The increased prevalence of pediatric obesity has led to an increased incidence and prevalence of type 2 diabetes mellitus (T2DM), as well as the intermediate condition of prediabetes, in youth. Given that the definitions for these conditions were established based on adult data, pediatric providers are struggling with the definition and significance of prediabetes in children.
Although this report addresses prediabetes as a precursor to T2DM in the child and adolescent with overweight/obese child, type 1 diabetes remains the most common cause of diabetes during childhood, and should be kept under consideration by the clinician regardless of the child’s body mass index (BMI). This is particularly true in youth less than 10 years of age and/or who are prepubertal.
The American Diabetes Association (ADA) defines diabetes as (1) a fasting (no caloric intake for at least 8 hours) glucose of >125 mg/dL, or (2) a 2-hour glucose on an oral glucose tolerance test (OGTT) of ≥200 mg/dL (in the absence of unequivocal hyperglycemia, the ADA recommends that the result should be confirmed with repeat testing), or (3) a random glucose of ≥200 mg/dL with the classic diabetes symptoms, or (4) a hemoglobin A1c (HbA1c) of ≥6.5% by an National Glycohemoglobin Standardization Program-certified device, standardized to the Diabetes Control and Complications Trial assay (in the absence of unequivocal hyperglycemia, the ADA recommends that the result should be confirmed with repeat testing).1
Impaired fasting glucose (fasting glucose of 100–125 mg/dL) and impaired glucose tolerance (IGT) (2-hour glucose of 140–199 mg/dL on an OGTT) are associated with increased risk of developing diabetes.1 Individuals with impaired fasting glucose, IGT, or both are included under the broad definition of prediabetes. With the updated diagnostic HbA1c criteria for diabetes in 2010, also came an HbA1c range associated with increased risk for diabetes: 5.7%−6.4%.1 The ADA states that the term “prediabetes” may be applied to this group and that those with HbA1c in the 6.0%−6.4% group are at particularly high risk for developing diabetes. In each of these conditions in adults (impaired fasting glucose, IGT, or elevated HbA1c in the prediabetes range), there is a continuum of risk that is curvilinear, with the diabetes risk increasing disproportionately to the HbA1c or absolute glucose value.1
Impaired fasting glucose and IGT are considered risk factors for both the development of diabetes and for cardiovascular disease (CVD). After adjustment for traditional CVD risk factors, a longitudinal, population-based study by Barr et al showed that adults with IGT and impaired fasting glucose had a 50%−60% greater 5-year mortality risk than those with normal glucose tolerance.2 In that study, 65% of CVD deaths occurred in those with known diabetes, newly diagnosed diabetes, impaired fasting glucose, or IGT at baseline. Up to 22.6% of adults with overweight have been shown to have prediabetes and approximately 5%−10% of adults with prediabetes will progress to diabetes within a year.3–5
Among US adolescents in the National Health and Nutrition Examination Survey cohort, 13.1% had impaired fasting glucose and 3.4% had IGT, with an overall prediabetes prevalence (not including HbA1c in this definition) of 16.1%. The prevalence of prediabetes was approximately 30% among adolescents with obesity, with 22.7% having impaired fasting glucose and 9.5% having IGT.6 The prevalence of prediabetes in adolescents has been increasing, with 1.8% having impaired fasting glucose in 1988–1994, 7.0% in 1999–2000, and 23% in 2007–2008.7 A school-based study screening eighth-grade minority children with multiple risk factors for diabetes found that 43% met the criteria for prediabetes using impaired fasting glucose and IGT definitions.8 However, youth have unique circumstances affecting the likelihood of progression of prediabetes to T2DM. Youth have differences in the degree of obesity, dietary choices, physical activity patterns, and other behaviors when compared with adults. In addition, elevations in hormones necessary to achieve final growth and physical development, particularly growth hormone and insulin-like growth factor-1, contribute strongly to the normal physiologic decrease in insulin sensitivity that occurs during puberty.9–11 In youth with multiple risk factors for the development of T2DM, dysglycemia can be associated with the pubertal decrease in insulin sensitivity. As growth hormone/insulin-like growth factor-1 levels decrease toward the end of puberty, insulin sensitivity improves.11 Thus, there is a high rate of spontaneous remission of prediabetes in youth with obesity when puberty ends. In a study of 547 youth age 14.5 ± 2.2 years (70% Hispanic), with baseline HbA1c in the prediabetes and T2DM range, 420 youth had a follow-up HbA1c available at a median of 12–22 months. Of those with follow-up HbA1c available, only 33% of those with baseline HbA1c of ≥6.5%, 8% of those with a baseline HbA1c of 6.0–6.4% and 4% of those with baseline HbA1c values of 5.7%−5.9%, had a follow-up HbA1c of ≥6.5%. There was a linear relationship overall in the cohort between worsening BMI and progression to T2DM. Thus, the risk for progression of HbA1c from the prediabetes to diabetes category was not equal amongst all youth with baseline HbA1c in the prediabetes range.12 In another study of 79 white youth with obesity 13.1 ± 2.1 years old with IGT, after 1 year only 32% still had IGT; 66% converted to normal glucose tolerance, 1 child had impaired fasting glucose, and 1 child developed T2DM.13
Although there is no direct evidence that early diagnosis of prediabetes improves the long-term outcome of diabetes in children and adolescents, there is indirect evidence that identifying prediabetes in youth may be valuable. Studies in adults indicate that lifestyle modification can prevent or delay the onset of T2DM.14 Thus, targeted screening of adolescents with risk factors for diabetes is currently recommended. However, one must exercise caution in extrapolating from adult data, because the phenotype of T2DM differs in youth from that of adults.15
Screening
The Pediatric Endocrine Society with the Endocrine Society and European Society of Endocrinology, and the ADA endorse screening for prediabetes/T2DM in high-risk youth.1,16,17 High risk is defined as (1) age ≥10 years or pubertal (if this occurs before age 10 years), and meeting the following weight criteria: BMI ≥85th percentile for age and sex on the standard Centers for Disease Control and Prevention growth charts, weight for height >85th percentile, or weight >120% of ideal (50th percentile) for height, and (2) the presence of at least 2 of the following risk factors: family history of T2DM in a first- or second-degree relative; minority race/ethnicity (Native American, black, Hispanic, Asian American, Pacific Islander); conditions or signs associated with insulin resistance (acanthosis nigricans, polycystic ovary syndrome, hypertension, dyslipidemia, small for gestational age); and maternal diabetes or gestational diabetes during the child’s gestation.17 In the ADA consensus statement published in 2000, it was recommended that screening be repeated every 2 years and clinical judgment should be used to test for prediabetes/T2DM in patients who do not fulfill these criteria. Appropriate screening tests for prediabetes/T2DM can include HbA1c, fasting plasma glucose (FPG), and/or OGTT.
