Abstract
Background:
There is limited understanding on the impact of the multidose medication packaging service (MDMPS).
Objectives:
The main objective of this study was to evaluate changes in medication adherence in patients using MDMPS compared to patients receiving standard medication packaging (control group). The other objectives were to determine the association between medication adherence and clinical outcomes, and to assess patients’/caregivers’ perceptions toward MDMPS.
Methods:
A retrospective cohort study was conducted among primary care patients in Singapore enrolled into MDMPS between 2012 and 2017. Eligible patients were taking at least five chronic medications, diagnosed with Hypertension, Hyperlipidemia and/or Type 2 Diabetes, with prescription records for at least six months before and after the index period. They were matched to control patients based on the type of comorbidities and medication adherence status. Medication Possession Ratio (MPR), glycated hemoglobin (HbA1c), blood pressure and low-density lipoprotein-cholesterol (LDL-C) of both groups were compared between baseline and at least six months post-index period. Interviewer-administered questionnaires were also conducted for MDMPS patients.
Results:
The MPR of MDMPS patients (n = 100) increased by 0.37% (P < .001) compared to the control group (n = 100). MDMPS patients with diabetes had reduced HbA1c by 0.1% after six months (P = .022) but was not significant after 12 months. No significant changes were seen in blood pressure and LDL-C between both groups. At least 50% of patients were highly satisfied with MDMPS.
Conclusion:
MDMPS can improve medication adherence. Further studies are needed to understand its clinical impact.
Keywords: multi-dose medication packaging, medication adherence, diabetes management
Introduction
Medication adherence is defined as the extent to which a patient conforms with the prescribed interval and dose of a dosing regimen.1 According to the World Health Organization, approximately 50% of patients in developed countries do not take their medications as prescribed.2 Treatment complexity and inconvenience are important modifiable factors contributing to poor medication adherence.3 For example, the probability of medication adherence decreased significantly as the treatment complexity increased among patients on oral hypoglycemic agents.4
Packaging aids such as pill boxes and multi-compartment adherence aids can help improve medication adherence.5 One meta-analysis showed a moderate effect size of 0.593 favoring the use of packaging aids in improving adherence.6 Such tools can help patients manage their medication better and comply to their treatment regimen, especially for those with poly-pharmacy.
ConviDose™ is a multi-dose medication packaging service (MDMPS) in Singapore to organize a patient’s oral medication in a ready-to-administer package and is prepared via a centralized automated system. Online Appendixes 1 and 2 show details of the MDMPS used. It is tailored to the patient’s medication regimen and helps to ensure that the right medication is administered to the patient at the right dose, right time, and in the right quantity. Primary care patients and/or caregivers of patients who may have difficulties in medication administration and/or medication handling are encouraged to enroll for the service.
However, unit-of-use packaging such as ConviDose™ has not been studied as extensively as multidrug punch cards or multi-compartment adherence aids, and is under-represented in existing systematic reviews studying the impact on medication adherence.5,6 Moreover, since the implementation of this service for primary care patients in Singapore in 2012, its impact on medication adherence has not been evaluated. Therefore, the primary objective of this study was to evaluate the changes in medication adherence in patients using this MDMPS between baseline, six and 12 months post MDMPS enrolment, as compared to patients receiving medications with standard packaging.
This study also aimed to determine if the changes in medication adherence were associated with improved clinical outcomes, namely systolic (SBP) and diastolic (DBP) blood pressure, low-density lipoprotein-cholesterol (LDL-C) and/or glycated hemoglobin (HbA1c). To understand the users’ experience, this study also sought to assess patients’/caregivers’ perceptions on the safety and effectiveness of MDMPS, as well as their satisfaction level toward MDMPS as a product and service.
Methodology
Study Design
This is a retrospective cohort study involving patients in nine primary care clinics under the National Healthcare Group Polyclinics (NHGP). The study was reviewed and approved by the NHG Institutional Review Board.
Patients enrolled into MDMPS during the index period of April 2012 to February 2017 were first identified by retrieving electronic prescriptions tagged with MDMPS. Eligible patients were on chronic medications for Hypertension, Type 2 Diabetes Mellitus, and/or Hyperlipidemia. They were dispensed with five or more oral chronic medications into multi-dose medication packaging. Chronic supplements and medications for chronic constipation were excluded because these may be collected by patients on an as-needed basis. These patients had baseline prescription records for similar chronic medications of at least six months before MDMPS enrolment, and prescription records of at least six months after the enrolment.
