Abstract
The use of COI barcodes for specimen identification and species discovery has been a useful molecular approach for the study of Anura. Here, we establish a comprehensive amphibian barcode reference database in a central area of South America, in particular for specimens collected in Mato Grosso do Sul state (Brazil), and to evaluate the applicability of the COI gene for species-level identification. Both distance- and tree-based methods were applied for assessing species boundaries and the accuracy of specimen identification was evaluated. A total of 204 mitochondrial COI barcode sequences were evaluated from 22 genera and 59 species (19 newly barcoded species). Our results indicate that morphological and molecular identifications converge for most species, however, some species may present cryptic species due to high intraspecific variation, and there is a high efficiency of specimen identification. Thus, we show that COI sequencing can be used to identify anuran species present in this region.
Keywords: Anura, Frog, Mato Grosso do Sul, DNA Barcode, COI, Brazil
Introduction
Anurans (Amphibia: Anura), commonly known as frogs and toads, are an extremely endangered group, with 30% of their species threatened (Vitt & Caldwell, 2014). This number is still considered underestimated given recent descriptions and the lack of studies evaluating and monitoring populations and/or determining the status of at least one-third of the known amphibian species (Bickford et al., 2007; Keever et al., 2009; IUCN, 2017; Vacher et al., 2020). The cryptic characters of many species often makes the correct identification of anuran species very difficult (e.g., Funk, Caminer & Ron, 2012), which has promoted the use of molecular approaches to discovery of new species and identification by non-specialists.
In Brazil, the largest country in South America, 1,136 species of the order Anura are known, of which approximately 70% are endemic (Segalla et al., 2019; AmphibiaWeb, 2020). This country contains a wide diversity of biomes from large forest areas such as the Amazon rainforest to open grasslands like the Pampas. Among its biomes, two were classified as biodiversity hotspots in the seminal article by Myers and collaborators: Atlantic forest and Cerrado (Myers et al., 2000). The Atlantic Forest is among the top five biomes labelled biodiversity hotspots, and is the most devastated biome in the country (Rezende et al., 2018). The Cerrado, the second largest biome in Brazil and the richest savannah in the world, constitutes the most extensive savannah region in South America. After the Atlantic Forest, the Cerrado is the Brazilian biome that has suffered the most changes due to human occupation (Colli, Vieira & Dianese, 2020). In addition to these biomes, other regions of the country are of great conservation importance for global species diversity, such as the Pantanal, the world’s richest tropical wetland area that is recognized by UNESCO as a World Heritage Site (Calheiros, De Oliveira & Padovani, 2012).
Considering the biological diversity of these environments, Mato Grosso do Sul is a Federative State in the central region of Brazil that consists of Atlantic forest, Cerrado, and Pantanal biomes. Given its privileged geographic positioning, several initiatives have improved the taxonomic knowledge and conservation of flagship species found in the State, e.g., the Hyacinth Macaw Project (Guedes, 2015). Moreover, other initiatives such as the Ecological-Economic Zoning and species inventories have been carried out to obtain new robust data about environmental threats and species lists for different taxa (Neves et al., 2017; Ferreira et al., 2017; Rodrigues et al., 2018). Until 2017, 86 anuran taxa, assigned to species level, were identified in Mato Grosso do Sul state (Souza et al., 2017). Although Souza et al. (2017) reported the presence of these species, some records have recently been questioned. Santos et al. (2019) suggested removing the species Boana crepitans (Wied-Neuwied 1924), Scinax similis (Cochran, 1952), Adenomera martinezi (Bokermann 1956) and Leptodactylus gracilis (Duméril & Bibron 1840). Brusquetti et al. (2014), suggested the incorrect identification of Lepidobatrachus asper Budgett, 1899 as Lepidobatrachus laevis Budgett, 1899 for the state.
In the last decades, the field of molecular systematics experienced remarkable progress that led to the development of standardized DNA sequences used for taxonomy (e.g., Koroiva et al., 2017; Guimarães et al., 2018). Among these genetic tools, we found the mitochondrial gene Cytochrome Oxidase I (mtCOI, Cox 1 or COI), which was first suggested by Hebert and colleagues, as a basis for creating a global species identification system, especially for animals (Hebert et al., 2003; Hebert, Ratnasingham & de Waard, 2003). Such system was proposed as an efficient tool in the face of the growing need for quick and easy methods to identify species and assist in the discovery of new taxonomic units, considering that only a fraction of biodiversity has been described and named (Mora et al., 2011).
This system called “DNA Barcode” or “DNA Barcoding” has gained a lot of attention from the scientific community. Since the article suggesting a standard threshold value of 1% in BOLD system (Ratnasingham & Hebert, 2007a), hundreds of papers testing DNA Barcode have been published in prestigious scientific journals, demonstrating the efficiency of this tool for biological identification (e.g Schindel & Miller, 2005; Park et al., 2011; Koroiva et al., 2017; Koroiva et al., 2018). In Latin America, the use of DNA Barcoding technique in anurans has been expanded in the last decade. Previous evaluations of the effective use of DNA Barcoding in the specimens identification suggest an accuracy above 75%, especially for anuran species present in the Amazon and the Atlantic Forest (Estupiñán et al., 2016; Lyra, Haddad & De Azeredo-Espin, 2017). Wide-scale DNA barcode surveys of anurans have also been performed in Colombia and Panama with confirmation of its ability for molecular identification (see Paz & Crawford, 2012; Guarnizo et al., 2015). However, in the main biomes of the central region of South America (e.g., Cerrado), molecular information on anurans is still scarce, limiting regional genetic data and the application of new molecular technologies (e.g., metabarcoding).