There is considerable debate surrounding what constitutes the optimal approach to screening for prediabetes in youth.18–20 The 2 most convenient tests, HbA1c and FPG, measure different aspects of overall glycemic control with poor correlation between HbA1c in the prediabetes range (5.7%−6.4%) and impaired fasting glucose (100–125 mg/dL).21 Epidemiologic studies in adults indicate that HbA1c and plasma glucose concentrations (FPG or 2-hour OGTT) identify overlapping but different groups of individuals.22 Moreover, FPG alone is a poor predictor of defects in glucose tolerance in youth, because many obese youth with IGT on OGTT have a FPG of <100 mg/dL.23 Thus, FPG alone may miss a significant proportion (approximately 70%) of youth with IGT detected by OGTT.24
An international expert committee has recommended using HbA1c for screening and diagnosis of prediabetes.25 These guidelines classify asymptomatic individuals as having diabetes if they have an HbA1c of ≥6.5% on 2 separate occasions and as having prediabetes if they have an HbA1c of 6.0%−6.4%.25 The ADA has endorsed classifying individuals with an HbA1c of 5.7%−6.4% as having prediabetes to include a broader range of at-risk individuals, so that interventions could be implemented earlier in the pathogenesis of diabetes.26 However, lowering the threshold brings with it the inclusion of more individuals who are unlikely to develop diabetes. Using HbA1c as a screening and diagnostic test has the benefits of being available as a point-of-care test that does not require fasting, can be done from a single finger stick, and is a less expensive and a much shorter procedure than a 2-hour OGTT. However, it is important to note that the usefulness of HbA1c is affected by pathologic conditions affecting red blood cell turnover. For example, sickle cell anemia decreases the lifespan of the red blood cell and can cause a lower HbA1c despite hyperglycemia.27 In addition, although HbA1c assays have been standardized, clinically significant intermethod variability still exists, such that diabetes classification between HbA1c methods remains poor (1 study of 2 methods for HbA1c found a concordance of diabetes classification of only 37%).28 Also, studies validating the use of HbA1c to detect risk for complications related to prediabetes/T2DM have solely been performed in adults, and may not be directly translatable to youth.
The OGTT can diagnose IGT earlier in the course of progressive dysglycemia than the HbA1c.20 Moreover, OGTT glycemic outcomes have also been associated with risk factors for the development of T2DM, CVD, and increased morbidity.29–32 However, the OGTT also requires fasting, takes ≥2 hours, and has poor reproducibility among youth with prediabetes, even under controlled research settings.24 Moreover, a high percentage of children with prediabetes diagnosed by OGTT revert to normal glucose tolerance over 1–5 years’ time, despite no intervention or weight loss. These limitations call into question the usefulness of this test for youth in clinical settings.13,33
As in adults, studies in youth indicate poor agreement between HbA1c, FPG, and OGTT in diagnosing prediabetes.20,34 One study found that 47% of youth with HbA1c in the prediabetes range (5.7%−6.4%) had OGTT results consistent with prediabetes; and 27% of youth with normal HbA1c levels had OGTT results consistent with prediabetes. Moreover, a large study of youth with overweight and obesity (n = 4848) showed only weak correlations between HbA1c and FPG (r = 0.18) and HbA1c and 2-hour OGTT glucose (r = 0.17).35 It is clear that these measures—FPG, OGTT, and HbA1c—reflect distinct aspects of carbohydrate metabolism and resulting glycemia, and that these are associated with the discrepancies with the other 2 measures. Moreover, in some youth, the 75 g of dextrose present in an OGTT is less than the glycemic load they typically consume in one sitting. Therefore, an adolescent could have normal glucose tolerance measured by OGTT, but have higher glucose consumption in their home diet, and this condition may be reflected in the HbA1c. When continuous glucose monitoring is used, HbA1c reflects average glucose and glucose area under the curve (AUC), whereas 2-hour glucose on OGTT reflects the peak glucose and percent time >140 and >200 mg/dL.36 Thus, one test may not be more “correct,” but may instead provide different information. It has been suggested that HbA1c is not sufficiently sensitive or specific enough to make the diagnosis of prediabetes alone, but should be used in combination with OGTT.37 However, it is not clear what the gold standard for prediabetes and diabetes diagnosis should be in youth. Thus, expert opinion and screening practice varies. Given the lack of consensus on an optimal screening test for prediabetes and T2DM in youth, the historical practice of using OGTT, and the effect of high red blood cell turnover on HbA1c levels in hemolytic anemias, one could consider screening those who have unique risk factors with both HbA1c and OGTT.
Recommendations for Prediabetes/Diabetes Screening
After preliminary obesity screening with HbA1c and/or FPG, who should be screened further and how? In our opinion, the OGTT may be beneficial in assessing high-risk patients who meet the following criteria: (1) HbA1c ≥6.0% or (2) FPG glucose ≥100 mg/dL on >2 occasions, or (3) significant symptoms of polyuria, polydipsia, and/or nocturia/enuresis (particularly new-onset or worsening). However, repeating the HbA1c over time may also be sufficient. The Figure shows a possible management algorithm.
Figure.
