Abstract
In this study, the detailed volatile compositions of Chinese herbaceous aroma-type Baijiu (HAB) were characterized by comprehensive two-dimensional gas chromatography-time of flight mass spectrometry (GC×GC-TOFMS). A total of 606 compounds were tentatively identified by similarity, mass spectral data, and retention indices, among which 247 compounds were positively verified by authentic standards. Esters were present in higher numbers (179), followed by aldehydes and ketones (111), and alcohols (81). In addition, there were also many terpenes (82), sulfides (37), furans (29), nitrogenous compounds (29), lactones (17), and so on. Meanwhile, the extraction effects of volatile components from different sample pretreatment methods (headspace solid-phase microextraction (HS-SPME), solid phase extraction (SPE), and stir bar sorptive extraction (SBSE)) for HAB were also revealed. The results indicated that HS-SPME has a better extraction effect on easily volatile compounds, such as alcohols and sulfides, especially for terpenes. SPE was particularly beneficial for the analysis of nitrogen-containing compounds; SBSE showed medium extraction ability for most types of compounds and was more suitable for the target analysis of trace content substances.
Keywords: GC×GC-TOFMS, HS-SPME, SPE, SBSE, Chinese herbaceous aroma-type Baijiu
1. Introduction
As a traditional indigenous spirit and the most distilled liquor produced globally [1], Baijiu plays an important role in the Chinese traditional food industry, with nearly 8 million kiloliters of production in 2019 [2]. Baijiu is made from sorghum as the main raw material, produced by a solid-state spontaneous fermentation process, which accumulates a complex community of microorganisms contributing to the generation of complex layers of flavor [3,4]. Due to the differences in production technology and aroma characteristics, Baijiu can be divided into different aroma-type categories, including soy sauce aroma type, light aroma-type, strong aroma-type, and herbaceous aroma-type Baijiu (HAB), etc. Among them, HAB is produced from sorghum mixed with more than 100 Chinese herbs [5], which gives the distillate a distinctive flavor and creates more aroma active substances.
Volatile compositions are often regarded as the main characteristics to determine Baijiu quality and are essential for consumers’ criteria; therefore, most studies focus on the identification of volatile components in Baijiu. Gas chromatography-flame ionization detection (GC-FID), gas chromatography-mass spectrometry (GC-MS), and other techniques have been gradually applied to the study of Baijiu, and hundreds of volatile components have been identified so far. Nevertheless, because the resolution of one-dimensional gas chromatography (1DGC) has a limit to separating mixtures including hundreds or even thousands of components, it is often necessary to combine normal phase chromatography and other complex pre-separation methods to assist in the separation and identification of volatile components in Baijiu samples [6,7]. Therefore, to meet the requirement of stronger separation energy, comprehensive two-dimensional gas chromatography (GC×GC) has emerged. GC×GC involves the combination of two capillary columns with different separation mechanisms through a single modulator. With properly selected orthogonal separation mechanisms, GC×GC allows for the separation of a large number of compounds in a single chromatographic run due to the added selectivity of the second column and inherently high peak capacity [8]. GC×GC has been a powerful technique for analyzing volatile components in highly complex samples [9,10], such as petroleum [11], environmental samples [12], essential oils [13], and wines [14]. Through acquisition of a large amount of data from samples based on GC×GC, significantly different compounds from different regions or varieties were recognized by means of multivariate analysis [15,16,17]. GC×GC has also been used to create sample-specific fingerprints for sample differentiation [18]; for example, Cordero et al. used GC×GC for the creation of two-dimensional (2D) fingerprints for roasted hazelnuts from different cultivars, varieties, and geographical origins [19]. In addition, Gracka et al. used GC×GC to monitor the changes in volatile compounds related to roasting conditions [20].
Despite the significant benefits offered by GC×GC for the separation and identification of volatiles, sample preparation is also a critical step when characterizing the volatile compositions in such a complex matrix. Headspace solid-phase microextraction (HS-SPME) has been by far the most applied sample preparation method in GC×GC, followed by solid phase extraction (SPE), and stir bar sorptive extraction (SBSE) [21]. A series of validated HS-SPME methods have been proposed for targeted analyses of volatile compounds in a variety of samples [22,23], while research found that HS-SPME has limited application for some influential high-boiling compounds [8]. Accordingly, to compensate for these shortcomings, SPE combined with GC×GC was utilized for the detailed investigation of particularly low-level semi-volatiles and obtained a satisfactory result in wine [24].
In the recent years, GC×GC has been gradually applied to the component analysis of soy sauce aroma-type Baijiu [25] and strong aroma-type Baijiu [26], and more than 1000 volatile components have been identified. However, there are few studies of HAB [27,28], and no systematic analysis using GC×GC has been performed so far. Hence, we analyzed HAB by means of comprehensive two-dimensional gas chromatography-time of flight mass spectrometry (GC×GC-TOFMS) for the purpose of overall characterization of volatiles and revealed the volatile compound profile, and potentially key aromatic compounds. Meanwhile, three pretreatment methods (HS-SPME, SPE, and SBSE) in combination with GC×GC-TOFMS were used for the first time to compare the extraction ability on a complex Baijiu sample and determine the biased analysis of some methods for certain groups of compounds.
2. Results and Discussion
2.1. GC×GC-TOFMS Separation and Identification of Volatile Components in HAB
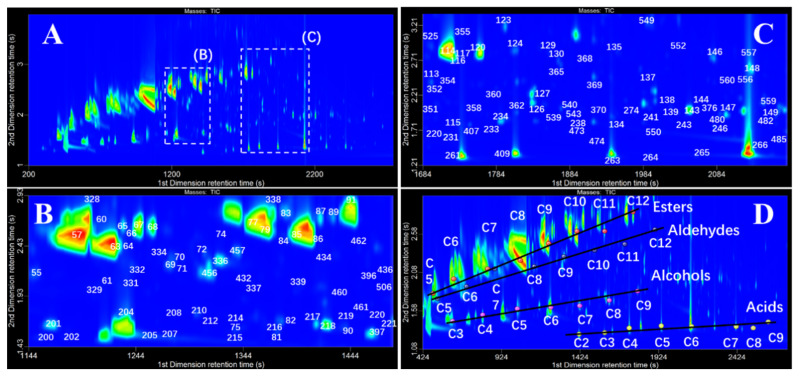
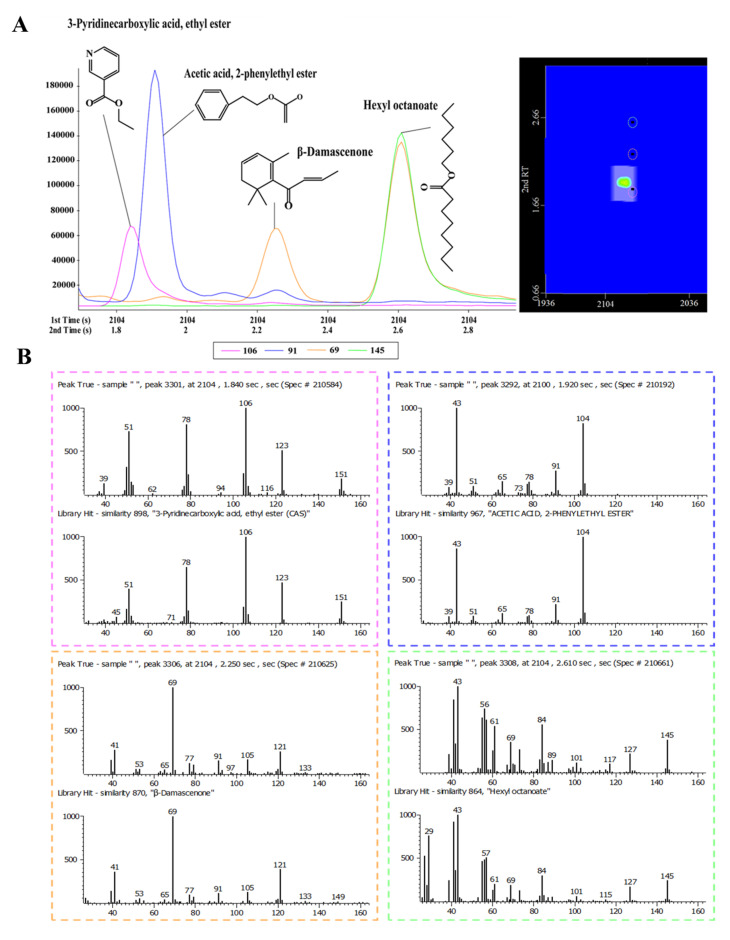
GC×GC-TOFMS was used for the overall characterization of volatile components in HAB in this study based on the higher capacity, significantly enhanced resolving power, and spectral deconvolution function. As Baijiu mainly consists of polar compounds, such as esters, alcohols, aldehydes, and acids, a (polar × medium-polar) column combination can be more beneficial to the separation [25]. Figure 1A is the 2D chromatogram obtained for HAB analyzed by HS-SPME combined with GC×GC-TOFMS. Ordered chromatograms of homologous series in HAB are observed using the above reversed-type column set. Baijiu has a complex matrix consisting of a large number of volatiles with wide-ranging physicochemical properties, and abundant coelution is observed on conventional 1DGC. This restriction is overcome in GC×GC by subjecting the sample to separation based on two different mechanisms, e.g., polarity in 1D and mid-polarity in 2D. Figure 1B,C illustrates the effectiveness of this approach for HAB analysis. Obviously, the number of compounds shown in this figure could not be separated using conventional 1DGC methods. In fact, only approximately 1/5 of the compounds detected here can be fully resolved in one dimension. As shown in Figure 2A, acetic acid 2-phenylethyl ester, 3-pyridinecarboxylic acid ethyl ester, hexyl octanoate, and β-damascenone were coeluted during 1DGC separation, and this overlapping peak makes qualitative and quantitative analysis difficult. Figure 2A shows that these four compounds are easily separated in the 2D plot. Analytes flowing from the 1st column were sequentially separated by 2nd columns with different retention mechanisms, and the interference of coeluted components was efficiently reduced. Figure 2B shows the mass spectra of the four compounds compared to the mass spectra in the NIST library, and the results indicate that identification of the compound is accurate.
