Abstract
Musical cueing has been widely utilised in post-stroke motor rehabilitation; however, the kinematic evidence on the effects of musical cueing is sparse. Further, the element-specific effects of musical cueing on upper-limb movements have rarely been investigated. This study aimed to kinematically quantify the effects of no auditory, rhythmic auditory, and melodic auditory cueing on shoulder abduction, holding, and adduction in patients who had experienced hemiparetic stroke. Kinematic data were obtained using inertial measurement units embedded in wearable bands. During the holding phase, melodic auditory cueing significantly increased the minimum Euler angle and decreased the range of motion compared with the other types of cueing. Further, the root mean square error in the angle measurements was significantly smaller and the duration of movement execution was significantly shorter during the holding phase when melodic auditory cueing was provided than when the other types of cueing were used. These findings indicated the important role of melodic auditory cueing for enhancing movement positioning, variability, and endurance. This study provides the first kinematic evidence on the effects of melodic auditory cueing on kinematic enhancement, thus suggesting the potential use of pitch-related elements in psychomotor rehabilitation.
Subject terms: Rehabilitation, Rehabilitation
Introduction
Stroke is a leading cause of long-term functional disability1, resulting in increased dependency2 and social isolation3,4 as well as decreased quality of life of patients5,6. Approximately 80% of stroke survivors have upper-limb dysfunction, varying from gross to complex and fine motor movements7. Post-stroke upper-limb rehabilitation remains challenging8–11, and the diverse approaches and methodologies have yielded controversial results11,12. Recently, physicians and researchers have explored the potential of using diverse types of sensory cueing as a complement to conventional, vision-oriented approaches for motor rehabilitation13–15. Auditory stimulation has especially shown beneficial effects in enhancing movement execution16–20. Such stimuli may be perceived from all directions and reach across distances, making them perceptible even when the patient is not consciously or attentively listening21. Further, they immediately increase the excitability and readiness of motor execution, entrain the periodicity of movement patterns, and allow individuals to anticipate and prepare for the forthcoming movement22,23.
Auditory-motor enhancement is believed to have multifaceted mechanisms. At the perceptual level, auditory stimuli reach the brain faster than visual and tactile stimuli24–26. The auditory system has a high temporal resolution27,28 and easily synchronises with the temporal periodicity of movements29,30. At the neuronal level, auditory cueing modulates neuromagnetic β oscillations31,32, recruits movement-specific motor neurons33, and increases the variability in musculoskeletal activation patterns34. Most important, neural substrates involved in auditory processing closely communicate with those modulating motor timing, sequencing, and execution35–37. This is evidenced by the presence of a cortico-subcortical network that involves the putamen, supplementary motor area, premotor cortex, and auditory cortex38–41. Such auditory-motor connectivity is well represented and adopted in clinical studies. For example, rhythmic auditory stimulation (RAS), one of the techniques used in neurologic music therapy, leads to enhanced lower- and upper-extremity movements in patients with neurological impairments42–44. Specific to the upper limbs, movement timing45 and movement trajectory smoothness46 and velocity45,47 were greatly improved when RAS training was used in previous studies.
However, research on pitch-related elements has been relatively sparse. Perception of pitch and melody is part of the intrinsic nature of humans, is based on tonotopic representation in the auditory cortex48–50, and is associated with space perception51,52. Thus, pitch and melody can provide a cognitive representation of movement in terms of spatial location and direction53. For example, ascending and descending melodic contours cue upward and downward movements. The effects of pitch-related elements have also been demonstrated in clinical studies. Patterned sensory enhancement utilises rhythmic, melodic, and harmonic elements to provide temporal, spatial, and dynamic information about the movement54,55 and has been applied to upper-limb rehabilitation55–61. However, the results have been inconsistent, which might be due to the non-specific use of musical elements. Moreover, to the best of our knowledge, element-specific effects have not been examined using a controlled experimental design to date.
Additionally, most previous studies employed conventional observations, rating scales, or questionnaires for evaluation56–60, which are inherently subjective and vary depending on the evaluator(s)62. These methodologies hardly allow the measurement of kinematic changes throughout upper-limb movements during auditory cueing; thus, more sophisticated methodologies are needed. In the present study, we employed inertial measurement units (IMUs) to quantify the kinematics of repetitive shoulder abduction, holding, and adduction movements in hemiparetic stroke patients. The purpose of the present study was to examine the effects of (1) no auditory cueing (NAC), (2) rhythmic auditory cueing (RAC), and (3) melodic auditory cueing (MAC) on the kinematic parameters of shoulder movement, including range of motion (ROM), minimum Euler angle (MIN), maximum Euler angle (MAX), duration, and root mean square error (RMSE).
Results
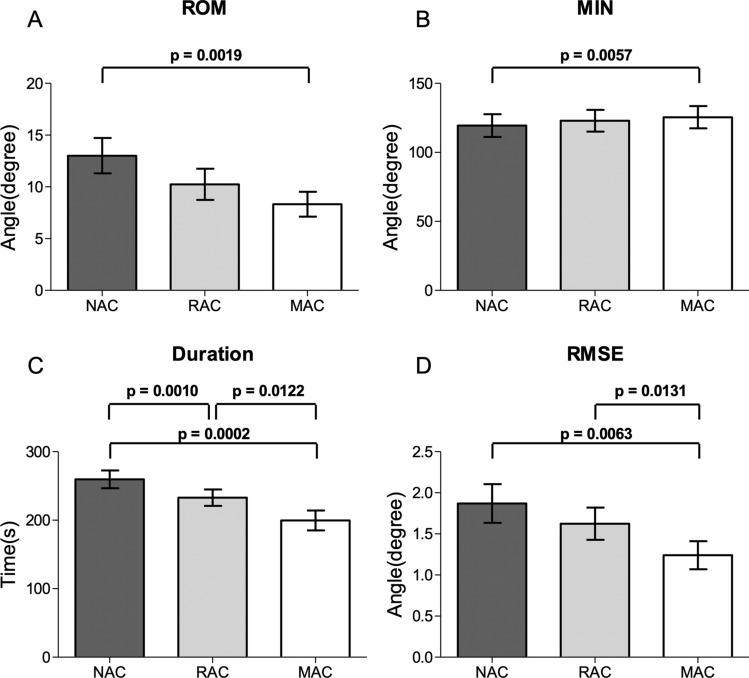
Table 1 shows the descriptive findings. During the holding phase, there existed a significant main effect of the cue on the ROM [F(2,30) = 8.801, p < 0.01] and MIN [F(2,30) = 9.087, p < 0.01] (Fig. 1). A pairwise post-hoc comparison with Bonferroni correction revealed that the ROM was greater in NAC than in MAC (p < 0.0167), whereas the MIN was higher in MAC than in NAC (p < 0.0167). A higher MIN indicated that MAC can assist in the maintenance of shoulder holding at a higher position. The smaller ROM observed in MAC than in NAC and RAC indicated that MAC helped maintain a less variable shoulder angle with less Euler angle drift.
Table 1.
