Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and the resulting disease, coronavirus disease 2019 (Covid-19), have spread to millions of persons worldwide. Multiple vaccine candidates are under development, but no vaccine is currently available. Interim safety and immunogenicity data about the vaccine candidate BNT162b1 in younger adults have been reported previously from trials in Germany and the United States.
Methods
In an ongoing, placebo-controlled, observer-blinded, dose-escalation, phase 1 trial conducted in the United States, we randomly assigned healthy adults 18 to 55 years of age and those 65 to 85 years of age to receive either placebo or one of two lipid nanoparticle–formulated, nucleoside-modified RNA vaccine candidates: BNT162b1, which encodes a secreted trimerized SARS-CoV-2 receptor–binding domain; or BNT162b2, which encodes a membrane-anchored SARS-CoV-2 full-length spike, stabilized in the prefusion conformation. The primary outcome was safety (e.g., local and systemic reactions and adverse events); immunogenicity was a secondary outcome. Trial groups were defined according to vaccine candidate, age of the participants, and vaccine dose level (10 μg, 20 μg, 30 μg, and 100 μg). In all groups but one, participants received two doses, with a 21-day interval between doses; in one group (100 μg of BNT162b1), participants received one dose.
Results
A total of 195 participants underwent randomization. In each of 13 groups of 15 participants, 12 participants received vaccine and 3 received placebo. BNT162b2 was associated with a lower incidence and severity of systemic reactions than BNT162b1, particularly in older adults. In both younger and older adults, the two vaccine candidates elicited similar dose-dependent SARS-CoV-2–neutralizing geometric mean titers, which were similar to or higher than the geometric mean titer of a panel of SARS-CoV-2 convalescent serum samples.
Conclusions
The safety and immunogenicity data from this U.S. phase 1 trial of two vaccine candidates in younger and older adults, added to earlier interim safety and immunogenicity data regarding BNT162b1 in younger adults from trials in Germany and the United States, support the selection of BNT162b2 for advancement to a pivotal phase 2–3 safety and efficacy evaluation. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04368728.)
Since the first cases of coronavirus disease 2019 (Covid-19) in Wuhan, China, in December 2019, pandemic illness has spread to millions of persons worldwide. An increased risk of severe disease and death has been noted among the elderly and among persons with preexisting medical conditions. No Covid-19 vaccines are currently available, and they are urgently needed to combat escalating cases and deaths worldwide.1
In response, BioNTech and Pfizer launched a coordinated program to compare four RNA-based Covid-19 pandemic vaccine candidates in umbrella-type clinical studies conducted in Germany (BNT162-01) and the United States (C4591001). The program was designed to support the selection of a single vaccine candidate and dose level for a pivotal international safety and efficacy trial. On the basis of initial clinical-trial results in Germany,2 two lipid nanoparticle–formulated,3 nucleoside-modified RNA (modRNA)4 vaccine candidates against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were evaluated in the phase 1 portion of the trial in the United States.5 One of these candidates, BNT162b1, encodes the SARS-CoV-2 receptor–binding domain, trimerized by the addition of a T4 fibritin foldon domain to increase its immunogenicity through multivalent display.6-8 The other candidate, BNT162b2, encodes the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the prefusion conformation9 and more closely mimic the intact virus with which the elicited virus-neutralizing antibodies must interact.10
Previous articles have described the assessment of BNT162b1, at multiple dose levels, in healthy adults 18 to 55 years of age.2,5 These studies indicated that dose levels of BNT162b1 that elicited an acceptable level of reactogenicity also efficiently elicited titers that were as high as those in a panel of SARS-CoV-2 human convalescent serum samples and that were broadly neutralizing across a panel of 17 SARS-CoV-2 pseudoviruses representing a diversity of circulating strains. BNT162b1 also elicited CD4+ type 1 helper T (Th1) cell responses and strong interferon-γ–producing and interleukin-2–producing CD8+ cytotoxic T-cell responses. This ability to elicit both humoral and cell-mediated antiviral mechanisms makes BNT162b1 a promising vaccine candidate.
Here, we report the full set of safety and immunogenicity data from the phase 1 portion of an ongoing randomized, placebo-controlled, observer-blinded, dose-escalation trial in the United States that was used to select the final vaccine candidate, as well as the comparison of the safety and immunogenicity of both vaccine candidates and additional phase 1 data that have been collected since candidate selection. These data include evaluation of the 10-μg, 20-μg, and 30-μg dose levels of BNT162b1 and BNT162b2 in adults 18 to 55 years of age and adults 65 to 85 years of age.
Methods
Trial Objectives, Participants, and Oversight
We assessed the safety and immunogenicity of three dose levels of BNT162b1 and BNT162b2. Healthy adults 18 to 55 years of age or 65 to 85 years of age were eligible for inclusion. Key exclusion criteria were known infection with human immunodeficiency virus, hepatitis C virus, or hepatitis B virus; an immunocompromised condition; a history of autoimmune disease; a previous clinical or microbiologic diagnosis of Covid-19; the receipt of medications intended to prevent Covid-19; any previous coronavirus vaccination; positive test for SARS-CoV-2 IgM or IgG at the screening visit; and positive nasal-swab results on a SARS-CoV-2 nucleic acid amplification test within 24 hours before the receipt of trial vaccine or placebo.
