Abstract
Background
Chronic periodontitis has an interplay between different species of bacteria found in dental biofilms act a crucial role in pathogenesis and disease progression. The existing antibacterial therapy is inadequate, associated with many side effects as well as evolving multidrug resistance. Hence, novel drugs development with minimum or no toxicity is an immediate priority.
Methods
Antibacterial efficacy of ethanolic extract of Matricaria aurea was tested against clinical isolates, ie. Treponema denticol, Tannerella forsythia, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis from the patients with chronic periodontitis. Zone of inhibition, the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) were investigated by well diffusion method and micro broth dilution assay using alamar blue. Anti-virulence properties of the extract, which include adherence property and the biofilm formation, were investigated by adherence as well as biofilm formation assay.
Results
Matricaria aurea extract showed potent inhibitory effect against pathogenic periodontal bacteria with the significant inhibitory zone (13–23 mm), MIC (0.39–1.56 mg/ml) as well as MBC (1.56–6.25 mg/ml). The M. aurea extract was able to inhibit bacterial adhesion ranged from 30 to 45%, 35 to 63% and 55 to 80% of MIC at MIC × 0.5, MIC × 1 and MIC × 2 respectively. Significant inhibition was found in biofilm formation to all the tested periodontal bacterial strains after the treatment with various concentrations of M. aurea extract for 24 and 48hrs.
Conclusion
These results reveal for the first time that the Matricaria aurea extract might be the source of various compounds to be applied for chronic periodontitis therapy, which might draw these valuable compounds to the subsequent phase of development of the drug.
Keywords: M. aurea, Red complex bacteria, Periodontal disease, Antibacterial activity, Biofilm
1. Introduction
Oral microflora, complex and diverse bacterial communities, plays a vital role in human health and diseases (Marsh and Martin, 2009). According to the Human Oral Microbiome database, around 1100 microbial communities with astounding diversity reside in various domains of the oral cavity (Floyd et al., 2010). Coaggregation between microbes plays significant characteristics in the colonization and dental plaque formation (Kolenbrander et al., 2006, Hannig and Hannig, 2009). Gingival plaque involves a critical role in periodontal disease involving both the hard and soft tissues, in due course leading to loss of a tooth. Additionally, plaque-induced periodontal infections may constitute potential endanger element for particular systemic sickness such as diabetes, cardiovascular disease, and inauspicious pregnancy outcomes as well as increased risk of various cancers. (Meyer et al., 2008, Fitzpatrick and Katz, 2010, Shamami et al., 2011, Nazir, 2017) which could cost around fifty-four billion USD per year (Tonetti et al., 2017). In Saudi Arabia, epidemiological data is still limited. A recent retrospective study proclaimed that several peoples suffering from periodontitis and gingivitis were 36.8% and 63.2%, respectively (Hossain et al., 2013).
The treatment of periodontitis is still a challenging task to the clinicians because of its poly-microbial etiology and complex clinical progression. Various important periodontal pathogenic bacteria such as Prevotella intermedia, Porphyromonas gingivalis (P. gingivalis), Peptostreptococcus migros, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Treponema denticol (T. denticol), Tannerella forsythia (T. forsythia), Eikenella corrodens and Campylobacter rectus are capable to the periodontal deterioration (Christina et al., 2013). However, the red complex, including three pathogens: T. forsythia, T. denticola, and P. gingivalis, as well as A. actinomycetemcomitans, which appears later in the biofilm formation, are considered as dominant pathogenic organisms in proceeding periodontitis (Kitti et al., 2015).
The available data has indicated herbal medicines as worthy substitutes to synthetic agents in the prophylactic and curative care of periodontal diseases on account of substantial natural effect, profound safety and decreased costs (Pandita et al., 2014, Kaur et al., 2016, Anand, 2017). The research in the field of herbals herbal science is still in its infancy concerning the clinical application of these agents in periodontics. Many types of herbs were described to be useful to treat the periodontitis (Shama et al., 2014). Also, there is a dramatically increased interest attributed to plants of the Matricaria genus (family Asteraceae), since its constituents have high therapeutic potencies as antimicrobial agents, antioxidant, anti-inflammatory, and analgesic (McKay and Blumberg, 2006, Abdoul-Latif et al., 2011, Neelma et al., 2014, Humaira et al., 2016). Matricaria chamomilla L (MTC), a herb of Genus Matricaria, also known as chamomile, is widely used, and one of the ancient medicinal plants globally. In vitro studies showed that MTC was efficacious against many Gram-positive and Gram-negative bacteria as well as 20 bacterial strains of Listeria monocytogenes (McKay and Blumberg, 2006, Ismail et al., 2011, Sourav et al., 2019).
