Abstract
Xenobiotic compounds are man-made compounds and widely used in dyes, drugs, pesticides, herbicides, insecticides, explosives, and other industrial chemicals. These compounds have been released into our soil and water due to anthropogenic activities and improper waste disposal practices and cause serious damage to aquatic and terrestrial ecosystems due to their toxic nature. The United States Environmental Protection Agency (USEPA) has listed several toxic substances as priority pollutants. Bacterial remediation is identified as an emerging technique to remove these substances from the environment. Many bacterial genera are actively involved in the degradation of toxic substances. Among the bacterial genera, the members of the genus Bacillus have a great potential to degrade or transform various toxic substances. Many Bacilli have been isolated and characterized by their ability to degrade or transform a wide range of compounds including both naturally occurring substances and xenobiotic compounds. This review describes the biodegradation potentials of Bacilli toward various toxic substances, including 4-chloro-2-nitrophenol, insecticides, pesticides, herbicides, explosives, drugs, polycyclic aromatic compounds, heavy metals, azo dyes, and aromatic acids. Besides, the advanced technologies used for bioremediation of environmental pollutants using Bacilli are also briefly described. This review will increase our understanding of Bacilli-mediated degradation of xenobiotic compounds and heavy metals.
Keywords: 4-Chloro-2-nitrophenol, naproxen, polycyclic aromatic hydrocarbons, cypermethrin, ibuprofen, Bacillus
Introduction
The genus Bacillus belongs to the family Bacillaceae that comprises of 293 species/subspecies (Patel and Gupta, 2020). This genus is characterized by a group of rod-shaped, Gram-positive, aerobic, or facultatively anaerobic, endospore-forming bacteria (Patel and Gupta, 2020). Members of the genus Bacillus are ubiquitous; they have been isolated from a variety of sources including soil, sewage sludge (Demharter and Hensel, 1989), ocean sediments (Ruger et al., 2000), saline water (Smibert and Krieg, 1994). They have the exceptional ability to grow very rapidly in high densities as well as to tolerate adverse environmental conditions. The genus Bacillus includes both non-pathogenic (free-living) and pathogenic (parasitic) species. Few examples of non-pathogenic species are Bacillus subtilis, B. licheniformis, B. amyloliquefaciens, and B. pumilus, which are closely related to each other. The pathogenic strains include B. anthracis, which causes anthracis in human beings and B. cereus that causes food poisoning (Claus and Berkeley, 1986).
Bacilli constitute a versatile group of bacteria, which have many applications in the field of health, environment, and agriculture. They produce several secondary metabolites including antibiotics and biosurfactants (Caulier et al., 2019). Furthermore, they are potential sources of industrial enzymes including lipases, proteases, alpha-amylase, and the BamH1 restriction enzyme (Latorre et al., 2016). Few species of Bacillus including B. thuringiensis, and some strains of B. sphaericus have insecticidal properties (Palma et al., 2014). Using the genetic engineering approach, the genes encoding insecticidal proteins in B. thuringiensis have been incorporated into corn and cotton plants to generate insect-resistance genetically modified crops (Jouzani et al., 2017). Some Bacillus species are ideal candidates for biological control due to their antagonistic activities against fungal and some bacterial pathogens (Wulff et al., 2002).
Bacilli are considered as potential bioremediator agents, which are capable of degrading several toxic substances (Arora et al., 2016; Singh and Singh, 2016; Xiao et al., 2017). Earlier studies have also been reported degradation of various xenobiotic compounds and heavy metals by the members of genus Bacillus (Birolli et al., 2016; Upadhyay et al., 2017; Arora et al., 2018; Díez-Méndez et al., 2019). Wang et al. (2019) reported the efficient biodegradation of petroleum hydrocarbons by B. subtilis BL-27. Viesser et al. (2020) isolated new petroleum-degrading strains of B. thuringiensis and B. subtilis from the rhizosphere of Panicum aquaticum. Both of these strains were able to utilize petroleum hydrocarbons as their sole source of carbon and energy. Bonifer et al. (2019) reported that B. pumilus B12 degrades poly-lactic acid that is the second most common biodegradable polymer found in commercial plastics. The transformation of 4-chloro-2-nitrophenol was extensively studied in many Bacilli (Arora et al., 2018). The ability of Bacilli to degrade polycyclic aromatic compounds, drugs, dyes, explosives have also been reported in the literature (Singh and Singh, 2016; Górny et al., 2019). These data indicated that Bacilli play a significant role in the biodegradation of toxic substances.
So far, several reviews have been published dealing with the biotechnological application of Bacilli (Bunk et al., 2010; Kumar et al., 2013; Jouzani et al., 2017; Sansinenea, 2019). Kumar et al. (2013) reviewed the significance of Bacilli for the production of biofuels, polyhydroxyalkanoates, and bioactive molecules. Sansinenea and Ortiz (2011) described the importance of secondary metabolites produced by Bacilli. Bunk et al. (2010) summarized the industrial applications of Bacillus megateriunm and other Bacilli. Sansinenea (2019) discussed the plant growth-promoting activities of Bacilli. Even though Bacill iare highly involved in biodegradation of various natural and xenobiotic compounds, a review on the biodegradation potential of Bacilli is rare. In the last decade, several researchers have been investigated the degradation abilities of Bacilli toward many toxic compounds. This review aims to summarize the role of Bacilli in the biodegradation process of various xenobiotic compounds and heavy metals.
Role of Bacillus Species in Biodegradation
Table 1 summarizes the role of various Bacilli in biodegradation of dyes, pesticides, herbicides, chlorophenols, nitrophenols, chloronitrophenols, heavy metals, drugs, explosives, crude oil waste, plastics, alkaline lignin, and other natural compounds. One of the following processes may involve in the degradation of toxic compounds by Bacilli: (i) Complete mineralization of toxic compounds, (ii) Co-metabolism of xenobiotics compounds. The mineralization involves complete utilization of toxic compounds by a Bacillus strain which utilized them as its sole source of carbon energy and converts them into CO2 and water (Arora et al., 2018). In the co-metabolism, Bacilli transform chemical compounds into other compounds which generally less toxic than parent compounds. Co-metabolism-based bioremediation is a non-growth linked biological process in which bacteria convert environmental pollutants to other substances in the presence of carbon source or growth substrate (Hazen, 2010). In this process, bacteria do not depend on the pollutants for growth and use non-specific enzymes to degrade environmental pollutants that do not support their growth (Hazen, 2010). In this section, the biodegradation potential of Bacilli toward a variety of xenobiotic compounds and heavy metals is discussed.
Table 1.
A list of members of the genus Bacillus, which have biodegradation potential.
| S. No. | Bacteria strain | Compounds | Remarks | References |
|---|---|---|---|---|
| A. Decolorization and transformation of Dyes | ||||
| 1. | Bacillus aryabhattai LN01 | Toluidine Blue | Decolorized Toluidine Blue in Lacaase-like and azoreductase-like reactions | Díez-Méndez et al., 2019 |
| 2 | Bacillus aryabhattai LN08 | Toluidine Blue, Remazol, and Brilliant Blue | Decolorized Toluidine Blue in Lacaase-like reaction and Remazol Brilliant Blue in Peroxidase-like reaction | Díez-Méndez et al., 2019 |
| 3. | Bacillus aryabhattai LN09 | Congo Red, Toluidine Blue, and Remazol Brilliant Blue | Decolorized Congo Red in Laccase-like reaction; Toluidine Blue in Azoreductase-like reaction and Remazol Brilliant Blue in Peroxidase like reaction | Díez-Méndez et al., 2019 |
| 4. | Bacillus aryabhattai LN10 | Toluidine Blue | Decolorized Toluidine Blue in Laccase-like and Azoreductase-like reactions | Díez-Méndez et al., 2019 |
| 5. | Bacillus aryabhattai LN15 | Congo Red, and Toluidine Blue | Decolorized Congo Red and Toluidine Blue in Laccase-like and Azoreductase-like reactions | Díez-Méndez et al., 2019 |
| 6. | Bacillus aryabhattai LN16 | Congo Red | Decolorized Congo Red by Peroxidase-like reaction | Díez-Méndez et al., 2019 |
| 7. | Bacillus megaterium LN30 | Congo Red | Decolorized Congo red by Azoreductase-like reaction | Díez-Méndez et al., 2019 |
| 8. | Bacillus aryabhattai LN37 | Congo Red, Toluidine Blue, and Remazol Brilliant Blue | Decolorized Congo red in Laccase like reaction; Remazol Brilliant Blue in Azoreductase-like reaction and Toluidine Blue in Laccase-like and Azoreducatase like reactions | Díez-Méndez et al., 2019 |
| 9. | Bacillus aryabhattai LN39 | Remazol Brilliant Blue, and Toluidine Blue | Decolorized Remazol Brilliant Blue and Toluidine Blue in Azoreductase-like reaction. | Díez-Méndez et al., 2019 |
| 10. | Bacillus aryabhattai LN41 | Remazol Brilliant Blue and Toluidine Blue | Decolorized Remazol Brilliant Blue and Toluidine Blue in Azoreductase like reduction | Díez-Méndez et al., 2019 |
| 11. | Bacillus aryabhattai LN49 | Remazol Brilliant | Decolorized Remazol Brilliant Blue in Laccase-like reaction | Díez-Méndez et al., 2019 |
| 12. | Bacillus aryabhattai LN61 | Toluidine Blue | Decolorized Toluidine Blue in Azoreductase-like reaction | Díez-Méndez et al., 2019 |
| 13. | Bacillus aryabhattai LN84 | Congo Red, and Remazol Brilliant Blue | Decolorized Congo Red in Laccase-like reaction and Remazol Brilliant Blue in Azoreductase and Laccase-like reactions | Díez-Méndez et al., 2019 |
| 14. | Bacillus aryabhattai LN87 | Toluidine Blue | Decolorized Toluidine Blue in Azoreductase and Laccase-like reactions | Díez-Méndez et al., 2019 |
| 15. | Bacillus aryabhattai LN88 | Toluidine Blue and Remazol Brilliant Blue | Decolorized Toluidine Blue in Peroxidase-like and Azoreducase-like reactions and Remazol Brilliant Blue in Azoreucatase-like reactions | Díez-Méndez et al., 2019 |
| 16. | Bacillus aryabhattai LN90 | Congo Red, Toluidine Blue, and Remazol Brilliant Blue | Decolorized Congo red in Laccase-like reaction; Toluidine Blue in Laccase-like and Azoredutase-like reactions and Remazol Brilliant Blue in Azoreductase-like reaction. | Díez-Méndez et al., 2019 |
| 17. | Bacillus sp. VUS | Brown 3REL | Transformed into 6,8-dichloro-quinazoline-4-ol and cyclopentanone. | Dawkar et al., 2008 |
| 18. | Bacillus sp. OY1-2 | Red B dye | Rapid biodegradation of Red B dye was observed in anoxic conditions as compared to aerobic conditions. | Li et al., 2004 |
| 19. | Bacillus firmus | Reactive Blue 160 | Decolorized dye (500 mg/l) and detoxify it. | Barathi et al., 2020 |
| 20. | Bacillus megaterium KY848339.1 | Acid red 337 dye | Degraded it via small aliphatic compounds and CO2 | Ewida et al., 2019 |
| 21. | Bacillus sp. BDN2 | Reactive Blue 160 | Degraded 65% dye | Balapurea et al., 2014 |
| 22. | Bacillus sp. BDN7 | Reactive Blue 160 | Degraded 80% within 12 h | Balapurea et al., 2014 |
| 23. | Bacillus sp. BDN8 | Reactive Blue 160 | Degraded 75% within 18 h | Balapurea et al., 2014 |
| 24. | Bacillus megaterium NCIM 2054 | Disperse Red 73 dye | 61% dye decolorization within 48 h | Kadam et al., 2014 |
| 25. | Bacillus cereus HJ-1 | Reactive Black B | Decolorized dye and detoxify it. | Liao et al., 2013 |
| 26. | Bacillus sp. YZU1 | Reactive Black 5 | 95% dye decolorization was observed in 120 h | Wang et al., 2013 |
| 27. | Bacillus sp. AK1 | Metanil Yellow | Degraded 200 mg/l dye within 27 h | Anjaneya et al., 2011 |
| 28. | Bacillus odyssey SUK3 | Reactive blue 59 | Decolorized dye (50 mg/l) completely within 60 h | Patil et al., 2008 |
| 29. | Bacillus cereus DC11q | Malachite green | Degraded to 4,4′-bis(dimethylamino)benzophenone and benzophenone | Deng et al., 2008 |
| 30. | Bacillus cereusDC11q | Acid Blue 25 | 95–98% dye (100 μM) decolorization within 6 h under anaerobic conditions | Deng et al., 2008 |
| 31. | Bacillus cereus DC11q | Basic Blue X-GRRL | Degraded via reduction of azo bonds. | Deng et al., 2008 |
| 32. | Bacillus fusiformis KMK5 | Disperse Blue 79, and Acid Orange 10 | Complete mineralization of dyes at the concentration of 1.5 g/L was observed within 48 h | Kolekar et al., 2008 |
| 33. | Bacillus subtilis IFO 13719 | Crystal Violet | Decolorized via 4,4'-bis(dimethylamino)benzophenone | Yatome et al., 1991 |
| 34. | Bacillus megaterium | Azo dye | Decolorized 98% dye | Shah et al., 2013 |
| 35. | Bacillus cereus | Azo dye | Decolorized 95% dye | Shah et al., 2013 |
| 36. | Bacillus pseudomycoides | Acid Black 24 | 96% of dye decolorization was achieved within 24 h. | Kumar et al., 2019 |
| 37. | Bacillus subtilis | Disperse yellow 211 | 80% dye (100 mg/) decolorization observed under optimum conditions. | Sharma et al., 2009 |
| 38. | Bacillus subtilis | Crystal violet | Decolorized dye (100 mg/l) effectively at pH 8 and temperature 35° C | Kochher and Kumar, 2011 |
| 39. | Bacillus cohnii MTCC 3616 | Direct Red-22 | 95% dye decolorization (5,000 mg l−1) was observed at 37° C and pH 9 in 4 h | Prasad and Rao, 2013 |
| 40. | Bacillus firmus | CI Direct Red 80 | Decolorized 50 mg/L of dye under anoxic conditions within 12 h | Ogugbue et al., 2012 |
| 41. | Bacillus licheniformis | Reactive Red 2 | Transformed it into 2, 4-dichloro-6-[(1H-indazol-5-ylimino)-methyl]-phenol, benzene sulfonamide, 1H indole and urea as final metabolites | Sudha and Balagurunathan, 2013 |
| 42. | Bacillus megaterium | Remazol Blue | Decolorized up to 5 mg/ml | Joshi et al., 2013 |
| 43. | Bacillus subtilis | RED M5B | Decolorization by the activity of peroxidase | Gunasekar et al., 2013 |
| 44. | Bacillus sp. VUS | Orange T4LL | transforms it into 4-methyl-2-o-tolylazo-benzene-1,3-diamine and [3-(phenyl-hydrazono)-cyclohexa-1,4-dienyl]-methanol | Dawkar et al., 2010 |
| 45. | Bacillus flexus | Remazol Black | Decolorized 100% of dye within 24 h | Saini et al., 2018 |
| 46. | Bacillus flexus | Direct Blue | Decolorized 100% of dye within 24 h | Saini et al., 2018 |
| 47. | Bacillus flexus | Acid Orange | Decolorized 100% of dye within 24 h | Saini et al., 2018 |
| B. Biodegradation of Pesticides, Herbicides, and Insecticides | ||||
| 48. | Bacillus subtilis strain 1D | Cypermethrin | Completely metabolized via cyclododecylamine, phenol, 3-(2,2-dichloroethenyl 2,2-dimethyl cyclopropane carboxylate,1-decanol, chloroacetic acid, acetic acid, cyclopentan palmitoleic acid, and decanoic acid | Gangola et al., 2018 |
| 49. | Bacillus sp. strain SG2 | Cypermethrin | Metabolized via Phenoxybenzaldehyde,2,2,3,3 tetramethylcyclopropanecarboxylic acid 4-propylbenzoate, 4-propylbenzaldehyde, phenol M-tert-butyl, and 1-dodecanol, | Pankaj et al., 2016 |
| 50. | Bacillus subtilis BSF01 | Cypermethrin | Metabolized via Phenoxybenzaldehyde, 2,2,3,3 tetramethylcyclopropanecarboxylic acid | Xiao et al., 2015 |
| 51. | Bacillus sp. AKD1 | Cypermethrin | Transformed Cypermethrin in presence of heavy metals | Tiwary and Dubey, 2016 |
| 52. | Bacillus sp. ISTDS2 | Cypermethrin | Metabolized Cypermethrin in soil microcosm via formation of cyclopropane, carboxylic acid, hydroxyacetonitrile, and benzene ethanamine | Sundaram et al., 2013 |
| 53. | Bacillus licheniformis B-1 | Cypermethrin | Degraded via 3-phenoxybenzoic acid | Lai et al., 2012 |
| 54. | Bacillus thuringiensis ZS-19 | Cyhalothrin | Degraded via α-hydroxy-3-phenoxy-benzeneacetonitrile, 3-phenoxyphenyl acetonitrile, N-(2-isoproxy-phenyl)-4-phenoxy-benzamide, 3-phenoxybenzaldehyde, 3-phenoxybenzoate, and phenol | Chen et al., 2015 |
| 55. | Bacillus cereus PU | Malathion | Degraded via malathion monocarboxylic and dicarboxylic acid | Singh et al., 2012 |
| 56. | Bacillus thuringiensis MOS-5 | Malathion | Degraded via malathion monocarboxylic and dicarboxylic acid | Zeinat et al., 2008 |
| 57. | Bacillus megaterium MCM B-423 | Monocrotophos | Degraded into carbon dioxide, ammonia, and phosphates | Bhadbhade et al., 2002 |
| 58. | Bacillus sp. N1 | Metribuzin | Used as a nitrogen source. | Zhang et al., 2014 |
| 59. | Bacillus alkalinitrilicus | Imidacloprid | Degraded via 6-chloronictinic acid nitrosamine | Sharma et al., 2014 |
