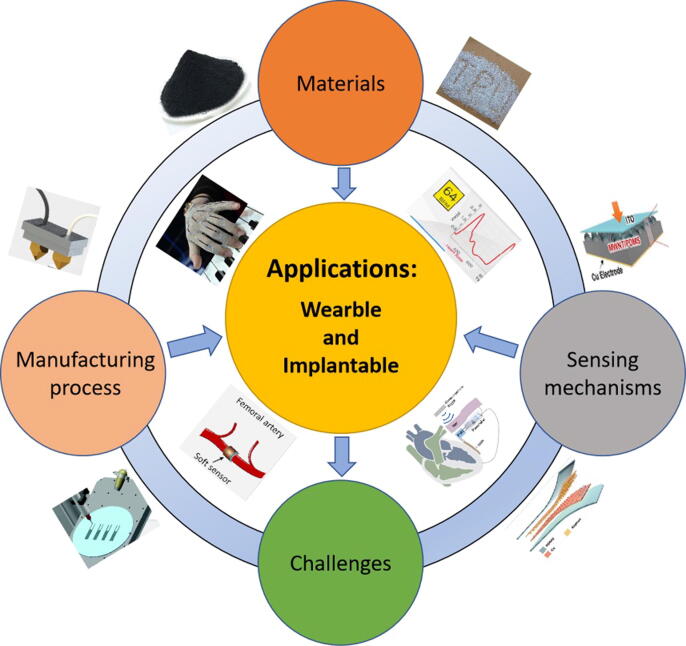
Graphical abstract
Keywords: Flexible force sensors, Health monitoring, Preventive medicine, Wearable, Implantable
Abstract
Background
In recent years, health monitoring systems (HMS) have aroused great interest due to their broad prospects in preventive medicine. As an important component of HMS, flexible force sensors (FFS) with high flexibility and stretch-ability can monitor vital health parameters and detect physical movements.
Aim of Review
In this review, the novel materials, the advanced additive manufacturing technologies, the selective sensing mechanisms and typical applications in both wearable and implantable HMS are discussed.
Key Scientific Concepts and important findings of Review
We recognized that the next generation of the FFS will have higher sensitivity, wider linear range as well as better durability, self-power supplied and multifunctional integrated. In conclusion, the FFS will provide powerful socioeconomic benefits and improve people's quality of life in the future.
Introduction
With the aging of the global population and the increase of aging-related diseases, there is a strong demand for personalized health care [1]. Preventive and personalized medical approaches can detect, diagnose and predict disease at their early stage [2], which will increase the cure rate, reduce the treatment costs and improve quality of life [3]. Many household medical devices such as electronic sphygmomanometers, portable electrocardiogram (ECG) monitors and blood glucose meters have been developed and widely used [4]. However, they are usually bulky, rigid, unwearable and unable to achieve multi-functionality and real-time detection. In recent years, the advancement of flexible electronic technology has promoted the development of regular and continuous healthcare monitoring [5]. As a human-interactive health monitoring device, flexible sensor (both wearable and implantable) can detect and measure various biological signals. Force sensors that convert mechanical forces such as pressure, tension, stress and strain into electrical signals are of great importance among the various types of flexible sensors [6]. Taking advantage of the force sensors, many mechanical stimuli generated by the human body, including muscle movement, blood pressure, pulse, etc. contain important health parameter signals, which can be easily captured by flexible force sensors [7]. Therefore, considerable efforts have been made in developing novel sensing materials, creative macrostructure, and rapid manufacturing strategies of flexible force sensors.
In this review, we will give an overview of the application of flexible force sensors in health monitoring. We first introduce the most commonly used materials for force sensors. We then discuss the recent additive manufacturing technologies used for fabrication of flexible force sensors. Next, we explore the sensing mechanisms allowing detection of mechanical force from physical activities. Having a comprehensive understanding on the design strategies, we present applications of flexible force sensors in the field of wearable and implantable health monitoring. Lastly, we outline the challenges currently faced by flexible force sensors and their prospects for the direction of development in the coming years. The structure of this review and the relations between each part are shown in Fig. 1.
Fig. 1.
Illustration of the structure of this review.
Materials
The materials used to fabricate flexible force sensors can be mainly divided into three categories: carbon-based materials, metal materials and polymer materials [8]. These three types of materials will be described in detail below.
Carbon-based materials
Carbon-based materials are widely used to manufacture flexible force sensors owing to their excellent electrical conductivity, versatile nanostructures and good biocompatibility. Carbon-based materials used in the manufacturing of flexible force sensors mainly include carbon black, carbon nanotubes (CNTs), graphene and graphene oxide (GO) [8], they are usually added to polymers to form conductive composites.
Carbon black
Carbon black (CB) belongs to an amorphous form of carbon, its structure is similar to disordered graphite [9]. It is a kind of low-cost conductive nanoparticles. By filling this particle in the elastic matrix, the mechanical strength, electrical conductivity of the composite materials will be enhanced [10]. Shintake et al. [10] used the layered casting method to cast the Ecoflex-CB conductive composite slurry into the silicone elastomer Ecoflex substrate, and then cured it through an oven. The produced Ecoflex-CB-based stretchable strain sensor showed high sensitivity (the range of gauge factor was approximately 1.62 ∼ 3.37), good stretch-ability (up to 500%), repeatability (cycle > 10000) and maintained good linearity and low hysteresis. Similarly, in order to obtain a piezoresistive sensor with high sensitivity and wide sensing range, Wang et al. [11] proposed to construct a piezoresistive sensor with a layered porous sensing architecture. They uniformly filled carbon black (CB) nanoparticles and NaCl in the thermoplastic polyurethane (TPU), the printed sensor was immersed in the solution to remove NaCl, and finally formed a multi-layer layered aperture sensing layer. Thanks to the filling of CB and the formation of multi-level layered aperture. The sensor had a sensitivity of 5.54 kPa−1, showing a wide pressure sensing range (10 Pa to 800 kPa) and excellent repeatability. Compared with other carbon-based materials, carbon black has a lower cost and good electrical conductivity, it will become a powerful material candidate in the field of wearable sensors.
Carbon nanotubes
Carbon nanotubes (CNTs) are allotropes of carbon, it can be envisioned as a single-layer or multi-layer graphene sheet rolled into a cylindrical shape [12]. Since multi-walled carbon nanotubes [13] (MWCNTs) and single-walled carbon nanotubes [14], [15] (SWCNTs) were reported in 1991 and 1993, CNTs research has expanded aggressively. CNTs have aroused great interest due to their low cost, extensible synthesis and excellent chemical stability [16]. Abshirini et al. [17] manufactured a strain sensor based on MWCNT/PDMS. The strain sensor exhibited high gauge factor (average GF of 4.3), large strain coefficient (30% maximum strain) and good stability (300 load-unload cycles) at low strain rates. Compared with MWCNT, SWCNT has a higher specific surface area, strength and uniformity. Gilshteyn et al. [18] used dry-transfer manufacturing method to produce a SWCNT/PDMS-based thin film strain sensor. Compared with the previously reported wet process, the sensor had higher transparency, conductivity and sensitivity (GF = 20.1 at 100% strain). Thanks to the strain variation of inter-tube tunneling resistance, carbon nanotubes have excellent strain sensing ability in piezoresistive composites. However, their defect is lack of durability. After hundreds of deformation cycles, their conductivity will decrease, which is caused by macroscopic cracks and peeling.
Graphene and graphene oxide
Graphene is a 2D carbon nanomaterial with atomic thickness, which has excellent electrical, optical, mechanical, thermal conductivity properties and extremely high specific surface area [19]. However, graphene is extremely difficult to handle for fabrication because there are no hydrophilic functional groups over its 2D plane structure. Graphene oxide (GO), derived from graphene, has a variety of oxygen-containing functional groups [20]. GO has unique viscoelasticity in aqueous solution, the high-concentration GO solution dissolved by ultrasound shows high viscosity and has good printability. Wang et al. [21] fabricated a flexible resistive strain sensor with a 3D conductive network structure. The sensor used an ultrasonic-induced method to uniformly distribute reduced graphene oxide (rGO) in TPU and form conductive paths. Thanks to the layered conductive network, the rGO/TPU strain sensor showed high sensitivity (the gauge factor was 11 under 10% strain), good repeatability (6000 cycles of tensile-release test) and fast response speed. Although a higher GF was obtained, its GF value was fixed under a certain strain range, and it cannot be adjusted according to different application environments. Wang et al. [22] proposed the idea ofadjusting the sensitivity of the sensor by precisely controlling the microstructure of the sensor (the specific microstructure is hexagon, triangle and grid structure), and produced a graphene/PDMS composite strain sensor with a porous structure. The sensitivity of the graphene/PDMS composite strain sensor can be adjusted in the range of 6–67 by changing the microstructure at a strain of 20%. This strain sensor with adjustable sensitivity can be widely used in the field of wearable health monitoring systems by adjusting the microstructure to adapt to the stress distribution of different body parts.
Metal materials
Metal is the most commonly used conductive material in flexible force sensors. The metal materials that have been used for FFS fabrication includes copper (Cu), silver (Ag), gold (Au), molybdenum (Mo), magnesium (Mg), zinc (Zn), aluminum (Al), Chromium (Cr), Nickel (Ni), titanium (Ti), etc. [23] And they are widely used in the forms of metal films, metal nanomaterials, liquid metals, metal oxides and Mxenes.
Metal film and metal nanomaterials
Metal films include gold film, silver film, copper film, zinc film, aluminum film, etc. Except the good conductivity, some metal films also have anti-corrosion and anti-interference functions. Hogas et al. [24] proposed a strain sensor manufacturing strategy that combines electrospinning and thin film deposition technology. Polymer nanofibers were first deposited onto a plastic carrier by electrospinning, and then metal films were deposited onto the nanofibers by thermal evaporation to obtain fine metal fibers. It was proved that the strain sensor had high sensitivity, but had relatively low signal noise ratio, so metal films are often used as electrode layers of flexible force sensors.
Metal nanomaterials include metal nanoparticles, metal nanowires, and metal nanosheets. In order to meet requirements such as flexibility, extensibility and transparency, metal nanomaterials of different geometries have been tested [23]. Among these metal nanomaterials, metal nanowires have attracted much attention due to their small diameter and high aspect ratio. In addition, thanks to the effect of nanoscale size, they also show excellent light transmittance and flexibility. Compared with metal nanoparticles as fillers, metal nanowires have very low loading capacity and are easy to form percolation channels [25]. Among the metal nanowires, the copper nanowires (CuNWs) [26], the gold nanowires (AuNWs) [27] and the silver nanowires (AgNWs) [28] show the desired properties of high stretchablity and conductivity. However, the copper nanowires are cheap but could be easily oxidized, and the gold nanowires have excellent properties but are expensive. The silver nanowires have excellent electrical conductivity and antibacterial properties, so they are widely used.