Patients who received standard medication packaging and did not enrol for MDMPS were screened to be part of the control group. Each patient in this group was identified and matched to a MDMPS patient based on their age, gender, baseline type of comorbidities, clinic location and index period of prescription fill.
They were also matched on their baseline medication adherence level. Medication Possession Ratio (MPR) was calculated to measure medication adherence, as often used in adherence studies in various countries including Singapore,7 in both MDMPS and control groups. MPR is defined as the ratio of the number of days of medication supplied within the refill interval to the number of days in refill interval.8 A MPR of 80% is conventionally considered as adherent,8 while a MPR lower than 80% was considered as poor adherence.
Intervention
Patients enrolled for MDMPS were dispensed up to two weeks of medications that were packed as per standard packaging procedures first, to allow for time for processing medications into multi-dose medication packaging. The patient’s electronic prescription would be tagged and sent to a centralized pharmacy where the medications were packed into sachets by specialized automated machines and subsequently vacuum-sealed into monthly packages.
Patients or their caregivers would return to the pharmacy to collect the sachets, where instructions were provided on how to use MDMPS appropriately. Chronic medications prescribed in subsequent consultations were processed the same way. The duration of medications packed via MDMPS was dependent on the prescribed duration from each consultation and was in sufficient amount to last until the next consultation.
Data Collection
Baseline demographic characteristics
Baseline patient characteristics such as gender, age, clinical diagnoses and location of clinic visit were retrieved from the NHGP electronic clinical documentation system and Computerized Patient Support System 2 (CPSS2). The number of chronic medications dispensed was obtained from NHG Pharmacy’s electronic dispensing system (iPHARM). This was calculated as the average number of medications prescribed per consultation for the past six months prior to the index date.
Primary outcome variable
Baseline MPR was calculated based on the baseline chronic medication prescription filling pattern for six months before the index date. Post-intervention MPR was calculated from six months and twelve months post the index date of prescription filling. Prescription filling patterns for calculating MPRs were extracted from iPHARM, manually recorded and tabulated.
Secondary outcome variables
Data from patients in both study groups were collected retrospectively from CPSS2.
The following variables were collected:
Baseline HbA1c, HbA1c measured six months and twelve months after index period
Baseline LDL-C and LDL-C measured twelve months after index period
Baseline SBP and DBP, SBP and DBP measured six months and twelve months after index period
The diagnostic method used to obtain HbA1c readings was standardized among all laboratories within the clinics. Similarly, all blood pressure measurements were done by validated blood pressure monitoring devices standardized in measuring stations within the clinics.
Interviewer-administered questionnaires were conducted with the patients/caregivers after the use of MDMPS to rate their perception on the effectiveness and safety of MDMPS (Online Appendix 3). The questionnaires also requested patients/caregivers to rate their level of satisfaction toward the product and service.
Statistical Analysis
Baseline demographic characteristics, number and type of comorbidities, number of chronic oral medications, medication adherence level and clinical parameters were compared between the two study groups using Chi-square tests and Mann–Whitney U tests. Changes in MPR and clinical parameters before and after the pre-specified index period were also compared between the 2 groups using Mann–Whitney U test.
The Generalized Estimating Equation (GEE) was used to compare the association between changes in MPR and clinical outcomes (ie, HbA1c, SBP and DBP) while adjusting for other covariates (ie, Gender, Ethnicity, Age, Time, Baseline Clinical Parameter, Baseline MPR, Baseline number of oral chronic medications and the use of MDMPS). Logistic regression was used to determine the association between changes in MPR and LDL-C. Questionnaire data were presented as percentages of responses.
All patients who met the inclusion criteria were analyzed. A conventional alpha of 0.05 was used, and a P-value less than .05 was considered statistically significant. All analyses were conducted using STATA software (Version 13, College Station, TX, StataCorp LP. StataCorp, USA) and SPSS software (Version 23, SPSS Inc., Chicago, IL, USA).
Results
Participants
From April 2012 to February 2017, 331 patients were enrolled into MDMPS, out of which 100 patients met all the inclusion criteria. Another 100 control patients were then identified and matched based on the pre-defined criteria.
The baseline demographics of both groups are shown in Table 1. The participants, with a median age of 78, were predominantly female and of Chinese ethnicity in both groups. Although there was a statistically significant difference in the overall number of baseline medications between the 2 groups, no significant differences were seen in the baseline number of medications for Hypertension, Hyperlipidemia or Type 2 Diabetes (P > .05).
Table 1.
