Abstract
The ongoing pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a substantial stressor that is greatly impacting environmental sustainability. Besides, the different pre-existing environmental stressors and coronavirus disease-2019 (COVID-19)-related stressors are further worsening the effects of the viral disease by inducing the generation of oxidative stress. The generated oxidative stress results in nucleic acid damage associated with viral mutations, that could potentially reduce the effectiveness of COVID-19 management, including the vaccine approach. The current review is aimed to overview the impact of the oxidative stress damage induced by various environmental stressors on COVID-19. The available data regarding the COVID-19-related stressors and the effects of oxidative stress damage induced by the chronic stress, exposure to free radicals, and malnutrition are also analyzed to showcase the promising options, which could be investigated further for sustainable control of the pandemic.
Keywords: COVID-19, Environmental stressors, Chronic stress, Oxidative stress damage, Viral mutations, Vaccine
Graphical abstract
Highlights
-
•
COVID-19 is associated with rising environmental stressors in stressed population.
-
•
Chronic stressful events and malnutrition are likely to generate oxidative stress.
-
•
Oxidative stress induces nucleic acid damage, leading to viral mutations.
-
•
The viral mutants could compromise the impact of immune system and vaccine.
-
•
COVID-19 management requires antioxidants, balanced diet, and healthy environment.
1. Introduction
Coronavirus disease-2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major public health problem of international emergency [1]. As of October 20, 2020, COVID-19 has caused the death of more than 1,122,758 humans, with nearly 41 million people infected worldwide [2]. To date, no antiviral therapy or vaccines have been approved against COVID-19 [3]. The characteristics of this pandemic, such as the rapidity of dissemination, uncertain knowledge, severity, and deaths among the caregivers, increase the potential psychological impact on healthcare professionals [4,5]. Furthermore, in regions where war is constantly raging, the populations have been victims for several years of various traumas, including repeated rapes used as a weapon of war [6], huge displacement [7], psychological stress [8], emergence of diseases (e.g., AIDS) [9], various co-infections [10], malnutrition [11], and several others. Among these, malnutrition is one of the main causes of the induction of oxidative stress, which alters the antioxidant protection mechanisms [12]. Moreover, such an affected population has relatively restricted access to comprehensive medical assistance and clean water, causing a chronically stressed population that is prone to several diseases [7]. The World Food Program (WFP) warns that the COVID-19 pandemic could double the number of people living with acute hunger by the end of 2020 [13]. Besides, the lockdown currently used as a strategy for minimizing the transmission of SARS-CoV-2 is also a source of divers' traumas [14,15]. Evidence supports that the population previously subjected to continuous traumatic events could be more vulnerable to distress when facing additional stressors [[16], [17], [18], [19], [20], [21]]. The aforementioned cocktail of various traumas is the endogenous and exogenous source of oxidative stress, the consequences of which on the pathogenesis of infections, especially viral, often results in the appearance of virulent mutants [22].
Mutations are the primary causes of genetic variations [23]. The human coronaviruses (HCoVs), including the SARS-CoV-2 and the severe acute respiratory syndrome coronavirus (SARS-CoV), are characterized by mutations, including the deletions in the coding and non-coding regions [24,25]; thereby, the possibilities leading to more or less virulent variants or mutants cannot be excluded. Additionally, a mutation in the spike (S) protein, such as D614G, of SARS-CoV-2 may increase its infectivity and make the vaccine less effective [26]. Moreover, an inappropriate immune response characterized by the excessive production of proinflammatory cytokines (cytokine storm), common in severe COVID-19, is another important source of endogenous oxidative stress resulting from the activated phagocytes [27]. The exogenous and endogenous sources of oxidative stress could potentially favor the system oxidants versus antioxidants; thereby, the increased production of reactive oxygen species (ROS) during the oxidative stress could promote viral multiplication [28]. The compensation for the pathogenic effects of viral replication requires the intact activities of detoxifying enzymes, hence highlighting the importance of exogenous antioxidants, a balanced diet, and a healthy environment. The current review aims to review the impact of oxidative stress damage induced by the chronic stressful environment on COVID-19. We describe the SARS-CoV-2 replication and progression of COVID-19 under oxidative stress as well as its prevention and therapeutic implications.
2. Environmental stressors
The environmental stressors refer to the environmental factors causing stress [29]. These include the biotic factors such as available food, the presence of predators, parasites, or interactions with conspecifics; as well as the abiotic factors such as available water, temperature, accessible sunlight, available oxygen, carbon dioxide concentration, air humidity, soil composition, wind, and different physicochemical agents [29,30]. The environmental stressors also include the cataclysmic (floods, earthquakes, major storms, volcanic eruptions, chemical plant accidents, nuclear power plant accidents, and toxic waste dumps), stressful life events (major changes in a residential environment or work), daily hassles (crowded classrooms, traffic congestions, and arguments with colleagues), ambient stressors (dust in an industrial area and noise), and war [30].
COVID-19 has shown a positive impact on the environment, such as by reducing noise pollution, improving air quality, and cleaning the beaches [31]. The latter were significantly associated with lockdown measures. On the other hand, the negative impacts, including the reduction of recycling, global economic activity, health system, education, and increase in waste production, are badly impacting the quality of human life [31]. During the pandemic of the COVID-19, most scientists, including the healthcare professionals have stopped their basic research to rather fully focus on COVID-19. Moreover, various hospitals have postponed their routine clinical trials and surgical operations involving tissue replacement and reconstructive surgeries, as the hospitals and the medical staff were devoted to treating the COVID-19 patients [32]. Thus, COVID-19 has created a great imbalance in the healthcare system worldwide. Several studies worldwide reported different COVD-19 related stressors [33,34]. In an anonymous investigation across Switzerland studying the impact of COVID-19 and confinement on mental health comprising of over 10,000 participants reported that 46.9% of the testified individuals showed an increased stress level during the lockdown versus 40% during the partial lockdown [35]. Moreover, the prevalence rate of severe depressive symptoms was 11.7% during the partial lockdown versus 9.1% during complete lockdown compared to the 3.4% as a prevalence rate before the COVID-19. In this study, a history of psychiatric disorder was considered as a risk factor for developing the depressive symptoms. The same tendencies of results were found in a study made in India during the