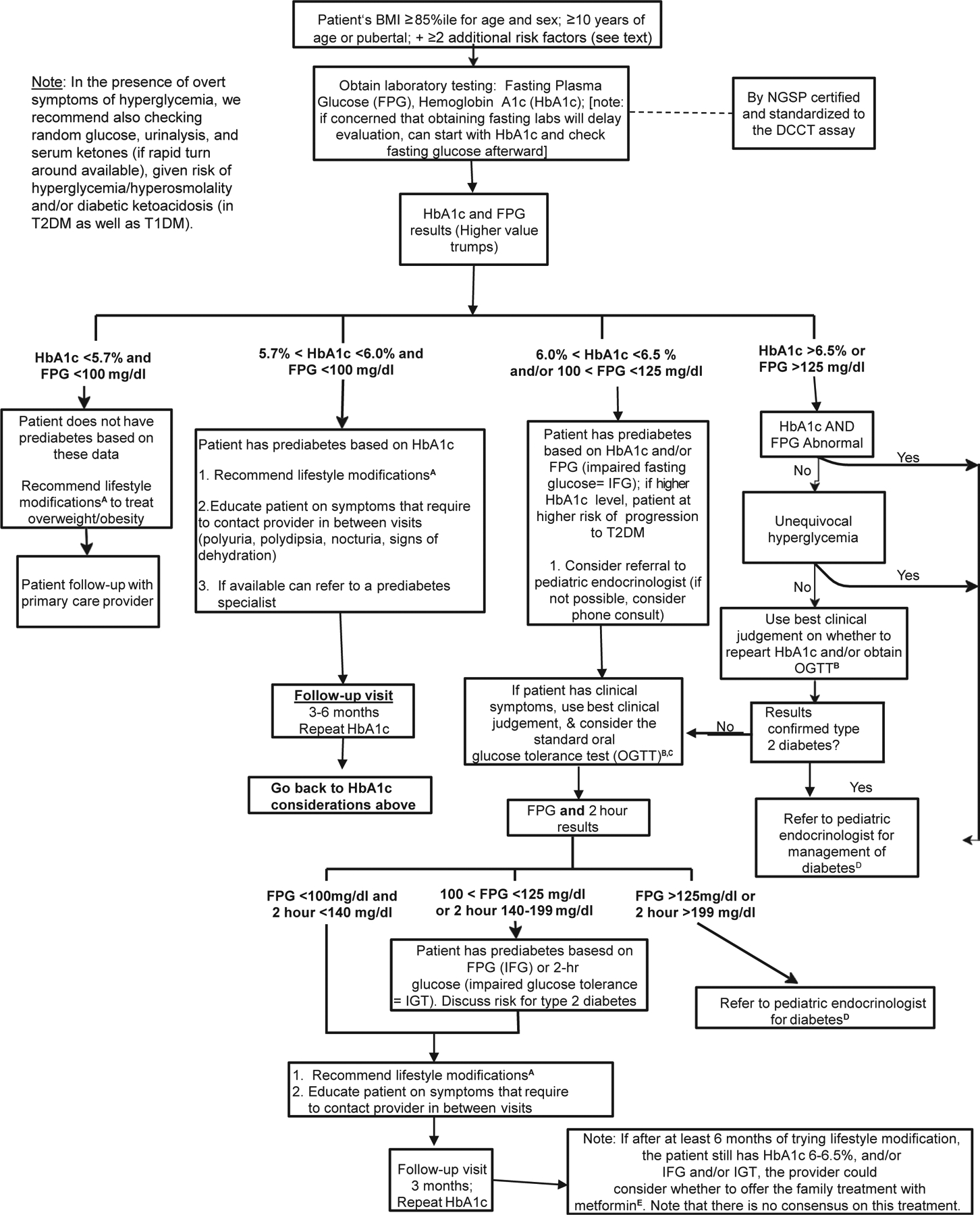
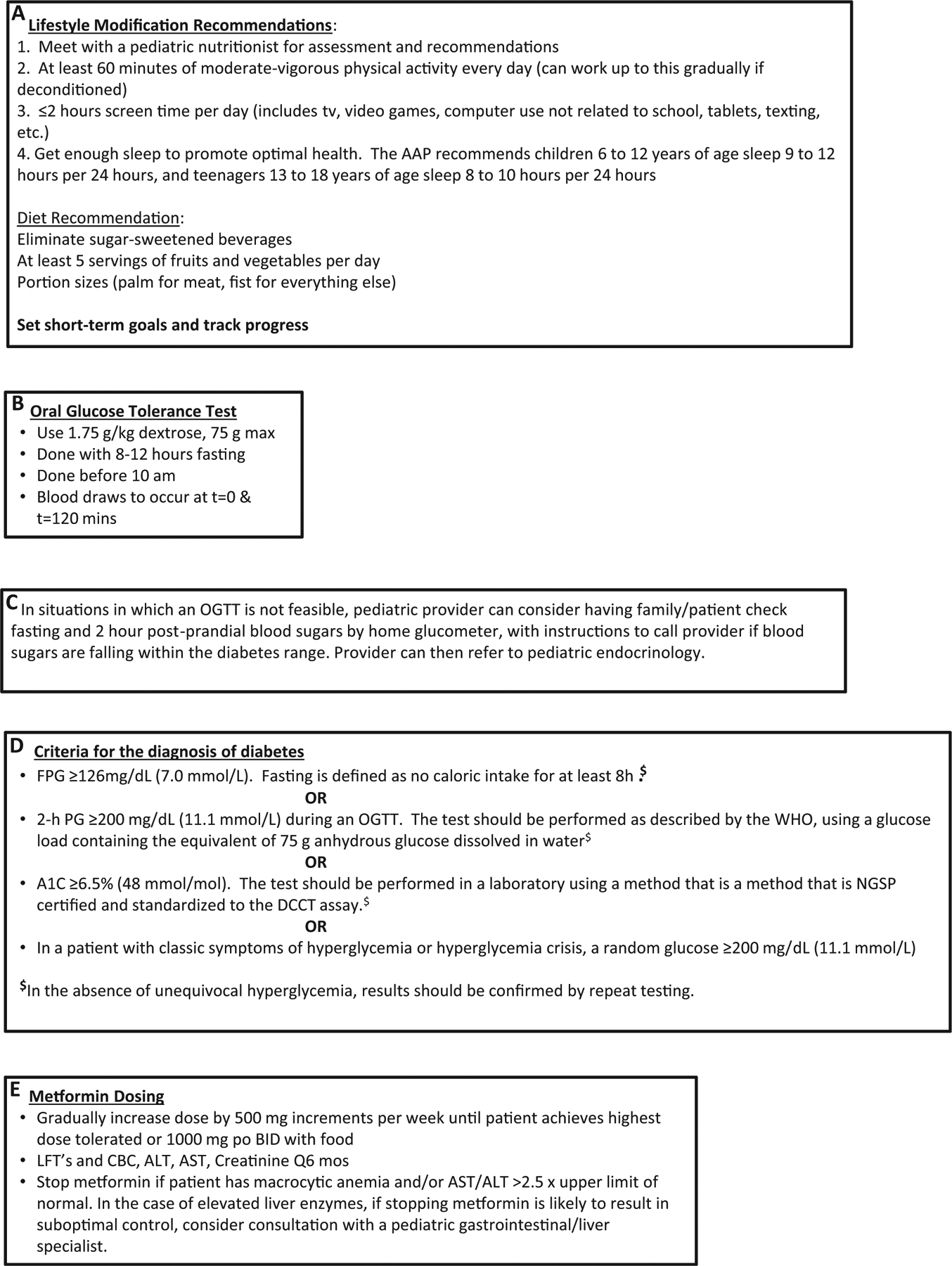
Possible management algorithm. The superscript references in the algorithm refer to the information on the next page. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; DCCT, Diabetes Control and Complications Trial; LFT, liver function tests; NGSP, National Glycohemoglobin Standardization Program; PG, plasma glucose; WHO, World Health Organization.