Patient Characteristics at Baseline.a
| Patient characteristics | Control group (n = 100) | Intervention (MDMPS) group (n = 100) | P-value | |
|---|---|---|---|---|
| Median age (IQR) | 77.5 (68.5-82.0) | 77.5 (69.0-82.0) | .951 | |
| Gender | Number of female patients | 59 | 59 | 1.000 |
| Number of male patients | 41 | 41 | ||
| Race | Number of Chinese patients | 81 | 81 | 1.000 |
| Number of non-Chinese patients | 19 | 19 | ||
| Number of patients with HTN | 100 | 100 | 1.000 | |
| Number of patients with HLD | 100 | 100 | 1.000 | |
| DM status | Number of patients without DM | 11 | 11 | 1.000 |
| Number of patients with DM | 89 | 89 | ||
| Use of insulin | Number of DM patients not on insulin | 69 | 69 | 1.000 |
| Number of DM patients on insulin | 20 | 20 | ||
| LDL-C b (mmol/L) | Number of patients with LDL-C <2.6 | 66 | 69 | .725 |
| Number of patients with LDL-C ≥2.6 | 33 | 31 | ||
| Median LDL-C (IQR) | 2.30 (1.9-2.8) | 2.22 (1.8-2.7) | .525 | |
| SBP c (mmHg) | Number of patients with SBP <140 | 52 | 53 | .887 |
| Number of patients with SBP ≥140 | 48 | 47 | ||
| Median SBP (IQR) | 138 (127-149) | 136 (128-150) | .582 | |
| DBP c (mmHg) | Number of patients with DBP <90 | 96 | 94 | .516 |
| Number of patients with DBP ≥90 | 4 | 6 | ||
| Median DBP (IQR) | 70 (62-77) | 70 (64-76) | .955 | |
| HbA1c d in patients with DM (mmol/L) | Number of patients with HbA1c ≤7.0 | 24 | 23 | .931 |
| Number of patients with HbA1c 7.1-9.0 | 48 | 47 | ||
| Number of patients with HbA1c >9.0 | 17 | 19 | ||
| Median HbA1c (IQR) | 7.6 (7.0-8.4) | 8 (7.0-8.7) | .330 | |
| MPR | Number of non-adherent patients | 21 | 21 | 1.000 |
| Number of adherent patients | 79 | 79 | ||
| Median MPR (IQR) | 90.02 (81.06-100.00) | 97.85 (83.46-100.00) | .039** | |
| Median number of chronic medications (IQR) | 7 (6-8) | 7 (6-9) | .041** | |
| Median number of HLD medications (IQR) | 1 (1-1) | 1 (1-1) | .916 | |
| Median number of HTN medications (IQR) | 2 (1-3) | 2 (2-3) | .189 | |
| Median number of OHGA for DM patients (IQR) | 2 (1-2) | 2 (1-2) | .632 | |
Abbreviations: DBP: diastolic blood pressure; DM: type 2 diabetes mellitus; HbA1c: glycated hemoglobin; HLD: hyperlipidemia; HTN: hypertension; LDL-C: low-density lipoprotein-cholesterol; MPR: medication possession ratio; OHGA: oral hypoglycemic agents; SBP: systolic blood pressure.
Data are presented in numbers unless stated otherwise.
Optimal LDL-C levels are less than 2.6 mmol/L in patients with HLD as per Adult Treatment Panel (ATP III) guidelines.9
Target SBP of less than 140 mmHg and target DBP of less than 90 mmHg in hypertensive patients as per Joint National Committee (JNC 8) guidelines.10
HbA1c of less than 7% in DM patients as per American Diabetes Association (ADA 2018) guidelines.11
P < .05.
Adherence and Clinical Outcomes
Table 2 shows the outcome comparisons between the control and MDMPS groups. There was an increase in MPR by 0.37% after six months in the MDMPS group, as compared to a decrease in MPR by 3.49% in the control group (P < .001). The overall MPR in the MDMPS group continued to show an increase of 0.41% compared to the control group (P < .001) after 12 months.
Table 2.