BioNTech was the regulatory sponsor of the trial. Pfizer was responsible for the trial design; for the collection, analysis, and interpretation of the data; and for the writing of the report. The corresponding author had full access to all the data in the trial and had final responsibility for the decision to submit the manuscript for publication. All the trial data were available to all the authors.
Trial Procedures
Using an interactive Web-based response technology system, we randomly assigned trial participants to groups defined according to the vaccine candidate, dose level, and age range. Groups of participants 18 to 55 years of age and 65 to 85 years of age were to receive doses of 10 μg, 20 μg, or 30 μg of BNT162b1 or BNT162b2 (or placebo) on a two-dose schedule; one group of participants 18 to 55 years of age was assigned to receive 100-μg doses of BNT162b1 or placebo. All the participants were assigned to receive two 0.5-ml injections of active vaccine (BNT162b1 or BNT162b2) or placebo into the deltoid, administered 21 days apart.
The first five participants in each new dose level or age group (with a randomization ratio of 4:1 for active vaccine:placebo) were observed for 4 hours after the injection to identify immediate adverse events. All the other participants were observed for 30 minutes. Blood samples were obtained for safety and immunogenicity assessments.
Safety
The primary end points in phase 1 of this trial were solicited local reactions (i.e., specific local reactions as prompted by and recorded in an electronic diary), systemic events, and use of antipyretic or pain medication within 7 days after the receipt of vaccine or placebo, as prompted by and recorded in an electronic diary; unsolicited adverse events and serious adverse events (i.e., those reported by the participants, without electronic-diary prompts), assessed from the receipt of the first dose through 1 month and 6 months, respectively, after the receipt of the second dose; clinical laboratory abnormalities, assessed 1 day and 7 days after the receipt of vaccine or placebo; and grading shifts in laboratory assessments between baseline and 1 day and 7 days after the first dose and between 2 days and 7 days after the second dose. Protocol-specified safety stopping rules were in effect for all the participants in the phase 1 portion of the trial. The full protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org. An internal review committee and an external data and safety monitoring committee reviewed all safety data.
Immunogenicity
Immunogenicity assessments (SARS-CoV-2 serum neutralization assay and receptor-binding domain [RBD]–binding or S1-binding IgG direct Luminex immunoassays) were conducted before the administration of vaccine or placebo, at 7 days and 21 days after the first dose, and at 7 days (i.e., day 28) and 14 days (i.e., day 35) after the second dose. The neutralization assay, which also generated previously described virus-neutralization data from trials of the BNT162 candidates,2,5 used a previously described strain of SARS-CoV-2 (USA_WA1/2020) that had been generated by reverse genetics and engineered by the insertion of an mNeonGreen gene into open reading frame 7 of the viral genome.11,12 The 50% neutralization titers and 90% neutralization titers were reported as the interpolated reciprocal of the dilutions yielding 50% and 90% reductions, respectively, in fluorescent viral foci. Any serologic values below the lower limit of quantitation were set to 0.5 times the lower limit of quantitation. Available serologic results were included in the analysis.
Immunogenicity data from a human convalescent serum panel were included as a benchmark. A total of 38 serum samples were obtained from donors 18 to 83 years of age (median age, 42.5 years) who had recovered from SARS-CoV-2 infection or Covid-19; samples were obtained at least 14 days after a polymerase chain reaction–confirmed diagnosis and after symptom resolution. Neutralizing geometric mean titers (GMTs) in subgroups of the donors were as follows: 90, among 35 donors with symptomatic infections; 156, among 3 donors with asymptomatic infection; and 618, in 1 donor who was hospitalized. Each serum sample in the panel was from a different donor. Thus, most of the serum samples were obtained from persons with moderate Covid-19 who had not been hospitalized. The serum samples were obtained from Sanguine Biosciences, the MT Group, and Pfizer Occupational Health and Wellness.
Statistical Analysis
We report descriptive results of safety and immunogenicity analyses, and the sample size was not based on statistical hypothesis testing. Results of the safety analyses are presented as counts, percentages, and associated Clopper–Pearson 95% confidence intervals for local reactions, systemic events, and any adverse events after the administration of vaccine or placebo, according to terms in the Medical Dictionary for Regulatory Activities, version 23.0, for each vaccine group. Summary statistics are provided for abnormal laboratory values and grading shifts. Given the small number of participants in each group, the trial was not powered for formal statistical comparisons between dose levels or between age groups.
Immunogenicity analyses of SARS-CoV-2 serum neutralizing titers, S1-binding IgG and RBD-binding IgG concentrations, GMTs, and geometric mean concentrations (GMCs) were computed along with associated 95% confidence intervals. The GMTs and GMCs were calculated as the mean of the assay results after the logarithmic transformation was made; we then exponentiated the mean to express results on the original scale. Two-sided 95% confidence intervals were obtained by performing logarithmic transformations of titers or concentrations, calculating the 95% confidence interval with reference to Student’s t-distribution, and then exponentiating the limits of the confidence intervals.
