Abstract
In 2002, China integrated hepatitis B vaccine (HepB) into its Expanded Program on Immunization (EPI) using HepB vaccine containing 5 µg of antigen. Although not recommended nationally, there was a common clinical practice in China of screening children for anti-HBs antibody level and giving a booster dose to HBV surface antigen (HBsAg)-negative children with non-protective anti-HBs antibody levels. We report an evaluation of the protective effectiveness of the 5 µg HepB vaccine and the serological response to the booster dose. We used data from a 2014 hepatitis B serological survey to determine HBsAg positivity and anti-HBs antibody levels among children who received and did not receive a booster dose. We determined HepB coverage from the Children Immunization Information Management System (CIIMS). We obtained and analyzed reports of acute Hepatitis B (AHB) during 2008–2014 obtained from the National Notifiable Disease Reporting System (NNDRS). The HBsAg-positive rate among children who had not received a booster dose was 0.41%, and did not increase with age (i.e., time since infant immunization). The anti-HBs positivity rate among the 6% of children who received a booster dose (88.41%) was higher than among those who had not received a booster (60.85%); anti-HBs antibody levels declined with age regardless of booster dose status. There was no statistically significant difference in HBsAg positivity between children who received a booster dose and those who did not. The AHB incidence among children born between 2002 and 2007 did not increase with age. Use of routine 5 µg HepB vaccine was not associated with an increase in AHB or of HBsAg positivity by time since vaccination, providing supportive evidence that individuals vaccinated with the 5 µg HepB vaccine do not need a booster dose. Although a booster dose was associated with increases in anti-HBs antibody levels, our study provided no evidence to support the need for this clinical practice. We should continue to strengthen serological monitoring of children, especially for those born to HBsAg positive mothers.
Subject terms: Immunology, Diseases, Health care, Medical research
Introduction
An estimated 257 million people were living with chronic hepatitis B virus (HBV) infection around the world in 20151. HBV has been highly endemic in China, as historically, vertical HBV transmission built a reservoir of approximately 86 million chronically infected persons, accounting for 30% of the global burden of chronic HBV2. Serosurveys conducted in 1979 and 1992 in China showed a 10% prevalence of HBV surface antigen (HBsAg) positivity3. Universal administration of a timely birth dose of hepatitis B vaccine (HepB) followed by two more doses during infancy has been the key strategy to prevent HBV transmission in China. Before 2002, HepB was available in China but was not included in the National Immunization Program (NIP); families had to pay out-of-pocket for the vaccine. In 2002, China included HepB into its Expanded Program on Immunization (EPI), making the vaccine available at no cost to families. HepB was administered at 0, 1, and 6 months of age using a 5 µg recombinant yeast-derived vaccine (brand name Hepatitis B Vaccine Made by Recombinant DNA Technique in Yeast; adjuvant: aluminum). In 2012, China suspended production of the 5 µg vaccine, replacing it with a 10 µg vaccine in 2014 after supplies of the 5 µg vaccine were exhausted. Thus, a 5 µg HepB vaccine was administered to infants born from 2002 to 2013.
The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC)4 do not recommend HepB booster vaccination in successfully immunized individuals. Some studies5 indicate that a full primary series of HepB confers protection against hepatitis B infection for long periods of time, even though anti-HBs antibody levels wane over time and eventually become undetectable in some successfully immunized individuals. However, it is controversial whether a decline in serum anti-HBs antibody levels implies reduced protection and the need for a booster dose6,7. China has a large number of persons living with HBV who therefore have potential to cause horizontal transmission of HBV. Horizontal transmission could be seen as an increase in acute hepatitis B (AHB) and HBsAg prevalence following vaccination during infancy. Evidence of horizontal transmission could indicate a need for a booster dose of HepB.