Recently, another herb of Genus Matricaria, Matricaria aurea (M. aurea), a native drug of Saudi Arabia has been explored for its therapeutic effect and has a potential of being the promising source of antimicrobials and antioxidant agent (Al-Mustafa and Al-Thunibat, 2008, Siddiqui, 2014). It has many similarities in chemical composition, mainly essential oils, flavones, and flavonoids as well as in traditional uses to the main species, Matricaria chamomilla (Singh et al., 2011). No research has been carried out so far to assess the antimicrobial efficacy of M. aurea on pathogenic bacteria and late colonizers advancing severe and acute periodontitis. Therefore, the current study is designed to evaluate the in vitro potency of M. aurea against pathogenic “Red Complex Bacteria,” i.e. T. denticola, T. forsythia, P. gingivalis as well as A. actinomycetemcomitans on clinical isolates of Saudi population with periodontal disease and to get future direction in developing promising therapeutic agents for the management of the systemic and periodontal infections linked to the disease.
2. Materials and methods
2.1. Preparation of plant extract
The dried flowers of M. aurea plant were purchased from the supermarket of Abha city of Asir region of Saudi Arabia. The plant sample was identified and confirmed by a taxonomist, Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia, for authenticity. A specimen sample of the plant was deposited for future reference at the Herbarium of Department of Biology, King Khalid University Abha, Saudi Arabia to obtain the voucher number (#48657). The drug was coarsely powdered with the help of Grinder for 10 s. The powdered material (50 gm) was packed in the muslin cloth and subjected to soxhlet extractor with absolute ethanol for continuous hot extraction for 72 hrs. Thereafter, ethanolic extract of M. aurea was filtered by muslin cloth followed by whatman-1 filter paper, and the filtrate was vaporized under the reduced pressure and temperature by rotary evaporator (Buchi Rotavapor R-200). The % yield of the extracts was calculated with respect to dried plant material, and it was found 14.52% w/w. The dried plant extracts were further re-dissolved in ethanol at the concentration of 0.2 g/ml, which was used for antibacterial susceptibility assay. Stock solutions were prepared, and a final working volume was achieved by diluting 2-fold dilution of the stock ranging from 0.2 g/ml to 50 μg/ml, which was used later for determination of MIC and MBC.
2.2. Collection of specimen and isolation of bacteria
Plaque specimens were collected from chronic periodontitis patients. The subgingival plaque specimens were collected by injecting Gracey-curette (Hu-Friedy, Chicago, USA). When curette touches the bottom of the periodontal pocket with no harm to soft tissues, a subgingival plaque was collected with a single perpendicular stroke. The plaque specimen was subsequently transferred to Sodium thioglycolate, an anaerobic transport media, (Sigma-Aldrich, Germany).
Red complex bacteria such as T. forsythia, P. gingivalis and T. denticola as well as A. actinomycetemcomitans, a molar incisor grade IV periodontitis was isolated on the selective media as described previously (Saquib et al., 2019). The bacterial strains were further incubated in an anaerobic jar (Don Whitley Scientific Ltd., West Yorkshire, UK) with an environment having 10% carbon dioxide, 10% hydrogen and 80% nitrogen at 37 °C for 3–7 days. The desire bacteria grown on the selective media were further identified based on the colony morphology, and use of biochemical tests for identification of species.
2.3. Antibacterial susceptibility assay by well diffusion method
For the early assessment of the tested bacteria for their susceptibility to the plant extract, the bacterial strains were grown-up to the logarithmic phase (O.D.610 of 0.4–0.6) in lysogenic broth (LB) broth. Consequently, the tested bacterial strains were further diluted in LB broth to a theoretic O.D.610 of 0.01. Agar well diffusion process was applied to make out the antibacterial efficacy of the extract (Magaldi et al., 2004). Briefly, wells of 6 mm diameter were formed in the LB agar by the cap of a sterile syringe and lawn culture was formed on the agar using the sterilized cotton swab from diluted culture. 20 μl of the extract (0.2 g/ml) and ethanol were transferred in triplicate wells of the Petri dish and were further incubated anaerobically for 24 h at 37 °C. The diameter of the clear zone of inhibition of bacterial growth, including the well diameter, was estimated in millimetres. Genuine zone of inhibition was analyzed by deducting the mean zone of inhibition by the extract from the mean zone of inhibition by the ethanol.