COVID-19 lockdown, which states that the lack of sufficient supplies to maintain the confinement was correlated with the development of depression, anxiety, and stress [14]. Furthermore, in a study conducted in the Israel-Gaza border, a region with repetitive shelling, comprising of 976 participants where 793 (81.3%) were exposed to traumatic events and 255 (31.5%) to continuous traumatic stress (CTS). The majority of participant reported COVID-19 related anxiety (84.4%), depression (85.9%), and peritraumatic stress (76.7%). Moreover, a small portion of participants developed anxiety (10.3%) and depression (10.1%). Additionally, 11.5% of peritraumatic stressed participants presented significant clinical symptoms of which 90.2% experienced prior trauma [16]. In another study, most of the traumatic events, including distress, were related to COVID-19. The peritraumatic stress symptoms, anxiety, and depression were found in participants exposed to CTS and trauma. Importantly, a high level of distress related to COVID-19 was observed in adult participants who faced child abuse [19]. These results show that the subjects previously exposed to trauma are more vulnerable to an additional stressor such as COVID-19. Similarly, among 290 Spanish participants, only people with chronic disease and those with age above 60 years developed stress, depression, or anxiety [36], while, in the study conducted in Saudi Arabia, out of 156 epilepsy patients, 71.2% testified a problem with sleep, 59.4% reported increased stress, and 29.5% showed an increased frequency of seizures [21]. The depression, anxiety, and stress were significantly higher in 76 psychiatric patients compared to the 109 healthy subjects used as the control in a study conducted in China [20]. Thereby it is important to provide the psychosocial tools to improve the emotional and social state of the vulnerable population concerning COVID-19. Besides, in the USA, among 742 co-parents, greater stress associated with COVID-19 was reported, predicting greater co-parent and family discord [37]. The level of stress was inversely proportional to the level of energy among 1003 parents, indicating the psychological inflexibility and flexibility [38]. Thereby, the COVID-19 related stressor can disrupt the parents' daily energy and sleep, and at the same time decreasing their ability to respond to difficult experiences flexibly and instead favoring more reactive and inflexible responses. Another study carried out in the USA and Canada reported that participants with anxiety (700) showed high COVID-19 related stress than the mood disorders (n = 368) and no mental disorder (n = 500). The COVID-19 stress scales expressed as adjusted mean and standard deviations were 52.4 (1.2) in anxiety, 45.1 (1.6) in mood disorders, and 41.7 (1.4) in no mental disorder group. There was no significant difference between mood disorders and no-mental disorder group [15]. Hence, the COVID-19 affected people with mood or anxiety disorders respond more negatively than those without a mental health disorder. Additionally, the high-level COVID-19 related stressors and child abuse are potentially associated with the depression and anxiety, as reported in 183 parents in a study conducted in the USA [39]. These results suggest the significant relationship of stressors and mental health risks with the COVID-19 and child abuse. In 1055 Canadian participants, the elevated neuroticism was found associated with the elevated levels of stress during the COVID-19 pandemic than period before [40]. A high level COVID19-related stress was also reported in France among students who did not change their habitations during the confinement [41]. Thereby, acquiring knowledge of the confinement impact could be used to decrease the negative effects of COVID-19 on chronically stressed population. Furthermore, in a study conducted in Bangladesh comprised of 340 participants, 86.6% developed stress related to COVID-19 resulting in short temper, sleep shortness, and chaos in family [42]; while in another study comprised of 1427 participants, 59.7%, 57.9%, and 33.7% reported mild to severe level of stress, depression, and anxiety, respectively [43]. Thus COVID-19 pandemic has created tension and fear among the Bangladeshi citizens associated with suicidal ideation. Similarly, the COVID 19 related stressors were also recorded in Pakistan. In this study, the media (i.e., print, electronic, and social media) was seen as one of the main sources of anxiety and stress in the population [44]. Therefore, media limitations and religious adaptation were used as a strategy to cope with stress. In Iraq, 268 physicians were included in a study to evaluate stress and insomnia, where 93.7% developed stress while 68.3% were sleepless [4]; while, in Turkey, among 442 physicians, 224, 286, and 182 developed anxiety, depression, and stress, respectively [5]. Mental disorders are more pronounced in young women than in men, and nurses than the doctors [45]. As with the SARS-CoV epidemic, it is feared that some caregivers, particular those fighting at frontline, will present, at a distance from the health crisis, psychiatric symptoms of various kinds: such as anxiety, depressive symptoms, acute stress, and post-traumatic stress disorder (PTSD) [46]. Indeed, a year after the 2003 epidemic, caregivers caring for infected patients had shown an increased prevalence of burnout, symptoms of PTSD, and psychological distress [47]. The recency of the pandemic does not provide data on the specific impact of COVID-19 and confinement on addictions; however, the literature supports the idea of an increased risk of addiction in short and medium-term. SARS-CoV outbreak was accompanied by increased alcohol use disorder three years later among the Beijing hospital workers [48]. This increased risk, observed in the primary care workers or those in the confinement, was mediated by symptoms of depression, PTSD, and early alcohol consumption as a coping strategy. Overall, these studies show the negative impact of COVID-19 on population, patients, and physicians, depicted by a high level of stress, depression, and anxiety associated with rising the measures of confinement.
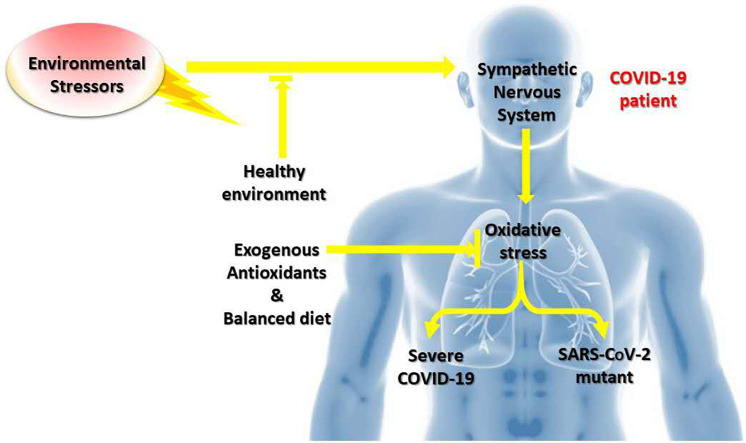
A stressful event causes a chain reaction that begins in the brain and results in the production of cortisol by the adrenal glands [49]. The cortisol activates two areas of brain: the cerebral cortex that reacts to the stressful stimulus and the hippocampus, which calms the reaction. In the case of high or chronic stress, the hippocampus saturated with cortisol can no longer ensure regulation. Cortisol invades the brain and sets up depression. It has been stated that stress also alters the immune response [50]. Moreover, the immune system has a function of regulating the negative emotional states such as stress via the conserved transcriptional response to adversity (CTRA) mediated by the sympathetic nervous system ‘fight-or-flight’ (Fig. 1 ) [51]. The CTRA pattern induces the high production of proinflammatory cytokines while suppressing the genes involved in the production of interferons and antibodies, thus leading to chronic inflammation, tissue damage, and vulnerability to viral infections [51]. Moreover, cytokines induce oxidative stress after the stimulation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) [52]. Studies have shown that major depression, chronic stress, acute anger episodes, and behavioral aspects of aggressive traits are depicted by the release of proinflammatory cytokines [53,54]. A study also reported that the chronic stress exposure promotes the oxidative stress [55].
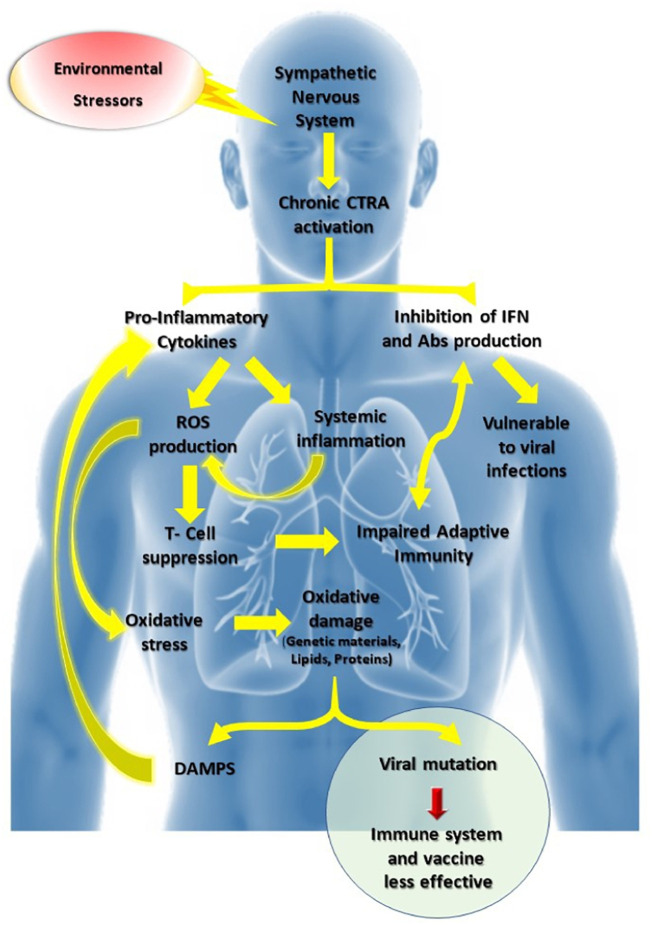
Fig. 1.
