Abstract
The progression of cardiovascular research is often impeded by the lack of reliable disease models that fully recapitulate the pathogenesis in humans. These limitations apply to both in vitro models such as cell-based cultures and in vivo animal models which invariably are limited to simulate the complexity of cardiovascular disease in humans. Implementing human heart tissue in cardiovascular research complements our research strategy using preclinical models. We established the Human Explanted Heart Program (HELP) which integrates clinical, tissue and molecular phenotyping thereby providing a comprehensive evaluation into human heart disease. Our collection and storage of biospecimens allow them to retain key pathogenic findings while providing novel insights into human heart failure. The use of human non-failing control explanted hearts provides a valuable comparison group for the diseased explanted hearts. Using HELP we have been able to create a tissue repository which have been used for genetic, molecular, cellular, and histological studies. This review describes the process of collection and use of explanted human heart specimens encompassing a spectrum of pediatric and adult heart diseases, while highlighting the role of these invaluable specimens in translational research. Furthermore, we highlight the efficient procurement and bio-preservation approaches ensuring analytical quality of heart specimens acquired in the context of heart donation and transplantation.
Abbreviations: ACE2, angiotensin converting enzyme 2; Ang II, angiotensin II; AT1R, Ang II type 1 receptor; CAD, coronary artery disease; CHD, congenital heart defects; CVD, cardiovascular diseases; DCM, dilated cardiomyopathy; EAT, epicardial adipose tissue; ECM, extracellular matrix; FA, fatty acid; FD, Fabry disease; HELP, Human Explanted Heart Program; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HOPE, Human Organ Procurement and Exchange program; LA/RA, left/right atria; LV/RV, left/right ventricles; LVAD, left ventricular assist device; MAHI, Mazankowski Alberta Heart Institute; MD, muscular dystrophy; MI, myocardial infarction; MMP, matrix metalloproteinase; NFC, non-failing control hearts; RAS, renin-angiotensin system; RNA-Seq, RNA-sequencing; TIMP, tissue inhibitor of metalloproteinase
Keywords: Tissue biobank, Human explanted heart, Heart failure, Pediatric heart diseases, Ventricular assist device, Translational research
Graphical abstract
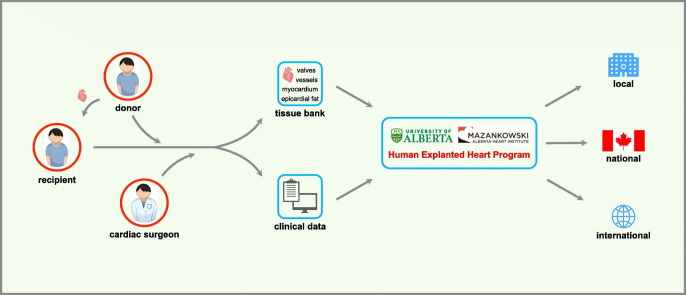
Illustration of the Human Explanted Heart Program (HELP) pathway as an integrative program that brings together clinicians and researchers aiming to unravel the underpinnings of cardiovascular disease and discover novel therapeutics. Our biobank contains tissue collections covering all sections of the explanted heart. A comprehensive review of the research participant clinical profile is also integrated into the analysis while complementary academic partnerships enabled us to broaden and diversify our approach to human heart disease.
Highlights
-
•
HELP - the largest research-integrated human heart transplant platform in Canada
-
•
Sizable specimen inventory of HELP enables research on a spectrum of heart diseases.
-
•
Systematic review of the methodology for HELP biobank management
-
•
Detailed evaluation on use of human hearts in translational cardiovascular medicine
1. Introduction
Cardiovascular diseases (CVD) remains the number one cause for mortality worldwide [1], which is projected to grow within the next decades resulting in enormous medical and societal burdens [2]. Heart failure (HF) is the final stage of structural and functional myocardial impairment due to varying etiologies [3]. It is characterized by activation of signaling pathways resulting in pathological remodeling, increased myocardial fibrosis, altered electrophysiology, and defective metabolite regulation leading to systolic and diastolic dysfunction [[4], [5], [6], [7], [8], [9], [10], [11]]. While medical and device therapies are effective against HF, many patients still progress to end-stage HF requiring left ventricular assist device (LVAD) and heart transplantation [[12], [13], [14], [15]]. However, organ supply from optimal donors severely limits transplantation as a therapeutic option, which causes high waitlist mortality [[16], [17], [18], [19], [20]]. Therefore, there is an urgent need for tailored and improved pharmacotherapies for HF.
Human heart biospecimens remain high on the agenda for mechanistic studies and drug discovery and validation when studying multifaceted human CVD [[21], [22], [23], [24]]. A biobank is a bio-depository that encompasses the collection and storage of biologic samples and associated biological and clinical data to be used for research [[25], [26], [27]]. Importantly, biobanking enables bi-directional approaches to discover novel targets for HF prospectively, or retrospectively verify existing hypotheses derived from previous experimental models. In this review, we outline the methodology and classification of our heart biobank, and we also illustrate the utility of our program for current and future applications to better elucidate the mechanism for human heart disease and to drive drug discovery.
2. Human Explanted Heart Program: overview & background
2.1. Tissue biobanking
The Mazankowski Alberta Heart Institute (MAHI) administers an extensive research-integrated human heart transplant program – the Human Explanted Heart Program (HELP). Established in 2010, HELP is a research partnership between basic scientists, clinicians, and clinician-scientists of the MAHI at the University of Alberta. The primary objective of this program was to serve as an integrative and sustainable platform for discovery, innovation, and improved patient care. Fundamental to this approach is the consistent collection of human explanted heart tissues over the past decade, such that our biobank now contains a breadth of heart diseases, which fosters ongoing collaborations [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37]]. Our biobank contains samples obtained from over 450 specimens with diverse phenotypes coupled with comprehensive clinical phenotypes. We have implemented quality control measures involving tissue collection, processing, biopreservation, and biobanking.
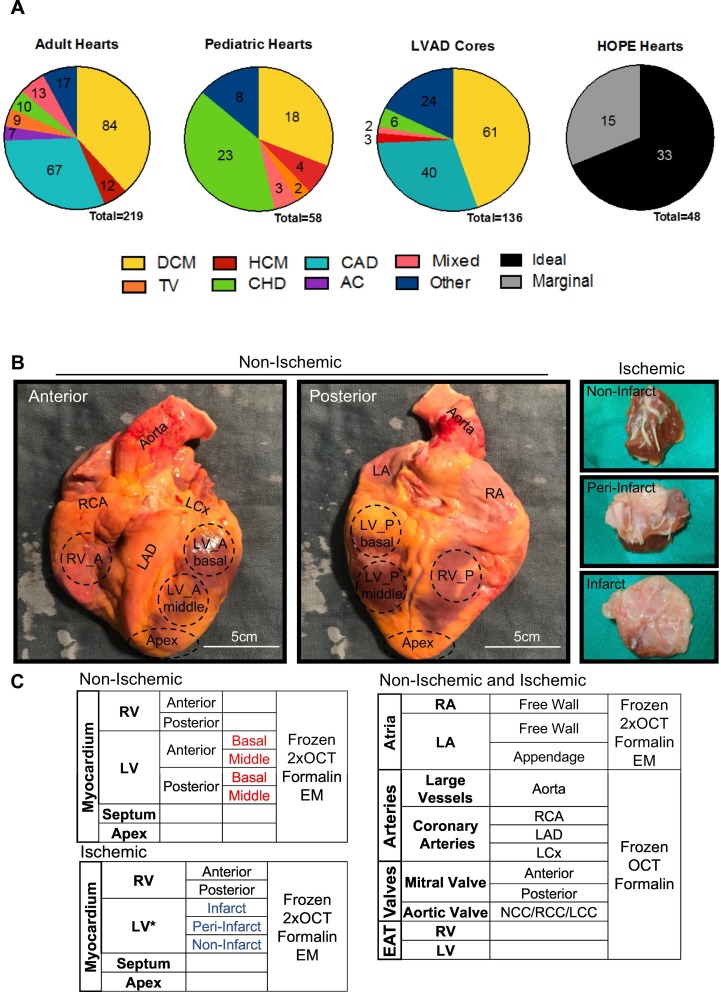
As of May 2020, the HELP heart biobank is primarily comprised of specimens representative of four categories: adult (n = 219) and pediatric (n = 58) failing native hearts, non-failing control hearts (NFC; n = 48), and left ventricular apical cores (n = 136; Fig. 1A). Specimen procurement is an ongoing effort, and as such our inventory continues to increase. For the adult cohort, dilated cardiomyopathy (DCM) remained the most common etiology, followed by coronary artery disease (CAD). Likewise, apical cores were collected from patients with similar etiologies at the time when they received left ventricular assist device (LVAD) as a bridge to heart transplant (Fig. 1A). Yet often, DCM in our inventory remains idiopathic or familial; we have collected a decent amount of specimens with identified cause(s) impairing the myocardium. Specifically, it includes exposure to toxins (i.e., illicit drugs, alcohol), anticancer chemotherapies (i.e., adriamycin), certain infectious diseases (i.e., viral myocarditis) and systemic conditions (e.g., transfusion iron-overload, peri-partum CM, diabetic and thyroid diseases). As for the pediatric specimens, congenital heart defects (CHD) constitute 40% of the collection with hypoplastic left heart syndrome (HLHS) and tetralogy of Fallot being the primary diagnoses (Fig. 1A). In addition to the HF specimens, HELP biobank has also compiled an inventory of the non-failing control (NFC) hearts that serve as the “control” group in the context of cardiovascular research. The NFCs consisted of non-diseased hearts from brain-dead donors obtained through the Human Organ Procurement and Exchange (HOPE) program, which were found unsuitable for transplantation due to various reasons but primarily due to blood type (ABO) and/or human leukocyte antigen (HLA) mismatch. Furthermore, the donor allografts were evaluated and stratified as ideal and marginal based on the reason the organ was declined for transplantation including assessment of clinical parameters (see Section 2.2 for more discussion; Figs. 1A; 2A).
Fig. 1.
Schematic of the collection and dissection of the explanted heart tissue and dissection. A. Etiology of heart disease in explanted hearts and apical cores. Numbers represent total hearts collected within that group as of May 2020. Mixed etiology describes patients that fit into multiple categories, such as DCM with CAD; other etiology describes conditions that do not fall into these categories, for example chemotherapy-induced and restrictive cardiomyopathies. B. Visual representation of the non-ischemic explanted heart sample collection (left) and the resultant tissue samples for the ischemic group (right). C. The range of distinct sample sections collected for each explanted heart with the myocardium collected differently according to the infarct area. DCM: dilated cardiomyopathy; CAD: coronary artery disease; HCM: hypertrophic cardiomyopathy; TV: transplant vasculopathy; CHD: congenital heart defects; AC: arrhythmogenic cardiomyopathy; LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery; LCC/RCC/NCC: left/right/non-coronary cusp, EAT: epicardial adipose tissue; OCT: optimal cutting temperature-mounted; EM: electron microscope.
