ABSTRACT
Hedgehog (HH) signaling, a critical developmental pathway, has been implicated in cancer initiation and progression. With vismodegib and sonidegib having been approved for clinical use, increasing numbers of HH inhibitors alone and in combination with chemotherapies are in clinical trials. Here we highlight the clinical research on HH antagonists and the genetics of response to these compounds in human cancers. Selectivity of HH inhibitors, determined by decreased pathway transcriptional activity, has been demonstrated in many clinical trials. Patients with advanced/metastatic basal cell carcinoma have benefited the most, whereas HH antagonists did little to improve survival rates in other cancers. Correlation between clinical response and HH gene expression vary among different cancer types. Predicting response and resistance to HH inhibitors presents a challenge and continues to remain an important area of research. New approaches combine standard of care chemotherapies and molecularly targeted therapies to increase the clinical utility of HH inhibitors.
KEYWORDS: Cancer, hedgehog, smoothened, SMO, GLI, vismodegib, sonidegib
Introduction
The Hedgehog (HH) signaling pathway is vital to the process of embryogenesis, including cell growth, differentiation and overall embryonic development. While essential for regulating development, abnormal activation of HH signaling and mutations in HH signaling genes have been linked to the development and progression of a number of human cancers, including basal cell carcinoma,1,2 medulloblastoma,3 breast cancer,4 pancreatic cancer,5,6 and many more.7–9 Based on the relationship between initiation and progression of cancer and Hedgehog signaling, targeted therapeutics have been developed to inhibit the pathway and its downstream effects. First-in-class HH inhibitors currently on the market include vismodegib (Genentech) and sonidegib (Sun Pharma). In this review, we will discuss the clinical research of these, as well as other in-development Hedgehog antagonists being used in a variety of human cancers in relation to their distinct genetic effects.
The hedgehog pathway
The HH pathway is comprised of a family of secreted morphogens that were first described in Drosophila in the early 1980s, and in mammals in the 1990s, as playing a crucial role in embryonic development, particularly organ and limb patterning.10–13 Nusslein-Volhard and Wieschaus10 were the first to name the hedgehog gene after noticing that when this gene is mutated, denticles (spiked cuticle that normally decorates only the anterior portion of fly body segments) were prevalent throughout the entire body (both anterior and posterior portions) of newly hatched larva. This continuous lawn of denticles suggested the spines of a hedgehog.
Vertebrate HH genes were first reported in 1993 when a collaborative effort involving three groups14–16 discovered that, unlike the fruit fly, which has a single hh gene, there are three related mammalian HH genes: Desert HH, Indian HH, and Sonic HH. Sonic HH, named after the title character in the popular video game series, is the best characterized of the three HH ligands as it appears to play a substantial role in the formation of several tissues and organs.17 In canonical HH signaling, these HH ligands are secreted by cells and interact with the 12-span transmembrane receptor Patched (PTCH), either in an autocrine or paracrine manner, thereby relieving PTCH-mediated inhibition of Smoothened (SMO), a 7-span transmembrane protein.18–20
In the absence of HH ligand, PTCH inhibits signal transduction through SMO, resulting in the cytoplasmic sequestration of the Glioma-associated oncogene (Gli) family of transcription factors (Gli-1, 2, 3).21,22 While sequestered, Gli is phosphorylated and ultimately marked for proteasomal degradation.23–25 At this point, the ultimate fates of the three Gli transcription factors diverge somewhat. Proteolysis of Gli-1 by the proteasome is a complete process whereas degradation of Gli-2 and Gli-3 is only partial. Gli-2 and Gli-3, unlike Gli-1, have activator and repressor domains. Upon proteolysis, Gli-2 and Gli-3 are cleaved into smaller transcriptional repressor fragments (Gli-2 R and Gli-3 R) with Gli-2 R being a much weaker repressor than Gli-3 R, which localize to the cell nucleus where it binds Gli-response elements to prevent HH signal transduction.25,26 The degradation of Gli is inhibited in the presence of HH ligand (Figure 1). When HH binds to PTCH, SMO becomes activated resulting in a signaling cascade that ultimately leads to the translocation of the full-length, activator forms of Gli into the nucleus where they transcribe HH target genes including PTCH1 and GLI1, thereby providing a mechanism whereby HH signaling is tightly regulated.27
Figure 1.
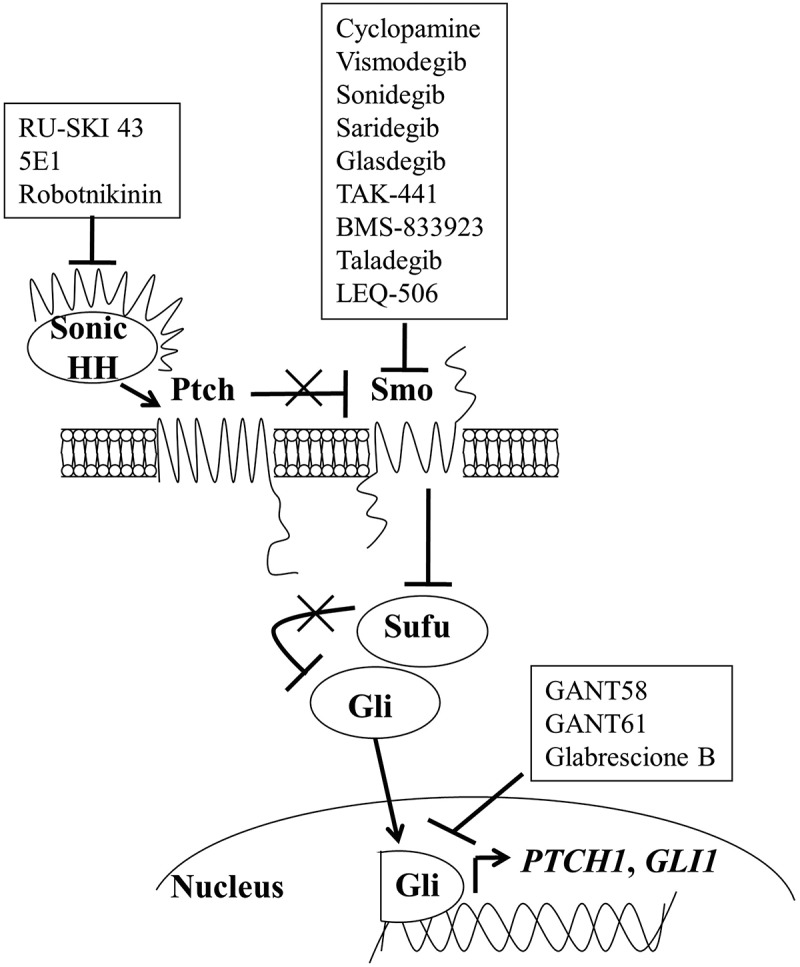
The hedgehog signaling pathway. In the presence of hedgehog ligand, repression of the SMO receptor by the PTCH receptor is relieved, allowing Gli transcription factors to translocate to the nucleus and transcribe hedgehog target genes. Pharmacologic inhibitors of SMO and Gli (shown in boxes) have been developed for cancer therapy
In contrast to the classic, canonical ligand-Patched-Smo regulated Hedgehog signaling; alternative, non-canonical activation of HH signaling has been shown in both the physiological and pathophysiological effects attributed to the pathway. Models of non-canonical activation of HH signaling include PTCH-dependent, Smo-independent (Type I) and Smo-dependent, PTCH-independent (Type II) signaling, as well as GLI activation driven by other pathways.28–30 Of particular interest related to the hedgehog inhibitors discussed here is PTCH-dependent, Smo-independent signaling and Smo-independent GLI activa-tion, as both would be refractory to Smo inhibition and may be difficult to distinguish from canonical HH signaling using traditional HH pathway assessments.