Results
Demographic Characteristics of the Participants
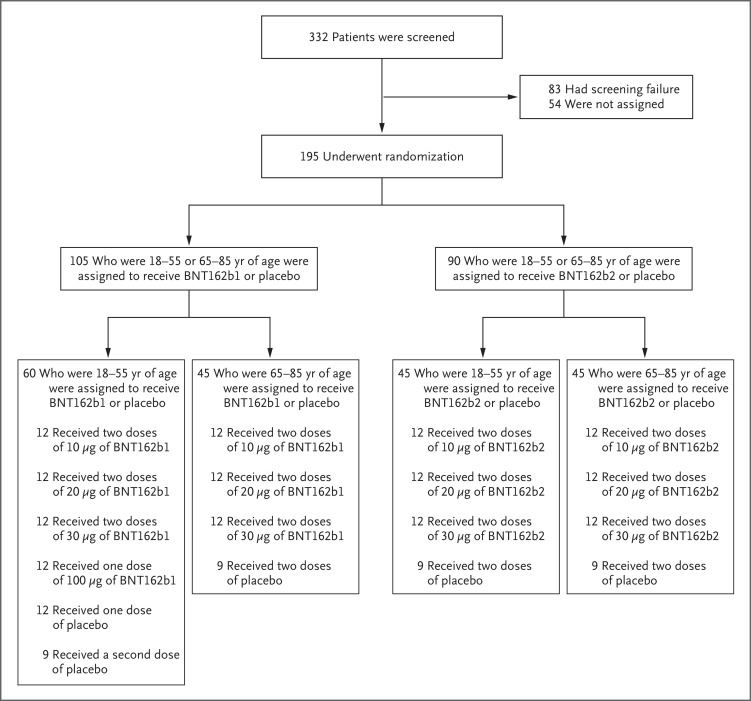
Between May 4, 2020, and June 22, 2020, a total of 332 healthy adults (men and nonpregnant women) underwent screening at four sites in the United States (two sites per vaccine candidate). A total of 195 participants were randomly assigned to 13 groups comprising 15 participants each; in each group, 12 participants received vaccine and 3 received placebo (Figure 1). In all groups but one, all the participants who underwent randomization received the assigned two doses of vaccine or placebo. Participants 18 to 55 years of age who had been assigned to receive 100 μg of BNT162b1 or placebo received one dose; the second dose was not administered because of reactogenicity in the participants who received active vaccine.5
Figure 1. Screening and Randomization of the Participants.
The 54 participants who were not assigned to a trial group were screened but did not undergo randomization because trial enrollment had closed. All the participants received two doses of the vaccine (BNT162b1 or BNT162b2) or placebo, except for the participants who were assigned to receive 100 μg of BNT162b1 or placebo, who received one dose.
The majority of participants were White (67 to 100%) and non-Hispanic (89 to 100%) (Table 1). More older women than older men participated. The median age among the younger participants was 35 years in the BNT162b1 group and 37 years in the BNT162b2 group; the median age among the older participants was 69 years and 68 years, respectively.
Table 1. Demographic Characteristics of the Participants, According to Vaccine Candidate and Age Group.*.
| Variable | Participants 18–55 Years of Age | Participants 65–85 Years of Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg | 20 μg | 30 μg | 100 μg | Placebo | Total | 10 μg | 20 μg | 30 μg | Placebo | Total | |
| BNT162b1 | |||||||||||
| No. of participants | 12 | 12 | 12 | 12 | 12 | 60 | 12 | 12 | 12 | 9 | 45 |
| Sex — no. (%) | |||||||||||
| Male | 7 (58) | 9 (75) | 6 (50) | 5 (42) | 7 (58) | 34 (57) | 4 (33) | 4 (33) | 4 (33) | 1 (11) | 13 (29) |
| Female | 5 (42) | 3 (25) | 6 (50) | 7 (58) | 5 (42) | 26 (43) | 8 (67) | 8 (67) | 8 (67) | 8 (89) | 32 (71) |
| Race — no. (%)† | |||||||||||
| White | 8 (67) | 11 (92) | 10 (83) | 11 (92) | 11 (92) | 51 (85) | 12 (100) | 11 (92) | 10 (83) | 9 (100) | 42 (93) |
| Black | 1 (8) | 1 (8) | 0 | 0 | 0 | 2 (3) | 0 | 1 (8) | 0 | 0 | 1 (2) |