Figure 1.
(A) Complete 2D contour plot; (B,C) present detailed portions of the contour plot to illustrate some of the identified compounds. Compound numbers correspond to Table S1. (D) GC × GC distribution of homologous series. Esters: ethyl propionate, ethyl butanoate, ethyl valerate, ethyl hexanoate, ethyl heptanoate, ethyl octanoate, ethyl nonanoate, and ethyl decanoate. Aldehydes: pentanal, hexanal, heptanal, octanal, nonanal, decanal, undecanal, and dodecanal. Alcohols: propanol, butanol, pentanol, hexanol, heptanol, octanol, and nonanol. Acids: acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, octanoic acid, nonanoic acid, and decanoic acid.
Figure 2.
(A) Four peaks shown in the two-dimensional chromatogram and modulated peaks of four compounds found in Chinese herbaceous aroma-type Baijiu. (B) Deconvoluted mass spectra of compounds.
Another advantage of GC×GC is the generation of structured chromatograms. The compounds with similar chemical structures will be grouped in a 2D plot. As shown in Figure 1D, the presence of four homologous series compounds was observed for some straight-chain esters, alcohols, acids, and aldehydes. The lines described in the graph show a tendency of organized distribution of these components in the 2D space, and labels represent the carbon atom number of the molecule. The organized distribution of homologous members can be predicted or confirmed, so it is very useful for reliable identification. Esters and alcohols have lower polarity, so they eluted early on the 1st column. Polar acid compounds were retained better in the 1D column, because of their strong polarity; they eluted at higher temperatures, so they eluted earlier on the second dimension and appeared at the bottom. However, apparent 2D tailing was observed for acid compounds, which may cause more coelutions and influence the accurate identification, especially of minor constituents. Tailing in the 2nd dimension was related to the incompatibility of the polar compounds with the mid-polar stationary phase used in 2D.
Nontargeted analysis is performed when it is desirable to have knowledge of all the components in a mixture. In this study, more than 3000 chromatographic peaks with signal-to-noise (S/N) ratios greater than 100 were recognized by deconvolution. Then, the deconvoluted mass spectra were compared with NIST 2014 and Wiley 9 spectral libraries using Chroma TOF4.61.1.0 software with a match value of 70% as the minimum requirement, and 1266 compounds were retained. Next, 472 unwanted search results were eliminated. Finally, a total of 606 compounds were verified by comparing retention indices and mass spectra to those of reference standards. Among them, 247 compounds were positively verified by authentic standards.
2.2. Comparison of Pretreatment Methods
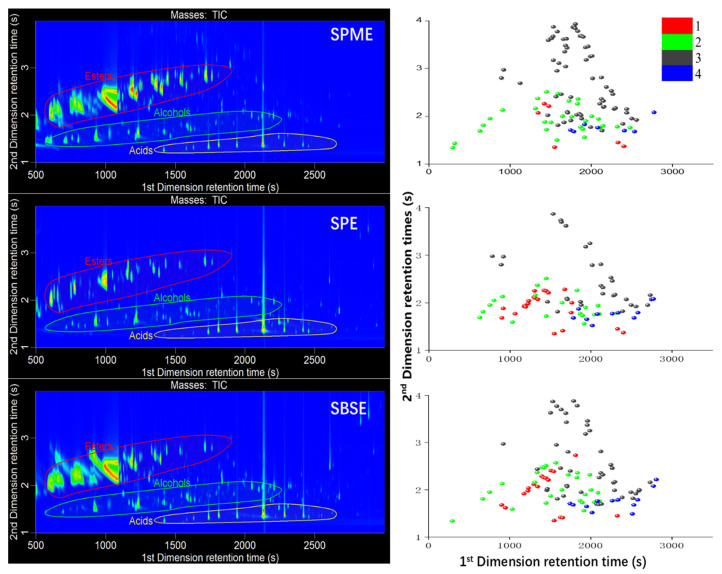
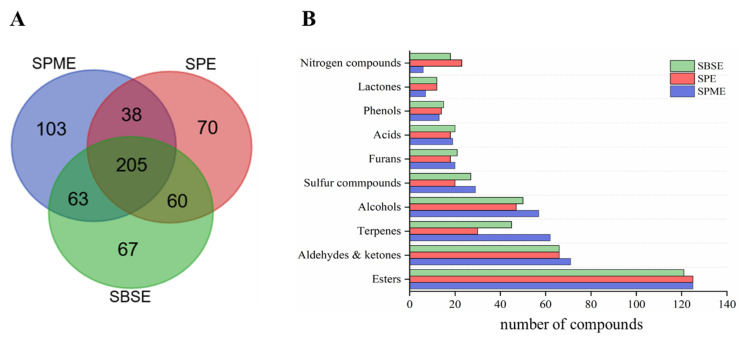
Figure 3 shows the 2D plot of HAB extracts obtained by three different pretreatment methods (HS-SPME, SPE, and SBSE). Table S1 lists the 606 compounds identified in this study, grouped according to different chemical classes. This result showed the most detailed characterization of volatile constituents in HAB for the first time. Figure 4 presents the correlation of HAB analytes identified by three pretreatment methods, and only 205 compounds were commonly identified, which shows the great difference among the three pretreatment methods. Table 1 compares the number of compounds identified in each class using HS-SPME, SPE, and SBSE.
Figure 3.
Total ion chromatogram (TIC) contour plot obtained from the HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS, and SBSE-GC×GC-TOFMS analysis of herbaceous aroma-type Baijiu, and 4 classes of compounds distributed in contour plot (red balls are nitrogenous compounds, green balls are sulfides, gray balls are terpenes, and blue balls are lactone compounds).
Figure 4.
Comparison of identification compounds obtained by HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS, and SBSE-GC×GC-TOFMS. (A) (Venn diagram) and (B) (Bar plot graph displaying compound distribution according to chemical class).
Table 1.
