Abstract
Background:
Diarrhea is a ubiquitous digestive system disease, leading to loss of fluid and electrolytes, and may be life-threatening, especially in children and adults who are immunosuppressed or malnourished. Berberine has a broad-spectrum antibiotic activity and is very widely used to treat diarrhea in China. No systematic review has been carried out to evaluate the evidence presented in clinical trials. The aim of this study was to assess the effectiveness and safety of berberine in diarrhea treatment among children and adults.
Methods:
Seven databases and two clinical trial registries were searched on 1 September 2019. Randomized controlled trials were included, where participants were diagnosed (first diagnosed) as having diarrhea according to clear diagnostic criteria. Berberine alone or in combination with Western medication as intervention were included. Subgroup analyses were conducted based on children or adults, acute or persistent diarrhea, infectious or noninfectious and treatment courses. Primary outcomes were clinical cure rate and duration of diarrhea. The GRADE tool was used to assess the quality of evidence.
Results:
A total of 38 randomized controlled trials were included involving 3948 participants (including 27 trials on 2702 children) were included. Compared with antibiotics, berberine plus antibiotics showed better results in both adults and in children in general, especially when given for 7 days or 3 days in acute infectious diarrhea of children. Compared with the control groups, using berberine alone or in combination with montmorillonite, probiotics, and vitamin B increased the clinical cure rate of diarrhea. The use of berberine alone or berberine combined with montmorillonite reduced the duration of hospitalization. Using berberine had significantly better laboratory indicators (isoenzyme, inflammatory factors, myocardial enzyme, and fecal trait) and fewer systemic symptoms than the no berberine groups. Overall, 22 of 27 trials on children used berberine as an enema. No deaths and serious adverse events were reported. The quality of evidence of included trials was moderate to low or very low. The impact of different dosages, frequencies and treatment durations on the outcomes was not evaluated due to insufficient number of trials.
Conclusion:
This review demonstrated that berberine was generally effective in improving clinical cure rates and shortening the duration of diarrhea compared with control groups. No severe adverse event was reported. However, there is still a lack of high-quality evidence for evaluating the efficacy and safety of berberine.
Trial registration:
PROSPERO CRD42020151001 (available from http://www.crd.york.ac.uk/PROSPERO/).
Keywords: antibiotic, berberine, diarrhea, inflammatory factors, isoenzyme, myocardial enzyme
Introduction
Diarrhea is defined as the passage of three or more loose or liquid stools per day (or more frequent passage than what is typical for the individual).1 It is a ubiquitous digestive system disease. Globally, there are nearly 1.7 billion cases of childhood diarrhea disease every year.2 Continued diarrhea can cause loss of fluid and electrolytes, and may even become life-threatening, especially in children and adults who are immunosuppressed or malnourished.3 Although not life-threatening for adults, diarrhea still has a high incidence.1 Diarrhea usually has intestinal tract noninfectious symptoms that could be caused by improper diet and malnutrition, and infectious symptoms that are usually caused by a variety of bacterial, viral, and parasitic organisms.4 According to the World Gastroenterology Organisation Global guidelines,1 the etiological diagnosis of diarrhea usually is based on medical history, symptoms, routine testing, and especially stool testing. In many countries, the goal of diarrhea treatment is to relieve symptoms and avoid complications.5 Commonly used treatments include probiotics,6 zinc,7 lactose-free formula,8 antibiotics, and antidiarrheal agents (such as montmorillonite).9
The main ingredient of berberine is C20H19NO5, an isoquinoline alkaloid belonging to the structural class of proto-berberines extracted from traditional Chinese herbal medicines, mainly rhizoma coptidis and rhizoma phellodendri,10,11 which have been used in China for more than 2000 years treating diarrhea.12 Pharmacological studies using doses of berberine (50–200 mg/kg per time, one time a day)13 substantially exceed those likely to occur in humans (0.1–0.3 g per time, three times a day) have demonstrated that berberine has a broad-spectrum antibiotic activity, including Vibrio cholera, Shigella and Pseudomonas14–16 and have approved that berberine has advantages in treating intestinal bacterial infections, including bacterial dysentery and viral infections.10,17–20 Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis can also be suppressed by berberine.21 In addition, berberine has broad prospects in the treatment of secretory diarrhea, it can inhibit the hypersecretion of electrolytes caused by cholera toxin.13 It has effects on reducing inflammation, inhibition of gastrointestinal motility, ameliorates impaired gastrointestinal function, and reducing intestinal secretion and exudation.21 Berberine can relax intestinal smooth muscle and prolong the residence time of intestinal contents to fully digest and absorb the intestinal contents to treat functional diarrhea.22 Therefore, berberine has a significant effect on infectious or noninfectious, secretory or exudative diarrhea.21 In clinical practice, berberine is sometimes combined with Western medicine in the treatment of diarrhea, such as montmorillonite, antibiotics, probiotics, vitamin B or anisodamine. However, there are no systematic reviews that have assessed efficacy and safety.
This systematic review aims to evaluate the effectiveness and safety of berberine alone or in combination with Western medicine in diarrhea treatment, and to provide evidence for clinical practice in this specific area.
Methods
This review was registered in PROSPERO (CRD42020151001), and the protocol is available at http://www.crd.york.ac.uk/PROSPERO/.
Eligibility criteria
This review included randomized controlled trials (RCTs) enrolling participants needed to be diagnosed (first diagnosed) with diarrhea according to clear diagnostic criteria, regardless of sex, age, and type of diarrhea (infectious, noninfectious, acute, and chronic). Diarrhea caused by drugs (such as antibiotics) or diseases (such as cancer) were excluded. All randomized controlled studies, regardless of language or publication date or state, were screened. Acute diarrhea was defined as a duration of diarrhea less than 14 days, while diarrhea was considered as persistent if lasting longer than 14 days.23 Usage of other Western medicine was permitted as long as they were antibiotics, fluid therapy (including oral rehydration salts, intravenous rehydration, electrolyte supplementation and correction of acid–base balance disorders), antipyretic, adjustment of diet/diet guidance/nutritional guidance, supplementation of trace elements and calories. Secondary diarrhea was excluded from this review. Based on real-world clinical practice, to include combinations from clinical practice to a large extent, we categorized interventions based on different combinations (berberine + montmorillonite; berberine + Bifidobacterium subtilis; berberine + montmorillonite + vitamin B; berberine + Bifidobacterium lactobacillus triple viable + montmorillonite; berberine + montmorillonite + anisodamine) in this systematic review.
Search strategy
Published studies were comprehensively searched in the following databases from their inception to April 2019: PubMed, the Cochrane Library, EMBASE, Chinese National Knowledge Infrastructure Database (CNKI), VIP, Wanfang, and SinoMed. We also searched for two clinical trial registration networks (ClinicalTrials.gov and Chinese Clinical Trial Registry). The detailed search strategy is provided in the Appendix.
Study outcomes
The primary outcome measures were clinical cure rate (as defined in each original study) and duration of diarrhea (day). Secondary outcomes included stool frequency (number of depositions per day), stool output (g or ml/kg per day), fecal trait improvement, absenteeism, intensity of antibiotic use, stool routine examination, stool bacterial culture, duration of hospitalization, duration of other symptoms (such as vomiting and fever), quality of life, recurrent diarrhea, laboratory indicators, death (all-cause and diarrhea-related), adverse events, and adverse drug reactions.
Study selection
The retrieved literature was imported into NoteExpress, and duplicate records were removed. Title/abstract screening and full-text screening were conducted by two reviewers (MK Yu and CH L) independently.
Any disagreement between the two reviewers was resolved through discussing with a third reviewer (or a senior author, YT Fei).
Data extraction
Data extraction was conducted by two pairs of reviewers (YM and CL, DP and QH) using a standardized, pre-piloted data extraction form, including authors information, characteristic of participants, details of interventions and controls, outcomes, and information related to study design. Two authors in each group independently and duplicated extracted data from each trial, cross-checked the data. Discrepancies were solved by discussing within the pair of reviewers or arbitrated by the senior author (YF) if necessary.