Dong et al. [29] developed a super-elastic piezoresistive sensor based on a composite conductive network and layered structure. The sensor was built based on a layered structure of natural sponge, and 2D reduced graphene oxide (rGO) and 1D AgNWs were used to form a cooperative conductive network. Polydopamine (pDA) was used both as a tackifier and a green reducing agent. The sensitivity of the piezoresistive sensor under the pressure range of 0–40 kPa was 0.016 kPa−1, and the gauge factor under the 0–60% strain range was 1.5. The sensor also showed good repeatability (>7000 load-unload cycles) and fast response speed (<54 ms). Because AgNW with high aspect ratio may be entangled with each other when stressed, the structure of the sponge is also relatively bulky, so its sensitivity is relatively low. Kwon et al. [30] produced a multi-axis stretchable resistance sensor based on nanocomposites. The sensor used polyurethane (PU) as an elastic substrate, while graphene nanosheets (GNPs) and silver nanoparticles (AgNPs) were used to build a collaborative conductive network. Benefit from the choice of two-dimensional nano-conductive materials and elastic matrix, the sensor exhibited high sensitivity (GF = 21.2 at 50% strain, GF = 30.2 at 100% strain, GF = 48.2 at 160% maximum strain), excellent stability (500 cycles) and fast response time (<72 ms). The review above shows that the combination of nano-metallic materials and carbon-based materials is very common, this is because the adhesion between nano-metallic materials and polymers is relatively weak, the addition of carbon-based materials will make them combined closer.
Liquid metals
Liquid metal is a kind of amorphous metal material that is liquid at room temperature, it has the highest consistency among all conductive materials, so it is very suitable for direct ink printing at room temperature. Eutectic gallium indium (EGaIn) and mercury (Hg) are the most commonly used liquid metal materials [31]. Mercury is a natural elemental liquid metal, which is not suitable for sensor manufacturing due to its high toxicity and harm to human health. However, EGaIn has been widely used in manufacturing flexible electronic components because of its biocompatibility [32]. Ali et al. [33] filled EGaIn liquid metal into PDMS with corona discharge method to build electrode channels of the sensor. After several sets of tests, the linear relationship of the average capacitance change was obtained. The sensitivity of the capacitive sensor was 0.11% MPa−1, and the gauge factor was 0.9975. Liquid metal will be widely used in the fields of wearable electronics, and biomedical due to its excellent resistivity, flexibility, fluidity and biocompatibility.
Metal oxides
Thanks to the high specific surface area, the capacity of metal oxides with microstructure and nanostructure morphology are two or three times higher than the carbon/graphite based materials [34], [35]. Meanwhile, the nano metal oxides have the advantages of good biocompatibility, rapid response speed and durability under different working conditions. Lee et al. [36] produced a highly durable textile-based wireless flexible strain sensor. Mixtures of RGO and CNTs were coated on PET textiles as the conductive part. The zinc oxide nanowires (ZnO NWs) were integrated into RGO and let grow freely. By controlling the growth time to change the aspect ratio (AR) of ZnO NWs, it was found that the GF of textile sensors based on ZnO-0 (without growth) was about 0.96, while the GF of textile sensors based on ZnO-3 (growth 3 h) was about 7.64, which was 1.67 times compared to the PET film (GF≈4.57). With the free growth of ZnO on RGO, the GF value has been greatly improved, showing that ZnO makes a great contribution to sensitivity. Inspired from this work, we can spray it on a large area of textiles and monitor the bending strain of the human body through wireless communication to obtain the health status of the human body.
Mxenes
MXenes, a graphene-like 2D conductive material composed of metal carbides and carbonitride, can be obtained by selective etching of a 3D layered compound MAX phase with a strong acid or a strong base. The chemical formula of MXenes is Mn+1Xn (where n = 1, 2, 3; M are early transition metal elements and X is carbon or nitrogen) [37]. Recently, flexible force sensors based on MXenes have been extensively studied. Sobolciak et al. [38] produced a electrospinning pads piezoresistive sensor based on MXene modified. They combined Ti3C2Tx into copolyamide (CO-PA) nanofibers (aspect ratio AR = 50) by means of electrospinning. The GF of the manufactured sensor could reach 4.5. MXenes has excellent conductivity, oxidation resistance and mechanical flexibility, which makes it widely used in the field of flexible electronics, but how to achieve fluorine-free preparation and expand its linear sensing range are still problems we need to consider.
Polymer materials
Polymer materials are often used as supporting materials for sensors. Many polymer matrix materials have been exemplified. Common flexible polymer materials include polydimethylsiloxane (PDMS) [39],polyimide (PI) [40], polyurethane (PU) [41], polyethylene terephthalate (PET) [42], polyvinylidene fluoride (PVDF) [43], parylene [44], polyethylene naphthalate (PEN) [45], etc. These flexible matrix materials are widely used due to their high tensile strength, good thermal conductivity, chemical stability, and ease of compounding with conductive materials [46].
The method of filling conductive active materials into an elastic polymer matrix is mostly used to manufacture resistive sensors and active electrodes. Table 1 summarizes the main characteristic parameters of resistive mechanical sensors based on different fillers and elastic substrates.
Table 1.
Summary of main characteristic parameters of resistive mechanical sensors based on different fillers and elastic substrates.
| Active fillers | Substrate | Sensitivity/GF | Response time | Stretchability/detection range | Reference |
|---|---|---|---|---|---|
| CB | Ecoflex | 1.62 ∼ 3.37 | / | 50%∼500% | [10] |
| CB and NaCl | TPU | 5.54 kPa−1 | / | 10 Pa ∼ 800 kPa | [11] |
| SWCNT | PDMS | 20.1 at 100% strain | / | 10%∼100% | [18] |
| MWCNT | PDMS | 4.3 | / | 0 ∼ 30% | [17] |
| MWCNT | TPU | 1.5–3 | / | 0 ∼ 50% | [47] |
| RGO | TPU | 10% strain: 11 100% strain:79 | 200 ms | 0 ∼ 200% | [21] |
| RGO | PDMS | 25.1 kPa –1 | / | 0 ∼ 2.6 kPa | [48] |
| Graphene | PDMS | 6 ∼ 67 | / | 50%∼60% | [22] |
| Graphene | PDMS | 1.2 kPa−1 | / | 5 Pa ∼ 25 kPa | [49] |
| metal films | Polymer nanofibers | 2 ∼ 200 | / | / | [24] |
| RGO and AgNWs | sponge | S = 0.016 kPa−1; GF = 1.5 | <54 ms | 0 ∼ 40 kPa; 0 ∼ 60% | [29] |
| GNPs and AgNPs | PU | GF = 21.2 at 50% strain; 30.2 at 100% strain; 48.2 at 160% strain | <72 ms | 0 ∼ 160% | [30] |
| ZnO NWs, RGO and CNTs | PET | 7.64 | / | 0 ∼ 40% | [36] |
| Ti3C2Tx | Copolyamide | 4.5 | / | / | [38] |
| EGaIn | Silicone (Dragon Skin 30) | 2.5 | / | 0 ∼ 100% | [50] |
| HEC/SPI/PANI | sponge | −0.29 | <140 ms | 0–345.25 kPa | [51] |
Manufacturing process of sensor
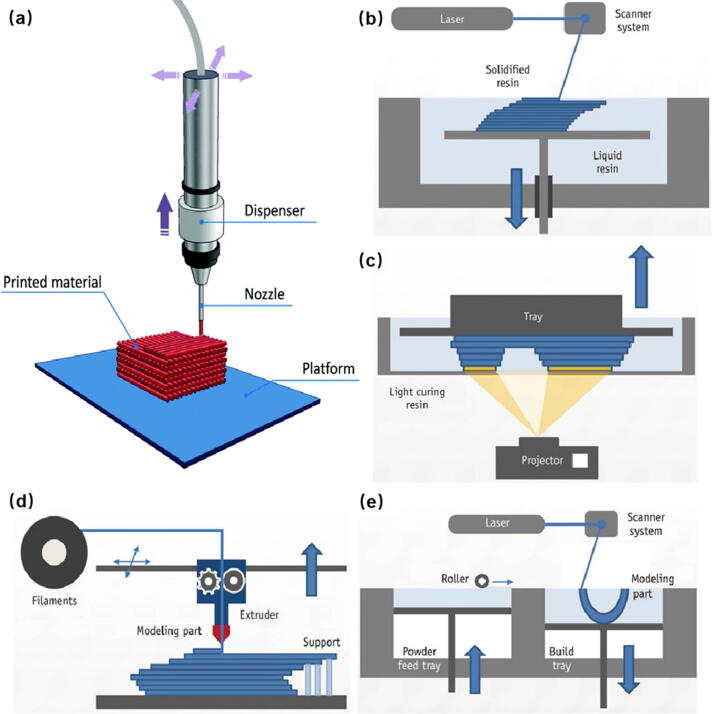
In recent years, as traditional manufacturing methods cannot meet people's needs for personalized and complex structures, 3D printing (3DP) technology, as an advanced additive manufacturing technology, is used to manufacture physical objects layer by layer from computer models [52], bringing unparalleled flexibility and simplicity to the manufacture of highly complex 3D objects. The typical 3D printing technologies applied to manufacture flexible force sensors mainly include direct ink writing (DIW), stereolithography (SLA), digital light processing (DLP), fused deposition modeling (FDM), and selective laser sintering (SLS) (Fig. 2) [53]. Each 3D printing technology has its own unique advantages and limitations (Table 2). In this section, we will discuss the working principles, advantages and disadvantages of these 3D printing strategies used for FFS fabrication.
Fig. 2.
The schematic of (a) DIW [54], (b) SLA, (c) DLP, (d) FDM and (e) SLS [55].
Table 2.