Comparison of volatile compounds detected in Chinese herbaceous aroma-type Baijiu using HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS, and SBSE-GC×GC-TOFMS.
| Class | Number of Compounds | |||
|---|---|---|---|---|
| SPME | SPE | SBSE | Total | |
| Esters | 125 | 125 | 121 | 179 |
| Aldehydes & Ketones | 71 | 66 | 66 | 111 |
| Terpenes | 62 | 30 | 45 | 82 |
| Alcohols | 57 | 47 | 50 | 81 |
| Sulfides | 29 | 20 | 27 | 37 |
| Furans | 20 | 18 | 21 | 29 |
| Nitrogenous compounds | 6 | 23 | 18 | 29 |
| Acids | 19 | 18 | 20 | 23 |
| Phenols | 13 | 14 | 15 | 18 |
| Lactones | 7 | 12 | 12 | 17 |
| Total | 409 | 373 | 395 | 606 |
HS-SPME is based on the establishment of partition equilibrium of the analytes between the polymeric stationary phase, which covers a fused silica fiber, and the matrix of the sample. It is a simple, rapid, and inexpensive technique in which the extraction and concentration processes are performed simultaneously; furthermore, only small sample volumes are required [29]. A total of 409 volatile compounds were identified by HS-SPME-GC×GC-TOFMS, including esters, alcohols, sulfides, and terpenes; however, lactones and nitrogenous compounds were poorly detected. The results indicated that HS-SPME was particularly beneficial for the analysis of volatile compounds, but defective for the extraction of some high-boiling volatile compounds.
SPE has also been reported to have the functions of extraction, enrichment, and rinsing. Abundant adsorbent material can provide high extraction capacity when properly optimized [21]. SPE is supposed to be the complementary nature of the extraction techniques for HS-SPME, which is best exploited for the analysis of semi-volatiles. Several peaks present at the end of the contour plot by SPE were not detected when using HS-SPME, such as γ-dodecalactone, ethyl cis-9-octadecenoate, ethyl linoleate, and ethyl vanillate. In addition, more nitrogenous compounds were detected when using SPE protocol. However, some volatile compounds may be lost, including terpenes and volatile sulfides, because the Baijiu sample was exposed to atmosphere during extraction.
SBSE was initially introduced in 1999 as a miniaturized and solvent-free extraction technique for aqueous samples. Compared with HS-SPME, SBSE provides greater analytical sensitivity and reaches much lower detection and quantification limits. The reason is that the enrichment factor for SBSE is higher than that of HS-SPME using the same stationary phase, because of the 50–250 times larger volume of extraction phase on the stir bar [30]. However, large amounts of stationary phase extracted excessive amounts of solute, thus overloading the GC×GC system (particularly the 2nd column). After split injection (20:1), more terpenes and sulfides were obtained than by the SPE method, due to the apolar adsorbent material PDMS. On the other hand, more high-boiling compounds, including higher fatty acid esters, acids, nitrogenous compounds, and lactones, were obtained.
2.3. Volatile Components in HAB
A total of 606 volatile compounds were identified in this study, among which esters were present in the highest number (179), followed by aldehydes and ketones (111), terpenes (82), alcohols (81), sulfides (37), furans (29), nitrogenous compounds (29), acids (23), phenols (18), and lactones (17).
2.3.1. Skeleton Components
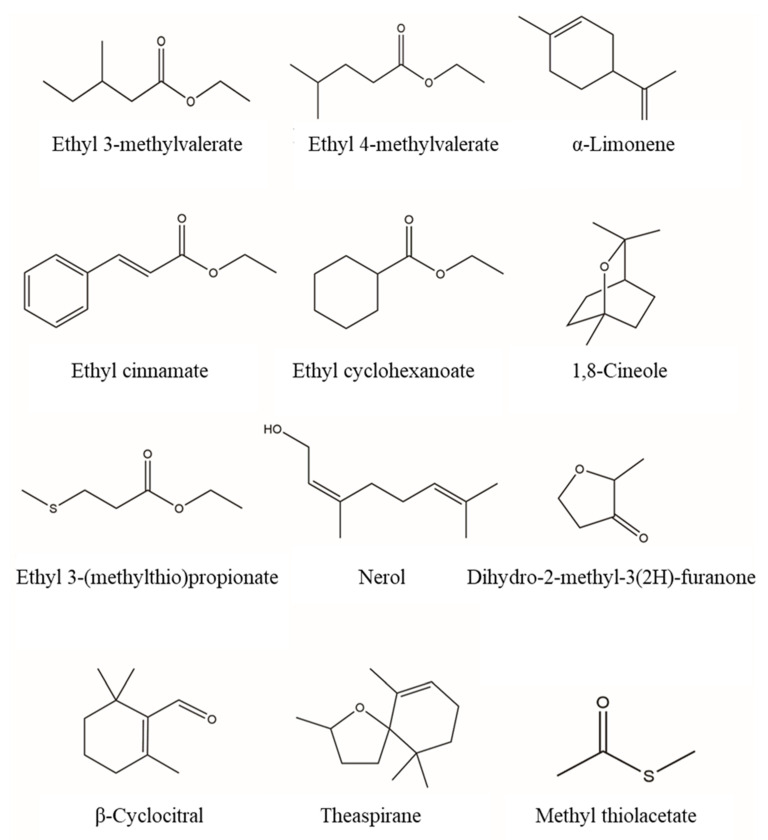
Esters, alcohols, acids, aldehydes, and ketones are the skeletal components of Chinese Baijiu. A total of 179 esters were detected in this study. Among them, ethyl esters were the most representative, and the ethyl esters homologous C2–C12 and C14–C18 were all detected. Ethyl acetate, ethyl butanoate, and ethyl hexanoate are the key aroma compounds in Baijiu, which mainly contribute to its fruity and sweet aroma. In addition, some compounds with very low odor thresholds that cannot be found in the previous literature also contribute to the overall aroma, for example (Figure 5), ethyl 3-methylvalerate has an odor threshold of 8 ng/L and contributes to a strawberry flavor [31]; ethyl cyclohexanoate has an odor threshold of 1 ng/L and reveals strawberry and anise aroma [32]; ethyl cinnamate is known for its honey and cinnamon flavor with an odor threshold of 1 μg/L [33].
Figure 5.
Chemical structure of some aroma compounds first reported in Chinese herbaceous aroma-type Baijiu.
Aldehydes and ketones were the second major categories of identified volatiles, amounting to a total of 111 compounds, and the homologous series of straight-chain aldehydes from C2–C12 were all detected. (E, Z)-2,6-nonadienal is the strongest aroma aldehyde compound in HAB and is described as having a strong cucumber aroma [27]; 3-methyl-butanal presents cocoa and almond aroma with an odor threshold of 0.5 μg/L [27]; 2,3-butanedione has been described as contributing a butter aroma with an odor threshold of 100 μg/L [33]; (E)-2-octenal is a key aroma odorant of Chinese chixiang aroma-type Baijiu, which has a fatty flavor with an odor threshold of 15.1 μg/L [27]. 3-Methyl-butanal, 2,3-butanedione and (E)-2-octenal were identified in this study for the first time.
Alcohols are highly volatile constituents of alcoholic beverages transformed from sugar during fermentation. Among them, 39 out of the 81 alcohols were confirmed using authentic standards, and the homologous series of saturated straight-chain primary alcohols from C3-C12 were all detected. 2-Butanol, 1-butanol, and 3-methyl-1-butanol have odor thresholds of 50, 2.73, and 179 mg/L, respectively, in Baijiu. They are also important aroma compounds in HAB, contributing to the fruit or mellow flavor [27].
A total of 23 acid compounds were identified in this study, most of which are saturated monocarboxylic fatty acids. The homologous series of straight-chain monocarboxylic fatty acids from C1–C10, C14, and C16 were all detected, among which butanoic acid, pentanoic acid, and hexanoic acid play an important role in the flavor of Baijiu, which contributes to rancid and cheesy odors [27]; 2-methyl butyric acid and phenylacetic acid have relatively low odor thresholds of 5.9 and 1.4 mg/L, respectively, and are the key food odorants (KFO) [34].
2.3.2. Terpenes
Many terpenoids have important physiological activities, although they exist with low contents in Baijiu. A total of 82 terpenoid compounds (Table 2) were detected in this study, including mono- and polyterpene hydrocarbons, alcohols, carbonyls, and esters. This represents a significant improvement in the number of terpenoids detected by GC×GC-TOFMS compared to a previous report (only 41 compounds) [28]. Terpenes are well-known varietal compounds of Vitis vinifera, and raw materials are important sources of terpenoids; furthermore, most terpenes exist in grapes, and their contribution to wine aroma is significant [35]. During the production process of HAB, more than 100 Chinese herbs are mixed in the raw materials, which create more terpenoid compounds and give Baijiu a distinctive flavor. In addition, substantial evidence also exists to show the formation of terpene-related compounds during fermentation and aging.
Table 2.