Quality assessment and publication bias
We assessed each included study’s risk of bias based on a modification of the Cochrane Risk of Bias tool, which consists of the following aspects: random sequence generation (selection bias); allocation concealment (selection bias); blinding (performance bias and detection bias); incomplete outcome data (attrition bias); selective reporting of outcomes and other bias. This modified tool has four response options for each aforementioned aspect: ‘low risk of bias,’ ‘probably low risk of bias,’ ‘probably high risk of bias,’ and ‘high risk of bias’.24 Publication bias was assessed using the funnel plot when there were more than 10 studies available for a certain outcome/comparison. In addition, we used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) system to grade the quality of evidence for primary outcomes.25
Data synthesis
Review Manager 5.3 was used. Continuous outcomes were analyzed by standardized mean difference (MD). We assessed the dichotomous variable by relative risk [(RR), Mantel–Haenszel RR]. The p-value and 95% confidence interval (CI) were obtained.
For those publications that failed to report any critical information, we tried to contact the corresponding author or first author to obtain the information via email or telephone calls.
Subgroup analysis and investigation of heterogeneity
The heterogeneity was assessed according to I² statistic. When heterogeneity was not significant (I2 < 50%), we used a fixed-effect model to synthesize the data. When heterogeneity was significant (I2 ⩾ 50%), the random-effects model was used.
The following potential sources of heterogeneity (ages; different drugs of berberine combined; a degree of diarrhea; route of administration; duration of treatment; type of primary disease; time of observation; drug dosage) were explored in subgroup analyses. We performed a sensitivity analysis to assess the robustness of the meta-analysis by excluding trials with poor methodological quality (those with insufficient randomization methods and trials with selective reporting bias).
Results
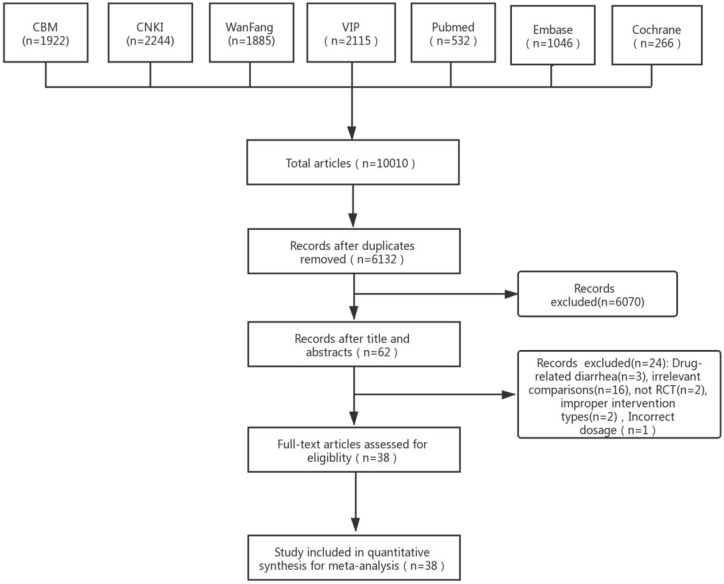
In total, we identified 10,010 studies through the searches, and a total of 38 studies were included (Figure 1).
Figure 1.
Study flow diagram.
CBM, SinoMed Database; CNKI, Chinese National Knowledge Infrastructure Database; VIP, VIP Chinese Science and Technique Journals Database; WanFang, Wanfang Database.
Study characteristics
Overall, 38 RCTs involving 3948 participants10,26–62 were included in this review. A total of 11 studies10,28,32,33,37,39,40,42,44 were conducted on adults (1246 of 3948, 31.56%), aged 16–76 years, with the remaining 27 studies26,27,29–31,34,35,37,38,41,43,45–62 conducted on children (2702 of 3948, 68.4%) aged from 2 months to 12 years. A total of 22 studies29,34,35,37,38,41,45–49,51–57,59–62 involved taking a berberine enema (all in children), and the other studies10,26–28,30–33,36,39,40,42–44,50,58 involved taking berberine orally. Sex was not reported in two studies.33,43 In the remaining 36 studies,10,26–32,34–42,44–62 the proportion of males to females was about 1:1.2. Overall, 31.6% studies were for infectious diarrhea, while 15.8% were mixed and 52.6% were not reported. A total of 19 studies were treating acute diarrhea, while 4 studies28,36,44,62 were mixed acute and persistent, 632,33,37,39,48,54 exclusively with persistent diarrhea, and 13 studies25,27,29–31,34,35,38,45,49,52,57,61 did not specify. The principle investigators of 37 studies came from a Western medicine hospital, and only 1 study came from a traditional Chinese medicine hospital. In the included studies that used antibiotics, 23 studies did not report the cause of diarrhea, 10 studies were due to virus of bacteria infection, 3 studies were due to bacteria infection, and 2 studies were virus infection.
The sample size ranged from 30 to 279 patients for each study, with a mean of 104 patients. Overall, 34 studies26–31,34–38,40–62 had two arms, 3 studies had three arms,32,33,39 and 1 trial had five arms.10 There were 14 studies that mentioned the inclusion criteria,10,28,33,39,42–44,48,51–53,58–60 and the remaining studies did not. The dosage and the frequency of oral berberine was 0.05–0.5 g and 2–3 times per day, while the dosage and the frequency of enema berberine was 10–20 mg and 1–2 times per day (Table 1).
Table 1.
Characteristics of the included 38 studies.
| Study ID | Sample T: (F), C: (F) |
Mean/range age (T/C) | Population | Acute/persistent/both/NR | Cause of diarrhea | Course of diarrhea (d) | Intervention | Route of administration | Control intervention | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|
| Berberine versus no berberine | ||||||||||
| Yang26 | T: 38 (16) C: 38 (17) |
T: 14.6 m C: 14.9 m |
Children | Acute | Virus or bacterial infection | NR | 1. BE: 0.2 g, 3 times/d, for 7 d 2. AMC: 30 mg/kg, 2 times/d, for 7 d* |
p.o. | AMC: 30 mg/kg, 2 times/d, for 7 d* | ①⑫ |
| Li27 | T: 35 (17) C: 35 (16) |
T: 2 y C: 1 y |
Children | NR | NR | NR | 1. BE: 0.2 g, 3 times/d, for 7 d 2. LOF: 200 ml, 1 times/d, for 7 d* |
p.o. | LOF: 200 ml, 1 times/d, for 7 d* | ①⑪⑫⑬ |
| Lin et al.28 | T: 49 (23) C: 49 (21) |
T: 36.61 y C: 37.03 y |
Adult | Both | Bacterial infection | T: 6.36 ± 2.58 d C: 6.44 ± 2.63 d |
1. BE: 0.3 g, 3 times/d 2. LOF: 500 ml 1 times/d* |