Summary of the advantages and disadvantages of different 3D printing technologies.
| 3D printing technology | Material types | Advantages | Disadvantages |
|---|---|---|---|
| DIW | Liquid inks | Low cost and easy construction; Applicability of multi-material printing | Relatively low printing speed and resolution; Difficult to control the viscosity of ink |
| SLA/DLP | Liquid photosensitive polymers | High resolution and rapid prototyping | Limited materials and complex devices; Material waste |
| FDM | Filaments | Low cost and simple operation; | Low resolution and slow print speed; Need high temperature processing |
| SLS | powders | Wide selection of materials; Good mechanical properties | Complex devices and shrinkage after cooling |
Direct ink writing (DIW)
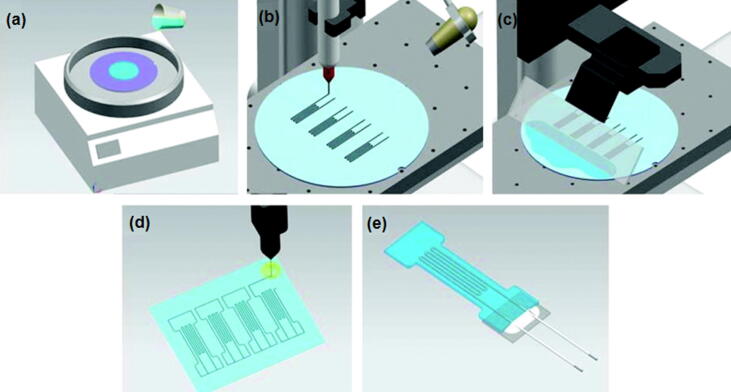
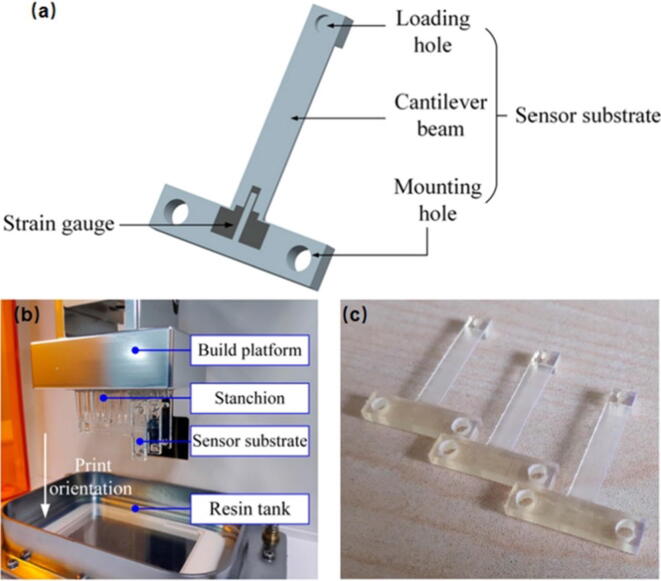
DIW is a kind of extrusion-based 3D printing technology. Owing to the shear thinning characteristics, the paste-like ink can deposit quickly upon squeezed from the nozzle, 3D objects are assembled layer by layer with uniform filaments during the deposition process (Fig. 2a). Most applicable materials including carbon-based materials, metals and polymers can be prescribed to use this printing technology for FFS fabrication. To use this technology, the ink must possess enough mechanical strength to maintain its shape after extrusion. The viscosity of the ink should be well controlled to avoid possible shape collapse and nozzle clogging during extrusion [52]. Kim et al. [50] reported a method for manufacturing eutectic gallium indium (EGaIn) soft sensors based on DIW technology (Fig. 3). They performed a series of tests on their performance and came to the conclusion that the DIW-based EGaIn strain sensors had reliable sensor signals at different loading speeds, little hysteresis effect, and moderate resistance up to 100% strain. In addition, the sensor was not broken up to 10,000 cycles under strain below 100%. Except for low cost and easy construction, DIW also has the capability of multi-material deposition which is suitable for the integrated forming of flexible substrate, sensitive and electrode layer.
Fig. 3.
Manufacturing process of EGaIn soft sensor based on DIW: (a) spin-coat the silicone layer on the wafer; (b) writing EGaIn on the silicone layer; (c) spreading uncured silicone material on the printed trace by rod coating; (d) trimming the silicone body into a desired shape by laser cutting, and (e) direct insertion and fixation of electrodes [50].
Stereolithography (SLA) and digital light processing (DLP)
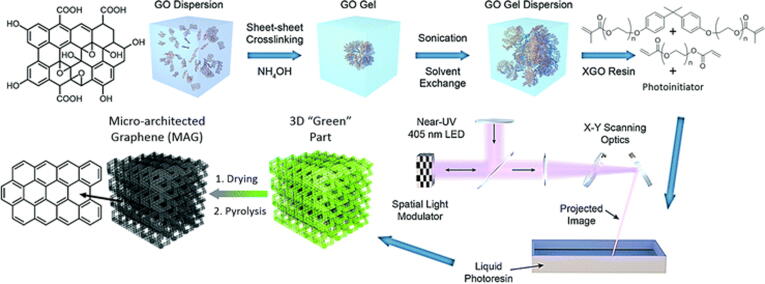
Photopolymerization based 3D printing process mainly includes stereolithography (SLA) and digital light processing (DLP) (Fig. 2b and Fig. 2c respectively). SLA typically use one or more discrete focused laser beams to solidify thin layers of a liquid photosensitive polymer resin to form 3D objects layer by layer. Hensleigh et al. [56] showed an effective way to manufacture 3D micro-architected graphene aerogel (Fig. 4), which will be used to fabricate electrodes for FFS. They designed and synthesized photocurable graphene oxide (GO) resins, which can be sequentially patterned using projection micro stereolithography (PμSL) technology to build complex layered 3D micro-architected graphene (MAG) components. The experimental results show that the 3D spatial feature size of MAG is about 10 µm, and the pore size of the strut microstructure is about 60 nm. In addition, MAG showed high surface area (130 m2·g−1) and high electrical conductivity (64 S·m−1). Compared with extrusion-based 3D printing technology, SLA technology can achieve higher resolution and faster printing speed.
Fig. 4.
Schematic diagram of the manufacturing process of micro-architected graphene (MAG) [56].
DLP uses mirror arrays and masks in a digital light processing system to expose and light cure a complete resin layer to form a 3D object, which greatly increases the productivity [53]. Liu et al. [57] produced a force sensor with a composite structure (Fig. 5a and Fig. 5c). The manufacture of the sensor used DLP and inkjet printing technology (Fig. 5b). They use inkjet printing to deposit poly (3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT/PSS) on the cured photosensitive resin to form the sensor. The sensor had a sensitivity of 2.92% N−1 and a GF of 0.98 under the range of 0–160 mN. Since the polymerization method of SLA technology is spot-wise, while DLP technology allows one layer of surface to be cured at a time, so DLP technology has a faster printing speed compared to SLA. With the development of the technology, DLP has exceeded the printing accuracy of SLA. However, DLP is only suitable for printing small parts due to the limited resolution of digital light mirrors. For printing large parts, SLA is still a better choice than DLP. In addition, due to the use of photosensitive polymer materials, both SLA and DLP face problems such as residual chemical waste and the potential cytotoxicity of light initiator [58].
Fig. 5.
(a) Structure diagram of the sensor (b) DLP-based desktop 3D printer (c) Optical photo of the sensor after UV curing [57].
Fused deposition modeling (FDM)
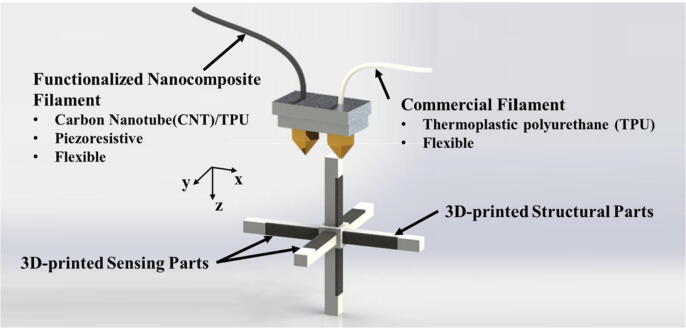
FDM is an extremely common 3D printing technology that melts and extrudes thermoplastic materials (filaments or particles) to form the final 3D object (Fig. 2d). This technology can be easily applied to a variety of composite materials by adding different fillers to the filaments [52]. Kim et al. [59] developed a 3D multiaxial force sensor based on FDM-based functionalized nanocomposite filaments (Fig. 6). The sensor had a unique 3D cube cross structure that enables force sensing from three axes (x, y, and z). The support structure and sensing layer of the sensor were made by melt extrusion of TPU filament and CNT/TPU nanocomposite filament respectively. The experiment result showed that when a force of 4 N was applied in the Z-axis direction of the sensor, the resistance of Ry and Rz decreased by 0.2% and 2% respectively. Due to its properties of simple operation and low costs, FDM has been widely used. However, there is a common contradiction between the printing precision and printing speed in this technology. In other words, if a faster printing speed is required, its resolution will be sacrificed [58].
Fig. 6.
3D printed schematic of multi-axis force sensor based on FDM [59].
Selective laser sintering (SLS)
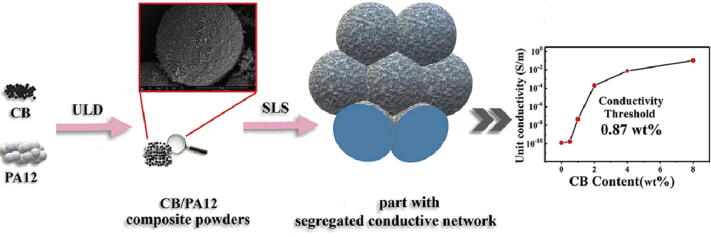
SLS is a powder-based 3D printing technology that uses moderate to highenergy lasers to sinter continuous thin layers of powder to produce the 3D object in layers (Fig. 2e) [52]. The laser selectively scans over the top of the power bed, the powder gets melt and solidified with the powder bed moving down one layer thickness each time, then another layer of powder is spread on the top of the power bed, this process in repeated until the 3D object is formed [58]. SLS can not only use metal powder particles, but also carbon-based powders, ceramics and polymers, or a combination of them [60]. For example, Hong et al. [61] mixed nano carbon black powder with polyamide 12 (PA12) powder, and successfully manufactured CB/PA12 composite parts based on SLS process (Fig. 7). Due to the formation of a 3D segregated conductive network, the composite component had an ultra-low conductive penetration threshold (0.87 wt%). SLS has a wide selection of materials, high material utilization, and the remaining powder can still be used. However, this technology requires complicated equipment, in addition, rapid cooling after high-temperature sintering may cause shrinkage and deformation of the printed parts.
Fig. 7.
Manufacturing process of the CB /PA12 composite parts [61].
Sensing mechanisms
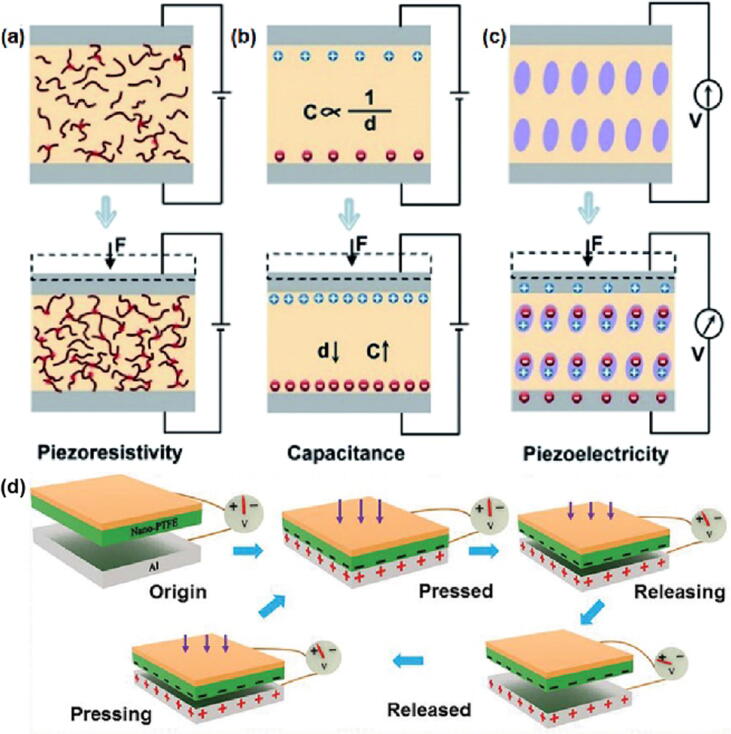
Human health status can be obtained by monitoring mechanical stimulation of human physiological activities through sensors. Mechanical stimuli including tension, pressure, torque, vibration, stress and strain, etc., can be converted into electrical parameters by flexible force-sensitive sensors through several representative sensing mechanisms [7]. According to the working mechanism, the flexible force-sensitive sensors can be divided into four typical types: resistive, piezoelectric, capacitive and triboelectric sensors [62] (Fig. 8).