2.4. Determination of MIC & MBC
MIC and MBC of the M. aurea extract were determined as described with modification (Wei et al., 2011). The concentrations of the plant extract applied for MICs and MBCs, on selected bacterial strains were exposed with a 2-fold dilution of plant extract ranged from 0.2 g/ml to 50 μg/ml. To determine the MIC, the culture of the bacterial strains grown-up to the logarithmic phase (O.D.610 0.4–0.6) was further diluted in LB broth to a theoretic O.D.610 of 0.01 to determine the MIC. Consequently, 180 μl culture of all bacterial strain was transferred into the wells of polystyrene sterile flat-bottom 96-well plates. 20 μl from the 2-fold dilution of the plant extract was loaded in triplicate wells. 20 μl of ethanol (5%) was loaded in triplicate wells considered as control. The plates were further incubated anaerobically for 24 h at 37 °C. After an incubation of 24 hrs, 20 μl of alamar blue (Thermo Fisher, USA) was loaded into each well, and pink color development was checked after each hour. The lowest concentration of the extract in a well that didn’t turn the color of the alamar blue to pink was read as MIC.
For the determination of MBC of the plant extract for the bacterial strains, 10 μl of the culture from the wells where the color of Alamar blue dye persists unchanged was sub-cultured on LB agar and incubated anaerobically for 24 hrs at 37 °C. The minimum concentration of the plant extract where no growth was found, studied as MBC of the plant extract for the examined strains.
2.5. Adherence assay
Adherence assay was done based on the procedure of Fazly et al. (2013). In brief, 100 μl of tested bacterial cells (O.D.610 of 0.01) grown in RPMI 1640 media and buffered with 0.165 M Mops (morpholinepropanesulphonic acid) at pH 7.0 (Sigma Aldrich, St. Louis, USA) were loaded into all the wells of 96 wells plate. Then the bacterial cells were treated with different concentrations (MIC × 0.5, MIC × 1, MIC × 2) of plant extract and incubated anaerobically for 6 hrs at 37 °C. Untreated bacterial cells were used in each set of investigation as the negative control. Consequently, incubation media was discarded, and every well was rinsed twice with 200 μl PBS to eliminate the non-adherent bacterial cells. A 100 μl of alamar blue at an absolute concentration (5%) in RPMI 1640 media were loaded into all the wells followed by incubation anaerobically at 37 °C for 6hr. Fluorescence indications were studied at 555Ex/585Em by a Synergy HT microplate reader (BioTek Instruments, WA, USA).
2.6. Biofilm formation
Biofilms were produced in sterile flat bottom 96 wells plates by the inoculating the tested bacterial cells suspensions (O.D.610 of 0.01) grown in RPMI 1640 media buffered with 0.165 M Mops pH 7.0 and incubated anaerobically for 6 hrs at 37 °C (Raut et al., 2013). After a 6 hrs of adhesion period, media was removed sensibly without any disturbance in the formation of biofilm. Then different concentrations of plant extract (MIC × 0.5, MIC × 1, MIC × 2) were make ready in the fresh RPMI 1640 media and loaded into the wells. Untreated bacterial cells were used in each set of investigation as a negative control. The 96 wells plate was further incubated anaerobically at 37 °C for 24 hrs and 48 hrs. Efficacies of tested plant extract on the formation of biofilm were assessed as described (Jin et al., 2004).
2.7. Time killing kinetic assay
The bacterial culture 180 μl (O.D.610 of 0.01) was treated with 20 μl of different concentration of plant extract (MIC × 0.5, MIC × 1, MIC × 2), to investigate the effect of plant extract on tested bacterial cells. The culture well with 20 μl of ethanol (5%) was considered as control. The plates were further incubated anaerobically at 37 °C and absorbance was measured at 610 nm after every 2 hrs in 96 well plate reader. The mean of the absorbance was plotted against time.
2.8. Statistical analysis
Each investigation was conducted thrice, and the results revealed as the mean ± SD. Statistical processes were achieved using Graphpad prism software − 6.0 (La Jolla, USA). Variations between the two groups were examined by the two-tailed Student’s t-test and p < 0.0001 (***) were studied statistically significant.
3. Results
3.1. Antibacterial activity of M. aurea extract
To determine the antibacterial activity of M. aurea extract, red complex bacterial strains causing periodontal disease such as T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans were treated. Susceptibility studies showed that M. aurea extract has higher antibacterial activity against T. forsythia than the other isolates (Table 1). A zone size above 8 mm was considered as significant for the sensitivity of the bacterial strains towards the tested M. aurea extracts. The bacterial strains where the zone size was above 8 mm were treated with distinctive concentrations of plant extracts to decide the MIC and MBC.
Table 1.
