Significance
Forest restoration has become a global conservation priority, particularly in the tropics where a significant proportion of remaining forest ecosystems are degraded. To achieve ambitious restoration targets via limited conservation funds, areas that will deliver the greatest biodiversity value must be prioritized. Here, we combine airborne laser scanning with an extensive camera trap dataset to target conservation and restoration across a degraded logged forest gradient. We demonstrate the importance of accounting for three-dimensional habitat structure when defining forest suitability and restoration potential for mammals. Consequently, we provide a robust quantitative framework to prioritize degraded forest restoration based on biodiversity considerations.
Keywords: ecological thresholds, LiDAR, occupancy, prioritization, forest degradation
Abstract
Tropical forest ecosystems are facing unprecedented levels of degradation, severely compromising habitat suitability for wildlife. Despite the fundamental role biodiversity plays in forest regeneration, identifying and prioritizing degraded forests for restoration or conservation, based on their wildlife value, remains a significant challenge. Efforts to characterize habitat selection are also weakened by simple classifications of human-modified tropical forests as intact vs. degraded, which ignore the influence that three-dimensional (3D) forest structure may have on species distributions. Here, we develop a framework to identify conservation and restoration opportunities across logged forests in Borneo. We couple high-resolution airborne light detection and ranging (LiDAR) and camera trap data to characterize the response of a tropical mammal community to changes in 3D forest structure across a degradation gradient. Mammals were most responsive to covariates that accounted explicitly for the vertical and horizontal characteristics of the forest and actively selected structurally complex environments comprising tall canopies, increased plant area index throughout the vertical column, and the availability of a greater diversity of niches. We show that mammals are sensitive to structural simplification through disturbance, emphasizing the importance of maintaining and enhancing structurally intact forests. By calculating occurrence thresholds of species in response to forest structural change, we identify areas of degraded forest that would provide maximum benefit for multiple high-conservation value species if restored. The study demonstrates the advantages of using LiDAR to map forest structure, rather than relying on overly simplistic classifications of human-modified tropical forests, for prioritizing regions for restoration.
Habitat degradation is pervasive in forest ecosystems, affecting ∼4 billion ha worldwide (1), with profound impacts on habitat suitability for wildlife and the delivery of ecosystem functions and services. The restoration of degraded forests has emerged as a global conservation priority, underwritten by the Bonn Challenge and New York Declaration on Forests, which seek to restore 350 million ha of forest by 2030 (2). Given limited conservation funding, it is imperative to maximize return on investment by targeting areas where interventions will have the greatest impact (i.e., optimize ecological benefits relative to opportunity and implementation costs). However, sophisticated frameworks to prioritize degraded forests for conservation and restoration are lacking, hindering the realization of ambitious policy targets (3).
Biodiversity underpins the ecological processes that facilitate forest regeneration (4), meaning that wildlife persistence and restoration are inextricably linked. For example, it is estimated that 90% of tropical tree species depend on interactions with vertebrates to complete their life cycle (5). Given the importance of biodiversity for maintaining forest quality and ecosystem stability, policy and management interventions that prioritize restoration based on wildlife retention are fundamental to achieving long-term restoration goals. This is paramount in the tropics where a significant proportion of the remaining forest extent is degraded, placing vertebrate taxa that use these regions at greater risk of extinction (6). Here, we introduce a framework based on high-resolution remote sensing and wildlife monitoring data to integrate biodiversity considerations into conservation and restoration planning for degraded forests in vulnerable tropical regions.
Selective logging is the principle driver of forest degradation across the tropics (7). Over a fifth of remaining forests have been logged, while an area of up to 600 million ha is currently designated as production forest (7, 8). Logged forests afford refuge to species of conservation concern (9) and play a pivotal role in protecting wildlife against the impacts of environmental change (10). Despite this, the conversion of degraded forests to agricultural land of limited ecological value is a common land-use trajectory across the tropics (9). Selecting which areas of degraded logged forest to protect or restore is hampered by the coarse classification of forest into logged vs. pristine categories (11). Such simplistic assessments overlook substantial spatial heterogeneity in levels of logging-induced degradation (12) and are often unable to provide specific recommendations to inform management and policy. To most effectively retain and enhance logged forests for biodiversity, we need to understand what habitat features species actively utilize.
Habitat selection is a nested hierarchical process describing home range establishment and episodic use of the home range to meet ecological demands (13). It is an adaptive process through which species balance reward (resource acquisition, mating opportunities) relative to risk (energy expenditure, predation) (14). It is generally assumed, therefore, that areas of habitat used preferentially by species convey the highest levels of ecological benefits to them (15). Forest structure is a key determinant of species diversity (16, 17). Logging results in the structural simplification of forest habitats (18); however, the extent to which structural alterations associated with logging influence habitat selection by wildlife remains poorly understood, particularly in a spatial context. This information is essential to delineate areas of forest that promote biodiversity retention and therefore, optimize the success of restoration initiatives.
Habitat selection models for species predominantly focus on a single spatial extent (13), potentially obscuring scale-dependent associations and hierarchical environmental interactions (14). These issues are exacerbated for rare and cryptic species that are observed too infrequently to quantify their habitat associations but are often most sensitive to forest degradation (19). Modern advances in statistical methods afford an analytical platform to overcome these challenges. Multispecies occupancy models provide robust parameter estimates for species infrequently encountered during biodiversity surveys while correcting for sampling bias (20). Moreover, the advent of multiscale occupancy models accounts for the complexity of habitat selection (21), but to date, applications have been limited to single-species approaches (22, 23). Thus, the formal integration of multispecies methods within a multiscale framework provides a powerful statistical tool to capture hierarchical habitat selection for vulnerable and rare species.
Efforts to characterize habitat selection to inform conservation are further hindered by multidimensionality in forest ecosystems. Tropical forests are three-dimensional (3D) environments composed of horizontal and vertical structural components. It is estimated that 75% of forest-dwelling vertebrates demonstrate some degree of arboreality, indicating that multidimensional interactions with vegetation structure are an important aspect of habitat selection (16, 17, 24). Nonetheless, structural complexity is rarely accounted for in conservation assessments due to challenges in measuring structural elements at scales appropriate to management. Airborne light detection and ranging (LiDAR) has emerged as a possible solution to these challenges and has the potential to significantly advance our understanding of the structural signature of logging on biodiversity. However, applications in degraded tropical regions are yet to catch up with these technological advances (16, 17). While LiDAR has been widely implemented in tropical forest carbon assessments (25), it has received much less attention for its potential to quantify 3D habitat associations, particularly for mammals (16), which occupy key trophic positions in tropical forest ecosystems and are a focus of global conservation efforts (4).