In China there was a voluntary practice of screening children for HBsAg and anti-HBs antibody levels at kindergarten and primary school entry and offering a booster dose to those negative for HBsAg and anti-HBs antibody. Using data from a 2014 national serological survey, we analyzed seropositivity rates of these two markers in 1–12-year-old children to assess for evidence of horizontal transmission and impact of the booster dose strategy on antibody levels. We evaluated the incidence of AHB by birth cohort to look for evidence of increased AHB in China. We report results of our analyses to provide evidence regarding the need for a booster dose for children vaccinated as infants with the 5 µg HepB vaccine.
Material and methods
Study design
The setting was mainland China. We assessed HBsAg prevalence by birth cohort using data from a national serological survey, and we assessed AHB incidence in the same birth cohorts to determine whether AHB or HBsAg prevalence were increasing after infancy. We compared anti-HBs antibody levels of children given a booster dose of HepB with those not given a booster dose to assess the impact of the booster dose on antibody levels.
Data sources and participants
Children who participated in a 2014 HBV serological survey, described elsewhere8, were included in this study who met the following criteria: (1) born in 2002 to 2013, (2) vaccinated with a 5 µg HepB vaccine as a birth dose and primary series; and (3) having all three doses administered by 12 months of age.
In China, many children were voluntarily tested to determine anti-HBs antibody levels before entering kindergarten or primary school. Children negative for anti-HBs antibody would be offered a booster dose of HepB vaccine. It was up to the parents to decide whether or not to accept the offer. Among the 6% of subjects in the 2014 serological survey who had received booster doses, 85.57% received a booster dose between 2 and 6 years of age, and 11.08% received a booster dose after 6 years of age.
Coverage levels of HepB1-OT (defined as the percentage of newborn infants who received a dose of HepB within 24 h of birth) and HepB3 (defined as the percentage of children who received 3 doses of HepB before reaching 12 months of age) by birth cohort were obtained from the Children Immunization Information System (CIIMS) managed by the Chinese Center for Disease Control and Prevention (China CDC).
We determined the incidence of AHB among children over 5 years of age in the 2002–2007 birth cohorts using data from the National Notifiable Disease Reporting System (NNDRS) reported from 2008 to 2014. For example, children in the 2002 birth cohort were 6 to 12 years old in 2008–2014, the years for which their AHB incidence was obtained from NNDRS.
Statistical analysis
Chi-square testing was used to compare HBsAg and anti-HBs seropositivity trends, and to compare seropositivity rates between booster and no-booster groups. P < 0.05 was considered statistically significant. We determined coverage of HepB1-OT and HepB3 from 2002 to 2013. AHB incidence was determined for each birth cohort.
Ethical reviews
The 2014 HBV serosurvey was approved by China CDC’s Ethical Review Committee. Ethical approval was not required for analysis of routinely obtained coverage and disease incidence data, as these data were obtained in aggregate form without personal identifying information.
Results
HBsAg and anti-HBs seropositivity
In total, 12,713 children from the 2014 serological survey were included in this study, and among these children, 7,663 (63.1%) were born to HBsAg-negative mothers, 313 (2.6%) were born to HBsAg-positive mothers, and 4167 (34.3%) were children born to mothers with unknown HBsAg status. Among all children, 11,401 (93.9%) had not received a booster dose, and 742 (6.1%) had. Among children receiving a booster dose, 480 (64.7%) received one booster dose and the rest received 2 or more doses. The practice of screening and administering booster doses decreased over time (Table 1).
Table 1.
Characteristics of the study population.