In regard to the restrictions and difficulties of morphological identification of anurans, molecular tools present a promising approach to solve this impediment in Neotropical species. In this study, we present a comprehensive DNA barcode library for the identification of anuran species in the central region of South America, in particular, for the state of Mato Grosso do Sul (Brazil). Although political borders have no biological basis, biodiversity knowledge produced in a state can direct contribute to the design and monitoring of public policies (Fernandes et al., 2017) and help the planning and management of resources (Pelayo-Villamil et al., 2018). Thus, our objectives were (i) to establish DNA barcode libraries for the Anura fauna of Mato Grosso do Sul state, (ii) to make a comparison between molecular (distance-based and tree-based) methods of species delimitation and traditional taxonomy, and (iii) to evaluate the accuracy of DNA barcoding in identifying specimens.
Materials & Methods
Ethics statement
This study was approved by the Brazilian Institute for Wildlife and Environment/Ministry of Environment (SISBIO license number 45889-1 and 49080-1). Protocols for collection and research were approved by the Animal Use Ethics Committee of the Federal University of Mato Grosso do Sul (CEUA/UFMS-1.1096/2019).
Data collection
A total of 104 anuran specimens of 48 species were collected from 13 municipalities in the state of Mato Grosso do Sul (from now on “MS”). Morphological identification of all specimens was done with the help of experts on anuran taxonomy and by consulting the Coleção Zoológica da Universidade Federal de Mato Grosso do Sul (ZUFMS-AMP). The collected specimens were killed using 5% lidocaine chlorohydrate, fixed with 10% formalin and then transferred to permanent storage in 70% ethanol. We also collected tissue samples and stored them in 100% ethanol before specimen fixation in formalin. For classification, we followed Frost (2020). Voucher specimens were deposited into the collections of the Coleção Zoológica da Universidade Federal de Mato Grosso do Sul, Brazil (ZUFMS-AMP/UFMS).
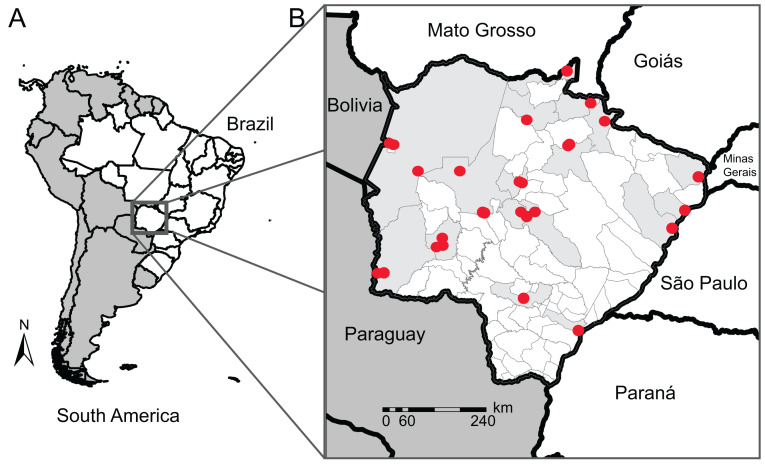
To improve the genetic database, we used sequences from neighboring states (São Paulo, Mato Grosso, Goiás, Paraná, and Minas Gerais) and countries (Bolivia and Paraguay) for species that we did not have sequences for in MS or for those with sequences from one single individual (singletons). COI data from traceable sequences available on GenBank and BOLD System repositories were also included in our reference library. These sequences belong to 22 anuran specimens of 11 species from 7 municipalities in MS sequenced by other authors, totaling 126 anuran specimens of 49 species from 17 municipalities (Fig. 1), and 74 anuran specimens of 17 species from neighboring Brazilian States or neighboring countries of MS. In this count, we sequenced four specimens from two species (Dendropsophus melanargyreus and Osteocephalus taurinus) collected in Mato Grosso state because these occur in MS state but we did not have access to tissue samples. All sequences were verified to represent the same region of COI gene and checked for indels and stop codons.
Figure 1. Map of our sampling locations in Mato Grosso do Sul state.
(A) Map of South America (dark gray) highlighting the geopolitical division of Brazil (white); (B) municipality division of Mato Grosso do Sul (MS) with the locality of the sampling sites (red dots) and their municipalities (light gray). Collection localities and other specimen information are available in Table S1.
Amplifying and sequencing
The total DNA from the samples was extracted using the Blood & Tissue DNA Mini Kit (Ludwig Biotec, Alvorada, Brazil) from a small piece of toe, muscle or liver tissue preserved in ethanol according to standard DNA barcoding methods for anurans. 658 bp were amplified from the 5′ region of the COI gene using the primers described in Lyra, Haddad & De Azeredo-Espin (2017):
T3-AnF1 –‘5- ATT AAC CCT CAC TAA AGA CHA AYC AYA AAG AYA TYG G-3′
T7-AnR1 –‘5- AAT ACG ACT CAC TAT AGC CRA ARA ATC ARA ADA RRT GTT G-3′
PCR conditions for amplification consisted of 1 × buffer (Colorless GoTaq® Flexi Buffer; Promega Corp., Madison, WI), 0.2 mM dNTP mix, 0.2 µM of each primer, 2mM MgCl2, 1U Taq polymerase (GoTaq® G2 hot start polymerase, Promega Corp., Madison, WI) and 2µl of template DNA, in a total reaction volume of 25 µl. The PCR cycling program was run as follows: initial denaturation step with 3 min at 95 °C, 35 cycles of denaturation for 20 s at 95 °C, annealing for 20 s at 50 °C and extension for 1 min at 60 °C, and final extension for 5 min at 60 °C (see Lyra, Haddad & De Azeredo-Espin, 2017). PCR products were purified with Ethanol/Sodium Acetate and directionally sequenced in ABI 3130 Genetic Analyzer (Applied Biosystems). COI sequencing was performed using T3 (5′- ATT AAC CCT CAC TAA AG - 3′) and T7 (5′- AAT ACG ACT CAC TAT AG - 3′) primers. The sequence data was uploaded to GenBank (accession numbers MT583090 to MT583197).