Effect of ethanolic extract of M. aurea on red complex bacterial strains causing periodontal disease.
| Organisms | Zone of inhibition (mm) Mean ± SD |
MIC (mg/ml) |
MBC (mg/ml) |
|---|---|---|---|
| P. gingivalis | 20 ± 1.20 | 0.78 | 3.12 |
| T. denticola | 15 ± 3.25 | 1.56 | 3.12 |
| T. forsythia | 23 ± 0.65 | 0.39 | 1.56 |
| A. actinomycetemcomitans | 13 ± 1.25 | 1.56 | 6.25 |
For the determination of the MIC and MBC, selected bacterial strains were exposed with the volume of plant extract described above, followed by an incubation of 24 h. The least concentration of the M. aurea extract where the color of the alamar blue dye persists unchanged was studied as the MIC. It is evident from table 1 that all of the bacterial strains were significantly susceptible to the secondary metabolites of M. aurea extract. The bacterial growth was inhibited with MIC ranged from 0.39 to 1.56 mg/ml, and MBC ranged from 1.56 to 6.25 mg/ml. These results were supported with the consequent higher zone of inhibition ranged from 13 to 23 mm.
3.2. M. aurea inhibits bacterial adhesion
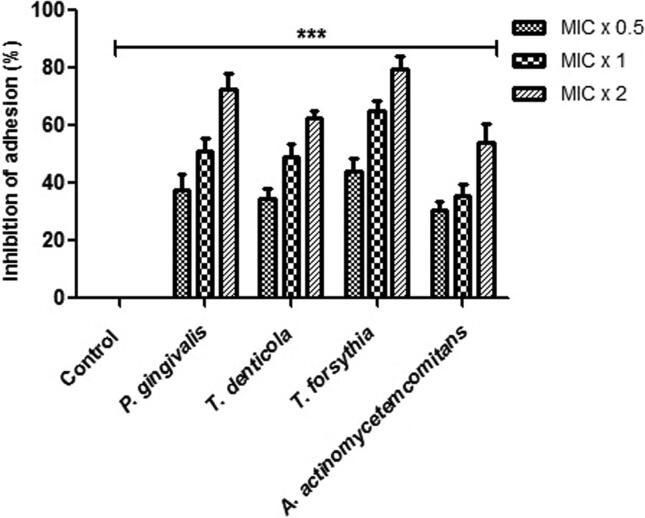
Adhesion assay was done using the alamar blue dye to assess the effect of M. aurea on tested bacterial adherence. After being exposed to the various concentration of plant extract, bacterial cells revealed inconsistent inhibition of bacterial adherence that specifies the effect was dependent on the concentration (Fig. 1). For all the bacterial strains such T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans, the tested plant extracts were able to inhibit bacterial adhesion ranged from 30 to 45%, 35 to 63% and 55 to 80% at MIC × 0.5, MIC × 1 and MIC × 2 respectively. The bacterial strains without any concentration of plants extract were designated as control. The results signified that these plant extracts were expressively efficient in diminishing tested bacterial adhesion, even at subinhibitory concentrations.
Fig. 1.
M. aurea extract inhibit the bacterial adhesion. Alamar Blue based polystyrene adhesion assay was used to evaluate the effect of M. aurea on P. gingivalis, T. denticola, T. forsythia and A. actinomycetemcomitans adherence. All the tested bacterial strains were exposed to MIC × 0.5, MIC × 1 and MIC × 2 values of plant extract for 6 hrs at 37 °C. Control bars indicate all untreated bacterial strains, accepted as 0% inhibition. Results are presented from three independent experiments using means ± SD. ***p < 0.0001.
3.3. M. aurea inhibits bacterial biofilm formation
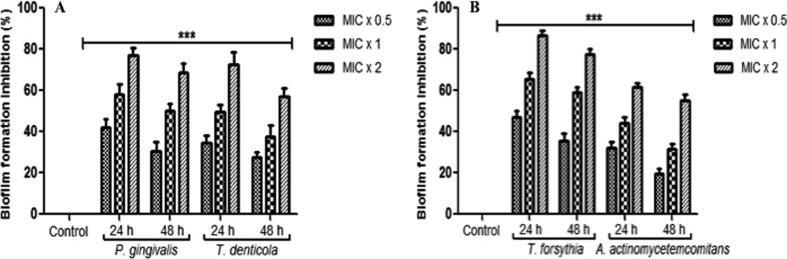
Interestingly, significant inhibition in the formation of biofilm was found in all the tested periodontal bacterial strains after the treatment with various concentrations of plant extract for 24 and 48 h (Fig. 2). After 24 h, at the concentrations of MIC × 0.5, MIC × 1 and MIC × 2 of the test entities in the biofilm formation inhibition to T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans was detected in the range of 32 to 47%, 44 to 65% and 60 to 86% respectively (Fig. 2A and B). In contrast, after 48 h, inhibition of biofilm formation at the different concentration of MIC × 0.5, MIC × 1 and MIC × 2 was detected in the range of 20 to 36%, 32 to 58% and 55 to 77% respectively (Fig. 2A and B). The bacterial strains without any concentration of plants extract were considered as control. Furthermore, the rate of biofilm formation inhibition by the plant extract was based on the concentration and treatment time as well. The findings exhibited that the plant extract significantly reduced the formation of biofilm in the tested bacterial cells.