Fig. 2.
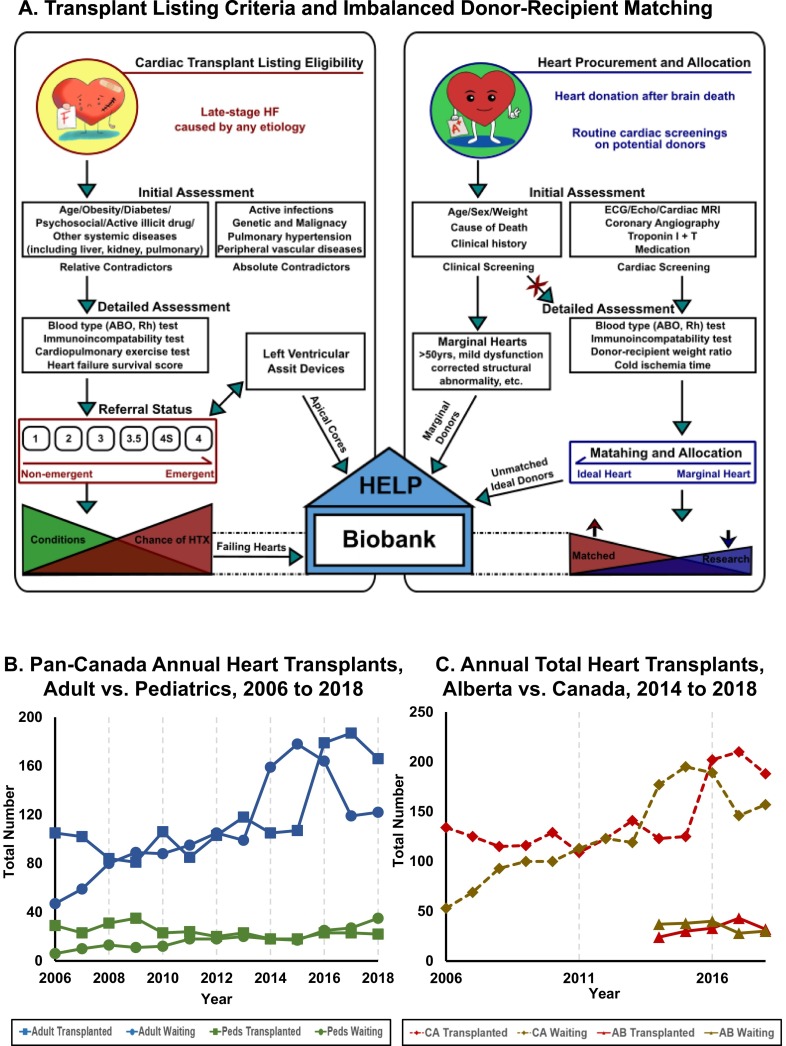
Transplant listing criteria and the relation to the Human Explanted Heart Program (HELP). A. Conceptual diagram depicting the heart transplant listing eligibilities for both recipients (left) and donors (right), and the sources of failing and non-failing hearts for HELP biobank. Left: Flow chart illustrating the eligibility, detailed evaluations, and referral status for being listed as heart transplant candidates. Right: The inclusion criteria, specific assessments, and types of donor hearts are delineated. Note that transplantations were limited by the scarcity of matched ideal donor hearts, with the potential to improve by extending the clinical acceptance of donor hearts. HELP biobank: Our heart pools are composed of native failing hearts from transplant recipients, the apical core from patients receiving an LVAD, and non-failing (namely marginal, and unmatched ideal) hearts. B. Imbalanced heart donor-recipient matching in Canada. The HF population was divided into adult (blue lines) and pediatric (green lines) subpopulations; accordingly, the statistics recorded the annual numbers of patients needing heart transplants (round marker) and the ones got transplanted (square marker) from 2006 to 2018. C. Clinical potentials of expanded application of “marginal hearts”. Annual total numbers of HF patients (including adult and pediatrics) were collected at both national (dash lines) and provincial (solid lines) levels. The data from 2014 to 2018 demonstrated the advantageous outcomes in terms of increased heart transplant cases (red lines) and simultaneously reduced number of patients awaiting (brown lines) at the national level, while the heart transplant rate remained relatively stable in Alberta during the past five years. Statistics were obtained from the Canadian Organ Replacement Register affiliated with the Canadian Institute for Health Information [39,40]. CA: Canada; AB: Alberta.
As of May 2020, over a total of 25,000 tissue samples from over 400 patients with HF, and over 50 healthy organ donors have been collected, catalogued and stored in the HELP biobank. Data collection from these hearts is based on the anatomic structure of the whole heart. Each heart was dissected into the left and right atria, left and right ventricles, interventricular septum, apex, coronary arteries, aorta, bicuspid and tricuspid valves, as well as the epicardial adipose tissue (Fig. 1B). We tailored our approach to the collection of myocardial samples and the dissection and collection of the myocardium was different based on ischemic versus non-ischemic etiologies. The myocardial specimens from the ischemic group were taken based on the position relative to the infarct area, while the myocardium from patients without myocardial infarction (MI) was procured based on anatomical location (basal, mid and apical regions) (Fig. 1B–C). The full-thickness biopsies of the myocardium and other structures were further divided longitudinally, followed immediately by freezing in liquid nitrogen as tissue samples and as tissue freezing medium (TFM)-embedded specimens. Samples were also collected for histological analysis and electron microscopy by being fixed in formalin or 2% glutaraldehyde, respectively.
2.2. Source of explanted failing and donor hearts
The failing explanted hearts in our collection comprise of the native hearts of transplant recipients. Therefore, our HELP inventory of specimens reflects the diagnoses seen in patients with advanced HF who are eligible for heart transplant (Figs. 1A; 2A) [18,38]. The high prevalence of cardiac diseases, together with improved survival in patients from advancements in medical and device therapies, have ultimately increased the number of patients with advanced HF. This has induced a supply-demand imbalance for cardiac transplantation, with the number of recipients far exceeding the number of available donor hearts (Fig. 2A). In Canada, for example, the annual number of HF patients (both adult and pediatric) on the waiting lists increased by almost three-fold from n = 47 adult and n = 6 pediatric in 2006 to n = 122 adult and n = 35 pediatric patients in 2018, according to the latest administrative data available from the longitudinal national database of the Canadian Organ Replacement Register (Fig. 2B) [[39], [40], [41]]. However, the number of heart transplants has climbed relatively slowly for adult patients with an upsurge only in the recent five years, and has even decreased for pediatric patients during the past thirteen years (Fig. 2B–C) [[39], [40], [41]].
To be eligible for heart transplantation, patients must pass rigorous criteria and thorough assessments, including considerations for relative or absolute contraindications (Fig. 2A) [18,42,43]. Following that, there are two types of assessments to guide the pre-transplant referral. The first includes compatibility tests for matching, such as blood types (e.g., ABO, Rh) and histocompatibility (i.e., HLA), whereas the second involves cardiopulmonary stress testing and prognostic assessments of HF survival (Fig. 2A) [18,43]. The other source of failing hearts (n = 9) in the HELP inventory is from patients who required re-transplantation due to transplant vasculopathy [33]. Heart transplantation cannot exist without organ donors. Occasionally, donor hearts are rejected for transplantation resulting in the organs being offered for research, thus our NFC collection via the HOPE program. This is a provincial organ procurement agency that facilitates the donation, allocation and recovery of the organs for transplantation, but also works jointly with HELP to coordinate the procurement of clinically exempted NFC hearts.
Following the procurement of donor organs, we classify the hearts into “ideal” or “marginal”, taking account of cardiac and extracardiac factors that prevented the donor hearts for being transplanted [[44], [45], [46], [47]]. To illustrate, a series of cardiac and clinical screenings (i.e., 12-lead electrocardiogram and echocardiogram) are performed routinely on the potential donors. Ideal donor hearts may be rejected from transplantation for extracardiac reasons (such as failed compatibility tests, mismatched donor-recipient size) and/or logistical limitations (i.e., extended cold ischemia time; Fig. 2A) [[44], [45], [46],48,49]. For the NFC classified as marginal hearts, cardiac (e.g., mild systolic dysfunction) and baseline clinical (namely advanced age and diabetes) conditions are contraindications for heart transplantation (Fig. 2A) [[44], [45], [46],48,49] in compliance to the nation-wide organ sharing and allocation agreement [18,38].
3. Human explanted heart procurement: technical features and quality control
3.1. Before collection
The HELP program encompasses Albertans and a wide coverage of out-of-province patients, including Saskatchewan, Manitoba, and northeastern British Columbia. Informed consent and/or assent were obtained from the patients (or power of attorney) in situations when patients were unable to consent or for pediatric patients less than seven years old. The HELP conforms to the ethical principles of the Declaration of Helsinki and has been approved by the institutional review committee and Health Research Ethics Board (HREB) at the University of Alberta. As for the non-diseased heart donors, informed consent was acquired from the family via the institutional HOPE protocol. The pre-collection preparation involved extensive labeling of aluminum foil in which dissected biopsies were wrapped and stored in −80 °C freezers or liquid N2, followed by aliquoting of different fixative solutions (e.g., formalin, 2% glutaraldehyde) for preserving tissues. This procedure was conducted by two trainees in the Alberta Cardiovascular and Stroke Research Center (ABACUS). The core laboratory within the ABACUS facility is a dedicated resource, which has allowed us to successfully launch this translational research project. The ABACUS facility has sufficient supplies and equipment to enable tissue collection, including a clean working bench with at least two scalpels, scissors, and forceps, as well as one reusable coronary artery probe with a bendable tip (d ~ 2 mm, for tracing the coronary arteries), metal plate, dissection block and surgical towels. Moreover, ABACUS is equipped with a large liquid N2 tank, ice maker machine, 4 °C fridge, and two freezers with the temperature set to either −80 °C or −20 °C. This setup is paramount to the rapid heart dissection and subsequent storage to maintain tissue integrity.
3.2. During collection
Two trainees with medical training background were on call 24/7 with the cardiac surgical team to procure each consecutively explanted heart. Trainees were informed about the transplant as soon as a donor heart became available, and they would be present on site to receive the explanted heart within 10 min of its explantation. Details of the dissection procedure varied slightly depending on the four collection types as indicated in the subsequent sections.