| Characteristic | No booster | Booster | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Birth year | ||||||
| 2012–2013 | 3567 | 97.43 | 94 | 2.57 | 3661 | 100.00 |
| 2010–2011 | 4195 | 96.50 | 152 | 3.50 | 4347 | 100.00 |
| 2008–2009 | 1429 | 92.13 | 122 | 7.87 | 1551 | 100.00 |
| 2006–2007 | 1126 | 87.97 | 154 | 12.03 | 1280 | 100.00 |
| 2004–2005 | 447 | 85.14 | 78 | 14.86 | 525 | 100.00 |
| 2002–2003 | 637 | 81.77 | 142 | 18.23 | 779 | 100.00 |
| HBsAg of mother | ||||||
| Negative | 7151 | 93.3 | 512 | 6.7 | 7663 | 100.00 |
| Positive | 279 | 89.1 | 34 | 10.9 | 313 | 100.00 |
| Unknown | 3971 | 95.3 | 196 | 4.7 | 4167 | 100.00 |
| Total | 11,401 | 93.9 | 742 | 6.1 | 12,143 | 100.00 |
The HBsAg-positivity rate among children who did not receive the booster was 0.41%, and It did not increase with age (χ2 trend = 0.698, p = 0.403). The HBsAg-positive rate among children born to HBsAg-positive mothers, HBsAg-negative mothers, and HBsAg-unknown mothers was 2.15%, 0.31%, and 0.48%, respectively. Children’s HBsAg positivity did not increase with age (χ2 Trend = 0.012, p = 0.911; χ2 Trend = 1.744, p = 0.187; χ2 Trend = 0.395, p = 0.53) (Table 2).
Table 2.
HBsAg seropositivity among children without booster doses.
| Birth year | HBsAg( +) mother | HBsAg(–) mother | HBsAg(unknown) mother | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | + | % | N | + | % | N | + | % | N | + | % | |
| 2012–2013 | 102 | 1 | 0.98 | 2265 | 7 | 0.31 | 1200 | 5 | 0.42 | 3567 | 13 | 0.36 |
| 2010–2011 | 100 | 2 | 2.00 | 2697 | 9 | 0.33 | 1398 | 6 | 0.43 | 4195 | 17 | 0.41 |
| 2008–2009 | 33 | 0 | 0.00 | 829 | 2 | 0.24 | 567 | 2 | 0.35 | 1429 | 4 | 0.28 |
| 2006–2007 | 21 | 2 | 9.52 | 692 | 2 | 0.29 | 413 | 4 | 0.97 | 1126 | 8 | 0.71 |
| 2004–2005 | 10 | 1 | 10.00 | 270 | 0 | 0.00 | 167 | 1 | 0.60 | 447 | 2 | 0.45 |
| 2002–2003 | 13 | 0 | 0.00 | 398 | 2 | 0.50 | 226 | 1 | 0.44 | 637 | 3 | 0.47 |
| Total | 279 | 6 | 2.15 | 7151 | 22 | 0.31 | 3971 | 19 | 0.48 | 11,401 | 47 | 0.41 |
The anti-HBs positivity rate among children who did not receive the booster was 60.85%, and the rate declined with age (χ2 Trend = 555.37, p < 0.001). The anti-HBs positivity rate among children born to HBsAg-positive mothers, HBsAg-negative mothers, and HBsAg-unknown mothers was 69.18%, 61.71%, and 58.70% and also declined with age (χ2 Trend = 6.025, p = 0.014; χ2 Trend = 348.02, p < 0.001; χ2 Trend = 197.91, p < 0.001 (Table 3).
Table 3.
Anti-HBs seropositivity among children without booster doses.
| Birth year | HBsAg( +) mother | HBsAg(–) mother | HBsAg(unknown) mother | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | + | % | N | + | % | N | + | % | N | + | % | ||
| 2012–2013 | 102 | 80 | 78.43 | 2265 | 1729 | 76.34 | 1200 | 874 | 72.83 | 3567 | 2683 | 75.22 | |
| 2010–2011 | 100 | 70 | 70.00 | 2697 | 1661 | 61.59 | 1398 | 856 | 61.23 | 4195 | 2587 | 61.67 | |
| 2008–2009 | 33 | 17 | 51.52 | 829 | 413 | 49.82 | 567 | 266 | 46.91 | 1429 | 696 | 48.71 | |
| 2006–2007 | 21 | 11 | 52.38 | 692 | 311 | 44.94 | 413 | 168 | 40.68 | 1126 | 490 | 43.52 | |
| 2004–2005 | 10 | 6 | 60.00 | 270 | 120 | 44.44 | 167 | 73 | 43.71 | 447 | 199 | 44.52 | |
| 2002–2003 | 13 | 9 | 69.23 | 398 | 179 | 44.97 | 226 | 94 | 41.59 | 637 | 282 | 44.27 | |
| Total | 279 | 193 | 69.18 | 7151 | 4413 | 61.71 | 3971 | 2331 | 58.70 | 11,401 | 6937 | 60.85 | |
The anti-HBs positivity rate among children who received boosters was 88.41% and declined with age (χ2 Trend = 14.62, p < 0.001). The anti-HBs positivity rate among children born to HBsAg-positive mothers, HBsAg-negative mothers, and HBsAg-unknown mothers was 91.18%, 89.26%, and 85.71%, respectively (Table 4).