Fig. 2.
M. aurea extract reduce the biofilm formation. (A) P. gingivalis and T. denticola (B) T. forsythia and A. actinomycetemcomitans, were incubated with MIC × 0.5, MIC × 1 and MIC × 2 values of M. aurea extract under biofilm growing conditions for 24 and 48 hrs. Control bars indicate all untreated bacterial strains, accepted as 0% inhibition. Results are presented from three independent experiments using means ± SD. ***p < 0.0001.
3.4. Effect on bacterial growth
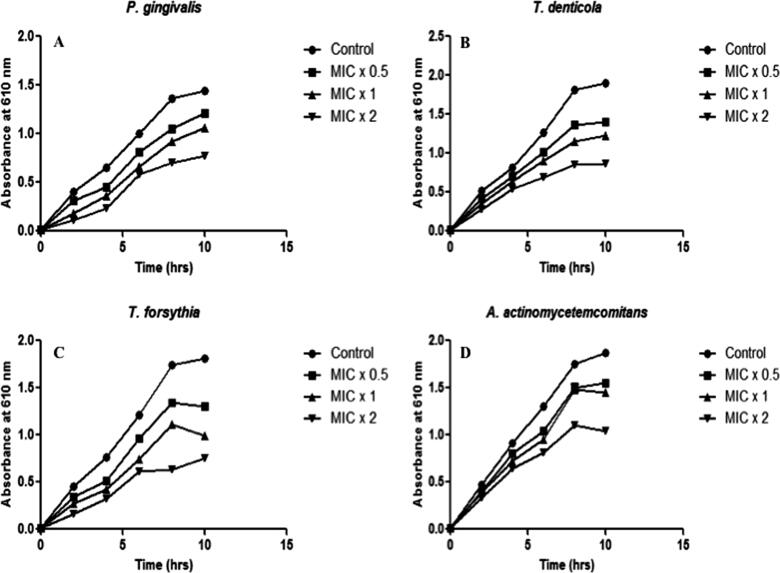
We executed real-time investigation of the plant extract on the bacterial growth at different time. To determine the time-killing kinetic, 180 μl of bacterial culture (OD610 of 0.01) treated with 20 μl of plant extract at the different concentration of MIC × 0.5, MIC × 1 and MIC × 2. The growth of bacteria was observed at the time intervals of 2 hrs (Fig. 3A–D). The bacterial strains growth was reduced upon treatment of plant extract at different concentrations. Time killing kinetics indicated a dose-dependent bactericidal effect of plant extract on tested bacteria. Our data distinctly suggest potent antibacterial activity of M. aurea against pathogenic periodontal bacteria.
Fig. 3.
Effect of M. aurea on bacterial growth kinetics. Representative bacterial strains of (A) P. gingivalis, (B) T. denticola, (C) T. forsythia, and (D) A. actinomycetemcomitans were treated with different concentrations (MIC × 0.5, MIC × 1 and MIC × 2) of ethanolic extract of M. aurea. Growth cycle of untreated organisms served as growth control. Absorbance at 610 nm was measured at regular time intervals of 2 h. Results are presented from three independent experiments using means ± SD. ***p < 0.0001.
4. Discussion
In this study, we observed that the antibacterial efficacy of the ethanolic flower extract of M. aurea collected from the southern region of Saudi Arabia. M. aurea is one of the ancient medicinal herb known as “star of the medicinal species”. As per our information, there is no study described an in vitro efficacy of M. aurea on periodontal pathogenic flora and its role in diminishing the virulence properties in a dose-dependent manner. The ethanolic extract of M. aurea showed potent antibacterial effect against the entire tested periodontal pathogenic flora such as T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans. The MIC and MBC ranged from 0.39 to 1.56 mg/ml and 1.56 to 6.25 mg/ml, respectively. The antibacterial effect of plant extract was found highest against T. forsythia (MIC = 0.39 mg/ml, MBC = 1.56 mg/ml), and lowest against A. actinomycetemcomitans (MIC = 1.56 mg/ml, MBC = 6.25 mg/ml). Earlier it was reported by the group of the authors that the essential oil, as well as Matricaria recutita aerial extract, have promising antimicrobial and antioxidants compounds, emphasizing that the plant is an essential natural source for future drug development (McKay and Blumberg, 2006, Ahmad, 2019). Our findings are also supported with the outcomes of the research that affirmed significant antimicrobial effect of ethanol, methanol and acetone extracts of C. zeylanicum against periodontal flora (Aneja et al., 2009).