| Asian | 3 (25) | 0 | 2 (17) | 1 (8) | 1 (8) | 7 (12) | 0 | 0 | 2 (17) | 0 | 2 (4) |
| Hispanic ethnic group — no. (%)† | 1 (8) | 0 | 1 (8) | 0 | 0 | 2 (3) | 0 | 0 | 0 | 1 (11) | 1 (2) |
| Age — yr‡ | |||||||||||
| Mean | 29.4±6.4 | 44.8±8.3 | 35.8±10.0 | 38.3±9.3 | 36.3±11.3 | 36.9±10.2 | 69.7±5.4 | 70.6±4.9 | 69.9±3.6 | 68.2±3.0 | 69.7±4.3 |
| Median (range) | 26.5 (24–42) |
49.0 (30–54) |
33.5 (23–52) |
38.0 (25–53) |
35.0 (19–54) |
35.0 (19–54) |
68.5 (65–82) |
69.0 (65–81) |
69.0 (65–77) |
68.0 (65–73) |
69.0 (65–82) |
| BNT162b2 | |||||||||||
| No. of participants | 12 | 12 | 12 | 0 | 9 | 45 | 12 | 12 | 12 | 9 | 45 |
| Sex — no. (%) | |||||||||||
| Male | 5 (42) | 6 (50) | 3 (25) | — | 5 (56) | 19 (42) | 2 (17) | 5 (42) | 6 (50) | 4 (44) | 17 (38) |
| Female | 7 (58) | 6 (50) | 9 (75) | — | 4 (44) | 26 (58) | 10 (83) | 7 (58) | 6 (50) | 5 (56) | 28 (62) |
| Race — no. (%)† | |||||||||||
| White | 11 (92) | 10 (83) | 9 (75) | — | 9 (100) | 39 (87) | 12 (100) | 12 (100) | 12 (100) | 9 (100) | 45 (100) |
| Black | 0 | 2 (17) | 1 (8) | — | 0 | 3 (7) | 0 | 0 | 0 | 0 | 0 |
| Asian | 1 (8) | 0 | 2 (17) | — | 0 | 3 (7) | 0 | 0 | 0 | 0 | 0 |
| Hispanic ethnic group — no. (%)† | 1 (8) | 1 (8) | 0 | — | 0 | 2 (4) | 0 | 0 | 0 | 0 | 0 |
| Age — yr‡ | |||||||||||
| Mean | 36.8±12.2 | 37.6±10.1 | 37.3±9.8 | — | 34.4±13.2 | 36.7±11.0 | 68.0±2.9 | 71.0±5.8 | 68.5±2.8 | 70.0±3.8 | 69.3±4.1 |
| Median (range) | 37.0 (21–53) |
38.0 (23–53) |
36.5 (23–54) |
— | 30.0 (19–53) |
37.0 (19–54) |
67.0 (65–73) |
68.5 (65–81) |
68.0 (65–74) |
69.0 (65–77) |
68.0 (65–81) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the participant.
The age of the participants was the age at the time of the injection.
Safety
Local Reactions
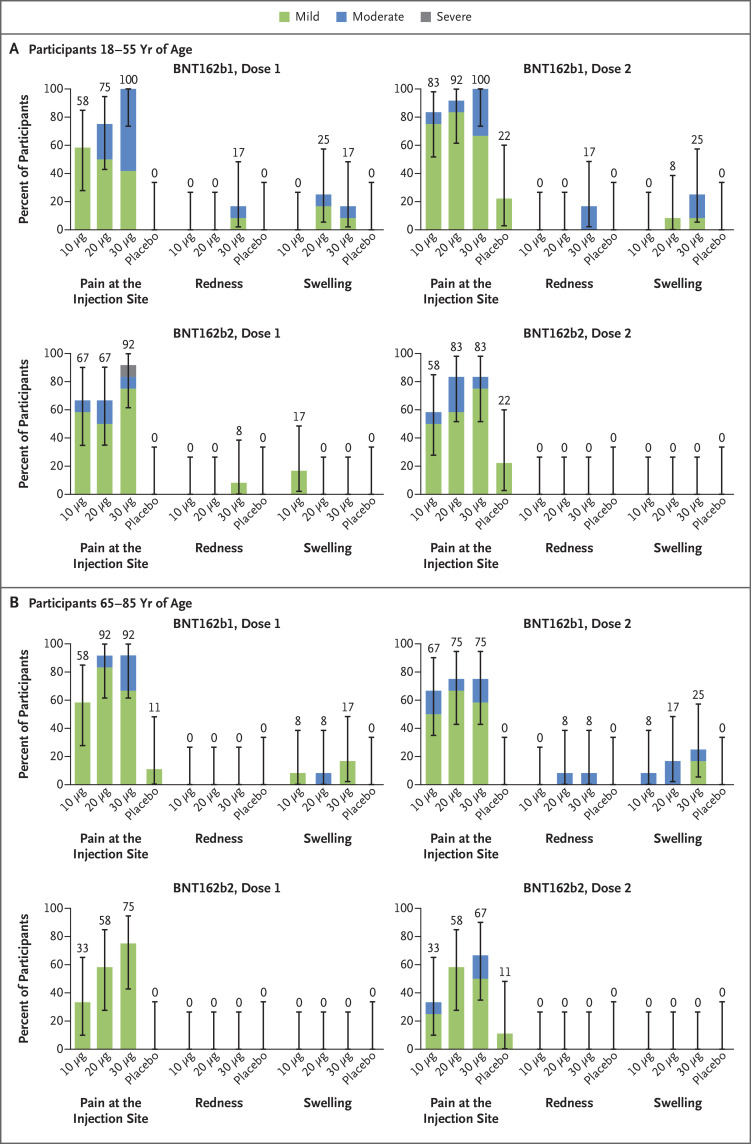
Participants 18 to 55 years of age who received 10 μg, 20 μg, or 30 μg of BNT162b1 reported mild-to-moderate local reactions, primarily pain at the injection site, within 7 days after an injection; the local reactions were more frequent after the second dose.2,5 BNT162b1 elicited local reactions in similar proportions of the participants in the younger age group and in the older age group. Among the older participants, mild-to-moderate injection-site pain was reported by 92% after the first dose and by 75% after the second dose (Figure 2). A similar pattern was observed after vaccination with BNT162b2. No older participant who received BNT162b2 reported redness or swelling. No participant who received either BNT162 vaccine candidate reported a grade 4 local reaction.