| 60. | Bacillus subtilis Y242 | Chlorpyrifos | 96% degraded within 48 h | El-Helow et al., 2013 |
| 61. | Bacillus pumilus NY97-I | Carbendazim | 87.76% degradation | Zhang et al., 2009 |
| 62. | Bacillus cereus WD-2 | Prochloraz-manganese | 90.7% degradation at pH 8. | Jiang et al., 2019 |
| 63. | Bacillus sp. DG-02 | Fenpropathrin | Transformed into 3,4-dihydroxybenzoic acid, 3,4-dimethoxyphenol, and phenol | Chen et al., 2014 |
| 64. | Bacillus aryabhattai strain VITNNDJ5 | Monocrotophos | Degraded via three routes. | Dash and Osborne, 2020 |
| 65. | Bacillus firmus | Fipronil | Degraded via fipronil sulfide, fipronil sulfone and fipronil amide. | Mandal et al., 2013 |
| 66. | Bacillus sp. TAP-1 | Triazophos | Co-metabolized via hydrolyzing insecticide triazophos | Tang and You, 2012 |
| 67. | Bacillus pumilus W1 | Organophosphates | Hydrolysis of organophosphates by enzyme encoding by opdA | Ali et al., 2012 |
| 68. | Bacillus subtilis DR-39 | Profenofos | 4-Bromo-2-chlorophenol was identified as a metabolite | Salunkhe et al., 2013 |
| 69. | Bacillus subtilis CS-126, | Profenofos | 4-Bromo-2-chlorophenol was identified as a metabolite | Salunkhe et al., 2013 |
| 70. | Bacillus subtilis TL-171 | Profenofos | 4-Bromo-2-chlorophenol was identified as a metabolite | Salunkhe et al., 2013 |
| 71. | Bacillus subtilis TS-204 | Profenofos | 4-Bromo-2-chlorophenol was identified as a metabolite | Salunkhe et al., 2013 |
| 72. | Bacillus sp. strain C5 | Methyl Parathion | Hydrolyzedmethyl parathion to 4-nitrophenol and other metabolites | Hao et al., 2014 |
| 73. | Bacillus pumilus C2A1 | Chlorpyrifos | Degraded via 3,5,6-trichloro-2-pyridinol | Anwar et al., 2009 |
| 74. | Bacillus subtilis MTCC 8561 | Endosulfan and Endosulfan sulfate | Used as sulfur source and transformed to endosulfan diol and endosulfan lactone | Kumar et al., 2014 |
| 75. | Bacillus subtilis HB-6 | Atrazine | Mineralized via hydroxyatrazine, cyanuric acid, and urea | Wang et al., 2014 |
| 76. | Bacillus badius ABP6 | Atrazine | Optimum conditions of the atrazine degradation were determined | Khatoon and Rai, 2020 |
| 77. | Bacillus megaterium strain Q3 | Quinclorac | Transformed to 3, 7-dichloro-8-methyl-quinoline, 3-chlorin-8-quinoline-carboxylic and 8-quinoline-carboxylic | Liu et al., 2014 |
| 78. | Bacillus licheniformis CY-012 | Fenvalerate | Co-metabolized via α-isopropyl-4-chlorobenzene acetic acid, 4-chlorobenzene acetic acid, 3-phenoxybenzyl alcohol, phenol, and benzoic acid. | Tang et al., 2018 |
| 79. | Bacillus sp. 4T | Esfenvalerate | Transformed to (i) 3-2-(4-chlorophenyl)-3-methylbutyric acid), (ii) phenoxybenzoic acid, (iii) 2-(3-hydroxyphenyl)acetic acid | Birolli et al., 2016 |
| 80. | Bacillus sp. 2B | Esfenvalerate | Transformed to (i) 3-2-(4-chlorophenyl)-3-methylbutyric acid), (ii) phenoxybenzoic acid, hydroxy phenoxybenzoic acid and 3-hydroxybenzoic acid(iii) 2-(3-hydroxyphenyl)acetic acid | Birolli et al., 2016 |
| 81. | Bacillus sp. P5CBNB | Esfenvalerate | Transformed to (i) 3-2-(4-chlorophenyl)-3-methylbutyric acid), (ii) phenoxybenzoic acid, hydroxy phenoxybenzoic acid | Birolli et al., 2016 |
| 82. | Bacillus sp. CBMAI 1833 | Esfenvalerate | Transformed to (i) 3-2-(4-chlorophenyl)-3-methylbutyric acid), (ii) phenoxybenzoic acid, hydroxy phenoxybenzoic acid (iii) 2-(3-hydroxyphenyl)acetic acid | Birolli et al., 2016 |
| 83. | Bacillus sp. DG-2 | 3-phenoxybenzoic acid | Degraded via 3-(2-methoxyphenoxy) benzoic acid, protocatechuate, phenol, and 3,4-dihydroxy phenol. | Chen et al., 2012a |
| C. Biodegradation and Biotransformation of Chlorophenol, Nitrophenol, and Chloronitrophenol | ||||
| 84. | Bacillus licheniformis strain SL10 | 2,4-Dichlorophenol | Degradation occurred via meta cleavage pathway of catechol or chlorocatechol | Chris Felshia et al., 2020 |
| 85. | Bacillus sp. MW-1 | 4-chloro-2-nitrophenol | Transformed to 5-chloro-2-methyl benzoxazole | Arora and Jain, 2012 |
| 86. | Bacillus subtilis RKJ 700 | 4-chloro-2-nitrophenol | Transformed to 5-chloro-2-methyl benzoxazole | Arora, 2012 |
| 87. | Bacillus cereus PC-1 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 1.0 mM | Arora et al., 2016 |
| 88. | Bacillus toyonensis PC-2 | 4-chloro-2-nitrophenol | Decolorized up to a concentration of 0.9 mM | Arora et al., 2016 |
| 89. | Bacillus thuringiensis PC-3 | 4-chloro-2-nitrophenol | Decolorized up to a concentration of 1.0 mM | Arora et al., 2016 |
| 90. | Bacillus firmus PC-4 | 4-chloro-2-nitrophenol | Decolorized up to a concentration of 0.8 mM | Arora et al., 2016 |
| 91. | Bacillus koreensis PC-5 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 0.6 mM | Arora et al., 2016 |
| 92. | Bacillus megaterium PC-6 | 4-chloro-2-nitrophenol | Decolorized up to a concentration of 1.5 mM | Arora et al., 2016 |
| 93. | Bacillus aryabhattai PC-7 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 2.0 mM | Arora et al., 2016 |
| 94. | Bacillus aerophilus PC-8 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 0.6 mM | Arora et al., 2016 |
| 95. | Bacillus siamensis PC-9 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 0.8 mM | Arora et al., 2016 |
| 96. | Bacillus amyloliquefaciens PC-10 | 4-chloro-2-nitrophenol | Decolorized up to concentration of 0.9 mM | Arora et al., 2016 |
| 97. | Bacillus subtilis MF447840 | 4-chlorophenol | Degraded 4-chlorophenol up to of 1,000 mg/L | Sandhibigraha et al., 2020 |
| 98. | Bacillus cereus PU | Trinitrophenol | Used trinitrophenol as nitrogen source and degraded via Hydride-Meisenheimer complex. | Singh et al., 2011 |
| D. Biodegradation of Polyaromatic hydrocarbons | ||||
| 99. | Bacillus subtilis 3KP | Naphthalene and Phenanthrene | Metabolized via hydroxy-2-naphthoic acid, salicylic acid, and pyrocatechol | Ni'matuzahroh et al., 2017 |
| 100. | Bacillus fusiformis | Naphthalene | Degraded via o-phthalic acid and benzoic acid, | Lin et al., 2010 |
| 101. | Bacillus cereus RKS4 | Naphthalene | Catechol and 2-naphthol were identified as major metabolites of naphthalene degradation. | Sonwani et al., 2019 |
| 102. | Bacillus sp. SBER3 | Anthracene and Naphthalene | Degraded 83.4% of anthracene and 75.1% of and naphthalene in 6 days. | Bisht et al., 2014 |
| 103. | Bacillus subtilis DM-04 | Pyrene | Used it as its carbon source and energy | Das and Mukherjee, 2007 |
| 104. | Bacillus subtilis BM-1 | Fluorene | Degrade 86% of 50 mg/L fluorine with 21 days | Salam and Obayori, 2014 |
| 105. | Bacillus amyloliquefaciens BR1 | Fluorene | Degrade 82% of 50 mg/L fluorine with 21 days | Salam and Obayori, 2014 |
| 106. | Bacillus subtilis BTM4i | Benza-pyrene | Utilized as a sole source of carbon and energy and degradation ability was chromosomally coded. | Lily et al., 2010 |
| 107. | Bacillus pumilus (MTCC 1002) | Pyrene | Co-metabolize 64% of 50 μg/ml pyrene via 9-methoxyphenanthrene and phthalate | Khanna et al., 2011 |
| E. Biotransformation and detoxification of heavy metals | ||||
| 108. | Bacillus sp. strain FM1 | Chromium | Completely reduced 100 mg/L Cr(VI) within 48 h | Masood and Malik, 2011 |
| 109. | Bacillus sp. strain KSUCr9a | Chromium | rapidly reduce up to 100 μM of Chromium within 24 h | Ibrahim et al., 2012 |
| 110. | Bacillus sphaericus AND 303 | Chromium | 300 μM Cr(VI) reduction by cell extracts (4.56 mg protein/mL) of strain AND303 | Pal et al., 2005 |
| 111. | Bacillus sp. FY1 | Chromium | Reduced 78–85% of Cr(VI) (100–200 mg/l) within 24 h | Xiao et al., 2017 |
| 112. | Bacillus sp. MNU16 | Chromium | Reduced 75% of Cr(VI) of 50 mg/L within 72 h. | Upadhyay et al., 2017 |
| 113. | Bacillus amyloliquefaciens CSB 9 | Chromium | Reduced Cr(VI) to Cr (III) that was immobilized to the bacterial cell surface and subsequent intercellular accumulation of Cr (III) along with the formation of coagulated cell precipitate | Das et al., 2014 |
| 114. | Bacillus cereus S612 | Chromium | Reduced chromate under aerobic conditions | Wang et al., 2015 |
| 115. | Bacillus cereus | Chromium | Reduced Cr(VI) to Cr (III). Cr(III) precipitates were accumulated on bacterial surfaces | Chen et al., 2012c |
| 116. | Bacillus sp. ES 29 | Chromium | A copper (Cu2+) stimulated soluble Cr(VI)-reducing enzyme reduced Cr(VI) to Cr (III) | Camargo et al., 2003 |
| 117. | Bacillus sp. MH778713 | Chromium | Accumulated up to 100 mg Cr(VI)/g of cells and tolerate up to 15,000 mg/L Cr (VI) | Ramírez et al., 2019 |
| 118. | Bacillus cereus TN10 | Chromium | Detected chromate transporters in the genome | Hossain et al., 2020 |
| 119. | Bacillus cereus 12-2 | Lead | Transformed Pb(II) into nanosized rod-shaped Ca2.5Pb7.5(OH)2(PO4)6 crystal | Chen et al., 2016 |
| 120. | Bacillus sp. KK-1 | Lead | Converted Pb(NO3)2 into lead sulfide (PbS) and lead silicon oxide (PbSiO3) | Govarthanan et al., 2013 |
| 121. | Bacillus cereus BPS-9 | Lead | Bioaccumulation of lead by biosorption | Sharma and Shukla, 2021 |
| 122. | Bacillus megaterium | Selenium | Reduced Se(IV) to red element Se (III) | Mishra et al., 2011 |
| 123. | Bacillus subtilis | Selenium | Proposed physiological mechanisms regulating the selenite reduction | Garbisu et al., 1995 |
| 124. | Bacillus selenatarsenatis SF-1 | Selenium | Reduced selenate to selenite through anaerobic respiration, and subsequently into elemental selenium | Kuroda et al., 2015 |
| 125. | Bacillus selenitireducens MLS10 | Selenium | Enzyme respiratory selenite [Se(IV)] reductase (Srr) was characterized. | Wells et al., 2019 |
| 126. | Bacillus cereus CM100B | Selenium | Produced selenium nanoparticles by transformation of toxic selenite (SeO32-) anions into red elemental selenium (Se0) under aerobic conditions | Dhanjal and Cameotra, 2010 |
| 127. | Bacillus mycoides strain SeITE01 | Selenium | Reduced selenite (SeO32-) anions into red elemental selenium (Se0) with the formation of selenium nanoparticles. | Lampis et al., 2014 |
| 128. | Bacillus thuringiensis | Uranium | Transformation from U(VI) into nano-uramphite | Pan et al., 2015 |
| 129. | Bacillus licheniformis SPB-2 | Copper | Reduced [Co(III)–EDTA]– to [Co(II)–EDTA]2– which was further absorbed by strain SPG-2 | Paraneeiswaran et al., 2015 |
| 130. | Bacillus firmus strain TE7 | Chromium and Arsenic | Reduced Cr(VI) to Cr (III) and oxidized As(III) to As(V) | Bachate et al., 2013 |
| 131. | Bacillus sp. strain Arzi | Mb | Reduced molybdate to molybdenum blue | Othman et al., 2013 |
| 132. | Bacillus thuringiensis OSM29 | Ni and Cu | Biosorption capacity of the strain OSM29 for the metallic ions was highest for Ni (94%) which was followed by Cu (91.8%). | Oves et al., 2013 |
| F. Degradation of Natural Compounds | ||||
| 133. | Bacillus macerans JJ-lb | Protocatechuate | Completely mineralized | Crawford et al., 1979 |
| 134. | Bacillus sp. | 3-Hydroxybenzoate | Completely mineralized via protocatechuate | Mashetty et al., 1996 |
| 135. | Bacillus brevis PHB-2 | 4-Hydroxybenzoate | Completely mineralized via protocatechuate | Crawford, 1976 |
| 136. | Bacillus circulans strain 3 | 4-Hydroxybenzoate | Completely mineralized via protocatechuate | Crawford, 1976 |
| 137. | Bacillus laterosporusPHB-7a | 4-Hydroxybenzoate | Completely mineralized via gentisate | Crawford, 1976 |
| 138. | Bacillus sp. B-1 | Cinnamic acid | Degraded via benzoic acid | Peng et al., 2003 |
| 139. | Bacillus sp. B-1 | 4-Coumaric acid | Degraded via 4-hydroxybenzoic acid | Peng et al., 2003 |
| 140 | Bacillus sp. B-1 | Ferculic acid | Metabolized via 4-hydroxy-3-methoxyphenyl-beta-hydroxypropionic acid, vanillin, and vanillic | Peng et al., 2003 |
| 141. | Bacillus pumilus W1 | Cholesterol degradation | Cholesterol as only carbon and energy | Wali et al., 2019 |
| 142. | Bacillus ligniniphilus L1 | Alkaline lignin | Degraded via three different pathway including gentisate pathway, benzoic acid pathway, and the β-ketoadipate pathway | Zhu et al., 2017 |
| G. Degradation of Explosives | ||||
| 143. | Bacillus sp. J8A2 | Pentaerythritol tetranitrate | Utilized it as a nitrogen source | Yerson and Christian, 2013 |
| 144. | Bacillus sp. SF | Trinitrotoluene | Transformed to hydroxylaminodinitrotoluene, 4-amino-2,6-dinitrotoluene; 2-amino-4,6-dinitrotoluene, different azoxy compounds, 2,6-diaminonitrotoluene and 2,4-diaminonitrotoluene. | Nyanhongo et al., 2008 |
| 145. | Bacillus cereus | Trinitrotoluene | Transformed into 2,4-dinitrotoluene and 4-aminodinitrotoluene derivates, | Mercimek et al., 2013 |
| 146. | Bacillus sp. ATCC51912 | Propylene glycol dinitrate | Sequentially denitrated to propylene glycol mononitrate and propylene glycol | Sun et al., 1996 |
| 147. | Bacillus sp. ATCC51912 | Glycerol trinitrates | Sequential denitration of glycerol trinitrates to glycerol via glycerol dinitrate isomers and glycerol mononitrate isomers | Meng et al., 1995 |
| H. Degradation of Drugs | ||||
| 148. | Bacillus thuringiensis B1 | Naproxen | Degraded via salicylic acid and catechol | Górny et al., 2019 |
| 149. | Bacillus thuringiensis B1 | Ibuprofen | Degraded it via hydroxyibuprofen | Marchlewicz et al., 2017 |
| 150. | Bacillus drentensis S1 | Acetaminophen | Degraded via 2-isopropyl-5-methylcyclohexanone and phenothiazine | Chopra and Kumar, 2020 |
| I. Degradation of other Xenobiotic compounds | ||||
| 151. | Bacillus salamalaya 139SI | Crude oil waste | Degraded 88% of the total petroleum hydrocarbons within 42 days in mineral media containing 1% of crude oil waste. | Ismail and Dadrasnia, 2015 |
| 152. | Bacillus sp. BCBT21 | Plastic bags | Produced extracellular hydrolase enzymes including lipase, carboxymethyl cellulase, xylanase, chitinase, and protease | Dang et al., 2018 |
| 153. | Bacillus pumilusB12 | Poly-lactic acid | Degraded plylacitc acid film within 48-h by the release of L-lactate monomers | Bonifer et al., 2019 |
| 154. | Bacillus sp. strain 4 | Pyridine | Used it sole C, N and energy source | Watson and Cain, 1975 |
Bacilli-Mediated Degradation of 4-Chloro-2-Nitrophenol
4-Chloro-2-nitrophenol is a chloro derivative of nitrophenol that is widely used for the synthesis of dyes, pesticides, drugs, and chemicals (Arora et al., 2018). Due to its wide range of applications, this compound has been detected in a variety of sources including industrial effluents. It is highly toxic to living beings and may cause methemoglobinemia in human beings. So far, several physicochemical and biological methods have been used for the 4-chloro-2-nitrophenol degradation (Bruhn et al., 1988; Beunink and Rehm, 1990; Saritha et al., 2007; Gharbani et al., 2010; Hashemi et al., 2017; Arora et al., 2018). In this sub-section, the role of Bacillus species in the 4-chloro-2-nitrophenol degradation is summarized.
Many Bacilli have been characterized for their ability to decolorize the yellow color of 4-chloro-2-nitrophenol in the presence of additional carbon source (Arora et al., 2018). A marine bacterium, Bacillus sp. MW-1 (Arora and Jain, 2012), and a soil bacterium, Bacillus subtilis RKJ 700 (Arora, 2012) decolorized and transformed 4-chloro-4-nitrophenol into 5-chloro-2-methylbenzoxazole via detoxification mechanism. In this mechanism, 4-chloro-2-nitrophenol initially reduced to 4-chloro-2-aminophenol, which is further acetylated to 4-chloro-2-acetaminophenol. The next step involves the conversion of 4-chloro-2-acetaminophenol to 5-chloro-2-methylbenzoxazole (Figure 1). Recently, ten bacterial strains belonging to Bacillus isolated from a wastewater sample showed decolorization of 4-chloro-2-nitrophenol in the presence of glucose. One of a bacterium, identified as Bacillus aryabhattai strain PC-7 decolorized 4-chloro-2-nitrophenol up to a concentration of 2.0 mM and transformed it into 5-chloro-2-methylbenzoxazole (Arora et al., 2016).
Figure 1.
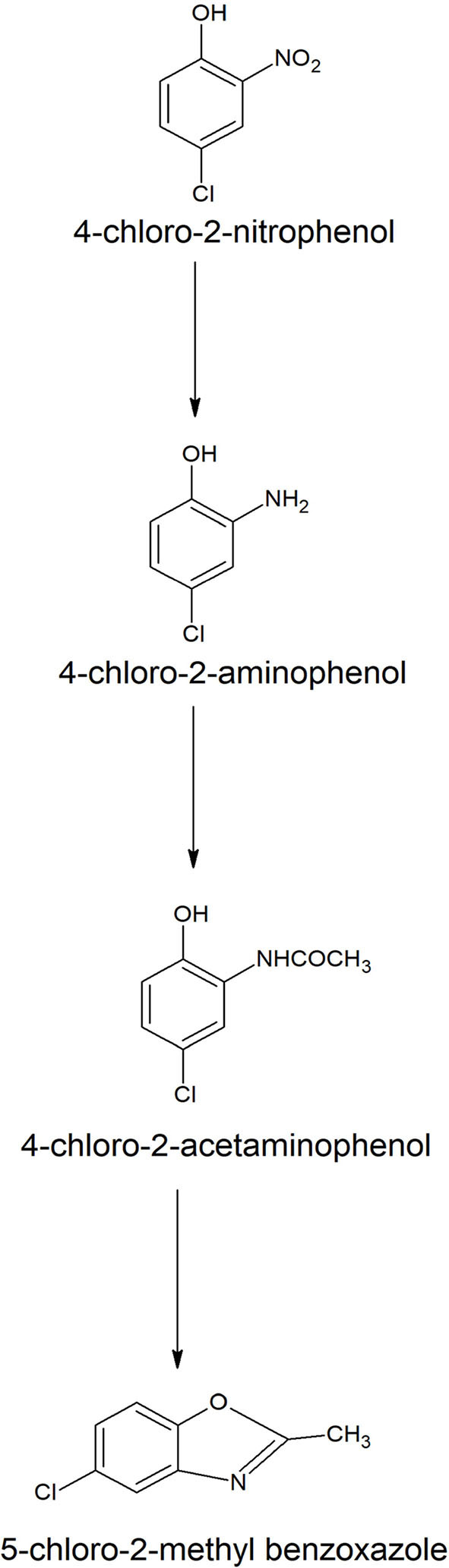
Biotransformation pathway of 4-chloro-2-nitrophenol in Bacillus spp. (Arora, 2012).
Besides Bacillus spp., several other bacteria are also capable of transforming 4-chloro-2-nitrophenol to 5-chloro-2-methylbenzoxazole. These bacteria belong to the genera Pseudomonas, Leuconostoc, and Paenibacillus (Arora et al., 2016). The memberes of genus Bacillus were unable to completely mineralize 4-chloro-2-nitrophenol, but they transformed 4-chloro-2-nitrophenol via a detoxification mechanism. The complete degradation of 4-chloro-2-nitrophenol was studied using an Exiguobacterium sp. PMA (Arora et al., 2012), a co-culture of Enterobacter cloacae and an Alcaligenes sp. TK-2 (Beunink and Rehm, 1990), and the genetically engineered bacterium, Pseudomonas sp. N31 (Bruhn et al., 1988).