Here, we couple high-resolution airborne LiDAR with bespoke multispecies multiscale Bayesian occupancy models to provide unprecedented insights into the conservation value of logged forests and demonstrate how species–habitat associations can be aligned with efforts to prioritize degraded forests for conservation and restoration. We examine the complexity of habitat selection in logged forests and assess degradation impacts on forest structure and biodiversity. We develop structural metrics from 3D plant area distributions to capture the horizontal and vertical components of forest architecture. Our appraisal was conducted in a region characterized by high levels of forest degradation in Borneo, where 46% of the remaining forest area is degraded, a figure that could increase to 88% based on land-use allocations to the timber estate (26).
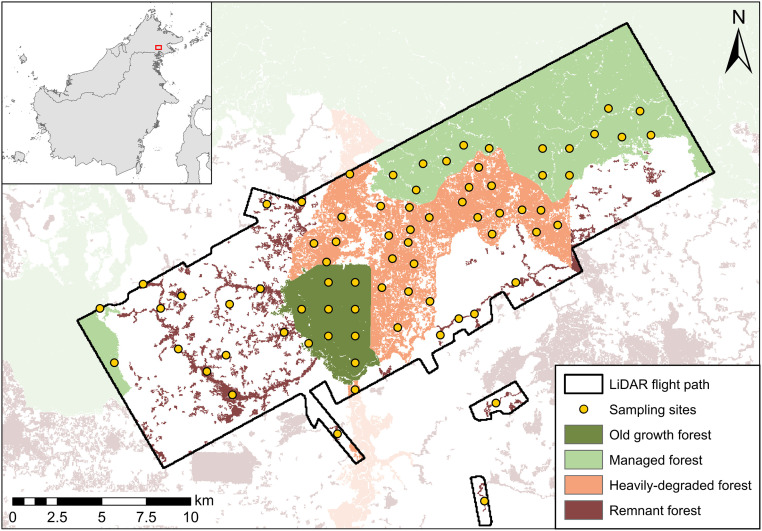
We assess forest structure deterioration across a logging-induced degradation gradient, comprising old growth forest (n = 10), managed forest (twice logged; n = 15), heavily degraded forest (repeatedly logged; n = 28), and remnant forest embedded within an oil palm matrix (n = 21) (Fig. 1). Integrating an extensive camera trap dataset (74 sampling locations, comprising two camera trap stations, n = 148; 5,472 camera trap nights) within a multiscale modeling framework, we explore how structural features influence hierarchical habitat selection by tropical biodiversity at the species and community levels. Throughout, we define occupancy as the probability that a sampling location is situated within the home range of at least one individual of a given species and specify probability of use as preferential habitat selection at the scale of the camera trap station, conditional on the home range being represented by the sampling location. By linking LiDAR-derived structural characteristics operating at different spatial extents to species detection data, we elucidate the forest architectural properties that characterize a home range and habitat preferences.
Fig. 1.
Map of the study site and sampling design showing the broader geographic context of the study site in Malaysia (Inset), the classification of forest across the disturbance gradient within the SAFE Project area, LiDAR flight path (black outline), and camera trap sampling locations (n = 74).
Our appraisal focuses on medium to large mammals, which have lost 70% of their original habitat across Southeast Asia (27). The development of effective conservation measures for threatened mammals has proved challenging due to a weak evidence base. Despite substantial value as conservation flagship species, basic ecological information is still lacking for many Southeast Asian vertebrates, 32% of which are considered data deficient (28). Given the scale of regional forest modification, interventions that recognize the potential value of degraded habitat are essential to safeguard Southeast Asia’s imperiled biodiversity.
Results and Discussion
The Structural Signature of Forest Degradation.
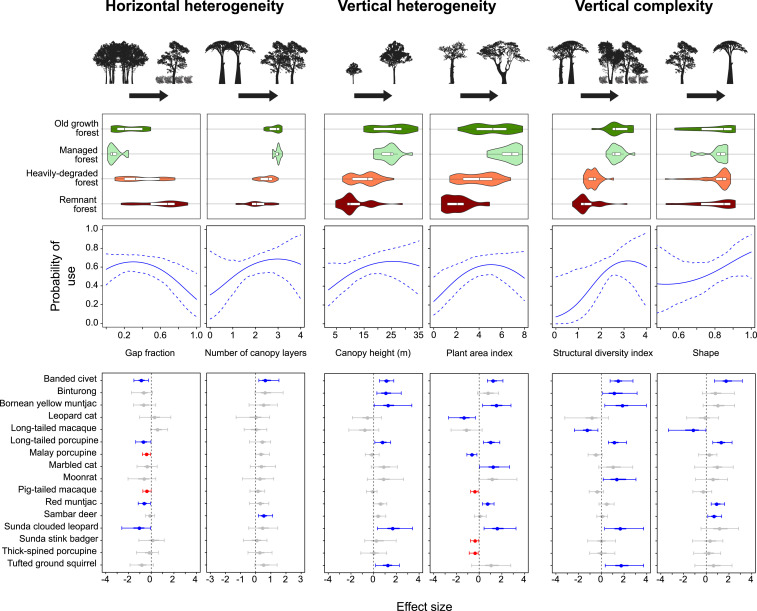
We quantified eight forest metrics from LiDAR point-cloud data, reflecting horizontal and vertical structure, vertical heterogeneity, and landscape context (Table 1 and SI Appendix, section S1.1) (16). Consistent patterns of habitat simplification relative to logging intensity were identified between the managed, heavily degraded, and remnant forest classes, demonstrated by a lack of overlap between Bayesian 95% credible intervals (BCIs) (Fig. 2 and SI Appendix, Table S1). Simplification was characterized by a lower height profile and reduced vegetation density, resulting in fewer environmental niches, fewer canopy pathways, and an increase in canopy gaps. This structural simplification is driven by the removal of large trees and damage to surrounding vegetation. In addition, intensive forestry causes soil compaction and eradication of the seedling community (29), which restricts the successional capacity of forests (30). Furthermore, forest remnants are susceptible to wind damage and altered microclimatic conditions, which lead to additional mortality of large trees in fragmented landscapes (31). While structural simplification associated with logging is well documented (32), we provide empirical evidence of progressive multidimensional architectural deterioration due to repeated logging and habitat fragmentation.