Figure 2. Local Reactions Reported within 7 Days after the Administration of Vaccine or Placebo, According to Age Group.
Panel A shows local reactions in participants 18 to 55 years of age, and Panel B those in participants 65 to 85 years of age. Injection-site (local) reactions were recorded in electronic diaries for 7 days after each injection. Pain at the injection site was graded as mild (does not interfere with activity), moderate (interferes with activity), severe (prevents daily activity), or grade 4 (led to an emergency department visit or hospitalization). Redness and swelling were graded as mild (2.0 to 5.0 cm in diameter), moderate (>5.0 to 10.0 cm in diameter), severe (>10.0 cm in diameter), or grade 4 (necrosis or exfoliative dermatitis for redness and necrosis for swelling). 𝙸 bars represent 95% confidence intervals. The numbers above the 𝙸 bars show the overall percentage of the participants in each group who reported the specified local reaction. No participant who received either vaccine candidate reported a grade 4 local reaction.
Systemic Events
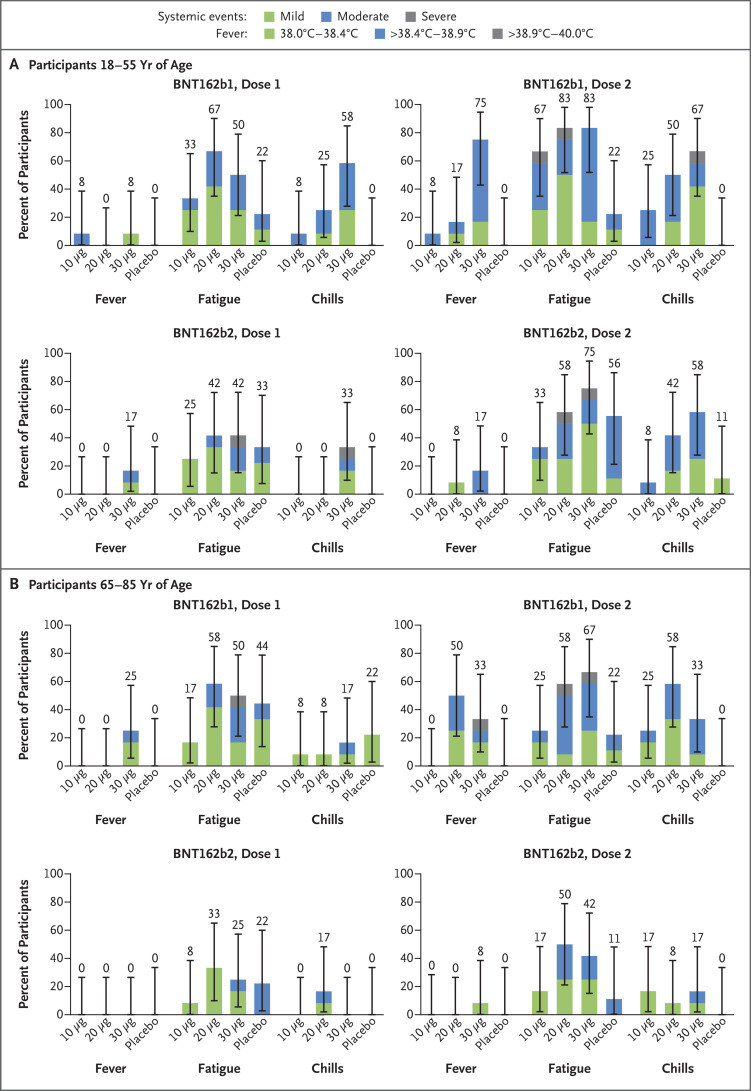
Participants 18 to 55 years of age who received 10 μg, 20 μg, or 30 μg of BNT162b1 frequently had mild-to-moderate fever and chills, with 75% of the participants reporting a temperature of 38.0°C or higher after the second 30-μg dose (Figure 3; and Fig. S1 in the Supplementary Appendix, available at NEJM.org).5 In participants 65 to 85 years of age who received BNT162b1, systemic events were milder than in the younger participants, although many older participants reported fatigue and headache after the first or second dose, and 33% reported a temperature of 38°C or higher after the second dose, including one older participant who reported a fever of 38.9 to 40.0°C (Figure 3 and Fig. S2). As was observed with local reactions, systemic events were dose-dependent (greater after the second dose than after the first dose) and transient. Symptoms generally peaked by day 2 after vaccination and resolved by day 7.
Figure 3. Selected Systemic Events Reported within 7 Days after the Administration of Vaccine or Placebo, According to Age Group.
