang J., Li L., Liu L. Cell. Physiol. Biochem. 2017;42(4):1657–1669. doi: 10.1159/000479411. [DOI] [PubMed] [Google Scholar]
- Fong J., Mortensen K. T., Nørskov A., Qvortrup K., Yang L., Tan C. H., Nielsen T. E., Givskov M. Front. Cell. Infect. Microbiol. 2019;8(443) doi: 10.3389/fcimb.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba S. P., Bhatnagar A. Curr. Opin. Toxicol. 2018;7:133–139. doi: 10.1016/j.cotox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann A., de Mello Martins A. G. G., Brengel C., Empting M., Hartmann R. W. ACS Chem. Biol. 2016;11(5):1279–1286. doi: 10.1021/acschembio.6b00117. [DOI] [PubMed] [Google Scholar]
- Maura D., Rahme L. G. Antimicrob. Agents Chemother. 2017;61(12):e01362. doi: 10.1128/AAC.01362-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari A., Noshiranzadeh N., Haghi F., Bikas R. Microb. Pathog. 2017;112:103–110. doi: 10.1016/j.micpath.2017.09.043. [DOI] [PubMed] [Google Scholar]
- Rajkumari J., Borkotoky S., Reddy D., Mohanty S. K., Kumavath R., Murali A., Suchiang K., Busi S. Microbiol. Res. 2019;226:19–26. doi: 10.1016/j.micres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Xu X.-J., Zeng T., Huang Z.-X., Xu X.-F., Lin J., Chen W.-M. J. Nat. Prod. 2018;81(12):2621–2629. doi: 10.1021/acs.jnatprod.8b00315. [DOI] [PubMed] [Google Scholar]
- Al-Youssef H., Ahmed A., Al-Shabib N. A., Laeeq S., Khan R. A., Mt R., Alsalme A., Alajmi M., Khan M., Husain F. Front. Microbiol. 2017;8:1675. doi: 10.3389/fmicb.2017.01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilburg Bernardes E., Charron-Mazenod L., Reading D. J., Reckseidler-Zenteno S. L., Lewenza S. Antimicrob. Agents Chemother. 2017;61(5):e01997. doi: 10.1128/AAC.01997-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Storek K. M., Ledvina H. E., Coulon C., Marmont L. S., Sadovskaya I., Secor P. R., Tseng B. S., Scian M., Filloux A., Wozniak D. J., Howell P. L., Parsek M. R. Proc. Natl. Acad. Sci. U. S. A. 2015;112(36):11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin K. M., Gordon V. D., Murakami K., Borlee B. R., Wozniak D. J., Wong G. C. L., Parsek M. R. PLoS Pathog. 2011;7(1):e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y., Borlee B. R., O'Connor J. R., Hill P. J., Harwood C. S., Wozniak D. J., Parsek M. R. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20632–20636. doi: 10.1073/pnas.1217993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Hu Y., Liu Y., Zhang J., Ulstrup J., Molin S. Environ. Microbiol. 2011;13(7):1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- van Tilburg Bernardes E., Charron-Mazenod L., Reading D. J., Reckseidler-Zenteno S. L., Lewenza S. Antimicrob. Agents Chemother. 2017;61(5):e01997-16. doi: 10.1128/AAC.01997-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterenko L. N., Zigangirova N. A., Zayakin E. S., Luyksaar S. I., Kobets N. V., Balunets D. V., Shabalina L. A., Bolshakova T. N., Dobrynina O. Y., Gintsburg A. L. J. Antibiot. 2016;69:422. doi: 10.1038/ja.2015.131. [DOI] [PubMed] [Google Scholar]