Hedgehog pathway inhibition and cancer therapy
In the last decade, several in vitro and in vivo studies have implicated aberrant HH signaling in a variety of cancers, either due to loss-of-function mutations in PTCH1 or gain-of-function mutations in SMO. These discoveries have led to ongoing research devoted to identifying and testing inhibitors of the HH pathway. Cyclopamine, a naturally occurring steroidal alkaloid, represents the first member of a class of small molecule compounds that selectively target SMO.31,32 This compound inactivates HH transcriptional activity by directly binding to SMO’s heptahelical bundle and inducing a conformational change similar to that induced by PTCH.33,34 Cyclopamine is so named due to its ability to induce holoprosencephaly (i.e., cyclopia) in the developing fetuses of pregnant sheep who had the misfortune of ingesting Veratrum californicum, the plant from which this compound is derived.35 Cyclopamine was first used as an anti-cancer agent to inhibit the proliferation of brain cancer cells in vitro.36 However, the clinical use of cyclopamine is hindered due to sub-optimal chemical stability, poor solubility in aqueous solutions, and a lack of HH specificity.37,38
Using cyclopamine as a “proof of concept,” SMO antagonists that are structurally distinct from cyclopamine and more selective for SMO have been developed by Genentech (GDC-0449/vismodegib),39 Novartis (LDE-225/sonidegib and LEQ-506), 40,41 Pfizer (PF-04449913/glasdegib),42 Millennium (TAK-441),43 Bristol-Myers Squibb (BMS-833923)44 and Eli Lilly (LY2940680/taladegib)45,46 pharmaceutical companies. In addition, Infinity Pharmaceuticals has taken cyclopamine and chemically modified it to make patidegib (IPI-926/saridegib), which is more selective for SMO and improves bioavailability.47–49 All of these compounds have been or are currently in clinical trials, either as single agents or in combination with other chemotherapy agents, for the treatment of a variety of cancers. Vismodegib and sonidegib were FDA-approved for the treatment of basal cell carcinoma in 2012 and 2015, respectively (see Table 1 for a list of hedgehog antagonists currently in clinical development). Response to SMO antagonists varies among different cancer types and the molecular basis for this differential response remains an active area of research. By elucidating this mechanism, it may be possible to identify cancer patients most likely to respond to pharmacological HH inhibition based on their tumor’s genetic or expression profile.
Table 1.
Smoothened antagonists currently under clinical investigation
| Route of | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Chemical | Generic Name | Brand Name | Developer | Administration | Clinical Trials | FDA-approved |
| Cyclopamine | Natural | n/a | n/a | n/a | n/a | None | No |
| GDC-0449 | Synthetic | vismodegib | Erivedge | Genentech | Oral | Phase I,II | Yes-2012 |
| LDE-225 | Synthetic | sonidegib | Odomzo | Novartis | Oral | Phase I,II | Yes-2015 |
| IPI-9261 | Semi-Synthetic | saridegib | n/a | Infinity | Oral | Phase I,II | No |
| PF-04449913 | Synthetic | glasdegib | n/a | Pfizer | Oral | Phase I,II | No |
| TAK-441 | Synthetic | n/a | n/a | Millennium | Oral | Phase I | No |
| BMS-833923 | Synthetic | n/a | n/a | Bristol-Myers Squibb | Oral | Phase I,II | No |
| LY2940680 | Synthetic | taladegib | n/a | Eli Lilly | Oral | Phase I,II | No |
| LEQ-5062 | Synthetic | n/a | n/a | Novartis | Oral | Phase I | No |
Clinical trials and genetic correlates
Primary literature and clinical trial registration data were reviewed to provide information on human clinical trials that examined cancer response to HH antagonism. All cancer types (both hematologic and non-hematologic/solid) and all clinical trial phases (I, II, III) were examined. Compounds designed to selectively target SMO (vismodegib, sonidegib, saridegib, etc.) were included, whereas compounds that inhibit HH signaling as part of an off-target effect were excluded from the selection (e.g., itraconazole). Studies must have included a genetic analysis component in which gene expression from available biopsies was compared to SMO inhibitor response (either pre- or post-treatment). Studies examining only gene expression or only drug/clinical response were excluded. Clinicaltrials.gov was used to identify SMO inhibitor compounds that are currently in clinical trials, as well as ongoing, and recently completed clinical trials; but the results of which have not yet been reported or published.
Fifteen clinical trials were located in PubMed using the inclusion/exclusion criteria described above (Table 2). Eight of the trials focused on vismodegib,50,51,57-62 two on sonidegib,52,63 two on patidegib (IPI-926), 53,54 one on PF-04449913 (glasdegib),55 one on TAK-441,56 and one on LY2940680 (taladegib).46 In addition, clinicaltrials.gov showed that two other SMO inhibitors, BMS-833923 and LEQ-506, are currently in clinical trials. Many published Phase I and II clinical trials demonstrated that SMO antagonists (vismodegib, sonidegib, patidegib, glasdegib, TAK-441) are selective for their target, as demonstrated by a decrease in GLI1 expression in patient tumor samples using mRNA or protein levels.46,52-59 These trials also found that SMO antagonists are well tolerated, with the side effects of dysgeusia, fatigue, muscle spasms and alopecia belonging to a drug class effect. Dose finding studies in solid tumors identified 150 mg/day vismodegib, 800 mg/day sonidegib, 160 mg/day patidegib, 100 mg/day glasdegib and 1600 mg/day TAK-441 as safe and effective doses.
Table 2.