Data analysis
We used GENEIOUS v 9.0.5 (https://www.geneious.com) to check the sequence quality of both strands through comparisons with their respective chromatograms, and to assemble and edit if necessary. Furthermore, we aligned sequences for each gene loci using Muscle v3.8.425 (Edgar, 2004) (module implemented in GENEIOUS v 9.0.5) with the default setting. To increase robustness in the homology statement and elevate matrix occupancy, long sequences were truncated to cover only the “Folmer” region of the COI gene. This is the most used region for DNA barcoding and covers 658 nt of the 5′-end of the gene (Koroiva & Kvist, 2018).
Species delimitation
Two methods were used to delimit species: distance-based and tree-based methods. As a distance-based method, we performed ABGD analyzes online (https://bioinfo.mnhn.fr/abi/public/abgd/), using K2P model and, since the program is optimized for COI gene, the default values of Pmin = 0.001 and Pmax = 0.10, steps = 20, and Nb bins = 20 were used. We also used a value of relative gap width (X), 0.75 to increase the sensitivity of the analysis (Puillandre et al., 2012). For this analysis, we used the K2P model because it is used in other barcoding studies (e.g., Hendrich et al., 2015; Hawlitschek et al., 2016). ABGD resulted in a stable genetic group count with a range of prior intraspecific values (P = 0.0234–0.0483) and results of these grouping are presented. For the latter analysis, the best-fit nucleotide substitution model was selected by jModeltest 2.1.7. (Darriba et al., 2012). GTR+I+G was the best fit model available.
For tree-based methods, we used the Poisson Tree Process (PTP and mPTP) as implemented in the PTP web servers (http://species.h-its.org/ and https://mptp.h-its.org) using default settings and removing outgroup sequences (Zhang et al., 2013; Kapli et al., 2017). We constructed a tree with RAxML (v 8.2.12) (Stamatakis, 2014) using GTR GAMMA I model and 1,000 bootstrap replicates. In addition, the computer program BEAST 1.8.4 and packages (Drummond & Rambaut, 2007) were used to infer an ultrametric tree (lognormal relaxed clock, GTR + Γ + G evolutionary model, ucld.mean parameter set to 1 and constant population size coalescent tree prior). Two independent runs of 1.3 108 generations were conducted remotely at CIPRES v 3.3. portal (Miller, Pfeiffer & Schwartz, 2010). Convergence was verified with Tracer v 1.6 and a maximum credibility tree was drawn using TreeAnotator 1.8.4, discarding the first 10% as burn-in. The GMYC analysis (Fujisawa & Barraclough, 2013) was run online (http://species.h-its.org/gmyc/), employing a single threshold method on standard parameters. Finally, a maximum likelihood (ML) tree was created to provide a graphic representation of the divergence pattern between species. ML tree was inferred using PhyML 3.0 (Guindon et al., 2010) website (http://www.atgc-montpellier.fr/phyml/) with 1,000 bootstrap replicates and GTR + I + G model. In all tree-based analysis, three species were used as outgroup (see Table S1).
Specimen identification
The success of identification was evaluated using the “nearest-neighbor” criterion (also known as “Best match” (BM)), “Best Close Match” (BCM) and “BOLD Identification Criterion” (BIC) analysis using the SPecies IDentity and Evolution (SPIDER v. 1.3) (Brown et al., 2012) in the R program (R Development Core Team, 2020). An in-depth description of each analysis can be found in Talaga et al. (2017) and Lavinia et al. (2017).
To evaluate the ability to identify species using DNA Barcoding gene, we applied the genetic distance threshold values of (i) 1%, as used in the BOLD system (Ratnasingham & Hebert, 2007b); (ii) function “threshVal” in SPIDER (Brown et al., 2012), (iii) 6%, as used and suggested by Lyra, Haddad & De Azeredo-Espin (2017); (iv) 8%, as used and suggested by Crawford, Lips & Bermingham (2010) (from now on “Crawford et al.”); (v) function “localMinima” in SPIDER (Brown et al., 2012); (vi) 10%, as used and suggested by Vences et al. (2005). Singletons were not used as queries. All analyses were performed using K2P model.
Results
Sampling and final data set
A total of 204 mitochondrial COI barcode sequences were obtained from 22 genera and 59 species (see Table S1). The data herein represents the first published DNA barcodes for 19 taxa (32%). Four species that were found and sequenced in this research were not in the species list for MS used as a parameter: Pseudis platensis, Ameerega berohoka, Proceratphrys dibernardoi, and Pristimantis dundeei. All sequences analyzed were greater than 416 bp. The average base pairs of the sequences was 624 bp and the median was 638 bp. Nine singletons were registered for the database: Rhinella scitula, Physalaemus marmoratus, Leptodactylus furnarius, Leptodactylys syphax, Trachycephaylus typhonius, Pseudopaludicola falcipes, Pseudopaludicola mystacalis, Scinax acuminatus and Boana caingua.
Species delimitation
Species delimitation analyzes provided varying numbers of possible species considering 59 species defined by morphological delimitation: 58 for ABGD-initial partition, 64 for ABGD-recursive partition, 77 for PTP, 53 for mPTP and 72 for GMYC (Fig. 2). Thus, the number of possible anuran species observed ranged from 58 to 77. The results of the species delimitation analysis revealed six conflicts with at least three methodologies disagreeing with the morphological delimitation.
Figure 2. PhyML tree of Anura species based on COI sequences obtained mainly from Mato Grosso do Sul State specimens.
The numbers above the internal nodes correspond to bootstrap values of only those higher than 50% are indicated. Black bars represent each species delimited by the following methods: Morph (Morphology), ABGDini (automatic barcoding gap discovery: initial partition), ABGDrec (automatic barcoding gap discovery: recursive partition), PTP (Poisson tree process), mPTP (multi-rate Poisson tree processes), and GMYC (General Mixed Yule Coalescent). Gray bars indicate divergence results from morphological identification.