Panel A shows systemic reactions in participants 18 to 55 years of age, and Panel B those in participants 65 to 85 years of age. Data on fever, chills, and fatigue are reported here. (Data on headache, vomiting, diarrhea, muscle pain, and joint pain are reported in Fig. S1.) Data on systemic events were recorded in electronic diaries for 7 days after each injection. The fever scale is shown in the key. Chills and fatigue were graded as being mild (does not interfere with activity), moderate (interferes somewhat with activity), severe (prevents daily activity), or grade 4 (led to an emergency department visit or hospitalization). 𝙸 bars represent 95% confidence intervals. The numbers above the 𝙸 bars show the overall percentage of participants in each group who reported the specified systemic event. No participant who received either vaccine candidate reported a grade 4 systemic event or a temperature higher than 40.0°C.
Systemic events in response to BNT162b2 were milder than those in response to BNT162b1 (Figure 3 and Figs. S1 and S2). For example, 17% of the participants 18 to 55 years of age and 8% of those 65 to 85 years of age reported fever (≥38.0 to 38.9°C) after the second dose of 30 μg of BNT162b2. Severe systemic events (fatigue, headache, chills, muscle pain, and joint pain) were reported in small numbers of younger recipients of BNT162b2, but no severe systemic events were reported by older recipients of this vaccine candidate. No participant who received either BNT162 vaccine candidate reported a grade 4 systemic event. After the first dose, systemic events that were reported by participants 65 to 85 years of age who received BNT162b2 were similar to those reported by participants who received placebo.
In both age groups and for both vaccine candidates, the use of antipyretic or pain medication increased with increasing dose level and with the number of doses administered. Fewer BNT162b2 recipients than BNT162b1 recipients reported using antipyretic or pain medication.
Adverse Events and Shifts in Laboratory Values
Through 1 month after the receipt of the second dose, adverse events that were considered by the investigators to be related to vaccine or placebo were reported by 50% of the participants 18 to 55 years of age who received 30 μg of BNT162b1, as compared with 8% of those who received placebo.5 Adverse events that were considered to be related to vaccine were reported by 17% of the participants 65 to 85 years of age who received 30 μg of BNT162b1 and by 25% of the participants 18 to 55 years of age who received 30 μg of BNT162b2. No participant 65 to 85 years of age who received 30 μg of BNT162b2 reported a related adverse event (Table S1).
No serious adverse events were reported, and no stopping rules were met as of the time of this report. The largest changes from baseline in laboratory values were transient decreases in lymphocyte counts, which resolved within 1 week after vaccination (Fig. S3) and which were not associated with clinical manifestations.
Immunogenicity
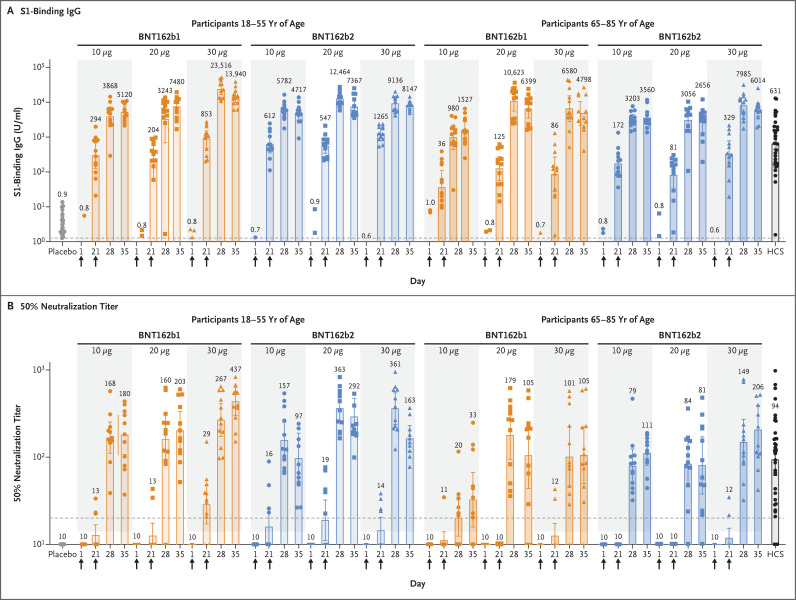
The serologic responses elicited by BNT162b1 and BNT162b2 were similar (Figure 4). Two serum samples, both from the group of participants 18 to 55 years of age who received 30 μg of BNT162b2, were obtained outside the specified time windows (one each at day 28 and day 35) and thus were excluded from the reported immunogenicity analysis. Antigen-binding IgG and virus-neutralizing responses to vaccination with 10 μg to 30 μg of BNT162b1 or BNT162b2 were boosted by the second dose in both the younger adults2,5 and the older adults. Both vaccines elicited generally lower antigen-binding IgG and virus-neutralizing responses in participants 65 to 85 years of age than in those 18 to 55 years of age. Higher doses appeared to elicit somewhat higher antibody responses.
Figure 4. Immunogenicity of BNT162b1 and BNT162b2.