p.o. | LOF: 500 ml 1 times/d* | ①⑨⑮ |
| Wei and Li29 | T: 15 C: 15 |
T: 3–13 y C: 3–13 y |
Children | NR | NR | NR | 1. BE: 10 mg/kg, 1 times/d, for 3 d 2. Antibiotic* |
p.r. | Antibiotic* | ① |
| Wang30 | T: 45 (21) C: 45 (20) |
T: 1.68 y C: 1.65 y |
Children | NR | Virus or bacterial infection | NR | 1. BE: 0.2 g, 3 times/d, for 7 d 2. LOF: 200 ml, 1 times/d, for 7 d* |
p.o. | LOF: 200 ml, 1 times/d, for 7 d* | ①⑪⑫⑬ |
| Cheng et al.31 | T: 29 (13) C: 29 (14) |
T: 1.60 y C: 1.64 y |
Children | NR | Virus or bacterial infection | NR | 1. BE: 0.2 g, 3 times/d, for 7 d 2. LOF: 200 ml, 1 times/d, for 7 d* |
p.o. | LOF: 200 ml, 1 times/d, for 7 d* | ①⑪⑫ |
| Luo32 | T1: 35 (16) T2: 31 (13) C: 33 (17) |
T1: 37.5 y T2: 32.1 y C: 34.4 y |
Adult | Persistent | NR | T1: 12.3 ± 1.1 m T2: 10.5 ± 2.3 m C: 11.9 ± 1.0 m |
1. BE: 0.2 g, 3 times/d, for 21 d 2. BLT: 0.63 g, 2 times/d, for 21 d |
p.o. | BLT: 0.63 g, 2 times/d, for 21 d | ①②⑮ |
| Ye33 | T1: 30 T2: 30 C: 30 |
T1: 19–67 y T2: 19–67 y C: 19–67 y |
Adult | Persistent | NR | NR | 1. BE: 0.2 g, 3 times/d, for 14 d 2. BLT: 0.63 g, 2 times/d, for 14 d |
p.o. | BLT: 0.63 g, 2 times/d, for 14 d | ①②⑮ |
| Zhou34 | T: 100 (48) C: 98 (53) |
T: 1.4 y C: 1.3 y |
Children | NR | Virus or bacterial infection | NR | 1. BE: <1 y 0.05 g; >1 y 0.1 g, 3 times/d 2. MN: <1 y 1 g, 3 times/d >1 y 2 g, 3 times/d 3. Antibiotic* |
p.r. | 1. MN: <1 y 1 g, 3 times/d >1 y 2 g, 3 times/d 2. Antibiotic* |
①⑨ |
| Huang et al.35 | T: 50 C: 50 |
T: 3–10 y C: 3–10 y |
Children | Acute | NR | NR | 1. BE: 0.1 g, 3 times/d, for 7 d 2. Amp:50 mg/kg, 2 times/d, for 7 d* |
p.r. | Amp:50 mg/kg, 2 times/d, for 7 d* | ① |
| Fu and Tan36 | T: 53 C: 53 |
T: 18–60 y C: 18–60 y |
Adult | Both | Virus or bacterial infection | ⩽ 21 d | 1. BE: 0.3 g, 3 times/d, for 5 d 2. Antibiotic* |
p.o. | Antibiotic* | ①⑮ |
| Huang and Zhang37 | T: 40 (21) C: 40 (27) |
T: 18.6 m C: 19.8 m |
Children | Persistent | Viral infection | NR | 1. BE: 10 mg/kg, 2 times/d, for 3 d 2. BLT: <12 m 0.5 g–1 g, 3 times/d; 12–72 m 0.5 g, 2 times/d, for 3 d* |
p.r. | BLT: <12 m, 0.5 g–1 g, 3 times/d; 12–72 m0.5 g, 2 times/d, for 3 d* | ①③④⑩ |
| Li38 | T: 60 C: 60 |
T: 4 m–2 y C: 4 m–2 y |
Children | NR | NR | NR | 1. BE: 20 mg/kg, 2 times/d, for 3 d 2. MN: NR, for 3 d* |
p.r. | MN: NR, for 3 d* | ①⑨ |
| Hu39 | T1: 20 T2: 20 C: 20 |
T1: 20–58 y T2: 20–58 y C: 20–58 y |
Adult | Persistent | NR | >2 m | 1. BE: 0.2 g, 3 times/d, for 14 d 2. BLT: 0.63 g, 2 times/d, for 14 d |
p.o. | BLT: 0.63 g, 2 times/d, for 14 d | ①⑮ |
| Zhao40 | T: 146 C: 133 |
T: 14–63 y C: 14–63 y |
Children | Acute | NR | NR | 1. BE: 0.2–0.3 g, 3 times/d, for NR 2. LOF: 500 ml, 1 times/d, for 3 d |
p.o. | LOF: 500 ml 1 times/d, for 3 d | ②⑩ |
| Xu41 | T: 84 C: 84 |
T: 4 m–2 y C: 4 m–2 y |
Children | Acute | Bacterial infection | ⩽14 d | 1. BE: 20 mg/kg, 2 times/d, for NR 2. Antibiotic* |
p.r. | Antibiotic* | ①② |
| Zhang42 | T: 30 (14) C: 30 (14) |
T: 18 y–65 y C: 18 y–65 y |
Adult | Acute | NR | ⩽2 d | 1. BE: 0.2 g, 3 times/d, for 7 d | p.o. | CPFX: 0.2 g, 3 times/d, for 7 d. | ⑦⑧⑮ |
| Dang43 | T: 57 C: 45 |
T: 2 y–12 y C: 2 y–12 y |
Children | Acute | NR | T: 48.4 ± 2.5h C: 47.9 ± 2.1h |
BE: 2–3 y 0.1 g, 4–6 y 0.1–0.15 7g, –9 y 0.15 g–0.2 g, 9–12 y 0.2 g–0.25 g, 3 times/d, for 3 d*. | p.o. | CEC: 20 mg-4o mg/kg, 3 times/d, for 3 d* | |
| Wu et al.44 | T: 80 (35) C: 80 (30) |
T: 44 y C: 47 y |
Adult | Both | NR | Acute: <5 d Chronic: 2 m–1 y |
BE: 0.5 g, 2 times/d* | p.o. | Fur: 0.1 g, 2 times/d* | ⑧⑮ |
| Khin-Maung and Nyunt-Nyunt-Wai12 | T: 45 (21) C: 45 (23) |
T: 38.8 y C: 37.2 y |
Adult | Acute | Bacterial infection | T: 10.6h C: 12.8h |
BE: 0.1 g, 4 times/d, for 3 d* | p.o. | Tet: 500 mg, 4 times/d, for 3 d* | ②⑯ |
| Berberine + montmorillonite versus no berberine + montmorillonite | ||||||||||
| Liu45 | T: 45 (22) C: 45 (21) |
T: 1.5 y C: 1.6 y |
Children | NR | Virus or bacterial infection | NR | 1. MN: NR, 1 times/d, for 3 d. 2. BE: 10 mg/kg, 1 times/d, for 3 d 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Wang46 | T: 40 (15) C: 30 (10) |
T: 5 m–6 y C: 5 m–6 y |
Children | Acute | NR | 2–7 d | 1. MN: <1 1.5 g, >1 y 3 g, 2 times/d, for 3 d. 2. BE: 10–20 mg/kg, 2 times/d, for 3 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Jin47 | T: 60 (18) C: 56 (20) |
T: 2 m–3 y C: 2 m–3 y |
Children | Acute | Viral infection | NR | 1. MN: 3 g, 2 times/d, for 3 d. 2. BE: 0.1 g, 2 times/d, for 3 d 3. Antibiotic* |
p.r. | Antibiotic* | ①② |
| Huang48 | T: 32 (15) C: 24 (11) |
T: 17.68 ± 1.64 m C: 16.82 m |
Children | Persistent | NR | T: 3.54 ± 0.43 m C: 3.32 ± 0.62 m |
1. MN: <1 3 1 g, –3 y 3–4.5 g, 2 times/d, for 5 d. 2. BE: <1 0.05–0.1 g, 1–3 y 0.1–0.2 g, 2 times/d, for 5 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Qiu49 | T: 52 (22) C: 36 (16) |
T: 6 m–3 y C: 6 m–3 y |
Children | NR | NR | NR | 1. MN: <1 1.5 g, 1–3 y 2 g, >3 y 3 g, 1 times/d, for 3 d. 2. BE: 10–15 mg/kg, 1 times/d, for 3 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Qin et al.50 | T: 60 C: 46 |
T: 18 y C: 18 y |
Adult | Acute | Bacterial infection | NR | 1. MN: 3 g, 3 times/d, for 3 d. 2. BE: 0.2–0.3 g, 3 times/d, for 3 d. |
p.o. | NOR: 0.3 g, 3 times/d, for 3 d* | ①⑮ |
| Gan51 | T: 51 (27) C: 51 (30) |
T: 5–23 m C: 5–24 m |
Children | Acute | NR | T: 2–13 d C: 2–14 d |
1. MN: <1 1.5 g ⩾1 y 3 g, 2 times/d, for 5 d. 2. BE: 10–15 mg/kg, 2 times/d, for 5 d 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Guo52 | T: 30 (11) C: 28 (8) |
