Significance
In plants, hormone signaling is, to some extent, controlled by transcriptional repressors that are degraded in a hormone-dependent manner. These repressor complexes consist of multiple proteins that assemble. MicroProteins are small single-domain proteins that are sequence-related to larger, multidomain proteins and act by disrupting protein complex formation. By forming protein complexes, or by sequestering signaling proteins, microProteins can act as effective modulators of protein activity. Our studies have discovered LITTLE NINJA, a NINJA-related microProtein that modulates JA signaling by attenuating the repression of JA-signaling. Transgenic plants ectopically expressing LITTLE NINJA are shorter and bushier than wild-type plants. Modulating LITTLE NINJA activity may provide strategies for future crop improvement and contribute to finding sustainable solutions for future agricultural production.
Keywords: microProteins, jasmonic acid, branching
Abstract
MicroProteins are small, often single-domain proteins that are sequence-related to larger, often multidomain proteins. Here, we used a combination of comparative genomics and heterologous synthetic misexpression to isolate functional cereal microProtein regulators. Our approach identified LITTLE NINJA (LNJ), a microProtein that acts as a modulator of jasmonic acid (JA) signaling. Ectopic expression of LNJ in Arabidopsis resulted in stunted plants that resembled the decuple JAZ (jazD) mutant. In fact, comparing the transcriptomes of transgenic LNJ overexpressor plants and jazD revealed a large overlap of deregulated genes, suggesting that ectopic LNJ expression altered JA signaling. Transgenic Brachypodium plants with elevated LNJ expression levels showed deregulation of JA signaling as well and displayed reduced growth and enhanced production of side shoots (tiller). This tillering effect was transferable between grass species, and overexpression of LNJ in barley and rice caused similar traits. We used a clustered regularly interspaced short palindromic repeats (CRISPR) approach and created a LNJ-like protein in Arabidopsis by deleting parts of the coding sentence of the AFP2 gene that encodes a NINJA-domain protein. These afp2-crispr mutants were also stunted in size and resembled jazD. Thus, similar genome-engineering approaches can be exploited as a future tool to create LNJ proteins and produce cereals with altered architectures.
To successfully respond to changing environmental conditions, plants have evolved sophisticated signaling systems. These signaling systems consist of proteins that form dynamic higher order structures and rely on protein-protein interactions as well as protein turnover. The formation of protein complexes can be effectively influenced by microProteins. MicroProteins are short single-domain proteins that are sequence-related to larger, often multidomain proteins, and they usually act by engaging the larger related proteins in different protein complexes to control biological processes (1, 2). MicroProtein-coding genes are products of genome evolution events involving either genome duplication or local amplifications followed by evolutionary trimming. Consequently, all microProteins that have been studied to date are sequence-related to the proteins they regulate (2). An example of microProtein function is the modulation of flowering and photomorphogenesis by small B-Box containing miP1a/b-type microProteins that are sequence-related to the CONSTANS (CO) transcription factor (3, 4). MiP1a/b-type microProteins evolved in dicotyledonous plants and besides the B-Box domain, these proteins have an additional TOPLESS-interaction domain. TOPLESS is a transcription corepressor protein that was initially identified to act in auxin signaling (5, 6). It has been shown that the flowering effect strongly depends on the ability of the microProteins to interact with TOPLESS, likely engaging CO in a repressor complex. Interestingly, another microProtein, the MINI ZINC FINGER2 also interacts physically with TOPLESS to control floral meristem termination (7).
TOPLESS does not only act in auxin signaling, but it is also a central component of jasmonic acid (JA) signaling where it engages in a JAZ/NINJA/TPL complex; JA is known as a plant defense hormone. In the absence of JA, the JAZ/NINJA/TPL complex interacts with transcription factors and converts these into repressors of gene expression. When JA is produced or ectopically applied, the CORONATINE INSENSITIVE 1 (COI1) JA-receptor recruits an ubiquitin-SCF-complex to degrade the JAZ-repressor. This degradation alleviates the repressive activity on the transcription factors that can in turn induce JA-responsive gene expression (8). Modulation of JA signaling in different plant species has been associated with altered plant performance such as increased tolerance to salt and drought and higher resistance to pathogens and pests (9). The uncoupling of JA signaling by mutating multiple genes encoding JAZ repressor proteins resulted in slower growing, stunted plants that were less fertile but more resistant to pathogens (10). These and other findings support a model in which JA adjusts defense and growth responses by rewiring the central carbohydrate metabolism.
Here we identified a microProtein with a role in controlling shoot branching. Shoot branching is regulated by different plant hormones and also by microProteins. One of these hormones are brassinosteroids (BRs), which mainly affect plant size through the regulation of cell elongation (11). In rice, BRs also induce bending of the leaf lamina, which can be mimicked by ectopic expression of a helix–loop–helix (HLH)-type microProtein (12, 13). Other plant hormones that have been implicated in controlling the development of side shoots are strigolactones, auxin, and cytokinin. For the latter two, the ratio of cytokinin to auxin has been proposed to be crucial, and recent discoveries support a hierarchical relationship in which cytokinin regulates auxin transport (14). Strigolactones act as suppressors of axillary meristem outgrowth. Hence mutations in Arabidopsis genes involved in the production and dissipation of the strigolactone-signal result in plants with a bushy appearance (15–18). Corresponding mutations in orthologous rice genes cause increased numbers of shoots (19, 20), suggesting that strigolactones are ancient branching regulators.
In this study, we used a computational approach to identify microProteins that exist in monocotyledonous plants and validated their biological activity by misexpressing synthetic variants in Arabidopsis as a heterologous host. LITTLE NINJA (LNJ) a microProtein related to NINJA proteins caused when overexpressed in Arabidopsis, Brachypodium, barley, and rice a reduction of plant size. Additionally, transgenic plants also appeared bushier. The sequence relationship to NINJA prompted us to investigate a possible role in JA signaling; in agreement, we found that LNJ can modulate JA-signaling. The latter effect is likely through the poisoning of the pool of NINJA proteins required for the repression of JA-signaling. Hormone profiling revealed substantial changes in transgenic plants that likely account for the bushy appearance. Our finding that the LITTLE NINJA effect is transferrable between distant cereal crops makes it a promising breeding target. Thus, our results have significant implications for the complex regulation of JA-mediated transcriptional regulation and its potential role in future crop improvement.
Results
Identification of LNJ, a Protein Isoform Related to NINJA.