Table 1.
Structural covariates quantified from LiDAR-derived point-cloud data (25 to 50 pulses m−2; aggregated at 20-m resolution), capturing three distinct axes of forest structure (horizontal structure, vertical structure, vertical heterogeneity)
| Structural axis and metric | Processing method | Spatial extent (m) | Description and justification |
| Horizontal structure | |||
| Gap fraction | CHM | 250 | Proportion of focal patch containing vegetation below 5 m in height, indicative of gaps in the forest canopy |
| Gap avoidance at understory level is documented for ungulates (33) and at canopy level for primates (34). Conversely, orangutans appear to favor areas adjacent to canopy gaps for nesting (35) | |||
| Vertical structure | |||
| No. of layers | PAD | 250 | No. of contiguous canopy layers within the vertical column, indicative of connectivity |
| Orangutans favor multilayered canopies when selecting nest sites (35) | |||
| Canopy height | CHM | 250 | Mean canopy height as derived from the CHM surface, indicative of forest maturity and level of disturbance |
| Mammals actively select forest areas with tall canopies, responding to higher productivity of foraging resources, seasonal refuge from environmental conditions, and structural opportunities for arboreal locomotion and den/nest site selection (34–37) | |||
| Plant area index | PAD | 500 | Plant area index, defined as the one-sided area of vegetation, inclusive of foliage, stems and branches, per unit ground area. Indicative of vegetation density throughout the vertical column |
| High vegetation density is favored by apex predators and mesocarnivores to improve hunting efficiency (33, 38). Dense vegetation may also provide important foraging opportunities, thermal cover, and antipredatory refuge for ungulates and secure denning sites for subordinate carnivores (39, 40) | |||
| Vertical heterogeneity | |||
| Structural diversity index | PAD | 500 | Composite measure of canopy height, vegetation density, and the distribution of plant matter throughout the vertical column. Calculated as the Shannon Index of the PAD. Indicative of the diversity of subcanopy environments (i.e., niche space) within the plant area distribution profile |
| Mesocarnivores select multistrata canopies with an equitable distribution of plant matter throughout the vertical column due to increased microhabitat availability and resource provisioning for prey species (39) | |||
| Shape | PAD | 500 | Morphological measurement of the relative distribution of vegetation within the canopy. Ratio of the canopy height with the maximum PAD and the 99th percentile of canopy height PAD |
| Canopy shape could influence primate habitat selection, although there is little evidence to support this from limited applications of this metric to date (34, 35) | |||
| Landscape context | |||
| Forest cover | CHM | 2,000 | Proportion of forest cover (forest defined as trees >10 m in height). Indicative of habitat extent and availability |
| Linked to mammal occurrence and abundance across the tropics, highlighting the importance of contiguous habitat (41, 42) | |||
| Canopy height variability | CHM | 2,000 | SD of canopy height, describing the variability of the vertical dimension. Indicative of canopy complexity and forest quality |
| Primates actively select uniform canopies characterized by low variability as interconnected canopies provide greater lateral connectivity that facilitates arboreal locomotion (34). Conversely, high canopy height variability provides greater structural complexity, which is an important determinant of den site selection by fishers (37) |
The covariates were derived from either CHMs or PAD distributions, estimated based on a one-dimensional Beer–Lambert-type model of light propagation through the canopy (43). We calculated landscape context covariates to describe forest extent and quality across broader spatial scales. Covariates were aggregated across spatial extents informed by scale optimization methods to characterize optimal scales of selection for predictors and determine sensitivity to spatial scale (SI Appendix, Table S2).
Fig. 2.
Habitat use by tropical forest mammals in response to the degradation of three structural axes: horizontal structure, vertical structure, and vertical heterogeneity (Table 1 has a formal description of structural covariates). Top represents structural modification across a tropical disturbance gradient. Violin plots depict the kernel density distribution of the data (colored shapes); wider sections indicate greater probability that structural characteristics within a disturbance class will take a given value. Box plots contained therein describe the median (central vertical line), interquartile range (outer vertical lines of the box), and BCI (thin horizontal lines). Middle demonstrates probability of use of the mammal community relative to structural alterations. Community trends are presented as predicted responses derived from posterior means (solid blue lines) and BCIs (dashed blue lines). Bottom denotes effect sizes for species-specific responses to structural modification. We present effect sizes for species parameters as posterior means (points) and BCIs (horizontal lines). Gray points and horizontal lines represent nonresponsive species, blue suggests influential unimodal effects, and red indicates influential nonlinear associations described by second-order polynomial terms. Effects for species-specific associations are considered substantial if the BCI does not overlap zero (vertical dashed black lines).
Multiscale Habitat Selection in Degraded Forest Ecosystems.
Landscape context covariates, indicative of forest availability (forest cover) and quality (canopy height variability), were important drivers of occupancy for 9 of 28 mammal species, representing 32% of the sampled community (SI Appendix, Figs. S1–S3). Habitat availability has been shown to be an important factor defining species persistence (44). However, our results indicate divergent species-specific responses, driven by differences between forest specialists (e.g., banded civet Hemilagus derbyanus: mean of posterior distribution = 0.83, BCI = 0.01 to 2.02; Bornean yellow muntjac Muntiacus atherodes: 1.14, 0.36 to 2.26) and taxa adapted to take advantage of resources in degraded or nonforest habitats (e.g., greater mouse deer Tragulus napu: −0.99, −1.78 to −0.28; leopard cat Prionailurus bengalensis: −1.27, −2.49 to −0.38). Species demonstrated a greater number of positive responses to forest quality (SI Appendix, Fig. S1), likely reflecting a greater abundance of resources typical of structurally complex habitats, such as fruit and browse availability for ungulates (45) and small mammal prey for carnivores (39). The contrasting influences of forest cover and quality may be indicative of the degree of habitat degradation across the study site, with old growth forests accounting for ∼8% of the landscape. Given the limited spatial extent of preferential habitat, species appear to be actively selecting areas that retained adequate structural quality to meet their ecological requirements. Our findings emphasize the importance of maintaining forest quality, as well as extent, in a region characterized by high levels of forest degradation. This concurs with evidence from elsewhere in the tropics (44).