Specimen identification
Using SPIDER (Table 1), we obtained 195 correct identifications and no incorrect identifications for “Best Match” approach. A value of 2.64% was proposed by “localMinima” function and the value that minimized the cumulative identification errors (“threshVal” approach) was 8.2%. For BCM approach, correct identification varied between 75.39% (147 correct at threshold value of 1%) and 98.46% (192 correct at threshold proposed by the “threshVal” approach, Crawford et al. and Vences et al.; 8.2%, 8.6% and 10%, respectively). For “BOLD identification criteria” analysis, the correct identification varied between 75.39% (147 correct at threshold value of 1%) and 95.38% (186 correct at threshold proposed by the “threshVal” approach and Crawford et al.; 8.2% and 8.6%, respectively).
Table 1. Summary of the results from the sequence-based identification simulations.
A species name was determined following three criteria: Best Match (BM), Best Close Match (BCM) and BOLD Identification Criterion (BIC). Six threshold values (1.00%, 2.64%, 6.00%, 8.20%, 8.60% and 10.00%) were used for the BCM and BIC approaches. The total number of identifications within each category (values in parenthesis) and the percentage they represent are based on 195 queries (singletons were not considered).
| Reference | Threshold value | Criterion | Correct | Incorrect | Ambiguous | No identification |
|---|---|---|---|---|---|---|
| “Nearest-neighbor” | – | BM | 100.00% (195) | 0.% (0) | ||
| BOLD’s threshold | 1.00% | BCM | 75.38% (147) | 24.62% (48) | ||
| BIC | 75.39% (147) | 24.62% (48) | ||||
| “localMinima” | 2.64% | BCM | 88.21% (172) | 11.79% (23) | ||
| BIC | 85.13% (166) | 3.08%(6) | 11.79%(23) | |||
| Lyra, Haddad & De Azeredo-Espin (2017) | 6.00% | BCM | 96.41% (188) | 3.59% (7) | ||
| BIC | 93.33% (182) | 3.08% (6) | 3.59% (7) | |||
| “threshVal” | 8.20% | BCM | 98.46% (192) | 1.54% (3) | ||
| BIC | 95.38% (186) | 3.08% (6) | 1.54% (3) | |||
| Crawford, Lips & Bermingham (2010) | 8.60% | BCM | 98.46% (192) | 1.54% (3) | ||
| BIC | 95.38% (186) | 3.08% (6) | 1.54% (3) | |||
| Vences et al. (2005) | 10.00% | BCM | 98.46% (192) | 1.54% (3) | ||
| BIC | 93.33% (182) | 5.13%(10) | 1.54%(3) |
Discussion
This library of DNA sequences represents a major advance for DNA barcodes to identify anurans from central South America. As advocated by Carstens et al. (2013), the application of multiple approaches should increase the reliability in the species identification, although it is necessary to recognize the limitations inherent in the choice of threshold values. Considering three or more methods of delimitation, six taxonomic inconsistencies (10.16%) had better evidence. The presence of complexes of closely related species and problems of taxonomic knowledge gaps may be associated with these results. Below, we discuss the most likely hypothesis for each taxon with disagreement.
Scinax squalirostris specimens were clustered into two main groups. Considering that the evaluated sequences are not from MS, other studies have already suggested that this species might contain cryptic species. Magalhães de (2012) suggests the presence of up to three species with divisions in the southern regions of South America, Atlantic forest and Brazil’s central area, which may explain its variation.
In Leptodactylus, specifically for Leptodactylus fuscus, the L. fuscus species group is the most diverse and widely distributed of the genus, with 30 currently recognized species (Sá et al., 2014). Camargo, De Sá & Heyer (2006) suggested the presence of three cryptic species in L. fuscus, which highlights this position. Despite our molecular results, as advocated by Struck et al. (2018), studies of cryptic species should be carried out using a multidimensional and interdisciplinary approach, requiring an integrated investigation of characters that can be used to differentiate species. In a recently accepted manuscript Magalhães et al. (in press), Leptodactylus latrans presents four cryptic species, which corroborates the variation found in the sequences obtained from GenBank. For L. labyrinthicus, Silva et al. (2017) demonstrate that there are limitations to morphological identification, and also suggest the existence of possible cryptic species.
Finally, in a phylogenetic analysis of the genus Osteocephalus, Jungfer et al. (2013) recognize five candidate cryptic species within O. taurinus. One of the candidate species occurs in the eastern region of the state of Mato Grosso, Brazil, and must occur in sympatry with O. taurinus sensu stricto, which explains the high genetic variability of this species. Furthermore, there was one case of merged species: Elachistoceleis bicolor and E. matogrosso. Elachistoceleis is a genus with very similar morphology (Almeida de et al., 2016), and few studies have molecularly evaluated its taxonomy (e.g., De Sá et al., 2012). Considering the conserved morphology, geographic proximity of these species, and that the main difference between them is based on coloration traits (Caramaschi, 2010), we believe that these reasons can cause bias in identification.
Regarding specimen identification, as registered by Raupach et al. (2015) in marine crustaceans from the North Sea and adjacent regions, the BM approach provided the greatest number of correct identifications. This statement should be considered with caution, considering that incomplete databases with few specimens per species may not represent accurate intraspecific genetic variation. Using the distance limits (BCM and BIC) we were able to highlight more cases of high intraspecific divergence (“No identification”) than interspecific divergence, indicating the potential for new species in the state and nearby regions. Recent descriptions of new species found in MS have indicated that this hypothesis appears to be correct (e.g., Pansonato et al., 2016). The threshold value of 8.6% suggested by Crawford, Lips & Bermingham (2010) who worked in a more restricted geographical area (highlands of central Panama), is close to that found for the ‘threshvall’ approach (8.2%). These values presented the best evaluations in both BCM and BIC. Regardless, such proximity to threshold values must be emphasized, as it cannot be considered a standard value to other regions.