This study is coherent with our previous report (Saquib et al., 2019), that exhibited a significant involvement between these bacterial strains and chronic periodontitis. It must be noticed that when T. forsythia was incorporated in the deterioration model, it was appreciably related to the diseases in a dosage-dependent approach. T. forsythia has been recognized to be intensely associated with other members of the red complex bacteria, T. denticola and P. gingivalis, both regarding quantity and prevalence of bacteria and this is wholly unsurprising (Byrne et al., 2009). The latest study revealed the substantial antibacterial activity of cinnamon oil against the oral pathobionts viz. S. mitis, S. mutans, S. salivarius, P. gingivalis, F. nucleatum and A. actinomycetemcomitans (Kim and Park, 2017). Additionally, the microbiological report described that ethanolic cinnamon extract was efficient in putting down the acid production and bacterial adhesion (Zainal-Abidin et al., 2013).
The progress of novel antibacterial agents is limited because of an inadequate number of recognized drug targets in bacteria (Wei et al., 2011). These aspects, in turn, intensify the resistance development to the existing antibacterial agents and emphasize the need for the development of new antibacterial drugs with various target sites. Based on these pieces of evidence, this study was aimed at main virulence factors in T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans, such as adherence and biofilm formation. Therefore, pointing the virulence factors might be a novel model towards the development of new and efficient antibacterial for pathogenic periodontal pathobionts.
The virulence property of the bacteria is explaining their capability to adhere to and formation of biofilm. This study also revealed that M. aurea inhibited the adhesion and biofilm formation. Adherence properties are directly related to the formation of biofilm and thus leads to plaque formation. Red complex bacterial cells have specialized type of adhesins proteins that enable the bacteria to adhere to the host cells (Steinberg, 2000). The adhesins of the periodontal bacteria encode host cell surface glycoproteins. From the findings of this study, it was noticed that the ethanolic extract of M. aurea significantly inhibited adherence in T. forsythia, P. gingivalis, T. denticola and A. actinomycetemcomitans in a dose-dependent approach with a range of 30 to 80%.
Furthermore, T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans adherence to host tissue leads to the formation of biofilm. Biofilm, vital virulence factors in periodontal bacteria, which enhances resistance to the most conventional antibacterial drugs (Cazzaniga et al., 2015). Moreover, numerous reports have been informed that biofilms are much resistant than planktonic cells for existing antibacterial treatment and the formation of the biofilm to the salivary pellicle leads to the formation of dental plaque (Marsh, 2006). In the present study, it was observed that the plant extract was significantly suppressing the formation of biofilm in a concentration dependent approach between 32–86% and 20–77% after the treatment of 24 hrs and 48 hrs respectively. The results of the current study support to assume that M. aurea targets the membrane adhesive proteins, which assist adherence, thus inhibiting adherence to the host cells, whereas consequently inhibiting biofilm formation.
A report by in-situ fluorescent hybridization has established that P. intermedium colonies are present in the top upper layer of dental plaque, although Treponemes is present outside the top layer (Zijnge et al., 2010). The proximity of these two bacteria can be suggestive of cell adherence. Additionally, many genes are associated with motility, transport, metabolism and plasma membrane proteins have revealed to be controlled in T. denticola in the occurrence of P. intermedia (Sarkar et al., 2014). Moreover, dentilisin formed by T. denticola can split the (C3) complement factor and the negative regulatory complement factor H (Miller et al., 2014). It is believable that their mutual proteolytic efficacies may be much efficient in moderating host immune reaction than independently. Nonetheless, it should be kept noticing that the interactions of bacteria within the biofilm are complex, and maximum strains are in associated with each other (Loozen et al., 2014).
This study showed the excellent antibacterial efficacy of the bioactive of M. aurea extract used in this study. M. aurea inhibits the adhesion and biofilm formation of the red-complex bacteria. Though, it requires further fractionation for compounds purification to define their antibacterial efficacy separately or in combination.
5. Conclusion
This study delivers new information about the antibacterial potential of M. aurea ethanolic flower extract against red-complex pathogenic periodontal flora. The ethanolic extract significantly diminished the pathogenicity of T. forsythia, T. denticola, P. gingivalis and A. actinomycetemcomitans in terms of adherence and biofilm formation. These results promote that the M. aurea extract might be the origin of an active compound to be utilized for the therapy of chronic periodontitis, which may draw these compounds to the next phase of development of the drug. More studies are required to be carried out in isolating the potential bioactive compounds exist in ethanolic extract of M. aurea flowers and to find its mechanism of action in curing chronic periodontitis.