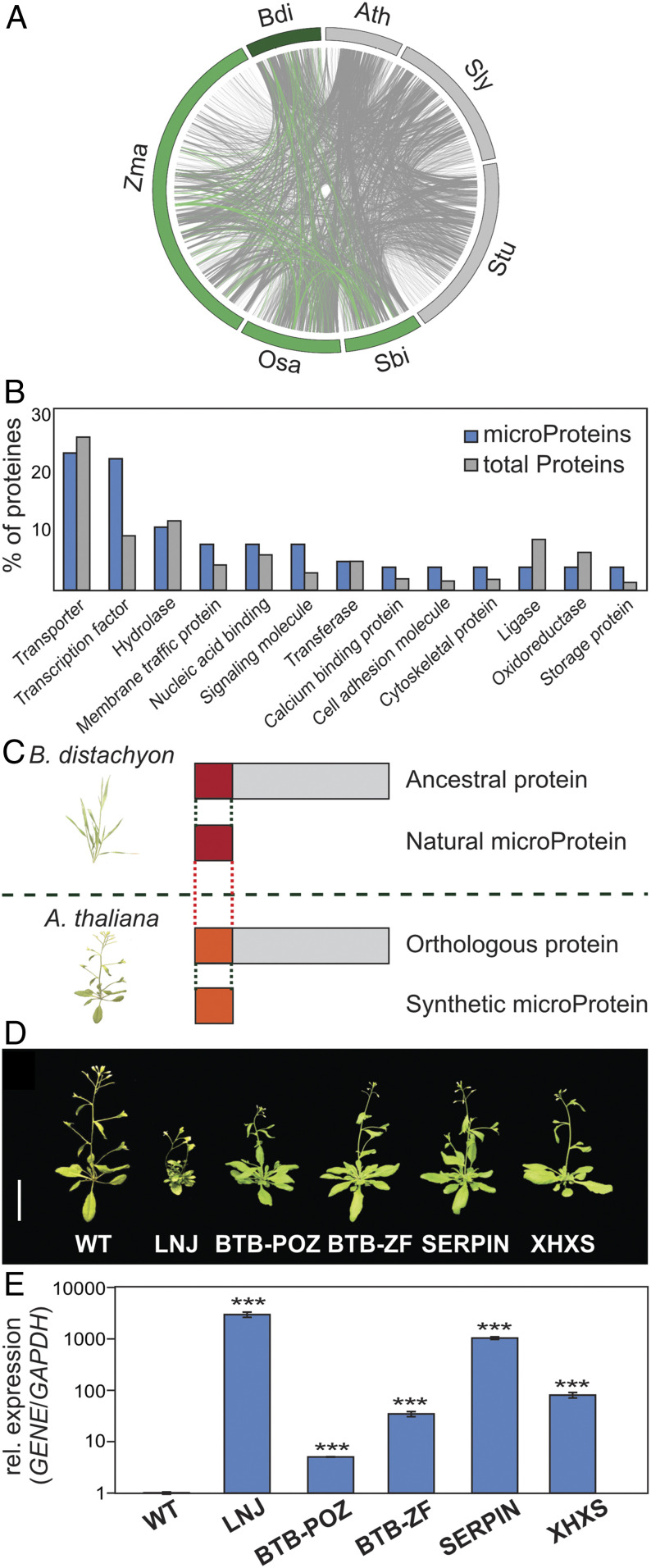
To identify new regulators of development, we employed a computational approach to detect monocot microProteins. Using the MiPFinder pipeline (21) we developed earlier, we first identified plant-specific microProteins and focused on the fraction that is present in monocotyledonous plants (Fig. 1A). Here, we focused on microProteins that can be identified in one of the monocotyledonous reference genomes (rice, sorghum, maize, or Brachypodium), but not in any of the dicotyledonous reference genomes (Arabidopsis, potato, tomato). In-depth analysis of this subset of microProteins encoded in the Brachypodium distachion genome revealed an enrichment for microProteins related to transcription factors and signaling molecules (Fig. 1B). We then decided to use transformation-efficient Arabidopsis plants to prescreen regulators of interest. In the Brachypodium genome, we found genes encoding microProteins as well as the corresponding ancestral, larger proteins. For the selected monocot-specific microProteins, we only found the ancestral larger proteins in Arabidopsis but not in the corresponding microProteins (Fig. 1C). This enabled us to develop so-called synthetic microProteins predicted to interfere with the ancestral proteins in Arabidopsis, potentially causing deviations from the normal growth behavior. In an initial screening, we overexpressed five such synthetic microProtein candidates in transgenic Arabidopsis plants. This strategy resulted in the identification of one candidate, which we named LNJ. Sequence analysis of LNJ revealed that it is closely related to the Arabidopsis ABI5-BINDING PROTEIN2 (AFP2) gene that encodes a protein containing a NINJA domain. Overexpression of full-length AFP2 has recently been shown to delay flowering while the afp2 mutant plants flowered earlier than wild-type (22). These phenotypes contrast what we observed in transgenic LNJ-OX plants that were stunted and lost apical dominance (Fig. 1D). This indicates that the dominant-negative function of LNJ differs from the biological functions of AFP2.
Fig. 1.
Identification of monocot-specific microProtein candidates and production of synthetic Arabidopsis microProteins. (A) Circos plot depicting evolutionary relationships of plant microProteins. Connections show conservation between species based on OrthoFinder. Dark-green arc indicates B. distachyon21-3 (Bdi). Green arcs indicate representative monocotyledonous plants; Zea mays (Zma), O. sativa (Osa), and Sorghum bicolor (Sbi). Gray arcs indicate representative dicotyledonous plants; Arabidopsis thaliana (Ath), Solanum lycopersicum (Sly), and Solanum tuberosum (Stu). Green lines specify the fraction of monocot-specific microProteins while gray lines specify microProteins present in all plant genomes. (B) Functional annotation of microProteins by protein classification using PANTHER (www.pantherdb.org/). The y axis indicates the percentage of unigenes in specific functional clusters. (C) Natural microProteins investigated here are monocot specific and possess conserved ancestral proteins in the Brachypodium genome. The Arabidopsis genome encode monocot-specific microProtein ancestors (orthologous protein). Based on the structure of the natural Brachypodium microProtein, Arabidopsis synthetic microProteins were designed. (D and E) Phenotype (D) and relative expression levels (E) of the transgenic plants overexpressing synthetic microProtein genes. Arabidopsis glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was used as reference gene for qRT-PCR and values were calibrated to the wild-type control (expression set to 1). Values are the means ± SD *P < 0.05, **P < 0.005, ***P < 0.0005 determined by Student’s t test. n = 3. (Scale bar, 10 cm.)
A further inspection of the LNJ origin uncovered that it is as expected not present in Arabidopsis, tomato, and potato, but it is also not monocot specific. Furthermore, LNJ is not encoded by an individual gene but is a potential splice isoform of a larger NINJA protein that had not been correctly annotated in the Brachypodium genome (SI Appendix, Fig. S1). Similar to Brachypodium, the same LNJ isoform is also annotated in maize, indicating that LNJ-like microProteins might be produced by alternative splicing. Inspection of the LNJ protein sequence in dicot genomes revealed that some species encode small LNJ-like microProteins as single copy genes. However, their evolutionary origin cannot be easily traced back and it seems that these microProteins evolved independently from different types of NINJA protein precursors.
The misexpression of other synthetic microProtein candidates produced phenotypically normal plants under standard growth conditions. All transgenic plants had high levels of synthetic microProtein messenger RNAs (mRNAs), supporting that they all were genuine overexpressors (Fig. 1E). Our strategy of using Arabidopsis as a heterologous host for ectopic synthetic microProtein expression proved to be successful and it can be used for future microProtein characterization of species where transformation is not easy or not yet possible.
Arabidopsis LNJ-OX Plants Resemble jaz-D Mutant Plants.
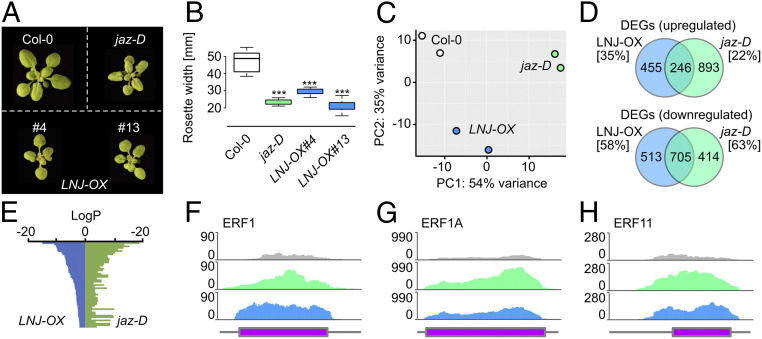
Having established that overexpression of LNJ in Arabidopsis causes a stunted growth phenotype resembling the recently described jazD decuple mutant (10), we assessed growth of the latter mutant in comparison to Col-0 wild-type and LNJ-OX plants. In agreement with previous findings (10), we observed a stunted growth phenotype of jazD bearing resemblance to LNJ-OX plants (Fig. 2 A and B). Multiple pathways have been described that can affect plant size. We therefore isolated RNA from Col-0 wild-type, jazD, and LNJ-OX plants to compare their individual transcriptomes (Dataset S1). Principle component analysis of the data revealed that individual replicates grouped together, indicating reproducible but distinct datasets (Fig. 2C). The analysis of differentially expressed genes (DEGs) identified 701 genes that were ectopically up-regulated in LNJ-OX plants and 1,139 genes that were highly expressed in jazD compared to Col-0 wild-type (Fig. 2D). In total, 246 genes were up-regulated in both LNJ-OX and jazD, corresponding to 35% and 22%, respectively. The analysis of down-regulated transcripts discovered 1,218 DEGs in LNJ-OX, 1,119 DEGs in jazD, and an overlap of 705 genes. The latter translates into an overlap of 58% in LNJ-OX and 63% in jazD (Fig. 2D). A comparative analysis of the down-regulated genes revealed also a significant overlap with regards to deregulated gene ontology categories (Fig. 2E). These results indicate that the stunted growth phenotype could be due to the deregulation of similar genes and is likely a result of ectopic JA signaling. Previous RNA sequencing (RNA-seq) studies of jazD have identified a number of ETHYLENE RESPONSE FACTOR (ERF) genes that were highly expressed in jazD (10). We checked the expression of three ERFs (ERF1, ERF1A, and ERF11) and found these to be strongly induced in both jazD and LNJ-OX plants (Fig. 2 F–H), further supporting the idea that these plants are affected in the same signaling pathway.