Finally, the use of a single value for DNA barcoding have been frequently questioned because several evolutionary factors must be considered in a community such as distinct mutational rates, which is related to their biologic aspects (e.g., size, growth rate, generation time and length), and the condition of the genetic difference in recently separated species (see Meyer & Paulay, 2005; Austerlitz et al., 2009). However, the use of databases and thresholds on regional scales has been encouraged considering that these approaches can increase identification accuracy (Bergsten et al., 2012). Here, we must highlight that the specimen identification capability is high (75.38–98.46%), even for congeneric species, such was also demonstrated by previous studies (e.g., Lyra, Haddad & De Azeredo-Espin, 2017), supporting the use of DNA Barcoding for species-level identification of Neotropical anurans.
Conclusions
Establishing an Anuran DNA barcode library for central region of South America, especially in Mato Grosso do Sul State, was a landmark to improve the taxonomy and biodiversity conservation in Brazil. Even without all the species, our results demonstrate the ability to identify most of them with the COI gene. Also, this database is an important baseline study for the application of mass sequencing tools for biomonitoring; a current trend in this area. Our results indicate that morphological and molecular identifications are converging for most species, however, some molecular evidence suggests the presence of cryptic species within certain species.
Supplemental Information
Shown in yellow are the estimate coordinates of the locality or city center, considering that these records did not have this information.
Acknowledgments
The authors acknowledge the researches from Mapinguari Lab (UFMS) (especially with Renata Serejo Martins Souza), Laboratório de Parasitologia Animal (UFMS) and Laboratório de Genética & Biodiversidade (UFOPA) for the assistance they have provided for this work. The authors also thank Angela M. Mendoza-Henao and one anonymous reviewer for comments that helped to improve the manuscript.
Funding Statement
Ricardo Koroiva received a Post-doctoral scholarship (PNPD/CAPES) from Coordenação de. Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES). Luís Reginaldo Ribeiro Rodrigues was financed by CAPES –Edital ProAmazônia (AUXPE 3318/2013). Diego José Santana received research fellowship (proc. 311492/2017-7) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This study was financed by the Coordenação de. Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -. Finance Code 001. Additional support was obtained from the Institutional Program of Internationalization sponsored by Coordination for the Improvement of Higher Education Personnel (Finance Code 001; CAPES-PrInt 41/2017 - Process 88881.311897/2018-01). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ricardo Koroiva conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Luís Reginaldo Ribeiro Rodrigues conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Diego José Santana conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Protocols for collection and research were approved by the Animal Use Ethics Committee of the Federal University of Mato Grosso do Sul (CEUA/UFMS) (approval CEUA/UFMS-1.1096/2019).
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
This study was conducted with the appropriate permission from the Brazilian Institute for Wildlife and Environment/Ministry of Environment (SISBIO/IBAMA license number 45889-1 and 49080-1).
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The list of specimens used in this study is available in a Supplemental File.
References
- Almeida de et al. (2016).Almeida de JPFA, Nascimento do FAC, Torquato S, Lisboa BS, Tiburcio ICS, Palmeira CNS, Lima de MG, Mott T. Amphibians of Alagoas State, northeastern Brazil. Herpetology Notes. 2016;9:123–140. [Google Scholar]
- AmphibiaWeb (2020).AmphibiaWeb AmphibiaWeb. 2020. https://amphibiaweb.org/index.html. [28 March 2020]. https://amphibiaweb.org/index.html
- Austerlitz et al. (2009).Austerlitz F, David O, Schaeffer B, Bleakley K, Olteanu M, Leblois R, Veuille M, Laredo C. DNA barcode analysis: a comparison of phylogenetic and statistical classification methods. BMC Bioinformatics. 2009;10:S10. doi: 10.1186/1471-2105-10-S14-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten et al. (2012).Bergsten J, Bilton DT, Fujisawa T, Elliott M, Monaghan MT, Balke M, Hendrich L, Geijer J, Herrmann J, Foster GN, Ribera I, Nilsson AN, Barraclough TG, Vogler AP. The effect of geographical scale of sampling on DNA barcoding. Systematic Biology. 2012;61:851–869. doi: 10.1093/sysbio/sys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford et al. (2007).Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution. 2007;22:148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2012).Brown SDJ, Collins RA, Boyer S, Lefort M-C, Malumbres-Olarte J, Vink CJ, Cruickshank RH. Spider: an R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Molecular Ecology Resources. 2012;12:562–565. doi: 10.1111/j.1755-0998.2011.03108.x. [DOI] [PubMed] [Google Scholar]
- Brusquetti et al. (2014).Brusquetti F, Jansen M, Barrio-Amorós C, Segalla M, Haddad CFB. Taxonomic review of Scinax fuscomarginatus (Lutz, 1925) and related species (Anura; Hylidae) Zoological Journal of the Linnean Society. 2014;171:783–821. doi: 10.1111/zoj.12148. [DOI] [Google Scholar]