Fig. 8.
The working principle of (a) resistive, (b) capacitive, (c) piezoelectric [63] and (d) triboelectric [64] sensors.
Resistive sensors
Resistive sensors can convert the pressure change into the resistance change. When the sensor is deformed under stress, the contact area of the conductive dielectric in the sensor changes, resulting in the change of its conductive channel, thus causing the resistance change [65] (Fig. 8a). According to different endurance signals, resistive sensors can be divided into strain type and piezoresistive type, which correspond to tension and pressure signals respectively. Due to biocompatibility and stability issues, most piezoresistive sensors used in human health monitoring are wearable devices, and there are relatively few reports on piezoresistive sensors in vivo. Wu et al. [51] developed an implantable piezoresistive sensor. They uniformly dispersed natural biocompatible conductive composite materials, including hydroxyethyl cellulose (HEC), soybean protein isolate (SPI) and polyaniline (PANI), in a natural sponge to form an HSPS piezoresistive sensor. The HSPS sensor exhibited a GF of −0.29, fast response speed (<0.14 s), excellent repeatability and stability. The resistive sensor has attracted much attention due to its simple structure, low cost, easy integration and relatively easy signal acquisition. But its working stability and power consumption are still issues we need to consider [66].
Capacitive sensors
Capacitive sensors are sensors that convert pressure change into capacitance change [67]. For a plate capacitor, the capacitance will change when the distance of the two plates get shorten under external force [68] (Fig. 8b). Yang et al. [69] developed a 3D micro-conformal graphene electrode with controllable microstructure and perfect shape retention. They sandwiched PDMS between the graphene electrode and the bottom electrode to make a capacitive sensor. The sensor showed high sensitivity (3.19 kPa−1) and fast response speed (30 ms). In addition, by adjusting the characteristic size of graphene or using double-layer graphene electrodes, the sensing capability of the capacitive sensor would be greatly improved. For example, a capacitive sensor using symmetric double micro-conformal graphene electrodes successfully achieved 7.68 kPa−1 sensitivity. Capacitive sensors are suitable for detecting small deflection changes, it has the merits of high spatial resolution and sensitivity, good frequency response, low power consumption and large dynamic range. However, problems such as junction capacitance and electromagnetic interference need to be resolved.
Piezoelectric sensors
Piezoelectric sensors use the piezoelectric characteristics of piezoelectric materials to convert the applied pressure into voltage signal [70]. This piezoelectric behavior is caused by the existence of an electric dipole moment. When the piezoelectric material is affected by the external force or external deformation in a certain direction, it produces electric polarization, resulting in opposite bound charges on the two surfaces. After the external force was removed, the polarization disappeared, forming the voltage difference (Fig. 8c) [71]. Piezoelectric sensors are widely used in the detection of dynamic pressure signals, but have encountered challenges in the monitoring of static pressure signals. Chen et al. [72] proposed a piezoelectric sensor with a heterostructure based on PbTiO3 nanowire/graphene, which was used for the piezoelectric induction of static measurement. Its working principle is that the strain-induced polarons in the piezoelectric PbTiO3 nanowires as charged impurities affect the carrier mobility of graphene, thereby achieving static piezoelectric induction. The sensor exhibited high sensitivity (9.4 × 10-3 kPa−1) and fast response speed (5–7 ms). Piezoelectric sensors have high sensitivity, and response speed and spontaneous generation of electrical signal. Meanwhile, they consume small amount of power and can even achieve self-supply [73]. However, because piezoelectric materials have certain pyroelectric properties, the polarization phenomenon is greatly affected by temperature, and the signal output is easy to drift with time [74].
Triboelectric sensors
Triboelectric sensors will generate charge after friction or contact under pressure. After release, the sensors are separated from each other to generate voltage difference, thus converting mechanical signals into electrical signal (Fig. 8d). Similar to piezoelectric sensors, triboelectric sensors produce electrical signals only when they are in contact and separated. Therefore, most triboelectric sensors are more suitable for dynamic sensing. The triboelectric nanogenerator (TENG) is a key part of triboelectric sensors, its working mechanism is the combination of triboelectric and electrostatic induction [75]. Currently, TENG have been widely studied and applied in low-power power sources and self-powered force sensors [76]. Liu et al. [64] reported a small, flexible and self-powered endocardial pressure sensor (SEPS) based on TENG, this sensor could convert blood flow energy in the heart cavity into electrical energy. SEPS were implanted into the left ventricle and atrium of pigs respectively. Experimental results showed that SEPS achieved excellent linearity (R2 = 0.997), super sensitivity (1.195 mV·mmHg−1) and maintained good response as well as mechanical stability in the body. Because TENG-based triboelectric sensors have the merits of low cost, diverse structure, stable power output and high energy conversion rate, it will have broad application prospects in the field of wearable and implantable electronic devices. However, triboelectric sensors based on TENG also face challenges such as biocompatibility, biodegradability, service life, miniaturization and integration.
Applications
With the advance of materials, processes and sensing technologies, various flexible force sensors are widely used in health monitoring systems. According to the working environment of these flexible force sensors, they can be divided into two categories: wearable and implantable sensors. Here, we will introduce some representative examples of flexible force sensors for health monitoring, which will greatly improve the development of preventive medicine business. Table 3 lists various physiological parameters monitored by flexible force sensors attached to different positions of the human body, and points out their possible application scenarios in health monitoring.
Table 3.
Applications of flexible force sensors in personal health monitoring.
| In vitro/in vivo | Position of human body | Measured parameter | Possible applications in human health monitoring |
|---|---|---|---|
| In vitro | Hand | motion | Monitoring hand recovery |
| Wrist | Pulse | Diagnose cardiovascular disease | |
| Upper arm | blood pressure | Hypertensive treatment | |
| Knee and elbow | Strain distribution | Postoperative rehabilitation; detection of posture and movement | |
| Foot | Pressure distribution | Gait detection and sport training | |
| Throat | Sound vibration | Voice recognition; diagnosing damaged vocal cords | |
| Face | Muscle movement | Facial expression recognition; diagnosis of facial paralysis | |
| in vivo | Cardiovascular | ECG | Prevent and treat cardiovascular diseases |
| Heart | Heart rhythm | Cardiac pacing and correction of sinus arrhythmia | |
| Bone | pressure | Treat osteoporosis and fractures; promote cell proliferation and differentiation; nerve tissue repair |
Wearable area
Wearable sensors usually attach sensors to the surface of the human body and assess the health status of human by monitoring various physiological parameters. In health assessment, the indicators to be detected can be divided into the following categories: (1) physical movements: including hands, arms, knees, etc. [23] and (2) vital signs: including heart rate, pulse, blood pressure, breathing, etc. [4].
Body movements monitoring
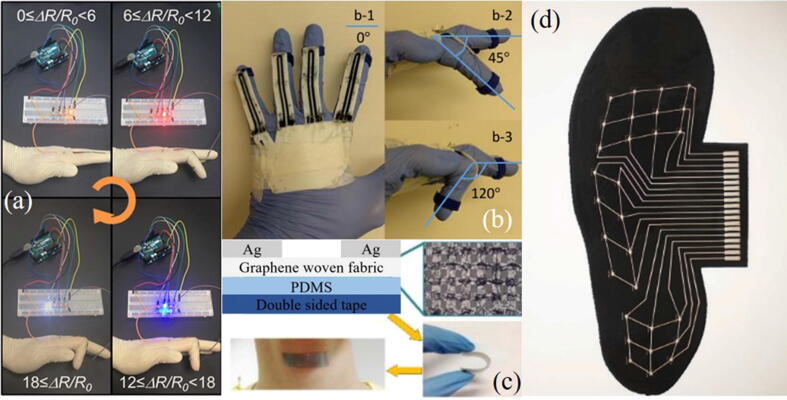
Body movement is an indispensable part of our daily life. Our bodies may suffer injuries such as fractures, torn ligaments, and atrophy of facial muscles in daily life. After surgery, if the patient lacks the appropriate medical equipment to monitor the affected area, it may cause secondary damage or even permanent damage to the healing site. Therefore, some researchers focus on developing flexible sensors for monitoring body movements. For example, Wajahat et al. attached a flexible sensor on the surface of the finger (Fig. 9a) [77] or integrated it on a glove-like device (Fig. 9b) [47]. When the finger was bent, straightened or coordinated moved with the fingers, the deformation of sensor would cause changes in the internal resistance or other electrical parameters. The changes can monitor the movement of the finger and can be used for the diagnosis of Parkinson disease and later rehabilitation monitoring.
Fig. 9.
(a) The change of the resistance of the wearable sensor under different bending degrees of the finger, that is, the change of the brightness of the LED [77]; (b) Schematic diagram of the change in the extension of the glove-type wearable sensor [47];(c) The sensor collecting throat muscle movement signals [81];(d) The image of the plantar sensor array [83].
Zhang et al. [78] synthesized a multifunctional conductive hydrogel with the polyacrylic acid, dopamine-functionalized hyaluronic acid and Fe3+. The substrate exhibited mechanically and electrically repeatable, thermoplastic and self-healing properties (98% recovery within 2 s). Taking advantage of the hydrogen bonding and metal coordination interactions between Fe3+. This new material can be used to detect different degrees of body movements (from breathing to knee flexion). In addition, MFH can also be considered as an ideal material in circuit maintenance and switch construction. Thus, these eco-friendly hydrogel sensors will become one of the candidates for smart wearable devices and human–machine interfaces in the coming years.
Except the movement of the limbs, the movement of facial and laryngeal muscles could also be monitored. Flexible sensors, attached on the forehead, chin and other parts, can recognize the patient's facial expressions [79], [80]. They can be used to identify and diagnose patients with facial paralysis. Many patients are unable to make sounds due to acquired disease. However, when trying to make sounds, the muscles in the patients' throat can still produce slight vibrations. Therefore, some researchers tried to collect weak muscle motion signals of the throat through strain sensors (Fig. 9c) [81]. After identification and classification, the signal can be used in the design of intelligent artificial throats [82]. In addition, performing gait analysis on the patients’ feet can monitor their postoperative rehabilitation. Valentine et al. [83]distributed the flexible sensor units according to the anatomical division of the soles of the feet and collected the pressure data (Fig. 9d). Compared with the limb sensing equipment, the feet sensing unit needs to be precisely divided and designed with different accuracy, which will be a major difficulty.