Fig. 2.
Ectopic expression of the Arabidopsis LNJ protein changes plant size and transgenic plants resemble jaz-D mutant plants. (A) Picture of representative 20-d-old seedlings. (B) Rosette width of wild-type Col-0, jaz-D, and two LNJ-OX transgenic plants. Box plots show the observed experimental data. Rosette width was determined after the transition to flowering. ***P < 0.0005 determined by Student’s t test. n = 11 to 13. (C) Principle component analysis (PCA) of the gene expression (regularized logarithm transformed count data) between the different RNA-seq libraries. Plotted is the percentage of variance for each component for each of the two biological samples per genotype. (D) Venn diagram showing the overlap of differentially expressed genes (log2FC > +1 and < −1, BH-adj. P value < 0.01) compared to the Col-0 wild-type. Numbers in brackets depict the overlap between the two datasets. (E) Comparative gene ontology analysis of genes down-regulated in LNJ-OX, and jaz-D shows a large overlap in genes and GO categories enriched in the two genetic backgrounds. (F–H) RNA-seq read coverages of representative candidate genes showing expression levels in the three different backgrounds.
LITTLE NINJA Is Functional in Brachypodium.
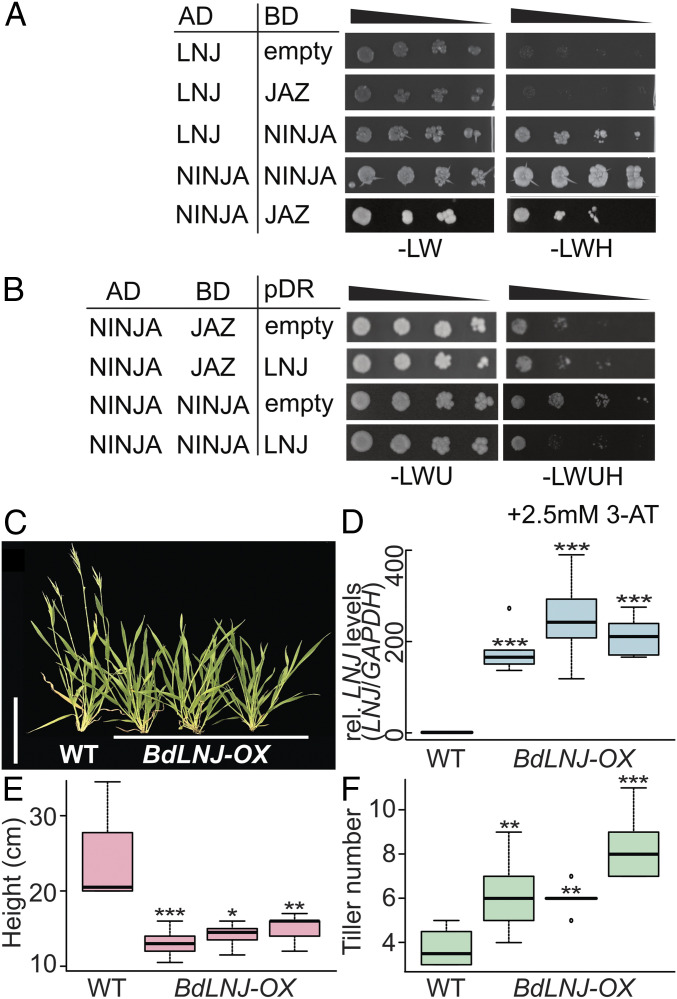
We investigated the sequence of LNJ and the BdLNJ microProtein in more detail and found that it contains the “Domain C” of NINJA proteins (SI Appendix, Fig. S2). NINJA proteins have known roles in jasmonic acid (JA) signal transduction and Domain C allows NINJA to interact with JAZ proteins (8). Similar to the situation in auxin signaling, JAZ/NINJA proteins recruit the TOPLESS (TPL) corepressor to transcription factors, here MYC-type factors that regulate JA response genes. Thus, downstream target genes are constantly repressed in the absence of JA. Once JA is present, it initiates the degradation of JAZ through the proteasome pathway, which results in the induction of JA-responsive genes (23, 24). Based on the protein sequence of LNJ, we hypothesized that it would influence jasmonic acid signaling either by shielding JA-responsive transcription factors from being repressed by the NINJA/TPL complex or by sequestering JAZ/NINJA/TPL directly (SI Appendix, Fig. S3). We tested in yeast whether LNJ interacted with either JAZ or NINJA and found an interaction with NINJA but not with JAZ (Fig. 3A). We also detected that NINJA can dimerize in yeast and LNJ can influence the dimeric state of NINJA repressors but not the heteromeric state of NINJA/JAZ (Fig. 3B). A negative influence on the formation of NINJA complexes could result in ectopic JA signaling activity in the absence of the hormone. Jasmonic acid has diverse roles in regulating developmental processes such as seed germination, root development, and senescence, but it is best known as a plant defense hormone (25).
Fig. 3.
LNJ interacts with NINJA and ectopic expression alters the shoot architecture of Brachypodium plants. (A) Yeast-two-hybrid interaction of LNJ (BRADI1g69970) fused to the Gal4 AD and the Gal4 BD and fusions of BD to JAZ (BRADI1g21490) and NINJA (BRADI2g11790). LNJ interacts with NINJA as indicated by growth on selective medium (−LWH). No interaction was observed between LNJ and JAZ but NINJA and JAZ interact and NINIJA can also homodimerize as indicated by growth on selective medium of yeast expressing both AD-NINJA and BD-NINJA or BD-JAZ. (B) Yeast-three-hybrid interaction assay. Yeast expressing AD fusions to NINJA and BD fusions to JAZ or NINJA were transformed with either pDR-empty or pDR-LNJ. NINJA/JAZ and NINJA/NINJA dimerization was observed on quadruple dropout medium in the presence of the empty vector, in the presence of LNJ; NINJA/JAZ heterodimerization remained unaffected but homodimerization of NINJA was weakened as indicated by a reduced ability to grow on the selective medium. (C) Picture of 4-wk-old plants of the wild-type Bd21-3 and three independent BdLNJ-OX lines grown under long day (LD) photoperiod (22/18 °C, 16 h light/8 h dark). (Scale bar, 10 cm.) (D) Relative expression levels of BdLNJ mRNA in wild-type and independent T3 transgenic plants determined by qRT-PCR. Expression levels, determined as relative quantities and normalized against Brachypodium GAPDH gene, are depicted as box plots, n = 3. (E) Quantification of plant height depicted as box plots in wild-type and three independent BdLNJ-OX lines grown under LD photoperiod. (F) Quantification of tiller number depicted as box plots in wild-type and three independent BdLNJ-OX lines grown under LD photoperiod. *P < 0.05, **P < 0.005, ***P < 0.0005 determined by Student’s t test. n = 15 to 20.
To determine whether ectopic expression of the Brachypodium LNJ (BdLNJ) would also affect growth and development of Brachypodium plants, we generated transgenic lines overexpressing the LNJ microProtein (BdLNJ-OX). Phenotypic analysis of transgenic T2 and T3 plants revealed a significant reduction of the height of adult plants that coincided with increased levels of transgene expression (Fig. 3 C–E). Besides having a small stature, BdLNJ-OX transgenic plants developed more axillary shoots compared to nontransgenic wild-type plants (Fig. 3F). Taken together, BdLNJ acts as a dominant master regulator of plant stature and leads to shorter, more branched Brachypodium plants.