Participants in groups of 15 received an injection with the indicated dose levels of one of either of the BNT162 vaccine candidates (12 participants) or placebo (3 participants) on days 1 and 21. Arrows indicate days of vaccination. Responses in the placebo recipients in each of the dose-level groups are combined. Serum samples were obtained before injection (on day 1) and on days 21, 28, and 35 after the first dose. The blood samples obtained on days 28 and 35 are those obtained 7 days and 14 days, respectively, after the second dose. Human coronavirus disease 2019 (Covid-19) or SARS-CoV-2 infection convalescent serum (HCS) samples were obtained from 38 donors at least 14 days after polymerase chain reaction–confirmed diagnosis and at a time when the donors were asymptomatic. Panel A shows the geometric mean concentrations of recombinant S1-binding IgG (lower limit of quantitation, 1.267; dashed line), and Panel B the 50% SARS-CoV-2–neutralizing geometric mean titers (lower limit of quantitation, 20; dashed line). On days that vaccine or placebo was administered, samples were obtained before the injection. Each data point represents a serum sample, and the top of each vertical bar represents the geometric mean with the 95% confidence interval (𝙸 bar). Data points associated with placebo, HCS samples, or the 10-μg dose of vaccine are shown as circles, those for the 20-μg dose as squares, and those for the 30-μg dose as triangles. The numbers above the bars show the geometric mean concentration or geometric mean titer in the group. All the vaccine groups had 12 valid results from samples that could be evaluated at each time point except for the following: among participants who received BNT162b2, 11 results from day 28 in younger participants who received 30 μg, 10 results from day 35 in younger participants who received 30 μg, and 11 results from day 35 in older participants who received 10 μg.
The highest neutralization titers were measured in samples obtained on day 28 (i.e., 7 days after the second dose) or on day 35 (i.e., 14 days after the second dose). Similar trends were observed for the 50% and 90% neutralizing titers (Fig. S4). The 50% neutralizing GMTs for the two vaccine candidates at the 30-μg dose level on day 28 or day 35 ranged from 1.7 to 4.6 times the GMT of the convalescent serum panel among participants 18 to 55 years of age and from 1.1 to 2.2 times the GMT of the convalescent serum panel among those 65 to 85 years of age. With 10 to 12 valid results per assay from samples that could be evaluated for each group at each time point, pair-wise comparisons are subject to error and have no clear interpretation.
Discussion
Previously reported data from vaccination with 10 μg or 30 μg of BNT162b1 in adults 18 to 55 years of age suggested that it could be a promising Covid-19 vaccine candidate.2,5 Consistent with our strategy to evaluate several RNA vaccine candidates and make a data-driven decision to advance the candidate with the best safety and immunogenicity profile, we compared clinical data obtained after vaccination with BNT162b1,2,5 which encodes the RBD, with data obtained after vaccination with BNT162b2, which encodes the full-length spike. The data presented here include those that guided our decision to advance BNT162b2 at the 30-μg dose level to the phase 2–3, international trial to evaluate its safety and efficacy in participants 18 to 85 years of age.
The primary consideration driving this decision was the milder systemic reactogenicity profile of BNT162b2, particularly in older adults, in the context of the similar antibody responses elicited by the two candidate vaccines. Short-lived decreases in postvaccination lymphocyte counts had no associated clinical effect, were observed across the age groups, and probably reflect a temporary redistribution of lymphocytes from the bloodstream to lymphoid tissues as a functional response to immune stimulation by the vaccine.13-16 The immune response and toxicity profile at the selected, relatively low, 30-μg dose level indicate that the BNT162b2 modRNA vaccine candidate has a favorable balance of reactogenicity and immunogenicity.17,18
The composition of the lipid nanoparticles, the formulation components, or the sequence selection for the vaccine RNA could influence the side-effect profile. The reason for the lower reactogenicity of BNT162b2 than of BNT162b1 is not certain, given that the two vaccine candidates share the same modRNA platform, RNA production and purification processes, and formulation of lipid nanoparticles. They differ in the nucleotide sequences that encode the vaccine antigens and in the overall size of the RNA constructs, which results in a number of RNA molecules in 30 μg of BNT162b1 that is approximately 5 times as high as that in 30 μg of BNT162b2. The nucleotide composition of RNA has been reported to affect its immune stimulatory activity and reactogenicity profile, and this is a possible explanation for the differences in these vaccine candidates.19
The immune responses elicited by BNT162b1 and BNT162b2 were similar. As has been observed with other vaccines and as is probably associated with immunosenescence,20,21 the immunogenicity of the two vaccine candidates decreased with age, eliciting lower overall humoral responses in adults 65 to 85 years of age than in those 18 to 55 years of age. Nevertheless, at 7 days and 14 days after the second dose, the 50% and 90% neutralizing GMTs that were elicited by 30 μg of BNT162b2 in older adults exceeded those of the convalescent serum panel. Antibody responses in both younger and older adults showed a clear benefit of a second dose.
This trial and interim report have several limitations. First, the relative importance of humoral and cellular immunity with regard to protection from Covid-19 has not yet been fully characterized. Although strong cell-mediated immune responses (Th1-biased CD4+ and CD8+) elicited by BNT162b1 have been observed and reported in the German trial,2 the cellular immune responses elicited by BNT162b2 are still being studied. Second, although the serum neutralizing responses that were elicited by the vaccine candidates relative to those elicited by natural infection are highly encouraging, the degree of protection against Covid-19 provided by this or any other benchmark is unknown. Third, the phase 1 portion of this trial tested many hypotheses and was not powered to make formal statistical comparisons. Fourth, the human convalescent serum panels that have been used by different vaccine developers are not standardized among laboratories, and each represents a unique distribution of donor characteristics and times of collection. Therefore, the serum panel that we used does not provide a well-controlled benchmark for comparisons of the serologic responses elicited by these two BNT162 vaccine candidates with those elicited by other Covid-19 vaccine candidates. Finally, the participants in this early-stage clinical trial were healthy and had limited racial and ethnic diversity as compared with the general population.