Vital signs monitoring
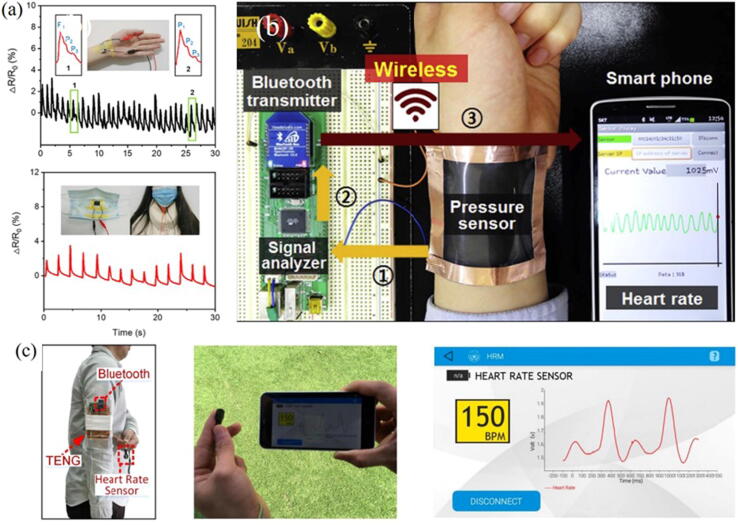
Monitoring vital signs, like heart rate, pulse, blood pressure and respiration are critical in clinical and medical care. Flexible sensors are usually attached to the patient's wrist, neck, upper arm and fingertips. Jiang et al. [84] proposed a flexible pressure sensor based on a layered 3D porous graphene oxide fiber fabric. The sensor showed excellent sensitivity under a strain range of 27% to 70%, a compression rate of 66% at a high strain coefficient (1668.48), and had good durability, low detection limit (1.17 Pa) and fast response time (30 ms) at all frequencies. By attaching this sensor to the human body, human motion, blood pressure and breathing could be detected (Fig. 10a). Shin et al. [85] successfully fabricated highly sensitive, wearable and wireless pressure sensors based on zinc oxide (ZnO) nanoneedle/polyvinylidene fluoride (PVDF) hybrid films. Thanks to its high dielectric constant, low polarization response time and excellent durability, this hybrid membrane could be used to monitor heart rate (Fig. 10b). In addition, the Bluetooth antenna based on RGO electrodes achieved high peak gain (2.70 dBi) and radiation efficiency (78.38%), suitable for transmitting wireless signals to smartphones. This approach provides a solution for the realization of continuous medical health monitoring, and the prospect to commercialize the product is promising.
Fig. 10.
(a) Wearable sensors for monitoring wrist activity and breathing during sports [84]; (b) Wireless sensor for heart rate monitoring through the wrist [85] ;(c) BSN system application demonstration [86].
Although the wearable sensor has excellent performance, the popularization of this technology is still limited by its complex structure, difficult material handling and dependence on external power supply. Therefore, some scholars have begun to work on the development of self-powered wireless body sensor network (BSN) systems. Lin et al. [86] developed a BSN system consist of a heart rate sensor, a Bluetooth module and a downy-structure-based triboelectric nanogenerator (D-TENG) (Fig. 10c). By converting the inertial energy of human walking into electrical energy, the maximum power it provided (2.28 mW) could drive a highly integrated BSN system. After processing the heart rate signal obtained by the sensor, it was wirelessly sent to the Bluetooth module of the external device and displayed on the personal mobile phone in real time. This method provides an effective way for instant heart rate monitoring.
Implantable area
There are many organs and tissues in the human body. If one of these tissues becomes diseased or its function is degraded, it will seriously damage human health. With traditional diagnosis and treatment, patients need to go to the hospital for diagnosis and treatment after the onset of disease. This may delay the disease and increase the difficulty of treatment. Preventive therapy using implanted devices can detect abnormal physiological parameters in advance. These abnormal physiological parameters can be monitored by the patient or their family members. Once an abnormality is found, the patient will be sent to the hospital for treatment in time, which will greatly improve the cure rate of the disease and reduce medical expenses. This idea encouraged more and more researchers to develop implantable sensors to monitor the internal health of the human body. Compared with wearable sensors, implantable sensors are generally miniature, biocompatible, and biodegradable. According to their functions, implantable electronic devices are generally divided into physiological parameter monitoring and adjuvant therapy.
Physiological parameter monitoring
Compared with human monitoring in wearable devices, the function of implantable devices is more focused on monitoring the health of specific organs or tissues, such as bone, cardiovascular, heart, and even brain.
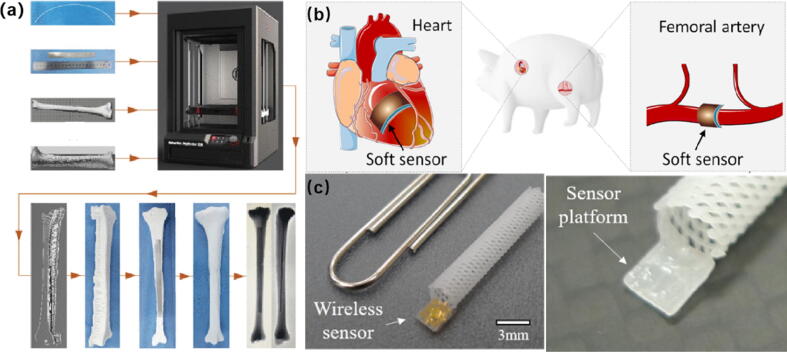
Artificial bone (AB) is usually used for bone repair surgery [87]. If the artificial bone is overloaded for a long time, its service life will be greatly reduced. Therefore, long-term continuous monitoring of the stress on AB is essential to assess the effect of postoperative rehabilitation. X-ray technology, high-quality computed tomography (CT) imaging and ultrasound examination are common non-invasive assessment methods [88]. However, frequent CT imaging will produce high-dose radiation, which is harmful to human health. In addition, the interference of soft tissue vibrations around bones reduces the accuracy of ultrasound testing. Ren et al. [89] proposed a 3D printed magnetoelastic sensor (MBS) (Fig. 11a), which used magnetoelastic material as a sensing element to monitor AB stress through wireless communication. This method has less harm to the patient's body and can realize long-term continuous monitoring, so it can be applied to the monitoring of implantable AB or prosthesis.
Fig. 11.
(a) Manufacturing method of AB [89]; (b) Sensors were implanted into the porcine heart and femoral arteries to monitor micro-pressure changes [91]; (c) Photo of the polymer scaffold with integrated wireless sensor [92].
Cardiovascular diseases, cerebral hemorrhage and other pathologies are increasingly common in the clinic. Accurate detection and measurement of small changes in the micro-pressure inside the human body (such as the heart, blood vessels, brain, etc.) is extremely challenging for the development of diagnostic biosensors for real-time monitoring of chronic diseases. The cuff sphygmomanometer is one of our most commonly used blood pressure measuring devices, which has the characteristics of simple operation, low risk, etc. [90]. However, Because of its non-portability, it is difficult to achieve continuous monitoring of blood pressure. This problem can be settled by implantable wireless sensors.
Li et al. [91] fabricated a flexible sensor based on organic nanofibers. The organic piezoelectric nanofibers (OPNs) was composed of PVDF and hydroxylamine hydrochloride (HHE), and had excellent piezoelectric stability and recoverability. The sensor showed excellent piezoelectric conversion efficiency, stability and recoverability, when the sensor was implanted on the cardiovascular wall of the pig’s heart and femoral artery (Fig. 11b), the weak pressure changes could be monitored and recorded. Abnormal cardiovascular diseases can thus be detected in advance. In addition, the sensor can also be used to monitor brain damage, hydrocephalus, glaucoma, and even tumor regeneration. Park et al. [92] fabricated a SU-8 wireless pressure sensor using a capacitive-inductive resonant circuit based on MEMS technology and a polymer smart stent based on 3D printing technology (Fig. 11c). Sensor and medicines were integrated into the smart stand to carry out medicine treatment and blood pressure monitoring for patients. Due to the biocompatibility of the PCL material used, after a period of medical treatment, the stent can be biodegraded and completely absorbed by the human body, this treatment will effectively prevent the formation of atherosclerosis. Although there is a slight deviation in the calculated blood pressure, this experiment still provides a basis for biocompatibility and measurement effect for the application of implantable sensors.
Adjuvant therapy
Many researches have shown that cell proliferation, adhesion and differentiation will be greatly affected by the biophysical properties of the extracellular matrix, such as nano-topography and bioelectricity. The surface nano-topography of biomaterial scaffolds can guide the formation of cytoskeletal tissue and focal adhesions (FA), thereby activating downstream cells to regulate cell behavior through cytomechanical transduction pathways. Zhang et al. [93] proposed a piezoelectric PVDF sensor with a nano-strip array structure. Sensors based on this material could generate surface piezoelectric potentials in the millivolt range. Experimental results showed that the nanomorphology and piezoelectricity could improve the proliferation, adhesion and neuron-like differentiation of rat bone marrow mesenchymal stem cells (rbMSCs). Furthermore, structure design of the biological nanomaterials will promote the repair of nerve tissue.
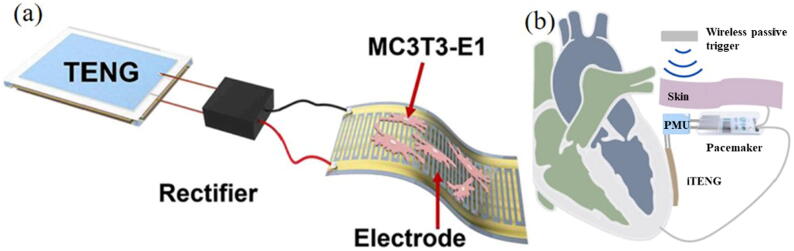
The main problem faced by the battery-powered implantable sensor is how to keep continuous power supply. When the battery is exhausted, it requires additional surgery to complete the replacement of the battery or the entire sensor, which would cause bleeding, inflammation and infection. Therefore, TENG has been developed as a potential biomechanical energy harvester for self-powering sensor in vivo. Tian et al. [94] proposed a flexible implantable electrical stimulator using TENG and flexible electrode (Fig. 12a). They implanted this electrical stimulation into the femur of rats. After a period of observation, they found that the stimulator can be self-powered by TENG from daily exercise of rats, and the osteoblasts had proliferated and differentiated to promote bone tissue regeneration. Electrical stimulator can also promote bone stabilization and reduce the occurrence of osteoporosis and fractures. This work made great progress in the clinical treatment of osteoporosis and fractures. Ouyang et al. [95] developed an implanted symbiotic pacemaker using TENG as power supply (Fig. 12b). They implanted it between the pig’s heart and pericardium, and successfully achieved cardiac pacing and the correction of arrhythmia. The experimental results showed that the open circuit voltage of TENG could reach 65.2 V, and the energy collected per heartbeat cycle was 0.495 μJ. Since the average threshold energy required by a pacemaker was 0.377 μJ, this symbiotic pacemaker can correct sinus arrhythmia and prevent its deterioration in a completely self-powered form.