- Calheiros, De Oliveira & Padovani (2012).Calheiros DF, De Oliveira MD, Padovani CR. Hydro-ecological processes and anthropogenic impacts on the ecosystem services of the pantanal wetland. In: Ioris AAR, editor. Tropical wetland management: the South-American pantanal and the international experience. Ashgate; 2012. pp. 29–58. [Google Scholar]
- Camargo, De Sá & Heyer (2006).Camargo A, De Sá RO, Heyer WR. Phylogenetic analyses of mtDNA sequences reveal three cryptic lineages in the widespread neotropical frog Leptodactylus fuscus (Schneider, 1799) (Anura, Leptodactylidae) Biological Journal of the Linnean Society. 2006;87:325–341. doi: 10.1111/j.1095-8312.2006.00581.x. [DOI] [Google Scholar]
- Caramaschi (2010).Caramaschi U. Notes on the taxonomic status of Elachistocleis ovalis (Schneider, 1799) and description of five new species of Elachistocleis Parker, 1927 (Amphibia, Anura, Microhylidae) Boletim do Museu Nacional. Nova Serie, Zoologia. 2010;527:1–30. [Google Scholar]
- Carstens et al. (2013).Carstens BC, Pelletier TA, Reid NM, Satler JD. How to fail at species delimitation. Molecular Ecology. 2013;22:4369–4383. doi: 10.1111/mec.12413. [DOI] [PubMed] [Google Scholar]
- Colli, Vieira & Dianese (2020).Colli GR, Vieira CR, Dianese JC. Biodiversity and conservation of the Cerrado: recent advances and old challenges. Biodiversity and Conservation. 2020;29:1465–1475. doi: 10.1007/s10531-020-01967-x. [DOI] [Google Scholar]
- Crawford, Lips & Bermingham (2010).Crawford AJ, Lips KR, Bermingham E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proceedings of the National Academy of Sciences. 2010;107:13777–13782. doi: 10.1073/pnas.0914115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sá et al. (2012).De Sá RO, Streicher JW, Sekonyela R, Forlani MC, Loader SP, Greenbaum E, Richards S, Haddad CFB. Molecular phylogeny of microhylid frogs (Anura: Microhylidae) with emphasis on relationships among New World genera. BMC Evolutionary Biology. 2012;12:241. doi: 10.1186/1471-2148-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond & Rambaut (2007).Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2004).Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estupiñán et al. (2016).Estupiñán RA, Ferrari SF, Gonçalves EC, Barbosa MSR, Vallinoto M, Cruz Schneider MP. Evaluating the diversity of Neotropical anurans using DNA barcodes. ZooKeys. 2016;637:89–106. doi: 10.3897/zookeys.637.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes et al. (2017).Fernandes GW, Vale MM, Overbeck GE, Bustamante MMC, Grelle CEV, Bergallo HG, Magnusson WE, Akama A, Alves SS, Amorim A, Araújo J, Barros CF, Bravo F, Carim MJV, Cerqueira R, Collevatti RG, Colli GR, da Cunha CN, D’Andrea PS, Dianese JC, Diniz S, Estrela PC, Fernandes MRM, Fontana CS, Giacomin LL, Gusmão LFP, Juncá FA, Lins-e Silva ACB, Lopes CRAS, Lorini ML, de Queiroz LP, Malabarba LR, Marimon BS, Junior BHM, Marques MCM, Martinelli BM, Martins MB, de Medeiros HF, Menin M, de Morais PB, Muniz FH, Neckel-Oliveira S, de Oliveira JA, Oliveira RP, Pedroni F, Penha J, Podgaiski LR, Rodrigues DJ, Scariot A, Silveira LF, Silveira M, Tomas WM, Vital MJS, Pillar VD. Dismantling Brazil’s science threatens global biodiversity heritage. Perspectives in Ecology and Conservation. 2017;15:239–243. doi: 10.1016/j.pecon.2017.07.004. [DOI] [Google Scholar]
- Ferreira et al. (2017).Ferreira WR, Hepp LU, Ligeiro R, Macedo DR, Hughes RM, Kaufmann PR, Callisto M. Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecological Indicators. 2017;72:365–373. doi: 10.1016/j.ecolind.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost (2020).Frost DR. Amphibian species of the world: an online reference. Version 6.1https://amphibiansoftheworld.amnh.org/index.php 2020
- Fujisawa & Barraclough (2013).Fujisawa T, Barraclough TG. Delimiting species using single-locus data and the generalized mixed yule coalescent approach: a revised method and evaluation on simulated data sets. Systematic Biology. 2013;62:707–724. doi: 10.1093/sysbio/syt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, Caminer & Ron (2012).Funk WC, Caminer M, Ron SR. High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings. Biological sciences. 2012;279:1806–1814. doi: 10.1098/rspb.2011.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnizo et al. (2015).Guarnizo CE, Paz A, Muñoz Ortiz A, Flechas SV, Méndez-Narváez J, Crawford AJ. DNA barcoding survey of anurans across the eastern cordillera of colombia and the impact of the andes on cryptic diversity. PLOS ONE. 2015;10:e0127312. doi: 10.1371/journal.pone.0127312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes (2015).Guedes NMR. Instituto Arara Azulhttps://www.institutoararaazul.org.br/?en. [28 March 2020];The Hyacinth Macaw Project—biology, management, and conservation. 2015
- Guimarães et al. (2018).Guimarães KLA, De Sousa MPA, Ribeiro FRV, Porto JIR, Rodrigues LRR. DNA barcoding of fish fauna from low order streams of Tapajós River basin. PLOS ONE. 2018;13:e0209430. doi: 10.1371/journal.pone.0209430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon et al. (2010).Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hawlitschek et al. (2016).Hawlitschek O, Morinière J, Dunz A, Franzen M, Rödder D, Glaw F, Haszprunar G. Comprehensive DNA barcoding of the herpetofauna of Germany. Molecular Ecology Resources. 2016;16:242–253. doi: 10.1111/1755-0998.12416. [DOI] [PubMed] [Google Scholar]