T: 5 m–2.5 y C: 5 m–2.5 y |
Children | NR | NR | NR | 1. MN:1.5–3 g, 1 times/d, for 5 d. 2. BE: 0.1–0.2 g, 2 times/d, for 5 d. |
p.r. | Antibiotic* | ① |
| Wang et al.53 | T: 48 (22) C: 42 (19) |
T: 6–38 m C: 5–40 m |
Children | Acute | Virus or bacterial infection | ⩽14 d | 1. MN: <1 1.5 g, 1–3 y 2 g, >3 y, 3 g, 2 times/d, for 5 d. 2. BE: 10–15 mg/kg, 2 times/d, for 5 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Zhu54 | T: 46 C: 56 |
T: 6 m–30 m C: 6 m–30 m |
Children | Persistent | NR | T: 15.85 ± 2.43 d C: 16.30 ± 1.87 d |
1. MN: 3 g, 2 times/d, for NR. 2. BE: 0.1 g, 2 times/d, for NR. 3. Antibiotic* |
p.r. | Antibiotic* | ①⑨ |
| Shi55 | T: 35 (19) C: 33 (20) |
T: 6–38 m C: 5–40 m |
Children | Acute | NR | NR | 1. MN: <1 1.5 g, 1–3 y 2 g, >3 y 3 g/times, 1 times/d, for 3 d. 2. BE: 10–15 mg/kg, 1 times/d, for 3 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Wan and Qin56 | T: 40 C: 40 |
T: 3 m–10 y C: 3 m–10 y |
Children | Acute | Virus or bacterial infection | From hours to days | 1. MN: 3 m–1 y 1.88 g1, –5 y 1.88–3.76 g6, –10 y 3.76–7.52 g, 2 times/d, for 3 d. 2. BE: 10 ml–15 ml, 1–2 times/d, for 3 d. 3. Antibiotic* |
p.r. | Antibiotic* | ① |
| Li57 | T: 63 C: 57 |
T: 12.11 m C: 12.11 m |
Children | NR | Virus or bacterial infection | T: 4.95 ± 1.68 d C: 6.27 ± 2.87 d |
1. MN:0.3 g, 2 times/d, for 3 d. 2. BE: 0.1 g, 2 times/d, for 3 d. 3. Antibiotic* |
p.r. | Antibiotic* | ①② |
| Berberine + Bifidobacterium subtilis versus no berberine + Bifidobacterium subtilis | ||||||||||
| Geng and Liu58 | T: 49 C: 49 |
T: 16 y–76 y C: 16 y–76 y |
Adult | Acute | NR | 3–14 d | 1. BS: 0.5 g, 3 times/d, for 3 d 2. BE: 4 g+100 ml water, 3 times/d, for 3 d* |
p.o. | MN: 3 g, 3 times/d, for 3 d* | ①③⑮ |
| Berberine + montmorillonite + vitamin B versus no berberine + montmorillonite + vitamin B | ||||||||||
| Lu59 | T: 49 C: 43 |
T: 6 m–3 y C: 6 m–3 y |
Children | Acute | NR | NR | 1. MN: 3 g, 2 times/d, for 3 d. 2. BE: 10 mg/kg, 2 times/d, for 3 d. 3. VB: NR, 2 times/d, for 3 d. 4. Antibiotic* |
p.r. | Antibiotic* | ① |
| Yi60 | T: 81 C: 81 |
T: 0–7 y C: 0–7 y |
Children | Acute | NR | ⩽14 d | 1. MN: <1 1.5 g, 1–3 y 2 g, >3 y 3 g, 2 times/d, for 3 d. 2. BE: 10 mg/kg, 2 times/d, for 3 d. 3. VB:NR, 2 times/d, for 3 d. 4. Antibiotic* |
p.r. | 1. Antibiotic*
2. MN: <1 1.5 g, 1–3 y 2 g, >3 y 3 g, 1 times/d, for 3 d. |
①⑧⑨ |
| Berberine + montmorillonite + Bifidobacterium lactobacillus triple viable versus no berberine + montmorillonite + Bifidobacterium lactobacillus triple viable | ||||||||||
| Han61 | T: 82 (36) C: 78 (33) |
T: 6 m–2 y C: 6 m–2 y |
Children | NR | NR | NR | 1. BLT: <1 y 0.21 g; 1–2 y 0.21–0.42 g, 2 times/d, for 3 d. 2. BE: 10–15 mg/kg, 2 times/d, for 3 d. 3. MN: <1 y 1.5 g; 1–2 y 3 g, 2 times/d, for 3 d. 4. Antibiotic* |
p.r. | Antibiotic* | ① |
| Berberine + montmorillonite + anisodamine versus no berberine + montmorillonite + anisodamine | ||||||||||
| Yu62 | T: 78 (35) C: 78 (32) |
T: 3 m–20 m C: 2 m–18 m |
Children | Both | NR | 3 d–2 m | 1. MN:3 g, 2 times/d, for 3 d. 2. BE: 0.1 2g, times/d, for 3 d. 3. AD: 3–5 mg, 2 times/d, for 3 d. |
p.r. | None | ① |
liquid therapy, adjustment of diet/diet guidance/nutritional guidance, supplementation of trace elements and calories.
Outcome measures: ① clinical cure rate (as mentioned by the study author); ② duration of diarrhea (day); ③ stool frequency, measured as the number of depositions per day; ④ fecal trait improvement; ⑤ absenteeism; ⑥ intensity of antibiotic use; ⑦ stool routine examination; ⑧ stool bacterial culture; ⑨ duration of hospitalization; ⑩ duration of other symptom such as vomiting, heat; ⑪ serum isoenzymes; ⑫ serum inflammatory factors; ⑬ serum myocardial enzymes; ⑭ recurrent diarrhea: repeat episodes of diarrhea in a defined period (as mentioned by the study author); ⑮ safety outcome (death: all-cause and diarrhea-related; adverse events; adverse drug reaction); ⑯ stool output, measured in g or ml/kg per day; and ⑰ quality of life.
AD, anisodamine; AMC, amoxicillin and clavulanate potassium; Amp, ampicillin; BE, berberine; BLT, Bifidobacterium lactobacillus triple viable; BS, Bifidobacterium subtilis; C, control group; CEC, cefaclor; CPFX, ciprofloxacin hydrochloride; d, day; F, female; Fur, furazolidone; LOF, levofloxacin; MN, montmorillonite; M, male; m, month; NR, not reported; NOR, norfloxacin; p.o., orally; p.r., enema; T, berberine group; Tet, tetracycline; VB, vitamin B; y, year.
Risk of bias in included studies
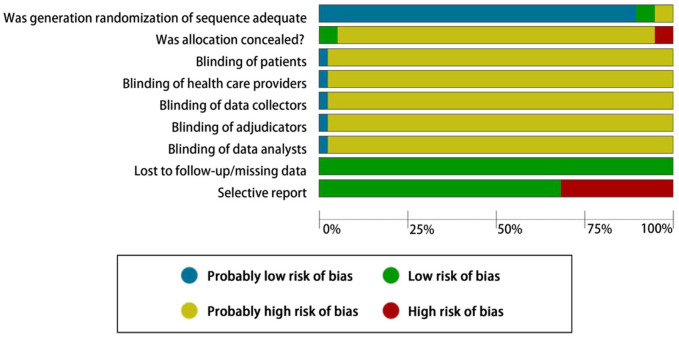
All studies were considered high risk of bias, mainly due to unclear concealment (36 of 38, 94.7%), no blinding (37 of 38, 97.3%) and selective reporting of outcomes (11 of 38, 28.9%; Figure 2).
Figure 2.
Risk of bias graph: review of author’s judgements about each risk of bias item presented as percentages across all included studies.
Effect estimates
Data analysis was performed according to the comparison. We could not summarize data or perform meta-analyses due to inclusion of just one study.
Primary outcomes
A total of 35 studies reported clinical cure rate, with a largely similar definition, that was the symptoms of diarrhea disappeared, and fecal trait and the stool frequency returned to normal within 48 h or 72 h or 100 h (as defined in each original study) after treatment.