Bacilli-Mediated Degradation of Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are those aromatic compounds which contain two or more fused aromatic rings in linear, angular, or cluster arrangements (Masih and Taneja, 2006). Examples are naphthalene, anthracene, fluorene, phenanthrene, fluoranthene, pyrene, and benzo[a]pyrene (Abdel-Shafy and Mansour, 2016). PAHs are toxic to the living world and some of them are considered as possible carcinogens. Therefore, the USEPA has listed 16 PAHS in its priority list of pollutants (Zelinkova and Wenzl, 2015). Major sources of PAHs pollution include fuel combustion, automobiles, spillage of petroleum products, waste incinerators, and industrial effluents (Abdel-Shafy and Mansour, 2016). In this section, the Bacilli-mediated degradation of a few PAHs is summarized.
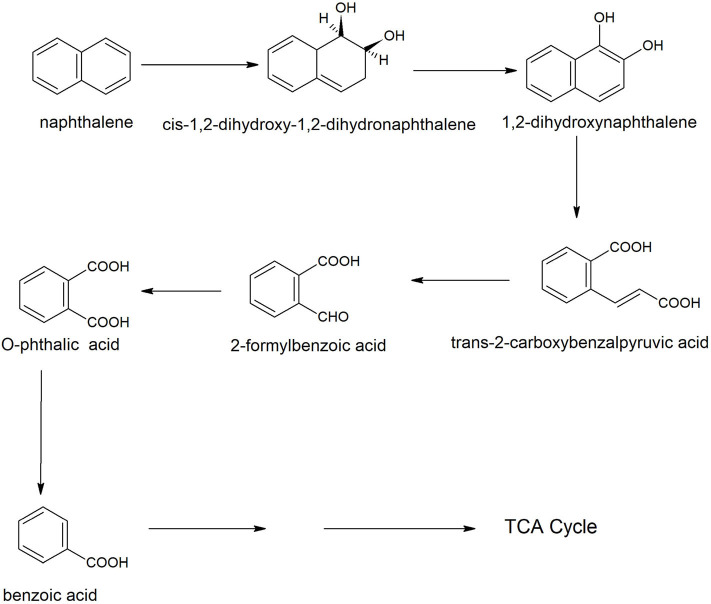
Naphthalene is the simplest example of polycyclic aromatic compounds. An early study on naphthalene degradation by B. cereus ATCC14579 showed the complete transformation of naphthalene to 1-naphthol (Cerniglia et al., 1984). A possible degradation pathway of naphthalene was studied in B. fusiformis BFN that was isolated from oil refining wastewater sludge (Lin et al., 2010). The naphthalene degradation was initiated with 1, 2-dioxygenation, resulting in the formation of cis-1,2-dihydroxy-1,2-dihydronaphthalene that dehydrogenated to 1,2-dihydroxynaphthalene. The ortho-ring cleavage of 1,2-dihydroxynaphthalene produced o-phthalic acid via the formation of trans-2-carboxybenzalpyruvic acid and 2-formyl benzoic acid (Figure 2). The phthalic acid decarboxylated to benzoic acid that further metabolized carbon dioxide and water. Ni'matuzahroh et al. (2017) studied the degradation of naphthalene and phenanthrene by B. subtilis 3KP that degraded them via the formation of 1-hydroxy-2-naphthoic acid, salicylic acid, and pyrocatechol. Sonwani et al. (2019) reported that B. cereus RKS4 degraded naphthalene via the formation of 2-naphthol and catechol. Annweiler et al. (2000) studied the degradation of naphthalene in B. thermoleovorans Hamburg 2 under thermophilic conditions (60° C). B. thermoleovorans Hamburg 2 utilized naphthalene as the sole source of carbon and energy and degraded it via formation of 1-naphthol, 2-naphthol, 2,3-dihydroxynaphthalene, 2-carboxycinnamic acid, phthalic acid, and benzoic acid, coumarin, 3-(2-Hydroxyphenyl)-propanoic acid, 2,3-dihydrocoumarin, 2-hydroxybenzoic acid (salicylic acid) and 2-carboxycinnamic acid.
Figure 2.
A degradation pathway of naphthalene in Bacillus fusiformis strain BFN (Lin et al., 2010).
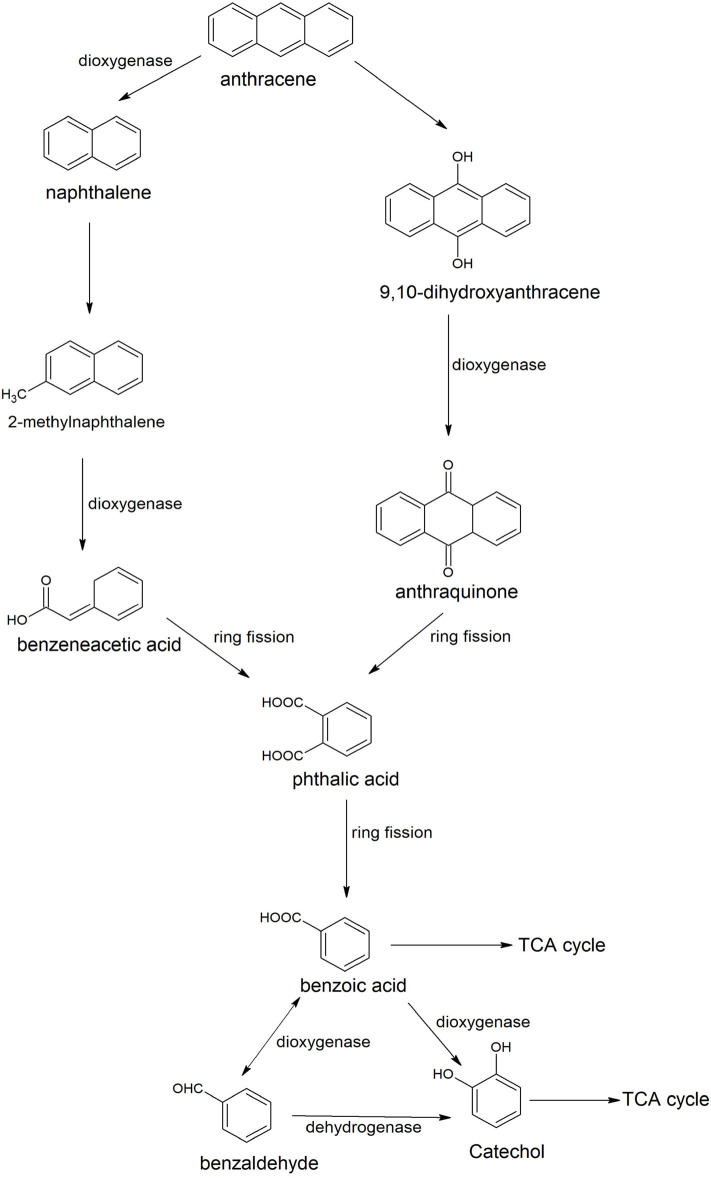
Anthracene is an integral part of many carcinogenic PAHs; therefore it has been detected easily in several contaminated sites of PAHs. Many Bacilli have been identified and characterized for degradation of anthracene. Examples are Bacillus sp. SBER3 (Bisht et al., 2014), B. cereus JMG-01 (Das et al., 2017), B. licheniformis MTCC 5514 (Swaathy et al., 2014), B. cereus S13 (Bibi et al., 2018), and B. badius D1 (Sarwade and Gawai, 2014). Das et al. (2017) studied the degradation pathway of anthracene for B. cereus JMG-01 that degraded 98% of 500 ppm anthracene. The anthracene degradation was initiated with the formation of naphthalene and naphthalene-2-methyl. In the next step, a dioxygenase enzyme catalyzed oxidation of naphthalene-2-methyl to benzene acetic acid. Further, benzene acetic acid underwent ring cleavage to produce phthalic acid and benzaldehyde. Benzaldehyde converted to catechol that degraded via either ortho or meta ring cleavage. Swaathy et al. (2014) reported the existence of two degradation pathways in biosurfactant mediated biodegradation of anthracene by B. licheniformis (MTCC 5514). One pathway proceeded with the formation of naphthalene, naphthalene 2-methyl, phthalic acid, and benzene acetic acid. Another pathway was initiated with dioxygenation of anthracene to produce di-hydroxy anthracene, which, further transformed to anthraquinone by a dioxygenase enzyme (Figure 3). Anthraquinone was further degraded with the formation of phthalic acid, benzaldehyde or benzoic acid, and catechol. Metabolites of both of the pathways (9, 10-dihydroxyanthracene, anthraquinone, benzene acetic acid, and catechol) were also reported in the anthracene degradation pathway of B. cereus S13 that utilized it as the sole source of carbon and energy (Bibi et al., 2018). Another pathway of anthracene was studied in an alkaliphilic bacterium B. badius D1 that was able to degrade anthracene at a concentration of 50 mg/100 ml at pH 9.0 (Sarwade and Gawai, 2014). In this pathway, anthracene was initially oxidized to 1, 2-dihydoxyanthracene that further oxidized (3Z)-4-[3-hydroxy (2-naphthyil)-2-oxobut-3-enoic acid with subsequent conversion to 2-hydroxynaphthoic acid, Further oxidation resulted in the formation of phthalic acid that was degraded via formation of simple aliphatic compounds.
Figure 3.
Degradation pathways of anthracene by Bacillus licheniformis MTCC 5514 (Swaathy et al., 2014).
Bacilli-Mediated Degradation of Pyrethroid Insecticides
Pyrethroid insecticides are synthetic pyrethroids which are analogs to natural pyrethrins extracted from Chrysanthemum cinerariaefolium (Cycoń and Piotrowska-Seget, 2016). Representative compounds of these pesticides are cyhalothrin, fenpropathrin, deltamethrin, cypermethrin, cyfluthrin, and bifenthrin (Zhan et al., 2020). They are used to control a broad spectrum of pests in households and agriculture fields. Due to their wide range of applications in agriculture fields, they have been spread into soil and water and create environmental problems because of their toxic nature (Zhan et al., 2020). Many Bacilli have been isolated and characterized for the degradation of several pyrethroids (Chen et al., 2012b; Cycoń and Piotrowska-Seget, 2016; Bhatt et al., 2020). In this section, Bacilli-mediated degradation of various pyrethroids is discussed.
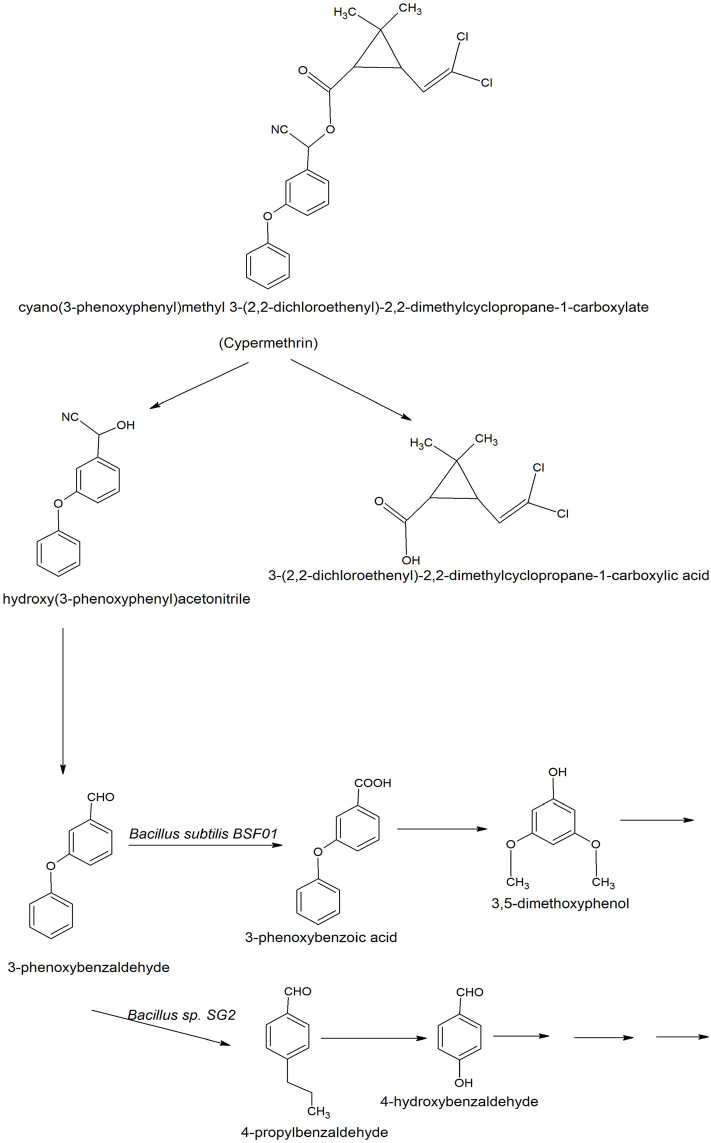
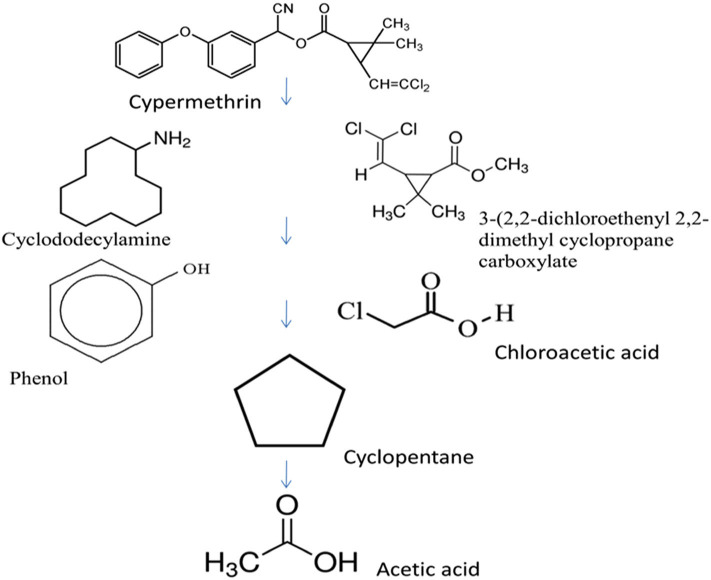
The degradation of cypermethrin is well-studied in some Bacilli including Bacillus sp. SG2 (Pankaj et al., 2016), B. subtilis BSF01 (Xiao et al., 2015), B. subtilis strain 1D (Gangola et al., 2018), Bacillus sp. AKD1 (Tiwary and Dubey, 2016), Bacillus sp. ISTDS2 (Sundaram et al., 2013) and B. licheniformis B-1 (Lai et al., 2012). The initial steps of degradation pathways of cypermethrin are common in Bacillus sp. SG2 and B. subtilis BSF01 (Xiao et al., 2015; Pankaj et al., 2016). Cypermethrin was initially transformed into two metabolites: α-hydroxy-3-phenoxy-benzene acetonitrile and 3-(2,2-dichloroethenyl)-2,2-dimethyl cyclopropanecarboxylate). The unstable compound, α-hydroxy-3-phenoxy-benzene acetonitrile was spontaneously transformed into 3-phenoxybenzaldehyde (Figure 4). Further degradation of 3-phenoxybenzaldehyde proceeded via a different route in Bacillus sp. SG2 and B. subtilis BSF01. In B. subtilis BSF01, the degradation of 3-phenoxybenzaldehyde proceeded via the formation of 3-phenoxybenzoic acid and 3, 5-dimethoxyphenol (Xiao et al., 2015). However, in Bacillus sp. SG2, 3-phenoxybenzaldehyde was further converted to 4-propylbenzaldehyde and then to 4-hydroxybenzoate that was transformed to phenyl ester of o-phenoxy benzoic acid (Pankaj et al., 2016). The phenyl ester of o-phenoxy benzoic acid was degraded via the formation of phenol-M-tert-butyl, phenol, and aliphatic hydrocarbons or short-chain compounds. Another pathway of degradation of cypermethrin was studied in B. subtilis strain 1D (Gangola et al., 2018). In this pathway, cypermethrin was initially transformed into 3-(2, 2-dichloro ethenyl)-2,2-dimethyl-cyclopropanecarboxylate and cyclododecylamine due to hydrolysis of the ester linkage (Figure 5). The unstable compound, cyclododecylamine oxidized to phenol which reacted with water to form cyclopentane that transformed into aliphatic compounds like acetic acid and decanoic acid. Another metabolite, 3-(2, 2-dichloro ethenyl)-2,2-dimethyl-cyclopropanecarboxylate was hydrolyzed to form chloroacetic acid (Gangola et al., 2018).
Figure 4.
Degradation pathways of cypermethrin in Bacillus sp. SG2 and Bacillus subtilis BSF01 (Xiao et al., 2015; Pankaj et al., 2016).
Figure 5.
Degradation pathways of cypermethrin in Bacillus subtilis strain 1D (adapted from Gangola et al., 2018).
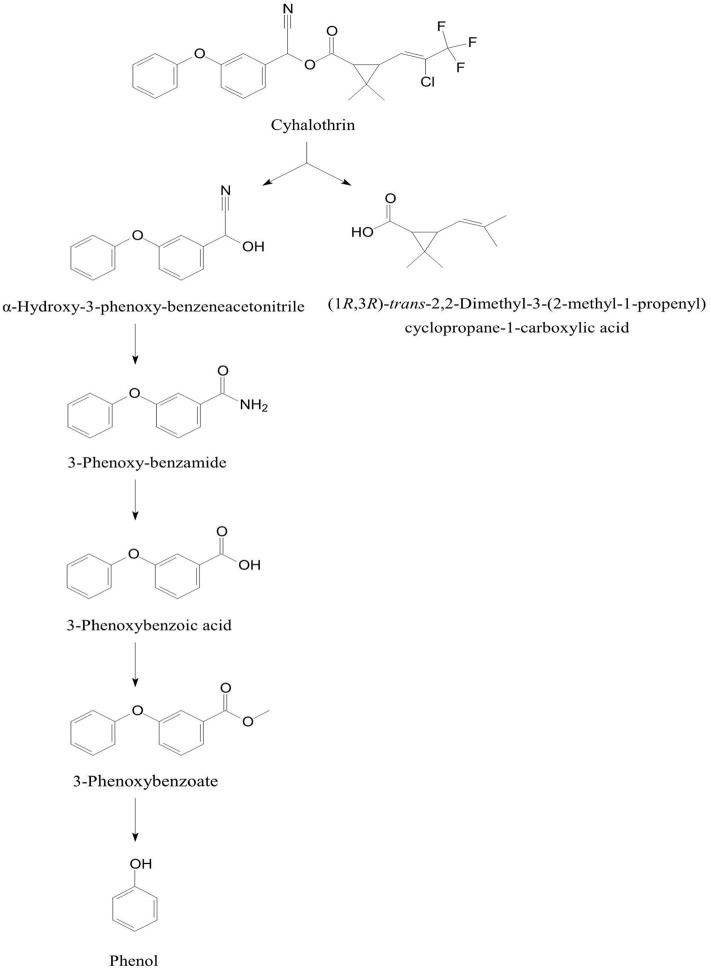
The degradation pathway of cyhalothrin [(RS)-α-Cyano-3-phenoxybenzyl-(Z)-(1RS,3RS)-(2-chloro-3,3, 3-trifluoro propenyl)-2,2-dimethylcyclopropanecarboxylate)] was studied in B. thuringiensis ZS-19 that initiated degradation of cyhalothrin by cleavage of the carboxyl ester linkage through hydrolysis to form α-hydroxy-3-phenoxy-benzeneacetonitrile and (1RS,3RS)-trans-2,2-dimethyl-(2-methyl-1-propenyl)cyclopropane-1-carboxylic acid (Chen et al., 2015). The α-hydroxy-3-phenoxy-benzeneacetonitrile was converted to 3-phenoxybenzote acid via 3-phenoxyphenyl acetonitrile, N-(2-isoproxy-phenyl)-4-phenoxy-benzamide, and 3-phenoxybenzaldehyde (Figure 6). Further degradation of 3-phenoxybenzoate was proceeded through cleavage of diaryl bond to produce and phenol that was degraded via aromatic ring cleavage (Chen et al., 2015).
Figure 6.
Degradation pathway of cyhalothrin in Bacillus thuringiensis ZS-19 (adapted from Chen et al., 2015).