Chronic stress could stimulate CTRA via the sympathetic nervous system leading to the induction of proinflammatory cytokines and suppression of genes involved in the production of antibodies and interferons, resulting in the vulnerability of viral infections. Proinflammatory cytokines induce chronic inflammation and ROS generation, thus producing an imbalanced oxidative stress response. The latter induces oxidative damage of endogenous molecules (nucleic acids, lipids, proteins), resulting in DAMPS that cause proinflammatory cytokine secretion (cytokine storm). For a person infected with a virus such as SARS-CoV-2, oxidative damage may result in viral mutations that could result in minimizing the effect of the immune system and vaccine. Besides, the overproduction of ROS suppresses the T lymphocyte response, which results in impaired adaptive immunity.
CTRA, conserved transcriptional response to adversity; IFN, interferon; ROS, reactive oxygen species; Abs, antibodies; DAMPS, damage-associated molecular patterns; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
It arises from the above studies that the pre-existing environmental stressors and measures to control the spread of COVID-19, such as confinement-induced stress, depression, and anxiety which make the populations and COVID-19 patients more vulnerable by altering their immune system and inducing oxidative stress. Thus, the importance of living in a healthy environment associated with the psychosocial care of the vulnerable population could minimize the adverse effects of COVID-19.
3. Oxidative stress
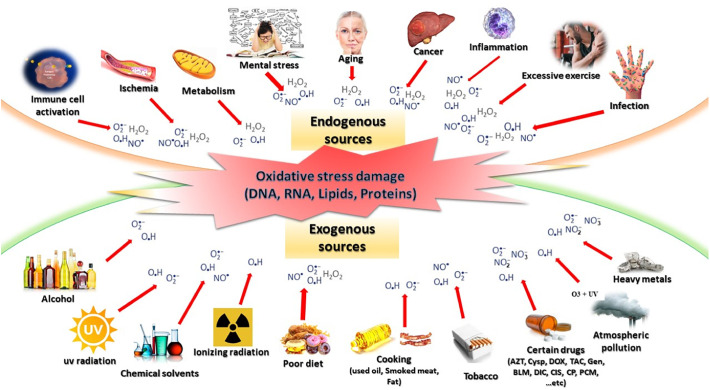
The oxidative stress is defined as an imbalance favoring the oxidative systems compared to the antioxidants, which gives rise to, particularly, toxic derivatives as ROS and reactive nitrogen species (RNS) [56]. ROS are oxygenated chemical species such as superoxide anion (O2 • −), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) [57]. These appear as the byproducts of normal oxygen metabolism, and thus play an important role in communication between the cells. In contrast, the RNS, including the nitric oxide radical (NO•), peroxynitrite (•NO3), and nitrogen dioxide (•NO2), play important physiological roles [56,57]. Both ROS and RNS could be generated from an exogenous or endogenous origin (Fig. 2 ). The essential causes of oxidative stress are either the mental stress, nutritional origin in the case of deficiencies in vitamins and trace elements, overloads in pro-oxidant factors, accidental origin (inflammation, infections, exposure to pro-oxidizing xenobiotics), and genetic origin [58].
Fig. 2.
Origin of reactive oxygen and reactive nitrogen species. These can be from endogenous sources such as mental stress, metabolism, and inflammation as well as exogenous sources such as poor diet, UV radiation, ionizing radiation, and atmospheric pollution. When these exogenous compounds penetrate the body, these are degraded or metabolized, resulting in the generation of ROS and RNS as the byproducts.
OH•, hydroxyl radical; O2•−, superoxide anion; H2O2, hydrogen peroxide; NO•, nitric oxide radical; •NO3, peroxynitrite; •NO2, nitrogen dioxide; cysp, cyclosporine; Tac, tacrolimus; Gen, gentamycin; BLM, bleomycin; Dox, doxorubicin; AZT, azidothymidine; Dic, diclofenac; PCM, paracetamol; Cis, cisplatin; CP, chlorpromazine.
The free radicals, including the ROS and RNS, perform several useful functions which, apart from phagocytosis, have been discovered [57]. These participate in the functioning of certain enzymes, transduction of cellular signals, immune defense against pathogens, destruction of tumor cells via apoptosis, cell cycle, cell differentiation, and regulation of capillary dilation. However, the imbalance favoring free radicals versus antioxidants is harmful to human health. Oxidative stress is involved in many diseases as a trigger or associated with disease complications [57]. The multiplicity of medical consequences of the stress is not surprising because, depending on the diseases, stress acts onto a particular tissue and cell type, and involves different radical species and associates with other factors such as variability and genetic abnormalities specific to each individual [59]. By revealing the abnormal biological molecules and overexpressing certain genes, oxidative stress could be the main initial cause or a trigger for several diseases. The paradox of free radicals in clinical biology is that these are extremely dangerous species, which are capable of causing a considerable number of diseases [56] such as respiratory disease, cancer, cardiovascular disease, neurological disease, rheumatoid arthritis, kidney disease, and delay of sexual maturation among others while being essential species for life. Oxidative stress causes an excess generation of ROS, which by binding to various cellular components such as DNA (deoxyribonucleic acid), RNA (ribonucleic acid), lipids, and proteins, disrupt the antioxidant defense systems, redox homeostasis, and interferes with the cellular signaling [57]. The activity of certain transcription factors is modified by the generation of ROS. Some, for example, nuclear factor κB (NF-κB), involved in pro-inflammatory cytokine and activator protein-1 (AP-1) involved in cell proliferation, differentiation, and death are activated, while others such as nuclear factor 1(NF1) involved in the regulation of cellular and viral genes transcription and specific protein-1 (SP-1) involved in response to DNA damage, cell growth, apoptosis, and cell differentiation, are inhibited [56].
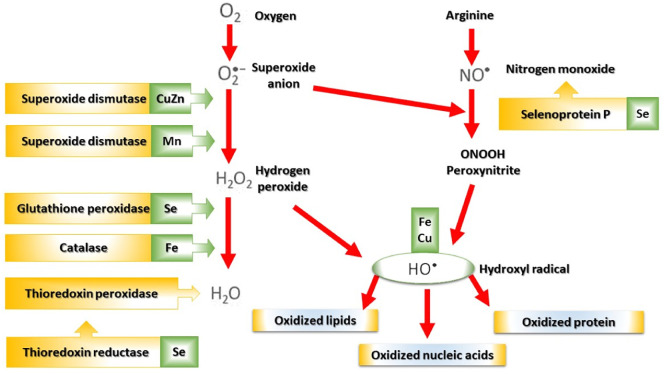
In the event of oxidative stress, cells use many antioxidant molecules grouped in enzymatic and non-enzymatic systems [60]. Three major types of antioxidant enzymes, including superoxide dismutases (SOD), glutathione peroxidases (GPxs), and catalases (CAT), are concerned (Fig. 3 ) [60]. SOD catalyzes the disappearance of superoxide radical through disproportionation, which results in the formation of hydrogen peroxide [61]. CAT converts the latter into water and oxygen [62], while GPxs couple the reduction of hydrogen peroxide with the oxidation of reduced glutathione (GSH) [63]. GSH is the most important of the endogenous non-enzymatic systems. The thiol function of GSH gives it a role of reducing agent towards certain ROS. Food-borne non-enzymatic antioxidants [60], such as vitamins (E, A, and C), flavonoids, carotenoids, hydroxycinnamic acids, allyl sulfides, and curcumin also exert important protective effects. However, in patients infected with viruses such as COVID-19, numerous studies have shown the deficiency of the intracellular and plasma anti-radical protective systems which alters the immune functions as well as deficits in SOD and CAT [64]; deficiency of reduced forms of glutathione (GSH) [65], deficiencies in trace elements (selenium and zinc) [66], vitamins, and sulfur amino acids (cysteine and cystine) [65].