Clinical cure rate
Comparison 1: berberine versus no berberine
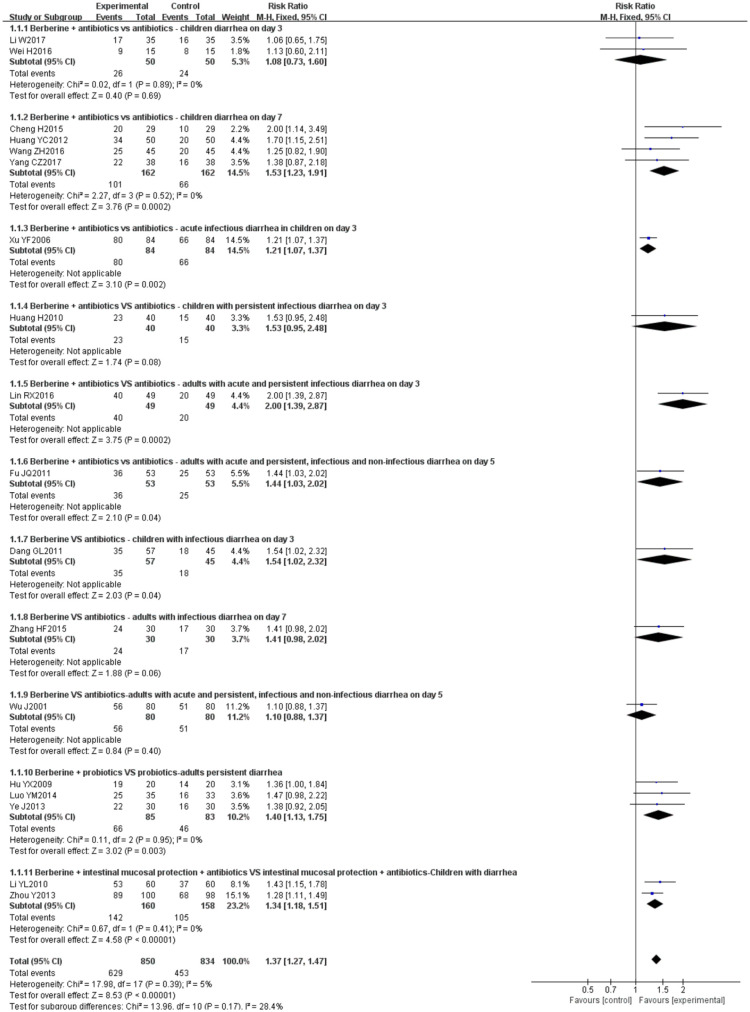
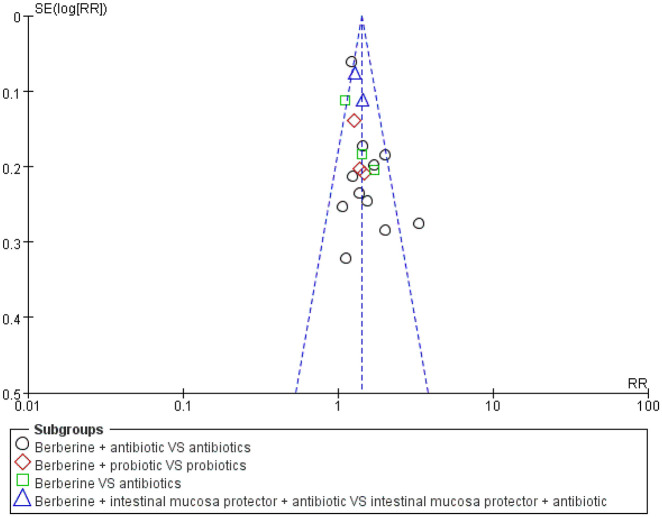
Overall, a meta-analysis from 17 studies26–39,41–44 showed that compared with no berberine groups, treatment with berberine resulted in a more significant number of patients being cured of diarrhea (RR 1.37, 95% CI 1.27–1.47, I2 = 5%, n = 1684, 17 trials, moderate certainty evidence; Figure 3). A funnel plot for these 17 studies appeared to be symmetrical, indicating no severe publication bias (Figure 4).
Figure 3.
Berberine versus no berberine clinical cure rate.
Figure 4.
Berberine versus no berberine clinical cure rate funnel plot.
In the subcomparison, that is, berberine + antibiotics versus antibiotics, there is insufficient evidence to prove that berberine has an advantage in treating children with diarrhea on day 3.28,29 One study37 found that berberine does not have an advantage in treating children with persistent infectious diarrhea on day 3. Other studies have reported advantages of berberine in treating diarrhea.26,28,30,31,36,39,41 In berberine versus antibiotics, a study43 reported that berberine could cure more children with infectious diarrhea compared with antibiotics on day 3. But there was no statistically significant between the experiment group and control group for adults in other studies.42,44 In berberine + probiotics versus probiotics, three studies32,33,39 showed that berberine has an advantage for adults with persistent diarrhea. In berberine + intestinal mucosa protection + antibiotics versus intestinal mucosa protection + antibiotics, the cure rate of berberine groups was significantly higher than the control group for children with diarrhea.34,38
A sensitivity analysis exploring the effect of randomization, allocation concealment (Appendix 1), and selective reporting bias (Appendix 2) did not change the result of the overall meta-analysis significantly, although it changed the results of some subgroup analyses.
Comparison 2: berberine + montmorillonite versus no berberine + montmorillonite
Overall, 13 studies45–57 evaluated clinical cure rates. Treatment with berberine and montmorillonite resulted in a more significant number of children being cured of acute diarrhea (RR 1.70, 95% CI 1.43 to 2.02, I2 = 55%; n = 1146, 13 trials, very-low-certainty evidence, Appendix 3). On visual inspection, the funnel plot was asymmetric, with most studies centered together on the upper left (Appendix 4).
In the subcomparison (berberine + montmorillonite + antibiotics versus antibiotics), 12 studies reported that berberine combined with montmorillonite and antibiotics had an advantage for children with diarrhea than antibiotics alone.45–49,51,57 In berberine + montmorillonite versus antibiotics, compared with the antibiotics group, the cure rate of berberine combined with montmorillonite and antibiotics was significantly higher for adults in acute diarrhea.50
A sensitivity analysis exploring the effect of selective reporting bias [Appendix 3(a)] did not change the result of the overall meta-analysis significantly.
Comparison 3: berberine + Bifidobacterium subtilis versus no berberine + Bifidobacterium subtilis; berberine + montmorillonite + vitamin B versus no berberine + montmorillonite + vitamin B; berberine + Bifidobacterium lactobacillus triple viable + montmorillonite versus no berberine + Bifidobacterium lactobacillus triple viable + montmorillonite; berberine + montmorillonite + anisodamine versus no berberine + montmorillonite + anisodamine
Compared with the control groups, using berberine in combinations with probiotics,58 vitamin B59,60 and Bifidobacterium lactobacillus triple viable61 increased the clinical cure rate of diarrhea (Appendices 5–7). There was no statistically significant benefit using berberine combined with montmorillonite and anisodamine62 (Appendix 8).
Duration of diarrhea
Comparison 1: berberine versus no berberine; berberine + montmorillonite versus no berberine + montmorillonite
Overall, five studies evaluated the duration of diarrhea. The use of berberine alone10,32,33 or berberine combined with montmorillonite47,57 has an advantage for diarrhea (low-certainty evidence) (Appendices 9 and 10). One of the studies10 measured the mean number of the duration of acute diarrhea in adults on day 3 (mean of 37.4 h in the berberine group versus 44.9 h in the control group).
Secondary outcomes
Stool frequency, stool output and fecal trait
The use of berberine alone37 or berberine combined with montmorillonite58 may improve the stool frequency, fecal trait, stool output (mean of 2.5 l in the berberine group versus 5.0 l in the control group)10 (Appendices 11–13).
Stool routine examination, stool bacterial culture and duration of hospitalization
The use of berberine alone42,44 (very-low-certainty evidence) or berberine combined with montmorillonite and vitamin B60 might have no effect on the improvement of the stool bacterial culture and the stool routine examination (Appendices 14–16).
There was evidence of benefit using berberine or berberine combined with montmorillonite28,38 (very-low-certainty evidence), vitamin B (mean of 2.5 d in the berberine group versus 5.0 d in the control group)60 (Appendices 17 and 18). There was significant heterogeneity (I2 = 99%). Due to the insufficient studies, we did not perform a subgroup analysis based on age and the route of administration to explain the statistical differences between the trials.
Duration of other symptoms, isoenzyme, inflammatory factors and myocardial enzyme
Using berberine also significantly had better laboratory tests results [isoenzyme-CK30,31 (low-certainty evidence), CK-MB27,30,31 (very-low-certainty evidence), inflammatory factors-INF-α26,27,30,31 (very-low-certainty evidence), IL-626,27,30,31 (very-low-certainty evidence), IL-1026,30,31 (very-low-certainty evidence) and myocardial enzyme-ALT26,27,31 (low-certainty evidence), AST27,30 (low-certainty evidence), LDH30] and fewer systemic symptoms37 (such as vomiting,40 heating40) than the control groups (Appendices 19–30).
Adverse events
The most commonly reported adverse effect was vomiting and rash, but were not serious. No deaths were reported in any of the included studies.
Discussion
Summary of our results
We conducted extensive literature searches and identified 38 studies (3948 participants, 27 pediatric trials with 2702 children) for analysis. Compared with antibiotics, berberine plus antibiotics showed better results in both adults and in children in general, especially when given for 7 days or 3 days in acute infectious diarrhea of children. Berberine was used orally in all adults and as enema in 2306 (2306 of 2702, 85.3%) children. We examined the use of berberine alone or berberine combined with montmorillonite, probiotics, vitamin B or anisodamine for diarrhea. Overall, compared with the control groups, using berberine alone or in combinations with montmorillonite, probiotics, and vitamin B increased the clinical cure rate of diarrhea. The use of berberine alone or berberine combined with montmorillonite may reduce the duration of hospitalization, improved the stool frequency and fecal trait. There was no evidence for the improvement of the culture of the stool bacteria due to use of berberine or berberine combined with montmorillonite and vitamin B. Using berberine also significantly had better laboratory tests results (isoenzyme, inflammatory factors and myocardial enzyme) and fewer systemic symptoms than the control groups. There was no evidence that these interventions had caused death or severe side effects. No study reported industry conflict of interest. However, the quality of evidence of included trials was moderate to low or very low.