Summary of smoothened antagonist clinical trials with genetic analysis
| Study by Author | Year | Cancer Type | Phase | Design | Treatment | Primary Endpoint | Target Inhibition | Ref # |
|---|---|---|---|---|---|---|---|---|
| Von Hoff, DDa | 2009 | Solid tumors | I | Open-label | Vismodegib | Safety | ↓ GLI1 pt, correlates w/co | 50 |
| LoRusso, PMa | 2011 | Solid tumors | I | Open-label | Vismodegib | Safety | ↓ GLI1 pt, correlates w/co | 51 |
| Rodon, J | 2014 | Solid tumors | I | Open-label | Sonidegib | Safety | ↓ GLI1 pt, no correlation w/co | 52 |
| Jimeno, A | 2013 | Solid tumors | I | Open-label | Saridegib | Safety | ↓ GLI1 pt, no correlation w/co | 53 |
| Bowles, DW | 2016 | SCC | I | Open-label | Saridegib + cetuximab | Safety | ↓ HH pathway pt, correlates w/co | 54 |
| Wagner, AJ | 2015 | Solid tumors | I | Open-label | Glasdegib | Safety | ↓ GLI1 pt, no correlation w/co | 55 |
| Goldman, J | 2015 | Solid tumors | I | Open-label | TAK-441 | Safety | ↓ GLI1 pt, no correlation w/co | 56 |
| Bendell, J. | 2018 | BCC | I | Open-label | Taladegib | Recommended Phase II | ↓ GLI1 pt, no correlation w/co | 46 |
| Tang, JYb | 2012 | BCC | II | R, DB | Vismodegib | Reduced incidence of new BCC | ↓ GLI1 pt, correlates w/co | 57 |
| Kaye, SB | 2012 | OC | II | Randomized | Vismodegib | PFS | No correlation | 58 |
| Berlin, J | 2012 | CRC | II | R, DB | Vismodegib + SOC | PFS | No correlation | 59 |
| Italiano, A | 2013 | CHS | II | Open-label | Vismodegib | Patients with non-PD at 6 months | No correlation | 60 |
| Kim, EJ | 2014 | PANC | II | Open-label | Vismodegib + SOC | PFS | ↓ GLI1 pt, no correlation w/co | 61 |
| Robinson, GW | 2015 | MB | II | R, DB | Vismodegib | PFS | Correlation between baseline gene expression and co | 62 |
| Migden, MRb | 2015 | BCC | II | Open-label | Sonidegib | Objective response rate | ↓ GLI1 pt, correlates w/co | 63 |
| Maughan | 2016 | PC | II | Open-label | Vismodegib | Pharmacodynamics | ↓ GLI1 pt, no correlation w/co | 64 |
aBoth studies are based on the same clinical trial
bServed as the basis for FDA approval
Abbreviations: BCC, basal cell carcinoma; CRC, colorectal cancer; CHS, chondrosacroma; OC, ovarian cancer; PANC, pancreatic cancer; PC, prostate cancer; SOC, standard of care; MB, medulloblastoma; SCC, squamous cell carcinoma; PD, progressive disease; R, DB, random, double blind; PFS, progression-free survival; pt, post-treatment; co, clinical outcome
Among the different cancer types examined, patients with locally advanced or metastatic BCC benefited the most from Smo antagonist therapy and, to a lesser extent, those with medulloblastoma. In Phase I clinical trials including patients with advanced tumors, vismodegib, sonidegib, and patidegib demonstrated objective or partial response in BCC patients compared to other advanced tumors (e.g. pancreatic, colorectal, lung, breast, and ovarian) .51–53 Later clinical trials for metastatic colorectal, ovarian, pancreatic, prostate, and non-small cell lung carcinoma showed vismodegib combined with chemotherapies did not provide clinical benefit compared to chemotherapy alone.58,59,61,64-67 In BCC patients, both vismodegib and sonidegib demonstrated objective disease control (with stable disease or tumor regression) in a majority (>90%) of patients.51–53,57,63 This clinical response was accompanied by a substantial decrease in GLI1 expression (>90%) in available biopsies post-treatment compared to pre-treatment. Progression-free survival was found to be significantly longer in medulloblastoma patients treated with vismodegib whose tumors expressed activating mutations in the HH pathway compared to those whose tumors did not have HH activation.62 Similarly, longer progression-free survival (>150 days) was associated with HH pathway downregulation in patients with squamous cell carcinoma after treatment with patidegib, whereas shorter progression-free survival was seen in patients with little or no change in HH pathway gene expression.54 Taken together, these studies suggest that gene expression (either before or after treatment) can correlate with HH antagonist clinical outcomes.
Conversely, other solid tumors (e.g. pancreatic, colorectal, lung) treated with SMO antagonists did not demonstrate a correlation between clinical response and genetic changes. Several of the clinical trials found that GLI1 mRNA expression was significantly decreased in available post-treatment biopsies (from 57–100% of patients), but there was no meaningful clinical response (e.g. progression-free survival) in these patients.52,53,55,56,61,64 For example, 57% of patients with metastatic, castration-resistant prostate cancer showed a reduction in expression of GLI1, GLI2 and PTCH1 with vismodegib treatment, but no clinical response.64 Of note, combination of vismodegib with standard of care (SOC) chemotherapy agents did not improve survival in patients with colorectal or pancreatic cancer compared to SOC chemotherapy alone.59,61 In addition, other clinical trials demonstrated that HH gene expression in biopsies taken from cancer patients before treatment (i.e., baseline expression) did not correlate with clinical response to SMO antagonists and, therefore, could not predict which patients might respond to this therapy.58–60 Glasdegib (PF-04449913) combined with chemotherapy in patients with acute myeloid leukemia and myelodysplastic syndrome did achieve a more than 2-fold change in expression in SMO and PTCH2,67–69 but failed to demonstrate significance between the molecular expression and clinical responsiveness. Recently, taladegib (LY2940680) began a Phase I clinical trials for advanced basal cell carcinoma (BCC), colon adenocarcinoma, and other advanced solid tumors.46 This study included both patients that had received a previous hedgehog inhibitor and treatment-naïve patients to determine if patients with prior hedgehog inhibitor exposure could be differentiated from treatment-naïve patients based on clinical response. Clinical response was observed for both treatment-naïve patients (68.8%) and those that had received a previous hedgehog inhibitor (35.5%); however, no statistically significant differences were observed amongst groups. Overall, GLI1 expression was inhibited by 92.3% compared to normal skin; however, % inhibition did not correlate with clinical response. Baseline Indian HH, Sonic HH, SMO, and GLI1 expression was evaluated in ovarian cancer patients,58 Indian HH, Sonic HH, SMO, PTCH1 and GLI1 in colorectal cancer patients61 and Sonic HH, SMO, GLI2, and GLI3 in chondrosarcoma patients.60 These molecular markers failed to correlate with clinical response.