Failing hearts: Before excision, the beating hearts were perfused with ice-cold standard cardioplegic solution via the aortic root after cross-clamping of the aorta by the operating surgeons. The hypothermic cardioplegic solution containing high potassium washed out the remaining blood within the coronaries circulation and maintained the excised heart at a low metabolic state. Importantly, it prevented further myocardial injury during the temporary ischemic period. Once removed, the hearts were placed in a container with cold saline, surrounded by ice, for immediate transportation to the ABACUS core laboratory, which is directly connected to the cardiac operating theaters. The hearts were then immediately dissected and processed by two trainees within 10 min while maintained cooled on ice, after a prompt heart weight measurement and macroscopic examination (Fig. 3, Fig. 4 ). One trainee led the systematic dissection while the other processed the collected tissues. The overall integrity of the removed native heart was maintained to obtain a conventional pathological assessment.
Fig. 3.
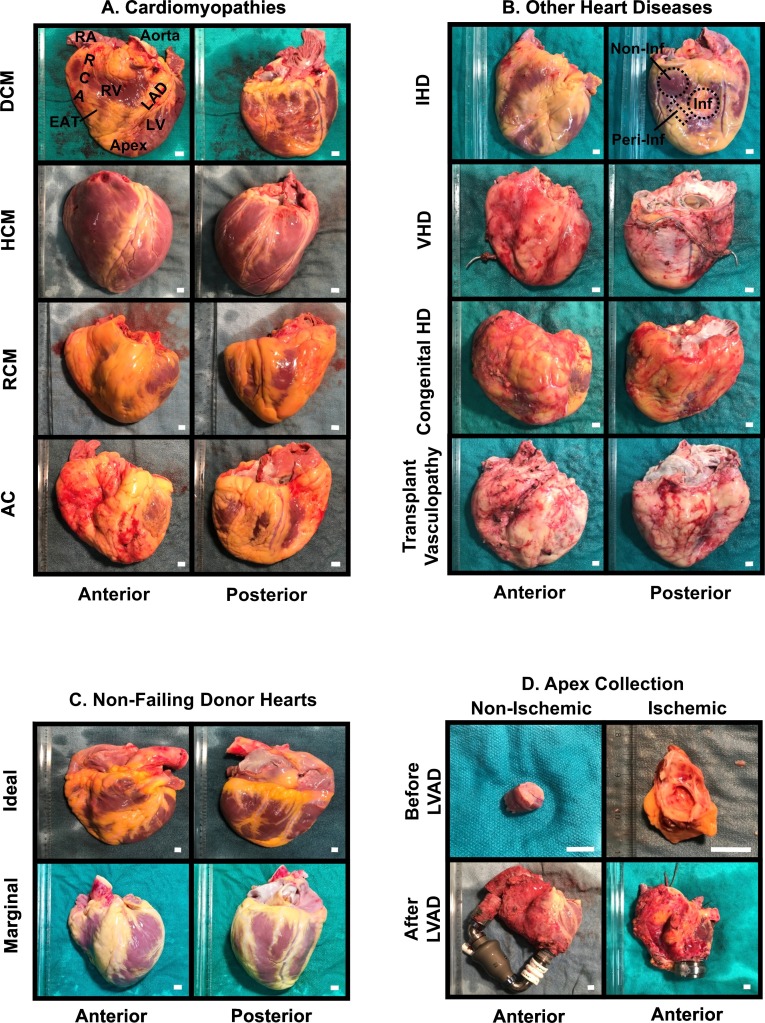
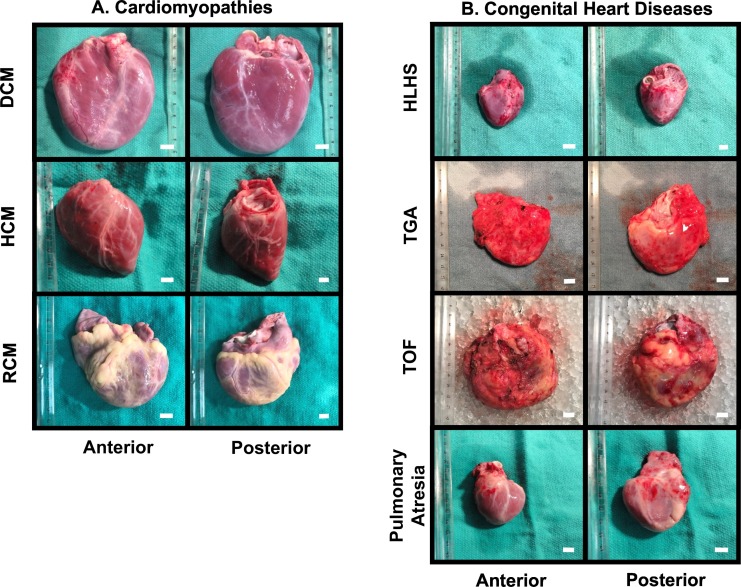
Adult human explanted heart gallery. A. Adult failing hearts with various types of cardiomyopathies including dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM) and arrhythmogenic cardiomyopathy (AC). B. Other etiologies that led to end-stage HF including ischemic heart disease (IHD), valvular heart disease (VHD), congenital heart defects (CHD) and transplant vasculopathy. C. Unmatched ideal hearts, and marginal hearts were harvested into our non-failing control pool. Age and gender-matched selection was adopted while seeking the best reference group in our research. D. Apex during LVAD insertion and native failing heart following transplant were both procured. Scale bar = 1 cm.
Fig. 4.
Pediatric human explanted heart gallery. A. The common types of cardiomyopathy contributing to pediatric HF such as dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), and restrictive cardiomyopathy (RCM). B. Congenital heart diseases with a variety of etiologies, such as hypoplastic left heart syndrome (HLHS), transposition of great artery (TGA), Tetralogy of Fallot (TOF), and pulmonary atresia. Scale bar = 1 cm.
Generally, all sections of the heart were harvested, including myocardium, coronary arteries, valves, larger vessels, and adipose tissues as described earlier in Section 2.1 (Figs. 1B; 3). Methodically, the myocardium was dissected into right and left atrium (both anterior and posterior walls) and its appendage, interventricular septum, left and right ventricles (anterior and inferolateral walls) and apex. The coronary arteries were divided into the left coronary arteries, left anterior descending artery and left circumflex artery, and right coronary artery. The valves included both atrioventricular (i.e., tricuspid and mitral) and semilunar (e.g., aortic and pulmonary) valves. Larger vessels contained ascending aorta, aortic arch and its branches (e.g., brachiocephalic trunk, left common carotid artery and left subclavian artery) and the descending aorta, when available. Lastly, the adipose tissues were procured from the epicardium from the left and right ventricles and around the epicardial coronary arteries. The ventricular free walls were collected based on either the physiological anatomy (apical, mid and basal segments) for the non-ischemic failing hearts and healthy donor hearts, or location relative to the pathological affected area (infarcted, peri-infarcted and non-infarcted regions) for hearts with CAD (Fig. 3A–C).
The tissue size that was obtained varied depending on the heart weight and shape, but the anatomical positions from where the tissues were captured remained consistent among samples. For instance, positions at approximately one-third and two-thirds below the aortic valves are where we captured the basal and middle ventricular free walls, respectively. As for the major coronary arteries, they were dissected with caution from the explanted hearts, and the vessels were dissected longitudinally to expose the endoluminal surface and cross-sectional landscape. Atherosclerotic lesions were identified and scored by inspecting through a dissecting microscope, and arteries would then be divided into 1.0- to 2.0-cm segments macroscopically designated as disease or disease-free segments. All samples were obtained as transmural biopsies and were further divided to be immediately flash-frozen or TFM-embedded frozen in liquid nitrogen. Meanwhile, the remaining full-thickness pieces were fixed in 10% formalin (containing approximate 4% formaldehyde) or 2% glutaraldehyde for long-term storage. As a bridge to transplantation, the LVAD-supported hearts were also collected when patients received heart transplantation. A 1.0-cm rim of fibrotic myocardium around the apical VAD insertion site was removed and discarded. These tissues offered the opportunity to study the effects of mechanical unloading on failing hearts (Fig. 3D) [34].
Non-failing hearts: The tissues were obtained in a similar fashion as noted above from organ donors deemed unsuitable for transplantation via the HOPE program. These hearts had no evidence of major functional or structural impairment and received 1 l of cold Celsior® cardioplegic solution into the coronary system following cross-clamping of the aorta (Fig. 3C). The excised donor hearts were placed in cold saline immediately following explanation and transported in ice-cool containers to the ABACUS core laboratory.
LVAD apical cores: As an integral part of the clinical care of HF patients [14,[50], [51], [52]], MAHI contains one of the largest VAD program in North America and involves both pediatric and adult patients (Fig. 1A). At the time of implantation of the LV apical inflow cannula, the apex was collected and divided into two halves: one frozen in liquid nitrogen and the other half further divided into two sections as formalin-fixed for pathological assessment and TFM-embedded for histological staining (Fig. 3D).
Pediatric failing hearts: A meticulous examination of the heart anatomy was conducted before each collection, as CHD were the leading causes of HF in pediatric cases, and they were characterized by various structural anomalies (Fig. 4). We have also collected some unique pediatric hearts with novel etiologies including Kawasaki disease. The tissues were also procured by two experienced team members within 10 min.
3.2.1. Cellular manipulation
Cryopreserved tissue has its limitation by virtue of the heterogeneous nature (as a mixture of diverse cell types) and the inability to perform experiments to examine specific cellular mechanisms or signaling pathways upon genetic or pharmacological manipulation. Alternatively, primary cells, which are derived and separated from tissue, provide an ideal model to assess the effects of drug candidates or chemical compounds in a specific cell type, such as cardiomyocytes, cardiofibroblasts and vascular smooth muscle cells. Further, adenoviral-based techniques can be utilized to inform potential candidates for gene therapy [32,[53], [54], [55], [56], [57], [58]].