Strengths and limitations
This is the first systematic review to provide evidence for the efficacy and safety of using berberine alone or in combination with Western medicine in diarrhea treatment. Berberine is the major active component of rhizoma coptidis and rhizoma phellodendron, which has been the most well-acknowledged traditional Chinese herb for diarrhea for over 2000 years. We used a modification of the Cochrane Risk of Bias Tool, which uses judgements of ‘probably high/low risk of bias’ to reduce the occurrence of ‘unclear’ to assist transparent judgements of the readers.23 We applied GRADE criteria to determine the certainty in the estimate of effect for our primary outcomes and plausible subgroups.25 We also evaluated the robustness of the meta-analysis by sensitivity analysis.
There are some limitations to our review. Excessive heterogeneity came to our notice in some of the comparisons; however, we did not have sufficient data and information to identify the source of heterogeneity. We contacted the authors of those studies that were missing critical information but did not receive any useful information from them. Missing essential information such as the course of the disease, absenteeism, the intensity of antibiotic use, quality of life and recurrent diarrhea, may reduce the guiding significance of the clinical practice. Because there is no blind design, most studies have produced a particular bias that affects the quality of evidence. The insufficient number of included studies in some comparisons affected the reliability of the results. We planned subgroup analysis based on dosage and treatment duration, however, the number of trials in each stratum was so limited that subgroup analysis could not be conducted as planned. Thus, the impact of different dosages, frequencies and treatment durations on the outcomes was not evaluated.
Implications for practice
The current data are promising but inconclusive. According to available evidence, berberine has a good effect on infectious, noninfectious, acute and persistent diarrhea. It can also be used to treat adults and children with diarrhea. There is also some evidence for the efficacy of berberine combined with montmorillonite in the treatment of diarrhea in children. The use of berberine and berberine in combination with montmorillonite appears to be an adjunctive therapy for diarrhea. Although some studies have demonstrated the efficacy of berberine and montmorillonite in combination with anisodamine, vitamin B or probiotics for diarrhea; however, due to the small number of studies and participants, we are not certain of this. Berberine enema treatments for childhood diarrhea were very outstanding. Montmorillonite slows down the peristaltic speed of the intestinal wall, improves the microcirculation of intestinal, and enhances the immunity of body.63 Vitamin B promotes metabolism, repairs the gastrointestinal mucosa and improves the function of the digestive system. Probiotics adjust the balance of intestinal flora, inhibit the production of endotoxins by harmful bacteria and maintain the normal physiological function of human intestines.37 Berberine combined with montmorillonite and vitamin B or probiotics can improve dyspepsia, reduce vomiting and restore the normal physiological function of the intestinal mucosal barrier.64
Because of the low bioavailability of berberine,38 it needs to be given three times a day for adults.65 Although it adds extra burden to the patients, it only has a high drug concentration in the intestine, and its blood drug concentration is almost negligible.38 Thus, berberine can reduce the risk of systemic side effects.
Acute diarrhea is often due to viruses or bacteria which are resistant to some antibiotics.66 Overall, 15 studies (Table 1) included in our review were infectious diarrhea; however, no pathogen was reported. We are unable to tell whether there were misuse of antibiotics. We suggest, in clinical practice, antibiotics should be prescribed according to local regulations to avoid as much as possible misuse and overuse (see Table 2 for summary of primary outcomes of RCTs on berberine for diarrhea).
Table 2.
Summary of primary outcomes of randomized controlled trials on berberine for diarrhea.
| Certainty assessment |
No. of participants |
(studies) |
Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Berberine | No berberine | Relative (95% CI) |
Absolute (95% CI) |
||
| berberine versus no berberine: clinical cure rate | ||||||||||||
| 18 | randomized trials | seriousa | not serious | not serious | not serious | not suspected | 629/850 (74.0%) | 453/834 (54.3%) |
RR 1.37
(1.27– 1.47) |
201 more per 1000
(from 147 more to 255 more) |
⨁⨁⨁◯ MODERATE |
CRITICAL |
| Berberine + montmorillonite versus no berberine + montmorillonite: clinical cure rate | ||||||||||||
| 13 | randomized trials | seriousa | seriousb | not serious | not serious | publication bias strongly suspectedd | 429/612 (70.1%) | 214/534 (40.1%) |
RR 1.70
(1.43– 2.02) |
281 more per 1000
(from 172 more to 409 more) |
⨁◯◯◯ VERY LOW |
CRITICAL |
| Berberine + montmorillonite + vitamin B versus no berberine + montmorillonite + vitamin B: clinical cure rate | ||||||||||||
| 2 | randomized trials | seriousa | not serious | not serious | seriousc | not suspected | 109/130 (83.8%) | 70/124 (56.5%) |
RR 1.49
(1.26– 1.77) |
277 more per 1000
(from 147 more to 435 more) |
⨁⨁◯◯ LOW |
CRITICAL |
| Berberine versus no berberine: duration of diarrhea | ||||||||||||
| 2 | randomized trials | seriousa | not serious | not serious | seriousc | not suspected | 65 | 63 | – | MD 3.25 lower (3.64 lower to 2.85 lower) | ⨁⨁◯◯ LOW |
CRITICAL |
| Berberine + montmorillonite versus no berberine + montmorillonite: duration of diarrhea | ||||||||||||
| 2 | randomized trials | seriousa | not serious | not serious | seriousc | not suspected | 123 | 113 | – | MD 1.63 lower (2.14 lower to 1.13 lower) | ⨁⨁◯◯ LOW |
CRITICAL |
CI, confidence interval; MD, mean difference (days); RR, risk ratio.
All the trials had a high risk of performance bias not blinding the participants. Methodological quality of these trials was graded as ‘high risk of bias’ due to the design of comparison is difficult to blind personnel and participants.
There is significant statistical heterogeneity indicating by a large I2 value.
For dichotomous outcomes, the total number of events is less than 300; for continuous outcomes, the total population size is less than 400; or pooled results included no effects.
Funnel plot was asymmetric.
Taking account of the over 30 years’ usage of berberine for diarrhea as an over-the-counter antimicrobial in a large Chinese population, the very low marketing price, the effectiveness we found, as well as the low incidence of adverse events, which was also addressed in a pharmacology review,10 berberine should be an attractive intervention for diarrhea.
The dosage of oral berberine for adults or children based on literature is consistent with the user’s manual, and for adults, is 0.1–0.3 g per time, three times a day,67 and for children, is based on the weight and age of the children (Table 3).67 Although we found that berberine can be used by enema from the included literature, no instruction could be found in the user’s manual. However no obvious adverse event was reported, either. Merits and risks of berberine enema need to be further explored.
Table 3.
The instructions of oral berberine for children.67
| Age | Weight | Dosage |
|---|---|---|
| 1–3 years | 10–15 kg | 0.05–0.1 g |
| 4–6 years | 16–21 kg | 0.1–0.15 g |
| 7–9 years | 22–27 kg | 0.15–0.2 g |
| 10–12 years | 28–32 kg | 0.2–0.25 g |
Implications for research
A large sample and multicenter well-designed clinical studies on berberine in specific population (children/adults), disease condition (infectious/noninfectious; acute/chronic) and treatment protocol (alone or in combination, orally taken or enema) are needed since berberine has shown therapeutic evidence or potentials in all above circumstances with seemingly low safety risk, and preclinical research evidence support. Economic analysis should be provided to guide practices in different countries or regions.
Conclusion
In conclusion, the current data are promising but inconclusive. Berberine alone or combined with montmorillonite may have benefit for diarrhea. There are few studies evaluating necessary outcomes in berberine for diarrhea, especially for absenteeism, the intensity of antibiotic use, quality of life and recurrent diarrhea. Due to the risk of bias and concern of publication bias, the above evidence was moderate to low or very low. A large sample and multicenter well-designed clinical studies are needed.