Consider referral of a youth with prediabetes (if diagnosed by HbA1c, particularly if HbA1c 6.0%−6.4%) to a pediatric provider specializing in the care of youth with prediabetes. In cases in which a referral is not available, consider telephone consultation with a pediatric endocrinologist and/or use best clinical judgement. There is no definitive consensus on how often the OGTT and/or HbA1c should be performed in youth with prediabetes. Testing may be repeated in 3–6 months or sooner if one suspects progression of dysglycemia for the following concerns: continued increase in BMI, patient continues to meets the prediabetes criteria as described, increase in HbA1c and/or FPG (but values remain below the diagnostic criteria for diabetes), patient has worsening or new symptoms of diabetes (as described).
Treatment
A summary of clinical trials investigating treatment of pre-diabetes in adults and youth is included in the Table.
Table.
Prediabetes trials in adults and youth
| Studies | Design | Participants | Intervention(s) | Follow-up | Primary Outcome(s) | Conclusions | |
|---|---|---|---|---|---|---|---|
| Adults | |||||||
| US Diabetes Prevention Program Trial14 | RCT | 3234 adults BMI ≥25 kg/m2 FPG 95–125 mg/dL IGT |
Placebo Intensive lifestyle modification Lifestyle recommendations + metformin |
2.8 y (mean) | Incidence of diabetes was 11.0 cases (placebo), 7.8 cases (metformin), 4.8 cases (lifestyle) per 100 person y Lifestyle reduced incidence by 58%, metformin by 31% |
Intensive lifestyle modification or treatment with metformin is associated with decreased rates of progression to diabetes. | |
| Finnish Diabetes prevention Study38 | RCT | 522 adults BMI ≥25 kg/m2 IGT |
Control Intensive lifestyle modification |
3.2 y (mean) | Incidence of diabetes was 23% (control) and 11% (intervention) | Intensive lifestyle modification is associated with decreased rates of progression to diabetes. | |
| Da Qing IGT and Diabetes Study39 | RCT | 577 adults IGT |
Control Diet Exercise Diet + exercise |
6 y (end point) | Incidence of diabetes was 68% (control), 44% (diet), 41% (exercise), 46% (diet + exercise) Diet reduced incidence by 31%, exercise by 46%, diet + exercise by 42% |
Diet and/or exercise is associated with decreased rates of progression to diabetes. | |
| Indian Diabetes Prevention Programme40 | RCT | 531 adults IGT |
Control Lifestyle modification Metformin Lifestyle + metformin |
2.5 y (mean) | Incidence of diabetes was 55% (control), 39% (lifestyle), 41% (metformin), 40% (lifestyle + metformin) To prevent one case of diabetes in 3 y period, 6.4 persons need the lifestyle-intervention, and 6.9 need metformin |
Intensive lifestyle modification or treatment with metformin is associated with decreased rates of progression to diabetes. No benefit to combination therapy was seen. | |
| Swedish Obese Subjects (SOS)41 | Bariatric surgery/matched controls | 1658 postsurgery, 1771 controls |
Bariatric surgery (nonstandardized) Usual care |
10 y (median) | Incidence of diabetes was 2.8 cases (control) and 0.68 cases (surgery) per 100 person y | Bariatric surgery was more efficient than usual care for preventing progression to diabetes. | |
| Troglitazone in Prevention of Diabetes (TRIPOD)42 | RCT | 266 adult Hispanic women Previous GDM |
Placebo + lifestyle recommendations Troglitazone (insulin sensitizer) + lifestyle recommendations |
2.5 y (mean) | Incidence of diabetes was 12% (control) and 5.4% (troglitazone) | Troglitazone decreased progression to diabetes in Hispanic women with previous GDM. | |
| Diabetes Reduction Assessment with ramipril and rosiglitazone Medication (DREAM)43 | RCT | 5269 adults IGT or impaired fasting glucose or both |
Placebo + lifestyle recommendations Rosiglitazone (insulin sensitizer) + lifestyle recommendations |
3.0 y (median) | Incidence of diabetes or death was 26% (placebo) and 12% (rosiglitazone) | Rosiglitazone decreased progression to diabetes. | |
| XENical in the prevention of diabetes in obese subjects (XENDOS)44 | RCT | 3305 adults BMI ≥30 kg/m2 NGT or IGT |
Placebo + lifestyle recommendations Orlistat + lifestyle recommendations |
4.0 y (end point) | Incidence of diabetes 9% (placebo) and 6% (orlistat) Orlistat reduced incidence by 37% |
Orlistat plus lifestyle changes decreased progression to diabetes. | |
| Outcome Reduction with an Initial Glargine Intervention (ORIGIN)45 | RCT | 12 537 adults CVD risk factors IGT or T2D |
Placebo or n-3 fatty acids + lifestyle recommendations Insulin glargine + lifestyle recommendation |
6.2 y (median) | Primary outcomes measures after treatment discontinued for 3 mo In those with IGT, incidence of diabetes 30% (glargine) and 35% (placebo) (OR, 0.80; 95% CI, 0.64–1.00) |
Insulin glargine decreased progression to diabetes, but was also associated with hypoglycemia and weight gain. | |
| Study TO Prevent Noninsulin-dependent diabetes mellitus (STOP-NIDDM)47 | RCT | 1368 adults BMI 25–40 kg/m2 Impaired fasting glucose IGT |
Placebo + lifestyle recommendations Acarbose + lifestyle recommendations |
3.3 y (mean) | Incidence of diabetes 42% (placebo) and 32% (acarbose) | Acarbose decreased progression to diabetes. | |
| Nepi ANtidiabetes StudY (NANSY)50 | RCT | 274 adults impaired fasting glucose | Placebo + lifestyle recommendations Glimepiride (insulin secretagogue) + lifestyle recommendations |
3.7 y | Incidence of diabetes 40% (placebo) and 30% (glimepiride); not statistically different | Glimepiride was not associated with decreased progression to diabetes. | |
| Prevention of Diabetes by Tolbutamide and Diet Regulation51 | RCT | 267 adult men IGT | Diet + tolbutamide (insulin secretagogue) Placebo + tolbutamide Diet No treatment |
10 y (end point) | Incidence of diabetes 29% (no treatment), 13% (diet), 0% (diet + tolbutamide) | Treatment with diet + tolbutamide might be associated with decreased progression to diabetes. | |
| Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR)52 | RCT | 9306 adults IGT CVD or CVD risk factors |
2 × 2 factorial design Placebo + either valsartan (angiotensin receptor blocker) or placebo and lifestyle modification Nateglinide (insulin secretagogue) + either valsartan or placebo and lifestyle modification |
5 y (median) | Incidence of diabetes 34% (placebo), 36% (nateglinide) | Natelinide was not associated with decreased progression to diabetes. | |
| Youth | |||||||