Enzymatic tissue digestion is commonly applied to isolate human cardiomyocytes and cardiofibroblasts with satisfactory yield and viability. Excised myocardial tissue was submerged in the cold formulated Krebs-Henseleit solution that supported cell growth [59]. Cardiomyocytes were separated using a modified five-step isolation procedure as previously described [53,[59], [60], [61], [62], [63], [64], [65], [66]], which successfully yield viable rod-shaped atrial and/or ventricular cardiomyocytes. The isolation of cardiofibroblasts was performed according to previously described methodology with slight modifications [[53], [54], [55],65,67]. In brief, 1-gram myocardium from the ventricular or atrial free walls of the explanted failing and non-failing hearts were carefully chopped into several hundred pieces and minced, followed by washing in Ca2+-free buffer (pH 7.4, RT, 9 min), and enzymatic digestion in collagenase buffer with the addition of collagenase II (275 u/ml), protease XXIV (1.2 u/ml), and (S)-(−)-blebbistatin (25 μM) at 37 °C. Next, the digested cell suspension was filtered, and the cardiomyocyte-containing homogenate was centrifuged at 20g for 3 min to pellet the cardiomyocytes and harvest cardiofibroblasts in the supernatant fraction simultaneously [53,[63], [64], [65]]. This step was repeated for multiple times under carbogen (95%O2/5% CO2), and cell suspensions were removed by gauze filtration. Cardiomyocytes were identified by α-sarcomeric actin and F-actin staining [53]. To enrich for cardiofibroblasts, the suspension was centrifuged for 10 min at 300g, and the resulting pellet was re-suspended in Dulbecco's modified Eagle's medium (DMEM) and centrifuged twice before seeded into pre-warmed culture dishes [53,67]. Fibroblast-specific markers, such as vimentin and discoidin domain receptor 2, can be used to identify cardiofibroblasts while α-smooth muscle actin staining is a reflection of the myofibroblast phenotype [53,68]. Despite the short-lived lifespan of primary cultures, overexpression or selective knockout/knockdown of specific genes using adenoviral vector allows for the manipulation of the isolated primary cells and to examine the genotype-phenotype association [53,60,61,69].
3.3. After collection
A tissue-based diagnosis in human heart failure has been shown to be more accurate than the clinical-based diagnosis. In fact, in both American and Canadian based studies, there is 17% clinical misdiagnosis rate with the omission of the patients with CAD, and there was a 30% misdiagnosis rate for non-ischemic cardiomyopathy [70,71]. Considering this, all obtained explanted hearts underwent a thorough pathological examination by our co-investigator, who is a board-licensed cardiac pathologist on a routine basis. A close correlation with the clinical features of every patient would be available, including a comparison with the clinical diagnosis.
Clinical database. Clinical phenotyping is another essential part of our program. The primary investigators (practicing clinicians) have collected a detailed clinical profile of our patients undergoing heart transplant and/or LVAD insertions via chart review. Our extensive database included demographics, comorbidities, medications, risk factors, electrophysiology, and imaging (e.g., echocardiography, cardiac magnetic resonance imaging, diagnostic coronary angiogram). Right heart catheterization data was obtained to evaluate the degree of pulmonary hypertension, pulmonary capillary wedge pressure (LV filling pressure), and the relationship between RV and LV function. The information collected best represents the patient medical condition at the time the heart tissue was acquired. A secured, uniform coding system and strict encryption were employed to maintain confidentiality.
Quality control for biobanking management. Quality control ensures transparency, traceability, and reproducibility across multiple heart collections, which is of pivotal importance in translational research to minimize introducing new variables and given the vast heterogeneity between individuals. We reiterated that the quality assurance standards were strictly implemented throughout the whole process, from preparation to experimentation.
Firstly, uniform coding (A = adult, P = pediatric, L = LV apical core, and H = non-diseased controls) and acronym (Ant = anterior side, Post = posterior side, Mid = middle wall, Base = basal wall, App = appendage, etc.) minimized the systematic errors that could result from mislabeling or misidentification. Temperature and cold ischemia time were the other two critical variables that were rigorously controlled. In addition, refrigerant cold packs were usually avoided as the solidification of fundamental cytoplasm at a temperature below the freezing point of water was presumed to damage the living tissue cells [72,73]. By maintaining hypothermic conditions, this procedure curtails analytical artifacts from potential proteolytic degradation and unstable post-translational modifications [[74], [75], [76], [77]], in conjunction with swift translocation (~1–2 min) and rapid processing (<10 min) after removal (Fig. 5A).
Fig. 5.
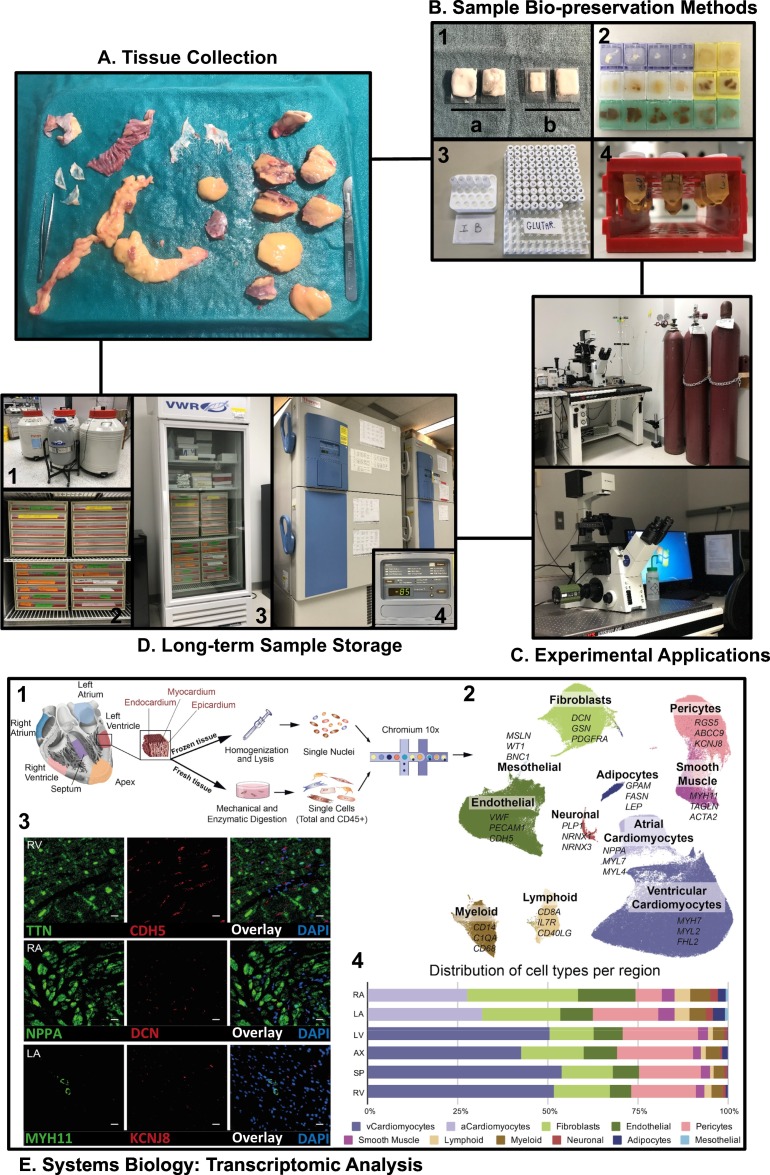
Quality control practices to manage and maintain the HELP biobank, and experimental applications. A. A visual representation of the dissected pieces of explanted heart tissues on a metal plate full of ice. B. Representative pictures showing optimized biopreservation methods leading to appropriately prepared TFM sample (1), paraffin-embedded blocks were systematically color-coded based on the structures (2), buffers for isolating cardiac cells or fixing tissues for histological examination (3), and embedded myocardial samples for ultrastructural study using an electron microscope (4). C. Experimental applications on the heart samples such as immunofluorescent light microscopy and confocal microscopy. D. A capture of the cryogenic long-term storage facility for appropriate depositing of different tissue samples. E. Systems biology analysis using advanced multi-omics techniques. Tissue sampling and processing (1), transcriptome from cells and nuclei delineating whole cardiac cell profiles by UMAP (2), spatial visualization of cell types probed by RNAscope (3), distribution of specific cell population after subclustering analysis (4). Modified from Litvinukova et al. [37] and reproduced with permission from Nature.
Secondly, we have gained plenty of experience to optimize the bio-preservation methods for subsequent quality-assured histopathological imaging (Fig. 5B). For example, OCT blocks were suspended on top of liquid nitrogen, after embedding the tissues in the cassettes with full TFM immersion, resulting in a slower freezing process without promoting crystallization or matrix cracking inside the tissues (Fig. 5B). For another, all the tissues were entirely soaked with different fixative buffers and nicely embedded in color-coded blocks for sectioning and mounting for immunofluorescent and immunohistochemical examinations (Fig. 5B–C); other details are omitted for the consideration of a concise body of work.
Thirdly, standard operating procedures for handling the frozen human heart specimens for RNA and protein extractions were well established. Cross-contamination between samples was avoided by decontaminating the cutting tools with 75% ethanol, followed by 10% bleach. To reduce the unpredictable impacts of warmth, samples were processed frozen with surrounding liquid nitrogen. Aliquots were prepared each time to avoid multiple freezing-thawing cycles, yet the total cycle times were tracked using the archival system that we developed for documenting sample usage, sharing, and retrieving information. Moreover, all frozen samples were safely stored in the cryogenic storage facility consisting of six −85 °C freezers and five liquid nitrogen tanks with a real-time temperature monitoring system (Fig. 5D).
4. Use and application of the explanted heart samples
The HELP program has offered the unique opportunity to study the correlates of tissue mechanisms in the context of the broader clinical phenotype of each patient. Specifically, we were able to acquire bio-molecular data from diseased myocardial samples to formulate and test hypotheses informed by our ongoing translational studies (i.e., sexually dimorphic responses post-MI, age-dependent or development-specific pathogenesis underlying DCM). Conversely, findings deciphered from the explanted hearts would be applied to gene-based approaches (e.g., adenovirus vector-mediated methods) and/or genetic models to manipulate specific pathways and to examine its downstream effects. As such, this bi-directional approach to translation research truly bridges a critical link to both basic science and to clinical medicine.
4.1. Systems biology
Multi-omics approaches, including genomics, transcriptomics, proteomics, and metabolomics were integrated into our analysis on the explanted human heart tissues. Compared with conventional techniques, systems biology provides a non-biased approach for the identification of novel tissue biomarkers and pathways implicated in CVD [[78], [79], [80]]. In this context, the multi-omics platform would facilitate the generation of a navigated “roadmap” connecting genotype to phenotype with potential clinical applicability for CVD patients.
Genomic analyses. The polygenetic nature of end-stage HF makes an unbiased, genome-wide analysis a useful tool to identify the underlying basis of heart disease [[81], [82], [83], [84]]. Slow progress has been made on understanding the genetic underpinnings of complex CVD due to confounding factors like extrinsic variables and genetic variation in susceptibility to disease [83]. Current technique for genotyping is based on gene panel based testing in which a predefined set of genes are examined in the diseased specimens. However, more advanced testing using whole-genome sequencing (WGS), for example, maps the entire genome, while whole-exome sequencing (WES) focuses on all the exon (protein-coding) regions in a genome. Though there has been a recent explosion in the number of genes responsible for cardiomyopathies, genotype-phenotype correlations are generally lacking, and studies on the underlying biological mechanisms for numerous mutations and gene variants need to be conducted [85].