Supplemental Material
Supplemental material, Appendix for Berberine for diarrhea in children and adults: a systematic review and meta-analysis by Mingkun Yu, Xuejing Jin, Changhao Liang, Fanlong Bu, Deng Pan, Qian He, Yang Ming, Paul Little, Hongbo Du, Shibing Liang, Ruixue Hu, Chengze Li, Yanhong Jessika Hu, Huijuan Cao, Jianping Liu and Yutong Fei in Therapeutic Advances in Gastroenterology
Acknowledgments
We are grateful to Dr. BY Lai and Dr. N Liang for their contributions in the manuscript.
Footnotes
Author contributions: YT Fei and MK Yu conceived and designed the review. MK Yu and FL Bu drafted the protocol. MK Yu and CH Liang were responsible for the searching, screening and selecting studies. CH Liang, Y Ming, D Pan and Q He participated in data extraction and assessed study quality. MK Yu and CZ Li made forms and pictures and performed the statistical analysis. MK Yu and XJ Jin drafted the manuscript. XJ Jin revised the article in its entirety. JP Liu, HB Du, SB Liang, RX Hu, Paul Little and Yanhong Jessika Hu were all involved in critically revising the manuscript. XJ Jin, Yanhong Jessika Hu and Paul Little were responsible for the refinement of the content and language of our article. HJ Cao re-checked and analyzed the article data. All authors have read and approved the final manuscript. All authors approved the final version of the article, including the authorship list.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by The National Key Research and Development Project of China, No. 2018YFE0102300.
PRISMA 2009 checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
ORCID iD: Mingkun Yu  https://orcid.org/0000-0002-5885-1612
https://orcid.org/0000-0002-5885-1612
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mingkun Yu, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Xuejing Jin, School of Public Health, University of Alberta, Edmonton, AB, Canada.
Changhao Liang, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Fanlong Bu, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Deng Pan, School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Qian He, School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Yang Ming, School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Paul Little, Primary Care, Population Sciences and Medical Education Unit, University of Southampton, Southampton, UK.
Hongbo Du, Gastroenterology Department, DongZhiMen Hospital, Beijing University of Chinese Medicine, Beijing, China.
Shibing Liang, College of Basic Medical Sciences, Shanxi University of Traditional Chinese Medicine, Taiyuan, Shanxi, China.
Ruixue Hu, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China; Center for Evidence-Based Chinese Medicine, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China.
Chengze Li, College of Integrated Traditional Chinese and Western Medicine, Binzhou Medical University, Yantai, Shandong Province, China.
Yanhong Jessika Hu, Department of Paediatrics, Murdoch Children’s Research Institute, The Royal Children’s Hospital, The University of Melbourne, Melbourne, Victoria, Australia.
Huijuan Cao, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Jianping Liu, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China.
Yutong Fei, Researcher, Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, School of Traditional Chinese Medicine, No. 11, North Third Ring Road, Chaoyang District, China.
Search strategy
PubMed, EMBASE and the Cochrane Library
#1 randomized controlled trial [pt]
#2 controlled clinical trial [pt]
#3 randomized[tiab]
#4 Randomly[tiab]
#5 Randomization[tiab]
#6 RCT[tiab]
#7 trial[tiab]
#8 groups[tiab]
#9 allocat*[tiab]
#10 blind procedure[tiab]
#11 Crossover procedure[tiab]
#12 Placebo[tiab]
#13 Single blind[tiab]
#14 Double blind[tiab]
#15 blind[tiab]
#16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#17 Berberi*[tiab]
#18 Berberine[Mesh]
#19 #17 OR #18
#20 #16 AND #19
CNKI, VIP Chinese Science and Technique Journals Database, Wanfang Database and SinoMed Database
#1 黄连素
#2 小檗碱
#3 小蘖碱
#4 盐酸黄连素
#5 #1 OR #2 OR #3 OR #4
#6 对照
#7 随机
#8 试验组
#9 干预组
#10 分组
#11 两组
#12 三组
#13 组间
#14 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 #5 AND #14
References
- 1. World Health Organization (WHO)/United Nations International Children’s Emergency Fund (UNICEF). Ending preventable child deaths from pneumonia and diarrhea by 2025. The Integrated Global Action Plan for Pneumonia and Diarhoea (GAPPD), www.who.int/maternal-child-adolescent/documents/global-actionplan-pneumoniadiarrhea/en/ (accessed 14 January 2014).
- 2. World Health Organization. Diarrhoeal disease [EB/OL], https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease (accessed 3 May 2017).
- 3. Das JK, Hadi YB, Salam RA, et al. Fly control to prevent diarrhoea in children. Cochrane Database Syst Rev 2018; 12: CD011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol 2013; 47: 12–20. [DOI] [PubMed] [Google Scholar]
- 5. Pérez-Gaxiola G, Cuello-García CA, Florez ID, et al. Smectite for acute infectious diarrhoea in children. Cochrane Database Syst Rev 2018; 4: CD011526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010; 2010: CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2016; 12: CD005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacGillivray S, Fahey T, McGuire W. Lactose avoidance for young children with acute diarrhoea. Cochrane Database Syst Rev 2013; 2013: CD005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qingwei Y. Clinical analysis of montmorillonite powder in treating diarrhoea in children. Psychological Monthly 2018; 10: 186. [Google Scholar]
- 10. Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine: an update review. Phytother Res 2016; 30: 1745–1764. [DOI] [PubMed] [Google Scholar]
- 11. Xiao PG, Xiao W, Xu LJ, et al. Coptis and Chinese herbal medicines containing berberine alkaloids. Mod Chin Med 2016; 18: 1381–1385. [Google Scholar]
- 12. Khin-Maung-U Myo-Khin, Nyunt-Nyunt-Wai, et al. Clinical trial of berberine in acute watery diarrhoea. Br Med J (Clin Res Ed) 1985; 291: 1601–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CQ, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract – a review of actions and therapeutic implications. Am J Chin Med 2014; 42: 1053–1070. [DOI] [PubMed] [Google Scholar]
- 14. Ukita T, Mizuno D, Tamura T. Studies on the antibacterial properties of berberiniumchloride. Jpn J Exp Med 1949; 20: 103–108. [PubMed] [Google Scholar]
- 15. Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol 1969; 15: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 16. Joshi PV, Shirkhedkar AA, Prakash K, et al. Antidiarrheal activity, chemical and toxicity profile of berberis aristata. Pharm Biol 2011; 49: 94–100. [DOI] [PubMed] [Google Scholar]
- 17. Rabbani GH, Butler T, Knight J, et al. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis 1987; 155: 979–984. [DOI] [PubMed] [Google Scholar]
- 18. More NV, Kharat KR, Kharat AS. Berberine from Argemone mexicana L exhibits a broad-spectrum antibacterial activity. Acta Biochim Pol 2017; 64: 653–660. [DOI] [PubMed] [Google Scholar]
- 19. Chen ZY, Wang MX, Tu YX, et al. Therapeutic effect of berberine on 125 cases of bacterial dysentery. J Nanjing Med Univ (Natural Sciences) 1959; 233–236. [Google Scholar]
- 20. Shi RY. Therapeutic effect of berberine on acute bacterial dysentery. J Peking Univ (Health Sciences) 1959; 153–155. [Google Scholar]
- 21. Cernakova M, Kostalova D. Antimicrobial activity of berberine — a constituent of Mahonia aquifolium. Folia Microbiol (Praha) 2002; 47: 375–378. [DOI] [PubMed] [Google Scholar]
- 22. Feng Y, Li Y, Chen C, et al. Inhibiting roles of berberine in gut movement of rodents are related to activation of the endogenous opioid system. Phytother Res 2013; 27: 1564–1571. [DOI] [PubMed] [Google Scholar]
- 23. Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics 2008; 121: 326–336. [DOI] [PubMed] [Google Scholar]
- 24. Guyatt G, Busse J. Methods commentary: risk of bias in randomized trials 1, http://distillercer.com/resources/methodological-resources/risk-of-bias-commentary/ (accessed 14 July 2015).