| Researching Effective Strategies to Improve Insulin Sensitivity in Children and Teenagers (RESIST)53 | RCT | 111 youth aged 10–17 y BMI ≥85th percentile Prediabetes or clinical features of insulin resistance |
Metformin Randomized to diet: (1) 55%–60% carb, 30% fat, 15% pro; (2) 40%–45% carb, 30% fat, 25%–30% pro 0 to 3 mo: intensive dietary intervention 4–6 mo: intensive exercise intervention plus dietary support |
6 mo | Insulin:glucose ratio decreased in cohort BMI decreased in cohort No differences between diet groups |
A structured program including both dietary and exercise support improves BMI in the short term. Assignment to moderate carbohydrate diet was not better than higher carbohydrate diet for markers of insulin sensitivity or BMI. | |
| Metformin Therapy in Obese Adolescents with Polycystic Ovary Syndrome and IGT54 | Open label | 15 female youth (mean age 14 y) IGT PCOS |
Metformin + lifestyle recommendations | 3 mo | Improved glucose tolerance on OGTT Increased clamp-measured insulin sensitivity |
Metformin improved insulin sensitivity and glucose tolerance in the short term in youth with PCOS. | |
| Metformin in obese children and adolescents (MOCA)55 | RCT | 151 youth 8–18 y BMI >98th percentile IGT or predesignated OGTT insulin levels |
Placebo + lifestyle recommendations Metformin + lifestyle recommendations |
6 mo | Metformin reduced BMI-SD score compared with placebo (mean difference, −0.1 SD; 95% CI, −0.18 to −0.02; P = .02). | Metformin moderately improved BMI-SD score in the short term. | |
| Metformin in pediatric patients with impaired glucose tolerance56 | RCT | 52 insured youth 4–17 y IGT |
Placebo + weight maintenance diet Metformin + weight maintenance diet |
3 mo | Percentage weight loss greater in the metformin group (−5.86% vs 2.75%), decrease in HOMA-IR, and HbA1c | Metformin moderately improved percent weight loss in the short term. | |
| Rosiglitazone in obese adolescents with IGT57 | RCT | 21 youth 13–18 y IGT or impaired fasting glucose and IGT |
Placebo + lifestyle recommendations Rosiglitazone + lifestyle recommendations |
4 mo | Rosiglitazone improved clamp-measured insulin sensitivity and was associated with 58% conversion to NGT; placebo was associated with 44% conversion to NGT | Rosiglitazone improved insulin sensitivity and glucose tolerance. However, rosiglitazone is no longer used in youth owing to safety concerns in adults. | |
| Safety and efficacy of orlistat in adolescents58 | Open label | 20 youth 12–17 y BMI >95th percentile ≥1 obesity-related condition |
Dietary instructions on 500 kcal-deficit/d and ≤30% calories from fat Orlistat TID + multivitamin supplement |
6 mo | BMI, cholesterol, fasting insulin, and glucose were lower after treatment intravenous glucose tolerance test-measured insulin sensitivity improved White youth had greater benefits than African American youth |
The true benefit of adding orlistat to dietary instruction cannot be concluded, but white youth seem to benefit more than African American youth. | |
| Restoring Insulin Secretion (RISE) Pediatric Medication Study60 | Comparative effectiveness RCT | 91 youth 10–19 y Tanner stage ≥2 BMI ≥85th percentile but ≤50 kg/m2 IGT or T2D diagnosed within 6 mo FPG ≥90 mg/dL Negative IA-2 and GAD autoantibodies |
Insulin glargine alone for 3 mo, titrated to fasting blood glucose of 80–90 mg/dL followed by metformin alone for 9 mo Metformin alone for 12 mo |
21 mo | FPG and 2-h OGTT glucose stable while on therapy Medication withdrawal associated with increase in BMI, HbA1c and OGTT glucose measures Progression beta-cell dysfunction in both groups No differences between groups |
Early treatment with insulin glargine followed by metformin or metformin alone did not stop the progressive beta-cell dysfunction in youth with IGT or recently diagnosed T2DM. | |
GDM, gestational diabetes mellitus; NGT, normal glucose tolerance; PCOS, polycystic ovary syndrome; RCT, randomized controlled trial; T2D, type 2 diabetes; 2hrPG, OGTT 2 hour plasma glucose. Impaired fasting glucose defined by a FPG of 110–125 mg/dL. IGT is defined by a 2 hour plasma glucose 140–199 mg/dL.
Studies in Adults
Multiple studies in adults suggest that the progression of pre-diabetes to T2DM can be prevented. The Finnish Diabetes Prevention Study of adults with IGT found that progression to T2DM in the lifestyle group was less than one-half of that in controls, and was related to the degree of lifestyle changes made and maintained ≥4 years after the intervention.38 The Diabetes Prevention Program of US adults with prediabetes found a 58% reduction in progression to T2DM with lifestyle vs 31% with metformin, although the 10-year follow-up indicated continued progression of the disease and need for continued follow-up.14 In Chinese adults with IGT, the Da Qing Study found that progression to diabetes was decreased 31% by diet, 46% by exercise, and 42% by combined diet and exercise over 6 years of follow-up.39 Furthermore, the Indian Diabetes Prevention Programme in Asian Indians found a relative risk reduction of progression to diabetes of 28.5% with lifestyle, 26.4% with metformin, and 28.2% with lifestyle combined with metformin.40
In the Swedish Obesity Surgery study, at ≤15 years after gastric bypass surgery, a protective effect on progression to diabetes persisted.41 Troglitazone improved insulin sensitivity but not glucose tolerance in Latino women with IGT and previous GDM in the Troglitazone in Prevention of Diabetes study. However, in the longer term, troglitazone delayed or prevented T2DM and preserved pancreatic β-cell function.42 In addition, the Diabetes REduction Assessment with ramipril and rosiglitazone Medication trial found that rosiglitazone significantly decreased incident T2DM and reversed prediabetes in adults with impaired fasting glucose or IGT or both.43 The XEnical in the prevention of Diabetes in Obese Subjects study found a 37.3% risk reduction with orlistat, driven by changes in the 21% of participants with initial IGT, despite similar weight loss in both groups.44 Finally, the Outcome Reduction with Initial Glargine INtervention found that insulin glargine titrated to keep the HbA1c at <6.5% decreased progression to diabetes, with persistent short-term benefits after withdrawal of therapy.45 Therefore, in adults, there is at least short-term evidence that weight loss, exercise, gastric bypass, metformin, thiazolidinediones, orlistat, and intensive glycemic control may all decrease progression from prediabetes to T2DM.