Many of the limitations cited above are being addressed in the international, phase 2–3 portion of this trial. In this later, pivotal part of the trial, we are assessing the safety and efficacy of two doses of 30 μg of BNT162b2 in up to 44,000 participants (randomly assigned in a 1:1 ratio to receive vaccine or placebo) from diverse backgrounds, including persons with stable chronic underlying health conditions, persons at increased risk owing to occupational exposure, and persons from racial and ethnic backgrounds at higher risk for severe Covid-19.22 We are conducting outreach to recruit trial participants from many backgrounds and are using U.S. Census data to locate trial sites in diverse communities.
Acknowledgments
We thank Carol Monahan and Deb Gantt (of Pfizer) for writing and editorial assistance with an earlier version of the manuscript; James Trammel (of Pfizer) for assistance with the statistical analysis in the generation of an earlier version of the manuscript; Tricia Newell, Nicole O’Regan, and Emily Stackpole (of ICON), for editorial assistance with an earlier version of the manuscript, which was funded by Pfizer; all the participants who volunteered for this trial; and the following persons for their contributions to this work: Angelica Kottkamp, Ramin Herati, Rebecca Pellet Madan, Mary Olson, Marie Samanovic-Golden, Elisabeth Cohen, Amber Cornelius, Laura Frye, Heekoung Youn, Baby Jane Fran, Kanika Ballani, Natalie Veling, Juanita Erb, Mahnoor Ali, Lisa Zhao, Stephanie Rettig, Hibah Khan, Harry Lambert, Kelly Hu, and Jonathan Hyde (all of New York University Langone Vaccine Center); Monica McArthur, Justin Ortiz, Rekha Rapaka, Linda Wadsworth, Ginny Cummings, Toni Robinson, Nancy Greenberg, Lisa Chrisley, Wanda Somrajit, Jennifer Marron, Constance Thomas, Kelly Brooks, Lisa Turek, Patricia Farley, Staci Eddington, Panagiota Komninou, Mardi Reymann, Kathy Strauss, Biraj Shrestha, Sudhaunshu Joshi, Robin Barnes, Roohali Sukhavasi, Myounghee Lee, Alyson Kwon, and Terry Sharp (all of the Center for Vaccine Development and Global Health, University of Maryland School of Medicine); Emily Pierce, Mary Criddle, Maryrose Laguio-Vila, Megan Helf, Madison Murphy, Maria Formica, and Sarah Korones (all of the University of Rochester and Rochester General Hospital); Amy Cline, Susan Parker, and Michelle Dickey (all of Cincinnati Children’s Hospital); Kristen Buschle (of Pfizer); Andrea Cawein, John L. Perez, Harpreet Seehra, Dina Tresnan, Robert Maroko, Helen Smith, Sarah Tweedy, Amy Jones, Greg Adams, Rabia Malick, Emily Worobetz, Erica Weaver, Liping Zhang, Carmel Devlin, Donna Boyce, Elisa Harkins Tull, Mark Boaz, Michael Cruz, and the staff of the Vaccines Clinical Assay Team and the Vaccines Assay Development Team (all of Cincinnati Children’s Hospital); and Corinna Rosenbaum, Christian Miculka, Andreas Kuhn, Ferdia Bates, Paul Strecker, and Alexandra Kemmer-Brück (all of BioNTech).
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on October 14, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by BioNTech and Pfizer.
Disclosure forms provide by the authors are available with the full text of the article at NEJM.org.
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020 (https://coronavirus.jhu.edu/map.html).
- 2.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020. September 30 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release 2015;217:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 2008;16:1833-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020. August 12 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 6.He Y, Zhou Y, Liu S, et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 2004;324:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Güthe S, Kapinos L, Möglich A, Meier S, Grzesiek S, Kiefhaber T. Very fast folding and association of a trimerization domain from bacteriophage T4 fibritin. J Mol Biol 2004;337:905-915. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol 1997;15:235-270. [DOI] [PubMed] [Google Scholar]
- 9.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A 2017;114(35):E7348-E7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Muruato A, Lokugamage KG, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe 2020;27(5):841-848.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muruato AE, Fontes-Garfias CR, Ren P, et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun 2020;11:4059-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster GR, Masri SH, David R, et al. IFN-α subtypes differentially affect human T cell motility. J Immunol 2004;173:1663-1670. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins RJ, Daczkowski NF, Kaptur PE, et al. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine 2013;31:3051-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017;376:330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai L, Davey R, Beck A, et al. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA 2015;313:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019;37:3326-3334. [DOI] [PubMed] [Google Scholar]
- 18.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondili M, Roux M, Vabret N, Bailly-Bechet M. Innate immune system activation by viral RNA: how to predict it? Virology 2016;488:169-178. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz N, Manalastas R Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet 2009;373:1949-1957. [DOI] [PubMed] [Google Scholar]
- 21.Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine 2010;28:3627-3631. [DOI] [PubMed] [Google Scholar]
- 22.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.