A total of 82 terpene compounds in Chinese herbaceous aroma-type Baijiu.
| NO. | Compounds | RT1 a | RT2 b | Similarity | LRIcal c | LRIlit d | Identification e |
|---|---|---|---|---|---|---|---|
| 1 | δ-3-Carene | 784 | 2.98 | 842 | 1139 | 1166 | RI, MS, Tent |
| 2 | α-Limonene | 892 | 2.8 | 913 | 1193 | 1200 | RI, MS, STD |
| 3 | 1,8-Cineole | 920 | 2.97 | 862 | 1207 | 1211 | RI, MS, Tent |
| 4 | Terpinolene | 1124 | 2.69 | 886 | 1306 | 1280 | RI, MS, STD |
| 5 | α-Thujone | 1340 | 2.44 | 790 | 1414 | 1431 | RI, MS, Tent |
| 6 | trans-Linalool oxide | 1460 | 1.99 | 925 | 1476 | 1483 | RI, MS, Tent |
| 7 | cis-Linalool oxide | 1464 | 2.02 | 921 | 1478 | 1454 | RI, MS, Tent |
| 8 | α-Longipinene | 1472 | 3.59 | 828 | 1483 | 1482 | RI, MS, Tent |
| 9 | α-Copaene | 1504 | 3.63 | 831 | 1500 | 1497 | RI, MS, Tent |
| 10 | Daucene | 1508 | 3.63 | 879 | 1502 | 1495 | RI, MS, STD |
| 11 | Longicyclene | 1528 | 3.67 | 889 | 1513 | 1497 | RI, MS, Tent |
| 12 | Theaspirane B | 1532 | 3.17 | 717 | 1515 | 1522 | RI, MS, Tent |
| 13 | Camphor | 1532 | 3.87 | 758 | 1515 | 1540 | RI, MS, STD |
| 14 | (−)-Camphor | 1564 | 2.41 | 949 | 1532 | 1532 | RI, MS, Tent |
| 15 | Vitispirane | 1576 | 2.95 | 853 | 1539 | 1527 | RI, MS, Tent |
| 16 | α-Gurjunene | 1580 | 3.77 | 903 | 1541 | 1529 | RI, MS, Tent |
| 17 | Linalool | 1588 | 1.81 | 947 | 1545 | 1552 | RI, MS, STD |
| 18 | Theaspirane | 1600 | 3.11 | 838 | 1552 | 1523 | RI, MS, Tent |
| 19 | α-Cedrene | 1628 | 3.74 | 877 | 1568 | 1571 | RI, MS, STD |
| 20 | Carvomenthone | 1628 | 2.46 | 753 | 1567 | 1552 | RI, MS, Tent |
| 21 | β-Funebrene | 1636 | 3.7 | 860 | 1572 | 1588 | RI, MS, Tent |
| 22 | Junipene | 1656 | 3.61 | 926 | 1583 | 1583 | RI, MS, Tent |
| 23 | d-Fenchyl alcohol | 1664 | 1.87 | 942 | 1586 | 1588 | RI, MS, Tent |
| 24 | α-trans-Bergamotene | 1672 | 3.35 | 903 | 1591 | 1583 | RI, MS, Tent |
| 25 | α-Guaiene | 1684 | 3.48 | 860 | 1598 | 1598 | RI, MS, Tent |
| 26 | β-Elemene | 1684 | 3.04 | 908 | 1598 | 1586 | RI, MS, Tent |
| 27 | Calarene | 1692 | 3.62 | 916 | 1602 | 1604 | RI, MS, STD |
| 28 | trans-Caryophyllene | 1700 | 3.43 | 949 | 1607 | 1581 | RI, MS, STD |
| 29 | Terpinen-4-ol | 1704 | 2.09 | 940 | 1608 | 1628 | RI, MS, STD |
| 30 | Isophorone | 1708 | 2.07 | 920 | 1610 | 1600 | RI, MS, STD |
| 31 | trans-Edulan | 1720 | 2.8 | 748 | 1617 | 1620 | RI, MS, Tent |
| 32 | β-Terpineol | 1748 | 1.91 | 862 | 1632 | 1616 | RI, MS, Tent |
| 33 | β-Cyclocitral | 1748 | 2.4 | 824 | 1632 | 1613 | RI, MS, STD |
| 34 | α-Patchoulene | 1776 | 3.86 | 819 | 1648 | 1640 | RI, MS, Tent |
| 35 | Alloaromadendrene | 1788 | 3.88 | 884 | 1655 | 1644 | RI, MS, Tent |
| 36 | β-Barbatene | 1800 | 3.84 | 746 | 1662 | 1667 | RI, MS, Tent |
| 37 | γ-Gurjunene | 1804 | 3.93 | 919 | 1664 | 1674 | RI, MS, Tent |
| 38 | Isoborneol | 1820 | 2.03 | 803 | 1671 | 1672 | RI, MS, Tent |
| 39 | α-Humulene | 1832 | 3.78 | 919 | 1679 | 1680 | RI, MS, Tent |
| 40 | l-Borneol | 1852 | 2.05 | 730 | 1689 | 1675 | RI, MS, Tent |
| 41 | α-Terpineol | 1872 | 2.02 | 958 | 1700 | 1700 | RI, MS, STD |
| 42 | γ-Amorphene | 1864 | 3.68 | 895 | 1696 | 1724 | RI, MS, Tent |
| 43 | Ledene | 1880 | 3.68 | 902 | 1705 | 1701 | RI, MS, Tent |
| 44 | trans-Borneol | 1880 | 1.95 | 924 | 1704 | 1679 | RI, MS, Tent |
| 45 | β-Chamigrene | 1900 | 3.66 | 864 | 1716 | 1702 | RI, MS, Tent |
| 46 | Valencene | 1928 | 3.44 | 899 | 1731 | 1726 | RI, MS, Tent |
| 47 | α-bisabolene | 1936 | 3.18 | 878 | 1735 | 1720 | RI, MS, STD |
| 48 | Germacrene A | 1956 | 3.37 | 839 | 1747 | 1743 | RI, MS, Tent |
| 49 | α-Chamigrene | 1960 | 3.46 | 851 | 1749 | 1753 | RI, MS, Tent |
| 50 | δ-Cadinene | 1988 | 3.25 | 932 | 1764 | 1753 | RI, MS, STD |
| 51 | β-Citronellol | 1992 | 1.77 | 889 | 1765 | 1771 | RI, MS, STD |
| 52 | 7 epi-a-Selinene | 2008 | 3.26 | 873 | 1775 | 1772 | RI, MS, Tent |
| 53 | α-Curcumene | 2016 | 2.79 | 881 | 1779 | 1788 | RI, MS, Tent |
| 54 | Nerol | 2072 | 1.7 | 845 | 1811 | 1821 | RI, MS, Tent |
| 55 | Isogeraniol | 2096 | 1.69 | 832 | 1827 | 1818 | RI, MS, Tent |
| 56 | β-Damascenone | 2104 | 2.26 | 910 | 1832 | 1827 | RI, MS, STD |
| 57 | Dihydro-β-ionone | 2124 | 2.36 | 835 | 1845 | 1854 | RI, MS, Tent |
| 58 | l-calamenene | 2124 | 2.81 | 946 | 1846 | 1838 | RI, MS, STD |
| 59 | Geraniol | 2132 | 1.7 | 872 | 1850 | 1851 | RI, MS, STD |
| 60 | trans-Geranylacetone | 2148 | 2.19 | 877 | 1861 | 1862 | RI, MS, STD |
| 61 | Geosmin | 2148 | 2.32 | 902 | 1861 | 1858 | RI, MS, STD |
| 62 | α-Ionone | 2156 | 2.2 | 846 | 1866 | 1866 | RI, MS, STD |
| 63 | α-Dehydro-himachalene | 2184 | 2.61 | 836 | 1885 | 1882 | RI, MS, Tent |
| 64 | α-Calacorene | 2248 | 2.53 | 898 | 1930 | 1904 | RI, MS, Tent |
| 65 | Palustrol | 2264 | 2.46 | 899 | 1941 | 1938 | RI, MS, Tent |
| 66 | trans-β-Ionone | 2280 | 2.15 | 854 | 1952 | 1953 | RI, MS, STD |
| 67 | cis-Jasmone | 2292 | 1.99 | 859 | 1961 | 1955 | RI, MS, STD |
| 68 | β-Caryophyllene oxide | 2296 | 2.17 | 792 | 1964 | 1990 | RI, MS, Tent |
| 69 | d-Nerolidol | 2388 | 1.84 | 921 | 2036 | 2010 | RI, MS, Tent |
| 70 | E-Nerolidol | 2392 | 1.82 | 926 | 2040 | 2054 | RI, MS, Tent |
| 71 | Epicubenol | 2436 | 2.07 | 765 | 2077 | 2078 | RI, MS, Tent |
| 72 | α-Corocalene | 2436 | 2.15 | 863 | 2077 | 2083 | RI, MS, Tent |
| 73 | Cubenol | 2436 | 2.07 | 787 | 2077 | 2071 | RI, MS, Tent |
| 74 | 6-Isocedrol | 2496 | 1.95 | 894 | 2135 | 2162 | RI, MS, Tent |
| 75 | α-Cedrol | 2496 | 1.95 | 877 | 2135 | 2127 | RI, MS, Tent |
| 76 | β-Bisabolol | 2520 | 1.82 | 728 | 2160 | 2151 | RI, MS, Tent |
| 77 | Torreyol | 2556 | 1.92 | 815 | 2197 | 2197 | RI, MS, Tent |
| 78 | α-Cadinol | 2556 | 1.92 | 810 | 2197 | 2217 | RI, MS, STD |
| 79 | α-Eudesmol | 2592 | 1.98 | 719 | 2237 | 2223 | RI, MS, Tent |
| 80 | β-Eudesmol | 2600 | 2 | 821 | 2246 | 2246 | RI, MS, Tent |
| 81 | Farnesol | 2700 | 1.95 | 846 | 2353 | 2351 | RI, MS, Tent |
| 82 | 9H-Fluorene | 2732 | 2.16 | 907 | 2386 | 2374 | RI, MS, Tent |
a RT1: retention time on the primary column. b RT2: retention time on the secondary column. c LRIcal: calculated linear retention indices. d LRIlit: literature linear retention indices obtained from the NIST library (https://webbook.nist.gov/chemistry/). e Identification: tentative identification (Tent.) based on retention indices (RI) and mass spectra (MS), positive identification based on retention times of authentic standards (STD).