Fig. 3.
Mode of action of the main antioxidant enzymatic systems and their metal cofactors. CuZn, copper-zinc; Mn, manganese; Se, selenium; Fe, iron; Cu, copper.
Oxidative stress induces immunopathological consequences by affecting the host's immune response [67]. Specifically, COVID-19 related oxidative stress is depicted by impaired adaptive immunity, which leads to severe disease associated with systemic tissue damage [68]. During viral infection, an oxidative aggression alters the structure and function of the circulating lymphocytes; for example, CD4+ lymphocytes are particularly affected [69]. Additionally, the intracellular oxidative stress at the origin of the lipid peroxidation could explain the weakening of the plasma membranes and loss of reactivity and viability of the lymphocytes [70]. Moreover, in the COVID-19 course, the oxidative stress impairs T cell response associated with reduced antiviral activity of CD8+ T cells and low titer of antibodies mediated by the B cells after interacting with the less efficient CD4+ T cells. The intracellular concentration of GSH, that is generally high in these cells, appears to be lowered in patients infected with the virus [71]. A decrease in the metabolic activity linked to a decrease in the intracellular antioxidant potential could explain the selective depletion of CD4+ lymphocytes and the paralysis of the immune system [71]. The participation of oxidative aggression in lymphocyte depletion can, therefore, arise from different mechanisms and, in particular, from apoptotic mechanisms [72]. In fact, the metabolic reactions related to oxidative stress are involved in the initiation of apoptosis, which brings into play various metabolic events. Apoptosis is one potential mechanism associated with T cell reduction during the COVID-19 course [68]. The existence of overproduction of H2O2 by the neutrophils of subjects infected with viruses is detected at the onset of the infection, and is independent of the number of CD4+ lymphocytes [73]. The elevated level of H2O2 in the COVID-19 patients is associated with the lung damage and oxidation of key constituents of innate immunity [64]. The H2O2 acts directly on the transcription factor NF-kB of T lymphocytes and macrophages, which induces cell death, while the set of free radicals promotes the secretion of TNFα, IL1, and IL6 by the monocytes, which act secondarily on the NF-kB [74]. The latter mechanism would be close to what is described during various opportunistic infections such as pneumocystosis, mycoplasma infections, which activate polynuclear neutrophils and monocytes-macrophages by antigenic stimulation and induce the production of ROS, which stimulate the production of cytokines such as TNFα [75]. The inhibition of activation of NF-kB factor by antioxidants supports these hypotheses. The severe COVID-19 is depicted by the higher neutrophil-lymphocytes ratio (NLR) associated with the elevated ROS level [76]. Since the self-regulating mechanism of ROS and RNS concentration is defective in virus-infected patients, the supply of exogenous antioxidants associated with a balanced diet should be included in the therapeutic strategy.
4. Oxidative stress damage and viral mutation
Free radicals are capable of generating oxidative damage at the levels of various molecular targets such as DNA, RNA, lipids, and proteins (Fig. 3) [56,77]. Mutation rates are defined as the probability that through viral genomic replication, a nucleotide is changed by substitution, deletion, insertion, inversion, or recombination [78]. During viral replication such as SARS-CoV-2, the mechanisms of genome expression disturbed by the high rate of free radicals activated by the oxidative stress would act on the proteins resulting from the translation of messenger RNAs, and would completely change the structure of the SARS-CoV-2 (Fig. 4 ). Such mutations may involve the non-structural proteins or structural proteins such as S protein, and would favor the evolution of the COVID-19 pandemic.
Fig. 4.
SARS-CoV-2 replication in the presence of oxidative stress. SARS-CoV-2 infects the host cell through interactions between its spike protein (S) and cell receptor, ACE2. The release of viral RNA through endosomes into the cytoplasm in the presence of oxidative stress may result in RNA mutation. After ARN mutation, the translation of viral polyprotein followed by proteolysis produces non-structural proteins (NSPs) and forms replication-transcription complex (RTC). The RTC drives the synthesis of (−) RNA. Full length (−) RNA copies of the genome provide templates for full-length (+) RNA genomes. Transcription further produces a subset of subgenomic RNAs, including those encoding the accessory and structural proteins. The oxidative stress may act on proteins resulting from the translation of messenger RNAs and could completely change the structure of the virus. The translated structural proteins (M, N, E, S) and genomic RNA are assembled into the viral nucleocapsid and envelope in the ER-Golgi intermediate compartment, which are subsequently released via exocytosis. The SARS-CoV-2 mutant would escape the antibody immune response.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; RNA, ribonucleic acid; (+) RNA, Positive-strand (5′-to-3′) RNA; (−) RNA, negative-strand (3′-to-5′) RNA; OH•, hydroxyl radical; O2−•, superoxide anion; H2O2, hydrogen peroxide; NO•, nitrogen monoxide; N, nucleocapsid protein; M, matrix protein; E, envelope protein; S, spike protein.
SARS-CoV-2 exhibits, like other coronaviruses, a relatively high ability of mutations which can lead to evolution of its S protein [26,79]. The S protein is essential for entry of SARS-CoV-2 into the host cell via the angiotensin-converting enzyme 2 (ACE2) [80]. During the first SARS-CoV outbreak, a mutation in the S protein allowed the infection to spread from the initial reservoir to the intermediate civet host and humans [81,82]. In the current pandemic, the D614G mutation became dominant by covering more than half of all viral sequences released [[82], [83], [84]]. There were also accompanying mutations [24,25]. Furthermore, the SARS-CoV-2 mutant increased the infectious potential associated with higher viral loads in the COVID-19 patients, indicating that the mutant or variant could probably cause higher viral entry into the cells and cause their subsequent replication in the respiratory tract cells [82,84]. Besides, the D614G mutation could increase the susceptibility to neutralization [82]. The term neutralization relates to the formation of the antigen-antibody complex which prevents the biological activity of the antigen [85]. The neutralization function is the main correlate of protection of numerous vaccines and serotherapies; thus, this could probably help improve the development of better vaccines and antibodies to prevent a future pandemic. Moreover, as in other RNA viruses [86,87], the SARS-CoV-2 mutant or variant may escape the antibody immunity induced by the vaccine. Thereby, the vaccine inducing innate immunity, cell-mediated immunity, and humoral immunity could be effective against the SARS-CoV-2 mutant or variant.
Lesions induced by the free radicals in genetic material such as oxidized bases, especially guanine, abasic sites, and single or double-strand breaks, are involved in many deleterious processes such as cell lethality, mutagenesis, carcinogenesis, or apoptosis [77]. The oxidative damage to DNA is varied and includes the oxidation of nucleic bases. Of the twenty basic lesions identified, the 8-hydroxy-2′-deoxyguanosine (8-OHdG) has been the most studied because of its frequency and potentially mutagenic nature [88]. The 8-OHdG could be used as a biological marker of oxidative damage to DNA while 8-isoprostaglandin F2α (IsoP) and 8 hydroxyguanosine (8-OxoG) reflect the lipid peroxidation and oxidative damage to RNA, respectively [55]. Moreover, malondialdehyde (MDA) could be used as a biomarker for the measurement of lipid peroxidation [89]. A high plasma concentration of MDA is observed during the viral infection [90]. The plasma MDA increases during the early stages of contamination, and its level gradually rises with the course of the disease. The correlation between the plasma MDA concentration and viral disease course has been elicited in different studies [91].