Outcome Comparisons between Control and MDMPS Groups.
| Outcomes | Control group (n = 100) Median (IQR) | MDMPS group (n = 100) Median (IQR) | P-value |
|---|---|---|---|
| Changes of MPR at 6 months | −3.49 (–19.31 to 2.30) | 0.37 (0.00 to 9.95) | <.001** |
| Changes of MPR at 12 months | −5.95 (–19.21 to 1.63) | 0.41 (0.00 to 8.67) | <.001** |
| Changes of LDL-C at 12 months | −0.14 (–0.43 to 0.20) | −0.1 (–0.31 to 0.27) | .413 |
| Changes of SBP at 6 months | −5 (–17 to 10) | −1.5 (–15 to 14) | .379 |
| Changes of SBP at 12 months | 0 (–9 to 9) | 2 (–8 to 16) | .228 |
| Changes of DBP at 6 months | 0 (-9 to 6) | −1.5 (–9 to 7) | .785 |
| Changes of DBP at 12 months | −1 (-8 to 7) | 0 (–7 to 5) | .764 |
| Changes of HbA1c at 6 months (DM patients) | 0.1 (–0.4 to 0.6) | −0.1 (–0.8 to 0.3) | .022** |
| Changes of HbA1c at 12 months (DM patients) | 0.1 (–0.6 to 0.6) | −0.2 (–1.0 to 0.5) | .287 |
Abbreviations: DBP: diastolic blood pressure; DM: type 2 diabetes mellitus; HbA1c: glycated hemoglobin; LDL-C: low-density lipoprotein-cholesterol; MPR: medication possession ratio; SBP: systolic blood pressure.
P < .05.
In terms of clinical outcomes, no significant differences in blood pressure and LDL-C levels were seen between the groups. There was a decrease in HbA1c in the MDMPS group of 0.1%, in contrast to the increase of 0.1% seen in the control group after six months (P = .022). The HbA1c decreased in the MDMPS group by 0.2% from baseline after twelve months (P = .287).
The association between the change in MPR in oral hypoglycemic agents (OHGAs) and HbA1c was also compared while adjusting for other covariates. As in Table 3, no significant association was seen between MPR of OHGAs and decrease in HbA1c in diabetic patients. Similarly, no association was seen between the change in MPR of antihypertensives and decrease in SBP and DBP as well (P > .05). However, changes in MPR in medication for hyperlipidemia were shown to be associated with a decrease in LDL-C (OR 1.04, P = .007).
Table 3.
Summary of Associations of Clinical Parameters as Outcomes with the Main Independent Variable (Changes of MPR) using GEE (for HbA1c, SBP and DBP) and Logistic Regression (for LDL-C).
| Clinical parameter | Factors | Odds ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Decrease in HbA1ca | Changes of MPR of OHGA | 1.02 (1.00-1.04) | .060 | |
| Group | Control | 1.00 | .497 | |
| MDMPS | 1.28 (0.63-2.60) | |||
| Decrease in SBPb | Changes of MPR of HTN medication | 0.99 (0.98-1.01) | .305 | |
| Group | Control | 1.00 | .411 | |
| MDMPS | 0.80 (0.47-1.36) | |||
| Decrease in DBPb | Changes of MPR of HTN medication | 0.99 (0.97-1.00) | .132 | |
| Group | Control | 1.00 | .870 | |
| MDMPS | 0.96 (0.55-1.65) | |||
| Decrease in LDL-Cc | Changes of MPR of HLD medication | 1.04 (1.01-1.07) | .007** | |
| Group | Control | 1.00 | .359 | |
| MDMPS | 0.65 (0.26-1.64) | |||
Abbreviations: DBP: diastolic blood pressure; DM: type 2 diabetes mellitus; GEE: generalized estimating equation; HbA1c: glycated hemoglobin; HLD: hyperlipidemia; HTN: hypertension; LDL-C: low-density lipoprotein-cholesterol; MPR: medication possession ratio; OHGA: oral hypoglycemic agent; SBP: systolic blood pressure.
Covariates in GEE: Time (6 or 12-month index period), gender, race, use of insulin, age, baseline HbA1c, baseline MPR of OHGA, baseline number of chronic medications and baseline number of OHGA.
Covariates in GEE: Time (6 or 12-month index period), gender, race, age, baseline SBP or DBP, baseline MPR of HTN medications, baseline number of chronic medications and baseline number of HTN medications.
Covariates in Logistic regression: Gender, race, age, baseline LDL-C, baseline MPR of HLD medications, baseline number of chronic medications and baseline number of HLD medications.
P < .05.