Linalool, geraniol, and β-citronellol are common terpenes in Baijiu and present floral aroma properties. The odor perception thresholds of these compounds are 13.1 μg/L, 120 μg/L, and 300 μg/L [36], respectively. Geosmin is known for its beet, earth aroma as an off-flavor compound, whose odor threshold is 0.1 μg/L in 46%vol ethanol aqueous solution [37]. β-Ionone is believed to be responsible for the characteristic violet and floral aroma, whose concentration in Baijiu is generally 0.3–2.2 μg/L, and the odor threshold in 46%vol ethanol aqueous solution is 1.3 μg/L [36]. In addition, a series of terpenes with low thresholds were also found in this study for the first time (as shown in Figure 5); for example, 1,8-cineole presents a fresh odor with an odor threshold of 0.26 μg/L, and β-cyclocitral has an odor threshold of 5 μg/L [38].
2.3.3. Sulfides
Volatile sulfides play a remarkable role in the aroma of food and beverages, even when present at low concentrations [39,40]. Recently, Wang et al. found that the imbalance of sulfides will lead to the off-odor in soy sauce aroma-type Baijiu; however, these compounds might contribute to the overall aroma of Baijiu at relatively low concentrations [41]. In this study, a total of 37 sulfides (Table 3) were identified, of which 28 compounds have not been reported before in Baijiu. Sulfides usually have a relatively low odor threshold and contribute to the onion, cabbage, and sulfur aroma. Dimethyl trisulfide is an important sulfide in Baijiu, and it is well known for the onion and cabbage aroma with an odor threshold of 0.36 μg/L [42]. Methional is characterized by cooked potatoes and has a perception threshold of 7.1 μg/L [42]. Several sulfides were first reported in HAB, and s-methyl ester butanethioic acid shows a sulfurous and cheesy aroma [43], 5-Methyl-2-formylthiophene is described as moldy odor, and 1,2,4-trithiolane contributes the roasted beef and sulfurous aroma.
Table 3.
A total of 37 sulfides in Chinese herbaceous aroma-type Baijiu.
| No | Compounds | RT1 a | RT2 b | Similarity | LRIcal c | LRIlit d | Identification e |
|---|---|---|---|---|---|---|---|
| 1 | Methanethiol | 292 | 1.34 | 985 | 669 | 643 | RI, MS, STD |
| 2 | Dimethyl sulfide | 316 | 1.43 | 895 | 750 | 774 | RI, MS, STD |
| 3 | Methyl thiolacetate | 628 | 1.69 | 814 | 1054 | 1052 | RI, MS, Tent |
| 4 | Dimethyl disulfide | 668 | 1.81 | 960 | 1077 | 1078 | RI, MS, STD |
| 5 | S-Methyl propanethioate | 752 | 1.95 | 749 | 1122 | 1131 | RI, MS, STD |
| 6 | Methyl ethyl disulfide | 804 | 2.05 | 736 | 1149 | 1141 | RI, MS, Tent |
| 7 | S-Methyl ester butanethioic acid | 908 | 2.13 | 835 | 1201 | 1198 | RI, MS, STD |
| 8 | Thiazole | 1032 | 1.59 | 907 | 1261 | 1259 | RI, MS, STD |
| 9 | Dimethyl trisulphide | 1312 | 2.16 | 966 | 1399 | 1400 | RI, MS, STD |
| 10 | S-Methyl hexanethioate | 1340 | 2.37 | 895 | 1414 | 1412 | RI, MS, Tent |
| 11 | Methyl pentyl disulfide | 1400 | 2.48 | 764 | 1445 | 1445 | RI, MS, Tent |
| 12 | 4,5-Dimethyl-2-isopropyl-thiazole | 1424 | 2.47 | 747 | 1457 | 1436 | RI, MS, Tent |
| 13 | Ethyl 2-(methylthio)acetate | 1428 | 1.88 | 902 | 1459 | 1484 | RI, MS, STD |
| 14 | Methional | 1448 | 1.72 | 826 | 1470 | 1480 | RI, MS, STD |
| 15 | 2-Pentyl-thiophene | 1448 | 2.51 | 893 | 1470 | 1452 | RI, MS, Tent |
| 16 | Furfuryl methyl sulfide | 1504 | 1.87 | 913 | 1499 | 1492 | RI, MS, Tent |
| 17 | 4,5-Dimethyl-2-isobutylthiazole | 1568 | 2.57 | 709 | 1534 | 1514 | RI, MS, Tent |
| 18 | 2-(Methylthio)ethanol | 1576 | 1.5 | 725 | 1538 | 1520 | RI, MS, Tent |
| 19 | Methyl propyl trisulfide | 1588 | 2.47 | 752 | 1545 | 1529 | RI, MS, Tent |
| 20 | Ethyl 3-(methylthio)propionate | 1644 | 2 | 961 | 1575 | 1580 | RI, MS, STD |
| 21 | 2,5-Dimethyl-1,3,4-trithiolane | 1724 | 2.32 | 865 | 1619 | 1618 | RI, MS, Tent |
| 22 | 3-(Methylthio)propyl acetate | 1760 | 1.99 | 752 | 1639 | 1627 | RI, MS, Tent |
| 23 | 2,4,5-Trithiahexane | 1828 | 2.26 | 895 | 1676 | 1662 | RI, MS, Tent |
| 24 | Methyl benzyl sulfide | 1836 | 2.36 | 932 | 1680 | 1665 | RI, MS, STD |
| 25 | 3-Thiophenecarboxaldehyde | 1868 | 1.77 | 711 | 1697 | 1687 | RI, MS, Tent |
| 26 | 2-Thiophenecarboxaldehyde | 1896 | 1.73 | 920 | 1713 | 1722 | RI, MS, STD |
| 27 | Methionol | 1916 | 1.56 | 914 | 1724 | 1721 | RI, MS, STD |
| 28 | 5-Methyl-2-formylthiophene | 1932 | 1.9 | 814 | 1733 | 1759 | RI, MS, Tent |
| 29 | Dimethyl tetrasulphide | 1988 | 2.32 | 727 | 1763 | 1750 | RI, MS, Tent |
| 30 | 1,2,4-Trithiolane | 2004 | 2 | 866 | 1772 | 1760 | RI, MS, Tent |
| 31 | 3-Acetylthiophene | 2044 | 1.75 | 752 | 1794 | 1772 | RI, MS, Tent |
| 32 | 2-Acetylthiophen | 2044 | 1.74 | 717 | 1794 | 1785 | RI, MS, STD |
| 33 | Furfuryl methyl disulfide | 2088 | 1.94 | 846 | 1822 | 1813 | RI, MS, Tent |
| 34 | 3-Methyl-2-thiophenecarbaldehyde | 2104 | 1.76 | 798 | 1832 | 1815 | RI, MS, Tent |
| 35 | 1-(2-Thienyl) propanone | 2144 | 1.8 | 714 | 1858 | 1840 | RI, MS, Tent |
| 36 | Benzothiazole | 2320 | 1.78 | 835 | 1981 | 1958 | RI, MS, STD |
| 37 | 2-Phenylthiophene | 2476 | 1.76 | 780 | 2114 | 2124 | RI, MS, STD |
a RT1: retention time on the primary column. b RT2: retention time on the secondary column. c LRIcal: calculated linear retention indices. d LRIlit: literature linear retention indices obtained from the NIST library (https://webbook.nist.gov/chemistry/). e Identification: tentative identification (Tent.) based on retention indices (RI) and mass spectra (MS), positive identification based on retention times of authentic standards (STD).