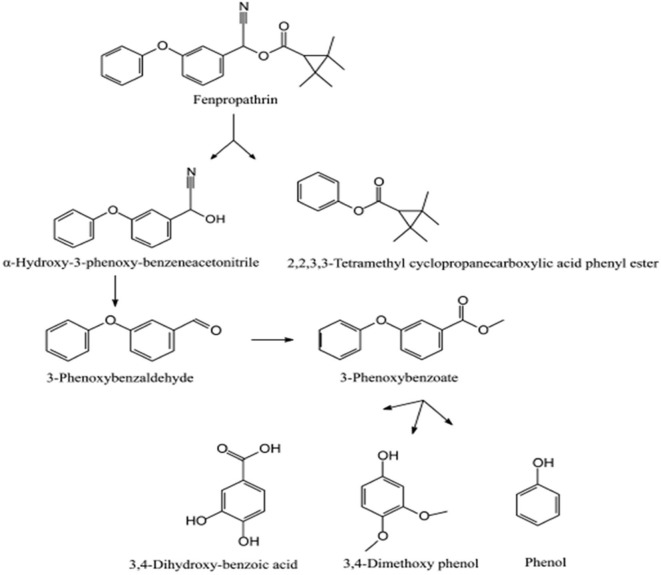
The degradation pathway of fenpropathrin(α-cyano-3-phenoxybenzyl 2,2,3,3-tetramethylcyclopropanecarboxylate) was studied in Bacillus sp. DG-02, isolated from a soil sample collected from the aerobic pyrethroid-manufacturing wastewater treatment system of China (Chen et al., 2014). Initially, fenpropathrin was converted to α-hydroxy-3-phenoxybenzeneacetonitrile and 2, 2, 3, 3-tetramethylcyclopropanecarboxylic acid phenyl ester due to cleavage of the carboxyl ester linkage (Figure 7). In the next step, unstable compound α-hydroxy-3-phenoxybenzeneacetonitrile was spontaneously transformed into 3-phenoxybenzaldehyde, which oxidized to 3-phenoxybenzoate. Subsequent degradation of 3-phenoxybenzoate produced 3, 4-dihydroxybenzoic acid, 3, 4-dimethoxyphenol, and phenol (Chen et al., 2014).
Figure 7.
Degradation pathway of fenpropathrin in Bacillus sp. DG-02 (Reprinted (adapted) from Chen et al., 2014). Copyright (2014) American Chemical Society.
Bacilli-Mediated Degradation of Organophosphorus Pesticides
Organophosphorus pesticides are a large group of chemicals that widely used for protecting crops, livestock from various pests (Sidhu G. K. et al., 2019). Commonly used organophosphates are malathion, parathion, methyl parathion, chlorpyrifos, diazinon, fenitrothion, dichlorvos, ethion, and monocrotophos (Sidhu G. K. et al., 2019). These compounds act as an inhibitor of an acetylcholinesterase enzyme that hydrolyzes the neurotransmitter acetylcholine found in both the peripheral and central nervous systems (Robb and Baker, 2020). This inhibition mechanism involves the phosphorylation of the serine hydroxyl group present on the active site of acetylcholinesterase (Robb and Baker, 2020). In this section, the role of Bacilli for the degradation of organophosphorus pesticides is discussed.
Many reports have been published dealing with the potential applications of Bacilli to degrade organophosphorus pesticides. Bhadbhade et al. (2002) reported mineralization of monocrotophos to carbon dioxide, ammonia, and phosphates by B. megaterium MCM B-423, isolated from soil exposed to monocrotophos. The enzymes, phosphatase, and esterase were involved in the monocrotophos degradation pathway, which proceeds via acetic acid, methylamine, and one unidentified metabolite. Dash and Osborne (2020) studied degradation pathways of monocrotophos by B. aryabhattai strain VITNNDJ5 in artificially contaminated soil and reported that B. aryabhattai may be degraded monocrotophos via three routes; one route proceeds with the hydrolysis of monocrotophos into dimethyl phosphate that was degraded further into phosphoric acid and acetic acid esters by hydrolase and monooxygenase enzymes. The second degradation pathway was initiated with the demethylation of monocrotophos to N-(hydroxymethyl) acetamide that was further degraded into acetamide. Acetamide converted into acetic that entered the TCA cycle. In the third route, monocrotophos, monocrotophos converted into orthophosphoric acid and acetic acid via formation of phosphonoacetate intermediate.
Another Bacillus sp. TAP-1 that was isolated from sewage sludge of a wastewater treating system of organophosphorus pesticide was capable of hydrolyzing high concentrations of triazophos (50–400 mg/l) (Tang and You, 2012). Salunkhe et al. (2013) reported the biodegradation of an organophosphorus insecticide, profenofos by four B. subtilis strains, namely, DR-39, CS-126, TL-171, and TS-204, isolated from grapevines or grape rhizosphere and 4-bromo-2-chlorophenol was identified as a metabolite. A marine Bacillus sp. strain C5 isolated from the China Bohai Sea produced an extracellular esterase that hydrolyzed methyl parathion to 4-nitrophenol and other metabolites (Hao et al., 2014). Anwar et al. (2009) reported that B. pumilus C2A1 isolated from a soil sample collected from the cotton field, degraded chlorpyrifos, and its first hydrolysis metabolite 3,5,6-trichloro-2-pyridinol. Strain C2A1 degraded maximum amounts of chlorpyrifos at alkaline pH (8.5) and high inoculums bacterial density. Pailan et al. (2015) isolated organophosphates-degrading bacterium, B. aryabhattai strain SanPS1 from a soil sample of an agricultural field located at Narigram in Burdwan district of West Bengal, India. Strain SanPS1 degraded parathion via the formation of 4-nitrophenol and 4-nitrocatechol.
Bacilli-Mediated Degradation of Organochlorine Pesticides
Organochlorine pesticides are a group of chlorinated compounds, which include DDT, methoxychlor, endosulfan, dieldrin, chlordane, toxaphene, mirex, kepone, lindane, and benzene hexachloride (Jayaraj et al., 2016). These compounds are widely distributed to the environment due to applications. In this section, Bacilli-mediated degradation of organochlorine pesticides is discussed.
B. subtilis MTCC 8561 utilized endosulfan and endosulfan sulfate as its sulfur sources and degraded both of them via the formation of endosulfan diol and endosulfan lactone (Kumar et al., 2014). Awasthi et al. (2003) also reported the degradation of alpha and beta isomers of endosulfan via the formation of endosulfan diol and endosulfan lactone using the co-culture of Bacillus sp. MTCC 4444 and Bacillus sp. MTCC 4445. Seralathan et al. (2014) postulated the role of cytochrome P450 BM3 of B. megaterium in biotransformation of endosulfan through in silico prediction approach. Kumar and Philip (2006) reported that the anaerobic degradation of endosulfan, endosulfan ether, and endosulfan lactone using mixed bacterial culture containing two strains of B. circulans and one strain of Staphylococcus sp. All three strains metabolized endosulfan via hydrolysis pathway with the formation of carbenium ions and/or ethylcarboxylates, which further converted into simple hydrocarbons (Kumar and Philip, 2006).
Bacilli-Mediated Degradation of Herbicides
Herbicides are chemical substances that are generally used to control the growth of unwanted plants (Herrera-Herrera et al., 2016). These are known as weed killers and divided into two categories: contact herbicides and systematic herbicides. Contact herbicides are localized in action and affect only the part of the plant that they touch (Herrera-Herrera et al., 2016). Examples are diclofop, dinoseb, diquat, and paraquat (Herrera-Herrera et al., 2016). Systemic herbicides may be translocated to other parts of the plants. Examples are atrazine, quinclorac, glyphosate 2,4-dichlorophenoxyacetic acid (2,4-D), and simazine (Herrera-Herrera et al., 2016). In this section, the role of Bacilli for the degradation of herbicides is discussed.
Bacillus subtilis HB-6 isolated from industrial wastewater utilized atrazine as its sole nitrogen source for growth and mineralized it via formation of hydroxyatrazine, cyanuric acid, and urea (Wang et al., 2014). The atrazine-degrading genes, trzN, atzB, and atzC which encode the enzymes to converting atrazine to cyanuric acid were detected in strain HB-6 (Wang et al., 2014). Liu et al. (2014) studied the degradation of a highly selective auxin herbicide, quinclorac (3,7-dichloro-8-quinoline-carboxylic) by B. megaterium Q3 isolated from the root of tobacco grown in quinclorac contaminated soil. Strain Q3 transformed quinclorac to 3, 7-dichloro-8-methyl-quinoline, 3-chlorin-8-quinoline-carboxylic and 8-quinoline-carboxylic (Liu et al., 2014).
Bacilli-Mediated Degradation of Drugs
Ibuprofen and naproxen are known as non-steroidal anti-inflammatory drugs and widely used to control mild to moderate pain, fever, inflammation, menstrual cramps, and types of arthritis (Marchlewicz et al., 2017). Due to the high consumption of these drugs, they have been detected in the effluents of several biological wastewater treatment systems as environmental pollutants (Marchlewicz et al., 2017). In this section, the Bacillus-medited degradation of ibuprofen and naproxen is discussed.
To date, only one species of Bacillus, i.e., B. thuringiensis B1 was able to degrade both Ibuprofen and naproxen (Marchlewicz et al., 2017; Górny et al., 2019). The effective degradation of both of these drugs occurred in the presence of glucose. B. thuringiensis B1 was able to degrade ibuprofen and naproxen up to concentrations of 25 mg/Land 12 mg/L, respectively.
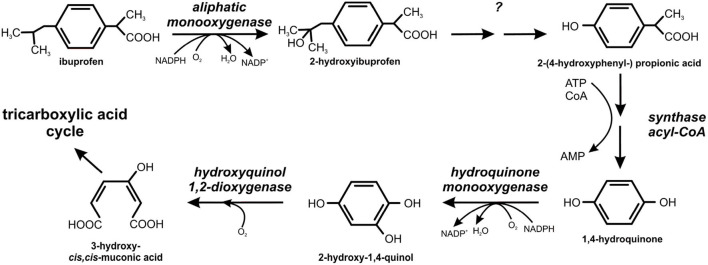
The degradation pathways of ibuprofen and naproxen were studied in B. thuringiensis B1. The first step of the ibuprofen degradation is hydroxylation of ibuprofen into 2-hydroxyibuprofen by aliphatic monooxygenase (Marchlewicz et al., 2017). The second step was the conversion of 2-hydroxyibuprofen to 2-(4-hydroxyphenyl-) propionic acid that was further transformed into 1,4-hydroquinone by acyl-CoA synthase/thiolase activity (Figure 8). In the next step, a hydroquinone monooxygenase catalyzed conversion of 1,4-hydroquinone to 2-hydroxy-1,4-quinol which cleaved to 3-hydroxy-cis, cis-muconic acid by hydroxyquinol 1,2-dioxygenase (Marchlewicz et al., 2017).
Figure 8.
Degradation pathway of ibuprofen in Bacillus thuringiensis B1 (adapted from Marchlewicz et al., 2017).
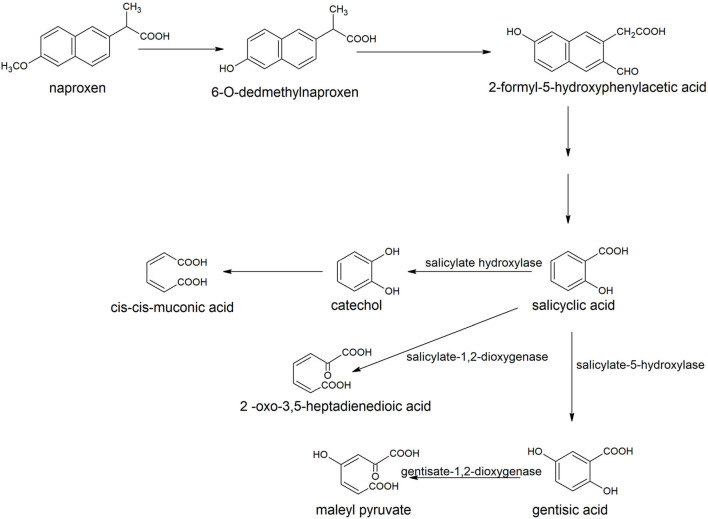
The degradation of naproxen was initiated with the transformation of naproxen into o-desmethylnaproxen by the action of tetrahydrofolate dependent O-demethylase (Górny et al., 2019). The next step involved the formation of 2-formyl-5-hydroxyphenylacetic that was converted to salicylic acid (Figure 9). Salicyclic acid hydroxylated to catechol or gentisic acid or can be cleaved to 2-oxo-3, 5-heptadienedioic acid (Górny et al., 2019).
Figure 9.
Degradation pathway of naproxen in Bacillus thuringiensis B1 (Górny et al., 2019).
Bacilli-Mediated Transformation of Heavy Metals
The bacterial remediation of heavy metals involves removals of heavy metals from aqueous solution and soil through biosorption, bioaccumulation, or biotransformation (Dixit et al., 2015). Biosorption is one of the important mechanisms for the removal of heavy metals, which involves the interaction of heavy metals with the functional groups present on bacterial surfaces (Igiri et al., 2018). Bioaccumulation is a metabolism-driven process in which the heavy metal ions pass across the cell membrane into the cytoplasm, accumulating inside the cells (Diep et al., 2018). Biotransformation involves conversation of one form of heavy metal to another form (Juwarkar and Yadav, 2010). In this subsection, the role of Bacilli in the bioremediation of various heavy metals is summarized.
Many Bacilli have been characterized for the bioreduction of chromium from Cr(VI) to Cr(III). Examples are Bacillus sp. strain FM1 (Masood and Malik, 2011), Bacillus sp. strain KSUCr9a (Ibrahim et al., 2012), B. sphaericus AND 303 (Pal et al., 2005), Bacillus sp. FY1 (Xiao et al., 2017), Bacillus sp. MNU16 (Upadhyay et al., 2017), B. amyloliquefaciens (Das et al., 2014), and B. cereus S612 (Wang et al., 2015). Several mechanisms have been proposed for chromium reduction and removal. Chen et al. (2012c) investigated the Cr(VI) uptake mechanism in B. cereus that reduced Cr(VI) into Cr(III). The reduced Cr(III) was coordinated with carboxyl and amido functional groups of the bacterial cell and the Cr(III) precipitates were accumulated on bacterial surfaces. Das et al. (2014) studied the mechanism of Cr(VI) reduction in B. amyloliquefaciens strain CSB 9 isolated from chromite mine soil of Sukinda, India. The reduced product Cr (III) was removed via surface immobilization and accumulated inside the bacterial cells. Bacillus sp. ES 29 produced copper (Cu2+) stimulated soluble Cr(VI)-reducing enzyme that reduced Cr(VI) to Cr(III)(Camargo et al., 2003).
The lead transformation from toxic Pb(II) to non-toxic lead compounds has been investigated in a few Bacillus strains. Chen et al. (2016) studied the transformation of Pb(II) into nanosized rod-shaped Ca2.5Pb7.5(OH)2(PO4)6 crystal in B. cereus 12-2, isolated from lead-zinc mine tailings. Initially, bacterial cells rapidly absorbed Pb(II) through the synergy of electrostatic attraction, ionic exchange, and chelating activity of functional groups present in bacterial cells. In the next step, enzyme-mediated Pb(II) transformation to rod-shaped crystalline minerals occurred inside the bacteria. Govarthanan et al. (2013) isolated and characterized an autochthonous bacterium, Bacillus sp. KK-1 for biomineralization of Pb in mine tailings. Strain KK-1 can convert Pb(NO3)2 into lead sulfide (PbS) and lead silicon oxide (PbSiO3). The ability of strain KK-1 to remove Pb was investigated in mine tailings. Strain KK-1 significantly reduced the exchangeable fraction of Pb and induced calcite in the precipitation of Pb ions.
The selenium reduction from Se(IV) to Se (III) is well-studied in Bacillus strains. Mishra et al. (2011) reported the reduction of Se(IV) to red-element Se (III) by two strains of B. megaterium. Garbisu et al. (1995) studied the physiological mechanisms regulating the selenite reduction in B. subtilis. They concluded that the reduction mechanism involves an inducible detoxification system, which deposited elemental selenium between the cell wall and the plasma membrane. Another mechanism was observed in a selenate reducing bacterium, B. selenatarsenatis SF-1, isolated from selenium-contaminated sediment (Kashiwa et al., 2001). Strain SF-1 reduced selenate to selenite and subsequently to non-toxic insoluble elemental selenium using lactate as an electron donor and selenate as an electron acceptor in an anaerobic condition. Elemental selenium was deposited both inside and outside of the cells. B. selenitireducens produced enzymes to reduce the oxidized forms of arsenic and selenium to their less toxic reduced forms (Wells et al., 2019). B. cereus CM100B and B. mycoidesstrain SeITE01 produced selenium nanoparticles (SNs) by transformation of toxic selenite () anions into red elemental selenium (Se0) under aerobic conditions. In this mechanism, initially, enzymatically reduced to selenium through redox reactions by the bacterial enzymes (membrane reductase) and later, selenium nanoparticles were generated due to the result of an Ostwald ripening mechanism (Dhanjal and Cameotra, 2010; Lampis et al., 2014).
The uranium transformation from U(VI) into nano-uramphite was studied in two B. thuringiensis strains isolated from uranium mine (Pan et al., 2015). The initial step involves the adsorption of U(VI) on the bacterial surface through coordinating with phosphate, -CH2, and amide groups. The next step involves the formation and accumulation of needle-like amorphous uranium compounds.
Paraneeiswaran et al. (2015) reported that B. licheniformis SPB-2 reduced [Co(III)–EDTA]− to [Co(II)–EDTA]2− which was further absorbed by strain SPG-2. B. firmus strain TE7, isolated from tannery effluent reduced Cr(VI) to Cr (III) and oxidized As(III) to As(V) (Bachate et al., 2013). Bacillus sp. strain A.rzi isolated from a metal-contaminated soil reduced molybdate to molybdenum blue (Othman et al., 2013). B. thuringiensis OSM29 isolated from the rhizosphere of cauliflower grown in soil irrigated consistently with industrial effluents was capable of removing several heavy metals including cadmium, chromium, copper, lead and nickel via biosorption (Oves et al., 2013). The biosorption capacity of the strain OSM29 for the metallic ions was highest for Ni (94%) which was followed by Cu (91.8%).
Bacilli-Mediated Transformation of Azo Dyes
Azo dyes are a large group of synthetic aromatic compounds which contain one or more azo groups (-N=N-) between organic residues. Based on the number of azo linkages, azo dyes are classified as monoazo, disazo, trisazo, and polyazo (Benkhaya et al., 2020). Few examples of azo dyes are Metanil Yellow, Navy Blue 2GL, Dye Orange T4LL, Reactive Red 2, Direct Red-22, Turquoise Blue dye, and Acid Black 24. These are widely used in the textile industry that is a major source of dye contamination. During the dyeing process, the textile industry discharged ~10% of the dyes into the wastewater (Easton, 1995). Apart from the textile industry, azo dyes are also used in food, paper printing, color photography, leather, and cosmetic industries (Chang and Lin, 2001). They are widely distributed in the environment due to improper discharge of dye into wastewater. These dyes are highly toxic to plants by inhibiting their photosynthesis. In the environment, they may generate mutagenic and carcinogenic amines due to microbial transformation (Chung and Cerniglia, 1992; Weisburger, 2002; Asad et al., 2007). Dye removal is an essential step for the treatment of dye-containing wastewater (Banat et al., 1996). Microbial dye degradation process has two steps; First is dye decolorization in which azoreductase-mediated cleavage of the azo bond (—N=N—) to give aromatic amines. The second step involves the degradation of aromatic amines into non-toxic compounds. In this sub-section, the role of Bacilli in dye decolorization is summarized.