- Hebert et al. (2003).Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, Ratnasingham & de Waard (2003).Hebert PDN, Ratnasingham S, de Waard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society B: Biological Sciences. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich et al. (2015).Hendrich L, Morinière J, Haszprunar G, Hebert PDN, Hausmann A, Köhler F, Balke M. A comprehensive DNA barcode database for Central European beetles with a focus on Germany: adding more than 3500 identified species to BOLD. Molecular Ecology Resources. 2015;15:795–818. doi: 10.1111/1755-0998.12354. [DOI] [PubMed] [Google Scholar]
- IUCN (2017).IUCN IUCN red list of threatened species. 2017. http://www.iucnredlist.org. [October 6 2017]. http://www.iucnredlist.org
- Jungfer et al. (2013).Jungfer K-H, Faivovich J, Padial JM, Castroviejo-Fisher S, Lyra MM, V. M. Berneck B, Iglesias PP, Kok PJR, MacCulloch RD, Rodrigues MT, Verdade VK, Torres Gastello CP, Chaparro JC, Valdujo PH, Reichle S, Moravec J, Gvoždík V, Gagliardi-Urrutia G, Ernst R, De la Riva I, Means DB, Lima AP, Señaris JC, Wheeler WC, F. B. Haddad C. Systematics of spiny-backed treefrogs (Hylidae: Osteocephalus): an Amazonian puzzle. Zoologica Scripta. 2013;42:351–380. doi: 10.1111/zsc.12015. [DOI] [Google Scholar]
- Kapli et al. (2017).Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T. Multi-rate poisson tree processes for single-locus species delimitation under maximum likelihood and Markov Chain Monte Carlo. Bioinformatics. 2017;33:1630–1638. doi: 10.1093/bioinformatics/btx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keever et al. (2009).Keever CC, Sunday J, Puritz JB, Addison JA, Toonen RJ, Grosberg RK, Hart MW. Discordant distribution of populations and genetic variation in a sea star with high dispersal potential. Evolution. 2009;63:3214–3227. doi: 10.1111/j.1558-5646.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Koroiva & Kvist (2018).Koroiva R, Kvist S. Estimating the barcoding gap in a global dataset of cox1 sequences for Odonata: close, but no cigar. Mitochondrial DNA Part A: DNA Mapping, Sequencing, and Analysis Jul. 2018;28:765–771. doi: 10.1080/24701394.2017.1357709. [DOI] [PubMed] [Google Scholar]
- Koroiva et al. (2017).Koroiva R, Pepinelli M, Rodrigues ME, Roque FDO, Lorenz-Lemke AP, Kvist S. DNA barcoding of odonates from the Upper Plata basin: database creation and genetic diversity estimation. PLOS ONE. 2017;12:e0182283. doi: 10.1371/journal.pone.0182283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroiva et al. (2018).Koroiva R, de Souza MS, Roque F de O, Pepinelli M. DNA barcodes for forensically important fly species in Brazil. Journal of Medical Entomology. 2018;55:1055–1061. doi: 10.1093/jme/tjy045. [DOI] [PubMed] [Google Scholar]
- Lavinia et al. (2017).Lavinia PD, Núñez Bustos EO, Kopuchian C, Lijtmaer DA, García NC, Hebert PDN, Tubaro PL. Barcoding the butterflies of southern South America: species delimitation efficacy, cryptic diversity and geographic patterns of divergence. PLOS ONE. 2017;12:e0186845. doi: 10.1371/journal.pone.0186845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra, Haddad & De Azeredo-Espin (2017).Lyra ML, Haddad CFB, De Azeredo-Espin AML. Meeting the challenge of DNA barcoding Neotropical amphibians: polymerase chain reaction optimization and new COI primers. Molecular Ecology Resources. 2017;17:966–980. doi: 10.1111/1755-0998.12648. [DOI] [PubMed] [Google Scholar]
- Magalhães de (2012).Magalhães de RF. Phylogeography in open areas of South America: origin and diversification of Scinax squalirostris (Anura, Hylidae). UFG 2012.
- Magalhães et al. (in press).Magalhães FM, Lyra ML, Carvalho TR, Baldo D, Bruschetti F, Burella P, Colli GR, Gehara MC, Giaretta AA, Haddad CFB, Langone JA, López JA, Napoli MF, Santana DJ, Sá RO De, Garda AA. Taxonomic review of South American Butter Frogs: phylogeny, biogeographic patterns, and species delimitation in the Leptodactylus latrans species group (Anura: Leptodactylidae) Herpetological Monographs. In Press. [Google Scholar]
- Meyer & Paulay (2005).Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLOS Biology. 2005;3:1–10. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Pfeiffer & Schwartz (2010).Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees. 2010 gateway computing environments workshop (GCE); 2010. pp. 1–8. [DOI] [Google Scholar]
- Mora et al. (2011).Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on earth and in the ocean? PLOS Biology. 2011;9:e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al. (2000).Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Neves et al. (2017).Neves MO, da Silva LA, Akieda PS, Cabrera R, Koroiva R, Santana DJ. A new species of poison frog, genus Ameerega (Anura: Dendrobatidae), from the southern amazonian rain forest. Salamandra. 2017;53:485–493. [Google Scholar]
- Pansonato et al. (2016).Pansonato A, Veiga-Menoncello ACP, Mudrek JR, Jansen M, Recco-Pimentel SM, Martins IA, Strüssmann C. Two new species of Pseudopaludicola (Anura: Leptodactylidae: Leiuperinae) from Eastern Bolivia and Western Brazil. Herpetologica. 2016;72:235–255. doi: 10.1655/Herpetologica-D-14-00047.1. [DOI] [Google Scholar]
- Park et al. (2011).Park HY, Yoo HS, Jung G, Kim CB. New DNA barcodes for identification of Korean birds. Genes and Genomics. 2011;33:91–95. doi: 10.1007/s13258-010-0089-3. [DOI] [Google Scholar]
- Paz & Crawford (2012).Paz A, Crawford AJ. Molecular-based rapid inventories of sympatric diversity: a comparison of DNA barcode clustering methods applied to geography-based vs clade-based sampling of amphibians. Journal of Biosciences. 2012;37:887–896. doi: 10.1007/s12038-012-9255-x. [DOI] [PubMed] [Google Scholar]