Transcriptomic analyses. The study of transcriptome examines all sets of transcribed RNA molecules from protein-coding messenger RNA (mRNA) to noncoding RNAs such microRNA and long non-coding RNA (lncRNA). The realization of genome-wide profiling of heart diseases necessitates the incorporation of transcriptomic analysis as an integral part, as incomplete interpretation arises without transcription linking genetic information and proteomic expression [80,86]. With regard to techniques, there are two approaches applied most frequently: hybridization-based microarrays and RNA-sequencing (RNA-Seq). Microarrays are tools that detect the expression of thousands of genes using chips that contain pre-determined probes [87], and hybridization happens when the complementary DNA (reversely transcribed from mRNA) binds to the DNA probes yielding a specific fluorescent color and gene expression is represented as the color intensity after normalized to the corresponding controls [88]. As for analysis on a more global scale, RNA-Seq is more readily employed, where high-throughput sequencing methods (i.e., next-generation sequencing technologies) are used to provide insights into the transcriptomes of explanted heart tissue in an unbiased manner including single-cell and single-nucleus analyses [[89], [90], [91]]. In addition, RNA-Seq analysis provides an improved and expanded RNA profiling with higher specificity and sensitivity, especially for less abundant transcripts. RNA expression profiling has provided new insight into disease pathogenesis including transcriptome signatures of DCM hearts with ventricular arrhythmia [36,[92], [93], [94], [95]]; however, full knowledge of the broader repertoire of healthy hearts' molecular and genetic landscape is fundamental. To achieve that, we profiled both cells and nuclei from six distinct cardiac anatomical regions (apex, septum, left/right atria and ventricles) of the non-failing donor hearts, and constructed a most comprehensive transcriptome atlas as the reference framework to advance mechanistic exploration into heart diseases (Fig. 5E) [36,37,[93], [94], [95], [96]].
Proteomic analyses. Proteomics offer the capability to capture protein structures from whole proteome sequencing, identify post-translationally modified peptides and monitor the subcellular protein-protein interactions [[97], [98], [99], [100]]. Among them, post-translational modifications, including phosphorylation, glycosylation, acetylation and ubiquitination add further diversity to the proteome, which may offer a targetable approach to HF management including the identification of novel biomarkers and therapeutic targets [101].
Metabolomics and correcting metabolic defects. Dysregulated metabolism contributes to the progression of myocardial dysfunction in obesity, diabetes and HF in part due to changes in mitochondrial dynamics post-MI, or varied epigenomic remodeling (e.g., histone modification, DNA methylation, non-coding RNAs in obese-induced cardiomyopathy) [11,32,102,103]. A switch in energy supply from glucose metabolism to fatty acid β-oxidation occurs in heart disease [11], and accordingly, inhibiting fatty acid β-oxidation and stimulating the use of other fuels such as glucose and ketones is a promising therapeutic approach in HF [11,104]. Pyruvate dehydrogenase kinase isozyme 4 and malonyl-CoA decarboxylase inhibitors are being developed as novel therapies [105], which can be readily tested in human HF cardiomyocytes to examine changes in metabolism. Our metabolomics approach showed elevated branch chain amino acids levels in explanted failing human hearts indicating impaired cardiac branch chain amino acid catabolism in human heart failure, while enhancing branch chain amino acids oxidation improved cardiac function in the failing heart [106].
4.2. Reverse remodeling of the failing human heart: impact of LVAD therapy
Extensive studies alleged the beneficial adaptive or reverse remodeling upon LVAD implantation, but several questions remain unanswered such as identification of markers of recovery, genetic basis underlying disparate outcomes among patients, and longitudinal effects of mechanical circulatory support [34,59,61,[107], [108], [109], [110], [111], [112]]. The HELP program offers the ability to investigate them comprehensively on a large scale. We would prioritize the apical tissue taken at the time of LVAD insertion and compare with the LV myocardium procured from the same patient undergoing subsequent transplantation. In addition, genetic or proteomic markers underlying RV failure, a persistent limitation of current LVAD therapy [113], would also be incorporated into our analysis.
4.3. Targeted approaches of the HELP program
4.3.1. Enhancing angiotensin-converting enzyme 2 and apelin pathways as potential novel therapy for human HF
Activation of the systemic RAS and the generation of angiotensin II (Ang II) by angiotensin-converting enzyme (ACE) is major driver of cardiovascular diseases [[114], [115], [116]]. In contrast, ACE2 functions as a pleiotropic monocarboxypeptidase, which generates Ang 1–7 from Ang II [[117], [118], [119]] leading to activation of the widely expressed Mas receptor in the CV system [55,119,120]. ACE2 is the receptor for the SARS-CoV-2 and is associated with myocarditis, microvascular dysfunction, LV dysfunction in COVID-19 patients [116,121,122]. Therapeutic strategies focus on maintaining the balance between the two arms by either enhancing ACE2 (e.g., administration of recombinant human ACE2, rhACE2) or suppressing Ang II actions (i.e. blockade of AT1R, or ACE inhibition) in the context of HF, whereby we have gained substantial understandings from rodent models combined with human tissue explants [55,116,[123], [124], [125], [126], [127]]. Interestingly, an alternative ACE-independent pathway for converting Ang I to Ang II was found in the heart driven by the serine proteinase, chymase family [128,129]. In accordance, there is incomplete suppression of plasma Ang II levels in patients who took ACE inhibitors chronically [[130], [131], [132]], and using the HELP platform we demonstrated increased chymase protein levels and activity in explanted DCM hearts which likely contributed to the elevated tissue Ang II despite ACE inhibition [133].
Apelin is an endogenous family of peptides which binds and activates the G protein-coupled receptor, apelin receptor [134]. Similar to the AT1R, the apelin receptor is highly expressed in the cardiovascular system [134] and the apelin axis is proposed to have the opposite effects to the Ang II/AT1R pathway [135]. Apelin mediates a positive inotropic effect and by activating eNOS results in mild vasodilation and afterload reduction [136,137]. Loss of apelin could lead to increased mortality, greater adverse remodeling post-MI, exacerbated myocardial damage in response to myocardial ischemia-reperfusion injury, and higher susceptibility to vascular events such as abdominal aortic aneurysm and peripheral arterial diseases [58,138,139]. Apelin levels are decreased in the coronary arteries from explanted hears with myocardial infarction [140] but increased in aortic samples obtained from patients with aortic aneurysm [58]. The metalloprotease, neprilysin, is a major physiological enzyme, apart from ACE2, that degraded apelin peptides and inactivated the apelinergic system providing important insight into the therapeutic benefit of sacubitril/valsartan therapy in HF [134,141]. Enhancing the apelin/apelin receptor axis has emerged as the novel pathway for the treatment of cardiovascular diseases, such as obesity-associated cardiac hypertrophy and contractile dysfunction [142].
4.3.2. Correcting the dysregulated extracellular matrix (ECM): role of TIMPs
At the tissue level, cardiomyocytes are surrounded and supported by the ECM, which maintains stability and the architectural integrity of the myocardium. The matrix metalloproteinases (MMPs) are the predominant proteases that regulate the ECM homeostasis by degrading the ECM proteins. This process can be blocked physiologically by the tissue inhibitor of metalloproteinases (TIMPs) [[143], [144], [145], [146], [147]]. Maladaptive myocardial remodeling is characterized by an overall imbalance in ECM turnover, which can result in excess accumulation or disruption of the ECM structural proteins, mainly collagens, leading to impaired systolic performance and diastolic dysfunction in failing hearts [35,[148], [149], [150]]. In addition, insufficient ECM remodeling can lead to LV dilation and rupture, resulting in a high proportion of early sudden death post-MI [[151], [152], [153]]. Myocardial TIMP-1, TIMP-3 and TIMP-4 were reduced in patients having CAD and DCM, [154,155], while TIMP2 markedly increased in later-stage DCM patients [156].
Despite similar phenotypes characterized as systolic dysfunction and eccentric ventricular dilation, pediatric DCM is distinct from adult DCM. Accordingly, there are no proven effective therapies for HF in pediatric patients with DCM [157,158]. We discovered disparate remodeling patterns of the fibrillar and non-fibrillar ECM components, such as glycosaminoglycans and proteoglycan that exists between the pediatric and adult DCM groups [35]. This finding may underlie the pathological basis for differential fibrotic reverse remodeling and distinct affinity to the transforming growth factor-β pathway [35], which provides insights as to why pediatric HF patients were less responsive to HF therapies based on clinical trials in adults. The use of explanted failing hearts from children and adults provides an important opportunity to examine the ECM remodeling and its relationship with advanced HF.
5. Future directions of HELP program
5.1. Investigation of inherited cardiomyopathies
Our large and diverse collection of samples gives us the opportunity to investigate the pathophysiology and genetics associated with various cardiomyopathies [[159], [160], [161], [162]]. We have the unique access to LVAD cores and explanted hearts of patients diagnosed with Fabry disease (FD), muscular dystrophy (MD) and other types of genetic cardiomyopathies. FD is an X-linked recessive lysosomal storage disorder, identifiable by the accumulation of glycosphingolipids, leading to multisystem disease [163]. Cardiac complications associated with FD include arrhythmias, LV hypertrophy, and diastolic dysfunction; progression of heart disease in FD results in HFpEF [163,164]. Assessment of human heart tissue through histological staining and genetic sequencing will provide critical insights into the adverse remodeling pathways associated with FD as well as the HFpEF phenotype. MD describes a family of inherited neuromuscular diseases with systemic manifestations [160]. Heart disease, characterized by cardiomyopathy and arrhythmias, is recognized as the primary cause of morbidity and mortality in these patients [[165], [166], [167]]. At the cellular level, Duchenne MD and limb-girdle MD are characterized by the absence or dysfunction of critical cytoskeletal proteins, which leaves cardiomyocytes vulnerable to contractile and shearing forces [165]. Importantly, the correlation of our tissue findings to clinical characteristics could serve to improve patient prognosis through a precision medicine-based approach.