Many of these solid tumors lack mutations in PTCH1 or SMO. These mutations activate HH signaling and are prevalent in tumors that respond to HH inhibitor treatments with corresponding decreases in GLI1.51 However, solid tumors lacking PTCH or SMO mutations may have increased expression of HH ligands or other components of the pathway (e.g., GLI1), which could be indicative of canonical or non-canonical signaling. So, while Smo antagonists failed to provide a correlation between clinical response and genetic changes in these solid tumors, non-canonical HH signaling may be important when interpreting these findings. Combinatorial or alternative therapies may target both canonical and non-canonical mechanisms of HH signaling and be a viable option for future studies
New approaches and future directions
Taken together, these clinical trials demonstrate that SMO antagonists are selective for their target in human cancers, can effectively reduce GLI1 expression, are well tolerated, but still have variable clinical efficacy. Clinical response to these compounds varied among different cancer types, with some cancers demonstrating a correlation between clinical outcomes and baseline HH gene expression or gene expression changes after treatment, whereas other cancers did not. BCCs, the most common type of skin cancer, appear to respond best to SMO antagonism compared to other cancer types, both in terms of HH pathway inhibition and clinical benefit. A majority of basal cell carcinomas are known to have loss of function mutations in PTCH1 or activating mutations in SMO, making HH signaling in these tumors ligand-independent.2 Tumors dependent upon HH ligand signaling (either in an autocrine or paracrine manner) appear to be less sensitive to SMO antagonists as single agents.58–60 In addition, there have been documented cases of resistance to SMO antagonists due to acquired mutations in the SMO protein that can change drug-binding affinity, diminish, or prevent the compounds from binding to the heptahelical bundle.70,71 Vismodegib and sonidegib bind to the same drug-binding pocket on the SMO protein, and new therapies are under development to target different domains of SMO.37,50,71,72 In vitro research has shown that the SMO mutation E518A (a glutamic acid to alanine change at amino acid 518 of SMO) increases the binding affinity for sonidegib, but reduces the affinity for vismogedib.73,74 SMO mutant D427H results in a disruption of sonidegib binding to SMO as a result of a conformation change in the transmembrane domain of SMO.75 This phenomenon is reported in patients as well, new mutations in SMO, not present in the pretreatment tumor, were observed in BCC patients with acquired resistance to vismodegib.76 SMO D473H prevents the effective binding of vismodegib and sonidegib, thus failing to inhibit SMO activity with either drug.49,75 In clinical specimens from BCC patients with resistance to Hedgehog antagonists, 50–69% had identified SMO mutations, which possibly confer resistance.77–79 Pietrobono and Stecca77 report numerous SMO mutations identified in human cancers and their role in drug resistance.80 Second generation SMO antagonists are currently under development with the goal of overcoming this resistance.41 Novartis’ LEQ-506 was developed to overcome the conformation change induced by the D473H mutation in SMO. It has not been fully tested for efficacy, but a Phase I clinical trial has been completed with no results yet available.75,81 The molecular basis for these resistance mechanisms continues to be studied, and may offer some explanation as to why certain cancers respond to Smo antagonists in both a targeted and clinically meaningful way, while others do not.
The use of SMO antagonists in HH ligand-dependent cancers may have some merit, as evidenced by the study from Kaye et al.58 which demonstrated a trend toward increased progression-free survival among ovarian cancer patients treated with vismodegib alone compared to placebo (7.5 months versus 5.8 months, respectively). Perhaps there exists a sub-population of ovarian cancer patients responsive to SMO antagonists that could be identified for future clinical trials, a concept that is currently being applied to other cancer types. A developing trend observed amongst clinical trials is the selection of drug regimen across a multiplicity of cancers based on the cancer or tumor’s molecular signature (basket or umbrella trials).82–87 Many of these trials include hedgehog inhibitors as a treatment option. Patients with recurrent brain tumors (young adults),82 medulloblastomas,84 refractory solid tumors, lymphomas, and multiple myeloma,83 and other advanced cancers85,86 are initially being analyzed for the molecular signature of their diseases, in order to give targeted single or combination therapies, which may include Hedgehog inhibitors. This sets the foundation for a broader examination of patient pharmacogenomics, possibly using cDNA microarrays or RNA sequencing,54,62 rather than quantifying the expression of a limited number of genes. In addition, changes at the epigenetic or protein level could be evaluated to better understand individual differences in response to SMO antagonists. If a molecular signature of response is identified, it may be used to screen for candidates who would gain the most benefit from HH inhibitor therapy, much the same way pharmacogenetic testing is now done for medications such as clopidogrel (CYP2C19), warfarin (CYP2C9), trastuzumab (HER2/neu) and imatinib (Bcr-abl).
Due to the limited therapeutic utility of SMO antagonists as single agents in cancer types outside of BCC and medulloblastoma, there is now movement toward combining HH inhibition with standard chemotherapy agents (Table 3). While initial published clinical trials examining this concept have yielded underwhelming results,59,61,88 there are numerous pre-clinical studies that suggest combination therapy could still be a useful strategy, possibly due to crosstalk between ligand-dependent HH signaling and other survival pathways.89 According to clinicaltrials.gov, vismodegib, sonidegib, patidegib/IPI-926, glasdegib/PF-04449913, BMS-833923 and taladegib/LY2940680 combined with other cancer therapeutic agents are currently under clinical investigation (Tables 3 and 4). These combination therapies are largely being conducted in patients with non-hematologic/solid tumors (pancreatic, lung and breast) in comparison to hematologic malignancies (acute myeloid leukemia, chronic myeloid leukemia and multiple myeloma). A pilot Phase I study combined cetuximab (an epidermal growth factor receptor antibody) and saridegib in patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC).54 Patient response correlated with expected changes in EGFR and HH-related genes as measured via RNA sequencing. Favorable patient outcomes correlated with a decrease in HH pathway genes, showing effective targeting of the HH pathway.
Table 3.
Clinical trials examining smoothened antagonists combined FDA approved drugs
| Smo Antagonist | Therapy (Target) | Cancer | Identifier |
|---|---|---|---|
| Vismodegib | Ribavirin±decitabine | AML | NCT02073838 |
| Vismodegib | Erlotinib (EGFR)±gemcitabine | Pancreatic | NCT00878163 |
| Vismodegib | Cisplatin+etoposide | SCLC | NCT00887159 |
| Vismodegib | Sirolimus (mTOR) | Pancreatic | NCT01537107 |
| Vismodegib | Pembrolizumab (PD-1) | BCC | NCT02690948 |
| Vismodegib | Temozolomide | Medulloblastoma | NCT01601184 |
| Vismodegib | Nivolumab (PD-1) | BCNS | NCT03767439 |
| Sonidegib | Ribociclib | Brain | NCT03434262 |
| Sonidegib | Azacitidine+decitabine | Leukemia | NCT02129101 |
| Sonidegib | Fluorouracil+leucovorin+oxaliplatin+irinotecan | Pancreatic | NCT01485744 |
| Sonidegib | Gemcitabine+Nab-paclitaxel | Pancreatic | NCT02358161 |
| Sonidegib | Docetaxel+prednisone | Prostate | NCT02182622 |
| Sonidegib | Lenalidomide | MM | NCT02086552 |
| Sonidegib | Nilotinib (tyrosine kinase) | CML | NCT01456676 |
| Sonidegib | Etoposide+cisplatin | SCLC | NCT01579929 |
| Sonidegib | Everolimus (mTOR kinase) | Esophageal | NCT02138929 |
| Sonidegib | Gemcitabine | Pancreatic | NCT01487785 |
| Sonidegib | Docetaxel | Breast | NCT02027376 |
| Sonidegib | Bortezomib | MM | NCT02254551 |
| Sonidegib | Gemcitabine+Nab-paclitaxel | Pancreatic | NCT01431794 |
| Sonidegib | Paclitaxel | Ovarian | NCT02195973 |
| Saridegib | Gemcitabine | Pancreatic | NCT01130142 |
| Glasdegib | Cytarabine+daunorubicin+decitabine | AML | NCT01546038 |
| Glasdegib | Azacitidine | AML | NCT02367456 |
| BMS-833923 | Dasatinib | CML | NCT01357655 |
| BMS-833923 | Cisplatin+capecitabine | Gastric/Esophageal | NCT00909402 |
| BMS-833923 | Carboplatin+etoposide | SCLC | NCT00927875 |
| BMS-833923 | Dasatinib | CML | NCT01218477 |
| Taladegib | Paclitaxel+carboplatin+radiation | Esophageal | NCT02530437 |
Abbreviations: AML, acute myeloid leukemia; EGFR, epidermal growth factor receptor; SCLC, small cell lung cancer; BCNS, basal cell Nevus syndrome; MM, multiple myeloma; CML, chronic myeloid leukemia
Table 4.