Table 4.
Anti-HBs seropositivity among children with booster doses.
| Birth year | HBsAg(+) mother | HBsAg(–) mother | HBsAg(unknown) mother | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | + | % | N | + | % | N | + | % | N | + | % | ||
| 2012–2013 | 5 | 5 | 100 | 69 | 62 | 89.86 | 20 | 20 | 100.00 | 94 | 87 | 92.55 | |
| 2010–2011 | 10 | 10 | 100 | 110 | 98 | 89.09 | 32 | 28 | 87.50 | 152 | 136 | 89.47 | |
| 2008–2009 | 5 | 4 | 80 | 83 | 80 | 96.39 | 34 | 29 | 85.29 | 122 | 113 | 92.62 | |
| 2006–2007 | 9 | 8 | 88.89 | 111 | 101 | 90.99 | 34 | 34 | 100.00 | 154 | 143 | 92.86 | |
| 2004–2005 | 0 | 0 | – | 56 | 52 | 92.86 | 22 | 17 | 77.27 | 78 | 69 | 88.46 | |
| 2002–2003 | 5 | 4 | 80 | 83 | 64 | 77.11 | 54 | 40 | 74.07 | 142 | 108 | 76.06 | |
| Total | 34 | 31 | 91.18 | 512 | 457 | 89.26 | 196 | 168 | 85.71 | 742 | 656 | 88.41 | |
None of the 742 children who received booster doses were positive to HBsAg. Among the 11,401 children who did not receive booster doses, the HBsAg positivity rate was 0.4%. There was no statistically significant difference in HBsAg positivity between the booster group and the no-booster group (χ2 = 3.1, P = 0.08). Regardless of the mother’s HBsAg status, there were no statistically significant differences in HBsAg positivity between the booster group and the no-booster group. Anti-HBs positivity in the booster-dose group was higher than in the no-booster group (Table 5).
Table 5.
Comparison of the HBsAg positive rate and anti-HBs positive rate between the booster group and the no booster group.
| Characteristics | HBsAg | Anti-HBs | |||||
|---|---|---|---|---|---|---|---|
| + | – | P | + | – | P | ||
| HBsAg( +) mother | Booster | 0 (0) | 34 (100) | P = 0.5(Fisher) | 31 (91.2) | 3 (8.8) | P = 0.007 |
| No booster | 6 (2.2) | 273 (97.8) | 193 (69.2) | 86 (30.8) | |||
| HBsAg(–) mother | Booster | 0 (0) | 512 (100) | P = 0.21 | 457 (89.3) | 55 (10.7) | P < 0.001 |
| No booster | 22 (0.3) | 7129 (99.7) | 4413 (61.7) | 2738 (38.3) | |||
| HBsAg(unknown) mother | Booster | 0 (0) | 196 (100) | P = 0.33 | 168 (85.7) | 28 (14.3) | P < 0.001 |
| No booster | 19 (0.5) | 3952 (99.5) | 2331 (58.7) | 1640 (41.3) | |||
| Total | Booster | 0 (0) | 742 (100) | P = 0.08 | 656 (88.4) | 86 (11.6) | P < 0.001 |
| No booster | 47 (0.4) | 11,354 (99.6) | 6937 (60.8) | 4464 (39.2) | |||
HepB1-OT and HepB3 coverage
From 2002 through 2013, 514,893,249 doses of 5 µg-HepB were administered to children in China. The mean HepB1-OT coverage was 86.7% (Range: 66.8–95.9%), and the mean HepB3 coverage was 96.2% (Range: 84.2–99.7%). Coverage increased year by year (Fig. 1).