2.3.4. Cyclic Components
Furans, phenols, and lactones were classified under this group. In this research, a total of 29 furans were detected. Compounds of 1-(2-furanyl)-ethanone, 5-methyl-furfural, 2-acetyl-5-methylfuran, and 2-furanmethanol have been described as contributing a honey, caramel odor [27]. Dihydro-2-methyl-3(2H)-furanone reveals sweet, bread and buttery aroma with a sensory threshold of 5 ng/L [44]. This kind of compound mainly contributes to the sweet aroma of Baijiu.
Eight out of the 18 phenols are first detected in HAB. 2-Methoxy-4-ethylphenol, 2-methoxy-4-methylphenol and 2-methoxy phenol were reported to be important aroma compounds in HAB, revealing smoke, sweet, and spice aroma with odor thresholds of 123 μg/L, 315 μg/L, and 13 μg/L, respectively. 2-Methyl-phenol and 3-ethyl-phenol are key food odorants [34], and they have not been reported in HAB before.
A total of 17 lactone compounds were detected in the current study, of which 12 were identified in Baijiu for the first time. γ-Caprolactone and γ-nonalactone present weak sweet, fruity aroma in HAB [27]. γ-Decalactone has been reported to contribute to peachy and fatty aroma with a threshold of 5000 μg/L [33]. γ-Dodecalactone is responsible for the sweet and floral aroma with a threshold of 7 μg/L [33]. γ-6-(Z)-dodecenolactone was first detected in Baijiu and is associated with sweet and fruity aroma, and its odor threshold is 700 ng/L [45].
2.3.5. Nitrogenous Components
In this study, a total of 29 nitrogenous compounds were detected in HAB, mainly consisting of pyrazines, pyrroles, and pyridines. Tetramethyl-pyrazine was previously described as a baked flavor in HAB [27]. Isopropylpyrazine was identified in Baijiu for the first time.
3. Materials and Methods
3.1. Reagents and Chemicals
A commercially available Dongjiu Baijiu (54% ethanol) was used, which was produced by Guizhou Dongjiu Co., Ltd. (Guizhou, China) according to the National Standard of Herbaceous Aroma-Type Baijiu (DB52/T550). All chemical standards with high-purity grade (GC grade) and C7-C30 n-alkane mixture were obtained from Sigma-Aldrich Co., Ltd. (Shanghai, China). Organic solvents of methanol (HPLC grade), ethanol (HPLC grade), and dichloromethane (HPLC grade) were purchased from J&K Scientific Co., Ltd. (Beijing, China). Sodium chloride (NaCl) and anhydrous sodium sulfate (Na2SO4) were purchased from China National Pharmaceutical Group Corp. Ultrapure water was obtained from a Milli-Q purification system (Millipore, Bedford, MA, USA).
3.2. Sample Extraction Methods
3.2.1. HS-SPME
An automatic headspace sampling system (MultiPurpose Sample MPS 2 with a SPME adaptor, from Gerstel Inc., Muülheim, Ruhr, Germany) with a 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (2 cm, Supelco Inc., Bellefonte, PA, USA) was used to extract the volatile compounds. Following a method presented in the literature [41], the Baijiu sample was diluted with ultrapure water to a final concentration of 10% ethanol by volume. A total of 10 mL diluted Baijiu sample was transferred into a 20 mL screw-capped vial and saturated with 3 g of NaCl. Then, the sample was equilibrated at 40 °C for 5 min and extracted for 40 min at the same temperature under stirring at a rotation speed of 250 rpm. The extracts were desorbed in a GC splitless injector port at 250 °C for 5 min.
3.2.2. SPE
SPE was based on a slightly modified method described by Chen et al. [46]. A total of 10 mL HAB was diluted with ultrapure water to 50 mL and saturated with 15 g of NaCl. The SPE cartridge (0.8 cm internal diameter, 12 mL internal volume, Sigma Aldrich, Shanghai, China) was consecutively conditioned using 20 mL of dichloromethane, 20 mL of methanol, and 30 mL of ultrapure water. A total of 50 mL diluted sample was passed through the Lichrolut EN cartridge at a flow rate of 2 mL/min. After the sample had been loaded, 30 mL ultrapure water was used to rinse the sorbent. Then, the sorbent was dried by letting the air pass through it (−0.6 bar, 10 min). Extracts were recovered by elution with 10 mL of dichloromethane, concentrated under a gentle stream of nitrogen to a final volume of 500 μL, and stored at −20 °C until analysis. Finally, 1 μL extracts were injected into the GC splitless injector port at 250 °C with 450 s acquisition delay.
3.2.3. SBSE
In this study, SBSE was carried out according to the description in the literature [47]. Stir bars (Twister) coated with PDMS (10 mm length × 1.0 mm thickness) were obtained from GERSTEL. Prior to use, the stir bar was conditioned for 30 min at 280 °C in a flow of helium. The Baijiu sample was diluted with ultrapure water to a final concentration of 10% ethanol by volume. A 10 mL diluted sample was saturated with 3 g NaCl in a 20 mL glass vial, and a stir bar was immersed in the sample for enriching the substance. Then, the sample was placed in an agitation plate at 25 °C and extracted at 800 rpm for 90 min. After extraction, the stir bar was removed with a magnetic rod (twister taking tool) and forceps, rinsed briefly with ultrapure water to remove ethanol, and dried with lint-free tissue, followed by placement in a sample holder for GC×GC-TOFMS analysis.
An automatic headspace sampling system was used to analyze the extracts in this study. The stir bar was placed in a glass thermal desorption liner and thermally desorbed by programming the twister desorption unit (TDU) from 35 °C heated at a rate of 700 °C/min to a final temperature of 270 °C and held for 5 min. TDU injection was in split ratio of 20:1 mode during thermal desorption. A cooled injection system (CIS4) was used in the GC×GC-TOFMS system. CIS4 was in solvent vent mode with a venting flow of 60 mL/min for 4.7 min at a venting pressure of 80 kPa. The initial temperature of CIS4 was kept at −60 °C for 0.2 min, ramped at a rate of 10 °C/s to a final temperature of 250 °C, and held for 3 min.
3.3. GC×GC-TOFMS Instrumentation
Experiments were performed on a LECO Pegasus® 4D GC×GC-TOFMS system (LECO Corp., St. Joseph, MI, USA). This instrument consisted of an Agilent 7890B GC (Agilent Technologies, Palo Alto, CA, USA) incorporating LECO’s thermal modulator (dual-stage quad-jet) and a secondary oven mounted inside the primary GC oven. The column set consisted of a 60 m × 0.25 mm × 0.25 μm DB-FFAP (Agilent Technologies, Palo Alto, CA, USA) primary column and a 1.5 m × 0.25 mm × 0.25 μm Rxi-17Sil MS secondary column (Restek, Bellefonte, PA, USA). Ultrahigh purity helium was used as the carrier gas at a constant flow of 1.00 mL/min. The separation was performed using the following temperature program: 45 °C kept for 3 min, ramped at 4 °C/min to 150 °C and held for 2 min; reaching 200 °C at 6 °C/min and 230 °C at 10 °C/min for 20 min. The secondary oven was operated at 5 °C higher than the primary oven throughout. The modulator was offset by +20 °C in relation to the primary oven. A modulation period of 4 s (hot pulse of 0.80 s) was used.