Descriptive statistics of kinematic parameters.
| Kinematic parameter | Abduction | Holding | Adduction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAC | RAC | MAC | NAC | RAC | MAC | NAC | RAC | MAC | |
| ROM | |||||||||
| M | 127.27 | 128.61 | 128.33 | 13.01 | 10.25 | 8.32 | 119.88 | 121.55 | 123.57 |
| SD | 53.58 | 52.33 | 53.35 | 11.84 | 10.42 | 8.32 | 56.91 | 54.82 | 55.91 |
| MIN | |||||||||
| M | 4.63 | 5.15 | 5.33 | 119.43 | 122.95 | 125.48 | 4.65 | 5.01 | 5.00 |
| SD | 10.53 | 12.41 | 11.12 | 57.00 | 54.63 | 55.98 | 9.89 | 12.52 | 10.85 |
| MAX | |||||||||
| M | 131.91 | 133.79 | 133.68 | 135.63 | 135.78 | 135.50 | 124.60 | 126.67 | 128.60 |
| SD | 54.48 | 52.29 | 53.79 | 54.74 | 52.10 | 54.22 | 57.87 | 54.38 | 56.14 |
| RMSE | |||||||||
| M | 11.91 | 12.97 | 12.78 | 1.87 | 1.62 | 1.24 | 13.37 | 13.08 | 13.69 |
| SD | 8.63 | 8.67 | 8.21 | 1.64 | 1.36 | 1.18 | 10.96 | 9.60 | 9.91 |
| Duration | |||||||||
| M | 295.44 | 300.36 | 312.59 | 259.60 | 232.90 | 199.63 | 330.24 | 351.84 | 373.36 |
| SD | 66.57 | 64.22 | 64.38 | 90.02 | 82.63 | 55.01 | 73.95 | 90.27 | 93.52 |
ROM range of motion, MIN minimum Euler angle, MAX maximum Euler angle, RMSE root mean square error, NAC no auditory cueing, RAC rhythmic auditory cueing, MAC melodic auditory cueing, M mean, SD standard deviation.
Figure 1.
Significant differences of kinematic parameters of movement. The graphs show means with standard errors. (A) ROM during the holding phase. (B) MIN during the holding phase. (C) Duration during the holding phase. (D) RMSE during the holding phase. P values are provided for significant differences (p < 0.0167).
Further, there existed a significant main effect of cue on the duration [F(2,30) = 22.028, p < 0.001]. A pairwise comparison showed that during the holding phase, the duration of shoulder movement was significantly longer in NAC than in RAC (p < 0.0167), in NAC than in MAC (p < 0.0167), and in RAC than in MAC (p < 0.0167). Interestingly, there also existed a significant main effect of cue on the duration [F(2,30) = 6.724, p < 0.01] in the adduction phase. Although the pairwise comparison yielded insignificant results for all pairs (p > 0.0167), the duration associated with MAC was the longest, followed by that associated with RAC and then NAC.
We also performed a movement variability (MV) analysis. General approaches to MV quantification in upper-limb movements are typically based on the distribution of angle, acceleration, and velocity63. During the holding phase, there was a significant main effect of cueing on the RMSE [F(2,30) = 8.109, p < 0.01]. A pairwise post-hoc comparison revealed significantly smaller RMSE values in MAC than in NAC (p < 0.0167) and RAC (p < 0.0167) (Fig. 1).
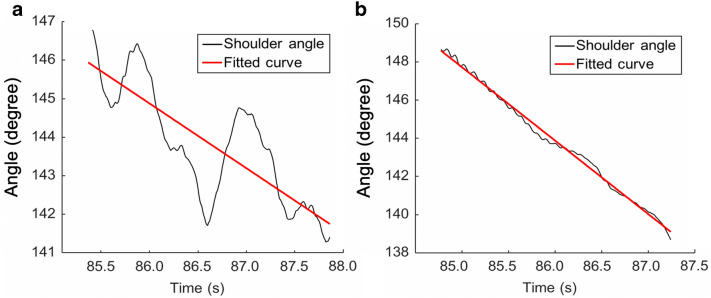
We calculated RMSE as a MV indicator because Euler angle values are sensitive for the measurement of movement acceleration and deceleration. Figure 2 shows the Euler angle variability between the affected and intact sides (black line) during the holding phase, as compared with the fitted curve (red line). The Euler angle of the affected side showed a sudden decrease after the arm approached the highest position and before the start of the adduction phase. This finding was similar to observations made in previous studies that investigated the stroke-specific kinematic features of shoulder movements63–66.
Figure 2.
Comparison of Euler angle data of shoulder movement. (A) Data obtained from severely affected patients’ shoulders. (B) Data obtained from moderately affected patients’ shoulders.
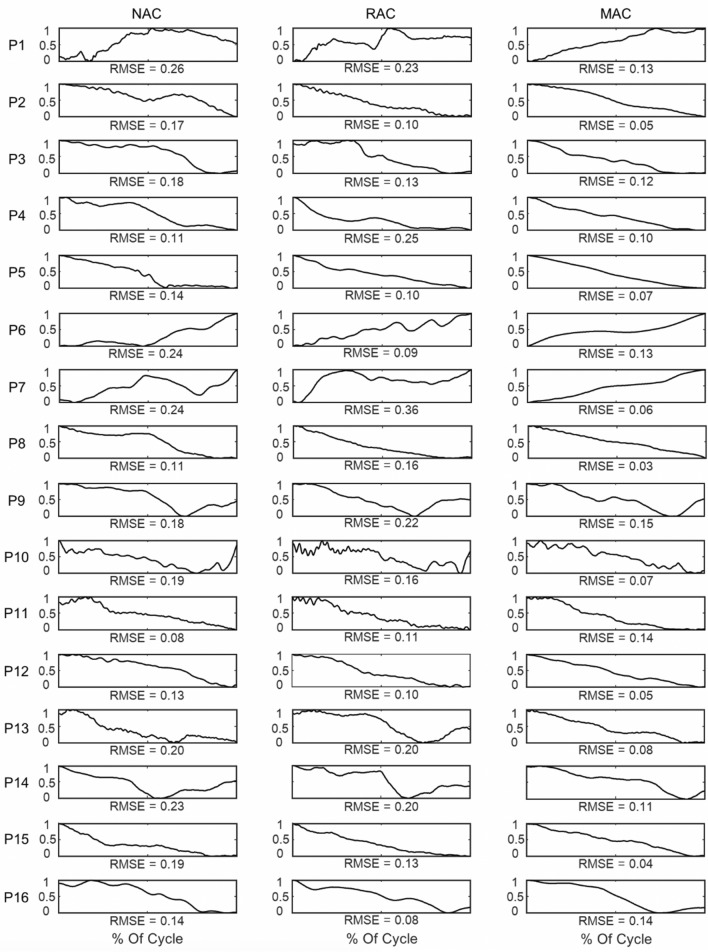
Further, we scrutinised the Euler angle profiles during the holding phase. Figure 3 shows representative Euler angle magnitude profiles. A visual inspection revealed that, in all patients except P2 and P11, less Euler angle profile variability was observed in MAC than in NAC. Moreover, in most patients except P2, P9, P10, P11, and P16, less Euler angle profile variability was observed in MAC than in RAC.
Figure 3.
Movement variability profile using Euler angle data. Representative RMSE profiles of the magnitudes of the Euler angle of 16 patients during the shoulder movement holding phase are shown. The x-axis displays 100% of the movement cycle: 0% and 100% are the shoulder movement holding times. The y-axis displays the normalised Euler angle values. Normalised Euler angle values were used to enhance the comparison among patients. The left column shows the NAC condition; the middle column shows the RAC condition; and the right column shows the MAC condition.
Discussion
In the present study, we used IMUs to measure the complex kinematic characteristics of shoulder abduction, holding, and adduction movements occurring in conjunction with NAC, RAC, and MAC. The findings revealed that MAC significantly increased the MIN and decreased the ROM and RMSE during the holding phase. MAC also decreased the duration of the holding phase. Taken together, these findings indicated that MAC enhances movement positioning, decreases movement variability, and increases movement anticipation and preparation.