Patient/Caregiver Questionnaire Data
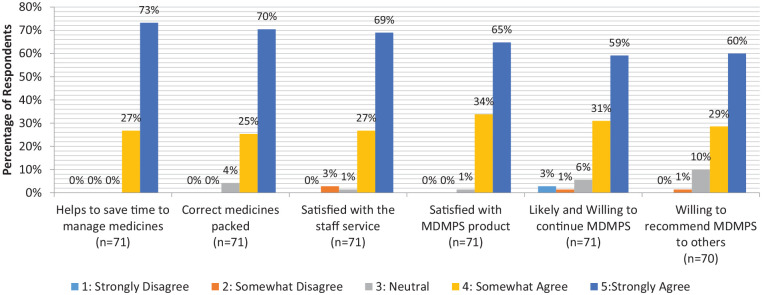
Based on Figure 1, the majority (73%) of patients strongly agreed that MDMPS helped them save time in managing their medications. Seventy percent of patients strongly agreed that the correct medications were packed in the right quantity. Sixty-nine percent of MDMPS patients strongly agreed that staff service was good, while 65% strongly agreed that the product was good. Fifty-nine percent of MDMPS patients strongly agreed that they would continue using the service, while 60% strongly agreed that they would recommend the service to others.
Figure 1.
MDMPS survey results.
Discussion
Medication Adherence
All patients enrolled in the MDMPS group had at least maintained or improved their adherence to medication. This is in contrast to the control group who showed a decrease in MPR. This improvement in medication adherence could be attributed to the simplification in administering a medication regimen via MDMPS, as unit-of-use sachets were easier to understand, as compared to standard medication packaging. Instead of having to read and follow instructions from multiple separate drug labels or boxes, patients on MDMPS only needed to refer to instructions on a single unit-of-use medication sachet. This result is consistent with other studies that have shown improvement in adherence with the use of medication packaging aids.5 In Conn’s meta-analysis on packaging interventions, data from 20 studies using blister packs resulted in a larger effect size of 0.804 on medication adherence, as compared to the effect size of 0.384 from the use of pill boxes.6
Clinical Outcomes
Among Type 2 Diabetes patients, there was a decrease in HbA1c in the MDMPS group, while the control group patients showed an increase in HbA1c after six months and twelve months. After subgroup analysis, it was found that patients on MDMPS were 28% more likely to have a decrease in HbA1c than control group patients. However, this result was not statistically significant. There were also no statistically significant associations between changes of MPR of oral hypoglycemic agents, or the use of insulin, and decrease in HbA1c. This is in contrast to a retrospective study involving approximately 32 000 patients that showed that patients non-adherent to certain OHGAs had a smaller decrease in HbA1c over a year compared to adherent patients. Medication adherent (MPR ≥ 80%) patients showed a baseline adjusted HbA1c decrease of approximately 1%.12
There was no statistical difference between the MDMPS and control groups in blood pressure parameters for hypertension. Subgroup analyses also showed no statistical significant association between the change in adherence to antihypertensive medications, or the use of MDMPS, and decrease in blood pressure parameters. There have been conflicting results in various studies in the association of changes in blood pressure parameters from baseline in those receiving adherence aids as compared to controls. In a randomized controlled trial by Jeannie et al, patients recruited in a pharmacy care program received their antihypertensive medication in blister packaging and showed a reduction in mean SBP of 3.3 mmHg from baseline within eight months compared to those who received usual care (P = .02).13 In a separate randomized controlled trial, there were no significant absolute differences in SBP or DBP from 47 patients using daily-dose medication packaging and 38 patients using traditional bottles.14
While patients between the two groups did not show any significant differences in changes in LDL-C, the change in MPR of hyperlipidemia medications was associated with decrease of LDL-C. Similar results have been concluded in other studies looking into the relationship between adherence of statins and changes to LDL-C, which involved much larger pools of patients.15,16 Multivariate analysis did not show any significant association between the use of MDMPS and decrease in LDL-C. One study showed that high adherence rates was associated with decrease in LDL-C levels only in patients on high intensity statins.17 This could potentially be a factor that influenced the LDL-C result independent of the use of MDMPS, which was not considered in the study.
Patient/Caregiver Surveys
Patient feedback was largely positive since the implementation of MDMPS. Based on Figure 1, 70% of patients who completed the survey were highly satisfied with the MDMPS product and service. Forty-two out of 71 patients who took up MDMPS were also willing to continue and recommend the service to others.
Not all patients were willing to continue or recommend MDMPS. A possible reason may be due to the inability to follow instructions on the sachets, as some patients had given feedback that the font was too small. The font size on the sachets were optimized to contain all the important information, as seen shown in online Appendix 1.