Many Bacillus strains have been characterized for decolorization of wastewater containing various azo dyes. Anjaneya et al. (2011) studied the decolorization of metanil yellow using a sulfonated azo dye decolourizing bacterium, Bacillus sp. AK1 that was isolated from dye contaminated soil sample collected from Atul Dyeing Industry, Bellary, India. Bacillus sp. AK1 decolorized metanil yellow (200 mg L−1) completely within 27h and transformed it into metanillic acid and p-aminodiphenylamine by the action of the azoreductase enzyme. Dawkar et al. (2009) studied the effects of inducers on the decolorization of a textile azo dye, navy blue 2GL by a Bacillus sp. VUS isolated from textile effluent contaminated soil. Strain VUS decolorized azo dye navy blue 2GL within 48 h under the static anoxic condition in yeast extract medium, whereas in the presence of CaCl2 it decolorized it only within 18 h. They reported that CaCl2 induced the activities of the enzymes involved in the decolorization of navy blue 2GL. 4-Amino-3-(2-bromo-4, 6-dinitro-phenylazo)-phenol and acetic acid 2-(-acetoxy-ethylamino)-ethyl ester were detected as the transformation products of dye decolorization. Bacillus sp. VUS also decolorized dye orange T4LL in static anoxic condition within 24 h and transformed it into 4-methyl-2-o-tolylazo-benzene-1,3-diamine and [3-(phenyl-hydrazono)-cyclohexa-1,4-dienyl]-methanol. Another bacterium, B. licheniformis decoulorized Reactive Red 2 and transformed it into 2, 4-dichloro-6-[(1H-indazol-5-ylimino)-methyl]-phenol, benzene sulfonamide, 1H indole and urea as final metabolites (Sudha and Balagurunathan, 2013). B. firmus immobilized within tubular polymeric gel completely decolorized 50 mg/L of CI Direct Red 80 under anoxic conditions within 12 h by transforming it into aromatic amine (Ogugbue et al., 2012). These aromatic amines were further degraded aerobically by the same strain within the subsequent 12 h.
Saleem et al. (2014) studied the effects of the various carbon sources, pH, temperature, and nitrogen sources on decolorization of pulp and paper industrial effluents by B. cereus. They observed that the optimum temperature and pH for decolorization were 45° C and 6.5, respectively. Maximum decolorization was observed when carbon and nitrogen sources were sucrose (0.5%) and ammonium sulfate (1%), respectively. Sharma et al. (2009) optimized process variables for decolorization of disperse yellow 211 by B. subtilis using Box–Behnken design and observed that the optimum conditions for maximum decolorization were 100 mg l−1 initial dye concentration, 7.0 pH and 32.5° C temperature. A crystal violet decolourizing bacterium, B. subtilis decolorized crystal violet (100 mg/L) effectively at pH 8 and temperature 35° C when starch and peptone were used as carbon and nitrogen sources, respectively (Kochher and Kumar, 2011). Gunasekar et al. (2013) reported the decolorization of reactive dye RED M5B by B. subtilis and observed that decolorization was due to the action of enzyme peroxidase produced by the organisms during its growth. Joshi et al. (2013) reported the decolorization of turquoise blue dye (Remazol Blue BB) by B. megaterium isolated from a sample collected from dye industries. This organism can decolorize turquoise blue dye up to a concentration of 5 mg/ml. Prasad and Rao (2014) reported decolorization of Acid Black 24 by B. halodurans MTCC 865 which was able to decolorize Acid Black within 6 hat pH 9 and 37° C with 5% NaCl under static conditions. Prasad and Rao (2013) reported aerobic decolorization of the textile azo dye Direct Red-22 by an obligate alkaliphilic bacterium B. cohnii MTCC 3616. This strain was able to decolorize Direct Red-22 (5,000 mg l1) with 95% efficiency at 37° C and pH 9 in 4 h under static conditions.
Bacilli-Mediated Degradation of Natural Aromatic Acids
Aromatic acids are a class of chemical compounds in which an organic acid attached to the aromatic ring. Examples are phenolic acids (3-Hydroxybenzoic acid. 4-Hydroxybenzoic acid and Salicylic acid) and Hydroxycinnamic acids (cinnamic, 4-coumaric, and ferulic acids). In this subsection, the role of Bacilli in biodegradation of various aromatic acids is summarized. B. macerans JJ-lb degraded protocatechuate via ring cleavage and subsequent enzymatic decarboxylation of the ring fission product (Crawford et al., 1979). Initially, protocatechuate-2,3-dioxygenase catalyzes the ring cleavage of protocatechuate to 5-carboxy-2-hydroxymuconic semialdehyde that is further decarboxylated to 2-hydroxymuconic semialdehyde. Mashetty et al. (1996) reported the degradation of 3-hydroxybenzoate by a Bacillus sp. that utilized it as the sole source of carbon and energy. This strain metabolized 3-hydroxybenzoic acid via protocatechuic acid that was further degraded via both the ortho- and meta-cleavage pathway. The enzyme activities for 3-hydroxybenzoate 4-hydroxylase, protocatechuate 3,4-dioxygenase, and protocatechuate 4,5-dioxygenase were detected in cell-free extracts. Crawford (1976) reported degradation pathways of 4-hydroxybenzoate in B. brevis PHB-2, B. circulans strain 3, and B. laterosporus PHB-7a. B. brevis PHB-2 and B. circulans strain 3 degraded 4-hydroxybenzoate via protocatechuate that was further degraded through ortho cleavage pathway or meta cleavage pathway. B. laterosporus PHB-7a converts 4-hydroxybenzoate to gentisate, which is further degraded by the glutathione-independent gentisic acid pathway. Peng et al. (2003) reported the degradation of cinnamic, 4-coumaric, and ferulic acids by thermophilic Bacillus sp. B-1. Strain B-1 degraded cinnamic acid via benzoic acid that was further degraded via catechol and its ring cleavage. The 4-coumaric acid degradation proceeded via 4-hydroxybenzoic acid that was further degraded via gentisic acid and its ring cleavage. The ferculic acid metabolized via 4-hydroxy-3-methoxyphenyl-beta-hydroxypropionic acid, vanillin, and vanillic acid as the intermediates. Bacillus sp. DG-2 degraded 3-phenoxybenzoic acid via 3-(2-methoxyphenoxy) benzoic acid, protocatechuate, phenol, and 3,4-dihydroxy phenol.
Bacilli-Mediated Degradation of Explosives
Bacilli play a critical role in the degradation of explosives such as nitrate esters, 2,4,6-Trinitrotoluene (TNT), Trinitrophenol (TNP). Denitration is the main step for the biodegradation of nitrate esters. Meng et al. (1995) studied the biotransformation of glycerol trinitrate by Bacillus sp. ATCC51912 that sequentially denitrated glycerol trinitrate to glycerol via the formation of glycerol dinitrate and glycerol mononitrate isomers. Similarly, Bacillus sp. ATCC51912 denitrated propylene glycol dinitrate to propylene glycol via propylene glycol mononitrate (Sun et al., 1996). Yerson and Christian (2013) isolated pentaerythritol tetranitrate (PETN)-degrading bacterium, Bacillus sp. J8A2 from mining environment. Strain J8A2 utilized PETN as its nitrogen source. Bacterial degradation of PENT generally initiated with sequential denitration of PENT to pentaerythritol via the intermediary formation of tri-, di-, and mononitrate pentaerythritol. An NADPH-dependent PETN reductase enzyme isolated from Bacillus sp. was capable of liberating nitrite from nitrate esters with the oxidation of NADPH.
Bacillus sp. can use TNP as a sole nitrogen source under aerobic conditions (Singh et al., 2011). TNPs has three electron-withdrawing nitro groups that prevent an initial oxidative attack on the aromatic ring. Therefore, the initial steps of TNP degradation are reductive. Bacilli degraded TNP by via hydrogenation to form a Meisenheimercomplex, hydride σ-complex (Singh et al., 2011).
Degradation of 2,4,6-Trinitrotoluene (TNT) by Bacillus sp. occurs also via the reductive route. B. cereus transformed TNT to 2,4-dinitrotoluene and 4-aminodinitrotoluene derivates and degraded 77% of 75 mg L−1, TNT within 96 h (Mercimek et al., 2013). Nyanhongo et al. (2008) reported that Bacillus sp. SF transformed TNT via an initial reduction mechanism to produce hydroxylaminodinitrotoluenes, 4-amino-2,6-dinitrotoluenes, 2-amino-4,6-dinitrotoluenes, different azoxy compounds, 2,6-diaminonitrotoluenes, and 2,4-diaminonitrotoluenes.
Pilot Scale Studies Using Bacilli
For biodegradation purposes, a pilot study plays a vital role before conducting the big scale degradation studies in fields. Chopra and Kumar (2020) examined the degradation of acetaminophen (N-acetyl-para-aminophenol) by B. drentensis strain S1 within the the pilot-scale anaerobic batch reactor. The ideal conditions include temperature 40° C, pH 7, 300 mg/L acetaminophen, and agitation speed 165 rpm (Chopra and Kumar, 2020). 2-Isopropyl-5-methylcyclohexanone and phenothiazine were identified metabolites of the acetaminophen degradation. Sonwani et al. (2019) studied the degradation of naphthalene in a pilot-scale integrated aerobic treatment plant and catechol and 2-naphthol were detected as the major intermediate metabolites. Fujita et al. (2002) studied the removal of toxic soluble selenium (selenite/selenate) using Bacillus sp. SF-1 in a continuous flow bioreactor under an anoxic condition. The outcomes indicated that both selenite and selenate were reduced to elemental selenium at long cell retention times. Sundar et al. (2011) successfully demonstrated the removal of trivalent chromium using Bacillus biofilms through a continuous flow reactor. Pan et al. (2014) used a mixture of planktonic cells and biofilms of B. subtilis for successful removal of Cr(IV) from Cr(IV)-containing wastewater in 10-L pilot-scale experiment. Kim et al. (2014) treated 80 tons of groundwater containing heavy metals using immobilized dead cells of B. drentensis in pilot-scale study and results demonstrated over 93% removal of Cu, Cd, Zn, and Fe. Narayanan et al. (2015) reported the production of laccase from B. subtilis MTCC 2414 for the study of decolorization of Yellow GR, Orange 3R, and T-Blue. They used guaiacol as a substrate under Submerged Fermentation Conditions for the production of laccase, which was immobilized with sodium alginate. The immobilized laccase exhibited optimum activity at pH 7 and temperature 35° C. Results of their studies showed that immobilized laccases degraded Yellow GR (81.72%), Orange 3R (77.2%), and T-Blue (78.55%) at higher efficiency as compared to free laccase. Several researchers investigated the pilot scale-production of commercial compounds using various wastes as substrates (Mohapatra et al., 2017). Yezza et al. (2004) studied the production of Bacillus thuringiensis-based biopesticides in fermenters using wastewater sludge as raw materials and results demonstrated high production of pesticides. Mohapatra et al. (2017) studied bioconversion of fish solid waste into polyhydroxybutyrate using the Bacillus subtilis-based submerged fermentation process. Barros et al. (2008) reported the production of biosurfactant by Bacillus subtilis on a pilot scale using cassava wastewater as substrate.
Advanced Technologies for Bioremediation of Xenobiotic Compounds and Heavy Metals Using Bacilli
This section briefly describes various current technologies used to enhance the bioremediation of xenobiotic compounds and heavy metals.
Metagenomics
Several xenobiotic-degrading enzymes stay undiscovered in light of the fact that a greater part of bacteria (99%) remain uncluturable in laboratory (Arora et al., 2010). In such a case, metagenomics plays a vital role to investigate novel microbial enzymes from whole network of microbial community. The metagenomic approach includes (i) the isolation and purification of DNA from a sample, (ii) cloning of DNA into appropriate vectors, (iii) the transformation of host cells with construct and (iv) functional and sequence based screening of constructed clones (Arora et al., 2010). The sequence-based approaches depend on already known sequences of the target gene and utilize bioinformatics tools. However, the function-based approaches do not include the involvement of metagenomic derived sequences and, in this way, may prompt to the invention of novel genes with desired functions. Several enzymes involved in biodegradation of various xenobiotic compounds have been identified by metagenomic studies of several environmental samples. Sidhu C. et al. (2019) identified novel 2,3-dihydroxybiphenyl 1,2-dioxygenase (BphC-SD3) and catechol 2,3-dioxygenase (C23O-RW1) from the metagenomic DNA isolated from sludge and river water samples. These enzymes were clones, expressed and purified to monitor their abilities to degrade various aromatic compounds. BphC-SD3 specifically oxidized 2,3-dihydroxybiphenyl, catechol, and 3-methylcatechol, whereas C23O-RW1 oxidized catechol, 4-chlorocatechol, 2,3-dihydroxybiphenyl and 3-methylcatechol. Suenaga et al. (2007) studied extradiol dioxygenases diversity in activated sludge used to treat coke plant wastewater by a metagenomic approach and identified 38 new extradiol dioxygenases that formed a new subfamily of extradiol dioxygenases. Singh et al. (2010) identified two flavin monooxygenases from an effluent treatment plant sludge metagenomic library which were involved in the oxidation of indole to a mixture of indigo and indirubin pigments. Nagayama et al. (2015) identified a multicomponent hydroxylase involved in the phenol degradation from a metagenomic library derived from soil sample artificially contaminated with aromatic compounds. Choi et al. (2018) identified and characterized the first metagenome-derived toxoflavin-degrading enzyme that was involved in biodegradation of toxoflavin and its derivatives including methyltoxoflavin, fervenulin, and reumycin. Ye et al. (2010) identified a muti-copper oxidase with laccase activity from activity-based functional screening of a metagenomic library from mangrove soil. The characteristic feature of this laccase was its strong alkaline activity and its high solubility.
Rational Designing
This protein engineering approach requires the knowledge of protein structure, function and mechanism to improve enzyme properties. Several xenobiotic-degrading enzymes of Bacilli have been improved using rational designing approach. Best studied example is laccase enzyme that catalyzes the oxidation of a variety of xenobiotic compounds, including diphenols, polyphenols, diamines, aromatic amines, and synthetic dyes. Mollania et al. (2011) used rational design approach to increase the thermal stability of laccase enzyme of Bacillus sp. HR03. They substituted Glu188 residue with 2 positive (Lys and Arg) and one hydrophobic (Ala) residues to obtain mutants. All variants exhibited strong thermal stability and thermal activation as compared to the wild-type. The 3-fold higher thermal activation and higher T50 (5° C) as compared to native enzyme was observed in the case of the Glu188Lys variant (Mollania et al., 2011). Rasekh et al. (2014) increased the tolerance of this laccase toward organic solvents by substitution of the Glu188 residue with non-polar (Ala, Ile, Leu, and Val) and positively charged (Lys and Arg) residues. All variants showed higher C50 values (organic solvent concentration at which 50% of enzyme activity remains) as compared to the wild type. Non-polar amino acid substitutions created more efficient mutants as they exhibited significantly increased C50 value and decreased thermo inactivation rate in the presence of organic solvents (Rasekh et al., 2014).
Another example of rational design to improve the enzyme activity is cytochrome P450 monooxygenase from Bacillus megaterium 3 (P450 BM3). Carmichael and Wong (2001) reported double mutation in P450 BM3 at R47L and Y51F to enhance its oxidation activity toward phenanthrene and fluoranthene. The mutants showed 40-folds and 10-folds oxidation activity toward phenanthrene and fluoranthene. Li et al. (2001) reported oxidation of polycyclic hydrocarbons such as naphthalene, fluorene, acenaphthene, acenaphthylene, and 9-methylanthracene by triple mutant of P450 BM3 at A74G/F87V/L188Q sites.
Directed Evolution
Directed evolution is an approach of protein engineering to improve the efficiency of proteins without a prior knowledge of amino acid sequences. It is based on the Darwinian principle of evolution and involves (i) the use of rapid molecular manipulations to mutate the target gene and (ii) the subsequent selection of the improved variants by screening (Arora et al., 2010). Using directed evolution, many xenobiotic-degrading genes have been improved for their properties. Best studied example is cytochrome P450 monooxygenase from Bacillus megaterium 3 (P450 BM3) that involves in oxidation of various aromatic compounds. Sideri et al. (2013) used directed evolution to generate mutants of P450 BM3 to hydoxylate chrysene and pyrene. Two rounds of random mutagenesis by error–prone PCR were used to generate mutants. Three mutants exhibited hydroxylation of chrysene and pyrene. These mutants hydroxylated chrysene in different positions and hydroxylate pyrene to 1-hydroxypyrene. Santos et al. (2019) reported that directed evolution of P450 BM3 to improve the hydroxylation activity toward six o-heterocycles; benzo-1,4-dioxane, phthalan, isochroman, 2,3-dihydrobenzofuran, benzofuran, and dibenzofuran. They screened in-house libraries of P450 BM3 to generate P450 BM3 CM1 (R255P/P329H) that was further underwent error–prone PCR, generating P450 BM3 GS2 (R255S/P329H/F331L). Another error-prone PCR of P450 BM3 GS-2 generated P450 BM3 GS3 (I122V/R255S/P329H/F331L). In next step, P450 BM3 WT was subjected to single site saturation mutagenesis (SSM) in the four identified positions and double SSM at positions I122 and R255, which provided the most active variants, P450 BM3 R255G and R255L.
Recombinant DNA Technology or Genetic Engineering
Genetic engineering or recombinant DNA technology includes multiple techniques used to cut up and join together DNA from various biological sources, and to introduce the resulting hybrid DNA into an organism so as to create new combinations of heritable genetic material (Rosenberg, 2017). Genetic engineering is a promising technique to enhance the potentials of microorganisms for the bioremediation of environmental pollutants (Ezezika and Singer, 2010). Genetically engineered bacteria are considered as potential candidates for bioremediation applications in soil, groundwater, and activated sludge (Sayler and Ripp, 2000). A list of few genetically engineered bacteria with their bioremediation applications is presented in Table 2.
Table 2.
A list of few genetically engineered bacteria invloved in bioremediation.
| Genetically engineered bacteria | Compound/heavy metal | Properties/application | References |
|---|---|---|---|
| Bacillus subtilis 168 | Arsenic | Expressed the arsenite S-adenosylmethionine methyltransferase gene from thermophilic algae, Cyanidioschyzon merolae. This bacterium involved in arsenic methylation and volatilization | Huang et al., 2015 |
| Rhodopseudomonas palustris | Mercury | Expressed mercury transport system and metallothionein for Hg2+ uptake | Deng and Jia, 2011 |
| Escherichia coli | Nickel | Expressed nickel-affinity transmembrane protiens and metallothionein for Ni2+ bioaccumulation | Deng et al., 2013 |
| Pseudomonas putida MC4-5222 | 1,2,3-Trichloropropane (TCP) | Expressed the haloalkane dehalogenase (DhaA31). More than 95% degradation of TCP was observed | Samin et al., 2014 |
| Pseudomonas fluorescens | Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | Expressed the RDX-metabolizing enzyme XplA to degrade RDX in the rhizosphere | Lorenz et al., 2013 |
| Pseudomonas putida KTUe | Organophosphates, pyrethroids, and carbamates | A scarless genome editing strategy was used to insert four pesticide degrading genes, vgb, and gfp. This bacterium completely degraded methyl parathion, chlorpyrifos, fenpropathrin, cypermethrin, carbofuran and carbaryl when concentration was 50 mg/L | Gong et al., 2018 |
| Cupriavidus necator JMP134-ONP | Nitrophenols | Inserted ortho-nitrophenol degradation operon (onpABC gene cluster). This bacterium was able to degrade two isomers of nitrophenols | Hu et al., 2014 |
Even though several genetically engineered Bacilli have been constructed for various industrial applications (Wang et al., 2006; Drejer et al., 2020), the bioremediation applications of genetically engineered Bacilli is very limited. Huang et al. (2015) constructed a genetically engineered B. subtilis 168 expressing the arsenite S-adenosylmethionine methyltransferase gene of thermophilic algae for bioremediation of arsenic. This genetically engineered bacterium was able to convert the inorganic As into dimethylarsenate and trimethylarsine oxide via methylation, and also able to volatilize substantial amounts of dimethylarsine and trimethylarsine (Huang et al., 2015). The rate of As methylation and volatilization increased with temperature from 37 to 50° C. However, wild type B. subtilis 168 lacks the properties of methylation and volatilization.
Genome-Editing Technologies
Genome-editing technologies are currently using to manipulate DNA by the engineered nucleases or molecular scissors, which have a wide range of applications in research fields of plants, animals, and microorganisms (Jaiswal et al., 2019). The process of genome editing is generally performed by genome editing tools and involves following steps (i) double standard break in targeted gene sequence (ii) repaired by homologous recombination using self-designed guide sequence complementary to targeted gene sequence (iii) error-prone non-homologous end joining (Jaiswal et al., 2019). The aim of using gene-editing tools is to develop a microbe with great potentials. Jaiswal et al. (2019) describe the role of the gene-editing tools such as Transcription-activators like effector nucleases (TALEN), clustered regularly interspaced short palindromic repeats (CRISPR-Cas), and zinc finger nucleases (ZFNs) to design bacteria with improved metabolic capabilities for enhancing the bioremediation of environmental pollutants.