First, the results during the holding phase showed that the MIN was significantly higher in MAC than in NAC and that the ROM was significantly greater in NAC than in MAC. These findings collectively indicated that the MIN of the shoulder was maintained at the highest position with less movement deviation in MAC. The enhancement observed in MAC occurred because the participants were provided with information about movement directions until they reached their maximum shoulder angle. These results are similar to those of previous studies that examined the effects of music cognition on movement, demonstrating that pitch contours can increase the cognitive awareness of spatial information during motion51 and actually enhance limb positioning during the execution of vertical movements67.
Second, the RMSE was significantly smaller in MAC than in NAC and RAC during the holding phase. These findings indicated that the observed Euler angle data, which can sensitively detect movement acceleration and deceleration, were best fitted to the predicted linear regression model in MAC; thus, MAC yielded the smallest RMSE. Note that during the holding phase, MAC involved an isochronous rhythm combined with a 740-Hz stationary contour, which is higher than the tone used in RAC (440 Hz, see Fig. 1). Thus, the closer RMSE values of the observed data to the predicted model in MAC was possibly due to the effect of high-pitched sequences embedded in the middle of a melodic contour on the cognitive representation of movement in terms of spatial location53, which allowed the participants to sustain their shoulders with less movement variability. In line with the current findings, a previous study reported that a melody cue was best for unconstrained point-to-point hand movements, prompting less variable hand movements from the initial to the final position within a given time period68. Moreover, previous studies have demonstrated that auditory cueing significantly decreases movement variability45,46,69–72. For example, Thaut et al. reported that the kinematic data obtained in auditory cueing fit the predicted model significantly better than those from a no auditory cueing condition. This result was possibly due to the entrainment of movement execution patterns with the patterns embedded in the rhythm46.
Third, the duration was significantly shorter in MAC than in NAC, in RAC than in NAC, and in MAC than in RAC. RAC consists of an isochronous rhythm, whereas MAC consists of pitch contours presented with an isochronous rhythm. In the current study, this rhythm pattern seemed to play a role in movement anticipation and preparation in both RAC and MAC. In a review study, Avanzino et al.73 reported that rhythm provides a temporal structure that enhances motor timing, motor sequencing, and movement control. Moreover, isochronous rhythm was reported to induce a temporal locking or entrainment process between motor movements and the external auditory rhythm22.
In addition, the shorter duration of RAC and MAC can be interpreted as a cognitive influence of musical cueing on anticipation and preparation for movement. Schaffert et al.74 proposed that auditory rhythm can prime the motor system and facilitate further anticipation of and preparation for cyclic movements75. Such influences, when observed in healthy and clinical (e.g. post-stroke) populations, manifest through the activation or establishment of alternative pathways76–78. The available findings implied that cognitive involvement in auditory processing (i.e. anticipation) plays a role in motor preparation and execution79,80. Importantly, as the pitch contours embedded in RAC are more salient to providing the structure of movement, patients are more likely to anticipate and prepare for the forthcoming movement. In general, music involves a high-order temporal organisation of sound events, of which allows individuals to make predictions about future events and raises expectations37. Previous studies that employed melodic feedback in post-stroke arm rehabilitation also reported improved smoothness of movement because of such anticipation81,82.
Collectively, MAC led to an effective, stable maintenance of the shoulder in the desired position during the holding phase. The closer fit observed in MAC indicated a smaller movement variability possibly due to the effect of pitch in addition to the effect of rhythm. The shorter movement time in MAC indicates that rhythm components play a role in movement anticipation and preparation and are more prominent when presented with melodic components. Compared with MAC, RAC showed significant enhancement only in movement time.
Our study had some limitations. We performed parameter extraction for the Euler angles of the sagittal axis because this axis provides more relevant information about our target movement. Obtaining similar data from two other axes and involving another body part (wrist, hand, neck, etc.) may provide a more comprehensive index for the movement features of the affected shoulder (e.g. post-stroke compensatory strategies). However, this study was a preliminary attempt to quantify the kinematic parameters of repetitive paretic shoulder abduction, holding, and adduction in post-stroke patients. As the effects of musical cueing on the kinematic changes of upper-limb movements have not yet been specified and the scope of their application has been limited, the present study provides important information about the specific roles of the elements as sensory cues and promotes a more appropriate use of the elements for upper-limb rehabilitation. Future studies are needed to find a more comprehensive index of the relationship between musical cueing and functional upper-limb movements and to develop a more efficient and targeted upper-limb patterned sensory enhancement protocol. Lastly, combining musical cueing with visually oriented virtual reality rehabilitation may yield better psychomotor rehabilitation.
In conclusion, auditory cueing led to enhanced movements, across all parameters, when compared with movements performed without auditory cueing in this study. In particular, MAC effectively increased the MIN but decreased the RMSE and duration in the holding phase. These findings seem to be associated with increased movement positioning, decreased movement variability, and enhanced movement anticipation and preparation. Such findings suggest the potential of pitch-related components for directing specific movement parameters and the potential use of musical cueing in psychomotor rehabilitation. Given that muscle endurance is necessary to allow smooth control of movements, which is typically impaired in stroke patients (as evidenced by their daily functional movements)83–85, the current findings can be used for the conclusive measurement of motor function recovery and for rehabilitation training86.
Methods
Participants
This study was approved by the Institutional Review Board at the National Rehabilitation Centre and performed in accordance with relevant guidelines and regulations. All participants provided written informed consent, in accordance with the Declaration of Helsinki. Informed consent to publish identifying images was also obtained from participants. Eighteen patients with hemiparetic stroke (Male = 10, Female = 8) volunteered to participate in the study and were recruited from the rehabilitation centre. The patients were eligible if they had upper-limb hemiparesis secondary to first-ever ischaemic or haemorrhagic stroke, Brunnstrom Stages of Motor Recovery scores > 2 for the affected proximal upper limb, the cognitive ability to understand and follow instructions, and the capability and willingness to participate in the experiment. Patients who had more than 3 months of regular involvement in musical activities and/or professional training and those with sensory impairments were excluded from the study. Two of the 18 patients were excluded because of unreliable signal-to-noise ratios. The average age of the patients was 49.78 (SD = 15.55) years, and the average time since stroke was 15.22 (SD = 12.82) months. The patients had an average of 12.28 (SD = 2.80) years of education, an average Brunnstrom Stage of Motor Recovery score of 4.67 (SD = 0.94), and an average shoulder abduction ROM of 138.33° (SD = 29.06).
Movement task and auditory stimuli
To examine the immediate effects of auditory cueing on paretic shoulder movements, we selected shoulder abduction, holding, and adduction movements as previous studies had reported the stroke-specific kinematic features of shoulder movements87–90. A visual avatar, from a third-person perspective, was provided to guide the movement. The avatar was programmed to raise its arms from the side to 180° (abduction phase, 3 s), maintain its arms at that position (holding phase, 3 s), and then slowly lower its arms back to the side (adduction phase, 3 s). The participants were instructed to perform the movements in conjunction with the avatar seen on the computer screen. The avatar was generated using a model of Rehab Master (Rehab Master, Seoul, Korea) and programmed using Unity (Unity Technologies, Copenhagen, Denmark).
Two types of auditory stimuli were used as auditory cues and presented via two stereo speakers. RAC consisted of a series of 50-ms tones with an inter-stimulus interval (ISI) of 200 ms (i.e. a beep sound on digital metronome at an audio frequency of 440 Hz). MAC consisted of a series of tones presented using the same isochronous time pattern as the RAC but at different frequencies. In the present study, RAC and MAC have definite pitches and the same isochronous time pattern, so they might also be labelled monotonic rhythm and melodic rhythm, respectively. However, we used the terms rhythmic and melodic in accordance with the terminologies used in the existing literature. RAC or RAS is a frequently used psychomotor rehabilitation technique. The rhythmic stimuli employed in these studies are mainly analogue or digital metronome beats69,76,91 comprising tones presented at the same pitch and with an isochronous time pattern. In addition, considering the general definition of melody as a combination of pitch and rhythm, it might be better to use the term MAC.