The TOFMS parameters included ion source of 230 °C and transfer line of 240 °C, electron energy of -70 volts, acquisition of 1430, mass range of 35–400 amu, and acquisition rate of 100 spectra/s.
3.4. Data Processing
ChromaTOF version 4.61.1.0 software (LECO Corp., St. Joseph, MI, USA) was used for peak finding, mass spectral deconvolution, peak area integration, and library searching. Automated peak finding and spectral deconvolution with a baseline offset of 0.5 and S/N of 100 after evaluating serval options (i.e., 25, 50, 100, 150, and 200). All analyses were performed in triplicate for each extraction method. The existence of the compound is considered reliable only when the number of detections is greater than 2 at the same retention time. Tentative identification was based on the comparison of mass spectra with the NIST 2014 and Weliy 9 databases using a minimum similarity value of 700 as the criterion, as well as experimentally determined linear retention indices compared to NIST library values. A series of n-alkanes were analyzed under the same conditions to determine first dimension linear retention indices (LRIs) for each compound. A maximum deviation of 30 between the experimental and literature RI values was used as the criterion. Some identification results may be consistent with MS and RI identification, but the 2nd dimensional retention time may not meet the linear distributions of homologous series. The ordered chromatograms of homologous series can also be used for identification. In addition, positive verification of 247 compounds (~41% of the total number) was based on comparison of retention time with authentic standards.
4. Conclusions
The combination of HS-SPME, SPE, and SBSE sample preparation methods coupled with GC×GC-TOFMS analysis enabled us to (tentatively and positively) identify 606 compounds in HAB. Many low content compounds that have never been reported before were identified for the first time. Especially for terpenes, 41 more compounds were identified than previously reported, which are important physiologically active substances in HAB. Furthermore, the contributions of some important compounds were studied in terms of aroma characteristics and odor thresholds. Meanwhile, the three extraction methods show distinct differences and biases for specific analytes. HS-SPME preferred the analysis of alcohols, sulfur-containing, and terpenes compounds; SPE generally revealed more high-boiling compounds, such as lactones and nitrogenous compounds; SBSE showed general extraction ability for all types of compounds, but too much adsorption led to chromatogram overload, making it suitable for the identification of trace content substances. Therefore, the analysis of volatiles in such a complex sample requires multiple pretreatment methods coupled with GC×GC-TOFMS. Importantly, the method can be applied to other alcoholic beverage systems for the determination of the specific kinds of volatile compounds. This approach proved beneficial for the analysis of terpenes, lactones, and sulfur containing compounds, which are important flavor contributors of Baijiu. In addition, the development of this technique laid a foundation for the quantitative determination of the content substances at very low levels (in the region of μg L−1 and lower). Based on the feasibility of accurate quantification, it is hoped that this method can be used to monitor the formation of key aroma substances in the production process of Baijiu.
Supplementary Materials
The following are available online, Table S1: Volatile compounds identified in Chinese herbaceous aroma-type Baijiu by HS-SPME-GC×GC-TOFMS, SPE-GC×GC-TOFMS and SBSE-GC×GC-TOFMS, Table S2: Peak area of 606 volatile compounds identified in Chinese herbaceous aroma-type Baijiu.
Author Contributions
L.W., Conceptualization, methodology, formal analysis, visualization, writing - review & editing; M.G., Validation, data curation; Z.L., investigation, writing—original draft; S.C., Conceptualization, validation, supervision, review & editing; Y.X., Supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 31530055), National Key R&D Program of China (no. 2018YFC1604100), Project funded by China Postdoctoral Science Foundation (no.2018M631971), National First-class Discipline Program of Light Industry Technology and Engineering (no. LITE2018-12), 111 Program of Introducing Talents (no. 111-2-06), and Sichuan Science and Technology Program (no. 2018JZ0033).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all chemicals used in this study are available from the authors.
References
- 1.Liu H.L., Sun B.G. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018;66:5425. doi: 10.1021/acs.jafc.8b00692. [DOI] [PubMed] [Google Scholar]
- 2.Analysis of National Baijiu Production and Growth from January to December 2019. [(accessed on 31 December 2019)]; Available online: https://bg.qianzhan.com/report/detail/459/200207-e8183426.html.
- 3.Jin G.Y., Zhu Y., Xu Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017;63:18–28. doi: 10.1016/j.tifs.2017.02.016. [DOI] [Google Scholar]
- 4.Wu J.H., Zheng Y., Sun B.G., Sun X.T., Sun J.Y., Zheng F.P., Huang M.Q. The Occurrence of Propyl Lactate in Chinese Baijius (Chinese Liquors) Detected by Direct Injection Coupled with Gas Chromatography-Mass Spectrometry. Molecules. 2015;20:19002–19013. doi: 10.3390/molecules201019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng X.W., Han B.Z. Baijiu, Chinese liquor: History, classification and manufacture. J. Ethnic Foods. 2016;3:19–25. doi: 10.1016/j.jef.2016.03.001. [DOI] [Google Scholar]
- 6.Fan W.L., Xu Y., Qian M.C. Flavor Chemistry of Wine and Other Alcoholic Beverages. ACS Publications; Washington, WA, USA: 2012. Identification of aroma compounds in Chinese “Moutai” and “Langjiu” liquors by normal phase liquid chromatography fractionation followed by gas chromatography/olfactometry; pp. 303–338. [Google Scholar]
- 7.Fan W.L., Qian M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006;54:2695–2704. doi: 10.1021/jf052635t. [DOI] [PubMed] [Google Scholar]
- 8.Weldegergis B.T., de Villiers A., McNeish C., Seethapathy S., Mostafa A., Górecki T., Crouch A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC x GC-TOFMS) Food Chem. 2011;129:188–199. doi: 10.1016/j.foodchem.2010.11.157. [DOI] [Google Scholar]
- 9.Amaral M.S.S., Marriott P.J. The Blossoming of Technology for the Analysis of Complex Aroma Bouquets—A Review on Flavour and Odorant Multidimensional and Comprehensive Gas Chromatography Applications. Molecules. 2019;24:2080. doi: 10.3390/molecules24112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaral M.S.S., Nolvachai Y., Marriott P.J. Comprehensive Two-Dimensional Gas Chromatography Advances in Technology and Applications: Biennial Update. Anal. Chem. 2020;92:85–104. doi: 10.1021/acs.analchem.9b05412. [DOI] [PubMed] [Google Scholar]
- 11.Frysinger G.S., Gaines R.B. Separation and identification of petroleum biomarkers by comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2001;24:87–96. doi: 10.1002/1615-9314(20010201)24:2<87::AID-JSSC87>3.0.CO;2-0. [DOI] [Google Scholar]
- 12.Focant J.F., Sjodin A., Patterson D.G. Improved separation of the 209 polychlorinated biphenyl congeners using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A. 2004;1040:227–238. doi: 10.1016/j.chroma.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Marriott P.J., Shellie R., Fergeus J., Ong R., Morrison P. High resolution essential oil analysis by using comprehensive gas chromatographic methodology. Flavour Fragr. J. 2000;15:225–239. doi: 10.1002/1099-1026(200007/08)15:4<225::AID-FFJ903>3.0.CO;2-#. [DOI] [Google Scholar]