To test if JA influences the size and branching of Brachypodium, we treated wild-type and BdLNJ-OX plants with 100 μM methyl jasmonate (MeJA) by spraying. Notably, the size of wild-type plants was strongly affected by the MeJA treatment and treated plants resembled BdLNJ-OX plants. On the contrary, BdLNJ-OX plants showed no further reduction in growth and thus appeared unresponsive to MeJA (SI Appendix, Fig. S4 A and B). We next analyzed whether the degrees of shoot branching of wild-type and BdLNJ-OX plants treated with either MeJA or a mock solution was affected. No increased numbers of axillary branches were observed in response to MeJA treatment (SI Appendix, Fig. S4C), indicative of additional or parallel functions of BdLNJ. We conclude that JA had an impact on plant size and the finding that BdLNJ-OX plants were not responsive to ectopic MeJA application supports the idea that JA signaling is affected in BdLNJ-OX transgenic plants, contributing to the phenotypic changes we observed.
LITTLE NINJA Affects JA Signaling in Brachypodium.
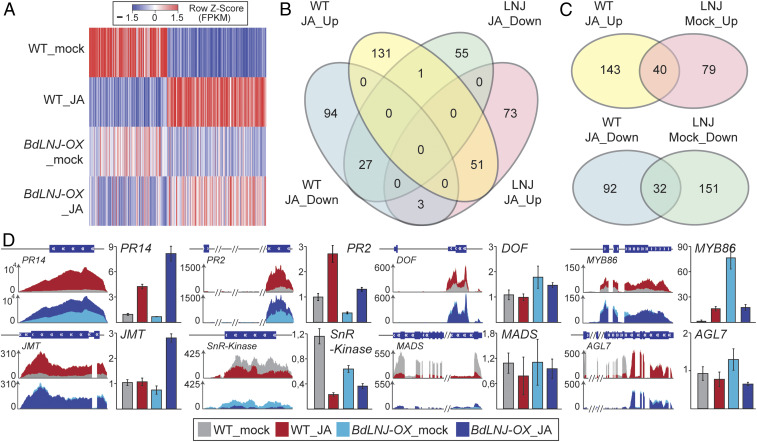
We had implicated BdLNJ in jasmonic acid signaling and therefore sought to determine whether transgenic BdLNJ-OX plants have an altered molecular response to JA. To test this, we performed a mRNA-sequencing experiment with RNA isolated from wild-type and transgenic BdLNJ-OX plants that were either treated with MeJA or a mock solution. Our analysis discovered 183 genes to be significantly up-regulated by MeJA treatment in wild-type plants and 127 genes to be up-regulated in transgenic BdLNJ-OX plants (Fig. 4 A and B and Dataset S2). On the contrary, we found 124 genes in wild-type and 183 genes in BdLNJ-OX plants to be down-regulated in response to MeJA (Fig. 4 A and B). The global analysis of the MeJA-influenced transcriptome in BdLNJ-OX plants compared to wild-type showed significant changes (Fig. 4 A and B). Interestingly, in contrast to Arabidopsis, we observed no significant transcriptional changes of JAZ genes in response to MeJA treatments. On the other hand, in-depth analysis of the 183 genes up-regulated by MeJA in wild-type revealed that as many as 40 genes (corresponding to around 22%) are ectopically induced in BdLNJ-OX plants (Fig. 4C). Similarly, for the 124 genes down-regulated by MeJA treatment of wild-type plants, we find 32 genes (corresponding to 26%) to be expressed at reduced levels in mock-treated BdLNJ-OX plants. Genes regulated by MeJa or by ectopic BdLNJ expression encode enzymes such as JASMONIC ACID METHYLTRANSFERASE (JMT), diverse transcription factors, such as the Brachypodium ortholog to MYB86, and enzymes such as β-1,3-GLUCANASE (Dataset S2). Some of these factors, such as JMT and β-1,3-GLUCANASE have established roles in jasmonic acid signaling in Arabidopsis (26, 27). We investigated gene expression changes that were initially observed by mRNA-seq by using qRT-PCR (Fig. 4D). We found that some genes change as detected by mRNA-seq, others such as JMT and the MADS genes did not show the expected changes, which could be a result of PCR bias. We conclude that LNJ affects jasmonic acid signaling, likely by interfering with the JAZ/NINJA/TPL repressor complex causing JA-response genes to be expressed as if plants were treated with MeJA.
Fig. 4.
BdLNJ globally affects jasmonic acid signaling. (A) Heat map of differentially expressed genes in wild-type Brachypodium (WT_mock/WT_JA) and BdLNJ-OX plant (LNJ-OX_mock/LNJ-OX_JA) with and without MeJA (mock/JA). The color scale indicates gene expression level (FPKM values). For each genotype and treatment, two biological samples were analyzed. (B) Venn diagram showing DEGs expressed in the four samples. WT indicates wild-type Brachypodium and LNJ indicates BdLNJ-OX plant. The number of genes of JA_UP or JA_Down indicates up-regulated or down-regulated genes in response to MeJA treatment. (C) Venn diagrams showing genes that were significantly induced after MeJA treatment (FDR ≤ 0.05). Induced genes were defined as transcripts with fold changes ≥2 based on gene expression levels between WT JA_UP and LNJ Mock_UP, between WT JA_Down and LNJ Mock_Down, respectively. (D) Validation of candidate genes identified by RNA-Seq using qRT-PCR. RNA-Seq read coverages of genes that are up- or down-regulated in response to MeJA treatment. Gene models depict exons in blue. QRT-PCR analysis of differential gene expression in MeJA treated wild-type Brachypodium and BdLNJ-OX plant, n = 3. RNA-seq read maps and qRT-PCRs show mock-treated wild-type Brachypodium in gray and wild-type MeJA-treated samples in red. The mock-treated BdLNJ-OX samples are shown in light blue and corresponding MeJA-treated samples in dark blue.
To investigate if the changes we observed by mRNA-seq correlate with metabolic alterations, we measured the abundance of the major plant hormones by mass spectrometry. The altered JA transcriptional network in plants ectopically expressing BdLNJ correlated in all three transgenic lines with significantly decreased levels of the active signaling compound JA-isoleucine. In addition, we observed overall perturbations in the hormone profiles of BdLNJ-OX plants, including decreased levels of cytokinins that could impact the indole-3-acetic acid (IAA):cytokinin ratio (SI Appendix, Fig. S5). A skewed IAA:cytokinin ratio might be causal for observed changes in shoot branching. In addition to the changes in hormone levels, we also observed a repression of genes in JA- and SA-related responses in transgenic BdLNJ-OX plants (SI Appendix, Fig. S6), which further supports a role for LNJ in hormone signaling. Taken together, BdLNJ acts as a modulator of JA-responsive gene regulation, accounts for around 25% of all JA-induced gene expression changes, and ectopic expression of LNJ affects plant hormone levels, indicating that LNJ is a regulator of JA signaling.
The LITTLE NINJA Effect Is Transferrable to Cereal Crops.