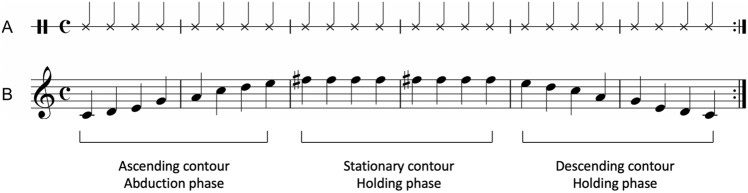
MAC consisted of ascending, stationary, and descending contours generated to musically demonstrate the angle of shoulder abduction, holding, and adduction, respectively. In typical participants, shoulder abduction involves three different types of muscle—the supraspinatus, deltoid, and trapezius and serratus anterior. The supraspinatus initiates from 0° to 15°, the deltoid functions from 15° to 90°, and the trapezius and serratus anterior are activated from 90° to 180°92. In addition, the glenohumeral joint contributes 90°–120° of shoulder abduction93,94. The range of shoulder movement (0°–180°) was divided into ten equal degrees, and a 12-tone scale (C4 to F#5) was assigned to each degree. As 0°, 15°, 90°, 120°, and 180° are important to indicate the muscle involvement in shoulder abduction, we selected the pitches that correspond to the degrees (C4, D4, A4, C5, and F5#). As 15° corresponds to the microtone between C4# and D4, we selected D. Additional tones were used to musically describe the spatial position and trajectory of shoulder movement, yielding an eight-tone ascending contour. The first four tones of the ascending contour (C4, D4, E4, and G4) were assigned to shoulder abduction from 0° to 89°, and the second four tones (A4, C5, D5, and E5) were assigned to shoulder abduction from 90° to 179°. The next eight tones of the stationary contour reflect the shoulder holding movement. As maximum effort is needed to hold the shoulder at the highest position (typically around 170°–180°), F5# was used to reflect a such effort. Figure 4 shows an example of RAC and MAC. The auditory stimuli were generated using a musical instrument digital interface synthesizer connected to a Logic Pro X (Apple, Cupertino, CA, USA) and programmed in accordance with the avatar movements using Unity (Unity Technologies, Copenhagen, Denmark).
Figure 4.
An example of auditory cueing. (A) Rhythmic auditory cueing. (B) Melodic auditory cueing.
IMUs and equipment setup
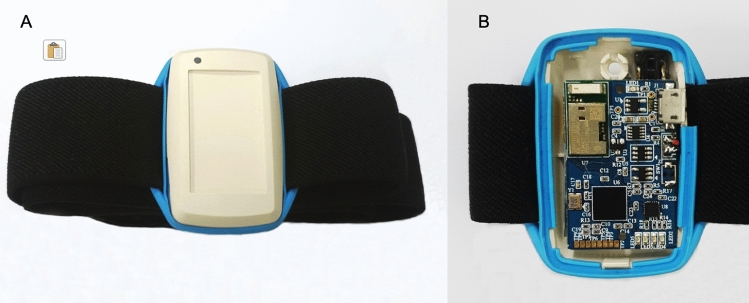
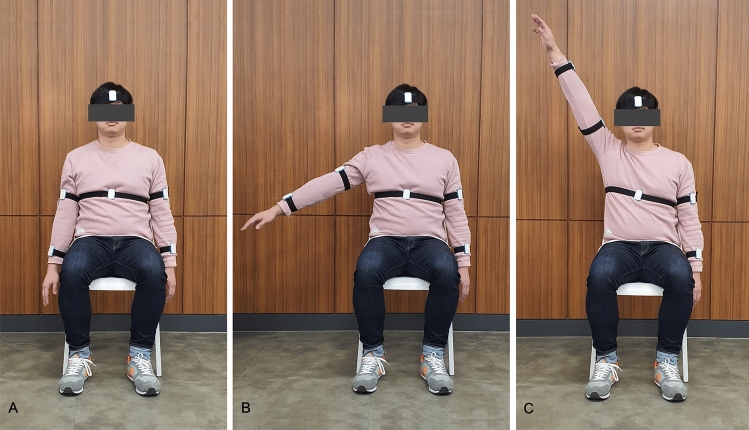
To measure and analyse the effects of cueing on the participants’ shoulder movements, we used spatiotemporal kinematic parameters. These parameters were provided by Hanyang IMU (H-IMU), which consists of an integrated 9-axis motion tracking sensor (MPU9250; InvenSense, San Jose, CA, USA), a microcontroller (MSP430F5338; Texas Instruments, Dallas, TX, USA), a Bluetooth module (PAN1321i; Panasonic, Osaka, Japan), and a lithium-ion battery (240 mAh, 3.7 V; DTP, Shenzhen, China). The device allows long-term data measurements (Fig. 5). A total of 6 H-IMUs were attached to the head, torso, arms, and forearms of each patient using Velcro straps and were positioned on the dorsal side of each arm and on the anterior portion of the head and torso. Signals associated with body movements were generated at < 20 Hz, such that a sampling frequency of 100 Hz was sufficient to detect all movements. Thus, all H-IMU signals were measured using a sampling rate of 100 Hz. The calculated Euler angles for each of the 6 H-IMUs were transmitted to a personal computer using Bluetooth communication. Figure 6 shows the H-IMUs and how they were attached to a participant.
Figure 5.
In-house-built H-IMU. (A) Cover closed. (B) Cover opened.
Figure 6.
An individual demonstrating shoulder abduction with the H-IMUs attached. (A) Arm at the side. (B) Raising one arm halfway. (C) Reaching the arm towards the head.
In each experiment, the participants underwent a practice period (P1) and an experimental period (P2). P1 comprised three shoulder abduction-holding-adduction trials, in which the three types of cueing were presented. The P1 period allowed the participants to become familiar with the movements and the different types of cueing. P2 comprised three blocks of three cueing conditions. Five trials, each consisting of abduction, holding, and adduction phases, were blocked with one of the three cueing conditions and randomly presented to the participant while avoiding the consecutive presentation of the same cueing condition. The blocks requiring the movement of the affected and unaffected shoulders were presented in an alternating manner.
Euler angle calculation
Micro-electromechanical system inertial sensors have a direct-current bias and gain error because of the direct-current noise caused by powering of the electric circuit and environmental noise. To minimise the error associated with these noises, we calibrated the sensors using an in-house-manufactured calibration jig with 9 degrees of freedom (DoFs)95. The calibrated data, c, can be obtained by multiplying the uncalibrated data, u, including the original error by the compensated gain value, K, through calibration and subtracting the offset error, b. These variables are defined in Eqs. (1–3), respectively. Finally, the calculated calibration equation is shown in Eq. (4)
| 1 |
| 2 |
| 3 |
| 4 |
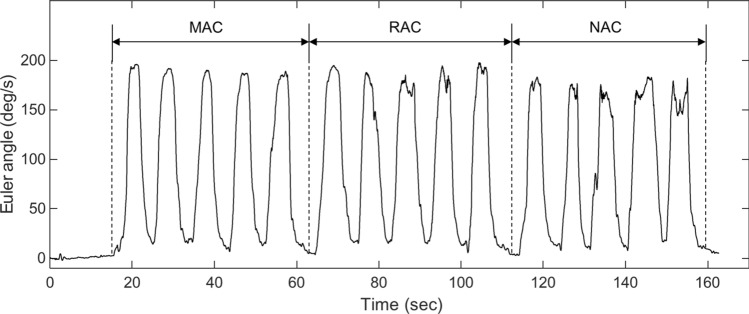
Thereafter, the Euler angles were calculated using a gradient descent algorithm to provide the quaternions based on tri-axis gyroscope, accelerometer, and magnetometer measurements, as shown in Eq. (5). Euler angles adopt the ‘ZYX’ rotation sequence to convert quaternion frame rotations in radians96,97. Figure 7 shows an example of the calculated Euler angles associated with the three types of cueing
| 5 |
where is the estimated orientation of the sensor frame relative to the earth frame.