Clinical trials examining smoothened antagonists combined with investigational compounds
| Smo Antagonist | Targeted Therapy (Target) | Cancer | Identifier |
|---|---|---|---|
| Vismodegib | RO4929097 (gamma-secretase/Notch) | Breast | NCT01071564 |
| Vismodegib | RO4929097 (gamma-secretase/Notch) | Sarcoma | NCT01154452 |
| Vismodegib | GSK2256098 (FAK/focal adhesional kinase) | Meningioma | NCT02523014 |
| Sonidegib | Buparlisib (PI3K) | BCC | NCT02303041 |
| Sonidegib | Buparlisib (PI3K) | Solid tumors | NCT01576666 |
Abbreviations: BCC, basal cell carcinoma
Clinical researchers are employing the knowledge gained from pre-clinical studies and combining HH inhibition with new molecules targeting multiple signaling pathways and targets (Tables 3 and 4). Examples include the use of nivolumab, a PD-1 inhibitor, which is being combined with vismogedib in patients with basal cell/Nevus Syndrome (BCNS).90 A novel FAK inhibitor, GSK2256098 was combined with vismogedib in patients with meningioma.91 Patients with advanced or metastatic BCC, who are either naïve to or refractory after SMO antagonist monotherapy, are being treated with sonidegib and buparlisib, a phosphoinositide 3-kinase inhibitor.92 Children and young adults with recurrent brain tumors are first having their brain tumors molecularly characterized, and then being stratified for combination therapies that include using sonidegib with ribociclib, a cyclin D1/CDK4 and CDK6 inhibitor.82 In a Phase II trial, ribavirin (a broad-spectrum anti-viral) and vismogedib are combined with and without decitabine in acute myeloid leukemia patients.93 The SMO inhibitor, BMS-833923, is currently being combined with cisplatin and capecitabine for inoperable, metastatic gastric adenocarcinomas,94 as well as carboplatin and etoposide in small cell lung cancer.95 A triple cocktail of azacitidine, decitabine, combined with sonidegib is currently being evaluated in patients with myeloid malignancies.96 While a monotherapeutic approach using SMO antagonists may not have been the most efficacious, combination therapy with chemotherapies (Table 3) or other investigational targeted therapies (Table 4), could provide an avenue for the continued and expanded use of HH inhibitors for other cancers, while circumventing refractory disease and drug resistance.
In addition to SMO antagonists, there is a growing body of pre-clinical research devoted to direct inhibitors of the downstream effectors of HH signaling, the Gli family of transcription factors. It is now understood that certain cellular pathways (e.g. PI3K, MAPK, Wnt, NF-κB, and TGFβ) can activate Gli transcription independently of the “classical” HH signaling pathway, also referred to as non-canonical signaling.97,98 Compounds such as GANT58, GANT61, and Glabrescione B, which can directly inhibit the binding of Gli1 and Gli2 to DNA, have been found to be effective against cancer cells both in vitro and in vivo.99,100 In an experiment performed by our lab, it was found that knockdown of both GLI1 and GLI2 using siRNA decreased ovarian cancer cell growth in vitro more than either knockdown alone (Figure 2). GANT61 (a Gli-1/Gli-2 antagonist) inhibited growth of ovarian cancer cells and decreased the expression of HH target genes (GLI1, GLI2, PTCH1) compared to vismodegib and sonidegib (Figure 3). Additional in vitro and in vivo studies have shown effectiveness of GANT61 in models of breast, lungs, and many other cancers.101–104 Collectively, these studies suggest that direct inhibitors of Gli would be useful in cases where downstream activation of Gli transcription occurs independently of upstream PTCH repression and/or SMO activation, scenarios in which SMO antagonists would potentially be no longer therapeutic.
Figure 2.
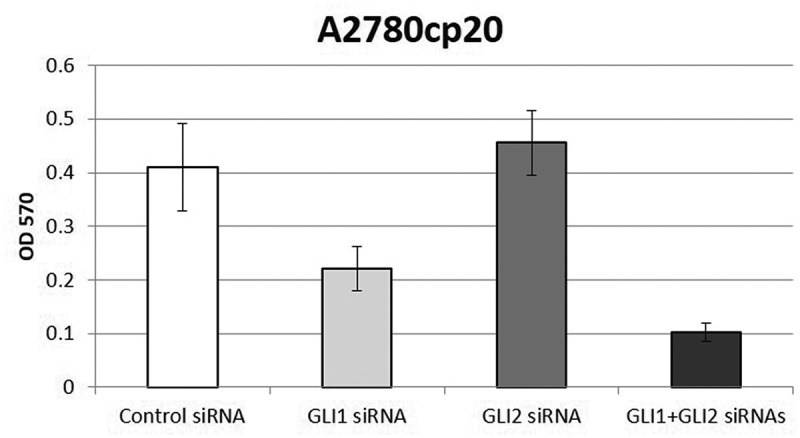
Combined knockdown of GLI1 and GLI2 decreases ovarian cancer cell growth more than individual knockdown alone in vitro. Platinum-resistant A2780cp20 ovarian cancer cells were grown for up to 6 days after transfection with control siRNA, GLI1 siRNA, GLI2 siRNA or combined GLI1+ GLI2 siRNAs. Cell viability was determined using an MTT assay.67
Figure 3.