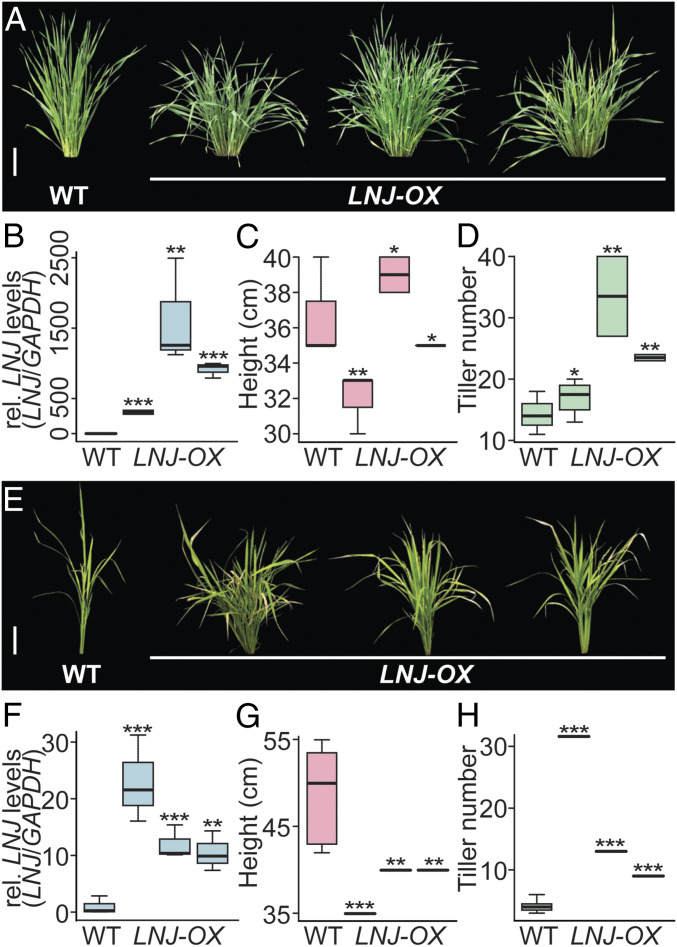
To understand whether BdLNJ acts as a universal regulator of plant architecture, we also generated transgenic barley and rice plants overexpressing BdLNJ. All transgenic barley plants that we generated overexpressed the transgene, but in comparison to Brachypodium were not consistently stunted (Fig. 5 A–C). We used the cultivar Golden Promise for transformation, which in comparison to Brachypodium is a domesticated crop plant and already shorter than wild, nondomesticated relatives. Golden Promise carries a loss-of-function mutation in HvDep1, an AGG3-type subunit encoding gene that positively regulates culm elongation and seed size in barley (28). When assessing the tiller numbers, we do however find significant increases in the number of axillary shoots and transgenic plants had a much bushier appearance. Thus, BdLNJ is functional in barley. Brachypodium and barley are, together with wheat, oat, and ryegrass, part of the Pooideae subfamily. To determine whether similar effects could also be achieved in a more distantly related crop plant, we generated transgenic rice (Oryzoideae) overexpressing BdLNJ. All transgenic plants that were recovered after transformation overexpressed the transgene and produced rice plants that were significantly stunted with increased numbers of tillers (Fig. 5 E–H). Taken together, these results indicate that even the growth behavior of domesticated germplasm can be further optimized by ectopic LNJ expression.
Fig. 5.
LNJ influences shoot architecture of crop plants. (A) Picture of 2-mo-old barley plants. Wild-type barley (H. vulgare cv. Golden Promise) and three independent LNJ-OX lines grown under LD photoperiod (20/15 °C, 16 h light/8 h dark). (Scale bar, 10 cm.) (B) Relative expression levels of LNJ mRNA in wild-type and independent T2 transgenic plants which were determined by qRT-PCR. Expression levels, normalized against barley GAPDH gene, are depicted as box plots; n = 3. (C and D) Quantification of height (C) and tiller number (D) are depicted as box plots in wild-type and three independent LNJ-OX lines grown under LD photoperiod. n = 8 to 15. (E) Picture of 3-mo-old representative rice plants. Wild-type rice (Oryza sativa cv. Nipponbare) and three independent LNJ-OX lines grown under SD photoperiod (28/25 °C, 12 h light/12 h dark). (Scale bar, 10 cm.) (F) Relative expression levels of LNJ mRNA in wild-type and independent T0 transgenic plants which were determined by qRT-PCR. Expression levels, determined as relative quantities and normalized against rice ACTIN gene, are depicted as box plots; n = 3. (G and H) Quantification of height (G) and tiller number (H) are depicted as box plots in wild-type and three independent LNJ-OX lines grown under SD photoperiod. *P < 0.05, **P < 0.005, ***P < 0.0005 determined by Student’s t test.
Disturbances of hormone pathways, especially jasmonic acid could potentially lead to an increased pathogen susceptibility. Therefore, we tested if transgenic LNJ-OX plants respond differently to pathogen attack. Inoculation with the barley pathogen Pyrenophora teres revealed no difference between wild-type barley plants and the transgenic LNJ-OX plants (SI Appendix, Fig. S7). Thus, it appears that plants with constitutive LNJ levels produce more tillers and are not compromised in their response to pathogen attack.
Creating a LITTLE NINJA Gene in Arabidopsis.
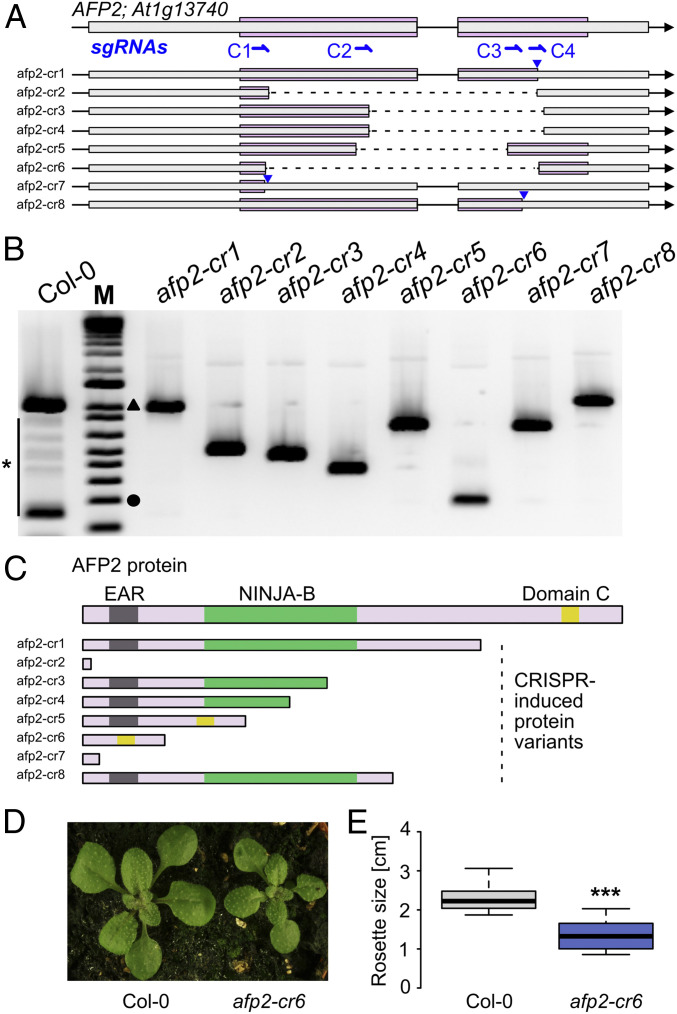
Overexpression of a LITTLE NINJA isoform of AFP2 caused a significant reduction of the rosette size in transgenic Arabidopsis plants (Fig. 2 A and B). This phenotype is different from the afp2 mutant or the overexpression of the full-length protein that has been shown to display alterations in flowering time (22). To test if a functional LITTLE NINJA protein could be engineered into the Arabidopsis genome, we employed a clustered regularly interspaced short palindromic repeats (CRISPR)/Cas-9 approach and designed single guide RNAs that would recognize regions after the translation start site and regions close to the Domain C (Fig. 6A) potentially cleaving out the EAR and NINJA domains. After transformation of the pooled single guide RNAs (sgRNAs), we obtained transgenic plants in which we detected chromosomal deletions in the AFP2 gene (Fig. 6B). Sequencing of the RNA isoforms revealed several AFP2 protein isoforms with altered domain composition (Fig. 6C) including one isoform (afp2-cr6) in which the reading frame was maintained but the entire amino-terminal region including the EAR and NINJA domains was deleted. Inspection of the afp2-cr6 T2 deletion mutants revealed a significant reduction in the rosette size of mutant compared to wild-type plants (Fig. 6 D and E), and afp2-cr6 mutants resembled jazD mutant plants. Together, these findings show that microProteins can be engineered from individual genes to establish regulatory feedback loops.
Fig. 6.
Creation of a LITTLE NINJA microProtein in Arabidopsis. (A) Gene model depicting the AFP2 locus with the coding sequence highlighted in pink. Four sgRNAs (C1 to C4) were designed and transformed as pools into Arabidopsis plants. Eight CRISPR-induced mutants (afp2-cr1-cr8) were identified. Dashed lines indicate deleted regions. Triangles depict point mutations. (B) RT-PCR showing amplification of the AFP2-coding region in wild-type and the eight afp2-CRISPR mutant plants. Star indicates spurious PCR products in wild-type. The circle depicts the 200 bp marker and the black triangle the 1.0 kb marker. (C) Protein model depicting the full-length AFP2 protein and the protein domains. The eight CRISPR mutants give rise to different protein isoforms. (D) Growth analysis of wild-type and afp2-cr6 T2 mutant plants revealed that afp2-cr6 plants showed reduced rosette sizes. (E) Rosette width of wild-type Col-0 and afp2-cr6 of 3-wk-old plants. Box plots show the observed experimental data. n = 8, ***P < 0.0005 determined by Student’s t test.