The excessive production of free radicals causing direct lesions of biological molecules but also secondary lesions due to the cytotoxic and mutagenic nature of the metabolites release has been reported, particularly during the lipids peroxidation [92]. The body can also react against these abnormal compounds by producing antibodies, where unfortunately, autoantibodies can lead to systemic autoimmunity diseases [93]. After mutations, the DNA or RNA can become highly immunogenic, and the antibodies produced could show varying antigen-binding characteristics. The patients with systemic lupus erythematosus have the DNA-derived immune complex in which 8-hydroxyguanosine has been detected [93]. This strengthens the evidence that ROS may be involved in its pathogenesis.
Several studies have shown an association between the exposure to exogenous sources of free radicals and the formation of oxidative stress damage markers such as MDA, 8-OHdG, and advanced oxidation protein products [94]. In a study evaluating the impact of chronic stress exposure on oxidative damage involving 48 post-menopausal women under the chronic stress showed higher 8-hydroxyguanosine (8-OxoG) level and oxidative damage to RNA compared to the control group [55]. Similar results were found with 33 chronically stressed post-menopausal caregivers [95]. In the animal model, the stressed rats exhibited higher levels of MDA, as well as higher activities of SOD, glutathione reductase (GR), and GPx [89]. Besides, the total antioxidant capacity was significantly lower than those of the non-stressed rats. The exogenous antioxidants (crocin and saffron) used in the latter study reversed theses parameters in the stressed rats and prevented the oxidative damage. Similar results were found in other chronically stressed rat model [96]. Furthermore, an oxidative stress induced mutations associated with the drug resistance in patients with chronic hepatitis B (CHB) and correlate with CHB progression [22]. Similarly, the study using mice reported that the increase in mutation load leading to carcinogenesis was associated with the oxidative stress induction [97]. In the respiratory infection, the Mycoplasma pneumonia induced ROS generation and DNA damage by double-strand breaks [98].
Overall, these studies show that the chronic stress and exposure to free radicals lead to oxidative stress damage associated with mutations in the case of viral infection. In order to avoid the mutations of SARS-CoV-2, which are likely to complicate the COVID-19 patient management, it would be wise to strengthen the immune system through the exogenous supply of free radical scavengers.
5. Malnutrition, oxidative stress damage, and viral mutation
Malnutrition is a pathophysiological condition resulting from the combined effect of over- or under-nutrition (deficiency or excess of calories and lack one or more nutrients) and other factors (genetic and inflammatory) [99]. Moreover, the lack of exogenous antioxidants favors the system oxidants of malnourished hosts resulting in oxidative stress damage. In 2019, 47 million children around the world were already suffering from the consequences of malnutrition [100]. According to the WFP report in 2020, the number of hungry or undernourished people in the world are above 820 million, or 1 in 9 people, and 132 million people live with acute hunger that approaches famine [101]. The COVID-19 infection increases the risk of malnutrition and food insecurity, secondary to lockdown [102]. The United Nations International Children's Emergency Fund (UNICEF) reports that in lockdown contexts, there have been 75–100% drop in the coverage of nutrition services [103]. The study conducted in 118 low- and middle-income countries found that the food crisis caused by the COVID-19 could increase the prevalence of moderate or severe weight loss among the children under five years by 14.3% [103]. Additionally, nearly 7 million more children could suffer from malnutrition this year globally due to the economic crisis linked to the Covid-19 pandemic; thereby, 80% of them are thought to live in Sub-Saharan Africa and South Asia [104]. Adult individuals in food insecurity situations are exposed to a mental health problem, depression, hypertension, and diabetics, while infants are exposed to asthma, anemia, anxiety, and poor health [105]. Malnutrition has long been associated with the increased susceptibility to infectious diseases [106]. This leads to the vicious cycle [107]; the nutritional deficiency alters the immune system, which in turn becomes susceptible to infections. Additionally, this condition deteriorates the nutritional state, which, in the long term, leads to protein-energy deficiency and may lead to death [108]. Conversely, the constantly activated immune system alters the nutritional status via certain cytokines acting on the cells, tissues, and organs [107]. In a retrospective study carried out in Wuhan, China, the malnutrition was a common clinical feature of severe COVID-19 death cases [109]. Moreover, the malnourished individuals have a rising risk of being admitted to the intensive care unit and of COVID-19 related mortality [110].
Different studies reveal the association of malnutrition with oxidative stress damage. In the animal model, the lipid peroxidation and DNA damage increased significantly among the second and third-degree malnourished rats compared to the control group (well-nourished rats) [12]. The mRNA expression, protein concentration, and antioxidant (SOD, GPx, and CAT) activity were inversely related to the oxidative damage in the malnourished rat group. In a model system of Se-deficient mice, the susceptibility to influenza virus infection and coxsackievirus was observed [111]. These mice develop myocarditis and severe pneumonia when infected with a mild strain of coxsackie and influenza virus, respectively. Mutations have been observed in influenza and coxsackievirus viral genome in Se-deficient mice. As in Se-deficient mice, GPx knockout mice also developed myocarditis when infected with the benign strain of coxsackie, thus highlighting the nutritional value of preventing the mutation. In the schizophrenia model, the rats associated with prenatal famine were depicted by a higher level of oxidative damage [112]. In the severe acute malnutrition (SAM) model, the hospitalized children with SAM (n = 100) exhibited oxidative stress depicted by significantly lower levels of Zn and GSH and elevated MDA compared to the healthy children (n = 100) used as a control group [113]. These studies highlight the significance of host nutrition not only for improving the antioxidant capacity and the immune response but also for averting the viral mutations that could rise the viral pathogenicity.
6. Concluding remarks
The COVID-19 pandemic interventions aimed to reduce the transmission of SARS-CoV-2 and the pre-existing environmental stressors are causing the stress-related public health problems, especially in a vulnerable population. Nevertheless, under the impact of oxidative stress, the course of COVID-19 becomes severe, thereby complicating the treatment. The SARS-CoV-2 S protein could be modified during the viral replication, thus may escape the antibody-based immunity of the host. Moreover, the oxidative stress would be at the origin of virulent variants or mutants, which would infect a large number of individuals in a population deprived of immunity against the mutant or variants. The emergence of different variants or mutants could, over time, make the vaccine less effective, and thus necessitating the revaccination of population. The vaccine approach, which seems to be the most effective, is that of complementing the vaccine by improving the innate immunity and cell-mediated immunity. Furthermore, in the situation of a region that lives from traumas generating oxidative stress, it is judicious to use COVID-19 treatment based on antioxidants, including vitamins and essential trace elements, which could potentially avoid the appearance of SARS-CoV-2 variants or mutants.
Authors' contribution
The manuscript was written through the contribution of all authors.