Figure 1.
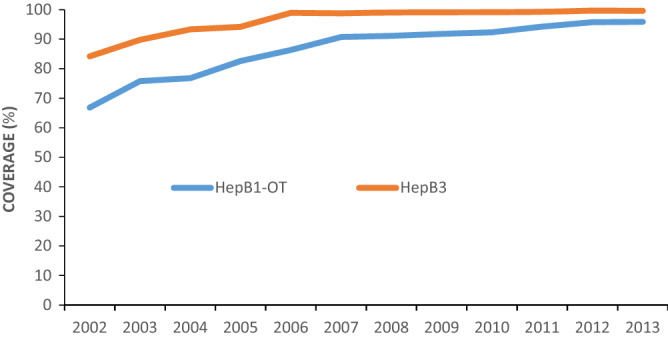
Annual HepB1-OT and HepB3 coverage in China, 2002–2013.
AHB incidence, ages six to twelve
Figure 2 shows the annual incidence of AHB by birth cohort during the years that the children birth cohorts were from 6 years old to up to 12 years of age. For example, one can see in the figure that the incidence of AHB did not increase with age among children born in 2002 from when they were 6 years old to when they were 12 years old (observation years 2008–2014; χ2 Trend = 0.273, p = 0.602). The χ2 trend test for the 2003 through 2006 birth cohorts also showed that there was no increasing trend of AHB (2003 χ2 trend = 0.238, p = 0.626; 2004 χ2 Trend = 0.164, p = 0.686; 2005 χ2 Trend = 0.322, p = 0.570; 2006 χ2 Trend = 0.79, p = 0.370).
Figure 2.
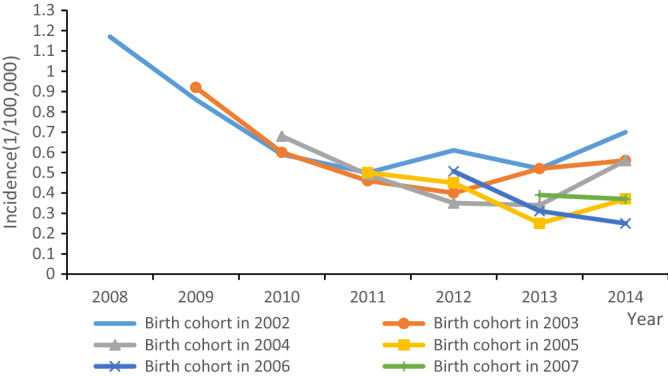
AHB incidence in the 2002 to 2007 birth cohorts.
Discussion
Our study showed that based on the 2014 nationwide hepatitis B serological survey, HBsAg seropositivity did not increase with age among children who received the hepatitis B vaccine birth dose and two more HepB doses during infancy. Additionally, national surveillance for AHB showed that the incidence of AHB did not increase among children between 6 and up to 12 years of age. We believe that this provides supportive evidence that protection from a 5 µg antigen HepB is maintained through childhood, indicating that booster doses of HepB are not indicated.
Our study also showed that the practice of screening for HBsAg and anti-HBs antibodies at kindergarten and primary school entry and offering HepB booster doses to children negative for both (e.g., uninfected children with no detectable anti-HBs) was associated with increases in anti-HBs seropositivity. That these children remained uninfected may show protection, but also may be related to immunity despite lack of antibodies or to a lack of a horizontal force of infection due to very little circulation of HBV due to the low endemicity of HBV among children (0.41%). We believe that our findings do not provide evidence in support of the screening and boosting strategy.