Inhibition of the HH signaling pathway decreases cell viability of platinum-resistant A2780cp20 ovarian cancer cells. (a) Cell viability after 96 hours of varying doses of HH inhibitor (SMO antagonists, cyclopamine, vismodegib, sonidegib; GLI antagonist, GANT61) treatment using an MTT assay. Data are representative of 3 independent experiments. (b) Gene expression of GLI1, GLI2, and PTCH1 was examined in A2780cp20 ovarian cancer cells treated with HH inhibitors for 24 hours compared to untreated controls. Date are representative of 3 independent experiments
Another possibility for therapeutic intervention of HH signaling is for tumors that demonstrate overexpression of the HH pathway, particularly HH ligands, but lack activating mutations in PTCH1, SMO, or other HH components. In these tumors, non-canonical HH signaling may be playing a role in tumorigenesis. Faria et al.29 showed PTCH-dependent, SMO-independent signaling was capable of activating protein kinase A (PKA) and Wnt signaling, and regulating SHH-mediated endocytosis. In a mouse model of small cell lung cancer, SHH overexpression induced chromosomal instability and promoted nuclear translocation of Cyclin B1 in cancer cells; whereas overexpression of constitutively active SMO did not.105 Canonical HH signaling has been shown to suppress Wnt signaling in colon cancer cells and normal colon cells; however, in colon cancer organoids enriched for cancer stem cells (CSC) there was an increase in active Wnt signaling and PTCH1 expression.28 These samples also expressed HH ligands and SMO, but lacked nuclear GLI expression. Additional experiments demonstrated that Wnt was activated via SHH-PTCH-dependent signaling that was independent of SMO or GLI, and necessary for the maintenance of colon cancer CSCs. Current molecules available that block the interaction between SHH and PTCH include: robotnikinin, a small molecule SHH inhibitor; 5E1 an SHH antibody, and RU-SKI 43, a small molecule inhibitor of hedgehog acyltransferase that blocks formation of functional SHH.106,107 Non-canonical HH signaling indicates another point in this pathway for potential development of cancer therapeutics.
The role of HH signaling plays in initiation and progression of human cancer make it an attractive target for cancer therapeutics. Many ongoing clinical trials highlight the importance of tracking molecular responses to drugs, whether in relation to patient response or acquired resistance. Continued inclusion of molecular and genetic analyses in clinical trials will be essential in determining response and efficacy of targeted therapies, as well as aiding in the design in the next generation of HH inhibitors.
Funding Statement
This work was supported by the NIDCR under Grants [K99/R00-DE023826; NIDCRT90-DE022736-01(DART)].
Disclosure of interest
The authors report no conflicts of interest.
References
- 1.Bale AE, Yu KP.. The hedgehog pathway and basal cell carcinomas. Hum Mol Genet. 2001;10(7):757–762. doi: 10.1093/hmg/10.7.757. [DOI] [PubMed] [Google Scholar]
- 2.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170(1):347–355. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64(17):6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67(5):2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niyaz M, Khan MS, Wani RA, Shah OJ, Mudassar S. Sonic hedgehog protein is frequently up-regulated in pancreatic cancer compared to colorectal cancer. Pathol Oncol Res. 2018;26(1):551–557. doi:10.1007/s12253-018-00564-2. [DOI] [PubMed] [Google Scholar]
- 7.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz I Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12(20 Pt 1):5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 9.Stecca B, Sanchez P, de Tribolet N, Radovanovic I, Ruiz I Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104(14):5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 11.Mohler J. Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics. 1988;120(4):1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13(1):14–21. doi: 10.1016/S0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 13.Weed M, Mundlos S, Olsen BR. The role of sonic hedgehog in vertebrate development. Matrix Biol. 1997;16(2):53–58. doi: 10.1016/S0945-053X(97)90072-X. [DOI] [PubMed] [Google Scholar]
- 14.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 15.Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75(7):1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 16.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 17.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 18.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384(6605):129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 19.Wilson CW, Chuang PT. New “hogs” in Hedgehog transport and signal reception. Cell. 2006;125(3):435–438. doi: 10.1016/j.cell.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci U S A. 1998;95(23):13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Platt KA, Censullo P, Ruiz I Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124(13):2537–2552. [DOI] [PubMed] [Google Scholar]
- 22.Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124(1):113–123. [DOI] [PubMed] [Google Scholar]
- 23.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108(6):823–835. doi: 10.1016/S0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci U S A. 2006;103(1):33–38. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26(9):3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100(4):423–434. doi: 10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 27.Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10(16):2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 28.Regan JL, Schumacher D, Staudte S, Steffen A, Haybaeck J, Keilholz U, Schweiger C, Golob-Schwarzl N, Mumberg D, Henderson D, et al. Non-canonical hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of colon cancer stem cells. Cell Rep. 2017;21(10):2813–2828. doi: 10.1016/j.celrep.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Faria AV, Akyala AI, Parikh K, Bruggemann LW, Spek CA, Cao W, Bruno MJ, Bijlsma MF, Fuhler GM, Peppelenbosch MP. Smoothened-dependent and -independent pathways in mammalian noncanonical Hedgehog signaling. J. Biol. Chem. 2019;294(25):9787–9798. doi: 10.1074/jbc.RA119.007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietrobono S, Gagliardi S, Stecca B. Non-canonical hedgehog signaling pathway in cancer: activation of GLI transcription factors beyond smoothened. Front Genet. 2019;10:556. doi: 10.3389/fgene.2019.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science.1998;280(5369):1603–1607.doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 32.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125(18):3553–3562. [DOI] [PubMed] [Google Scholar]
- 33.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 34.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binns W, James LF, Shupe JL, Everett G. A congenital cyclopian-type malformation in lambs induced by maternal ingestion of a range plant, veratrum californicum. Am J Vet Res. 1963;24:1164–1175. [PubMed] [Google Scholar]
- 36.Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz I Altaba A. The sonic hedgehog-gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128(24):5201–5212. [DOI] [PubMed] [Google Scholar]
- 37.Tay EY, Teoh YL, Yeo MS. Hedgehog pathway inhibitors and their utility in basal cell carcinoma: a comprehensive review of current evidence. Dermatol Ther (Heidelb). 2018;9(1):33–49. doi:10.1007/s13555-018-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkler JD, Isaacs A, Holderbaum L, Tatard V, Dahmane N. Design and synthesis of inhibitors of Hedgehog signaling based on the alkaloid cyclopamine. Org Lett. 2009;11(13):2824–2827. doi: 10.1021/ol900974u. [DOI] [PubMed] [Google Scholar]
- 39.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Gould SE, Guichert O, Gunzner JL, Halladay J, et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19(19):5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 40.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, et al. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1(3):130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peukert S, He F, Dai M, Zhang R, Sun Y, Miller-Moslin K, McEwan M, Lagu B, Wang K, Yusuff N, et al. Discovery of NVP-LEQ506, a second-generation inhibitor of smoothened. Chem Med Chem. 2013;8(8):1261–1265. doi: 10.1002/cmdc.201300217. [DOI] [PubMed] [Google Scholar]