Funding source
This work was supported by the National Natural Science Foundation of China (Grant No. 21774039) and the National Key Research and Development Program of China (2018YFE0123700).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Coronavirus cases. 2020. https://www.worldometers.info/coronavirus/
- 3.Dos Santos W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed. Pharmacother. 2020;129:110493. doi: 10.1016/j.biopha.2020.110493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiou N., Delfabbro P., Balzan R. COVID-19-related conspiracy beliefs and their relationship with perceived stress and pre-existing conspiracy beliefs. Pers Individ Dif. 2020;166 doi: 10.1016/j.paid.2020.110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbay R.Y., Kurtulmuş A., Arpacıoğlu S., Karadere E. Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020;290 doi: 10.1016/j.psychres.2020.113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denov M., Lakor A.A. When war is better than peace: the post-conflict realities of children born of wartime rape in northern Uganda. Child Abus. Negl. 2017;65:255–265. doi: 10.1016/j.chiabu.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Kassem I.I. Refugees besieged: the lurking threat of COVID-19 in Syrian war refugee camps. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempuraj D., Ahmed M.E., Selvakumar G.P., Thangavel R., Raikwar S.P., Zaheer S.A., Iyer S.S., Burton C., James D., Zaheer A. Psychological stress–induced immune response and risk of Alzheimer’s disease in veterans from operation enduring freedom and operation Iraqi freedom. Clin. Ther. 2020;42:974–982. doi: 10.1016/j.clinthera.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katamba A., Ogwang M.D., Zamar D.S., Muyinda H., Oneka A., Atim S., Jongbloed K., Malamba S.S., Odongping T., Friedman A.J., Spittal P.M., Sewankambo N.K., Schechter M.T. Cango Lyec (healing the elephant): HIV incidence in post-conflict Northern Uganda. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staruch R.M.T., Hettiaratchy S. Warzone trauma and surgical infections. Surg. 2019;37:58–63. doi: 10.1016/j.mpsur.2018.12.001. [DOI] [Google Scholar]
- 11.Han C., Hong Y.-C. Fetal and childhood malnutrition during the Korean War and metabolic syndrome in adulthood. Nutrition. 2019;62:186–193. doi: 10.1016/j.nut.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Gavia-García G., González-Martínez H., Miliar-García Á., Bonilla-González E., Rosas-Trejo M. de L.Á., Königsberg M., Nájera-Medina O., Luna-López A., González-Torres M.C. Oxidative damage and antioxidant defense in thymus of malnourished lactating rats. Nutrition. 2015;31:1408–1415. doi: 10.1016/j.nut.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 13.World Food Program COVID-19 will double number of people facing food crises unless swift action is taken. 2020. https://www.wfp.org/news/covid-19-will-double-number-people-facing-food-crises-unless-swift-action-taken
- 14.Rehman U., Shahnawaz M.G., Khan N.H., Kharshiing K.D., Khursheed M., Gupta K., Kashyap D., Uniyal R. Depression, anxiety and stress among Indians in times of Covid-19 lockdown. Community Ment. Heal. J. 2020 doi: 10.1007/s10597-020-00664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asmundson G.J.G., Paluszek M.M., Landry C.A., Rachor G.S., McKay D., Taylor S. Do pre-existing anxiety-related and mood disorders differentially impact COVID-19 stress responses and coping? J. Anxiety Disord. 2020;74:102271. doi: 10.1016/j.janxdis.2020.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahav Y. Psychological distress related to COVID-19 – the contribution of continuous traumatic stress. J. Affect. Disord. 2020 doi: 10.1016/j.jad.2020.07.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S.G., Staudenmeyer A.H., Wickham R., Firmender W.M., Fields L., Miller A.B. War-exposed newcomer adolescent immigrants facing daily life stressors in the United States. Int. J. Intercult. Relat. 2017;60:120–131. doi: 10.1016/j.ijintrel.2017.03.002. [DOI] [Google Scholar]
- 18.Valderrama J., Hansen S.K., Pato C., Phillips K., Knowles J., Pato M.T. Greater history of traumatic event exposure and PTSD associated with comorbid body dysmorphic disorder in a large OCD cohort. Psychiatry Res. 2020;289 doi: 10.1016/j.psychres.2020.112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsur N., Abu-Raiya H. COVID-19-related fear and stress among individuals who experienced child abuse: the mediating effect of complex posttraumatic stress disorder. Child Abus. Negl. 2020 doi: 10.1016/j.chiabu.2020.104694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao F., Tan W., Jiang L., Zhang L., Zhao X., Zou Y., Hu Y., Luo X., Jiang X., McIntyre R.S., Tran B., Sun J., Zhang Z., Ho R., Ho C., Tam W. Do psychiatric patients experience more psychiatric symptoms during COVID-19 pandemic and lockdown? A case-control study with service and research implications for immunopsychiatry. Brain Behav. Immun. 2020;87:100–106. doi: 10.1016/j.bbi.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhotani A., Siddiqui M.I., Almuntashri F., Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xianyu J., Feng J., Yang Y., Tang J., Xie G., Fan L. Correlation of oxidative stress in patients with HBV-induced liver disease with HBV genotypes and drug resistance mutations. Clin. Biochem. 2018;55:21–27. doi: 10.1016/j.clinbiochem.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Abdullahi I.N., Emeribe A.U., Ajayi O.A., Oderinde B.S., Amadu D.O., Osuji A.I. Implications of SARS-CoV-2 genetic diversity and mutations on pathogenicity of the COVID-19 and biomedical interventions. J Taibah Univ Sci. 2020 doi: 10.1016/j.jtumed.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D., Zhang Z., Chu F., Li Y., Jin L., Zhang L., Gao G.F., Wang F.-S. Genetic variation of SARS coronavirus in Beijing hospital. Emerg. Infect. Dis. 2004;10:789–794. doi: 10.3201/eid1005.030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zharko Daniloski N.E.S. 2020. Xinyi Guo, the D614G Mutation in SARS-CoV-2 Spike Increases Transduction of Multiple Human Cell Types, BioRxiv. [DOI] [Google Scholar]
- 27.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reshi M.L., Su Y.-C., Hong J.-R. RNA viruses: ROS-mediated cell death. Int J Cell Biol. 2014;2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., Soares J.W. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guski R. Int. Encycl. Soc. Behav. Sci. Elsevier; 2001. Environmental stress and health; pp. 4667–4671. [DOI] [Google Scholar]
- 31.Manan S., Ullah M.W., Guo Z., Yang G. Impact of COVID-19 on environment sustainability. ES Energy Environ. 2020 doi: 10.30919/esee8c378. [DOI] [Google Scholar]
- 32.Shafiee A., Moradi L., Lim M., Brown J. Coronavirus disease 2019: a tissue engineering and regenerative medicine perspective. Stem Cells Transl. Med. 2020 doi: 10.1002/sctm.20-0197. sctm.20-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Quervain . 2020. The Swiss Corona Stress Study. [DOI] [Google Scholar]