Patterns in probability of use revealed the structural properties that constitute quality habitat and help maintain ecological processes. Looking at the mammal community as a whole, forest structure was a key determinant of probability of use, highlighting the importance of mature, connected forest habitat containing a breadth of environmental niches for mammal persistence (Fig. 2).
At the species level, species–habitat structure associations were evident for 16 of the 28 mammals assessed (57% of the sampled community) (Fig. 2 and SI Appendix, Figs. S4–S9). In general, species were most responsive to structural measures that captured the inherent multidimensionality of the forest environment, emphasizing the importance of recognizing the 3D signature of habitat degradation in management and policy. Plant area index throughout the vertical column was the strongest predictor of probability of use (Fig. 2 and SI Appendix, Table S2). For arboreal ambush predators, such as the Sunda clouded leopard Neofelis diardi, dense vegetation provides cover that increases hunting efficiency through visual or locomotive obstruction, as shown previously for lions (38). Conversely, vegetation density and distribution may provide refuges for prey species such as ungulates, particularly when engaged in vulnerable behaviors such as resting or rumination (46). Mammals actively selected forest areas with taller canopies and a greater breadth of environmental niches (Fig. 2), which are characteristic properties of late-successional stands (47). Mature, diverse forests demonstrate higher primary productivity (48), affording greater resources to primary consumers such as the Bornean yellow muntjac. Moreover, tall trees are fruiting oases for frugivorous species like the binturong Arctictis binturong, as has been demonstrated for species with similar dietary preferences (34). Forests with late-successional characteristics also accumulate leaf litter at a faster rate, attracting a diverse, abundant invertebrate community (49) that may encourage the persistence of insectivorous mammals such as the banded civet.
To date, a limited understanding of the structural features of logged forests that promote species persistence has restricted our capacity to capitalize on conservation opportunities within the vast global timber estate. Here, we identify consistent active selection of structurally complex environments by mammals at fine spatial scales indicative of episodic habitat use to meet ecological demands, revealing a causal mechanism for the negative effects of forest degradation on mammal persistence. This emphasizes the importance of maintaining and/or restoring structurally intact forests for biodiversity conservation. Taken as a whole, our results confirm that species will track resources at successively lower hierarchical levels of habitat selection in degraded forests to overcome limitations at the preceding level (14). Here, the mammal community was more responsive to changes in the structural environment at the scale of probability of use, presumably because resources were limited throughout the home range to the extent that species tracked relevant structural variations at progressively finer scales. Moreover, these findings suggest the potential for negative feedback loops in degraded systems. Mammals occupy key ecological roles in tropical forests; thus, active avoidance of heavily degraded areas could potentially affect the resilience of these systems, preventing natural postdisturbance recovery and leaving ecosystems in a state of arrested succession and ultimately, defaunation (4).
Prioritizing Degraded Forests for Restoration and Conservation.
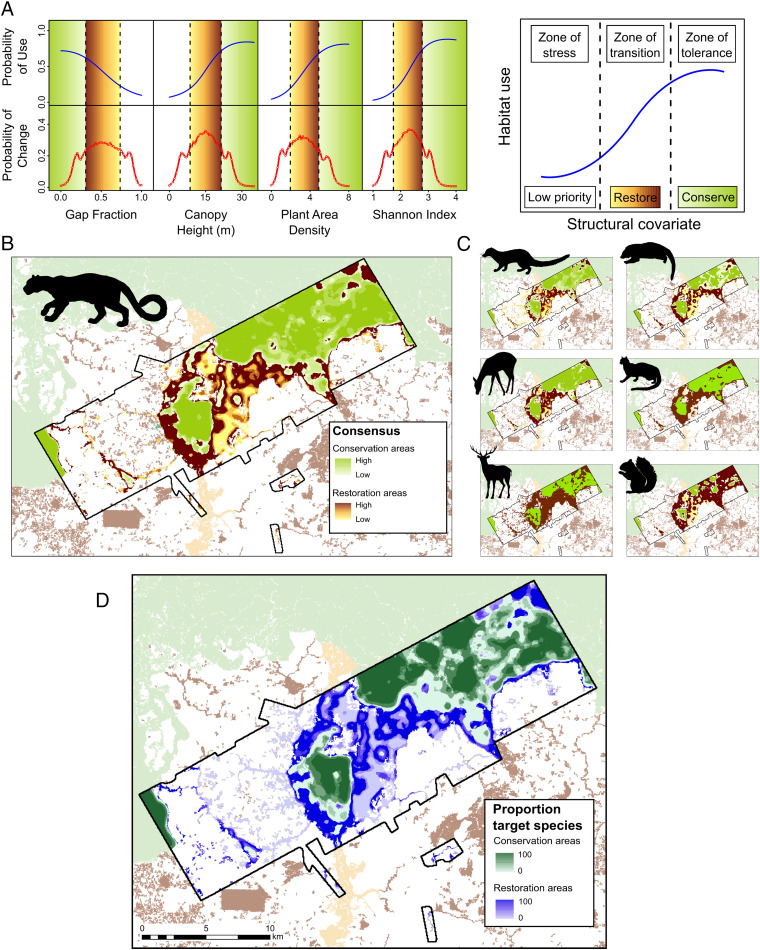
The capacity to identify and prioritize areas of degraded forests for improved management is imperative to inform biodiversity conservation and restoration objectives. To achieve this, we employed Bayesian change point analysis to detect thresholds in forest structural properties, based on records of active habitat selection by tropical mammals. Thresholds were applied to partition species response curves into three distinct occurrence states: 1) zones of tolerance—high probability of use and low rate of change, representing optimal conservation areas; 2) zones of transition—variable probability of use and high rate of change, ideal for restoration as they offer substantial gains in species persistence per unit management effort; and 3) zones of stress—low probability of use and low rate of change, thus low priority for any habitat intervention (Fig. 3A).
Fig. 3.
A spatial delineation of conservation and restoration priority areas for high-conservation value mammals, defined as endemic or classified as threatened (vulnerable/endangered/critically endangered) by the IUCN (banded civet, binturong, Bornean yellow muntjac, marbled cat, sambar deer, Sunda clouded leopard, and tufted ground squirrel), based on records of active habitat selection. Using the Sunda clouded leopard as an example, response curves for each structural covariate (blue lines) were partitioned into occurrence states (dashed vertical black lines), corresponding to priority conservation and restoration areas using Bayesian change point analysis. Areas of the curve exhibiting the highest rate of change in occupancy (peaks in the probability of change; red line graphs) were deemed optimal restoration (yellow–brown gradient), while areas characterized with high stable occurrence were deemed optimal conservation areas (green gradient) (A). Agreement between structural covariates was visualized in a consensus map (B). This process was replicated for the remaining six other species (C). Single-species consensus maps were combined to produce a multispecies zonation indicating taxonomic agreement between proposed conservation/restoration areas. Forest areas only qualified for intervention in areas of highest consensus for each species (D).