- 42.Munchhof MJ, Li Q, Shavnya A, Borzillo GV, Boyden TL, Jones CS, LaGreca SD, Martinez-Alsina L, Patel N, Pelletier K, et al. Discovery of PF-04449913, a potent and orally bioavailable inhibitor of smoothened. ACS Med Chem Lett. 2012;3(2):106–111. doi: 10.1021/ml2002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi T, Oguro Y, Tanaka T, Shiokawa Z, Tanaka Y, Shibata S, Sato Y, Yamakawa H, Hattori H, Yamamoto Y, et al. Discovery of the investigational drug TAK-441, a pyrrolo[3,2-c]pyridine derivative, as a highly potent and orally active hedgehog signaling inhibitor: modification of the core skeleton for improved solubility. Bioorg Med Chem. 2012;20(18):5507–5517. doi: 10.1016/j.bmc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Akare UR, Bandaru S, Shaheen U, Singh PK, Tiwari G, Singare P, Nayarisseri A, Banerjee T. Molecular docking approaches in identification of high affinity inhibitors of human SMO receptor. Bioinformation. 2014;10(12):737–742. doi: 10.6026/97320630010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497(7449):338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendell J, Andre V, Ho A, Kudchadkar R, Migden M, Infante J, Tiu RV, Pitou C, Tucker T, Brail L, et al. Phase I study of LY2940680, a smo antagonist, in patients with advanced cancer including treatment-naive and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24(9):2082–2091. doi: 10.1158/1078-0432.CCR-17-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, Yu LC, Behnke ML, Nair SJ, Hagel M, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926). J Med Chem. 2009;52(14):4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 48.Lauressergues E, Heusler P, Lestienne F, Troulier D, Rauly-Lestienne I, Tourette A, Ailhaud MC, Cathala C, Tardif S, Denais-Lalieve D, et al. Pharmacological evaluation of a series of smoothened antagonists in signaling pathways and after topical application in a depilated mouse model. Pharmacol Res Perspect. 2016;4(2):e00214. doi: 10.1002/prp2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migden MR, Chang ALS, Dirix L, Stratigos AJ, Lear JT. Emerging trends in the treatment of advanced basal cell carcinoma. Cancer Treat Rev. 2018;64:1–10. doi: 10.1016/j.ctrv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 51.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, Sarantopoulos J, Mahalingam D, Shou Y, Moles MA, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20(7):1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 53.Jimeno A, Weiss GJ, Miller WH Jr., Gettinger S, Eigl BJ, Chang AL, Dunbar J, Devens S, Faia K, Skliris G, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19(10):2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowles DW, Keysar SB, Eagles JR, Wang G, Glogowska MJ, McDermott JD, Le PN, Gao D, Ray CE, Rochon PJ, et al. A pilot study of cetuximab and the hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;53:74–79. doi: 10.1016/j.oraloncology.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner AJ, Messersmith WA, Shaik MN, Li S, Zheng X, McLachlan KR, Cesari R, Courtney R, Levin WJ, El-Khoueiry AB. A phase I study of PF-04449913, an oral hedgehog inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015;21(5):1044–1051. doi: 10.1158/1078-0432.CCR-14-1116. [DOI] [PubMed] [Google Scholar]
- 56.Goldman J, Eckhardt SG, Borad MJ, Curtis KK, Hidalgo M, Calvo E, Ryan DP, Wirth LJ, Parikh A, Partyka J, et al. Phase I dose-escalation trial of the oral investigational Hedgehog signaling pathway inhibitor TAK-441 in patients with advanced solid tumors. Clin Cancer Res. 2015;21(5):1002–1009. doi: 10.1158/1078-0432.CCR-14-1234. [DOI] [PubMed] [Google Scholar]
- 57.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaye SB, Fehrenbacher L, Holloway R, Amit A, Karlan B, Slomovitz B, Sabbatini P, Fu L, Yauch RL, Chang I, et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin Cancer Res. 2012;18(23):6509–6518. doi: 10.1158/1078-0432.CCR-12-1796. [DOI] [PubMed] [Google Scholar]
- 59.Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19(1):258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 60.Italiano A, Le Cesne A, Bellera C, Piperno-Neumann S, Duffaud F, Penel N, Cassier P, Domont J, Takebe N, Kind M, et al. GDC-0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute single-arm Phase II collaborative study. Ann Oncol. 2013;24(11):2922–2926. doi: 10.1093/annonc/mdt391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20(23):5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, et al. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from Phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–2654. doi: 10.1200/JCO.2014.60.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kudchadkar R, Trefzer U, Gogov S, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 64.Maughan BL, Suzman DL, Luber B, Wang H, Glavaris S, Hughes R, Sullivan R, Harb R, Boudadi K, Paller C, et al. Pharmacodynamic study of the oral hedgehog pathway inhibitor, vismodegib, in patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2016;78(6):1297–1304. doi: 10.1007/s00280-016-3191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belani CP, Dahlberg SE, Rudin CM, Fleisher M, Chen HX, Takebe N, Velasco MR Jr., Tester WJ, Sturtz K, Hann CL, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN cancer research group (E1508). Cancer. 2016;122(15):2371–2378. doi: 10.1002/cncr.30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, et al. Randomized Phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33(36):4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cortes JE, Douglas Smith B, Wang ES, Merchant A, Oehler VG, Arellano M, DeAngelo DJ, Pollyea DA, Sekeres MA, Robak T, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. 2018;93(11):1301–1310. doi: 10.1002/ajh.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, Montesinos P, Pollyea DA, DesJardins P, Ottmann O, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389. doi:10.1038/s41375-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savona MR, Pollyea DA, Stock W, Oehler VG, Schroeder MA, Lancet J, McCloskey J, Kantarjian HM, Ma WW, Shaik MN, et al. Phase Ib study of glasdegib, a hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high-risk MDS. Clin Cancer Res. 2018;24(10):2294–2303. doi: 10.1158/1078-0432.CCR-17-2824. [DOI] [PubMed] [Google Scholar]
- 70.Metcalfe C, de Sauvage FJ. Hedgehog fights back: mechanisms of acquired resistance against Smoothened antagonists. Cancer Res. 2011;71(15):5057–5061. doi: 10.1158/0008-5472.CAN-11-0923. [DOI] [PubMed] [Google Scholar]
- 71.Danial C, Sarin KY, Oro AE, Chang AL. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22(6):1325–1329. doi: 10.1158/1078-0432.CCR-15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peer E, Tesanovic S, Aberger F. Next-generation hedgehog/GLI pathway inhibitors for cancer therapy. Cancers (Basel). 2019;11(4):538–558. doi:10.3390/cancers11040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen L, Aria AB, Silapunt S, Lee HH, Migden MR. Treatment of advanced basal cell carcinoma with sonidegib: perspective from the 30-month update of the BOLT trial. Future Oncol. 2018;14(6):515–525. doi: 10.2217/fon-2017-0457. [DOI] [PubMed] [Google Scholar]
- 74.Wang C, Wu H, Evron T, Vardy E, Han GW, Huang XP, Hufeisen SJ, Mangano TJ, Urban DJ, Katritch V, et al. Structural basis for Smoothened receptor modulation and chemoresistance to anticancer drugs. Nat Commun. 2014;5:4355. doi: 10.1038/ncomms5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu J, Li JJ, Song LT, Zhai HL, Wang J, Zhang XY. Molecular modeling study on resistance of WT/D473H SMO to antagonists LDE-225 and LEQ-506. Pharmacol Res. 2018;129:491–499. doi: 10.1016/j.phrs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 76.Brinkhuizen T, Reinders MG, van Geel M, Hendriksen AJ, Paulussen AD, Winnepenninckx VJ, Keymeulen KB, Soetekouw PM, van Steensel MA, Mosterd K. Acquired resistance to the Hedgehog pathway inhibitor vismodegib due to smoothened mutations in treatment of locally advanced basal cell carcinoma. J Am Acad Dermatol. 2014;71(5):1005–1008. doi: 10.1016/j.jaad.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Atwood SX, Sarin KY, Whitson RJ, Li JR, Kim G, Rezaee M, Ally MS, Kim J, Yao C, Chang AL, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27(3):342–353. doi: 10.1016/j.ccell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pricl S, Cortelazzi B, Dal Col V, Marson D, Laurini E, Fermeglia M, Licitra L, Pilotti S, Bossi P, Perrone F. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2015;9(2):389–397. doi: 10.1016/j.molonc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharpe HJ, Pau G, Dijkgraaf GJ, Basset-Seguin N, Modrusan Z, Januario T, Tsui V, Durham AB, Dlugosz AA, Haverty PM, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015;27(3):327–341. doi: 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pietrobono S, Stecca B. Targeting the oncoprotein smoothened by small molecules: focus on novel acylguanidine derivatives as potent smoothened inhibitors. Cells. 2018;7(12). doi: 10.3390/cells7120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.A dose finding and safety study of oral LEQ506 in patients with advanced solid tumors. Available from: https://ClinicalTrials.gov/show/NCT01106508.