5.2. Epicardial adipose tissue and its relationship with heart disease
Adipose tissue of the heart can be divided into two distinct subsets: the epicardial adipose tissue (EAT) and the pericardial adipose tissue (PAT). The EAT is a visceral fat depot with anatomic proximity to the myocardium, located between the visceral pericardium and myocardium [[168], [169], [170], [171]]. In fact, the close relationship between the myocardium and EAT is exemplified in that they share a common microcirculation. Healthy EAT accounts for approximately 15% of total cardiac mass and primarily resides in the atrioventricular and interventricular grooves (Fig. 3C). However, in diseased states, EAT volume increases and expands to cover the ventricles and the entire epicardial surface [168,171,172] (Fig. 3A–B). Physiologically, the EAT maintains fatty acid (FA) homeostasis to both mobilize FA for oxidation, which in general meets 50–70% of the metabolic demand of the heart, as well as sequester excess FA to circumvent lipotoxicity [172,173]. Further, at physiological volumes, the EAT secretes anti-inflammatory cytokines and adipokines, including adiponectin, leptin, and apelin [174]. However, the pathological progression of CAD and HF is associated with a shift in the EAT secretasome, favoring a proinflammatory cytokine profile and excess release of FA [169,171,174,175]. Importantly, cardiac adipose tissue is limited in laboratory mice, and when present is restricted to the atrioventricular groove [28,176]. Therefore, this highlights the utility of our program to obtain and study EAT from explanted human hearts, offering an optimal platform to study EAT tissue and to unravel its close connection with human heart disease.
5.3. Cardiac electrophysiology and ventricular optical mapping
Ventricular arrhythmias and sudden cardiac death account for 50% of the mortality in patients with advanced HF [177]. Our studies have provided an improved understanding of the mechanism of ventricular fibrillation and the role of signaling pathways and late sodium current [178,179]. The MiCAM Ultima camera system incorporates novel image sensors with high-speed image acquisition while retaining maximal quantum efficiency, and the high signal-to-noise ratio allows for detection of activation times from the first derivative of optical signals during ventricular fibrillation without spatial averaging. We have recently described the genetic and electrophysiological characteristics of a patient with familial Long QT syndrome caused by missense mutations in KCNH2 [180]. The ability to characterize the electrophysiological changes at a cellular level coupled with the optical mapping of the electrical activation of the whole heart will offer unique insights into the mechanism of ventricular arrhythmias.
6. Conclusions
There are limitations at different stages of the HELP and HOPE programs that need to be acknowledged. Prior to collection, we are limited both by the time of organ procurement and the general heterogeneity of patients. HOPE donors spend an average of 4 days in hospital prior to the declaration of brain death while receiving ongoing therapy. The issue of heterogeneity is intrinsic to working with human samples; comorbidities, lifestyle, and pharmaceutical interventions in HF may affect experimental results. However, access to clinical data allows us to assess specimens and perform subsequent subgrouping. Further, the pathological effects of the adrenergic storm (associated with brain death) on the donor hearts can alter the tissue characteristics in the NFC hearts we have collected. Indeed, some of the data acquired from the control group might not be true representatives of the healthy heart in vivo. Following collection, the challenge of working exclusively with frozen clinical specimens or short-lived primary cardiomyocytes limits the analysis and exploration of dynamic biological mechanisms, which could be unraveled when combined with animal models. Lastly, our patient cohorts were all at end-stage HF and thus we captured a single time point of the entire disease course. Therefore, despite the limitations of our program, our human explanted heart tissue biobank constitutes a unique platform for cardiovascular translational research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the professional and multi-disciplinary collaborations between all the HELP team members, as well as the support from altruistic donors and their families. Also, we acknowledge the funding from the Canadian Institutes of Health Research (CIHR), Canada Research Chair (CRC) and Heart & Stroke Foundation to GYO. HZ is supported by the China Scholarship Council (CSC) Award.
References
- 1.Roth G.A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin E.J. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Ziaeian B., Fonarow G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016;13(6):368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E., Bristow M.R. Congestive heart failure: fifty years of progress. Circulation. 2000;102(20 Suppl 4):Iv14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 5.Hill J.A., Olson E.N. Cardiac plasticity. N. Engl. J. Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 6.Hunter J.J., Chien K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999;341(17):1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 7.Coronel R. Electrophysiological changes in heart failure and their implications for arrhythmogenesis. Biochim. Biophys. Acta. 2013;1832(12):2432–2441. doi: 10.1016/j.bbadis.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Oudit G.Y. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J. Mol. Cell. Cardiol. 2001;33(5):851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H. Role of iron metabolism in heart failure: from iron deficiency to iron overload. Biochim. Biophys. Acta. 2019;1865(7):1925–1937. doi: 10.1016/j.bbadis.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Oudit G.Y. L-type Ca 2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003;9(9):1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk G.D. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 12.Rossignol P. Heart failure drug treatment. Lancet. 2019;393(10175):1034–1044. doi: 10.1016/S0140-6736(18)31808-7. [DOI] [PubMed] [Google Scholar]
- 13.Kaye D.M., Krum H. Drug discovery for heart failure: a new era or the end of the pipeline? Nat. Rev. Drug Discov. 2007;6(2):127–139. doi: 10.1038/nrd2219. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter M.S. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 15.Drazner M.H. A new left ventricular assist device — better, but still not ideal. N. Engl. J. Med. 2018;378(15):1442–1443. doi: 10.1056/NEJMe1802639. [DOI] [PubMed] [Google Scholar]
- 16.Abouna G.M. Organ shortage crisis: problems and possible solutions. Transplant. Proc. 2008;40(1):34–38. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 17.Zaroff J.G. Maximizing use of organs recovered from the cadaver donor: cardiac recommendations. J. Heart Lung Transplant. 2002;21(11):1153–1160. [Google Scholar]
- 18.Isaac D. Cardiac Transplantation: Eligibility and Listing Criteria in Canada 2012. 2011. https://www.ccs.ca/en/cctn-resources Available from:
- 19.Rajab T.K., Singh S.K. Donation after cardiac death heart transplantation in America is clinically necessary and ethically justified. Circ. Heart Fail. 2018;11(3) doi: 10.1161/CIRCHEARTFAILURE.118.004884. [DOI] [PubMed] [Google Scholar]
- 20.Kirk R. ISHLT consensus statement on donor organ acceptability and management in pediatric heart transplantation. J. Heart Lung Transplant. 2020;39(4):331–341. doi: 10.1016/j.healun.2020.01.1345. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen Q., Lim K.R.Q., Yokota T. Genome editing for the understanding and treatment of inherited cardiomyopathies. Int. J. Mol. Sci. 2020;21(3):733. doi: 10.3390/ijms21030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strong A., Musunuru K. Genome editing in cardiovascular diseases. Nat. Rev. Cardiol. 2017;14(1):11–20. doi: 10.1038/nrcardio.2016.139. [DOI] [PubMed] [Google Scholar]
- 23.Perel P. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334(7586):197. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Worp H.B. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3) doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewitt R., Watson P. Defining biobank. Biopreserv. Biobank. 2013;11(5):309–315. doi: 10.1089/bio.2013.0042. [DOI] [PubMed] [Google Scholar]
- 26.Bartels P., Kotze A. Wildlife biomaterial banking in Africa for now and the future. J. Environ. Monit. 2006;8(8):779–781. doi: 10.1039/b602809h. [DOI] [PubMed] [Google Scholar]
- 27.Paskal W. Aspects of modern biobank activity - comprehensive review. Pathol. Oncol. Res. 2018;24(4):771–785. doi: 10.1007/s12253-018-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V.B. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65(1):85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitler K.M. Cardiac Med1 deletion promotes early lethality, cardiac remodeling, and transcriptional reprogramming. Am. J. Physiol. Heart Circ. Physiol. 2017;312(4):H768–H780. doi: 10.1152/ajpheart.00728.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall D.D. Ectopic expression of Cdk8 induces eccentric hypertrophy and heart failure. JCI insight. 2017;2(15) doi: 10.1172/jci.insight.92476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X. Endothelial and cardiomyocyte PI3Kβ divergently regulate cardiac remodelling in response to ischaemic injury. Cardiovasc. Res. 2019;115(8):1343–1356. doi: 10.1093/cvr/cvy298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponce Jessica M. Stress-induced cyclin C translocation regulates cardiac mitochondrial dynamics. J. Am. Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.119.014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masoud A.G. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Invest. 2019;130(1):94–107. doi: 10.1172/JCI128469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamuri S.S. Differential impact of mechanical unloading on structural and nonstructural components of the extracellular matrix in advanced human heart failure. Transl. Res. 2016;172:30–44. doi: 10.1016/j.trsl.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Jana S. Disparate remodeling of the extracellular matrix and proteoglycans in failing pediatric versus adult hearts. J. Am. Heart Assoc. 2018;7(19) doi: 10.1161/JAHA.118.010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parajuli N. Determinants of ventricular arrhythmias in human explanted hearts with dilated cardiomyopathy. Eur. J. Clin. Investig. 2015;45(12):1286–1296. doi: 10.1111/eci.12549. [DOI] [PubMed] [Google Scholar]
- 37.Litviňuková M. Cells of the adult human heart. Nature. 2020 doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddad H. Canadian Cardiovascular Society Consensus Conference update on cardiac transplantation 2008: executive summary. Can. J. Cardiol. 2009;25(4):197–205. doi: 10.1016/s0828-282x(09)70061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canadian Organ Replacement Register Organ Replacement in Canada: CORR Annual Statistics. 2019. https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2019 Available from:
- 40.Canadian Organ Replacement Register e-Statistics on Organ Transplants, Waiting Lists and Donors. 2019. https://www.cihi.ca/en/e-statistics-on-organ-transplants-waiting-lists-and-donors Available from:
- 41.Canadian Society of Transplantation Transplant Programs & OPOS. 2020. https://www.cst-transplant.ca/cgi/page.cgi/transplant-programs-opos.html Available from:
- 42.Conway J., Dipchand A.I. Heart transplantation in children. Pediatr. Clin. N. Am. 2010;57(2):353–373. doi: 10.1016/j.pcl.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Mehra M.R. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J. Heart Lung Transplant. 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Hu X.J. Status on heart transplantation in China. Chin. Med. J. 2015;128(23):3238–3242. doi: 10.4103/0366-6999.170238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abouna G.M. The use of marginal-suboptimal donor organs: a practical solution for organ shortage. Ann. Transplant. 2004;9(1):62–66. [PubMed] [Google Scholar]