Figure 7.
Kinematic recording of hemiparetic shoulder abduction, holding, and adduction movements across three types of cueing.
Movement analysis
We extracted the kinematic parameters of movement during the three phases (abduction, holding, and adduction) of shoulder movement. Parameter extraction was performed on the Euler angles obtained from the frontal plane around the sagittal axis, which is the axis that provides more relevant information about the abduction, holding, and adduction movements of the shoulder. Figure 8 shows the points that were included in the movement analysis. The start point (SP) and end point (EP) indicate the initiation of the abduction phase and the cessation of the adduction phase, respectively, based on the minimum Euler angle values. Fiducial points (FP1, FP2) were defined to correspond with the initiation and cessation of the holding phase; FP1 represents the maximum point on the Euler angle graph for one cycle.
Figure 8.
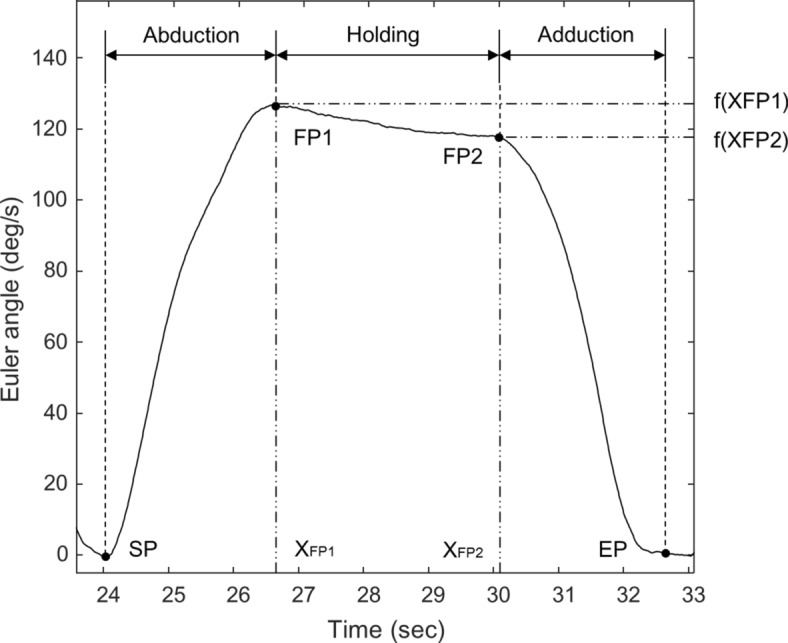
Sample raw Euler angle data obtained from one trial of shoulder abduction, holding, and adduction. SP start point, EP end point, FP fiducial point.
We selected FP2 values that satisfy the equations below, in which f(x) represents the angle, x represents the time point, and i represents the peak points between FP1 and EP. FP2 was not easy to define because the signal-to-noise ratios were extreme in all participants. Based on our preliminary experimentation and simulations, we set a threshold (α) for the between-peak slope (− 0.03) and applied this to all data sets. Small peak points, with peak values below − 0.03, were not considered to be reliable FP2 values (6, 7)
| 6 |
| 7 |
Based on previous studies in clinical populations that revealed the meaning of Euler angle values relative to motor movements98–100, we calculated the kinematic parameters using Euler angles (Table 2).
Table 2.
Spatiotemporal features.
| Kinematic parameter | Formula | Description |
|---|---|---|
| ROM | ||
| MIN | ||
| MAX | ||
| RMSE | Calculated using a linear regression model | |
| Duration | Time spent to complete one phase of each trial |
Euler angle values obtained during one phase of each trial, n number of samples for the phase, linear regression value, t time point, MIN minimum Euler angle of the phase, MAX maximum Euler angle of the phase, RMSE root mean square error.
ROM
Shoulder movement is characterised by many DoFs and a wide ROM at the joints101. The ROM is important for assessing joint range, control, strength, and willingness to perform a movement102. The clinical implication of the kinematic ROM has recently been expanded to the determination of diagnoses and the success of therapeutic interventions. Also, previous studies have reported the validity and reliability of IMUs for measuring parameters of shoulder joint motion, such as the ROM103–105. In the present study, the ROM refers to the difference between the MIN and MAX, using the anatomical DoFs for human limbs64,106, and was calculated for each of the three movement phases. In the abduction and adduction phases, the ROM reflects the largest Euler angle, as arm raising was initiated from approximately 0°. During the holding phase, the ROM reflects the variance among the Euler angles.
Movement variability (MV)
General approaches to MV quantification in upper-limb movements are typically based on the distribution of characteristic values, such as angle, acceleration, and velocity63. Movement variability is associated with the variety of coordination patterns used to complete tasks65,66. For the current study, we calculated RMSEs using a linear regression model and considered the parameter as an indicator of MV. The reason that we considered RMSE as a measure of MV was that the Euler angle values measure movement acceleration and deceleration. Thus, the RMSE represents the difference between the generated linear regression model (fitted curve) and the obtained Euler angle data, which indicate the ideal movement trajectory and the raw data associated with shoulder movement, respectively. In the current study, MV during the abduction and adduction phases might indicate movement smoothness, while MV during the holding phase might indicate movement endurance.
Movement time (MT)
MT refers to the time required to execute a movement and is defined as the interval between movement onset and movement offset, representing temporal efficiency107–109. In the present study, MT included the duration of shoulder abduction (time between the onset of arm raising and attaining the maximum shoulder angle, SP to FP1), shoulder holding (time of maintaining the maximum shoulder angle, FP1 to FP2), and shoulder adduction (time between the onset of arm lowering and back to the initial position, FP2 to EP; see Fig. 8 for details). In general, MT is associated with muscle endurance, which is the ability of a muscle to sustain repetitive isometric or isotonic contractions110.
Statistical analysis
The kinematic parameters from the affected side were used and categorised into the three phases of shoulder movement (abduction, holding, and adduction). A one-way repeated measures analysis of variance was performed to compare the kinematic parameters across the three cueing conditions (NAC, RAC, and MAC). All analyses were conducted using R (R Project for Statistical Computing, Vienna, Austria).
Acknowledgements
This study was supported by a National Research Foundation of Korea Grant funded by the Korean Government (NRF-2018S1A5A2A03034582).