Discussion
Here we report the identification of the LNJ microProtein involved in the modulation of jasmonic acid signaling. We initially identified LNJ as a potentially monocot-specific microProtein, which means that we did not identify LNJ or LNJ-related proteins in our reference dicot genomes (here Arabidopsis, tomato, and potato). Closer analysis of the LNJ protein sequence using Basic Local Alignment Search Tool (BLAST) searches revealed, however, that LNJ-like proteins can be identified in other dicot genomes and it is thus not monocot specific. We initially identified LNJ because of a misannotation of a gene encoding a larger NINJA-domain protein where the first exon was excluded (SI Appendix, Fig. S1). To study the loss of LNJ function in Brachypodium, we also attempted to generate respective mutants but it is not yet possible to target a specific splice isoform because both isoforms are identical except for their length. Introduction of CRISPR guide RNAs related to LNJ, which will affect both the long and the short isoforms, resulted in the isolation of only few transgenic calli that after shoot-induction failed to induce roots and perished during the regeneration process (SI Appendix, Fig. S8). Thus, it is currently unclear whether an LNJ-type protein is formed in Brachypodium and what its function would be. The prediction that LNJ-type microProteins may exist in different plant genomes as individual genes suggests that the mechanism of modulating or buffering JA signaling might be evolutionary conserved.
We provide evidence that the Brachypodium LNJ protein acts as a dominant regulator of plant stature. Ectopic LNJ expression alters JA signaling, which is likely partially responsible for the growth phenotypes that we have observed. This is in agreement with a recent metabolomic analysis of a barley brassinosteroid-insensitive mutant that showed a semidwarf phenotype and had altered levels of JA and gibberellic acid (29). The dwarf phenotypes we observed could therefore at least be partially due to disturbances in the hormone homeostasis. Our hormone profiling comparing Brachypodium wild-type and LNJ-OX transgenic plants demonstrated that several hormones are significantly different. The level of JA-Ile (isoleucine), the active signaling compound of jasmonic acid, was reduced in LNJ-OX plants compared to wild-type (SI Appendix, Fig. S5). This negative correlation could be a consequence of inducing JA-signaling in the absence of the hormone and a concomitant negative feedback that curbs JA biosynthesis. In addition, it is well established that the ratio of auxin to cytokinin has a profound impact on plant development and morphogenesis. The observation that the levels of both auxin and different cytokinins were considerably altered (SI Appendix, Fig. S5) suggests that the branching phenotype might be a consequence of the simultaneous alteration of different hormone levels. The analysis of RNA-sequencing data (Dataset S2), however, does not contain obvious candidates that were induced in BdLNJ-OX plants and involved in specific hormonal signaling pathways. Interestingly, in comparison to Arabidopsis (8, 30), we did not detect changes in JAZ expression in response to MeJA treatment in Brachypodium. This could either indicate that we missed the timepoint of JAZ induction in our experiments or that JA-signaling is different in grasses.
The finding that the function of LNJ is transferrable between crop plants (here, rice and barley) indicates that LNJ can function as a general growth regulator, at least in the BEP (Bambusoideae-Ehrhartoideae-Pooideae) clade. Interestingly, overexpression of LNJ did not result in an increased pathogen resistance, a trait that had been observed in higher order jaz mutants (10).
Recently, a promoter editing approach using CRISPR/Cas9 has been used to increase phenotypic diversity, resulting in edited plants with altered expression levels of candidate genes (31). As we have shown in Arabidopsis, LITTLE NINJA-like proteins can be created by deleting parts of the coding sequence (Fig. 6 A–D). Similar genome-engineering approaches in crop plants can be used to create one or more LITTLE NINJA proteins. Additional promoter-hacking approaches could then lead to crop plants with enhanced levels of LNJ expression that will likely correlate with altered architectural traits.
The comparison of LNJ-OX and jazD mutant plants revealed that both plants show reduced growth (Fig. 2). However, jazD mutants are also less fertile than wild-type plants and more resistant to pathogens, and these traits have not been observed in LNJ-OX plants. Thus, it seems that despite the phenotypic similarities, differences exist that could result from interferences with different signaling pathways. Molecularly, we detected a significant overlap of commonly deregulated genes, which suggests that LNJ could be used as a tool to affect JA signaling in a cell type-specific fashion. Since it is challenging to remove 10 JAZ genes in a given cell type, but it is feasible to misexpress one LNJ, tissue- or cell-specific JA responses could be dissected using LNJ-like isoforms.
Finally, because of its functional conservation, LITTLE NINJA-like proteins can be engineered and could be used as a universal tool to control architectural traits of many major crops including wheat, maize, sorghum, and sugar cane.
Materials and Methods
Plant Material, Growth Conditions, Measurements of Agronomic Traits.
Seeds of the Arabidopsis Col-0 ecotype were stratified at 4 °C for 3 d and transplanted into soil and grown at 22 °C in a growth chamber with a 16 h/8 h (day/night) photoperiod and ∼ 65% relative humidity (RH).
B. distachyon inbred line Bd21-3 was stratified at 4 °C for 5 d and transplanted into soil and grown at 24/16 °C in a growth chamber with a 16 h/8 h (day/night) photoperiod and ∼ 60% RH. Plant height was determined by measuring stem lengths of plants 4 wk after germination.
The barley cultivar used in this study is Golden Promise (Hordeum vulgare). Barley seeds from wild-type plants and the transgenic plants imbibed in darkness for 3 d at 4 °C and transplanted into soil. Plants were grown at 20 °C and a 16 h photoperiod (400 to 500 μE), followed by 8 h at 15 °C without light and a relative humidity of 65%.
The rice cultivar used in the experiments is Nipponbare (Oryza sativa). Rice seeds from the control plants and the transgenic plants were imbibed in darkness for 3 d at 4 °C and then were grown at 28 °C, and a 12 h photoperiod (350 μE), followed by 12 h at 25 °C without light and a RH (80% for day, 75% for night). For disease-resistant assays, barley plants were treated with P. teres as described earlier (32).
Arabidopsis transgenic plants (T3) were grown at 22 °C. Phenotype analysis was performed with eight individual plants per line. Brachypodium plants of 4-wk-old plants (T3) were grown at 24/16 °C. Height and tiller number were analyzed with 15 to 20 individual plants per line. Total spikelet numbers were determined when the seeds were harvested. The 2-mo-old barley plants (T2) with individual plants per line (8–15) were measured for height and tiller number. The height and tiller number were determined for T0 transgenic rice plants.
Identification and Classification of MicroProtein Candidates.
Identification of B. distachyon microProtein candidates was done with MiPFinder v1.1 (21) using B. distachyon v3.1 genome (33), and conservation in other genomes was determined by OrthoFinder v0.3.0 (34) using published microProtein lists (21). Only microProtein candidates containing interaction domains according to Pfam v28 (pfam.xfam.org/) and iPfam v1.0 (35) were retained. Evolutionary conservation of microProtein candidates was visualized using Circos v0.68 (36).
Functional annotations were assigned to the most significant putative ancestor of each microProtein candidate family using Protein Analysis through Evolutionary Relationships (PANTHER v13.1; www.pantherdb.org/). One-sided Fisher’s exact test for overrepresentation was performed with R version 3.4.4 against all annotated proteins of the B. distachyon v3.1 genome.
Generation of Transgenic Plants.