- 46.Zaroff J.G. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28–29, 2001, Crystal City, Va. Circulation. 2002;106(7):836–841. doi: 10.1161/01.cir.0000025587.40373.75. [DOI] [PubMed] [Google Scholar]
- 47.Costanzo M.R. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010;29(8):914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 48.Kilic A. Donor selection in heart transplantation. J. Thorac. Dis. 2014;6(8):1097–1104. doi: 10.3978/j.issn.2072-1439.2014.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittwer T., Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl. Int. 2008;21(2):113–125. doi: 10.1111/j.1432-2277.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 50.Rose E.A. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 51.VanderPluym C.J. The use of ventricular assist devices in pediatric patients with univentricular hearts. J. Thorac. Cardiovasc. Surg. 2011;141(2):588–590. doi: 10.1016/j.jtcvs.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Feldman D. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J. Heart Lung Transplant. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Das S.K. Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci. Rep. 2015;5:18132. doi: 10.1038/srep18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo D. Loss of PI3Kγ enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ. Res. 2010;107(10):1275–1289. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- 55.Zhong J. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(7):717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. (18 p following 728) [DOI] [PubMed] [Google Scholar]
- 56.Shen M. Loss of smooth muscle cell disintegrin and metalloproteinase 17 transiently suppresses angiotensin II-induced hypertension and end-organ damage. J. Mol. Cell. Cardiol. 2017;103:11–21. doi: 10.1016/j.yjmcc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Shen M. Cell-specific functions of ADAM17 regulate the progression of thoracic aortic aneurysm. Circ. Res. 2018;123(3):372–388. doi: 10.1161/CIRCRESAHA.118.313181. [DOI] [PubMed] [Google Scholar]
- 58.Wang W. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc. Natl. Acad. Sci. U. S. A. 2019;116(26):13006–13015. doi: 10.1073/pnas.1900152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dipla K. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97(23):2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 60.Williams M.L. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109(13):1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 61.Perrino C. Dynamic regulation of phosphoinositide 3-kinase-gamma activity and beta-adrenergic receptor trafficking in end-stage human heart failure. Circulation. 2007;116(22):2571–2579. doi: 10.1161/CIRCULATIONAHA.107.706515. [DOI] [PubMed] [Google Scholar]
- 62.Nuss H.B., Houser S.R. Voltage dependence of contraction and calcium current in severely hypertrophied feline ventricular myocytes. J. Mol. Cell. Cardiol. 1991;23(6):717–726. doi: 10.1016/0022-2828(91)90981-q. [DOI] [PubMed] [Google Scholar]
- 63.Bird S.D. The human adult cardiomyocyte phenotype. Cardiovasc. Res. 2003;58(2):423–434. doi: 10.1016/s0008-6363(03)00253-0. [DOI] [PubMed] [Google Scholar]
- 64.del Monte F. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100(23):2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connell T.D., Rodrigo M.C., Simpson P.C. Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 66.Guo G.R. A modified method for isolation of human cardiomyocytes to model cardiac diseases. J. Transl. Med. 2018;16(1):288. doi: 10.1186/s12967-018-1649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neuss M. Isolation and characterisation of human cardiac fibroblasts from explanted adult hearts. Cell Tissue Res. 1996;286(1):145–153. doi: 10.1007/s004410050683. [DOI] [PubMed] [Google Scholar]
- 68.Doppler S.A. Cardiac fibroblasts: more than mechanical support. J. Thorac. Dis. 2017;9(Suppl. 1):S36–s51. doi: 10.21037/jtd.2017.03.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee C.S. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4(2):43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardehali H. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am. Heart J. 2004;147(5):919–923. doi: 10.1016/j.ahj.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Luk A. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can. J. Cardiol. 2009;25(2):e48–e54. doi: 10.1016/s0828-282x(09)70484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazur P. Freezing of living cells: mechanisms and implications. Am. J. Phys. 1984;247(3 Pt 1):C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 73.Michel S.G. Innovative cold storage of donor organs using the Paragonix Sherpa Pak ™ devices. Heart Lung Vessel. 2015;7(3):246–255. [PMC free article] [PubMed] [Google Scholar]
- 74.Jewell S.D. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am. J. Clin. Pathol. 2002;118(5):733–741. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 75.Spruessel A. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques. 2004;36(6):1030–1037. doi: 10.2144/04366RR04. [DOI] [PubMed] [Google Scholar]
- 76.Grizzle W.E., Bell W.C., Sexton K.C. Issues in collecting, processing and storing human tissues and associated information to support biomedical research. Cancer Biomark. 2010;9(1–6):531–549. doi: 10.3233/CBM-2011-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang J. Effects of ischemia on gene expression. J. Surg. Res. 2001;99(2):222–227. doi: 10.1006/jsre.2001.6195. [DOI] [PubMed] [Google Scholar]
- 78.Leon-Mimila P., Wang J., Huertas-Vazquez A. Relevance of multi-omics studies in cardiovascular diseases. Front. Cardiovasc. Med. 2019;6:91. doi: 10.3389/fcvm.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson C. Integrated multiomics approach to identify genetic underpinnings of heart failure and its echocardiographic precursors: Framingham heart study. Circ. Genom. Precis. Med. 2019;12(12) doi: 10.1161/CIRCGEN.118.002489. [DOI] [PubMed] [Google Scholar]
- 80.Rau C.D., Lusis A.J., Wang Y. Systems genetics for mechanistic discovery in heart diseases. Circ. Res. 2020;126(12):1795–1815. doi: 10.1161/CIRCRESAHA.119.315863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Donnell C.J., Nabel E.G. Genomics of cardiovascular disease. N. Engl. J. Med. 2011;365(22):2098–2109. doi: 10.1056/NEJMra1105239. [DOI] [PubMed] [Google Scholar]
- 82.Lee D.S. Association of parental heart failure with risk of heart failure in offspring. N. Engl. J. Med. 2006;355(2):138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 83.Ganesh S.K. Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association. Circulation. 2013;128(25):2813–2851. doi: 10.1161/01.cir.0000437913.98912.1d. [DOI] [PubMed] [Google Scholar]
- 84.Lopes L.R., Elliott P.M. Genetics of heart failure. Biochim. Biophys. Acta. 2013;1832(12):2451–2461. doi: 10.1016/j.bbadis.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Dainis A.M., Ashley E.A. Cardiovascular precision medicine in the genomics era. JACC Basic Transl. Sci. 2018;3(2):313–326. doi: 10.1016/j.jacbts.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedrotty D.M., Morley M.P., Cappola T.P. Transcriptomic biomarkers of cardiovascular disease. Prog. Cardiovasc. Dis. 2012;55(1):64–69. doi: 10.1016/j.pcad.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Napoli C. Microarray analysis: a novel research tool for cardiovascular scientists and physicians. Heart. 2003;89(6):597–604. doi: 10.1136/heart.89.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo G., Zegar C., Giordano A. Advantages and limitations of microarray technology in human cancer. Oncogene. 2003;22(42):6497–6507. doi: 10.1038/sj.onc.1206865. [DOI] [PubMed] [Google Scholar]
- 89.Pawlak M., Niescierowicz K., Winata C.L. Decoding the heart through next generation sequencing approaches. Genes. 2018;9(6):289. doi: 10.3390/genes9060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang K.C. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129(9):1009–1021. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 2020;22(1):108–119. doi: 10.1038/s41556-019-0446-7. [DOI] [PubMed] [Google Scholar]
- 92.Jo B.S. Data of methylome and transcriptome derived from human dilated cardiomyopathy. Data Brief. 2016;9:382–387. doi: 10.1016/j.dib.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hall C.L. RNA sequencing-based transcriptome profiling of cardiac tissue implicates novel putative disease mechanisms in FLNC-associated arrhythmogenic cardiomyopathy. Int. J. Cardiol. 2020;302:124–130. doi: 10.1016/j.ijcard.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haywood M.E. Transcriptome signature of ventricular arrhythmia in dilated cardiomyopathy reveals increased fibrosis and activated TP53. J. Mol. Cell. Cardiol. 2020;139:124–134. doi: 10.1016/j.yjmcc.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sweet M.E. Transcriptome analysis of human heart failure reveals dysregulated cell adhesion in dilated cardiomyopathy and activated immune pathways in ischemic heart failure. BMC Genomics. 2018;19(1):812. doi: 10.1186/s12864-018-5213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jo B.S. Methylome analysis reveals alterations in DNA methylation in the regulatory regions of left ventricle development genes in human dilated cardiomyopathy. Genomics. 2016;108(2):84–92. doi: 10.1016/j.ygeno.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Sharma P., Cosme J., Gramolini A.O. Recent advances in cardiovascular proteomics. J. Proteome. 2013;81:3–14. doi: 10.1016/j.jprot.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindsey M.L. Transformative impact of proteomics on cardiovascular health and disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):852–872. doi: 10.1161/CIR.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 99.Gramolini A.O. Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses. Mol. Cell. Proteomics. 2008;7(3):519–533. doi: 10.1074/mcp.M700245-MCP200. [DOI] [PubMed] [Google Scholar]
- 100.Van Eyk J.E. Overview: the maturing of proteomics in cardiovascular research. Circ. Res. 2011;108(4):490–498. doi: 10.1161/CIRCRESAHA.110.226894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lam M.P., Ping P., Murphy E. Proteomics research in cardiovascular medicine and biomarker discovery. J. Am. Coll. Cardiol. 2016;68(25):2819–2830. doi: 10.1016/j.jacc.2016.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhabyeyev P. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc. Res. 2013;97(4):676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y., Ren J. Epigenetics and obesity cardiomyopathy: from pathophysiology to prevention and management. Pharmacol. Ther. 2016;161:52–66. doi: 10.1016/j.pharmthera.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Neubauer S. The failing heart—an engine out of fuel. N. Engl. J. Med. 2007;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 105.Lopaschuk G.D. Metabolic abnormalities in the diabetic heart. Heart Fail. Rev. 2002;7(2):149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- 106.Uddin G.M. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 2019;18(1):86. doi: 10.1186/s12933-019-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y.Y. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation. 2001;104(10):1147–1152. doi: 10.1161/hc3501.095215. [DOI] [PubMed] [Google Scholar]
- 108.McCarthy P.M. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann. Thorac. Surg. 1995;59(3):609–613. doi: 10.1016/0003-4975(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 109.Nakatani S. Left ventricular echocardiographic and histologic changes: impact of chronic unloading by an implantable ventricular assist device. J. Am. Coll. Cardiol. 1996;27(4):894–901. doi: 10.1016/0735-1097(95)00555-2. [DOI] [PubMed] [Google Scholar]
- 110.Burkhoff D. Left ventricular assist device-induced reverse ventricular remodeling. Prog. Cardiovasc. Dis. 2000;43(1):19–26. doi: 10.1053/pcad.2000.7190. [DOI] [PubMed] [Google Scholar]