Fig. 12.
(a) The schematic diagram of a cell stimulation device [94]; (b) Schematic diagram of a wireless symbiotic cardiac pacemaker system [95].
Challenges
High-performance, reliable medical monitoring systems require flexible sensors with a variety of properties, including basic properties (e.g. sensitivity, linearity and durability) and specific properties (e.g. self-powered, wireless communication, biocompatibility and biodegradability). Although continuous improvements in materials, manufacturing processes and sensing mechanisms have further stimulated the application of flexible force sensors in the field of healthcare, there are still many challenges in its development.
Sensitivity and linearity
Sensitivity and linearity are important parameters for sensors. In order to have high sensing gauge factor, one common method is to choose functional materials with large piezoresistive or piezoelectric coefficient. Excellent linearity helps signal detecting and processing. However, the high sensitivity and wide working range of flexible sensors can hardly be satisfied simultaneously. Furthermore, these key performance parameters still depend on conducting tests after sensors being produced. It is difficult to maintain the consistency of the characteristics for different batches of sensor devices. Microstructure design of nanomaterials has been proved to be one of the effective ways. Shi et al. [49] proposed a pressure sensor array with bionic structure of lotus leaf by casting PDMS precursor and spraying graphene onto its surface to form conductive elements. It has been proved that the structure could increase the contact area of graphene material, thus improve the sensitivity of the pressure sensor. Pang et al. [48] developed a graphene-based pressure sensor with a randomly distributed spin microneedle structure. The microneedle structure is clarified to improve the compression performance of the sensor, and the random distribution expands the linear range of the sensor. However, the quantitative relationship between sensor structure and sensitivity has not been established, which should be done in the future.
Durability
Durability determines the sensor's sustainable life. At present, flexibility properties are still limited to sensitive components and substrate materials. Other components in sensing system such as energy storage devices, signal processing and transmission circuits are still made of rigid materials. Under continuous mechanical load, rigid electric components will be damaged or separated from the substrate. Functional materials may also encounter the problem of periodic instability after considerable loading–unloading cycles. Buckling, breaking and ever peeling may occur then. For example, after about 1000 strain cycles of 0% to 2%, the peak resistance of a graphene woven fabric (GWF) film decreased by 1/4 as a result of weak interface interaction between the functional film and the flexible PDMS substrate [96]. One possible method to improve durability is to equip the flexible device with external packaging to reduce core component exposure and physical damage. In addition, using mechanically gradient substrates may reduce the fracture caused by differences in mechanical properties of materials. In particular, the tissue fluid surrounding the sensor may cause corrosion of the implanted functional material. Thus, how to improve the durability of flexible sensors especially implantable sensors is still challenging.
Biocompatibility and biodegradability
No matter wearable or implantable sensors, we need to consider the biocompatibility and biodegradability of functional materials. For example, the potential toxicity of carbon-based materials remains a concern of the medical community [97], experimental results showed that excessive carbon nanotubes injected into the lungs of mice will cause pathogenicity similar to asbestos [96]. Thus we need to further research the immune response for sensors used in different situations and define the biocompatibility standard for functional materials [98]. Meanwhile, flexible materials that can be absorbed by human body can be used as the substrates for sensors. For example, silk fibroin has adjustable dissolution and biodegradation rate, and it can be attached to the surface of organs [99]. Many microbial degradants or cell uptakes, like cellulose paper, gelatin and starch, are also candidates for substrate materials.
Manufacturing methods of flexible sensors
In recent years, flexible force sensors have developed rapidly in materials and processes, but there are still some limitations in terms of cost, manufacturing efficiency and performance consistency. Many flexible force sensors are still in the laboratory stage and cannot be mass-produced. Although the production capacity of some functional materials has been increased and the cost was reduced, some parameters of the current manufacturing process cannot be accurately controlled, thus, the sensing performance of different batches of flexible devices cannot be guaranteed. The combination of direct writing technology and low temperature deposition provides a new method for the preparation of flexible sensors [100]. The direct writing technology has the advantage to print a wide range of materials and the printing process is simple, while the low temperature forming environment provides a novel solidification strategy and ensures the forming accuracy. For devices with complex internal structures, organic solvents can be used as supporting materials to maintain structural stability in the printing process through solvent crystallization. During the freeze-drying process after forming, solvent sublimation can form a large number of micropores in the conductive wall, which is expected to further improve the mechanical and electrical properties of the sensor [11]. With the continuous development of sensor manufacturing technology, mass production of flexible sensors will be achieved in the near future.
Multifunctional induction
Health monitoring systems are often required to test multiple physiological parameters to obtain comprehensive health information. The measurement of different parameters can be realized by adding different types of sensing equipment. However, it may increase the equipment volume and the difficulty of wiring. Therefore, the development of a multi-function sensor capable of detecting different stimuli at the same time is particularly important. For example, Hua et al. [101] proposed a highly stretchable and compliant matrix network (SCMN). As a multi-sensory electronic skin, SCMN could detect multiple parameters at the same time, including temperature, in-plane strain, relative humidity (RH), ultraviolet (UV), magnetic field pressure and proximity. In addition, SCMN also had an adjustable sensing range and large area scalability. The creation of SCMN has laid a solid foundation for the development of multi-functional monitoring system in the field of health monitoring. However, there are still some problems with multi-function monitoring. For example, during signal acquisition, mutual interference in multi-function monitoring may cause data distortion. Therefore, how to enhance the recognition ability of functionally integrated sensors under synchronous excitation is particularly important.
Conclusion
In this review, we introduce the materials, manufacturing technologies, sensing mechanisms and applications of flexible force sensors in the field of health monitoring, and summarize the challenges that flexible force sensors face during development. Firstly, the materials used to manufacture flexible force sensors can be divided into three categories: carbon-based materials, metal materials and polymer materials. For wearable sensors, the development of nanomaterials and effective structural design have become important directions for further improvement, because it will lead to higher sensitivity and linearity. For implantable sensors, more attention should be paid to the material's biocompatibility, biodegradation rate and potential toxicity. Secondly, we should adopt advanced manufacturing techniques to improve the mechanical properties of the sensor. The current advanced 3D printing technologies used to manufacture flexible force sensors include direct ink writing, stereolithography, digital light processing, fused deposition modeling and selective laser sintering, an improvement to these existing 3D printing technologies, has proven to be an effective approach, for example, direct ink writing can be used in combination with low temperature deposition to improve the curing speed of materials and the accuracy of prototyping. Next, we should adopt smart sensing mechanisms, which include resistive, capacitive, piezoelectric and triboelectric. How to make the existing sensing mechanism more stable and reliable and develop new sensing mechanism should be the future research directions. The continuous advancement of new materials, advanced manufacturing technologies and smart sensing mechanisms has greatly prompted the improvement of the performance of flexible force sensors, these high-performance sensors will be widely used in wearable and implantable health monitoring fields. For wearable sensors, the monitoring of body movement and vital signs has been found to be an applicable scenario, the development of miniaturized and multifunctional integrated wearable electronics could be one of the future research directions. For implantable areas, physiological parameter monitoring and adjuvant therapy are applicable scenarios. Long-term challenges include energy supply and data transmission. Although piezoelectric and triboelectric sensors can generate electricity, how to make energy harvesting greater than consumption is the problem that should be addressed in the future. Radio frequency (RF) circuits have proved to be an effective way of data transmission, but how to achieve lossless transmission of signals is a problem. In summary, flexible force sensors are promising for health monitoring, and their application in personalized and preventive medicine will provide powerful socio-economic benefits and improve people's quality of life.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Jiangsu Provincial Key R&D Program (BE2018731, BE2018010), National Natural Science Foundation of China (NSFC 51705259, 51805272), Jiangsu Province Graduate Practice Innovation Program(SJCX20_0430), China Postdoctoral Science Foundation (2020M671450) and Jiangsu Planned Projects for Postdoctoral Research Funds (2020Z042).
Biographies
Ming Cheng is a M.S. candidate in school of electrical and automation engineering, Nanjing Normal University. His research focuses on 3D printing and flexible sensors.
Guotao Zhu is a M.S. candidate in school of electrical and automation engineering, Nanjing Normal University. His research direction is 3D printing and wireless sensing system.
Feng Zhang got his Ph.D. degree from the State University of New York at Buffalo (UB) in 2018. From Nov. 2018 to Mar. 2019, he worked as a short term Postdoctoral researcher in the department of Mechanical and Aerospace Engineering with Dr. Deborah Chung. In May of 2019, he joined Nanjing Normal University, and is a member of Jiangsu Key Laboratory of 3D Printing Equipment and Manufacturing. His current research interests include developing novel 3D printing methods for fabrication of various porous objects.
Wen-lai Tang is currently a lecturer in the Nanjing Normal University and the Jiangsu Key Laboratory of 3D Printing Equipment and Manufacturing. He received the B.S. degree from the Changzhou University, and the Ph.D. degree from the Southeast University, China. His current research interests include 3D printing, microfluidics and micromachining.
Jian-ping Shi received his B.S. degree in Mechanical Engineering in 2010 and M.S. degree in Electronic Engineering in 2013 from Nanjing Normal University, he received his Ph.D. degree from Southeast University. He is a Lecturer at Nanjing Normal University and Jiangsu Key Laboratory for 3D Printing Equipment and Manufacturing. His research interests include 3D printing methods and 3D bio-printing for tissue engineering.
Ji-quan Yang is currently a professor of Nanjing Normal University, dean of Electrical and Automation Engineering College, director of Jiangsu Key Laboratory of 3D Printing Equipment and Manufacturing, chairman of Nanjing Intelligent Advanced Equipment Industry Research Institute. He is mainly engaged in the application and industrialization of 3D printing technology.
Li-ya Zhu is currently a lecturer with Nanjing Normal University and the Jiangsu Key Laboratory of 3D Printing Equipment and Manufacturing. She received the B.S. degree from the Wuhan University of Technology, and the Ph.D. degree from the Nanjing University of Aeronautics and Astronautics, China. Her current research interests include 3D printing, flexible electronics, sensors and energy harvesting devices.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Shi Jianping, Email: jpshi@njnu.edu.cn.
Li-ya Zhu, Email: 61193@njnu.edu.cn.