The synthetic microProtein-OX plants were generated by amplification DNA encoding conserved domains of Arabidopsis orthologous protein using primers listed in the SI Appendix, Table S1. The PCR-amplified fragments were cloned into pDONR207 (Invitrogen) and recombined into pJAN33 binary vector, which was constructed from the pPAM (GenBank acc. no. AY027531), has a FLAG tag at N terminus for protein detection, and has Gateway recombination sites to facilitate the cloning of target gene fragments. These constructs driven by the double CAMV35S promoter, were used to transform Col-0 to generate overexpression lines using Agrobacterium tumefaciens-mediated transformation with floral dipping. The resulting transgenic T1 lines were screened by qPCR for synthetic microProtein-overexpressing lines. Homozygous microProtein-overexpressing T3 lines were used for phenotypic analyses.
To generate LNJ-OX plants, the LNJ coding sequence was amplified from Brachypodium immature spikelet complementary DNA (cDNA) using primers listed in the SI Appendix, Table S1 and cloned into pDONR207 (Invitrogen). The coding sequence was subsequently recombined into binary destination vector pIPKb003 (GenBank: EU161569) for overexpression. Transgenic Brachypodium plants were obtained by Agrobacterium-mediated transformation (37). Briefly, immature embryos were prepared and calli were induced on Linsmaier and Skoog (LS) basal medium supplemented with 2.5 mg/L 2,4-D. Calli were cocultivated with the Agrobacterium AGL1 strain containing the binary plasmid. Transgenic calli were selected on hygromycin medium. After shoot and root regeneration, the resulting plantlets were transferred to a regular greenhouse for continued growth and seed collection. Transgenic T2 lines were analyzed for growth phenotypes and LNJ expression levels. Increased branching correlated with elevated LNJ expression levels. Finally, homozygote T3 progeny was obtained that showed increased tilling.
The BdLNJ-OX plasmid was also transformed into barley (H. vulgare cv. Golden Promise) and we generated >15 independent transgenic lines (T0) by A. tumefaciens-mediated embryo transformation (38). Transgenic plants were tested by analyzing the levels of LNJ expression by qPCR. Transgenic T2 plants with high LNJ levels were subsequently used for the scoring of agronomic traits.
Finally, we also transformed the BdLNJ-OX construct in rice (Nipponbare) and obtained >20 independent transgenic lines. For rice transformation, we induced immature embryonic calli on CHU (N6) medium supplemented with vitamins and 2 mg/L 2,4-D. After cocultivation with the Agrobacterium AGL1 strain containing the binary plasmid, the transgenic calli were selected on hygromycin medium. Transgenic plantlets were obtained by inducing shoots and roots and subsequently used for trait scoring. Branching was analyzed as described before (39).
The afp2-CRISPR deletion mutants were generated using the pKI1.1R vector system (40). Specific single guide RNAs were designed using CRISPR-P (41). To achieve mutants with larger deletions in the coding region, individual pKI1.1R vectors containing different gene-specific sgRNAs were pooled and transformed into wild-type Col-0. The red fluorescence seed coat marker was used for selection of transgenic seeds and PCR was used to detect larger deletions.
Yeast-Two and -Three Hybrid Experiments.
For yeast-two-hybrid analysis, fusions of the Gal4-binding domain (BD) to respective proteins were achieved by cloning the respective coding sequence into the pGBKT7-GW vector. BD-fusion constructs were transformed into the pJ69-4α yeast strain. Fusions to the Gal4-activation domain (AD) were achieved by cloning respective coding sequences into the pGADT7-GW vector. AD-fusions were transformed into the YM4271 MATa strain. The presence of the plasmids in the strains was verified by PCR, and positive strains were mated for 2 d at 28 °C and then selected on dropout media lacking tryptophan and leucine. Positive colonies were screened on selective triple dropout medium (-Trp, -Leu, -His). For the yeast-three-hybrid assay, the BdNINJA coding sequence was recombined into a pGBKT7-GW vector and BdLNJ into the pDRf1-GW yeast expression vector. The empty vectors or the vectors containing the respective coding sequences were cotransformed into the pJ69-4α yeast strain, and positive colonies were selected on dropout media lacking Trp and Ura. The pGADT7-BdNINJA vector was transformed into a YM4271 MATa strain and selected on dropout media without Leu. The presence of the plasmids in the strains was verified by PCR and positive strains for each transformation were mated for 2 d at 28 °C and then selected on dropout media without Trp, Leu, and Ura. Positive colonies were screened on selective media without Trp, Leu, Ura, and His with additional 2.5 mM 3-aminotriazole.
Analysis of Gene Expression.
Total RNAs were isolated from leaves of Arabidopsis, Brachypodium, barley, and rice plants using a Spectrum Plant Total RNA Kit according to the manufacturer’s instructions (Sigma). For LNJ and synthetic microProtein expression, rosette leaves of 4-wk-old plants grown at 22 °C were collected for RNA preparation. For Brachypodium, barley, and rice LNJ gene expression, RNA was prepared from leaves of 4-wk-old plants grown in each appropriate growth condition.
Total RNAs were also prepared from leaves of 4-wk-old Brachypodium plants treated by methyl jasmonate for analysis of gene expression in response to JA. For the MeJA application, we followed a treatment regime published earlier (42). In brief, three independent plant lines with 12 individuals each were treated after 4 wk by spraying with methyl jasmonate (100 μM in 0.15% Tween) or a mock solution (0.15% Tween). Treatments were performed over an interval of 3 d over a period of 2 wk. After the fifth application, plants were harvested for RNA analysis 30 min after treatment and growth and development changes were recorded. Gene expression analysis was carried out by qRT-PCR for which we converted 1 μg of total RNA into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). All qRT-PCR reactions were performed in triplicates on a CFX384 Real-Time System Cycler (Bio-Rad) with KAPA SYBR FAST qPCR Master Mix Bio-Rad iCycler (KAPA Biosystem) according to the manufacturer’s instructions. We used a two-step amplification procedure (50 cycles à 95 °C for 5 s and 60 °C for 30 s). Relative expression levels were calculated using the ΔΔCT method. Oligonucleotide sequences are listed in the SI Appendix.
RNA-Seq and Bioinformatics Analysis.
The 4-wk-old Brachypodium leaves from the wild-type, BdLNJ-OX plants (n = 12 plants for each sample) were collected to extract the total RNA. For Arabidopsis, leaves of 20-d old soil-grown Arabidopsis plants were collected for RNA extraction. In total, we collected two biological replicates for each genotype and treatment. The mRNA library preparation and sequencing was performed at Novogene Company Limited (Hong Kong).
Sequencing libraries were prepared using the Illumina TruSeq RNA Library Preparation Kit according to the manufacturer’s recommendations. Libraries were sequenced on the Illumina HiSeq2000 platform, and between 17 and 21 million read pairs per sample were obtained. Approximately 377 million paired-end reads were loaded into Galaxy version 15.05.rc1 (43–45), and quality was assessed using FastQC (version 0.10.1). Tophat2 (version 2.0.9) aligned above 85% of read pairs of each sample correctly to Brachypdium genome. Galaxy’s Cufflinks package (version 0.0.7) was employed for differentially expressed gene calling (cutoff q-value of 0.05) (46). Gene expression levels were calculated as FPKM (Fragments Per Kilobase Million), and differentially expressed genes were chosen based on a false discovery rate (FDR) ≤ 0.001 and ≥2 fold change. A heat map of differentially expressed genes on FPKM values was visualized by the online tool Heatmapper (www.heatmapper.ca) using average linkage clustering and Euclidean distance measurement. All data, including the processed data files, have been submitted to the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/): accession number GSE124493.
MeJA Treatment.
For mMeJA treatment, wild-type Brachypodium Bd21-3 and BdLNJ-OX plants were grown in the growth chamber with a growth condition described as above. For the control group, plants were sprayed with 50 mL of 0.15% Tween-20 solution (labeled as “Mock”). For the MeJA treatment group, plants were sprayed with 50 mL of a 100 μM MeJA solution (95% MeJA dissolved in 0.15% Tween-20 solution; labeled as “JA”). About 8 plants each line, 4-wk-old-plants were treated every 2 d for a week (3 times).
Phytohormone Analysis by UHPLC/TQ-MS.