Author contributions
E.J. conceived and designed the experiments; J.-H.S. and J.-Y.L. recruited the participants and arranged the individual experiments; S.K. developed the computerised protocol; J.-Y.L. performed the experiments; S.K., E.J., J.L., and I.-Y.K. analysed the data; E.J., S.K., I.-Y.K., J.L., and J.-H.S. interpreted the data; S.K. prepared the tables and figures; E.J., J.-H.S., and S.K. wrote the manuscript; E.J., J.-H.S., S.K., J.L., and I.-Y.K. edited the manuscript; and all authors read and approved the final manuscript.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hong K-S, et al. Stroke Statistics in Korea: Part I. Epidemiology and risk factors: A report from the Korean Stroke Society and Clinical Research Center for Stroke. J. Stroke. 2013;15:2–20. doi: 10.5853/jos.2013.15.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollock A, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014;2014:CD010820. doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haun J, Rittman M, Sberna M. The continuum of connectedness and social isolation during post stroke recovery. J. Aging Stud. 2008;22:54–64. doi: 10.1016/j.jaging.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.White JH, Attia J, Sturm J, Carter G, Magin P. Predictors of depression and anxiety in community dwelling stroke survivors: A cohort study. Disabil. Rehabil. 2014;36:1975–1982. doi: 10.3109/09638288.2014.884172. [DOI] [PubMed] [Google Scholar]
- 5.King JM, Hux K. Attention allocation in adults with and without aphasia: Performance on linguistic and nonlinguistic tasks. J. Med. Speech. Lang. Pathol. 1996;4:245–256. [Google Scholar]
- 6.Franceschini M, La Porta F, Agosti M, Massucci M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur. J. Phys. Rehabil. Med. 2010;46:389–399. [PubMed] [Google Scholar]
- 7.Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing. 2009;38:260–266. doi: 10.1093/ageing/afp020. [DOI] [PubMed] [Google Scholar]
- 8.Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Contributions of altered stretch reflex coordination to arm impairments following stroke. J. Neurophysiol. 2010;104:3612–3624. doi: 10.1152/jn.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messier S, Bourbonnais D, Desrosiers J, Roy Y. Kinematic analysis of upper limbs and trunk movement during bilateral movement after stroke. Arch. Phys. Med. Rehabil. 2006;87:1463–1470. doi: 10.1016/j.apmr.2006.07.273. [DOI] [PubMed] [Google Scholar]
- 10.Van Dokkum L, et al. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabil. Neural Repair. 2014;28:4–12. doi: 10.1177/1545968313498514. [DOI] [PubMed] [Google Scholar]
- 11.Pulman J, Buckley E, Clark-Carter D. A meta-analysis evaluating the effectiveness of two different upper limb hemiparesis interventions on improving health-related quality of life following stroke. Top. Stroke Rehabil. 2013;20:189–196. doi: 10.1310/tsr2002-189. [DOI] [PubMed] [Google Scholar]
- 12.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 13.Lam P, et al. A haptic-robotic platform for upper-limb reaching stroke therapy: Preliminary design and evaluation results. J. Neuroeng. Rehabil. 2008;5:1–13. doi: 10.1186/1743-0003-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urra O, Casals A, Jane R. The impact of visual feedback on the motor control of the upper-limb. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Society EMBS. 2015;2015:3945–3948. doi: 10.1109/EMBC.2015.7319257. [DOI] [PubMed] [Google Scholar]
- 15.Hatem SM, et al. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016;10:1–22. doi: 10.3389/fnhum.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaut MH, Abiru M. Rhythmic auditory stimulation in rehabilitation of movement disorders: A review of current research. Music Percept. 2010;27:263–269. doi: 10.1525/mp.2010.27.4.263. [DOI] [Google Scholar]
- 17.Spaulding SJ, et al. Cueing and gait improvement among people with Parkinson’s disease: A meta-analysis. Arch. Phys. Med. Rehabil. 2013;94:562–570. doi: 10.1016/j.apmr.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Ghai S, Ghai I, Effenberg AO. Effect of rhythmic auditory cueing on aging gait: A systematic review and meta-analysis. Aging Dis. 2018;9:901–923. doi: 10.14336/AD.2017.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, Y. et al. A real-time, multimodal biofeedback system for stroke patient rehabilitation. in Proceedings of the 14th Annual ACM International Conference on Multimedia, MM 2006 501–502 (ACM, 2006). 10.1145/1180639.1180745.
- 20.Duff M, et al. An adaptive mixed reality training system for stroke rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18:531–541. doi: 10.1109/TNSRE.2010.2055061. [DOI] [PubMed] [Google Scholar]
- 21.McCormick EJ, Sanders MS. Human Factors in Engineering and Design. New York: McGraw-Hill, Inc.; 1993. [Google Scholar]
- 22.Schaefer RS. Auditory rhythmic cueing in movement rehabilitation: Findings and possible mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014;369:20130402. doi: 10.1098/rstb.2013.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaut MH, Hoemberg V. Neurologic music therapy in neuropsychological rehabilitation. Neuropsychol. Rehabil. Int. Handb. 2017;2:414–424. [Google Scholar]
- 24.Spidalieri G, Busby L, Lamarre Y. Fast ballistic arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. II. Effects of unilateral dentate lesion on discharge of precentral cortical neurons and reaction time. J. Neurophysiol. 1983;50:1359–1379. doi: 10.1152/jn.1983.50.6.1359. [DOI] [PubMed] [Google Scholar]
- 25.Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function. IEEE Eng. Med. Biol. Mag. 1999;18:101–108. doi: 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- 26.Nombela C, Hughes LE, Owen AM, Grahn JA. Into the groove: Can rhythm influence Parkinson’s disease? Neurosci. Biobehav. Rev. 2013;37:2564–2570. doi: 10.1016/j.neubiorev.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.van Noorden L, Moelants D. Resonance in the perception of musical pulse. Int. J. Phytoremed. 1999;21:43–66. [Google Scholar]
- 28.Grahn JA. Neural mechanisms of rhythm perception: current findings and future perspectives. Top. Cogn. Sci. 2012;4:585–606. doi: 10.1111/j.1756-8765.2012.01213.x. [DOI] [PubMed] [Google Scholar]
- 29.Grahn JA. See what I hear? Beat perception in auditory and visual rhythms. Exp. Brain Res. 2012;220:51–61. doi: 10.1007/s00221-012-3114-8. [DOI] [PubMed] [Google Scholar]
- 30.Repp BH, Su YH. Sensorimotor synchronization: A review of recent research (2006–2012) Psychon. Bull. Rev. 2013;20:403–452. doi: 10.3758/s13423-012-0371-2. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka T, Trainor LJ, Large EW, Ross B. Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J. Neurosci. 2012;32:1791–1802. doi: 10.1523/JNEUROSCI.4107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross B, Barat M, Fujioka T. Sound-making actions lead to immediate plastic changes of neuromagnetic evoked responses and induced β-band oscillations during perception. J. Neurosci. 2017;37:5948–5959. doi: 10.1523/JNEUROSCI.3613-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochester L, Baker K, Nieuwboer A, Burn D. Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: Selective responses to internal and external cues. Mov. Disord. 2011;26:430–435. doi: 10.1002/mds.23450. [DOI] [PubMed] [Google Scholar]
- 34.Miller RA, Thaut MH, McIntosh GC, Rice RR. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1996;101:1–7. doi: 10.1016/0013-4694(95)00209-X. [DOI] [PubMed] [Google Scholar]
- 35.Besson M, Chobert J, Marie C. Transfer of training between music and speech: Common processing, attention, and memory. Front. Psychol. 2011;2:1–12. doi: 10.3389/fpsyg.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janata P, Tillmann B, Bharucha JJ. Listening to polyphonic music recruits domain-general attention and working memory circuits. Cogn. Affect. Behav. Neurosci. 2002;2:121–140. doi: 10.3758/CABN.2.2.121. [DOI] [PubMed] [Google Scholar]
- 37.Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: Auditory-motor interactions in music perception and production. Nat. Rev. Neurosci. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- 38.Chen JL, Zatorre RJ, Penhune VB. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. Neuroimage. 2006;32:1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- 39.Tecchio F, Salustri C, Thaut MH, Pasqualetti P, Rossini PM. Conscious and preconscious adaptation to rhythmic auditory stimuli: A magnetoencephalographic study of human brain responses. Exp. Brain Res. 2000;135:222–230. doi: 10.1007/s002210000507. [DOI] [PubMed] [Google Scholar]
- 40.Grahn JA, Rowe JB. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 2009;29:7540–7548. doi: 10.1523/JNEUROSCI.2018-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giovannelli F, et al. Role of the dorsal premotor cortex in rhythmic auditory-motor entrainment: A perturbational approach by rTMS. Cereb. Cortex. 2014;24:1009–1016. doi: 10.1093/cercor/bhs386. [DOI] [PubMed] [Google Scholar]
- 42.Hurt CP, Rice RR, McIntosh GC, Thaut MH. Rhythmic auditory stimulation in gait training for patients with traumatic brain injury. J. Music Ther. 1998;35:228–241. doi: 10.1093/jmt/35.4.228. [DOI] [PubMed] [Google Scholar]
- 43.Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: Randomized trials of gait improvement. Clin. Rehabil. 2003;17:713–722. doi: 10.1191/0269215503cr668oa. [DOI] [PubMed] [Google Scholar]
- 44.Thaut MH, McIntosh GC, Prassas SG, Rice RR. Effect of rhythmic auditory cuing on temporal stride parameters and EMG patterns in normal gait. Neurorehabil. Neural Repair. 1992;6:185–190. doi: 10.1177/136140969200600403. [DOI] [Google Scholar]
- 45.Malcolm MP, Massie C, Thaut M. Rhythmic auditory-motor entrainment improves hemiparetic arm kinematics during reaching movements: A pilot study. Top. Stroke Rehabil. 2009;16:69–79. doi: 10.1310/tsr1601-69. [DOI] [PubMed] [Google Scholar]
- 46.Thaut MH, Kenyon GP, Hurt CP, McIntosh GC, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia. 2002;40:1073–1081. doi: 10.1016/S0028-3932(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 47.Wallis, I. et al. Real-time sonification of movement for an immersive stroke rehabilitation environment. in Proceeding of the International Community on Auditory Display 497–503 (2007).