By linking the species–habitat relationships to extensive LiDAR habitat maps, covering 40,150 ha, we were able to estimate occurrence states for multiple species from the structural covariates (SI Appendix, Table S3). At the species level, consensus across covariates reveals priority areas for conservation (i.e., tolerance zones) and restoration (i.e., transition zones). Moreover, spatial agreement between areas prioritized for multiple species indicates where interventions will be most optimal (i.e., of benefit to the most species). For example, adopting a conservative approach whereby only areas of high consensus (i.e., full agreement between all structural measures) qualified for management, the highly threatened Sunda clouded leopard would benefit from 6,767 ha (16.7%) of the landscape prioritized for conservation and 4,415 ha (10.7%) for restoration (Fig. 3B). Combining this information with findings from six other high-conservation value species (either endemic or classified as threatened [vulnerable/endangered/critically endangered] by the International Union for the Conservation of Nature [IUCN]: banded civet, binturong, Bornean yellow muntjac, marbled cat, sambar deer Rusa unicolor, and tufted ground squirrel Rheithrosciurus macrotis) (Fig. 3C and SI Appendix, Figs. S14–S20), conservation activities would be best targeted to 11,300 ha (27.4%) and restoration would be best targeted to 16,410 ha (39.7%) of the landscape (Fig. 3D and SI Appendix, Table S4).
Logged forests have been proposed as a cost-effective strategy to expand the existing protected area network to connect pristine habitats (10). The most extensive areas to prioritize for conservation were in old growth (1,680 ha, 14.9%) and managed forests (7,899 ha, 69.8%). However, within these classes, optimal habitat for all seven target species covered only 443 and 1,747 ha (26.3 and 22.1%), respectively (SI Appendix, Table S5). These findings illustrate the challenge of identifying conservation areas that maximize species representation, even when only a fraction of the mammal community is considered. Collectively, our results provide further evidence of declining conservation value with increasing logging intensity (50). We therefore advocate reduced-impact logging as a preventative measure to maintain forest structural integrity and reconcile production and conservation (51).
There is a growing concern that many tropical countries lack the capacity to fulfill their international restoration commitments (52). Our framework provides a methodology to direct restoration activities to optimize biodiversity conservation outcomes and support restoration initiatives such as the Bonn Challenge and New York Declaration on Forests. Restoration opportunities were predominantly identified in managed (5,612 ha, 34.2%) and heavily degraded forests (7,046 ha, 42.9%). However, areas that would universally benefit all target species were again rare (managed forest: 1,747 ha, 6.8%; heavily degraded forest: 1,988 ha, 28.2%) (SI Appendix, Table S5). This demonstrates the potential for ecological trade-offs during the implementation of restoration initiatives, reinforcing the need for restoration planning to avoid perverse management outcomes. Based on economic data available elsewhere in Borneo (53), combined restoration and opportunity costs for the study landscape would be financially prohibitive (average net present value: US $943 ha−1, equating to US >$5 million for the entire landscape). It is therefore essential that any forest restoration efforts are deployed in such a way that they optimize conservation value for associated biodiversity, including mammals. Based on our findings, we believe that buffering pristine conservation areas and enhancing connectivity between them are most likely to maximize species representation and returns on investment within our study system. Applying these principles over much larger spatial scales also serves as an effective climate change mitigation measure for wildlife conservation (10).
Here, we demonstrate the use of a robust prioritization framework that can identify priority areas for habitat restoration and conservation, ensuring that biodiversity is better integrated into land management decision making. Moreover, our methodology has the potential to deliver important cobenefits due to documented spatial concordance between areas of high biodiversity and those offering climate change mitigation and water security (54). However, we recognize that restoration is a holistic process containing a significant socioeconomic dimension (55) that is not captured by our framework. Our approach maximizes benefits for highly threatened species prioritized by conservation but like all approaches, may lead to trade-offs between addressing various goals (53). While our approach focused on species of conservation concern to guide restoration planning, the study system could be restricted to taxonomic groups/species that underpin ecological processes if the recovery of ecosystem functions is the ultimate goal of restoration. Although we have shown the value of our approach at the landscape scale, it could equally be applied to direct conservation policy at regional and global scales. Recent proposals by the Sabah government to increase protected area coverage by 5%, coupled with the state-wide availability of LiDAR data (25), provide an unparalleled opportunity to mobilize a collaborative network of species occurrence data and fully integrate biodiversity considerations into the conservation agenda. Moreover, the launch of NASA’s Global Ecosystem Dynamics Investigation promises to increase the scope of LiDAR coverage to global scales (56). Capitalizing on these developments could greatly enhance the limited ecological understanding of biodiversity across a pantropical gradient of forest degradation.
Methods
Study Landscape.
Fieldwork was undertaken at the Stability of Altered Forest Ecosystems (SAFE) Project (https://www.safeproject.net/) and neighboring oil palm estates in Sabah, Malaysian Borneo. The SAFE Project area is nested within the Kalabakan Forest Reserve (KFR; 4°33′N, 117°16′E), comprising lowland and hill dipterocarp forest. A legacy of commercial logging has resulted in a heterogeneous forest stand (Fig. 1). Between 1978 and 2008, the KFR experienced multiple logging rotations (cumulative extraction rate = 179 m3 ha−1) (11). Similarly, the neighboring Ulu Segama Forest Reserve underwent two logging rounds (cumulative extraction rate = 150 m3 ha−1) with more stringent size quotas. In contrast, Brantian–Tantulit Virgin Jungle Reserve (VJR) retains near-pristine old growth forest, with some past encroachment on the western and southern borders. The disturbance gradient is representative of transitional degradation states seen elsewhere on Borneo and much of tropical Southeast Asia.
Mammal Surveys and Sampling Design.