- 82.SJDAWN: St. jude children’s research hospital Phase 1 study evaluating molecularly-driven doublet therapies for children and young adults with recurrent brain tumors. Available from: https://ClinicalTrials.gov/show/NCT03434262.
- 83.Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors, lymphomas, or multiple myeloma (The MATCH screening trial). Available from: https://ClinicalTrials.gov/show/NCT02465060.
- 84.A clinical and molecular risk-directed therapy for newly diagnosed medulloblastoma. Available from: https://ClinicalTrials.gov/show/NCT01878617.
- 85.The drug rediscovery protocol (DRUP trial). Available from: https://ClinicalTrials.gov/show/NCT02925234.
- 86.A Phase II randomized study comparing the efficacy and safety of targeted therapy or cancer immunotherapy versus platinum-based chemotherapy in patients with cancer of unknown primary site. Available from: https://ClinicalTrials.gov/show/NCT03498521.
- 87.Canadian profiling and targeted agent utilization trial (CAPTUR). Available from: https://ClinicalTrials.gov/show/NCT03297606.
- 88.Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, et al. A Phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas. 2016;45(3):370–375. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brechbiel J, Miller-Moslin K, Adjei AA. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat Rev. 2014;40(6):750–759. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Nivolumab with vismodegib in patients with basal cell nevus syndrome. Available from: https://ClinicalTrials.gov/show/NCT03767439.
- 91.Vismodegib and FAK inhibitor GSK2256098 in treating patients with progressive meningiomas. Available from: https://ClinicalTrials.gov/show/NCT02523014.
- 92.Sonidegib and buparlisib in treating patients with advanced or metastatic basal cell carcinoma. Available from: https://ClinicalTrials.gov/show/NCT02303041.
- 93.Ribavirin and hedgehog inhibitor with or without decitabine in AML. Available from: https://ClinicalTrials.gov/show/NCT02073838.
- 94.A study of BMS-833923 with cisplatin and capecitabine in inoperable, metastatic gastric, gastroesophageal, or esophageal adenocarcinomas. Available from: https://ClinicalTrials.gov/show/NCT00909402.
- 95.A study of BMS-833923 with carboplatin and etoposide followed by BMS-833923 alone in subjects with extensive-stage small cell lung cancer. Available from: https://ClinicalTrials.gov/show/NCT00927875.
- 96.Azacitidine and sonidegib or decitabine in treating patients with myeloid malignancies. Available from: https://ClinicalTrials.gov/show/NCT02129101.
- 97.Shevde LA, Samant RS. Nonclassical hedgehog-GLI signaling and its clinical implications. Int J Cancer. 2014;135(1):1–6. doi: 10.1002/ijc.28424. [DOI] [PubMed] [Google Scholar]
- 98.Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget. 2015;6(16):13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104(20):8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Infante P, Mori M, Alfonsi R, Ghirga F, Aiello F, Toscano S, Ingallina C, Siler M, Cucchi D, Po A, et al. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. Embo J. 2015;34(2):200–217. doi: 10.15252/embj.201489213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, Bonanno E, Di Stefano E, Frajese GV, Orlandi A, et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 2016;7(8):9250–9270. doi: 10.18632/oncotarget.7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang L, Walter V, Hayes DN, Onaitis M. Hedgehog-GLI signaling inhibition suppresses tumor growth in squamous lung cancer. Clin Cancer Res. 2014;20(6):1566–1575. doi: 10.1158/1078-0432.CCR-13-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oladapo HO, Tarpley M, Sauer SJ, Addo KA, Ingram SM, Strepay D, Ehe BK, Chdid L, Trinkler M, Roques JR, et al. Pharmacological targeting of GLI1 inhibits proliferation, tumor emboli formation and in vivo tumor growth of inflammatory breast cancer cells. Cancer Lett. 2017;411:136–149. doi: 10.1016/j.canlet.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Po A, Silvano M, Miele E, Capalbo C, Eramo A, Salvati V, Todaro M, Besharat ZM, Catanzaro G, Cucchi D, et al. Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma. Oncogene. 2017;36(32):4641–4652. doi: 10.1038/onc.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szczepny A, Rogers S, Jayasekara WSN, Park K, McCloy RA, Cochrane CR, Ganju V, Cooper WA, Sage J, Peacock CD, et al. The role of canonical and non-canonical Hedgehog signaling in tumor progression in a mouse model of small cell lung cancer. Oncogene. 2017;36:5544–5550. doi: 10.1038/onc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng LF, Stanton BZ, Maloof N, Wang X, Schreiber SL. Syntheses of aminoalcohol-derived macrocycles leading to a small-molecule binder to and inhibitor of Sonic Hedgehog. Bioorg Med Chem Lett. 2009;19(22):6319–6325. doi: 10.1016/j.bmcl.2009.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maun HR, Wen X, Lingel A, de Sauvage FJ, Lazarus RA, Scales SJ, Hymowitz SG. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285(34):26570–26580. doi: 10.1074/jbc.M110.112284. [DOI] [PMC free article] [PubMed] [Google Scholar]