In contrast, the Acarbose for Prevention of T2DM trial found acarbose to be as effective as metformin in preventing T2DM, but its gastrointestinal side effects were problematic,46 and acarbose failed to prevent worsening of fasting glucose in early T2DM in the Early Diabetes Intervention Program.47 Sulfonylureas initially increase insulin secretion, but may accelerate secondary β-cell failure,48,49 and had mixed effects in two T2DM prevention trials.50,51 Similarly, nateglinide failed to prevent progression to diabetes in the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research study.52 Thus, acarbose, sulfonylureas, and meglitinides appear less promising for T2DM prevention in adults.
Studies in Youth
Owing to unique features of T2DM in youth, pediatric data are critical. The Researching Effective Strategies to Improve Insulin Sensitivity in Children and Teenagers study included 111 overweight/obese 10- to 17-year-olds with prediabetes and/or clinical features of insulin resistance, who received metformin and either a high carbohydrate or moderate carbohydrate/increased protein diet for 6 months, plus an exercise intervention from months 4 to 6.53 The insulin sensitivity index increased at 3 months and the insulin to glucose ratio and BMI decreased at 6 months, but there were no significant differences between the diet groups.53 Fifteen adolescents with polycystic ovary syndrome and IGT receiving 3 months of metformin had improvement in IGT, clamp-derived insulin sensitivity, and androgen concentrations.54 In the Metformin in Obese Children and Adolescents placebo-controlled trial of 151 obese 8- to 18-year-olds with hyperinsulinemia and/or impaired fasting glucose or IGT, 6 months of metformin was associated with a significant decrease in BMI-SD score at 6 months, and within the metformin group, there were reductions in fasting glucose, alanine aminotransferase, and adiponectin:leptin ratio.55 In a placebo-controlled study in fifty-two 4- to 17-year-old Mexican children with IGT, 12 weeks of metformin induced a greater improvement in weight, resistin, Homeostatic Model Assessment of Insulin Resistance-calculated measure of insulin resistance (HOMA-IR) and HbA1c than placebo.56 Of note, 4 prepubertal and some normal weight youth were included, calling into question the IGT diagnosis.56 In 21 adolescents with obesity with IGT, more youth in the rosiglitazone group reverted to NGT vs placebo (58% vs 44%), associated with an increase in hyperinsulinemic-euglycemic clamp-derived insulin sensitivity and β-cell function (doubling in the disposition index measured by OGTT).57 Twenty adolescents with obesity-related comorbid conditions treated with 6 months of orlistat had improvement in BMI, total and LDL cholesterol, frequently sampled intravenous glucose tolerance test-derived insulin sensitivity and HOMA-IR, with a greater effect in white participants.58 However, orlistat tolerability is often very poor owing to the high rate of flatulence and other gastrointestinal symptoms. Savoye et al59 published the 6-month results of their Bright Bodies Healthy Lifestyle program, a randomized control trial of pubertal adolescents with “elevated” 2-hour blood glucose levels during an OGTT. Investigators found that intensive lifestyle intervention resulted in more favorable reductions in 2-hour glucose and improved insulin sensitivity, compared with controls. Thus, in sum, the limited available data in youth show that while on treatment, metformin and rosiglitazone (not approved for use in children) improve insulin sensitivity and possibly β-cell function, and that orlistat improves insulin sensitivity, suggesting potential benefits of these drugs on preventing short-term progression from prediabetes to T2DM while on treatment. Furthermore, there is evidence that intensive lifestyle intervention also improved 2-hour postprandial glucose during an OGTT and insulin sensitivity. However, the majority of these treatment trials did not last >6 months, the durability of the intervention was not tested after stopping therapy, and the known poor reproducibility in OGTT data makes interpretation of the responses difficult.
The multicenter National Institute of Diabetes and Digestive and Kidney Diseases-funded Restoring Insulin Secretion (RISE) study enrolled 91 youth and 355 adults with IGT or recently diagnosed T2DM.60 The 91 youth were pubertal, overweight/obese 10- to 19-year-olds, with IGT (60%) or T2DM of <6 months duration (40%), and were randomized to either 3 months of insulin glargine (titrated to a target fasting glucose of 80–90 mg/dL, average dose 0.7 U/kg/day) followed by 9 months of 1000 mg twice daily of metformin daily or to 12 months of 1000 mg twice daily metformin daily alone. β-Cell function (insulin sensitivity paired with β-cell responses) was assessed by a hyperglycemic clamp at baseline, 12 months (on treatment), and 15 months (3 months off treatment), with OGTTs performed at these timepoints as well. No significant differences were observed between treatment groups at baseline, 12 months, or 15 months in β-cell function, BMI percentile, HbA1c, fasting glucose, or OGTT 2-hour glucose results. In both treatment groups, clamp-measured β-cell function was significantly lower at 12 and 15 months vs baseline. HbA1c improved only transiently at 6 months within both groups. BMI was higher in the glargine followed by metformin vs metformin alone group between 3 and 9 months. After medication withdrawal at 12 months, participants in both groups experienced progressive decline in β-cell function as FPG, 2-hour glucose, HbA1c, and BMI increased during a 9-month follow-up period off medications. Only 5% of participants discontinued the interventions, and both treatments were well -tolerated with good compliance. Therefore, the beneficial effects of metformin and glargine insulin on β-cell function seen in previous adult studies were not seen in youth, and neither metformin nor glargine had a durable impact after withdrawal of medication for prevention of diabetes progression in youth.
Potential explanations for the different effect of metformin and glargine in youth vs prior studies in adults can also be gleaned from the RISE study. When the 91 youth and 355 adults from RISE were compared at baseline, hyperglycemic clamp-derived insulin sensitivity was significantly (46%) lower in youth, youth had significantly greater hyperglycemic clamp-derived acute and steady-state C-peptide and insulin responses to glucose and arginine-stimulated C-peptide and insulin responses, and youth had significantly lower insulin clearance.61 After adjustment for insulin sensitivity, all β-cell responses remained significantly greater in youth and insulin clearance was also reduced in youth. The same participants also underwent a 3-hour OGTT.62 Fasting, 2-hour glucose, and incremental glucose AUC were similar in both age-groups, but youth had an approximately 50% lower 1/fasting insulin, 75% higher incremental C-peptide AUC, and more than 2-fold higher insulinogenic index. Two-hour C-peptide and insulin concentrations, incremental C-peptide AUC, and incremental insulin AUC were all higher in youth. C-peptide and insulin responses remained significantly greater in youth after adjustment for insulin sensitivity. Thus, youth had increased insulin resistance and a greater hyperinsulinemic response to glucose compared with adults. Moreover, when comparing treatment responses, youth in the insulin followed by metformin arm gained weight, whereas adults lost weight once metformin was begun, and overall in youth, β-cell function deteriorated during treatment and after treatment withdrawal, whereas in adults, β-cell function improved or remained stable during treatment, but was not sustained after treatment withdrawal.63 These age-related differences may contribute to declining β-cell function and/or impact responses to glucose-lowering interventions, arguing for future longitudinal studies to determine whether these features of youth contribute to a more rapid decline in β-cell function in youth with dysglycemia.