- 48.Mathias SR, Micheyl C, Bailey PJ. Stimulus uncertainty and insensitivity to pitch-change direction. J. Acoust. Soc. Am. 2010;127:3026–3037. doi: 10.1121/1.3365252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semal C, Demany L. Individual differences in the sensitivity to pitch direction. J. Acoust. Soc. Am. 2006;120:3907–3915. doi: 10.1121/1.2357708. [DOI] [PubMed] [Google Scholar]
- 50.Kang R, et al. Development and validation of the University of Washington clinical assessment of music perception test. Ear Hear. 2009;30:411–418. doi: 10.1097/AUD.0b013e3181a61bc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tubul ZEN. Musical parameters and children’s images of motion. Music. Sci. 2010;14:89–111. doi: 10.1177/10298649100140S207. [DOI] [Google Scholar]
- 52.Larson S. Musical Forces: Motion, Metaphor, and Meaning in Music. Bloomington: Indiana University Press; 2012. [Google Scholar]
- 53.Thaut MH. The future of music in therapy and medicine. Ann. N. Y. Acad. Sci. 2005;1060:303–308. doi: 10.1196/annals.1360.023. [DOI] [PubMed] [Google Scholar]
- 54.Thaut MH, Trimarchi PD, Parsons LM. Human brain basis of musical rhythm perception: Common and distinct neural substrates for meter, tempo, and patter. Brain Sci. 2014;4:428–452. doi: 10.3390/brainsci4020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamb J. The Effect of Patterned Sensory Enhancement on Hemiparetic Upper Limb Kinematics. Fort Collins: Colorado State University; 2012. [Google Scholar]
- 56.Hamburg J, Clair AA. The effects of a movement with music program on measures of balance and gait speed in healthy older adults. J. Music Ther. 2003;40:212–226. doi: 10.1093/jmt/40.3.212. [DOI] [PubMed] [Google Scholar]
- 57.Hamburg J, Clair AA. The effects of a Laban-based movement program with music on measures of balance and gait in older adults. Act. Adapt. Aging. 2004;28:17–33. [Google Scholar]
- 58.Mathews RM, Clair AA, Kosloski K. Keeping the beat: Use of rhythmic music during exercise activities for the elderly with dementia. Am. J. Alzheimers. Dis. Other Demen. 2001;16:377–380. doi: 10.1177/153331750101600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Konski M, Bane C, Hettinga J, Krull K. Comparative effectiveness of exercise with patterned sensory enhanced music and background music for long-term care residents. J. Music Ther. 2010;47:120–136. doi: 10.1093/jmt/47.2.120. [DOI] [PubMed] [Google Scholar]
- 60.Clark IN, Baker F, Taylor NF. The effects of live patterned sensory enhancement on group exercise participation and mood in older adults in rehabilitation. J. Music Ther. 2012;49:180–204. doi: 10.1093/jmt/49.2.180. [DOI] [PubMed] [Google Scholar]
- 61.Wang TH, et al. A home-based program using patterned sensory enhancement improves resistance exercise effects for children with cerebral palsy: A randomized controlled trial. Neurorehabil. Neural Repair. 2013;27:684–694. doi: 10.1177/1545968313491001. [DOI] [PubMed] [Google Scholar]
- 62.FitzGerald JJ, Lu Z, Jareonsettasin P, Antoniades CA. Quantifying motor impairment in movement disorders. Front. Neurosci. 2018;12:202. doi: 10.3389/fnins.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thies SB, et al. Movement variability in stroke patients and controls performing two upper limb functional tasks: A new assessment methodology. J. Neuroeng. Rehabil. 2009;6:2. doi: 10.1186/1743-0003-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Baets L, van der Straaten R, Matheve T, Timmermans A. Shoulder assessment according to the international classification of functioning by means of inertial sensor technologies: A systematic review. Gait Posture. 2017;57:278–294. doi: 10.1016/j.gaitpost.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 65.Miller RH, Chang R, Baird JL, Van Emmerik REA, Hamill J. Variability in kinematic coupling assessed by vector coding and continuous relative phase. J. Biomech. 2010;43:2554–2560. doi: 10.1016/j.jbiomech.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Haken H, Kelso JAS, Bunz H. A theoretical model of phase transitions in human hand movements. Biol. Cybern. 1985;51:347–356. doi: 10.1007/BF00336922. [DOI] [PubMed] [Google Scholar]
- 67.Kohn D, Eitan Z. Moving music: Correspondences of musical parameters and movement dimensions in children’s motion and verbal responses. Music Percept. 2016;34:40–55. doi: 10.1525/mp.2016.34.1.40. [DOI] [Google Scholar]
- 68.Flash T, Hogan N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985;5:1688–1703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitall J, Waller SMC, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.STR.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 70.Luft AR, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: A randomized controlled trial. J. Am. Med. Assoc. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeong S, Kim MT. Effects of a theory-driven music and movement program for stroke survivors in a community setting. Appl. Nurs. Res. 2007;20:125–131. doi: 10.1016/j.apnr.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Chen JL, Fujii S, Schlaug G. The use of augmented auditory feedback to improve arm reaching in stroke: A case series. Disabil. Rehabil. 2016;38:1115–1124. doi: 10.3109/09638288.2015.1076530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avanzino L, et al. Time processing and motor control in movement disorders. Front. Hum. Neurosci. 2016;10:631. doi: 10.3389/fnhum.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaffert N, Janzen TB, Mattes K, Thaut MH. A review on the relationship between sound and movement in sports and rehabilitation. Front. Psychol. 2019;10:244. doi: 10.3389/fpsyg.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thaut MH, McIntosh GC, Hoemberg V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015;5:1185. doi: 10.3389/fpsyg.2014.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bella SD, et al. Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci. Rep. 2017;7:42005. doi: 10.1038/srep42005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braunlich K, et al. Rhythmic auditory cues shape neural network recruitment in Parkinson’s disease during repetitive motor behavior. Eur. J. Neurosci. 2019;49:849–858. doi: 10.1111/ejn.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hömberg V. Evidence based medicine in neurological rehabilitation: A critical review. Acta Neurochir. Suppl. 2005;93:3–14. doi: 10.1007/3-211-27577-0_1. [DOI] [PubMed] [Google Scholar]