Genomics
Genomic studies are a powerful tool for the study of microorganisms capable of degrading environmental pollutants (Rodríguez et al., 2020). Next-Generation sequencing technology has been widely used for the whole-genome sequences of various organisms. The whole genomes of several xenobiotic-degrading Bacilli have been sequenced using Next-Generation sequencing technology, and several genes and proteins involved in biodegradation have been identified through gene predictions and annotation of the Bacilli genomes. Hossain et al. (2020) identified chromate transporters in the genome of a chromium-reducing bacterium, B. cereus TN10 isolated from tannery effluent. Chromate transporters are involved in chromium resistance and play a role in the efflux of cytoplasmic chromate. He et al. (2010) identified a putative chromate transport operon, two chromate transporters, azoreductase gene, and four nitroreductase genes in Bacillus cereus SJ1 which may be involved chromate resistance and chromate reduction. The genome of B. cereus S612 contains genes encoding multidrug efflux pumps and reductases that are potentially related to chromium resistance and reduction (Wang et al., 2015). Genome analysis of zearalenone-degrading Bacillus velezensis ANSB01E revealed the presence of genes coding peroxiredoxin and alpha/beta hydrolase, which may be involved in zearalenone degradation (Guo et al., 2020).
Bioinformatics Tools
Bioinformatics approaches including biodegradative databases, pathway prediction systems, and protein-structure predicting tools may be used for biodegradation studies (Arora and Bae, 2014). Biodegradative databases provide information about pollutants, their degradation pathways, bacteria, genes, and enzymes in their degradation (Arora and Bae, 2014). Examples of these databases are the EAWAG Biocatalysis/Biodegradation Database (EAWAG-BBD), a database of biodegradative oxygenases (OxDBase), Biodegradation Network-Molecular Biology database (Bionemo), MetaCyc, and BioCyc (Arora and Bae, 2014). The structure of enzymes involved in biodegradation of environmental pollutants in Bacilli can be predicted by online structure prediction tools such as Iterative Threading Assembly Refinement server (I-TASSER) (Yang and Zhang, 2015), SWISS-MODEL (Waterhouse et al., 2018), and optimized protein fold RecognitION (ORION) (Ghouzam et al., 2015).
Conclusion
Many Bacilli have been isolated and characterized for degradation of various environmental pollutants including chloronitrophenols, dyes, drugs, pesticide, explosives, polycyclic aromatic compounds, heterocyclic aromatic compounds, and heavy metals. The biochemical characterization of degradation pathways of various environmental pollutants was extensively studied in Bacilli. The genes involved in the degradation of various xenobiotic compounds have been identified from the genome sequences of various xenobiotic degrading Bacilli. Further studies on cloning and expression of these genes would be useful to understand the mechanism of biodegradation. The construction of genetically engineered Bacilli with improved degradation efficiency will be useful for biodegradation applications. Furthermore, genome editing tools may be used to develop more efficient Bacilli for the bioremediation of pollutants. Bioinformatics tools such as databases, pathway prediction systems, and protein structure predicting tools are useful to determine the fate of environmental pollutants in the fields.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author acknowledges the Department of Biotechnology, India to provide him Ramalingaswami Re-entry Fellowship.
References
- Abdel-Shafy H. I., Mansour M. S. (2016). A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 25, 107–123. 10.1016/j.ejpe.2015.03.011 [DOI] [Google Scholar]
- Ali M., Naqvi T. A., Kanwal M., Rasheed F., Hameed A., Ahmed S. (2012). Detection of the organophosphate degrading gene opdA in the newly isolated bacterial strain Bacillus pumilus W1. Anal. Microbiol. 62, 233–239. 10.1007/s13213-011-0251-4 [DOI] [Google Scholar]
- Anjaneya O., Souche S. Y., Santoshkumar M., Karegoudar T. B. (2011). Decolorization of sulfonated azo dye metanil yellow by newly isolated bacterial strains: Bacillus sp. strain AK1 and Lysinibacillus sp. strain AK2. J. Hazard. Mater. 190, 351–358. 10.1016/j.jhazmat.2011.03.044 [DOI] [PubMed] [Google Scholar]
- Annweiler E., Richnow H. H., Antranikian G., Hebenbrock S., Garms C., Franke S., et al. (2000). Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl. Environ. Microbiol. 66, 518–523. 10.1128/AEM.66.2.518-523.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S., Liaquat F., Khan Q. M., Khalid Z. M., Iqbal S. (2009). Biodegradation of chlorpyrifos and its hydrolysis product 3, 5, 6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J. Hazard. Mater. 168, 400–405. 10.1016/j.jhazmat.2009.02.059 [DOI] [PubMed] [Google Scholar]
- Arora P. K. (2012). Decolourization of 4-chloro-2-nitrophenol by a soil bacterium, Bacillus subtilisRKJ 700. PLoS ONE 7:e52012. 10.1371/journal.pone.0052012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. K., Bae H. (2014). Integration of bioinformatics to biodegradation. Biol. Proced. Online. 16:8. 10.1186/1480-9222-16-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. K., Jain R. K. (2012). Biotransformation of 4-chloro-2-nitrophenol into 5-chloro-2-methylbenzoxazole by a marine Bacillus sp. strain MW-1. Biodegradation 23, 325–331. 10.1007/s10532-011-9512-y [DOI] [PubMed] [Google Scholar]
- Arora P. K., Sharma A., Mehta R., Shenoy B. D., Srivastava A., Singh V. P. (2012). Metabolism of 4-chloro-2-nitrophenol in a gram-positive bacterium, Exiguobacterium sp. PMA. Microb. Cell. Fact. 11:150. 10.1186/1475-2859-11-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. K., Srivastava A., Garg S. K., Singh V. P. (2018). Recent advances in degradation of chloronitrophenols. Bioresour. Techol. 250, 902–909. 10.1016/j.biortech.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Arora P. K., Srivastava A., Singh V. P. (2010). Application of monooxygenases in dehalogenation, desulphurization, denitrification and hydroxylation of aromatic compounds. J. Bioremed. Biodegrad. 1:112 10.4172/2155-6199.1000112 [DOI] [Google Scholar]
- Arora P. K., Srivastava A., Singh V. P. (2016). Diversity of 4-chloro-2-nitrophenol-degrading bacteria in a waste water sample. J. Chem. 2016:7589068 10.1155/2016/7589068 [DOI] [Google Scholar]
- Asad S., Amoozegar M. A., Pourbabaee A. A., Sarbolouki M. N., Dastgheib S. M. M. (2007). Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour. Technol. 98, 2082–2088. 10.1016/j.biortech.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Awasthi N., Singh A. K., Jain R. K., Khangarot B. S., Kumar A. (2003). Degradation and detoxification of endosulfan isomers by a defined co-culture of two Bacillus strains. Appl. Microbiol. Biotechol. 62, 279–283. 10.1007/s00253-003-1241-7 [DOI] [PubMed] [Google Scholar]
- Bachate S. P., Nandre V. S., Ghatpande N. S., Kodam K. M. (2013). Simultaneous reduction of Cr (VI) and oxidation of As (III) by Bacillus firmus TE7 isolated from tannery effluent. Chemosphere 90, 2273–2278. 10.1016/j.chemosphere.2012.10.081 [DOI] [PubMed] [Google Scholar]
- Balapurea K. H., Jainb K., Chattarajb S., Bhatta N. S., Madamwarb D. (2014). Co-metabolic degradation of diazo dye—reactive blue 160 by enriched mixed cultures BDN. J. Hazard. Mater. 279, 85–95. 10.1016/j.jhazmat.2014.06.057 [DOI] [PubMed] [Google Scholar]
- Banat I. M., Nigam P., Singh D., Marchant R. (1996). Microbial decolorization of textile-dye-containing effluents: a review. Bioresour. Technol. 58, 217–227. 10.1016/S0960-8524(96)00113-7 [DOI] [Google Scholar]
- Barathi S., Karthik C., Selvaraj N., Padikasan I. A. (2020). Biodegradation of textile dye reactive blue 160 by Bacillus firmus (Bacillaceae: Bacillales) and non-target toxicity screening of their degraded products. Toxicol. Rep. 7, 16–22. 10.1016/j.toxrep.2019.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F. F., Ponezi A. N., Pastore G. M. (2008). Production of biosurfactant by Bacillus subtilis LB5a on a pilot scale using cassava wastewater as substrate. J. Ind. Microbiol. Biotechnol. 35, 1071–1078. 10.1007/s10295-008-0385-y [DOI] [PubMed] [Google Scholar]
- Benkhaya S., M'rabet S., El Harfi A. (2020). Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6:e03271. 10.1016/j.heliyon.2020.e03271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beunink J., Rehm H. J. (1990). Coupled reductive and oxidative degradation of 4-chloro-2-nitrophenol by a co-immobilized mixed culture system. Appl. Microbiol. Biotechnol. 34, 108–115. 10.1007/BF00170933 [DOI] [PubMed] [Google Scholar]
- Bhadbhade B. J., Sarnik S. S., Kanekar P. P. (2002). Biomineralization of an organophosphorus pesticide, monocrotophos, by soil bacteria. J. Appl. Microbiol. 93, 224–234. 10.1046/j.1365-2672.2002.01680.x [DOI] [PubMed] [Google Scholar]
- Bhatt P., Huang Y., Zhang W., Sharma A., Chen S. (2020). Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 8:223. 10.3390/microorganisms8020223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi N., Hamayun M., Khan S. A., Iqbal A., Islam B., Shah F., et al. (2018). Anthracene biodegradation capacity of newly isolated rhizospheric bacteria Bacillus cereus S13. PLoS ONE 13:e0201620. 10.1371/journal.pone.0201620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birolli W. G., Borges E. M., Nitschke M., Romão L. P., Porto A. L. (2016). Biodegradation pathway of the pyrethroid pesticide esfenvalerate by bacteria from different biomes. Water Air Soil Poll. 227:271 10.1007/s11270-016-2968-y [DOI] [Google Scholar]
- Bisht S., Pandey P., Kaur G., Aggarwal H., Sood A., Kumar V., et al. (2014). Utilization of endophytic strain Bacillus sp. SBER3 for biodegradation of polyaromatic hydrocarbons (PAH) in soil model system. Eur. J. Soil. Biol. 60, 67–76. 10.1016/j.ejsobi.2013.10.009 [DOI] [Google Scholar]
- Bonifer K. S., Wen X., Hasim S., Phillips E. K., Dunlap R. N., Gann E. R., et al. (2019). Bacillus pumilus B12 degrades polylactic acid and degradation is affected by changing nutrient conditions. Front. Microbiol. 10:2548. 10.3389/fmicb.2019.02548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn C., Bayly R. C., Knackmus H. J. (1988). The in vivo construction of 4-chloro-2-nitrophenol assimilatory bacteria. Arch. Microbiol. 150, 171–177. 10.1007/BF00425158 [DOI] [Google Scholar]
- Bunk B., Biedendieck R., Jahn D., Vary P. S. (2010). Bacillus megaterium and other bacilli: industrial applications, in Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology, Vol 1. ed Flickinger M. C. (Wiley: Hoboken; ), 1–15. 10.1002/9780470054581.eib063 [DOI] [Google Scholar]
- Camargo F. A. O., Okeke B. C., Bento F. M., Frankenberger W. T. (2003). In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu 2+. Appl. Microbial. Biotechnol. 62, 569–573. 10.1007/s00253-003-1291-x [DOI] [PubMed] [Google Scholar]
- Carmichael A. B., Wong L. L. (2001). Protein engineering of Bacillus megaterium CYP102: the oxidation of polycyclic aromatic hydrocarbons. J. Biochem. 268, 3117–3125. 10.1046/j.1432-1327.2001.02212.x [DOI] [PubMed] [Google Scholar]
- Caulier S., Nannan C., Gillis A., Licciardi F., Bragard C., Mahillon J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 10:302. 10.3389/fmicb.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Evans F. E. (1984). Evidence for an arene oxide-NIH shift pathway in the transformation of naphthalene to 1-naphthol by Bacillus cereus. Arch. Microbial. 138, 283–286. 10.1007/BF00410891 [DOI] [PubMed] [Google Scholar]
- Chang S. J., Lin Y. C. (2001). Decolorization kinetics of recombinant Escherichia coli strain harboring azo dye decolorization determinants for Rhodococcus sp. Biotechnol. Lett. 23, 631–636. 10.1023/A:1010306114286 [DOI] [Google Scholar]
- Chen S., Chang C., Deng Y., An S., Dong Y. H., Zhou J., et al. (2014). Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agri. Food Chem. 62, 2147–2157. 10.1021/jf404908j [DOI] [PubMed] [Google Scholar]
- Chen S., Deng Y., Chang C., Lee J., Cheng Y., Cui Z., et al. (2015). Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci. Rep. 5:8784. 10.1038/srep08784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Hu W., Xiao Y., Deng Y., Jia J., Hu M. (2012a). Degradation of 3-phenoxybenzoic acid by a Bacillus sp. PLoS ONE 7:e50456. 10.1371/journal.pone.0050456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Luo J., Hu M., Lai K., Geng P., Huang H. (2012b). Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Biores. Technol. 110, 97–104. 10.1016/j.biortech.2012.01.106 [DOI] [PubMed] [Google Scholar]
- Chen Z., Huang Z., Cheng Y., Pan D., Pan X., Yu M., et al. (2012c). Cr (VI) uptake mechanism of Bacillus cereus. Chemosphere 87, 211–216. 10.1016/j.chemosphere.2011.12.050 [DOI] [PubMed] [Google Scholar]
- Chen Z., Pan X., Chen H., Guan X., Lin Z. (2016). Biomineralization of Pb (II) into Pb-hydroxyapatite induced by Bacillus cereus 12-2 isolated from lead–zinc mine tailings. J. Hazard. Mater. 301, 531–537. 10.1016/j.jhazmat.2015.09.023 [DOI] [PubMed] [Google Scholar]
- Choi J. E., Nguyen C. M., Lee B., Park J. H., Oh J. Y., Choi J. S., et al. (2018). Isolation and characterization of a novel metagenomic enzyme capable of degrading bacterial phytotoxin toxoflavin. PLoS ONE 13:e0183893. 10.1371/journal.pone.0183893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra S., Kumar D. (2020). Characterization, optimization and kinetics study of acetaminophen degradation by Bacillus drentensis strain S1 and waste water degradation analysis. Bioresour. Bioprocess. 7, 1–18. 10.1186/s40643-020-0297-x [DOI] [Google Scholar]
- Chris Felshia S., Ashwin Karthick N., Thilagam R., Gnanamani A. (2020). Elucidation of 2, 4-Dichlorophenol degradation by Bacillus licheniformis strain SL10. Environ. Technol. 20241, 366–377. 10.1080/09593330.2018.1498923 [DOI] [PubMed] [Google Scholar]
- Chung K. T., Cerniglia C. E. (1992). Mutagenicity of azo dyes: structure–activity relationships. Mutat. Res. 277, 201–220. 10.1016/0165-1110(92)90044-A [DOI] [PubMed] [Google Scholar]
- Claus D., Berkeley R. C. W. (1986). Genus Bacillus Cohn 1872, 174AL, in Bergey's Manual of Systematic Bacteriology, eds Sneath P. H. A., Mair N. S., Sharpe M. E., Holt J. G. (Baltimore: Williams and Wilkins; ), 1105–1139. [Google Scholar]
- Crawford R. L. (1976). Pathways of 4-hydroxybenzoate degradation among species of Bacillus. J. Bacteriol. 127, 204–210. 10.1128/JB.127.1.204-210.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Bromley J. W., Perkins-Olson P. E. (1979). Catabolism of protocatechuate by Bacillus macerans. Appl. Environ. Microbiol. 37, 614–618. 10.1128/AEM.37.3.614-618.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycoń M., Piotrowska-Seget Z. (2016). Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microbiol. 7:1463. 10.3389/fmicb.2016.01463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T. C. H., Nguyen D. T., Thai H., Nguyen T. C., Tran T. T. H., Le V. H., et al. (2018). Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci.: Nanosci. Nanotech. 9:015014 10.1088/2043-6254/aaabaf [DOI] [Google Scholar]
- Das K., Mukherjee A. K. (2007). Differential utilization of pyrene as the sole source of carbon by Bacillus subtilis and Pseudomonas aeruginosa strains: role of biosurfactants in enhancing bioavailability. J. Appl. Microbial. 102, 195–203. 10.1111/j.1365-2672.2006.03070.x [DOI] [PubMed] [Google Scholar]
- Das M., Bhattacharya A., Banu S., Kotoky J. (2017). Enhanced biodegradation of anthracene by Bacillus cereus strain JMG-01 isolated from hydrocarbon contaminated soils. Soil Sediment Contam. 26, 510–525. 10.1080/15320383.2017.1357111 [DOI] [Google Scholar]
- Das S., Mishra J., Das S. K., Pandey S., Rao D. S., Chakraborty A., et al. (2014). Investigation on mechanism of Cr (VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 96, 112–121. 10.1016/j.chemosphere.2013.08.080 [DOI] [PubMed] [Google Scholar]
- Dash D. M., Osborne J. W. (2020). Biodegradation of monocrotophos by a plant growth promoting Bacillus aryabhattai (VITNNDJ5) strain in artificially contaminated soil. Int. J. Environ. Sci. Technol. 17, 1475–1490. 10.1007/s13762-019-02432-1 [DOI] [Google Scholar]
- Dawkar V. V., Jadhav U. U., Ghodake G. S., Govindwar S. P. (2009). Effect of inducers on the decolorization and biodegradation of textile azo dye navy blue 2GL by Bacillus sp. VUS. Biodegradation 20, 777–787. 10.1007/s10532-009-9266-y [DOI] [PubMed] [Google Scholar]
- Dawkar V. V., Jadhav U. U., Jadhav S. U., Govindwar S. P. (2008). Biodegradation of disperse textile dye Brown 3REL by newly isolated Bacillus sp. VUS. J. Appl. Microbiol. 105, 14–24. 10.1111/j.1365-2672.2008.03738.x [DOI] [PubMed] [Google Scholar]
- Dawkar V. V., Jadhav U. U., Tamboli D. P., Govindwar S. P. (2010). Efficient industrial dye decolorization by Bacillus sp. VUS with its enzyme system. Ecotoxicol. Environ. Saf. 73, 1696–1703. 10.1016/j.ecoenv.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Demharter W., Hensel R. (1989). Bacillus thermocloaceae sp. nov., a new thermophilic species from sewage sludge. Syst. Appl. Microbiol. 11, 272–276. 10.1016/S0723-2020(89)80025-6 [DOI] [Google Scholar]
- Deng D., Guo J., Zeng G., Sun G. (2008). Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11. Int. Biodeterior. Biodegr. 62, 263–269. 10.1016/j.ibiod.2008.01.017 [DOI] [Google Scholar]
- Deng X., He J., He N. (2013). Comparative study on Ni2+-affinity transport of nickel/cobalt permeases (NiCoTs) and the potential of recombinant Escherichia coli for Ni2+ bioaccumulation. Bioresour. Technol. 130, 69–74. 10.1016/j.biortech.2012.11.133 [DOI] [PubMed] [Google Scholar]
- Deng X., Jia P. (2011). Construction and characterization of a photosynthetic bacterium genetically engineered for Hg2+ uptake. Bioresour. Technol. 102, 3083–3088. 10.1016/j.biortech.2010.10.051 [DOI] [PubMed] [Google Scholar]
- Dhanjal S., Cameotra S. S. (2010). Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Fact. 9:52. 10.1186/1475-2859-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep P., Mahadevan R., Yakunin A. F. (2018). Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 6:157. 10.3389/fbioe.2018.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez-Méndez A., García-Fraile P., Solano F., Rivas R. (2019). The ant lasius niger is a new source of bacterial enzymes with biotechnological potential for bleaching dye. Sci. Rep. 9:15217. 10.1038/s41598-019-51669-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Malaviya D., Pandiyan K., Singh U. B., Sahu A., Shukla R., et al. (2015). Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7, 2189–2212. 10.3390/su7022189 [DOI] [Google Scholar]