In summary, clinical trials investigating the treatment of prediabetes in youth to date are relatively small, compared with those in adults, and most have only short-term outcomes. Youth on active treatment with multidisciplinary intensive lifestyle modification, metformin or other insulin sensitizers, and weight loss medications not routinely used in pediatrics have modestly improved insulin sensitivity and derive potential weight loss or weight stabilizing benefits of these interventions in the short term. For patients with persistent HbA1c of 6.0%−6.4%, and/or impaired fasting glucose and/or IGT, providers often consider treatment with metformin, however, there is no consensus on this treatment (Figure). Based on the available trial results, the ADA panel of experts in 2007 recommended that only metformin be considered as drug therapy for adults with impaired fasting glucose/IGT.5 Metformin is also recommended as a choice of first-line treatment for youth and adults with polycystic ovary syndrome and with T2DM. In the Diabetes Prevention Program, the subsets of the study cohort that had substantially increased benefit from metformin were those adults <60 years of age and those with a BMI of ≥35 kg/m2. Therefore, the ADA also recommended that metformin be limited to such adults. The ADA has not recommended the use of metformin in youth with prediabetes; moreover, owing to insufficient data and the recent data from the RISE study, they also do not support the use of insulin for prediabetes in youth. Studies have shown that metformin, calorie restriction, and exercise all activate the cellular energy sensor AMP-activated protein kinase, which improves glucose uptake and fatty acid oxidation in muscle, glucose uptake, glycolysis and fatty acid oxidation in the heart, and is anorexigenic in the hypothalamus, resulting in decreased food intake. They also suppress hepatic fatty acid synthesis and gluconeogenesis, fatty acid synthesis and lipolysis in adipose tissue, and pancreatic insulin secretion. However, after 1 year, metformin diffuses across the blood–brain barrier, and stimulates orexigenic receptors in the hypothalamus, resulting in increased appetite.64 Thus, it is possible that favorable effects of metformin on appetite may be short lived. The only currently universally accepted treatment for prediabetes is lifestyle modification through increased physical activity, improved nutrition, and decreased sedentary activity. A detailed description of recommended lifestyle changes is beyond the scope of this report, but brief suggestions are included in the Figure. It is strongly recommended that all youth with prediabetes have access to an age-appropriate multidisciplinary intensive lifestyle modification program.
Follow-Up Care
There is no consensus recommendation on how frequently the pediatric patient with prediabetes should be seen. Current practice largely depends on the clinical setting and the goals for the visits. As stated, prediabetes, especially in the 6.0%−6.4% range, likely puts a child at increased risk for diabetes and CVD. Therefore, the need for intensive lifestyle intervention with improved nutrition and increased exercise in such youth should be stressed. Some pediatric providers see prediabetes patients every 3 months, as with a child with diabetes, whereas others see patients with pre-diabetes every 6 months. If possible, a multidisciplinary team, including a dietitian, an exercise specialist and a behavioral health specialist, should be gathered. In the setting of a weight management clinic, visits may be significantly more frequent.
Follow-Up Summary
Based on expert opinion, once prediabetes is identified, at least annual rescreening for diabetes is recommended unless there is a change that indicates a need for earlier retesting (weight change, symptoms of hyperglycemia, increased HbA1c, etc).
Summary
Prediabetes in youth has become a more frequent challenge facing patients, families, and providers alike. As we learn more about the natural history of prediabetes and T2DM, investigators have found distinct differences between these conditions in youth and adults, making extrapolation of adult practice problematic. Moreover, there is a lack of evidence-based management and treatment guidelines for prediabetes in youth. Although current approaches may differ, best clinical practice indicates that providers should screen at-risk youth for prediabetes, and intervene with intensive lifestyle modification through improved nutrition and exercise. In some cases, pharmacologic intervention may also be warranted, but always in the context of lifestyle and behavioral changes.
Acknowledgments
We appreciate the support and advice of Dr Erinn Rhodes (E.R. is the Site Principal Investigator for a clinical trial sponsored by AstraZeneca and was formerly Site Principal Investigator for a clinical trial sponsored by Merck); Dr Morey Haymond; and the Pediatric Endocrinology Society, who initially identified the need for quality improvement in the area of pediatric prediabetes.
Glossary
- ADA
American Diabetes Association
- AUC
Area under the curve
- BMI
Body mass index
- CVD
Cardiovascular disease
- FPG
Fasting plasma glucose
- HbA1c
Hemoglobin A1c
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance-calculated measure of insulin resistance
- IGT
Impaired glucose tolerance
- OGTT
Oral glucose tolerance test
- RISE
Restoring Insulin Secretion
- T2DM
Type 2 diabetes mellitus
Footnotes
The authors declare no conflicts of interest.
References
- 1.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S13–28. [DOI] [PubMed] [Google Scholar]
- 2.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–7. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KMV. Estimated number of adults with prediabetes in the US in 2000 - Opportunities for prevention. Diabetes Care 2003;26:645–9. [DOI] [PubMed] [Google Scholar]
- 4.Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of Type 2 diabetes in England and its association with baseline impaired fasting glucose: The Ely study 1990–2000. Diabet Med 2007;24: 200–7. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance - Implications for care. Diabetes Care 2007;30:753–9. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 2009;32:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinhas-Hamiel O, Zeitler P. Prediabetes in children and adolescents: what does it mean?. http://www.medscape.com/viewarticle/776457. 2013. Accessed August 5, 2019.