References
- 1.Yao S., Swetha P., Zhu Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv Healthcare Mater. 2018;7(1):1700889. doi: 10.1002/adhm.201700889. [DOI] [PubMed] [Google Scholar]
- 2.Ng P.C., Murray S.S., Levy S. An agenda for personalized medicine. Nature. 2009;461(7265):724–726. doi: 10.1038/461724a. [DOI] [PubMed] [Google Scholar]
- 3.Tricoli A., Nasiri N., De S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv Funct Mater. 2017;27(15):1605271. [Google Scholar]
- 4.Liu Y., Wang H., Zhao W. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors. 2018;18(2) doi: 10.3390/s18020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y., Yu L., Yeo J.C. Flexible Hybrid Sensors for Health Monitoring: Materials and Mechanisms to Render Wearability. Adv Mater. 2019 doi: 10.1002/adma.201902133. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Fan X., Chen S.-C. Emerging Technologies of Flexible Pressure Sensors: Materials, Modeling, Devices, and Manufacturing. Adv Funct Mater. 2019;29(12):1808509. [Google Scholar]
- 7.Gu Y., Zhang T., Chen H. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res Lett. 2019;14(1):263. doi: 10.1186/s11671-019-3084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang P., Ji Z., Zhang X. Recent advances in direct ink writing of electronic components and functional devices. Progr Addit Manuf. 2018;3(1):65–86. [Google Scholar]
- 9.Huang J.-C. Carbon black filled conducting polymers and polymer blends. Adv Polym Tech. 2002;21(4):299–313. [Google Scholar]
- 10.Shintake J., Piskarev E., Jeong S.H. Ultrastretchable Strain Sensors Using Carbon Black-Filled Elastomer Composites and Comparison of Capacitive Versus Resistive Sensors. Adv Mater Technol. 2018;3(3):1700284. [Google Scholar]
- 11.Wang Z.Y., Guan X., Huang H.Y. Full 3D Printing of Stretchable Piezoresistive Sensor with Hierarchical Porosity and Multimodulus Architecture. Adv Funct Mater. 2019;29(11):8. [Google Scholar]
- 12.Chen K., Gao W., Emaminejad S. Printed Carbon Nanotube Electronics and Sensor Systems. Adv Mater. 2016;28(22):4397–4414. doi: 10.1002/adma.201504958. [DOI] [PubMed] [Google Scholar]
- 13.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58. [Google Scholar]
- 14.Iijima S., Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363(6430):603–605. [Google Scholar]
- 15.Bethune D.S., Kiang C.H., De Vries M.S. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature. 1993;363(6430):605–607. [Google Scholar]
- 16.Shi J., Li X., Cheng H. Graphene Reinforced Carbon Nanotube Networks for Wearable Strain Sensors. Adv Funct Mater. 2016;26(13):2078–2084. [Google Scholar]
- 17.Abshirini M., Charara M., Liu Y.T. 3D Printing of Highly Stretchable Strain Sensors Based on Carbon Nanotube Nanocomposites. Adv Eng Mater. 2018;20(10):9. [Google Scholar]
- 18.Gilshteyn E.P., Romanov S.A., Kopylova D.S. Mechanically Tunable Single-Walled Carbon Nanotube Films as a Universal Material for Transparent and Stretchable Electronics. ACS Appl Mater Interfaces. 2019;11(30):27327–27334. doi: 10.1021/acsami.9b07578. [DOI] [PubMed] [Google Scholar]
- 19.Secor E.B., Prabhumirashi P.L., Puntambekar K. Inkjet Printing of High Conductivity, Flexible Graphene Patterns. The J Phys Chem Lett. 2013;4(8):1347–1351. doi: 10.1021/jz400644c. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y., Murali S., Cai W. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv Mater. 2010;22(35):3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.L., Hao J., Huang Z.Q. Flexible electrically resistive-type strain sensors based on reduced graphene oxide-decorated electrospun polymer fibrous mats for human motion monitoring. Carbon. 2018;126:360–371. [Google Scholar]
- 22.Wang Z.Y., Zhang Q., Yue Y.N. 3D printed graphene/polydimethylsiloxane composite for stretchable strain sensor with tunable sensitivity. Nanotechnology. 2019;30(34):10. doi: 10.1088/1361-6528/ab1287. [DOI] [PubMed] [Google Scholar]
- 23.Costa J.C., Spina F., Lugoda P. Flexible Sensors-From Materials to Applications. Technologies. 2019;7(2):81. [Google Scholar]
- 24.Hogas I, Fosalau C, Zet C. A new strain sensor based on electrospinning and thin film technologies. A new strain sensor based on electrospinning and thin film technologies. In: 2016 International Conference and Exposition on Electrical and Power Engineering (EPE), 20-22 Oct. 2016. P. 576–80.
- 25.Zhao Q., Zhao M., Qiu J. One Dimensional Silver-based Nanomaterials: Preparations and Electrochemical Applications. Small. 2017;13(38):1701091. doi: 10.1002/smll.201701091. [DOI] [PubMed] [Google Scholar]
- 26.Ahn K., Kim K., Kim J. Thermal conductivity and electric properties of epoxy composites filled with TiO2-coated copper nanowire. Polymer. 2015;76:313–320. [Google Scholar]
- 27.Gong S., Zhao Y., Yap L.W. Fabrication of Highly Transparent and Flexible NanoMesh Electrode via Self-assembly of Ultrathin Gold Nanowires. Adv Electron Mater. 2016;2(7):1600121. [Google Scholar]
- 28.Chung W.-H., Kim S.-H., Kim H.-S. Welding of silver nanowire networks via flash white light and UV-C irradiation for highly conductive and reliable transparent electrodes. Sci Rep. 2016;6(1):32086. doi: 10.1038/srep32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong X., Wei Y., Chen S. A linear and large-range pressure sensor based on a graphene/silver nanowires nanobiocomposites network and a hierarchical structural sponge. Compos Sci Technol. 2018;155:108–116. [Google Scholar]
- 30.Kwon S.N., Kim S.W., Kim I.G. Direct 3D Printing of Graphene Nanoplatelet/Silver Nanoparticle-Based Nanocomposites for Multiaxial Piezoresistive Sensor Applications. Adv Mater Technol. 2019;4(2):9. [Google Scholar]
- 31.Wang X., Liu J. Recent Advancements in Liquid Metal Flexible Printed Electronics: Properties, Technologies, and Applications. Micromachines. 2016;7(12):206. doi: 10.3390/mi7120206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickey M.D., Chiechi R.C., Larsen R.J. Eutectic Gallium-Indium (EGaIn): A Liquid Metal Alloy for the Formation of Stable Structures in Microchannels at Room Temperature. Adv Funct Mater. 2008;18(7):1097–1104. [Google Scholar]
- 33.Ali S., Maddipatla D., Narakathu B.B. Flexible Capacitive Pressure Sensor Based on PDMS Substrate and Ga–In Liquid Metal. IEEE Sens J. 2019;19(1):97–104. [Google Scholar]
- 34.Jiang J., Li Y., Liu J. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv Mater. 2012;24(38):5166–5180. doi: 10.1002/adma.201202146. [DOI] [PubMed] [Google Scholar]
- 35.Manjakkal L., Szwagierczak D., Dahiya R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog Mater Sci. 2020;109:31. [Google Scholar]
- 36.Lee T., Lee W., Kim S.-W. Flexible Textile Strain Wireless Sensor Functionalized with Hybrid Carbon Nanomaterials Supported ZnO Nanowires with Controlled Aspect Ratio. Adv Funct Mater. 2016;26(34):6206–6214. [Google Scholar]
- 37.Sobolčiak P., Ali A., Hassan M.K. 2D Ti3C2Tx (MXene)-reinforced polyvinyl alcohol (PVA) nanofibers with enhanced mechanical and electrical properties. PLoS ONE. 2017;12(8) doi: 10.1371/journal.pone.0183705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobolčiak P., Tanvir A., Sadasivuni K.K. Piezoresistive Sensors Based on Electrospun Mats Modified by 2D Ti3C2Tx MXene. Sensors. 2019;19(20):4589. doi: 10.3390/s19204589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo B., Lerberghe L.M.V., Motsegood K.M. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J Microelectromech Syst. 2000;9(1):76–81. [Google Scholar]
- 40.Liaw D.-J., Wang K.-L., Huang Y.-C. Advanced polyimide materials: Syntheses, physical properties and applications. Prog Polym Sci. 2012;37(7):907–974. [Google Scholar]
- 41.Ahmed N., Kausar A., Muhammad B. Advances in Shape Memory Polyurethanes and Composites: A Review. Polym-Plast Technol Eng. 2015;54(13):1410–1423. [Google Scholar]
- 42.Goh C.S., Tan S.C., Ngoh S.L. Surface Treatment of Polyethylene Terephthalate (PET) Film for Lamination of Flexible Photovoltaic Devices. Energy Procedia. 2012;15:428–435. [Google Scholar]
- 43.Xin Y., Zhu J., Sun H. A brief review on piezoelectric PVDF nanofibers prepared by electrospinning. Ferroelectrics. 2018;526(1):140–151. [Google Scholar]
- 44.Seymour J.P., Langhals N.B., Anderson D.J. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed Microdevices. 2011;13(3):441–451. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedia E.L., Murakami S., Kitade T. Structural development and mechanical properties of polyethylene naphthalate/polyethylene terephthalate blends during uniaxial drawing. Polymer. 2001;42(17):7299–7305. [Google Scholar]
- 46.Lu Y., Biswas M.C., Guo Z. Recent developments in bio-monitoring via advanced polymer nanocomposite-based wearable strain sensors. Biosens Bioelectron. 2019;123:167–177. doi: 10.1016/j.bios.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 47.Christ J.F., Aliheidari N., Potschke P. Bidirectional and Stretchable Piezoresistive Sensors Enabled by Multimaterial 3D Printing of Carbon Nanotube/Thermoplastic Polyurethane Nanocomposites. Polymers. 2019;11(1):16. doi: 10.3390/polym11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang Y., Zhang K., Yang Z. Epidermis Microstructure Inspired Graphene Pressure Sensor with Random Distributed Spinosum for High Sensitivity and Large Linearity. ACS Nano. 2018;12(3):2346–2354. doi: 10.1021/acsnano.7b07613. [DOI] [PubMed] [Google Scholar]
- 49.Shi J.D., Wang L., Dai Z.H. Multiscale Hierarchical Design of a Flexible Piezoresistive Pressure Sensor with High Sensitivity and Wide Linearity Range. Small. 2018;14(27):7. doi: 10.1002/smll.201800819. [DOI] [PubMed] [Google Scholar]
- 50.Kim S., Oh J., Jeong D. Consistent and Reproducible Direct Ink Writing of Eutectic Gallium-Indium for High-Quality Soft Sensors. Soft Rob. 2018;5(5):601–612. doi: 10.1089/soro.2017.0103. [DOI] [PubMed] [Google Scholar]
- 51.Wu P., Xiao A., Zhao Y. An implantable and versatile piezoresistive sensor for the monitoring of human-machine interface interactions and the dynamical process of nerve repair. Nanoscale. 2019;11(44):21103–21118. doi: 10.1039/c9nr03925b. [DOI] [PubMed] [Google Scholar]
- 52.Guo H., Lv R., Bai S. Recent advances on 3D printing graphene-based composites. Nano Mater Sci. 2019;1(2):101–115. [Google Scholar]
- 53.Liu C., Huang N., Xu F. 3D Printing Technologies for Flexible Tactile Sensors toward Wearable Electronics and Electronic Skin. Polymers. 2018;10(6):629. doi: 10.3390/polym10060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambrosi A., Pumera M. 3D-printing technologies for electrochemical applications. Chem Soc Rev. 2016;45(10):2740–2755. doi: 10.1039/c5cs00714c. [DOI] [PubMed] [Google Scholar]
- 55.Kim G., Lee S., Kim H. Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology. Korean J Radiol. 2016;17:182. doi: 10.3348/kjr.2016.17.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hensleigh R.M., Cui H., Oakdale J.S. Additive manufacturing of complex micro-architected graphene aerogels. Mater Horiz. 2018;5(6):1035–1041. [Google Scholar]
- 57.Liu M., Zhang Q., Shao Y. Research of a Novel 3D Printed Strain Gauge Type Force Sensor. Micromachines. 2018;10(1):20. doi: 10.3390/mi10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nadgorny M., Ameli A. Functional Polymers and Nanocomposites for 3D Printing of Smart Structures and Devices. ACS Appl Mater Interfaces. 2018;10(21):17489–17507. doi: 10.1021/acsami.8b01786. [DOI] [PubMed] [Google Scholar]
- 59.Kim K., Park J., Suh J.-H. 3D printing of multiaxial force sensors using carbon nanotube (CNT)/thermoplastic polyurethane (TPU) filaments. Sens Actuat, A. 2017;263:493–500. [Google Scholar]
- 60.Han T., Kundu S., Nag A. 3D Printed Sensors for Biomedical Applications: A Review. Sensors. 2019;19(7):1706. doi: 10.3390/s19071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong R., Zhao Z., Leng J. Two-step approach based on selective laser sintering for high performance carbon black/ polyamide 12 composite with 3D segregated conductive network. Compos B Eng. 2019;176 [Google Scholar]
- 62.Li S, Zhang Y, Wang Y, et al. Physical sensors for skin‐inspired electronics. InfoMat.