- Diepold A., Armitage J. P. Philos. Trans. R. Soc., B. 2015;370(1679):20150020. doi: 10.1098/rstb.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. R. Crit. Care Med. 2011;39(9):2193–2194. doi: 10.1097/CCM.0b013e318221742d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharajah A., Buyck J. M., Sundin C., Tulkens P. M., Mingeot-Leclercq M.-P., Van Bambeke F. Antimicrob. Agents Chemother. 2017;61(6):e02566. doi: 10.1128/AAC.02566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko J., Westerlund-Wikström B. Biology. 2013;2(4):1242–1267. doi: 10.3390/biology2041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. M., Schmiel D. H., Miller V. L. Proc. Natl. Acad. Sci. U. S. A. 1999;96(11):6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corech R., Rao A., Laxova A., Moss J., Rock M. J., Li Z., Kosorok M. R., Splaingard M. L., Farrell P. M., Barbieri J. T. J. Clin. Microbiol. 2005;43(8):3956–3962. doi: 10.1128/JCM.43.8.3956-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinaud M., Chabert J., Faudry E., Neumann E., Lemaire D., Pastor A., Elsen S., Dessen A., Attree I. J. Biol. Chem. 2005;280(43):36293–36300. doi: 10.1074/jbc.M508089200. [DOI] [PubMed] [Google Scholar]
- Kurahashi K., Kajikawa O., Sawa T., Ohara M., Gropper M. A., Frank D. W., Martin T. R., Wiener-Kronish J. P. J. Clin. Invest. 1999;104(6):743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. R. Nat. Rev. Microbiol. 2009;7:654. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareechon C., Zmina S. E., Karmakar M., Pearlman E., Rietsch A. Cell Host Microbe. 2017;21(5):611–618.e5. doi: 10.1016/j.chom.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani S. H., Engel J. Proc. Natl. Acad. Sci. U. S. A. 2006;103(42):15605–15610. doi: 10.1073/pnas.0605949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai P., Berry L., Moussouni M., Bleves S., Blanc-Potard A.-B. PLoS Pathog. 2019;15(6):e1007812. doi: 10.1371/journal.ppat.1007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr T. L., Wolfgang M. C. Mol. Microbiol. 2006;62(3):631–640. doi: 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- Maura D., Ballok A. E., Rahme L. G. Curr. Opin. Microbiol. 2016;33:41–46. doi: 10.1016/j.mib.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschiol S., Normark S., Henriques-Normark B., Subtil A. BMC Microbiol. 2009;9(1):75. doi: 10.1186/1471-2180-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal A. K. J., Sundin C., Blocker A. J. J. Bacteriol. 2009;191(2):563–570. doi: 10.1128/JB.01004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist P.-A., Gylfe Å., Hägglund U., Lindström P., Norberg-Scherman H., Sundin C., Elofsson M. Bioorg. Med. Chem. Lett. 2012;22(10):3550–3553. doi: 10.1016/j.bmcl.2012.03.096. [DOI] [PubMed] [Google Scholar]
- Sheremet A. B., Zigangirova N. A., Zayakin E. S., Luyksaar S. I., Kapotina L. N., Nesterenko L. N., Kobets N. V., Gintsburg A. L. BioMed Res. Int. 2018;2018:5810767. doi: 10.1155/2018/5810767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharajah A., Mingeot-Leclercq M.-P., Van Bambeke F. Trends Pharmacol. Sci. 2016;37(9):734–749. doi: 10.1016/j.tips.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan M. C., Linington R. G., Auerbuch V. Antimicrob. Agents Chemother. 2012;56(11):5433–5441. doi: 10.1128/AAC.00975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremet A. B., Zigangirova N. A., Zayakin E. S., Luyksaar S. I., Kapotina L. N., Nesterenko L. N., Kobets N. V., Gintsburg A. L. BioMed Res. Int. 2018;2018:13. doi: 10.1155/2018/5810767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepali K., Lee H.-Y., Liou J.-P. J. Med. Chem. 2019;62(6):2851–2893. doi: 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- Miao E. A., Ernst R. K., Dors M., Mao D. P., Aderem A. Proc. Natl. Acad. Sci. U. S. A. 2008;105(7):2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. C., Whiteley M. Microbiol. Spectrum. 2015;3(4) doi: 10.1128/microbiolspec.MBP-0003-2014. [DOI] [PubMed] [Google Scholar]