- 36.Picaza Gorrochategi M., Eiguren Munitis A., Dosil Santamaria M., Ozamiz Etxebarria N. Stress, anxiety, and depression in people aged over 60 in the COVID-19 outbreak in a sample collected in Northern Spain. Am. J. Geriatr. Psychiatry. 2020;28:993–998. doi: 10.1016/j.jagp.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daks J.S., Peltz J.S., Rogge R.D. Psychological flexibility and inflexibility as sources of resiliency and risk during a pandemic: modeling the cascade of COVID-19 stress on family systems with a contextual behavioral science lens. J Context. Behav Sci. 2020;18:16–27. doi: 10.1016/j.jcbs.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peltz J.S., Daks J.S., Rogge R.D. Mediators of the association between COVID-19-related stressors and parents’ psychological flexibility and inflexibility: the roles of perceived sleep quality and energy. J Context. Behav Sci. 2020;17:168–176. doi: 10.1016/j.jcbs.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown S.M., Doom J.R., Lechuga-Peña S., Watamura S.E., Koppels T. Stress and parenting during the global COVID-19 pandemic. Child Abus. Negl. 2020 doi: 10.1016/j.chiabu.2020.104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Lithopoulos A., Zhang C.-Q., Garcia-Barrera M.A., Rhodes R.E. Personality and perceived stress during COVID-19 pandemic: testing the mediating role of perceived threat and efficacy. Pers Individ Dif. 2020;110351 doi: 10.1016/j.paid.2020.110351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husky M.M., Kovess-Masfety V., Swendsen J.D. Stress and anxiety among university students in France during Covid-19 mandatory confinement. Compr. Psychiatry. 2020;102 doi: 10.1016/j.comppsych.2020.152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam S.M.D.-U., Bodrud-Doza M., Khan R.M., Haque M.A., Mamun M.A. Exploring COVID-19 stress and its factors in Bangladesh: a perception-based study. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeasmin S., Banik R., Hossain S., Hossain M.N., Mahumud R., Salma N., Hossain M.M. Impact of COVID-19 pandemic on the mental health of children in Bangladesh: a cross-sectional study. Child Youth Serv. Rev. 2020;117 doi: 10.1016/j.childyouth.2020.105277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munawar K., Choudhry F.R. Exploring stress coping strategies of frontline emergency health workers dealing Covid-19 in Pakistan: a qualitative inquiry. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J.Z., Han M.F., Luo T.D., Ren A.K., Zhou X.P. Mental health survey of medical staff in a tertiary infectious disease hospital for COVID-19. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38:192–195. doi: 10.3760/cma.j.cn121094-20200219-00063. [DOI] [PubMed] [Google Scholar]
- 46.Chong M.-Y., Wang W.-C., Hsieh W.-C., Lee C.-Y., Chiu N.-M., Yeh W.-C., Huang O.-L., Wen J.-K., Chen C.-L. Psychological impact of severe acute respiratory syndrome on health workers in a tertiary hospital. Br. J. Psychiatry. 2004;185:127–133. doi: 10.1192/bjp.185.2.127. [DOI] [PubMed] [Google Scholar]
- 47.Maunder R.G., Lancee W.J., Balderson K.E., Bennett J.P., Borgundvaag B., Evans S., Fernandes C.M.B., Goldbloom D.S., Gupta M., Hunter J.J., McGillis Hall L., Nagle L.M., Pain C., Peczeniuk S.S., Raymond G., Read N., Rourke S.B., Steinberg R.J., Stewart T.E., VanDeVelde-Coke S., Veldhorst G.G., Wasylenki D.A. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg. Infect. Dis. 2006;12:1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu P., Liu X., Fang Y., Fan B., Fuller C.J., Guan Z., Yao Z., Kong J., Lu J., Litvak I.J. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS outbreak. Alcohol Alcohol. 2008;43:706–712. doi: 10.1093/alcalc/agn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thau L., Gandhi J., Sharma S. Physiology, cortisol. 2020. http://www.ncbi.nlm.nih.gov/pubmed/30855827 [PubMed]
- 50.Capitanio J.P., Cole S.W. Social instability and immunity in rhesus monkeys: the role of the sympathetic nervous system. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchida Y., Kitayama S., Akutsu S., Park J., Cole S.W. Optimism and the conserved transcriptional response to adversity. Health Psychol. 2018;37:1077–1080. doi: 10.1037/hea0000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 53.Kiecolt-Glaser J.K., Preacher K.J., MacCallum R.C., Atkinson C., Malarkey W.B., Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D., Rebusi N., Heshmati M., Aleyasin H., Warren B.L., Labonté B., Horn S., Lapidus K.A., Stelzhammer V., Wong E.H.F., Bahn S., Krishnan V., Bolaños-Guzman C.A., Murrough J.W., Merad M., Russo S.J. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aschbacher K., O’Donovan A., Wolkowitz O.M., Dhabhar F.S., Su Y., Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxidative Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel R., Rinker L., Peng J., Chilian W.M. React. Oxyg. Species Living Cells. InTech; 2018. Reactive oxygen species: the good and the bad. [DOI] [Google Scholar]
- 58.Günther M., Al Nimer F., Piehl F., Risling M., Mathiesen T. Susceptibility to oxidative stress is determined by genetic background in neuronal cell cultures. ENeuro. 2018;5 doi: 10.1523/ENEURO.0335-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang I.-J., Karmaus W. Oxidative stress-related genetic variants may modify associations of phthalate exposures with asthma. Int. J. Environ. Res. Public Heal. 2017;14:162. doi: 10.3390/ijerph14020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moussa Z., Judeh Z.M.A., Ahmed S.A. Free Radic. Med. Biol. IntechOpen; 2020. Nonenzymatic exogenous and endogenous antioxidants. [DOI] [Google Scholar]
- 61.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A.-F., Teixeira M., Valentine J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nandi A., Yan L.-J., Jana C.K., Das N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019;2019:1–19. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bayindir M., Bayindir E.E. Synergic viral-bacterial co-infection in catalase-deficient COVID-19 patients causes suppressed innate immunity and lung damages due to detrimental elevation of hydrogen peroxide concentration. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3648292. [DOI] [Google Scholar]
- 65.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 66.Fedele D., De Francesco A., Riso S., Collo A. Obesity, malnutrition and trace elements deficiency in the covid-19 pandemic: an overview. Nutrition. 2020:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moro-García M.A., Mayo J.C., Sainz R.M., Alonso-Arias R. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front. Immunol. 2018;9:339. doi: 10.3389/fimmu.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Itri R., Junqueira H.C., Mertins O., Baptista M.S. Membrane changes under oxidative stress: the impact of oxidized lipids. Biophys. Rev. 2014;6:47–61. doi: 10.1007/s12551-013-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lima J.E. 2018. The Role of Glutathione in Viral Diseases of the Central Nervous System. (Glutathione Heal. Dis., InTech). [DOI] [Google Scholar]
- 72.Formichi P., Radi E., Branca C., Battisti C., Brunetti J., Da Pozzo P., Giannini F., Dotti M.T., Bracci L., Federico A. Oxidative stress-induced apoptosis in peripheral blood lymphocytes from patients with POLG-related disorders. J. Neurol. Sci. 2016;368:359–368. doi: 10.1016/j.jns.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 73.Galani I.E., Andreakos E. Neutrophils in viral infections: current concepts and caveats. J. Leukoc. Biol. 2015;98:557–564. doi: 10.1189/jlb.4VMR1114-555R. [DOI] [PubMed] [Google Scholar]
- 74.Pieniazek A., Gwozdzinski K. Apoptosis and necrosis induced by hydrogen peroxide and cyanate in human lymphocytes. Free Radic. Biol. Med. 2017;108:S92. doi: 10.1016/j.freeradbiomed.2017.04.302. [DOI] [Google Scholar]