Phytohormones were extracted from around 200 mg (fresh weight, FW) plant material ground in liquid nitrogen with 1.25 mL 80% (vol/vol) methanol containing 4 μL of internal standard mix containing 88 pmol (2H5)tZ and 216 pmol (2H6)ABA (abscisic acid) (both purchased from OlChemIm). Samples were thoroughly vortexed, incubated for 30 min at 4 °C, and centrifuged (20.000 g, 4 °C, 15 min). Supernatants were passed through C18 columns (Phenomenex, Strata C18-E, 8B-S001-FBJ) after pre-equilibration with three times 3 mL 80% (vol/vol) methanol, and flow-throughs were collected and kept on ice. Extraction was repeated with 1.25 mL 80% (vol/vol) methanol and second extracts were passed through the same columns. The combined extracts were concentrated using a SpeedVac. The residues were dissolved in 1 mL 20% (vol/vol) methanol by brief sonication and filtered (MultiScreenHTS; EMD Millipore, cat no. MSGVN 2250). Phytohormones were analyzed by ultra-performance liquid chromatography-triple quadruple mass spectrometer (UHPLC/TQ-MS) on an AdvanceTM-UHPLC/EVOQTMElite-TQ-MS instrument (Bruker) equipped with a C-18 reversed phase column (Kinetex 1.7 u XB-C18, 10 cm × 2.1 mm, 1.7 µm particle size, Phenomenex) by using a 0.05% formic acid in water (vol/vol), pH 4.0 (solvent A)–methanol (solvent B) gradient at a flow rate of 0.4 mL/min at 40 °C. The gradient applied was as follows: 10 to 50% B (15 min), 50% (2 min), 50 to 100% B (0.1 min), 100% B (2.9 min), 100 to 10% B (0.1 min), and 10% B (5 min). Compounds were ionized by electrospray ionization with a spray voltage of +4,500 V and −4,000 V in positive and negative mode, respectively; heated probe temperature was 350 °C, cone temperature was 300 °C. Quantification was based on response factors relative to (2H5)tZ (positive mode) and (2H6)ABA negative mode. The individual hormones were monitored based on the following multiple reaction monitoring (MRM) transitions: (2H5)tZ, (+) 225 > 137 [15 V]; (2H6)ABA, () 269 > 159 [7 V]; ABA, (−) 263 > 153 [7 V]; 1-aminocyclopropane-1-carboxylic acid, (+) 102 > 56 [15 V]; indole-3-acetic acid, (+) 176 > 130 [10 V]; indole-3-aldehyde, (+) 146 > 118 [15 V]; isopentenyladenine-riboside, (+) 336 > 2014 [15 V]; JA-Ile, (−) 322 > 130 [17 V]; SA, (−) 137 > 93 [20 V]; trans-zeatin, (+) 220 > 136 [15 V]; tZ7G/tZ9G/tZOG, (+) 382 > 220 [17 V]; tZR, (+) 352 > 220 [15 V]; tZROG, (+) 514 > 382 [15 V]. tZ7G, tZ9G, and tZOG were distinguished based on retention times in comparison to those of known standards.
Supplementary Material
Acknowledgments
Work in the laboratory was funded by the European Research Council (ERC-StG miPDesign, 336295), the Independent Research Fund Denmark (DFF-FNU, Adaptogenomics), the NovoNordisk Foundation (NNF18OC0034226), and start-up funding of Copenhagen Plant Science Centre through the University of Copenhagen. We thank the anonymous reviewers from a previous submission, Hans Thordal-Christensen, Gregg Howe, and Michael Broberg Palmgren for critically reading and improving our manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.V.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005198117/-/DCSupplemental.
Data Availability.
RNA-seq data have been deposited in Gene Expression Omnibus (accession no. GSE124493 and GSE155487).
References
- 1.Staudt A.-C., Wenkel S., Regulation of protein function by “microProteins”. EMBO Rep. 12, 35–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eguen T., Straub D., Graeff M., Wenkel S., MicroProteins: Small size-big impact. Trends Plant Sci. 20, 477–482 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Graeff M. et al., MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet. 12, e1005959 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav A. et al., The B-Box-Containing MicroProtein miP1a/BBX31 regulates photomorphogenesis and UV-B protection. Plant Physiol. 179, 1876–1892 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long J. A., Ohno C., Smith Z. R., Meyerowitz E. M., TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312, 1520–1523 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Szemenyei H., Hannon M., Long J. A., TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384–1386 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Bollier N. et al., At-mini zinc finger2 and sl-inhibitor of meristem activity, a conserved missing link in the regulation of floral meristem termination in Arabidopsis and tomato. Plant Cell 30, 83–100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauwels L. et al., NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464, 788–791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasternack C., Feussner I., The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 69, 363–386 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Guo Q. et al., JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 115, E10768–E10777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szekeres M. et al., Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85, 171–182 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A. et al., BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 151, 669–680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L.-Y. et al., Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21, 3767–3780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldie T., Leyser O., Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 177, 803–818 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett T. et al., The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 16, 553–563 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Booker J. et al., MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 14, 1232–1238 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Sorefan K. et al., MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 17, 1469–1474 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stirnberg P., van De Sande K., Leyser H. M., MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129, 1131–1141 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa S. et al., Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46, 79–86 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Zou J. et al., Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.). Planta 222, 604–612 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Straub D., Wenkel S., Cross-species genome-wide identification of evolutionary conserved MicroProteins. Genome Biol. Evol. 9, 777–789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang G. et al., ABI5-BINDING PROTEIN2 coordinates CONSTANS to delay flowering by recruiting the transcriptional corepressor TPR2. Plant Physiol. 179, 477–490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chini A. et al., The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Thines B. et al., JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Jin H., Zhu Z., Temporal and spatial view of jasmonate signaling. Trends Plant Sci. 22, 451–454 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Seo H. S. et al., Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. U.S.A. 98, 4788–4793 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C., Zhang L., Duan J., Miki B., Wu K., HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 17, 1196–1204 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wendt T. et al., HvDep1 is a positive regulator of culm elongation and grain size in barley and impacts yield in an environment-dependent manner. PLoS One 11, e0168924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villette C., Zumsteg J., Schaller H., Heintz D., Non-targeted metabolic profiling of BW312 Hordeum vulgare semi dwarf mutant using UHPLC coupled to QTOF high resolution mass spectrometry. Sci. Rep. 8, 13178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta I. F. et al., Role of NINJA in root jasmonate signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 15473–15478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Leal D., Lemmon Z. H., Man J., Bartlett M. E., Lippman Z. B., Engineering quantitative trait variation for crop improvement by genome editing. Cell 171, 470–480.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Lyngs Jorgensen H. J., Andresen H., Smedegaard-Petersen V., Control of Drechslera teres and other barley pathogens by preinoculation with Bipolaris maydis and Septoria nodrum. Phytopathology 86, 602–607 (1996). [Google Scholar]
- 33.International Brachypodium Initiative , Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463, 763–768 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Emms D. M., Kelly S., OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn R. D., Marshall M., Bateman A., iPfam: Visualization of protein–protein interactions in PDB at domain and amino acid resolutions. Bioinformatics 21, 410–412 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Krzywinski M. et al., Circos: An information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel J., Hill T., High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep. 27, 471–478 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Holme I. B., Dionisio G., Madsen C. K., Brinch-Pedersen H., Barley HvPAPhy_a as transgene provides high and stable phytase activities in mature barley straw and in grains. Plant Biotechnol. J. 15, 415–422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y. C. et al., Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 31, 848–852 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Tsutsui H., Higashiyama T., pKAMA-ITACHI vectors for highly efficient CRISPR/Cas9-Mediated gene knockout in Arabidopsis thaliana. Plant Cell Physiol. 58, 46–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei Y. et al., CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 7, 1494–1496 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Napoleão T. A. et al., Methyl jasmonate and salicylic acid are able to modify cell wall but only salicylic acid alters biomass digestibility in the model grass Brachypodium distachyon. Plant Sci. 263, 46–54 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Blankenberg D. et al., . “Galaxy: A web-based genome analysis tool for experimentalists” in Current Protocols in Molecular Biology, (Wiley, 2010), Vol. 19, p. 19.10.11-19.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giardine B. et al., Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 15, 1451–1455 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goecks J., Nekrutenko A., Taylor J.; Galaxy Team , Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11, R86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trapnell C. et al., Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited in Gene Expression Omnibus (accession no. GSE124493 and GSE155487).