- 79.Ijmker T, Lamoth CJC. Gait and cognition: The relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012;35:126–130. doi: 10.1016/j.gaitpost.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 80.Wittwer JE, Webster KE, Hill K. Effect of rhythmic auditory cueing on gait in people with Alzheimer disease. Arch. Phys. Med. Rehabil. 2013;94:718–724. doi: 10.1016/j.apmr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Scholz DS, et al. Sonification as a possible stroke rehabilitation strategy. Front. Neurosci. 2014;8:332. doi: 10.3389/fnins.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholz DS, et al. Sonification of arm movements in stroke rehabilitation: A novel approach in neurologic music therapy. Front. Neurol. 2016;7:106. doi: 10.3389/fneur.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milner TE. A model for the generation of movements requiring endpoint precision. Neuroscience. 1992;49:487–496. doi: 10.1016/0306-4522(92)90113-G. [DOI] [PubMed] [Google Scholar]
- 84.Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J. Physiol. 1993;469:673–691. doi: 10.1113/jphysiol.1993.sp019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc. Natl. Acad. Sci. 1999;96:4645–4649. doi: 10.1073/pnas.96.8.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rohrer B, et al. Movement smoothness changes during stroke recovery. J. Neurosci. 2002 doi: 10.1523/jneurosci.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price CI, Rodgers H, Franklin P, Curless RH, Johnson GR. Glenohumeral subluxation, scapula resting position, and scapula rotation after stroke: A noninvasive evaluation. Arch. Phys. Med. Rehabil. 2001;82:955–960. doi: 10.1053/apmr.2001.23826. [DOI] [PubMed] [Google Scholar]
- 88.Meskers CGM, Koppe PA, Konijnenbelt M, Veeger DJEJ, Janssen TWJ. Kinematic alterations in the ipsilateral shoulder of patients with hemiplegia due to stroke. Am. J. Phys. Med. Rehabil. 2005;84:97–105. doi: 10.1097/01.PHM.0000150792.26793.E9. [DOI] [PubMed] [Google Scholar]
- 89.Niessen MH, et al. Proprioception of the shoulder after stroke. Arch. Phys. Med. Rehabil. 2008;89:333–338. doi: 10.1016/j.apmr.2007.08.157. [DOI] [PubMed] [Google Scholar]
- 90.Niessen M, et al. Kinematics of the contralateral and ipsilateral shoulder: A possible relationship with post-stroke shoulder pain. J. Rehabil. Med. 2008;40:482–486. doi: 10.2340/16501977-0201. [DOI] [PubMed] [Google Scholar]
- 91.Kim JR, et al. Effects of rhythmic auditory stimulation during hemiplegic arm reaching in individuals with stroke: An exploratory study. Hong Kong J. Occup. Ther. 2014 doi: 10.1016/j.hkjot.2014.11.002. [DOI] [Google Scholar]
- 92.Miniato MA, Caire MJ. Anatomy, Shoulder and Upper Limb. StatPearls: Shoulder; 2018. [PubMed] [Google Scholar]
- 93.Rundquist PJ, Dumit M, Hartley J, Schultz K, Finley MA. Three-dimensional shoulder complex kinematics in individuals with upper extremity impairment from chronic stroke. Disabil. Rehabil. 2012;34:402–407. doi: 10.3109/09638288.2011.607214. [DOI] [PubMed] [Google Scholar]
- 94.Barnes CJ, Van Steyn SJ, Fischer RA. The effects of age, sex, and shoulder dominance on range of motion of the shoulder. J. Shoulder Elb. Surg. 2001;10:242–246. doi: 10.1067/mse.2001.115270. [DOI] [PubMed] [Google Scholar]
- 95.Madgwick, S. O. Automated calibration of an accelerometers, magnetometers and gyroscopes: A feasibility study. Tehc Rep, x-io Technol. Ltd. 1–11, Bristol, UK. http://www.x-io.co.uk/ (report and demonstration video) (2010).
- 96.Madgwick, S. O. H., Harrison, A. J. L. & Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. in IEEE International Conference on Rehabilitation Robotics 1–7 (2011). 10.1109/ICORR.2011.5975346. [DOI] [PubMed]
- 97.Diebel J. Representing attitude: Euler angles, unit quaternions, and rotation vectors. Matrix. 2006;58:1–35. [Google Scholar]
- 98.Hall LC, Middlebrook EE, Dickerson CR. Analysis of the influence of rotator cuff impingements on upper limb kinematics in an elderly population during activities of daily living. Clin. Biomech. 2011;26:579–584. doi: 10.1016/j.clinbiomech.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Brookham RL, Cudlip AC, Dickerson CR. Examining upper limb kinematics and dysfunction of breast cancer survivors in functional dynamic tasks. Clin. Biomech. 2018;55:86–93. doi: 10.1016/j.clinbiomech.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 100.Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain. 2009;132:2151–2160. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- 101.Gates DH, Walters LS, Cowley J, Wilken JM, Resnik L. Range of motion requirements for upper-limb activities of daily living. Am. J. Occup. Ther. 2016;70:7001350010p1–7001350010p10. doi: 10.5014/ajot.2016.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aizawa J, et al. Ranges of active joint motion for the shoulder, elbow, and wrist in healthy adults. Disabil. Rehabil. 2013;35:1342–1349. doi: 10.3109/09638288.2012.731133. [DOI] [PubMed] [Google Scholar]
- 103.Rettig O, Krautwurst B, Maier MW, Wolf SI. Definition of anatomical zero positions for assessing shoulder pose with 3D motion capture during bilateral abduction of the arms. BMC Musculoskelet. Disord. 2015;16:383. doi: 10.1186/s12891-015-0840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brookham RL, Wong JM, Dickerson CR. Upper limb posture and submaximal hand tasks influence shoulder muscle activity. Int. J. Ind. Ergon. 2010;40:337–344. doi: 10.1016/j.ergon.2009.11.006. [DOI] [Google Scholar]
- 105.Jaspers E, et al. Upper limb kinematics: Development and reliability of a clinical protocol for children. Gait Posture. 2011;33:279–285. doi: 10.1016/j.gaitpost.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 106.El-Gohary M, McNames J. Shoulder and elbow joint angle tracking with inertial sensors. IEEE Trans. Biomed. Eng. 2012;59:2635–2641. doi: 10.1109/TBME.2012.2208750. [DOI] [PubMed] [Google Scholar]
- 107.Wu CY, Chen CL, Tang SF, Lin KC, Huang YY. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2007;88:964–970. doi: 10.1016/j.apmr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: A randomized controlled study. Clin. Rehabil. 2007;21:1075–1086. doi: 10.1177/0269215507079843. [DOI] [PubMed] [Google Scholar]
- 109.Wu CY, Lin KC, Chen HC, Chen IH, Hong WH. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: A kinematic study of motor control mechanisms. Neurorehabil. Neural Repair. 2007;21:460–466. doi: 10.1177/1545968307303411. [DOI] [PubMed] [Google Scholar]
- 110.Kerr A, Rowe P. An Introduction to Human Movement and Biomechanics. New York: Elsevier; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.