- 75.Lai J.-F., Zindl C.L., Duffy L.B., Atkinson T.P., Jung Y.W., van Rooijen N., Waites K.B., Krause D.C., Chaplin D.D. Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to mycoplasma pneumoniae. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo C., Ding P., Xie C., Ye C., Ye M., Pan C., Cao X., Zhang S., Zheng S. Potential application of the oxidative nucleic acid damage biomarkers in detection of diseases. Oncotarget. 2017;8:75767–75777. doi: 10.18632/oncotarget.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanjuán R., Domingo-Calap P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016;73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W., Wong S.-K., Li F., Kuhn J.H., Huang I.-C., Choe H., Farzan M. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J. Virol. 2006;80:4211–4219. doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., Hou C., Wang H., Liu J., Yang D., Xu Y., Cao Z., Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L.-F., Shi Z., Zhang S., Field H., Daszak P., Eaton B. Review of bats and SARS. Emerg. Infect. Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi P.-Y., Plante J., Liu Y., Liu J., Xia H., Johnson B., Lokugamage K., Zhang X., Muruato A., Zou J., Fontes-Garfias C., Mirchandani D., Scharton D., Kalveram B., Bilello J., Ku Z., An Z., Freiberg A., Menachery V., Xie X., Plante K., Weaver S. 2020. Spike Mutation D614G Alters SARS-CoV-2 Fitness and Neutralization Susceptibility., BioRxiv 2020. [DOI] [Google Scholar]
- 83.Leung J.T., K., Pei Y., Leung G.M., Lam T.T., Wu . 2020. Empirical Transmission Advantage of the D614G Mutant Strain of SARS-CoV-2, MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C., Angyal A., Brown R.L., Carrilero L., Green L.R., Groves D.C., Johnson K.J., Keeley A.J., Lindsey B.B., Parsons P.J., Raza M., Rowland-Jones S., Smith N., Tucker R.M., Wang D., Wyles M.D. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert P.-H., Liu M., Siegrist C.-A. Can successful vaccines teach us how to induce efficient protective immune responses? Nat. Med. 2005;11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 86.Simek M.D., Rida W., Priddy F.H., Pung P., Carrow E., Laufer D.S., Lehrman J.K., Boaz M., Tarragona-Fiol T., Miiro G., Birungi J., Pozniak A., McPhee D.A., Manigart O., Karita E., Inwoley A., Jaoko W., Dehovitz J., Bekker L.-G., Pitisuttithum P., Paris R., Walker L.M., Poignard P., Wrin T., Fast P.E., Burton D.R., Koff W.C. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar R., Qureshi H., Deshpande S., Bhattacharya J. Broadly neutralizing antibodies in HIV-1 treatment and prevention. Ther. Adv. Vaccines Immunother. 2018;6:61–68. doi: 10.1177/2515135518800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bandegi A.R., Rashidy-Pour A., Vafaei A.A., Ghadrdoost B. Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull. 2014;4:493–499. doi: 10.5681/apb.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olaniyan M.F., Ojediran T.B., Olayinka G.S. Evidence of systemic responses to viral pathogens using malondialdehyde, tumor necrosis factor alpha and superoxide dismutase. Int J Clin Exp Physiol. 2020;7:18–21. doi: 10.5530/ijcep.2020.7.1.5. [DOI] [Google Scholar]
- 91.Khedr M.A., El-Araby H.A., Konsowa H.A.-S., Sokar S.S., Mahmoud M.F., Adawy N.M., Zakaria H.M. Glutathione peroxidase and malondialdehyde in children with chronic hepatitis C. Clin Exp Hepatol. 2019;5:81–87. doi: 10.5114/ceh.2019.83161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pratt D.A., Tallman K.A., Porter N.A. Free radical oxidation of polyunsaturated lipids: new mechanistic insights and the development of peroxyl radical clocks. Acc. Chem. Res. 2011;44:458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pisetsky D.S. Evolving story of autoantibodies in systemic lupus erythematosus. J. Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ściskalska M., Zalewska M., Grzelak A., Milnerowicz H. The influence of the occupational exposure to heavy metals and tobacco smoke on the selected oxidative stress markers in smelters. Biol. Trace Elem. Res. 2014;159:59–68. doi: 10.1007/s12011-014-9984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aschbacher K., Kornfeld S., Picard M., Puterman E., Havel P.J., Stanhope K., Lustig R.H., Epel E. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samarghandian S., Farkhondeh T., Samini F., Borji A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem. Res. Int. 2016;2016:1–7. doi: 10.1155/2016/2645237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melis J.P.M., Kuiper R.V., Zwart E., Robinson J., Pennings J.L.A., van Oostrom C.T.M., Luijten M., van Steeg H. Slow accumulation of mutations in Xpc−/− mice upon induction of oxidative stress. DNA Repair (Amst) 2013;12:1081–1086. doi: 10.1016/j.dnarep.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun G., Xu X., Wang Y., Shen X., Chen Z., Yang J. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect. Immun. 2008;76:4405–4413. doi: 10.1128/IAI.00575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saunders J., Smith T., Stroud M. Malnutrition and undernutrition. Medicine (Baltimore) 2019;47:152–158. doi: 10.1016/j.mpmed.2018.12.012. [DOI] [Google Scholar]
- 100.UNICEF, WHO World Bank Group. Joint malnutrition estimates, 2020 edition. 2020. https://www.who.int/publications/i/item/jme-2020-edition
- 101.World Food Program 2020 - Global report on food crises. 2020. https://www.wfp.org/publications/2020-global-report-food-crises
- 102.Huizar M.I., Arena R., Laddu D.R. The global food syndemic: the impact of food insecurity, malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.UNICEF Situation tracking for COVID-19 socio-economic impacts. 2020. https://data.unicef.org/resources/rapid-situationtracking-covid-19-socioeconomic-impacts-data-viz/ June, 2020.
- 104.Headey D., Heidkamp R., Osendarp S., Ruel M., Scott N., Black R., Shekar M., Bouis H., Flory A., Haddad L., Walker N. Standing together for nutrition consortium, impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet. 2020;396:519–521. doi: 10.1016/S0140-6736(20)31647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spoede E., Corkins M.R., Spear B.A., Becker P.J., Gunnell Bellini S., Hoy M.K., Piemonte T.A., Rozga M. Food insecurity and pediatric malnutrition related to under- and overweight in the United States: an evidence analysis center systematic review. J. Acad. Nutr. Diet. 2020;20:30283–30285. doi: 10.1016/j.jand.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 106.Farhadi S., Ovchinnikov R. The relationship between nutrition and infectious diseases: a review. J. Biomed. Biotechnol. 2018;2:168. doi: 10.4103/bbrj.bbrj_69_18. [DOI] [Google Scholar]
- 107.Bourke C.D., Berkley J.A., Prendergast A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–398. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.França T., Ishikawa L., Zorzella-Pezavento S., Chiuso-Minicucci F., da Cunha M., Sartori A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009;15:374–390. doi: 10.1590/S1678-91992009000300003. [DOI] [Google Scholar]
- 109.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Handu D., Moloney L., Rozga M., Cheng F.W. Malnutrition care during the COVID-19 pandemic: considerations for registered dietitian nutritionists. J. Acad. Nutr. Diet. 2020 doi: 10.1016/j.jand.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beck M.A. Antioxidants and viral infections: host immune response and viral pathogenicity. J. Am. Coll. Nutr. 2001;20:384S–388S. doi: 10.1080/07315724.2001.10719172. discussion 396S-397S. [DOI] [PubMed] [Google Scholar]
- 112.Xu F., Li X., Niu W., Ma G., Sun Q., Bi Y., Guo Z., Ren D., Hu J., Yuan F., Yuan R., Shi L., Li X., Yu T., Yang F., He L., Zhao X., He G. Metabolomic profiling on rat brain of prenatal malnutrition: implicated for oxidative stress and schizophrenia. Metab. Brain Dis. 2019;34:1607–1613. doi: 10.1007/s11011-019-00468-3. [DOI] [PubMed] [Google Scholar]
- 113.Shrivastava K., Gaur A., Ambey R. Oxidative stress in children with severe acute malnutrition between 6 months to 5 years of age. Int. J. Contemp. Pediatr. 2018;5:1327. doi: 10.18203/2349-3291.ijcp20182434. [DOI] [Google Scholar]