- 25. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang CZ. Efficacy of amoxicillin and clavulanate potassium combined with berberine in the treatment of diarrhea in children and its effect on serum inflammatory factors. Clin Med 2017; 37: 111–112. [Google Scholar]
- 27. Li W. The effect of levofloxacin combined with berberine in the treatment of infantile diarrhea and its effect on myocardial enzymes, isoenzymes and serum inflammatory factors. Everybody Health (First Ten Day Edition) 2017; 11: 154–155. [Google Scholar]
- 28. Lin RX, Lin CF, Xu Y. Study of berberine combined with levofloxacin in the treatment of adult patients with infectious diarrhea. J Colorectal Anal Surg 2016; 22: 658–661. [Google Scholar]
- 29. Wei H, Li F. Therapeutic effect of berberine enema on 30 children with diarrhea. Med Front 2016; 6: 358–359. [Google Scholar]
- 30. Wang ZH. Effect of levofloxacin combined with berberine on diarrhea in children and on myocardial enzymes and serum inflammatory factors. Modern Digestion and Interventional Diagnosis and Treatment 2016; 21: 323–325. [Google Scholar]
- 31. Chen H, Liu QS, Le QS, et al. Effect of berberine combined with levofloxacin on myocardial enzymes, isozymes and serum inflammatory factors in children with diarrhea. Chin J Biochem Pharmaceuticals 2015; 35: 97–99. [Google Scholar]
- 32. Luo YM. Feasibility and safety of Peifeikang and berberine in the treatment of chronic diarrhea. Clin Res Tradit Chin Med 2014; 6: 110–111. [Google Scholar]
- 33. Ye J. Clinical efficacy analysis and evaluation of Peifeikang combined with berberine in the treatment of chronic diarrhea. Chin Med Innov 2013; 10: 51–53. [Google Scholar]
- 34. Zhou Y. Efficacy observation of montmorillonite powder combined with berberine retention enema in the treatment of infantile diarrhea. China Health Nutr (Mid Issue) 2013: 614. [Google Scholar]
- 35. Huang YC, Lai XQ, Luo LH. Clinical observation of berberine enteral enema on infantile infectious diarrhea. Asia Pac Tradit Med 2012; 8: 137. [Google Scholar]
- 36. Fu JQ, andTan YZ. Clinical observation on diarrhea treated with berberine hydrochloride. Mod J Integr Tradit Chin West Med 2011; 20: 3567–3568. [Google Scholar]
- 37. Huang H, Zhang JR. Clinical observation of Jinshuangqi combined with berberine enema in the treatment of infantile autumn diarrhea. Chin Community Doct 2010; 12: 84–85. [Google Scholar]
- 38. Li YL. Observation on the effect of berberine hydrochloride retention enema in the treatment of infantile diarrhea. Nurs Pract Res 2010; 7: 33–34. [Google Scholar]
- 39. Hu YX. Observation on the clinical efficacy of Peifeikang combined with berberine in the treatment of chronic diarrhea. Chin Med Front 2009; 4: 43. [Google Scholar]
- 40. Zhao L. Therapeutic effect of berberine combined with levofloxacin on acute infectious diarrhea in summer. World Health Dig 2007; 4: 46–47. [Google Scholar]
- 41. Xu YF. Observation and nursing of berberine retention enema in the treatment of infantile infectious diarrhea. J Qiqihar Med Coll 2006; 1751–1752. [Google Scholar]
- 42. Zhang HF. Therapeutic effect of berberine on 30 cases of infectious diarrhea. Health Care Today 2015; 75–75. [Google Scholar]
- 43. Dang GL. Evaluation of risk and efficacy of berberine in the treatment of infectious diarrhea in children. Journal of Yan’an University (Medical Science Edition) 2011; 9: 52–53. [Google Scholar]
- 44. Wu J, Ding Y, Li SF, et al. Efficacy and in vitro antibacterial activity of compound berberine in the treatment of acute and chronic diarrhea. China J New Drugs Clin Med 2001; 360–362. [Google Scholar]
- 45. Liu H. Evaluation of the clinical effect of conventional therapy with montmorillonite and berberine enema in the treatment of infantile diarrhea. Diet Health 2015; 2: 49. [Google Scholar]
- 46. Wang YX. Observation of the effect of reserved therapy with Smecta and berberine enema used in the treatment of pediatric diarrhea. J Front Med 2013; 5: 122–123. [Google Scholar]
- 47. Jin XH. Clinical observation on the effect of berberine hydrochloride combined with montmorillonite powder retention enema on infantile autumn diarrhea. Chinese Medical Guide 2012; 10: 268–269. [Google Scholar]
- 48. Huang HH. Therapeutic effect of enema on 56 children with chronic diarrhea. Chin Pediatr Integr Tradit West Med 2011; 3: 149–150. [Google Scholar]
- 49. Qiu LL. Discussions of clinical treatment realization in infants suffered diarrhea. Chin J Mod Drug Appl 2010; 4: 79–80. [Google Scholar]
- 50. Qin HL, Feng L, Geng NG. Efficacy observation on adult acute diarrhea treated with montmorillonite and berberine. Chinese and Foreign Medical Research 2010; 8: 32–33. [Google Scholar]
- 51. Gan YL. Comparation and nursing on efficacy of pediatric diarrhea treated with montmorillonite and berberin enema. Chin J Mod Nurse 2009; 15: 51–53. [Google Scholar]
- 52. Guo XH. Efficacy observation on adjuvant therapy with montmorillonite and berberine enema in infant patients with diarrhea. Chin J Mod Drug Appl 2009; 3: 137–138. [Google Scholar]
- 53. Wang HQ, Wang HX, Guo XM, et al. Therapeutic effect of berberine and montmorillonite enema on children with diarrhea. Chinese Journal of Misdiagnostics 2009; 9: 7852–7853. [Google Scholar]
- 54. Zhu HL. Reserved therapy with montmorillonite and berberine enema in the treatment of persistent diarrhea in infants and young children. Zhejiang Prev Med 2009; 21: 65–82. [Google Scholar]
- 55. Shi ZL. Efficacy observation on pediatric diarrhea treated with montmorillonite and berberine. China Med Herald 2008; 5: 66. [Google Scholar]
- 56. Wan YL, Qin JY. Therapeutic effect of berberine and Smecta retention enema on children with diarrhea. J Mod Med Health 2007; 23: 1517. [Google Scholar]
- 57. Liu H. Evaluation of the clinical effect of conventional therapy with berberine combined with montmorillonite enema used in the treatment of infantile diarrhea. Diet Health 2015; 2: 49. [Google Scholar]
- 58. Li H, Zhang LX, Zou JY. Observation on clinical effect of beberine combined with montmorillonite enema in the treatment of diarrhea in children. J Nurs Sci 2004; 58–59. [Google Scholar]
- 59. Geng Y, Liu XL. Effect observation of berberine combined with Bifidobacterium subtilis enema in acute diarrhea. China Med Pharm 2012; 2: 76–79. [Google Scholar]
- 60. Lu M. Observation on the therapeutic effect of berberine enema in the treatment of infantile diarrhea. Chinese and Foreign Health Abstracts, Clinician Edition 2008; 5: 87. [Google Scholar]
- 61. Yi Q. Therapeutic effect of berberine, montmorillonite and vitamin B enema on children with diarrhea. China Mod Doct 2008; 46: 89–90. [Google Scholar]
- 62. Han ZZ. Clinical observation on 82 cases of pediatric diarrhea treated by berberine combined with Bifidobacterium Lactobacillus triple viable and montmorillonite enema. Inner Mongolia Med J 2013; 45: 591–592. [Google Scholar]
- 63. Kang H, Yang LF, Zheng LR. Observation on retention enema of montmorillonite powder in the treatment of infantile diarrhea. Med Theory Pract 2005; 18: 1193–1194. [Google Scholar]
- 64. Song SY, Wang QJ. Observation on the curative effect of probiotics combined with compound vitamin B powder on autumn and winter diarrhea. Chin J Microecology 1999; 4: 70–71. [Google Scholar]
- 65. National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press, 2015, pp.1243–1244. [Google Scholar]
- 66. Yin X, Gu X, Yin T, et al. Study of enteropathogenic bacteria in children with acute diarrhoea aged from 7 to 10 years in Xuzhou, China. Microb Pathog 2016; 91: 41–45. [DOI] [PubMed] [Google Scholar]
- 67. China Medical Information Platform. Berberine Hydrochloride [EB/OL]. https://www.dayi.org.cn/drug/1146785#0,2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for Berberine for diarrhea in children and adults: a systematic review and meta-analysis by Mingkun Yu, Xuejing Jin, Changhao Liang, Fanlong Bu, Deng Pan, Qian He, Yang Ming, Paul Little, Hongbo Du, Shibing Liang, Ruixue Hu, Chengze Li, Yanhong Jessika Hu, Huijuan Cao, Jianping Liu and Yutong Fei in Therapeutic Advances in Gastroenterology