- 8.Baranowski T, Canada A, Cullen K, Jago R, Missaghian M, Thompson D, et al. Presence of diabetes risk factors in a large US eighth-grade cohort. Diabetes Care 2006;29:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60: 759–63. [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Jacobs DR Jr, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–44. [DOI] [PubMed] [Google Scholar]
- 11.Moran A, Jacobs DR Jr, Steinberger J, Cohen P, Hong CP, Prineas R, et al. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 2002;87:4817–20. [DOI] [PubMed] [Google Scholar]
- 12.Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes 2018;19:199–204. [DOI] [PubMed] [Google Scholar]
- 13.Kleber M, Lass N, Papcke S, Wabitsch M, Reinehr T. One-year follow-up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabet Med 2010;27:516–21. [DOI] [PubMed] [Google Scholar]
- 14.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbloom A, Arslanian S, Brink S, Conschafter K, Jones KL, Klingensmith G, et al. Type 2 diabetes in children and adolescents. Diabetes Care 2000;23:381–9. [DOI] [PubMed] [Google Scholar]
- 18.Kester LM, Hey H, Hannon TS. Using hemoglobin A1c for prediabetes and diabetes diagnosis in adolescents: can adult recommendations be upheld for pediatric use? J Adolesc Health 2012;50:321–3. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–52.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowicka P, Santoro N, Liu HB, Lartaud D, Shaw MM, Goldberg R, et al. Utility of Hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–10. [DOI] [PubMed] [Google Scholar]
- 24.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vangeepuram N, Townsend K, Arniella G, Goytia C, Horowitz CR. Recruitment in Clinical Versus Community-Based Sites for a Pilot Youth Diabetes Prevention Program, East Harlem, New York, 2011–2012. Prev Chronic Dis 2016;13:E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001;47: 153–63. [PubMed] [Google Scholar]
- 28.Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes 2014;15:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libman IM, Barinas-Mitchell E, Bartucci A, Chaves-Gnecco D, Robertson R, Arslanian S. Fasting and 2-hour plasma glucose and insulin: relationship with risk factors for cardiovascular disease in overweight nondiabetic children. Diabetes Care 2010;33:2674–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao Q, Dekker JM, de Vegt F, Nijpels G, Nissinen A, Stehouwer CD, et al. Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 2004;57:590–6. [DOI] [PubMed] [Google Scholar]
- 31.Qiao Q, Jousilahti P, Eriksson J, Tuomilehto J. Predictive properties of impaired glucose tolerance for cardiovascular risk are not explained by the development of overt diabetes during follow-up. Diabetes Care 2003;26:2910–4. [DOI] [PubMed] [Google Scholar]
- 32.Qiao Q, Pyorala K, Pyorala M, Nissinen A, Lindstrom J, Tilvis R, et al. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 2002;23:1267–75. [DOI] [PubMed] [Google Scholar]
- 33.Kleber M, deSousa G, Papcke S, Wabitsch M, Reinehr T. Impaired glucose tolerance in obese white children and adolescents: three to five year follow-up in untreated patients. Exp Clin Endocrinol Diabetes 2011;119:172–6. [DOI] [PubMed] [Google Scholar]
- 34.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehehalt S, Körner A, Schweizer R, Liesenkötter KP, Partsch CJ, Blumenstock G, et al. Low association between fasting and OGTT stimulated glucose levels with HbA1c in overweight children and adolescents. Pediatr Diabetes 2017;18:734–41. [DOI] [PubMed] [Google Scholar]
- 36.Chan CL, McFann K, Nadeau KJ, Newnes L, Zeitler PS, Kelsey MM, eds. The Relationship between A1c, 2hr Plasma Glucose, and Continuous Glucose Monitoring-determined Glycemic Patterns in Obese Adolescents 73rd Annual American Diabetes Association Scientific Sessions 2013. Chicago, IL: Diabetes: Supplement, ADA Meeting; 2013. [Google Scholar]
- 37.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- 39.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97. [DOI] [PubMed] [Google Scholar]
- 41.Carlsson LMS, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, et al. Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N Engl J Med 2012;367:695–704. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–803. [DOI] [PubMed] [Google Scholar]
- 43.Gerstein HC, Yusuf S, Holman RR, Bosch J, Anand S, Avezum A, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–105. [DOI] [PubMed] [Google Scholar]
- 44.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27: 856. [DOI] [PubMed] [Google Scholar]
- 45.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung HJ, Maggioni AP, et al. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med 2012;367:319–28. [DOI] [PubMed] [Google Scholar]
- 46.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7. [DOI] [PubMed] [Google Scholar]
- 47.Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, Baron A, et al. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes Care 2006;29:2095–101. [DOI] [PubMed] [Google Scholar]
- 48.Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43. [DOI] [PubMed] [Google Scholar]
- 50.Lindblad U, Lindberg G, Mansson NO, Ranstam J, Tyrberg M, Jansson S, et al. Can sulphonylurea addition to lifestyle changes help to delay diabetes development in subjects with impaired fasting glucose? The Nepi ANtidiabetes StudY (NANSY). Diabetes Obes Metab 2011;13:185–8. [DOI] [PubMed] [Google Scholar]
- 51.Sartor G, Schersten B, Carlstrom S, Melander A, Norden A, Persson G. Ten-year follow-up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes 1980;29: 41–9. [DOI] [PubMed] [Google Scholar]
- 52.Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1463–76. [DOI] [PubMed] [Google Scholar]
- 53.Garnett SP, Gow M, Ho M, Baur LA, Noakes M, Woodhead HJ, et al. Optimal macronutrient content of the diet for adolescents with prediabetes; RESIST a randomised control trial. J Clin Endocrinol Metab 2013;98:2116–25. [DOI] [PubMed] [Google Scholar]
- 54.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab 2002;87:1555–9. [DOI] [PubMed] [Google Scholar]
- 55.Kendall D, Vail A, Amin R, Barrett T, Dimitri P, Ivison F, et al. Metformin in obese children and adolescents: the MOCA trial. J Clin Endocrinol Metab 2013;98:322–9. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Diaz RA, Talavera JO, Pool EC, Ortiz-Navarrete FV, Solorzano-Santos F, Mondragon-Gonzalez R, et al. Metformin decreases plasma resistin concentrations in pediatric patients with impaired glucose tolerance: a placebo-controlled randomized clinical trial. Metabolism 2012;61:1247–55. [DOI] [PubMed] [Google Scholar]
- 57.Cali AM, Pierpont BM, Taksali SE, Allen K, Shaw MM, Savoye M, et al. Rosiglitazone improves glucose metabolism in obese adolescents with impaired glucose tolerance: a pilot study. Obesity (Silver Spring) 2011;19:94–9. [DOI] [PubMed] [Google Scholar]
- 58.McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Frazer TE, et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab 2004;17:307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014;37:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.RISE Consortium. Impact of insulin and metformin versus metformin alone on beta-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41: 1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41: 1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.RISE Consortium, RISE Consortium Investigators. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on beta-cell function: comparison of responses in youth and adults. Diabetes 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 2014;7:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