To characterize the mammal community, we collected detection/nondetection data using camera traps deployed between June 2015 and August 2017 (57), following protocols described in Deere et al. (58). Remotely operated digital cameras (Reconyx HC500) were deployed across 74 sampling locations, separated by a mean distance of 1.6 km, and randomly stratified to capture the degradation gradient relative to logging intensity using the Putz and Redford (59) classification scheme: old growth forest (VJR), managed forest (Ulu Segama Forest Reserve), and heavily degraded forest (KFR). We also sampled remnant forest embedded within an oil palm matrix, differentiated from heavily degraded forest due to isolation and increased exposure to anthropogenic stressors.
Sampling locations comprised two camera trap stations, positioned up to 250 m apart depending on the terrain and availability of forest cover (mean = 185 m), resulting in a total of 148 deployments. Cameras were unbaited, positioned at a standardized height (ca. 30 cm), and preferentially placed above flat surfaces, targeting low-resistance travel routes and randomized locations simultaneously to maximize detections. Accounting for theft, vandalism, malfunction, and animal damage, data were obtained from 126 stations distributed across 74 sampling locations. Cameras were deployed for a minimum of 42 consecutive nights per camera station, yielding a total survey effort of 5,427 camera trap nights.
LiDAR Methods and Structural Covariates.
To characterize the structural properties of the landscape, LiDAR surveys were conducted in November 2014 by the Natural Environment Research Council’s Airborne Research Facility. LiDAR is an active remote sensor that emits a laser pulse from an aircraft toward a target object and quantifies distance based on the time elapsed between emission and reflection (16). Surveys employed a Leica ALS50-II sensor attached to a Dornier 228–201 light aircraft, flown at an elevation of 1,400 to 2,400 meters above sea level and a velocity of 120 to 240 knots. The sensor produced pulses at a frequency of 120 kHz, encompassing a scan angle of 12° and a footprint of 40 cm, resulting in a point-cloud density of 25 to 50 points m−2. Concurrent ground surveys using a Leica base station facilitated accurate georeferencing of the point cloud.
To quantify structural metrics, point-cloud data were subjected to two processing procedures. Initially, ground and nonground returns were partitioned from the point cloud, using the former to generate a 1-m-resolution digital elevation model (DEM). We constructed a canopy height model (CHM) of similar resolution by normalizing nonground returns and subtracting ground observations derived from the DEM. To develop a 3D insight into canopy structure, plant area density (PAD) distributions were generated from point-cloud data using a one-dimensional Beer–Lambert approximation for the propagation of LiDAR pulses through the canopy (43). We provide a detailed description of LiDAR processing methods in SI Appendix, section S1.1.
We employed Bayesian linear models to determine differences in forest structural properties across a degradation gradient (SI Appendix, section S1.2 has model specification details). Structural covariates were extracted as mean values across buffer radii corresponding to optimal scales of habitat use (SI Appendix, Table S1).
Modeling Framework.
We developed a multispecies extension to Bayesian multiscale occupancy models to explore occupancy and probability of use by medium–large terrestrial mammals relative to LiDAR-derived structural covariates. We specified models of the form
Occupancy (ψ), probability of use (ϑ), and detection probabilities (p) were modeled on the logit scale with random intercepts (α0, β0, δ0) and slopes (α1–2, β1–2, δ1–3) for each species (i). We modeled occupancy of species i, at sampling location j (ψi,j), as a function of forest cover and canopy height variability at coarse spatial scales (buffer radii: 1, 1.5, 2 km). We assessed probability of use of species i, within sampling location j, at camera trap station l (ϑi,j,l) at finer spatial scales (radii: 10, 25, 50, 100, 150, 250, 500 m) relative to covariates associated with our three structural axes (“Structure”) (Table 1) and incorporated second-order polynomial terms (“Structure2”) to account for nonlinear responses. Due to analytically prohibitive levels of multicollinearity (|r| > 0.7; generalized variance inflation factor > 5), independent models were constructed for each structural predictor (Table 1) (N = 6). We implemented temporal random effects (ε) for both the occurrence and probability-of-use models, addressing unmeasured interannual variation due to sampling across multiple years (“Year”). We modeled detection probability of species i, at sampling location j, at camera trap station l across temporal replicates k (pi,j,l,k) as a function of structural and sampling covariates presumed to influence the observation process, including sampling intensity (“Trap Effort”), obstructing vegetation features in the camera trap detection zone (“PAD Herb”; plant area index values extracted from 2 to 5 m within the vertical column, broadly corresponding to the herbaceous layer), and alternative pathways in the vertical column (i.e., number of layers: “Nlay”) (Table 1). Detection covariates were extracted across a fixed buffer of 25 m, corresponding to the detection zone of our camera trap models. Prior to analysis, all continuous covariates were centered and standardized to place them on a comparable scale and improve model convergence. We outline a formal model description, including specification details and predictive performance checks, in SI Appendix, sections S2.1 and S2.2.
We constructed 126 models to identify the most influential structural covariates and inform scale optimization methods (SI Appendix, section S2.1). We ranked competing models using Watanabe Akaike information criterion (SI Appendix, Table S2), a within-sample model selection criteria analogous to Akaike information criterion and robust to latent parameters (60). We report findings for occupancy and detection parameters corresponding to the overall best-fitting model, presenting the results according to the highest-ranked spatial scale associated with that structural covariate. Throughout, we consider parameters influential if their BCI did not overlap zero.
Delineating Restoration and Conservation Priority Areas.
Focusing on seven high-conservation value species, we implemented change point analysis to link abrupt shifts in the occurrence state to specific forest structural attributes. Using the “bcp” package in R, we employed a Bayesian algorithm (10,000 iterations, 2,000 burn-in) to identify upper- and lower-transition zone thresholds (61), characterized by high rates of change in probability of use relative to spatial variation in structural covariates. Thresholds were used to partition species response curves into three distinct occurrence states (zone of stress: below the lower threshold; zone transition: between the lower and upper thresholds; zone of tolerance: above the upper threshold), each associated with a specific management intervention (low priority, restoration priority, and conservation priority, respectively) (Fig. 3A). This protocol was embedded within a spatially explicit framework to prioritize degraded forests for conservation and restoration. For each species, thresholds were implemented to reclassify LiDAR-derived maps of significant structural covariates, which were averaged to generate single-species consensus maps delineating priority conservation and restoration areas based on levels of agreement between structural covariates (Fig. 3 B and C). The species-specific prioritization maps were reclassified according to areas of high consensus (i.e., full agreement between all structural predictors) and averaged across focal taxa to produce a multispecies zonation illustrating the proportion of target species that would benefit from management action (Fig. 3D).