Author’s contribution
IA, SW, NN, AD, MYS and SSA designed the study. IA, SW and AD performed and supervised the plant collections. IA, SW, SI and SS performed laboratory investigations. IA, MMA, SI and SS analysed the data. IA, NN, SW, SI, MYS and SSA drafted and thoroughly reviewed the manuscript. Each authors approved the final manuscript.
Declaration of Competing Interest
The authors reveal no conflicts of interest concerning the work reported in this article.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through General Research Project under grant number (GRP-49-41).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Irfan Ahmad, Email: irfancsmmu@gmail.com.
Ayed A. Dera, Email: ayedd@kku.edu.sa.
Mohammad Y Alshahrani, Email: moyahya@kku.edu.sa.
Mohammad Mahtab Alam, Email: mmalam@kku.edu.sa.
References
- Abdoul-Latif F.M., Mohamed N., Edou P., Ali A.A., Djama S.O., Obame L.C., Bassole I.H.N., Dicko M.H. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L. from Djibouti. J. Med. Plants Res. 2011;5(9):1512–1517. [Google Scholar]
- Ahmad I. Antibacterial activity of Nepeta deflersiana extracts against pathogenic Gram-positive bacteria. Res. J. Biotechnol. 2019;14(7):43–51. [Google Scholar]
- Al-Mustafa A.H., Al-Thunibat O.Y. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci. 2008;11:351–358. doi: 10.3923/pjbs.2008.351.358. [DOI] [PubMed] [Google Scholar]
- Anand B. Herbal therapy in periodontics: a review. J. Res. Pharm. Sci. 2017;3(5):1–7. [Google Scholar]
- Aneja K.R., Joshi R., Sharma C. Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J. Pharm. Res. 2009;2:1387–1390. [Google Scholar]
- Byrne S.J., Dashper S.G., Darby I.B., Adams G.G., Hoffmann B., Reynolds E.C. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol. Immunol. 2009;24:469–477. doi: 10.1111/j.1399-302X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- Cazzaniga G., Ottobelli M., Ionescu A., Garcia-Godoy F., Brambilla E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am. J. Dent. 2015;28(6):311–320. [PubMed] [Google Scholar]
- Christina P., Velitchka D.P., Vladimir P. Microbiology of periodontal diseases. A review. Biotechnol. Biotechnol. Equip. 2013;27:3754–3759. [Google Scholar]
- Fazly A., Jain C., Dehner A.C., Issi L., Lilly E.A., Ali A., Cao H., Fidel P.L., Jr., Rao R.P., Kaufman P.D. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc. Natl. Acad. Sci. 2013;110:13594–13599. doi: 10.1073/pnas.1305982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick S.G., Katz J. The association between periodontal disease and cancer: a review of the literature. J. Dent. 2010;38:83–95. doi: 10.1016/j.jdent.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Floyd E.D., Tuste C., Jacques I., Bruce J.P., Anne C.R.T., Wen-Han Y., Abirami L., William G.W. The human oral microbiome. J. Bacteriol. 2010;192(19):5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig C., Hannig M. The oral cavity - a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Invest. 2009;13:123–139. doi: 10.1007/s00784-008-0243-3. [DOI] [PubMed] [Google Scholar]
- Hossain M.Z., Fageeh H., Elagib M. Prevalence of Periodontal Diseases among Patients Attending the Outpatient Department at the College of Dentistry, King Khalid University, Abha, Saudi Arabia. City Dent. College J. 2013;10(1):9–12. [Google Scholar]
- Humaira R., Mona S.A., Dina A.S. Antimicrobial Activity and Chemical Composition of Flowers of Matricaria aurea a Native Herb of Saudi Arabia. Int. J. Pharmacol. 2016;12(6):576–586. [Google Scholar]
- Ismail M.C., Ibrahim K.M., Lateef N.S. Effect of flower extracts of Matricaria chamomilla L. on some bacteria causing eye infections. Diyala J. Pure Sci. 2011;7(3):97–109. [Google Scholar]
- Jin Y., Samaranayake L.P., Samaranayake Y., Yip H.K. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral. Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kaur A., Kapoor D., Soni N., Gill S. Phytodentistry – a boon??? Arch. Dent. Med. Res. 2016;2(4):35–41. [Google Scholar]
- Kim H., Park J. In vitro evaluation of anti-caries effect of cinnamon extracts on oral pathogens. Biomed. Res. 2017;28:2848–2853. [Google Scholar]
- Kitti T., Supawadee J., Orawan C., Yingampa G. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia co-infection are associated with severe periodontitis in a Thai population. PLoS ONE. 2015;10(8):e0136646. doi: 10.1371/journal.pone.0136646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P.E., Palmer R.J., Rickard A.H., Jakubovics N.H., Chalmers N.I., Diaz P.I. Bacterial inter-actions and successions during plaque development. Periodontology. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Loozen G., Ozcelik O., Boon N., De Mol A., Schoen C., Quirynen M.T. Inter-bacterial correlations in subgingival biofilms: a large-scale survey. J. Clin. Periodontol. 2014;41:1–10. doi: 10.1111/jcpe.12167. [DOI] [PubMed] [Google Scholar]
- Magaldi S., Mata-Essayag S., Hartung C.C., Perez C., Colella M.T., Olaizola C., Ontiveros Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004;8(1):39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Marsh P.D. Dental plaque as a biofilm and a microbial community-implications for health and disease. BMC Oral Health. 2006;6:S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, P.D., Martin, M.V., 2009. Oral Microbiology, fifth ed., Churchill Livingstone.