- 14.Robinson A.L., Boss P.K., Heymann H., Solomon P.S., Trengove R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A. 2011;1218:504–517. doi: 10.1016/j.chroma.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 15.He Y.X., Liu Z.P., Qian M.C., Yu X.W., Xu Y., Chen S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. 2020;331:127335. doi: 10.1016/j.foodchem.2020.127335. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Zeng Z.D., Zhao C.X., Kong H.W., Lu X., Xu G.W. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A. 2013;1313:245–252. doi: 10.1016/j.chroma.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Welke J.E., Zanus M., Lazzarotto M., Pulgati F.H., Zini C.A. Main differences between volatiles of sparkling and base wines accessed through comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection and chemometric tools. Food Chem. 2014;164:427–437. doi: 10.1016/j.foodchem.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Stilo F., Tredici G., Bicchi C., Robbat A.J., Morimoto J., Cordero C. Climate and Processing Effects on Tea (Camellia sinensis L. Kuntze) Metabolome: Accurate Profiling and Fingerprinting by Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry. Molecules. 2020;25:2447. doi: 10.3390/molecules25102447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordero C., Liberto E., Bicchi C., Rubiolo P., Schieberle P., Reichenbach S.E., Tao Q. Profiling food volatiles by comprehensive two-dimensional ga schromatography coupled with mass spectrometry: Advanced fingerprinting approaches for comparative analysis of the volatile fraction of roasted hazelnuts (Corylus avellana L.) from different orig. J. Chromatogr. A. 2010;1217:5848–5858. doi: 10.1016/j.chroma.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Gracka A., Jeleń H.H., Majcher M., Siger A., Kaczmarek A. Flavoromics approach in monitoring changes in volatile compoundsof virgin rapeseed oil caused by seed roasting. J. Chromatogr. A. 2016;1428:292–304. doi: 10.1016/j.chroma.2015.10.088. [DOI] [PubMed] [Google Scholar]
- 21.Tranchida P.Q., Maimone M., Purcaro G., Dugo P., Mondello L. The penetration of green sample-preparation techniques in comprehensive two-dimensional gas chromatography. Trac-Trends Anal Chem. 2015;71:74–84. doi: 10.1016/j.trac.2015.03.011. [DOI] [Google Scholar]
- 22.Schurek J., Portoles T., Hajslova J., Riddellova K., Hernandez F. Application of head-space solid-phase microextraction coupled to comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the determination of multiple pesticide residues in tea samples. Anal. Chim. Acta. 2008;611:163–172. doi: 10.1016/j.aca.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Purcaro G., Tranchida P.Q., Conte L., Obiedzinska A., Dugo P., Dugo G., Mondello L. Performance evaluation of a rapid-scanning quadrupole mass spectrometer in the comprehensive two-dimensional gas chromatography analysis of pesticides in water. J. Sep. Sci. 2011;34:2411–2417. doi: 10.1002/jssc.201100085. [DOI] [PubMed] [Google Scholar]
- 24.Weldegergis B.T., Crouch A.M., Gorecki T., de Villiers A. Solid phase extraction in combination with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the detailed investigation of volatiles in South African red wines. Anal. Chim. Acta. 2011;701:98–111. doi: 10.1016/j.aca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhu S.K., Lu X., Ji K.H., Guo K.F., Li Y.L., Wu C.Y., Xu G.W. Characterization of flavor compounds in Chinese liquor Moutai by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta. 2007;597:340–348. doi: 10.1016/j.aca.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Yao F., Yi B., Shen C., Tao F., Liu Y., Lin Z., Xu P. Chemical analysis of the Chinese liquor Luzhoulaojiao by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Sci. Rep. 2015;5:9553. doi: 10.1038/srep09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan W.L., Hu G.Y., Yan X.U., Jia Q.Y., Ran X.H. Analysis of Aroma Components in Chinese Herbaceous Aroma Type Liquor. J. Food Sci. Biotechnol. 2012;31:8. [Google Scholar]
- 28.Fan W.L., Hu G.Y., Xu Y. Quantification of Volatile Terpenoids in Chinese Medicinal Liquor Using Headspace-Solid Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Food Sci. 2012;33:110–116. [Google Scholar]
- 29.Castro R., Natera R., Duran E., Garcia-Barroso C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008;228:1–18. doi: 10.1007/s00217-008-0900-4. [DOI] [Google Scholar]
- 30.Ochiai N., Sasamoto K., Ieda T., David F., Sandra P. Multi-stir bar sorptive extraction for analysis of odor compounds in aqueous samples. J. Chromatogr. A. 2013;1315:70–79. doi: 10.1016/j.chroma.2013.09.070. [DOI] [PubMed] [Google Scholar]
- 31.Campo E., Ferreira V., Lopez R., Escudero A., Cacho J. Identification of three novel compounds in wine by means of a laboratory-constructed multidimensional gas chromatographic system. J. Chromatogr. A. 2006;1122:202–208. doi: 10.1016/j.chroma.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Campo E., Cacho J., Ferreira V. Multidimensional chromatographic approach applied to the identification of novel aroma compounds in wine-Identification of ethyl cyclohexanoate, ethyl 2-hydroxy-3-methylbutyrate and ethyl 2-hydroxy-4-methylpentanoate. J. Chromatogr. A. 2006;1137:223–230. doi: 10.1016/j.chroma.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Francis I.L., Newton J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005;11:114–126. [Google Scholar]
- 34.Dunkel A., Steinhaus M., Kotthoff M., Nowak B., Krautwurst D., Schieberle P., Hofmann T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014;53:7124–7143. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- 35.Coelho E., Rocha S.M., Delgadillo I., Coimbra M.A. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta. 2006;563:204–214. [Google Scholar]
- 36.Wang L., Hu G.Y., Lei L.B., Lin L., Wang D.Q., Wu J.X. Identification and Aroma Impact of Volatile Terpenes in Moutai Liquor. Int. J. Food Prop. 2016;19:1335–1352. [Google Scholar]
- 37.Du H., Fan W.L., Xu Y. Characterization of Geosmin as Source of Earthy Odor in Different Aroma Type Chinese Liquors. J. Agric. Food Chem. 2011;59:8331–8337. doi: 10.1021/jf201171b. [DOI] [PubMed] [Google Scholar]
- 38.Buttery R.G., Teranishi R., Ling L.C., Turnbaugh J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990;38:336–340. [Google Scholar]
- 39.Roland A., Schneider R., Razungles A., Cavelier F. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 2011;111:7355–7376. doi: 10.1021/cr100205b. [DOI] [PubMed] [Google Scholar]
- 40.Song X.B., Zhu L., Jing S., Li Q., Ji J., Zheng F.P., Zhao Q.Z., Sun J.Y., Chen F., Zhao M.M., et al. Insights into the Role of 2-Methyl-3-furanthiol and 2-Furfurylthiol as Markers for the Differentiation of Chinese Light, Strong, and Soy Sauce Aroma Types of Baijiu. J. Agric. Food Chem. 2020;68:7946–7954. doi: 10.1021/acs.jafc.0c04170. [DOI] [PubMed] [Google Scholar]
- 41.Wang L.L., Fan S.S., Yan Y., Yang L., Chen S., Xu Y. Characterization of Potent Odorants Causing a Pickle-like Off-Odor in Moutai-Aroma Type Baijiu by Comparative Aroma Extract Dilution Analysis, Quantitative Measurements, Aroma Addition, and Omission Studies. J. Agric. Food Chem. 2020;68:1666–1677. doi: 10.1021/acs.jafc.9b07238. [DOI] [PubMed] [Google Scholar]
- 42.Sha S., Chen S., Qian M., Wang C., Xu Y. Characterization of the Typical Potent Odorants in Chinese Roasted Sesame-like Flavor Type Liquor by Headspace Solid Phase Microextraction–Aroma Extract Dilution Analysis, with Special Emphasis on Sulfur-Containing Odorants. J. Agric. Food Chem. 2017;65:123–131. doi: 10.1021/acs.jafc.6b04242. [DOI] [PubMed] [Google Scholar]
- 43.Du X.F., Plotto A., Baldwin E., Rouseff R. Evaluation of Volatiles from Two Subtropical Strawberry Cultivars Using GC-Olfactometry, GC-MS Odor Activity Values, and Sensory Analysis. J. Agric. Food Chem. 2011;59:12569–12577. doi: 10.1021/jf2030924. [DOI] [PubMed] [Google Scholar]
- 44.Sunarharum W.B., Williams D.J., Smyth H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014;62:315–325. doi: 10.1016/j.foodres.2014.02.030. [DOI] [Google Scholar]
- 45.Siebert T.E., Barker A., Barter S.R., Lopes M.A.D.B., Herderich M.J., Francis I.L. Analysis, potency and occurrence of (Z)-6-dodeceno-γ-lactone in white wine. Food Chem. 2018;256:85–90. doi: 10.1016/j.foodchem.2018.02.094. [DOI] [PubMed] [Google Scholar]
- 46.Chen S., Xu Y., Qian M.C. Aroma Characterization of Chinese Rice Wine by Gas Chromatography-Olfactometry, Chemical Quantitative Analysis, and Aroma Reconstitution. J. Agric. Food Chem. 2013;61:11295–11302. doi: 10.1021/jf4030536. [DOI] [PubMed] [Google Scholar]
- 47.Fan W.L., Shen H.Y., Xu Y. Quantification of volatile compounds in Chinese soy sauce aroma type liquor by stir bar sorptive extraction and gas chromatography-mass spectrometry. J. Sci. Food Agric. 2011;91:1187–1198. doi: 10.1002/jsfa.4294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.