- 111.Heerdt P.M. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102(22):2713–2719. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 112.Drakos S.G. Reverse remodeling during long-term mechanical unloading of the left ventricle. J. Mol. Cell. Cardiol. 2007;43(3):231–242. doi: 10.1016/j.yjmcc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 113.Argiriou M. Right heart failure post left ventricular assist device implantation. J. Thorac. Dis. 2014;6(Suppl. 1):S52–S59. doi: 10.3978/j.issn.2072-1439.2013.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zaman M.A., Oparil S., Calhoun D.A. Drugs targeting the renin-angiotensin-aldosterone system. Nat. Rev. Drug Discov. 2002;1(8):621–636. doi: 10.1038/nrd873. [DOI] [PubMed] [Google Scholar]
- 115.Wang W. Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 2012;17(4–5):683–691. doi: 10.1007/s10741-011-9259-x. [DOI] [PubMed] [Google Scholar]
- 116.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142(5):426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 117.Crackower M.A. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110(6):737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 118.Oudit G.Y. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc. Med. 2003;13(3):93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 119.Patel V.B. Role of the ACE2/Angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bodiga S. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc. Res. 2011;91(1):151–161. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oudit G.Y. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gheblawi M. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo J. Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar–Kyoto rats and spontaneously hypertensive rats. Exp. Physiol. 2013;98(1):109–122. doi: 10.1113/expphysiol.2012.067165. [DOI] [PubMed] [Google Scholar]
- 124.Oudit G.Y. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007;75(1):29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 125.Patel V.B. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1–7 in experimental heart failure in angiotensin-converting enzyme 2–null mice. Hypertension. 2012;59(6):1195–1203. doi: 10.1161/HYPERTENSIONAHA.112.191650. [DOI] [PubMed] [Google Scholar]
- 126.Oudit G.Y., Penninger J.M. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr. Heart Fail. Rep. 2011;8(3):176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 127.Patel V.B. Antagonism of angiotensin 1–7 prevents the therapeutic effects of recombinant human ACE2. J. Mol. Med. (Berl.) 2015;93(9):1003–1013. doi: 10.1007/s00109-015-1285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urata H. Angiotensin II-forming pathways in normal and failing human hearts. Circ. Res. 1990;66(4):883–890. doi: 10.1161/01.res.66.4.883. [DOI] [PubMed] [Google Scholar]
- 129.Urata H. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J. Biol. Chem. 1990;265(36):22348–22357. [PubMed] [Google Scholar]
- 130.Juillerat L. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension. 1990;16(5):564–572. doi: 10.1161/01.hyp.16.5.564. [DOI] [PubMed] [Google Scholar]
- 131.van de Wal R.M. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int. J. Cardiol. 2006;106(3):367–372. doi: 10.1016/j.ijcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 132.Roig E. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur. Heart J. 2000;21(1):53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 133.Basu R. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J. Am. Coll. Cardiol. 2017;69(7):805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 134.Zhong J.C. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta. 2017;1863(8):1942–1950. doi: 10.1016/j.bbadis.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 135.Kazemi-Bajestani S.M. Targeting the ACE2 and apelin pathways are novel therapies for heart failure: opportunities and challenges. Cardiol. Res. Pract. 2012;2012:823193. doi: 10.1155/2012/823193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pitkin S.L. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010;62(3):331–342. doi: 10.1124/pr.110.002949. [DOI] [PubMed] [Google Scholar]
- 137.Japp A.G. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121(16):1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Z.Z. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension. 2017;70(6):1165–1175. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 139.Liang D. Therapeutic efficacy of apelin on transplanted mesenchymal stem cells in hindlimb ischemic mice via regulation of autophagy. Sci. Rep. 2016;6:21914. doi: 10.1038/srep21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang W. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J. Am. Heart Assoc. 2013;2(4) doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McKinnie S.M. The metalloprotease neprilysin degrades and inactivates Apelin peptides. Chembiochem. 2016;17(16):1495–1498. doi: 10.1002/cbic.201600244. [DOI] [PubMed] [Google Scholar]
- 142.Ceylan-Isik A.F. Apelin administration ameliorates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell. Cardiol. 2013;63:4–13. doi: 10.1016/j.yjmcc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 143.Baker A.H., Edwards D.R., Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 144.Moore L. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail. Rev. 2012;17(4–5):693–706. doi: 10.1007/s10741-011-9266-y. [DOI] [PubMed] [Google Scholar]
- 145.Kandalam V. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124(19):2094–2105. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 146.Basu R. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc. Res. 2013;98(3):360–371. doi: 10.1093/cvr/cvt067. [DOI] [PubMed] [Google Scholar]
- 147.Sakamuri S. Absence of Tissue Inhibitor of Metalloproteinase-4 (TIMP4) ameliorates high fat diet-induced obesity in mice due to defective lipid absorption. Sci. Rep. 2017;7(1):6210. doi: 10.1038/s41598-017-05951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spinale F.G. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 2007;87(4):1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 149.Zannad F., Rossignol P., Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail. Rev. 2010;15(4):319–329. doi: 10.1007/s10741-009-9143-0. [DOI] [PubMed] [Google Scholar]
- 150.Takawale A. Tissue inhibitor of matrix metalloproteinase-1 promotes myocardial fibrosis by mediating CD63-integrin β1 interaction. Hypertension. 2017;69(6):1092–1103. doi: 10.1161/HYPERTENSIONAHA.117.09045. [DOI] [PubMed] [Google Scholar]
- 151.Pouleur A.-C. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122(6):597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 152.Jugdutt B.I. Preventing adverse remodeling and rupture during healing after myocardial infarction in mice and humans. Circulation. 2010;122(2):103–105. doi: 10.1161/CIRCULATIONAHA.110.969410. [DOI] [PubMed] [Google Scholar]
- 153.Jugdutt B.I., Dhalla N.S. Springer Science & Business Media; 2013. Cardiac Remodeling: Molecular Mechanisms. [Google Scholar]
- 154.Li Y.Y. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98(17):1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 155.Tummalapalli C.M., Heath B.J., Tyagi S.C. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J. Cell. Biochem. 2001;80(4):512–521. doi: 10.1002/1097-4644(20010315)80:4<512::aid-jcb1005>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 156.Thomas C.V. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 157.Shaddy R.E. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 158.Hsu D.T. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122(4):333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shah S. Novel dominant-negative mutation in cardiac troponin I causes severe restrictive cardiomyopathy. Circ. Heart Fail. 2017;10(2) doi: 10.1161/CIRCHEARTFAILURE.116.003820. [DOI] [PubMed] [Google Scholar]
- 160.Miskew Nichols B. Advanced dilated cardiomyopathy in a patient with Hutterite limb-girdle muscular dystrophy: use of a left ventricular assist device. Circ. Heart Fail. 2018;11(4) doi: 10.1161/CIRCHEARTFAILURE.118.004960. [DOI] [PubMed] [Google Scholar]
- 161.Yogasundaram H. Glycogen storage disease because of a PRKAG2 mutation causing severe biventricular hypertrophy and high-grade atrio-ventricular block. Circ. Heart Fail. 2016;9(8) doi: 10.1161/CIRCHEARTFAILURE.116.003367. [DOI] [PubMed] [Google Scholar]
- 162.Yogasundaram H. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can. J. Cardiol. 2014;30(12):1706–1715. doi: 10.1016/j.cjca.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 163.Yogasundaram H. Clinical features, diagnosis, and management of patients with Anderson-Fabry cardiomyopathy. Can. J. Cardiol. 2017;33(7):883–897. doi: 10.1016/j.cjca.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 164.Yogasundaram H. Elevated inflammatory plasma biomarkers in patients with Fabry disease: a critical link to heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2018;7(21) doi: 10.1161/JAHA.118.009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nikhanj A. Cardiac intervention improves heart disease and clinical outcomes in patients with muscular dystrophy in a multidisciplinary care setting. J. Am. Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nikhanj A. Comparison of usefulness of cardiac resynchronization therapy in patients with type 1 myotonic dystrophy with versus without left bundle branch block. Am. J. Cardiol. 2019;124(11):1770–1774. doi: 10.1016/j.amjcard.2019.08.039. [DOI] [PubMed] [Google Scholar]
- 167.Nikhanj A. Ventricular tachycardia in patients with type 1 myotonic dystrophy: a case series. Eur. Heart J. Case Rep. 2019;3(2) doi: 10.1093/ehjcr/ytz095. (p. ytz095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iacobellis G., Willens H.J. Echocardiographic epicardial fat: a review of research and clinical applications. J. Am. Soc. Echocardiogr. 2009;22(12):1311–1319. doi: 10.1016/j.echo.2009.10.013. (quiz 1417-8) [DOI] [PubMed] [Google Scholar]
- 169.Talman A.H. Epicardial adipose tissue: far more than a fat depot. Cardiovasc. Diagn. Ther. 2014;4(6):416–429. doi: 10.3978/j.issn.2223-3652.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity. 2009;17(4):625. doi: 10.1038/oby.2008.575. (author reply 626-7) [DOI] [PubMed] [Google Scholar]
- 171.Rafeh R. Targeting perivascular and epicardial adipose tissue inflammation: therapeutic opportunities for cardiovascular disease. Clin. Sci. (Lond.) 2020;134(7):827–851. doi: 10.1042/CS20190227. [DOI] [PubMed] [Google Scholar]
- 172.Corradi D. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004;13(6):313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 173.Iacobellis G., Bianco A.C. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011;22(11):450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patel V.B. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail. Rev. 2017;22(6):889–902. doi: 10.1007/s10741-017-9644-1. [DOI] [PubMed] [Google Scholar]
- 175.Mazurek T. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 176.Yamaguchi Y. Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARgamma activation. Proc. Natl. Acad. Sci. U. S. A. 2015;112(7):2070–2075. doi: 10.1073/pnas.1417232112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Al-Khatib S.M. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018;72(14):e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 178.Witkowski F.X. Spatiotemporal evolution of ventricular fibrillation. Nature. 1998;392(6671):78–82. doi: 10.1038/32170. [DOI] [PubMed] [Google Scholar]
- 179.Zhabyeyev P. Inhibition of PI3Kinase-α is pro-arrhythmic and associated with enhanced late Na(+) current, contractility, and Ca(2+) release in murine hearts. J. Mol. Cell. Cardiol. 2019;132:98–109. doi: 10.1016/j.yjmcc.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 180.Mihic A. Trafficking defect and proteasomal degradation contribute to the phenotype of a novel KCNH2 long QT syndrome mutation. PLoS One. 2011;6(3):e18273. doi: 10.1371/journal.pone.0018273. [DOI] [PMC free article] [PubMed] [Google Scholar]