- McKay D.L., Blumberg J.B. A review of bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother. Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- Meyer M.S., Joshipura K., Giovannucci E., Michaud D.S. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.P., McDowell J.V., Bell J.K., Goetting-Minesky M.P., Fenno J.C., Marconi R.T. Analysis of the complement sensitivity of oral treponemes and the potential influence of FH binding, FH cleavage and dentilisin activity on the pathogenesis of periodontal disease. Mol. Oral. Microbiol. 2014;29:194–207. doi: 10.1111/omi.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- Neelma M., Ayesha S.I., Imran A., Rasheeda B., Nadia S., Faiza S., Shagufta N. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa Belladonna and Matricaria Chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014;11(5):111–117. doi: 10.4314/ajtcam.v11i5.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita V., Patthi B., Singla A., Singh S., Malhi R., Vashishtha V. Dentistry meets nature – role of herbs in periodontal care: a systematic review. J. Indian Assoc. Public Health Dent. 2014;1(3):148–156. [Google Scholar]
- Raut J.S., Shinde R.B., Chauhan N.M., Karuppayil S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling. 2013;29:87–96. doi: 10.1080/08927014.2012.749398. [DOI] [PubMed] [Google Scholar]
- Saquib S., Alqahtani N., Ahmad I., Kader A., Al-Shahrani S., Asiri E. Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontopathic bacteria: An in vitro microbiological study. Antibiotics. 2019;8(89):1–12. doi: 10.3390/antibiotics8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J., McHardy I.H., Simanian E.J., Shi W., Lux R. Transcriptional responses of Treponema denticola to other oral bacterial species. PLOS One. 2014;9:e88361. doi: 10.1371/journal.pone.0088361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shama N.S., Prasanna K.R., Joshna A., Lakshmi S.T. Effect of herbs on periodontitis – a serious gum infection. Int. J. Pharmacol. Res. 2014;4(1):17–22. [Google Scholar]
- Shamami M.S., Sadighi S.M., Amini S. Periodontal disease and tooth loss as risks for cancer: a systematic review of the literature. Iran J. Cancer Prev. 2011;4(4):189–198. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui N.A. Chemical constituents of essential oil from flowers of Matricaria aurea grown in Saudi Arabia. Indian J. Drugs. 2014;2:164–168. [Google Scholar]
- Singh O., Khanam Z., Misra N., Srivastava M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011;5(9):82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourav D., Barbara H., Silvija S., Stela J., Aleksandar S., Tamas K. Antimicrobial activity of chamomile essential oil: effect of different formulations. Molecules. 2019;24(23):4321. doi: 10.3390/molecules24234321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Studying plaque biofilms on various dental surfaces. In: An Y.H., Friedman R.J., editors. Handbook of Bacterial Adhesion: Principles, Methods, and Applications. Humana Press; New Jersey, USA: 2000. pp. 353–370. [Google Scholar]
- Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- Wei J.R., Krishnamoorthy V., Murphy K., Kim J.H., Schnappinger D., Alber T., Christopher M.S., Kyu Y.R., Eric J.R. Depletion of antibiotic targets has widely varying effects on growth. Proc. Natl. Acad. Sci. 2011;108(10):4176–4181. doi: 10.1073/pnas.1018301108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal-Abidin Z., Mohd-Said S., Abdul-Majid F.A., Mustapha W.A.W., Jantan I. Anti-bacterial activity of cinnamon oil on oral pathogens. The Open Conf. Proce. J. 2013;4:12–16. [Google Scholar]
- Zijnge V., van Leeuwen M.B., Degener J.E., Abbas F., Thurnheer T., Gmur R., Harmsen H.J.M. Oral biofilm architecture on natural teeth. PLOS One. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]