Study Strengths and Limitations
This study focused on how MDMPS as a medication packaging aid could improve medication adherence of the majority of patients seen in the Singapore primary care setting who have Hypertension, Hyperlipidemia and/or Type 2 Diabetes. This study considered and controlled for multiple variables to identify if the use of MDMPS could be translated to impactful clinical outcomes for the patients using them. Moreover, the study captured patients’ experiences on the use of medication packaging aids, which is rarely seen in existing literature.
However, there were some limitations. Firstly, there might be other factors that result in poorer medication adherence that were not accounted for in this retrospective analysis. Secondly, for control group patients, it was not assessed whether they were on medications from other healthcare institutions. Thirdly, socioeconomic status and patients’ perceptions of their medical conditions were not considered in this study that could have possibly affected the patients’ medication adherence. Fourthly, MPR is based on prescription refills and may not necessarily translate to actual adherence.
Conclusion
This retrospective cohort study showed the potential of using MDMPS to improve medication adherence among patients with the common chronic conditions (Hypertension, Hyperlipidemia and/or Type 2 Diabetes) seen in primary care. It also showed largely positive feedback in terms of the product and service from its users. A larger sample size with a prospective study design may be better suited to assess whether MDMPS can contribute toward improvements in clinical outcomes. In the long term, it may also be beneficial to conduct a cost effectiveness analysis to better understand the full health benefits for patients who enroll into MDMPS.
Supplemental Material
Supplemental material, Appendices_MDMPS for Evaluating the Impact of Outpatient Multi-Dose Medication Packaging Service (MDMPS) on Medication Adherence and Clinical Outcomes by Pratibha Nair, Kok Wai Kee, Choon Siong Mah and Eng Sing Lee in Journal of Primary Care & Community Health
Footnotes
Authors’ Note: The study has been presented as posters in the Asia Pacific Primary Care Research Conference 2019 and Singapore Health and Biomedical Congress 2019.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by the Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme (Ref No: CGAug16C019). The funding body did not have any role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
ORCID iD: Pratibha Nair  https://orcid.org/0000-0002-1821-2496
https://orcid.org/0000-0002-1821-2496
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44-47. [DOI] [PubMed] [Google Scholar]
- 2. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollack M, Chastek B, Williams SA, Moran J. Impact of treatment complexity on adherence and glycemic control: an analysis of oral antidiabetic agents. J Clin Outcomes Manag. 2010;17:257-265. [Google Scholar]
- 5. Boeni F, Spinatsch E, Suter K, Hersberger KE, Arnet I. Effect of drug reminder packaging on medication adherence: a systematic review revealing research gaps. Syst Rev. 2014;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conn VS, Ruppar TM, Chan KC, Dunbar-Jacob J, Pepper GA, De Geest S. Packaging interventions to increase medication adherence: systematic review and meta-analysis. Curr Med Res Opin. 2015;31:145-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai YF, Neo JK, Cheen MH, Kong MC, Tai BC, Ng HJ. Comparison of medication adherence and treatment persistence between new oral anticoagulant and warfarin among patients. Ann Acad Med Singap. 2016;45:12-17. [PubMed] [Google Scholar]
- 8. Tang KL, Quan H, Rabi DM. Measuring medication adherence in patients with incident hypertension: a retrospective cohort study. BMC Health Serv Res. 2017;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486. [DOI] [PubMed] [Google Scholar]
- 10. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S55-S64. [DOI] [PubMed] [Google Scholar]
- 12. Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose-lowering therapies and associations with 1-Year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563-2571. [DOI] [PubMed] [Google Scholar]
- 14. Schneider PJ, Murphy JE, Pedersen CA. Impact of medication packaging on adherence and treatment outcomes in older ambulatory patients. J Am Pharm Assoc. 2008;48:58-63. [DOI] [PubMed] [Google Scholar]
- 15. Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28:595-599. [DOI] [PubMed] [Google Scholar]
- 16. Vupputuri S, Joski PJ, Kilpatrick R, et al. LDL cholesterol response and statin adherence among high-risk patients initiating treatment. Am J Manag Care. 2016;22:e106-e115. [PubMed] [Google Scholar]
- 17. Vodonos A, Ostapenko I, Toledano R, et al. Statin adherence and LDL cholesterol levels. Should we assess adherence prior to statin upgrade? Eur J Intern Med. 2015;26:268-272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendices_MDMPS for Evaluating the Impact of Outpatient Multi-Dose Medication Packaging Service (MDMPS) on Medication Adherence and Clinical Outcomes by Pratibha Nair, Kok Wai Kee, Choon Siong Mah and Eng Sing Lee in Journal of Primary Care & Community Health