That anti-HBs concentrations declined year by year in boosted and non-boosted children was observed in other study9. Although anti-HBs positivity decreased with age, protection against HBV is associated with immune memory, which persists beyond the time when anti-HBs disappears10–12.
Whether a booster dose of HepB is necessary for children born to HBsAg-positive mothers remains controversial. Some research indicated that individuals who were born to HBsAg-positive mothers had significantly increased risk of developing chronic HBV infection in adulthood, in which case booster doses may be appropriate for children born to HBsAg-positive mothers13. However, a 30-year prospective observational study performed in Hong Kong that initially enrolled 1,086 infants born to HBsAg-positive mothers and vaccinated after birth, showed that none developed chronic infection after 3 years of age14. In our study, although the HBsAg positive rate (2.15%) was significantly higher among children born to HBsAg-positive mothers than among children born to HBsAg-negative or HBsAg-unknown mothers within 1–12 years after primary vaccination, the HBsAg positivity rate did not increase with age. Our findings, therefore, do not provide evidence supporting use of a booster dose of HepB for children born to HBsAg-positive mothers.
China’s AHB surveillance data showed that the incidence of AHB in children in the birth cohorts of 2002 to 2007 also did not increase with age. We believe that this was due to implementation of the national vaccination program for newborns using a 5 µg HepB vaccine. The Hep3 coverage rate was greater than 80% since 2002. Apparently, the persistence of immunity from the 5 µg HepB was protective.
Six percent of children in the study received booster doses. China’s Ministry of Health does not recommend booster vaccination of children who received 3-doses of the 5 µg recombinant yeast-grown vaccine. Among the children who received booster doses, the HBsAg positivity rate was zero. This was likely because children were voluntarily tested for hepatitis B markers before entering kindergarten and primary school, and only if HBsAg and anti-HBs were both negative, would a booster dose be given. HBsAg positive children would not receive a booster dose. As one would anticipate, the anti-HBs positive rate was significantly higher in the booster group than in the no-booster group, a finding shown by others15,16. There was no statistically significant difference in HBsAg positivity between the two groups. A study from Taiwan showed that booster vaccination against hepatitis B did not confer additional protection against becoming an HBsAg carrier17.
Strengths of the study are that the data were nationally-representative with large sample sizes, including for the 2014 sero-epidemiological survey. Limitations are that the longest follow-up available is 12 years and that the HBsAg status of mothers in the serological survey were from self-report, leading to the high proportion of unknown HBsAg status mothers.
This study showed that in spite of subsequent decline of detectable serum anti-HBs, a full primary course of 5 µg HepB conferred apparently complete protection against acute clinical disease—evidence supportive of not using a screening/booster dose strategy for all infants. This is different than for infants born to HBsAg positive women. The HBsAg positivity rate among women of childbearing age is still relatively high18,19, and infants born to HBsAg-positive mothers who are HBsAg negative with anti-HBs levels < 10 mIU/mL after having received a complete, 3-dose HepB vaccine series should be identified by post-vaccination serological 1–2 months after the third dose of HepB vaccine. Infants born to HBsAg positive women who did not respond to HepB should be revaccinated with a second 3-dose HepB vaccine series. Periodic serological surveys and AHB surveillance among children in the 2002 to 2012 birth cohorts should continue to be strengthened.
Supplementary information
Acknowledgements
The study was funded by the Major Science and Technology Special Project of China’s 13th 5-Year Plan (Grant Nos. 2018ZX10721202 and 2017ZX10105015) and Emerging and Re-emerging infectious Diseases program in the United States and China (Hepatitis B standardized monitoring project). Special thanks to Lance Rodewald, for his support in providing constructive comments and polishing the English of the manuscript.
Author contributions
N.M. and F.W. wrote the main manuscript text and H.Z. and X.S. prepared Figs. 1–2. G.Z. prepared Tables 1–4. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75338-5.