- Drejer E. B., Chan D. T. C., Haupka C., Wendisch V. F., Brautaset T., Irla M. (2020). Methanol-based acetoin production by genetically engineered Bacillus methanolicus. Green Chem. 22, 788–802. 10.1039/C9GC03950C [DOI] [Google Scholar]
- Easton J. (1995). The dye maker's view, in Colour in Dyehouse Effluent, ed Cooper P. (Bradford: Society of Dyers and Colourists; ), 9–21. [Google Scholar]
- El-Helow E. R., Badawy M. E. I., Mabrouk M. E. M., Mohamed E. A. H., El-Beshlawy Y. M. (2013). Biodegradation of chlorpyrifos by a newly isolated Bacillus subtilis strain Y242. J. Bioreme. 17, 113–123. 10.1080/10889868.2013.786019 [DOI] [Google Scholar]
- Ewida A. Y., El-Sesy M. E., Abou Zeid A. (2019). Complete degradation of azo dye acid red 337 by Bacillus megaterium KY848339.1 isolated from textile wastewater. Water Sci. 33, 154–161. 10.1080/11104929.2019.1688996 [DOI] [Google Scholar]
- Ezezika O. C., Singer P. A. (2010). Genetically engineered oil-eating microbes for bioremediation: prospects and regulatory challenges. Technol. Soc. 32, 331–335. 10.1016/j.techsoc.2010.10.010 [DOI] [Google Scholar]
- Fujita M., Ike M., Kashiwa M., Hashimoto R., Soda S. (2002). Laboratory-scale continuous reactor for soluble selenium removal using selenate-reducing bacterium, Bacillus sp. SF-1. Biotechnol. Bioeng. 80, 755–761. 10.1002/bit.10425 [DOI] [PubMed] [Google Scholar]
- Gangola S., Sharma A., Bhatt P., Khati P., Chaudhary P. (2018). Presence of esterase andlaccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 8:12755. 10.1038/s41598-018-31082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbisu C., Gonzalez S., Yang W. H., Yee B. C., Carlson D. L., Yee A., et al. (1995). Physiological mechanisms regulating the conversion of selenite to elemental selenium by Bacillus subtilis. BioFactors 5, 29–37. [PubMed] [Google Scholar]
- Gharbani P., Khosravi M., Tabatabaii S. M., Zare K., Dastmalchi S., et al. (2010). Degradation of trace aqueous 4-chloro-2-nitrophenol occurring in pharmaceutical industrial wastewater by ozone. Int. J. Environ. Sci. Technol. 7, 377–384. 10.1007/BF03326147 [DOI] [Google Scholar]
- Ghouzam Y., Postic G., de Brevern A. G., Gelly J. C. (2015). Improving protein fold recognition with hybrid profiles combining sequence and structure evolution. Bioinformatics. 31, 3782–3789. 10.1093/bioinformatics/btv462 [DOI] [PubMed] [Google Scholar]
- Gong T., Xu X., Dang Y., Kong A., Wu Y., Liang P., et al. (2018). An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates. Sci. Total. Environ. 628, 1258–1265. 10.1016/j.scitotenv.2018.02.143 [DOI] [PubMed] [Google Scholar]
- Górny D., Guzik U., Hupert-Kocurek K., Wojcieszyńska D. (2019). A new pathway for naproxen utilisation by Bacillus thuringiensis B1 (2015b) and its decomposition in the presence of organic and inorganic contaminants. J. Environ. Manag. 239, 1–7. 10.1016/j.jenvman.2019.03.034 [DOI] [PubMed] [Google Scholar]
- Govarthanan M., Lee K. J., Cho M., Kim J. S., Kamala-Kannan S., Oh B. T. (2013). Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere 90, 2267–2272. 10.1016/j.chemosphere.2012.10.038 [DOI] [PubMed] [Google Scholar]
- Gunasekar V., Gowdhaman D., Ponnusami V. (2013). Biodegradation of reactive red M5B dye using Int. J. Chem.Tech. Res. 5, 131–135. [Google Scholar]
- Guo Y., Zhou J., Tang Y., Ma Q., Zhang J., Ji C., et al. (2020). Characterization and genome analysis of a zearalenone-degrading Bacillus velezensis strain ANSB01E. Curr. Microbiol. 77, 273–278. 10.1007/s00284-019-01811-8 [DOI] [PubMed] [Google Scholar]
- Hao J., Liu J., Sun M. (2014). Identification of a marine Bacillus strain C5 and parathion-methyl degradation characteristics of the extracellular esterase B1. Bio Med. Res. Int. 2014:863094. 10.1155/2014/863094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S., Arfaeinia H., Sharafi H., Moradi M., Ehsanifar M. (2017). Efficient degradation of 4-chloro-2-nitrophenol using photocatalytic ozonation with nano-zinc oxide impregnated granular activated carbon (ZnO–GAC). Desalination Water Treat. 93, 145–151. 10.5004/dwt.2017.21427 [DOI] [Google Scholar]
- Hazen T. C. (2010). Cometabolic bioremediation, in Handbook of Hydrocarbon and Lipid Microbiology, ed Timmis K. N. (Berlin; Heidelberg: Springer; ), 2505–2514. 10.1007/978-3-540-77587-4_185 [DOI] [Google Scholar]
- He M., Li X., Guo L., Miller S. J., Rensing C., Wang G. (2010). Characterization and genomic analysis of chromate resistant and reducing Bacillus cereus strain SJ1. BMC Microbiol. 10:221. 10.1186/1471-2180-10-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Herrera A. V., Asensio-Ramos M., Herna'ndez-Borges J., Rodriguez-Delgado M. A. (2016). Pesticides and herbicides: types, uses, and determination of herbicides, in: Encyclopedia of Food and Health, eds Finglas P. M., Toldrá F. (Oxford, UK: Academic; ), 326–332. 10.1016/B978-0-12-384947-2.00536-5 [DOI] [Google Scholar]
- Hossain M., Tasnim S., Safa A., Rayhan A. B. H., Khan M. T. I. A., Bulbul N., et al. (2020). Draft genome sequence of bacillus cereus tn10, a chromium-resistant and-reducing strain isolated from tannery effluent. Microbiol. Resour. Announc. 9, e00603–e00620. 10.1128/MRA.00603-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Jiang X., Zhang J. J., Zhou N. Y. (2014). Construction of an engineered strain capable of degrading two isomeric nitrophenols via a sacB-and gfp-based markerless integration system. Appl. Microbial. Biotechnol. 98, 4749–4756. 10.1007/s00253-014-5567-0 [DOI] [PubMed] [Google Scholar]
- Huang K., Chen C., Shen Q., Rosen B. P., Zhao F. J. (2015). Genetically engineering Bacillus subtilis with a heat-resistant arsenite methyltransferase for bioremediation of arsenic-contaminated organic waste. Appl. Environ. Microbiol. 81, 6718–6724. 10.1128/AEM.01535-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. S., Elbadawi Y. B., El-Tayeb M. A., Al-Salamah A. A. (2012). Hexavalent chromium reduction by novel chromate resistant alkaliphilic Bacillus sp. strain KSUCr9a. Afr. J. Biotechnol. 11, 3832–3841. 10.5897/AJB11.3026 [DOI] [Google Scholar]
- Igiri B. E., Okoduwa S. I., Idoko G. O., Akabuogu E. P., Adeyi A. O., Ejiogu I. K. (2018). Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J. Toxicol. 2018:2568038. 10.1155/2018/2568038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail S., Dadrasnia A. (2015). Biotechnological potential of Bacillus salmalaya 139SI: a novel strain for remediating water polluted with crude oil waste. PLoS ONE 10:e0120931. 10.1371/journal.pone.0120931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Singh D. K., Shukla P. (2019). Gene editing and systems biology tools for pesticide bioremediation: a review. Front. Microbiol. 10:87. 10.3389/fmicb.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj R., Megha P., Sreedev P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 9, 90–100. 10.1515/intox-2016-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Tang M., Chen J., Yang Y. (2019). Identification and degradation characteristics of Bacillus cereus strain WD-2 isolated from prochloraz-manganese-contaminated soils. PLoS ONE 14:e0220975. 10.1371/journal.pone.0220975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B., Kabariya K., Nakrani S., Khan A., Parabia F. M., Doshi H. V., et al. (2013). Biodegradation of turquoise blue dye by Bacillus megaterium isolated from industrial effluent. Am. J. Environ. Protec. 1, 41–46. 10.12691/env-1-2-5 [DOI] [Google Scholar]
- Jouzani G. S., Valijanian E., Sharafi R. (2017). Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 101, 2691–2711. 10.1007/s00253-017-8175-y [DOI] [PubMed] [Google Scholar]
- Juwarkar A. A., Yadav S. K. (2010). Bioaccumulation and biotransformation of heavy metals, in Bioremediation Technology, ed Fulekar M.H. (Dordrecht: Springer; ), 266–284. 10.1007/978-90-481-3678-0_9 [DOI] [Google Scholar]
- Kadam A. A., Kulkarni A. N., Lade H. S., Govindwar S. P. (2014). Exploiting the potential of plant growth promoting bacteria in decolorization of dye disperse red 73 adsorbed on milled sugarcane bagasse under solid state fermentation. Inter. Biodet. Biodeg. 86, 364–371. 10.1016/j.ibiod.2013.10.012 [DOI] [Google Scholar]
- Kashiwa M., Ike M., Mihara H., Esaki N., snd Fujita M. (2001). Removal of soluble selenium by a selenate-reducing bacterium Bacillus sp. SF-1. BioFactors 14, 261–265. 10.1002/biof.5520140132 [DOI] [PubMed] [Google Scholar]
- Khanna P., Goyal D., Khanna S. (2011). Pyrene degradation by Bacillus pumilus isolated from crude oil contaminated soil. Polycycl. Aromat. Comp. 31, 1–15. 10.1080/10406638.2010.542792 [DOI] [Google Scholar]
- Khatoon H., Rai J. P. N. (2020). Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnol. Rep. 26:e00459. 10.1016/j.btre.2020.e00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Lee M., Wang S. (2014). Heavy metal removal in groundwater originating from acid mine drainage using dead Bacillus drentensis sp. immobilized in polysulfone polymer. J. Environ. Manage. 146, 568–574. 10.1016/j.jenvman.2014.05.042 [DOI] [PubMed] [Google Scholar]
- Kochher S., Kumar J. (2011). Microbial decolourization of crystal violet by Bacillus subtilis. Biol. Forum. 3, 82–86. [Google Scholar]
- Kolekar Y. M., Pawar S. P., Gawai K. R., Lokhande P. D., Shouche Y. S., Kodam K. M. (2008). Decolorization and degradation of disperse blue 79 and acid orange 10, by Bacillus fusiformis KMK5 isolated from the textile dye contaminated soil. Bioresour. Technol. 99:8999. 10.1016/j.biortech.2008.04.073 [DOI] [PubMed] [Google Scholar]
- Kumar A., Bhoot N., Soni I., John P. J. (2014). Isolation and characterization of a Bacillus subtilis strain that degrades endosulfan and endosulfan sulfate. 3 Biotech 4, 467–475. 10.1007/s13205-013-0176-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Philip L. (2006). Enrichment and isolation of a mixed bacterial culture for complete mineralization of endosulfan. J. Environ. Sci. Health. B 41, 81–96. 10.1080/03601230500234935 [DOI] [PubMed] [Google Scholar]
- Kumar N., Sinha S., Mehrotra T., Singh R., Tandon S., Thakur I. S. (2019). Biodecolorization of azo dye acid black 24 by Bacillus pseudomycoides: process optimization using box behnken design model and toxicity assessment. Bioresou. Technol. Rep. 8:1003 10.1016/j.biteb.2019.100311 [DOI] [Google Scholar]
- Kumar P., Patel S. K., Lee J.-K., Kalia V. C. (2013). Extending the limits of Bacillus for novel biotechnological applications. Biotechnol. Adv. 31, 1543–1561. 10.1016/j.biotechadv.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Kuroda M., Ayano H., Sei K., Yamashita M., Ike M. (2015). Draft genome sequence of Bacillus selenatarsenatis SF-1T, a promising agent for bioremediation of environments contaminated with selenium and arsenic. Genome Announc. 3, e01466–14. 10.1128/genomeA.01466-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W., Liu S. L., Zhao N., Yuan H. Y. (2012). Isolation, identification and characterization of a cypermethrin-degrading strain. Food Sci. 21, 157–163. 10.3389/fmicb.2019.01778 [DOI] [Google Scholar]
- Lampis S., Zonaro E., Bertolini C., Bernardi P., Butler C. S., Vallini G. (2014). Delayed formation of zero-valent selenium nanoparticles by Bacillus mycoides SeITE01 as a consequence of selenite reduction under aerobic conditions. Microb. Cell Fact. 13:35. 10.1186/1475-2859-13-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J. D., Hernandez-Velasco X., Wolfenden R. E., Vicente J. L., Wolfenden A. D., Menconi A., et al. (2016). Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 3:95. 10.3389/fvets.2016.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. G., Lalman J. A., Biswas N. (2004). Biodegradation of Red B dye by Bacillus sp. OY1-2. Environ. Technol. 25, 1167–1176. 10.1080/09593332508618384 [DOI] [PubMed] [Google Scholar]
- Li Q. S., Ogawa J., Schmid R. D., Shimizu S. (2001). Engineering cytochrome P450 BM-3 for oxidation of polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 67, 5735–5739. 10.1128/AEM.67.12.5735-5739.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. S., Hung C. H., Chao S. L. (2013). Decolorization of azo dye reactive black B by Bacillus cereus strain HJ-1. Chemosphere 90, 2109–2114. 10.1016/j.chemosphere.2012.10.077 [DOI] [PubMed] [Google Scholar]
- Lily M. K., Bahuguna A., Dangwal K., Garg V. (2010). Optimization of an inducible, chromosomally encoded benzo [a] pyrene (BaP) degradation pathway in Bacillus subtilis BMT4i (MTCC 9447). Ann. Microbiol. 60, 51–58. 10.1007/s13213-009-0010-y24031437 [DOI] [Google Scholar]
- Lin C., Gan L., Chen Z. L. (2010). Biodegradation of naphthalene by strain Bacillus fusiformis (BFN). J. Hazar. Mater. 182, 771–777. 10.1016/j.jhazmat.2010.06.101 [DOI] [PubMed] [Google Scholar]
- Liu M., Luo K., Wang Y., Zeng A., Zhou X., Luo F., et al. (2014). Isolation, identification and characteristics of an endophytic quinclorac degrading bacterium Bacillus megaterium Q3. PLoS ONE 9:e108012. 10.1371/journal.pone.0108012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A., Rylott E. L., Strand S. E., Bruce N. C. (2013). Towards engineering degradation of the explosive pollutant hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine in the rhizosphere. FEMS Microbiol. Lett. 340, 49–54. 10.1111/1574-6968.12072 [DOI] [PubMed] [Google Scholar]
- Mandal K., Singh B., Jariyal M., Gupta V. K. (2013). Microbial degradation of fipronil by Bacillus thuringiensis. Ecotoxicol. Environ. Saf. 93, 87–92. 10.1016/j.ecoenv.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Marchlewicz A., Guzik U., Smułek W., Wojcieszyńska D. (2017). Exploring the degradation of ibuprofen by Bacillus thuringiensis B1 (2015b): the new pathway and factors affecting degradation. Molecules 22:1676. 10.3390/molecules22101676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashetty S. B., Manohar S., Karegoudar T. B. (1996). Degradation of 3-hydroxybenzoic acid by a Bacillus species. Indian J. Biochem. Biophys. 33, 145–148. [PubMed] [Google Scholar]
- Masih A., Taneja A. (2006). Polycyclic aromatic hydrocarbons (PAHs) concentrations and related carcinogenic potencies in soil at a semi-arid region of India. Chemosphere 65, 449–456. 10.1016/j.chemosphere.2006.01.062 [DOI] [PubMed] [Google Scholar]
- Masood F., Malik A. (2011). Hexavalent chromium reduction by Bacillus sp. strain FM1 isolated from heavy-metal contaminated soil. Bull. Environ. Contam. Toxicol. 86, 114–119. 10.1007/s00128-010-0181-z [DOI] [PubMed] [Google Scholar]
- Meng M., Sun W. Q., Geelhaar L. A., Kumar G., Patel A. R., Payne G. F., et al. (1995). Denitration of glycerol trinitrate by resting cells and cell extracts of Bacillus thuringiensis/cereus and Enterobacter agglomerans. Appl. Environ. Microbiol. 61, 2548–2553. 10.1128/AEM.61.7.2548-2553.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercimek H., Dincer S., Guzeldag G., Ozsavli A., Matyar F. (2013). Aerobic biodegradation of 2,4,6-trinitrotoluene (TNT) by Bacillus cereus isolated from contaminated soil. Microb. Ecol. 66, 512–523. 10.1007/s00248-013-0248-6 [DOI] [PubMed] [Google Scholar]
- Mishra R. R., Prajapati S., Das J., Dangar T. K., Das N., Thatoi H. (2011). Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from bhitarkanika mangrove soil and characterization of reduced product. Chemosphere 84, 1231–1237. 10.1016/j.chemosphere.2011.05.025 [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Sarkar B., Samantaray D. P., Daware A., Maity S., Pattnaik S., et al. (2017). Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ. Technol. 38, 3201–3208. 10.1080/09593330.2017.1291759 [DOI] [PubMed] [Google Scholar]
- Mollania N., Khajeh K., Ranjbar B., Hosseinkhani S. (2011). Enhancement of a bacterial laccase thermostability through directed mutagenesis of a surface loop. Enzyme. Microb. Technol. 49, 446–452. 10.1016/j.enzmictec.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Nagayama H., Sugawara T., Endo R., Ono A., Kato H., Ohtsubo Y., et al. (2015). Isolation of oxygenase genes for indigo-forming activity from an artificially polluted soil metagenome by functional screening using Pseudomonas putida strains as hosts. Appl. Microbiol. Biotechnol. 99, 4453–4470. 10.1007/s00253-014-6322-2 [DOI] [PubMed] [Google Scholar]
- Narayanan M. P., Murugan S., Eva A. S., Devina S. U., Kalidass S. (2015). Application of immobilized laccase from Bacillus subtilis MTCC 2414 on decolourization of synthetic dyes. Res. J. Microbiol. 10, 421–432. 10.3923/jm.2015.421.432 [DOI] [Google Scholar]
- Ni'matuzahroh T., Pramadita A. R. A., Pratiwi I. A., Salamun F., Sumarsih S. (2017). Biodegradation of naphthalene and phenanthren by Bacillus subtilis 3KP. AIP Conf. Proc. 1854:20026 10.1063/1.4985417 [DOI] [Google Scholar]
- Nyanhongo G. S., Aichernig N., Ortner M., Steiner W., Guebitz G. M. (2008). A novel environmentally friendly 2, 4, 6-trinitrotoluene (TNT) based explosive. Maced. J. Chem. Chem. Eng. 27, 107–116. [Google Scholar]
- Ogugbue C. J., Morad N., Sawidis T., Oranusi N. A. (2012). Decolorization and partial mineralization of a polyazo dye by Bacillus firmus immobilized within tubular polymeric gel. 3 Biotech 2, 67–78. 10.1007/s13205-011-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman A. R., Bakar N. A., Halmi M. I. E., Johari W. L. W., Ahmad S. A., Jirangon H., et al. (2013). Kinetics of molybdenum reduction to molybdenum blue by Bacillus sp. strain A. rzi. BioMed. Res. Int. 2013:371058. 10.1155/2013/371058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oves M., Khan M. S., Zaidi A. (2013). Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi. J. Biol. Sci. 20, 121–129. 10.1016/j.sjbs.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailan S., Gupta D., Apte S., Krishnamurthi S., Saha P. (2015). Degradation of organophosphate insecticide by a novel Bacillus aryabhattai strain SanPS1, isolated from soil of agricultural field in Burdwan, West Bengal, India. Intern. Biodet. Biodeg. 103, 191–195. 10.1016/j.ibiod.2015.05.006 [DOI] [Google Scholar]
- Pal A., Dutta S., Paul A. K. (2005). Reduction of hexavalent chromium by cell-free extract of Bacillus sphaericus AND 303 isolated from serpentine soil. Curr. Microbiol. 51, 327–330. 10.1007/s00284-005-0048-4 [DOI] [PubMed] [Google Scholar]