- Pelayo-Villamil et al. (2018).Pelayo-Villamil P, Guisande C, Manjarrés-Hernández A, Jiménez LF, Granado-Lorencio C, García-Roselló E, González-Dacosta J, Heine J, González-Vilas L, Lobo JM. Completeness of national freshwater fish species inventories around the world. Biodiversity and Conservation. 2018;27:3807–3817. doi: 10.1007/s10531-018-1630. [DOI] [Google Scholar]
- Puillandre et al. (2012).Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology. 2012;21:1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2020).R Development Core Team . Vienna: R Foundation for Statistical Computing; 2020. [Google Scholar]
- Ratnasingham & Hebert (2007a).Ratnasingham S, Hebert PDN. BOLD: the barcode of life data system ( http://www.barcodinglife.org) Molecular Ecology Notes. 2007a;7:355–364. doi: 10.1111/j.1471-8286.2006.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnasingham & Hebert (2007b).Ratnasingham S, Hebert PDN. BARCODING: bold: the barcode of life data system ( http://www.barcodinglife.org) Molecular Ecology Notes. 2007b;7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach et al. (2015).Raupach MJ, Barco A, Steinke D, Beermann J, Laakmann S, Mohrbeck I, Neumann H, Kihara TC, Pointner K, Radulovici A, Segelken-Voigt A, Wesse C, Knebelsberger T. The application of DNA barcodes for the identification of marine crustaceans from the North Sea and adjacent regions. PLOS ONE. 2015;10:e0139421. doi: 10.1371/journal.pone.0139421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende et al. (2018).Rezende CL, Scarano FR, Assad ED, Joly CA, Metzger JP, Strassburg BBN, Tabarelli M, Fonseca GA, Mittermeier RA. From hotspot to hopespot: an opportunity for the Brazilian Atlantic Forest. Perspectives in Ecology and Conservation. 2018;16:208–214. doi: 10.1016/j.pecon.2018.10.002. [DOI] [Google Scholar]
- Rodrigues et al. (2018).Rodrigues ME, Moura EB, Koroiva R, Borges ACP, Roque F de O. Survey of Dragonflies (Odonata) in palm swamps of cerrado hotspot. Entomological News. 2018;128:24–38. doi: 10.3157/021.128.0104. [DOI] [Google Scholar]
- Sá et al. (2014).Sá RO de, Grant T, Camargo A, Heyer WR, Ponssa ML, Stanley E. Systematics of the Neotropical Genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology. 2014;9:S1–S100. doi: 10.2994/SAJH-D-13-00022.1. [DOI] [Google Scholar]
- Santos et al. (2019).Santos CC, Ragalzi E, Valério-Junior C, Koroiva R. Anuran species composition from Chaco and Cerrado areas in central Brazil. Oecologia Australis. 2019;23:1027–1052. doi: 10.4257/oeco.2019.2304.25. [DOI] [Google Scholar]
- Schindel & Miller (2005).Schindel DE, Miller SE. DNA barcoding a useful tool for taxonomists. Nature. 2005;435:17–17. doi: 10.1038/435017b. [DOI] [PubMed] [Google Scholar]
- Segalla et al. (2019).Segalla MV, Caramaschi U, Cruz CAG, Garcia PCA, Grant T, Haddad CFB, Santana DJ, Toledo LF, Langone JA. Brazilian Amphibians: list of species. Herpetologia Brasileira. 2019;8:65–98. [Google Scholar]
- Silva et al. (2017).Silva IMG da, Prette del ACH, Werneck F, Garda A, Lemmon EM, Lemmon A, Colli G. Filogeografia dos grupos Leptodactylus labyrinthicus e Leptodactylus vastus na diagonal seca da América do Sul. Anais do VIII Congresso Brasileiro de Herpetologia; 2017. p. 61288. [Google Scholar]
- Souza et al. (2017).Souza FL, Prado CPA, Sugai JLMM, Ferreira VL, Aoki C, Landgref-Filho P, Strüssmann C, Ávila RW, Rodrigues DJ, Albuquerque NR, Terra J, Uetanabaro M, Béda AF, Piatti L, Kawashita-Ribeiro RA, Delatorre M, Faggioni GP, Demczuk SDB, Duleba S. Diversidade de anfíbios do Estado de Mato Grosso do Sul, Brasil. Iheringia. Série Zoologia. 2017;107:e2017152. doi: 10.1590/1678-4766e2017152. [DOI] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck et al. (2018).Struck TH, Feder JL, Bendiksby M, Birkeland S, Cerca J, Gusarov VI, Kistenich S, Larsson K-H, Liow LH, Nowak MD, Stedje B, Bachmann L, Dimitrov D. Finding evolutionary processes hidden in cryptic species. Trends in Ecology & Evolution. 2018;33:153–163. doi: 10.1016/j.tree.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Talaga et al. (2017).Talaga S, Leroy C, Guidez A, Dusfour I, Girod R, Dejean A, Murienne J. DNA reference libraries of French Guianese mosquitoes for barcoding and metabarcoding. PLOS ONE. 2017;12:e0176993. doi: 10.1371/journal.pone.0176993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher et al. (2020).Vacher J, Chave J, Ficetola FG, Sommeria-Klein G, Tao S, Thébaud C, Blanc M, Camacho A, Cassimiro J, Colston TJ, Dewynter M, Ernst R, Gaucher P, Gomes JO, Jairam R, Kok PJR, Lima JD, Martinez Q, Marty C, Noonan BP, Nunes PMS, Ouboter P, Recoder R, Rodrigues MT, Snyder A, Marques-Souza S, Fouquet A. Large-scale DNA-based survey of frogs in Amazonia suggests a vast underestimation of species richness and endemism. Journal of Biogeography. 2020;47(8):1781–1791. doi: 10.1111/jbi.13847. [DOI] [Google Scholar]
- Vences et al. (2005).Vences M, Thomas M, Bonett RM, Vieites DR. Deciphering amphibian diversity through DNA barcoding: chances and challenges. Philosophical transactions of the Royal Society B: Biological Sciences. 2005;360:1859–1868. doi: 10.1098/rstb.2005.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt & Caldwell (2014).Vitt LJ, Caldwell JP. Herpetology. Elsevier; 2014. Frogs; pp. 471–522. [DOI] [Google Scholar]
- Zhang et al. (2013).Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shown in yellow are the estimate coordinates of the locality or city center, considering that these records did not have this information.
Data Availability Statement
The following information was supplied regarding data availability:
The list of specimens used in this study is available in a Supplemental File.