Supplementary Material
Acknowledgments
This study was funded by UK Natural Environment Research Council (NERC) Grants NE/K016407/1 and NE/K016377/1 and an NERC EnvEast PhD studentship (N.J.D.). G.G.-A. is the recipient of Discovery Early Career Research Award DE160100904 from the Australian Research Council. We thank the Sabah Biodiversity Council, the Sabah Forest Department, Yayasan Sabah, Sime Darby, and Benta Wawasan for permitting access. We are indebted to Jamiluddin Jami, Esther L. Baking, Arnold James, Mohd. Mustamin, Ampat Siliwong, Sabidee Mohd. Rizan, Jessica K. Haysom, and Najmuddin Jamal for field assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001823117/-/DCSupplemental.
Data Availability.
Data associated with this manuscript are available for download from the Zenodo online repository (species detection data for 28 medium–large mammals: http://doi.org/10.5281/zenodo.4010757; spatial delineations of LiDAR-derived structural covariates: http://doi.org/10.5281/zenodo.4020697).
References
- 1.Watson J. E. M. et al., The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Chazdon R. L. et al., A policy‐driven knowledge agenda for global forest and landscape restoration. Conserv. Lett. 10, 125–132 (2017). [Google Scholar]
- 3.Crouzeilles R. et al., A new approach to map landscape variation in forest restoration success in tropical and temperate forest biomes. J. Appl. Ecol. 56, 2675–2686 (2019). [Google Scholar]
- 4.Gardner C. J., Bicknell J. E., Baldwin-Cantello W., Struebig M. J., Davies Z. G., Quantifying the impacts of defaunation on natural forest regeneration in a global meta-analysis. Nat. Commun. 10, 4590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi Y., Gardner T. A., Goldsmith G. R., Silman M. R., Zelazowski P., Tropical forests in the Anthropocene. Annu. Rev. Environ. Resour. 39, 125–159 (2014). [Google Scholar]
- 6.Barlow J. et al., The future of hyperdiverse tropical ecosystems. Nature 559, 517–526 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Asner G. P. et al., Selective logging in the Brazilian Amazon. Science 310, 480–482 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Blaser J., Sarre A., Poore D., Johnson S., “Status of tropical forest management” (ITTO Tech. Series 38, International Tropical Timber Organization, Yokohama, Japan, 2011).
- 9.Edwards D. P. et al., Selective‐logging and oil palm: Multitaxon impacts, biodiversity indicators, and trade‐offs for conservation planning. Ecol. Appl. 24, 2029–2049 (2014). [PubMed] [Google Scholar]
- 10.Struebig M. J. et al.; Borneo Mammal Distribution Consortium , Targeted conservation to safeguard a biodiversity hotspot from climate and land-cover change. Curr. Biol. 25, 372–378 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Struebig M. J. et al., Quantifying the biodiversity value of repeatedly logged rainforests: Gradient and comparative approaches from Borneo. Adv.Ecol. Res. 48, 183–224 (2013). [Google Scholar]
- 12.Berry N. J., Phillips O. L., Ong R. C., Hamer K. C., Impacts of selective logging on tree diversity across a rainforest landscape: The importance of spatial scale. Landsc. Ecol. 23, 915–929 (2008). [Google Scholar]
- 13.McGarigal K., Wan H. Y., Zeller K. A., Timm B. C., Cushman S. A., Multi-scale habitat selection modeling: A review and outlook. Landsc. Ecol. 31, 1161–1175 (2016). [Google Scholar]
- 14.Mayor S. J., Schneider D. C., Schaefer J. A., Mahoney S. P., Habitat selection at multiple scales. Ecoscience 16, 238–247 (2009). [Google Scholar]
- 15.Mosser A., Fryxell J. M., Eberly L., Packer C., Serengeti real estate: Density vs. fitness-based indicators of lion habitat quality. Ecol. Lett. 12, 1050–1060 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Davies A. B., Asner G. P., Advances in animal ecology from 3D-LiDAR ecosystem mapping. Trends Ecol. Evol. 29, 681–691 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Simonson W. D., Allen H. D., Coomes D. A., Applications of airborne LiDAR for the assessment of animal species diversity. Methods Ecol. Evol. 5, 719–729 (2014). [Google Scholar]
- 18.Pinard M. A., Putz F. E., Retaining forest biomass by reducing logging damage. Biotropica 28, 278–295 (1996). [Google Scholar]
- 19.Brodie J. F. et al., Correlation and persistence of hunting and logging impacts on tropical rainforest mammals. Conserv. Biol. 29, 110–121 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Dorazio R. M., Royle J. A., Estimating size and composition of biological communities by modeling the occurrence of species. J. Am. Stat. Assoc. 100, 389–398 (2005). [Google Scholar]
- 21.Nichols J. D. et al., Multi‐scale occupancy estimation and modelling using multiple detection methods. J. Appl. Ecol. 45, 1321–1329 (2008). [Google Scholar]
- 22.Crosby A. D., Porter W. F., A spatially explicit, multi-scale occupancy model for large-scale population monitoring. J. Wildl. Manage. 82, 1300–1310 (2018). [Google Scholar]
- 23.Mordecai R. S., Mattsson B. J., Tzilkowski C. J., Cooper R. J., Addressing challenges when studying mobile or episodic species: Hierarchical Bayes estimation of occupancy and use. J. Appl. Ecol. 48, 56–66 (2011). [Google Scholar]
- 24.Oliveira B. F., Scheffers B. R., Vertical stratification influences global patterns of biodiversity. Ecography 42, 249 (2018). [Google Scholar]
- 25.Asner G. P. et al., Mapped aboveground carbon stocks to advance forest conservation and recovery in Malaysian Borneo. Biol. Conserv. 217, 289–310 (2018). [Google Scholar]
- 26.Gaveau D. L. A. et al., Four decades of forest persistence, clearance and logging on Borneo. PLoS One 9, e101654 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A., Kent J., Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Li B. V., Hughes A. C., Jenkins C. N., Ocampo-Peñuela N., Pimm S. L., Remotely sensed data informs red list evaluations and conservation priorities in Southeast Asia. PLoS One 11, e0160566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinard M., Barker M., Tay J., Soil disturbance and post-logging forest recovery on bulldozer paths in Sabah, Malaysia. For. Ecol. Manage. 130, 213–225 (2000). [Google Scholar]
- 30.Bischoff W. et al., Secondary succession and dipterocarp recruitment in Bornean rain forest after logging. For. Ecol. Manage. 218, 174–192 (2005). [Google Scholar]