- Palma L., Muñoz D., Berry C., Murillo J., Caballero P. (2014). Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6, 3296–3325 10.3390/toxins6123296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Chen Z., Chen F., Cheng Y., Lin Z., Guan X. (2015). The mechanism of uranium transformation from U (VI) into nano-uramphite by two indigenous Bacillus thuringiensis strains. J. Hazard. Mater. 297, 313–319. 10.1016/j.jhazmat.2015.05.019 [DOI] [PubMed] [Google Scholar]
- Pan X., Liu Z., Chen Z., Cheng Y., Pan D., Shao J., et al. (2014). Investigation of Cr (VI) reduction and Cr (III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633. Water Res. 55, 21–29. 10.1016/j.watres.2014.01.066 [DOI] [PubMed] [Google Scholar]
- Pankaj Sharma A., Gangola S., Khati P., Kumar G., Srivastava A. (2016). Novel pathway of cypermethrin biodegradation in a Bacillus sp. strain SG2 isolated from cypermethrin-contaminated agriculture field. 3 Biotech 6:45 10.1007/s13205-016-0372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraneeiswaran A., Shukla S. K., Prashanth K., Rao T. S. (2015). Microbial reduction of [Co (III)–EDTA]– by Bacillus licheniformis SPB-2 strain isolated from a solar salt pan. J. Hazard. Mater. 283, 582–590. 10.1016/j.jhazmat.2014.09.058 [DOI] [PubMed] [Google Scholar]
- Patel S., Gupta R. S. (2020). A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 70, 406–438. 10.1099/ijsem.0.003775 [DOI] [PubMed] [Google Scholar]
- Patil P. S., Shedbalkar U. U., Kalyani D. C., Jadhav J. P. (2008). Biodegradation of reactive Blue 59 by isolated bacterial consortium PMB11. J.Indust. Microbiol. Biotechnol. 35, 1181–1190. 10.1007/s10295-008-0398-6 [DOI] [PubMed] [Google Scholar]
- Peng X., Misawa N., Harayama S. (2003). Isolation and characterization of thermophilic bacilli degrading cinnamic, 4-coumaric, and ferulic acids. Appl. Environ. Microbiol. 69, 1417–1427. 10.1128/AEM.69.3.1417-1427.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. A., Rao K. B. (2013). Aerobic biodegradation of Azo dye by Bacillus cohnii MTCC 3616; an obligately alkaliphilic bacterium and toxicity evaluation of metabolites by different bioassay systems. Appl. Microbiol. Biotechnol. 97, 7469–7481. 10.1007/s00253-012-4492-3 [DOI] [PubMed] [Google Scholar]
- Prasad A. A., Rao K. B. (2014). Aerobic biodegradation of azo dye acid black-24 by Bacillus halodurans. J. Environ. Biol. 35, 549–54. [PubMed] [Google Scholar]
- Ramírez V., Baez A., López P., Bustillos M. D. R., Villalobos M. A., Carreño R., et al. (2019). Chromium hyper-tolerant Bacillus sp. MH778713 assists phytoremediation of heavy metals by mesquite trees (Prosopis laevigata). Front. Microbiol. 10:1833. 10.3389/fmicb.2019.01833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasekh B., Khajeh K., Ranjbar B., Mollania N., Almasinia B., Tirandaz H. (2014). Protein engineering of laccase to enhance its activity and stability in the presence of organic solvents. Eng. Life Sci. 14, 442–448. 10.1002/elsc.201300042 [DOI] [Google Scholar]
- Robb E. L., Baker M. B. (2020). Organophosphate Toxicity, in StatPearls [Internet]. (Treasure Island, FL: StatPearls Publishing; ). [PubMed] [Google Scholar]
- Rodríguez A., Castrejón-Godínez M. L., Salazar-Bustamante E., Gama-Martínez Y., Sánchez-Salinas E., Mussali-Galante P., et al. (2020). Omics approaches to pesticide biodegradation. Curr. Microbiol. 77, 545–563. 10.1007/s00284-020-01916-5 [DOI] [PubMed] [Google Scholar]
- Rosenberg E. (2017). It's in Your DNA: From Discovery to Structure, Function and Role in Evolution, Cancer and Aging. Chapter 10:-Genetic Engineering (Academic Press: ), 81–93. 10.1016/B978-0-12-812502-1.00010-X [DOI] [Google Scholar]
- Ruger H.-J., Fritze D., Sproer C. (2000). New psychrophilic and psychrotolerant Bacillus marinus strains from tropical and polar deep-sea sediments and emended description of the species. Int. J. Syst. Evol. Microbiol. 50, 1305–1313. 10.1099/00207713-50-3-1305 [DOI] [PubMed] [Google Scholar]
- Saini S., Battan B., Maan S., Sharma J. (2018). Decolourization of dyes by Alcaligenes faecalis and Bacillus flexus isolated from textile effluent. Indian J. Exp. Biol. 56, 820–826. [Google Scholar]
- Salam L. B., Obayori O. S. (2014). Fluorene biodegradation potentials of Bacillus strains isolated from tropical hydrocarbon-contaminated soils. Afr. J. Biotechnol. 13, 1554–1559. 10.5897/AJB2013.13598 [DOI] [PubMed] [Google Scholar]
- Saleem M., Ahmad S., Ahmad M. (2014). Potential of Bacillus cereus for bioremediation of pulp and paper industrial waste. Ann. Microbiol. 64, 823–829. 10.1007/s13213-013-0721-y [DOI] [Google Scholar]
- Salunkhe V. P., Sawant I. S., Banerjee K., Rajguru Y. R., Wadkar P. N., Oulkar D. P., et al. (2013). Biodegradation of profenofos by Bacillus subtilis isolated from grapevines (Vitis vinifera). J. Agric. Food Chem. 61, 7195–7202. 10.1021/jf400528d [DOI] [PubMed] [Google Scholar]
- Samin G., Pavlova M., Arif M. I., Postema C. P., Damborsky J., Janssen D. B. (2014). A Pseudomonas putida strain genetically engineered for 1,2,3-trichloropropane bioremediation. Appl. Environ. Microbiol. 80, 5467–5476. 10.1128/AEM.01620-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhibigraha S., Chakraborty S., Bandyopadhyay T., Bhunia B. (2020). A kinetic study of 4-chlorophenol biodegradation by the novel isolated bacillussubtilis in batch shake flask. Environ. Eng. Res. 25, 62–70. 10.4491/eer.2018.416 [DOI] [Google Scholar]
- Sansinenea E. (2019). Bacillus spp.: as plant growth-promoting bacteria, in Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms, eds Singh H., Keswani C., Reddy M., Sansinenea E., García-Estrada C. (Singapore: Springer; ), 225–237. 10.1007/978-981-13-5862-3_11 [DOI] [Google Scholar]
- Sansinenea E., Ortiz A. (2011). Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 33, 1523–1538. 10.1007/s10529-011-0617-5 [DOI] [PubMed] [Google Scholar]
- Santos G. D. A., Dhoke G. V., Davari M. D., Ruff A. J., Schwaneberg U. (2019). Directed evolution of P450 BM3 towards functionalization of aromatic O-Heterocycles. Int. J. Mol. Sci. 20:3353. 10.3390/ijms20133353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saritha P., Aparna C., Himabindu V., Anjaneyulu Y. (2007). Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 149, 609–614. 10.1016/j.jhazmat.2007.06.111 [DOI] [PubMed] [Google Scholar]
- Sarwade V., Gawai K. (2014). Biodegradation of aniline by alkaliphilic strain Bacillus badius D1. IOSRJ. Environ. Sci. Toxicol. Food Technol. 8, 71–78. 10.9790/2402-08527178 [DOI] [Google Scholar]
- Sayler G. S., Ripp S. (2000). Field applications of genetically engineered microorganisms for bioremediation processes. Curr. Opin. Biotechnol. 11, 286–289. 10.1016/S0958-1669(00)00097-5 [DOI] [PubMed] [Google Scholar]
- Seralathan M. V., Sivanesan S., Bafana A., Kashyap S. M., Patrizio A., Krishnamurthi K., et al. (2014). Cytochrome P450 BM3 of Bacillus megaterium—A possible endosulfan biotransforming gene. J. Environ. Sci. 26, 2307–2314. 10.1016/j.jes.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Shah M. P., Patel K. A., Nair S. S., Darji A. M. (2013). Potential effect of two Bacillus spp on decolorization of azo dye. J. Bioremed.Biodeg. 4:199 10.4172/2155-6199.1000199 [DOI] [Google Scholar]
- Sharma B., Shukla P. (2021). Lead bioaccumulation mediated by Bacillus cereus BPS-9 from an industrial waste contaminated site encoding heavy metal resistant genes and their transporters. J. Hazar. Mater. 401:123285. 10.1016/j.jhazmat.2020.123285 [DOI] [PubMed] [Google Scholar]
- Sharma P., Singh L., Dilbaghi N. (2009). Optimization of process variables for decolorization of disperse yellow 211 by Bacillus subtilis using box–behnken design. J. Hazard. Mater. 164, 1024–1029. 10.1016/j.jhazmat.2008.08.104 [DOI] [PubMed] [Google Scholar]
- Sharma S., Singh B., Gupta V. K. (2014). Assessment of imidacloprid degradation by soil-isolated Bacillus alkalinitrilicus. Environ. Monit. Assess. 186, 7183–7193. 10.1007/s10661-014-3919-y [DOI] [PubMed] [Google Scholar]
- Sideri A., Goyal A., Di Nardo G., Tsotsou G. E., Gilardi G. (2013). Hydroxylation of non-substituted polycyclic aromatic hydrocarbons by cytochrome P450 BM3 engineered by directed evolution. J. Inorg. Biochem.120, 1–7. 10.1016/j.jinorgbio.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Sidhu C., Solanki V., Pinnaka A. K., Thakur K. G. (2019). Structure elucidation and biochemical characterization of environmentally relevant novel extradiol dioxygenases discovered by a functional metagenomics approach. mSystems 4, e00316–e00319. 10.1128/mSystems.00316-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu G. K., Singh S., Kumar V., Dhanjal D. S., Datta S., Singh J. (2019). Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit. Rev. Environ. Sci. Technol. 49, 1135–1187. 10.1080/10643389.2019.1565554 [DOI] [Google Scholar]
- Singh A., Chauhan N. S., Thulasiram H. V., Taneja V., Sharma R. (2010). Identification of two flavin monooxygenases from an effluent treatment plant sludge metagenomic library. Bioresour. Technol. 101, 8481–8484. 10.1016/j.biortech.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Singh B., Kaur J., Singh K. (2011). 2, 4, 6-Trinitrophenol degradation by Bacillus cereus isolated from a firing range. Biotechnol. Lett. 33, 2411–2415. 10.1007/s10529-011-0726-1 [DOI] [PubMed] [Google Scholar]
- Singh B., Kaur J., Singh K. (2012). Biodegradation of malathion by Brevibacillus sp. strain KB2 and Bacillus cereus strain PU. World. J. Microbiol. Biotechnol. 28, 1133–1141. 10.1007/s11274-011-0916-y [DOI] [PubMed] [Google Scholar]
- Singh B., Singh K. (2016). Bacillus: as bioremediator agent of major environmental pollutants, in Bacilli and Agrobiotechnology, eds Islam M., Rahman M., Pandey P., Jha C., Aeron A. (Cham: Springer; ), 35–55. 10.1007/978-3-319-44409-3_2 [DOI] [Google Scholar]
- Smibert R. M., Krieg N. R. (1994). Phenotypic characterization, in Methods for General and Molecular Bacteriology, eds. Gerhardt P., Murray R. G. E., Wood W. A., Krieg N. R. (Washington, DC: American Society for Microbiology; ), 617–620–622. [Google Scholar]
- Sonwani R. K., Giri B. S., Singh R. S., Rai B. N. (2019). Studies on optimization of naphthalene biodegradation using surface response methodology: kinetic study and performance evaluation of a pilot scale integrated aerobic treatment plant. Process Saf. Environ. 132, 240–248. 10.1016/j.psep.2019.10.004 [DOI] [Google Scholar]
- Sudha D., Balagurunathan R. (2013). Biodegradation of reactive red 2 azo dye by Bacillus licheniformis isolated from textile effluent contaminated site. Inter. J Curr. Res. Rev. 5, 1–9. [Google Scholar]
- Suenaga H., Ohnuki T., Miyazaki K. (2007). Functional screening of a metagenomic library for genes involved in microbial degradation of aromatic compounds. Environ. Microbiol. 9, 2289–2297. 10.1111/j.1462-2920.2007.01342.x [DOI] [PubMed] [Google Scholar]
- Sun W. Q., Meng M., Kumar G., Geelhaar L. A., Payne G. F., Speedie M. K. (1996). Biological denitration of propylene glycol dinitrate by Bacillus sp. ATCC 51912. App. Microbiol Biotechnol. 45, 525–529. 10.1007/BF00578466 [DOI] [PubMed] [Google Scholar]
- Sundar K., Sadiq I. M., Mukherjee A., Chandrasekaran N. (2011). Bioremoval of trivalent chromium using Bacillus biofilms through continuous flow reactor. J. Hazard. Mater. 196, 44–51. 10.1016/j.jhazmat.2011.08.066 [DOI] [PubMed] [Google Scholar]
- Sundaram S., Das M. T., Thakur I. S. (2013). Biodegradation of cypermethrin by Bacillus sp. in soil microcosm and in-vitro toxicity evaluation on human cell line. Inter. Biodeter. Biodeg. 77, 39–44. 10.1016/j.ibiod.2012.11.008 [DOI] [Google Scholar]
- Swaathy S., Kavitha V., Pravin A. S., Mandal A. B., Gnanamani A. (2014). Microbial surfactant mediated degradation of anthracene in aqueous phase by marine Bacillus licheniformis MTCC 5514. Biotechnol. Rep. 4, 161–170. 10.1016/j.btre.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Liu B., Shi Y., Zeng C. Y., Chen T. T., Zeng L., et al. (2018). Isolation, identification, and fenvalerate-degrading potential of Bacillus licheniformis CY-012. Biotechnol. Biotechnol. Equip. 32, 574–582. 10.1080/13102818.2018.1438210 [DOI] [Google Scholar]
- Tang M., You M. (2012). Isolation, identification and characterization of a novel triazophos-degrading Bacillus sp. (TAP-1). Microbiol. Res. 167, 299–305. 10.1016/j.micres.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Tiwary M., Dubey A. K. (2016). Cypermethrin bioremediation in presence of heavy metals by a novel heavy metal tolerant strain, Bacillus sp. AKD1. Int. Biodeterior. Biodegr. 108, 42–47. 10.1016/j.ibiod.2015.11.025 [DOI] [Google Scholar]
- Upadhyay N., Vishwakarma K., Singh J., Mishra M., Kumar V., Rani R., et al. (2017). Tolerance and reduction of chromium (VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front. Plant Sci. 8:778. 10.3389/fpls.2017.00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viesser J. A., Sugai-Guerios M. H., Malucelli L. C., Pincerati M. R., Karp S. G., Maranho L. T. (2020). Petroleum-tolerant rhizospheric bacteria: isolation, characterization and Bioremediation potential. Sci. Rep. 10:2060. 10.1038/s41598-020-59029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali H., Rehman F. U., Umar A., Ahmed S. (2019). Cholesterol degradation and production of extracellular cholesterol oxidase from Bacillus pumilus W1 and Serratia marcescens W8. BioMed. Res. Int. 2019:1359528. 10.1155/2019/1359528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Boukhalfa H., Ware D. S., Reimus P. W., Daligault H. E., Gleasner C. D., et al. (2015). Genome sequence of a chromium-reducing strain, Bacillus cereus S612. Genome Announc. 3, e01392–15. 10.1128/genomeA.01392-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Lin J., Lin J., Wang W., Li S. (2019). Biodegradation of petroleum hydrocarbons by Bacillus subtilis BL-27, a strain with weak hydrophobicity. Molecules 24:3021. 10.3390/molecules24173021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang J., Song F., Wu J., Feng S., Huang D. (2006). Engineered Bacillus thuringiensis GO33A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol. 72, 924–930. 10.1007/s00253-006-0390-x [DOI] [PubMed] [Google Scholar]
- Wang J., Zhu L., Wang Q., Wang J., Xie H. (2014). Isolation and characterization of atrazine mineralizing Bacillus subtilis strain HB-6. PLoS ONE 9:e107270. 10.1371/journal.pone.0107270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. W., Liang J. S., Liang Y. (2013). Decolorization of reactive black 5 by a newly isolated bacterium Bacillus sp. YZU1. Int. Biodeterior. Biodegr. 76, 41–48. 10.1016/j.ibiod.2012.06.023 [DOI] [Google Scholar]
- Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., et al. (2018). SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G. K., Cain R. B. (1975). Microbial metabolism of the pyridine ring. metabolic pathways of pyridine biodegradation by soil bacteria. Biochem. J. 146, 157–172. 10.1042/bj1460157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger J. H. (2002). Comments on the history and importance of aromatic and heterocyclic amines in public health. Muta. Res. 506–507, 9–20. 10.1016/S0027-5107(02)00147-1 [DOI] [PubMed] [Google Scholar]
- Wells M., McGarry J., Gaye M. M., Basu P., Oremland R. S., Stolz J. F. (2019). Respiratory selenite reductase from Bacillus selenitireducens strain MLS10. J. Bacteriol. 201, e00614–e00618. 10.1128/JB.00614-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff E. G., Mguni C. M., Mansfeld-Giese K., Fels J., Lübeck M., Hockenhull J. (2002). Biochemical and molecular characterization of Bacillus amyloliquefaciens, B. subtilis and B. pumilus isolates with distinct antagonistic potential against Xanthomonas campestris pv. campestris. Plant Pathol. 51, 574–584. 10.1046/j.1365-3059.2002.00753.x [DOI] [Google Scholar]
- Xiao W., Ye X., Yang X., Zhu Z., Sun C., Zhang Q., et al. (2017). Isolation and characterization of chromium (VI)-reducing Bacillus sp. FY1 and Arthrobacter sp. WZ2 and their bioremediation potential. Bioremediat. J. 2, 100–108. 10.1080/10889868.2017.1282939 [DOI] [Google Scholar]
- Xiao Y., Chen S., Gao Y., Hu W., Hu M., Zhong G. (2015). Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbiol.Biotechnol. 99. 2849–2859. 10.1007/s00253-014-6164-y [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang Y. (2015). I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43, W174–W181. 10.1093/nar/gkv342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatome C., Ogawa T., Matsui M. (1991). Degradation of crystal violet by Bacillus subtilis. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 26, 75–87. 10.1080/10934529109375621 [DOI] [Google Scholar]
- Ye M., Li G., Liang W. Q., Liu Y. H. (2010). Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression. Appl. Microbial. Biotechnol. 87, 1023–1031. 10.1007/s00253-010-2507-5 [DOI] [PubMed] [Google Scholar]
- Yerson D., Christian A. (2013). Biodegradation of the explosive pentaerythritol tetranitrate (PETN) by bacteria isolated from mining environments. Rev. Peru. Biol. 20, 145–150. [Google Scholar]
- Yezza A., Tyagi R. D., Valero J. R., Surampalli R. Y., Smith J. (2004). Scale-up of biopesticide production processes using wastewater sludge as a raw material. J. Ind. Microbiol. Biotechnol. 31, 545–552. 10.1007/s10295-004-0176-z [DOI] [PubMed] [Google Scholar]
- Zeinat K. M., Nashwa A. H., Mohamed A. I., Sherif E. N. (2008). Biodegradation and detoxification of malathion by of Bacillus thuringiensis MOS-5. Aust. J. Basic. Appl. Sci. 2, 724–732. [Google Scholar]
- Zelinkova Z., Wenzl T. (2015). The occurrence of 16 EPA PAHs in food–a review. Polycycl. Aromat. Compd. 35, 248–284. 10.1080/10406638.2014.918550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H., Huang Y., Lin Z., Bhatt P., Chen S. (2020). New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 182, 109–138. 10.1016/j.envres.2020.109138 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Hou Z., Wu X., Gao H., Sun F., et al. (2014). Biodegradation of triazine herbicide metribuzin by the strain Bacillus sp. N1. J. Environ. Sci. Health B 49, 79–86. 10.1080/03601234.2014.844610 [DOI] [PubMed] [Google Scholar]
- Zhang L. Z., Qiao X. W., Ma L. P. (2009). Influence of environmental factors on degradation of carbendazim by Bacillus pumilus strain NY97–1. Int. J. Environ. Res. Public Health 38, 309–317. 10.1504/IJEP.2009.027231 [DOI] [Google Scholar]
- Zhu D., Zhang P., Xie C., Zhang W., Sun J., Qian W. J., et al. (2017). Biodegradation of alkaline lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 10:44. 10.1186/s13068-017-0735-y [DOI] [PMC free article] [PubMed] [Google Scholar]