- 63.Zang Y., Zhang F., Di C.-A. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater Horiz. 2015;2(2):140–156. [Google Scholar]
- 64.Liu Z., Ma Y., Ouyang H. Transcatheter Self-Powered Ultrasensitive Endocardial Pressure Sensor. Adv Funct Mater. 2019;29(3):10. [Google Scholar]
- 65.Jian M., Xia K., Wang Q. Flexible and Highly Sensitive Pressure Sensors Based on Bionic Hierarchical Structures. Adv Funct Mater. 2017;27(9):1606066. [Google Scholar]
- 66.Gong S., Schwalb W., Wang Y. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat Commun. 2014;5(1):3132. doi: 10.1038/ncomms4132. [DOI] [PubMed] [Google Scholar]
- 67.Li T., Luo H., Qin L. Flexible Capacitive Tactile Sensor Based on Micropatterned Dielectric Layer. Small. 2016;12(36):5042–5048. doi: 10.1002/smll.201600760. [DOI] [PubMed] [Google Scholar]
- 68.Heikenfeld J., Jajack A., Rogers J. Wearable sensors: modalities, challenges, and prospects. Lab Chip. 2018;18(2):217–248. doi: 10.1039/c7lc00914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J., Luo S., Zhou X. Flexible, Tunable, and Ultrasensitive Capacitive Pressure Sensor with Microconformal Graphene Electrodes. ACS Appl Mater Interfaces. 2019;11(16):14997–15006. doi: 10.1021/acsami.9b02049. [DOI] [PubMed] [Google Scholar]
- 70.Yu P., Liu W., Gu C. Flexible Piezoelectric Tactile Sensor Array for Dynamic Three-Axis Force Measurement. Sensors. 2016;16(6):819. doi: 10.3390/s16060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K.-I., Son J.H., Hwang G.-T. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv Mater. 2014;26(16):2514–2520. doi: 10.1002/adma.201305659. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z., Wang Z., Li X. Flexible Piezoelectric-Induced Pressure Sensors for Static Measurements Based on Nanowires/Graphene Heterostructures. ACS Nano. 2017;11(5):4507–4513. doi: 10.1021/acsnano.6b08027. [DOI] [PubMed] [Google Scholar]
- 73.Hu W., Zhang C., Wang Z.L. Recent progress in piezotronics and tribotronics. Nanotechnology. 2018;30(4) doi: 10.1088/1361-6528/aaeddd. [DOI] [PubMed] [Google Scholar]
- 74.Zhong-Hua Z., Bao-Yuan S.U.N., Min Q. Influence of Multiple Piezoelectric Effects on Static Performance of Piezoelectric Sensors. Piezoelectr Acoustooptics. 2009;31(3):360–363. [Google Scholar]
- 75.Fan F.-R., Tian Z.-Q., Lin Wang Z. Flexible triboelectric generator. Nano Energy. 2012;1(2):328–334. [Google Scholar]
- 76.Liu Z., Li H., Shi B.J. Wearable and Implantable Triboelectric Nanogenerators. Adv Funct Mater. 2019;29(20):19. [Google Scholar]
- 77.Wajahat M., Lee S., Kim J.H. Flexible Strain Sensors Fabricated by Meniscus-Guided Printing of Carbon Nanotube-Polymer Composites. ACS Appl Mater Interfaces. 2018;10(23):19999–20005. doi: 10.1021/acsami.8b04073. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z., Gao Z.L., Wang Y.T. Eco-Friendly, Self-Healing Hydrogels for Adhesive and Elastic Strain Sensors, Circuit Repairing, and Flexible Electronic Devices. Macromolecules. 2019;52(6):2531–2541. [Google Scholar]
- 79.Su M., Li F., Chen S. Nanoparticle Based Curve Arrays for Multirecognition Flexible Electronics. Adv Mater. 2016;28(7):1369–1374. doi: 10.1002/adma.201504759. [DOI] [PubMed] [Google Scholar]
- 80.Waller B.M., Cray J.J., Jr., Burrows A.M. Selection for universal facial emotion. Emotion. 2008;8(3):435–439. doi: 10.1037/1528-3542.8.3.435. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Yang T., Lao J. Ultra-sensitive graphene strain sensor for sound signal acquisition and recognition. Nano Res. 2015;8(5):1627–1636. [Google Scholar]
- 82.Zhang Z., Tian H., Lv P. High-performance sound source devices based on graphene woven fabrics. Appl Phys Lett. 2017:110(9). [Google Scholar]
- 83.Valentine A.D., Busbee T.A., Boley J.W. Hybrid 3D Printing of Soft Electronics. Adv Mater. 2017;29(40):8. doi: 10.1002/adma.201703817. [DOI] [PubMed] [Google Scholar]
- 84.Jiang X., Ren Z., Fu Y. Highly Compressible and Sensitive Pressure Sensor under Large Strain Based on 3D Porous Reduced Graphene Oxide Fiber Fabrics in Wide Compression Strains. ACS Appl Mater Interfaces. 2019;11(40):37051–37059. doi: 10.1021/acsami.9b11596. [DOI] [PubMed] [Google Scholar]
- 85.Shin K.-Y., Lee J.S., Jang J. Highly sensitive, wearable and wireless pressure sensor using free-standing ZnO nanoneedle/PVDF hybrid thin film for heart rate monitoring. Nano Energy. 2016;22:95–104. [Google Scholar]
- 86.Lin Z., Chen J., Li X. Triboelectric Nanogenerator Enabled Body Sensor Network for Self-Powered Human Heart-Rate Monitoring. ACS Nano. 2017;11(9):8830–8837. doi: 10.1021/acsnano.7b02975. [DOI] [PubMed] [Google Scholar]
- 87.Savolainen S., Usenius J.P., Hernesniemi J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir. 1994;129(1–2):54–57. doi: 10.1007/BF01400873. [DOI] [PubMed] [Google Scholar]
- 88.Jessen K.A., Shrimpton P.C., Geleijns J. Dosimetry for optimisation of patient protection in computed tomography. Appl Radiat Isotopes: Including Data, Instrument Methods Use Agric, Indus Med. 1999;50(1):165–172. doi: 10.1016/s0969-8043(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 89.Ren L.M., Yu K., Tan Y.S. Wireless and Passive Magnetoelastic-Based Sensor for Force Monitoring of Artificial Bone. IEEE Sens J. 2019;19(6):2096–2104. [Google Scholar]
- 90.Hunyor S.N., Flynn J.M., Cochineas C. Comparison of performance of various sphygmomanometers with intra-arterial blood-pressure readings. Br Med J. 1978;2(6131):159–162. doi: 10.1136/bmj.2.6131.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li T., Feng Z.Q., Qu M.H. Core/Shell Piezoelectric Nanofibers with Spatial Self-Orientated beta-Phase Nanocrystals for Real-Time Micropressure Monitoring of Cardiovascular Walls. ACS Nano. 2019;13(9):10062–10073. doi: 10.1021/acsnano.9b02483. [DOI] [PubMed] [Google Scholar]
- 92.Park J., Kim J.K., Kim D.S. Wireless pressure sensor integrated with a 3D printed polymer stent for smart health monitoring. Sens Actuat B-Chem. 2019;280:201–209. [Google Scholar]
- 93.Zhang X.D., Cui X., Wang D.C. Piezoelectric Nanotopography Induced Neuron-Like Differentiation of Stem Cells. Adv Funct Mater. 2019;29(22):10. [Google Scholar]
- 94.Tian J., Shi R., Liu Z. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy. 2019;59:705–714. [Google Scholar]
- 95.Ouyang H., Liu Z., Li N. Symbiotic cardiac pacemaker. Nat Commun. 2019;10:10. doi: 10.1038/s41467-019-09851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang T., Wang W., Zhang H. Tactile Sensing System Based on Arrays of Graphene Woven Microfabrics: Electromechanical Behavior and Electronic Skin Application. ACS Nano. 2015;9(11):10867–10875. doi: 10.1021/acsnano.5b03851. [DOI] [PubMed] [Google Scholar]
- 97.Poland C.A., Duffin R., Kinloch I. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 98.Oberdorster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 99.De Volder M.F.L., Tawfick S.H., Baughman R.H. Carbon Nanotubes: Present and Future Commercial Applications. Science. 2013;339(6119):535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Q.Q., Zhang F., Medarametla S.P. 3D Printing of Graphene Aerogels. Small. 2016;12(13):1702–1708. doi: 10.1002/smll.201503524. [DOI] [PubMed] [Google Scholar]
- 101.Hua Q.L., Sun J.L., Liu H.T. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat Commun. 2018;9:11. doi: 10.1038/s41467-017-02685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]