References
- 1.Global hepatitis report, 2017.https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- 2.Global health sector strategy on viral hepatitis 2016–2021.https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
- 3.Hadler SC, Fuqiang C, Averhoff F, Taylor T, Fuzhen W, Li L, Xiaofeng L, Weizhong Y. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine. 2013;31(Suppl 9):J66–72. doi: 10.1016/j.vaccine.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Hepatitis B questions and answers for health professionals. 2018 https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm. Accessed 29 October 2018.
- 5.FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccination: a completed schedule enough to control HBV lifelong? Milan, Italy, 17–18 November 2011. Vaccine. 2013;31(4):584–590. doi: 10.1016/j.vaccine.2012.10.101. [DOI] [PubMed] [Google Scholar]
- 6.Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35(46):6302–6307. doi: 10.1016/j.vaccine.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 7.Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, Twu SJ, Su IJ, Huang LM, Lee CY. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology (Baltimore, MD) 2004;40(6):1415–1420. doi: 10.1002/hep.20490. [DOI] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China; Chinese Center for Disease Control and Prevention. The national report of hepatitis B serosurvey of people aged 1–29 years in China in 2014. 1–154 (People’s Medical Publishing House, Beijing, 2018) (in Chinese).
- 9.Van Damme P. Long-term protection after hepatitis B vaccine. J. Infect. Dis. 2016;214(1):1–3. doi: 10.1093/infdis/jiv750. [DOI] [PubMed] [Google Scholar]
- 10.Hu YC, Yeh CC, Chen RY, Su CT, Wang WC, Bai CH, Chan CF, Su FH. Seroprevalence of hepatitis B virus in Taiwan 30 years after the commencement of the national vaccination program. PeerJ. 2018;6:e4297. doi: 10.7717/peerj.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu CL, Liu P, Chen T, Ni Z, Lu LL, Huang F, Lu J, Sun Z, Qu C. Presence of immune memory and immunity to hepatitis B virus in adults after neonatal hepatitis B vaccination. Vaccine. 2011;29(44):7835–7841. doi: 10.1016/j.vaccine.2011.07.098. [DOI] [PubMed] [Google Scholar]
- 12.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum. Vac. Immunother. 2012;8(7):896–904. doi: 10.4161/hv.19989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Chen T, Lu LL, Wang M, Wang D, Yao H, Fan C, Qi J, Zhang Y, Qu C. Adolescent booster with hepatitis B virus vaccines decreases HBV infection in high-risk adults. Vaccine. 2017;35(7):1064–1070. doi: 10.1016/j.vaccine.2016.12.062. [DOI] [PubMed] [Google Scholar]
- 14.Lin AW, Wong KH. Long-term protection of neonatal hepatitis B vaccination in a 30-year cohort in Hong Kong. J. Hepatol. 2013;59(6):1363–1364. doi: 10.1016/j.jhep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Bassal R, Markovich MP, Weil M, Shinar E, Carmeli Y, Dan M, Sofer D, Mendelson E, Cohen D, Shohat T. Prevalence of anti-hepatitis B surface antibodies among children and adolescents vaccinated in infancy and effect of booster dose administered within a pilot study. Epidemiol. Infect. 2017;145(14):2890–2895. doi: 10.1017/S0950268817002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J. Infect. Dis. 2016;214(1):16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 17.Chang YC, Wang JH, Chen YS, Lin JS, Cheng CF, Chu CH. Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study. BMC Public Health. 2014;14:991. doi: 10.1186/1471-2458-14-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang AL, Qiao YP, Wang LH, Fang LW, Wang F, Jin X, Qiu J, Wang XY, Wang Q, Wu JL, et al. Integrated prevention of mother-to-child transmission for human immunodeficiency virus, syphilis and hepatitis B virus in China. Bull. World Health Organ. 2015;93(1):52–56. doi: 10.2471/BLT.14.139626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Cui F, Gong X. Staus of the hepatitis B virus surface antigen and e antigen prevalence among reproductive women in China. Chin. J. Vac. Immun. 2010;16(6):496–499. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