- 31.Laurance W. F., Delamônica P., Laurance S. G., Vasconcelos H. L., Lovejoy T. E., Rainforest fragmentation kills big trees. Nature 404, 836 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer M. et al., Mapping the structure of Borneo’s tropical forests across a degradation gradient. Remote Sens. Environ. 176, 84–97 (2016). [Google Scholar]
- 33.Lone K. et al., Living and dying in a multi‐predator landscape of fear: Roe deer are squeezed by contrasting pattern of predation risk imposed by lynx and humans. Oikos 123, 641–651 (2014). [Google Scholar]
- 34.Davies A. B., Ancrenaz M., Oram F., Asner G. P., Canopy structure drives orangutan habitat selection in disturbed Bornean forests. Proc. Natl. Acad. Sci. U.S.A. 114, 8307–8312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies A. B., Oram F., Ancrenaz M., Asner G. P., Combining behavioural and LiDAR data to reveal relationships between canopy structure and orangutan nest site selection in disturbed forests. Biol. Conserv. 232, 97–107 (2019). [Google Scholar]
- 36.Mateo-Sánchez M. C. et al., Seasonal and temporal changes in species use of the landscape: How do they impact the inferences from multi-scale habitat modeling? Landsc. Ecol. 31, 1261–1276 (2016). [Google Scholar]
- 37.Zhao F., Sweitzer R., Guo Q., Kelly M., Characterizing habitats associated with Fisher den structures in the Southern Sierra Nevada, California using discrete return LiDAR. For. Ecol. Manage. 280, 112–119 (2012). [Google Scholar]
- 38.Davies A. B., Tambling C. J., Kerley G. I., Asner G. P., Effects of vegetation structure on the location of lion kill sites in African thicket. PLoS One 11, e0149098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreira-Arce D. et al., Mesocarnivores respond to fine-grain habitat structure in a mosaic landscape comprised by commercial forest plantations in southern Chile. For. Ecol. Manage. 369, 135–143 (2016). [Google Scholar]
- 40.Ewald M., Dupke C., Heurich M., Müller J., Reineking B., LiDAR remote sensing of forest structure and GPS telemetry data provide insights on winter habitat selection of European roe deer. Forests 5, 1374–1390 (2014). [Google Scholar]
- 41.Boron V. et al., Richness, diversity, and factors influencing occupancy of mammal communities across human-modified landscapes in Colombia. Biol. Conserv. 232, 108–116 (2019). [Google Scholar]
- 42.Wearn O. R. et al., Mammalian species abundance across a gradient of tropical land-use intensity: A hierarchical multi-species modelling approach. Biol. Conserv. 212, 162–171 (2017). [Google Scholar]
- 43.Stark S. C. et al., Amazon forest carbon dynamics predicted by profiles of canopy leaf area and light environment. Ecol. Lett. 15, 1406–1414 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Barlow J. et al., Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Brodie J. F., Giordano A., Lack of trophic release with large mammal predators and prey in Borneo. Biol. Conserv. 163, 58–67 (2013). [Google Scholar]
- 46.Brodie J. F., Brockelman W. Y., Bed site selection of red muntjac (Muntiacus muntjak) and sambar (Rusa unicolor) in a tropical seasonal forest. Ecol. Res. 24, 1251–1256 (2009). [Google Scholar]
- 47.Peña‐Claros M., Changes in forest structure and species composition during secondary forest succession in the Bolivian Amazon. Biotropica 35, 450–461 (2003). [Google Scholar]
- 48.Apps C. D., McLellan B. N., Woods J. G., Proctor M. F., Estimating grizzly bear distribution and abundance relative to habitat and human influence. J. Wildl. Manage. 68, 138–152 (2004). [Google Scholar]
- 49.Ewers R. M. et al., Logging cuts the functional importance of invertebrates in tropical rainforest. Nat. Commun. 6, ncomms7836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burivalova Z., Sekercioğlu C. H., Koh L. P., Thresholds of logging intensity to maintain tropical forest biodiversity. Curr. Biol. 24, 1893–1898 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Bicknell J. E., Struebig M. J., Edwards D. P., Davies Z. G., Improved timber harvest techniques maintain biodiversity in tropical forests. Curr. Biol. 24, R1119–R1120 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Fagan M. E., Reid J. L., Holland M. B., Drew J. G., Zahawi R. A., How feasible are global forest restoration commitments? Conserv. Lett. 13, e12700 (2020). [Google Scholar]
- 53.Budiharta S. et al., Restoring degraded tropical forests for carbon and biodiversity. Environ. Res. Lett. 9, 114020 (2014). [Google Scholar]
- 54.Brancalion P. H. et al., Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 5, eaav3223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chazdon R. L., Landscape restoration, natural regeneration, and the forests of the Future1. Ann. Mo. Bot. Gard. 102, 251–257 (2017). [Google Scholar]
- 56.Dubayah R. et al., The global ecosystem dynamics investigation: High-resolution laser ranging of the Earth’s forests and topography. Sci. Remote Sens. 1, 100002 (2020). [Google Scholar]
- 57.Deere N. J., Maximizing the value of forest restoration for tropical mammals by detecting three-dimensional habitat associations. Zenodo . 10.5281/zenodo.4010757. Deposited 9 January 2020. [DOI] [PMC free article] [PubMed]
- 58.Deere N. J. et al., Implications of zero‐deforestation commitments: Forest quality and hunting pressure limit mammal persistence in fragmented tropical landscapes. Conserv. Lett. 13, e12701 (2020). [Google Scholar]
- 59.Putz F. E., Redford K. H., The importance of defining “forest”: Tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica 42, 10–20 (2010). [Google Scholar]
- 60.Broms K. M., Hooten M. B., Fitzpatrick R. M., Model selection and assessment for multi-species occupancy models. Ecology 97, 1759–1770 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Huggett A. J., The concept and utility of “ecological thresholds” in biodiversity conservation. Biol. Conserv. 124, 301–310 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this manuscript are available for download from the Zenodo online repository (species detection data for 28 medium–large mammals: http://doi.org/10.5281/zenodo.4010757; spatial delineations of LiDAR-derived structural covariates: http://doi.org/10.5281/zenodo.4020697).